UNIT 17: IONIC AND METALLIC BONDS
Key Unit competence: Describe how properties of ionic
compounds and metals are related to the
nature of their bondingIntroductory Activity
Consider the following figures and answer the related questions
1. Figure 1 shows materials commonly used at home. If you reflect back
around your house/home you will see hundreds of objects made from
different kinds of materials.
a) Observe the objects (in picture) and classify them according to
the materials they are made of.
b) Have you ever wondered why the manufacturers choose the
material they did for each item?
c) Why are frying saucepans made of metals and dishes, cups and
plates often made of glass and ceramic?
d) Could dishes be made of metal? And saucepans made of
ceramic and glass?2. Figure 17.1 (b) shows the electric conductivity of distilled water,
solid table salt and a solution of a table salt respectively.
a) Use the diagrams A, B and C to explain the observations from
the set up.
i. No light is given out by bulb in A
ii. No light is given out by bulb in B
iii. Light is given out in C
b) Suppose that you have a 30 cm bar made of table salt. Suggest
the change, if there is any, that can occur and deduce the
property related, when this salt bar is:
i. Dropped from a table of 1 m high to the floor
ii. Immersed in water found in a water bath.
iii. Dry heated to 100oC17.1. Explanations of why atoms of elements form bonds
Activity 17.1
Consider Chlorine (Cl, Z = 17) and Argon (Ar, 18) atoms of the elements
of Period 3 in the Periodic Table.
d) Which of these atoms is more reactive?
e) Suggest the reasons for your answer in (a) in terms of the
electronegativity and electronic structure.
f) Choose, between Chlorine and Argon, which one has lower energy
potential.The atoms of most elements form chemical bonds because the atoms become
more stable when bonded together. Electric forces attract neighboring atoms
to each other, making them stick together.In atoms, electrons are arranged into complex layers called shells. For most
atoms, the outermost shell is incomplete, and the atom shares electrons with
other atoms to fill the shell.The type of chemical bond maximizes the stability of the atoms that form it.
An ionic bond, where one atom essentially donates an electron to another,
forms when one atom becomes stable by losing its outer electrons and the
other atoms become stable (usually by filling its valence shell) by gaining the
electrons.Covalent bonds form when sharing atoms results in the highest stability.
Other types of bonds besides ionic and covalent chemical bonds exist, too.
Atoms with incomplete shells are said to have high potential energy;
atoms whose outer shells arefull have low potential energy. In nature,
objects with high potential energy “seek” a lower energy, becoming more
stable as a result. Atoms form chemical bonds to achieve lower potential
energy.Application activity 17.1
Explain why atoms of elements form bonds
17.2. Gain of stability by losing and gaining electrons
Activity 17.2
Observe the electronic configuration of the following atoms in groups and
discuss the following questions.
a) How many electrons does sodium have on its outer most shell?
i. How many electrons does Sodium need to be stable?
ii. What is the easiest way for Sodium to be stable?b) How many electrons does Chlorine have on its outer most shell?
i. How many electrons does Chlorine need missing to get stability?
ii. What is the easiest for Chlorine to be stable?c) Does Neon need more electrons to be stable? If Yes why? If no
why?Like people always relate and connect to others depending on their values,
interests and goals so does unstable atoms. They combine together to
achieve stability. We know that noble gases are the most stable elements in
the periodic table. They have a filled outer electron energy level.When an atom loses, gains, or shares electrons through bonding to achieve
a filled outer electron energy level, the resulting compound is often more
stable than individual separate atoms.• Neutral sodium has one valence electron. When it loses this electron
to chlorine, the resulting Na+ cation has an outermost electron energy
level that contains eight electrons. It is isoelectronic (same electronic
configuration) with the noble gas neon.• On the other hand, chlorine has an outer electron energy level that
contains seven electrons. When chlorine gains sodium’s electron, it
becomes an anion that is isoelectronic with the noble gas argon. The
fact that atoms need to form bonds with other atoms by loosing or
gaining electrons to attain stability is called the octet rule; this means
to have 8 electrons at the outermost shell.It is easiest to apply the “Octet Rule” to predict whether two atoms will form
bonds and how many bonds they will form. Most atoms need 8 electrons
to complete their outer shell. So, an atom that has 2 outer electrons will
often form a chemical bond with an atom that lacks two electrons to be
“complete”. The octet rule states that elements gain or lose electrons to
attain an electron configuration of the nearest noble gas. Octet comes from
Latin language meaning “eight”.Note that the “Duet Rule” is also applied. The noble gas HELIUM has two
electrons (a doublet) in its outer shell, which is very stable. Hydrogen only
needs one additional electron to attain this stable configuration, while lithium
needs to lose one.Low atomic weight elements (the first twenty elements) are most likely to
adhere to the Octet Rule. For example,• A sodium atom has one lone electron in its outer shell.
• A chlorine atom, in contrast, is short one electron to fill its outer shell.
• Sodium readily donates (looses) its outer electron (forming the Na+ ion,
since it then has one more proton than it has electrons), while chlorine
readily accepts (gains) a donated electrons(making the Cl- ion, since
chlorine is stable when it has one more electron than it has protons).• Sodium and chlorine form an ionic bond with each other, to form table
salt or sodium chloride.Application activity 17.2
d) State the following Rule?
i. Octet Rule
ii. Duet Ruleb) Answer to the following questions
i. Does sodium need to gain electron than chlorine? (Yes or No)
ii. Explain the target of sodium when it is seeking to lose electron
and chlorine to gain electron.c) Which of the following is stable? Explain why?
i. Na+ ii. Na iii.Cl iv.Cl-17.3. Ionic bonding
Activity 17.3
In the Ordinary Level, you learnt that there exist three main types of
chemical bonding namely, covalent, ionic and metallic.b) Recall the definition of the ionic bond.
c) State the properties of a table salt and use it to generalize the
properties of ionic compounds. (Appearance, Solubility in water and
in petrol, Temperature required to melt, Electrical conductivity of solid
and aqueous solution)17.3.1. Concept of ionic bonding
The ionic bond is formed by complete transfer of electrons from one to
the other, being a metal atom and the other a non-metal atom.Due to the atoms are neutral:
• When an atom gives up an electron, it is positively charged, forming
what is called a positive ion or cation. The positive charge of a
monovalent cation is equal in magnitude but opposite to that of the
electron (1,602 x 10-19 C) sign.• If an atom captures one electron, it will be negatively charged, thereby
forming a negative ion or anion. The negative charge of a monovalent
anion is therefore the same as the electron.When two counter-ions have been formed, i.e. a cation and an anion, they
attract each other through electrostatic forces, and so, they can form a
stable molecule.These electrostatic attractive forces (sometimes called Coulomb
forces) are therefore responsible for the formation of ionic compounds.Suppose the simple case of sodium chloride or common salt. The sodium
atom (Z = 11) has a single (unpaired) electron in orbital 3s, somewhat
isolated from other pairs; while in the chlorine atom, there is also a single
(unpaired) electron, but in this case it is in the 3pz orbital, other 3s, 3px and
3py orbitals are being inhabited by respective pairs of electrons.The alone electron passes from sodium to chlorine; which, besides forming
a pair with the electron in 3pz orbital, it will be found surrounded by other
couples.All the atoms of the alkali (group 1) metals have an external electronic
configuration type ns1, i.e., with a single electron in the outermost orbit. This
electron, which is often called valence electron is quite far from the core
(the nucleus), which is separated also by the other electrons, called internal
(electrons), which largely core-shielding attraction on said valence electron.So, it is quite easy to remove this electron, for which a little energy is spent.
This is why the ionization energy, which, for the alkali metals, is very small.When an alkali metal atom has been easily removed its valence electron,
however, it is difficult to remove a second electron, as their ionization
energy is very high. Therefore, the alkali metal cations are relatively easily
monovalent (M+).For the group 13 elements (B, Al, Ga, In, Tl), it costs much energy to remove
valence electrons, which, in this case, are the type ns2np1, so it is difficult.
Hence, they form trivalent cations, except heavier atom of Tl that can form
in certain cases, monovalent cation.The elements of groups 14, 15, 16 and 17 of the Periodic Table, as well as
the noble gases (group 18), the ionization energy is increasing, so it is very
difficult for these elements to form positive ions.• Only the heaviest elements of group 14, tin and lead, form, in some
cases, divalent cations (loss of two of the valence electrons np2). The
rest of the elements of these groups form covalent bonds or negative
ions.• The atoms of group 17 elements of the Periodic Table (halogens) have
an outer electron configuration ns2np5, that is, they lack one electron
to complete the p orbitals and thus to form the electron configurationof noble gas that follows in the same period. Therefore, it is easy to
understand that if one of these atoms is joined by a new electron; a more
stable configuration is obtained, shedding energy in this process. This
energy is called electron affinity, which for the halogens is high. These
elements will form in a relatively easily way, monovalent anions.• These anions have no tendency to take a second electron, so it would
have to stand, alone in an outermost orbit, without the nucleus exercised
about it any attractive force. Halogens form only monovalent anions.• Atoms of oxygen family elements (group 16 of the Periodic Table) are
missing two electrons to complete the external orbitals np and acquire
the noble gas configuration. Therefore, these elements tend to form
divalent ions, although in this process the energy balance is slightly
negative.• Nitrogen family elements (group 15 of the periodic table are hardly
trivalent anion; while carbon group (group 14) is almost impossible
the form tetravalent anions. Therefore, the compounds of the nitrogen
family are largely covalent part and those of carbon family are typically
covalent.17.3.2. Ionic bond formation
Once the oppositely charged ions form when electrons are transferred from
one atom to another, they are attracted by their positive and negative
charges (by electrostatic forces) and form an ionic compound. Ionic bonds
are also formed when there is a large electronegativity difference between
two atoms. This difference causes an unequal sharing of electrons such that
one atom completely loses one or more electrons and the other atom
gains one or more electrons. For example, in the creation of an ionic bond
between a metal atom, sodium (electronegativity = 0.93) and a non-metal,
fluorine (electronegativity = 3.98).Let us take a look of how sodium and fluorine bond to form sodium
fluoride.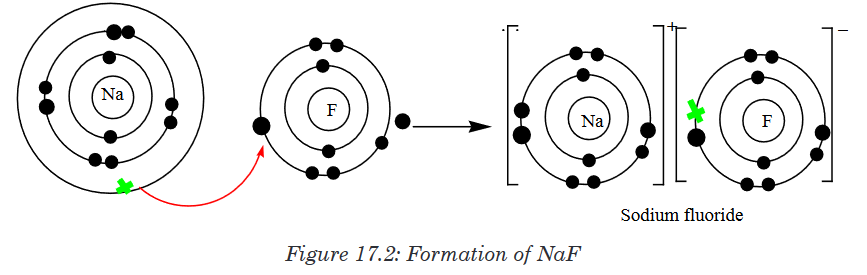
The curved arrow between sodium and fluorine atoms represents the
transfer of an electron from a sodium atom to a fluorine atom to form
oppositely charged ions. These two ions are strongly attracted to each
other because of their opposite charges. A bond is now formed and the
resulting compound is called Sodium fluorideAnother example of ionic bonding formation is the formation of magnesium
oxide.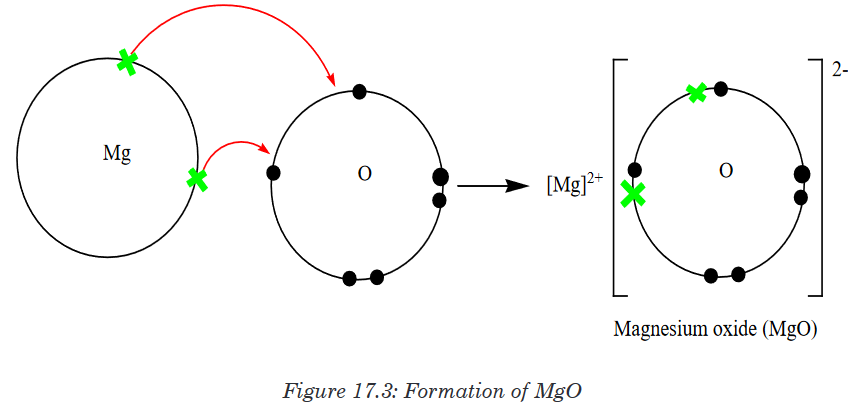
17.3.3. Properties of ionic compounds
Here are the properties shared by the ionic compounds. Notice that the
properties of ionic compounds relate to how strongly the positive and
negative ions attract each other in an ionic bond.1. They have high melting points and high boiling points
In an ionic lattice, there are many strong electrostatic attractions between
oppositely charged ions. We therefore expect that ionic solids will have
high melting points. On melting although the regular lattice is broken down,
there will still be significant attractions between the ions in the liquid. This
should result in high boiling points also.The factors which affect the melting point of an ionic compound are:
• The charge on the ions of ionic compound: “The greater the charge,
the greater the electrostatic attraction, the stronger the ionic bond, the
higher the melting point.”For example, Melting Point of NaCl is 801 oC and that of MgO is
2,800 oC.• The size of the ions of ionic compound: “Smaller ions can pack
closer together than larger ions so the electrostatic attraction is greater,
the ionic bond is stronger, the melting point is higher.” For example,
Melting Point of NaF is 992 oC and that of CsF is 2,800 oC.2. Most ionic compounds are soluble in water
This is because the electrostatic forces of the polar water molecules are
stronger than the electrostatic forces keeping the ions together. When an
ionic compound like NaCl is added to water, water molecules attract the
positive and negative salt ions. Water molecules surround each ion and move
the ions apart from each other. The separated ions dissolve in water. There
are several exceptions, however, where the electrostatic forces between the
ions in an ionic compound are strong enough that the water molecules cannot
separate them. Despite these few limitations, water’s ability to dissolve ionic
compounds is one of the major reasons it is so vital to life on Earth. Ionic
compounds are generally insoluble in non-polar solvents like kerosene.3. They are hard and brittle
Ionic crystals are hard because the positive and negative ions are strongly
attracted to each other and difficult to separate, however, ionic solids are
brittle.When a stress is applied to the ionic lattice, the layers shift slightly. The layers
are arranged so that each cation is surrounded by anions in the lattice. If the
layers shift then ions of the same charge will be brought closer together. Ions
of the same charge will repel each other, so the lattice structure breaks down
into smaller pieces.4. They conduct electricity when molten or dissolved in water
In order for a substance to conduct electricity, it must contain mobile particles
capable of carrying charge.• Solid ionic compounds do not conduct electricity because the ions
(charged particles) are locked into a rigid lattice or array. The ions
cannot move out of the lattice, so the solid cannot conduct electricity.• When is molten, the ions are free to move out of the lattice structure.
– Cations (positive ions) move towards the negative electrode
(cathode): M+ + e- → M– Anions (negative ions) move towards the positive electrode (anode):
X- → X + e-• When the ionic compound is dissolved in water to form an aqueous
solution, the ions are released from the lattice structure and are free
to move so the solution conducts electricity just like the molten (liquid)
ionic compound.5. They form crystals
Ionic compounds form crystal lattices rather than amorphous solids. Although
molecular compounds form crystals, they frequently take other forms but
molecular crystals typically are softer than ionic crystals. At an atomic level,
an ionic crystal is a regular structure, with the cation and anion alternating
with each other and forming a three-dimensional structure based largely on
the smaller ion evenly filling in the gaps between the larger ion.Application activity 17.3
1. The diagram below represents a part of the structure of sodium
chloride. The ionic charge is shown on the centre of only one of the
ions.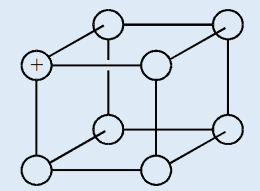
a) On the diagram, mark the charges on the four negative ions.
b) What change occurs to the motion of the ions in sodium chloride
when it is heated from room temperature to a temperature below
its melting point?c) Sodium chloride can be formed by reacting sodium with chlorine.
A chloride ion has one more electron than a chlorine atom. In
the formation of sodium chloride, from where does this electron
come?2. Draw diagrams to illustrate the formation of ionic compounds in the
following substances:
a) Calcium chloride
b) Sodium peroxide
c) Iron (III) chloride
d) Sodium sulphide3. Solid sodium chloride and solid magnesium oxide are both held
together by ionic (electrovalent) bonds.a) Using s, p and d notation write down the symbol for and the
electronic configuration of (i) a sodium ion; (ii) a chloride ion; (iii)
a magnesium ion; (iv) an oxide ion.b) Explain what holds sodium and chloride ions together in the solid
crystalc) Sodium chloride melts at 1074 K; magnesium oxide melts at 3125
K. Both have identical structures. Why is there such a difference in
their melting points?17.4. Metallic bonding
Activity 17.4
1. Give three examples of substances which are malleable, ductile,
good conductor of heat and electricity, and having a characteristic
luster. Here you can use a dictionary or other searching tools to find
the meaning for any unfamiliar word.2. Suggest another property, apart from those given, of the substances
you have given in (1).3. Choose from the examples given in (1), one which is most common
and well known.a) This substance is seen to be composed by atoms of one element.
Which one?b) Use a labeled drawing to show the internal structure of that kind
of substance.A metallic bond is a type of chemical bond formed between positively charged
atoms in which the free electrons are shared among a lattice of cations. In
contrast, covalent and ionic bonds form between two discrete (separate)
atoms.Metallic bonding is the main type of chemical bonds that forms between
metal atoms (pure metals and alloys and some metalloids). A metal is a
lattice of positive metal ‘ions’ in a ‘sea’ of delocalised electrons.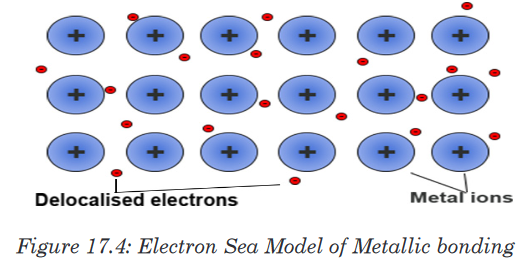
17.4.1. How metallic bonds work
The outer energy levels of metal atoms (the s and p orbitals) overlap. At least
one of the valence electrons participating in a metallic bond is not shared
with a neighbor atom, nor is it lost to form an ion. Instead, the electrons form
what may be termed an “electron sea” in which valence electrons are free
to move from one atom to another. Metallic bonding refers to the interaction
between the delocalised electrons and the metal nuclei.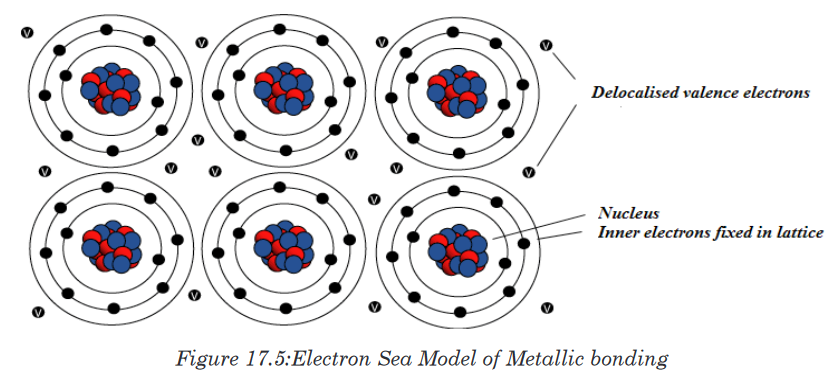
The electron sea model is an oversimplification of metallic bonding.
Calculations based on electronic band structure or density functions are
more accurate. Metallic bonding may be seen as a consequence of a
material having many more delocalized energy states than it has delocalized
electrons (electron deficiency), so localized unpaired electrons may become
delocalized and mobile. The electrons can change energy states and move
throughout a lattice in any direction.Bonding can also take the form of metallic cluster formation, in which
delocalized electrons flow around localized cores. Bond formation depends
heavily on conditions. For example, hydrogen is a metal under high pressure!
As pressure is reduced, bonding changes from metallic to non-polar covalent.17.4.2. Physical properties of metals
Because electrons are delocalized around positively-charged nuclei, metallic
bonding explains many properties of metals.1. Electrical Conductivity
Most metals are excellent electrical conductors because the electrons in the
electron sea are free to move and carry charge. For example, electric wires
in our homes are made of aluminium and copper. They are good conductorof electricity. Electricity flows most easily through gold, silver, copper and
aluminium. Gold and silver are used for fine electrical contacts in computers.2. Thermal Conductivity
Metals conduct heatbecause the free electrons are able to transfer energy
away from the heat source and because vibrations of atoms (phonons) move
through a solid metal as a wave. Cooking utensils and water boilers are also
made of iron, copper and aluminium, because they are good conductors of
heat.3. Ductility
Metalstend to be ductile or able to be drawn into thin wires because local
bonds between atoms can be easily broken and also reformed. Single atoms
or entire sheets of them can slide past each other and reform bonds. Wires
are mainly made from copper, aluminium, iron and magnesium.4. Malleability
Metals are often malleable or capable of being molded or pounded into a
shape, again because bonds between atoms readily break and reform. This
ability to bend or be shaped without breaking occurs because the electrons
simply slide over each other instead of separating. The binding force between
metals is non-directional, so drawing or shaping a metal is less likely to
fracture it. Electrons in a crystal may be replaced by others. Gold and Silver
metals are the most malleable metals. They can be hammered into very fine
sheets. Thin aluminium foils are widely used for safe wrapping of medicines,
chocolates and food material.5. Metallic Luster
Metals tend to be shiny or display metallic luster. They are opaque once a
certain minimum thickness is achieved.The electron sea reflects photons off the smooth surface therefore there is an
upper frequency limit to the light that can be reflected. Silver is a very good
reflector. It reflects about 90% of the light falling on it. All modern mirrors
contain a thin coating of metals. Due to their shiny appearance they can be
used in jewellery and decorations.Application activity 17.4
1. Magnesium has a higher melting and boiling point than sodium. This
can be explained in terms of the electronic structures, the packing,
and the atomic radii of the two elements.a) Explain why each of these three things causes the magnesium
melting and boiling points to be higher.b) Explain why metals are good conductors of electricity.
c) Explain why metals are also good conductors of heat.
2. Pure metals are usually malleable and ductile.
a) Explain what those two words mean.b) If a metal is subjected to a small stress, it will return to its original
shape when the stress is removed. However, when it is subjected to
a larger stress, it may change shape permanently. Explain, with the
help of simple diagrams why there is a different result depending on
the size of the stress.c) When a piece of metal is worked by a blacksmith, it is heated to a high
temperature in a furnace to make it easier to shape. After working
it with a hammer, it needs to be re-heated because it becomes too
difficult to work. Explain what is going on in terms of the structure of
the metal.d) Why is brass harder than either of its component metals, copper
and zinc?Skills lab 17
Experiment to demonstrate the malleability and ductility of metals
Materials: Wires, nails, hammer, piece of cloth.Procedure:
1. Wrap the material to be test in a heavy plastic or cloth to avoid pieces
flying from the material.2. Place the material on a flat hard surface
3. Use a harmer to pound the material flat
4. Record your observations as malleable or non-malleable.
End unit assessment 17
1. State whether the following statement is True or False. Justify
your answer. “Sodium Chloride has a higher melting point than
Magnesium Oxide”.2. Why are ionic compounds brittle?
3. Why do ionic compounds have high melting points?
4. What happens when an electric current is passed through a solution
of an ionic compound?5. This question is about metallic bonding.
a) Describe the bonding that is present in metals.
b) Explain how the bonding and structure lead to the typical metallic
properties of electrical conductivity and malleability.
c) Suggest a reason why aluminium is a better conductor of
electricity than magnesium.6. Silver and sodium chloride melt at similar temperatures. Give two
physical properties of silver which are different from those of sodium
chloride and, in each case, give one reason why the property of
silver is different from that of sodium chloride.7. This question is about calcium oxide (CaO).
a) Describe the nature and strength of the bonding in solid calcium
oxide.
b) Use the kinetic theory to describe the changes that take place
as calcium oxide is heated from 25°C to a temperature above its
melting point.
c) State two properties of calcium oxide that depend on its bonding.
