UNIT 12: THE CHEMICAL BASIS OF LIFE
Key Unit competence: Explain the use of biological molecules in
living organism.Introductory Activity 12

Analyze all foods in the figure above and answer the questions below:
a). Among the foods observed in figure, plan a list for your menu for
breakfast, lunch and supper. Justify your choices.
b). Are there foods you missed on the list which you prefer to eat?
Why?
c). List the nutrients our body gains from different food and justify
why all are needed.12.1. Biological molecules
Activity 12.1
1. Discuss chemical elements, sub-units of different types of
carbohydrates, lipids and proteins.
2. What do you know about the function of water to living organisms?12.1.1. The chemical elements that make up carbohydrates,
lipids and proteinsa. Carbohydrates
Carbohydrates comprise a large group of organic compounds which contain
carbon, hydrogen and oxygen. The word carbohydrate indicates that these
organic compounds are hydrates of carbon. Their general formula is
.In carbohydrates the ration hydrogen-oxygen is usually 2:1.
Carbohydrates are divided into three groups including the monosaccharide
(single sugars), disaccharides (double sugars) and polysaccharides (many
sugars). The most common monosaccharide of carbohydrates is glucose
with molecular formula C6 H12 O6 .
Lipids
Lipids are a broad group of naturally occurring molecules which include
waxes, sterols, fat soluble vitamins (such as vitamins A, D, E and K),
monoglycerides, diglycerides, triglycerides, Phospholipids and others.
Lipids are made by carbon, hydrogen and oxygen, but the amount of oxygen
in lipids is much smaller than in carbohydrates.
Lipids are grouped into fats which are solid at room temperature and oils
which are liquid at room temperature.b. Proteins
Proteins are organic compounds of large molecular mass. For example,
the hemoglobin has a molecular mass of 64500. In addition to carbon,
hydrogen and oxygen, proteins always contain nitrogen, usually Sulphur and
sometimes phosphorus.c. Water
Living organisms contain between 60% and 90% of water, the remaining
being the dry mass. Water is made up of only two elements, Hydrogen and
Oxygen.The function of water is defined by its physical and chemical properties that
differ from those of most liquids and make it effective in supporting life.12.1.2. The sub-units that make up biological molecules
a. Sub-units of Carbohydrates
In carbohydrates the following three categories are identified:
Monosaccharides , disaccharides and polysaccharides.i. Monosaccharides
Monosaccharides are the smallest subunits and are made up of single
sugar molecules. Monosaccharides are the sugars like galactose, fructose
and glucose with a general formula C6 H12 O6 , and these typically take on
a ring-shaped structure.All monosaccharides are reducing sugars capable of acting as a reducing
agents because they have a free aldehyde group or a free ketone group.Sources of Monosaccharides:
• Glucose: Fruits and vegetables are natural sources of glucose. It’s also
commonly found in syrups, candy, honey, sports drinks, and desserts.
• Fructose: The primary natural dietary source of fructose is fruit, which
is why fructose is commonly referred to as fruit sugar.
• Galactose: The main dietary source of galactose is lactose, the sugar
in milk and milk products, such as cheese, butter, and yogurt.ii. Oligosaccharides
These are complex carbohydrate chains made up of two to twenty
simple sugars joined together with a covalent bond. The most common
oligosaccharide is the disaccharide, and examples of this include sucrose,
maltose and lactose whose general formula is C12 H22 O11 .A disaccharide is the sugar formed when two monosaccharides are joined
by glycosidic bond. Like monosaccharides, disaccharides are soluble in
water, have sweet taste, and they are reducing sugars because they are
able to reduce Copper II Sulfate of benedict solution directly by heating
into copper II oxide except sucrose which is non-reducing sugar which are
unable to reduce the copper ions in Benedict’s solution.This makes the color of Benedict’s solution to persist when this sugar is
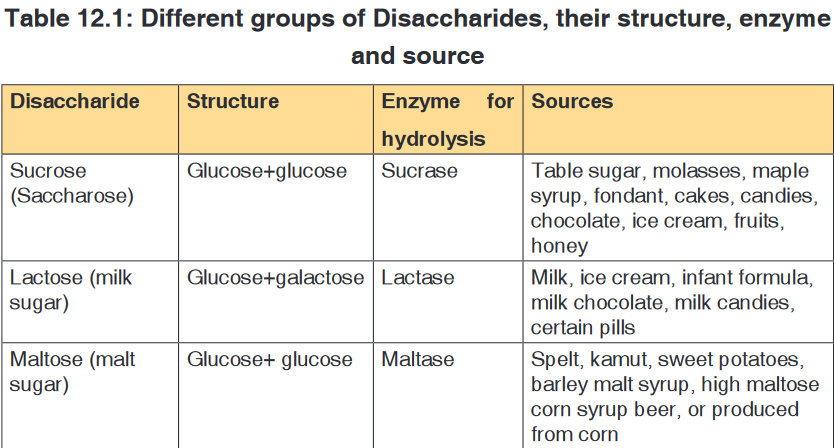
boiled with it.Sucrose is made up of two monosaccharides: Glucose and fructose
Maltose is made up of two monosaccharides: Glucose and glucose
Lactose is made up of two monosaccharides: Glucose and galactoseIn maltose ring, the two ring of glucose are bonded by the -1, 4-glycosidic
bond while in sucrose the glucose and fructose are bonded by -1,
2-glycosidic bond.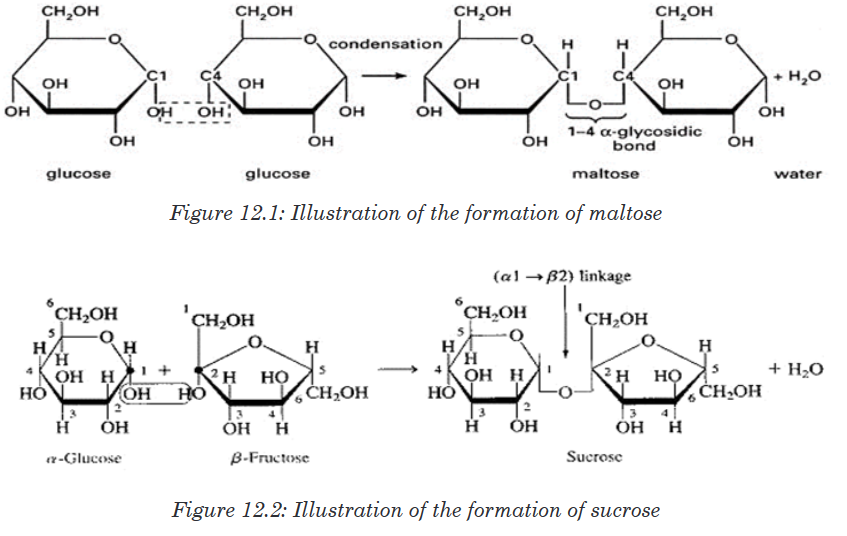
iii. Polysaccharides
These are known for their ability to store energy and are made up of long
chains of glucose sugars. The most common polysaccharides are starch
(sugar of plant tissues), glycogen (glucose in the human liver and muscles),
cellulose (structural polysaccharide in plants; which acts as a dietary fiberwhen consumed), chitin (sugar found in exoskeleton of arthropods) and
peptidoglycan (sugar found in the bacteria cell membrane).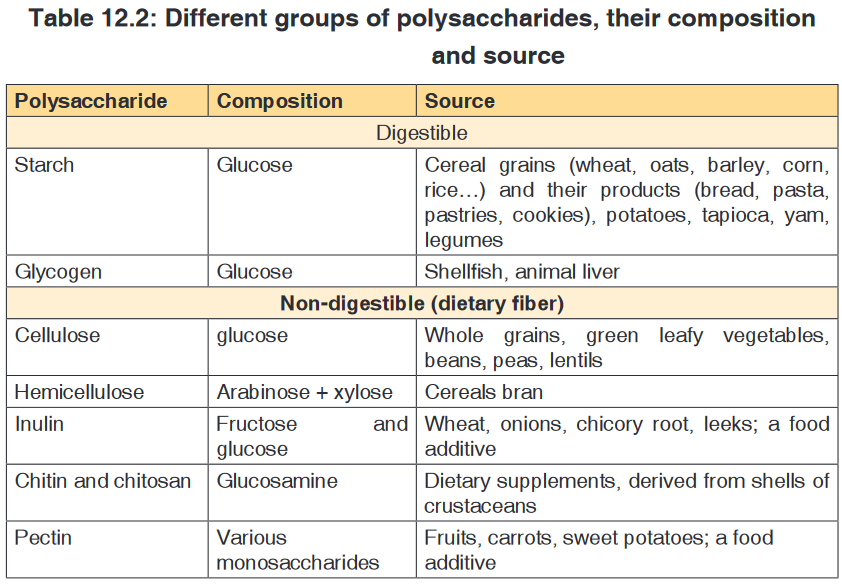
b. Sub-units of Proteins
These are also referred to as macro-nutrients. The protein are also called
body- building food.The protein molecules are made up of small units called amino acids joined
together like links in a chain.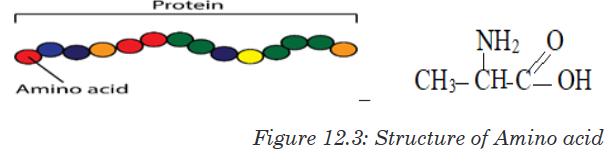
There are 21 different amino acids and each has its own chemical name.
Different proteins are made when different numbers and types of amino acids
combine through a covalent peptide bond. Proteins are therefore known as
polypeptides.
Examples of proteins
a). Collagen, myosin and elastin found in meat,
b). Caseinogen, lactalbumin, lacto globulin found in milk,c). Avalbumin, mucin and liporitellin found in eggs,
d). Zein found in maizeThe 21 different amino acids found in protein are:
Arginine, Serine,Selenocysteine, Leusine, Histidine,Threonine, Glycine,
Methionine, Lysine, Asparagine, Proline, Phenylalanine, Aspartic acid,
Glutamine, Alanine, Tyrosine, Glutamic acid, Cysteine,Valine, Tryptophan,
Isoleucine.
They are used to repair, to build, to maintain our bodies; to make muscles
and to make breast milk during lactation period. The proteins are classified
into two categories: animal or complete proteins and plant proteins or
incomplete proteins.c. Sub-units of lipids
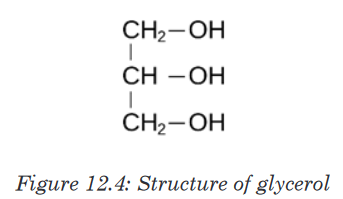
Lipids are made by two components namely glycerol and fatty acids. The
chemical formula for glycerol is C3 H8 O3 .Structural formula of glycerolis
Sources and classification of lipids
Fats and oils are obtained from both the plants and animals. And fat is
present in food either as visible fat or invisible fat.Visible fat is the one that is easily seen or detected in food for example; fat
in meat, butter, margarine, lard, suet and cooking fat and oil.
Invisible fat is the part of food that is not easily seen for example fat with
in lean meat, egg yolk, flesh of oily fish, groundnuts, soya beans, avocado
and fat found in prepared foods, for example, pastry, cakes, biscuits, French
fries, pancakes, croquettes.
Lipids are of different types as it is summarized in the following table.

The following are three main types of lipids: Triglycerides, phospholipids and
steroids:
• Triglycerides: These are lipids that are obtained from cooking oils,
butter and animal fat. They are made up with: one molecule of glycerol
and three molecules of fatty acids bonded together by Ester bonds.
The triglycerides play the role like storing energy they have thermal
insulation and protective properties
• Sterols: These are lipids that include steroid hormones like testosterone
and oestrogen, cholesterol that is formed four carbon-based rings and
it helps in regulation of fluid and strength of the cell membrane.• Phospholipids: They are made up of one molecule of glycerol, two
molecules of fatty acids and one phosphate group. The phospholipids
form a molecule that is part hydrophobic, part hydrophilic, ideal for
basis of cell surface membranes
Functions of lipids
• Fats are a source of energy. They supply energy to the body more than
carbohydrates and proteins.
• Fat surrounds and protects important organs of the body such as
the kidney and the heart, however too much fat around the organs is
dangerous as it slows down their functioning.• Fat forms an insulating layer beneath the skin to help keep us warm
by preserving body heat and it also protects the skeleton and organs.
• Fat provides a source of fat soluble vitamins A, D, E and K in the body.
• Fat is a reserve of energy for long term storage and can be used if
energy intake is restricted.
• Fat in foods provides texture and flavour in foods and it helps to make
it palatable.Food containing fat provides a feeling of satiety or fullness after a meal as
fat is digested slowly.12.1.3. The structure of proteins and their function
Activity 12.1.3
1. From the books make a research on proteins and answer to the
following questions:
a). What are different structures of proteins?
b). Differentiate globular proteins and fibrous proteins.
2. Take a plastic cord, create the bulk on it and suppose that those are
monomers of a long chain of polymer (the whole cord), heat it using
a Bunsen burner or another source of fire. Discuss the change that
takes place.Proteins are organic compounds of large molecular mass ranging up to
40,000,000 for some viral proteins but more typically several thousand. For
example, the hemoglobin has a molecular mass of 64,500. Proteins are
polymers of amino acids and they are not truly soluble in water, but form
colloidal suspensions. In addition to carbon, hydrogen and oxygen, proteins
always contain nitrogen, usually Sulphur and sometimes phosphorus.
Whereas there are relatively few carbohydrates and fats, the number of
proteins is limitless. Coined by a Dutch chemist Mulder the word protein
etymologically means “of the first importance” due to the fundamental role
they play in living cells.a. Amino acids
Amino acids are group of over a hundred chemicals of which around 20
commonly occur in proteins. They always contain a basic group, the amine
group (-NH2) and an acid group (-COOH) together with -R group side chain
(Figure 12.14). All the amino acid differs one to another by the structure of
their side chain.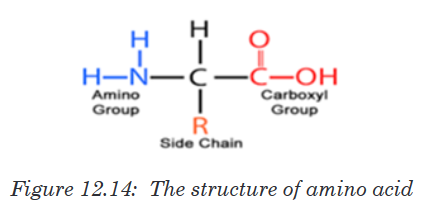
Amino acids are divided into two categories including essential amino acid
and non-essential amino acid. Essential amino acids are those amino acids
which cannot be synthesized by the body. They include isoleucine, leucine,
lysine, methionine, phenylalanine, threonine, tryptophan, valine, arginine
and histidine. Non –essential amino acids are synthesized by the organism.
They include alanine, asparagine, aspartic acid, cysteine, glutamine,
glutamic acid, glycine, proline, serine and tyrosine. The simplest amino acid
is glycine with H as -R group (Figure 12.15. a). The other one is Alanine with
–CH3 as -R group (Figure 12.15.b). All 20 amino acids can be found in diet
from animals such as meat, eggs, milk, fish…but diet from plant lack one or
two essential amino acids such plant are beans, soy beans…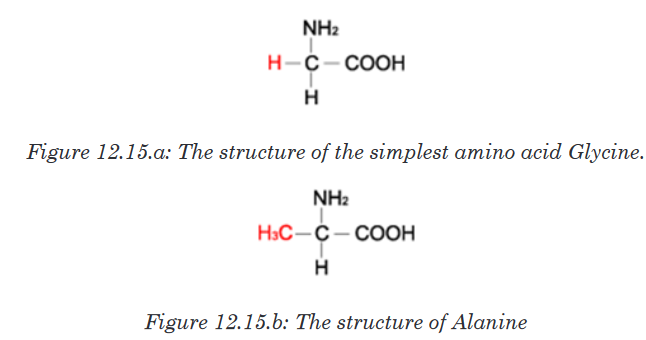
When an amino acid is exposed to basic solution, it is deprotonated (release
of a proton H+) to became negative carboxylate COO -while in acid solution
it is protonated (gains of a proton H+) to became ammonium positive ion
-NH3 +(Figure 12.16.a and Figure 12.16.b).
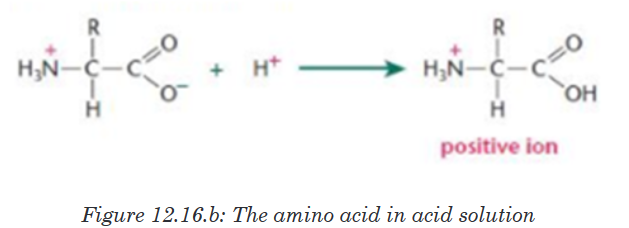
At a physiological pH, usually around 7, the amino acid exists as ZWITTERION
(from German means hermaphrodite) it is a molecule with two different
charges (positive and negative) at the same time (Figure 12.17).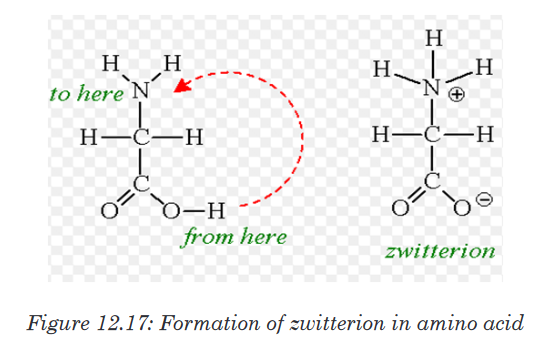
b. Formation and breakage of peptide bond
The formation of peptide bond follows the same pattern as the formation
of glycosidic bond in carbohydrates and ester bond in fats. A condensation
reaction occurs between the amino group of one amino acid and the carboxyl
group of another, to form a dipeptide (Figure 12.18).
A peptide bond is formed between two amino acids to form a dipeptide
molecule, if three amino acids are assembled together it is a tripeptide, four
amino acids form a tetrapeptide and so on. A long chain of amino acid it is
called a polypeptide. The polypeptide chain or oligopeptide comprise more
than 50 amino acids joined together by a peptide bond.During digestion, proteins are hydrolyzed and give their monomer amino
acids small molecules that can be diffused in the wall of intestine to the
organism. In hydrolysis the peptide bond break down by the addition of a
water molecule (Figure 12.19).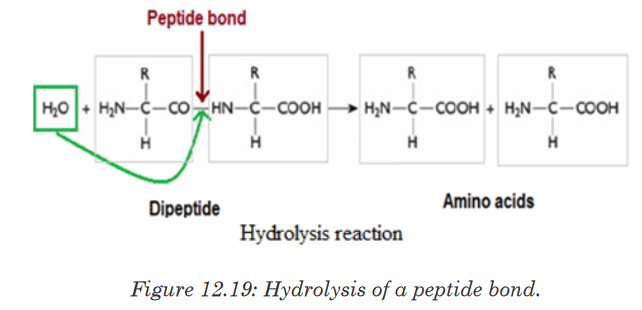
c. Structure and denaturation of proteins
The long chain of polypeptide can take different form according to its
molecular weight and the types of bond that hold together atoms and
molecules, those form are described as primary, secondary, tertiary and
quaternary structure. A human has tens of thousands of different proteins,
each with a specific structure and function. Proteins in fact are the most
structurally sophisticated molecules known. Consistent with their diverse
functions, they vary extensively in structure, each type of protein having a
unique three-dimensional shape.Structure of proteins
i. Primary structure of proteins
Primary structure of a protein is the sequence of amino acid in a linear shape,
the amino acid are joined together with the peptide bond.The alteration of
this linear sequence (change of the shape) can inhibit the proper function
of the protein as well as the function of the protein depending on its three
dimensional shape.
ii. Secondary structure of proteins
The regular arrangement of amino acids in primary structure can induce
the interaction of the back bone of the polypeptide chain (side chain) by
hydrogen bonds. Those side chains are coiled and folded in the patterns
that contribute to the protein’s overall shape. One such secondary structure
is α-helix and sometime β-pleated sheet.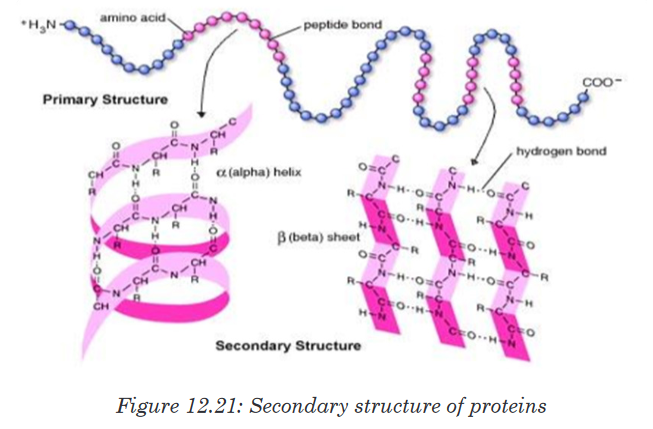
iii. Tertiary structure of proteins
In addition to hydrogen and peptide bond in primary and secondary structure,
the tertiary structure of protein has other types of interaction called hydrophobic
interaction. Once the amino acid side chains are close together, Van der
Waals interactions hold it together, and their stability depends on ionic bond
between positively and negatively charged R groups. The cumulative effect
of those week interactions reinforced with the covalent bonds called disulfide
bridges (-S-S) give the protein a unique shape.
iv. Quaternary structure of proteins
Quaternary structure involves different polypeptide chains into one functional
three dimensional molecules. For example, the protein that bind oxygen in
red blood cells (Hemoglobin) is made by four polypeptide subunits, two of
the same kind (α chains) and two of another kind (β chains). Both α and β
sub-units primarily are α helical secondary structure with anon polypeptide
chain iron that binds oxygen called Haeme.
Globular proteinsuch as haemoglobin and enzymes are soluble in water.
In coiling, the hydrophobic R group is dressed toward the inside while the
hydrophilic is addressed toward the outside. The opposite is observed in
fibrous protein such as collagen and keratin which are insoluble in water.
Such protein may contain another group of compound which is not an amino
acid called prosthetic group for example the iron (haeme) is prosthetic group
in haemoglobin, magnesium in green pigment of plant (chlorophyll) is also a
prosthetic group.Protein denaturation
Protein denaturation is a mechanism by which quaternary, tertiary and
secondary structure of protein changes their shape due to external stress
called agent or factor of denaturation. Protein denaturation may be temporary
or permanent due to a variety of factors. The agent of denaturation may
include heat, changes in pH, Ultra Violet (UV°) rays, high salt concentration
and heavy metals. Cooked egg is an example of a denatured protein due to
the heat. This also explains why excessively high fever disease is fatal to the
organism because protein in the blood denature at high temperature. The
agents of denaturation will denature protein causing the loose of its shape
and hence its ability to function.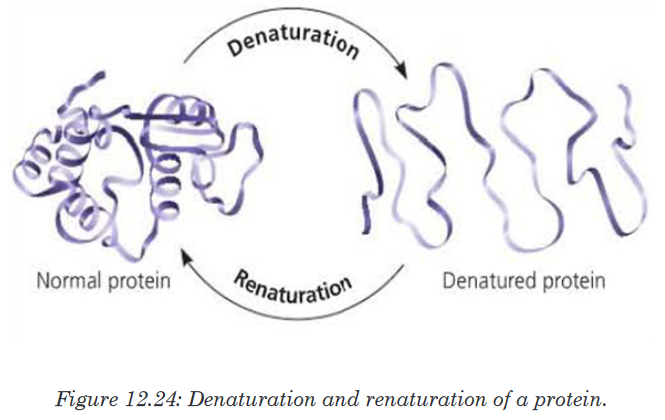
d. Functions of proteins.
• Proteins such as lipase, pepsin and protease act as enzymes as they
play a crucial role in biochemical reaction where they act as catalysts.
• Proteins play an important role in coordination and sensitivity (hormones
and pigments).• Proteins have a transport functions. Example: Haemoglobin transport
oxygen
• Proteins in the cell membrane facilitate the transport of substance
across the cell membrane.
• Proteins provide a mechanical support and strength.
• Proteins such as myosin and actin are involved in movement.
• Proteins play the role of defense of the organisms. Example: Antibodies
are proteins12.1.4. Molecular structure and functions of polysaccharides,
glycogen and celluloseActivity 12.1.4
1. Based on the meaning of monosaccharide, what is the meaning of
polysaccharide?2. Classify the following compound into polysaccharide, monosaccharide
and disaccharide
a). Glucose, fructose and galactose
b). Lactose, sucrose, and maltose
c). Starch, cellulose and glycogen3. Use glucose to form any polysaccharide of your choice
In the same way that two monosaccharides may combine in pairs to give
a disaccharide, many monosaccharides may combine by condensation
reactions to form a polysaccharide. The number of monosaccharides that
combine is variable and the chain produced may be branched or unbranched.
Polysaccharide are many but the most known are starch, glycogen and
cellulose.a. Starch
Starch is made up of two components: amylose and amylopectin. Amylose
is a linear unbranched polymer of 200 to 1500 α-glucose units in a repeated
sequence of α-1,4-glucosidic bonds. The amylose chain coils into helix held
by hydrogen bonds formed between hydroxyl groups. A more compact shape
is formed. The amylose helices are entangled in the branches of amylopectin
to form a complex compact three dimensional starch molecule.Amylopectin is a branched polymer of 200 to 200,000 α-glucose units per
starch molecule. The linear chains of α-glucose units are held together by
α-1,4-glucosidic bonds. Branches occur at intervals of approximately 25 to 30where α-1,6-glucosidic bonds occur. Starch grains are found in chloroplast,
potato tubers, cereals and legumes. Starch is insoluble in cold water. It is
digested by salivary amylase and pancreatic amylase into maltose and the
latter is hydrolyzed by maltase enzyme to form glucose. Therefore, diabetic
people should avoid tubers since they are rich in starch which in turn gives
glucose.
b. Glycogen
Glycogen is often called animal starch because it is a major polysaccharide
storage material in animals and fungi. The brain and other tissues require
constant supply of blood glucose for survival. Some tissues particularly the
liver and skeletal muscles store glycogen in the form that can be rapidly
mobilized to form glucose. Like starch, glycogen is made up of α-glucose
and exists as granules. It is similar to amylopectin in structure but it has
shorter chains (10-20glucose unit) and is more highly branched.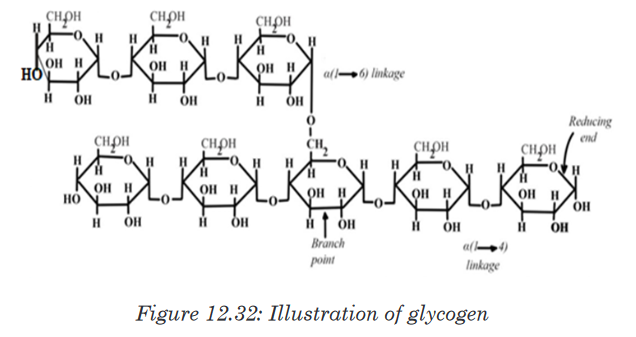
c. Cellulose
Cellulose is the structural polysaccharide in plant cell wall. It is found in
vegetables and fruits but cannot be hydrolyzed by enzymes in the human
digestive system. Cellulose is composed of long unbranched chains of up
to 10,000 β-glucose units linked by β-1,4-glucosidic bonds. Each β-glucose
unit is related to the next by a rotation of 180°C with OH groups projecting
outwards on either side of the chain.Cellulose chains run parallel to one another. Unlike amylopectin and
glycogen molecules, there are no side chains (no branch) in the cellulose.
This allows the linear chains to lie close together. Many H-bonds are formed
between the OH groups of adjacent chains. The chains group together to
form microfibrils arranged in larger bundles of macrofibrils. The fibrils give
the plant cell their high tensile strength and rigidity. The layers of fibrils are
permeable to water and solutes.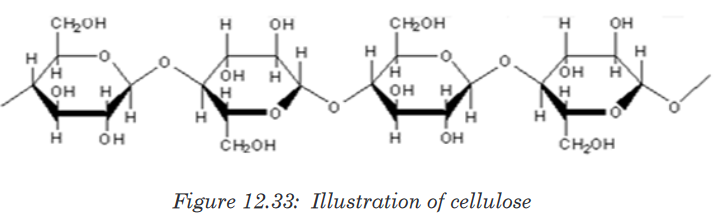
12.1.5. Isomerism of monosaccharide and formation of
glycosidic bond
Monosaccharides are group of sweet and soluble crystalline molecules of
relatively low molecular mass. They are named with the suffix –ose. The
general formula for a monosaccharide is (CH2 O) , with n the number of
carbon atoms. The simplest monosaccharide has n=3 and it is a triose
sugar. When n = 5, this is a pentose sugar, and when n = 6, this is a hexose
sugar. The two common pentose sugars are ribose and deoxyribose, while
the most known hexose is glucose. Its molecular formula is C6H12O6. It is
the most important simple sugar in human metabolism called simple sugar
or monosaccharide because it is one of the smallest units which has the
characteristics of this class of carbohydrates.a. Isomerism and ring formation
Monosaccharides can exist as isomers. The isomer is defined as each of
two or more compounds with the same formula but a different arrangement
of atoms in the molecule and different properties. The isomer can also be
each of two or more atomic nuclei that have the same atomic number andthe same mass number but different energy states. For example, glucose,
fructose and galactose share the same molecular formula which is C6H12O6
however they differ by their structural formulae as follow:
One important aspect of the structure of pentoses and hexoses is that the
chain of carbon atoms is long enough to close up on itself and form a more
stable ring structure. This can be illustrated using glucose as an example.
When glucose forms a ring, carbon atom number 1 joins to the oxygen on
carbon atom number 5.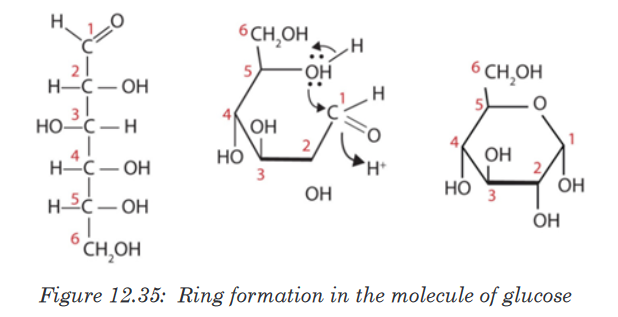
All hexoses sugars can exist as straight-chain structures but they tend to
form ring structures. Glucose, fructose, galactose can exist in ring structures.
Ring monosaccharides are said to be alpha (α) if the -OH group located
on carbon 1 is below the ring and beta (β) when the -OH group is above
the ring. The molecule of glucose for example can exist as alpha and beta
glucose denoted by α-glucose and β-glucose.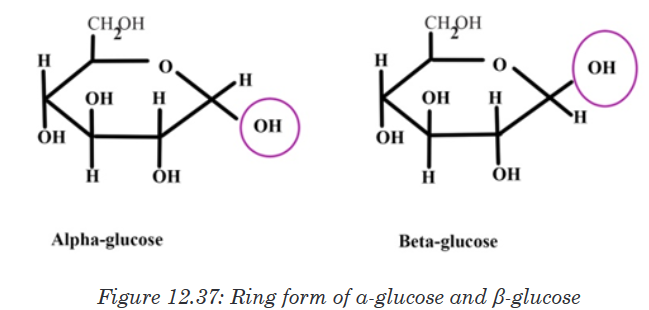
b. Formation and breakdown of glycosidic bonds
Monosaccharides may combine together in pairs to give a disaccharide
(double-sugar). The union involves the loss of a single molecule of water
and is therefore a condensation reaction. The bond which is formed is
called a glycosidic bond. It is usually formed between carbon atom1of one
monosaccharide and carbon atom 4 of the other, hence it is called a -1, 4-
glycosidic bond. Any two monosaccharides may be linked together to form a
disaccharide of which maltose, sucrose and lactose are the most common.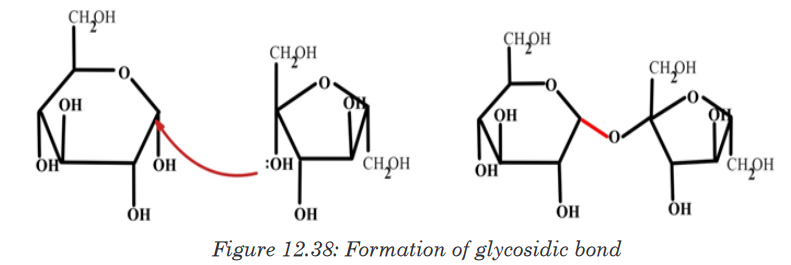
The addition of water under suitable conditions is necessary if the disaccharide
is to be split into its constituent monosaccharide. This is called hydrolysis
water-breakdown or more accurately, breakdown by water.Application activity 12.1
1. Provided with different kinds of biological molecules such as
carbohydrates, proteins, lipids, make a table to show their food source
you always take and suggest their functions.
2. Explain what is meant the essential amino acids
3. Describe the formation of a peptide bond?
4. Alanine is an amino acid with -CH3 as a side chain. Writes its structural
formulae.
5. Most of the plant lacks one or more of the essential amino acids
needed by the body explain how a vegetarian can obtain the essential
amino acids.
6. What are the structures of proteins?
7. How do we call the bond in a dipeptide?
8. What type of reaction is involved in the formation of glucose from
starch?
9. Use the type of reaction above to form glucose from sucrose molecule
10. Describe how the glycosidic bond is formed.
11. Describe the major types of starch12.2. Test for the presence of different biological molecules
in variety of contextActivity 12.2
You are given solutions containing different food stuffs including maize
flour, vegetable cooking oil, and egg white sugar cane liquid and passion
fruit. Using prior knowledge of biological molecules to suggest the type of
biological molecule in each one of them. Suggest the chemical tests used
to identify each of the molecules.2.2.1. Test for carbohydrates
Activity 12.2.1
Materials required:
Starch powder, Irish potatoes juice, prepared porridge, Iodine solution,
beakers, droppers, source of heat and test tubesA.Test for starch
Procedure
• Mix 1g of starch powder with 100ml of water
• Boil the mixture while stirring; then cool the solution
• Boil the mixture while stirring; then cool the solution
• Put 2ml of starch solution in a test tube labeled 1, 2ml of Irish potato
juice in a test tube labeled 2 and 2ml of prepared porridge in a test
tube labeled 3
• In each test tube put 2 drops of Iodine solution and shake
• Record your observation and draw a conclusionB.Test for reducing sugar
Requirements:
Glucose powder, beaker and test tube, Benedict solution, Bunsen burner,
droppers.
Procedure:
• In the beaker mix 1cm3 of water and 1g of glucose powder.
• Pour the prepared solution of glucose in a test tube and
• Add 2ml of benedict’s solution and heat
• Record your observation.Biological molecules are grouped into organic molecules including
carbohydrates, proteins, lipids, nucleic acids and vitamins. They also contain
inorganic molecules such as minerals and water. The first four organic
molecules are called macromolecules because they are required in organism
in large quantity. Carbohydrates including starch, reducing and non-reducing
sugars appear in this category and are the main energy producers in the
organisms. Others, including lipids and proteins are needed for building
organisms while vitamins protect the organisms against diseases.We need to ensure that what we take from diet have all required biological
molecules.
a. Test for starch
Carbohydrates such as starch are tested by mixing a sample with 2-4 drops
of iodine or Lugol’s solution. If the sample contains starch the solution
will turn from a yellow to brown color to a dark purple/dark blue (Figure
12.10). The color change is due to a chemical reaction between the large
carbohydrate molecule and the iodine ions. If the sample does not contain
starch the solution remains yellow-brown.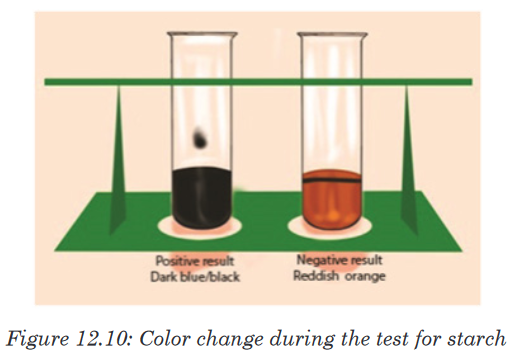
b. Testing for reducing and non-reducing sugar
The presence of reducing sugar can be tested by using benedict reagent.
Benedict solution has copper ions that have a light blue color. When this
solution is heated in the presence of simple reducing sugars such as
glucose, the blue color of copper ions changes from a light green color to
rusty orange-brown color (Figure 12.11).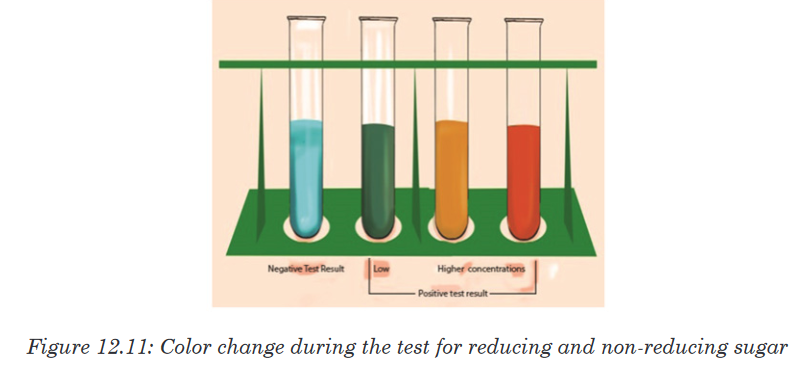
If the color of Benedict reagent persists, the sugar tested is not a reducing
sugar. Note that there is no special reagent to test for non-reducing sugar, but
by the addition of HCl, non-reducing sugars can be hydrolyzed to reducing
sugars. To test the presence of reducing sugars, a solution of sodium
hydroxide is needed to neutralize the acidity because Benedict reagent
works better in neutral solution.12.2.2. Test for proteins
Activity 12.2.2
Requirements
Milk, eggs, soybeans, test tubes, beakers, mortar for crushing beans, 1%
NaOH or 1% KOH solution, 0.1M of CuSO4 solution and Millon’s reagent.Procedure
• Extract the white fluid from an egg (albumen)
• Prepare an extra of soya bean and 10ml of fresh milk
• Put 2ml of albumen solution in a test tube labelled A1 and 2ml in A2
• Put 2ml of milk solution in a test tube labelled B1 and 2ml in B2
• Put 2ml of soya bean solution in a test tube labelled C1 and 2ml in
C2
• Put 1ml of KOH or NaOH solution in each of the test tubes A1, B1,
and C1. Shake the mixture and add 1ml of CuSO4 solution in each
(A1, B1, and C1) test tube
• Put 1ml of Millon’s reagent in each of test tubes (A2, B2, and C2).
Shake the mixture and thereafter boil the three test tubes (A2, B2,
and C2).
• Record and interpret your observations.The Biuret reagent is used to test for the presence of proteins. It contains
copper ions with blue characteristic color. During the copper ions react with
protein molecules and causes the biuret solution to turn from a light blue
color to purple if proteins are present.
The test can also be done by using Millon’s reagent, which in the presence
of proteins, the Millon reagent changes from colorless to pink.12.2.3. Test for lipids
Activity 12.2.3
Laboratory experiments
Use olive oil to carry out the following experiments
To 2 cm3 of olive oil in the test tube:
• Add 5 cm3 of ethanol followed by 5 drops of water.
• Shake the mixture and record your observation.
To another test tube containing 2 cm3 of olive oil:
• Add 5 drops of Sudan III solution
• Shake thoroughly and examine the mixture in the test tube after few
minute and record your observationsThe presence of lipids can be determined using Sudan III indicator, which is
fat-loving molecules that are colored. During the test for a solution containing
lipids, two results are likely to be found: there is either the separation of
layers indicating the levels of water and lipid, or the dye migrates toward
one of the layers. If the mixtures are all water soluble, the conclusion is that
the lipids are not present. In this case, the Sudan III indicator will form small
micelles/droplets and disperse throughout the solution.A positive result indicates the lipid layers sitting on top of the water layer with
a red-orange color. When using ethanol for testing lipids the presence of the
color changes from colorless to milky (emulsion test).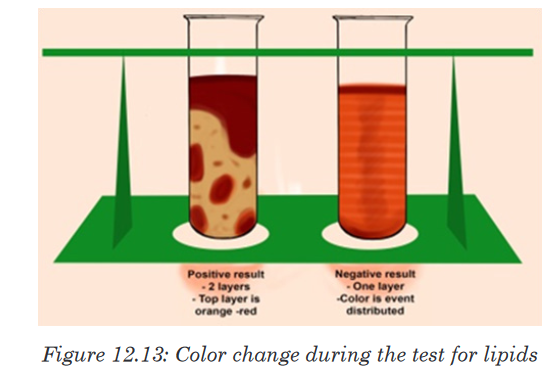
12.2.4. Test for vitamin C (Ascorbic Acid)
Activity 12.2.4
Squeeze the orange fruits to extract the juice and carry out the following
test.
Vitamin C is tested by using DCPIP (Dichlophenol Indophenol). Its positive
(presence of vitamin C) test decolorizes DCPIP, while the negative (absence
of vitamin C) test is indicated by the persistence blue color of DCPIP.12.3. The structure of DNA
Activity 12.3
1. Discuss the following in the class.
Nucleotide sequences make DNA, and DNA makes you. If DNA is
a double stranded structure, how do the two strands in DNA join or
stick together to form double stranded structure?
Make a report on it and present to the class.A basic unit nucleotide of DNA (Deoxyribo Nucleic Acid) is made up of
pentose sugar(Deoxyribose) , a nitrogenous base, and a phosphate sugar.
However, a combination of only a pentose sugar and nitrogenous base,
without phosphate group, is called nucleoside.Pentose sugar + Nitrogenous base + Phosphate group =Nucleotide
Pentose sugar + Nitrogenous base=Nucleoside
In DNA, bases are covalently bonded to the 1’ carbon of the pentose sugar.
The purine and pyrimidines bases attached to pentose sugar from different
positions of their nitrogen bases. Purine bases use the 9th position of nitrogen
to attach with 1’ carbon of pentose sugar, while pyrimidine bases use the 1st
position of nitrogen to attach with 1’ carbon of pentose sugar.In both DNA, the phosphate group (PO42–) attaches to the 5’ carbon of
pentose sugar. Thus, by attaching phosphate group to a nucleoside yields a
nucleoside phosphate or nucleotide. The complex of deoxyribose, nitrogenous
base and phosphate group is called DNA nucleotide (a deoxyribonucleotide).
Phosphodiester Bond formation
Two nucleotides are covalently joined together by a bond called
phosphodiester bond. In phosphodiester bond, the phosphate group, which
is attached on 5’ of one nucleotide, forms a bond with the 3’ carbon of another
nucleotide. In this way, many phosphodiester bonds are formed between
sugar and phosphate groups. The repeated sugar-phosphate-sugar-
phosphate backbone is a strong one. Because of this strong backbone, DNA
is a stable structure.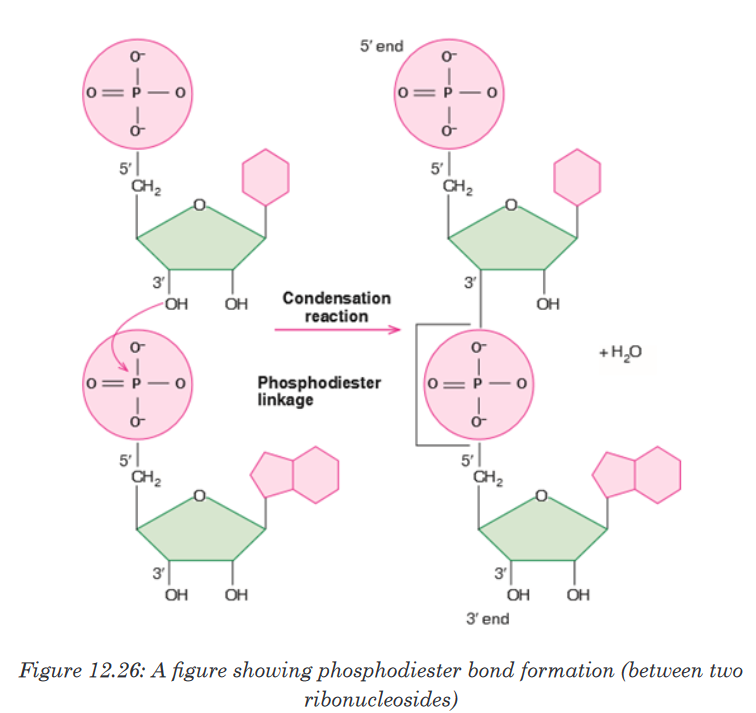
In 1953, James D. Watson, an American molecular biologist, and Francis
H.C.Crick, a British molecular biologist, proposed a model for the physical
and chemical structure of the DNA molecule. Today, their model is known
as double helix model of DNA or simply the Structure of DNA. The main
features of Watson and Crick double helix model of DNA are:1. Two polynucleotide chains wind around each other in a right-hand
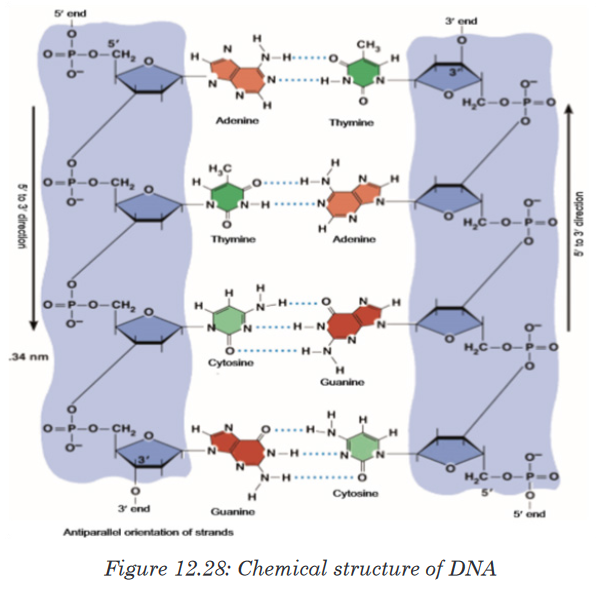
double helix.2. The two polynucleotide chains run side-by-side in an antiparallel fashion.
This means that one strand of DNA will orient itself in a 5’ -3’ direction,
whereas, the other strand will orient itself alongside the first one in a 3’-5’
direction. In this way, the two strands are oriented in opposite directions3. On one hand, the sugar-phosphate backbones lie outside of the double
helix. On the other hand, the bases orient themselves toward the central
axis of the double helix structure. The bases of one strand are bonded
with the bases of the other strand of double helix by hydrogen bonds.
These bonds are weak chemical bonds. Since hydrogen bonds are
relatively weak bonds, the two strands can be easily separated by heatingthe DNA. The bonding of these bases in the double helical structure
follows the Chargaff’s base pairing rules. For example—Adenine (A) will
form a hydrogen bond with Thymine (T). Similarly, Guanine (G) will form
a hydrogen bond with Cytosine (C). This specific base paring is called
complementary base pairing.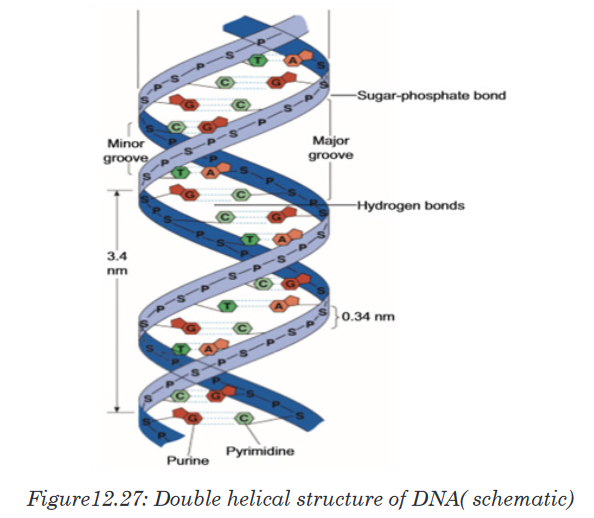
4. The distance between adjacent bases is 0.34 nm in the DNA helix. A
complete turn of the helix takes 3.4 nm. One complete turn, which is
360° turn, accommodates 10 base pairs (bp). And the diameter of the
helix is 2 nm.5. There are major and minor grooves in the double helix. The two sugar-
phosphate backbones of the double helix are not equally spaced from
one another along the helical axis, because of the way the base bind with
each other. As a result, there is an unequal size of grooves between the
backbones. The wider groove is called major groove; rich in chemical
information. The narrower groove is called minor groove; less rich in
chemical information.DNA is also Described as a Twisted Ladder Structure
A typical ladder has two long wooden or metal side strands or pieces between
which a series of rungs or bars are set in suitable distances. In the structure
of DNA, the pentose sugars and phosphate groups make up the “long two
side strands or pieces” of a typical ladder. And the A-T and G-C base pairs
which are bonded by hydrogen bonds make up the“rungs or bars”of a typical ladder. But unlike a typical ladder which is straight,
the two strands of DNA are twisted into spiral. Scientists call this a double
helix. DNA also folds and coils itself into more complex shapes. The coiled
shape makes it very small. In fact, it is small enough to easily fit inside any
of our cells. If a DNA from a cell is unfolded, it would stretch out to a length
of about six feet. The structural twisted nature of DNA has been attributed to
enhance its stability and strength. Thus, for these simple similarities with a
typical ladder, DNA is also referred as a twisted ladder structure.
Application activity 12.3
i. Watson and Crick proposed the model of ....................................
ii. Enzyme ..................................... maintains the length of telomere.
iii. ...................................... can be used to cure cancer.
iv. .................................. bonds are seen in both DNA and RNA.
v. ...................................... directs synthesis of proteins in the body12.4. Water and Enzymes
Activity 12.4.a
You are provided with three groups of enzymes: Group A Group B Group
C Enzymes Maltase and lactase Dehydrogenase and oxidase Pepsin
and renin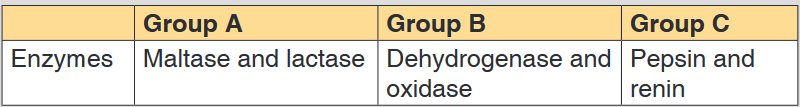
Make a research to find out:
a). Specific role of each of the six enzymes mentioned above
b). Criterion followed to name enzymes of group A, B and C respectivelyActivity 12.4.b
1. What is the medium of reaction in the organisms?
2. If two people are boiling the same quantity of cooking oil and
water, which one could evaporate first? Explain your choice.12.4.1. Water
Living organisms contain between 60% and 90% of water, the remaining
being the dry mass. The scientist accepts that life originates from water
and most of animals live in water. The function of water is defined by its
properties mainly: Its physical properties, solvent properties, heat capacity,
surface tension and freezing points. The physical and chemical properties of
water differ from those of most other liquids but make it uniquely effective in
supporting living activities.a). Physical properties of water
Water has the high boiling point (100°C) compared to other liquid due to the
hydrogen bond that exists among molecules of water. This help the water to
exist on the surface in a liquid state otherwise it would evaporate.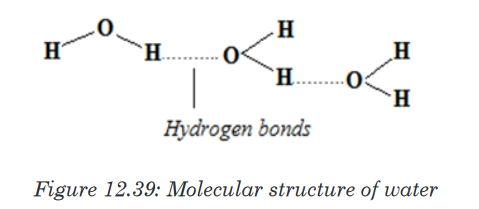
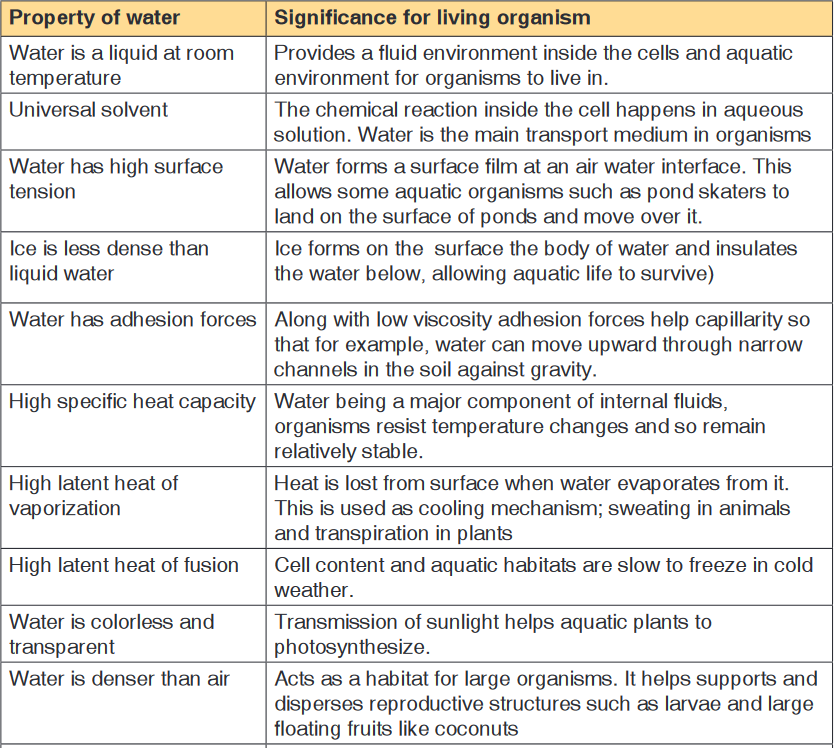
Table 12.4: Biological significance of the physical properties of water


b. Solvent properties of water
Water is a polar molecule due to its chemical arrangement of hydrogen
and oxygen atom in asymmetric shape instead of being linear. Most of the
substance that are transported in the blood is dissolved in the plasma, the
fluid part of the blood. Water occupies around 92% of the constituents of
plasma. Thus the oxygen atom has a positive charge and hydrogen atom
net positive charges. This is of great importance because all negative and
positive ions are attracted by water. Therefore, water is a good solvent
because the ionic solids and polar molecules are dissolved in it.
c. Heat capacity and latent heat of vaporization
Large changes of heat results in a comparatively small rise in water
temperature this explain why water has a high heat capacity compared to
other liquid. The high heat capacity is defined as the amount of heat required
to raise the temperature of 1gram to 10C.The high thermal capacity of water
make the ideal environment for life in plant and animals because it helps in
maintaining the temperature even if there will be environmental fluctuations
in temperature. The biological importance of this is that the range of
temperatures in which biochemical processes can proceed is narrow.The latent heat of vaporization is a measure of heat energy needed to cause
the evaporation of a liquid, which means to change from water liquid to
water vapor. During vaporization the energy transferred to water molecules
correspond to the loss of energy in the surroundings which therefore cool
down. During sweating and transpiration living organisms use vaporization
to cool down.i. Surface tension
The surface tension of water results from its polar nature, and is defined
as the ability of the external surface of the liquid to resist to external force
due to cohesive nature of its molecules. The high surface tension of
water and the cohesion force play a vital role in capillarity thus help the
transport of substance in vessels (tracheid of plant) to the stems and to
fulfill the blood in the cardiovascular vessels. Water being the second liquid
with high surface tension after mercury its surface tension is lowered by
the dissolution of ions and molecules and tend to collect at the interface
between its liquid phase and other.ii. Freezing points
Oppositely to other liquid water expand as it freezes, under 40 C
temperatures the hydrogen bond becomes more rigid but more open.
This explains why the solid water (ice) is less dense than the liquid water
and why the ice floats over water rather than sinking. When the bodies of
water freeze the ice float over the liquid act as an insulator and prevent
water below it from freezing. This protects the aquatic organisms so that
they can survive the winter.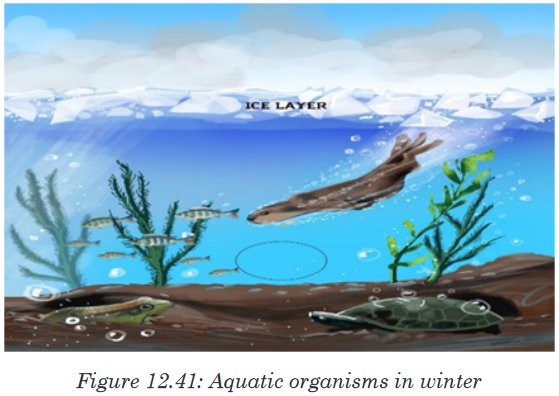
d. Functions of water
• Turgidity of plant cell which increase their size is due to the availability
of water.
• The transport of substances (minerals, nutrients in plant and animals)
is done in water.
• Excretion of waste product
• Support for hydrostatic skeleton.• Temperature regulation in plant and animals(transpiration)
• Seed germination by breaking down the seed coat
• Medium for biochemical reaction.12.4.2. Enzymes, their characteristics and actions
Activity 12.4.2a
You are provided with three groups of enzymes:

Make a research from text book or internet to find out:
a). What is the specific role of each of the six enzymes mentioned
above?
b). What criterion was followed to name enzymes of group A, B and C
respectively?a. Criteria for naming enzymes
Enzymes are biological catalyst produced by a living organism to control
the speed of specific biochemical reactions (metabolism) by reducing its
activation energy.First of all, Individual enzymes are named by adding -ase to the name
of the substrate with which they react. The enzyme that controls urea
decomposition is called urease; those that control protein hydrolyses are
known as proteases.A second way of naming enzymes refers to the enzyme commission number
(EC number) which is a numerical classification scheme for enzymes based
on the chemical reactions they catalyse.As a system of enzyme nomenclature, every EC number is associated
with a recommended name for the respective enzyme catalysing a specific
reaction. They include:• Oxidoreductases catalyse redox reactions by the transfer of hydrogen,
oxygen or electrons from one molecule to another. Example: Oxidase
catalyses the addition of oxygen to hydrogen to form water.• Glucose + oxygen glucose oxidase (→Oxidase )gluconic acid +water
• Hydrolase catalyses the hydrolysis of a substrate by the addition of
water.• Sucrose + water (→Hydrolase )glucose+ fructose
• Ligases catalyze reactions in which new chemical bonds are formed
and use ATP as energy source.• Amino acid + tRNA (→(ligase) )amino acid-tRNA complex.
• Transferases catalyze group transfer reactions. The transfer occurs
from one molecule that will be the donor to another molecule that will
be the acceptor. Most of the time, the donor is a cofactor that is charged
with the group about to be transferred. Example: Hexokinase used in
glycolysis.• Lyases catalyze reactions where functional groups are added to break
double bonds in molecules or the reverse where double bonds are
formed by the removal of functional groups. For example: Fructose
bisphosphate aldolase used in converting fructose 1,6-bisphospate to
G3P and DHAP by cutting C-C bond.• Isomerases catalyze reactions that transfer functional groups within a
molecule so that isomeric forms are produced. These enzymes allow
for structural or geometric changes within a compound. Sometime the
interconverstion is carried out by an intramolecular oxidoreduction.
In this case, one molecule is both the hydrogen acceptor and donor,
so there’s no oxidized product. The lack of a oxidized product is the
reason this enzyme falls under this classification. The subclasses are
created under this category by the type of isomerism. For example:
phosphoglucose isomerase for converting glucose 6-phosphate to
fructose 6-phosphate. Moving chemical group inside same substrate.A third way of naming enzymes is by their specific names e.g. trypsin and
pepsin are proteases. Pepsin, trypsin, and some other enzymes possess, in
addition, the peculiar property known as autocatalysis, which permits them
to cause their own formation from an inert precursor called zymogen.b. Characteristics of enzymes
Activity 12.4.2b
Requirement:
Three test tubes, match box, about 1g of liver, 1g of sands, 1% H2 O2 and
MnO2 powder.
Procedure:
• Label three test tubes A, B and C respectively.
• Put about 0.1 g of MnO2 powder in test tube A and 1g of liver in
tube B and 0.1g of sand in tube C.• Pour 5 ml of H2O2 (hydrogen peroxide) in each tube. What do you
observe?
• Place a glowing splint in the mouth parts of each test tube. What do
you observe?
Questions
1. Explain your observations.
2. Write down the chemical equation of the reaction taking place in
tube A and B
3. Carry out your further research to find out the characteristics of
enzymesThe following are main characteristics of enzymes
1. Enzymes are protein in nature: all enzymes are made up of proteins.2. Enzymes are affected by temperature. They work best at specific
temperatures; for example, enzymes found in human bodies work best
at 37oC. This is called the optimum temperature.• Very low temperatures inactivate enzymes. Therefore enzymes are
not able to catalyse reactions temperatures beyond the optimum
temperature denature enzymes. The structure of the protein molecule
is destroyed by heat.3. Enzymes work best at specific pH. Different enzymes have a given
specific pH at which they act best.
This pH is called optimum pH. Some enzymes work best at low pH (acidic
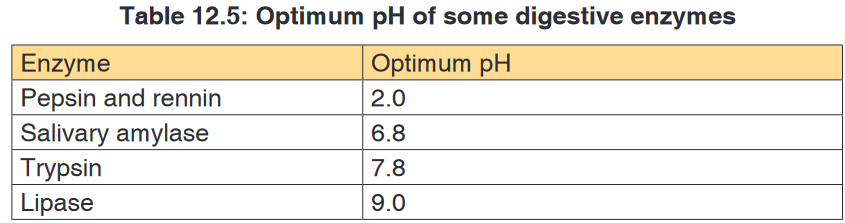
medium) while others work best at high pH (alkaline medium).Most enzymes in the human body for instance work best at neutral or
slightly alkaline pH. Examples are: lipases, peptidases and amylase. A
few enzymes like pepsin that digests proteins in the stomach works best
at an acidic pH of 2.4. Enzymes remain unchanged after catalysing a reaction. Enzymes
are catalysts and can therefore be used over and over again in small
amounts without being changed.5. Enzymes catalyse reversible reactions. This means that they can change
a substrate into products and the products back to the original substrate.
6. Enzymes are substrate-specific. This means that an enzyme can only
catalyse one reaction involving aparticular substrate. This is because
they have active sites which can only fit to a particular substrate whose
shape complements the active site. For example, pepsin works on
proteins but not on fats or starch.7. Enzymes work rapidly. Enzymes work very fast in converting substrates
into products. The fastest known enzyme is catalase, which is found in
both animal and plant tissues.8. Enzymes are efficient. This is best described by the fact that:
• They are required in very small amounts.
• They are not used up in a reaction and can therefore be used
repeatedly.9. Enzymes are globular proteins.
10. Enzymes lower the activation energy (Ea) required for reactions to take
place. In many chemical reactions, the substrate will not be converted to
a product unless it is temporarily given some extra energy. This energy
is called activation energy i.e. the minimum energy required the make a
reaction take place.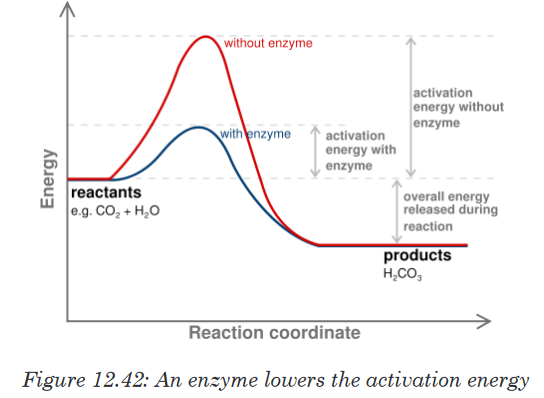
An enzyme provides a reaction surface for a reaction to take place. This
is normally a hollow or cleft in the enzyme which is called the active site,
but it is normally hydrophobic in nature rather than hydrophilic. An enzyme
provides a reaction surface and a hydrophilic environment for the reaction
to take place.A very small amount of enzymes is needed to react with a large amount of
substrate. The turnover number of an enzyme is the number or reactions
an enzyme molecule can catalyse in one second. Enzymes have a highturnover number e.g. the turnover number of catalase is 200,000 i.e. one
molecule of enzyme catalase can catalyse the breakdown of about 200,000
molecules of hydrogen peroxide per second into water and oxygen at body
temperature.A cofactor is the best general term to describe the non-protein substances
required by an enzyme to function properly. This term covers both organic
molecules and metal ions. A co-enzyme is an organic molecule that acts as
a cofactor. A prosthetic group is a cofactor that is covalently bound to the
enzyme.12.4.3. Factors affecting enzyme action
Activity 12.4.3
You will need
Eight test tubes containing 2 cm3 starch solution, amylase solution, and
cold water (ice) water bath, iodine solution, HCl solution, and droppers
Procedure:
1. Label your test tubes A-D as follows:
2. Add 1 cm3 of starch solution to each test tube
3. Keep tube A and B in cold (ice) and tube C and D in the water bath
at 350C for 5 minutes.4. Add 1 cm3 of 1M HCl on test tubes B and D, then shake the mixture
to stir.5. Add 1 cm3 of amylase solution on each test tube. Shake and therefore
keep A and B in cold and C and D in water bath for 10 minutes.6. Take a sample from each tube and mix it with one drop of iodine.
Use a different tile for each test tube. Record and interpret your
observation and then draw a conclusion.Enzymes activities can be limited by a number of factors such as the
temperature, the pH, the concentration of the substrate or the enzyme itself
and the presence of inhibitors.i. Temperature
At zero temperature, the enzyme cannot work because it is inactivated. At
low temperatures, an enzyme-controlled reaction occurs very slowly.The molecules in solution move slowly and take a longer time to bind to
active sites. Increasing temperature increases the kinetic energy of the
reactants. As the reactant molecules move faster, they increase the number
of collisions of molecules to form enzyme-substrate complex.At optimum temperature, the rate of reaction is at maximum. The enzyme is
still in active state. The optimum temperature varies with different enzymes.
The optimum temperature for enzymes in the human body is about 37oC.
When the temperature exceeds the optimum level, the enzyme is denatured.The effect is irreversible. However, some species are thermophilic that is
they better work at high temperatures; others are thermophobic, that is they
better work at low temperatures. For example, some thermophilic algae and
bacteria can survive in hot springs of 60oC.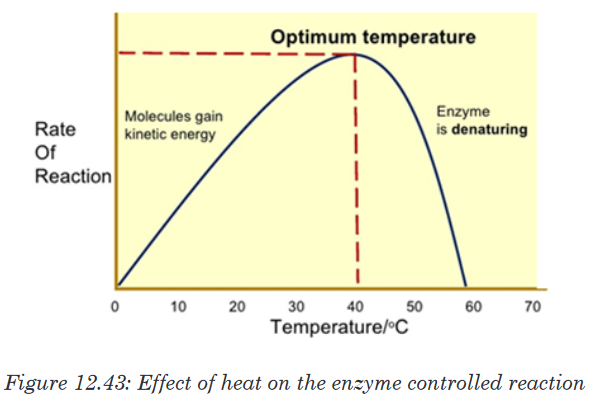
The rate doubles for each 10°C rise in temperature between 0°C and 40°C.
The temperature coefficient Q10 is the number which indicates the effect of
rising the temperature by 10°C on the enzyme-controlled reaction. The Q10
is defined as the increase in the rate of a reaction or a physiological process
for a 10°C rise in temperature. It is calculated as the ratio between rate of
reaction occurring at (X + l0)°C and the rate of reaction at X °C. The Q10 at
a given temperature x can be calculated from: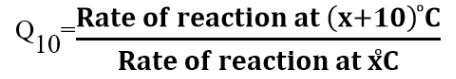
Worked out example
The rate of an enzyme-controlled reaction has been recorded at different
temperatures as follows:
This means that the rate of the reaction doubles if the temperature is raised
from 30oC to 40oCBe aware that not all enzymes have an optimum temperature of 40°C. Some
bacteria and algae living in hot springs (e.g. Amashyuza in Rubavu) are
able to tolerate very high temperatures. Enzymes from such organisms are
proving useful in various industrial applications.ii. The pH
Most enzymes are effective only within a narrow pH range. The optimum pH
is the pH at which the maximum rate of reaction occurs. Below or above the
optimum pH the H+ or OH- ions react with functional groups of amino acids
in the enzyme which loses its tertiary structure and become natured.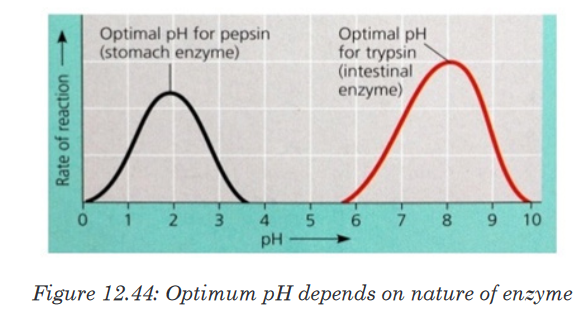
Different enzymes have different pH optima (look in the table).
iii. Enzyme concentration
The rate of an enzyme-catalyzed reaction is directly proportional to the
concentration of the enzyme if substrates are present in excess concentration
and no other factors are limiting.
iv. Substrate concentration
At low substrate concentration, the rate of an enzyme reaction increases with
increasing substrate concentration. The active site of an enzyme molecule
can only bind with a certain number of substrate molecules at a given time.
At high substrate concentration, there is saturation of active sites and the
velocity of the reaction reaches the maximum rate.
v. Inhibitors
The inhibitors are chemicals or substances that prevent the action of an
enzyme. An inhibitor binds to an enzyme and then decreases or stops its
activity. There are three types of inhibitors:
a). Competitive inhibitors are molecules that have the similar shape
as the substrate. They are competing with the substrate to the active
site of the enzyme e.g. O2 compete with CO2 for the site of RuBP-
carboxylase.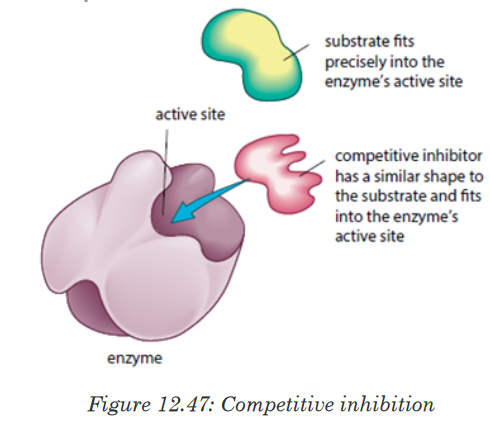
b). Non-competitive inhibitors are molecules that can be fixed to the
other part of enzyme (not to the active site) so that they change the
shape of active site, due to this the substrate cannot bind to the
active sit of the enzyme.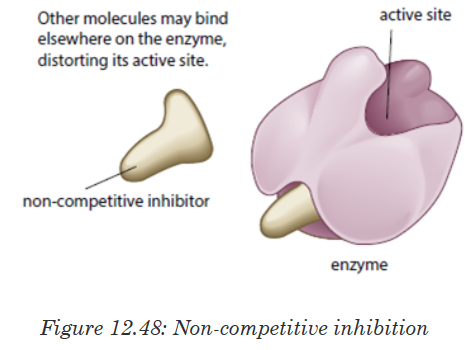
c). End product inhibitor or Allosteric inhibitor or Allostery.
This is a chain enzymatic metabolic pathway where the final end product acts
as an allosteric reversible inhibitor for the first, the second or the third step
in the metabolic pathway. The shape of an allosteric enzyme is altered by
the binding of the end product to an allosteric site. This decreases enzymatic
activity. By acting as allosteric inhibitors of enzymes in an earlier metabolic
pathway, the metabolites can help to regulate metabolism according to the
needs of organisms. This is an example of negative feedback.This often happen when few enzymes are working on a large number
of substrate e.g. ATP is an end-product inhibitor of the enzyme PFK
(Phosphofructokinase) in glycolysis during cell respiration. The end-product
inhibitor leads to a negative feedback.
The products of enzyme-catalysed reactions are often involved in the
feedback control of those enzymes. Glucose-1-phosphate is the product
formed from this enzyme-catalysed reaction. As its concentration increases,
it increasingly inhibits the enzyme.Note: Reversible and irreversible inhibition
Competitive inhibitor is reversible inhibitor as it binds temporarily to the
active site. It can be overcome by increasing the relative concentration of
the substrate. Some non-competitive inhibitors are reversible, that is, if the
inhibitor binds temporarily and loosely to the allosteric site. Some inhibitors
have very tightly, often, by forming covalent bonds with enzyme.The nerve gas DIPF (DiIsopropylPhosphoFluoridate) is an irreversible
inhibitor. It binds permanently with enzyme acetylcholisterase, altering
its shape. The enzyme cannot bind with and break down its substrate
acetylcholine (neurotransmitter). Acetylcholine molecules accumulate in the
synaptic cleft.Nerve impulses cannot be stopped causing continuous muscle contraction.
This leads to convulsions, paralysis and eventually death.Many pesticides such as organophosphate pesticides act as irreversible
enzyme inhibitors. Exposure to pesticides can produce harmful effects to
the nervous and muscular systems of humans. Heavy metal ions such as
Pb2+, Hg2+, Ag+, As+ and iodine-containing compounds which combine
permanently with sulphydryl groups in the active site or other parts of the
enzyme cause inactivation of enzyme.This usually disrupts disulphide bridges and cause denaturation of the
enzyme.12.4.4. Importance of enzymes in living organisms
Activity 12.4.4
Elaborate your ideas about the need for different enzymes in living
organisms.Without enzymes, most of the biochemical reactions in living cells at body
temperature would occur very slowly at not at all. Enzyme can only catalyze
reactions in which the substrate shape fits that of its active siteThere are thousands upon thousands of metabolic reactions that happen
in the body that require enzymes to speed up their rate of reaction, or will
never happen. Enzymes are very specific, so nearly each of these chemical
reactions has its own enzyme to increase its rate of reaction. In addition, the
organism has several areas that differ from one another by the pH. Therefore,
the acid medium requires enzymes that work at low pH while other media
are alkaline and therefore require enzymes that work at high pH. In addition
to digestion, enzymes are known to catalyze about 4,000 other chemical
reactions in your body. For example, enzymes are needed to copy genetic
material before your cells divide.Enzymes are also needed to generate energy molecules called ATP, move
fluid and nutrients around the insides of cells and pump waste material out
of cells. Most enzymes work best at normal body temperature – about 98
degrees Fahrenheit – and in an alkaline environment. As such, high fevers
and over-acidity reduce the effectiveness of most enzymes. Some enzymes
need co-factors or co-enzymes to work properly.12.4.5. Mode of action of enzymes
Activity 12.4.5
There are two main hypotheses that explain the more of action of an
enzyme on its substrate: the lock and key hypothesis and the induced-fit
hypothesis. Carry out a research to find the relevance of each.Enzymes do not change but substrates are converted to products. A substrate
is a molecule upon which an enzyme acts. In the case of a single substrate,
the substrate binds with the enzyme active site to form an enzyme-substrate
complex. Thereafter the substrate is transformed into one or more products,
which are then released from the active site.This process is summarized as follows:

Whereby: E = enzyme, S = substrate(s), ES = Complex Enzyme-Substrate
and P= product (s). There are two main hypotheses explaining the mechanism
of enzyme action:a. The lock and key hypothesis by Emil Fischer
In this hypothesis the substrate is the key and enzyme is the lock. In otherwise
the active site is exactly complementary to the shape of the substrate.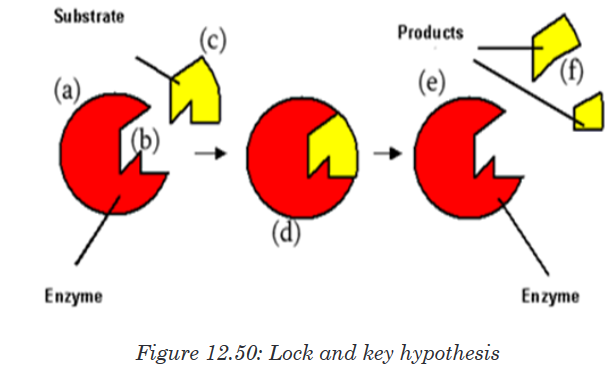
b. The induced-fit hypothesis by Daniel Koshland
The induced-fit hypothesis is a modified version of the lock and key hypothesis
and is more widely accepted hypothesis. In this hypothesis, the active site
is flexible and is not exactly complementary to the shape of the substrate.An enzyme collides with the substrate molecule.The substrate binds to the
active site. The bindings induce a slight change in the shape of the enzyme
to enclose the substrate making the fit more precise. The active site now
becomes fully complementary with the substrate as the substrate binds to
the enzyme.
Application activity 12.4
1. Fill the blank with appropriate terms: Enzymes are
biological ____________________ produced by
___________________________ cells. Enzymes reduce the
amount of ____________________ energy required for reactions
to occur. They consist of globular ____________________ with
_______________________ structure.
2. Answer the following questions:
a). What is the main role of enzymes?
b). What would happen if there are no enzymes in the cell? State any
four properties of enzymes.Skills lab 12
Test the components of the food and beverages produced by different
companies in our country. Here, you will need to use the knowledge of food
test techniques.test techniques.End unit assessment 12
1. Biological molecules are divided into (Choose the correct answer):
a). Organic molecules and inorganic molecules
b). Carbohydrates and starch
c). Lipids, carbohydrates and water
d). Carbohydrates, food and potatoes
2. Name the reagents that are used to test for the following food
substances
a). Lipids
b). Starch
c). Reducing sugar
3. Some drops of fresh pineapple fruit juice are added drop by drop to
DCPIP solution. The deep blue color of the DCPIP quickly fades.
a). Explain why the blue colour disappeared?
b). What is the importance of this food substance to the human body?
4. Write the formula of a monosaccharide with 3 atoms of carbon
5. Compare the structure of fat(triglycerides)and the phospholipids
6. Give two examples of how carbohydrates are used in the body.
7. The formula for a hexose is C6H12O6 or (CH2O)6. What would be the
formula of?a). Triose
b). Pentose
8. Distinguish between:
a). Alpha glucose and beta glucose
b). Glycogen and cellulose
c). Amylopectin and amylose
9. The drug can cleave the covalent bond between two sulfur atoms
of non-adjacent amino acids. Which level of protein can be affected
the most if the drug is mixed with primary, secondary, tertiary and
quaternary structure of proteins.
10. State the property of water that allows each of the following to take
place. In each case, explain its importance:a). The cooling of skin during sweating
b). The transport of glucose and ions in a mammal
c). Much smaller temperature fluctuations in lakes and oceans than
in terrestrial (land-based) habitats.
11. Construct the table that organize the following terms and label the
columns and rows.
Phosphodiester linkages Monosaccharide polypeptides
Peptide bonds Nucleotides Triacylglycerol
Glycosidic linkages Amino acids Polynucleotides
Ester linkages fatty acids Polysaccharides
12. Explain what happen during protein denaturation?
13. Enzymes are biocatalysts.
a). What is the meaning of the following terms elated to enzyme
activity?
i. Catalyst
ii. Activation energy
iii. Lock and key
iv. Q10
b). Why are there hundreds of different enzymes in a cell?
c). How do enzymes reduce the activation energy of a reaction?
14. Enzyme activity is related to a number of factors.
a). Explain why enzymes work faster at high temperatures
b). Describe what happens to the enzyme structure if the temperature
is raised well above the optimum temperature.
c). How are enzymes affected by pH?
d). Why do different enzymes have a different optimum pH?
e). What is the difference between a reversible and irreversible
enzyme inhibitor?15. Some bacteria and algae can survive in the boiling waters of hot
springs. Enzymes from these organisms are used in industrial
processes. Why are these enzymes useful?16. The following set data show the effect of temperature on the
completion time of an enzyme reaction.
a). Plot the data on a graph
b). What is the optimum temperature of this reaction?
c). Describe the shape of the graph between 10 and 400 C
d). Calculate the rate of increase between 20 and 300 C.
