UNIT 19: COVALENT BOND AND MOLECULAR STRUCTURES

As you can see from the picture above, Oxygen is the big buff creature
with the tattoo of “O” on its arm. The little bunny represents a Hydrogen
atom. The blue and red bow tied in the middle of the rope, pulled by the
two creatures represents the shared pair of electrons, a single bond.Because the Hydrogen atom is weaker, the shared pair of electrons will be
pulled closer to the Oxygen atom.1. Suggest the measure used in Chemistry to describe the strength
dedicated to oxygen, the stronger.2. Suppose that the rope being pulled represents a single covalent bond.
The electron contributed by hydrogen, the weaker, will be transferred
to oxygen the stronger. If not, why?3. Suppose again that we have two oxygens. They have the same
strengths. What will happen to the pulled rope, or the shared pair of
electrons?4. Suggest a reason why, from the figure, one oxygen needed sharing
with two hydrogens to form water.5. Conclude about the possible types of covalent bonds.
19.1. Theories on the formation of covalent bond
Activity 19.1
1. Referring to the types of bonding already known, let us base on the
electrovalent (ionic) bonding. Suppose that potassium metal and
chlorine atom combine to form potassium chloride. Recall that each
of those atoms engages in that bonding to get stability. How atoms
in electrovalent bonding get the stability?2. In covalent bonding, the same purpose remains but each atom
contributes the same number of electrons to share in bonding. This
is achieved by atoms of equal or close electronegativities.a) Draw the diagram to show the outer Bohr energy level for each
of the following: Hydrogen (Z = 1), chlorine (Z = 17), potassium
(Z = 19) and nitrogen (Z = 7).b) Basing on the information given, construct the H2, Cl2 and NH3
molecules by only showing the electrons on the outermost shell.
Be informed that only the unpaired (single) electrons need to
participate in the bonding. The paired ones are stable.The sharing of pair of electrons between two atoms is referred to as a
covalent bond. Normally, each atom that is participating in the covalent
bond formation, contributes equal number of electrons to form pair(s) of
electrons. The pair of electrons shared between the atoms is also known as
bond pair.The bond pair is strongly attracted by the nuclei of two atoms and thus by
reducing the potential energy of them. This is the driving force of formation of
covalent bond, which stabilizes the two atoms.If two atoms share only one bond pair, that bond is referred to as a single
bond. If two bond pairs are shared, that is known as a double bond. Likewise,
a triple bond is formed when the atoms share three bond pairs.A covalent bond is formed between two atoms when their electronegativity
difference is less than 1.7 on Pauling’s scale. Usually it is formed between
two non-metals. For example;• H2 molecule: The electronegativity difference is zero.
• Cl2 molecule: The electronegativity difference is zero.
• Hydrogen chloride (HCl): The electronegativity difference between
them is 3.5 - 2.1 = 1.4.
• Ammonia (NH3): The electronegativity difference between them is
3.0 - 2.1 = 0.9.
• H2O molecule: The electronegativity difference between them is
3.5 - 2.1 = 1.4.To explain the formation of covalent bond, a simple qualitative model was
developed by Gilbert Newton Lewis in 1916.According to this model:
• Octet rule:The inert gas atoms with 8 electrons in their outer shell
(also known as valence shell) are highly stable. The Helium atom with2 electrons in its outer shell is also stable.
• Hence every atom tries to get nearest inert gas configuration by sharing
electrons.
• The bond formed due to sharing of electrons is otherwise known as a
covalent bond.
• Only the electrons in the valence shell are contributed for sharing.
The inner electrons, which are also known as core electrons do not
participate in the bond formation.
• In the formation of covalent bond between two atoms, each atom
contributes its valence electrons to form pair(s) of electrons, which in
turn is/are shared by both of them.• Due to sharing of electrons, each atom gets nearest inert gas
configuration.• Covalency: The number of electrons contributed by the atom of an
element in the formation of covalent compound is known as covalency
of that element.• In Lewis dot model, the electrons in the valence shell of the atom
are shown as dots around it.19.1.1. Lewis structures using octet rule
Lewis structures (also known as Dot and cross structures, Lewis dot diagrams,
Lewis dot formulas, Lewis dot structures, and electron-dot structures) are
diagrams that show the bonding between atoms of a molecule and the
lone pairs of electrons that may exist in the molecule. A Lewis structure
can be drawn for any covalently bonded molecule, as well as coordination
compounds.The Lewis Structure was named after Gilbert Newton Lewis, who introduced
it in his 1916 article “The Atom and the Molecule”.Lewis structures extend the concept of the electron dot diagram by adding
lines between atoms to represent shared pairs in a chemical bond.Lewis structures show each atom and its position in the structure of the
molecule using its chemical symbol. Lines are drawn between atoms that
are bonded to one another (pairs of dots canbe used instead of lines).
Excess electrons that form lone pairs are represented as pairs of dots, and
are placed next to the atoms.How to draw Lewis Structures
Let us use the nitrate ion (NO3-) as a typical example. An outline of how to
determine the “best” Lewis structure for NO3- is given below:1. Determine the total number of valence electrons in a molecule.

2. Draw a skeleton for the molecule which connects all atoms using
only single bonds. In simple molecules, the atom with the most
available sites for bonding is usually placed central. The number of
bonding sites is determined by considering the number of valence
electrons and the ability of an atom to expand its octet.As you become better, you will be able to recognize that certain groups
of atoms prefer to bond together in a certain way!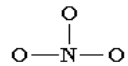
3. Of the 24 valence electrons in NO3-, 6 were required to make the
skeleton. Consider the remaining 18 electrons and place them so
as to fill the octets of as many atoms as possible (start with
the most electronegative atoms first then proceed to the more
electropositive atoms).
4. Are the octets of all the atoms filled? If not then fill the remaining
octets by making multiple bonds (make a lone pair of electrons,
located on a more electronegative atom, into a bonding pair of
electrons that is shared with the atom that is electron deficient).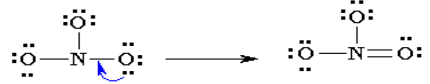
5. Check that you have the lowest formal charges (F.C.) possible for
all the atoms, without violating the octet rule; F.C. = (valence e-) - (1/2
bonding e-) - (lone electrons).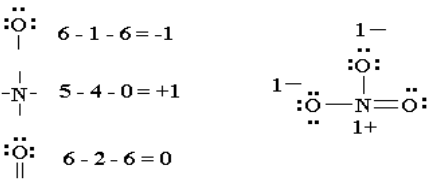
6. Thus the Lewis structure of NO3- ion can be written in the following
ways: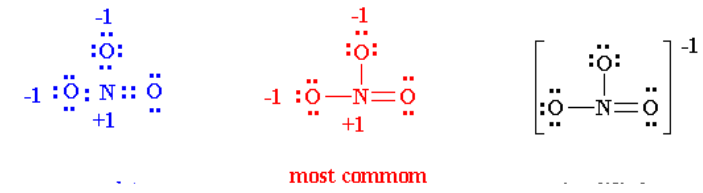
19.1.2. Lewis structures of unusual compounds that do not obey
Octet RuleThere are three general ways in which the octet rule breaks down:
• Molecules with an odd number of electrons
• Molecules in which an atom has less than an octet
• Molecules in which an atom has more than an octet1. Odd number of electrons
Consider the example of the Lewis structure for the molecule nitrous oxide
(NO):
• Total electrons: 6 + 5 = 11
• Bonding structure:
• Octet on “outer” element:
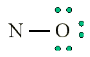
• Remainder of electrons (11-8 = 3) on “central” atom:
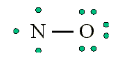
There are currently 5 valence electrons around the nitrogen. A double bond
would place 7 around the nitrogen, and a triple bond would place 9 around
the nitrogen. We appear unable to get an octet around each atom.2. Less than an octet (most often encountered with elements of Boron
and Beryllium)Consider the example of the Lewis structure for boron trifluoride (BF3):
• Add electrons (3 x 7) + 3 = 24
• Draw connectivities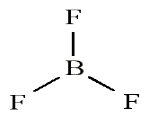
• Add octets to outer atoms:
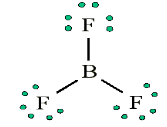
• Add extra electrons (24 – 24 = 0) to central atom:
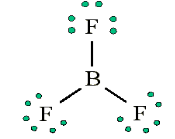
• Does central electron have octet? No, it has 6 electrons. Add a multiple
bond (double bond) to see if central atom can achieve an octet: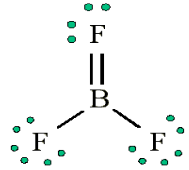
The central Boron now has an octet (there would be three resonance Lewis
structures).However, in this structure with a double bond the fluorine atom is sharing
extra electrons with the boron.The fluorine would have a positive ‘+’ partial charge, and the boron a
negative ‘-’ partial charge, this is inconsistent with the electronegativities
of fluorine and boron. Thus, the structure of BF3 with single bonds, and 6
valence electrons around the central boron is the most likely structure.BF3 reacts strongly with compounds which have an unshared (lone) pair
of electrons which can be used to form a bond with the boron. Example:
Reaction of BF3 with ammonia.
3. More than an octet (most common example of exceptions to the
Octet Rule)
PCl5 is a legitimate compound, whereas NCl5 is not.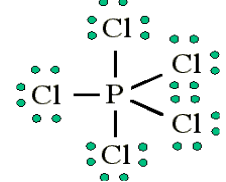
Expanded valence shells are observed only for elements in period 3 (i.e.
n=3) and beyond.The ‘octet’ rule is based upon available ns and np-orbitals for valence
electrons (2 electrons in the s orbitals, and 6 in the p orbitals). Beginning
with the n=3 principle quantum number, the d-orbitals become available
(l=2).“The orbital diagram for the valence shell of phosphorous is:

“Third period elements occasionally exceed the octet rule by using their
empty d-orbitals to accommodate additional electrons.”Size is also an important consideration: “The larger the central atom, the larger
the number of electrons which can surround it”. Expanded valence shells
occur most often when the central atom is bonded to small electronegative
atoms, such as F, Cl and O.Example: Draw the Lewis structure for ICl4-
• Count up the valence electrons: 7 + (4 x 7) + 1 = 36 electrons
• Draw the connectivities: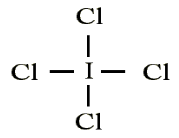
• Add octet of electrons to outer atoms:
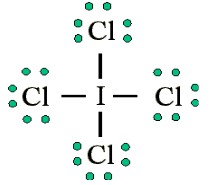
• Add extra electrons (36-32=4) to central atom:
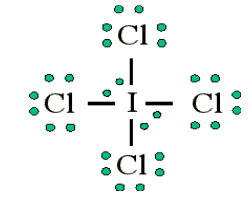
The ICl4- ion thus has 12 valence electrons around the central Iodine (in the
5d orbitals)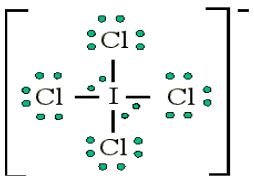
Other examples include: PCl5 and SF6
Application activity 19.1
1. Make a clear definition of the covalent bond.
2. For each of the following, write the electron configuration and
choose one which violates the Octet Rule?
19.2. Coordinate or dative covalent bond
Activity 19.2
The covalent bonding refers to the sharing of electrons to form a bond
pair. There is a special type of covalence where the electrons shared are
given by only one of the bonding atoms.This special type of covalent bonding is described to be “dative”.
Formulate a succinct definition of the “dative covalent bond”.
A dative covalent bond, or coordinate bond is a type of covalent bonding
(i.e., electron sharing) where the shared electron pair(s) are completely
provided by one of the participants in the union, and not by contributions
from the two of them.The contributors of these shared electrons are either neutral molecules
which contain lone pair(s) of electrons on one of their atoms, or negatively
charged groups (radicals) with free electrons to donate. Examples of these
are: H2O, NH3 and CN−.Examples of coordinate bonding:
In the reaction between ammonia and hydrogen chloride a coordinate bond
takes place forming solid ammonium chloride.
NH3 + HCl → NH4Cl
In this reaction the hydrogen ion from the hydrogen chloride leaves its
electrons and gets transferred to the lone pair of electrons on the ammonia
molecule forming ammonium ions (NH4+). This is known as a coordinate
bonding.Seeing that the hydrogen has left its electrons, the chloride will therefore
have a negative charge while the ammonium will have a positive charge.
The diagram below shows the reaction: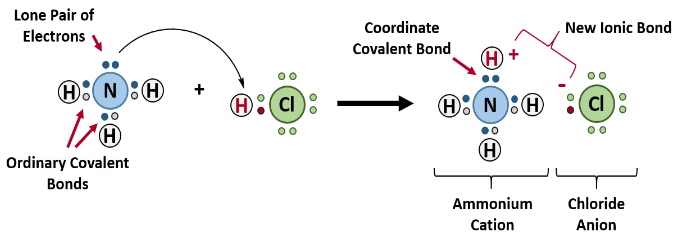
Figure 19.2: Reaction of ammonia with hydrogen chloride
Note: The complete compounds eventually formed comprises of the three
types of bonding, i.e., covalent, co-ordinate and electrovalent. In NH4Cl:
Formation of NH3 (covalency); formation of NH4+ (co-ordinate or dative
bonding); and formation of NH4Cl (electrovalency).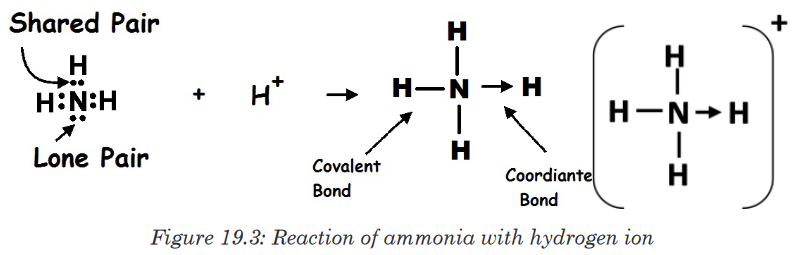
Dative covalent bonds are represented on drawings as an “arrow”, which
usually points from the atom donating the lone pair to the atom accepting it.Another example would be the reaction between ammonia and boron
trifluoride. Boron trifluoride is said to be electron deficient meaning it has
3 pairs of electrons at its bonding level but it is capable of having four pairs.
In this reaction the ammonia is used to supply this extra lone pair.A coordinate bond is formed where the lone pair from the nitrogen moves
toward the boron. The end containing the nitrogen will therefore become
more positive while the boron end will become more negative because it has
received electrons.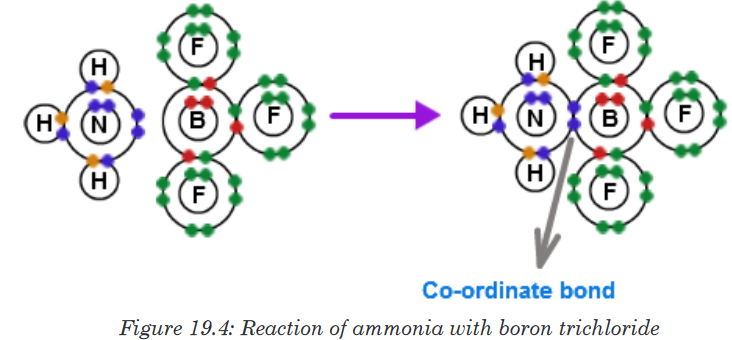
Application activity 19.2
1. Give the difference and the similarity between a dative covalent
bond and the normal covalent bond.2. An aluminium chloride molecule reacts with a chloride ion to form
the AlCl4− ion.a) Name the type of bond formed in this reaction.
b) Explain how this type of bond is formed in the AlCl4− ion.3. Co-ordinate bonding can be described as dative covalency.
a) In this context, what is the meaning of each of the terms covalency
and dative?
b) Write an equation for a reaction in which a co-ordinate bond is
formed.19.3. Overlap of atomic orbitals to form covalent bonds
Activity 19.3
There are different theories describing the formation of a covalent bond.
For your own, basing on the knowledge of the composition of the atom,
formulate a paragraph which describe how two neutral atoms can be
bound together to form a covalent bond (Include the way there will be
attracted or repelled and what make them to remain together)Covalent bonding occurs when atoms share electrons (Lewis Model),
concentrating electron density between nuclei. The build-up of electron
density between two nuclei occurs when a valence atomic orbital of one
atom merges with that of another atom (Valence Bond Theory).The orbitals share a region of space, i.e. they overlap. The overlap of orbitals
allows two electrons of opposite spin to share the common space between
the nuclei, forming a covalent bond.The simplest case to consider is the hydrogen molecule, H2. When we say
that the two electrons from each of the hydrogen atoms are shared to form
a covalent bond between the two atoms, what we mean in Valence Bond
Theory terms is that the two spherical 1s orbitals overlap, allowing the two
electrons to form a pair within the two overlapping orbitals.
These two electrons are now attracted to the positive charge of both of the
hydrogen nuclei, with the result that they serve as a sort of “chemical glue”
holding the two nuclei together.How far apart are the two nuclei? That is a very important issue to consider.
• If they are too far apart, their respective 1s-orbitals cannot overlap,
and thus no covalent bond can form - they are still just two separate
hydrogen atoms.• As they move closer and closer together, orbital overlap begins to
occur, and a bond begins to form. This lowers the potential energy of the
system, as new, attractive positive-negative electrostatic interactions
become possible between the nucleus of one atom and the electron
of the second. However, something else is happening at the same
time: as the atoms get closer, the repulsive positive-positive interaction
between the two nuclei also begins to increase.• When the two nuclei are‘too close’, we have a very unstable, high-
energy situation.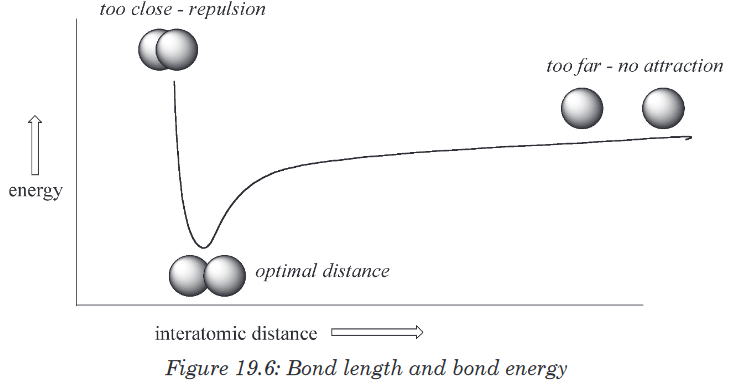
There is a defined optimal distance between the nuclei in which the potential
energy is at a minimum, meaning that the combined attractive and repulsive
forces add up to the greatest overall attractive force. This optimal inter-
nuclear distance is the bond length. For the H2 molecule, this distance is
74x10-12 meters, or 0.74 Å (Å means angstrom, or 10-10 meters).Likewise, the difference in potential energy between the lowest state (at
the optimal inter-nuclear distance) and the state where the two atoms are
completely separated is called the bond energy. For the hydrogen molecule,
this energy is equal to about 104 kcal/mol.Every covalent bond in a given molecule has a characteristic length and
strength. Most covalent bonds in organic molecules range in strength from
just under 100 kcal/mol (for a carbon-hydrogen bond in ethane, for example)
up to nearly 200 kcal/mol.Application activity 19.3
1. Explain the following terms:
a) Bond length
b) Bond energy
2. Account for the Valence Bond Theory.
3. Describe how the Lewis and Valence Bond Theories complement
each other in explaining the formation of a covalent bond.19.4. The concept of valence bond theory and formation of
(σ) and (π) bondsActivity 19.4
1. Recall and draw the shapes of s-orbital and p-orbital.
2. Using drawing, try to show different possible combinations of those
orbitals overlapping.Earlier we saw that covalent bonding requires the sharing of electrons
between two atoms, so that each atom can complete its valence shell. But
how does this sharing process occur? Remember that we can only estimate
the likelihood of finding an electron in a certain area as a probability. In
chemistry, valence bond (VB) theory is one of two basic theories—along
with molecular orbital (MO) theory—that use quantum mechanics to explain
chemical bonding.According to this theory, a covalent bond is formed when two orbitals overlap
(share the same space) to produce a new combined orbital containing two
electrons of opposite spin.The valence bond theory was proposed by Heitler and London to explain
the formation of covalent bond quantitatively using Quantum Mechanics.
Later on, Linus Pauling improved this theory by introducing the concept of
hybridization.The main postulates of this theory are as follows:
• A covalent bond is formed by the overlapping of two half filled valence
atomic orbitals of two different atoms.
• The electrons in the overlapping orbitals get paired and confined
between the nuclei of two atoms.
• The electron density between two bonded atoms increases due to
overlapping. This confers stability to the molecule.
• Greater the extent of overlapping, stronger is the bond formed.
• The direction of the covalent bond is along the region of overlapping of
the atomic orbitals i.e., covalent bond is directional.
• There are two types of covalent bonds based on the pattern of
overlapping as follows:i. σ-bond: The covalent bond formed due to overlapping of atomic
orbital along the inter nucleus axis is called σ-bond. It is a stronger
bond and cylindrically symmetrical. Depending on the types of
orbitals overlapping, the σ-bond is divided into following types:
σs-s bond ii. π-bond:
ii. π-bond:  The covalent bond formed by sidewise overlapping of
The covalent bond formed by sidewise overlapping of
atomic orbitals is called π- bond. In this bond, the electron density
is present above and below the inter-nuclear axis. It is relatively a
weaker bond since the electrons are not strongly attracted by the
nuclei of bonding atoms.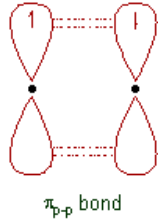
Note: The ‘s’ orbitals can only form σ-bonds, whereas the p, d& f orbitals can
form both σ and π-bonds.Examples:Formation of covalent bonds in oxygen and hydrogen chloride
molecules.
1. O2 molecule:
• The electronic configuration of O in the ground state is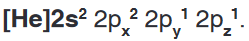
• The half filled 2py orbitals of two oxygen atoms overlap along the
inter-nuclear axis and form σp-p bond.
• The remaining half filled 2pz orbitals overlap laterally to form a πp-p
bond.
• Thus a double bond (one σp-p and one πp-p) is formed between two
oxygen atoms.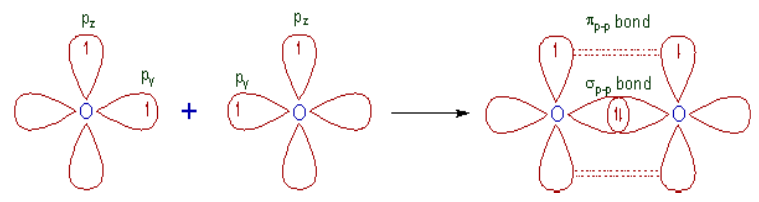

• The half filled 1s orbital of hydrogen overlap with the half filled 3pz
atomic orbital of chlorine atom along the inter-nuclear axis to form a
σs-p bond.
Need for modification of valence bond theory
The old version of Valence Bond Theory is limited to diatomic (like N2) and
binary (like HCl) molecules only. It could not explain the structures and bond
angles of molecules with more than three atoms. For example: It could not
explain the structures and bond angles of H2O, NH3 etc.However, in order to explain the structures and bond angles of these
molecules, Linus Pauling modified the Valence Bond Theory using
hybridization concept.Note: Both types of overlapping orbitals can be related to bond order.
• Single bonds have one sigma bond,
• Double bonds consist of one σ and one π-bond,
• Triple bonds contain one σ and two π-bonds.Application activity 19.4
1. In chemistry, valence bond (VB) theory is one of two basic theories—
along with molecular orbital (MO) theory—that use quantum
mechanics to explain chemical bonding. In one sentence, describe
how the covalent bond is formed, according to this theory.2. Explain why the Valence Bond Theory needed to be modified.
3. Describe the types of covalent bond basing on the pattern of
overlapping.19.5. Hybridisation and its types
Activity 19.5
Just as animals can be cross bred to form hybrids (having the
characteristics of each of the intermixed animal), atomic orbitals also can
be intermixed. Make a research to formulate a definition of hybridization
and state the common types of hybridization of the atomic orbitals.19.5.1. Definition of hybridisation
The concept hybridization involves the “cross breeding” of atomic orbitals
to create “new” orbitals. Hence the use of the term “hybrid”: Think of a
hybrid animal which is a cross breed of two species.Hybridization is the process of “intermixing of atomic orbitals of nearly same
energies to form same number of identical and degenerate (having equivalent
energies) new type of orbitals”. Orbitals which are formed in hybridization
process are called hybrid orbitals.During hybridization, the atomic orbitals with different characteristics are
mixed with each other. Hence there is no meaning of hybridization between
same type of orbitals i.e., mixing of two ‘s’ orbitals or two ‘p’ orbitals is not
called hybridization. However orbital of ‘s’ type can mix with the orbitals of
‘p’ type or of ‘d’ type.Keep in mind that only the orbitals of nearer energy values can participate in
the hybridization. Based on the type and number of orbitals, the hybridization
can be subdivided into following types.19.5.2. Types of hybridization
1. sp-Hybridization
Intermixing of one ‘s’ and one ‘p’ orbitals of almost equal energy to give two
identical and degenerate hybrid orbitals is called ‘sp’ hybridization.
These two sp-hybrid orbitals are arranged linearly at by making 180o of
angle.
They possess 50% ‘s’ and 50% ‘p’ character.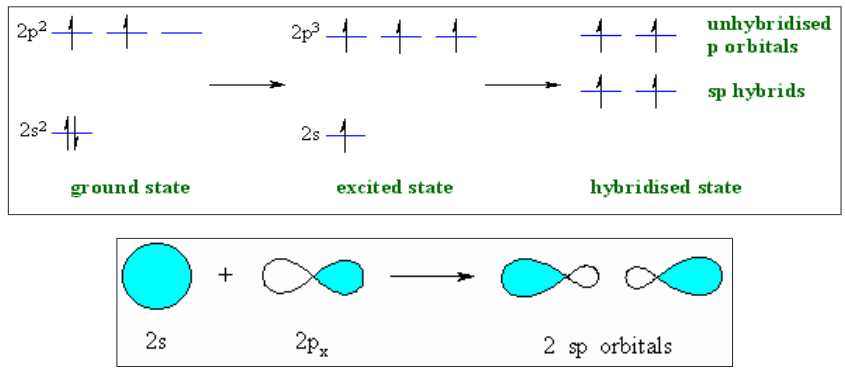
2. sp2-Hybridization
Intermixing of one ‘s’ and two ‘p’ orbitals of almost equal energy to give
three identical and degenerate hybrid orbitals is known as sp2 hybridization.
The three sp2 hybrid orbitals are oriented in trigonal planar symmetry at
angles of 120o to each other.The sp2 hybrid orbitals have 33.3% ‘s’ character and 66.6% ‘p’ character.
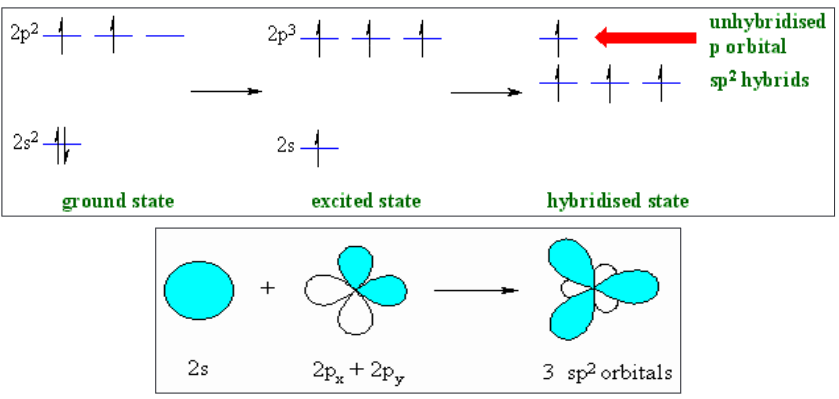
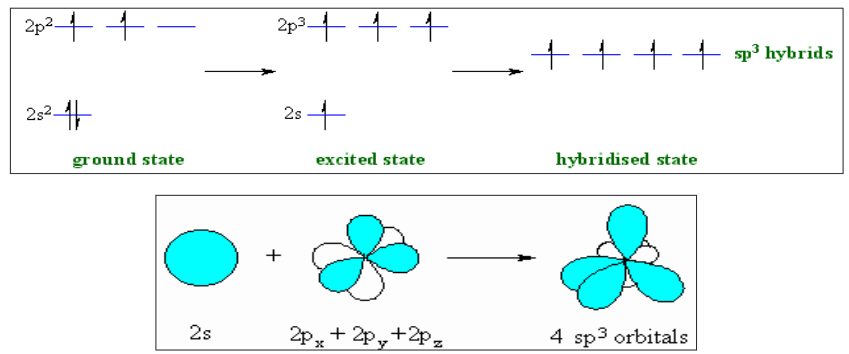
3. sp3-Hybridization
In sp3 hybridization, one ‘s’ and three ‘p’ orbitals of almost equal energy
intermix to give four identical and degenerate hybrid orbitals.These four sp3 hybrid orbitals are oriented in tetrahedral symmetry with
109o28’ angle with each other.The sp3 hybrid orbitals have 25% ‘s’ character and 75% ‘p’ character.
4. sp3d-Hybridization
In sp3d hybridization, one ‘s’, three ‘p’ and one ‘d’ orbitals of almost equal
energy intermix to give five identical and degenerate hybrid orbitals, which
are arranged in trigonal bipyramidal symmetry.Among them, three are arranged in trigonal plane and the remaining two
orbitals are present above and below the trigonal plane at right angles.The five sp3d hybrid orbitals have 20% ‘s’, 60% ‘p’ and 20% ‘d’ characters.

5. sp3d2-Hybridization
Intermixing of one ‘s’, three ‘p’ and two ‘d’ orbitals of almost same
energy by giving six identical and degenerate hybrid orbitals is called
sp3d2 hybridization.These six sp3d2 orbitals are arranged in octahedral symmetry by making 90o
angles to each other. This arrangement can be visualized as four orbitals
arranged in a square plane and the remaining two are oriented above and
below this plane perpendicularly.
Conditions for the hybridization
• Orbitals of same element should take part in the hybridization.
• There should be minimum difference between the orbitals undergoing
hybridization.Characteristics of Hybridization
• During hybridization the number of hybrid orbitals formed is equal to
the number of atomic orbitals involved in hybridization.
• Hybrid orbitals form more stable and stronger bonds than pure atomic
orbitals.
• Hybridization does not take place in isolated atoms and possible in
those atoms which are prior to participate in chemical bonding.Application activity 19.5
1. What is meant by hybridization?
2. Use a table to state five types of the common types of hybridization
and for each; give the name of the shape and bond angles they
present.19.6. VSEPR theory
Activity 19.6
Find out the ball and stick molecular models in the laboratory and try to
construct the following molecules: H2O, HCl, CH4, CO2 and NH3
• For each of the molecule constructed, try to draw it in your exercise
book as it appears.
In order to predictthe geometry of molecules, Nyholm and Gillespie
developed a qualitative model known as Valence Shell Electron Pair
Repulsion Theory (VSEPR Theory). The basic assumptions of this theory
are summarized below.1. The electron pairs in the valence shell around the central atom of a
molecule repel each other and tend to orient in space so as to minimize
the repulsions and maximize the distance between them.2. There are two types of valence shell electron pairs namely, (i) Bond
pairs and (ii) Lone pairs.• Bond pairs are shared by two atoms and are attracted by two nuclei.
Hence they occupy less space and cause less repulsion. It is also
called “sharing pair”.• Lone pairs are not involved in bond formation and are in attraction with
only one nucleus. Hence they occupy more space. As a result, the lone
pairs cause more repulsion.
The order of repulsion between different types of electron pairs is as
follows:Lone pair - Lone pair > Lone Pair - Bond pair > Bond pair - Bond pair
Note: The bond pairs are usually represented by a solid line, whereas
the lone pairs are represented by a lobe with two electrons.
3. In VSEPR theory, the multiple bonds are treated as if they were single
bonds. The electron pairs in multiple bonds are treated collectively
as a single super pair. The repulsion caused by bonds increases with
increase in the number of bonded pairs between two atoms i.e., a triple
bond causes more repulsion than a double bond which in turn causes
more repulsion than a single bond.4. The shape of a molecule can be predicted from the number and type
of valence shell electron pairs around the central atom. When the
valence shell of central atom contains only bond pairs, the molecule
assumes symmetrical geometry due to even repulsions between them.
However the symmetry is distorted when there are also lone pairs along
with bond pairs due to uneven repulsion forces.5. Primary & Secondary effects on bond angle and shape:
i. The bond angle decreases due to the presence of lone pairs, which
cause more repulsion on the bond pairs and as a result the bond
pairs tend to come closer.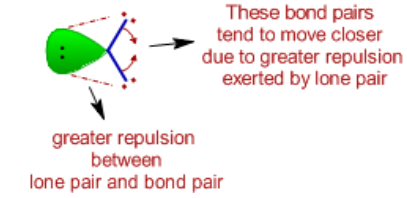
ii. The repulsion between electron pairs increases with increase
in electronegativity of central atom and hence the bond angle
increases. The bond pairs are closer and thus by shortening the
distance between them, which in turn increases the repulsion. Hence
the bonds tend to move away from each other.
However the bond angle decreases when the electronegativities of
ligand atoms are more than that of central atom. There is increase
in the distance between bond pairs since they are now closer to
ligand atoms. Due to this, they tend to move closer resulting in the
decrease in bond angle.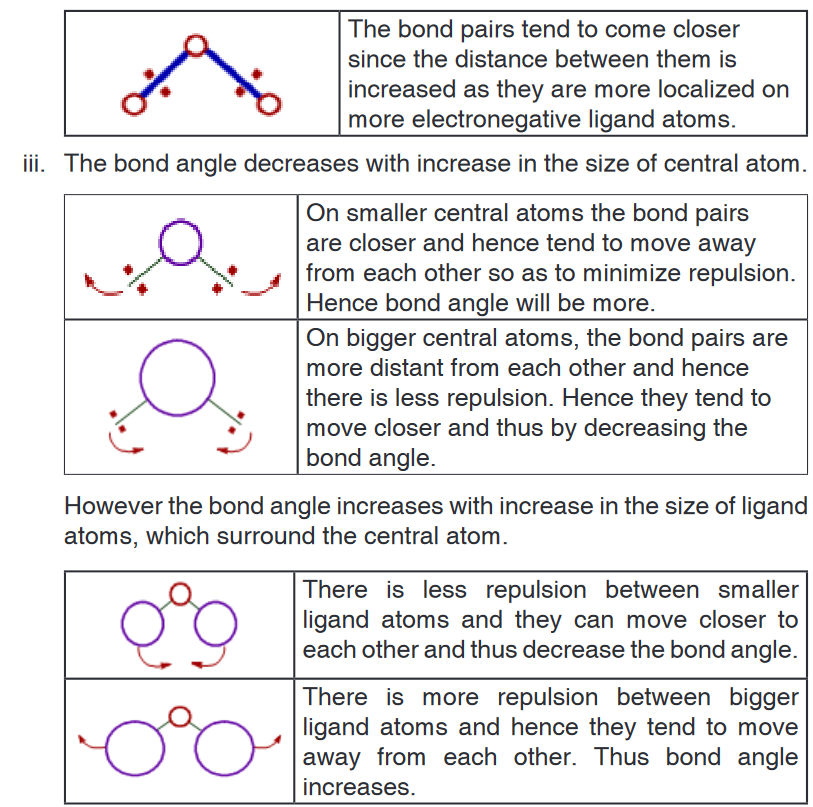
iv. The bond angles are also changed when multiple bonds are present.
It is due to uneven repulsions.6. When there are two or more resonance structures, the VSEPR theory is
applicable to any of such contributing structure.The shape of molecule and also the approximate bond angles can be
predicted from the number and type of electron pairs in the valence shell of
central atom as tabulated below.In the following table the molecule is represented by “AXE” notation, where
A = Central atom
X = Ligand atom bonded to the central atom either by a single bond or
by multiple bond; indicating a bond pair.
E = Lone paiNote:
• The sum of number of ligand atoms (X) and number of lone pairs (E) is
also known as steric number.
• The bond pairs are shown as green colored thick lines, whereas the
lone pairs are shown as point charges using green colored lobes.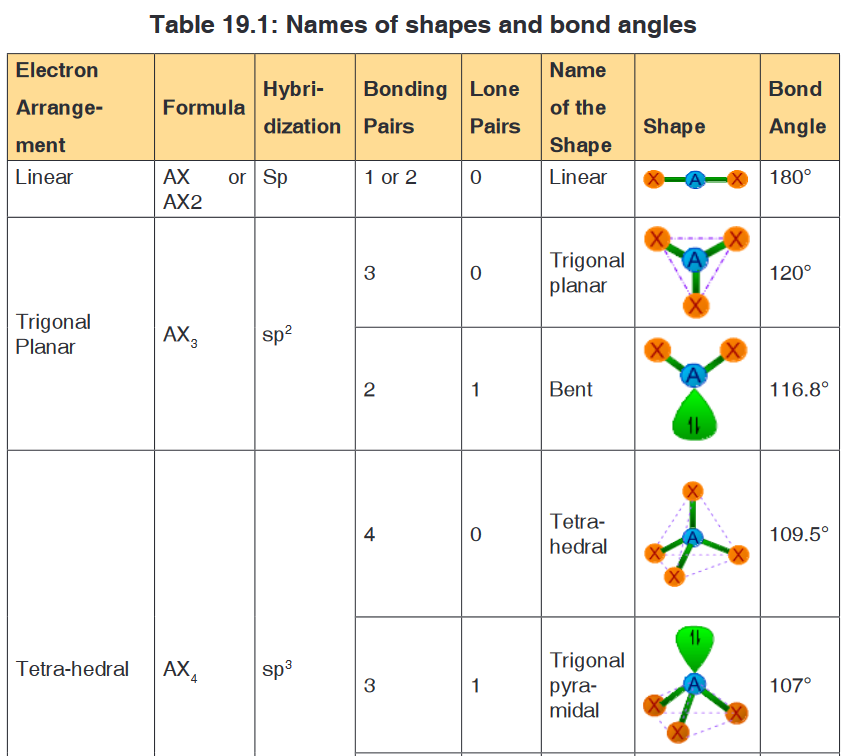

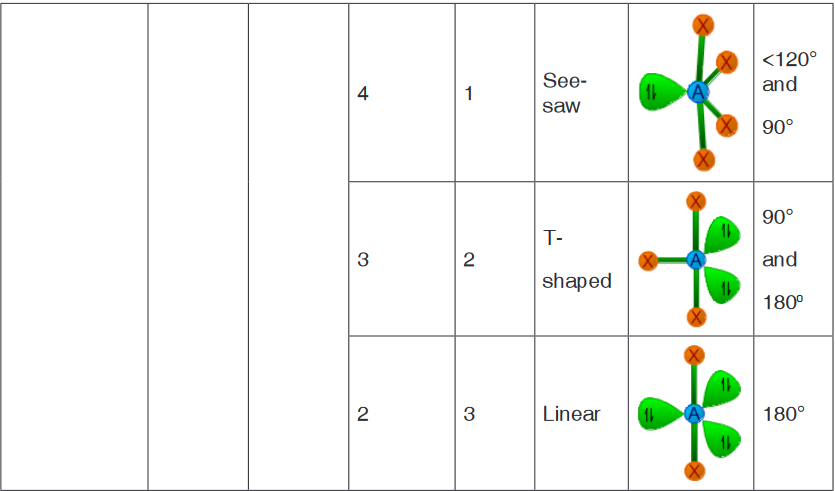

Determination of shape of a molecule
• The first step in determination of shape of a molecule is to write the
Lewis dot structure of the molecule.• Then find out the number of bond pairs and lone pairs in the valence
shell of central atom.• While counting the number of bond pairs, treat multiple bonds as if
they were single bonds. Thus electron pairs in multiple bonds are to be
treated collectively as a single super pair.• Use the above table to predict the shape of molecule based on steric
number and the number of bond pairs and lone pairs.The following table shows some examples for each type of shapes:
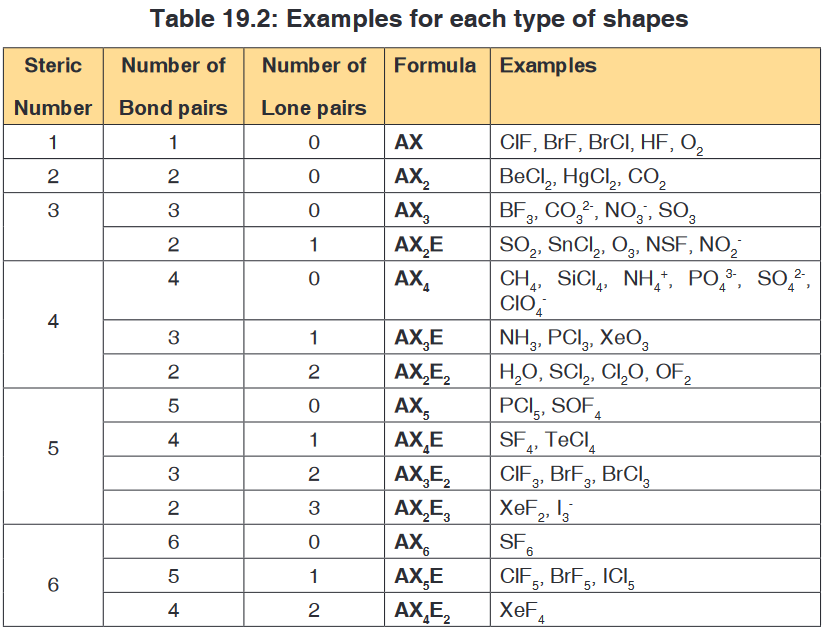
Worked examples
1. Methane (CH4)
• The Lewis structure of methane molecule is:
• There are 4 bond pairs around the central carbon atom in its valence
shell. Hence it has tetrahedral shape with 109o28’ of bond angles.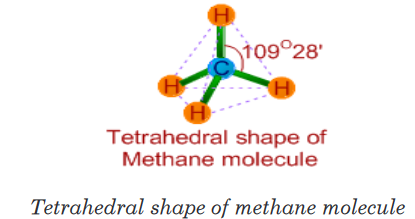
2. Ammonia (PCl3)
• The Lewis structure of ammonia indicates there are three bond pairs
and one lone pair around the central nitrogen atom.
• Since the steric number is 4, its structure is based on tetrahedral
geometry. However, its shape is pyramidal with a lone pair on nitrogen
atom.• The bond angle is decreased from 109o28’ to 107o48’ due to repulsion
caused by lone pair on the bond pairs.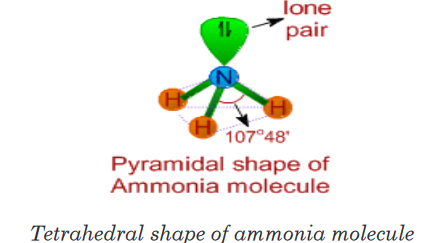
3. Water (H2O)
• It is evident from the Lewis structure of water molecule; there are two
bond pairs and two lone pairs in the valence shell of oxygen. Hence
its structure is based on tetrahedral geometry. However its shape is
angular with two lone pairs on oxygen.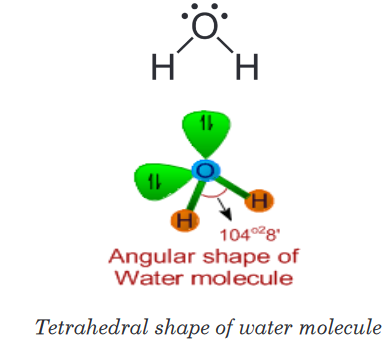
• The bond angle is decreased to 104o28’ due to repulsions caused by
lone pairs on bond pairs. It can be noted that the bond angle decreases
with increase in the number of lone pairs on the central atom.4. Formaldehyde (HCHO)
There are three bond pairs around the central carbon atom. The double
bond between C and O is considered as a single super pair. Hence the
shape of the molecule is trigonal planar and the bond angles are expected
to be equal to 120o.However, the C=O exerts more repulsion on the C-H bond pairs. Hence the
<H-C-H bond angle will be less than 120o and the <H-C-O is greater than
120o.
Application activity 19.6
1. There are two types of valence shell electron pairs such as Bond
pairs and Lone pairs.
a) State and explain these electron pairs.
b) Show the increasing order of repulsion between different types
of electron pairs.2. Questions about covalent bonding.
a) Sketch the shapes of each of the following molecules, showing
any lone pairs of electrons. In each case, state the bond angle(s)
present in the molecule and name the shape.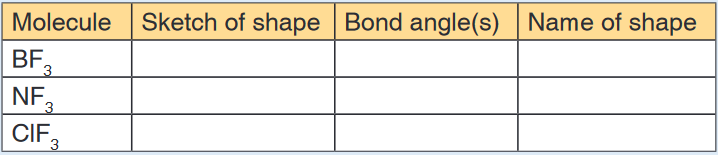
b) State the types of intermolecular force which exist, in the liquid
state, between pairs of BF3 molecules and between pairs of NF3
molecules.
c) Name the type of bond which you would expect to be formed
between a molecule of BF3 and a molecule of NF3. Explain how
this bond is able to form.3. The following diagram shows a hydrogen peroxide molecule.

a) On the diagram above, draw the lone pairs, in appropriate positions,
on the oxygen atoms.b) Indicate, on the diagram, the magnitude of one of the bond angles.
c) When considering electron pair repulsions in molecules, why does a
lone pair of electrons repel more strongly than a bonding pair?4. Phosphorus and nitrogen are in Group V of the Periodic Table and both
elements form hydrides. Phosphine, PH3, reacts to form phosphonium
ions, , in a similar way to that by which ammonia, NH3, forms ammonium
ions,a) Give the name of the type of bond formed when phosphine reacts
with an H+ion. Explain how this bond is formed.b) Draw the shapes, including any lone pairs of electrons, of a phosphine
molecule and of a phosphonium ion. Give the name of the shape
of the phosphine molecule and state the bond angle found in the
phosphonium ion.5. The shape of the molecule BCl3 and that of the unstable molecule CCl2
are shown below.
a) Why is each bond angle exactly 120° in BCl3?
b) Predict the bond angle in CCl2 and explain why this angle is different
from that in BCl3
c) Give the name which describes the shape of molecules having bond
angles of 109° 28’.
Give an example of one such molecule.
6. The shape of the XeF4 molecule is shown below.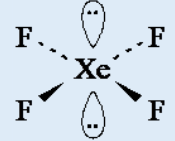
a) State the bond angle in XeF4
b) Suggest why the lone pairs of electrons are opposite each other in
this molecule.c) Name the shape of this molecule, given that the shape describes
the positions of the Xe and F atoms only.7. Copy and complete the following table:

8. Boron, nitrogen and oxygen form fluorides with molecular formulae
BF3,NF3 and OF2a) Draw the shape of each molecule showing the position of lone pairs
if any.b) Give the bond angle in each case, explaining your reasons.
19.7. Polarity of the covalent bond in relation to difference
in electronegativity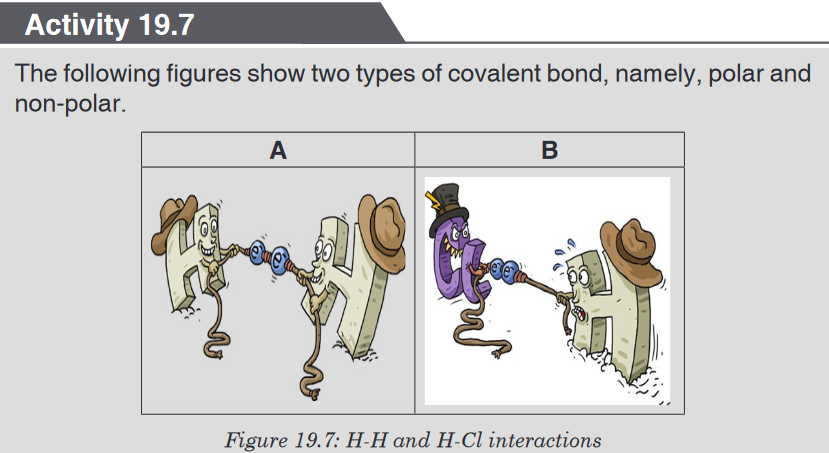
1. Covalent bond is formed between two atoms with similar or close
ability to attract electrons towards themselves, and this is the reason
why they share electrons without being transferred.a) What is the name of the measure used to compare that ability?
b) When the strengths of both atoms are equal, the covalent bond
will be non-polar. Is figure A represents the polar or non-polar
bond? Why?c) Look at the figure B. The atom, in the zone with more electrons,
will have a partial negative charge. In which zone can we label the
partial negative charges?2. Experiment to study the behaviour of water on the charged species.
• Fill a burette with water.
• Open the tap and bring a charged ebonite rod close to the stream of
water running from the jet.
• Observe the direction of water and using a drawing, note the change,
if there is any.A quantity termed ‘electronegativity’ is used to determine the polarity of
the covalent bond; whether a given bond will be non-polar covalent, polar
covalent, or ionic.Electronegativity is defined as the ability of an atom in a particular molecule to
attract electrons to itself (the greater the value, the greater the attractiveness
for electrons).Fluorine is the most electronegative element (electronegativity = 4.0), the
least electronegative is Caesium (electronegativity = 0.7).The bond pair is equally shared in between two atoms when the
electronegativity difference between them is zero or nearer to zero. In this
case, neither of the atoms gets excess of electron density and hence carry no
charge. This is called non-polar covalent bond.However, when there is a considerable difference in the electronegativity,
the bond pair is no longer shared equally between the atoms. It is shifted
slightly towards the atom with higher electronegativity by creating partial
negative charge (represented by δ-) over it, whereas, the atom with less
electronegativity gets partial positive charge (represented by δ+). This type
of bond is also referred to as polar covalent bond.We can use the difference in electronegativitybetween two atoms to gauge
the polarity of the bonding between them.In F2 the electrons areshared equally between the atoms, the bond is non-
polar covalent.In HF the fluorine atom has greater electronegativity than the hydrogen
atom. The sharing of electrons in HF is unequal: the fluorine atom attracts
electron density away from the hydrogen (the bond is thus a polar covalent
bond). The H-F bond can thus be represented as: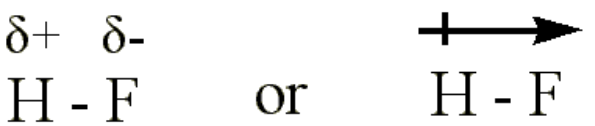
The ‘+’ and ‘-’ symbols indicate partial positive and negative charge
respectively.The arrow indicates the “pull” of electrons off the hydrogen and towards the
more electronegative atom, fluorine.In LiF the much greater relative electronegativity of the fluorine atom
completely strips the electron from the lithium and the result is an ionic bond
(no sharing of the electron).Note:The following is the general thumb rule for predicting the type of bond
based upon electronegativity differences:
• If the electronegativities are equal (i.e. if the electronegativity difference
is 0), the bond is non-polar covalent.• If the difference in electronegativities between the two atoms is greater
than 0, but less than 2.0, the bond is polar covalent.• If the difference in electronegativities between the two atoms is 2.0, or
greater, the bond is ionic.Using the examples used above, we can predict the type of bond as follows:
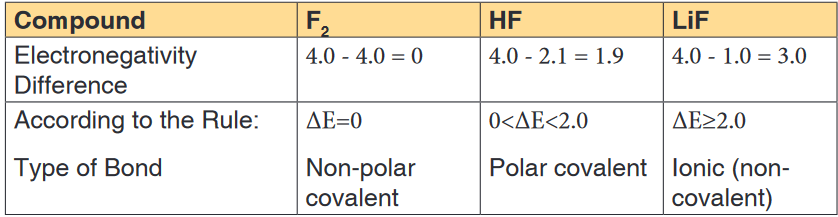
Note: A non-polar molecule is one in which the electrons are distributed
more symmetrically and thus does not have an abundance of charges at
the opposite sides. The charges all cancel out each other. Examples of non-
polar molecules include diatomic molecules, CH4, CO2, C2H4, cyclohexene,
CCl4, etc.Application activity 19.7
1. State what is meant by the term polar bond.
2. Sulphuric acid is a liquid that can be represented by the formula
drawn below.3. The two substances CS2 and HCN have linear molecules but CS2
molecules are non-polar while HCN molecules are polar; explain why
these molecules have different polarities. Support your explanation
with appropriate diagrams.4. A negatively charged rod was brought near a jet of water running
out from a burette. The jet of water was deflected as shown: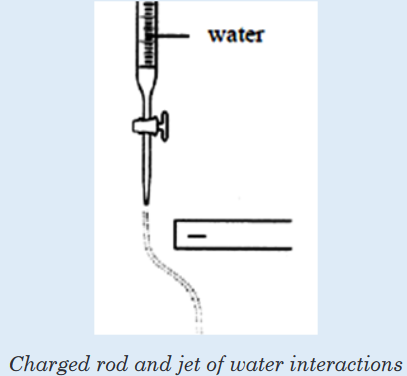
a) With reference to the structure of water, explain why the jet of
water was deflected.b) State the effect on the jet of water if the negatively charged rod is
replaced by a positively charged rod. Explain your answer.c) If hexane is used instead of water and a negatively charged rod
is brought near the liquid jet, would the liquid jet be deflected?
Explain your answer.19.8. Physical properties of covalent structures
Activity 19.8
1. You are provided with the following chemical compounds: PH3,
CO2, H2O and SiO2, Cl2, Br2 and I2 and graphite.
a) Classify them into solid, liquid and gas.
b) What type of bond which held together atoms in the given
compounds they have in common?
c) Deduce from (b) the name describing all those compounds.d) Some of them have very high melting points. Suggest the reason
for this.2. State some points that you know about diamond.
Covalent bonds involve the sharing of electrons so that all atoms have “full
outer shells”. Sometimes in a Covalent Bond, both shared electrons come
from the same atom. This is known as a Dative Covalent Bond. This often
results in the formation of charged molecules.
Covalently bonded substances fall into two main types:
• Simple molecular structures
• Giant covalent structures.19.8.1. Simple molecular structures
Substances composed of relatively small covalently bonded structures are
called Simple Molecular Structures. These contain only a few atoms held
together by strong covalent bonds and can be further categorised into two
types: Individual (which are usually gases like carbon dioxide) and molecular
(which are usually solids like iodine).The physical properties
1. Low melting and boiling points
Simple Molecular Structures tend to have low melting and boiling points
since the forces between molecules (intermolecular forces, which are van
der Waals forces) are quite weak. Little energy is required to separate
the molecules.2. Poor electrical conductivity
There are no charged particles (ions or electrons) delocalized throughout
the molecular crystal lattice to conduct electricity. They cannot conduct
electricity in either the solid or molten state.3. Solubility
They tend to be quite insoluble in water, but this depends on how
polarized the molecule is. The more polar the molecules, the more water
molecules will be attracted to them (some may dissolve in water as a
result of forming hydrogen bonds with it). Molecular crystals tend to
dissolve in non-polar solvents such as alcohol.4. Soft and low density
Van der Waals forces are weak and non-directional. The lattice is readily
destroyed and the crystals are soft and have low density.19.8.2. Giant covalent structures
Sometimes covalently bonded structures can form giant networks, known as
Giant Covalent Structures or Macromolecular Structures.Giant covalent structures contain very many atoms, each joined to adjacent
atoms by covalent bonds. The atoms are usually arranged into giant regular
lattices - extremely strong structures because of the many bonds involved.Properties of giant covalent structures
• Very high melting points. This is because a lot of strong covalent
bonds must be broken. Graphite, for example, has a melting point of
more than 3,600°C.
• Variable electrical conductivity. Diamond does not conduct electricity,
whereas graphite contains free electrons so it does conduct electricity.
Silicon is a semi-conductor – it is midway between non-conductive and
conductive.1. Physical properties of diamond
Diamond is a form (allotrope) of carbon in which each carbon atom is
joined to four other carbon atoms, forming a giant covalent structure. As
a result, diamond is very hard and has a high melting point. It does not
conduct electricity. Diamond is tetrahedral face-centered cubic.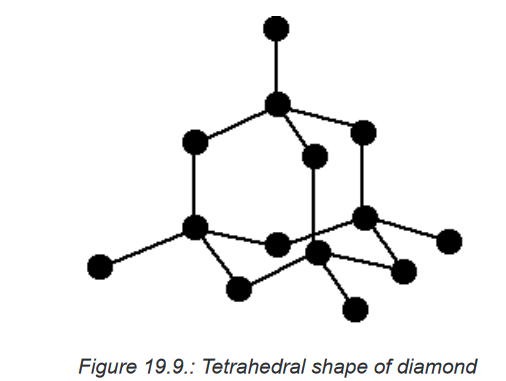
The physical properties of diamond are outlined below.
• It has a very high melting point (almost 4,000°C). Very strong
carbon-carbon covalent bonds have to be broken throughout the
structure before melting occurs.• It is very hard. This is again due to the need to break very strong
covalent bonds operating in 3-dimensions.• It does not conduct electricity. All the electrons are held tightly
between the atoms, and are not free to move.• It is insoluble in water and organic solvents. There are no possible
attractions which could occur between solvent molecules and carbon
atoms which could outweigh the attractions between the covalently
bound carbon atoms.2. Physical properties of graphite
Graphite is another form (allotrope) of carbon in which the carbon atoms
form layers. These layers can slide over each other, so graphite is much
softer than diamond. Each carbon atom in a layer is joined to only three
other carbon atoms in hexagonal rings.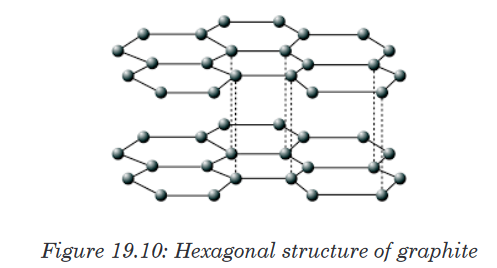
The physical properties of graphite are oulined below.
• It has a high melting point, similar to that of diamond. In order to
melt graphite, it isn’t enough to loosen one sheet from another. You
have to break the covalent bonding throughout the whole structure.• It has a soft, slippery feel, and is used in pencils and as a dry
lubricant for things like locks. You can think of graphite rather
like a pack of cards - each card is strong, but the cards will slide
over each other, or even fall off the pack altogether. When you use
a pencil, sheets are rubbed off and stick to the paper.• It has a lower density than diamond. This is because of the
relatively large amount of space that is “wasted” between the sheets.• It is insoluble in water and organic solvents - for the same reason
that diamond is insoluble. Attractions between solvent molecules
and carbon atoms will never be strong enough to overcome the
strong covalent bonds in graphite.• It conducts electricity. The delocalised electrons are free to move
throughout the sheets. If a piece of graphite is connected into a
circuit, electrons can fall off one end of the sheet and be replaced
with new ones at the other end.3. Physical properties of silicon dioxide
Silica, which is found in sand, has a similar structure to diamond. It is
also hard and has a high melting point, but contains silicon and oxygen
atoms, instead of carbon atoms. Silica or SiO2 is tetrahedral. The fact
that it is a semi-conductor makes it immensely useful in the electronics
industry: most transistors are made of silica.
The physical properties of diamond are oulined below.
• It has a high melting point - varying depending on what the particular
structure is (remember that the structure given is only one of three
possible structures), but around 1700°C. Very strong silicon-oxygen
covalent bonds have to be broken throughout the structure before
melting occurs.• It is hard. This is due to the need to break the very strong covalent
bonds.• It does not conduct electricity. There are not any delocalised
electrons. All the electrons are held tightly between the atoms, and
aren’t free to move.• It is insoluble in water and organic solvents. There are no possible
attractions which could occur between solvent molecules and the
silicon or oxygen atoms which could overcome the covalent bonds in
the giant structure.Application activity 19.8
1. What is meant by the statement that “the electrons in diamond are
localized, whereas graphite has delocalised electrons”.2. Which electrons in graphite are delocalised? How do they affect the
properties of graphite?3. Why is it easy to rub away carbon atoms from graphite?
4. Why is graphite used as lubricant?
5. Diamond and graphite are both forms of carbon.
• Diamond is able to scratch almost all other substances, whereas
graphite may be used as a lubricant.
• Diamond and graphite both have high melting points.
a) Explain each of these properties of diamond and graphite in
terms of structure and bonding.
b) Give one other difference in the properties of diamond and
graphite.6. Iodine and diamond are both crystalline solids at room temperature.
a) Identify one similarity in the bonding, and one difference in the
structures, of these two solids.
b) Explain why these two solids have very different melting points.7. Silicon dioxide has a macromolecular structure. Draw a diagram
to show the arrangement of atoms around a silicon atom in silicon
dioxide. Give the name of the shape of this arrangement of atoms
and state the bond angle.19.9. Intermolecular forces
Activity 19.9
Experiment: To investigate boiling point and to determine the
relation between boiling point and intermolecular forces.Apparatus
• Water, cooking oil (sunflower oil), Glycerine, nail polish remover,
methylated spirits
• Test-tubes and a beaker
• Hot plateMethod
Methylated spirits and nail polish remover are highly flammable. They
will easily catch fire if left near an open flame. For this reason they must
be heated in a water bath. This experiment must be performed in a well
ventilated room.• Place about 20 mL of each substance given in separate test-tubes.
• Half-fill the beaker with water and place on the hot plate.
• Place the test-tubes in the beaker.
• Observe how long each substance takes to boil. As soon as a
substance boils, remove it from the water bath.Results and questions
1. Write down the order in which the substances boiled, starting with
the substance that boiled first and ending with the substance that
boiled last.2. Suggest the explanation for the above order.
Now let us talk about the intermolecular forces that exist between molecules.
Intermolecular forces are much weaker than the intramolecular forces of
attraction but are important because they determine the physical properties
of molecules like their boiling point, melting point, density, and enthalpies of
fusion and vaporization.19.9.1. Definition, types and origin of intermolecular forces
Intermolecular forces are the forces between molecules forces that bind
them together.Intermolecular forces are like the glue that holds molecules together. There
are strong and weak forces; the stronger the force, the more energy is
required to break those molecules apart from each other.Intermolecular forces include (listed from weakest to strongest):
• Van der Waals dispersion forces
• Van der Waals dipole-dipole interactions
• Hydrogen bondingSo, if two molecules are only connected using van der Waals dispersion
forces, then it would require very little energy to break those molecules apart
from each other. On the other hand, if two molecules are connected using
ionic bonds, it takes a whole lot more energy to break those two apart.1. Van der Waals Dispersion Forces
Van der Waals dispersion forces, also called London forces, occur
due to instantaneous dipoles. At any given moment the electrons in a
molecule or atom may not be evenly distributed around the molecule.
If more electrons are on the left side of the molecule than on the right
side, then there will be a slight (partial) negative charge on the left side
of the molecule. The side with fewer electrons will have a slight (partial)
positive charge.These momentary, partial, positive and negative charges are attracted
to each other (like the positive and negative ends on a magnet). This
causes momentary bonds between molecules. We can already see why
these bonds would be so weak, because they only last for a little while.Van der Waals dispersion forces increase as the atomic size increases.
This means that larger molecules will feel more force, thus increasing
the intermolecular forces. So if we have two molecules that are exactly
the same except that one is bigger than the other (such as methane and
ethane), then the intermolecular forces of the bigger one will be stronger
than for the smaller one.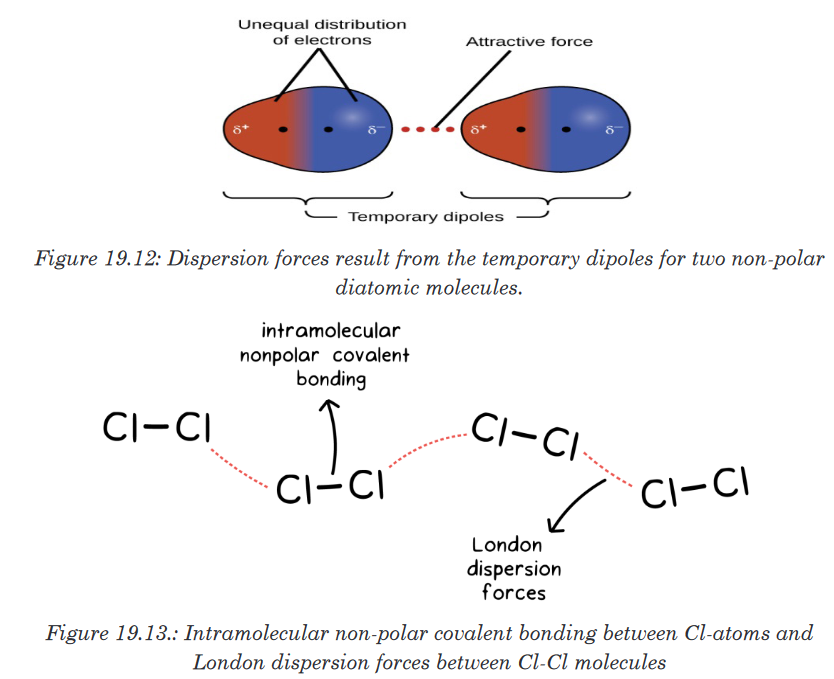
2. Van der Waals Dipole-Dipole Interactions
A partial positive charge and a partial negative charge can be created
between two atoms when there is a difference in electronegativity. These
interactions are called van der Waals dipole-dipole interactions.For example, carbon is less electronegative than oxygen, creating a
partial positive on carbon and a partial negative on oxygen. The dipole
interactions are stronger than the dispersion forces because the oxygen
will almost always have slightly more electrons than the carbon, instead
of constantly changing. There still is not a full negative charge on the
oxygen, or a full positive charge on the carbon. But the partial positive and
negative charges are still enough to attract opposite charges together.The higher the difference in electronegativity, the strong the dipole-
dipole interactions will be. So compounds with a higher electronegativity
difference will have strong intermolecular forces.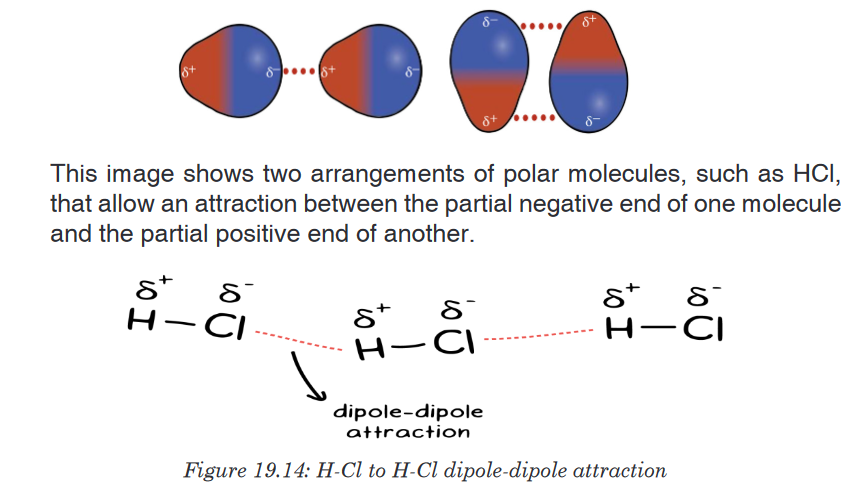
3. Hydrogen bonding
This is a special kind of dipole-dipole interaction that occurs between
a hydrogen atom bonded to a high electronegative atom, specifically
either an oxygen, nitrogen, or fluorine atom. The partially positive end of
hydrogen is attracted to the partially negative end of the oxygen, nitrogen,
or fluorine of another molecule. A hydrogen bond is usually represented
as a dotted line between the hydrogen and the unshared electron pair of
the other electronegative atom.Hydrogen bonding is a relatively strong force of attraction between
molecules, and considerable energy is required to break hydrogen
bonds. This explains the exceptionally high boiling points and melting
points of compounds like water and hydrogen fluoride.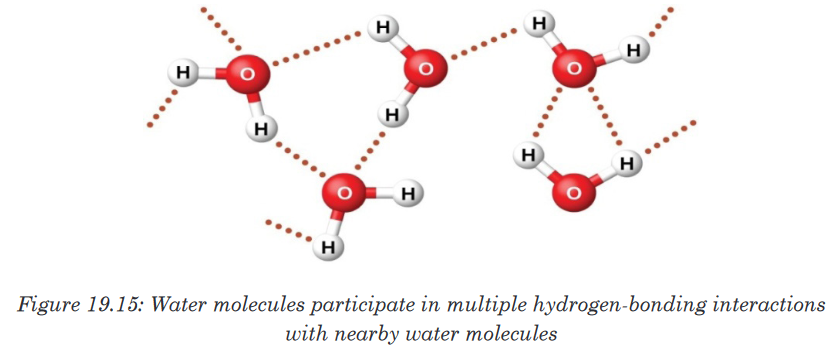
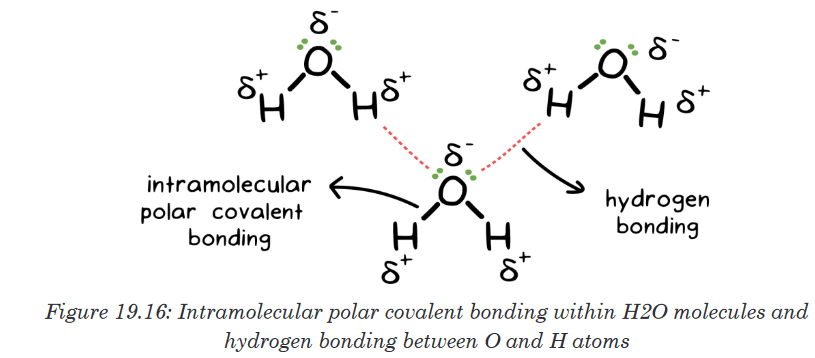
19.9.2. Effect of intramolecular forces on physical properties of
certain moleculesIntermolecular forces control how well molecules stick together. This affects
many of the measurable physical properties of substances:• Melting and Boiling Points
– If molecules stick together more, they will be tougher to break apart
– Stronger intermolecular forces → higher melting and boiling points• Viscosity
– Viscosity is a measure of how well substances flow.
– Stronger intermolecular forces → higher viscosity.• Surface Tension
– Surface tension is a measure of the toughness of the surface of a
liquid
– Stronger intermolecular forces → higher surface tension.• Vapour Pressure
– This is a small amount of gas that is found above all liquids.
– Stronger intermolecular forces → Lower vapour pressure.Note: If you are asked to rank molecules in order of melting point, boiling
point, viscosity, surface tension or vapour pressure, what they are actually
asking is for you to rank them by strength of intermolecular forces (either
increasing or decreasing).Here is the strategy for this:• Look for molecules with hydrogen bonding. They will have the
strongest intermolecular forces.• Look for molecules with dipoles. These will have the next strongest
intermolecular forces.• Larger molecules will have stronger London dispersion forces.
These are the weakest intermolecular forces but will often be the
deciding factor in multiple choice questions.If we use this trend to predict the boiling points for the lightest hydride for
each group, we would expect NH3 to boil at about −120 °C, H2O to boil at
about −80 °C, and HF to boil at about −110 °C. However, when we measure
the boiling points for these compounds, we find that they are dramatically
higher than the trends would predict, as shown in the figure below. The
stark contrast between our naïve predictions and reality provides compelling
evidence for the strength of hydrogen bonding.
These exhibit anomalously high boiling points due to hydrogen bonding.
Hydrogen bonding is important in many chemical and biological processes.
It is responsible for water’s unique solvent capabilities. Hydrogen bondshold complementary strands of DNA together, and they are responsible
for determining the three-dimensional structure of folded proteins including
enzymes and antibodies.
1. An Example: Water
Since oxygen is more electronegative than hydrogen, oxygen pulls the
shared electrons more closely to itself. This gives the oxygen atom a slightly
more negative charge than either of the hydrogen atoms. This imbalance is
called a dipole, causing the water molecule to have a positive and negative
side, almost like a tiny magnet. Water molecules align so the hydrogen on
one molecule will face the oxygen on another molecule. This gives water a
greater viscosity and also allows water to dissolve other molecules that have
either a slightly positive or negative charge.2. Protein Folding
Protein structure is partially determined by hydrogen bonding. Hydrogen
bonds can occur between a hydrogen on an amine and an electronegative
element, such as oxygen on another residue. As a protein folds into place, a
series of hydrogen bond “zips” the molecule together, holding it in a specific
three-dimensional form that gives the protein its particular function.
3. DNA
Hydrogen bonds hold complementary strands of DNA together. Nucleotides
pair precisely based on the position of available hydrogen bond donors
(available, slightly positive hydrogens) and hydrogen bond acceptors
(electronegative oxygens). The nucleotide thymine has one donor and one
acceptor site that pairs perfectly with the nucleotide adenine’s complementary
acceptor and donor site. Cytosine pairs perfectly with guanine through three
hydrogen bonds.Application activity 19.9
1. Define the following and give an example of each:
a) Dispersion force
b) Dipole-dipole attraction
c) Hydrogen bond
2. The table below shows the boiling points of some other hydrogen
halides.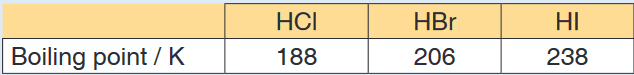
a) Explain the trend in the boiling points of the hydrogen halides from
HCl to HI.b) Give one reason why the boiling point of HF is higher than that of
all the other hydrogen halides.3. The types of intermolecular forces in a substance are identical whether
it is a solid, a liquid, or a gas. Why then does a substance change
phase from a gas to a liquid or to a solid?4. Why do the boiling points of the noble gases increase in the order He
< Ne < Ar < Kr < Xe?5. Neon and HF have approximately the same molecular masses. Explain
why the boiling points of Neon and HF differ.6. Arrange each of the following sets of compounds in order of increasing
boiling point temperature:
a) HCl, H2O, SiH4
b) F2, Cl2, Br2
c) CH4, C2H6, C3H8
d) O2, NO, N27. The molecular mass of butanol, C4H9OH, is 74.14; that of ethylene
glycol, CH2(OH)CH2OH, is 62.08, yet their boiling points are 117.2 °C
and 174 °C, respectively. Explain the reason for the difference.8. On the basis of intermolecular attractions, explain the differences in
the boiling points of n–butane (−1 °C) and chloroethane (12 °C), which
have similar molar masses.9. On the basis of dipole moments and/or hydrogen bonding, explain in
a qualitative way the differences in the boiling points of acetone (56.2
°C) and 1-propanol (97.4 °C), which have similar molar masses.10. The melting point of H2O(s) is 0 °C. Would you expect the melting
point of H2S(s) to be −85 °C, 0 °C, or 185 °C? Explain your answer.11. Explain why a hydrogen bond between two water molecules is weaker
than a hydrogen bond between two hydrogen fluoride molecules.12. Under certain conditions, molecules of acetic acid, CH3COOH, form
“dimers,” pairs of acetic acid molecules held together by strong
intermolecular attractions:
Draw a dimer of acetic acid, showing how two CH3COOH molecules
are held together, and stating the type of intermolecular force that is
responsible.13. Proteins are chains of amino acids that can form in a variety of
arrangements, one of which is a helix. What kind of intermolecular
force is responsible for holding the protein strand in this shape? On
the protein image, show the locations of the intermolecular forces that
hold the protein together: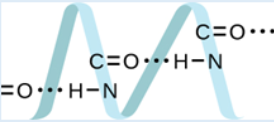
14. Identify the intermolecular forces present in the following solids:
a) CH3CH2OH
b) CH3CH2CH3
c) CH3CH2Cl
15. Explain why non-polar molecules usually have much lower surface
tension than polar ones.
1. Pour water into a small jar that has a tight-fitting lid until the jar is about
a third full.2. Add an equal amount of vegetable oil to the jar. Cover the jar tightly.
3. Shake the jar vigorously for 20 seconds. Observe the contents.
4. Allow the jar to sit undisturbed for 1 minute. Observe again.
5. Remove the top and add 3 drops of liquid detergent.
6. Cover the jar and repeat Steps 3 and 4.
a) Based on your observations, write an operational definition of
detergent.
b) How might your observations relate to chemical bonds in the
detergent, oil and water molecules?
c) Demonstrate the action of soaps and detergents to your family.
d) Pour some vegetable oil on a clean cloth and show how a detergent
solution can wash the oil away better than water alone can.
e) Explain to your family the features of soap and detergent molecules
in terms of their chemical bonds.End unit assessment 19
1. Which of the following statements is false about VSEPR theory?
a) The geometry of a molecule is determined by the number of
electron groups on the central atom.
b) The geometry of the electron groups is determined by minimizing
repulsions between them.
c) A lone pair, a single bond, a double bond, a triple bond and a
single electron - each of these is counted as a single electron
group.
d) Bond angles may depart from the idealized angles because lone
pairs of electrons take up less space than bond pairs.
e) The number of electron groups can be determined from the Lewis
structure of the molecule.2. Choose the best answer. The correct dot formulation for nitrogen
trichloride has:
a) 3 N-Cl bonds and 10 lone pairs of electrons.
b) 3 N=Cl bonds and 6 lone pairs of electrons.c) 1 N-Cl bond, 2 N=Cl bonds and 7 lone pairs of electrons.
d) 2 N-Cl bonds, 1 N=Cl bond and 8 lone pairs of electrons.
e) 3 N-Cl bonds and 9 lone pairs of electrons.
3. Choose the molecule that is incorrectly matched with the electronic
geometry about the central atom.
a) CF4 - tetrahedral
b) BeBr2 - linear
c) H2O - tetrahedral
d) NH3 - tetrahedral
e) PF3 - pyramidal
4. Choose the correct answer. A π(pi) bond is the result of the
a) Overlap of two s orbitals.
b) Overlap of an s and a p orbital.
c) Overlap of two p orbitals along their axes.
d) Sidewise overlap of two parallel p orbitals.
e) Sidewise overlap of two s orbitals.5. Choose the missing answer. The F-S-F bond angles in SF6 are ______.
a) 109o28’
b) 120o only
c) 90o and 120o6. Draw a complete line-bond or electron-dot formula for acetic acid and
then decide which statement is incorrect.
a) One carbon is described by sp2 hybridization.
b) The molecule contains only one πbond.
c) The molecule contains four lone pairs of valence electrons.
d) One carbon is described by sp3 hybridization.
e) Both oxygens are described by sp3 hybridization.7. The equation below shows the reaction between boron trifluoride and
a fluoride ion. BF3 + F− → BF4−a) Draw diagrams to show the shape of the BF3 molecule and the
shape of the BF4− ion. In each case, name the shape. Account for
the shape of the BF4− ion and state the bond angle present.b) In terms of the electrons involved, explain how the bond between
the BF3 molecule and the F− ion is formed. Name the type of bond
formed in this reaction.8. Draw the shape of a molecule of BeCl2and the shape of a molecule of
Cl2O. Show any lone pairs of electrons on the central atom. Name the
shape of each molecule.9. Ammonia, NH3, reacts with sodium to form sodium amide, NaNH2, and
hydrogen.a) Draw the shape of an ammonia molecule and that of an amide
ion, NH2−. In each case show any lone pairs of electrons.
b) State the bond angle found in an ammonia molecule.
c) Explain why the bond angle in an amide ion is smaller than that in
an ammonia molecule.10. The table below shows the electronegativity values of some elements.
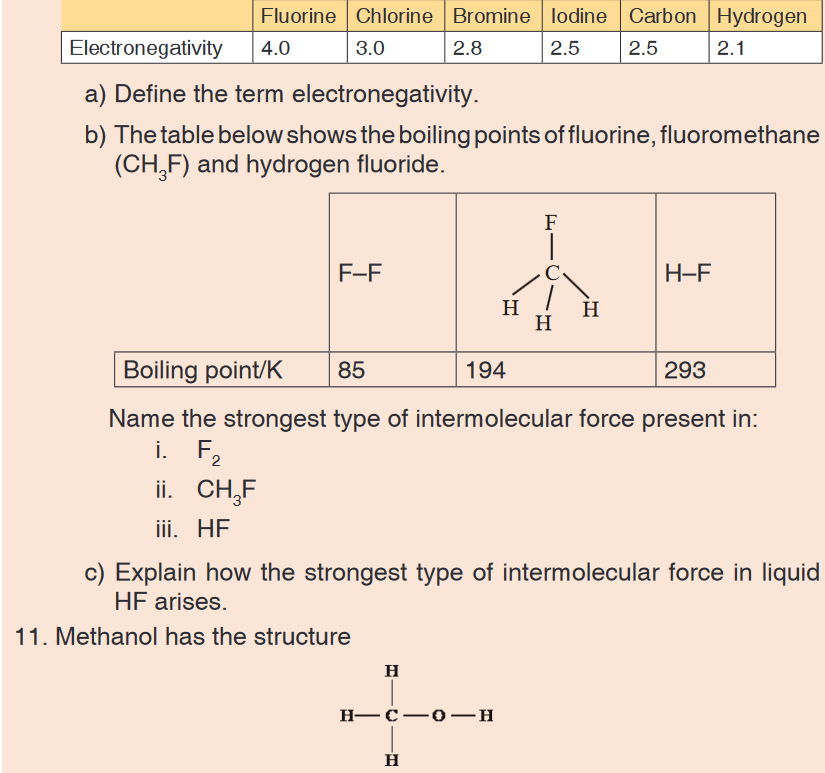
a) Explain why the O–H bond in a methanol molecule is polar.
b) The boiling point of methanol is +65 °C; the boiling point of oxygen is
–183 °C. Methanol and oxygen each have a Mr value of 32. Explain,
in terms of the intermolecular forces present in each case, why the
boiling point of methanol is much higher than that of oxygen.12. The table below gives the boiling points, Tb, of some hydrogen halides.
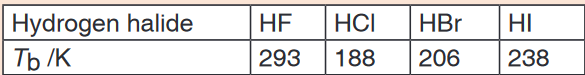
a) By referring to the types of intermolecular force involved, explain why
energy must be supplied in order to boil liquid hydrogen chloride.
b) Explain why the boiling point of hydrogen bromide lies between
those of hydrogen chloride and hydrogen iodide.
c) Explain why the boiling point of hydrogen fluoride is higher than that
of hydrogen chloride.
d) Draw a sketch to illustrate how two molecules of hydrogen fluoride
interact in liquid hydrogen fluoride.13. Figure below shows some data concerned with halogens.
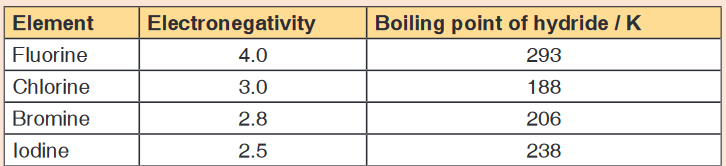
a) Define the term electronegativity.
b) Explain the trend in boiling points from hydrogen chloride to hydrogen
iodide.
c) Explain why hydrogen fluoride does not fit this trend.14. The oxygen atoms in the sulphate ion surround the sulphur in a regular
tetrahedral shape.
a) Write the formula of the ion.
b) State the O–S–O. bond angle.15. (a) State one feature which molecules must have in order for hydrogen
bonding to occur between them.
b) Give the name of the type of intermolecular bonding present in
hydrogen sulphide, H2S, and explain why hydrogen bonding does
not occur.
c) Account for the much lower boiling point of H2S (–61 °C) compared
with that of water (100 °C).16. Protein molecules are composed of sequences of amino acid molecules
that have joined together, with the elimination of water, to form long
chains. Part of a protein chain is represented by the graphical formula
given below.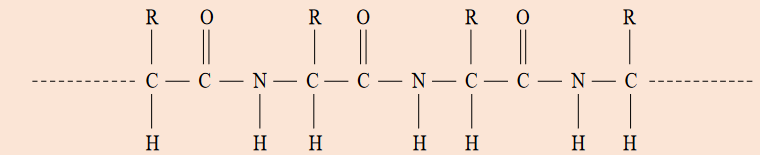
Explain the formation of hydrogen bonding between protein molecules.
17. The diagram below shows how a water molecule interacts with a
hydrogen fluoride molecule.
a) What is the value of the bond angle in a single molecule of water?
b) Explain your answer to part (a) by using the concept of electron
pair repulsion.
c) Name the type of interaction between a water molecule and a
hydrogen fluoride molecule shown in the diagram above.
d) Explain the origin of the + charge shown on the hydrogen atom in
the diagram.
e) When water interacts with hydrogen fluoride, the value of the bond
angle in water changes slightly. Predict how the angle is different
from that in a single molecule of water and explain your answer.18. Phosphorus exists in several different forms, two of which are white
phosphorus and red phosphorus. White phosphorus consists of
P4molecules, and melts at 44°C. Red phosphorus is macromolecular,
and has a melting point above 550°C.
a) Explain what is meant by the term macromolecular.
b) By considering the structure and bonding present in these two
forms of phosphorus, explain why their melting points are so
different.
