UNIT 9: EFFECT OF X-RAYS
Key unit competence: BAnalyze and evaluate the effects of x-rays.
My goals• Explain the production of X-rays
• State the properties of X-rays.
• Explain the origin and characteristic features of an x-ray spectrum.
• Outline the applications of X-rays in medicine, industries, and scientific
research
• Solve problems involving accelerating potential and minimum
wavelength of X-rays.
• Recognize how the intensity and quality of X-rays can be controlled.
• Appreciate the use of X-rays in medicine and industryWhen a person goes to the hospital with pain in her/his chest, or with anINTRODUCTORY ACTIVITY
internal fracture of the bone, physicians do normally recommend the patient
to pass by radiology service. Hence try to answer the following questions:1. Why do physicians recommend patients to pass by radiology service?9.1 PRODUCTION OF X-RAYS AND THEIR PROPERTIES
2. Radiology means that there are radiations. Discuss different types of
radiations that are found in there?
3. Discuss the production of X-ray radiations.
4. What are the positive and negative effects of X-ray radiation on thehuman body?
ACTIVITY 9.1: Investigating the production of X-rays
Read the following text and answer the questions that follow.
Discovery of X-rays: Becquerel’s discovery wasn’t the only important
accidental one. In the previous year W.C. Roentgen unexpectedly
discovered X-rays while studying the behavior of electrons in a
high voltage vacuum tube. In that instance, a nearby material was made to
fluoresce. Roentgen named them X- rays because he didn’t know what
they were.
Within twenty years of this discovery, diffraction patterns produced
using X-rays on crystal structures had begun to show the finer structure
of crystals while, at the same time, giving evidence that X-rays had a
wave nature. Since then, X-ray radiation has become an indispensable
imaging tool in medical science.
Questions:1. What do you understand by X-rays?9.1.1 X-ray production
2. How are X-rays produced?3. Where are X-rays used?
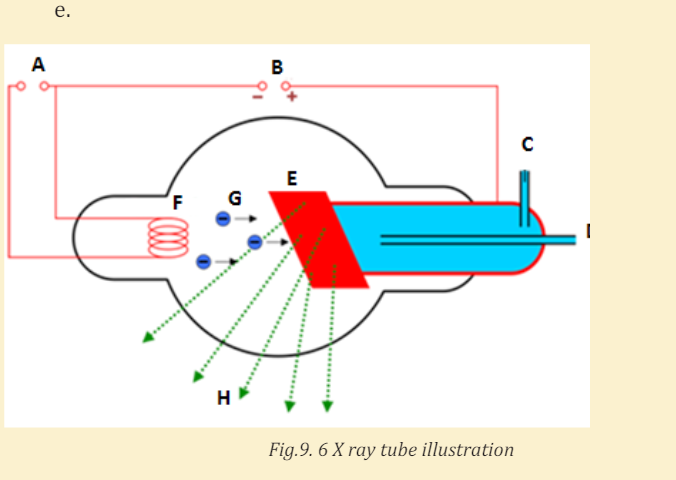
X-rays are produced when fast moving electrons strike matter (see Fig.9.1).
They were first produced in 1895 by Wilhelm Rontgen (1845-1923), using anapparatus similar in principle to the setup shown in Fig.9.1.
Electrons are emitted from the heated cathode by thermionic emission and are
accelerated toward the anode (the target) by a large potential difference V. The
bulb is evacuated (residual pressure 10−7 atm or less), so that the electrons can
travel from the cathode to the anode without colliding with air molecules. It
was observed that when V is a few thousands volts or more, a very penetratingradiation is emitted from the anode surface.
The above figure is an illustration of the Coolidge tube which is the most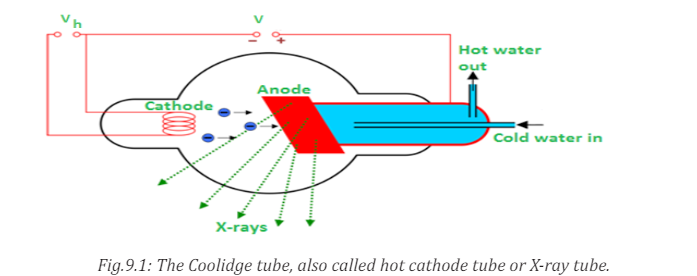
widely used device for the production of X-rays. The electrons are produced by
thermionic effect from filament, which is the cathode of the tube, heated by an
electric current. These electrons are accelerated towards a metal target that isthe anode due to the high potential voltage between the cathode and the anode.
The target metals are normally Tungsten or Molybdenum and are chosen
because they have high melting point and higher atomic weights. The accelerated
electrons interact with both electrons and nuclei of atoms in the target and
a mysterious radiation is emitted. This radiation was referred to as X-rays.
About 98% of the energy of the incident electron is converted into heat that isevacuated by the cooling system and the remaining 2% come out as X-rays.
9.1.2 Types of X-rays
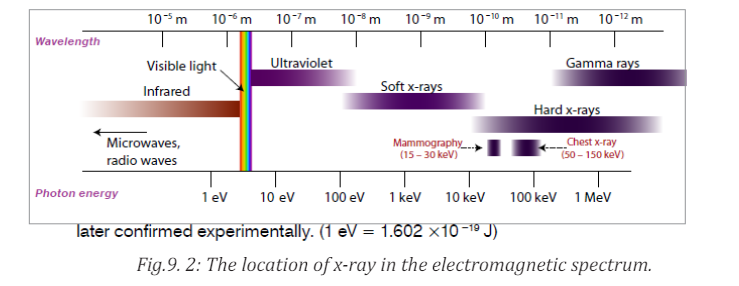
Sometimes X-rays are classified according to their penetrating power. Two
types are mentioned:• Hard X-rays: those are X-rays on upper range of frequencies or
shorter wavelength. They have greater energy and so they are more
penetrating.
• Soft X-rays: they are X-rays on lower range of frequencies or
longer wavelength. They have lower energy and they have very low
penetrating power. The Fig.9.2 below shows the relative location of thedifferent types of X-rays.
Hard X-rays are produced by high accelerating potential. They have high
penetrating power and short wavelength while soft X-rays are produced
by lower accelerating potential, have relatively low penetrating power andrelatively long wavelength.
9.1.3 Properties of X-rays
ACTIVITY 9.2: Understanding the pros and cons of X-rays
Make intensive research on the production and the properties of
X-rays, then write a report about your findings.
The following are the main properties of X-rays:a. X-rays can penetrate through most substances. However, their penetratingFrom the above characteristics it can be seen that X-rays have the properties
power is different.
b. X-ray can produce fluorescence in different substances.
c. X-rays can blacken photographic plate. The degree of blackening depends
upon the intensity of x-rays incident upon the plate. Thus, X-ray intensity
can be measured with the help of photographic plates.
d. X-rays ionize the gas through which they travel. The ionizing power
depends on the intensity of the x-ray beam. Thus, X-ray intensity can also
be measured by measuring their ionizing power.
e. X-rays are not deflected by electric or magnetic fields. This proves that
unlike cathode rays or positive rays they are not a beam of charged
particles.
f. X-rays travels on a straight lines like ordinary light.
g. X-ray are both reflected and refracted.
h. X-rays can be diffracted with the help of crystalline substances. They canalso be polarized.
that are common to all electromagnetic radiations.9.1.4 Checking my progress
1. Describe the process by which X-rays are produced.
2. Discuss and describe the types of X-rays?
3. What is the meaning of the X in X-ray?4. How are X-rays different from other electromagnetic radiations?
9.2 THE ORIGINS AND CHARACTERISTIC FEATURES OF AN
X-RAY SPECTRUM
ACTIVITY 9.3: investigating the X-ray spectrum
During the production of X-rays, a high voltage must be applied across
the x rays tube to produce enough acceleration of electrons towards the
target.
Search internet, then discuss and explain the relationship between theapplied
9.2.1 Variation of the X-ray intensity with wavelength
Depending on the accelerating voltage and the target element, we may find
sharp peaks superimposed on a continuous spectrum as indicated on Fig.9.3.
These peaks are at different wavelengths for different elements; they form whatis called a characteristic x-ray spectrum for each target element.
X-rays of different wavelengths are emitted from X-ray tube. If the intensity is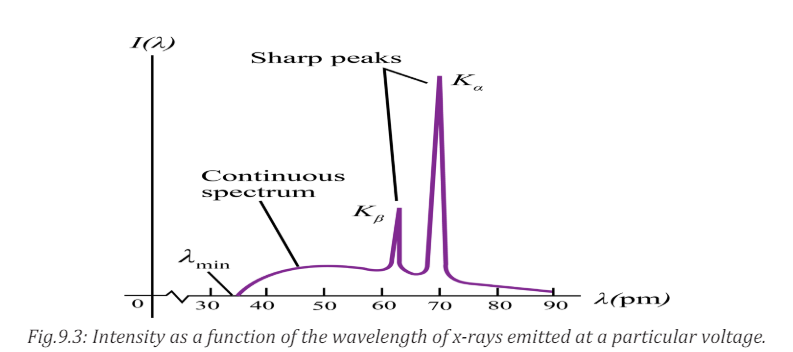
measured as a function of the wavelength and the variation is plotted graphically
then a graph of the nature shown on the figure above is obtained.The graph has
the following features:a. Minimum wavelength9.2.2 Origin of the continuous spectrum
b. Continuous spectrumc. Characteristic peaks
It is known that when charged particles such as electrons are accelerated or
decelerated they emit electromagnetic radiation of different frequencies.
In doing so a part of their kinetic energy is transformed in the energy of the
emitted radiation. Electrons inside the x-ray tube decelerate upon hitting the
target and as a result they emit electromagnetic radiations with a continuous
distribution of wavelength starting from a certain minimum wavelength. This
mechanism of producing electromagnetic radiation from an accelerated ordecelerated electron is called bremsstrahlung.
The energy of the emitted photon is given by

The maximum energy of the emitted photons is therefore equal to the energy
of the incident electron:
Where
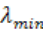 is the minimum wavelength, V is the potential difference between
is the minimum wavelength, V is the potential difference between
anode and cathode and e the charge of the electron.If V is measured in volts we get
As the many electrons in the X-ray are decelerated differently, this will result in
a continuous spectrum of the emitted wavelengths.
It can be observed from the above Fig.9.4 that, for different values of the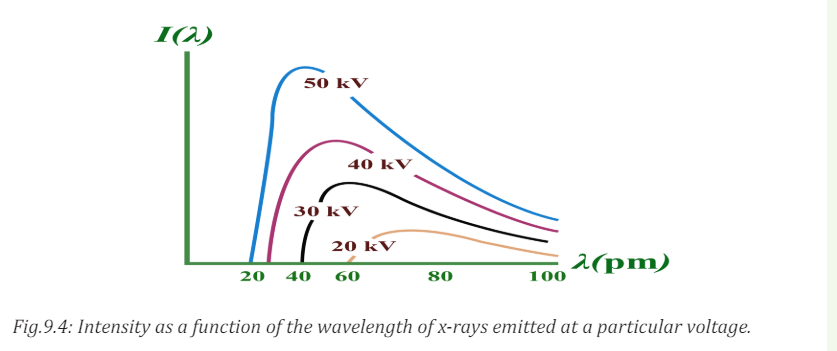
accelerating voltage, the minimum wavelength decreases with increasing
potential difference and for a given wavelength the intensity is higher when thepotential difference is higher.
9.2.3 Origin of characteristic lines
The peaks observed in wavelengths distribution curves as shown in Fig. 9.4
are spectral lines in the X-ray region. Their origin lies in the transition between
energy levels in the atoms of the target.The electrons in the atoms are arranged
in different atomic shell. Of these, the first two electrons occupy the K-shell
followed by 8 electrons in the L-shell, 18 electrons in the M-shell and so on
until the electron in the target are used up. A highly accelerated electron may
penetrate atom in the target and collide with an electron in K-shell. If such
electron is knocked out it will leave an empty space that is immediately filled
up by another electron probably from the L-shell or M-shell. This transitionwill be accompanied by the emission of the excess energy as a photon.
The energy of the emitted photon is a characteristic of the energy levels in theparticular atom and is given by
For a transition between K and L-shells.
Thus the energy of the emitted photon depends on the binding energies in the
K and L shells and hence the x-ray spectral lines have definite frequencies and
wavelengths which are characteristic of the target atom.
For a given target material more than one spectral lines are observed astransitions may occur between different energy levels.
The X-ray lines originating from the transition between the different electron
levels are usually labelled by the symbols α, β, γ, etc.
From L-level to K-level transition produces Kα-line
From M-level to K-level transition produces Kβ
–line
From M-level to L-level transition produces Lα –line
From N-level to L- level transition produces Lβ –line
9.2.4 Checking my progress
1. What is the characteristic of X-ray characteristic peak radiation?
2. How is X-ray continuum produced via bremsstrahlung?
3. X-rays are generated when a highly accelerated charged particle such
aselectrons collide with target material of an X-ray tube. The resulting
X-rays have two characteristics: the continuous X-rays (also called white
X-rays) and characteristic X-rays peaks. The wavelength distribution and
intensity of continuous X-rays are usually depending upon the applied
voltage and a clear limit is recognized on the short wavelength side.
a. Estimate the speed of electron before collision when applied voltage is
30kV and compare it with the speed of light in vacuum.
b. In addition, establish the expression of the shortest wavelength limit
λmin of X-rays generated with the applied voltage V. it is obtained whenthe incident electron loses all its energy in a single collision.
9.3 APPLICATIONS AND DANGERS OF X-RAYS
ACTIVITY 9.4: investigating the X-ray uses and dangers
1. Using the historical background of X-ray discovery, what are the
uses of X-rays in real life?
2. Discuss the dangers that X-rays may cause when they are used in awrong way.
X-rays have many practical applications in medicine and industry. Because X-ray
photons are of such high energy, they can penetrate several centimetres of solid
matter. Hence they can be used to visualize the interiors of materials that areopaque to ordinary light, such as broken bones or defects in structural steel.
9.3.1 In medicine
X-ray imaging utilizes the ability of high frequency electromagnetic waves to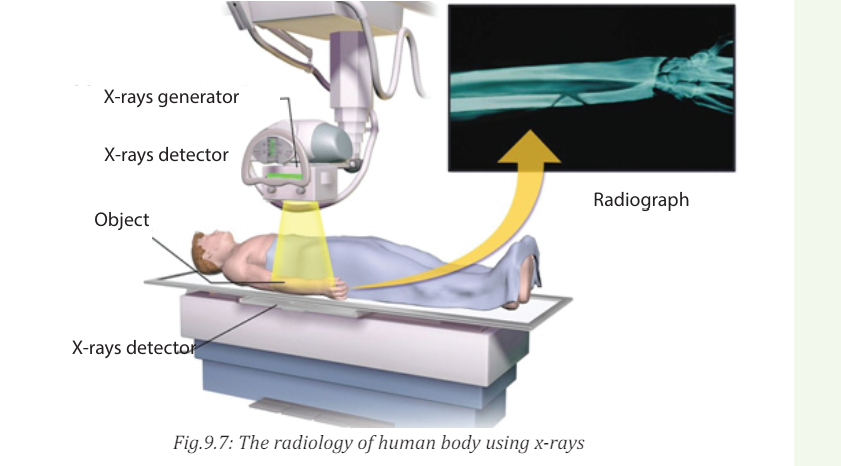
pass through soft parts of the human body largely unimpeded. For medical
applications, parts of the human body are exposed to moderated X-rays
intensity and images are produced in similar way as light on a photographic
plate or digital recorder to produce a radiograph (See Fig.9.7).
By rotating both source and detector around the patient’s body a “slice” image
can be produced in what is called computerized tomography (CT). Although CT
scans expose the patient to higher doses of ionizing radiation the slice imagesproduced make it possible to see the structures of the body in three dimensions.
In 1895, the Dutch Wilhelm Roentgen (See Fig.9.8) discovered that light energy
could be used to take photographs through substances such as paper, cloths
and wood. Roentgen also discovered that this invisible form of light energy,
called X-rays could be used to take the pictures of structures inside the body asshown in Fig. below. Bone tissue appears clearly on an X-rays.
The object to be visualized is placed between an X-ray source and an electronic
detector (like that used in a digital camera) or a piece of photographic film
(Fig.9.8 or Fig.9.8B). The darker area in the recorded images by such a detector,
the greater the radiation exposure. Bones are much more effective X-ray
absorbers than soft tissue, so bones appear as light areas. A crack or air bubbleallows greater transmission and shows as a dark area.
A widely used and vastly improved x-ray technique is computed tomography;
the corresponding instrument is called a CT scanner. The x-ray source produces
a thin, fan-shaped beam that is detected on the opposite side of the subject by an
array of several hundred detectors in a line. Each detector measures absorption
along a thin line through the subject. The entire apparatus is rotated around
the subject in the plane of the beam, and the changing photon-counting rates of
the detectors are recorded digitally. A computer processes this information and
reconstructs a picture of absorption over an entire cross section of the subject.
In the middle 1970, CT (Computer Tomography) scanning machines were
introduced in human medicine.
X-rays are also used in the following:• Killing of cancerous cells
• Radiography is also used in industry for examining potentially damaged
machinery to ascertain the cause of damage and to verify castings orwelded joints
• X-rays are used to study the structure of crystals (crystallography).
• When a handgun is fired, a cloud of gunshot residue (GSR) is ejected from
the barrel. The x-ray emission spectrum of GSR includes characteristic
peaks from lead (Pb), antimony (Sb), and barium (Ba). If a sample taken
from a suspect’s skin or clothing has an x-ray emission spectrum with
these characteristics, it indicates that the suspect recently fired a gun.9.3.2 Examining luggage cargo and security
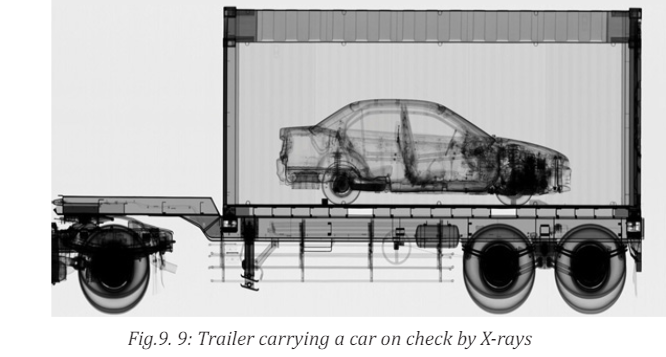
X-rays are being used in airports to examine luggage for weapons or bombs.
Note that the metal detector that you walk through in the airport does not X-ray
you. It uses magnetic waves to detect metal objects. X-rays are also being usedto examine cargo luggage for illegal or dangerous material as in Fig.9.9.
9.3.3 In industry

They can be used to detect structural problems and cracks in metals
that cannot be seen from the outside. X-rays are used on commercial airplanes,
bridges metals and pipe lines, to make sure there are no stress fracturesor other dangerous cracks in the material.
9.3.4 In scientific research• X-ray diffraction provides one of the most important tools for examining
the three-dimensional (3D) structure of biological macromolecules
and cells.
• They are also used in crystallography, where X-ray diffraction and
scattered waves show the arrangement of atoms in the crystal.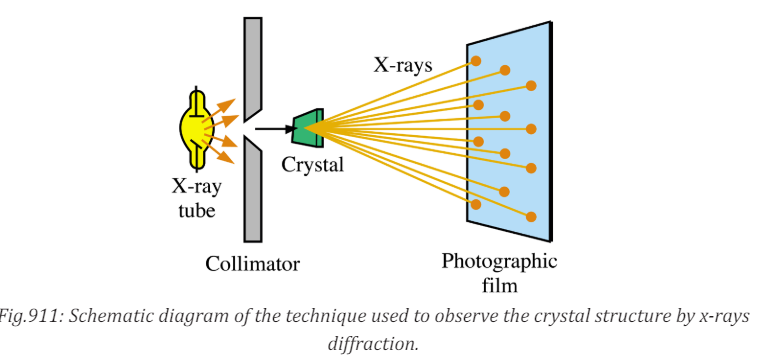
The array of spots formed on the film is called a Laue pattern and show the
atom structure of the crystal.
9.3.5 Dangers of X-rays• X rays cause damage to living tissues. As X-ray photons are absorbed
in tissues, their energy breaks molecular bonds and creates highly
reactive free radicals (such as neutral H and OH), which in turn can
disturb the molecular structure of proteins and especially genetic
material. Young and rapidly growing cells are particularly susceptible,
which is why X-rays are useful for selective destruction of cancer cells.
• Because X-rays can kill living cells, they must be used with extreme care.
When improperly used they can cause severe burns, cancer, leukemia,
and cataracts. They can speed aging, reduce immunity to disease, and
bring about disastrous changes in the reproductive cells.
• Lead screens, sheets of lead-impregnated rubber, and leaded glass are
used to shield patients and technicians from undesired radiation.• The effect of X-ray radiations is cumulative. That is, many minor doses9.3.6 Safety precaution measures of dangers caused by X-rays
over a number of years is equivalent to a large dose at one time.
• Unnecessary exposure to x-rays should be avoided. MRI (Magnetic
Resonance Imaging) uses magnets and sound energy to form pictures
of the internal organs without exposing patients to harmful X-rays.
• When they are used in hospitals, the sources should be enclosed in
lead shields.
• A careful assessment of the balance between risks and benefits ofradiation exposure is essential in each individual case.
Medical and dental X-rays are of very low intensity, so that the hazard is
minimized. However, X-ray technicians who go frequently behind the lead
shield while operating X-rays need to be protected because of the frequency
of exposure. A person can receive many medical or dental X-rays in a year with
very little risk of getting cancer from it. In fact, exposure to natural radiationsuch as cosmic rays from space poses a greater risk.
The following are some of the precautions:i. Protective suits and wears such as gloves and eye glasses made of lead are9.3.7 Checking my progress
used always when handling these radiations. These shields protect the
workers from X-ray exposure.
ii. Workers who operate equipment’s that use X-rays must wear special
badges which detect the amount of radiation they are exposed to.
iii. Food and drinks are not allowed in places where X-radiations are present.
iv. Experiments that involve these radiations (X-rays) substances should be
conducted in a room surrounded by thick concrete walls or lead shields.
v. Equipment that use X-rays should be handled using remote-controlledmechanical arms from a safe distance.
1. How do we create different X-ray images in medicine?9.4 PROBLEMS INVOLVING ACCELERATING POTENTIAL AND
2. What are the dangers that may be caused by using excessive dose ofX-rays?
MINIMUM WAVELENGTH.
9.4.1 Accelerating potential and minimum wavelength
ACTIVITY 9.5: Calculation of accelerating potential in X-ray tube
An x-rays tube operates at 30 kV and the current through it is 2.0 mA.
Calculate:a. The electrical power outputWhen a high voltage with several tens of kV is applied between two electrodes,
b. The number of electrons striking the target per second.
c. The speed of the electrons when they hit the targetd. The lower wavelength limit of the X-rays emitted.
the high-speed electrons with sufficient kinetic energy is drawn out from the
cathode and collides with the anode. The electrons rapidly slow down and lose
kinetic energy. Since the slowing down patterns(method of losing kinetic
energy)varies with electrons, continuous X-rays with various wavelength
are generated. When an electron loses all its energy in a single collision, the
generated X-ray has the maximum energy (or the shortest wavelength
). The value of the shortest wave length limit can be estimated from the
accelerating voltage V between electrodes.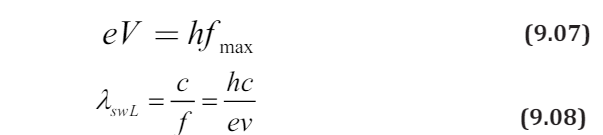
Because X-rays are emitted by accelerated charges, x-rays are electromagnetic
waves. Like light, X-rays are governed by quantum relationships in their
interaction with matter. Thus, we can talk about X-ray photons or quanta, and
the energy of an X-ray photon is related to its frequency and wavelength in thesame way as for photons of light,

Typical X-ray wavelengths are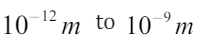 . X-ray wavelength can be
. X-ray wavelength can be
measured quite precisely by crystal diffraction techniques. X-ray emission
is the inverse of the photoelectric effect. In photoelectric emission there is a
transformation of the energy of a photon into the kinetic energy of an electron,
in X-ray production there is a transformation of the kinetic energy of an electron
into energy of a photon. In X-ray production we usually neglect the work
function of the target and the initial kinetic energy of the boiled off electrons
because they are very small in comparison to the other energies.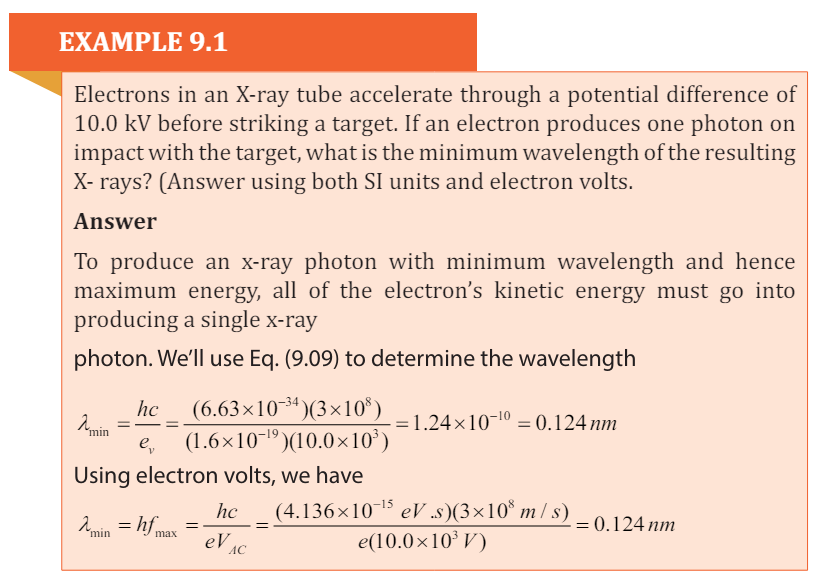
Bragg’s Law
According to W. L. Bragg ( (Weseda, Mastubara, & Shinoda, 2011), X-ray
diffraction can be viewed as a process that is similar to reflection from planes of
atoms in the crystal. In Bragg’s construct, the planes in the crystal are exposed
to a radiation source at a glancing angle θ and X rays are scattered with an angle
of reflection also equal to θ. The incident and diffracted rays are in the same
plane as the normal to the crystal planes (Fig.9. 4).
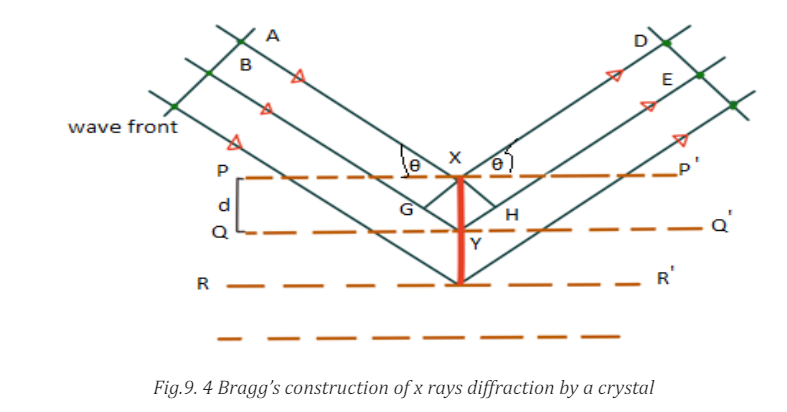
Constructive interference occurs only when the path difference between rays
scattered from parallel crystal planes would be an integer number of wavelengths
of the radiation. When the crystal planes are separated by a distance d, the path
length difference would be 2dsin θ. Thus, for constructive interference to occurthe following relation must hold true.

The above derivation assumes that phase differences between wavelengths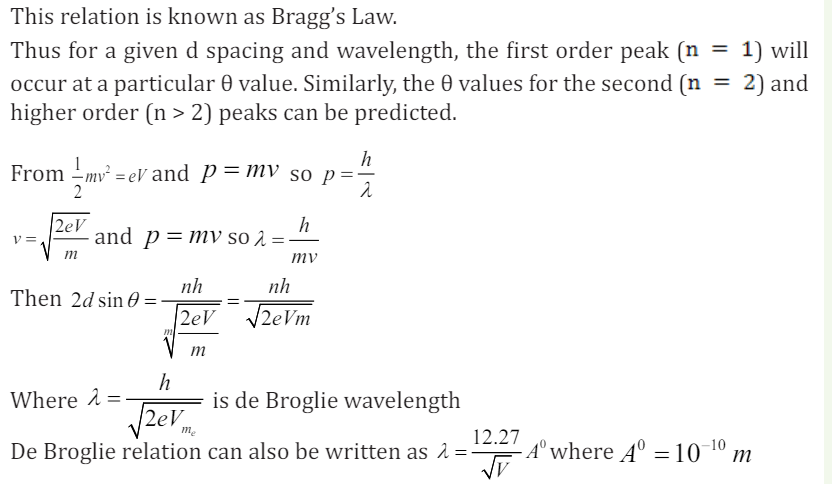
scattered at different points depend only on path differences. It is assumed that
there is no intrinsic phase change between the incident and scattered beams or
that this phase change is constant for all scattering events.
9.4.2 Checking my progress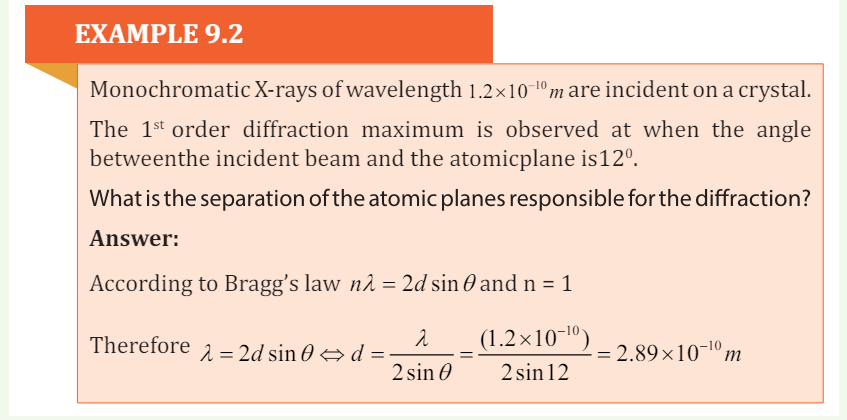
1. Calculatea. Strength of the electric field E,2. Crystal diffraction experiment can be performed using X-rays, or
b. Force on the electron F,
c. Acceleration a of electron, when a voltage of 10 kV is applied between
two electrodes separated by an interval of 10 mm.
electrons accelerated through appropriate voltage. Which probe has
greater energy?(For quantitative comparison,take the wavelength of the
probe equalto1Å, which is of theorderofinter- atomicspacinginthelattice)(me = 9.11×10−31kg).
END UNIT ASSESSMENT 9

a. There are two main components of this x-ray spectrum: a broad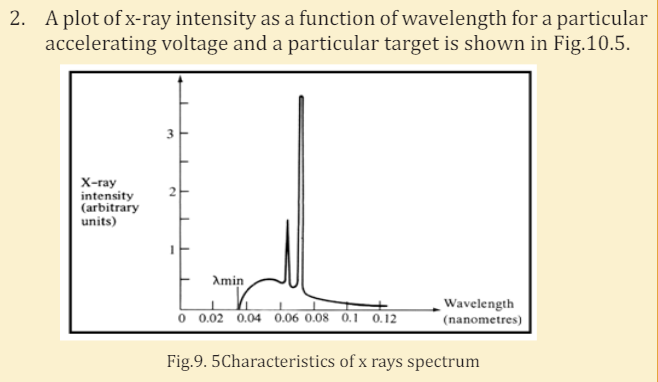
range of x-ray energies and a couple of sharp peaks. Explain how
each of these arises.
b. What is the origin of the cut-off wavelength λmin of the Fig.9.5 shown
below? Why is it an important clue to the photon nature of x-rays?
c. What would happen to the cut-off wavelength if the accelerating
voltage was increased? What would happen to the characteristic
peaks? Use a sketch to show how this spectrum would look if the
accelerating voltage was increased.
d. What would happen to the cut-off wavelength if the target was
changed, keep the same accelerating voltage? What would happen
to the characteristic peaks? Use a sketch to show how the spectrum
would look if some other target material was used, but the
accelerating voltage was kept the same.
3. Electrons are accelerated from rest through a p.d of 10 kV in an x ray
tube. Calculate:
I. The resultant energy of the electrons in eV.II. The wavelength of the associated electron waves.
III. The maximum energy and the minimum wavelength of the x ray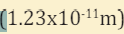
radiation generated (assume
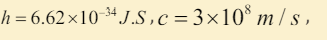
(1.6 10 ,1.24 10 ) J m − − × × .
4. Monochromatic X-ray of wavelength 10 1.2 ×−10 m are incident on a crystal.
The1st order diffraction maximum is observed at when the angle between
the incident beam and the atomic plane is 120.What is the separation of the atomic planes responsible for the diffraction?
5. An x-ray operates at 30 kV and the current through it is 2.0 mA. Calculate:
I. The electrical power output
II. The number of electrons striking the target per second.
III. The speed of the electrons when they hit the target
IV. The lower wavelength limit of the x-rays emitted.
6. An x-ray machine can accelerate electrons of energies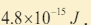 . The
. The
shortest wavelength of the x- rays produced by the machine is found
to be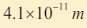 Use this information to estimate the value of the plank
Use this information to estimate the value of the plankconstant.
7. The spacing between Principal planes of Nacl crystal is 0 2.82 A . It is found
that the first order Bragg diffraction occurs at an angle of 100
. What is the
wavelength of the x rays?
8. What is the kinetic energy of an electron with a de Broglie wavelength of
0.1 nm. Through what p.d should it be accelerated to achieve this value?
9. You have decided to build your own x-ray machine out of an old television
set. The electrons in the TV set are accelerated through a potential difference
of 20 kV. What will be the λmin for this accelerating potential?
10. A tungsten target (Z = 74) is bombarded by electrons in an x-ray tube.
The K, L, and M atomic x-ray energy levels for tungsten are -69.5, -11.3 and
-2.30 keV, respectively.
a. Why are the energy levels given as negative values?
b. What is the minimum kinetic energy of the bombarding electrons
that will permit the production of the characteristic Kα and Kβ
lines of tungsten?
c. What is the minimum value of the accelerating potential that will
give electrons this minimum kinetic energy?
d. What are the Kα and Kß wavelengths?
11. Using the following illustration figure Fig.10.6, label each part marked byletter from A to H and explain the function of each part A, B, C, D, E, F and H.