Key Unit CompetenceDescribe how protein structure is related to function and the role of water as a special molecule with extrardinary properties that make life possible.
Learning objectives
By the end of this unit, I should be able to:
–– Describe the structure of an amino acid and the formation and breakage of a peptide bond.
–– Describe the primary, secondary, tertiary and quaternary structure of proteins.
–– Describe the molecular structure of hemoglobin as an example of a globular protein.
–– Describe the functions with an emphasis on iron in the hemoglobin molecule.
–– Explain the effect of heat, pH and chemicals on protein structure.
–– Explain how hydrogen bonding occurs between water molecules and relate the properties of water to its roles in living organisms.
–– Devise an experiment to investigate the effect of temperature, pH and chemicals on the structure of protein.
–– Relate the structure of globular and fibrous proteins to their functions.
–– Investigate the effect of lowering temperature on water.
–– Distinguish between collagen molecules and collagen fibres
–– Appreciate the importance of globular and fibrous proteins in biological processes such as the transport of gases and providing support for tissues.
–– Express that protein structure is central to many aspects of biology, such as enzymes, antibodies and muscle contraction.
–– Acknowledge that water is a special molecule with extraordinary properties that make life possible on this planet.
Introductory activity
1. What are proteins?
2. What do you understand by universal solvent in living organisms?
8.1 Proteins
Proteins are organic compounds of large molecular mass. For example, the hemoglobin has a molecular mass of 64500. In addition to carbon, hydrogen and oxygen, proteins always contain nitrogen, usually sulphur and sometimes phosphorus. Proteins are polymers of amino acids and they are not truly soluble in water, but form colloidal suspensions.
8.1.1. Amino acids
Amino acids are group of over a hundred chemicals of which around 20 commonly occur in proteins. They always contain a basic group, the amine group (-NH2) and a carboxylic acid group(-COOH) together with -R group or side chain (Figure 8.1). All the amino acid differs one to another by the structure of their side chain.
Amino acids are divided into two categories: essential amino acid and non-essential amino acid. Essential amino acids are those amino acids which cannot be synthesized by the body. Non –essential amino acids are synthesized by the organism. All 20 amino acids can be found in diet from plant and animal tissues.
Amphoteric nature of an amino acid
When an amino acid is exposed to basic solution, it is deprotonated (release of a proton H+) to became negative carboxylate COO -while in acid solution it is protonated (gains of a proton H+) to became ammonium positive ion -NH3 +(Figure 8.1.3.a and Figure 8.1.3.b).
At a physiological pH, usually around 7, the amino acid exists as ZWITTERION (from German means hermaphrodite) it is a molecule with two different charges (positive and negative) at the same time (Figure 8.1.4).
8.1.2. Formation and breakage of peptide bond
The formation of peptide bond follows the same pattern as the formation of glycosidic bond in carbohydrates and ester bond in fats. A condensation reaction occurs between the amino group of one amino acid and the carboxyl group of another, to form a dipeptide (fig 8.5).
A peptide bond is formed between two amino acids to form a dipeptide molecule. If three amino acids are assembled togetherthey form a tripeptidewhile four amino acids form a tetrapeptide and so on. A long chain of amino acid it is called a polypeptide. The polypeptide chain or oligopeptide comprise more than 50 amino acids joined together by peptide bonds.
During digestion, proteins are hydrolyzed to give amino acids that can be diffuse across the wall of intestine into blood stream. In hydrolysis the peptide bond breaks down by the addition of a water molecule (Figure 8.5).
Self-assessment 8.1
1. Explain what are essential amino acids?
2. Describe the formation of a peptide bond?
3. At physiological pH, the amino acid exists as zwitterions. What is a zwitterion?
4. Alanine is an amino acid with -CH3 as a side chain. Write its structural formulae.
5. Most plants lack one or more of the essential amino acids needed by the body. Explain how a vegetarian can obtain the essential amino acids.
8.2 Structure and denaturation of proteins
Activity 8.2
1. From the books make a research on proteins and answer to the following questions:
a. What are different structures of proteins?
b. Differentiate globular proteins and fibrous proteins.
2. Take a plastic rope cord, create the nodes bulk on it and suppose that those are
monomers of a long chain of polymer (the whole cord). Heat it using a Bunsen
burner or another source of fire. Discuss the change that takes place.
8.2.1. Structure of proteins
The long chain of polypeptide can take different forms according to its molecular weight and the types of bond that hold together atoms and molecules.
a. Primary structure of proteins
Primary structure of a protein is the sequence of amino acid thatis made up of the polypeptide chain or chains.
Ribonuclease is an enzyme found in pancreatic juice, which hydrolyses (digests) RNA. Notice that at one end of the amino acid chain there is an –NH3+ group, while at the other end there is a –COO− group. These are known as the amino and carboxyl ends, or the N and C terminals, respectively. (Adapted from Cambridge International AS and A Level Biology Course Book Fourth Edition)
b. Secondary structure of proteins
The regular arrangement of amino acids in primary structure can induce the interaction of the back bone of the polypeptide chain (side chain) by hydrogen bonds. Those side chains are coiled and folded in the patterns that contribute to the protein’s overall shape. One such secondary structure is α-helix and sometime β-pleated sheet (Figure 8.8).
c. Tertiary structure of proteins
In addition to hydrogen and peptide bond in primary and secondary structure, the tertiary structure of protein has other types that include:
–– Hydrophobic interaction
–– Ionic bond between positively and negatively charged r groups.
–– Disulfide bridges (-s-s)
d. Quaternary structure of proteins
Quaternary structure involves more than one polypeptide chain chemically bonded to each other. The quaternarystructure refers to the way in which these polypeptide chains are arranged in the protein. Examples, Hemoglobin that is composed of:
–– Four polypeptide subunits, two α- chains and two β- chains. Both α and β subunits primarily are α helical secondary structure polypeptide chain with 140 amino acids.
–– Haeme composed of iron that binds with oxygen.
Collagen: this is a fibrous protein consisting of three helical polypeptides that are supercoiled to form a rope like structure of great strength
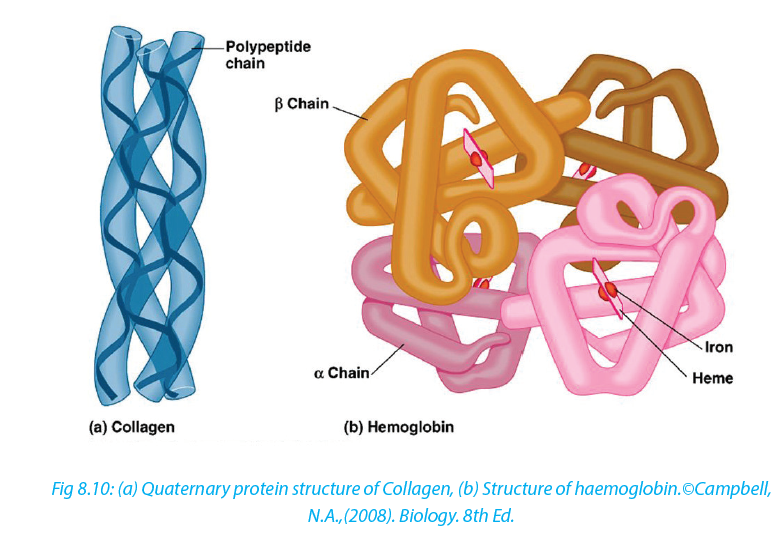
Globular protein
These are polypeptide chains that are tightly folded to form a spherical shape. Many globular proteins are folded so that their hydrophobic groups are on the inside of the molecule and the hydrophilic groups face outwards making these proteins soluble in water.
Properties of globular protiens:
–– They are spherical in shape
–– Physiologically active
–– Soluble in water.
–– May contain prosthetic group for example the iron (haeme)
–– Examples include hemoglobin and enzymes.
8.2.2. Protein denaturation
Protein denaturation is a process by which protein changes shape due to breakage
of bonds holding the polypeptide chains. Protein denaturation may be temporary or permanent.
The agent of denaturation can be caused by;
–– Extremely high temperatures beyond optimum,
–– changes in pH,
–– Ultra Violet (UV) rays,
–– High salt concentration and heavy metals.
8.2.3. Functions of proteins.
–– Proteins such as lipase, pepsin and protease act as enzymes as they play a crucial role in biochemical reaction where they act as catalysts.
–– Proteins play an important role in coordination and sensitivity (hormones and pigments).
–– Proteins have a transport functions. Example: Haemoglobin transport oxygen
–– Proteins in the cell membrane facilitate the transport of substance across the cell membrane.
–– Proteins provide a mechanical support and strength.
–– Proteins such as myosin and actin are involved in movement.
–– Proteins play the role of defense of the organisms. Example: Antibodies are proteins
8.3 Water
Activity 8.3
1. What is the medium of reaction in the organisms?
2. If two people are boiling the same quantity of cooking oil and water, which one could evaporate first? Explain your choice.
Living organisms contain between 60% and 90% of water, the remaining being the dry mass. The function of water is defined by its physical and chemical properties that differ from those of most liquids and make it effective in supporting life.
8.3.1 Biological significance of the physical properties of water
Functions of water
–– Turgidity of plant cell which increases their size is due to the availability of water.
–– The transport of substances (minerals, nutrients in plant and animals) that are dissolved in water.
–– Excretion of waste product
–– Support for hydrostatic skeleton.
–– Temperature regulation in plant and animals
–– Seed germination by breaking down the seed coat
–– Medium for biochemical reaction.
Self-assessment 8.3
1. State the functions of water in animals
2. What do you understand by heat capacity?
3. Relate the high heat capacity of water to its biology functions.
4. Describe and explain how aquatic organisms live below frozen water
bodies
End of unit assessment 8
1. Certain drugs can break the covalent bond between two sulfur atoms of nonadjacent amino acids. Which level of protein that can be affected most if the drug is mixed with primary,secondary,tertiary and quaternary structure of proteins?
2. Complete the following statements by appropriate terms:
a. The formation of large molecules from small repeating units is called ………reaction.
b. A carbohydrate(polysaccharide)that is formed by the plant as a reserve food supply and made up of only glucose molecules covalently bonded
together is……...
3. State the property of water that allows each of the following to take place. In each case,explain its importance:
a. The cooling of skin during sweating
b. The transport of glucose and ions in a mammal
c. Much smaller temperature fluctuations in lakes and oceans than in terrestrial (land-based) habitats.
4. Construct a three column table and relate the following terms with arrows to
indicate the correct match.
Phosphodiester linkages Monosaccharide Polypeptides
Peptide bonds Nucleotides Triacylglycerol
Glycosidic linkages Amino acids Polynucleotides
Ester linkages Fatty acids Polysaccharides
5. Explain what happens during protein denaturation?
