Topic outline
UNIT 1 SOUND WAVES
Key unit competence: Analyze the effects of sound waves in elastic medium
My goals
• Describe how sound propagates through a substance
• Give the characteristics of sound.
• Relate loudness and pitch to amplitude and frequency
• Carry out calculations relating decibels and intensity
• Establish relationship between characteristics of notes and sound
waves
• Explain beats and establish beat frequency
• Explain Doppler – Fizeau effect.
• Give examples of musical pipe instruments.
• Explain Doppler – Fizeau effect.
• Give examples of musical pipe instruments.
• Establish the fundamental frequency and 2nd harmonic, 3rd harmonic,
in vibrating strings and in pipes
INTRODUCTORY ACTIVITY
1. Most people like to listen to music, but hardly anyone likes to listen to
noise. What is the difference between a musical sound and noise?
2. A guitarist plays any note. The sound is made by the vibration of the
guitar string and propagates as a wave through the air and reaches your
ear. Which of the following statement is the right?
• The vibration on the string and the vibration in the air have the
same wavelength.
• They have the same frequency.
• They have the same speed.
• None of the above is the same in the air as it is on the string.
Questions
a. Explain the meaning of underlined terms used in the text above
b. Do you think, it was 100% correct for Claudette to relate sound waves to
light waves. Explain.
c. There is somewhere where she was asked to discuss the different media
in which sound waves can travel. Discuss these different media and talk
about velocity of sound waves in the stated media.
d. In one of the paragraph, Claudette said that the laws governing reflection
and refraction of sound waves were similar to those of light. Can you
explain these laws (Use diagrams where possible)
e. Assuming that you were an interviewer and the interview was out of
80.What mark would you award Claudette? Why?
1.1. CHARACTERISTICS AND PROPERTIES OF SOUND WAVES
ACTIVITY 1.1: Properties of sound
Read the scenario below and answer the questions that follow.
On an interview for Physics placement in a certain school in Rwanda,
Claudette a S.6 leaver who had applied for the job was asked about
sound waves during the interview. She was asked to state the properties
of sound waves. Confidently, she responded that the properties are
reflection, refraction, diffraction and interference. This was enough
to make Claudette pass the first level of the interview.
However, in the second step, she was required to discuss different media
in which sound waves can propagate. Claudette started discussing
these different media. What surprised the interviewer was Claudette’s
ability to relate sound waves to other kinds of waves stating that these
waves behaves the same way when they pass from one medium toanother.
Looking at Claudette’s face, the interviewer asked her to discuss the laws
governing reflection and refraction of sound waves. With a smile, she
started by saying that since sound waves have the same properties as for
light; these laws therefore do not change.
As she was attempting to state them, the interviewer stopped her and
congratulated her upon her confidence and bravery she showed in the room.
She was directly told that she was successful and she was given the job.
Claudette is now working as assistant S2 Physics teacher and doubles as a
Physics laboratory attendant.
1.1.1 Properties of sound waves
Most of us start our lives by producing sound waves! We spend much of our
life surrounded by objects which produce sound waves. Most machines in
use vibrate and produce sound so the only sure way to silence them would
be to put them in vacuum where there would be no surrounding medium for
the vibrating surfaces of the machine to push against, hence no sound waves.
Some physiologists are concerned with how speech is produced, how speech
impairment might be corrected, how hearing loss can be alleviated.
Sound is associated with our sense of hearing and, therefore, with the physiology
of our ears that intercept the sound and the psychology of our brain which
interprets the sensations that reach our ears. Sound waves are longitudinal
mechanical waves that can travel through solids, liquids, or gases.
As the sound wave propagates, many interactions can occur, including reflection,
refraction, diffraction and interference. When a sound wave hits a surface, a
part of the energy gets scattered while a part of it is absorbed. Absorption is
the phenomenon of the wave where the energy of sound wave gets transformed
from one form to another. The high frequency sound waves are more easily
absorbed than low frequency sounds. It happens most with the soft materials.
1.1.2 Characteristics of sound waves
ACTIVITY 1.2: Characteristics of sound waves
1. How to calculate the speed of sound waves in different materials.
2. How to calculate the intensity of a sound wave.
3. From the Fig.1.2, can you hear the ultrasound waves that a bat usesfor echolocation? Why or why not?
Fig.1. 2: Range of frequencies heard by various animals and human (Randall & Knight.,-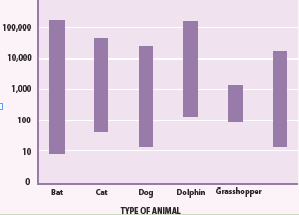
Physics for scientists and engineers: Stategic approach., 2008)
Usually, the characteristics used to describe waves are period, frequency,
wavelength, and amplitude.
a. Frequency ranges
Any periodic motion has a frequency, which is the number of complete cycles
in a second and a period which is the time used to complete one cycle. While
the frequency is measured in Hertz (Hz), the period is measured in seconds (s).
For a wave, the frequency is the number of wave cycles that pass a point in a
second. A wave’s frequency equals the frequency of the vibrating source
producing the wave.
Sound waves are classified into three categories that cover different frequency
ranges:
• Audible soundlies within the range of sensitivity of the human ear.
They can be generated in a variety of ways, such as musical instruments,
human voices, or loudspeakers. It is almost impossible to hear sounds
outside the range of 20 Hz to 20 kHz. These are the limits of audibility
for human beings but the range decreases with age.
• Infrasonic waveshave frequencies below the audible range. They are
sound waves with frequencies that are below 20 Hz limit. Some animals
such as elephants can use infrasonic waves to communicate with each
other, even when they are separated by many kilometers. Rhinoceros
also use infrasonic as low as 5 Hz to call one another.
• Ultrasonic waves have frequencies above the audible range. They
are sound waves whose frequencies are higher than 20 KHz. You may
have used a “silent” whistle to retrieve your dog. The ultrasonic sound
emitted by that device is easily heard by dogs, although humans cannot
detect it at all. Ultrasonic waves are also used in medical imaging.
Many animals hear a much wider range of frequencies than human beings do.
For example, dog whistles vibrate at a higher frequency than the human ear
can detect, while evidence suggests that dolphins and whales communicate at
frequencies beyond human hearing (ultrasound) (Cutnell & Johnson, 2006).
b. Wavelength
Wavelength is the distance covered by a wave in a period. It is represented by
the separation between a point on one wave and a similar point on the next
cycle of the wave. For a transverse wave, wavelength is measured between
adjacent crests or between adjacent troughs. For a longitudinal wave such as
sound wave, wavelength is the distance between adjacent compressions or
rarefaction.
c. Speed of sound
For a periodic wave, the shape of the string at any instant is a repeating pattern.
The length of one complete wave pattern is the distance from one crest to the
next or from one trough to the next or from any point to the corresponding point
on the next repetition of the wave shape. We call this distance the wavelength
of the wave, denoted by the Greek letter lambda (λ).
The wave pattern travels with constant speed and advances a distance of one
wavelength in a time interval of one period T. So the wave speed is given by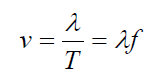
where f is the frequency of the wave.
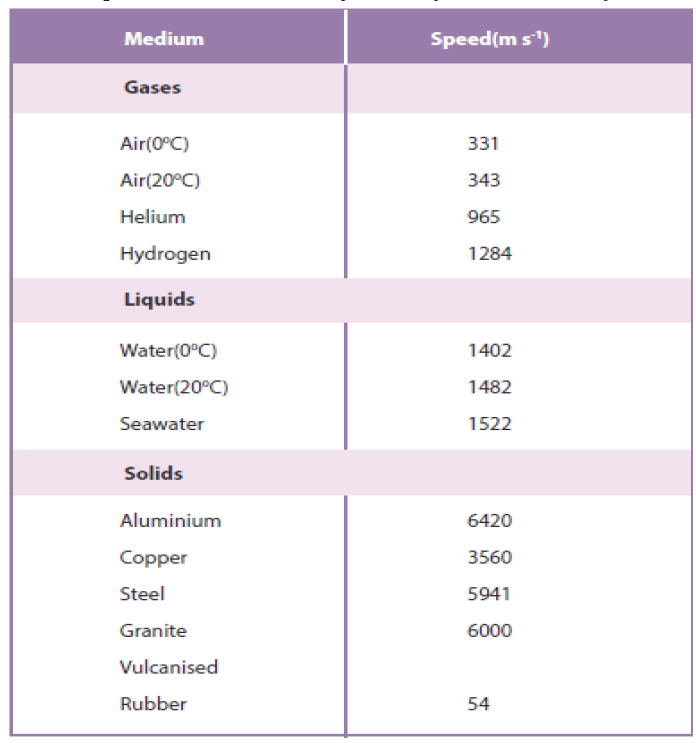
Sound travels faster in liquids and solids than in gases, since the particles in
liquids and solids are closer together and can respond more quickly to the
motion of their neighbors. As examples, the speed of sound is 331 m/s in
air,1500 m/s in water and 5000 m/s in iron (though these can change dependingon temperature and pressure). Sound does not travel in vacuum.
Example 1.1: Wavelength of musical sound
Example 1.1 Wavelength of a musical sound
1) Sound waves can propagate in air. The speed of the sound depends
on temperature of the air; at 200 C it is 344 m/ s it is. What is the
wavelength of a sound wave in air if the frequency is 262 Hz (the
approximate frequency of middle C on a piano)?Answer:
Using Equation of wave (1.01):
Factors which affect the velocity of sound in air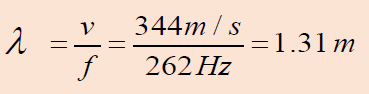
• The speed of sound waves in a medium depends on the compressibility
and density of the medium. If the medium is a liquid or a gas and has a
bulk modulus Band density ρ , the speed of sound waves in that mediumis given by:
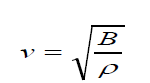 (1.02)
(1.02)• It is interesting to compare this expression with the equation
the wave speed depends on an elastic property of the medium (bulk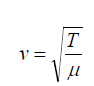 applicable to transverse waves on a string. In both cases,
applicable to transverse waves on a string. In both cases,
modulus B or tension in the string T) and on an inertial property of the
medium (the density ρ or linear mass μ ).
In fact, the speed of all mechanical waves follows an expression of thegeneral form
• For longitudinal sound waves in a solid rod of material, for example, (1.03)
(1.03)
the speed of sound depends on Young’s modulus Y and the density ρChanges of pressure have no effect on the velocity of sound in air.
Sir Isaac Newton showed that:
• In accordance with Boyle’s law, if the pressure of a fixed mass of air is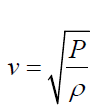 (1.04)
(1.04)
doubled, the volume will be halved. Hence the density will be doubled.
Thus at constant temperature, the ratio P⁄ρ
will always remain constant
no matter how the pressure may change. The speed of sound increases
with temperature . If the air temperature increases at constant pressure
the air will expand according to Charles’ law, and therefore become
less dense. The ratio P⁄ρ
will therefore increase, and hence the speed of
sound increases with temperature. For sound traveling through air, therelationship between wave speed and medium temperature is
Where v0 331m/ s = is the speed of sound in air(at 0 degree Celsius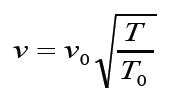 ( 1 . 0 5 )
( 1 . 0 5 )
and normal pressure) .
• The speed of sound in air at standard temperature and pressure (25
oC, 760 mm of mercury) is 343 m/s. It is determined by how often the
air molecules collide. The speed of sound increases by about 6 m/s ifthe temperature increases by 10 oC (Glencoe, 2005).
d.Amplitude
The amplitude of a wave is the maximum displacement of the medium from its
rest position. The amplitude of a transverse wave is the distance from the rest
position to a crest or a trough. The more energy a wave has, the greater is its
amplitude.
1.1.3 Checking my progress
1. The correct statement about sound waves is that:
a. They are transverse waves
b. They can be polarized
c. They require material medium to propagate
2. Sound travels in
a. Air b. Wate c. Iron d. All of these
3. Two men talk on the moon. Assuming that the thin layer of gases on the
moon is negligible, which of the following is the right answer:
a. They hear each other with lower frequency
b. They hear each other with higher frequency
c. They can hear each other at such frequency
d. They cannot hear each other at all
4. Do you expect an echo to return to you more quickly on a hot day or a
cold day?
a. Hot day. b. Cold day. c. Same on both days.
5. A sound wave is different than a light wave in that a sound wave is:
a. Produced by an oscillating object and a light wave is not.
b. Not capable of traveling through a vacuum.
c. Not capable of diffracting and a light wave is.
d. Capable of existing with a variety of frequencies and a light wave
has a single frequency.
6. A spider of mass 0.30 g waits in its web of negligible mass see Fig. below.
A slight movement causes the web to vibrate with a frequency of about
15 Hz.
Fig.1. 3 A spider of mass waits in its web
a. Estimate the value of the spring stiffness constant k for the web
assuming simple harmonic motion.
b. At what frequency would you expect the web to vibrate if an insectof mass 0.10 g were trapped in addition to the spider?
1.2 PRODUCTION OF STATIONARY SOUND WAVESACTIVITY 1.3: Production of stationary sound waves

Fig.1. 4: A guitarist.
Look at the Fig.1.4 of guitarist and then answer the following question.
1. How do vibrations cause sound?
2. What determines the particular frequencies of sound produced by
an organ or a flute?
3. How resonance occurs in musical instruments?
4. How to describe what happens when two sound waves of slightlydifferent frequencies are combined?
1.2.1 Sound in pipes
The source of any sound is vibrating object. Almost any object can vibrate
and hence be a source of sound. For musical instruments, the source is set
into vibration by striking, plucking, bowing, or blowing. Standing waves (also
known as stationary waves are superposition of two waves moving in opposite
directions, each having the same amplitude and frequency) are produced and
the source vibrates at its natural resonant frequencies.
The most widely used instruments that produce sound waves make use of
vibrating strings, such as the violin, guitar, and piano or make use of vibrating
columns of air, such as the flute, trumpet, and pipe organ. They are called wind
instruments. We can create a standing wave:
• In a tube, which is open on both ends. The open end of a tube is
approximately a node in the pressure (or an antinode in the longitudinal
displacement).
• In a tube, which is open on one end and closed on the other end. The
closed end of a tube is an antinode in the pressure (or a node in the
longitudinal displacement).
In both cases a pressure node is always a displacement antinode and vice versa.
a. Tube of length L with two open ends
An open pipe is one which is open at both ends. The length of the pipe is thedistancebetween consecutive antinodes. But the distance between consecutive
antinode is
The longest standing wave in a tube of length L with two open ends has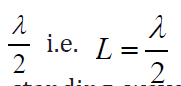 (1.06)
(1.06)
displacement antinodes (pressure nodes) at both ends. It is called thefundamental.
Notes with higher frequencies than fundamental can be obtained from the pipe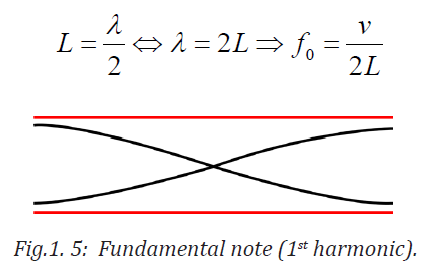
by blowing harder. The stationary wave in the open pipe has always an antinodeat each end.
The next longest standing wave in a tube of length L with two open ends is the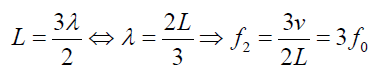
second harmonic (first overtone). It also has displacement antinodes at eachend.
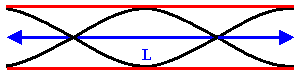
Fig.1. 6: First overtone (second harmonic).
The second overtone is obtained from Fig. 1.6 and is the third harmonic.
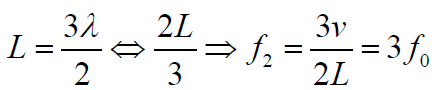
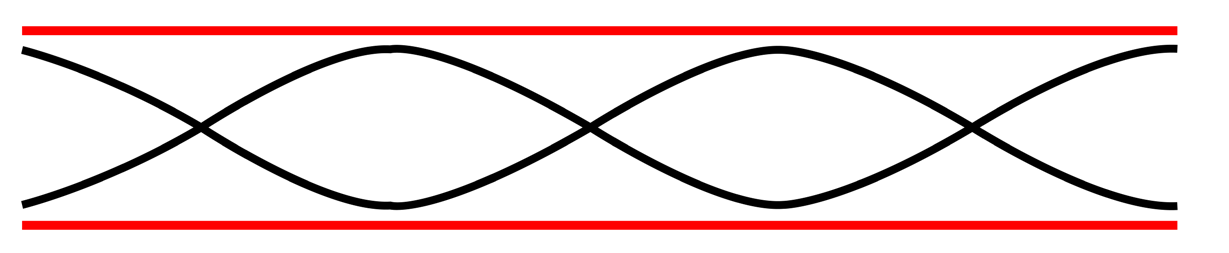
Fig.1. 7: Second overtone (third harmonic).
An integer number of half wavelength has to fit into the tube of length L:
For a tube with two open ends, all frequencies fn− 1 =nf0 with n equal to an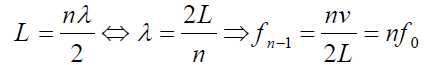 (1.07)
(1.07)
integer are natural frequencies.
The frequency f of fundamental note emitted by a vibrating string of length L,mass per unit length m and under tension T is given by
Example 1.3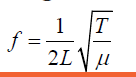 (1.08)
(1.08)
The fundamental frequency of a pipe that is open at both ends is 594 Hz.
a. How long is this pipe?
b. Find the wavelength. Assume the temperature is 20oc
c. Determine the fundamental frequency of the flute when all holes are
covered and the temperature is 10 °C instead of 20 °C?Answer :
Quick check 1.1: Standing sound waves are produced in a pipe that is 1.20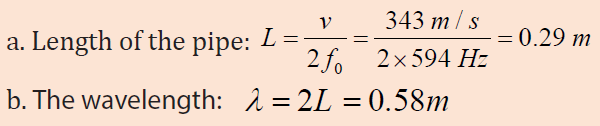
m long. For the fundamental and first two overtones, determine the locations
along the pipe (measured from the left end) of the displacement nodes and
the pressure nodes if the pipe is open at both ends.
b. Tube of length L with one open end and one closed end.
The longest standing wave in a tube of length L with one open end and one
closed end has a displacement antinode at the open end and a displacement
node at the closed end.This is the fundamental.
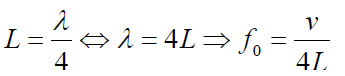 (1.09)
(1.09)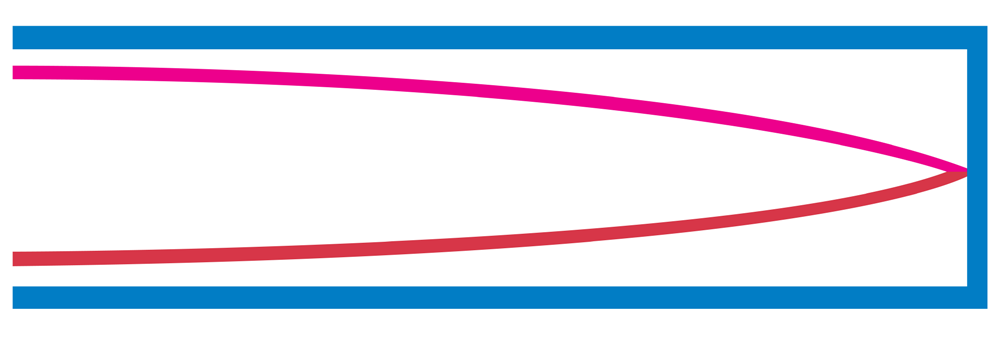
Fig.1. 8: Fundamental note (1st harmonic).
The next longest standing wave in a tube of length in a tube of length L with one
open end and one closed end is the third harmonic (second overtone). It alsohas a displacement antinode at one end and a node at the other.
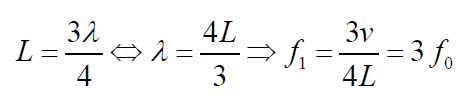 (1.10)
(1.10)
Fig.1. 9: First overtone (third harmonic)
The next longest standing wave in a tube of length L with one open end and oneclosed end is the second overtone (fifth harmonic).
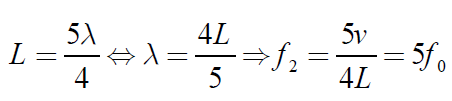 (1.11)
(1.11)
Fig.1. 10: Second overtone (fifth harmonic)
An odd-integer number of quarter wavelength has to fit into the tube of length L.

For a tube with one open end and one closed end, frequencies
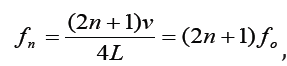 with n equal to an odd integer are natural frequencies.
with n equal to an odd integer are natural frequencies.
Only odd harmonics of the fundamental are natural frequencies.
Another way to analyze the vibrations in a uniform tube is to consider
a description in terms of the pressure in the air. Where the air in a wave
is compressed, the pressure is higher, whereas in a wave expansion (or
rarefaction), the pressure is less than normal. We call a region of increased
density a compression; a region of reduced density is a rarefaction.
The wavelength is the distance from one compression to the next or from onerarefaction to the next.
Fig.1. 11: Pressure variation in the air: Graphs of the three simplest modes of vibration (standing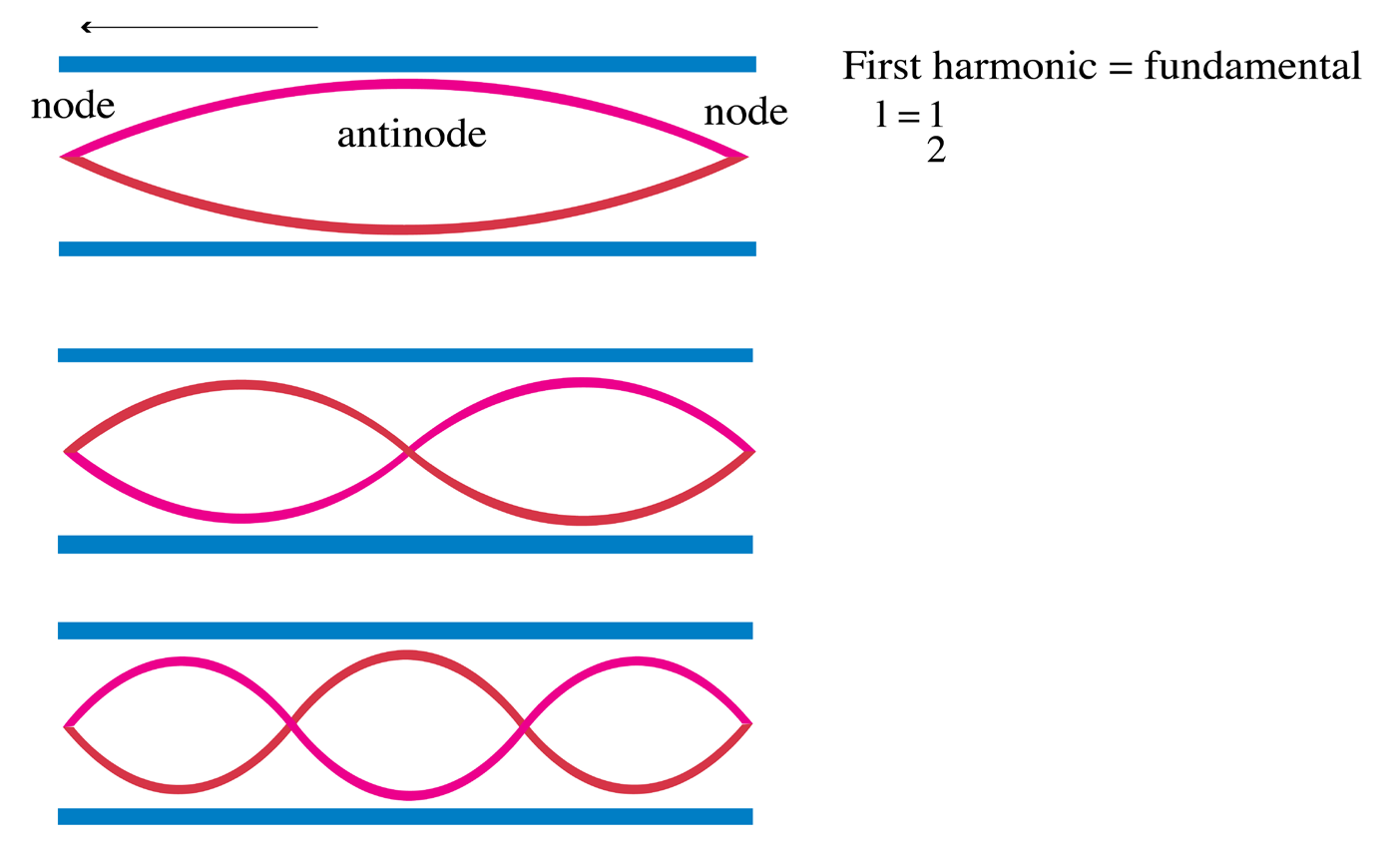
waves) for a uniform tube open at both ends (“open tube”).
The open end of a tube is open to the atmosphere. Hence the pressure variation
at an open end must be a node: the pressure does not alternate, but remains atthe outside atmospheric pressure as shown in Fig.1.12.
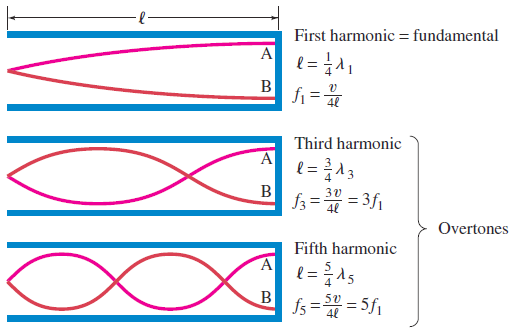
Fig.1. 12: Modes of vibration (standing waves) for a tube closed at one end (“closed tube”).
If a tube has a closed end, the pressure at that closed end can readily alternate
to be above or below atmospheric pressure. Hence there is a pressure antinode
at a closed end of a tube. There can be pressure nodes and antinodes within the
tube as shown in Fig.1.12.Example 1.4
1. A section of drainage culvert 1.23 m in length makes a howling noise
when the wind blows.
a. Determine the frequencies of the first three harmonics of the
culvert if it is open at both ends. Take v = 343 m/s as the speed
of sound in air.
b. What are the three lowest natural frequencies of the culvert if
it is blocked at one end?
c. For the culvert open at both ends, how many of the harmonics
present fall within the normal human hearing range (20 Hz to
17 000 Hz)?
Answer
a. The frequency of the first harmonic of a pipe open at both
ends is Because both ends are open, all harmonics are present;thus,
Quick check 1.2: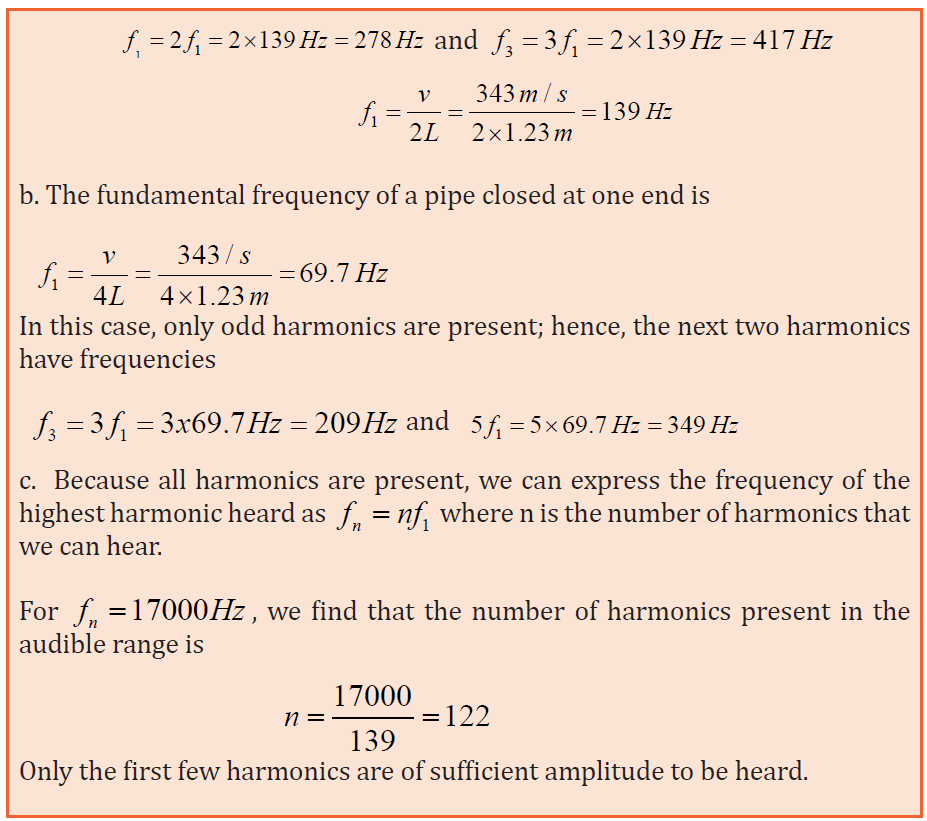
Standing sound waves are produced in a pipe that is 1.20 m long. For the
fundamental and first two overtones, determine the locations along the pipe
(measured from the left end) of the displacement nodes and the pressure
nodes if the pipe is closed at the left end and open at the right end.
1.2.2 Vibrating strings
The string is a tightly stretched wire or length of gut. When it is struck, bowed
or plucked, progressive transverse waves travel to both ends, which are fixed,
where they are reflected to meet the incident waves. A stationary wave pattern
is formed for waves whose wavelengths fit into the length of the string, i.e.
resonance occurs.
If you shake one end of a cord (slinky) and the other end is kept fixed, a
inverted. The frequencies at which standing waves are produced are the natural
frequencies or resonant frequencies of the cord. A progressive sound wave (i.e.
a longitudinal wave) is produced in the surrounding air with frequency equal
to that of the stationary transverse wave on the string.
Now let consider a cord stretched between two supports that is plucked like
a guitar or violin string. Waves of a great variety of frequencies will travel in
both directions along the string, will be reflected at the ends, and will be travel
back in the opposite direction. The ends of the string, since they are fixed, will
be nodes.
The lowest frequency, called the fundamental frequency, corresponds to one
antinode (or loop) and corresponds to whole length of the string i.e, L= λ⁄2the
other natural frequencies are called overtones or harmonics. The next mode
after the fundamental has two loops and is called the second harmonic or first
overtone and so on.
Fundamental note (first harmonic):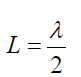 (1.13)
(1.13)
The frequency: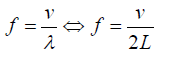 (1.14)
(1.14)It was stated that the speed of a transverse wave travelling along a string is
given by
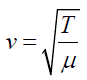
The frequency of the vibration is given by:
First overtone (second harmonic) of a string plucked in the middle corresponds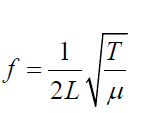 (1.15)
(1.15)
to a stationary wave which has nodes at the fixed ends and antinode in the
middle. If is λ1
the wave length it can be seen that: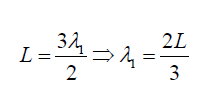 (1.16)
(1.16)
The frequency of fist overtone is given by: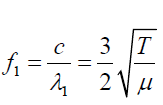
In order to find the frequency f of each vibration we use equation: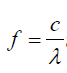
and we see that (1.17)
(1.17)
where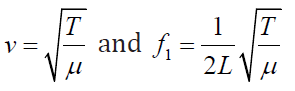 (1.18)
(1.18)
Consider a string of length L fixed at both ends, as shown in Fig.1.12. Standing
waves are set up in the string by a continuous superposition of wave incident
on and reflected from the ends.
Note that there is a boundary condition for the waves on the string. The ends of
the string, because they are fixed, must necessarily have zero displacement andare, therefore, nodes by definition.
Fig.1. 13: Fundamental and first two overtones: (a) A string of length L fixed at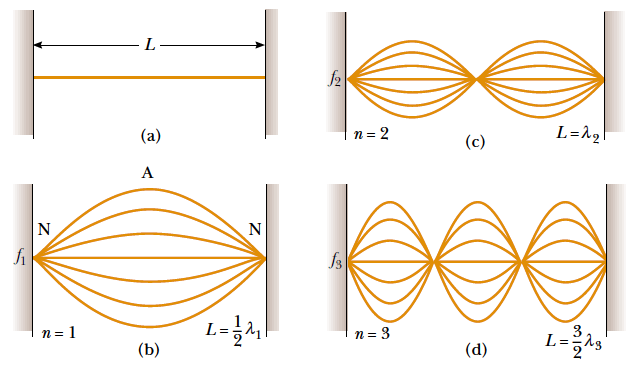
both ends.
The normal modes of vibration form a harmonic series: (b) the fundamental
note (first harmonic); (c) First overtone (second harmonic); (d) the secondovertone (third harmonic) (Halliday, Resneck, & Walker, 2007).
Quick check 1.3: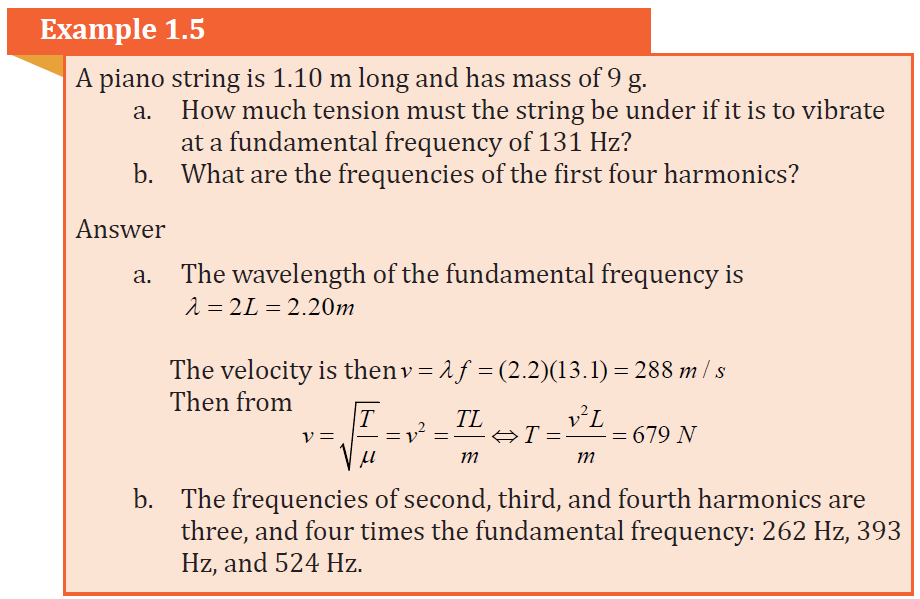
Middle C on a piano has a fundamental frequency of 262 Hz, and the first A
above middle C has a fundamental frequency of 440 Hz.
a. Calculate the frequencies of the next two harmonics of the C string.
b. If the A and C strings have the same linear mass densityμ and
length L, determine the ratio of tensions in the two strings.
c. With respect to a real piano, the assumption we made in (b) is only
partially true. The string densities are equal, but the length of the A
string is only 64 % of the length of the C string. What is the ratio of
their tensions?
1.2.3. Wave Interference and Superposition
a. Wave interference
Up to this point we’ve been discussing waves that propagate continuously in
the same direction. But when a wave strikes the boundaries of its medium, all
or part of the wave is reflected.
When you yell at a building wall or a cliff face some distance away, the sound
wave is reflected from the rigid surface and you hear an echo. When you flip the
end of a rope whose far end is tied to a rigid support, a pulse travels the length
of the rope and is reflected back to you. In both cases, the initial and reflected
waves overlap in the same region of the medium. This overlapping of waves is
called interference.
In general, the term “interference” refers to what happens when two or more
waves pass through the same region at the same time Fig.1.14 shows an exampleof another type of interference that involves waves that spread out in space.
Fig.1. 14: Two speakers driven by the same amplifier: Constructive interference occurs at point P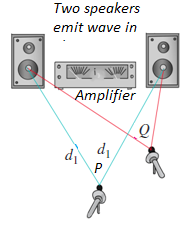
and destructive interference occurs at Q.
Two speakers, driven in phase by the same amplifier, emit identical sinusoidal
sound waves with the same constant frequency. We place a microphone at
point P in the figure, equidistant from the speakers. Wave crests emitted from
the two speakers at the same time travel equal distances and arrive at point
P at the same time; hence the waves arrive in phase, and there is constructive
interference.
The total wave amplitude at P is twice the amplitude from each individual wave,
and we can measure this combined amplitude with the microphone.
Now let’s move the microphone to point Q, where the distances from the
two speakers to the microphone differ by a half-wavelength. Then the two
waves arrive a half-cycle out of step, or out of phase; a positive crest from one
speaker arrives at the same time as a negative crest from the other. Destructive
interference takes place, and the amplitude measured by the microphone is
much smaller than when only one speaker is present. If the amplitudes from
the two speakers are equal, the two waves cancel each other out completely at
point Q, and the total amplitude there is zero.
b. The principle of superposition
Combining the displacements of the separate pulses at each point to obtain
the actual displacement is an example of the principle of superposition: “When
two waves overlap, the actual displacement of any point on the string at any
time is obtained by adding the displacement the point would have if only the
first wave were present and the displacement it would have if only the second
wave were present”.
In other words, the wave function y(t, x) that describes the resulting motion in
this situation is obtained by adding the two wave functions for the two separatewaves:
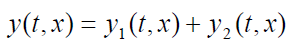 (1.19)
(1.19)
As we saw with transverse waves, when two waves meet they create a third
wave that is a combination of the other two waves. This third wave is actually
the sum of the two waves at the points where they meet. The two original
waves are still there and will continue along their paths after passing through
each other. After passing the third wave no longer exists. Its amplitude has themagnitude
ACTIVITY 1.4: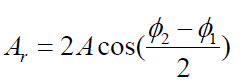 (1.21)
(1.21)
Problem 1
The Adventures of Marvin the Mouse: You and your friend are walking
down by the pool when you hear a cry for help. Poor Marvin the Mouse
has fallen into the pool and needs your help. The sides of the pool are
to slippery for Marvin to climb out but there is an inner tube anchored
in the center of the pool. Oh no! The sides of the inner tube are too
slippery and high for Marvin to climb. He’s getting tired and can’t swim
to the sides; he has just enough energy to float by the inner tube. Having
studied about waves, you and your friend take up positions on opposite
sides of the pool. How did you help Marvin get safely onto the inner
tube?
Problem 2: Dance club designer
You are the designer of a new Dance Club. You have been informed that
you need to design the club in such a way that the telephone is placed
in a location that allows the customers to hear the people on the other
side. The phone company states that they can only put the phone line
in at a point 20 m from the stage. Develop a model which allows the
customers to use the phone with the least amount of trouble given that
the phone must be placed at a distance of 20 m, (2/3 the room size),
from the stage. This will be an area where there will be virtually no
sound.
c. Resonance of sound
We have seen that a system such as a taut string is capable of oscillating in one
or more normal modes of oscillation. If a periodic force is applied to such a
system, the amplitude of the resulting motion is greater than normal when the
frequency of the applied force is equal to or nearly equal to one of the natural
frequencies of the system. This phenomenon is known as resonance. Although
a block–spring system or a simple pendulum has only one natural frequency,
standing-wave systems can have a whole set of natural frequencies.
Because oscillating systems exhibits large amplitude when driven at any of
its natural frequencies, these frequencies are often referred to as resonance
frequencies. Fig.1.15 shows the response of an oscillating system to various
driving frequencies, where one of the resonance frequencies of the system isdenoted by f0
Fig.1. 15: Graph of the amplitude versus driving frequency for oscillating system. The amplitude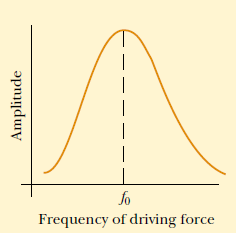
is a maximum at the resonance frequency. Note that the curve is not symmetric (Halliday, Resneck,& Walker, 2007)
One of our best models of resonance in a musical instrument is a resonance
tube. This is a hollow cylindrical tube partially filled with water and forced into
vibration by a tuning fork (Fig.1.16). The tuning fork is the object that forced
the air, inside the resonance tube, into resonance.
Fig.1. 16: Turning fork forcing air column into resonance
As the tines of the tuning fork vibrate at their own natural frequency, they
created sound waves that impinge upon the opening of the resonance tube.
These impinging sound waves produced by the tuning fork force air inside
of the resonance tube to vibrate at the same frequency. Yet, in the absence of
resonance, the sound of these vibrations is not loud enough to discern.
Resonance only occurs when the first object is vibrating at the natural frequency
of the second object. So if the frequency at which the tuning fork vibrates is not
identical to one of the natural frequencies of the air column inside the resonance
tube, resonance will not occur and the two objects will not sound out together
with a loud sound. But the location of the water level can be altered by raising
and lowering a reservoir of water, thus decreasing or increasing the length of
the air column.
So by raising and lowering the water level, the natural frequency of the air in
the tube could be matched to the frequency at which the tuning fork vibrates.
When the match is achieved, the tuning fork forces the air column inside of
the resonance tube to vibrate at its own natural frequency and resonance is
achieved. The result of resonance is always a big vibration - that is, a loud sound.
A more spectacular example is a singer breaking a wine glass with her amplified
voice. A good-quality wine glass has normal-mode frequencies that you can
hear by tapping it.
If the singer emits a loud note with a frequency corresponding exactly to one of
these normal-mode frequencies, large-amplitude oscillations can build up and
break the glass (Fig. 1.17)
Fig.1. 17: Some singers can shatter a wine glass by maintaining a certain frequency of their voice
for seconds, (a) Standing-wave pattern in a vibrating wine glass. (b) A wine glass shattered by theamplified sound of a human voice
d. Beats and its phenomena
Beats occur when two sounds-say, two tuning forks- have nearly, but not exactly,
the same frequencies interfere with each other. A crest may meet a trough at one
instant in time resulting in destructive interference. However at later time the
crest may meet a crest at the same point resulting in constructive interference.
To see how beats arise, consider two sound waves of equalamplitudes and
slightly different frequencies as shown on the figure below.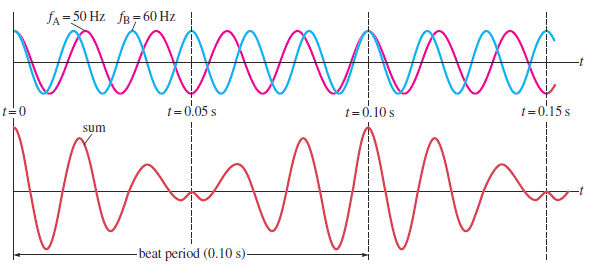
Fig.1. 18: Beats occur as a result of the superposition of two sound waves of slightly differentfrequencies (Cutnell & Johnson, 2006).
In 1.00 s, the first source makes 50 vibrations whereas the second makes 60. We
now examine the waves at one point in space equidistant from the two sources.
The waveforms for each wave as a function of time, at a fixed position, are shown
on the top graph of Fig. 1.19; the red line represents the 50 Hz wave, and the
blue line represents the 60 Hz wave. The lower graph in Fig. 1.18 shows the sum
of the two waves as a function of time. At the time the two waves are in phase
they interfere constructively and at other time the two waves are completely
out of phase and interfere destructively. Thus the resultant amplitude is large
every 0.10 s and drops periodically in between. This rising and falling of the
intensity is what is heard as beats. In this case the beats are 0.10 s apart. The
beat frequency is equal to the difference in frequencies of the two interfering
waves.
Consider two sound waves of equal amplitude traveling through a medium with
slightly different frequencies f1 and f2atchosen point x = 0:utnell & Johnson, 2006).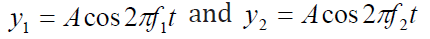
Using the superposition principle, we find that the resultant wave function atthis point is
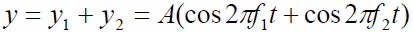
The trigonometric identity
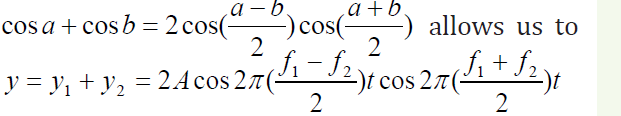 write the expression for y as
write the expression for y asWe see that the resultant sound for a listener standing at any given point has
an effective frequency equal to the average frequency given by the expression:
The frequency of the beats is equal to the difference in the frequencies of the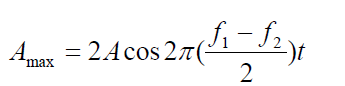 (1.22)
(1.22)two sound waves:
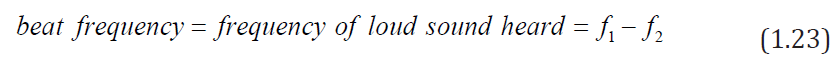
The interference pattern varies in such a way that a listener hears an alternation
between loudness and softness. The variation from soft to loud and back to soft
is called a Beat. The phenomena of beats can be used to measure the unknownfrequency of a note.
Example 1.6
Two identical piano strings of length 0.750 m are each tuned exactly to
440 Hz. The tension in one of the strings is then increased by 1.0%. If they
are now struck, what is the beat frequency between the fundamentals ofthe two strings?
Answer:
We find the ratio of frequencies if the tension in one string is 1.0% largerthan the other:
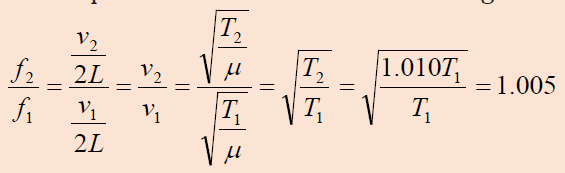 Thus,
Thus,the frequency of the tightened string is
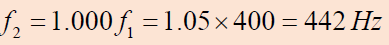
and the beat frequency is
Quick check 1.4: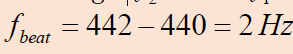
A tuning fork produces a steady 400 Hz tone. When this tuning fork is struck
and held near a vibrating guitar string, twenty beats are counted in five
seconds. What are the possible frequencies produced by the guitar string?
1.2.4 Checking my progress
1. Is the wavelength of the fundamental standing wave in a tube open
at both ends greater than, equal to, or less than the wavelength of the
fundamental standing wave in a tube with one open end and one closed
end?
2. You blow across the opening of a bottle to produce a sound. What must
be the approximate height of the bottle for the fundamental note to be a
middle C (1.29 m)?
3. Two loudspeakers are separated by 2.5 m. A person stands at 3.0 m from
one and at 3.5 m from the other one. Assume a sound velocity of 343
m/s.What is the minimum frequency to present destructive interference
at this point? Calculate the other two frequencies that also produce
destructive interference.
4. How would you create a longitudinal wave in a stretched spring? Would
it be possible to create a transverse wave in a spring?
5. In mechanics, massless strings are often assumed. Why is this not a good
assumption when discussing waves on strings?
6. Draw the second harmonic (The second lowest tone it can make.) of a
one end fixed, one end open pipe. Calculate the frequency of this mode
if the pipe is 53.2 cm long, and the speed of sound in the pipe is 317 m/s.
7. Calculate the wavelengths below. The length given is the length of the
waveform (The picture)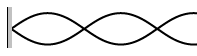
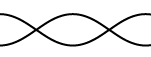
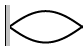
L = 45 cm L = 2.67 m L = 68 cm
8. A guitar string is 64 cm long and has a fundamental Mi frequency of
330 Hz. When pressing in the first fret (nearest to the tuning keys) see
fig. the string is shortened in such a way that it plays a Fa note having a
frequency of 350 Hz. Calculate the distance between this first fret and thenut necessary to get this effect.
9. Why is a pulse on a string considered to be transverse?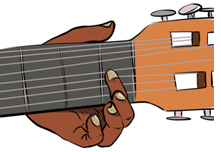
10. A guitar string has a total length of 90 cm and a mass of 3.6 g. From the
bridge to the nut there is a distance of 60 cm and the string has a tension
of 520 N. Calculate the fundamental frequency and the first two over
tones
1.3 CHARACTERISTICS OF MUSICAL NOTES
ACTIVITY 1.5: Characteristics of musical notes
The physical characteristics of a sound wave are directly related to
the perception of that sound by a listener. Before you read this section
answer these questions. As you read this section answer again these
questions. Compare your answer.
1. What is the difference between the sound of whistle and that of
drum?
2. Can you tell which musical instrument is played if the same note is
played on different instrument without seeing it? Explain
3. How can you calculate the intensity of a sound wave?
A musical note is produced by vibrations that are regular and repeating,
i.e. by periodic motion. Non-periodic motion results in noise which is not
pleasant to the ear. Many behaviors of musical note can be explained using a
few characteristics: intensity and loudness, frequency and pitch, and quality or
timber.
1.3.1. Pitch and frequency
The sound of a whistle is different from the sound of a drum. The whistle
makes a high sound. The drum makes a low sound. The highness or lowness of
a sound is called its pitch. The higher the frequency, the higher is the pitch. The
frequency of an audible sound wave determines how high or low we perceive
the sound to be, which is known as pitch.
Frequency refers to how often something happens or in our case, the number
of periodic, compression-rarefaction cycles that occur each second as a sound
wave moves through a medium and is measured in Hertz (Hz) or cycles/second.
The term pitch is used to describe our perception of frequencies within the
range of human hearing.
If a note of frequency 300 Hz and note of 600 Hz, are sounded by a siren, the
pitch of the higher note is recognized to be an upper octave of the lower note.
The musical interval between two notes is an upperoctave if the ratio of their
frequencies is 2:1. It can be shown that the musical interval between two notes
depends on the ratio of their frequencies, and not on the actual frequencies.
Whether a sound is high-pitched or low-pitched depends on how fast something
vibrates. Fast vibrations make high-pitched sounds. Slow vibrations make lowpitched
sounds.
Do not confuse the term pitch with frequency. Frequency is the physical
measurement of the number of oscillations per second. Pitch is a psychological
reaction to sound that enables a person to place the sound on a scale from high
to low, or from treble to bass. Thus, frequency is the stimulus and pitch is the
response. Although pitch is related mostly to frequency, they are not the same.
A phrase such as “the pitch of the sound” is incorrect because pitch is not a
physical property of the sound.The octave is a measure of musical frequency.
1.3.2 Intensity and amplitude
A police siren makes a loud sound. Whispering makes a soft sound. Whether a
sound is loud or soft depends on the force or power of the sound wave. Powerful
sound waves travel farther than weak sound waves. To talk to a friend across
the street you have to shout and send out powerful sound waves. Your friend
would never hear you if you whispered.
A unit called the decibel measures the power of sound waves. The sound waves
of a whisper are about 10 decibels. Loud music can have a level of 120 decibels
or more. Sounds above 140 decibels can actually make your ears hurt. The
energy carried by a sound wave is proportional to the square of its amplitude.
The energy passing a unit area per unit time is called the intensity of the wave.
The intensity of spherical sound wave at a place p is defined as the energy per
second per m2, or power per m2 flowing normally through an area at X. i.e
So the unit of intensity is W /m2 where r is the distance from the source for a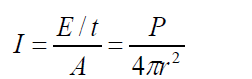 (1.25)
(1.25)spherical wave
Sound intensity level
To the human ear the change in loudness when the power of a sound increases
from 0.1 W to 1.0 W is the same as when 1 W to 10 W. The ear responds to the
ratio of the power and not to their difference. We measure sound level intensity
in terms of “decibels”. The unit bel is named after the inventor of the telephone,
Alexander Graham Bell (1847–1922). The decibel is a “relative unit” which is
actually dimensionless, comparing a given sound to a standard intensity whichrepresents the smallest audible sound:
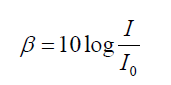 (1.26)
(1.26)Where
the softest audible sound (threshold of human hearing), while 80 dB (i.e.,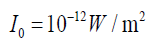 at 1000 Hz is the reference intensity. 0 dB thus represents
at 1000 Hz is the reference intensity. 0 dB thus represents
moderately loud music) represents an intensity which is one hundred milliontimes greater.
Example 1.8
Two identical machines are positioned the same distance from a worker.
The intensity of sound delivered by each machine at the location of
the worker is . Find the sound level heard by the worker (a) when one
machine is operating and (b) when both machines are operating.
Answer
a. The sound level at the location of the worker with one
machine operating is
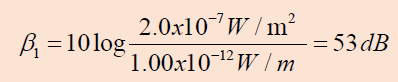
b. When both machines are operating, the intensity is doubled
to ; therefore, the sound level now is
From these results, we see that when the intensity is doubled,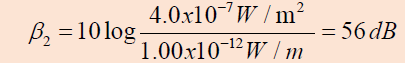
the sound level increases by only 3 dB.
Quick check 1.4:
A point source emits sound waves with an average power output of 80.0 W.
a. Find the intensity 3.00 m from the source.
b. Find the distance at which the sound level is 40 dB.
ACTIVITY 1.6: Noise or music
Most people like to listen to music, but hardly anyone likes to listen to
noise.
1. What is the physical difference between musical sound and noise?2. What is the effect of noise to human being?
The physical characteristics of a sound wave are directly related to the
perception of that sound by a listener. For a given frequency the greater the
pressure amplitude of a sinusoidal sound wave, the greater the perceived
loudness. The loudness or softness of sound depends on the intensity of the
sound wave reaching the person concerned. Loudness is a subjective quantity
unlike intensity.Sound that is not wanted or unpleasant to the ear is called
noise. High intensity can damage hearing.The higher the intensity, the louder is
the sound. Our ears, however, do not respond linearly to the intensity. A wave
that carries twice the energy does not sound twice as loud.
1.3.3 Quality or timbre
If the same note is sounded on the violin and then on the piano, an untrained
listener can tell which instrument is being used, without seeing it. We would
never mistake a piano for flute. We say that the quality or timbre of note is
different in each case. The manner in which an instrument is played strongly
influences the sound quality. Two tones produced by different instruments
might have the same fundamental frequency (and thus the same pitch) but
sound different because of different harmonic content. The difference in sound
is called tone color, quality, or timbre. A violin has a different timbre than a
piano.
1.3.4 Checking my progress
1. Complete each of the following sentences by choosing the correct term
from the word bank: loudness, pitch, sound quality, echoes, intensity
and noise
a. The ------------ of a sound wave depends on its amplitude
b. Reflected sound waves are called ---------------------------
c. Two different instruments playing the same note sound different
because of ------------------
2. Plane sound wave of frequency 100 Hz fall normally on a smooth wall. At
what distances from the wall will the air particles have:
a. Maximum
b. Minimum amplitude of vibration?
Give reasons for your answer. The speed of sound in air may be taken as 340
m/s
3. A boy whistles a sound with the power of 0.5x10-4w . What will be his
sound intensity at a distance of 5m?
4. Calculate the intensity level equivalent to an intensity 1 nW/m2
5. If the statement is true, write true. If it is false, change the underlined
word or words to make the statement true.
a. Intensity is mass per unit volume.
b. Loudness is how the ear perceives frequencyc. Music is a set of notes that are pleasing
1.4 APPLICATIONS OF SOUND WAVES
ACTIVITY 1.7: Doppler Effect and uses of sound waves
1. Why does the pitch of a siren change as it moves past you?
2. How is Doppler’s effect used in communication with satellites?
3. Explain how is the Doppler’s effect used in Astronomy?
4. People use sound for other things other than talking and making
music. In your own word, give more examples and explanations to
support this statement.
1.4.1 The Doppler Effect
Doppler’s effect is the apparent variation in frequency of a wave due to therelativemotion of the source of the wave and the observer.

Fig.1. 19 C.J.Doppler (Douglass, PHYSICS, Principles with applications., 2014)
The effect takes its name from the Austrian Mathematician Christian Johann
Doppler (1803-1853), who first stated the physical principle in 1842. Doppler’s
principle explains why, if a source of sound of a constant pitch is moving toward
an observer, the sound seems higher in pitch, whereas if the source is moving
away it seems lower. This change in pitch can be heard by an observer listeningto the whistle of an express train from a station platform or another train.
Fig.1. 20: An observer O (the cyclist) moves with a speed vOtoward a stationary point source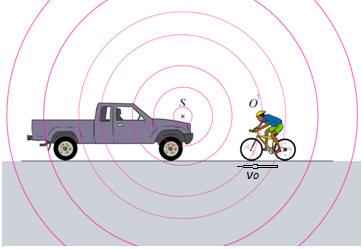
S, the horn of a parked truck. The observer hears a frequency f’ that is greater than the sourcefrequency.
The wavelength is shortened by an amount vsT , where T is the period
of the wave. This is simply due to the motion of the source. Since the
“received” wavelength (λ r )is related to the “source” wavelength by
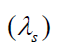
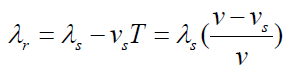
Knowing the velocity of the moving source of wave ( vs ), you can use the
equation v = λf to convert the wavelength equations to solve for frequency.
The received frequency is related to the source frequency by
Hence the frequency you hear is higher than the frequency emitted by the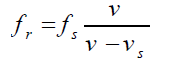
approaching source.
Example 1.9
If a source emits a sound of frequency 400 Hz when at rest, then when
the source moves toward a fixed observer with a speed of 30 m/s, what
frequency does the observer hears knowing that the speed of a sound in
air at room temperature is 343m/s?
AnswerThe observer hears a frequency of
As the source passes you and recedes, the “speed of approach” vs becomes
negative, and the frequency you hear becomes lower than the frequency emitted
by the now receding source.The frequency of the wave will be:
In this case if a source vibrating at 400 Hz is moving away from a fixed observer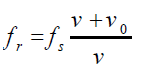
at 30 m/s, the later will hear a frequency of about
a. When the source is stationary but you are approaching it at a speed vo.
The Doppler’s effect also occurs when the source is at rest and the observer is
in motion. If the observer is travelling toward the source the pitch is higher; and
if the observer is travelling away from the source, the pitch is lower.
With a fixed source and moving observer, the distance between wave crests, the
wavelengthλ , is not changed. But the velocity of the crests with respect to the
observer is changed. If the observer is moving toward the source, the speed of
the wave relative to the observer is v′ = v + v0Hence, the new frequency is
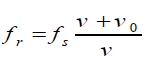
If the observer is moving away from the source, the relative velocity is o v'= v − v
and
Example 1.10
1. The siren of a police car at rest emits at a predominant frequency
of 1600 Hz. What frequency will be heard if you were moving with
speed of 25 m/s?
a. Toward it?
b. Away from it?Answer
b. If both the source and receiver are moving
If both the source and receiver are moving and vs and vo are the speeds withwhich they are approaching each other (respectively), the Doppler shift is
c. Here v is the speed of sound in air; vr is the speed of the listener (substituted (1.31)
(1.31)
as positive if he moves towards the source, as negative if he moves away from
the source), and vs is the speed of the source (reckoned as positive if it movestowards the listener, as negative if it moves away from the listener.
Example 1.11
A car, sounding a horn producing a note of 500 Hz, approaches and
passes a stationary observer O at a steady speed of 20 m/s. Calculate the
change in pitch of the note heard by O (speed of sound is 340 m/s)Answer:
For convenience, we can write Doppler’s effect equation as a single
equation that covers all cases of both source and observer in motion:
The upper signs apply if source and/or observer move toward each other. The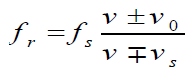
lower signs apply if they are moving apart. The word toward is associated with
an increase in observed frequency. The words away from are associated with a
decrease in observed frequency. Although the Doppler’s effect is most typically
experienced with sound waves, it is a phenomenon that is common to all waves.
For example, the relative motion of source and observer produces a frequency
shift in light waves. The Doppler’s effect is used in police radar systems to
measure the speeds of motor vehicles. Likewise, astronomers use the effect to
determine the speeds of stars, galaxies, and other celestial objects relative to
the Earth.
Example 1.12
As an ambulance travels east down a highway at a speed of 33.5 m/s, its
siren emits sound at a frequency of 400 Hz. What frequency is heard by
a person in a car traveling west at 54.6 m/s
a. As the car approaches the ambulance and
b. As the car moves away from the ambulance?
Answer
As the ambulance and car approach each other, he person in the car
hears the frequency
a. As the vehicles recede from each other, the person hears the frequency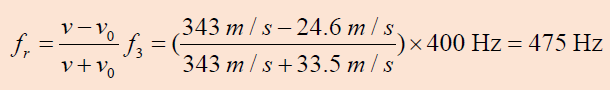
The change in frequency detected by the person in the car is 475 Hz - 338 Hz
= 137 Hz, which is more than 30% of the true frequency.
b. Suppose the car is parked on the side of the highway as the ambulance
speeds by. What frequency does the person in the car hear as the
ambulance (a) approaches and (b) recedes?
Answer
(a) 443 Hz. (b) 364 Hz.The motion of the source of sound affects its pitch.
Quick check 1.5:
Middle C on the musical scale has a frequency of 264 Hz. What is the
wavelength of the sound wave?
1.4.2 Uses of Ultrasonic
a. Echolocation
Some marine mammals, such as dolphin, whales, and porpoises use sound
waves to locate distant objects. In this process, called echolocation, a dolphin
produces a rapid train of short sound pulses that travel through the water,
bounce off distant objects, and reflect back to the dolphin. From these echoes,
dolphins can determine the size, shape, speed, and distance of their potential
prey. Experiments have shown that at distance of 114 m, a blindfolded dolphin
can locate a stainless-steel sphere with a diameter of 7.5 cm and can distinguish
between a sheet of aluminum and a sheet of copper (Cutnell & Johnson, 2006).
The Ultrasonic waves emitted by a dolphin enable it to see through bodies of
other animals and people (Fig.1.21). Skin muscles and fat are almost transparent
to dolphins, so they see only a thin outline of the body but the bones, teeth and
gas-filled cavities are clearly apparent. Physical evidence of cancers, tumors,
heat attacks, and even emotional shake can all be seen by dolphin. What is more
interesting, the dolphin can reproduce the sonic signals that paint the mental
image of its surroundings, and thus the dolphin probably communicates its
experience to other dolphins. It needs no words or symbol for fish, for example,but communicates an image of the real thing.
Fig.1. 21: The Ultrasonic waves emitted by a dolphin enable it to see through bodies of other
animals and people.
Bats also use echo to navigate through air.Bats use ultrasonic with frequenciesup to 100 kHz to move around and hunt (Fig.1.23).
Fig.1. 22 Bats use ultrasonic with frequencies up to 100 kHz to move around and hunt.
The waves reflect off objects and return the bat’s ears. The time it takes for the
sound waves to return tells the bat how far it is from obstacles or prey. The bat
uses the reflected sound waves to build up a picture of what lies ahead. Dogs,
cats and mice can hear ultrasound frequencies up to 450 kHz. Some animals
not only hear ultrasound but also use ultrasonic to see in dark.
b. In medicine
The sonogram is device used in medicine and exploits the reflected ultrasound
to create images. This pulse-echo technique can be used to produce images of
objects inside the body and is used by Physicians to observe fetuses. Ultrasound
use a high frequency in the range of 1 MHz to 10 MHz that is directed into the
body, and its reflections from boundaries or interfaces between organs and
other structures and lesions in the body are then detected. (Michael, Loannis,
& Martha, 2006)
Tumors and other abnormal growths can be distinguished; the action of
heart valves and the development of a foetus (Fig.1.24) can be examined; and
information about various organs of the body, such as the brain, heart, liver, and
kidneys, can be obtained.
Although ultrasound does not replace X-rays, for certain kinds of diagnosis it is
more helpful. Some tissues or fluid are not detected in X-ray photographs, but
ultrasound waves are reflected from their boundaries. Echoes from ultrasound
waves can show what is inside the body. Echo is a reflection of sound off thesurface of an object.
Fig.1. 23: Ultrasound image as an example of using high-frequency sound waves to see within the
human body (Douglass, PHYSICS, Principles with applications., 2014).
In medicine, ultrasonic is used as a diagnostic tool, to destroy diseased tissue,
and to repair damaged tissue.Ultrasound examination of the heart is known as
echocardiography.
c. Sonar
The sonar or pulse-echo technique is used to locate underwater objects and to
determine distance. A transmitter sends out a sound pulse through the water,
and a detector receives its reflection, or echo, a short time later. This time interval
is carefully measured, and from it the distance to the reflecting object can be
determined since the speed of sound in water is known. The depth of the sea
and the location of sunken ships, submarines, or fish can be determined in this
way. Sonar also tells how fast and what direction things are moving. Scientists
use sonar to make maps of the bottom of the sea. An analysis of waves reflected
from various structures and boundaries within the Earth reveals characteristic
patterns that are also useful in the exploration for oil and minerals.
Radar used at airports to track aircraft involves a similar pulse-echo technique
except that it uses electromagnetic (EM) waves, which, like visible light, travel
with a speed of 3 ×108 m/s.
One reason for using ultrasound waves, other than the fact that they are
inaudible, is that for shorter wavelengths there is less diffraction so the beam
spreads less and smaller objects can be detected.
1.4.3 Uses of infrasonic
Elephants use infrasonic sounds waves to communicate with one another. Their
large ears enable them to detect these low frequency sound waves which have
relatively long wavelengths. Elephants can effectively communicate in this way
even when they are separated by many kilometers. Some animals, such as thisyoung bat-eared fox, have ears adapted for the detection of very weak sounds.
Fig.1. 24: Some animals, such as this young bat-eared fox, have ears adapted for the detection of
very weak sounds.
1.4.4 Checking my progress
For question 1 to 2: Choose the letter of the best answer
1. Bats can fly in the dark without hitting anything because
a. They are flying mammals
b. Their night vision is going
c. They are guided by ultrasonic waves produced by them
d. Of no scientific reason
2. Bats and dolphins use echolocation to determine distances and find
prey.
What characteristic of sound waves is most important for echolocation?
a. Sound waves reflect when they hit a surface
b. Sound waves spread out from a source
c. Sound waves diffract around corner
d. Sound waves interfere when they overlap
3. Discuss application of sound waves in medicine and navigation
4. Explain how sonar is used to measure the depth of a sea
5. a. What is meant by Doppler Effect?
b. A police car sound a siren of 1000 Hz as it approaches a stationary
observer at a speed of 33.5 m/s. What is the apparent frequency
of the siren as heard by the observer if the speed of sound in air is
340 m/s.
c. Give one application of the Doppler Effect.
END UNIT ASSESSMENT 1
A. Multiple choices question
For question 1 to 6, choose the letter of the best answer
1. Which of the following affects the frequency of wave?
a. Reflection
b. Doppler effect
c. Diffraction
d. All of the above
2. Consider the following statements:
I. Recording of sound on tapes was first invented by Valdemar
Poulsen.
II. Audio tapes have magnetic property.
III. The tapes may also be made of PVC (Polyvinyl-chloride)Of
these statements:
a. I, II, and III all are correct.
b. I, II, and III all are wrong
c. I and II are correct, III is wrong
d. I and II are wrong, III is correct
3. Nodes are
a. Positions of maximum displacement
b. Positions of no displacement
c. A position between no displacement and maximum
displacement
d. None of these
4. Sound waves are
a. Transverse waves characterized by the displacement of air
molecules.
b. Longitudinal waves characterized by the displacement of air
molecules.
c. Longitudinal waves characterized by pressure differences.
d. Both (B) and (C).
e. (A), (B), and (C).
5. In which of the following is the wavelength of the lowest vibration
mode the same as the length of the string or tube?
a. A string.
b. A tube closed at one end.
c. All of the above.
d. An open tube.
e. E. None of the above.
6. When a sound wave passes from air into water, what properties of the
wave will change?
a. Frequency.
b. Wave speed.
c. Both frequency and wavelength.
d. Wavelength.
e. Both wave speed and wavelength.
B. Structured questions
1. Does the phenomenon of wave interference apply only to sinusoidal
waves? Explain.
2. As oppositely moving pulses of the same shape (one upward, one
downward) on a string pass through each other, there is one instant at
which the string shows no displacement from the equilibrium position
at any point. Has the energy carried b traveling in opposite directions
on the same string reflect from each other? Explain.
4. When two waves interfere, can the amplitude of the resultant wave be
greater than the amplitude of any of the two original waves? Under
which conditions?
5. When two waves interfere constructively or destructively, is there any
gain or loss in energy? Explain.
6. Explain why your voice seems to sound better than usual when you sing
in the shower.
7. An airplane mechanic notices that the sound from a twin-engine aircraft
rapidly varies in loudness when both engines are running. What could
be causing this variation from loud to soft?
8. Explain how a musical instrument such as a piano may be tuned by using
the phenomenon of beats.
9. Fill in the gap
a. As a sound wave or water ripple travels out from its source, its -----
--------- decreases.
b. The vibrating air in a/an ----------------------------- has displacement
antinodes at both ends.
c. For a /an ……………., the fundamental corresponds to a wavelength
four times the length of the tube.
d. The ……………….. refers to the change in pitch of a sound due to
the motion either of the source or of the observer. If source and
observer are approaching each other, the perceived pitch is …….. If
they are moving apart, the perceived pitch is …………….
10. A bat, moving at 5.00 m/s, is chasing a flying insect. If the bat emits a
40.0 kHz chirp and receives back an echo at 40.4 kHz, at what speed
is the insect moving toward or away from the bat? (Take the speed of
sound in air to be v = 340 m/s.)
11. If you hear the horn of the car whose frequency is 216 Hz at a frequency
of 225 Hz, what is their velocity? Is it away from you or toward you? The
speed of sound is 343 m/s
12. You run at 12.5 m/s toward a stationary speaker that is emitting a
frequency of 518 Hz. What frequency do you hear? The speed of sound
is 343 m/s
13. If you are moving and you hear the frequency of the speaker at 557
Hz, what is your velocity? Is it away from or toward the speaker? The
speed of sound is 343 m/s
C. Essay type question
20. Read the following text and answer the question
Researchers have known for decades that whales sing complicated songs.
Their songs can last for 30 min and a whale may repeat the song for two or
more hours. Songs can be heard at a distances of hundreds of kilometers.
There is evidence that whales use variations in the songs to tell other whales
about the location of food and predators. Only the male whales sing, which
has led some researchersto think that songs are also used to attract a male.
The whale songs may be threatened by noise pollution. in the past 50 years,
ocean noise has increased due to human activities. Goods are transported
across the ocean in larger ships than ever before. Large ships use bigger
engines. They produce low-frequency noise by stirring up air bubbles with
their propellers. Unfortunately, whales also use low-frequency sound in their
songs, perhaps because these sounds carry further than high-frequency
sounds in the ocean. Propeller noise from large ships is loud enough to
interfere with whale songs at a distance of 20 km.
Question: Are regulations needed to protect whales from noise?
In your own words, describe the major issue that needs to be resolved about
ocean noise pollution. List three arguments for those who think regulations
should require large ships to reduce noise pollution. List three arguments forthose who think regulations are not necessary.
UNIT 2 APPLICATION OF PHYSICS IN AGRICULTURE

Fig.2. 1: A farmer spraying rice
Key unit competence: Evaluate applications of Physics in Agriculture.
My goals
• Describe the atmosphere and its constituents.
• Outline variation of atmospheric pressure, air density and water
vapour with altitude.
• Evaluate how heat and mass transfers occur in the atmosphere.
• Apply knowledge of physics to illustrate changes in water vapour
atmospheric pressure, and air with altitude.
• Evaluate and interpret physical properties of soil (soil Texture and
structure).
• Evaluate why air, temperature and rainfall limit economical activities
in Agriculture.
• Explaining how mechanical weathering and soil erosion impact
economic activities in agriculture.
• Explaining clearly how agrophysics plays an important role in the
limitation of hazards to agricultural objects and environment in ourcountry.
INTRODUCTORY ACTIVITY: Role of machines in agriculture
It is very important to know the role of Physics in agriculture and
environment. Knowledge in physics can contribute more in the limitation of
hazards in agriculture and the environment based on suitable programs of
transformation and modernization of agriculture in our country.
Look at the image given in Fig.2.1
a. What do you observe? Is there any application of prior knowledge
of Physics learnt before applied on the image? Justify your answer?
b. Can you suggest the role of machines in agriculture? How do they
contribute
in the rapid development of the country programs
of transformation and modernization of agriculture? Knowing
different stages of growing plants activities, suggest which stages
mostly benefit the use of technology!
Plan it! To get started, brainstorm about your prior knowledge on applications
of physics in agriculture and try to suggest answers to questions given abovebased on your understanding.
2.1 ATMOSPHERE AND ITS CONSTITUENTS
2.1.1 Atmosphere
ACTIVITY 2.1: How the atmosphere protect life on the earth
a. Brainstorm and write short notes on constituents of atmosphere
and explain clearly how the atmosphere of Earth protects life on
earth.
b. Why should you care about protecting the atmosphere and
minimise the long-term changes in the climate?
c. What can be the use of atmospheric knowledge in evaluating andimproving agricultural activities?
The atmosphere of earth is the layer of gases, commonly known as air that
surrounds the earth. This layer of gases is retained by Earth’s gravity. The
atmosphere of Earth protects life on Earth by absorbing ultraviolet solar
radiations that cause cancers and other diseases, warming the surface
through heat retention (greenhouse effect) and reducing temperature extremes
between day and night. It also contain the oxygen which human beings, animals
and plants are using, The atmospheric knowledge can be helpful in evaluating
and improving the quality of soils and agricultural products as well as the
technological processes.
2.1.2 Composition of the Atmosphere
The atmosphere is composed of a mixture of several gases in differing amounts.
The permanent gases whose percentages do not change from day to day are
nitrogen, oxygen and argon. By volume, dry air contains 78.0% nitrogen, 21%
oxygen, 0.9% argon, 0.04% carbon dioxide, and small amounts of other gases
called trace gases 0.1% as shown in Fig.2.2
Gases like carbon dioxide, nitrous oxides, methane, and ozone are trace gases
that account for about a tenth of one percent of the atmosphere.and plants are using, The atmospheric knowledge can be helpful in evaluating
and improving the quality of soils and agricultural products as well as the
technological processes.
2.1.2 Composition of the Atmosphere
The atmosphere is composed of a mixture of several gases in differing amounts.
The permanent gases whose percentages do not change from day to day are
nitrogen, oxygen and argon. By volume, dry air contains 78.0% nitrogen, 21%
oxygen, 0.9% argon, 0.04% carbon dioxide, and small amounts of other gases
called trace gases 0.1% as shown in Fig.2.2
Gases like carbon dioxide, nitrous oxides, methane, and ozone are trace gases
that account for about a tenth of one percent of the atmosphere.

Fig.2. 2Composition of the atmosphere
Air also contains a variable amount of water vapor, on average around 1% at
sea level, and 0.4% over the entire atmosphere. Water vapor is unique in that
its concentration varies from 0-4% of the atmosphere depending on where you
are and what time of the day it is. In the cold, dry Arctic regions water vapor
usually accounts for less than 1% of the atmosphere, while in humid, tropical
regions water vapor can account for almost 4% of the atmosphere. Water vapor
content is very important in predicting weather.
Air content and atmospheric pressure vary at different layers, and air suitable
for use in photosynthesis by terrestrial plants and breathing of terrestrial
animals is found only in earth’s troposphere.
The composition of the atmosphere, among other things, determines its ability
to partly absorb and transmit sunlight radiations and trap infrared radiations,leading to potentially minimise the long-term changes in climate.
2.1.3 Layers of the atmosphere.
ACTIVITY 2.2: Classifying layers of the atmosphere
Materials For class demonstration:
• Chalk board or dry erase board mounted on a wall
• Chalk or dry erase marker
• 2-meter piece of string
• 1000 ml (1 litre) graduated cylinder
• Four bags of fish gravel or coloured sand (different colours)
Procedures
Case1:
1. Use a model to explore how far the earth’s atmosphere extends
above the surface of the earth and learn about the thickness of the
different layers of the atmosphere.
2. How far do you think the atmosphere extends above us? Tie a dry
eraser marker or a piece of chalk to one end of the string. Standing
next to the board, place your foot on the free end of the string and
draw an arc on the board with a radius of about 1.2 m. Your foot
represents the center of the earth. The arc represents the surfaceof the Earth.

Fig.2. 3 A person demonstrating the layers of the atmosphere
3. Suggest how far the earth’s atmosphere would extend above the
surface in this drawing. Mark your suggestions on the board above
the chalk/marker line. Note that it is found that over 90% of the
earth’s atmosphere is within about 12km of the earth’s surface. The
distance from the centre of the earth to its surface equals about6361 km.
Case 2:
1. Use a 1000 ml (1 litter) graduated cylinder and represent the layers by
using the following amounts of fish gravel or coloured sand found in the
photo and table below.
2. Choose what colour you want for each atmospheric layer. Keep in
mind these are relative proportions and not exact points of departure
for the different layers. In this scale model, each millilitre of volume
represents one kilometre of atmosphere layer thickness (for example,
the troposphere is 10 km thick and is represented by 10 ml of sand orgravel in the graduated cylinder).
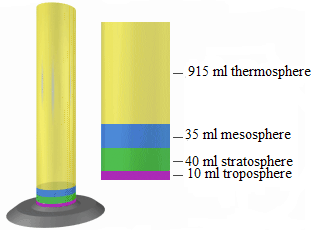
Fig.2. 4: Color coding represented in cylinder with the corresponding layers
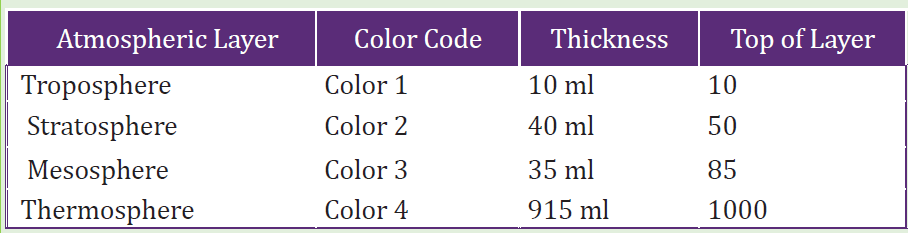
Table 2. 1: Atmospheric layers with color code and thickness
Questions
1. What atmospheric layers are represented by the different colors?
2. What atmospheric layer do we live in?
3. How much thicker is the stratosphere compared to the troposphere?
4. How much thicker is the thermosphere compared to all the other layers
combined?
5. Where in this model would you expect to find clouds?
6. Where in this model would you expect to find mounts Everest and
Kalisimbi?
7. Where in this model would you expect to find a satellite?
8. Where in this model would you expect to find the space shuttle?9. Where in this model is the ozone layer?
Earth’s atmosphere has a series of layers, each with its own specific
characteristics and properties. Moving upward from ground level, these layers
are named the troposphere, stratosphere, mesosphere, thermosphere and
exosphere.
The above activity demonstrates the relative thickness of the thin section of the
atmosphere that includes the troposphere and stratosphere. These layers are
essential to all life on Earth. Over 99% of the mass of the Earth’s atmosphere
is contained in the two lowest layers: the troposphere and the stratosphere.
Most of the Earth’s atmosphere (80 to 90%) is found in the troposphere, the
atmospheric layer where we live (Cunningham & William, 2000).
This layer, where the Earth’s weather occurs, is within about 10 km of the
Earth’s surface. The stratosphere goes up to about 50 km. Gravity is the reasonwhy atmosphere is more dense closer to the Earth’s surface.
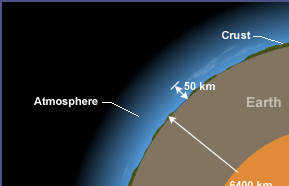
Fig.2. 5: Illustration of layers from the core to the atmosphere
While we may think of the atmosphere as a vast ocean of air around us, it is very
thin relative to the size of the earth. The “thickness” of the atmosphere, or the
distance between the earth’s surface and the “top” of the atmosphere, is not an
exact measure. Although air is considered as a fluid, it does not have the same
well-defined surface as water does. The atmosphere just fades away into space
with increasing altitude.
Description of layers of earth’s atmosphere
A. Troposphere
This is the lowest layer of our atmosphere starting at ground level; it extends
upward to about 10 km above the sea level. We live in the troposphere, and
nearly all weather occurs in this lowest layer. Most clouds appear here, mainlybecause 99% of the water vapor in the atmosphere is found in the troposphere.
Air pressure drops and temperatures get colder, as you climb higher in the
troposphere.
B. Stratosphere
The stratosphere extends from the top of the troposphere to about 50 km above
the ground. The infamous ozone layer is found within the stratosphere. Ozone
molecules in this layer absorb high-energy ultraviolet (UV) light from the sun,
converting the ultraviolet energy into heat.
Unlike the troposphere, the stratosphere actually gets warmer the higher
you go! That trend of rising temperatures with altitude means that air in the
stratosphere lacks the turbulence and updrafts of the troposphere beneath.
Commercial passenger jets fly in the lower stratosphere, partly because this
less-turbulent layer provides a smoother ride.
C. Mesosphere
It extends upward to a height of about 85 km above our planet. Most meteors
burn up in the mesosphere. Unlike the stratosphere, temperatures once again
grow colder as you rise up through the mesosphere. The coldest temperatures
in earth’s atmosphere, about -90° C, are found near the top of this layer. The air
in the mesosphere is far too thin to breathe; air pressure at the bottom of this
layer is well below 1% of the pressure at sea level, and continues dropping as
you go higher.
D. Thermosphere
High-energy X-rays and ultraviolet radiations from the Sun are absorbed in the
thermosphere, raising its temperature to hundreds or at times thousands of
degrees. Many satellites actually orbit Earth within the thermosphere! Variations
in the amount of energy coming from the Sun exert a powerful influence on
both the height of the top of this layer and the temperature within it. Because of
this, the top of the thermosphere can be found anywhere between 500 km and
1 000 km above the ground. Temperatures in the upper thermosphere can
range from about 500 ° C to 2 000 °C or even higher.
E. Exosphere
Although some experts consider the thermosphere to be the uppermost layer of
our atmosphere, others consider the exosphere to be the actual “final frontier”
of Earth’s gaseous envelope. As you might imagine, the “air” in the exosphere is
very thin, making this layer even more space-like than the thermosphere.
In fact, air in the exosphere is constantly “leaking” out of earth’s atmosphere
into outer space. There is no clear-cut upper boundary where the exosphere
finally fades away into space. Different definitions place the top of the exosphere
somewhere between 100 000 km and 190 000 km above the surface of earth.
The latter value is about halfway to the moon!
F. Ionosphere
The ionosphere is not a distinct layer like the others mentioned above.
Instead, the ionosphere is a series of regions in parts of the mesosphere and
thermosphere where high-energy radiation from the sun has ionized atoms
and molecules. The ions formed in this way are responsible of the naming ofthis region as the ionosphere and endowing it with some special properties.
ACTIVITY 2.3: Classifying layers of the atmosphere
Observe scale models of the atmosphere on fig.3.3 below and its layers.
Note the height of earth’s atmosphere as compared to the size of
the planet overall and the relative thickness of each of the four main
layers of the atmosphere. Interpret the graph and Use it to answerquestions below:
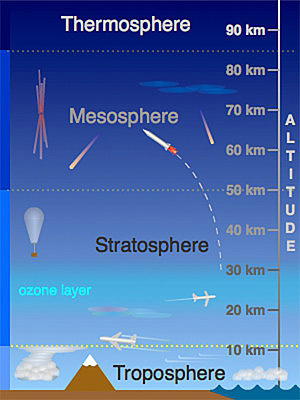
Fig.2. 6: Layers of the atmosphere.
Questions:
a. Outline the economic activities taking place in the layers
represented above?
b. Why is it very important to have ozone layer in the layers
close to the earth surface?
c. Explain why it is advisable to travel in troposphere and
stratosphere than in other layers of the atmosphere?
d. Explain clearly why the rocket and aeroplanes decide to movein the corresponding layers of the atmosphere?
2.1.4 Checking my progress
1. Use the fruit question suggested in the procedure and respond in writing
or with a picture instead of simply orally in class. Think of the fruit as the
size of the Earth and the skin of the fruit represents the thickness of the
atmosphere. Make a labelled diagram in your note book illustrating the
most important point of the lesson and the reason why the atmosphere
is considered as the skin of the fruit?
2. Imagine that you are in an orbit around a planet one half the size and
mass of the Earth. Explain how I would expect the atmosphere of the new
planet to be different from my planet?
3. What is the structure and composition of the atmosphere?
4. How does solar energy influence the atmosphere?
5. How does the atmosphere interact with land and oceans?
6. Outline two most important layers that are essential to all life on earth?
Explain clearly to support your answer.
2.2 HEAT AND MASS TRANSFER
2.2.1 Modes of Heat Transfer
ACTIVITY 2.4: Describing the different modes of heat transfer
in the atmosphere
Brainstorm on modes of heat transfer and explain clearly how eachaffects agricultural activities?
Heat transfer is concerned with the exchange of thermal energy through a
body or between bodies which occurs when there is a temperature difference.
When two bodies are at different temperatures, thermal energy transfers from
the one with higher temperature to the one with lower temperature. Naturally
heat transfers from hot to cold. A small amount of the energy that was directed
towards the earth from the sun is absorbed by the atmosphere, a larger amount
(about 30%) is reflected back to space by clouds and the Earth’s surface, and
the remaining is absorbed at the planet surface and then partially released as
heat. Energy is transferred between the Earth’s surface and the atmosphere
in a variety of ways, including radiation, conduction, and convection. The
figure below uses a camp stove to summarize the various mechanisms of heat
transfer. If you were standing next to the camp stove, you would be warmed by
the radiation emitted by the gas flame. A portion of the radiant energy generated
by the gas flame is absorbed by the frying pan and the pot of water.
By the process of conduction, this energy is transferred through the pot and pan.
If you reached for the metal handle of the frying pan without using a potholder,
you would burn your fingers! As the temperature of the water at the bottom
of the pot increases, this layer of water moves upward and is replaced by cool
water descending from above. Thus convection currents that redistribute thenewly acquired energy throughout the pot are established.

Fig.2. 7: Camp stove to summarize the various mechanisms of heat transfer.
As in this simple example using a camp stove, the heating of the Earth’s
atmosphere involves radiation, conduction, and convection, all occurring
simultaneously. A basic theory of meteorology is that the Sun warms the ground
and the ground warms the air. This activity focuses on radiation, the process
by which the Sun warms the ground. Energy from the Sun is the driving force
behind weather and climate.
Quick check 2.1
What do trees, snow, cars, horses, rocks, centipedes, oceans, the atmosphere,
and you have in common?
Each one is a source of radiation to some degree. Most of this radiation is
invisible to humans but that does not make it any less real.
Radiation is the transfer of energy by electromagnetic waves. The transfer of
energy from the Sun across nearly empty space is accomplished primarily by
radiation. Radiation occurs without the involvement of a physical substance
as the medium. The Sun emits many forms of electromagnetic radiation invarying quantities.
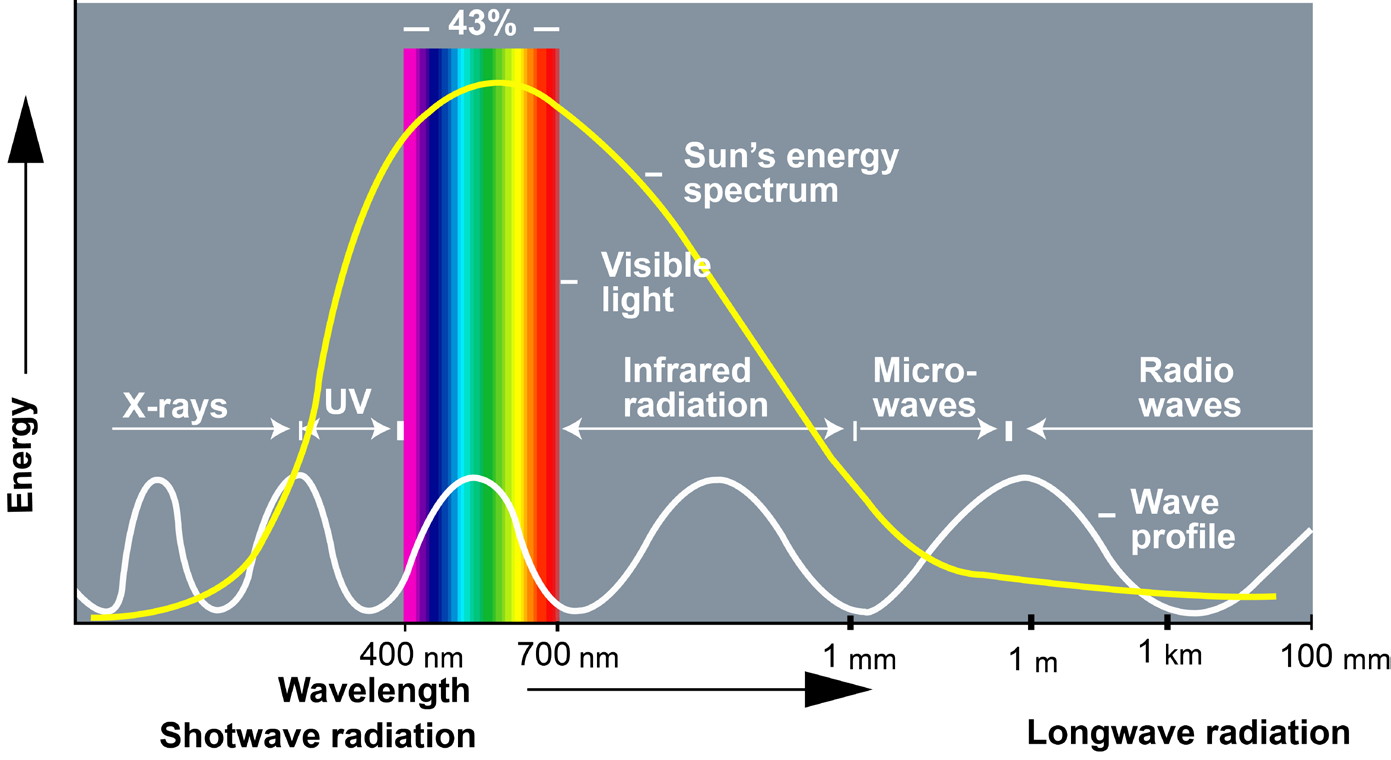
Fig.2. 8: The spectrum of electromagnetic radiations emitted by the sun
About 43% of the total radiant energy emitted from the Sun is in the visible
part of the spectrum. The bulk of the remainder lies in the near-infrared (49%)
and ultraviolet (7%) bands. Less than 1% of solar radiation is emitted as x-rays,
gamma rays, and radio waves.
A perfect radiating body emits energy in all possible wavelengths, but the wave
energies are not emitted equally in all wavelengths; a spectrum will show a
distinct maximum in energy at a particular wavelength depending upon the
temperature of the radiating body. As the temperature increases, the maximum
radiation occurs at shorter wavelengths.
The hotter the radiating body, the shorter the wavelength of maximum radiation.
For example, a very hot metal rod will emit visible radiation and produce a
white glow. On cooling, it will emit more of its energy in longer wavelengths
and will glow a reddish color. Eventually no light will be given off, but if you
place your hand near the rod, the infrared radiation will be detectable as heat.
The amount of energy absorbed by an object depends upon the following:
• The object’s absorptivity, which, in the visible range of wavelengths, is
a function of its color
• The intensity of the radiation striking the object
Every surface on Earth absorbs and reflects energy at varying degrees, based
on its color and texture. Darker-colored objects absorb more visible radiation,
whereas lighter-colored objects reflect more visible radiation. These conceptsare clearly discussed in unit two.
2.2.2 Environmental heat energy and mass transfer
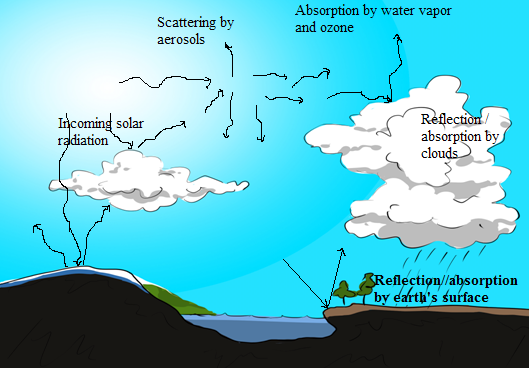
Fig.2. 9: Illustration of interactions of solar radiations with different constituents of the atmosphere.
Practically all of the energy that reaches the earth comes from the sun.
Intercepted first by the atmosphere, a small part is directly absorbed,
particularly by certain gases such as ozone and water vapor. Some energy is
also reflected back to space by clouds and the earth’s surface.
In the atmosphere, convection includes large- and small-scale rising and
sinking of air masses.. These vertical motions effectively distribute heat and
moisture throughout the atmospheric column and contribute to cloud and
storm development where rising motion occurs) and dissipation where sinkingmotion occurs.
2.2.3 Water vapour in the atmosphere.
ACTIVITY 2.5: Impact of water vapor in agricultural activities
1. Brainstorm on water vapor in the atmosphere and explain clearly
how it impacts on agricultural activities?
2. Does water vapor play an important role in the atmosphere? Justify
your answer with clear reasons.
When water vapor condenses onto a surface, a net warming occurs on that
surface. The water molecule brings heat energy with it. In turn, the temperature
of the atmosphere drops slightly. In the atmosphere, condensation
produces clouds, fog and precipitation (usually only when facilitated by cloud
condensation nuclei).The role of water vapor in the atmosphere
Water vapor plays a dominant role in the radiative balance and the hydrological
cycle. It is a principal element in the thermodynamics of the atmosphere as it
transports latent heat and contributes to absorption and emission in a number
of bands. It also condenses into clouds that reflect and adsorb solar radiation,
thus directly affecting the energy balance.
In the lower atmosphere, the water vapor concentrations can vary by orders of
magnitude from place to place.
2.2.4 Variation in Atmospheric Pressure
Variation with height or vertical variation: The pressure depends on the
density or mass of the air. The density of air depends on its temperature,
composition and force of gravity. It is observed that the density of air decreases
with increase in height so the pressure also decreases with increase in height.
Horizontal variation of pressure: The horizontal variation of atmospheric
pressure depends on temperature, extent of water vapor, latitude and land and
water relationship.
Factors affecting atmospheric pressure:
1. Temperature of air
2. Altitude
3. Water vapor in air
4. Gravity of the earth.
Effect of atmospheric pressure in agricultural activities
The pressure exerted by the atmosphere of the earth’s surface is called
atmospheric pressure. Generally, in areas of higher temperature, the
atmospheric pressure is low and in areas of low-temperature the pressure
is high. Atmospheric pressure has no direct influence on crop growth. It is,
however an important parameter in weather forecasting.
2.2.5 Air density and water vapour with altitude
ACTIVITY 2.6: Effect of air density in the atmosphere
Brainstorm on air density and explain clearly its affects in the earth’s
atmosphere?
The density of air (air density) is the mass per unit volume of earth’s
atmosphere. Air density, like air pressure, decreases with increasing altitude.It also changes with variation in temperature and humidity. At sea level and at
15 °C air has a density of approximately 1.225 kg/m3.
Air density and the water vapor content of the air have an important effect
upon engine performance and the takeoff characteristics of air-craft. Some of
the effects these two factors have upon engine takeoff, and the methods for
computing these elements from a meteorological standpoint. Pressure altitude,
density altitude, vapor pressure, and specific humidity in the atmosphere are
determined using a Density Altitude Computer.
Pressure altitude: Pressure altitude is defined as the altitude of a given
atmospheric pressure in the standard atmosphere. The pressure altitude of
a given pressure is, therefore, usually a fictitious altitude, since it is equal to
true altitude only rarely, when atmospheric conditions between sea level and
the altimeter in the aircraft correspond to those of the standard atmosphere.
Aircraft altimeters are constructed for the pressure-height relationship thatexists in the standard atmosphere.
2.2.6 Checking my progress
1. Why does atmospheric pressure change with altitude?
2. The graph below gives an indication of how pressure varies non-linearlywith altitude. Use the graph to answer the following questions:
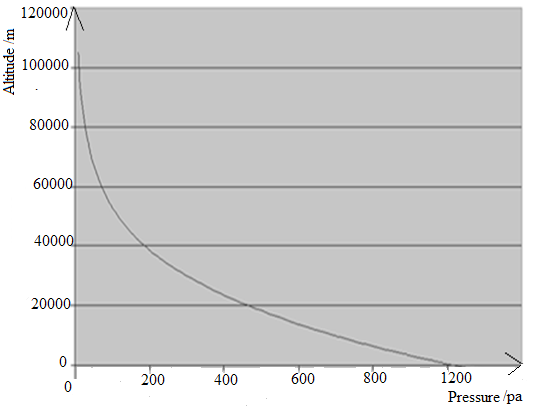
Fig.2. 10: A graph of altitude vs pressure
a. Explain what happens to pressure if the altitude reduces?
b. Estimate the atmospheric pressure when someone is at an altitude of40 km above sea level.
2.3 PHYSICAL PROPERTIES OF SOIL
ACTIVITY 2.7: How the surface of earth reflects and absorbs heat
Perform the activity to investigate how different surfaces of the
earth reflect and absorb heat and apply this knowledge to realworld
situations. It justifies that the physical characteristics of the
Earth’s surface affect the way that surface absorbs and releases heat
from the Sun.Materials needed in demonstration
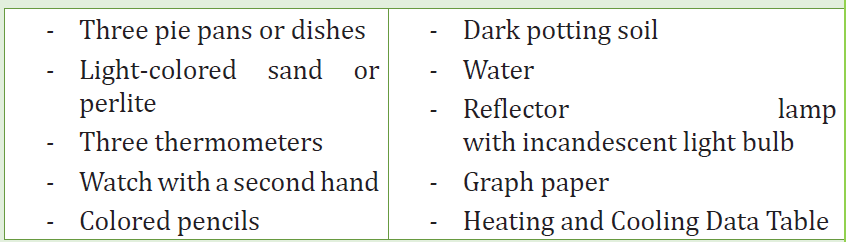
Procedures
1. a. Brainstorm on the already known concepts about how the color
and type of material affects how hot it gets in the sunshine. Try to
think about these questions. When it is a hot day, what color shirt
would you wear to keep cool and why? During the summer, what
would it feel like to walk on gravel with no shoes?
b. In performing activity, explore how different types of surfaces
found at the earth’s surface (such as sand, soil, and water) heat
up when the sun’s energy reaches them, and how they cool down
when out of the sunshine.
c. Note that this experiment uses materials to model sunshine
and earth materials. Observe the materials and realize how each
material relates to the earth system. (The lamp represents the
sun in this model. The sand represents beaches, sand dunes, and
rocks. The potting soil represents large areas of soil outdoors. And
the water represents lakes, rivers, and the ocean.)
2. Fill the pie pans to the same level, one with dark soil, one with light
sand, and one with water.
3. Place the pie pans on a table or desk and position the lamp about30.48 cm above them. (Do not turn on the lamp yet.)
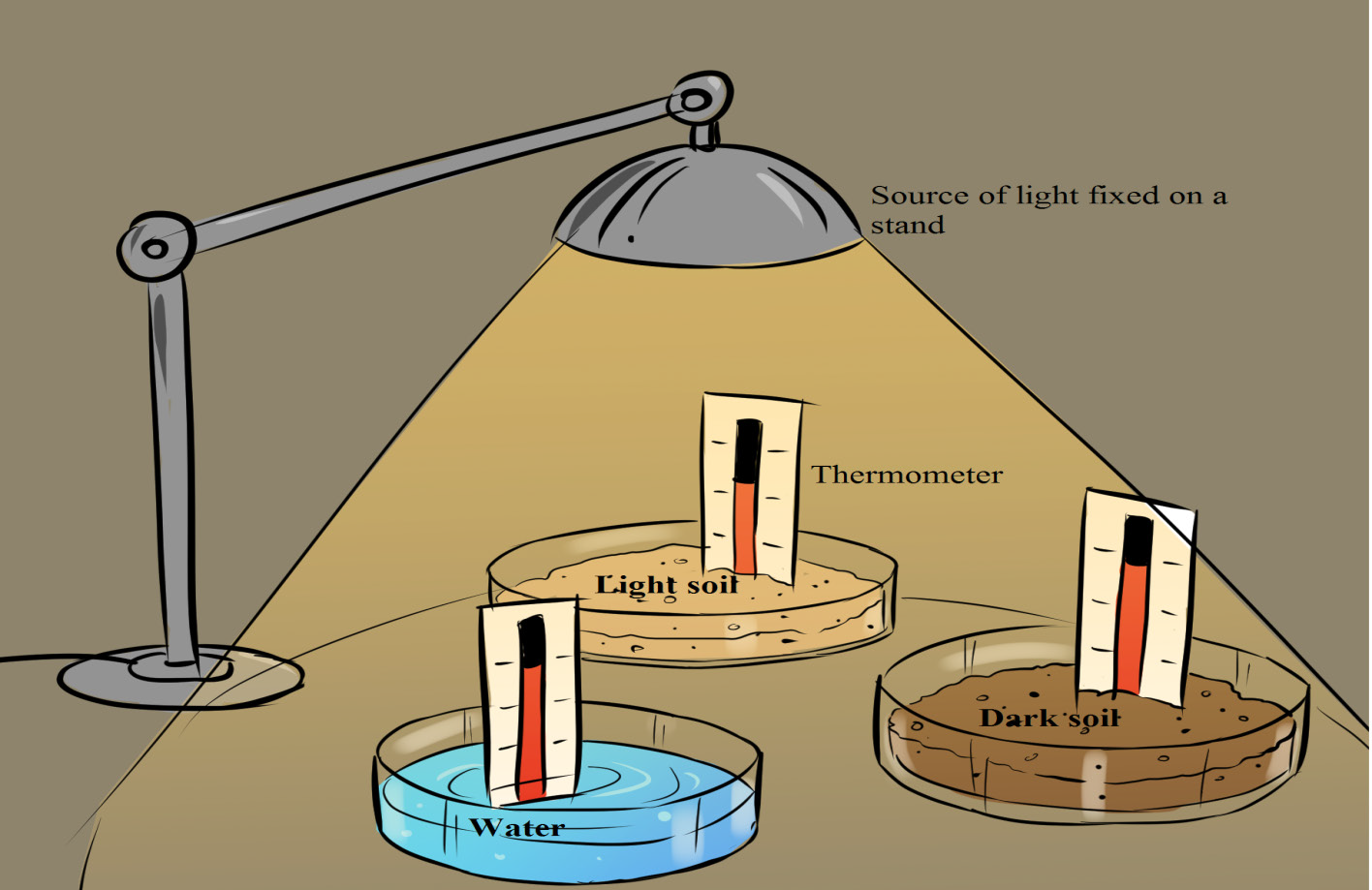
Fig.2. 11: Arrangement of pie pans to investigate the absorption of solar radiation.
Checking skills Questions
1. Which material absorbed more heat in the first ten minutes?
2. Which material lost the most heat in the last ten minutes?
3. Imagine that it is summer and that the Sun is shining on the ocean and
on a stretch of land.
a. Which one will heat up more during the day?
b. Which one will cool more slowly at night? Explain.
4. Imagine three cities in the desert, all at about the same altitude and
latitude. Which city would likely have the highest average summer air
temperature and why?
• One city (A) is surrounded by a dark-colored rocky surface.
• Another city (B) is surrounded by a light-colored sandy surface.
• The third city (C) is built on the edge of a large man-made desert
lake.
5. The Earth’s surface tends to lose heat in winter. Which of the above cities
would have the warmest average winter temperature? Why?
6. Since the Sun is approximately 93 million miles from the Earth and space
has no temperature, how do we get heat from the Sun?
Physical properties of a soil that affect a plant’s ability to grow include: Soil
texture, which affects the soil’s ability to hold onto nutrients (cation exchange
capacity) and water. Texture refers to the relative distribution of the different
sized particles in the soil. It is a stable property of soils and, hence, is used in
soil classification and description.Soil structure, which affects aeration, waterholding
capacity, drainage, and penetration by roots and seedlings, among other
things. Soil structure refers to the arrangement of soil particles into aggregates
and the distribution of pores in between. It is not a stable property and is
greatly influenced by soil management practices.
2.3.1 Soil texture
ACTIVITY 2.8
Soil texture is determined by three proportions of the soil. Brainstorm
and try to answer questions using knowledge gained.
a. Outline three proportions of the soil?
b. What does the underlined word mean?
Soil texture, or the ‘feel’ of a soil, is determined by the proportions of sand, silt,
and clay in the soil. When they are wet, sandy soils feel gritty, silky soils feel
smooth and silky, and clayey soils feel sticky and plastic, or capable of being
moulded. Soils with a high proportion of sand are referred to as ‘light’, and
those with a high proportion of clay are referred to as ‘heavy’.
The names of soil texture classes are intended to give you an idea of their
textural make-up and physical properties. The three basic groups of texture
classes are sands, clays and loams.A soil in the sand group contains at least
70% by weight of sand. A soil in the clay group must contain at least 35% clay
and, in most cases, not less than 40%. A loam soil is, ideally, a mixture of sand,
silt and clay particles that exhibit light and heavy properties in about equal
proportions, so a soil in the loam group will start from this point and theninclude greater or lesser amounts of sand, silt or clay.
2.3.2 Soil structure
ACTIVITY 2.9
a. It is known that soil structure contains soil particles and pores
and is classified under physical properties of soil. Brainstorm and
write short notes on soil structure.
Use the knowledge gained in part (a) above to answer questions in part (b).
b. (i) List the elements found in soil particles.
(ii)What do the underlined words mean?
(iii)Explain the role of pores in soil structure that improves
capillary action in plant growth.
Structure is the arrangement of primary sand, silt and clay particles into
secondary aggregates called peds or structural units which have distinct shapes
and are easy to recognize. These differently shaped aggregates are called the
structural type.
There are 5 basic types of structural units:
• Platy: Plate-like aggregates that form parallel to the horizons like
pages in a book. This type of structure may reduce air, water and root
movement.
• Blocky: Two types--angular blocky and sub angular blocky. These
types of structures are commonly seen in the B horizon. Angular is
cube-like with sharp corners while sub angular blocky has rounded
corners.
• Prismatic: Vertical axis is longer than the horizontal axis. If the top
is flat, it is referred to as prismatic. If the top is rounded, it is called
columnar.
• Granular: Peds are round and pourous, spheroidal. This is usually the
structure of A horizons.
• Structureless: No observable aggregation or structural units.
Good soil structure means the presence of aggregations which has positive
benefits for plant growth. These benefits arise from the wider range of pore
sizes which result from aggregation. The nature of the pore spaces of a soil
controls to a large extent the behavior of the soil water and the soil atmosphere.
It influences the soil temperature. All these affect root growth, as does the
presence of soil pores of appropriate size to permit root elongation. Favorable
soil structure is therefore crucial for successful crop development. The
destruction of soil structure may result in a reduction in soil porosity and/or
change to the pore size distribution.
Soil structure refers to the arrangement of soil particles (sand, silt and clay)
and pores in the soil and to the ability of the particles to form aggregates.
Aggregates are groups of soil particles held together by organic matter or
chemical forces. Pores are the spaces in the soil. The pores between the
aggregates are usually large (macro pores), and their large size allows good
aeration, rapid infiltration of water, easy plant root penetration, and good
water drainage, as well as providing good conditions for soil micro-organisms
to thrive. The smaller pores within the aggregates or between soil’s particles
(micro pores) hold water against gravity (capillary action) but not necessarily
so tightly that plant cannot extract the water.
A well-structured soil forms stable aggregates and has many pores (Fig.3.12 A).
it is friable, easily worked and allows germinating seedlings to emerge and to
quickly establish a strong root system. A poorly structured soil has either few
or unstable aggregates and few pore spaces (Fig.3.12 B). This type of soil can
result in unproductive compacted or waterlogged soils that have poor drainage
and aeration. Poorly structured soil is also more likely to slake and to becomeeroded.
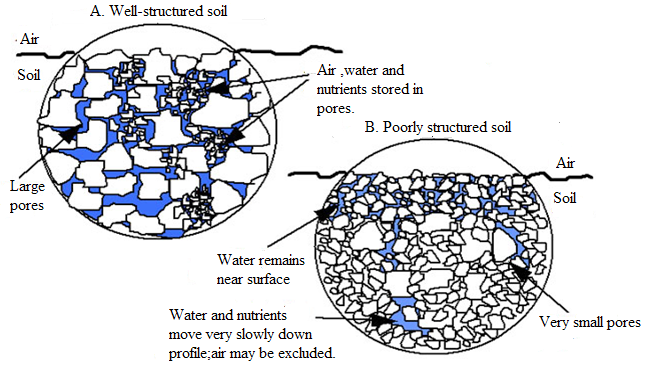
Fig.3. 12: Different soil structures: well structured and poorly structured soil.
2.3.3 Checking my progress:
1. Explain the physical properties of Soil and explain clearly how each
impact agricultural activities?
2. (a) Explain how the weathering of rocks contributes to soil formation.
(b) Explain the following terms as used in the context of soil and plant
growth.
I. Well structured soil
II. Poor structured soil
(c) The following table shows the water content of three soil samples.Use the table to answer questions that follows:
Analytical Questions: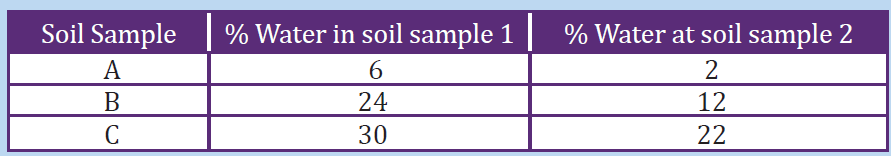
I. What is the percentage of available water in sample A?
II. Which sample would be the most suitable for a crop suffering a
drought during the growing season?
III. Which sample would be the most suitable for a crop growing
during a rainy season?
(d) Describe an experiment to compare the capillarity of two contrastingsoils.
2.4 MECHANICAL WEATHERING
2.4.1 Concepts of mechanical weathering
ACTIVITY 3.10:Exploring Mechanical Weathering
Mechanical rock weathering is an important part of the formation of
both soils and new rocks, and an important part of the entire rock cycle.
The activity explores what causes rocks to break down.
Materials:
• Coffee can with lid
• Rocks• Dark-coloured construction paper

Fig.2. 13 Coffee can with lid
Procedures
Place a handful of rocks on a piece of dark-coloured construction paper.
Observe the rocks and take notes on their appearance. Place the rocks
in a coffee can. Put the lid on the can and shake the can forcefully for 2
minutes, holding the lid tightly shut. Pour the rocks onto the construction
paper. Observe them and take notes on any changes in their appearance.
Use the skills gained above to answer the following questions:
d. What happened to the rocks and why?e. What forces in nature might affect rocks in similar ways?
Briefly explain what causes mechanical weathering?
Earth’s surface is constantly changing. Rock is integrated and decomposed,
moved to lower elevations by gravity, and carried away by water, wind, or
ice. When a rock undergoes mechanical weathering, it is broken into smaller
and smaller pieces of sediment and dissolved minerals; each retaining the
characteristics of the original material. The result is many small pieces from a
single large one.
Weathering takes place in two ways: physical weathering and chemical
weathering. Physical and chemical weathering can go on at the same time.
Weathering is thus the response of Earth’s materials to change environment.
Weathering is the first step in the breakdown of rock into smaller fragments.
This process is critical to the formation of landscapes and many other geological
processes. Our discussion will focus on mechanical weathering. Mechanical
weathering is the physical breaking up of rocks into smaller pieces.
2.4.2 Causes of mechanical weathering
Temperature change
ACTIVITY 3.11:Effects of temperature on mechanical weathering
a. Brainstorm on the effects of temperature in mechanical weathering
and explain clearly its impacts on soil formation and agricultural
activities?
b. Explain how thermal expansion and contraction affect mineral
composition?
As the water evaporates, the salt is left behind. Over time, these salt deposits build
up, creating pressure that can cause rocks to split and weaken. Temperature
changes also affect mechanical weathering. As temperatures heat up, therocks themselves expand.

Fig.2. 14 Illustration of mechanical weathering
Temperature is an essential part of rock creation, modification and destruction.
Heating a rock causes it to expand, and cooling causes it to contract. Repeated
swelling and shrinking of minerals that have different expansion and
contraction rates should exert some stress on the rock’s outer shell.
Thermal expansion and contraction of minerals
Thermal expansion is the tendency of matter to change in shape, area, and
volume in response to a change in temperature. Thermal expansion due to
the extreme range of temperatures can shatter rocks in desert environments.
Temperature is a monotonic function of the average molecular kinetic energy
of a substance. When a substance is heated, the kinetic energy of its molecules
increases. Thus, the molecules begin vibrating more and usually maintain a
greater average separation.
Materials which contract with increasing temperature are unusual; this effect
is limited in size, and only occurs within limited temperature ranges. The
relative expansion (also called strain) divided by the change in temperature is
called the material’s coefficient of thermal expansion and generally varies
with temperature. Materials expand or contract. when subjected to changes in
temperature. Most materials expand when they are heated, and contract when
they are cooled. When free to deform, concrete will expand or contract due
to fluctuations in temperature. Concrete expands slightly as temperature rises
and contracts as temperature falls. Temperature changes may be caused by
environmental conditions or by cement hydration.
Thermal expansion and contraction of concrete varies primarily with aggregate
type (shale, limestone, siliceous gravel and granite), cementitious material
content, water cement ratio, temperature range, concrete age, and ambient
relative humidity. Of these factors, aggregate type has the greatest influence on
the expansion and contraction of concrete.
Quick Check 2.2
1. How does climate affect the rate of weathering?
2. What is the process that breaks down rocks?
a. Effects of temperature and moisture changes on weathering
At high elevations, cold night time temperatures during much of the year can
produce relentless freeze-thaw cycles. This process explains the presence of
broken boulders and stony fragments that litter mountaintops. And, the minerals
in volcanic rock that formed at the highest temperatures and pressures are themost vulnerable to chemical weathering at Earth’s surface.
In many locations, changes in temperature and moisture content of the
environment cause significant physical weathering. When rock is warmed,
it expands; when it cools, it contracts. In some regions, rocks are heated to
relatively high temperatures during the day and then cooled to much lower
temperatures during the night. The constant expansion and contraction of the
rocks may result in pieces being broken off. Temperature also affects the land
as the cool nights and hot days always cause things to expand and contract.
That movement can cause rocks to crack and break apart.
The most common type of mechanical weathering is the constant freezing, and
thawing of water. In liquid form, water is capable of penetrating holes, joints,
and fissures within a rock. As the temperature drops below zero celcius, this
water freezes. Frozen water expands compared to its liquid form. The result is
that the holes and cracks in rocks are pushed outward. Even the strongest rocks
are no match for this force.
As temperatures heat up, the rocks themselves expand. As the temperatures
cool down, rocks contract slightly. The effect can be the weakening of the rock
itself which induces mechanical weathering. It breaks rock into smaller pieces.
These smaller pieces are just like the bigger rock, but smaller. That means the
rock has changed physically without changing its composition. The smaller
pieces have the same minerals, in just the same proportions as the original
rock.
b. Ice wedging
There are many ways that rocks can be broken apart into smaller pieces.
Ice wedging is the main form of mechanical weathering in any climate that
regularly cycles above and below the freezing point (Fig.2.15). Ice wedging
works quickly, breaking apart rocks in areas with temperatures that cycle aboveand below freezing in the day and night.
Fig.2. 15 Ice wedging.
Explanation of figure 3.15:
(A) water seeps into cracks and fractures in rock, (B) when the water freezes, it
expands about 9% in volume, which wedges apart the rock, (C) with repeated
freeze cycles, rock breaks into pieces.
Ice wedging breaks apart so much, rocks with large piles of broken rock are
seen at the base of a hillside, as rock fragments separate and tumble down. Ice
wedging is common in Earth’s polar regions and mid latitudes, and also at higher
elevations in the mountains. Water has the unique property of expanding about
9% when it freezes. This increase in volume occurs because, as ice form, the
water molecules arrange themselves into a very open crystalline structure. As a
result, when water freezes, it expands and exerts a tremendous outward force.
This can be verified by completely filling a container with water and freezing it.
After many freezing cycle, the rock is broken into pieces. This process is called
frost wedging also as known as Freeze-thaw weathering as shown in Fig.3.13.
This occurs when water gets into the small holes and gaps in rocks. If the water
in the gap freezes, it expands, splitting the existing gaps into wider cracks. When
the water thaws, the wider gaps allow even more water to enter the rock and
freeze. Frost wedging can repeat over months or years, turning microscopic
gaps in the rock into large cracks. Ice has more volume than liquid water, so
the cracks are forced wider. Then, more water accumulates in the cracks the
next day, which freeze at night to widen the cracks further. When this happens
repeatedly, the rock eventually breaks apart along the crevices.
Frost heaving, a similar process to frost wedging, occurs when a layer of ice
forms under loose rock or soil during the winter, causing the ground surface to
bulge upward. When it melts in the spring, the ground surface collapses.
c. Abrasion
ACTIVITY 2.12: Importance of abrasion in real life situations
With the help of knowledge gained in the concepts above, explain how
abrasion is formed and suggest its importance in real life situations?
The word ‘abrasion’ literally means scraping of the surface of an object. This
is exactly what happens with abrasion of rocks. Weathering by abrasion is
responsible for the creation of some of the largest deserts in the world. The
rock’s surface is exposed to blown sands - high velocity winds which blowthroughout the day while carrying large sand particle
The sand blasts against the surfaces of the rocks, undercutting and deflating
them. As a result, smaller rock particles are formed, which when exposed to
further sand abrasion become sand particles themselves.
Abrasion makes rocks with sharp or jagged edges smooth and round. If you
have ever collected beach glass or cobbles from a stream, you have witnessed
the work of abrasion (Fig.2.16 below). Rocks on a beach are worn down byabrasion as passing waves cause them to strike each other

Fig.2. 16 Smooth round rocks
In abrasion, one rock bumps against another rock. The following are the causes
of abrasion;
• Gravity causes abrasion as a rock tumbles down a mountainside or cliff.
• Moving water causes abrasion as particles in the water collide and
bump against one another.
• Strong winds carrying pieces of sand can sandblast surfaces.
• Ice in glaciers carries many bits and pieces of rock. Rocks embedded
at the bottom of the glacier scrape against the rocks.
Therefore abrasion occurs when the surface of rocks is exposed to water or
wind. These elements can carry tiny particles of sediment or rock that then
collide against the rock’s surface. When these particles rub against the rock’s
surface, they break off tiny pieces of the rock. Over time, abrasion can wear
down and smooth extremely large sections of the rock.
d. Biological activity
Weathering is also accomplished by the activities of organisms, including
plants, burrowing animals, and humans. Plant roots in search of minerals and
water grow into fractures, and as the roots grow they wedge the rock apart .
Burrowing animals further break down the rock by moving fresh material to
the surface, where physical and chemical process can more effectively attack
it. Decaying organisms also produce acids, which contribute to chemical
weathering .
2.4.3 Factors influencing the type and rate of rock weathering
ACTIVITY 2.13
Brainstorm and classify clearly the factors affecting the rate of weathering?
Several factors influence the type and rate of rock weathering. By breaking a rock
into smaller pieces, the amount of surface area exposed to chemical weathering
is increased. The presence or absence of joints can be significant because they
influence the ability of water to penetrate the rock. Other important factors
include the mineral makeup of rocks and climate.
a. Climate
The amount of water in the air and the temperature of an area are both part of
an area’s climate. Moisture speeds up chemical weathering. Weathering occurs
fastest in hot, wet climates. It occurs very slowly in hot and dry climates. Without
temperature changes, ice wedging cannot occur. In very cold, dry areas, there
is little weathering.
b. Surface area
Most weathering occurs on exposed surfaces of rocks and minerals. The more
surface area a rock has, the more quickly it will weather. When a block is cut
into smaller pieces, it has more surface area. So, therefore, the smaller pieces of
a rock will weather faster than a large block of rock
c. Rock composition
Headstones of granite, which is composed of silicate minerals, are relatively
resistant to chemical weathering. The minerals that crystallize first form under
much higher temperatures than those which crystallize last. Consequently,
the early formed minerals are not as stable at Earth’s surface, where the
temperatures are different from the environment in which they formed. Olivine
crystalizes first and is therefore the least resistant to chemical weathering,
whereas quartz, which crystallizes last, is the most resistant.
d. Pollution speeds up weathering
Factories and cars release carbon dioxide and other gases into the air. These
gases dissolve in the rainwater, causing acid rain to form. Acid rain contains
nitric and sulfuric acid, causing rocks and minerals to dissolve faster.
e. Soil erosion and soil deposition
ACTIVITY 2.14: Soil erosion
Look at the figure below that represents soil erosion. Carefully studythe figure and answer the following questions:

Fig.2. 17 The erosive force of wind on an open field
a. What do you think caused water to get contaminated as shown in
fig below
b. How often have you seen water looking like that in your area.
What was the cause?
c. What scientific phenomena that explains the washing away of the
top soil.
d. how does the phenomena explained in c) above affect agriculture?
e. Suggest possible measures that can be taken to reduce or stop the
phenomenon explained in c) above
Soil covers most land surfaces. Along with air and water, it is one of our most
indispensableresources. Soil is a combination of mineral and organic matter.
Soil erosion is a naturally occurring process that affects all landforms. In
agriculture, soil erosion refers to the wearing away of a field’s topsoil by the
natural physical forces of water and wind or through forces associated with
farming activities.
Erosion is incorporation and transportation of material by a mobile agent,
usually water, wind, or ice. Erosion whether it is by water and wind, involves
three distinct actions – soil detachment, movement and deposition. Topsoil,
which is high in organic matter, fertility and soil life, is relocated elsewhere
“on-site” where it builds up over time or is carried “off-site” where it fills in
drainage channels. Soil erosion reduces cropland productivity and contributes
to the pollution of adjacent watercourses, wetlands and lakes. Soil erosion
can be a slow process that continues relatively unnoticed or can occur at an
alarming rate, causing serious loss of topsoil.
Soil compaction, low organic matter, loss of soil structure, poor internal drainage
and soil acidity problems are other serious soil degradation conditions that canaccelerate the soil erosion process.
Fig.2. 18 The erosive force of water from concentrated surface water runoff.
Deposition is the geological process in which sediments, soil and rocks are
added to a landform or land mass. Wind, ice, and water, as well as sediment
flowing via gravity, transport previously eroded sediment, which, at the loss of
enough kinetic energy in the fluid, is deposited, building up layers of sediment.
2.4.4 Checking my progress
The pictures A and B are of two geographical features. Look and carefullystudy the pictures to answer questions below.
Fig.2. 18 Illustration of geographical features
a. Interpret the images above and Use your observation to suggest names
of the corresponding geographical features in the image above?
b. Do you have such geographical features in your district or neighboring
districts? Use your observation to explain clearly the two geographical
features occurring in the image above?
c. Explain the causes for each geographical feature occurring above?
d. Can the geographical features identified above impact agriculture in
our communities? Explain with clear facts to support your decision.
e. What are moral and ethical issues associated with the geographicalfeatures given above?
END UNIT ASSESSMENT 2
A. Multiple choices questions
For question 1 to 5, choose the letter of the best answer
1. It is known that earth’s atmosphere has a series of layers, each with
its own specific characteristics and properties? The following is the
appropriate layer where we live.
a. Thermosphere
b. Troposphere
c. Stratosphere
d. Mesosphere
2. Consider the following statements:
I. The atmosphere of Earth protects life on Earth by absorbing
ultraviolet solar radiation, warming the surface through
greenhouse effect and reducing temperature extremes between
day and night.
II. X-rays and ultraviolet radiation from the Sun are absorbed in the
thermosphere.
III. The stratosphere extends from the top of the thermosphere to
about 50 km above the ground.
Of these statements:
a. I, II, and III are correct.
b. I, II and III are wrong
c. I and II are correct but III is wrong
d. I and III are wrong but II is correct
3. Agrophysics is defined as
a. The branch of science dealing with study of matter and energy
and their mutual relation.
b. The branch of science dealing with communication devices to
measure and collect information about physical conditions in
agricultural and natural environments.
c. The branch of natural sciences dealing with the application of
physics in agriculture and environment.
d. None of these
B. Structured Questions
1. Write the missing word or words on the space before each number.
For items (a)-(i)
a. ___speeds up chemical weathering.
b. Weathering happens ______ in hot, wet (humid) climates.
c. Weathering occurs very slowly in _______ and ______ climates.
e. In very ________ and ________ areas, there is little weathering.
f. Most weathering occurs on ____________________of rocks and minerals
g. The ________ surface area a rock has, the quicker it will weather.
h. Some minerals resist weathering. _________________ is a mineral that
weathers slowly.
i. Rocks made up of minerals such as feldspar, ______, and iron, weather
more quickly.
2. If the statement is true, write true. If it is false, change the underlined word
or words to make the statement true.
a. Water vapor is very important in predicting weather.
b. Temperature is a reason why atmosphere is more dense close to the
earth’s surface.
c. Agrophysics plays an important role in the limitation of hazards to
agricultural objects and environment.
d. Energy is transferred between the earth surface and planet in a variety
of ways.
e. As the temperature increases in the atmosphere, the minimum radiation
occurs at short wavelengths.
3. Write a sentence describing the relationship between each pair of terms.
I. Gravity, atmosphere
II. Temperature, rocks.
4. Marry wants to make agrophysics journal. She says, “My journal will be
focused on advances in sensors and communication devices to measure and
collect information about physical conditions in agricultural and natural
environments”. Evaluate Marry’s statement.
5. With the help of two clear examples on each, explain clearly howtemperature and water vapor impact agricultural activities using the table.
6. Complete the chart below. If the left column is blank, give the correct
term. If the right column is blank, give an example of economic activitiestaking place in the corresponding layer if possible.
7. How do climate impact agricultural activities?
8. Explain briefly the role of machines in agriculture in rapid development of
the country towards suitable programs of transformation and modernization
of agriculture?
9. Knowing different stages of growing plants in our daily agriculture
activities, explain clearly which stages mostly benefit the use of technology?
10. Cracks in rocks widen as water in them freezes and thaws. How does this
affect the surface of Earth?
11. Name the four factors that can hasten or speed up the process of
weathering.
12. How is weathering different from erosion?
13. How can increasing the surface area of rock hasten or speed up the
process of weathering
14. Human activities are responsible for enormous amounts of mechanical
weathering, by digging or blasting into rock to build homes, roads, and
subways or to quarry stone. Suggest measures that can be taken to minimize
mechanical weathering caused by human activities?
C. Essay type questions
15. Design and conduct your own research into the influence of surfaces on
temperature comparing earth surfaces that interest them (such as colored
soils, dry and wet soils, grass, dry leaves, or different types of coverings
such as plastic or aluminum foil). Compare the data with these new surfaces
compared to the given surfaces (water, light soil, dark soil). Note that the
data may not be comparable due to variations in experimental design, suchas differences in light bulb temperature and height of the lamp.
UNIT 3 FOSSIL AND NON-FOSSIL FUEL AND POWER PRODUCTION
Key unit competence: By the end of this chapter, I should be able to evaluate
fossil and non-fossil fuel for power production.
Unit Objectives:
By the end of this unit I will be able to;
• explain the concept of fossil and no-fossil fuels and their use in power
production properly.
• explain the differences between fossil and no-fossil fuels properly.
• explain Nuclear fuel and nuclear fission and their use in energy
production and associated dangers properly.
• explain the environmental problems of fossil fuels and suggest theirsolution clearly.

3.0 INTRODUCTORY ACTIVITY
Fossil fuel is a source of conventional or non-renewable energy. There are
many examples of fossil fuels which we use in our daily lives. In fact, most
of the energy that we consume comes from fossil fuels. Coal, petroleum
and natural gas are called fossil fuels. Millions of years ago, during the
carboniferous age, due to the change in atmospheric conditions and other
changes, the forests were destroyed and they were fossilized. With the action
of bacteria and other microorganisms on the surface of the earth, these trees
and other vegetations were decayed and disintegrated. Years after these
trees were available in solid, liquid and gaseous state. The solid form is coal.It is the most widely used form of fossil fuel for domestic purposes.
ACTIVITY 3.1: The Atmosphere
Crossword puzzle: Fill the missing words in the crossword puzzle given
below.
Down
1. __________ _________ refers to the rise in the world’s average
temperature due to air pollution.
2. _________ _______ are gases in the atmosphere that absorb and
emit radiation, causing the greenhouse effect.
3. ______________ is a mixture of smoke and fog in the atmosphere.
4. ____________ ________is a non-renewable source of energy
formed from the remains of dead plants and animals.
Across
5. ______ _____ is the reduction of the amount of ozone
6. The water sources and the land are polluted by ______ ________when exhaust gases dissolve in the rain.
ACTIVITY 3-2: Pollution
Word splashThe following are the key words we learn about air pollution.
3.1. FOSSIL FUELS AND NON-FOSSIL FUELS
3.1.1 Fossil Fuels
Fossil fuels are hydrocarbons, primarily coal, fuel oil or natural gas, formed
from the remains of dead plants and animals. In common dialogue, the term
‘fossil fuel’ also includes hydrocarbon-containing natural resources that are notderived from animal or plant sources.

Fig. 3.1. Fossil fuels in nature
Coal, oil and natural gas are called ‘fossil fuels’ because they have been formed
from the fossilized remains of prehistoric plants and animals. Fossil fuels are
non-renewable energy source since they take millions of years to form. They
ultimately get their energy from the sun.
Types of Fossil Fuels
Coal
Coal is a hard, black coloured rock-like substance formed when dead plants
were subjected to extreme heat and pressure for millions of years. It is made up
of carbon, hydrogen, oxygen, nitrogen and varying amounts of sulphur. There
are two ways to mine coal: surface mining and underground mining.
Natural Gas
Natural gas is formed from the remains of tiny sea animals and plants that
died millions of years ago. The gas then became trapped in layers of rock-like
water in a wet sponge. Raw natural gas is a mixture of different gases. Its main
ingredient is methane. The strange smell of natural gas (like rotten eggs) comes
from a chemical added by the companies. It is called mercaptan. This is added
to detect the gas leakage.
Oil (Petroleum)
Oil is formed from the remains of animals and plants that died millions of years
ago. The organic material was then broken down into hydrogen and carbon
atoms and a sponge-like rock was formed, full of oil.
Oil cannot be used as it is when it is drawn from the ground. Oil refineries clean
and separate the oil into various fuels and byproducts. The most important of
these is gasoline.
Fossil fuels are used to generate electrical energy in a series of energytransformations as shown in Fig.6.2.
3.1.2 Non-fossil fuels
Non-fossil fuels are alternative sources of energy or renewable sources of
energy that do not rely on burning up limited supplies of coal, oil or natural
gas. Examples of these fuels include: nuclear energy, wind or water generated
energy and solar power. These tend to be renewable energy sources, or means
of generating power that can be utilized indefinitely.
Non-fossil fuels are considered to be extremely important for power creation.
This is because they are usually renewable energy sources that could be tapped
for hundreds of years and not run out. In addition, energy production using
nonfossil-based fuels usually generates much less pollution than fossil-based
energy sources.
3.2 Storage and transportation of different types of fossil
fuels
3.2.1 Coal
Types of coal
• Peat
• Lignite
• Semi bituminous
• Bituminous
• Anthracite
Means of transporting coal
• Transportation by rail
• Transportation by ropeways
• Transportation by sea or river
• Road transport
• Transport by pipeline
Coal storage
Storage of coal is undesirable because it costs more as there is:
• Risk of spontaneous combustion,
• Weathering,
• Possibility of loss and deterioration during storage,
• Interest on capital cost of coal lying dormant,
• Cost of protecting the stored coal from deterioration.
Types of coal storage
1. Dead storage:
This storage supplies the coal to places where there is a shortage of coal in
plant due to failure of normal supply of coal. This is a long-term storage and
comprises 10% of annual consumption, so, it requires protection against
weathering and spontaneous combustion.
2. Living storage:
It supplies coal to plant for day-to-day usage. The capacity of live storage is less
than that of dead storage. It is usually stored in vertical cylindrical bunkers or
coal basins or silos, e.g. coal is transferred to boiler grate. Bunkers are normally
diamond-shaped cross-section storage areas made up of steel or reinforced
concrete.
Purpose of dead coal storage of coal
• To prevent shutdown of power plant in case of failure of normal
supplies of coal due to coal strike, failure of the transport system, etc.
• To permit choice of purchase allowing management to take advantage
of seasonal market conditions.
Means of coal storage
1. Storage in coal heaps
It is required to:
• Keep coal at low temperature (>70oC).
• Prevention of air circulation from bottom of coal piles.
• Proper drainage of rainy water to prevent weathering–drainage should
not be rapid to prevent washing of coal.
Hence, ground used for stocking should be dry and levelled for proper drainage.
It should have concrete floor to prevent flow of air from bottom. Coal is piled up
to a height of about 10 m to 12 m in layers of 15 cm to 30 cm.
In dead storage, coal pile is sealed by asphalt, fine coal dust, bituminous or
other coating materials.
2. Underwater storage
Possibility of slow oxidation and spontaneous combustion can be completelyeliminated by storing coal under water.

Fig. 3.3. Coal dead storage
Site selection for coal dead storage
• The storage should be free from standing water
• If well drainage is not available, artificial drainage should be provided.
• It should be free from all foreign materials like wood, paper rags, waste
oil or materials having low ignition temperature.
• Handling cost should be minimum.
• Pile should build up in successive layers and be compact.
• Pile should be dressed to prevent entry of rainy water.
• Alternative drying and wetting should be avoided.
• Stoker size coal should be oil treated to prevent absorption of water
and oxygen.
• Side of pile should not be steep.
• Air may circulate freely through pile for proper ventilation to keep
temperatures low.
• Hot surfaces or boiler blow down or hot water or steam pipe and tanks
should be kept far from coal storage
• Hot bright days should be avoided.
• There should be provision for temperature measurement at different
points.
• Conical piling should be avoided.
• Fire fighting equipment should be easily available.
Coal Transfer
Equipments used in coal transfer are:
A: Belt conveyor
It can transfer large quantities of coal over large distance economically. It haslow initial cost and ensures low power consumption.
Fig. 3.4. Belt conveyor
Advantages:
• Economical, low power consumption
• Large capacity
• Rate of coal transfer rapidly change
• Low maintenance cost
Disadvantages
• Not suitable for shorter distance and inclination > 200.
• Not suitable for dust particles and slurry.
B: Flight conveyor
It is used when coal is discharged at different points in bins situated below the
conveyor. All parts are made of steel and iron, so it can handle hot materials. It
is totally enclosed, so dust of coal can get transferred. It can transfer coal at high
inclination
Fig. 3.5. Flight conveyor
Advantages
• It requires small head room.
• Speed and material transfer rate can easily change.
• It can handle hot materials also.
Disadvantages
• High wear and tear, so, it has short life.
• High maintenance required.
• Speed is limited up to 300 m/min due to abrasive action of material.
• High power consumption per unit of material transfer.C: Screw conveyor

Fig. 3.6. Screw conveyor
• It is used for shorter distance.
• It is totally enclosed from atmosphere.
• Coal dust can also be transferred easily.
• It is generally used in metering of coal.
• Driving mechanism is attached at the end of the shaft.
• Diameter: 15 cm to 50 cm.
• Speed: 70 rpm to 120 rpm.
• Capacity: 125 tones/h (max)
Advantage
• Cheap initial cost.
• Simple and compact.
• Dust tight.
• It can transfer coal at high inclination also.
• Most suitable for short distance.
Disadvantages
• High power consumption.
• Length is limited up to 30 m.
• High maintenance due to high wear and tear.
D: Bucket elevator
It is used for vertical lifts. Buckets are fixed on chain which moves on two wheelsor sprockets. Buckets are loaded at bottom and discharged at top.

Fig. 3.7. Bucket elevator
E: Grab bucket elevator
• It is used for lifting as well as transfer material.
• It can be used with crane or tower.
• Initial cost is high but operating cost is less.
• It is used when another arrangement is not possible.
• Bucket capacity: 2 to 3 m3
• Distance: 60 m• Capacity: 100 tonnes/h.

Fig. 3.8. Grab bucket elevator
3.2.2 Transporting Natural Gas and Crude Oil
Transporting natural gas and crude oil thousands of miles through pipelines is
the safest method of transportation. The transportation system for natural gas
consists of a complex network of pipelines, designed to transport natural gas
from its origin to the areas of high natural gas demand quickly and efficiently.
In general, pipelines can be classified in three categories depending on the
purpose:
Gathering pipelines
These are smaller interconnected pipelines forming complex networks with
the purpose of bringing crude oil or natural gas from several nearby wells to
a treatment plant or processing facility. In this group, pipelines are usually
short — a couple of hundred metres — and with small diameters. Also subsea
pipelines for collecting product from deep water production platforms areconsidered gathering systems.

Fig. 3.9. Gathering pipelines
Transportation pipelines
These are long pipes with large diameters, moving products (oil, gas, refined
products) between cities, countries and even continents. These transportation
networks include several compressor stations in gas lines or pump stations forcrude and multi-products pipelines.

Fig. 3.10. Transportation pipelines
Distribution pipelines
These are composed of several interconnected pipelines with small diameters,
used to take the products to the final consumer. Feeder lines to distribute gas
to homes and business downstream, and pipelines at terminals for distributingproducts to tanks and storage facilities, are included in this group.

Fig. 3.11. Distribution pipelines
3.3 Advantages and disadvantages of fossil fuels
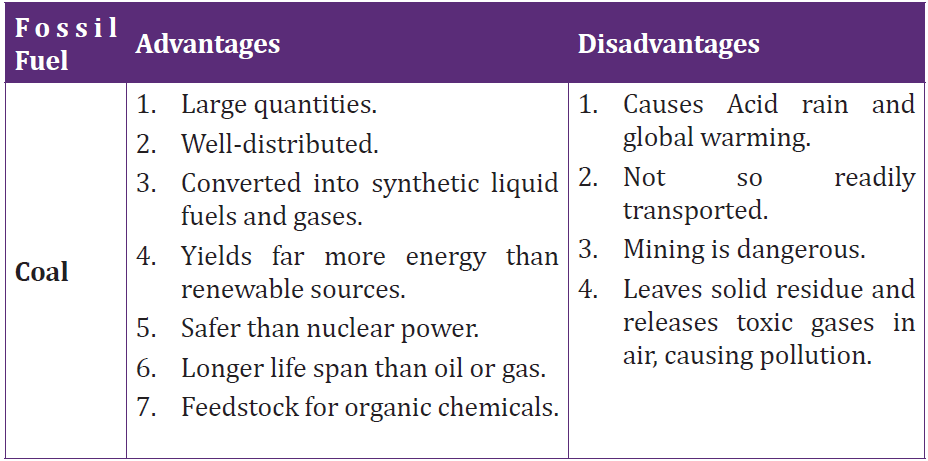
3.4 Energy production using fossil fuels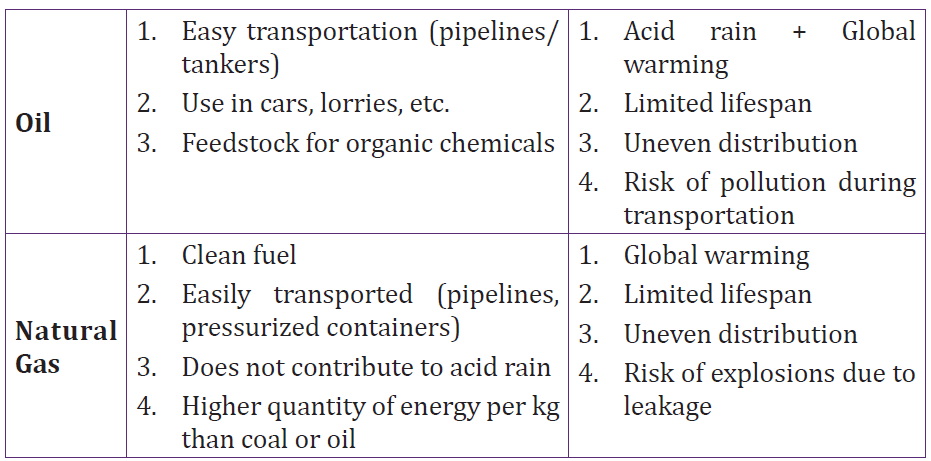
A fossil-fuel power station is a power station which burns fossil fuels, such as
coal, natural gas or petroleum to produce electricity. Central station fossil-fuelpower plants are designed on a large scale for continuous operation.
Fig. 3.12. Fossil fuel power plant
There are two main cycles in a power plant; the steam cycle and the gas turbine
cycle. The steam cycle relies on the Rankine cycle in which high pressure and
high temperature steam raised in a boiler is expanded through a steam turbine
that drives an electric generator. The generator then transforms mechanical
energy into electrical energy which is distributed for local use.
The steam gives up its heat of condensation to a heat sink, such as water from
a river or a lake and the condensate can then be pumped back into the boiler
to repeat the cycle. The heat taken up by the cooling water in the condenser is
dissipated mostly through cooling towers into the atmosphere.
3.5 Nuclear fuel and nuclear fission
Nuclear fuel is any material that can be consumed to derive nuclear energy.
The nuclear fuel can be made to undergo nuclear fission chain reactions in a
nuclear reactor. The most common nuclear fuels are 235U (uranium 235) and
239Pu (plutonium 239). Not all nuclear fuels are used in fission chain reactions.
Nuclear fission is a process, by which a heavy nucleus splits into two or
more simpler pieces. This process releases a lot of energy.
When a neutron strikes an atom of uranium, the uranium nucleus splits into
two lighter atoms and releases heat simultaneously. Fission of heavy elements
is an exothermic reaction which can release large amounts of energy both aselectromagnetic radiation and as kinetic energy of the fragments.
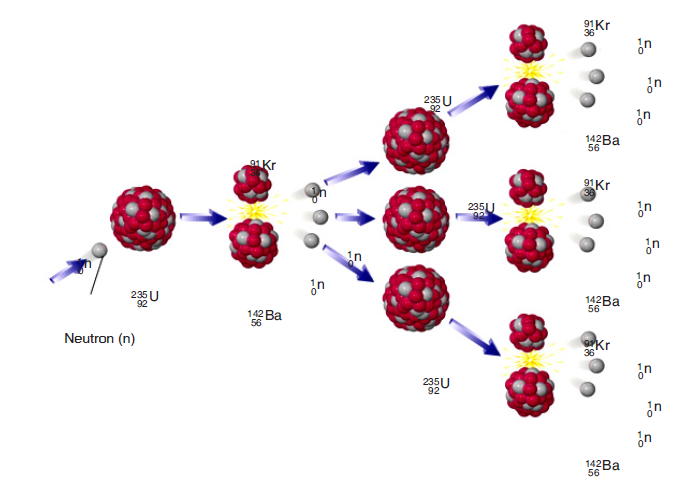
Fig. 3.13. Fission of Uranium 235
A chain reaction refers to a process in which neutrons released in fission
produce an additional fission in at least one further nucleus. This nucleus in
turn produces neutrons, and the process continues. If the process is controlled
it is used for nuclear power or if uncontrolled it is used for nuclear weapons.
Fig.3.13 illustrates a chain reaction of uranium 235.The equation of reaction is:
3.6 Controlled fission (power production)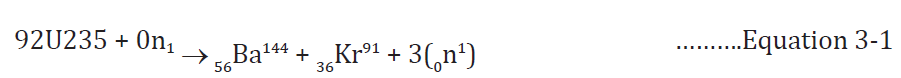
Of the three neutrons, liberated during a fission reaction, only one triggers a
new reaction and the others are simply captured. The system is in equilibrium.
One fission reaction leads to one new fission reaction, which leads to onemore, and so on. This is known as controlled fission.
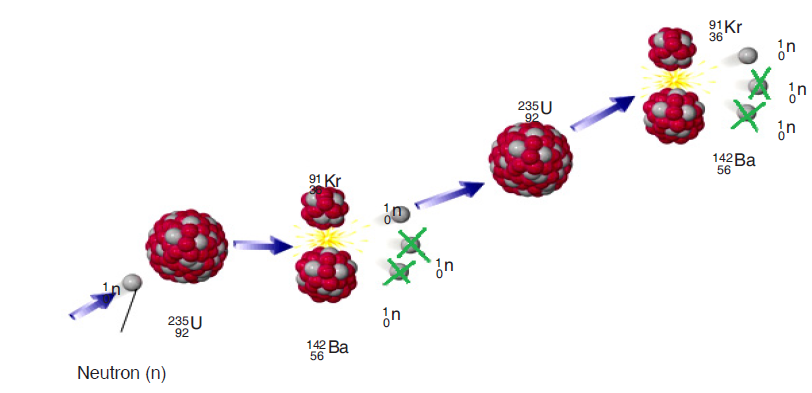
Fig. 3.14. Controlled fission reaction
In a nuclear power station, the uranium is first formed into pellets and then into
long rods. The uranium rods are kept cool by submerging them in water. When
they are removed from the water, a nuclear reaction takes place causing heat
production. The amount of heat required is controlled by raising and lowering
the rods. If more heat is required, the rods are raised further out of the water
and if less heat is needed, they are lowered further into it.
3.7 Uncontrolled fission (nuclear weapons)
A fission reaction which is allowed to proceed without any moderation (by
removal of neutrons) is called an uncontrolled fission reaction. Here more and
more neutrons are given out and cause more fission reactions, thus, releasing
large amounts of energy. An uncontrolled fission reaction is used for nuclearbombs.
Using the energy released from the nuclear fission of uranium-235, an explosive
device can be made by simply positioning two masses of U-235 so that they can
be forced together quickly enough to form a critical mass and result in a rapid,
uncontrolled fission chain reaction.
This is not an easy task to accomplish. First, you must obtain enough uranium
which is highly enriched to over 90% U-235, since natural uranium is only
0.7% U-235. This enrichment is an exceptionally difficult task, a fact that has
helped control the proliferation of nuclear weapons. Once the required mass is
obtained, it must be kept in two or more pieces until the moment of detonation.
Then the pieces must be forced together quickly and in such a geometry that
the generation time for fission is extremely short. This leads to an almost
instantaneous build up of the chain reaction, creating a powerful explosion
before the pieces can fly apart. Two hemispheres which are explosively forced
into contact, can produce a bomb, such as the one detonated at Hiroshima in1945.
Fig. 3.15. Nuclear atomic bomb of Uranium 235.
3.8 Impacts of nuclear weapons
There are five immediate destructive effects from a nuclear explosion:
1. The initial radiation, mainly gamma rays;
2. An electromagnetic pulse, which in a high altitude explosion can knock out
electrical equipment over a very large area;
3. A thermal pulse, which consists of bright light (even many miles away) and
intense heat equal to that at the centre of the sun);
4. A blast wave that can flatten buildings; and
5. Radioactive fallout, mainly in dirt and debris that is sucked up into the
mushroom cloud and then falls to earth.
There are three long-term effects of a nuclear explosion:
1. Delayed radioactive fallout, which gradually fall over months and even years
to the ground, ofen in rain;
2. A change in the climate (possibly by lowering of the earth’s temperature
over the whole hemisphere which could ruin agricultural crops and cause
widespread famine);
3. A partial destruction of the ozone layer, which protects the earth from
the sun’s ultraviolent rays. If ozone layer is depleted, unprotected Caucasians
would get an incapacitating sunburn within 10 minutes, and people would
suffer a type of snow blindness from the rays which, if repeated, would lead
to permanent blindness. Many animals would suffer the same fate.
3.9 Energy transformations in a nuclear power station
In a nuclear power plant, Nuclear Steam Supply System (NSSS) consists of a
nuclear reactor and all of the components necessary to produce high pressuresteam, which will be used to turn the turbine for the electrical generator.
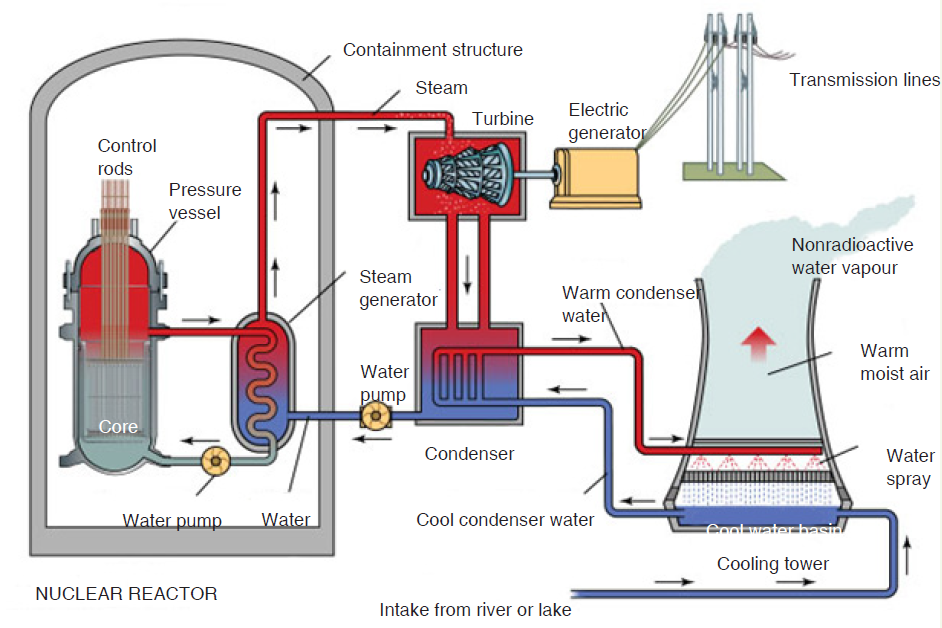
Fig. 3.16. Nuclear power plant
The nuclear reactor contains some radioactive isotopes like uranium which
undergo fission reaction when bombarded with some neutrons and a large
amount of heat energy is evolved. This heat energy converts water into steam,
which is piped to the turbine. In the turbine, the steam passes through the
blades, which spins the electrical generator, resulting in a flow of electricity.
After leaving the turbine, the steam is converted (condensed) back into water
in the condenser. The water is then pumped back to the nuclear reactor to be
reheated and converted back into steam.
3.10 Problems associated with the production of nuclear
power
• The problem of radioactive waste is still unsolved. The waste
from nuclear energy is extremely dangerous and it has to be carefully
looked after for several thousand years (10,000 years according to
United States Environmental Protection Agency standards).
• High risks: Despite a generally high security standard, accidents
can still happen. It is technically impossible to build a plant with
100% security. A small probability of failure will always last. The
consequences of an accident would be absolutely devastating both for
human beings and the nature. The more nuclear power plants (and
nuclear waste storage shelters) are built, the higher is the probability
of a disastrous failure somewhere in the world.
• Nuclear power plants as well as nuclear waste could be preferred
targets for terrorist attacks. Such a terrorist act would have
catastrophic effects for the whole world.
• During the operation of nuclear power plants, radioactive waste is
produced, which, in turn, can be used for the production of nuclear
weapons. In addition, the same is used to design nuclear power plants
can to a certain extent be used to build nuclear weapons (nuclear
proliferation).
• The energy source for nuclear energy is Uranium. Uranium is a
scarce resource; its supply is estimated to last only for the next 30 to
60 years depending on the actual demand.
• The timeframe needed for formalities, planning and building of a new
nuclear power generation plant, is in the range of 20 to 30 years in the
western democracies. In other words, it is an illusion to build new
nuclear power plants in a short time.
3.11 Environmental problems of fossil fuels
Climate Change/Global Warming and Greenhouse Effect
The earth’s atmosphere allows a lot of sunlight to reach the earth’s surface
but reflects much of that light back into space. Some gases trap more sunlight,
therefore, less light is reflected back into space. These gases are called
Greenhouse Gases, because the effect is like being in a plant glasshouse,
or in a car with the windows wound up. The result is a gradual increase in
the earth’s temperature or Global Warming. The major greenhouse gases are
carbon dioxide, methane, nitrous oxide and chlorofluorocarbons (CFCs).
The main man made causes are thought to be carbon dioxide and methane from
factory, power station and car emissions, the waste products of respiration, the
mining of fossil fuels and the breakdown of plant matter in swamps. The longterm
effects may include melting of glaciers and a rise in sea level and a global
change in climate and type of vegetation.
‘Hole’ in the Ozone Layer
Ozone is a gas in the earth’s upper atmosphere whose chemical formula is O3.
Ozone acts to block out much of the sun’s ultraviolet radiation which causes
skin cancer and contributes to the fluctuations of global climatic conditions
that affect the environment. Above Antarctica, there is a thinner layer of ozone
caused by the destruction of ozone gas by emissions of chlorofluorocarbons
and hydrochlorofluorocarbons which are propellants in pressure-pack spray
cans and refrigerants in refrigerators and air-conditioning units.
Acid Rain
When gases, such as sulphur dioxide and nitrogen oxides react with water in
the atmosphere to form sulphuric acid and nitric acid, they form an acidic ‘rain’
which can destroy vegetation. Some of these gases are from natural sources,
such as lightning, decomposing plants and volcanoes. However, much of these
gases are the result of emissions from cars, power stations, smelters and
factories.
Air Pollution
Air pollution is the release of excessive amounts of harmful gases (e.g. methane,
carbon dioxide, sulphur dioxide, nitrogen oxides) as well as particles (e.g.
dust of tyre, rubber, lead from car exhausts) into the atmosphere. To reduce
emissions, the Australian government has legislated that all new cars should
use unleaded petrol and have catalytic converters fitted to the exhausts.
Water Pollution
1. Sewage is the household waste water. Many detergents contain phosphates
which act as plant fertilisers. When these phosphates and the sewerage
reach rivers, they help water plants to grow in abundance, reducing the
dissolved oxygen in the river water. The result is death of aquatic animals
due to suffocation by the algal blooms. This harmful effect is called
eutrophication. Eutrophication is also caused by excessive use of fertilizers
in agricultural fields and subsequent surface run-off.
2. Biodegradable detergents are more environment-friendly because they are
readily broken down to harmless substances by decomposing bacteria.
3. Suspended solids in water, such as silt reduce the amount of light that
reaches the depths of the water in lakes and rivers. This reduces the ability
of aquatic plants to photosynthesise and reduce the plant and animal life.
Turbidity is the measure of ‘cloudiness’ or the depth to which light can
reach in water.
Introduced Species
They are species of plants or animals that have migrated or been brought to
Australia. Many fit into the natural ecosystems and are kept in control by natural
predators and parasites. However, some become pests as they are well-adapted
to that environment, readily obtain nutrients and lack of natural predators or
parasites. Examples include rabbits, foxes, carp and prickly pear cactus plant.
Biological Control
It is an environment-friendly method to control these pests by the introduction
of species-specific, living organisms to control their numbers. Successful
examples include the myxoma virus and the calici virus for rabbits, and the
cactoblastis moth feeding on the prickly pear. Unsuccessful examples include
the introduction of the cane toad to reduce the numbers of natural cane beetles.
Biological Magnification
It is the accumulation in body tissues of certain chemicals, such as DDT,
pesticides and mercury. The higher it moves along the food chain, the greater
is the accumulation, sometimes to such toxic levels, which causes birth defects
and even death.
Soil Salinity
Soil salinity has increased greatly since the widespread logging of trees by
farmers. Deep tree roots normally draw water from the underground water
table. However, when logging of trees occurs, the water table rises close to
the surface bringing with it, salt from rocks. This makes the soil salty so that
vegetation cannot grow effectively. The result is loss of vegetation and erosion.
Population Explosion
It is the rapid increase in population in developing countries causing famine,
and also in developed countries causing more demand for energy and with
that, it increases pollution and destruction of the environment.
ACTIVITY3-3: Sources of Pollution
Aim: the aim of this activity is to find out the causes of pollution.
Procedure: analyse the figure below and answer the questions thatfollow
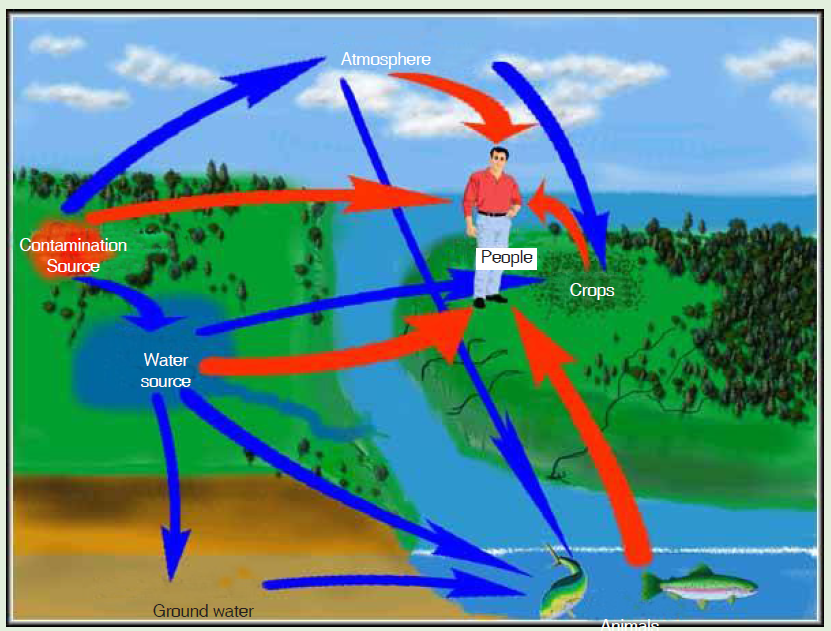
Fig. 3.17. Effects of poorly deposited nuclear wastes.
a. Outline some sources of water and air pollution shown on the figure.
b. Explain how each of the cause in (a) affect the environment.
c. Give and explain any other sources of air and/ or water pollution you
know.
d. Explain how air and water pollutions can be reduced.
ACTIVITY 3-4: Wate Pollution
Aim: to investigate the effect of water contamination
Source: internet and textbooks or journals.
Background Information
1. Scientists have studied the influence of chlorine on organic
materials in water supplies. Some of the chlorine reacts with this
organic material to form chloroform and other chlorine-containing
chemicals. Research has shown that some chlorine-containing
chemicals can increase the risk of cancer.
2. Working with your group, find out more about the benefits and
costs of using chlorine in the water supply. Have each member of
your group research information on one of the following:
a. The risk to health of not treating water supplies with chlorine
b. The risk to health of using chlorine in water treatment
c. Alternatives to using chlorine for water treatment
d. Scientific research underway on chlorine use
e. What (if anything) is used to treat your local water supply
Support Your Opinion
3. When you have finished your research, share your information
with your group. Design a presentation to summarize your group’s
findings. Be prepared to share your group’s findings with the rest
of the class.
4. Do you think that the amount of chlorine in our water should be
increased at certain times of the year? Give reasons to support youropinion
3.12 Safety issues and risks associated
with nuclear power
3.12.1 Nuclear Meltdown
A nuclear meltdown is an informal term for a severe nuclear reactor accidentthat results in core damage from overheating.

Fig. 3.18. Reactor meltdowns at Fukushima Daiichi.
A nuclear meltdown occurs when a nuclear power plant system or component
fails so the reactor core becomes overheat and melts. Usually, this occurs due to
the lack of coolant that decreases the temperature of the reactor. The commonly
used coolant is water but sometimes a liquid metal, which is circulated past the
reactor core to absorb the heat, is also used. In another case, a sudden power
surge that exceeds the coolant’s cooling capabilities causes an extreme increase
in temperature which leads to a meltdown. A meltdown releases the core’s
highly radioactive and toxic elements into the atmosphere and environment.
The causes of a meltdown occur due to:
A: A loss of pressure control
The loss of pressure control of the confined coolant may be caused by the failure
of the pump or having resistance or blockage within the pipes. This causes the
coolant to cease flow or insufficient flow rate to the reactor; thus, the heat
transfer efficiency decreases.
B: A loss of coolant
A physical loss of coolant, due to leakage or insufficient provision, causes a
deficit of coolant to decrease the heat of the reactor. A physical loss of coolant
can be caused by leakages. In some cases, the loss of pressure control and
the loss of coolant are similar because of the systematic failure of the coolant
system.
C: An uncontrolled power excursion
A sudden power surge in the reactor is a sudden increase in reactor reactivity.
It is caused by an uncontrolled power excursion due to the failure of the
moderator or the control that slows down the neutron during chain reaction.
A sudden power surge will create a high and abrupt increase of the reactor’s
temperature, and will continue to increase due to system failure. Hence, the
uncontrollable increase of the reactor’s temperature will ultimately lead to a
meltdown.
3.12.2 Nuclear (Radioactive) Wastes
Nuclear wastes are radioactive materials that are produced after the nuclear
reaction. Nuclear reactors produce high-level radioactive (having high levels
of radioactivity per mass or volume) and low-level (having low levels of
radioactivity) wastes. The wastes must be isolated from human contact for avery long time in order to prevent radiation.

Fig. 3.19. High level waste being stored in underground repository.
The ‘high-level wastes’ will be converted to a rock-like form and placed in a
natural habitat of rocks, deep underground. The ‘low-level wastes’, on the other
hand, will be buried in shallow depths (typically 20 feet) in soil.
A number of incidents have occurred when radioactive material was disposed
improperly, where the shielding during transport was defective, or when the
waste was simply abandoned or even stolen from a waste store.
The principal risks associated with nuclear power arise from health effects
of radiation, which can be caused due to contact with nuclear wastes. This
radiation consists of sub-atomic particles travelling at or near the velocity of
light (186,000 miles per second). They can penetrate deep inside the human
body where they can damage biological cells and thereby initiate a cancer. Ifthey strike sex cells, they can cause genetic diseases in progeny
END UNIT ASSESSMENT 3
1. Why should solar energy be harnessed to take care of our electric
power needs?
2. How do we confirm that the ‘greenhouse effect’ is real?
3. How does acid rain destroy forests and fish?
4. Is it possible to eliminate the air pollution from coal burning?
5. Radioactivity can harm us by radiating from sources outside our
bodies, by being taken in with food or water or by being inhaled
into our lungs. But we consider only one of these pathways. Why is
it so?
6. Cancers from radiation may take up to 50 years to develop, and
genetic effects may not show up for a hundred years or more. How,
then, can we say that there will be essentially no health effects from
the Three Mile Island accident?
7. Air pollution may kill people now, but radiation induces genetic
effects that will damage future generations. How can we justify our
enjoying the benefits of nuclear energy while future generations
bear the suffering from it?
8. Can the genetic effects of low-level radiation destroy the human
race?
9. Isn’t the artificial radioactivity created by the nuclear industry,
more dangerous than the natural radiation which has always been
present?
10. Can radiation exposure to parents cause children to be born with two
heads or other such deformities?
11. Can a reactor explode like a nuclear bomb?
12. If reactors are so safe, why don’t home owners’ insurance policies cover
reactor accidents? Does this mean that insurance companies have no
confidence in them?
13. How is radioactive waste disposed off?
14. How long will the radioactive waste be hazardous?
15. How will we get rid of reactors when their useful life is over?
Fossil fuels are hydrocarbons, primarily coal, fuel oil or natural gas, formed
from the remains of dead plants and animals.
Types of Fossil Fuels
• Coal
• Natural Gas
• Oil (Petroleum)
Types of coal storage
• Dead storage
• Living storage
Means of coal storage
• Storage in coal heaps
• Underwater storage
Energy production using fossil fuels
A fossil-fuel power station is a power station which burns fossil fuel, such as
coal, natural gas or petroleum to produce electricity.
Nuclear fuel and nuclear fission
Nuclear fuel is any material that can be consumed to derive nuclear energy.
Controlled fission (power production)
When a fission reaction leads to a new fission reaction, which leads to another
one and so on, it is called controlled fission. The amount of heat required is
controlled by raising and lowering the rods in the reactor.
Uncontrolled fission (nuclear weapons)
A fission reaction whereby the reaction is allowed to proceed without any
moderation (by removal of neutrons) is called an uncontrolled fission reaction.
An uncontrolled fission reaction is used for nuclear bombs.
Problems associated with the production of nuclear power
• problem of radioactive waste.
• high risks.
• targets for terrorist attacks.
• nuclear weapons.
• uranium is a scarce resource.
• illusion to build new nuclear power plants.
Environmental problems of fossil fuels
Climate Change / Global Warming and Greenhouse Effect
The earth’s atmosphere allows a lot of sunlight to reach the earth’s surface, but
reflects much of that light back into space.
The result is a gradual increase in the earth’s temperature or Global Warming.
‘Hole’ in the Ozone Layer
Ozone acts to block out much of the sun’s ultraviolet radiation which causes
skin cancer and contributes to the fluctuations of global climatic conditions
that affect the environment.
Acid Rain
When gases, such as sulphur dioxide and nitrogen oxides react with water in
the atmosphere to form sulphuric acid and nitric acid, they form an acidic ‘rain’
which can destroy vegetation.
Air Pollution
Air pollution is the release into the atmosphere of excessive amounts of harmful
gases as well as particles.
Other environmental problems of fossil fuels include:
• Biological Control
• Biological Magnification
• Introduced Species
• Soil Salinity• Population Explosion
UNIT 4:ATOMIC NUCLEI AND RADIOACTIVE DECAY
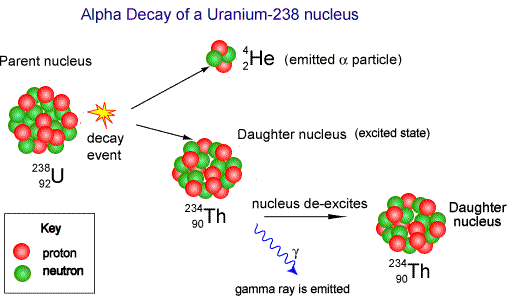
Fig.4. 1: Sign of radiation precaution
Key unit competence: Analyse atomic nuclei and radioactivity decay
My goals
• Define atomic mass and atomic number
• Identify the constituents of a nucleus
• Explain Einstein’s mass-energy relation.
• Define nuclear fusion and fission.
• Analyze determinations of a mass of nuclei by using Bainbridge mass
spectrometer.
• Derive the relationship between decay constant and half-life.
• Determine the stability of a nuclei.
• Describe properties of different radiations.
• Describe creation of artificial isotopes.
• Identify the application of radioactivity in life.
• Plot a graph of binding energy against nucleon and explain its features.
• Calculate the decay rate of unstable isotopes.
• Appreciate the safety precautions to be taken when handling radioactive
materials.
• Appreciate that the nucleus of an atom and quantum system has discreteenergy levels.
INTRODUCTORY ACTIVITY
In different places like industries, hospitals, and other sensitive places, there
are different posts that caution someone about dangerous substances one
may encounter if care is not taken. Among the reasons why these places bare
such instruction is because of chemicals and radiations that are used in such
places which may be harmful if not handled with care.
1. Discuss some of the safety signs you have ever seen in any hospitals or
industry if you have ever visited one.
2. Why do you think there is a need to put those signs in such places?
3. It is believed that there are some of diseases that are treated using
radioactive substances. Can you state some of the radiations used to
treat some diseases.
4. There are natural men made radioactive substances. All of these are
used for different purposes. What are some of negative effects of these
radiations to (i) man , (ii) environment
5. Some countries like Iran are affected by these radiations. Imagine you
were a resident of that country, what would you do to protect yourselffrom such effects of radioactive substances.
4.1 ATOMIC NUCLEI-NUCLIDE
4.1.1 Standard representation of the atomic nucleusACTIVITY 4.1: Investigating the stable and unstable nicleus
Fig.4. 2 The standard representation of an atom nucleus
Observe the Fig.4.1 above of an atom and answer to the questions that
follow:
1. What do numbers A and Z stand for?
2. Describe the relation between the two numbers and their meanings.
3. When do we say that an atom is stable or unstable?
4. Explain clearly the meaning of isotopes. Give an example of isotopesyou know.
A nucleus is composed of two types of particles: protons and neutrons. The
only exception is the ordinary hydrogen nucleus, which is a single proton. We
describe the atomic nucleus by the number of protons and neutrons it contains,
using the following quantities:
a. The atomic number or the number of protons Z in the nucleus (sometimes
called the charge number).
b. The neutron number or the number of neutrons N in the nucleus.
c. The mass number or the number of nucleons in the nucleus,
A = Z + N. (4.01)
In representing nuclei, it is convenient to use the symbol AZX
to show how many
protons and neutrons are present in the nucleus. X represents the chemical
symbol of the element. For example, 5626 Fe nucleus has mass number 56 and
atomic number 26. It therefore, contains 26 protons and 30 neutrons.
When no confusion is likely to arise, we omit the subscript Z because the
chemical symbol can always be used to determine Z. Therefore, 5626 Fe is the same
as 56 Fe and can also be expressed as “iron-56.” Each type of atom that containsa unique combination of protons and neutrons is called nuclide.
4.1.2 Classification
Depending on the combinations of protons and neutrons in the nucleus,
nuclides can be classified in the following 3 categories:
a. Isotopes: These are nuclei of a particular element that contain the same
number of protons but different numbers of neutrons. Most elements
have a few stable isotopes and several unstable, radioactive isotopes.Example of isotopes:
Therefore, the chemical properties of different isotopes of an element are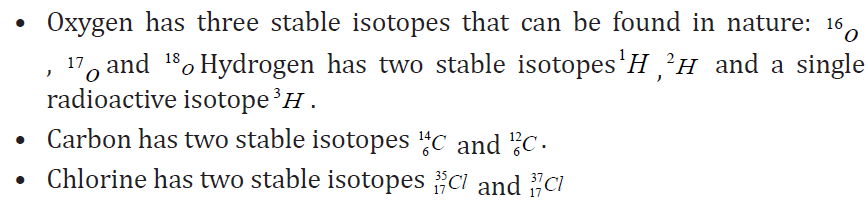
identical but they will often have great differences in nuclear stability. For
stable isotopes of light elements, the number of protons will be almost equal to
the number of neutrons. Physical properties of different isotopes of the same
element are different and therefore they cannot be separated by chemical
methods i.e. only physics methods such as the centrifugation method can be
used to separate different isotopes of an element.
b. Isobars: these are nuclei which have the same mass number but differentnumber of protons Z or neutrons N.
c. Isotones: these are nuclei in which the number of neutrons is the same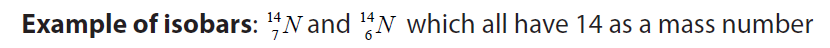
but the mass number A and the atomic number Z differ
4.1.3 Units and dimensions in nuclear physics
The standard SI units used to measure length, mass, energy etc. are too large
to use conveniently on an atomic scale. Instead appropriate units are chosen.
• The length: The unit of length in nuclear physics is the femtometer.
1 fm =10−15 m
This unit is called Fermi in the honor of the Italian Americano physicists whodid a lot of pioneering work in nuclear physics.
• The mass: The unit used to measure the mass of an atom is called the
atomic mass unit, abbreviated “amu or u” and is defined as a1⁄12 the
mass of an atom of carbon-12. Since mass in grams of one carbon-12atom is its atomic mass (12) divided by Avogadro’s number gives.
• Nuclear masses can be specified in unified atomic mass units (u).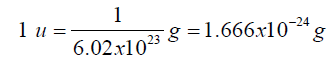
On this scale:
• A neutral 126 C atom is given the exact value 12.000000 u.
• A neutron then has a measured mass of 1.008665 u,
• A proton 1.007276 u,
• A neutral hydrogen 11H
atom (proton plus electron) 1.007825 u
Energy: the SI unit used for energy that is Joule is too large. In nuclear physics
the appropriate unit used for energy is an electronvolt (eV). An electron volt
(eV) isdefined as the energy transferred to a free electron when it is accelerated
trough a potential difference of one volt. This means that
1eV =1.6022×10−19 C×1V =1.6022×10−19 J
It is also a common practice in nuclear physics to quote the rest mass energy
calculated using ,
E = mc2 (4.02)
Since the mass of a proton is mp =1.67262 ×10 −27kg =1.007276 u, then 1 u is equal toThis is equivalent to energy in MeV of
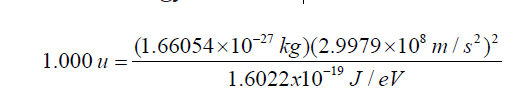
• The time: the time involved in nuclear phenomena is of the order of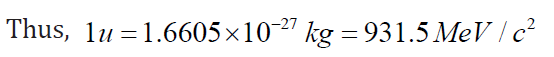
10-20s to million or billion years.
• Nuclear radius: various types of scattering experiments suggest that
nuclei are roughly spherical and appear to have the same density. Thedata are summarized in the expression called Fermi model.
Where r0 =12 fm =1.2 × 10−15 m and A is the mass number of the nucleus
The assumption of a constant density leads to the estimate of the mass densitywhich is obtained by considering.
This high density can explain why ordinary particles cannot go through the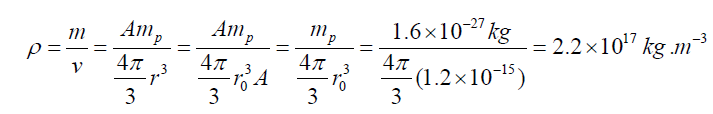
nucleus as highlighted by Rutherford experiments. The same density was only
observed in neutron stars. The nuclear mass can be determined using a mass
spectrometer.
4.1.4 Working principle a mass spectrometer
The figure below highlights the working principle of a typical mass spectrometer
used to separate charges of different masses. It can be used to differentiateisotopes of a certain element.
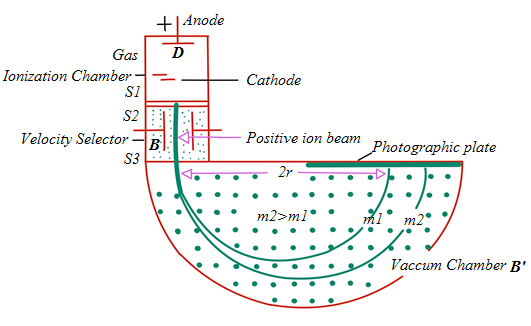
Fig.4. 3: Bainbridge mass spectrometer
Ions are formed in ionization chamber and accelerated towards the cathode.
The beam passes through the cathode and is focused by the collimating slits S1
and S2. The beam is then passed through a velocity selector in which electric
and magnetic fields are applied perpendicular to each other. The ion moves in
straight line path for which both the forces acting on it are equal
qE = qvB
The velocity of ion which passes un-deflected through the velocity selector isthen given by
The ions then reach the vacuum chamber where they are affected by the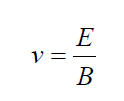
magnetic field alone and then move in circular paths; the lighter ions
alone and then move in circular paths; the lighter ions
having the larger path radius. If the mass of an ion is m, its charge q and itsvelocity v then
The radius of the path in the deflection chamber is directly proportional to the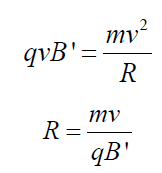
mass of the ion. The detection is done by photographic plate when the ions fall
on it. The fig. 4.5 shows the recorded mass spectrum for a gas containing three
isotopes. Note the wider line for the mass m1, showing its relatively greaterabundance.
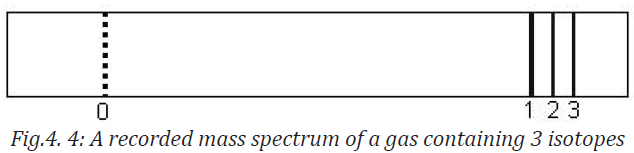
4.1.5 Checking my progress
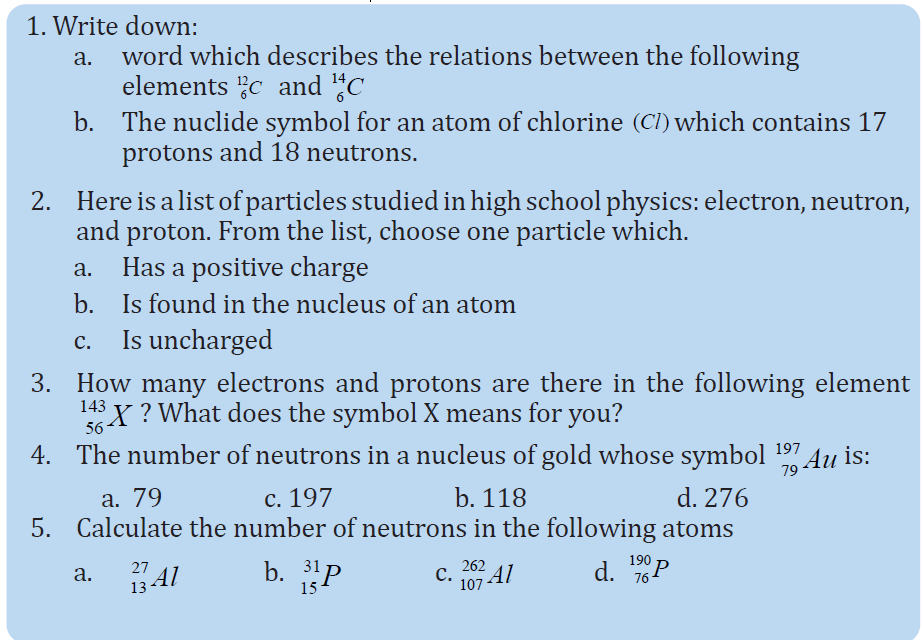
4.2 MASS DEFECT AND BINDING ENERGY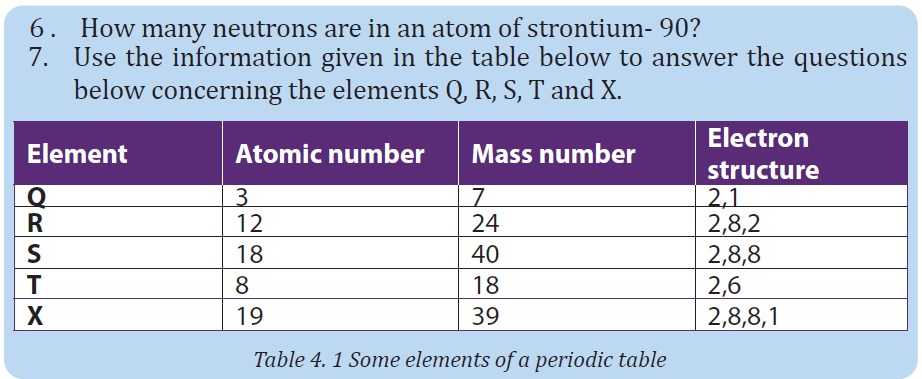
4.2.1 Mass defect
ACTIVITY 5.2: Select the words in the following puzzle
Observe the puzzle below:
1. Discover 8 different words related to particle Physics hidden in the
puzzle below, and write them in your notebook.2. Use them to formulate a meaningful sentence
3. Complete the sentences below using the words you discovered in
the puzzle
a. An …….is the SI unit of energy
c. The ………..of nucleons is greater than the mass of a nucleus.
d. The atom releases ………when its nucleus is formed from its
constituent particles
e. The binding energy per nucleon gives an indication of the …………
of the nucleus.
f. The surprising suggestion that energy and mass are equivalent
was made by ……in 1905.
4. Discuss and explain the meaning of the following expression as used in
physics
a. Mass defect c. Electronvoltb. Biding energy d. Stable nuclides
The nucleus is composed of protons that are positively charged and neutrons
that are neutral. The question is what is holding these particles together in
this tiny space?
Experiences have demonstrated that the mass of a nucleus as a whole is always
less than the sum of the individual masses of protons and neutrons composing
that nucleus.
The difference between the two measurements is called mass defect Δm . For anucleus
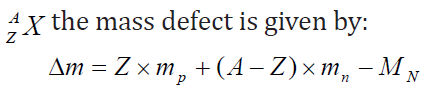
4.2.2 Einstein mass-energy relation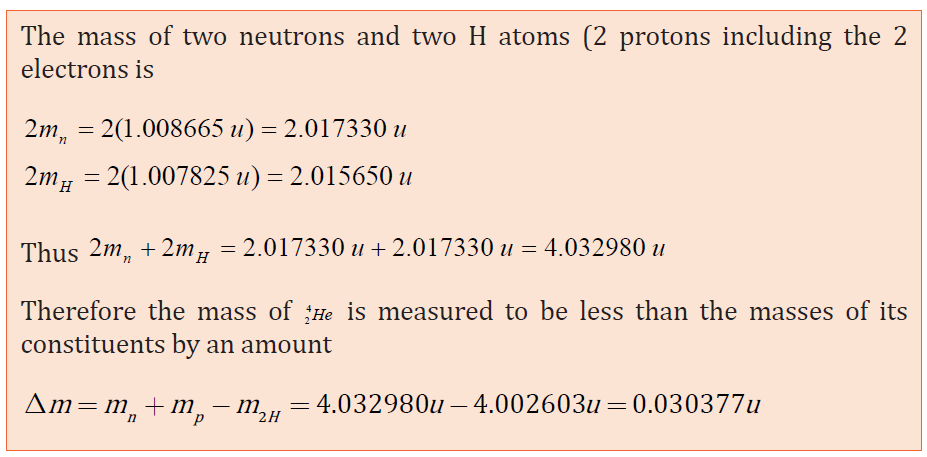
In 1905, while developing his special theory of relativity, Einstein made the
surprising suggestion that energy and mass are equivalent. He predicted that if
the energy of a body changes by an amount of energy E, its mass changes by an
amount m given by the equation
E = mc2 (4.09)
Where c is the speed of light and m mass of a body
Everyday examples of energy gain are much too small to produce detectable
changes of mass.
4.2.3 Binding energy
The mass of a nucleus is less than the combined mass of its protons and
neutrons (nucleons). The missing mass is called the mass defect. This observed
mass defect represent a certain amount of energy in the nucleus known as the
binding energy b E and calculated using the Einstein formula as:
ΔE = Δmc2 (4.10)
where c is the speed of light and Δm the mass defect.The binding energy for a nucleus containing Z protons and N neutrons is defined as
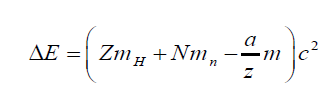
The binding energy is the energy released when a nucleus is formed from its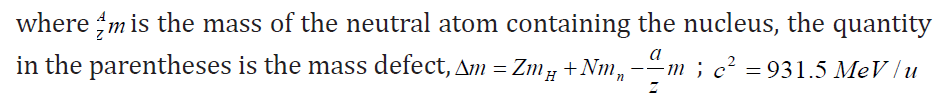
constituent particles or the energy required to break up (to split) the nucleus
into protons and neutrons. Protons and electrons are held together in the
nucleus of an atom by the strong nuclear force. So if we imagine splitting a
nucleus up into its separate protons and neutrons, it would require energy,
because we would need to overcome the strong nuclear force.
4.2.4 Binding energy per nucleon and stability
Instead of looking at the total binding energy of a nucleus, it is often more useful
to consider the binding energy per nucleon. This is the total biding energydivided by the total number of nucleons.
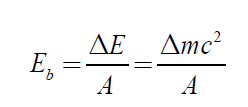
A plot of binding energy per nucleon Eb/A as a function of mass number A for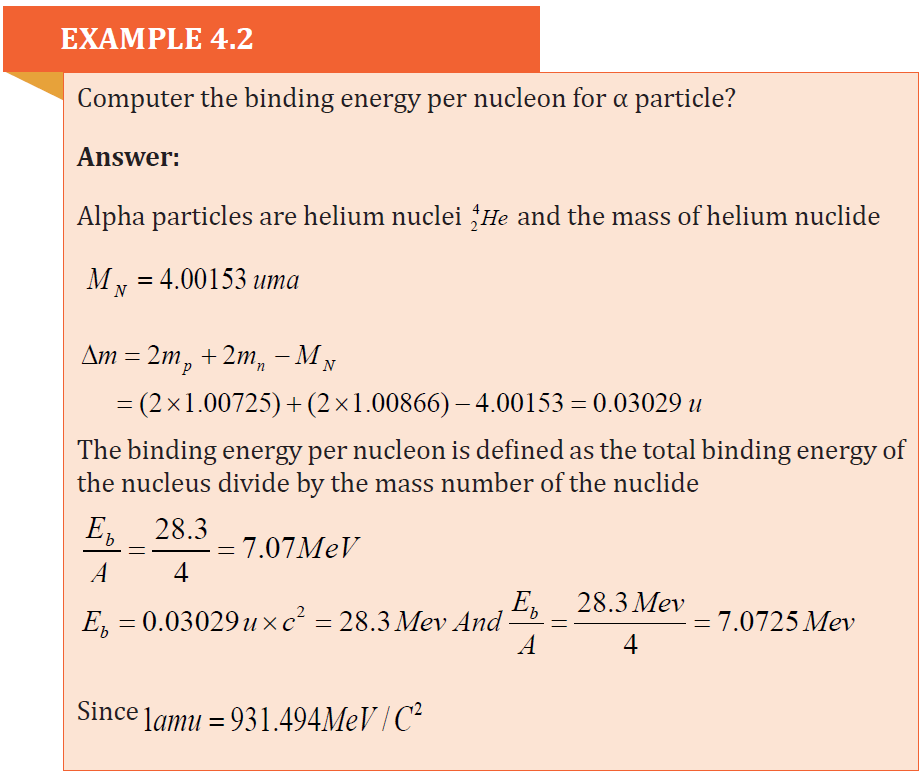
various stable nuclei is shown on Fig. 5.6.

Fig.4. 5: The graph of binding energy per nucleon of the known elements (Giancoli D. C., 2005)
The nuclear binding energy per nucleon for light element increases with the
mass number until a certain maximum is reached at around A = 56 and then
after it almost saturate. The fact that there is a peak in the binding energy per
nucleon curve means that either the breaking of heavier nucleus (fission) or
the combination of lighter nuclei (fusion) will yield the product nuclei with
greater binding energy per nucleon and therefore more stable.
As an example if a nucleus like is 23892U split into two fragments of nearly equal
masses, the two fragments will have higher binding energy per nucleon than
the original. The excess energy is released as useful energy and this process
called fission is the basis of electricity production in a nucleus plant.
If two light elements combine their nuclei in one nucleus, the formed nucleuswill have a greater binding energy per nucleon than the originals.
This process is called nuclear fusion and can only take place at a very high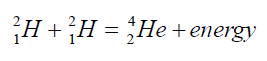
temperature. It is the source of energy in the sun and other stars. The fusion is
more energetic than the fission.
The binding energy per nucleon therefore gives an indication of the stability
of the nucleus. A high binding energy per nucleon indicates a high degree ofstability – it would require a lot of energy to take these nucleons apart.
4.2.5 Checking my Progress
4.3 RADIOACTIVITY AND NUCLEAR STABILITY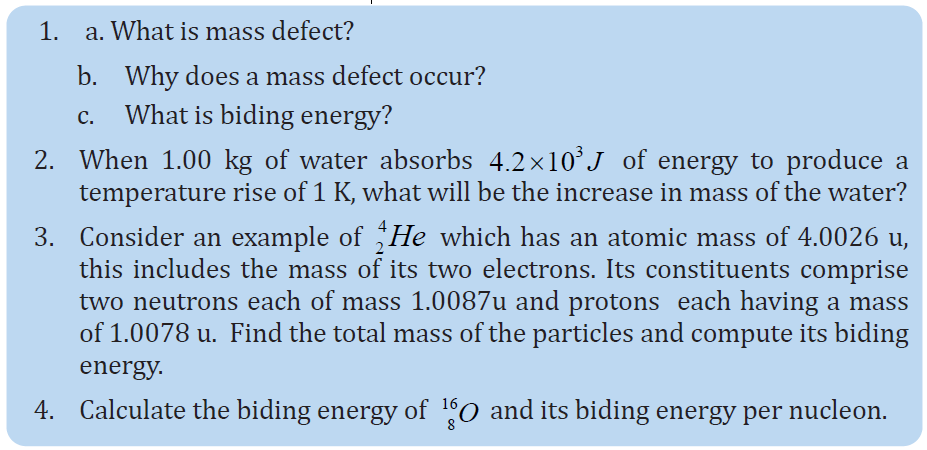
ACTIVITY 4.3: Investigating radioactivity
During the World War II, its final stage was marked by the atomic bombing
on Nagasaki and Hiroshima towns in Japan (Fig.5.6). Observe the image andread the text provided below before answering the following questions.

Fig.4. 6: The atomic bomb in Nagasaki (Japan in 1945)
In August 1945, after four years of world war, united States B-29 bomber,
dropped the atomic bomb over the cities of Hiroshima on August 6th 1945.
70.000 people died in 9 seconds, and the city of Hiroshima was leveled. 3 days
after as second bomb was dropped in Nagasaki, Japan with the same devastating
results. The bombing killed over 129.000 people.
The bomb released cataclysmic load of energy. The ones who were close enough
to see the blast lost their eyes. It was the last thing they ever saw. The bright
light of what the blinded them. The black of their eyes, the retina, melted away.
The radiation received by the body is equivalent today’s thousands of x-rays.
The human body can’t absorb unlimited radiation. It falls apart because the
cells are dying of radiation poisoning, if the radiation is intense enough, it looks
like a urn. Layers of the skin begin to fall off. The body vital functioning began
to slow down until it stops.
1. Describe and discuss the phenomena happening on two images.
2. From the text, show that the atomic bomb of Hiroshima was very harmful
to human body.
3. What are the types of radiations should be there?
4. Stable isotopes do not emit radiations. What is the name of materials which
emit radiations? Describe them.
5. What are the possible main radioisotopes used to produce energy in figure
above?
6. Which processes are used to generate such heavy energy? Describe any one
of your choice
Radioactivity is one of the dynamic properties of nuclei, in this process the
system makes a transition from a high energy state to a low energy by emitting
α and β-particles or γ-rays. This process happens naturally and is not affected by
any external agent such as pressure, temperature or electric and magnetic fields.
The α-particles are Helium nuclei and can be stopped by a piece of paper while
β-particles are either electron or positron. There are high energetic particles
and can pass through one cm thick aluminum sheet. γ-rays are electromagnetic
radiations and can be stopped by several inches of lead.
4.3.1 Radioactive decay of a single parent
Nucleus decay is a random process and the rate of disintegration is proportional
to the number of available radioactive nuclides. Let us analyses the simple case
where the first daughter nuclide is stable. Suppose that at time t, there are N
radioactive nuclide and dN is the number of nuclide disintegrating within a
time dt. As the rate of disintegration is proportional to the number of nuclides
present in the radioactive substance, we get
where λ, the proportionality constant, is called the radioactive constant.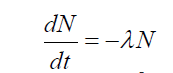
This constant depends on the nature of the radioactive substance. The negative
sign shows that an increase in disintegration rate will decrease the number of
radioactive nuclides which are present. From this we can establish the formulaof radioactive decay:
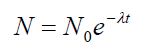
where it assumed that the initial number of radioactive nuclide is equal to N0.
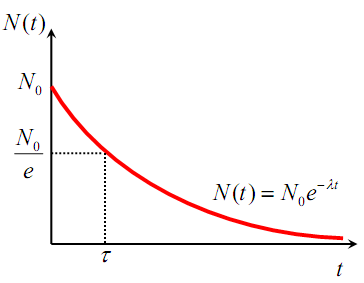
Fig.4. 7: Illustration of the radioactive decay law
If we consider the activity A of a radioactive sample which is the number of
decay events in a unit time we obtain a similar expression for the radioactivedecay law but expressed in terms of activity of the radioactive substance:
where A0 is the initial activity of the radioactive source. Another parameter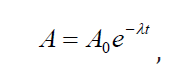
useful in characterizing nuclear decay is the half-life T1⁄2 .
The half-life of a radioactive substance is the time interval during which halfof a given number of radioactive nuclei decay. Therefore the half time period is
Finally,one shows that the mean-life of a nuclide or the average life period of a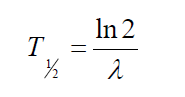
nuclide is related to the radioactive constant by
In general, after n half-lives, the number of un-decayed radioactive nuclei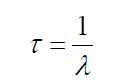
remaining is
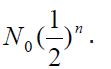
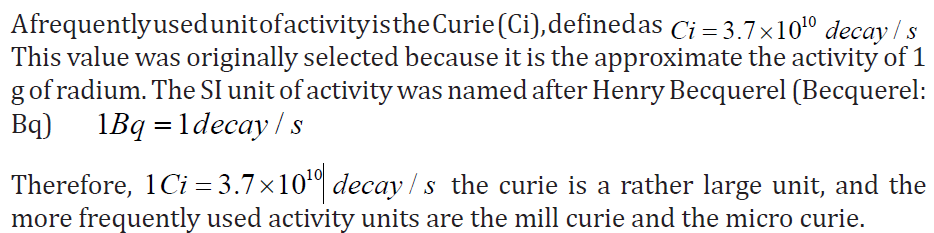
4.3.2 Characteristics of radioactive substances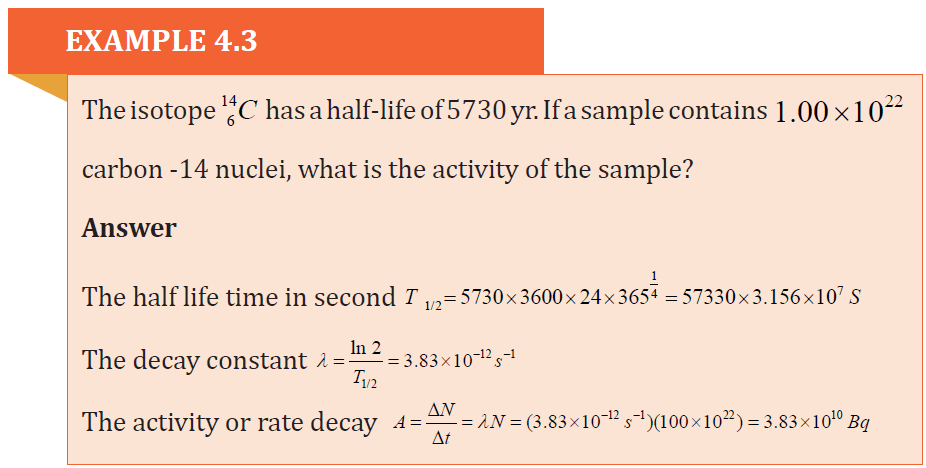
Radioactive substances (nuclides) present one or more of the following features
• The atom of radioactive elements are continually decaying into simpler
atoms as a result of emitting radiation
• The radiations from radioactive elements produce bright flashes of
light when they strike certain compounds. The compound fluoresce.
For example, rays from radium cause zinc sulphide to give off light in
the dark. For this reason, a mixture of radium and zinc sulphide is used
to make luminous paints.
• They cause ionization of air molecules. The radiations from radioactive
substances knock out electrons from molecules of air. This leaves the
gas molecules with a positive charge.
• Radiations from radioactive substances can penetrate the heavy black
wrapping around a photographic film. When the film is developed, it
appears black where the radiations struck the film.
• Radiations from radioactive substances can destroy the germinating
power of plants seeds, kill bacteria or burn or kill animals and plants.
Radiations can also kill cancers.
A. Properties of emitted radiationsSome of their properties are summarized and shown in the table below:
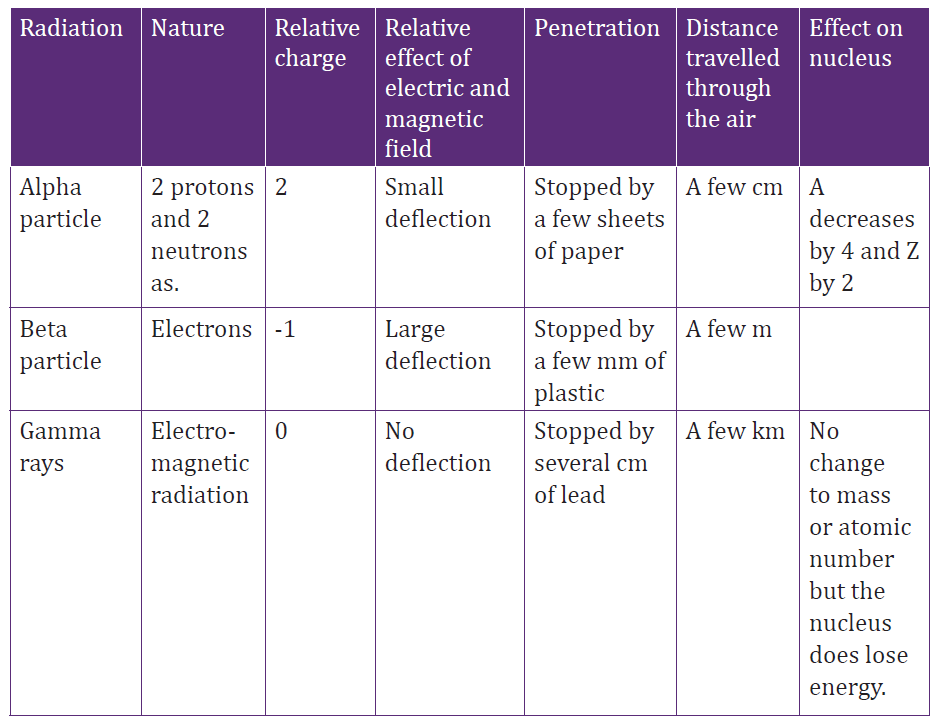
Table 4. 3 Properties of different types of radiations
a. Alpha decay ( 42He )
If one element changes into another by alpha decay, the process is called
transmutation. For alpha emission to occur, the mass of the parent must be
greater than the combined mass of the daughter and the alpha particle.
In the decay process, this excess of mass is converted into energy of other forms
and appears in the form of kinetic energy of both the daughter nucleus and
the alpha particle. Most of kinetic energy is carried away by the alpha particle
because it is much less massive than the daughter nucleus. The momentum is
conserved in this process.
The isotope whose natural radioactive decay involves the emission of alpha
particles usually have a relative atomic mass greater than 210 (Ar>210). They
have too much mass to be stable and give out alpha particles to form smallerand more stable atoms.
b. Beta decay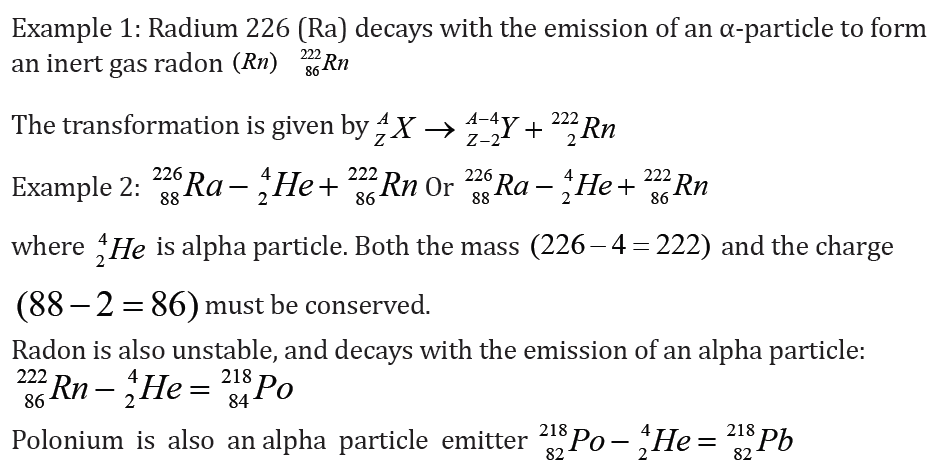
The isotopes whose radioactive decay involves the emission of beta particles
often have a relative atomic mass less than 210 (Ar < 210). Beta particles are
usually emitted from heavier nuclei that have too many neutrons compared
with the number of protons.
I. Negative β-decayIn this process of negative β-decay an electron and an antineutrino are emitted.
The emitted electron results from the following reaction where a neutron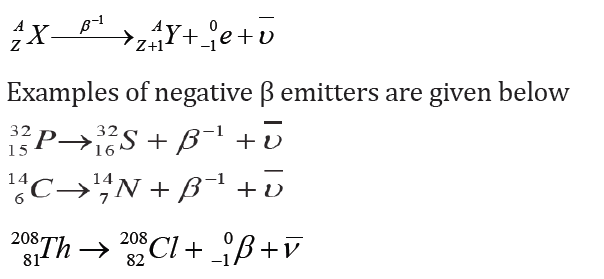
changes into a proton and an electron is emitted from the nucleus as a betaparticle:
The conservation of charges and mass number is maintained. The daughter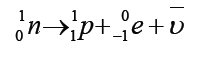
nuclide may be in an excited state and will become stable after emitting a γ-ray.
II. Positive β-decayIn this process the positron and the neutrino are emitted.
This positive decay is different from an electron capture which takes place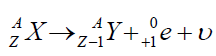
when an electron that is close to the nucleus recombines with a proton in thenucleus producing a neutron and a neutrino:
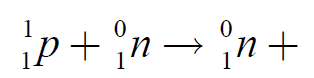
The equation of decay of the electron capture is:
The daughter nuclide is seem to be the same as that we could have been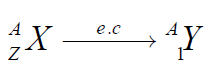
produced if a positron has been emitted. The rearrangement of the remaining
Z-1electrons will lead to emission of characteristic x-ray of the daughter
nucleus. In few case both positron and electron capture may happen.
C. Gamma decay (Y)
Very often a nucleus that undergoes radioactive decay is left in an excited
energy state. The nucleus can then undergo a second decay to a lower energy
state by emitting one or more photons. Unlike α and β decay, γ decay results
in the production of photons that have zero mass and no electric charge. The
photons, emitted in such process, are called gamma rays and they have very
high energy.
If an atom of a material Y emits a γ ray (γ photon), then the nuclear reaction canbe represented symbolically as
Gamma emission does not result in any change in either Z or A.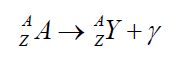
Notes:
• A transmutation does not occur in gamma decay. When an alpha
particles and beta particles are emitted, gamma rays are often emitted
at the same time. When a radioisotope emits gamma rays, it become
more stable because it loses energy.
• In both alpha and beta decay, the new element formed is called thedaughter isotope.
• Gamma rays are like X-rays. Typical gamma rays are of a higher
frequency and thus higher energy than X-rays.
• Deviations of alpha, beta and gamma radiations due to electric field and
magnetic field ( See Fig.4.9). It can be seen unlike gamma-rays, alpha
and -particles are affected by the presence of electric and magnetic
fields since these particles are charged. Gamma-rays are not affectedby these fields.
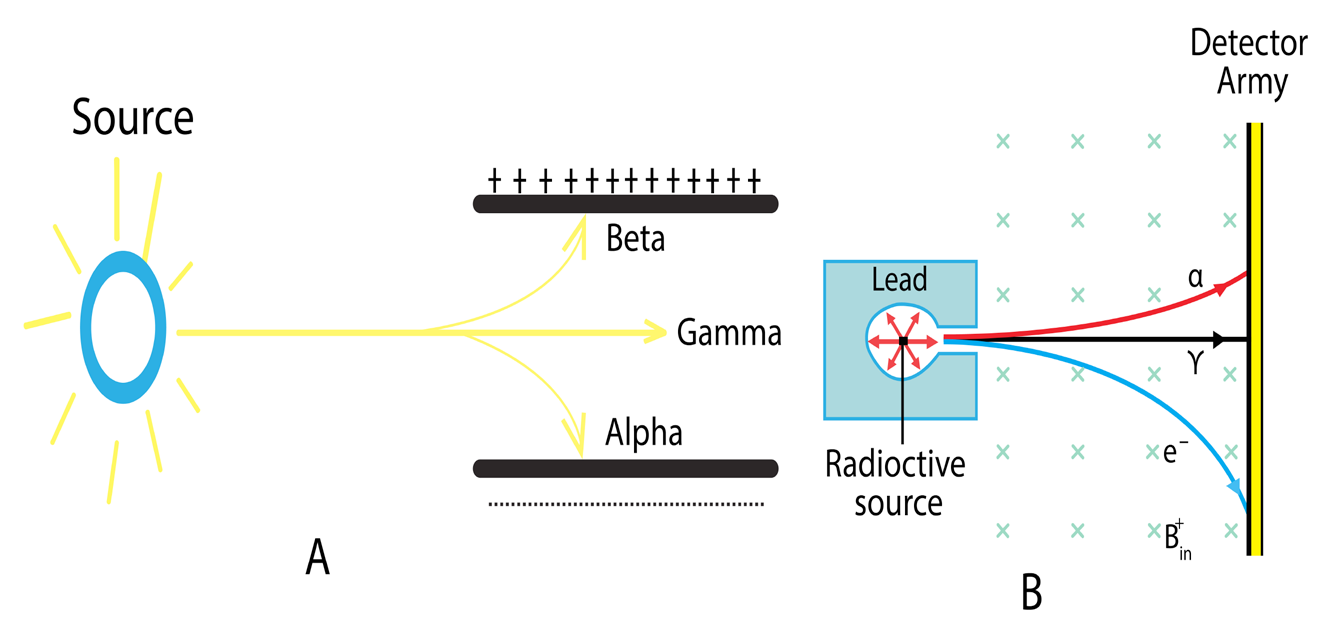
Fig.4. 8: Deviation of radiation particles in an electric field
4.3.3 Nuclear fission and Fusion
a. Nuclear fission
Heavy unstable nuclides can be broken up to produce energy in a process
called nuclear fission. When uranium decays naturally, alpha and beta particles
are emitted. However, when uranium-235 is bombarded by neutrons it forms
uranium-236. Uranium-236 is unstable and breaks down, splitting into two
large particles and emitting three neutrons. The fission of 235U by thermalneutrons can be represented by the reaction
where 236U∗ is an intermediate excited state that lasts for approximately10-12s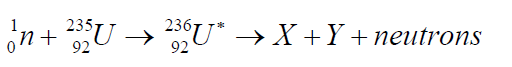
before splitting into medium-mass nuclei X and Y, and these are called fissionfragments.
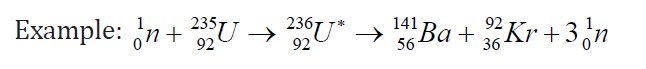
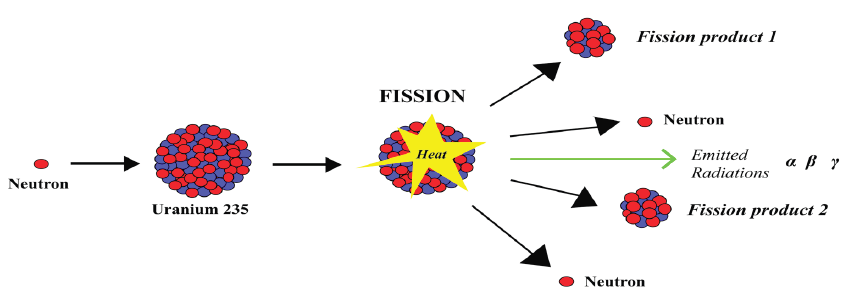
Fig.4. 9: Fission diagram illustration
When the exact masses of the final products are added together, the sum is
found to be appreciably less than the exact masses of the uranium-235 and the
original neutron. This difference in mass Δm appears as energy given by
Δm = Δmc2
Another important point arises! The three neutrons released may collide with
other nuclides and split them also resulting in cascade reactions. In this way,
chain reactions occur and as a result, the quantity of energy released can be
very large. A few kilogram of uranium can produce as much heat energy as
thousands of tons of coal.
Advantages and disadvantages of nuclear fission
The nuclear fission produces a huge amount of energy. This energy can
be released in a controlled manner in nuclear power station and be used in
driving steam turbines that produce electric power. However, when produced
in uncontrolled manner it will result in the fabrication of atomic bomb that may
release a large amount of heat energy and damaging radiations. The emitted
radiations have both short term and long term effect on the living things.
b. Nuclear fusion
When lighter nuclides merge together in a process called fusion, energy isproduced and mass is lost. For example:
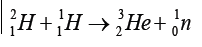
These reactions occur in the core of a star and are responsible for the outpouring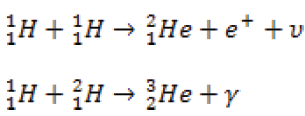
of energy from the star.
The sum of the exact masses of the helium atom is less than the sum of exact
masses of the four hydrogen atoms. This lost mass is released as energy. It is
thought that the sun’s energy is produced by nuclear fusion.
4.3.4 Radiation detectors
ACTIVITY 4.4: Smoke detector bellow
Observe the diagram of a smoke detector bellow then answer to thequestions that follow:
Fig.4. 10: illustration diagram of a smoke detector
1. Name the components labeled A, B, C and D on the smoke detector
above?
2. What is meant by smoke detector?
3. Describe a functioning of a smoke detector.
4. Design an inventory of other radiation detectors you know
Experiments in Nuclear and Particle Physics depend upon the detection of
primary radiation/particle and that of the product particles if any. The detection
is made possible by the interaction of nuclear radiation with atomic electrons
directly or indirectly.
a. Classification of radiation detectors
There are a variety of other radioactive detectors that we may convenientlyclassify into two classes: Electrical and Optical detectors.
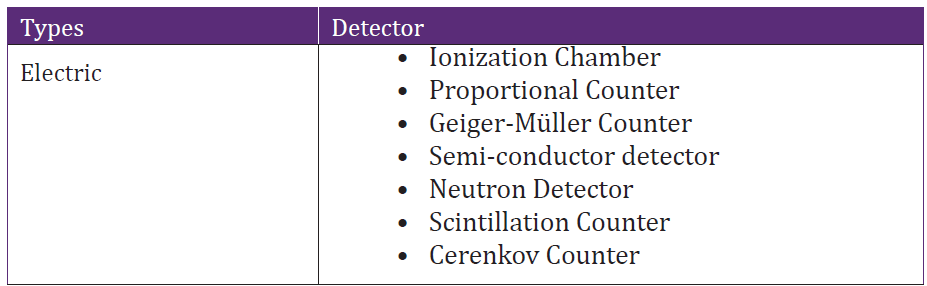

Table4. 1 Classification of radiation detectors
b. Working principle of an ionization chamber
Conventionally, the term “ionization chamber” is used exclusively to describe
those detectors which collect all the charges created by direct ionization within
the gas through the application of an electric field. Ionization chamber is filled
with inert gases at low pressure. In the chamber there are two electrodes,
namely, the cathode and the anode which are maintained at a high potentialdifference as shown on the figure below
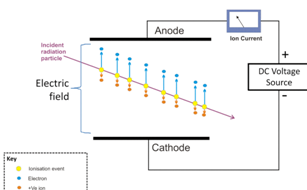
Fig,4.11: visualization of ion chamber operation
When radiation enters the chamber, it ionizes the gas atoms creating negative
and positive charges. The negative charges or electrons are attracted by the
anode while positive ions are attracted by the cathode; this produces the current
in the outside circuit depending on the strength and the type of radiation. The
current produced is quite small and dc amplified electrometers are used tomeasure such small currents.
4.3.5 Checking my progress
In the following exercises (1 to 4), choose the best answer and explain your
choice
1. Which of the following is an electron?
a. Neutrino c. Photon
b. Gamma particle d. Beta particle
2..Which of the following most accurately describe radioactive decay?
a. Molecules spontaneously break apart to produce energy
b. Atoms spontaneously break apart to produce energy beta decay, alpha
decay and positron emission are all forms of radioactive decay. Energy is
released because the atoms are converted to a more stable energy
c. Protons and neutrons spontaneously break apart to produce energy
d. Electrons spontaneously break apart to produce energy
3. Which of the following is true concerning the ratio neutrons to protons in
stable atoms?
a. The ratio for all stable atoms is 1:1.
b. The ratio for small stable atoms is 1:1, and the ratio for large stable atom is
greater than 1:1. As atomic weight goes up, the ratio of neutrons to protons
for stable atoms increases up to as much as 1.8:1 ratio.
c. The ratio for large stable atom is 1:1, and the ratio for small stable atoms
is greater than 1:1.
d. There is no correlation between the stability of the atom and its neutron
to proton ratio.
4. Polonium-218 undergoes one alpha decay and two beta decays to make
a. Polonium-214 c. Bismuth-214
b. Plomb-214 d. Plomb-210
5. a) Compare (i) the charge possessed by alpha, beta and gamma radiations
(ii) The penetrating power of these radiations
6. a.What is meant by the term (i) radioactive decay? (ii) Half-life of a radioactive
substance?
b. A 32 g sample of radioactive material was reduced to 2 g in 96 days. What is its
half-life? How much of it will remain after another 96 days?
7. 212Be Decays to 208Th by α-emission in 34% of the disintegration and to212Ra
by β-emission in 66% of the disintegration. If the total half value period is 60.5
minutes, find the decay constants for alpha and beta and the total emission.
8. If a radioactive material initially contains 3.0milligrams of Uranium234U , how
much it will contain after 150,000 years? What will be its activity at the end ofthis time?
4.4 APPLICATION OF RADIOACTIVITY
ACTIVITY 5.5: Use of nuclear energy to generate electricity
Fig.4. 12: Nuclear power plant functioning mechanism diagram.
Many people disagree to the use of nuclear power to generate our
electricity, even though the safety record of nuclear industry is extremely
good. Observe clearly the image diagram of the nuclear power plant
(Fig.4.12) and answer to the questions that follow
1. Why do you think people disagree to the use of nuclear power
station?
2. What are the main parts of the power plant station observed in
Fig.5.12?
3. Analyze and explain the steps of energy transformation from reactor
to generator
4. Write a brief explanation on the advantages and disadvantages of
using nuclear energy as a source of electricity if any.
5. Use internet and your library or any other resources to find out
about the other application of radionuclides in our daily life
People would not have fear of radiations when controlled in certain manner.
Radioisotopes and nuclear power process have been used and produced
improvement in various sectors. These includes: consumer products, food and
agriculture, industry, medicine and scientific research, transport, water
resources and the environment. The following are some descriptive examples
among others.
4.4.1 Industry
Different materials we use at home are manufactured in industry and made of
different radioactive materials. The dosage of use of radioactive substance is
thus controlled so that they are not harmful to human body.
Gamma radiation and beta radiation from radio-isotopes can be used to
monitor the level of the material inside the container. The penetrating power
of gamma rays is used to detect hidden flow in metal castings. Beta rays are
used to measure the thickness of various flat objects (the mass absorbed by the
object is proportional to its thickness).
In the textile industries, irradiation with beta radiations fixes various chemicals
onto cotton fibers. This produces for instance permanent press clothing. Again,
radioactive materials can be used as tracers to investigate the flow of liquids inchemical factories.
Fig.4. 13 Testing the level of a liquid in a container using radiations
If there is a sudden decrease in the amount of radiation reaching the detector,
which will happen when the container is full, then this can be used as a signal
to switch off the flow of substance into the container. A similar method is used
to monitor the thickness of sheets of plastic, metal and paper in production.
4.4.2 Tracer studies
Tracer techniques can be used to track where substances go to and where leaks
may have occurred. Leaks in gas pipes or oil pipes can be detected by using this
technique. Tracer techniques are also used in medicine to treat thyroid glands
which can be underactive or over active. The activity of the thyroid gland can
be monitored by the patient being injected with or asked to drink radioactive
iodine. The radioactivity in the vicinity of the thyroid gland is then checked to
see how much of the radioactive iodine has settled in the area around the gland.
4.4.3 Nuclear power stations
Nuclear power stations control a large amounts of energy released when
Uranium-235undergoes nuclear fission. The energy released by this controlled
chain reaction, is then used to produce electricity.
4.4.4 Nuclear fusion
In nuclear fusion, the nuclei of elements with a very low atomic number are fused
together to make heavier elements. When this takes place, it is accompanied by
a very large release of energy. In the sun, hydrogen nuclei are fusing together
all the time to make helium nuclei. This is also the process by which a hydrogen
bomb works.
4.4.5 Medicine
ACTIVITY 4.6: Radionuclides in MedicineObserve the figure below and suggest answers to the question below
Fig.4. 14: A gamma camera assembly. The photons emitted in the patients are detected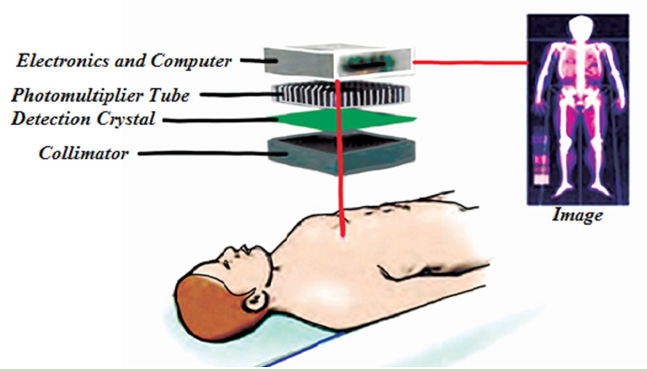
by the photomultiplier tubes. A computer monitor displays the image computed fromthe photomultiplier signals.
1. Who is this person (a man or a woman)?
2. Where is he?
3. What does the image on the right represent?
4. What do you think the patient is suffering from?
5. Using the knowledge acquired in optics, what kind of light
propagation observed there?
6. Does the imaging use reflection or refraction? Why?7. What should be the name of radiation being used in this imaging?
Nuclear medicine has revolved treatment of different disease of the century. It
consists on the use of nuclear properties of radioactive substances in diagnosis,
therapy and research to evaluate metabolic, physiologic and pathologic
conditions of human body. Today, nuclear medicine is currently used in the
diagnosis, treatment and prevention of many serious sicknesses.
Cancer cells are more easily killed by radiation than healthy cells. In medicine,
penetrating gamma rays of cobalt-60 sources are used for this purpose. Other
cancers such as skin cancers are treated by less penetrating beta radiation from
strontium-90 source. Surgical instruments can also be sterilized using gamma
radiation.
4.4.6 Food preservation
The preservation of food uses gamma rays is spreading worldwide. Treating
food with gamma rays can:
• Slow down the ripening of some fruits and the sprouting of potatoes.
In both cases, this helps storage and increases the half-life of the food.
• Kill highly dangerous micro-organisms such as salmonella
• Kill micro-organisms that spoil food
4.4.7 Radiocarbon dating
There are three isotopes of carbon: carbon-12, carbon-13 and carbon-14. The
isotope carbon-14 is radioactive and has half-life of 5760 years. To estimate
the age of a biological sample, radiocarbon146C is used as a radioactive
nuclide. Nucleus decay is independent of the physical or chemical condition
imposed on the elements. This can be used to measure the ages of biological
samples by considering the ratio of 146Cwhich is radioactive and 126C in dead
species. Radioactive carbon is produced when cosmic rays interact withair atoms to produce neutrons and these neutrons interact with nitrogen
The half-life period of 146Cis equal to 5760 years. The carbon reacts with oxygen
to produce CO2 and plants combine this CO2 with water in the process of
photosynthesis to manufacture their food. Therefore plants and animals are
radioactive. When plants or animals die, the 146Cin them keeps on decaying
without any new intake. The ratio of 146C and 126C will therefore be different in
dead and leaving plants and animals. The age of the dead plant or animal can beestimated by measuring this ratio.
4.4.8 Agricultural uses
In agriculture, radionuclides are used as tracers for studying plants, insect
and animals. For example, phosphorus-32 can be added to plant fertilizer.
Phosphorus is absorbed by plants and its distribution can be measured.
Radiation has been used in South American to detect and control the screw
worm fly pest. A large number of the male of the species were exposed to
gamma radiation. When the males were released back into the wild and mated
with wild females, sterile eggs resulted and no new flies were born.
The points of photosynthesis in a leaf are revealed by growing it in air containing
carbon-14. The presence of this radioactive nuclide in the leaf is the revealed
by putting the leaf onto a photographic plate and letting it take its own picture.4.4.9 Checking my progress
1. Suggest different uses of radionuclides in (i) Medicine (ii) food and
agriculture
2. In our daily life, we are exposed to radiations of different types mainly
in materials we use.
a. Make an inventory of all of the devices in your home that may have
(contain) a radioactive substance.
b. What is the origin of these radiations in the materials highlighted
above?
c. Explain the purpose of radioactive material in the device.
d. Then make research to find out how the objects shown in Fig.5.15 useradiation in their manufacture.

Fig.4. 15
4.5 HAZARDS AND SAFETY PRECAUTIONS OF WHEN
HANDLING RADIATIONSACTIVITY 4.7: Investigating the safety in a place with radiations
Fig.4. 16: Radiation effects on human body according to the exposure.
The image above (Fig.4.16) shows different side effects of radiation on
human body according to the exposure time taken. With reference to
section 4.3 and activity 4.3, answer to the following questions:
1. What are the dangers of radiations you may observe?
2. Analyze measures should be taken for radiation users?
4.5.1 Dangers of radioactivity
• Both beta particles and gamma rays can pass easily in the skin and can
easily destroy or even kill cells, causing illness.
• They can cause mutations in a cell’s DNA, which means that it cannot
reproduce properly, which may lead to diseases such as cancer.
• Alpha particles cannot pass through the skin. However, they are
extremely dangerous when they get inside your body. This can happen
if you inhale radioactive material.
4.5.2 Safety precautions when Handling Radiations
The precautions taken by workers who deal with radioactive materials are:
• Wearing protective suits
• Wearing radiation level badges
• Checking the radiation level regularly
• Using thick lead-walled containers for transporting radioactive
materials
• Using remote control equipment from behind thick glass or lead walls
to handle radioactive material
• They should be held with forceps and never touched with hands.
• No eating, drinking or smoking where radioactive materials are in use
• Wash your hands thoroughly after exposure of to any radioactive
materials
• Any cuts in the body should be covered before using radioactive sources
• Arrange the source during experiments such that the radiation window
points away from your body• There are ten golden rules for working safely with radioactivity.


END UNIT ASSESSMENT 4
A. Multiple choice questions
Instructions: Write number 1 to 5 in your notebook. Beside each
number, write the letter corresponding to the best choice
1. Radionuclides
a. Are those nuclides having more neutrons than protons
b. May emit X-rays.
c. Decay exponentially
d. May be produced in a cyclotron
2. Concerning Compton Effect:
a. There is interaction between a photon and a free electron.
b. The larger the angle through which the photon is scatted, the
more energy it loses.
c. The wavelength change produced depends upon the
scattering material.
d. High energy radiation is scatted more than lower energy
radiations.
e. The amount of scattering that occurs depends on the electron
density of the scattering material.
3. Classical physics offered a satisfactory explanation for
a. The diffraction of electrons by crystals
b. The deflection of charged particles in an electric field
c. The intensity spectrum of black body radiation
d. The photoelectric effect
e. Matter waves
4. When investigating β decay, the neutrino was postulated to explain
a. Conservation of the number of nucleons
b. Counteracting the ionizing effect of radiation
c. Conservation of energy and momentum
d. The production of antiparticles
e. The energy to carry away the β particles.
5. Gamma radiations differ from α and β emissions in that
a. It consist in photons rather than particles having nonzero
rest mass
b. It has almost no penetrating ability
c. Energy is not conserved in the nuclear decays producing it
d. Momentum is not conserved in the nuclear decays producing it
e. It is not produced in the nucleus
6. The process represented by the nuclear equation is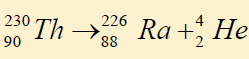
a. Annihilation c. β decay e. γ decay
b. α decay d. pair production
7. Write number (i) to (iii) in your note book. Indicate beside each number
whether the corresponding statement is true (T) or false (F). If it is false,
write a corrected version.
I. An alpha particle is also called a hydrogen nucleus
II. The neutrino was suggested to resolve the problem of conserving
energy and momentum in β decay.
III. The amount of energy released in a particular α or β decay is found
by determining the mass difference between the products and the
parent. A mass-energy equivalence calculation then gives the energy.
IV. The average biding energy per nucleon decreases with the increasing
atomic mass number
8. A radioactive source emits radiations alpha, beta and gamma a shown
below: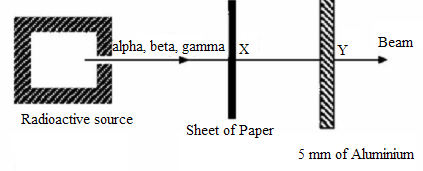
Fig.4. 17 Absorption of radiation
The main radiation(s) in the beam at X and Y are
9. The energy released by the nuclear bomb that destroyed Hiroshima was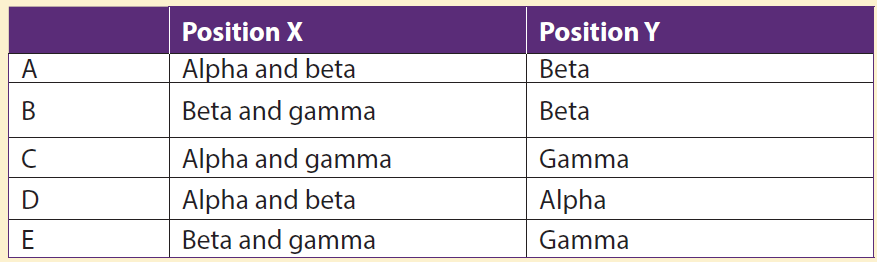
equivalent to 12.4 kilotons of TNT. This is equivalent to 9.1 × 1026 MeV.
The mass that was Converted into energy in this explosion was (ConvertMeV in Joules, use E=mc2)
10. In the decay scheme (Conserve charge and electron lepton number) the
blanks should contain
11. Complete the following sentences by using a word, number and an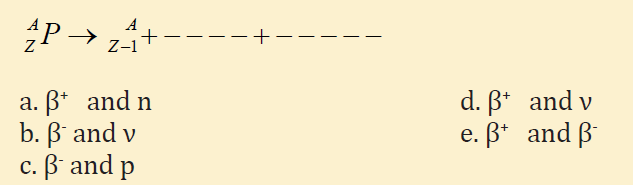
equation where necessarya. The half-life in years of the decay represented by the graph in fig.4.18
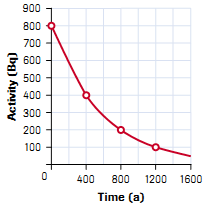
Fig.4. 18 Half life carve
b. When an animal or plant dies, no more _______________is taken in and
that which is present undergoes radioactive decay. If we measure the
amount of carbon-14 left, it is possible to determine the________ of the
sample.
c. If an atom of material Y emits a gamma ray (gamma photon), then thenuclear reaction can be represented symbolically as_________________
B. Structured questions
12. Prepare a table summarizing the three types of radioactive emission.
Classify each type under the following headings: Type of Emission,
Mass, Charge, penetrating Power and Ionization Ability.
13. Copy the following table in your notebook and answer the questionsthat follow
14. Give the value of x and y in each of the following equations
15. Give the value of x and y in each of the reaction classify each as α , β ,
or γ decay
16. The half-life of carbon14 is 5730 years the mass of certain sample of
this isotope is800 μg . Graph the activity for the first 5 half-lives.
17. Beams of a, b- and g radiation of approximately the same energy passthrough electric and magnetic fields as shown below.
a. Show the path taken by each particle in the two fields. Why do
they follow these paths?
b. Which particle is the most penetrating? Explain your answer.
c. Which has the highest ionizing power?
d. How are β − ,β + and electrons different?
e. How are x-rays, y rays and photons different?
18. We are exposed to radiation all the time, indoors and outdoors. This is
called background radiation.
a. Give two examples of sources of this background radiation.
b. Which organ generally receives the most background radiation, and
why?
c. There is some concern at the moment that pilots and flight attendants
may have significantly higher exposures to radiation than the normal
exposure rates for the general public.
d. Why do pilots have a higher exposure to radiation than most other
people?
19. Nuclei can decay by emitting particles which can change the energy, mass
and charge of the nucleus.
a. How is α decay possible when the α particle must pass an energy
barrier which is greater than the energy of the particle? Describe the
process involved.
b. If isotope A emits α particles with greater energy than isotope B (of
the same element), which will have the longer half-life?
c. How can a nucleus change its charge without emitting a chargedparticle?
C. Question of research
21. Using the information in radioactivity and making an internet search
or/ and using other sources of information, consolidate your skills in
other hazardous materials you may meet in your area. Then completethe table below (not exhaustive):
Table 4. 7 Precaution signs
UNIT 5 APPLICATIONS OF OPTICAL FIBER IN TELECOMMUNICATION SYSTEMS.
Key unit competence: Differentiate optical fiber transmission and other
transmitting systems.
My goals
• Explain the functioning of optical fiber
• Explain attenuation in optical fiber
• Identify and explain the components of optical fiber system
• Solve problem related to attenuation giving answers in decibels
• Describe telecommunication system
• Describe functions of amplifiers in optical fiber transmission
• Distinguish optical fiber and other telecommunication systems
INTRODUCTORY ACTIVITY
Investigating the use of optical fiber in RWANDA
Rwanda plans to connect three million people to the World Wide Web as
part of the “Internet for All” project. The project is a World Economic Forum
initiative that aims to connect 25 million new Internet users in Kenya,
Uganda, South Sudan and Rwanda by 2019.
This goal will partly be achieved by addressing the challenges of affordability,
digital skills gap, lack of local content and limited infrastructure, which are
hindering growth in the use of Internet across the region (http://www.threastafrican.co.ke, 2017)

Fig.5. 1: The installation and use of optical fiber in Rwanda
1. Observe the images A, B and C (Fig.5.1) and describe what you can see.
2. What are the uses of optical fiber in transmission of signals?
3. How do optical fibers function? In which field?
4. Discuss other applications of optical fibers.
5.1 PRINCIPLES OF OPERATIONS OF OPTICAL FIBERSACTIVITY 5.1: Total internal reflection in optical fiber.
Fig.5. 2: The total internal reflection in the optical fiber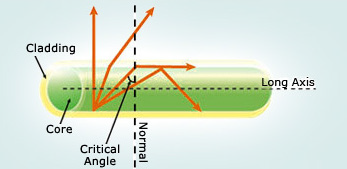
Given the illustration above (Fig.5.2), one can see different rays insidethe optical fiber.
As the angle of incidence in the core increases, as the angle of refraction
increases more until it becomes right angle at a certain value of incidence
angle called critical angle. Discuss:
1. What do you understand by the term critical angle?
2. What causes the total internal reflection?
3. Discuss different fields where total internal reflection can be useful.
5.1.1 Definition
An optical fiber (fiber optics) is a medium for carrying information from one
point to another in the form of light. It uses a flexible, transparent fiber made
by drawing glass or plastic and has a diameter slightly thicker than that of
a human hair. They are arranged in bundles called optical cables and can be
used to transmit signals over long distances. Fiber optics continues to be used
in more and more applications due to its inherent advantages over copperconductors.

Fig.5. 3: An optical cable and a bundle of optical fibers
An optical fiber is made of 3 concentric layers:
• Core: This central region of the optical fiber is made of silica or doped
silica. It is the light transmitting region of the fiber.
• Cladding: This is the first layer around the core. It is also made of
silica, but not with the same composition as the core. This creates an
optical waveguide which confines the light in the core by total internal
reflection at the core-cladding interface.
• Coating: The coating is the first non-optical layer around the cladding.
The coating typically consists of one or more layers of polymer thatprotect the silica structure against physical or environmental damage.
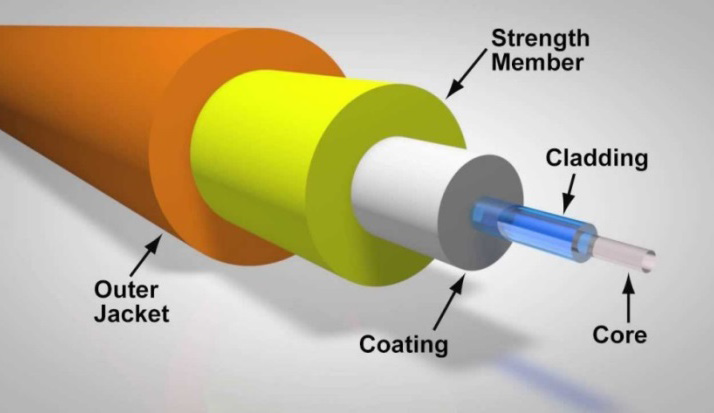
Fig.5. 4: the structure of optical fiber
The light is guided down the core of the fiber by the optical cladding which has
a lower refractive index. Remember that the refractive index is the ratio of the
velocity of light in a vacuum to its velocity in a specified medium. Then light is
trapped in the core through total internal reflection. The other outer parts that
are the strength member and the outer jacket, serve as protectors.
Connecting two optical fibers is done by fusion splicing or mechanical splicing.
It requires special skills and interconnection technology due to the microscopic
precision required to align the fiber cores.
5.1.2 Refractive index of light
When light falls at the interface (boundary) of two media, it is partially reflected
and partially refracted. As it passes from one medium to another it changes itsdirection.
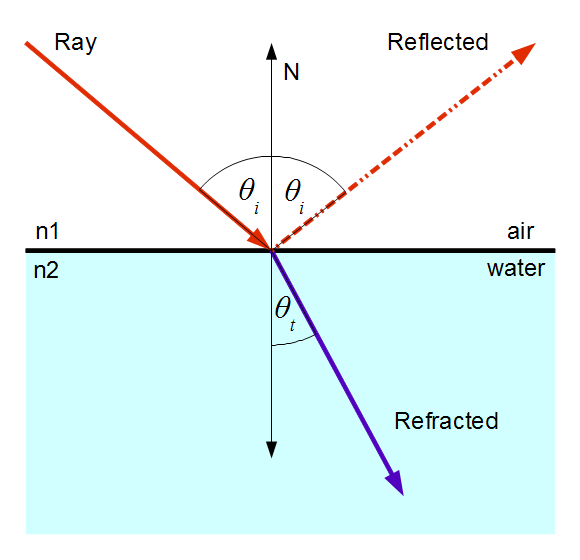
Fig.5. 5: Refraction of light from air to water and water to air for comparison.
The change in its direction is associated with the change in velocity. The ratio of
the speed of light in the vacuum c (or air) and that of light in a certain mediumv is called the absolute refractive index n.
5.1.3 Total internal reflection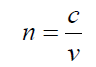
When light passes from one a medium of higher index of refraction into a medium
of lower refractive index the light bends away from the normal as indicated on
Fig.6.6. A weak internally reflected ray is also formed and its intensity increasesas the incident angle increases.
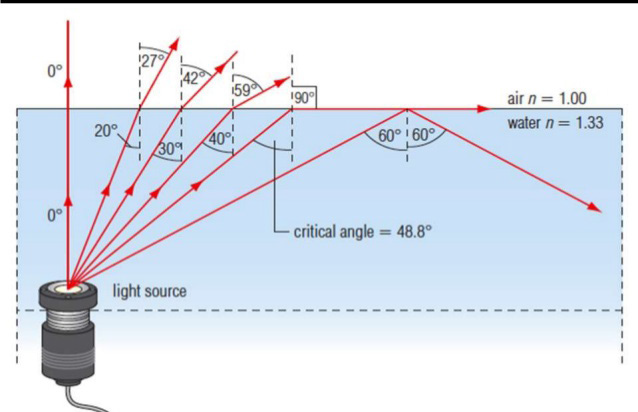
Fig.5. 6: Illustration of total internal reflection
Increasing the angle of incidence increases the angle of refraction and at a
particular incidence, the angle of refraction reaches the 90°. This particular
incident angle is called the critical angle θc. As the incident angle exceeds the
critical angle, the incident beam reflects on the interface between the 2 media
and return in the first medium. This effect is called total internal reflection.For any two media, using Snell’s law the critical angle is calculated using the
expression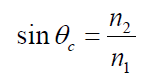
where n1 and n2 are respectively the refractive indices of the first and second
media.θc increases when approaches n1 .
EXAMPLE 5.1
Applying the above relation to the critical ray at a glass-air boundary we
have where index of glass is ng =1.50.
Answer
A beam of light is propagating through diamond, n = 2.42 and strikes a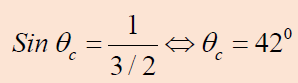
diamond-air interface at an angle of incidence of 28°.
Will part of the beam enter the air or will the beam be totally refracted
at the interface?
Repeat part (a) assuming that diamond is surrounded by water, n = 1.33
Answer:
Since 28° is greater thanθC , total internal reflection will occur, there is norefraction.
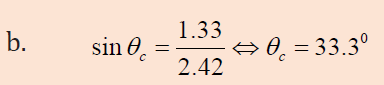
Since 28° is less than θC some light will undergo refraction into the water.
Application:
An optical fiber is basically made of 2 types of glass put together in a concentric
arrangement so the middle is hollow. The inner circle of glass also called the
Core consists of a glass of higher refractive index than the outside layer asindicated on fig.5.4.
Fig.5. 7: Total internal reflection in optical fiber as the angle of incidence θ is greater than the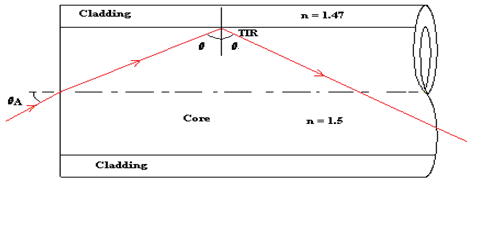
critical angle.
The outer layer of glass, which is also known as the optical cladding, does not
carry light but is essential to maintain the critical angle of the inner glass. The
underlying main physics concept behind the functioning of an optical fiber is a
phenomenon known as total internal reflection.
Any light entering the fiber will meet the cladding at an angle greater than
the critical angle. If light meets the inner surface of the cladding or the core -
cladding interface at greater than or equal to critical angle then total internal
reflection (TIR) occurs. So all the energy in the ray of light is reflected back into
the core and none escapes into the cladding. The ray then crosses to the other
side of the core and, because the fiber is more or less straight, the ray will meet
the cladding on the other side at an angle which again causes the total internal
reflection. The ray is then reflected back across the core again and again until it
reaches the end of the optical fiber.
Maximum angle of incidence
The maximum angle of incidence in air for which all the light is total reflected
at the core-cladding is given by: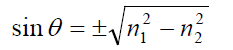
EXAMPLE 6.2
1. An optical fibre consists of an inner material (the fiber) with refractive
index nf and an outer material of lower refractive index nc, known ascladding, as in Fig. 6.6 below.
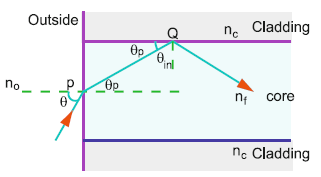
Fig.5. 6
a. What is the purpose of cladding?b. Show that the maximum acceptance angle θmax is given
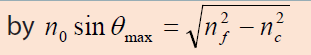
c. Discuss two main fiber loss mechanisms.
Answer
The purpose of the cladding is to improve the transmission efficiency
of the optical fibre. If cladding is not used then the signal is attenuated
dramatically.
Let a ray be incident at an angle θ , Fig.6.6, the angle of refraction at P
being θp Let C be the critical angle at Q, interface of core and cladding
(in this case θC =θmax )
Refraction from air to core:
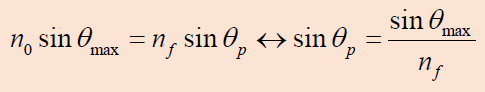
This shows that there is a maximum angle of acceptance cone outside of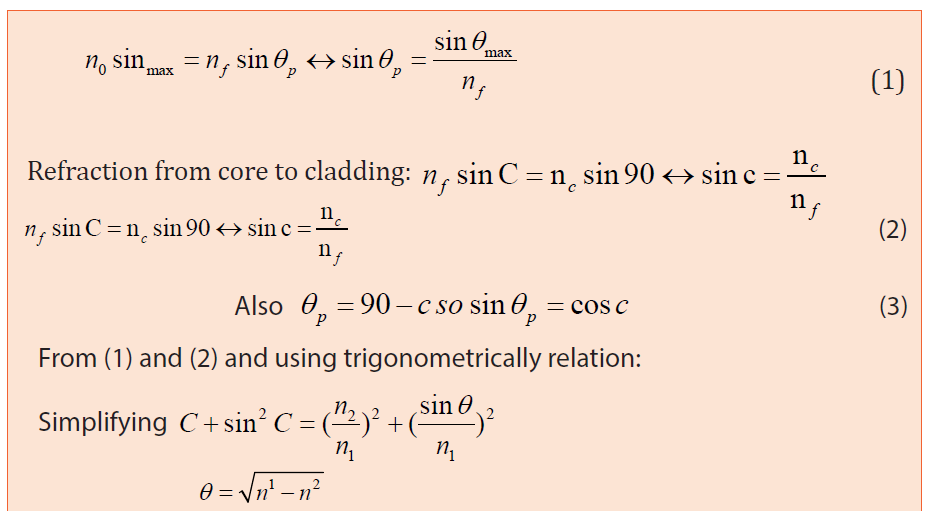
which entering rays will not be totally reflected within the fiber. For the
largest acceptance cone, it is desirable to choose the index of refraction of the
cladding to be as small as possible. This is achieved if there is no cladding at
all. However, this leads to other problems associated with the loss of intensity.
d. The transmission is reduced due to multiple reflections and the absorption
of the fibre core material due to impurities.
2. A step-index fiber 0.01 cm in diameter has a core index of 1.53 and a
cladding index of 1.39. See Fig.5.7. Such clad fibers are used frequently inapplications involving communication, sensing, and imaging.
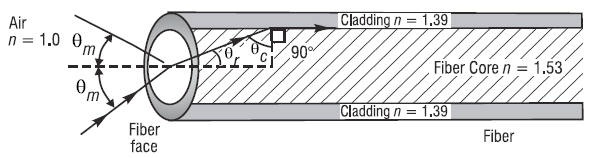
Fig.5. 7
What is the maximum acceptance angle θmfor a cone of light rays incident on
the fiber face such that the refracted ray in the core of the fiber is incident onthe cladding at the critical angle?
5.1.4 Checking my progress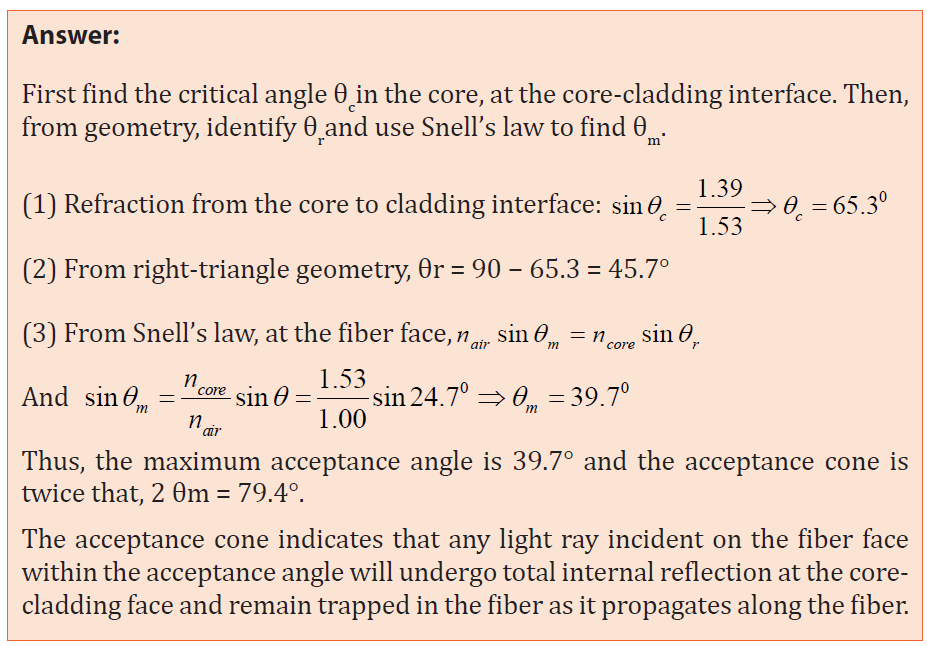
1. Operation of optical fiber is based on:
a. Total internal reflection
b. Total internal refraction
c. Snell’s law
d. Einstein’s theory of reality
e. None of the above
2. When a beam of light passes through an optical fiber
a. Rays are continually reflected at the outside(cladding) of the fiber
b. Some of the rays are refracted from the core to the cladding
c. The bright beam coming out of the fiber is due to the high refractive
index of the core
d. The bright beam coming out of the fiber is due to the total internal
reflection at the core-cladding interface
e. All the rays of light entering the fiber are totally reflected even at very
f. small angles of incidence
3. A laser is used for sending a signal along a mono mode fiber because
a. The light produced is faster than from any other source of light
b. The laser has a very narrow band of wavelengths
c. The core has a low refractive index to laser light
d. The signal is clearer if the cladding has a high refractive index
e. The electrical signal can be transferred quickly using a laser
4. Given that the refractive indices of air and water are 1 and 1,33,
respectively, find the critical angle.
5. The frequency of a ray of light is 6.0x1014 Hz and the speed of light in air is
3x108 m/s. the refractive index of the glass is 1.5.
a. Explain the meaning of refracting index
b. A ray of light has an angle of incidence of 30° on a block of quartz and an
angle of refraction of 20°. What is the index of refraction of the quartz?
6. A beam of light passes from water into polyethylene (n = 1.5). If θi = 57.5°,
what is the angle of refraction?
7.
a. What is the critical angle when light is going from a diamond (n= 2.42)
to air?
b. Using the answer to (a), what happens when:
I. The angle of incidence is less than that angle?II. The angle of incidence is more than that angle
5.2 TYPES OF OPTICAL FIBERS
ACTIVITY 5.2: Investigating the types of optical fiber.
Use search internet and discuss different types of optical fiber. Then,
differentiate them according to their respective uses.
There are three main types of Optical Fibers: Monomode (or single
mode), Multimode and special purpose optical fibers.
5.2.1 Monomode fibers
Those are Fibers that support a single mode and are called single-mode
fibers (SMF). Single-mode fibers are used for most communication links longerthan 1 000 m.

Fig.5. 8: Structure of monomode or single-mode optical fiber
In the monomode fiber, the core is only about 8 μm in diameter, and only
the straight through transmission path is possible, i.e. one mode. This type,
although difficulty and expensive to make, is being used increasingly. For short
distances and low bit-rates, multimode fibers are quite satisfactory. Following
the emergence of single-mode fibers as a viable communication medium in
1983, they quickly became the dominant and the most widely used
fiber type within Telecommunications. Major reasons for this situation are
as follows:
1. They exhibit the greatest transmission bandwidths and the lowest losses of
the fiber transmission media.
2. They have a superior transmission quality over other fiber types
because of the absence of modal noise.
3. They offer a substantial upgrade capability (i.e. future proofing) for future
wide- bandwidth services using either faster optical transmitters or
receivers or advanced transmission techniques (e.g. coherent technology,).
4. They are compatible with the developing integrated optics technology.
5. The above reasons 1 to 4 provide confidence that the installation of singlemode
fiber will provide a transmission medium which will have adequate
performance such that it will not require replacement over its anticipated
lifetime of more than 20 years. (John, 2009)
5.2.2 Multimode fiers
In multimode fier, light travels through the fier following diffrent light paths
called “modes” as indicated on Fig.5.9. Those are fiers that support many
propagation paths. A multi-mode optical fier has a larger core of about 50 μm,
allowing less precise, cheaper transmitters and receivers to connect to it aswell as cheaper connectors
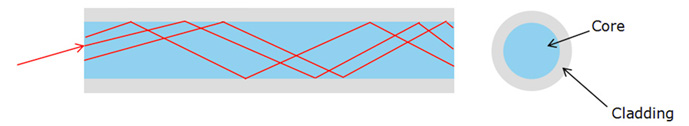
Fig.5. 9 Multimode optical fiber
The propagation of light through a multimode optical fiber is shown on Fg.
5.9. However, a multi-mode fiber introduces multimode distortion, which
often limits the bandwidth and length of the link. Furthermore, because of its
higher dopant content, multi-mode fibers are usually expensive and exhibit
higher attenuation.
There are two types of multi-mode optical fibers: multimode step-index andmultimode graded index (see Fig.5.10)
Fig.5. 10: Step index and graded index multimode optical fibers illustration.
• In step-index multimode type, the core has the relatively large
diameter of 50μm and the refractive index changes suddenly at the
cladding. The wide core allows the infrared to travel by several paths
or modes. Paths that cross the core more often are longer, and signals
in those modes take longer to travel along the fiber. Arrival times at the
receiver are therefore different for radiation of the same pulse, 30ns
km-1, being a typical difference. The pulse is said to suffer dispersion,
it means that it is spread out.
• In the graded index multimode type, the refractive index of the glass
varies continuously from a higher value at the center of the fiber to a
low value at the outside, so making the boundary between core and
the cladding indistinct. Radiation following a longer path, travel faster
on average, since the speed of light is inversely proportional to the
refractive index. The arrival times for different modes are the about
the same (to within 1ns km-1) and all arrive more or less together at the
receiving end. Dispersion is thereby much reduced.
5.2.3 Special-purpose optical fiber
Some special-purpose optical fiber is constructed with a non-cylindrical
core and/or cladding layer, usually with an elliptical or rectangular crosssection.
These include: polarization-maintaining fiber and fiber designed to
suppress whispering gallery mode propagation.
• Polarization-maintaining fiber is a unique type of fiber that is
commonly used in fiber optic sensors due to its ability to maintain the
polarization of the light inserted into it.
• Photonic-crystal fiber is made with a regular pattern of index
variation. It is often in the form of cylindrical holes that run along the
length of the fiber. Such fiber uses diffraction effects in addition to total
internal reflection, to confine light to the fiber’s core.
5.2.4 Checking my progress
1. Fiber optics is best known for its application in long-distance
telecommunications.
a. True
b. False
2. Choose the basic types of optical fiber:
a. Single-mode e. Multi-mode
b. X-mode f. A and C
c. Microwave-mode g. B and D
d. Graded-index mode h. A and E
3. Single-mode fiber has the advantage of greater bandwidth capability. It
has the disadvantage of:
a. Being harder to bend
b. Smaller mechanical tolerances in connectors and splices
c. Being difficult to couple light into
d. B and C
e. None of the above
4. Describe with the aid of simple ray diagrams:
a. The multimode step index fiber;
b. The single-mode step index fiber.
c. Compare the advantages and disadvantages of these two types of fiberfor use as an optical channel.
5.3 Mechanism of attenuation
ACTIVITY 5.3: Light transmission analysis in optical fiber
Fig.5. 11 The images to show the attenuation in optical fiber
Observe the image clearly, and answer to the following questions:
1. Does all the light from the source getting to the destination?
2. What do you think is causing the loss in light transmission?
3. What can be done to minimize that loss in the optical fibers above?
Attenuation in fiber optics, also known as transmission loss, is the reduction
in intensity of the light beam (or signal) as it travels through the transmission
medium. Over a set distance, fiber optic with a lower attenuation will allow
more power to reach its receiver than a fiber with higher attenuation.
Attenuation can be caused by several factors both extrinsic and intrinsic:
• Intrinsic attenuation is due to something inherent to the fiber such as
impurities in the glass during manufacturing. The interaction of such
impurities with light results in the scattering of light or its absorption.
• Extrinsic attenuation can be caused by macro bending and
microlending. A bent imposed on an optical fiber produce a strain in
that region of the fiber and affects its refractive index and the critical
angle of the light ray in that area. Macrobending that is a large-scale
bent and microbending which is a small-scale bent and very localized
are external causes that result in the reduction of optical power.
Attenuation coefficients in fiber optics usually are expressed decibels per
kilometer (dB/km) through the medium due to the relatively high quality of
transparency of modern optical transmission media. It is observed that the
attenuation is a function of the wavelength of the light. The attenuation αtot (λ )
at wavelength λ of a fiber between two cross-sections, 1 and 2, separated bydistance Lis defined, as
where
P1 λ optical power at the cross-section 1, and
P2 λ the optical power
at the cross-section 2. Attenuation is an important limiting factor in the
transmission of a digital signal across large distances. Thus, much research has
gone into both limiting the attenuation and maximizing the amplification of the
optical signal.
5.3.1 Light scattering and absorption
In the light transmission of signals through optical fibers, attenuation occurs
due to light scattering and absorption of specific wavelengths, in a manner
similar to that responsible for the appearance of color.
a. Light scatteringScattering losses
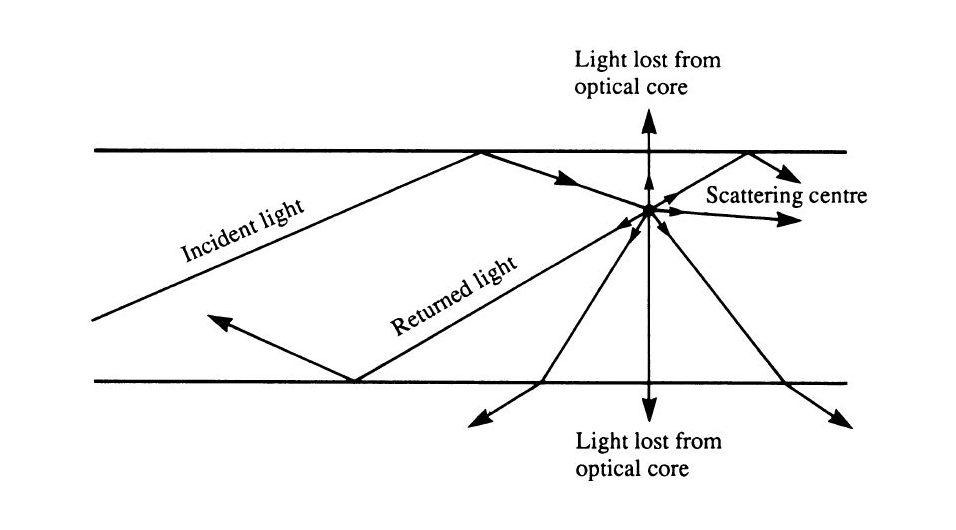
Fig.5. 12: Light scattering in optical fiber
The propagation of light through the core of an optical fiber is based on total
internal reflection of the light wave. Rough and irregular surfaces, even at the
molecular level, can cause light rays to be reflected in random directions as it
is illustrated on Fig.5.12. This is called diffuse reflection or scattering, and it is
typically characterized by wide variety of reflection angles.
Light scattering depends on the wavelength of the light being scattered.
Thus, limits to spatial scales of visibility arise, depending on the frequency
of the incident light-wave and the physical dimension (or spatial scale) of the
scattering center, which is typically in the form of some specific micro-structural
feature. Since visible light has a wavelength of the order of one micrometer (one
millionth of a meter) scattering centers will have dimensions on a similar
spatial scale. Thus, attenuation results from the incoherent scattering of light
at internal surfaces and interfaces.
b. Light absorption
Material absorption is a loss mechanism related to the material composition
and fiber fabrication process. This results in the dissipation of some transmitted
optical power as heat in the waveguide. Absorption is classified into two basic
categories: Intrinsic and extrinsic absorptions. (John, 2009)
Intrinsic absorption: is caused by basic fiber material properties. If an optical
fiber is absolutely pure, with no imperfections or impurities, ten all absorption
will be intrinsic. Intrinsic absorption in the ultraviolet region is caused bands.
Intrinsic absorption occurs when a light particle (photon) interacts with an
electron and excites it to a higher energy level.
5.3.2 Measures to avoid Attenuation
The transmission distance of a fiber-optic communication system has
traditionally been limited by fiber attenuation and by fiber distortion.
• Repeaters: Repeaters convert the signal into an electrical signal, and
then use a transmitter to send the signal again at a higher intensity
than was received, thus counteracting the loss incurred in the previous
segment. They mostly used to be installed about once every 20 km.
• Regenerators: Optical fibers link, in common with any line
communication system, have a requirement for both jointing and
termination of the transmission medium. When a communications
link must span at a larger distance than existing fiber-optic technology
is capable of, the signal must be regenerated at intermediate points
in the link by optical communications repeaters called regenerators.
An optical regenerator consists of optical fibers with special coating
(doping). The doped portion is pumped with a laser. When the
degraded signal comes into the doped coating, the energy from the
laser allows the doped molecules to become lasers themselves. The
doped molecules then emit a new strong light signal with the same
characteristics as the incoming weak signal. Basically, the regenerator
is a laser amplifier for the incoming signal.
• Optical Amplifiers: Another approach is to use an optical
amplifier which amplifies the optical signal directly without having to
convert the signal into the electrical domain. It is made by doping a
length of fiber with the rare-earth mineral erbium and pumping it with
light from a laser with a shorter wavelength than the communications
signal (typically 980 nm). Amplifiers have largely replaced repeaters in
new installations.
5.3.3 Checking my progress
1. True or False: One of the reasons fiber optics hasn’t been used in more
areas has been the improvement in copper cable such as twisted pair.
2. True or False: With current long-distance fiber optic systems using
wavelength-division multiplexing, the use of fiber amplifiers has
become almost mandatory.
3. Fiber optics has extraordinary opportunities for future applications
because of its immense bandwidth.
a. True
b. False
4. a. What do we mean by attenuation in optical fibers?
c. State two ways in which energy is lost in optical fibers.
d. If a fiber loses 5% of its signal strength per kilometer, how much ofits strength would be left after 20 km?
5.4 OPTICAL TRANSMITTER AND OPTICAL RECEIVER
ACTIVITY 5.4: Investigating the signal sources and signal receiver
for optic fibers
1. With the basic information you know about the functioning process
of optical fiber, answer to the following questions.
2. Where does the light that is transmitted into the optical fiber core
medium come from?
3. What are the type compositions of the light signal propagating into
optical fiber?
4. Discuss and explain the function principle of signal generators and
signal receivers of light from optical fibers.
The process of communicating using fier-optics involves the following basic
steps:
1. Creating the optical signal involving the use of a transmitter, usually from
an electrical signal.
2. Relaying the signal along the fier, ensuring that the signal does not
become too distorted or weak.
3. Receiving the optical signal.4. Converting it into an electrical signal
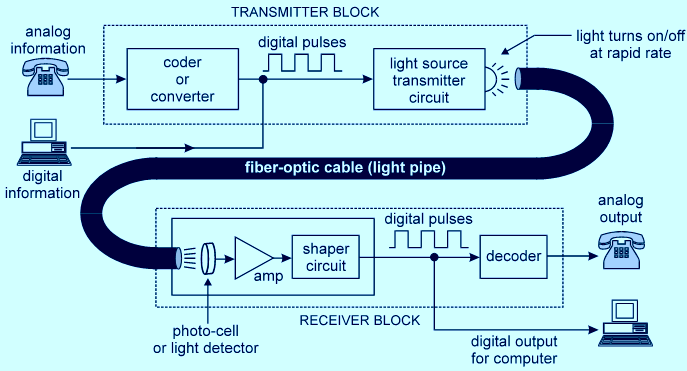
Fig.5. 13: Optical fiber communication mechanism (Transmitter and receiver blocks).
5.4.1 Transmitters
The most commonly used optical transmitters are semiconductor devices
such as light-emitting diodes (LEDs) and laser diodes. The difference between
LEDs and laser diodes is that LEDs produce incoherent light, while laser diodes
produce coherent light. For use in optical communications, semiconductor
optical transmitters must be designed to be compact, efficient and reliable,
while operating in an optimal wavelength range and directly modulated at high
frequencies (see Fig.5.13: Transmitter block).
In its simplest form, a LED is a forward-biased p-n junction, emitting
light through spontaneous emission, a phenomenon referred to
as electroluminescence. The emitted light is incoherent with a relatively wide
spectral width of 30–60 nm. LED light transmission is also inefficient, with
only about 1% of input power, or about 100 microwatts, eventually converted
into launched power which has been coupled into the optical fiber. However, due
to their relatively simple design, LEDs are very useful for low cost applications.
5.4.2 The Optical Receivers
The main component of an optical receiver is a photodetector (photodiode)
which converts the infrared light signals into the corresponding electrical
signals by using photoelectric effect before they are processed by the decoder
for conversion back into information. The primary photo detectors fortelecommunications are made from Indium gallium arsenide (see Fig.5.13).
The photodetector is typically a semiconductor-based photodiode. Several
types of photodiodes include p-n photodiodes, p-i-n photodiodes, and avalanche
photodiodes. Metal-semiconductor-metal (MSM) photodetectors are also used
due to their suitability for circuit integration in regenerators and wavelengthdivision
multiplexers.
5.4.3 Checking my progress
1. Circle the three basic components in a fiber optic communications system.
a. Telescope e. Maser fiber
b. Transmitter f. Optical fiber
c. Receiver G. Alternator
d. Surveillance satellites
2. Information (data) is transmitted over optical fiber by means of:
a. Light d. Acoustic waves
b. Radio waves e. None of the above
c. Cosmic rays
3. Connectors and splices add light loss to a system or link.
a. True
b. False
4. Do fibers have losses?
5.5. USES OF OPTICAL FIBERS
ACTIVITY 6.5: Applications of fiber optics in telecommunication
and in medicine
Use the internet or the library to investigate the applications of optical
fiber in medicine and telecommunication systems.
5.5.1. Telecommunications Industry
Optical fibers offer huge communication capacity. A single fiber can carry the
conversations of every man, woman and child on the face of this planet, at the
same time, twice over. The latest generations of optical transmission systems
are beginning to exploit a significant part of this huge capacity, to satisfy the
rapidly growing demand for data communications and the Internet.
The main advantages of using optical fibers in the communications industry
are:
1. A much greater amount of information can be carried on an optical fiber
compared to a copper cable.
2. In all cables some of the energy is lost as the signal goes along the cable.
The signal then needs to be boosted using regenerators. For copper cable
systems these are required every 2 to 3km but with optical fiber systems
they are only needed every 50km.
3. Unlike copper cables, optical fibers do not experience any electrical
interference. Neither will they cause sparks so they can be used in explosive
environments such as oil refineries or gas pumping stations.
4. For equal capacity, optical fibers are cheaper and thinner than copper
cables and that makes them easier to install and maintain.
5.5.2 Medicine Industry
The advent of practicable optical fibers has seen the development of much
medical technology. Optical fibers have paved the way for a whole new field of
surgery, called laproscopic surgery (or more commonly, keyhole surgery), which
is usually used for operations in the stomach area such as appendectomies.
Keyhole surgery usually makes use of two or three bundles of optical fibers.
A “bundle” can contain thousands of individual fibers”. The surgeon makes a
number of small incisions in the target area and the area can then be filled with
air to provide more room.
One bundle of optical fibers can be used to illuminate the chosen area, and
another bundle can be used to bring information back to the surgeon. Moreover,
this can be coupled with laser surgery, by using an optical fiber to carry the
laser beam to the relevant spot, which would then be able to be used to cut the
tissue or affect it in some other way.
5.5.3 Checking my progress
The basic unit of digital modulation is:
a. Zero c. A and Bb. One d. None of the above
5.6 ADVANTAGES AND DISADVANTAGES OF OPTICAL FIBERS
ACTIVITY 5.6: Advantages and disadvantages of optical fibers
Use search internet or your library to investigate the advantages and
disadvantages of fiber optics.
Although there are many benefits to using optical fibers, there are also
some disadvantages. Both are discussed below:fiber in medicine and
telecommunication systems.
5.6.1 Advantages
• Capacity: Optical fibers carry signals with much less energy loss than
copper cable and with a much higher bandwidth. This means that
fibers can carry more channels of information over longer distances
and with fewer repeaters required.
• Size and weight: Optical fiber cables are much lighter and thinner than
copper cables with the same bandwidth. This means that much less
space is required in underground cabling ducts. Also they are easier for
installation engineers to handle.
• Security: Optical fibers are much more difficult to tap information from
undetected; a great advantage for banks and security installations.
They are immune to electromagnetic interference from radio signals,
car ignition systems, lightning etc. They can be routed safely through
explosive or flammable atmospheres, for example, in the petrochemical
industries or munitions sites, without any risk of ignition.
• Running costs: The main consideration in choosing fiber when
installing domestic cable TV networks is the electric bill. Although
copper coaxial cable can handle the bandwidth requirement over the
short distances of a housing scheme, a copper system consumes far
more electrical power than fiber, simply to carry the signals.
5.6.2 Disadvantages
• Price: In spite of the fact that the raw material for making optical fibers,
sand, is abundant and cheap, optical fibers are still more expensive per
metre than copper. Having said this, one fiber can carry many more
signals than a single copper cable and the large transmission distances
mean that fewer expensive repeaters are required.
• Special skills: Optical fibers cannot be joined together (spliced) as an
easily as copper cable and requires additional training of personnel
and expensive precision splicing and measurement equipment.
5.6.3 Checking my progress
1. List two advantages of using optical fiber. __________________________
2. The replacement of copper wiring harnesses with fiber optic cabling
will increase the weight of an aircraft.
a. Trueb. False
END UNIT ASSESSMENT 5
1. a. An endoscope uses coherent and non−coherent fiber bundle
I. State the use of the coherent bundle and describe its arrangement
of fibers.
II. State the use of the non−coherent bundle and describe its
arrangement of fibers.
b. Each fiber has a core surrounded by cladding. Calculate the critical
angle at the core−cladding interface.
Refractive index of core = 1.52
Refractive index of cladding = 1.
2. (a)Fig.5.9 shows a ray of light travelling through an individual fiber
consisting of cladding and a core. One part has a refractive index of1.485 and the other has a refractive index of 1.511.
Fig.5. 9: Light transmission in optical fiber.
I. State which part of the fiber has the higher refractive index and
explain why.
II. (ii) Calculate the critical angle for this fiber.
(b) The figure below shows the cross-section through a clad opticalfiber which has a core of refractive index 1.50.
Complete the graph below to show how the refractive index changes
with the radial distance along the line ABCD in the figure above.
Fig.5. 11: Axes for the half life decay curve
3.
a. What do we mean by attenuation in optical fibers?
b. State two ways in which energy is lost along the length of an
optical fiber.
c. If a fiber loses 5% signal strength per km, how much strength
would be left after 20 km?
4. Estimate the length of time it would take a fiber optic system to
carry a signal from the UK to the USA under the Atlantic. (Take c =
2 x 108 m/s in the cable. Estimate the length of the cable under the
sea.
a. Estimate the length of time it would take a microwave signal to
travel from the UK to the USA a satellite Enk. (Geosynchronous
satellites orbit at a height of about 36 000 Ian above the Earth’s
surface.b. Which would give less delay in a telephone conversation?
UNIT 6 BLOCK DIAGRAM OF TELECOMMUNICATION
Key unit competence: Construct and analyze block diagram of
telecommunication systems.
My goals
• Identify parts of a block diagram of telecommunication system.
• Differentiate oscillator, modulator and amplifier.
• Outline the function of a microphone and antenna.
• Describe terms applied in telecommunication systems
• Construct, analyse and judge block diagrams of a telecommunication
system.
• Realise that parts of a telecommunication system are dependent
INTRODUCTORY ACTIVITY
Investigating the function of wireless microphone
Materials:
• Wireless microphone set
• Amplifier and mixer
• Connecting wires
• Speaker
Procedure:
Connect the full sound system such that the signal will be transmitted to the
speakers using wireless microphone.
Questions:
1. How is your voice getting to the speakers?
2. Where else this system is used?3. What are advantages and disadvantages of communication?
6.1. OPERATING PRINCIPLE OF MICROPHONE
ACTIVITY 6.1: Investigating the function of a microphone
Take the case of two people talking on telephone (see Fig.6.1). Observethe image below and answer to the following questions:

Fig.6. 1 People talking on telephone
1. Discuss the functioning process of a telephone.2. Differentiate the functions of a microphone from that of a speaker.
Telecommunication in real life is the transmission of signals and other types
of data of any nature by wire, radio, optical or other electromagnetic systems of
communication. Telecommunication occurs when the exchange of information
between communicating participants includes the use of signs or other
technologically based materials such as telephone, TV set, radio receiver, radio
emitter, computer, and so on. All can be done either mechanically, electrically
or electronically.
The use of microphones began with the telephone in the nineteenth century.
The requirements were basically those of speech intelligibility, and the carbon
microphone, developed early in that art, is still used in telephones today.
Particles of carbon are alternately compressed and relaxed by the diaphragm
under the influence of sound pressure, and the resulting alternation of
resistance modulates the current proportionally to the change in resistance.
Carbon microphones are noisy; they have limited dynamic range and produce
high levels of distortion. However, none of these defects is really serious in its
application to telephony.
Operating principle of microphones
A microphone converts sound vibrations into electrical entity. Basically a
microphone has a diaphragm which moves when sound pressure pushes it. This
movement can be converted into proportional voltage using several possibletransducers.
Fig.6. 2: The outer and internal view of a microphone
A transducer is a device which receives electrical, mechanical or acoustic
waves from one medium and converts them into related waves for a similar or
different medium. Thus, it can be said that a microphone is a transducer that
converts acoustical sound energy into electrical energy. Its basic function is
therefore to convert sound energy into electrical audio signals which can be
used for further processing. Microphones are classified based on constructionand directivity.
6.2 CHANNELS OF SIGNAL TRANSMISSION
ACTIVITY 6.2: Investigating signal transmission
Basing on the activity 6.1, explain and discuss how the voices are
transmitted from our mouth to telephone and then to the receiver’s
telephone
An audio frequency (acronym: AF) or audible frequency is characterized as a
periodicvibration whose frequency is audible to the average human. The SI unit
of frequency is the hertz (Hz). It is the property of sound that most determines
pitch. The generally accepted standard range of audible frequencies is 20Hz
to 20 kHz, although the range of frequencies that individuals hear is greatly
influenced by environmental factors. Frequencies below 20 Hz are generally
felt rather than heard, assuming the amplitude of the vibration is great enough.
Frequencies above 20 kHz can sometimes be sensed by young people. High
frequencies are the first to be affected by hearing loss due to age and/or
prolonged exposure to very loud noises.
Modulation is the process of superimpose to a low frequency signal (original
signal) a high frequency signal (carrier signal) for transmission. The resulting
signal is a modulated or radio signal.
6.2.1 Amplitude modulation (AM)
It is a type of modulation, where the amplitude of the carrier wave is changed
in accordance with the intensity of the signal. However, the frequency and thephase shift of the modulated wave remains the same.
Fig.6. 3 A graphs of amplitude modulation
Note that the amplitudes of both positive and negative half-cycles of carrier
wave are changed in accordance with the signal. For instance, when the signal
is increasing in the positive sense, the amplitude of carrier wave also increases.
On the other hand, during negative half-cycle of the signal, the amplitude of
carrier wave decreases. Amplitude modulation is done by an electronic circuitcalled modulator.
6.2.2 Frequency modulation (FM).
It is a type of modulation, where the frequency of the carrier wave is changed
in accordance with the intensity of signal. The amplitude and the phase shift of
the modulated wave remain the same. The frequency variations of carrier wave
depend upon the instantaneous amplitude of the original signals. The carrier
frequency increases and decreases respectively to its positive and negative peakvalues as the voltage of the original signal seem to approach its peak values.
Fig.6. 4: Process of FM transmission
Comparison of amplitude modulation and frequency modulation
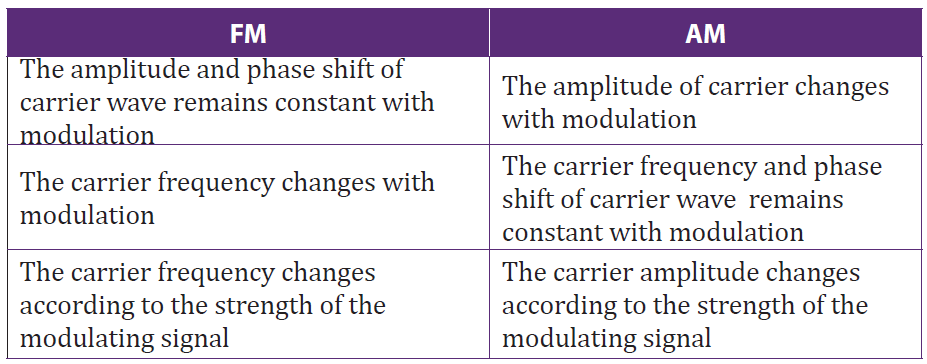
Table 6. 1: Comparison between FM and AM
6.2.3 Short wave (SW)
A short wave is any wave whose frequency ranges between 300 kHz and 3 MHz.
In transmission, these waves are used for very long distance communication as
their bands can be reflected or refracted from the ionosphere by an electricallycharged layer with atoms in the atmosphere.
Fig.6. 5: Short wave illustration
The short waves directed at an angle into the sky can be reflected back to Earth at
great distances, beyond the horizon. This called sky wave or skip propagation.
These waves are used for radio broadcasting of voice and music to shortwave
listeners over very large areas; sometimes entire continents or beyond. They
are also used for military communication, diplomatic communication, and twoway
international communication by amateur radios enthusiasts for hobby.
6.2.4 Medium wave (MW)
Medium wave (MW) is the part of the medium frequency (MF) radio band
used mainly for AM radio broadcasting. It is the original radio broadcasting
band, in use since the early 1920’s. It is typically used by stations serving a local
or regional audience. At night, medium wave signals are no longer absorbed by
the lower levels of the ionosphere, and can often be heard hundreds or even
thousands of miles away.
For Europe the MW band ranges from 526.5 kHz to 1606.5 kHz, using channels
spaced every 9 kHz, and in North America an extended MW broadcast band
ranges from 525 kHz to 1705 kHz, using 10 kHz spaced channels.
6.2.5 Checking my progress
1. In transmission, the range of short waves are between
a. Radio wave and microwave
b. X-rays and gamma rays
c. Infrared and visible light
d. Infrared and ultraviolet
2. Where Short waves can be used?
3. Explain what is meant by Medium wave (MW)
4. Distinguish between Amplitude modulation and frequency modulation
6.3 CARRIER WAVE AND MODULATOR
6.3.1 Concept of carrier wave modulation
ACTIVITY 6.3: Modulation techniquesWhat are applications of such a system shown in the below figure?
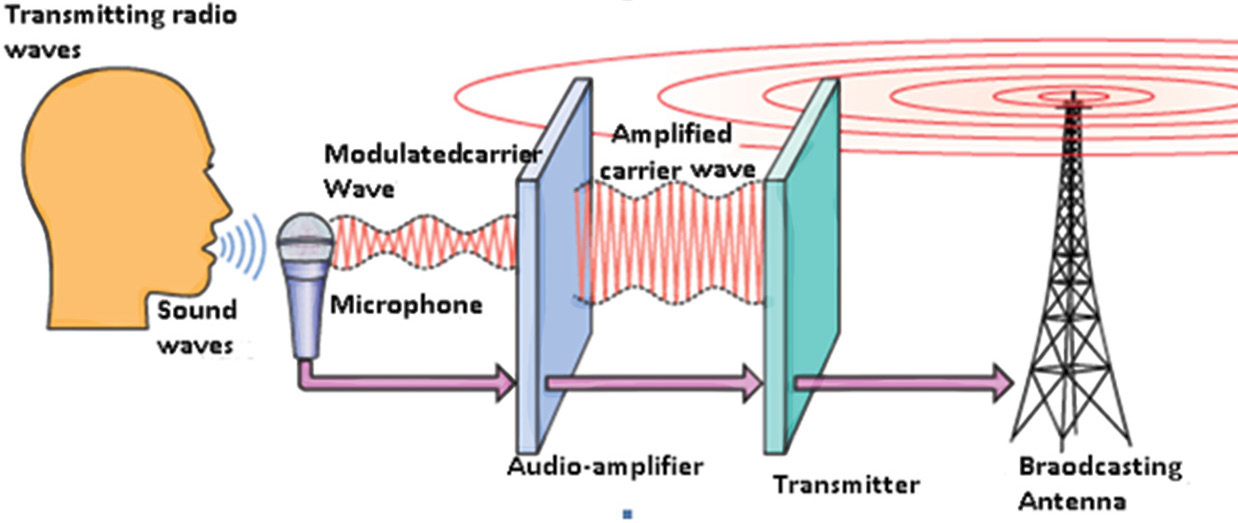
Fig.6. 6: Radio wave transmission
Observe the mechanism above (Fig.6.6) and answer to the following
questions
1. Analyze the provided figure and explain the transmission process
used there.2. What are applications of such a system shown in the above figure?
Modulation is the process of varying the characteristics of carrier signal with
the modulating signal or modulation is defined as the superimposition of low
frequency baseband signal (modulating signal) over high frequency carrier
signal by varying different parameters of the carrier signals (see Fig.6.6). Based
on the types of parameters that are varied in proportion to the baseband (low
frequency) signal, modulation is of different types. In digital modulation, the
message signal is converted from analog into digital. In digital modulation
techniques, the analog carrier signal is modulated by discrete signal. The carrier
wave is switched on and off to create pulses such that signal is modulated.
Low frequency signal (Baseband) communication is not commonly used for
distance communication. Low frequency baseband signals, having low energy,
if transmitted directly will get distorted. So baseband signal must be modulated
with high frequency signal to increase the range of transmission.
6.3.2 Checking my progress
1. In………transmission, the carrier signal modulated so that its amplitude
varies with the changing amplitudes of the modulating signal
a. AM c. FM
b. PM d. None of the above?
2. Distinguish between analog signal and digital signal?
3. What is meant by carrier wave in telecommunication?4. What is the application of a carrier wave in a telecommunication system?
6.4 OSCIALLATOR, RADIO FREQUENCY AMPLIFIER AND
POWER AMPLIFIER
ACTIVITY 6.4: Investigating what is an oscillator and a radio
frequency amplifier
Make an intensive research on the properties and function of an
oscillator and radio frequency amplifier. According to your findings,
answer to the following questions:
1. Explain a radio frequency amplifier and state its importance in
telecommunication?
2. What do you understand by an oscillator in telecommunication
system? Discus its importance?3. Describe other uses of oscillator and radio frequency amplifier.
6.4.1 Oscillator
Oscillators are electronic circuits that produce a periodic waveform on its
output with only the DC supply voltage as an input. The output voltage can
be either sinusoidal or non-sinusoidal, depending on the type of oscillator, thus,
the outputs signals can be sine waves, square waves, triangular waves, and sawtooth waves.
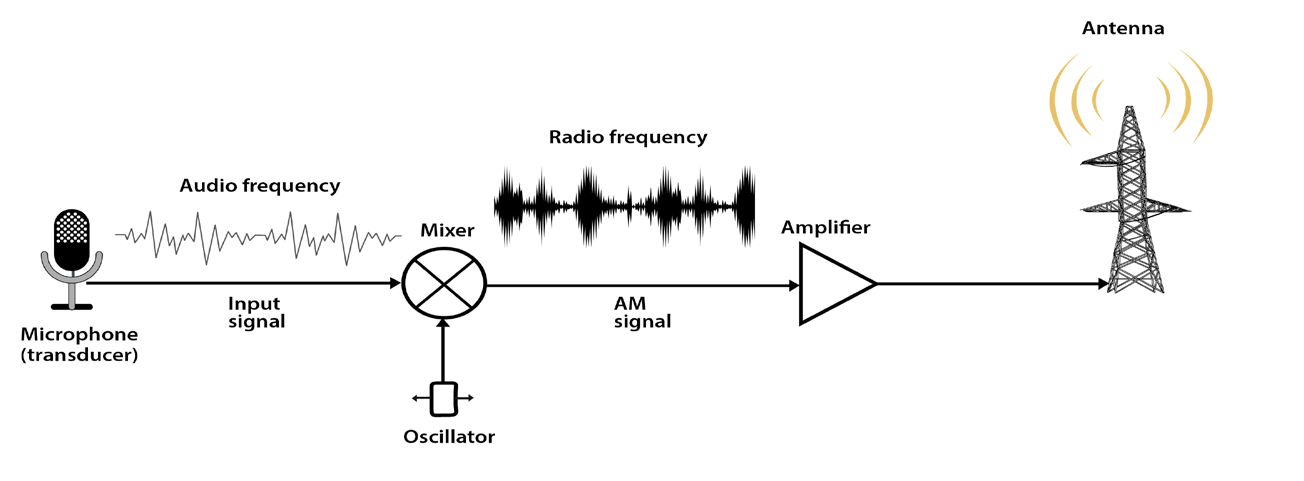
Fig.6.7: Basic function of oscillator and radio frequency amplifier in telecommunication system
The oscillation in any circuit will depend on the following properties:
• Amplification of the used amplifier
• A frequency determining device (receiver/ transmitter)
• Signal regeneration (Positive feedback)
Factors which may fluctuate the operating frequency
• Long time of operation
• Heat that is generated along the operation
• Operating point of the active elements
• Frequency dropper elements (capacitors, inductors)
• Change in total opposition faced by the alternating current (impedance)
Oscillators may be classified in three ways, including:
a. The design principle used where we have a positive and a negative
feedback oscillators,
b. The frequency range of the signal over which they are used:
• Audio Frequency (AF) oscillators (frequency range is 20 Hz to 20 kHz)
• Radio Frequency (RF) oscillators (frequency range is 20 kHz to 30
MHz)
• Video Frequency oscillators (frequency range is dc to 5 MHz)
• High Frequency (HF) oscillators (frequency range is 1.5 MHz to 30
MHz)
• Very High Frequency (VHF) oscillators (frequency range is 30 MHz to
300 MHz)
c. The nature of generated signals where we have:
• Sinusoidal Oscillators: These are known as harmonic oscillators and
are generally LC tuned-feedback or RC tuned-feedback type oscillator
that generates a sinusoidal waveform which is of constant amplitude
and frequency.
• Non-sinusoidal Oscillators: These are known as relaxation oscillators
and generate complex non-sinusoidal waveforms that changes very
quickly from one condition of stability to another such as square-wave,triangular-wave or sawtooth-wave type waveforms
Fig.6. 8: Types of signals output of an oscillator
The oscillators have a variety of applications. In some applications we need
voltages of low frequencies, in others of very high frequencies. For example
to test the performance of a stereo amplifier, we need a signal of variable
frequency in the audio range (20 Hz-20 KHz). Next to amplifiers, oscillators
are the most important analog circuit block. Oscillators can be found in almost
every imaginable electronic system. For example all radio receiving systems
must have a local oscillator. All transmitting systems require oscillators to
define the carrier frequency. Similarly, most digital systems are clocked and
require a master clock oscillator to operate. Signal sources, which are essential
for testing electronic systems, are also precise oscillators whose frequency and
amplitude can be accurately set according to the requirement.
6.4.2 Radio frequency amplifier
An amplifier is an electronic device which can increase the amplitude or the
power of the input signal to its input parts, without the needs of modifying
the form of that signal. Mostly, these devices are used in telecommunication,
especially in receivers. Any amplifier has an active element, more often
transistors, though there may exist also resistors, inductors and capacitors.
Classes of amplifiers
There exist two classes: Capacitor coupled amplifiers and transformer
coupled amplifiers. The two are used in multistage amplifiers, that is when
we connect two stage amplifiers using a capacitor and when we connect two
stages amplifiers using a transformer, we get a capacitor coupled amplifier and
a transformer coupled amplifier respectively.
Characteristics of RF amplifier
1. It may require or not a wide bandwidth signal to amplify
2. The output signal from the RF amplifier may or not be linear
3. They require to operate at a narrow bandwidth
4. They can use filters to reduce bandwidth5. To tune the circuit, the resonant frequency is set to
All electronic devices have an inductive reactance and capacitive reactances. The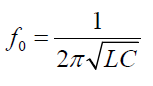
latter are vary as the frequency fluctuates. Normally, as the frequency increases,
the inductive reactance increases but capacitive reactance decreases. Then
the circuit will be called self-resonate at point, where the two characteristics
mentioned above become equal.
In signal processing, we need to realize as many operations as possible so
that we arrive to a signal that fits the transmission standards. The signal to be
modulated is referred to as a baseband signal. The carrier signal needs to be a
higher frequency than the baseband. A RF amplifier is a device which amplifies
the baseband signal. However, devices such as Oscillators, Mixers, Multipliers
and frequency synthesizers can be used to meet the above conditions.
6.4.3 Power Amplifier
Signals are amplified in several stages (Fig.6.9). The initial stages are small
signal amplifiers, they are designed to give good voltage gain, so they are called
voltage amplifiers. At the final stage, the signal becomes large, the large-signalamplifier is called power amplifier, as it is designed for good power gain.
Fig.6. 9 The functioning mechanism of power amplifier
The Fig.6.10 shows that the power amplifiers are classified according to the
conduction angle they produced. Conduction angle measures the portion of
the input cycle that is reproduced at the output of a power amplifier. If the
conduction angle is 360°, which means that all of the input cycle is reproduced,the amplifier is called class A amplifier.
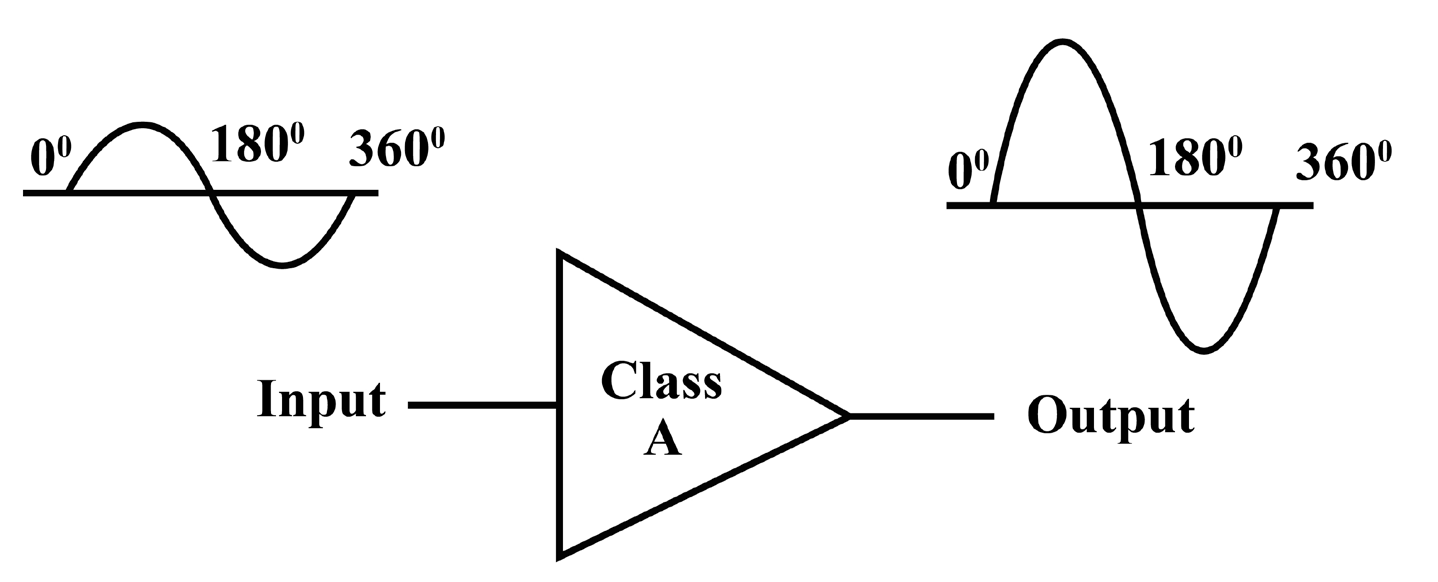
Fig.6. 10: The conduction angle of power amplifier
Every amplifier has a DC equivalent circuit and an AC equivalent circuit.
Because of this, it has two load lines : a Dc load line and an AC load line.
6.4.4 Checking my progress
1. State the classifications of oscillators according to Frequency Band of
the Signals
2. Explain what is mean by Oscillator
3. The figure is about transmission of signals in telecommunication. Studyit carefully and label it.
6.5 ANTENNAS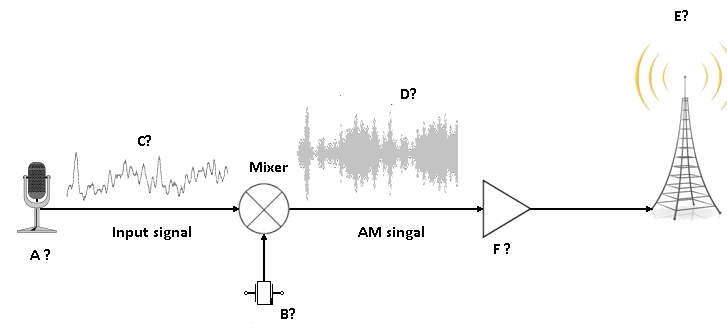
ACTIVITY 6.5: Defining types of antennas
Observe clearly the images on the fig. 6.11 below and answer thequestions that follow:
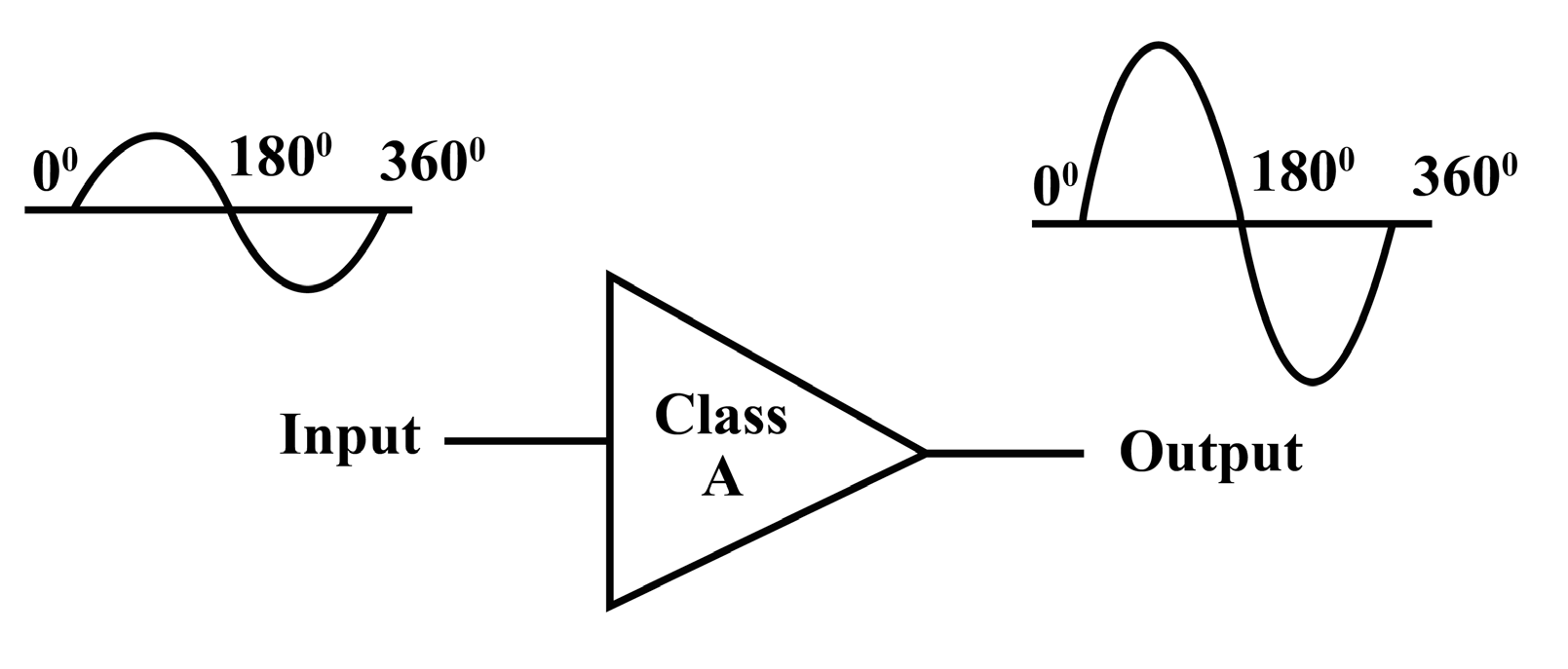
Fig.7. 11 Different types of antenna
1. Describe the different the types of antenna shown in the Fig.7.11
above.
2. Discuss other different types of antenna you know.
3. Discuss and explain the function principle of an antenna.
An antenna or aerial is an electrical device connected (often through a
transmission line) to the receiver or transmitter which converts electric power
into radio waves, and vice versa. It is usually used with a radio transmitter
or radio receiver. In transmission, a radio transmitter supplies an oscillating
radio frequency electric current to the antenna’s terminals, and the antenna
radiates the energy from the current as electromagnetic waves (radio waves).
In reception, an antenna intercepts some of the power of an electromagnetic
wave in order to produce a tiny voltage at its terminals, which is fed to a receiver
to be amplified.
Antennas are essential components of all equipment which are used in radio.
They are used in broadcasting systems, broadcast television systems, two-way
radio systems, communications receiver’s systems, radar systems, cell phones
systems, and satellite communications systems, garage door openers systems,
wireless microphones systems, Bluetooth enabled devices systems, wireless
computer networks systems, baby monitors systems, and Radio Frequency
Identification (RFID) tags systems on merchandise etc.
6.5.1 Types of antennas
There are a very large variety of antennas used in telecommunication. Here we
can discuss at least four types of antenna among others.
Wire antennas
The wire antennas are dipole, monopole, loop antenna, helix and are usually
used in personal applications, automobiles, buildings, ships, aircrafts and supercrafts.
Aperture antennas
These are horn antennas and waveguide opening and they are usually used inaircrafts and space crafts because they are flush-mounted.
Fig.6. 13: A horn antenna with aperture field distribution
Reflector antennas
These are parabolic reflectors and corner reflectors and they are high gain
antennas usually used in radio astronomy, microwave communication andsatellite tracking.

Fig.6. 14: Reflector antenna
Array antennas
These are also called Yagi-Uda antennas or micro-strip patch arrays or
aperture arrays, slotted waveguide arrays. They are suitable for very high gainapplications with added advantage, such as, controllable radiation pattern.

Fig.6. 15: Array antenna.
6.5.2 Checking my progress
1. What is meant by an antenna in telecommunication system?2. State and explain at least two types of antenna
6.6 BLOCK DIAGRAMS OF TELECOMMUNICATIONACTIVITY 6.6: Investigating communication block
Information: Information is any entity or form that resolves uncertainty or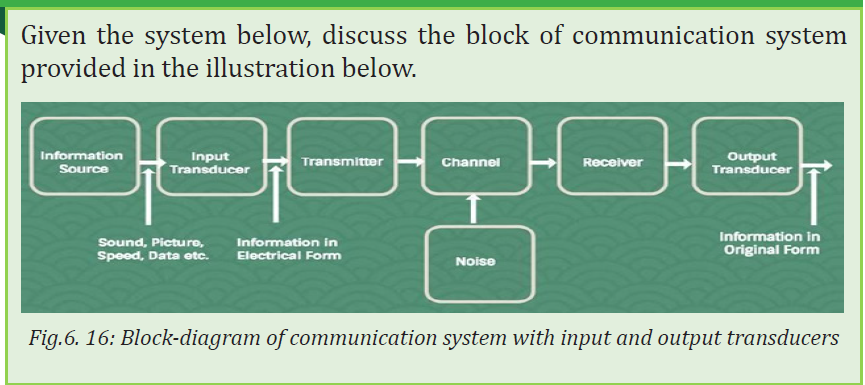
provides the answer to a question of some kind. It is thus related to data and
knowledge, as data represents values attributed to parameters, and knowledge
signifies understanding of real things or abstract concepts. Message: A message
is a term standing for information put in an appropriate form for transmission.
Each message contains information. A message can be either analog message
(a physical time variable quantity usually in smooth and continuous form) or
a digital message (anordered sequence of symbols selected from finite set of
elements) as shown in Fg.6.19.
• Analog message: a physical time-variable quantity usually in smooth
and continuous form.
• Digital message: ordered sequence of symbols selected from finite set
of elements.
A signal is a mathematical function representing the time variation of a physical
variable characterizing a physical process and which, by using various models,
can be mathematically represented. In telecommunication, the message is also
known as a signal and the signal is transmitted in an electrical or voltage form.( i.e Signal ≈ Message)
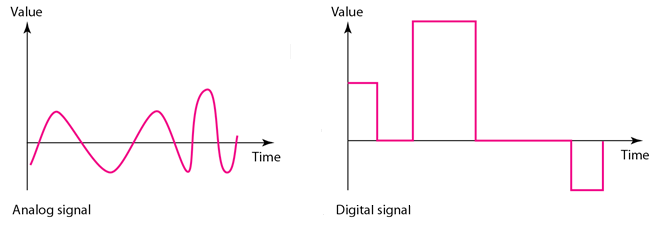
Fig.6. 17: Analog signal and digital signal representation diagram
COMPARISON OF AN ANALOG SIGNAL TO A DIGITAL SIGNAL
As discussed in the previous section, we can have the summary of differencesbetween analog signal and digital signal (see Table 6.2)
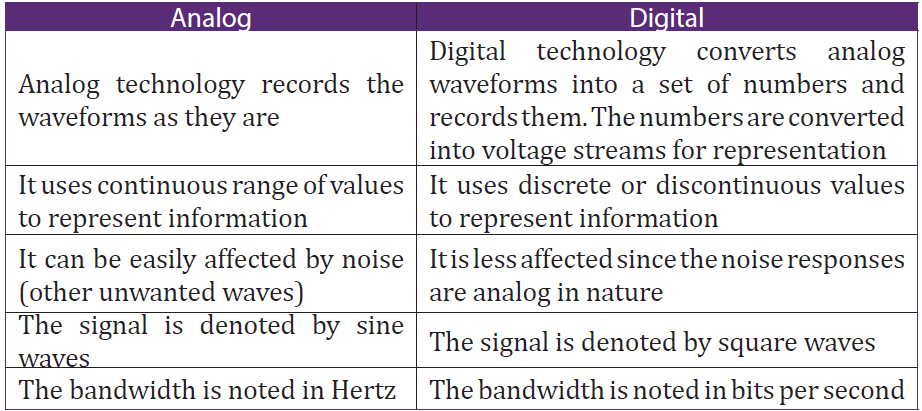
Table 6. 2: Comparison of an analog signal to a digital signal
SOME ELEMENTS OF BLOCK DIAGRAM OF TELECOMMUNICATION
1. Transmission channel which is the electric medium that bridges the
distance from source to destination
2. The receiver to convert the received signal in a form appropriate for the
output transducer after amplifying, filtering, demodulating and decoding it
3. Output transducer to convert the output electrical signal the desired
message form
4. Modulation is defined as the process by which some characteristics (i.e.
amplitude, frequency, and phase) of a carrier are varied in accordance with
a modulating wave.
5. Encoding is the process of coding the message and changes it in the
language understandable by the transmitter. This operation is realized at
the transmitting end
6. Demodulation is the reverse process of modulation, which is used to
get back the original message signal. Modulation is performed at the
transmitting end whereas demodulation is performed at the receiving end
7. Decoding is the reverse process of encoding to retrieve the original
message and make it human understandable message. It is realized at the
receiving end
8. Antennas which are aerials used to transmit and receive the signals.
9. The oscillators which are the sources of carrier signals which are used to
modulate and help the original signal to reach the destination
10. The signal normally, must be raised at a level that will permit it to reach itsdestination. This operation is accomplished by amplifiers
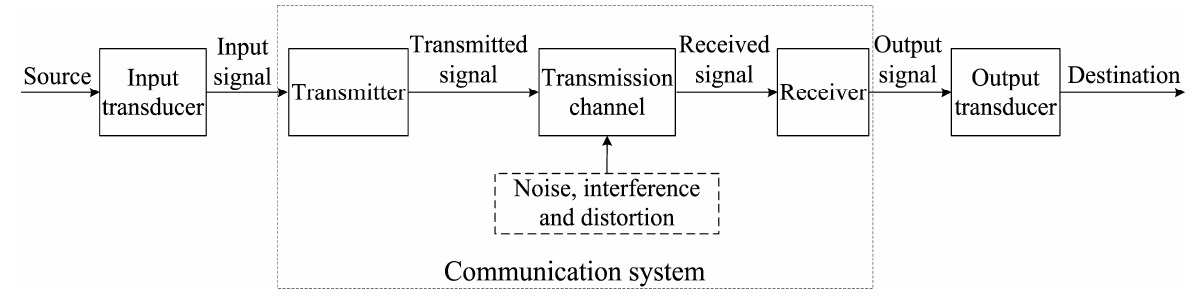
Fig.6. 18: Block diagram of telecommunication
6.6.1 Simple radio transmitter
A radio transmitter consists of several elements that work together to generate
radio waves that contain useful information such as audio, video, or digital
data. The process by which a radio station transmits information is outlined inFig. 6.21.
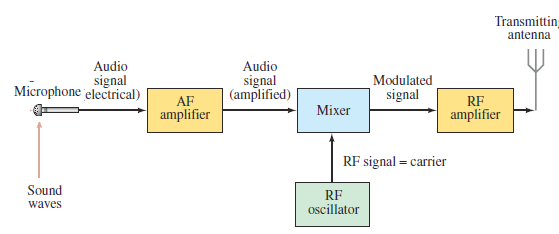
Fig.6. 19: Block diagram of a radio transmitter
• Power supply: Provides the necessary electrical power to operate the
transmitter.
• The audio (sound) information is changed into an electrical signal
of the same frequencies by, say, a microphone, a laser, or a magnetic
read write head. This electrical signal is called an audio frequency
(AF) signal, because the frequencies are in the audio range (20 Hz to
20 000 Hz).
• The signal is amplified electronically in AF amplifier and is then mixed
with a radio-frequency (RF) signal called its carrier frequency, which
represents that station. AM radio stations have carrier frequencies
from about 530 kHz to 1700 kHz. Today’s digital broadcasting uses the
same frequencies as the pre-2009 analog transmission.
• The Modulator or Mixer adds useful information to the carrier wave.
The mixing of the audio and carrier frequencies is done in two ways.
- In amplitude modulation (AM), the amplitude of the highfrequency
carrier wave is made to vary in proportion to the
amplitude of the audio signal, as shown in Fig.6.3. It is called
“amplitude modulation” because the amplitude of the carrier is
altered (“modulate” means to change or alter).
- In frequency modulation (FM), the frequency of the carrier wave
is made to change in proportion to the audio signal’s amplitude,
as shown in Fig.7.4. The mixed signal is amplified further and
sent to the transmitting antenna (Fig.6.13.C), where the complex
mixture of frequencies is sent out in the form of EM waves.
• Amplifier: Amplifies the modulated carrier wave to increase its power.
The more powerful the amplifier, the more powerful the broadcast.
In digital communication, the signal is put into digital form which modulates the
carrier. A television transmitter works in a similar way, using FM for audio and
AM for video; both audio and video signals are mixed with carrier frequencies.
6.6.2 Simple radio receiver
A radio receiver is the opposite of a radio transmitter. It uses an antenna to
capture radio waves, processes those waves to extract only those waves that are
vibrating at the desired frequency, extracts the audio signals that were added
to those waves, amplifies the audio signals, and finally plays them on a speaker.
Now let us look at the other end of the process, the reception of radio and TV
programs at home. A simple radio receiver is graphed in Fig. 6.22. The EMwaves sent out by all stations are received by the antenna.
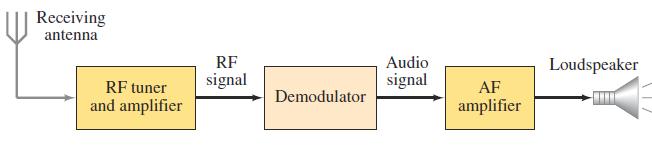
Fig.6. 20 Block diagram of a simple radio receiver
The signalantennadetect and send the radio waves,to the receiver are very
small and contain frequencies from many different stations. The receiver uses
a resonant LC circuit to select out a particular RF frequency (actually a narrow
range of frequencies) corresponding to a particular station.
A simple way of tuning a station is shown in Fig.6.23. When the wire of antenna
is exposed to radio waves, the waves induce a very small alternating current inthe antenna.
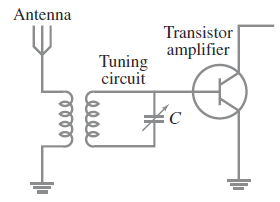
Fig.6. 21: Simple tuning stage of a radio.
A particular station is “tuned in” by adjusting the capacitance C and/or
inductance L so that the resonant frequency of the circuit equals that of the
station’s carrier frequency. R.F. Amplifier: A sensitive amplifier that amplifies
the very weak radio frequency (RF) signal from the antenna so that the signal
can be processed by the tuner.
R.F. Tuner: A circuit that can extract signals of a particular frequency from a
mix of signals of different frequencies. On its own, the antenna captures radio
waves of all frequencies and sends them to the RF amplifier, which dutifully
amplifies them all. Unless you want to listen to every radio channel at the same
time, you need a circuit that can pick out just the signals for the channel you
want to hear. That’s the role of the tuner.
The tuner usually employs the combination of an inductor (for example, a coil)
and a capacitor to form a circuit that resonates at a particular frequency. This
frequency, called the resonant frequency, is determined by the values chosen
for the coil and the capacitor. This type of circuit tends to block any AC signals
at a frequency above or below the resonant frequency.
You can adjust the resonant frequency by varying the amount of inductance
in the coil or the capacitance of the capacitor. In simple radio receiver circuits,
the tuning is adjusted by varying the number of turns of wire in the coil. More
sophisticated tuners use a variable capacitor (also called a tuning capacitor) tovary the frequency.
6.6.3 Wireless Radio Communication
Let us now discuss the basic principles of wireless radio communications.
We shall mainly concentrate on the principle of amplitude modulation and
demodulation. The simplest scheme of wireless communication would be
to convert the speech or music to be transmitted to electric signals using a
microphone, boost up the power of the signal using amplifiers and radiate the
signal in space with the air of an antenna. This would constitute the transmitter.
At the receiver end, one could have a pick-up antenna feeding the speech ormusic signal to an amplifier and a loud speaker. (See Fig.6.24)

Fig.6. 22 Wireless radio communication
The above scheme suffers from the following drawbacks:
i. EM waves in the frequency range of 20 Hz to 20 kHz (audio-frequency
range) cannot be efficiently radiated and do not propagate well in space.
ii. Simultaneous transmission of different signals by different transmitters
would lead to confusion at the receiver.
In order to solve these problems; we need to devise methods to convert or
translate the audio signals to the radio-frequency range before transmission and
recover the audio-frequency signals back at the receiver. Different transmitting
stations can then be allotted slots in the radio-frequency range and a single
receiver can then tune into these transmitters without confusion.
The frequency range 500 kHz to 20 MHz is reserved for amplitude-modulated
broadcast, which is the range covered by most three band transistor radios. The
process of frequency translation at the transmitter is called modulation. The
process of recovering the audio-signal at the receiver is called demodulation. Asimplified block diagram of such a system is shown in the below figure.
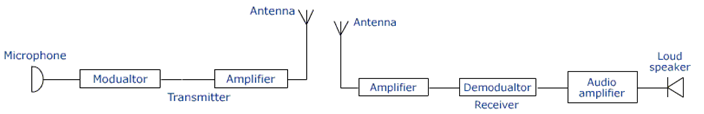
Fig.6. 23 Block diagram of radio transmitter and receiver
6.6.4 Checking my progress
1. What is the importance of power amplifier in simple radio transmitter.
2. What do you understand by the following terms.
3. Analog message
4. Digital message
5. Draw a circuit diagram of a simple radio receiver
END UNIT ASSESSMENT 6
A. Multiple choices
1. One of the following is used for satellite communication
a. Radio waves c. Microwaves
b. Light waves d. All of these
2. Amplitude –modulated radio waves are received by a tuned radiofrequency
( trf) Receiver. The receiver has a suitable detector
circuit in order to
a. Amplifier the carrier waves
b. Amplifier the audio-frequencies carried
c. Rectifier the carrier waves
d. detect the carrier waves
e. Transfer the audio-frequencies to the radio-frequency
amplifier
B. Structured questions
3. What do you understand by the following terms?
a. Amplifier
b. Modulator
4. What is meant by telecommunication system?
5. Draw a labeled diagram showing the elements of radio transmitter
C. Essay question
6. Recently, the government of Rwanda decided to replace analog
system of communication by digital system of communication.
Debate about this government policy
7. Explain briefly positive impact of telecommunication in developmentof a country like Rwanda.
UNIT 7 NATURE OF PARTICLES AND THEIR INTERACTIONS
Key unit competence: Organize the properties and basic principles of quarks.
My goals
• The key varieties of fundamental subatomic particles and how they
were discovered.
• Distinguish between fundamental particles and composite particles
• Distinguish between particles and antiparticles
• Describe how antimatter can be used as a source of energy
• State some applications for elementary particles
• Compare matter and antimatter
• The four ways in which subatomic particles interact with each other.
• Analyze the structure of protons, neutrons, and other particles can beexplained in terms of quarks
INTRODUCTORY ACTIVITY
Investigating the elementary particles discovery
In the study of matter description and energy as well as their interactions;
the fascinating thing of discovery is the structure of universe of unknown
radius but still to know the origin of matter one need to know about small
and smallest composites of matter. The smallest particle was defined to be
electron, proton, and neutron. But one can ask:
1. Are electron, proton and neutron the only particle that can define the
origin of matter?
2. What are other particles matter is composed of?3. Describe and discuss how particles interact with energy to form matter
7.1 ELEMENTARY PARTICLES.
7.1.1 Introduction
ACTIVITY 7.1: Investigate the presence of smaller particles
1. Use internet and retrieve the definition and the information about
elementary particles, and then answer to the following questions.
2. What does elementary particle physics talk about?
3. What are the elementary particles found through your research?
4. Discuss and explain the use of knowledge about the elementaryparticles.
Particle physics, also known as high-energy physics, is the field of natural
science that pursues the ultimate structure of matter.
The protons and neutrons are collectively called hadrons, were considered
as elementary particles until 1960. We now know that they are composed of
more fundamental particles, the quarks. Electrons remain elementary to
this day. Muons and τ-leptons, which were found later, are nothing but heavy
electrons, as far as the present technology can tell, and they are collectively
dubbed leptons.
Quarks and leptons are the fundamental building blocks of matter. The
microscopic size that can be explored by modern technology is nearing. The quarks and leptons are elementary at this level (Nagashima, 2013).
Particle physics is the study of the fundamental constituents of matter and
their interactions. However, which particles are regarded as fundamental have
changed with time as physicists’ knowledge has improved. Modern theory called
the standard model attempts to explain all the phenomena of particle physics
in terms of the properties and interactions of a small number of particles of
three distinct types (see Fig.7.1):
• Two families of fermions (of spin ½): leptons and quarks• One family of bosons (of spin 1)
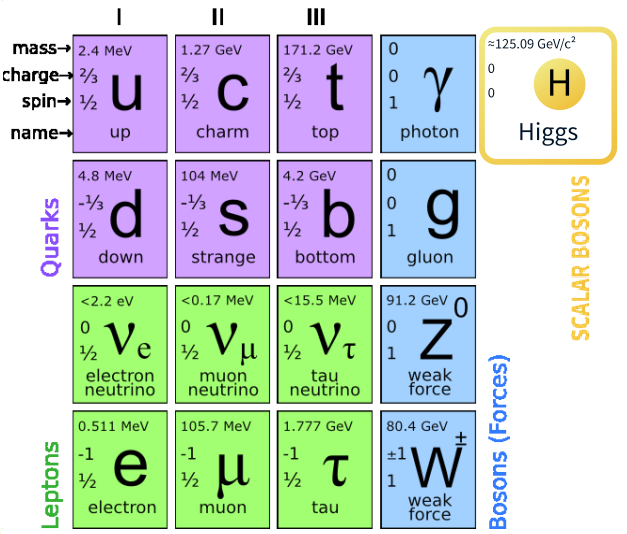
Fig.7. 1 Fundamental Standard model of elementary particle
I, II and III represent the first, second and the third generations. In addition,
at least one spin-0 particle, called the Higgs boson, is postulated to explain the
origin of mass within the theory, since without it all the particles in the model
are predicted to have zero mass (see Fig.7.1).
All the particles of the standard model are assumed to be elementary; i.e. they
are treated as point particles, without internal structure or excited states. The
most familiar example of a lepton is the electron (the superscript denotes the
electric charge), which is bound in atoms by the electromagnetic interaction,
one of the four fundamental forces of nature. A second well-known lepton is
the electron neutrino, which is a light, neutral particle observed in the decay
products of some unstable nuclei (the so-called β-decays). The force responsiblefor the β-decay of nuclei is called the weak interaction.
7.1.2 Checking my progress
1. Particles that make up the family of Hadrons are:
a. Baryons and mesons c. Protons and electrons
b. Leptons and baryons d. Muons and Leptons
2. Using the elementary particles, Complete the following sentences
I. One family of bosons of spin 1 called__________ which act as ‘force
carriers’ in the theory
II. Two fermions of spin 1/2 called_________ and ________
3. The first antiparticle found was the
a. Positron c. Quark
b. Hyperons d. baryon4. Explain what is meant by particle physics?
7.2 CLASSIFICATION OF ELEMENTARY PARTICLES.
ACTIVITY 7.1: Classes of elementary particles
Based on the previous introduction section, reread the text and the
answer to the following questions.1. What are the types of elementary particles?There are three properties that describe an elementary particle ‘’mass,’’2. What properties are based on to classify elementary particles?
‘’charge’’ and ‘’spin’’. Each property is assigned as number value. These
properties always stay the same for an elementary particle.• Mass (m): a particle has mass if it takes energy to increase its speed or
to accelerate it. The values are given in MeV/C2
. This comes from special
relativity, which tells us that energy equals mass times the square of
the speed of light. 2 E mc = × . All particles with mass are affected by
gravity even particles with no mass like photon.
• Electric charge (Q): particles may have positive, negative charge or
none. If one particle has a negative charge and another particle has a
positive, the two particles are attracted to each other. If particles have
a similar charge, they repel each other. At a short distance this force
is much stronger than the force of gravity which pulls all particles
together. An electron has a charge -1 and a proton has a charge +1. A
neutron has average charge 0. Normal quarks have charge of 2/3 or-1/3
Spin: the angular momentum or constant turning of particles has a7.2.1 Classification of particles by mass
particular value, called its spin number. Spin for elementary particle
is 0, 1 or . The spin property only donates the presence of angular
. The spin property only donates the presence of angularmomentum.
The most basic way of classifying particles is by their mass. The heaviest
particles are the hadrons and the lightest one is the leptons.
As seen the diagram above hadrons group is divided into baryons and mesons.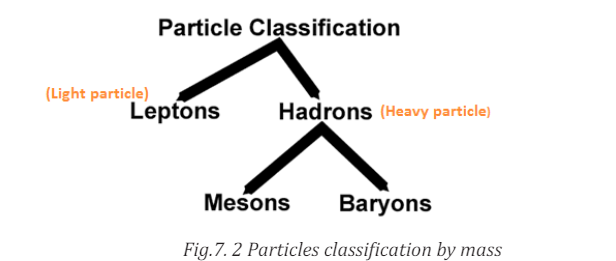 Baryons are the heaviest particles and are followed by mesons.
Baryons are the heaviest particles and are followed by mesons.
Hadrons are composite particles made of quarks held together by the strong
force in a similar way as molecules are held together by electromagnetic force.
They are subjected to the strong nuclear force and are not fundamental particlesas they are made up of quarks.
Baryons are composite sub-atomic particle made up of 3 quarksEx: Protons and neutrons.
(triquarks are distinct from mesons which are composed of one quark and
one antiquark). Baryon comes from Greek word which means “heavy”.
The protons are only stable baryons; all other baryons eventually decayinto proton.
• Mesons are hadrons sub-atomic particles made up of one quark andEach pion has quark and one anti-quark therefore is a meson.one anti-quark bound together by strong interaction. Ex: Pion and kaon
It is the lightest meson and generally the lightest hadrons. They are unstable.
Leptons do not interact via the strong force. They carry electric charge also interactvia the weak nuclear force. They include electron, muons, tau and three the types
of neutrino: the electron neutrino (νE), the muon neutrino (νμ
) and the tau neutrinovτ .
In summary, leptons are subjected to the weak nuclear force and they do not
feel the strong nuclear force.
Examples: Electron, muons and neutrino.
7.2.2 Classification of particles by spin.
The spin classification determines the nature of energy distribution in a
collection of particles. Particles of integer spin obey Bose-Einstein statistics
whereas those of half-integer spin behave according to Fermi-Dirac statistics
as shown in the following chart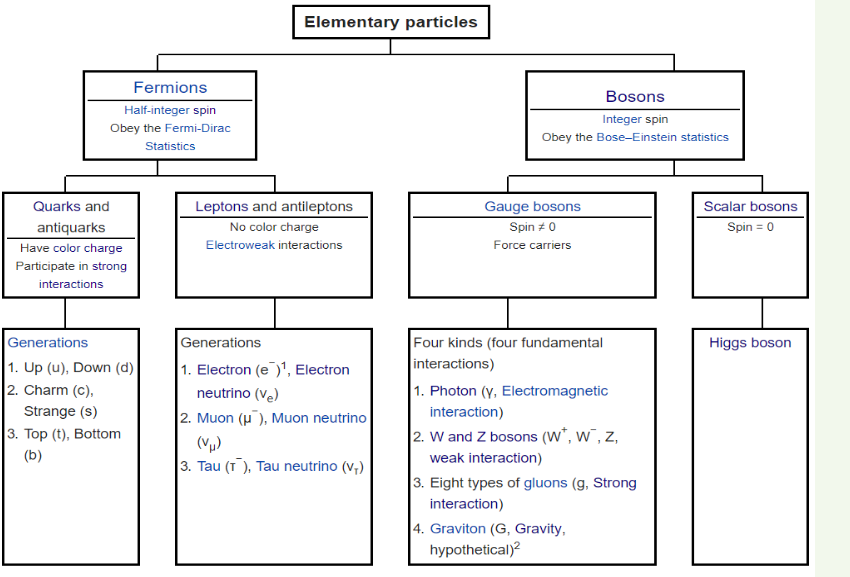
Fermions are particles which have half-integer spin and therefore are
constrained by the Pauli Exclusion Principle (see Section 7.4). It includes
electrons, protons and neutrons.
The fact that electrons are fermions is foundational to the buildup of the
periodic table of elements since there can be only one electron for each state
in an atom (only one electron for each possible set of quantum numbers).
The fermion nature of electrons also governs the behavior of electrons in a
metal where at low temperatures all the low energy states are filled up to a
level called the Fermi energy. This filling of states is described by Fermi-Dirac
statistics.
7.2.3 Checking my progress
7.3 ANTI PARTICLE AND PAULI’S EXCLUSION PRINCIPLE7.3.1 Concept of particle and antiparticle
ACTIVITY 7.3Discuss the following terms:There are two important points about pair production. The first is that you need
1. Particle
2. Antiparticle
to collect energy to produce the electron-positron pair. You need the equivalent
rest mass of energy that is the amount of energy contained in the both particle
and anti-particle when at rest. The energy converted to mass is ‘lost’ or fully
‘’bound’’ until the particle is annihilated and the energy can be recovered. The
second thing is that it needs a correct environment. The process does not occur
unless certain conditions are present.
Viewing the phenomena as a creative process we can say a threshold amountof energy is sacrificed in a correct context to manifest a pair of particle with
a physical mass. It can be said something was created out of nothing. That is
before the interaction, no particles with mass existed. After interaction, there
were two particles with mass. Hence something was created out of nothing.
But this can be said only because of the perspective taken when viewing the
process.
For every charged particle of nature, whether it is one of the elementaryparticles of the standard model, or a hadron, there is an associated particle
of the same mass, but opposite charge, called its antiparticle.
This result is a necessary consequence of combining special relativity withquantum mechanics. This important theoretical prediction was made by Dirac
and follows from the solutions of the equation he first wrote down to describe
relativistic electrons
7.3.2 Pauli’s exclusion principle,Pauli’s exclusion principle is a quantum mechanical principle which states
that:
“Two or more identical fermions (particles with half-integer spin) cannot
occupy the same quantum state simultaneously.”
In case of electrons in atoms it can be stated as follows: it is impossible for two
electrons of a poly-electron atom to have the same values of the four quantum
numbers:
The principle quantum number, the angular momentum quantum number
(l), the magnetic quantum number (ml) and the spin quantum number (ms).
For example, if two electrons reside in the same orbital and if their msmust be
different and thus like electrons must have opposite half integer spin projections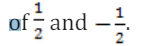
This principle was formulated by Austrian physicist Wolfgang Pauli in 1925 for
electrons, and later extended to all fermions with his spin–statistics theorem of
1940.
Particles with an integer spin, or bosons, are not subject to the Pauli Exclusion
Principle: any number of identical bosons can occupy the same quantum state,
for instance, photons produced by a laser and Bose–Einstein condensate.
The Pauli Exclusion Principle describes the behavior of all fermions (particles
with “half-integer spin”), while bosons (particles with “integer spin”) are
subject to other principles. Fermions include elementary particles such
7.3.3 Checking my progress1. What do you understand by antiparticle?
2. State Pauli’s exclusion principle?
3. Why Pauli’s exclusion Principle is known as exclusion?
7.4 FUNDAMENTAL INTERACTIONS BY PARTICLE EXCHANGE
ACTIVITY 7.4: Fundamental interaction
Using internet, discusses the fundamental interactions in terms of
exchange particles, then find the relation between the following concepts.
1. Gravitational forces
2. electroweak force,
3. Strong force and
4. Weak forces.
7.4.1 Forces and Interactions
have recognized three basic forces:• The gravitational force is an inherent attraction between two masses.In the 1860s, the Scottish physicist James Clerk Maxwell developed a theory
Gravitational force is responsible for the motion of the planets and
Stars in the Universe. It is carried by Graviton. By Newton’s law of
gravitation, the gravitational force is directly proportional to the
product of the masses and inversely proportional to the square of
the distance between them. Gravitational force is the weakest force
among the fundamental forces of nature but has the greatest large−
scale impact on the universe. Unlike the other forces, gravity works
that unified the electric andmagnetic forces into a single electromagnetic force.
Maxwell’s electromagnetic force was soon found to be the “glue” holding atoms,
molecules, and solids together. It is the force between charged particles such
as the force between two electrons, or the force between two current carrying
wires. It is attractive for unlike charges and repulsive for like charges. Theuniversally on all matter and energy, and is universally attractiveelectromagnetic force obeys inverse square law. It is very strong compared
• The electric force is a force between charges
• The magnetic force, which is a force between magnets or between
magnetic body and ferromagnetic body.
to the gravitational force. It is the combination of electrostatic and magnetic forces.
The discovery of the atomic nucleus, about 1910, presented difficulties that
could not be explained by either gravitational or electromagnetic forces.
The atomic nucleus is an unimaginably dense ball of protons and neutrons.
But what holds it together against the repulsive electric forces between the
protons? There must be an attractive force inside the nucleus that is stronger
than the repulsive electric force. This force, called the strong force, is the force
that holds the protons and neutrons together in the nucleus of an atom. It is the
strongest of all the basic forces of nature. It, however, has the shortest range,
of the order of 10−15 m. This force only acts on quarks. It binds quarks together
to form baryons and mesons such as protons and neutrons. The strong force is
mediated or carried by Gluons. Quarks carry electric charge so they experienceelectric and magnetic forces.
In the 1939, physicists found that the nuclear radioactivity called beta decay
could not be explained by either the electromagnetic or the strong force. Careful
experiments established that the decay is due to a previously undiscovered
force within the nucleus. The strength of this force is less than either the strong
force or the electromagnetic force, so this new force was named the weak
force. Weak nuclear force is important in certain types of nuclear process such
as β-decay. This force is not as weak as the gravitational force. The weak force
acts on both leptons and quarks (and hence on all hadrons). The weak force is
carried by W+, W- and Z. Leptons – the electrons, muons and tau – are chargedso they experience electric and magnetic forces.
Of these, our everyday world is controlled by gravity and electromagnetism. The
strong force binds quarks together and holds nucleons (protons & neutrons)
in nuclei. The weak force is responsible for the radioactive decay of unstablenuclei and for interactions of neutrinos and other leptons with matter.
By 1940, the recognized forces of nature (fundamental forces)were four:• Gravitational forces between masses,
• Electromagnetic forces resulting from the combination of electric and
magnetic fields,
• Strong force (nuclear force) between subatomic particles,
• Weak forces that arise in certain radioactive decay processes.By 1980, Sheldon Glashow, Abdus Salam, and Steven Berg developed a theory
that unifies electromagnetism and weak force into electroweak force. Hence,
our understanding of the forces of nature is in terms of three fundamentalforces:
• The gravitational force,
• The electroweak force,• The strong force.
The Table 7.1 below summaries the fundamental forces and force carriers.
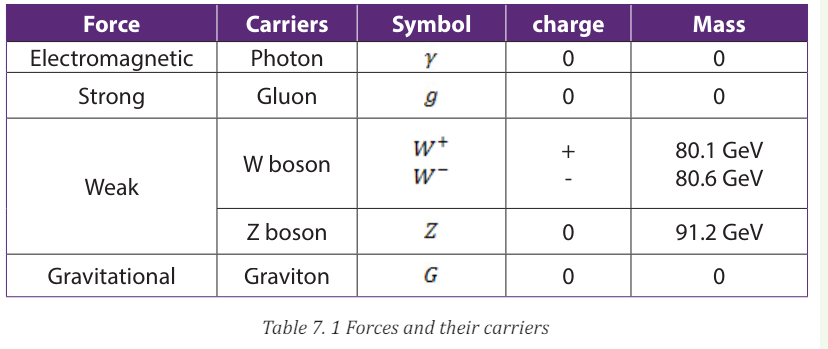
• W boson: short-lived elementary particle; one of the carriers of the
weak nuclear force
• Z boson: short-lived elementary particle; one of the carriers of the
weak nuclear force
• Graviton: the hypothetical particle predicted to carry the gravitationalforce
All the forces of nature should be capable of being described by single theory.
But only at high energies should be the behavior of the forces combines, this is
called unification. We can compare the relative strengths of the electromagnetic
repulsion and the gravitational attraction between two protons of unit chargeusing the above equations.
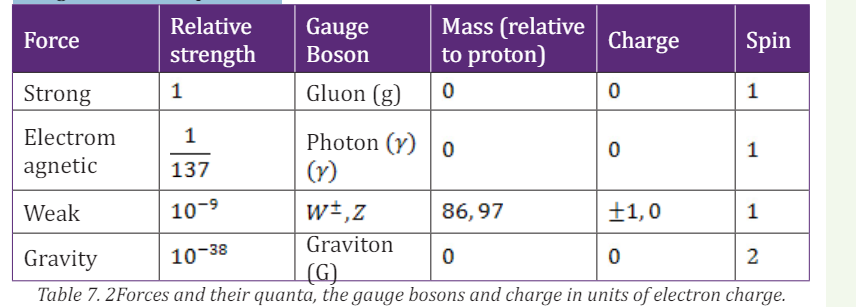
EXAMPLE 7.1
Thus the gravitational is the weakest of the fundamental forces. These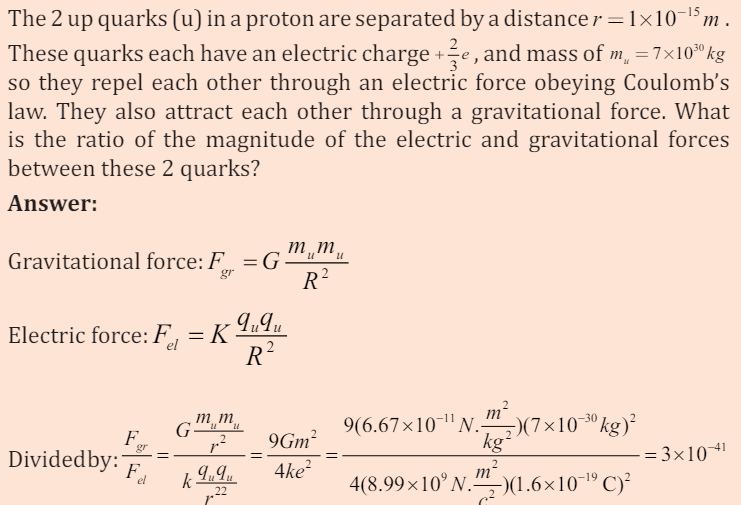
interactions and their relative strengths are summarized in Table 7.1
7.4.2 Checking my progress
1. Particles that interact by the strong force are called:
a. Leptons c. Muons
b. hadrons d. Electrons2. Name the four fundamental interaction and the particles that mediate each
7.5 UNCERTAINTY PRINCIPLE AND PARTICLE CREATION
7.5.1 The concept of uncertainty principle
ACTIVITY 7.5: Investigation of particle creation and position.
Basing on the knowledge and skills obtained from the previous sections
of this unit, use internet to find the meaning of the particle creation.
a. Is it possible to know the exact location of an elementary particle?b. Discuss and explain your findings
The discovery of the dual wave–particle nature of matter forces us to re-evaluatethe kinematic language we use to describe the position and motion of a particle.
In classical Newtonian mechanics we think of a particle as a point. We can
describe its location and state of motion at any instant with three spatial
coordinates and three components of velocity. But because matter also has a
wave aspect, when we look at the behaviour on a small enough scale comparable
to the de Broglie wavelength of the particle we can no longer use the Newtonian
description. Certainly no Newtonian particle would undergo diffraction likeelectrons do.
To demonstrate just how non Newtonian the behaviour of matter can be, let’s
look at an experiment involving the two-slit interference of electrons (Fig.7.4).
We aim an electron beam at two parallel slits, just as we did for light. (The
electron experiment has to be done in vacuum so that the electrons don’t collidewith air molecules.)
What kind of pattern appears on the detector on the other side of the slits?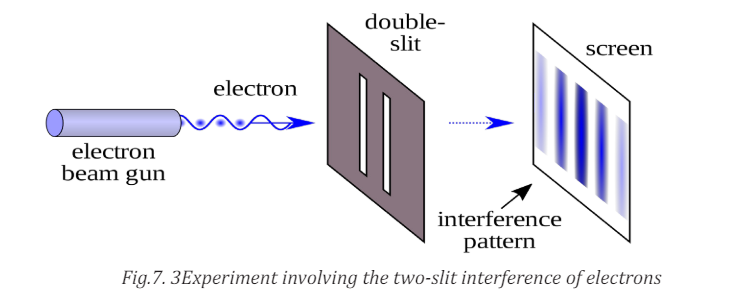
The answer is: exactly the same kind of interference pattern we saw for photons.
Moreover, the principle of complementarily, tells us that we cannot apply the
wave and particle models simultaneously to describe any single element of
this experiment. Thus we cannot predict exactly where in the pattern (a wave
phenomenon) any individual electron (a particle) will land. We can’t even ask
which slit an individual electron passes through. If we tried to look at where the
electrons were going by shining a light on them that is, by scattering photons
off them the electrons would recoil, which would modify their motions so thatthe two-slit interference pattern would not appear.
Just as electrons and photons show the same behaviour in a two-slit interference
experiment, electrons and other forms of matter obey the same Heisenberguncertainty principles as photons do:
Heisenberg uncertainty principle for position and momentum is given by
This is a mathematical statement of the Heisenberg uncertainty principle Or
it is sometimes called, the indeterminacy principle. It tells us that we cannot
measure both the position and momentum of an object precisely at the sametime.
The uncertainty principle relates energy and time, examining this as follows.
The object to be detected has an uncertainty in position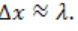 the photon that
the photon that
detects it travels with speed c, and it takes a time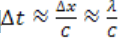 to pass through
to pass throughthe distance of uncertainty.
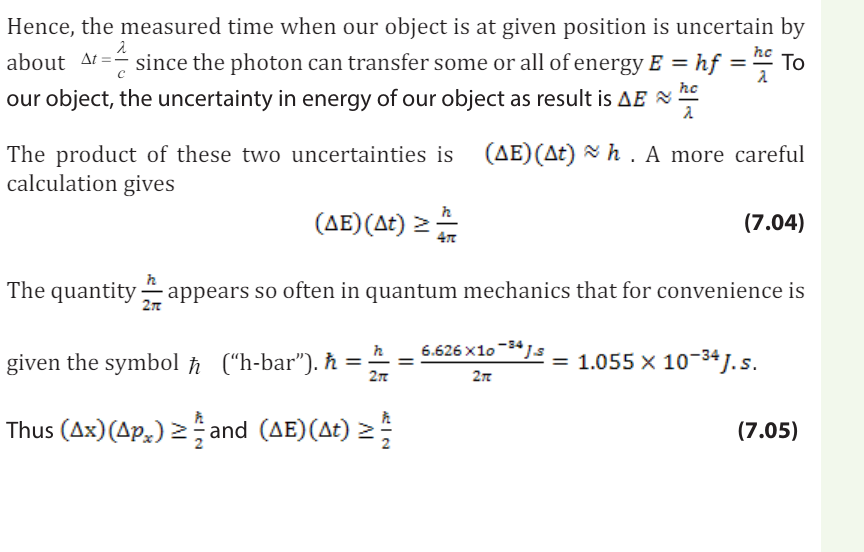
7.5.2 Checking my progress
1. The idea of uncertainty is used in many contexts; social, economic
and scientific. People often talk about uncertain times, and when you
perform a measurement you should always estimate the uncertainty
(sometimes called the error). In physics the Heisenberg Uncertainty
relation has a very specific meaning.a. Write down the Heisenberg uncertainty relation for position and2. An electron is confined within a region of width 11 5 10 m − × (Roughly
momentum.
b. Explain its physical significance.
c. Does the Heisenberg uncertainty principle need to be considered
when calculating the uncertainties in a typical first year physics
experiment? Why or why not?
d. Discuss the following statement: the uncertainty principle places a
limit on the accuracy with which a measurement can be made. Do
you agree or disagree, and why?
the Bohr radius)a. Estimate the minimum uncertainty in the component of the
electron’s momentum.
b. What is the kinetic energy of an electron with this magnitude of
momentum? Express your answer in both joules and electronvolts.
7.6 MATTER AND ANTIMATTER (PAIR PRODUCTION AND
ANNIHILATION)ACTIVITY 7.5: Describing the matter and antimatter
Use internet to describe the following concepts:
1. Matter and give examples of matter particles
2. Antimatter and give examples of antimatter particles
3. Pair production
4. Annihilation7.6. 1 Introduction
Matter is a substance that has mass and takes up a space by having a volume. This
include atoms and anything made up of these but no other energy phenomena
or wave such as light or sound. Everything around you is made up of matter
and is composed of particles including the fundamental fermions (quarks,
leptons, antiquarks and antileptons) which generally are matter particles andantimatter particles.
Antimatter is a material composed of the antiparticle to the corresponding
particle or ordinary particles. In theory a particle and its antiparticle have the
same mass as one another but opposite electric charge and other differences in
quantum numbers. Neutrons have antineutrons, electrons have positrons and
neutrons have antineutrons as their respective antimatter. It was once thought
that matter would neither be created nor destroyed. We know that energy andmass are interchangeable.
7.6.2 Pair production and annihilation
Pair production is a crucial example that photon energy can convert into
kinetic energy as well as rest mass energy. Schematic diagram about the process
of pair production is shown in Fig.7.5. The high-energy photon that has energy
hf loses its entire energy when it collides with nucleus. Then, it makes pair ofelectron and positron and gives kinetic energy to each particle.
Annihilation: When a particle collides with its antiparticle, the two annihilate
each other with their mass being entirely converted into energy by the process
called ‘’Annihilation’’
These particles and anti-particles can meet each other and annihilate one
another (See Fig.7.6). In each case the particle and its antiparticle annihilate
each other, releasing a pair of high energy gamma photons.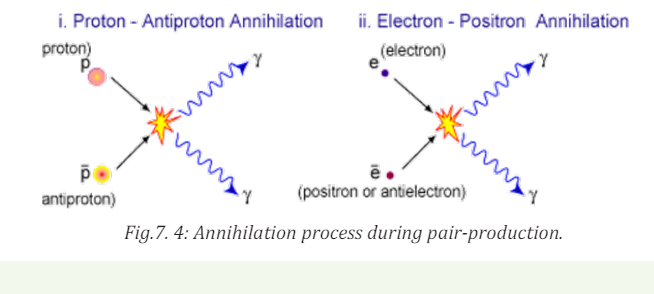
In this example, a proton and an anti-proton meet each other and annihilate,
producing high energy gamma rays in the form of photons. Rest mass, charge,
momentum and energy are conserved. They can also be produced from a highenergy photon, this is called pair production.
7.6.3 Application of antimatter
Antimatter as a form of antiparticle of sub atomic particles has a variety of
applications:• Positron emission tomography can be used to potentially treat cancer.
• Stored antimatter can be used for interplanetary and inter stellar
travel.
• Antimatter reactions have practical applications in medical energy.
• Antimatter has been considered as a trigger mechanism for nuclear
weapons because whenever antimatter meets its corresponding
matter the energy is released by annihilation.7.6.4 Checking my progress
1. Antimatter as a form of sub atomic particlesa. Electron2. The process in which a particle and antiparticle unite annihilate each
b. proton
c. matter
d. antiparticle
e. none of them is correct
other and produce one or more photons is called………
3. What happens when matter and antimatter collide?4. Compare matter and antimatter
END UNIT ASSESSMENT 7
A. Multiple choices
1. The positron is called the antiparticle of electron, because ita. Has opposite charge and Annihilates with an electron2. Beta particles are
b. Has the same mass
c. Collides with an electron
d. Annihilates with an electrone. Neutrons3. If gravity is the weakest force, why is it the one we notice most?
f. Protons
g. Electrons
h. Thermal neutronsa. Our bodies are not sensitive to the other forces.B. Structured questions
b. The other forces act only within atoms and therefore have no
effect on us.
c. Gravity may be “very weak” but always attractive, and the
Earth has enormous mass. The strong and weak nuclear
forces have very short range. The electromagnetic force has a
long range, but most matter is electrically neutral.
d. At long distances, the gravitational force is actually stronger
than the other forces.
e. The other forces act only on elementary particles, not on
objects our size.
4. According to the classification of elementary particles by mass.Complete the following figure
5.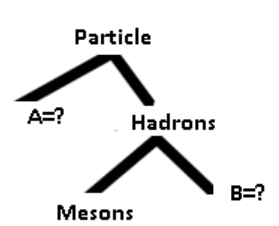
I. State two differences between a proton and a positron.
II. A narrow beam of protons and positrons travelling at the same
speed enters a uniform magnetic field. The path of the positrons
through the field is shown in Fig.7.7. Sketch on this figure thepath you would expect the protons to take.
III. Explain why protons take a different path to that of the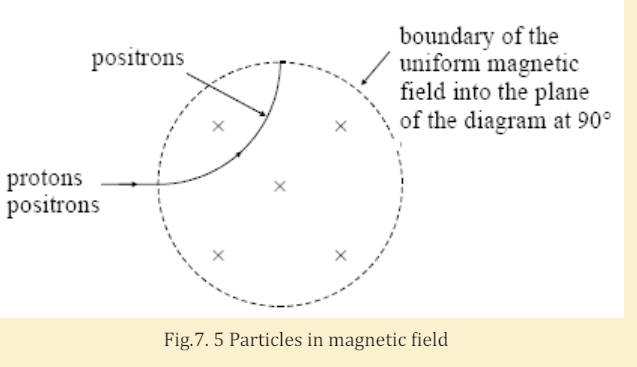
positrons.
6. A positron with kinetic energy 2.2 MeV and an electron at rest
annihilate each other. Calculate the average energy of each of the
two gamma photons produced as a result of this annihilation.
C. Essay question
7. Describe briefly the following particle-terms terms: π -meson,muon, neutrino, antiparticle, hadrons and lepton.
UNIT 8: PROPERTIES AND BASIC PRINCIPLES OF QUARKS
Key unit competence: Organize the properties and basic principles of quarks.
My goals• List types of quarks, identify quarks, antiquarks and hadrons (baryonsINTRODUCTORY ACTIVITY
and mesons)
• Define baryon number and state the law of conservation of baryon
number.
• Interpret the baryon number and apply the law of conservation of
baryon number
• State colors of quarks and gluons.
• Explain how color forms bound states of quarks.• Formulate the spin structure of hadrons (baryon and mesons)
In the study of matter description and energy as well as their interactions;
the fascinating thing of discovery is the structure of universe of infinite size
but still there is a taskto know the origin of matter. The smallest particle was
defined to be electron, proton, and neutron. But one can ask:
1. What particles are components of matter?2. Describe and discuss how particles interact with energy to form matter.
8.1 INTRODUCTION
ACTIVITY 8.1: Investigating about elementary particles
Considering the knowledge and skills obtained from unit 8, about
the study of elementary particles, discuss and explain the following
questions:
1. Discuss the major groups of elementary particles
2. Explain and analyze the family of quarks and their interactions.3. Why should we learn about elementary particles?
Particle physics is the field of natural science that pursues the ultimate
structure of matter. This is possible in two ways. One is to look for elementary
particles, the ultimate constituents of matter at their smallest scale, and the
other is to clarify what interactions are acting among them to construct matter
as we see them. The exploitable size of microscopic objects becomes smaller as
technology develops. What was regarded as an elementary particle at one time
is recognized as a structured object and relinquishes the title of “elementary
particle” to more fundamental particles in the next era. This process has beenrepeated many times throughout the history of science (Nagashima, 2013).
In the 19th century, when modern atomic theory was established, the exploitable
size of the microscopic object was and the atom was “the elementary particle”.
Then it was recognized as a structured object when J.J. Thomson extracted
electrons in 1897 from matter in the form of cathode rays. Its real structure
(the Rutherford model) was clarified by investigating the scattering pattern ofα-particles striking a golden foil (See Fig 9.1).
In 1932, Chadwick discovered that the nucleus, the core of the atom, consisted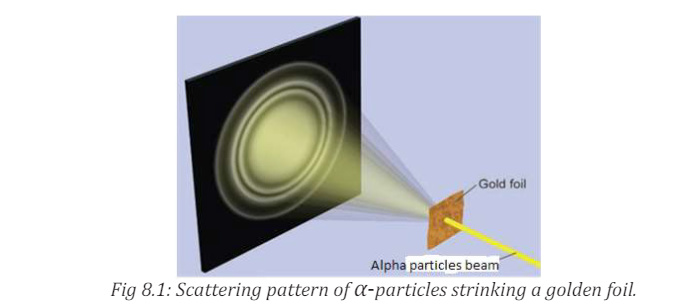
of protons and neutrons. In the same year, Lawrence constructed the first
cyclotron. In 1934 Fermi proposed a theory of weak interactions. In 1935
Yukawa proposed the meson theory to explain the nuclear force acting amongthem.
It is probably fair to say that the modern history of elementary particles began
around this time. The protons and neutrons together with their companion
pions, which are collectively called hadrons, were considered as elementary
particles until 1960. We now know that they are composed of more fundamental
particles, the quarks. Electrons remain elementary to this day. Muons and
τ-leptons, which were found later, are nothing but heavy electrons, as far as the
present technology can tell, and they are collectively dubbed leptons. Quarks
and leptons are the fundamental building blocks of matter. The microscopic
size that can be explored by modern technology is nearing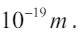 The quarks
The quarks
and leptons are elementary at this level (Nagashima, 2013). Some compositeparticles as stated by (Hirsch, 2002) are summarized in the tables below
8.1. Checking my progress
1. Each hadron consists of a proper combination of a few elementary
components called
a. Photons. c. Quarks.
b. Vector bosons. d. Meson-baryon pairs.
2. Which of the following is not conserved in a nuclear reaction?
a. Nucleon number. c. Charge
b. Baryon number. d. All of the above are
c. Conserved.
3. The first antiparticle found was the
a. Positron. c. Quark.
b. Hyperon. d. Baryon.
8.2 TYPES OF QUARKS
ACTIVITY 9.2: Investigating Quark particles
Use search internet and find the explanation about quarks and types ofquarks
Aquark is a type of elementary particle and a fundamental constituent of
matter. Quarks combine to form composite particles called hadrons, the most
stable of which are protons and neutrons, the components of atomic nuclei.
Due to a phenomenon known as color confinement, quarks are never directly
observed or found in isolation; they can be found only within hadrons, such as
baryons (of which protons and neutrons are examples) and mesons. For this
reason, much of what is known about quarks has been drawn from observations
of the hadrons themselves (Douglass, PHYSICS, Principles with applications.,
2014).
Quarks have various intrinsic properties, including electric charge, mass, color
charge, and spin. Quarks are the only elementary particles in the Standard
Model of particle physics to experience all four fundamental interactions,
also known as fundamental forces (electromagnetism, gravitation, strong
interaction, and weak interaction (see section 8.5), as well as the only known
particles whose electric charges are not integer multiples of the elementary
charge. There are six types of quarks, known as flavors: up, down, strange,
charm, top, and bottom (see Fig. 8.2). Up and down quarks have the lowestmasses of all quarks.
The heavier quarks rapidly change into up and down quarks through a process
of particle decay (the transformation from a higher mass state to a lower mass
state). Because of this, up and down quarks are generally stable and the most
common in the universe, whereas strange, charm, bottom, and top quarks can
only be produced in high energy collisions (such as those involving cosmic rays
and in particle accelerators). For every quark flavor there is a corresponding
type of antiparticle, known as an antiquark, that differs from the quark only in
that some of its properties have equal magnitude but opposite sign (Nagashima,2013).
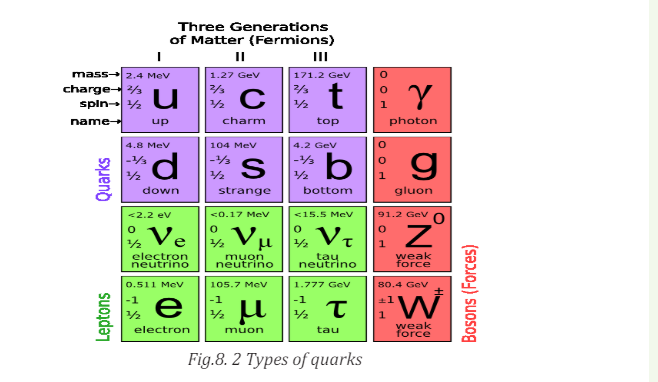
8.2.1 Checking my progress
1. A proton is made up of
a. One up quark and two down quarks
b. An up quark and down antiquark
c. Two up quarks and a down quark
d. Strange quark and an anti-strange quark
2. Particles that are un affected by strong nuclear force are
a. Protons c. Neutrons
b. Leptons d. Bosons
3. Particle which explains about mass of matter is called
a. Higgs boson c. Leptons
b. Protons d. Neutrons
4. Describe the types and the characteristics of the quarks as well as their
interaction properties.
8.3 BARYON NUMBER, LEPTON NUMBER AND THEIR LAWS OFCONSERVATION
ACTIVITY 8.3: Investigating about particle numbers
Use search internet and retrieve the meaning of the following property
of elementary particles.• Baryon numbers and• Lepton numbers
One of the important uses of high energy accelerators is to study the interactions
of elementary particles with each other. As a means of ordering this sub-nuclear
world, the conservation laws are indispensable. The law of conservation of
energy, of momentum, of angular momentum, and of electric charge is found tohold precisely in all particle interactions.
A study of particle interactions has revealed a number of new conservation laws
which (just like the old ones) are ordering principles. They help to explain why
some reactions occur and others do not. For example, the following reactionshave never been found to occur:

Even though charge, energy, and so on are conserved
 means an antiproton and
means an antiproton and  means the reaction does not occur). To understand why such a reaction does
means the reaction does not occur). To understand why such a reaction does
not occur, physicists hypothesized a new conservation law, the conservationof baryon number.
Thus the law of conservation of baryon number states that: “Whenever a nuclear
reaction or decay occurs, the sum of baryon numbers before the process mustequal the sum of the baryon numbers after the process.”
Baryon number is a generalization of nucleon number, which is conserved in
nuclear reaction and decays. All nucleons are defined to have baryon number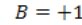 , and all antinucleons (antiprotons, antineutrons) have
, and all antinucleons (antiprotons, antineutrons) have  . All
. All
other types of particles, such as photons, mesons, and electrons and otherleptons have
The reaction (9.01) shown above does not conserve baryon number since the .
.
left side B = +1+1 = +2 , and the right-hand side has B = +1+1−1 = +1 On
the other hand, the following reaction does conserve B and does occur if theincoming proton has sufficient energy
As indicated,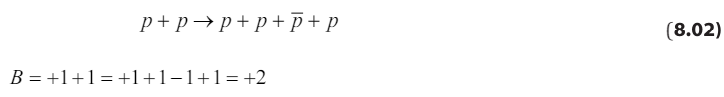
 on both sides of this equation. From these and other
on both sides of this equation. From these and other
reactions, the conservation of baryon number has been established as basicprinciple of physics.
Also useful are the conservation laws of the three lepton numbers, associated
with weak interactions including decays, in ordinary decay, an electron or
decay, an electron or
positron is emitted along with a neutrino or antineutrino. In a similar type of
decay, a particle known as or mu meson, or muo, can be emitted instead of
an electron. The muon seems to be much like an electron, except its mass is
207 times larger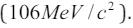 The neutrino
The neutrino that accompanies an emitted
that accompanies an emitted
electron is found to be different from the neutrino that accompanies an
that accompanies anemitted muon. Each of these neutrinos has an antiparticle
The law of conservation of electron-lepton number states that: “The sum of the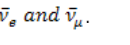
electron-lepton numbers before reaction or decay must equal the sum of the
electron-lepton numbers after the reaction or decay.”In ordinary decay we have for example,, a second quantum number, muon
lepton number, is conserved. The and are assigned and and havewhereas other
particles have, too is conserved in interaction and decays. Similarly assignment
can be made for the tau lepton number associated with the Lepton and itsneutrino,
Keep in mind that antiparticles have not only opposite electric charge fromtheir particles, but also opposite
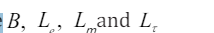
For example, neutrino has B = 1 an antineutrion has B = −1while all the

The particle predicted by Yukawa was discovered in cosmic rays by C.F Powell
and G. Ochialini in 1947, and is called the π or pi meson, or simple called pion.
The incident proton from the accelerator must have sufficient energy to produce
the additional mass of the free pion. Baryon number conservation keeps the
proton stable, since it forbids the decay of the proton to e.g. a 0 π and a + π eachof which have baryon number of zero.
8.3.1 Checking my progress
8.4 SPIN STRUCTURES OF HADRONS (HADRONS AND MESONS)
ACTIVITY 8.4: Investigating the structure of elementary particles1. Use search internet and find the structure of elementary particles:
Hadrons and mesons.
2. Discuss and explain your findings in a brief summary aboutstructure of hadrons.
There are hundreds of hadrons, on the other hand, and experiments indicate
they do have an internal structure. In 1963, M. Gell-Mann and G. Zweig
proposed that none of the hadrons, not even the proton and neutron, are
more fundamental, point like entities called, somewhat whimsically, quarks.
Today, the quark theory is well accepted, and quarks are considered the trulyelementary particles, like leptons. The three quarks originally proposed we
labeled u, d, s and have the names up, down and strange. The theory today hassix quarks, just as there are six leptons based on presumed symmetry in nature,
The other three quarks are called charmed, bottom and top (see Fig.8.2). The
theory names apply also to new properties of each (quantum numbers c, t, b)
that distinguish the new quarks from the old quarks (see Table 9.1below), andwhich (like strangeness) are conserved in strong, but not weak, interactions.
Table 8. 1 Properties of Quarks (Antiquarks have opposite sign Q, B. S, c, b and t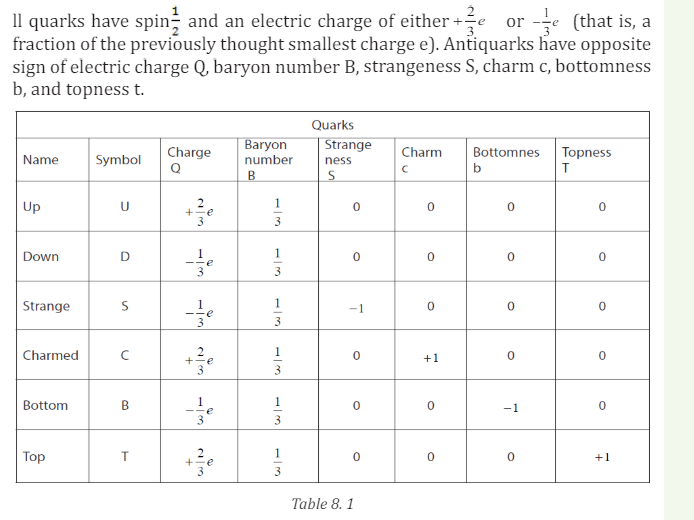
All hadrons are considered to be made up of combinations of quarks, and their
properties are described by looking at their quark content. Mesons consist of
quark-antiquark pair (See Table 8.2).For example, a + π meson is a ud combination: note that for the ud pair,
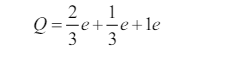

After the quark theory was proposed, physicists began looking for those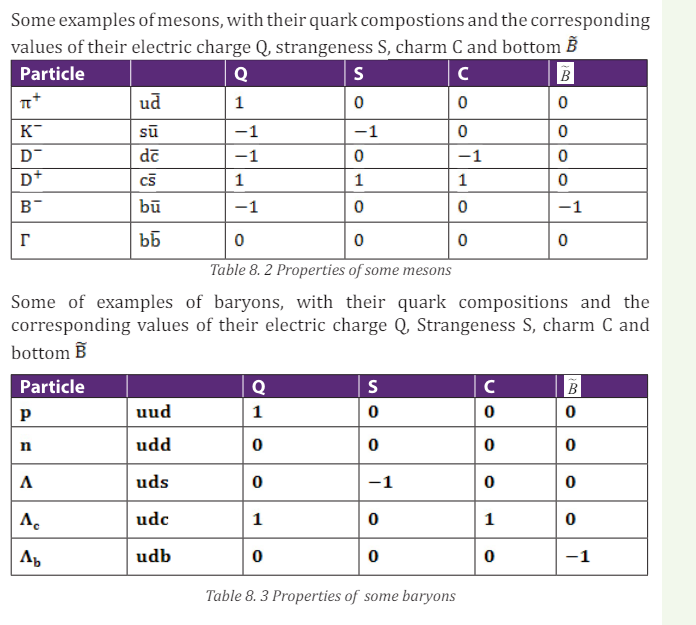
fractionally charged particles, but direct detection has not been successful.
Current models suggest that quarks may be so tightly bound together that they
may not ever exist singly in the free State. But observations of very high energy
electrons scattered off protons suggest that protons are indeed made up ofconstituents.
Today, the truly elementary particles are considered to be the six quarks, the
six leptons and the gauge bosons that carry the fundamental forces. See Table
9.4 where the quarks and leptons are arranged in three “generations.” Ordinary
matter-atoms made of protons, neutrons, and electrons are contained in the
“first generation”. The others are thought to have existed in the very early
universe, but are seen by us today at powerful accelerators or in cosmic rays.
All of the hundreds of hadrons can be accounted for by combinations of the sixquarks and six antiquarks.
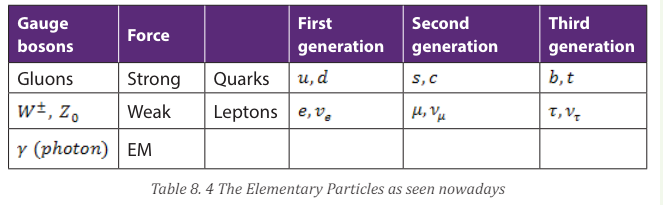
Note that the quarks and leptons are arranged into three generations each
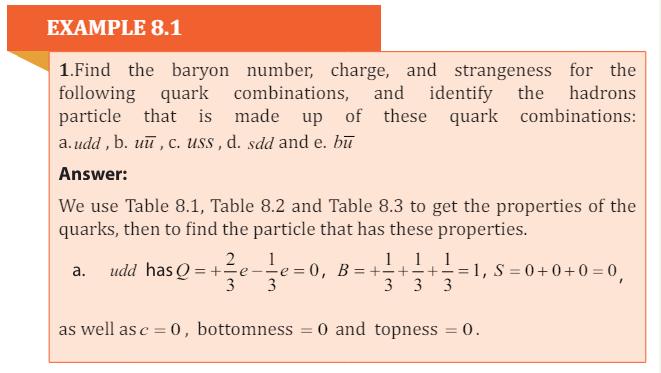
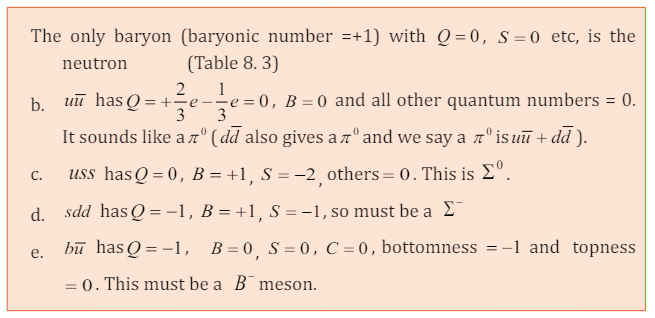
8.4.1 Checking my progress
8.5 COLOR IN FORMING OF BOUND STATES OF QUARKS
ACTIVITY 8.5: Investigating the bound state of an atom
Take the case of an electronic configuration of hydrogen atom. Make
the illustration and then contrast the interaction between electron andproton and bound state of elementary particles.
8.5.1 Bound state of quarks
In the hydrogen atom configuration, the proton is located at centre while
electron moves around it at a speed of about 1% the speed of light. The protonis heavy while the electron is light (See Fig.8.3)
This is the simplest example of what physicists call a “bound state”. The word
“state” basically just meaning a thing that hangs around for a while, and the
word “bound” meaning that it has components that are bound to each other, asspouses are bound in marriage.
The inside of the proton itself is more like a commune packed full of single
adults and children: pure chaos. It too is a bound state, but what it binds is
not something as simple as a proton and an electron, as in hydrogen, or even a
few dozen electrons to an atomic nucleus, as in more complicated atoms such
as gold, but zillions (meaning “too many and too changeable to count usefully”)
of lightweight particles called quarks, antiquarks and gluons. It is impossible
to describe the proton’s structure simply, or draw simple pictures, because it’s
highly disorganized. All the quarks and antiquarks and gluons (see Fig.8.4)
inside are rushing around as fast as possible, at nearly the speed of light(Strassler, 2011).
Fig.8. 4 Snapshot of a proton: Imagine all of the quarks (up, down, and strange:
u, d, s), antiquarks (u, d, s with a bar on top), and gluons (g) zipping around near
the speed of light, banging into each other, and appearing and disappearing
(Strassler, 2011). You may have heard that a proton is made from three quarks
but this is not true. In fact there are billions of gluons, antiquarks, and quarksin a proton.
8.5.2 Color in forming of bound states of quarks.
In the standard model of Quantum Chromodynamics (QCD) and the electroweak
theory (Giancoli D. C., Physics: principals with application, 2005), not long after
the quark theory was proposed, it was suggested that quarks have another
property (or quality) called color, or ‘color charge’ (analogous to electric
charge). The distinction between the six quarks (u, d, s, c, b, t) was referred toas flavors.
According to the theory, each of the flavors of quark can have three colors,
usually designated red, green and blue. These are the three primary colors
which, when added together in equal amounts, as on a TV screen, produce
white. Note that the names ‘color’ and ‘flavor’ have nothing to do with our
sense, but are purely whimsical as are other names, such as charm, in this new
field.The antiquarks are colored antired, antigreen and antiblue. Baryons are
made up of three quarks, one of each color. Mesons consist of quark-antiquark
pair of a particular color and its anti color. Both baryons and mesons are thuscolorless or white.
Originally, the idea of quark color was proposed to preserve the Pauli exclusion
principle. Not all particles obey the exclusion principle. Those that do, such
as electrons, protons and neutrons, are called fermions. Those that don’t are
called bosons. These two categories are distinguished also in their spin: bosons
have integer spin (0, 1, etc) whereas fermions have half-integer spin, usual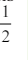
as for electrons and nucleons, but other fermions have spin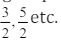
Matter is made up mainly of fermions, but the carriers of forces ( and
gluons) are all bosons. Quarks are fernions they have spin 2
1 and therefore
should obey the exclusion principle. Yet for three particular baryons (uuu, ddd,
and sss), all three quarks would have the same quantum numbers, and at least
two quarks have their spin in the same quantum numbers, and at least twoquarks have their spin in the same direction (since there are only two choices,
This would seem to violate the exclusion principle; but if quarks have an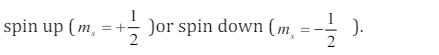
additional quantum number (color), which is different for each quark, it would
serve to distinguish them and allow the exclusion principle to hold. Although
quark color, and the resulting threefold increase in the number of quarks, was
originally an adhoc idea, it also served to bring the theory into better agreement
with experiment, such as predicting the correct lifetime of the π 0 meson. The
idea of color soon became a central feature of the theory as determining theforce binding quarks together in hadron.
8.5.3 Colour as component of quarks and gluons
ACTIVITY 8.6: Investigating the origin of color
When a metal like iron is heated red-hot, one can observe the change
in color. As the energy increases, as the color changes. Use the same
experiment and discuss on the following questions
1. As the color changes in the metal, what are the scientific reasons
behind that?
2. Explain the matter −energy interaction and their consequences3. What is color in the field of elementary particles?
The attractive interactions among quarks are mediated by massless spin
bosons called gluons in much the same way that photons mediate the
electromagnetic interaction or that pions mediated the nucleon–nucleon force in theold Yukawa theory (Nagashima, 2013).
Particles were classified into two categories:
Quarks and leptons have an intrinsic angular momentum called spin, equal to
a half-integer ( ) of the basic unit and are labeled as fermions. Fermions obey
) of the basic unit and are labeled as fermions. Fermions obey
the exclusion principle on which the Fermi-Dirac distribution function is based.
This would seem to forbid a baryon having two or three quarks with the same
flavor and same spin component. To avoid this difficulty, it is assumed that each
quark comes in three varieties, which are called color: red, green, and blue. The
exclusion principle applies separately to each color. Particles that have zero or
integer spin are called bosons. Bosons do not obey the exclusion principle and
have a different distribution function, the Bose-Einstein distribution.• A baryon always contains one red, one green, and one blue quark, soSimilar processes occur in mesons such as pions:
the baryon itself has no net color.
• Each gluon has a color–anticolor combination (for example, blue–
antired) that allows it to transmit color when exchanged, and color is
conserved during emission and absorption of a gluon by a quark.
• The gluon-exchange process changes the colors of the quarks in such
a way that there is always one quark of each color in every baryon.
The color of an individual quark changes continually as gluons areexchanged.
• The quark–antiquark pairs of mesons have canceling color andThe gluon is then absorbed by the antiblue antiquark, converting it to an antired
anticolor (for example, blue and antiblue), so mesons also have no net
color. Suppose a pion initially consists of a blue quark and an antiblue
antiquark.
• The blue quark can become a red quark by emitting a blue–antiredvirtual gluon.
antiquark (Fig. 8.8). Color is conserved in each emission and absorption,
but a blue–antiblue pair has become a red–antired pair. Such changes occur
continually, so we have to think of a pion as a superposition of three quantumstates:
• Blue–antiblue,In terms of quarks and gluons, these mediating virtual mesons are quark–
• Green–antigreen, and• Red–antired.
antiquark systems bound together by the exchange of gluons.
Fig.8. 5 (a) A pion containing a blue quark and an antiblue antiquark. (b) The
blue quark emits a blue–antired gluon, changing to a red quark. (c) The gluon is
absorbed by the antiblue antiquark, which becomes an antired antiquark. The
pion now consists of a red–antired quark–antiquark pair. The actual quantum
state of the pion is an equal superposition of red–antired, green antigreen, andblue–antiblue pairs.
8.5.4 Checking my progess
1. Label the illustration below and analyze the interaction between itsparticles
Define and describe the following key concept:
I. Color charge:
II. Gluons
III. Quantum chromodynamics
2. Which one of the following sets of color combinations is added in color
vision in TV’?
a. Red, green and blue c. White. red and yellow
b. Orange, back and violet d. Yellow, green and blue
3. What are the color composition of
a. Gluons
b. Mesonc. Baryon
END UNIT ASSESSMENT 8
A. Multiple choices
1. A proton is made up of
a. One up quark and two down quarks
b. An up quark and down antiquark
c. Two up quarks and a down quark
d. Strange quark and an antistrange quark
2. Particles that are unaffected by strong nuclear force are
a. Protons c. Neutrons
b. Teptons d. Bosons
3. Particle which explains about mass of matter is calleda. Higgs boson c. Protons4. A conservation law that is not universal but applies only to certain
b. Leptons d. Neutrons
kinds of interactions is conservation of:a. Lepton number d. Charge5. In quantum electrodynamics (QED), electromagnetic forces are
b. Baryon number e. Strangeness
c. Spin
mediated bya. the interaction of electrons.
b. hadrons.
c. D. the weak nuclear interaction.
d. action at a distance.e. E. the exchange of virtual photons.6. Conservation laws that describe events involving the elementary
particles include the conservation of energy.a. All of these are correct.7. The conservation law violated by the reaction
b. electric charge.
c. baryon and lepton numbers.d. linear and angular momentum.
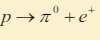 is the
is the
conservation ofa. Charge.8. Particles that participate in the strong nuclear interaction are called
b. Energy.
c. Linear momentum.
d. Lepton number and baryon number.
e. Angular momentum.a. NeutrinosB. Structured questions
b. Hadrons
c. Leptons
d. Electrons
e. Photons
9. In the table cross-word below, find at least fifteen names associated to
elementary particles. Among them, select ones that represents quarks,leptons or radiations.
10.a. Making massive particles: Relatively massive particles like the proton
and neutron are made of combinations of three quarks.
I. What is the charge on the combination uuu?
II. What is the charge on the combination uud?
III. What is the charge on the combination udd?
IV. What is the charge on the combination ddd?
b. There are four compound particles here
I. Which combination has the right charge to be a proton?II. Which combination has the right charge to be a neutron?
III. There is a particle called the which has a charge of –1e. Which
which has a charge of –1e. Whichquark combination could be the
IV. There is a particle called the ∆++ which has a charge of + 2e. Which
quark combination could be the ∆− ?
V. A neutron can be changed to a proton if one quark changes ‘flavour’.
What change is needed? What charge must be carried away if thishappens?
c. Making mesons
Other, lighter ‘middle-weight’ particles called mesons can be made from
pairs of quarks. But they have to be made from a special combination: a
quark and an antiquark. There are now four particles to play with: Up
quark u: charge +2/3 e, Down quark d: charge –1/3 e, Antiup quark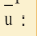
charge –2/3 e. Antidown quark
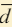 : charge + 1/3 e.
: charge + 1/3 e.

UNIT 9: EFFECT OF X-RAYS
Key unit competence: BAnalyze and evaluate the effects of x-rays.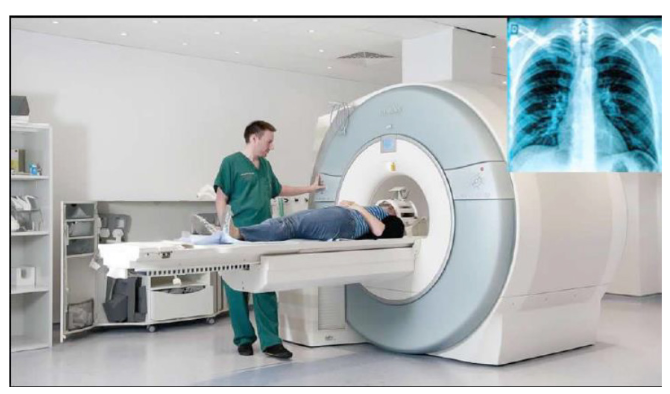
My goals• Explain the production of X-rays
• State the properties of X-rays.
• Explain the origin and characteristic features of an x-ray spectrum.
• Outline the applications of X-rays in medicine, industries, and scientific
research
• Solve problems involving accelerating potential and minimum
wavelength of X-rays.
• Recognize how the intensity and quality of X-rays can be controlled.
• Appreciate the use of X-rays in medicine and industryWhen a person goes to the hospital with pain in her/his chest, or with anINTRODUCTORY ACTIVITY
internal fracture of the bone, physicians do normally recommend the patient
to pass by radiology service. Hence try to answer the following questions:1. Why do physicians recommend patients to pass by radiology service?9.1 PRODUCTION OF X-RAYS AND THEIR PROPERTIES
2. Radiology means that there are radiations. Discuss different types of
radiations that are found in there?
3. Discuss the production of X-ray radiations.
4. What are the positive and negative effects of X-ray radiation on thehuman body?
ACTIVITY 9.1: Investigating the production of X-rays
Read the following text and answer the questions that follow.
Discovery of X-rays: Becquerel’s discovery wasn’t the only important
accidental one. In the previous year W.C. Roentgen unexpectedly
discovered X-rays while studying the behavior of electrons in a
high voltage vacuum tube. In that instance, a nearby material was made to
fluoresce. Roentgen named them X- rays because he didn’t know what
they were.
Within twenty years of this discovery, diffraction patterns produced
using X-rays on crystal structures had begun to show the finer structure
of crystals while, at the same time, giving evidence that X-rays had a
wave nature. Since then, X-ray radiation has become an indispensable
imaging tool in medical science.
Questions:1. What do you understand by X-rays?9.1.1 X-ray production
2. How are X-rays produced?3. Where are X-rays used?
X-rays are produced when fast moving electrons strike matter (see Fig.9.1).
They were first produced in 1895 by Wilhelm Rontgen (1845-1923), using anapparatus similar in principle to the setup shown in Fig.9.1.
Electrons are emitted from the heated cathode by thermionic emission and are
accelerated toward the anode (the target) by a large potential difference V. The
bulb is evacuated (residual pressure 10−7 atm or less), so that the electrons can
travel from the cathode to the anode without colliding with air molecules. It
was observed that when V is a few thousands volts or more, a very penetratingradiation is emitted from the anode surface.
The above figure is an illustration of the Coolidge tube which is the most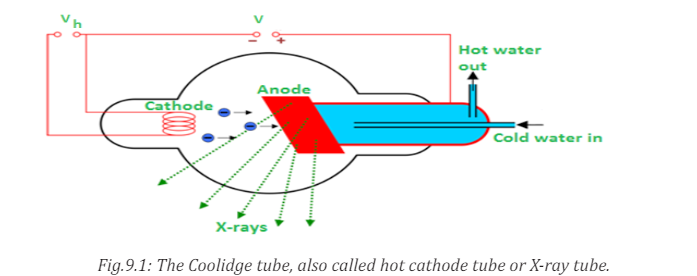
widely used device for the production of X-rays. The electrons are produced by
thermionic effect from filament, which is the cathode of the tube, heated by an
electric current. These electrons are accelerated towards a metal target that isthe anode due to the high potential voltage between the cathode and the anode.
The target metals are normally Tungsten or Molybdenum and are chosen
because they have high melting point and higher atomic weights. The accelerated
electrons interact with both electrons and nuclei of atoms in the target and
a mysterious radiation is emitted. This radiation was referred to as X-rays.
About 98% of the energy of the incident electron is converted into heat that isevacuated by the cooling system and the remaining 2% come out as X-rays.
9.1.2 Types of X-rays
Sometimes X-rays are classified according to their penetrating power. Two
types are mentioned:• Hard X-rays: those are X-rays on upper range of frequencies or
shorter wavelength. They have greater energy and so they are more
penetrating.
• Soft X-rays: they are X-rays on lower range of frequencies or
longer wavelength. They have lower energy and they have very low
penetrating power. The Fig.9.2 below shows the relative location of thedifferent types of X-rays.
Hard X-rays are produced by high accelerating potential. They have high
penetrating power and short wavelength while soft X-rays are produced
by lower accelerating potential, have relatively low penetrating power andrelatively long wavelength.
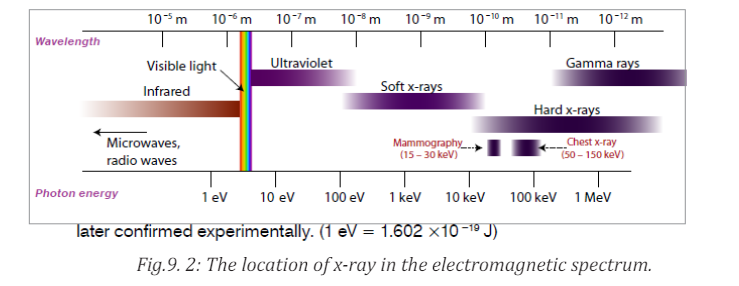
9.1.3 Properties of X-rays
ACTIVITY 9.2: Understanding the pros and cons of X-rays
Make intensive research on the production and the properties of
X-rays, then write a report about your findings.
The following are the main properties of X-rays:a. X-rays can penetrate through most substances. However, their penetratingFrom the above characteristics it can be seen that X-rays have the properties
power is different.
b. X-ray can produce fluorescence in different substances.
c. X-rays can blacken photographic plate. The degree of blackening depends
upon the intensity of x-rays incident upon the plate. Thus, X-ray intensity
can be measured with the help of photographic plates.
d. X-rays ionize the gas through which they travel. The ionizing power
depends on the intensity of the x-ray beam. Thus, X-ray intensity can also
be measured by measuring their ionizing power.
e. X-rays are not deflected by electric or magnetic fields. This proves that
unlike cathode rays or positive rays they are not a beam of charged
particles.
f. X-rays travels on a straight lines like ordinary light.
g. X-ray are both reflected and refracted.
h. X-rays can be diffracted with the help of crystalline substances. They canalso be polarized.
that are common to all electromagnetic radiations.9.1.4 Checking my progress
1. Describe the process by which X-rays are produced.
2. Discuss and describe the types of X-rays?
3. What is the meaning of the X in X-ray?4. How are X-rays different from other electromagnetic radiations?
9.2 THE ORIGINS AND CHARACTERISTIC FEATURES OF AN
X-RAY SPECTRUM
ACTIVITY 9.3: investigating the X-ray spectrum
During the production of X-rays, a high voltage must be applied across
the x rays tube to produce enough acceleration of electrons towards the
target.
Search internet, then discuss and explain the relationship between theapplied
9.2.1 Variation of the X-ray intensity with wavelength
Depending on the accelerating voltage and the target element, we may find
sharp peaks superimposed on a continuous spectrum as indicated on Fig.9.3.
These peaks are at different wavelengths for different elements; they form whatis called a characteristic x-ray spectrum for each target element.
X-rays of different wavelengths are emitted from X-ray tube. If the intensity is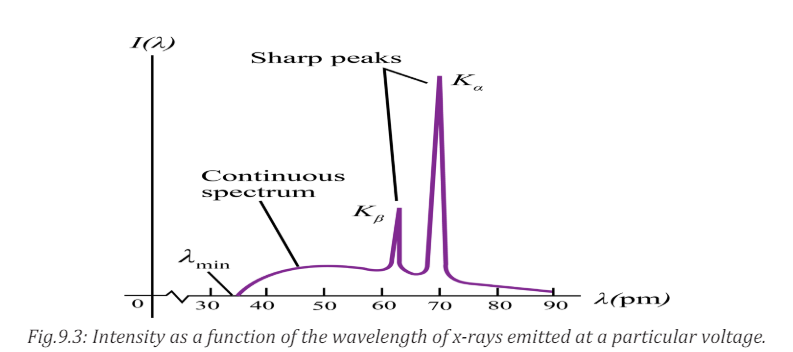
measured as a function of the wavelength and the variation is plotted graphically
then a graph of the nature shown on the figure above is obtained.The graph has
the following features:a. Minimum wavelength9.2.2 Origin of the continuous spectrum
b. Continuous spectrumc. Characteristic peaks
It is known that when charged particles such as electrons are accelerated or
decelerated they emit electromagnetic radiation of different frequencies.
In doing so a part of their kinetic energy is transformed in the energy of the
emitted radiation. Electrons inside the x-ray tube decelerate upon hitting the
target and as a result they emit electromagnetic radiations with a continuous
distribution of wavelength starting from a certain minimum wavelength. This
mechanism of producing electromagnetic radiation from an accelerated ordecelerated electron is called bremsstrahlung.
The energy of the emitted photon is given by

The maximum energy of the emitted photons is therefore equal to the energy
of the incident electron:
Where
 is the minimum wavelength, V is the potential difference between
is the minimum wavelength, V is the potential difference between
anode and cathode and e the charge of the electron.If V is measured in volts we get
As the many electrons in the X-ray are decelerated differently, this will result in
a continuous spectrum of the emitted wavelengths.
It can be observed from the above Fig.9.4 that, for different values of the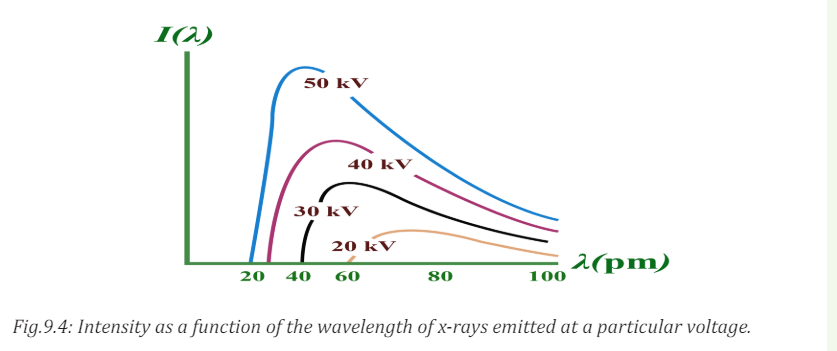
accelerating voltage, the minimum wavelength decreases with increasing
potential difference and for a given wavelength the intensity is higher when thepotential difference is higher.
9.2.3 Origin of characteristic lines
The peaks observed in wavelengths distribution curves as shown in Fig. 9.4
are spectral lines in the X-ray region. Their origin lies in the transition between
energy levels in the atoms of the target.The electrons in the atoms are arranged
in different atomic shell. Of these, the first two electrons occupy the K-shell
followed by 8 electrons in the L-shell, 18 electrons in the M-shell and so on
until the electron in the target are used up. A highly accelerated electron may
penetrate atom in the target and collide with an electron in K-shell. If such
electron is knocked out it will leave an empty space that is immediately filled
up by another electron probably from the L-shell or M-shell. This transitionwill be accompanied by the emission of the excess energy as a photon.
The energy of the emitted photon is a characteristic of the energy levels in theparticular atom and is given by
For a transition between K and L-shells.
Thus the energy of the emitted photon depends on the binding energies in the
K and L shells and hence the x-ray spectral lines have definite frequencies and
wavelengths which are characteristic of the target atom.
For a given target material more than one spectral lines are observed astransitions may occur between different energy levels.
The X-ray lines originating from the transition between the different electron
levels are usually labelled by the symbols α, β, γ, etc.
From L-level to K-level transition produces Kα-line
From M-level to K-level transition produces Kβ
–line
From M-level to L-level transition produces Lα –line
From N-level to L- level transition produces Lβ –line
9.2.4 Checking my progress
1. What is the characteristic of X-ray characteristic peak radiation?
2. How is X-ray continuum produced via bremsstrahlung?
3. X-rays are generated when a highly accelerated charged particle such
aselectrons collide with target material of an X-ray tube. The resulting
X-rays have two characteristics: the continuous X-rays (also called white
X-rays) and characteristic X-rays peaks. The wavelength distribution and
intensity of continuous X-rays are usually depending upon the applied
voltage and a clear limit is recognized on the short wavelength side.
a. Estimate the speed of electron before collision when applied voltage is
30kV and compare it with the speed of light in vacuum.
b. In addition, establish the expression of the shortest wavelength limit
λmin of X-rays generated with the applied voltage V. it is obtained whenthe incident electron loses all its energy in a single collision.
9.3 APPLICATIONS AND DANGERS OF X-RAYS
ACTIVITY 9.4: investigating the X-ray uses and dangers
1. Using the historical background of X-ray discovery, what are the
uses of X-rays in real life?
2. Discuss the dangers that X-rays may cause when they are used in awrong way.
X-rays have many practical applications in medicine and industry. Because X-ray
photons are of such high energy, they can penetrate several centimetres of solid
matter. Hence they can be used to visualize the interiors of materials that areopaque to ordinary light, such as broken bones or defects in structural steel.
9.3.1 In medicine
X-ray imaging utilizes the ability of high frequency electromagnetic waves to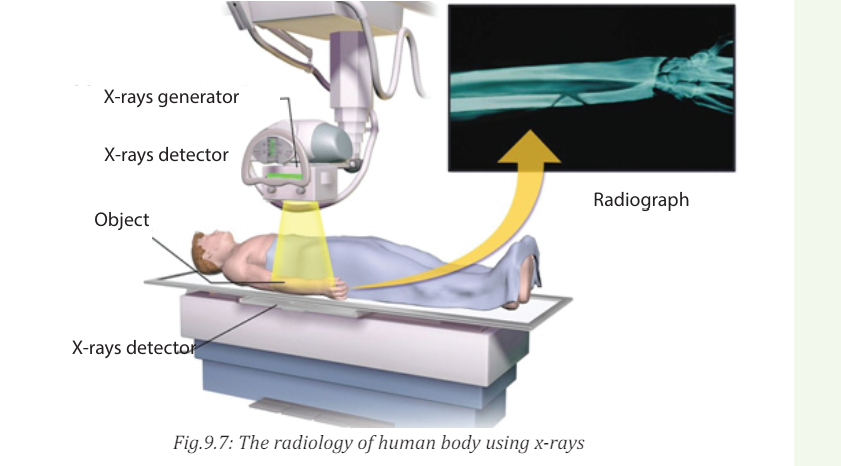
pass through soft parts of the human body largely unimpeded. For medical
applications, parts of the human body are exposed to moderated X-rays
intensity and images are produced in similar way as light on a photographic
plate or digital recorder to produce a radiograph (See Fig.9.7).
By rotating both source and detector around the patient’s body a “slice” image
can be produced in what is called computerized tomography (CT). Although CT
scans expose the patient to higher doses of ionizing radiation the slice imagesproduced make it possible to see the structures of the body in three dimensions.
In 1895, the Dutch Wilhelm Roentgen (See Fig.9.8) discovered that light energy
could be used to take photographs through substances such as paper, cloths
and wood. Roentgen also discovered that this invisible form of light energy,
called X-rays could be used to take the pictures of structures inside the body asshown in Fig. below. Bone tissue appears clearly on an X-rays.
The object to be visualized is placed between an X-ray source and an electronic
detector (like that used in a digital camera) or a piece of photographic film
(Fig.9.8 or Fig.9.8B). The darker area in the recorded images by such a detector,
the greater the radiation exposure. Bones are much more effective X-ray
absorbers than soft tissue, so bones appear as light areas. A crack or air bubbleallows greater transmission and shows as a dark area.
A widely used and vastly improved x-ray technique is computed tomography;
the corresponding instrument is called a CT scanner. The x-ray source produces
a thin, fan-shaped beam that is detected on the opposite side of the subject by an
array of several hundred detectors in a line. Each detector measures absorption
along a thin line through the subject. The entire apparatus is rotated around
the subject in the plane of the beam, and the changing photon-counting rates of
the detectors are recorded digitally. A computer processes this information and
reconstructs a picture of absorption over an entire cross section of the subject.
In the middle 1970, CT (Computer Tomography) scanning machines were
introduced in human medicine.
X-rays are also used in the following:• Killing of cancerous cells
• Radiography is also used in industry for examining potentially damaged
machinery to ascertain the cause of damage and to verify castings orwelded joints
• X-rays are used to study the structure of crystals (crystallography).
• When a handgun is fired, a cloud of gunshot residue (GSR) is ejected from
the barrel. The x-ray emission spectrum of GSR includes characteristic
peaks from lead (Pb), antimony (Sb), and barium (Ba). If a sample taken
from a suspect’s skin or clothing has an x-ray emission spectrum with
these characteristics, it indicates that the suspect recently fired a gun.9.3.2 Examining luggage cargo and security
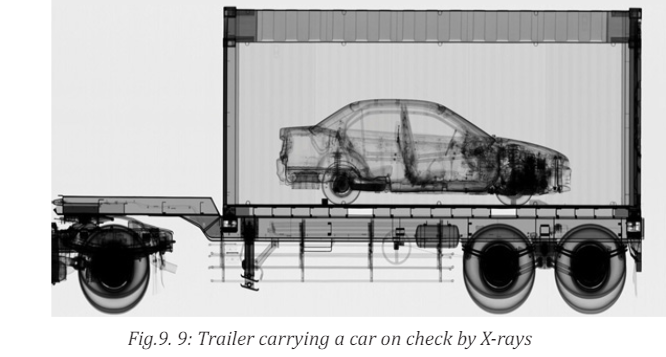
X-rays are being used in airports to examine luggage for weapons or bombs.
Note that the metal detector that you walk through in the airport does not X-ray
you. It uses magnetic waves to detect metal objects. X-rays are also being usedto examine cargo luggage for illegal or dangerous material as in Fig.9.9.
9.3.3 In industry

They can be used to detect structural problems and cracks in metals
that cannot be seen from the outside. X-rays are used on commercial airplanes,
bridges metals and pipe lines, to make sure there are no stress fracturesor other dangerous cracks in the material.
9.3.4 In scientific research• X-ray diffraction provides one of the most important tools for examining
the three-dimensional (3D) structure of biological macromolecules
and cells.
• They are also used in crystallography, where X-ray diffraction and
scattered waves show the arrangement of atoms in the crystal.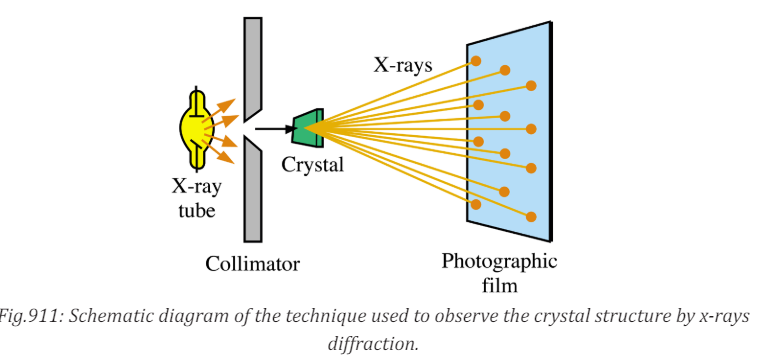
The array of spots formed on the film is called a Laue pattern and show the
atom structure of the crystal.
9.3.5 Dangers of X-rays• X rays cause damage to living tissues. As X-ray photons are absorbed
in tissues, their energy breaks molecular bonds and creates highly
reactive free radicals (such as neutral H and OH), which in turn can
disturb the molecular structure of proteins and especially genetic
material. Young and rapidly growing cells are particularly susceptible,
which is why X-rays are useful for selective destruction of cancer cells.
• Because X-rays can kill living cells, they must be used with extreme care.
When improperly used they can cause severe burns, cancer, leukemia,
and cataracts. They can speed aging, reduce immunity to disease, and
bring about disastrous changes in the reproductive cells.
• Lead screens, sheets of lead-impregnated rubber, and leaded glass are
used to shield patients and technicians from undesired radiation.• The effect of X-ray radiations is cumulative. That is, many minor doses9.3.6 Safety precaution measures of dangers caused by X-rays
over a number of years is equivalent to a large dose at one time.
• Unnecessary exposure to x-rays should be avoided. MRI (Magnetic
Resonance Imaging) uses magnets and sound energy to form pictures
of the internal organs without exposing patients to harmful X-rays.
• When they are used in hospitals, the sources should be enclosed in
lead shields.
• A careful assessment of the balance between risks and benefits ofradiation exposure is essential in each individual case.
Medical and dental X-rays are of very low intensity, so that the hazard is
minimized. However, X-ray technicians who go frequently behind the lead
shield while operating X-rays need to be protected because of the frequency
of exposure. A person can receive many medical or dental X-rays in a year with
very little risk of getting cancer from it. In fact, exposure to natural radiationsuch as cosmic rays from space poses a greater risk.
The following are some of the precautions:i. Protective suits and wears such as gloves and eye glasses made of lead are9.3.7 Checking my progress
used always when handling these radiations. These shields protect the
workers from X-ray exposure.
ii. Workers who operate equipment’s that use X-rays must wear special
badges which detect the amount of radiation they are exposed to.
iii. Food and drinks are not allowed in places where X-radiations are present.
iv. Experiments that involve these radiations (X-rays) substances should be
conducted in a room surrounded by thick concrete walls or lead shields.
v. Equipment that use X-rays should be handled using remote-controlledmechanical arms from a safe distance.
1. How do we create different X-ray images in medicine?9.4 PROBLEMS INVOLVING ACCELERATING POTENTIAL AND
2. What are the dangers that may be caused by using excessive dose ofX-rays?
MINIMUM WAVELENGTH.
9.4.1 Accelerating potential and minimum wavelength
ACTIVITY 9.5: Calculation of accelerating potential in X-ray tube
An x-rays tube operates at 30 kV and the current through it is 2.0 mA.
Calculate:a. The electrical power outputWhen a high voltage with several tens of kV is applied between two electrodes,
b. The number of electrons striking the target per second.
c. The speed of the electrons when they hit the targetd. The lower wavelength limit of the X-rays emitted.
the high-speed electrons with sufficient kinetic energy is drawn out from the
cathode and collides with the anode. The electrons rapidly slow down and lose
kinetic energy. Since the slowing down patterns(method of losing kinetic
energy)varies with electrons, continuous X-rays with various wavelength
are generated. When an electron loses all its energy in a single collision, the
generated X-ray has the maximum energy (or the shortest wavelength
). The value of the shortest wave length limit can be estimated from the
accelerating voltage V between electrodes.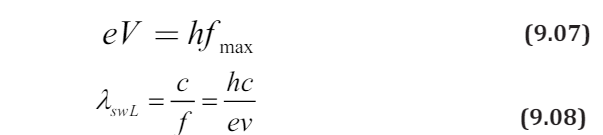
Because X-rays are emitted by accelerated charges, x-rays are electromagnetic
waves. Like light, X-rays are governed by quantum relationships in their
interaction with matter. Thus, we can talk about X-ray photons or quanta, and
the energy of an X-ray photon is related to its frequency and wavelength in thesame way as for photons of light,

Typical X-ray wavelengths are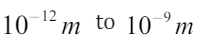 . X-ray wavelength can be
. X-ray wavelength can be
measured quite precisely by crystal diffraction techniques. X-ray emission
is the inverse of the photoelectric effect. In photoelectric emission there is a
transformation of the energy of a photon into the kinetic energy of an electron,
in X-ray production there is a transformation of the kinetic energy of an electron
into energy of a photon. In X-ray production we usually neglect the work
function of the target and the initial kinetic energy of the boiled off electrons
because they are very small in comparison to the other energies.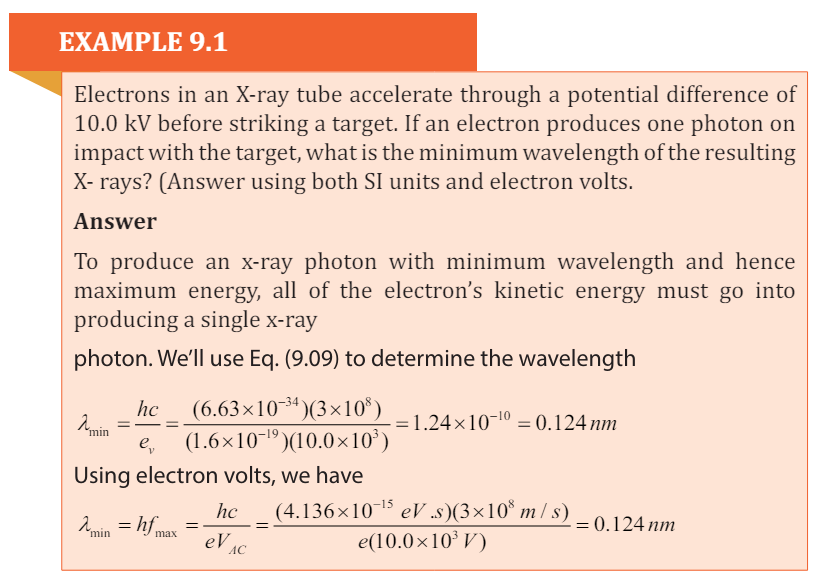
Bragg’s Law
According to W. L. Bragg ( (Weseda, Mastubara, & Shinoda, 2011), X-ray
diffraction can be viewed as a process that is similar to reflection from planes of
atoms in the crystal. In Bragg’s construct, the planes in the crystal are exposed
to a radiation source at a glancing angle θ and X rays are scattered with an angle
of reflection also equal to θ. The incident and diffracted rays are in the same
plane as the normal to the crystal planes (Fig.9. 4).
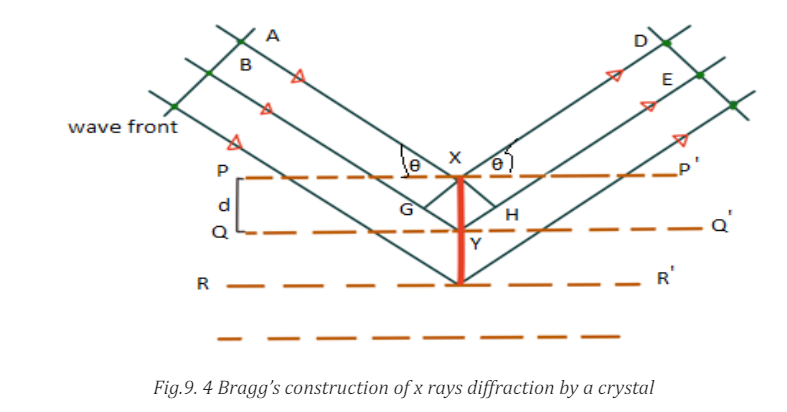
Constructive interference occurs only when the path difference between rays
scattered from parallel crystal planes would be an integer number of wavelengths
of the radiation. When the crystal planes are separated by a distance d, the path
length difference would be 2dsin θ. Thus, for constructive interference to occurthe following relation must hold true.

The above derivation assumes that phase differences between wavelengths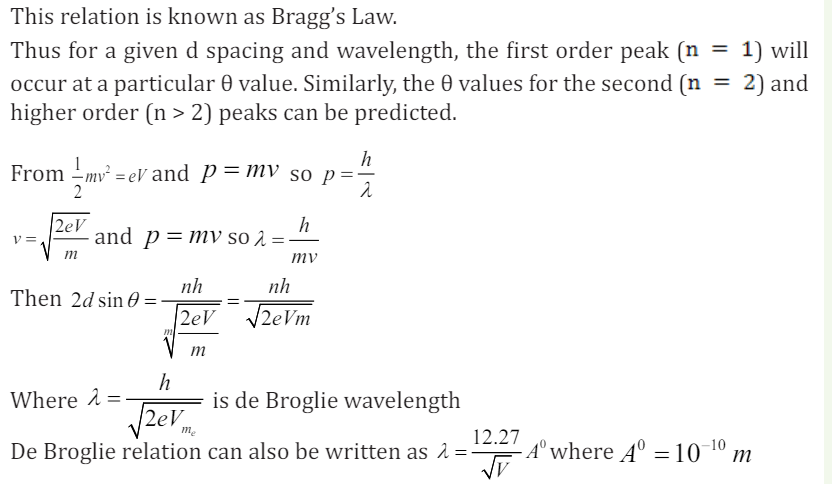
scattered at different points depend only on path differences. It is assumed that
there is no intrinsic phase change between the incident and scattered beams or
that this phase change is constant for all scattering events.
9.4.2 Checking my progress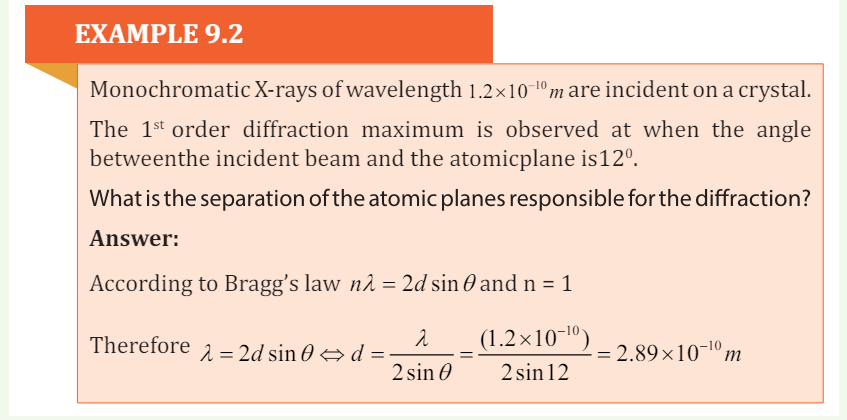
1. Calculatea. Strength of the electric field E,2. Crystal diffraction experiment can be performed using X-rays, or
b. Force on the electron F,
c. Acceleration a of electron, when a voltage of 10 kV is applied between
two electrodes separated by an interval of 10 mm.
electrons accelerated through appropriate voltage. Which probe has
greater energy?(For quantitative comparison,take the wavelength of the
probe equalto1Å, which is of theorderofinter- atomicspacinginthelattice)(me = 9.11×10−31kg).
END UNIT ASSESSMENT 9

a. There are two main components of this x-ray spectrum: a broad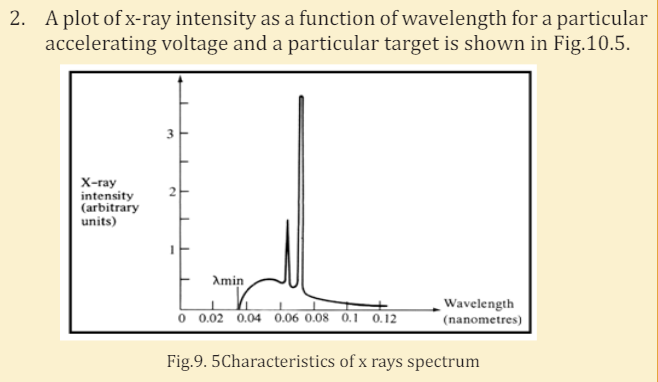
range of x-ray energies and a couple of sharp peaks. Explain how
each of these arises.
b. What is the origin of the cut-off wavelength λmin of the Fig.9.5 shown
below? Why is it an important clue to the photon nature of x-rays?
c. What would happen to the cut-off wavelength if the accelerating
voltage was increased? What would happen to the characteristic
peaks? Use a sketch to show how this spectrum would look if the
accelerating voltage was increased.
d. What would happen to the cut-off wavelength if the target was
changed, keep the same accelerating voltage? What would happen
to the characteristic peaks? Use a sketch to show how the spectrum
would look if some other target material was used, but the
accelerating voltage was kept the same.
3. Electrons are accelerated from rest through a p.d of 10 kV in an x ray
tube. Calculate:
I. The resultant energy of the electrons in eV.II. The wavelength of the associated electron waves.
III. The maximum energy and the minimum wavelength of the x ray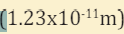
radiation generated (assume
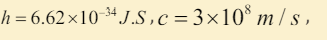
(1.6 10 ,1.24 10 ) J m − − × × .
4. Monochromatic X-ray of wavelength 10 1.2 ×−10 m are incident on a crystal.
The1st order diffraction maximum is observed at when the angle between
the incident beam and the atomic plane is 120.What is the separation of the atomic planes responsible for the diffraction?
5. An x-ray operates at 30 kV and the current through it is 2.0 mA. Calculate:
I. The electrical power output
II. The number of electrons striking the target per second.
III. The speed of the electrons when they hit the target
IV. The lower wavelength limit of the x-rays emitted.
6. An x-ray machine can accelerate electrons of energies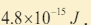 . The
. The
shortest wavelength of the x- rays produced by the machine is found
to be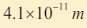 Use this information to estimate the value of the plank
Use this information to estimate the value of the plankconstant.
7. The spacing between Principal planes of Nacl crystal is 0 2.82 A . It is found
that the first order Bragg diffraction occurs at an angle of 100
. What is the
wavelength of the x rays?
8. What is the kinetic energy of an electron with a de Broglie wavelength of
0.1 nm. Through what p.d should it be accelerated to achieve this value?
9. You have decided to build your own x-ray machine out of an old television
set. The electrons in the TV set are accelerated through a potential difference
of 20 kV. What will be the λmin for this accelerating potential?
10. A tungsten target (Z = 74) is bombarded by electrons in an x-ray tube.
The K, L, and M atomic x-ray energy levels for tungsten are -69.5, -11.3 and
-2.30 keV, respectively.
a. Why are the energy levels given as negative values?
b. What is the minimum kinetic energy of the bombarding electrons
that will permit the production of the characteristic Kα and Kβ
lines of tungsten?
c. What is the minimum value of the accelerating potential that will
give electrons this minimum kinetic energy?
d. What are the Kα and Kß wavelengths?
11. Using the following illustration figure Fig.10.6, label each part marked byletter from A to H and explain the function of each part A, B, C, D, E, F and H.
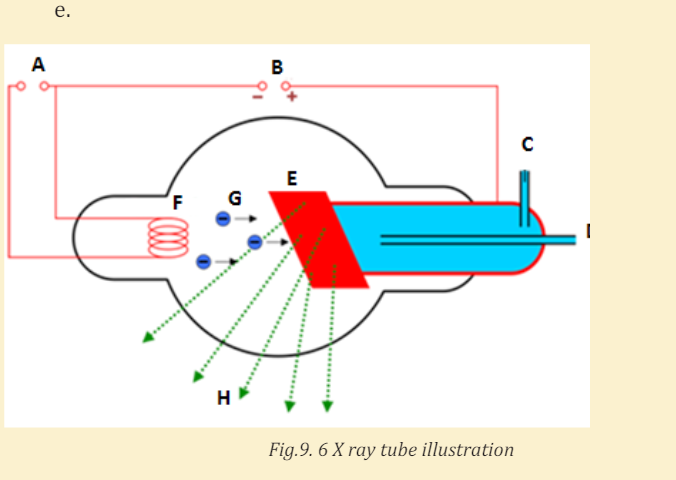
UNIT 10: EFFECT OF LASER
Key unit Competence: Analyze the applications of LASER.
My goals• Define a laser beam
• Explain the stimulated emission of light
• Explain the spontaneous emission of light
• Analyse the mechanism to produce LASER beam
• Explain laser properties
• Explain and describe monochromatic and coherent sources of light
• Analyse a LASER light as a source of coherent light.
• Explain the principle and uses of Laser.
• Outline applications of LASER
• Analyse applications and dangers of LASER beam• Analyse precautional measures of the negative effects of Laser.
INTRODUCTORY ACTIVITY
A man has tied all forms of advancements from traditional methods of solving
problems to advanced methods by use of different technologies. Among
other technological advancements, discovery of Laser that is a part of visible
light under electromagnetic waves has had a great impact in solving many of
our problems.a. What do you understand by Electromagnetic waves?10.1 CONCEPT OF LASER
b. Discuss at least four (4) characteristics of Electromagnetic waves
c. In your own words, discuss how these electromagnetic waves are
produced.
d. Are all kinds of these electromagnetic waves have the same energy? If
Yes why? If No, why not?
e. Basing on what you know about these electromagnetic waves, what
could be positive uses of these waves. Also discuss negative effects of
electromagnetic waves.f. How are electromagnetic waves related to LASERS?
ACTIVITY 10.1a. From your own understanding, explain how a LASER light isThe acronym LASER stands for Light Amplifier by Stimulated Emission of
produced.
b. Does production, need source of energy like electricity. Explain
your reasoning.
c. In energy levels, particles are either in ground or excited states.
Is laser formed when particles or electrons are in ground orexcited states? Explain your reasoning.
Radiation. This expression means that the light is formed by stimulating a
material’s electrons to give out the laser light or radiation.
The laser is a device that uses the ability of some substances to absorb
electromagnetic energy and re-radiate it, as a highly focused beam of
monochromatic and synchronized wavelength radiation. In 1953 Charles H.
Townes, with graduate students James P. and Herbert J., produced the first
Microwave Amplifier by Stimulated Emission of Radiation (MASER),
as a device operating in the same way as a laser, but amplifying microwave
radiations.
This system could release stimulated emissions without falling to the ground
state, and thus maintaining a population inversion. A laser is a device that
emits light through a process of optical amplification based on the stimulated
emission of electromagnetic radiation. That is, the laser is a light source that
produces a beam of highly coherent and very nearly monochromatic lightbecause of cooperative emission from many atoms.
10.1.1 Absorption, Spontaneous emission and Stimulated emission
ACTIVITY 10.2
1. Using scientific explanations, Explain the meaning of the following
terms
I. Absorption
II. Stimulated emission
III. Spontaneous emission
2. Electrons can jump from excited to ground state; does it absorb or
radiate energy. Explain your reasoning.
3. Write an equation that would be used to calculate the energy radiated
by an electron when it jumps from one energy level to another. Explain
each term used in the equation.4. What do you understand by the term population inversion?
a. Absorption
During the process of absorption, a photon from the source is destroyed andthe atom which was at the ground state is promoted to the excited state.
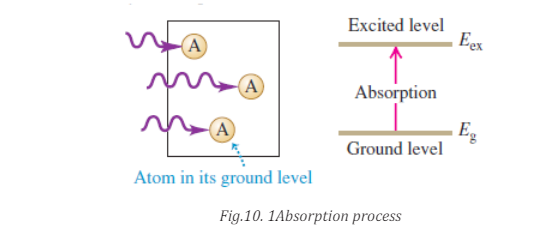
In normal cases the excited states are less populated than the ground state.
b. Spontaneous emission.
An atom or an electron can move from one energy level to another. A photon is
released when an electron moves from a higher energy level to a lower energy
level. The release of photon (a particle of light) is called spontaneous emission.
At the excited state, an atom will drop to a lower level by emitting a photon
of radiation in a process called spontaneous emission. It emits the photon
spontaneously after an average time τ called the spontaneous lifetime of the
level. This time depends on the atomic species; some levels have long lifetime
measured in seconds, whereas others are relatively short on the order of
nanoseconds or less. This lifetime determines the ability of the emitting atomto store energy and will affect the efficiency of sources.
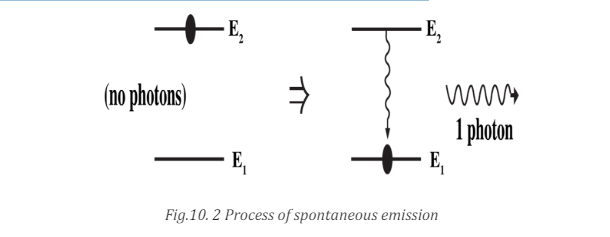
frequency as the atomic frequency, there is a finite probability that this wave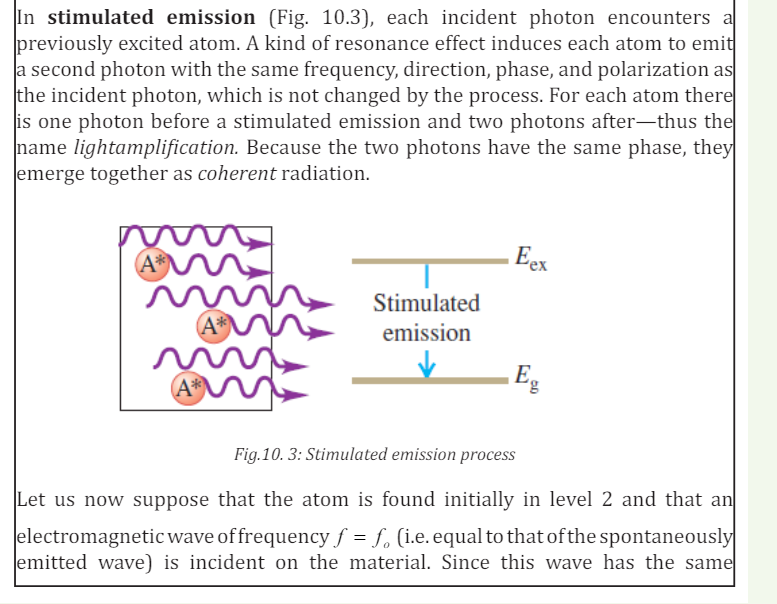
will force the atom to undergo the transition E2 → E1 .
In this case the energy difference between the two levels is emitted in the form
of electromagnetic wave that adds to the incident one. This is the phenomenon
of stimulated emission. There is a fundamental difference between the
spontaneous and stimulated emission processes because in spontaneous
emission one photon is emitted and in stimulated emission both incident and
emitted photons are observed.
10.1.2 Laser principle
The principle of operation remains the same though there is a wide range of
lasers. Laser action occurs in three stages: photon absorption, spontaneous
emission, and stimulated emission. The particle of the material, which undergoes
the process of excitation, might be an atom, molecule, or ion depending on the
laser material. This principle is based on the principle of stimulated emission of
radiation, the theory that was discussed by Einstein in 1917.The whole concept
was discussed in the previous section.
The photon emitted during stimulated emission has the same energy as the
incident photon and it is emitted in the same direction as the latter, thus, getting
two coherent photons. If these two coherent photons are incident on other
two atoms in E2, then it will result in emission of two more photons and hence
four coherent photons of the same energy are emitted. The process continuesleading to doubling of the present number of photons.
If the process is made to go on chain, we ultimately can increase the intensity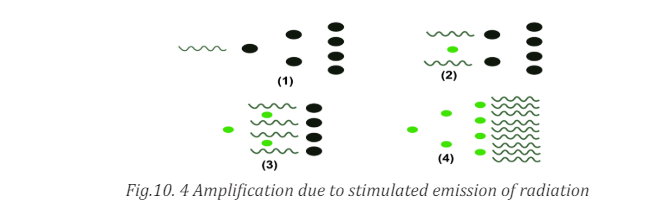
of coherent radiation enormously. In figure above, such amplification of the
number of the coherent photons due to stimulated emission is shown.
The necessary condition for this type of amplification of light intensity by
stimulated emission of radiation is that number of atoms in the upper energy
state E2 must be sufficiently increased.
10.1.3 Population inversion
Population inversion: This is the process of increasing excited electrons in
higher energy levels. This is the redistribution of atomic energy levels thattakes place in a system so that laser action can occur.
There are different methods of achieving population inversion in atomic states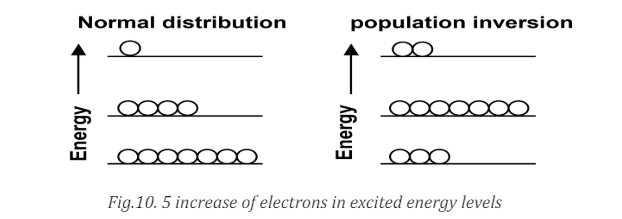
that is essential requirement to produce laser beam.
Normally, most of the atoms in a medium are in the ground state of energy E0
.There are four different methods of making these atoms to excited states.
i. Excitation with the help of photons. If the atoms are exposed to an
electromagnetic radiation of high frequency, then there is selective
absorption of energy and thus atoms are raised to excited state.
ii. Excitation by electrons. This method is used in some gas lasers. Electrons
are released from the atoms due to high voltage electric discharge
through a gas. These electrons are then accelerated to high velocities
due to high electric field inside a discharge tube. When they collide with
neutral gas atoms, a fraction of these atoms are raised to excited state
e + X → X ∗+ e Where X is an atom in ground state and ∗ X is an atom inexcited state
iii. Inelastic collision between atoms. If a gas contains two different two
different kinds of atoms X and Y, then during electric discharge through
the gas some of the atoms are raised to excited state.
iv. Excitation by chemical energy. Sometimes, an atom or a molecule can be a
product of a chemical reaction and can be produced in its excited state. An
example is hydrogen combining with fluorine to form hydrogen fluorideHF that is in excited state.
10.1.4 Laser structure
ACTIVITY 10.3
1. From what you know about LASER, what could be the components
of laser
2. Are all parts on laser Light Similar? Explain your reasoning.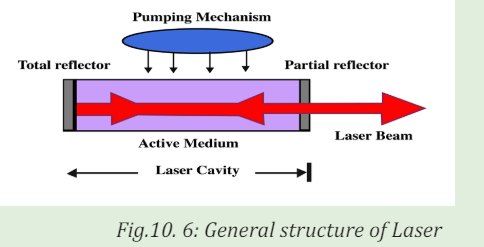
In general case laser system consists of three important parts: Active medium
or amplifying medium, the energy source referred to as the pump or pumpsource and the optical resonator consisting of mirrors or system of mirrors.
Pumping Mechanism.
Pumping is the process of supplying energy to the laser medium to excite to
the upper energy levels. To have this mechanism, it depends on the existence of
interactions between light from pump source to constituents of active medium.
Usually, pump sources can be: electrical discharges, flash lamps, arc lamps,
light from another laser, chemical reactions and even explosive devices. Most
common lasers use electrical or optical pumping. The type of pump source useddepends essentially on the gain medium.
Active Medium
The active medium is the major determining factor of the wavelength of
operation, and other properties of the laser. The gain medium is excited by the
pump source to produce a population inversion, and it is where the spontaneous
and stimulated emission of photons take place, leading to the phenomenon of
optical gain or amplification. The gain medium may be a solid crystal like a
ruby, a liquid dye, gases like CO2 or He-Ne or semiconductors. The gain medium
for some lasers like gas lasers is closed by a window under the Brewster’s angleto allow the beam to leave the laser tube.
Optical resonator or Optical cavity
The optical resonator or optical cavity is a system of two parallel mirrors placed
around the gain medium that provide reflection of the light beam. Light from
the medium produced by the spontaneous emission is reflected by the mirrors
back into the medium where it may be amplified by the stimulated emission.
Mirrors are required for most lasers to increase the circulating power within
the cavity to the point where gains exceed losses, and to increase the rate of
stimulated emission. One of the mirrors reflects essentially 100% of the light,
while the other less than 100% and transmits the remainder. Mirrors can be
plane, spherical or a combination of both. Here represented are the commoncavities configuration that can be used:
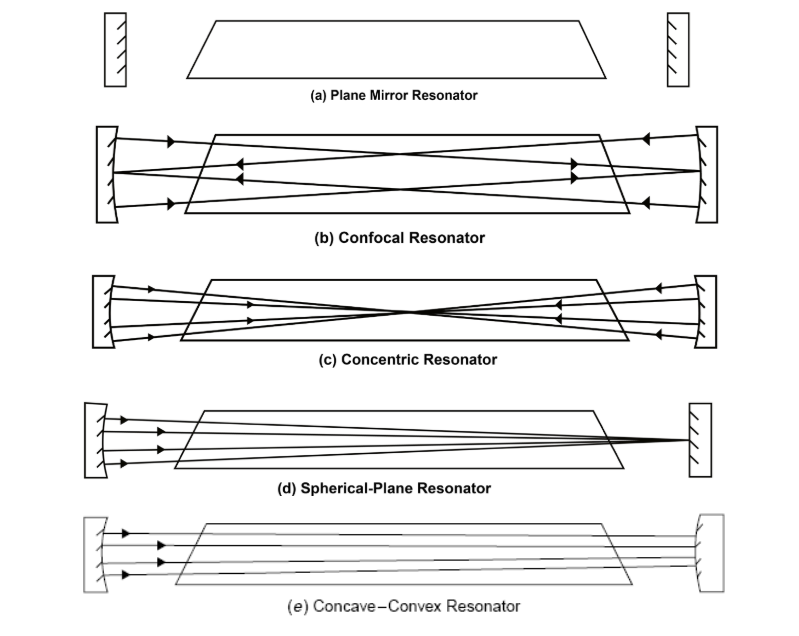
10.1.5 Checking my progress
1. What do you understand by the term LASER?
2. Write in full the acronym L.A.S.E.R
3. In your own words, explain how laser light is produced.
4. Explain the meaning of population inversion and discuss how an atom
can be put into excited state.
5. What is the energy of the laser light that propagates with a frequency
of 1010 Hz in gaseous medium. (Given that the plank’s constant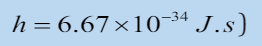
6. What are the three major components of laser?7. Using diagrams, explain all the types of optical cavity.
10.2 PROPERTIES OF LASER LIGHT
ACTIVITY 10.4
a. Using the ideas about electromagnetic radiations, what are
characteristics of laser light?
b. Do you think all different kinds of laser light have the same
properties? Give reasons to support your answer.
The laser light is not like any other light emitted by usual sources found in
nature. This special light emitted by the laser, has three properties according
to its usefulness in many applications: Coherence, Monochromaticity andCollimation or Directionality.
10.2.1 Coherence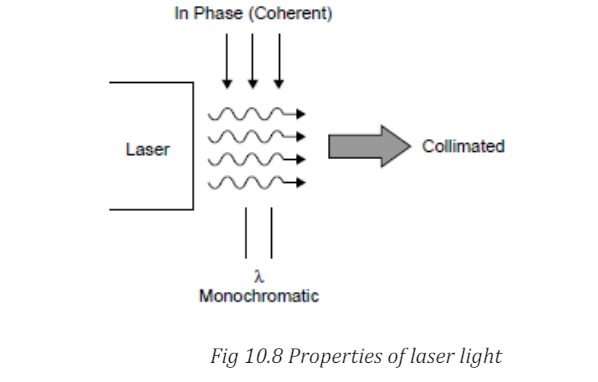
Coherence is the most interesting property of laser light. All photons
emitted, are exactly in the same phase, they are all crest and valley at the
same time. It is brought about by the mechanism of the laser itself in which
photons are essentially copied.The good temporal coherence is essentially for
Interferometry like in Holography. Coherence is not trivial and is brought aboutby the amplification mechanism of the laser.
10.2.2 Monochromaticity
Monochromaticity is the ability of the laser to produce light that is at one
wavelength λ. It is a requirement for coherence since photons of different
wavelengths cannot be coherent. When white light is dispersed through a
prism, you note that it is composed of an infinite number of wavelengths of
light covering the entire visible spectrum as well as into the UV and IR regions.
However, no light source is perfectly monochromatic. Lasers tend to be relatively
monochromatic and this depends on the type of laser. Monochromatic output,
or high frequency stability, is of great importance for lasers being used inInterferometry.
10.2.3 Collimation or Directionality
Collimation or directionality is the property of laser light that allows it to stay in
one direction at the strait line, confined beam for large distances. This property
makes it possible to use the laser as a level in construction or to pinpoint
speeders on a highway. This highly directional laser light is determined by themechanism of the laser itself.
10.2.4 Checking my progress
1. Choose the correct group of terms that are properties of laser light.
a. Coherent, unpolarized, monochromatic, high divergence
b. Monochromatic, low divergence, polarized, coherent
c. Polychromatic, diffuse, coherent, focused
d. Monochromatic, birefringent, nonpolarized, coherent
2. Which of the following properties of laser light enables us to use it to
measure distances with great precision?
a. All the light waves emitted by the laser have the same direction
b. The light waves are coherent
c. The light waves are monochromatic
d. The individual waves effectively work like a single wave with very
large amplitude.
3. Explain how coherence, monochromatic and collimation are
interconnected.
4. All light in laser light are produced and found to be in the same phase.
How does this help in the formation of 3D images?
5. Laser light can be used as a level. Which special feature that makes it be
used
10.2.4 Checking my progress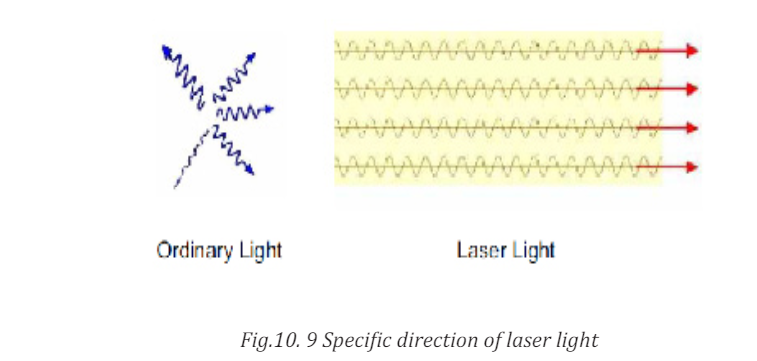
1. Choose the correct group of terms that are properties of laser light.
a. Coherent, unpolarized, monochromatic, high divergence
b. Monochromatic, low divergence, polarized, coherent
c. Polychromatic, diffuse, coherent, focused
d. Monochromatic, birefringent, nonpolarized, coherent
2. Which of the following properties of laser light enables us to use it to
measure distances with great precision?
a. All the light waves emitted by the laser have the same direction
b. The light waves are coherent
c. The light waves are monochromatic
d. The individual waves effectively work like a single wave with very
large amplitude.
3. Explain how coherence, monochromatic and collimation are
interconnected.
4. All light in laser light are produced and found to be in the same phase.
How does this help in the formation of 3D images?
5. Laser light can be used as a level. Which special feature that makes it be
used
10.3 APPLICATIONS AND DANGERS OF MISUSE OF LASER
10.3.1 Applications of lasers.ACTIVITY 10.5
a. Having studied LASERS, where do you think in real life LASERS
are helpful?
b. From your experience, have you ever used LASER light?
c. Other than using it by yourself, what are other places where laserlight is applied
There are many interesting uses for lasers, depending on the special characteristic
being applied. Laser Diodes are used in a wide range of applications. Partial
lists of those applications include:
i. They are used in common consumer devices such as DVD players, bar code
scanners; CD ROM drivers; laser disc and other optical storage drivers;
laser printers and laser fax machines; sighting and alignment scopes;
measurement equipment; free space communication systems; pump
source for other lasers; high performance imagers; and typesetters. CD
players have lasers. Light from the laser in CD player reflects off patterns
on CD’s surface. The reflected light is converted to a sound wave.
ii. Laser beams can be used in diverse fields of science and technology. Like
in the control of motion of moving objects like aircrafts or missiles. This
method thus makes it possible for a missile to hit a certain target.
iii. Because of high directional property, lasers are used to measure distances
accurately. A laser beam is sent and the time taken for it to be reflected
back is measured. Using this idea, the distance can thus be measured.
iv. Because laser beam can be focused into a small spot, it can thus be used
to cut minute holes onto a material.
v. The very high intensity of laser beam means that the amplitude of the
corresponding electromagnetic wave is very large. So it is possible to
investigate the non linear optical properties of different materials with
the help of laser light.
vi. Lasers are also used in industry for cutting materials such as metal and
cloths. and welding materials
vii. Doctors use lasers for surgery and various skin treatments
viii. They are used in military and law enforcement devices for marking targets
and measuring range and speed.
ix. Laser lighting displays use laser light as an entertainment medium.
x. Lasers also have many important applications in scientific research .
In a tabular way, we can have a summary of different types of lasers and their
applications.The following are types of lasers and their Applications
a. Gas Lasers: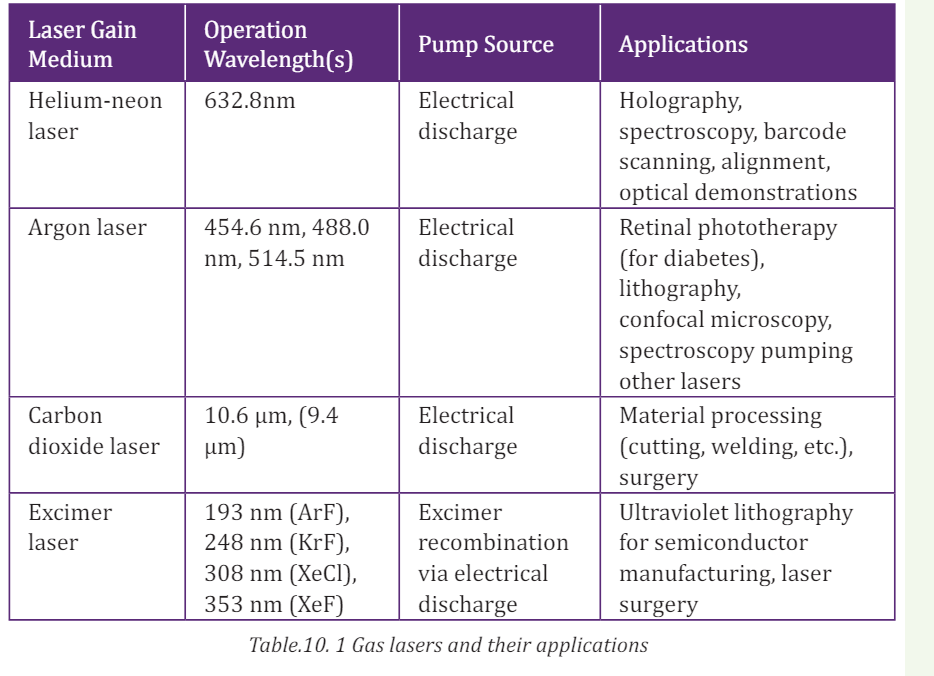
b. Solid State Lasers:
10.3.2 Dangers of lasers
ACTIVITY 10.6
Laser light is used in many areas like industries, offices, airports and
many other places. Do you think long exposure of laser light is harmful?
1. Why do you think so?
2. What makes these lasers harmful if mis-used? Give a scientificreasoning
You should be careful when dealing with lasers, because they can have a negative
impact when exposed to your body. Among other negative effects, some of them
are discussed below .
i. If directly exposed to our skin, it burns the skin
ii. When absorbed by skin, Laser light reacts with body cells causing cancer.
iii. Because of their high energy, it affects eyes if exposed to them
iv. Lasers can affect cells of a human being. This leads to mutation
Because of the negative effects of lasers, care must be taken to avoid all therisks of being affected by lasers.
10.3.3 Precaution measuresACTIVITY 11.7
a. Observe the picture above clearly. Using scientific reasoning
explain why the people performing the activity above are putting
on protective wear as shown.
b. Building on what you have discussed in a) above, what precautional
measures can you take to avoid negative effects of LASERS if at allyou were working in a place exposed to them.
The following are some of the measures one can take to avoid the negative
effects of lasers.
i. For any one working in places where there are incidences of being exposed
to laser light, one should wear protective clothes, glasses and shoes so
that there is no direct exposure of these radiations on to the body.
ii. One should minimize the time of working with lasers.
iii. Areas that are exposed to these radiations should be warning signs and
labels so that one can be aware of places/areas where laser light is used.
iv. Safe measures like Use of remote control should be used to avoid direct
exposure of these radiations (LASER light).
v. People should be given trainings on how to handle lasers.vi. There should also access restrictions to laboratories that use laser
10.3.4 Checking my progress
1. Discuss all the negative effects of laser light.
2. Using vivid examples, explain how one can prevent him or herself of all
dangers caused by laser light.
3. We have seen that laser light is good and at the same time bad. Using
your personal judgement, which side outweighs the other. Give scientific
reasons.
4. Depending on your judgement in (3) do you think man should continueusing laser light?
END UNIT ASSESSMENT 1
A. Multiple choice
Copy the questions below to your exercise and chose the best alternative
that answers the question.
1. What does the acronym LASER stand for?
a. Light Absorption by Stimulated Emission of Radiation
b. Light Amplification by Stimulated Emission of Radiation
c. Light Alteration by Stimulated Emission of Radiation
2. The acronym MASER stands for?
a. Microwave Amplification by Stimulated Emission of Radiation
b. Molecular Absorption by Stimulated Emission of Radiation
c. Molecular Alteration by Stimulated Emission of Radiation
d. Microwave amplification by Stimulated Emission of Radio
waves
3. What is one way to describe a Photon?
a. Solid as a rock
b. A wave packet
c. A torpedo
d. Electromagnetic wave of zero energy
4. Which of the following determines the color of light?
a. Its intensity
b. Its wavelength
c. Its source
d. Some information missing
5. Among the three examples of laser listed below, which one is
considered “eye safe”?
a. Laser bar-code scanners
b. The excimer laser
c. Communications lasers
d. YAG
6. Why are lasers used in fiber optic communications systems
a. The government has mandated it
b. They can be pulsed with high speed data
c. They are very inexpensived. They are not harmful
7. Lasers are used in CDs and DVDs. What type of laser is used in these
players?
a. Semiconductor
b. YAG
c. Alexandrited. All the above
8. The best reason why lasers used in “Laser Printers” is
a. They can be focused down to very small spot sizes for high
resolution
b. They are cheap
c. They are impossible to damage
d. They are locally available
9. As wavelength gets longer, the laser light can be focused to…
a. Larger spot sizes
b. Smaller spot sizes
c. Large and small spot sizes
d. None of the above
10. Among the following, which color of laser has the shortest wavelength?a. Yellow c. Blue
b. Red d. Green
11. What property of laser light is used to measure strain in roadways?
a. Intensity
b. Power
c. Coherence
d. All the above
12. What is the type of laser used most widely in industrial materials
processing applications?
a. Dye Laser c. YAG laser
b. Ruby Laser d. Carbon Dioxide Laser
13. Why are lasers used for cutting materials
a. It never gets dull d. It has a small “heat affected zone”
b. Accuracy e. Smoother cuts
c. Repeatability f. All of the above
14. The Excimer laser produces light with what wavelength?
a. Visible
b. Ultraviolet
c. Infrared
d. All the above.
15. Most lasers are electrically inefficient devices.
a. True
b. False
16. Chemical lasers use………………. to produce their beams.
a. Excessive amounts of electrical power
b. Small amounts of electrical power
c. No electrical power
d. Other lasers
17. What type of laser could cause skin cancer if not used properly?
a. Red semiconductor laser c. Blue semiconductor
b. Excimer laser d. YAG laser.
B. Structured questions
18.
a. What do you understand by term LASER?
b. Depending on the nature and what laser is made of, Laser is
classified into different types. Discuss at least 5 types of lasers.
19 The following are basic characteristics of laser light. With clear
explanation, what does each imply as connected to laser light.
a. Coherence
b. Monochromaticity
c. Collimation
20. a. With the aid of diagram Explain the meaning of the following terms
I. Spontaneous Absorption of light
II. Stimulated Emission cause harm if mis-used In what ways is
laser light harmful.
III. Spontaneous Emission
IV. Population inversion
b. Laser light have been employed in different areas. This has helped
man in solving different problems. What are some of the areas
where laser light is employed.
c. Though laser light is very important in different activities, it canalso
UNIT 11: MEDICAL IMAGING
Key unit Competence: Analyze the processes in medical imaging.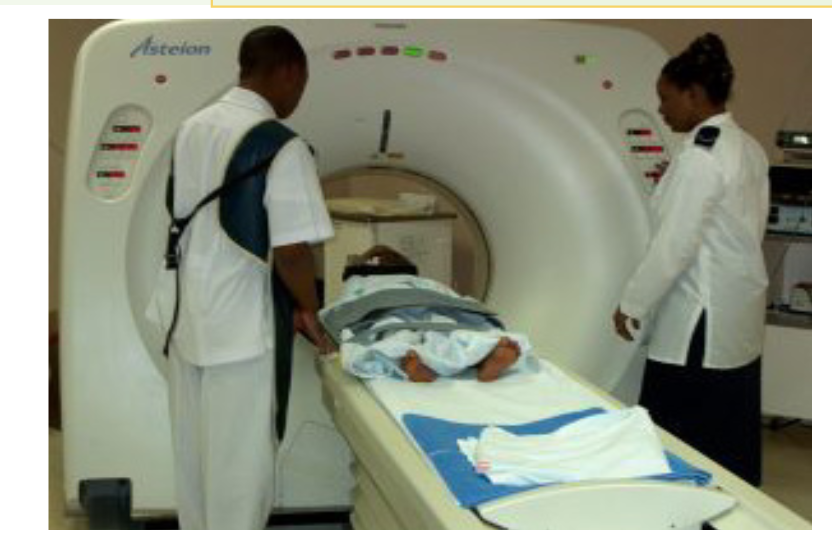
Learning objectives:• Outline specific purposes of imaging techniquesINTRODUCTORY ACTIVITY
• Explain the effects of various imaging techniques for particular
purposes.
• Explain the basic functioning principles of major medical imaging
techniques• Identify advantages and disadvantages of medical imaging techniques
Investigation on the use of medical imaging techniques:
Years ago, the only way to get information from inside of human bodies was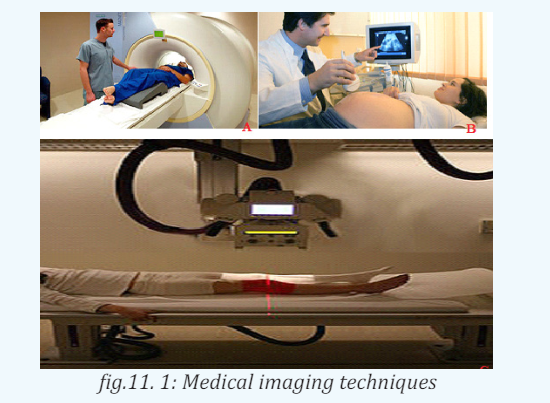
through surgery. In modern medicine, medical imaging has undergone major
advancements and this ability to obtain information about different parts of
the human body has many useful clinical applications.
Using information provided on the above pictures, answer to the following
questions:
1. Observe the image A, B, and C (Fig.12.1) and describe what is happening.
2. Suggest the technique that is being used for each image?
3. Explain the working principle of the mentioned techniques in question2?
11.1 X-RAY IMAGING.
11.1.1 Interaction of X-rays with matter.
a. Introduction
In unit 10, we learnt that X-rays are electromagnetic radiation produced by
focusing a beam of high energy electron on a target material in x-ray tube. Since
the major part of the energy of the electrons is converted into heat in the target
(only about 1% will appear as X-rays), the target material should have a high
melting point and good heat conduction ability. To get a high relative amount of
X-ray energy, the anode material should be of high atomic number. Tungsten is
the dominating anode material and is in modern X-ray tubes often mixed withRhenium.
In X-ray diagnostics, radiation that is partly transmitted through and partly
absorbed in the irradiated object is utilized. An X-ray image shows the variations
in transmission caused by structures in the object of varying thickness, densityor atomic composition.
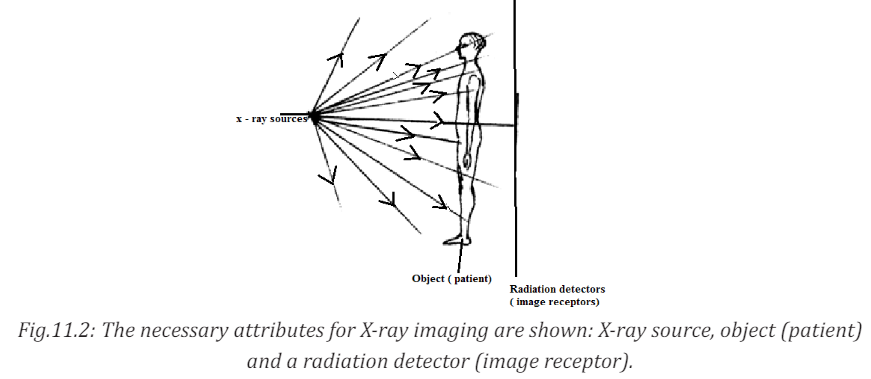
b. Attenuation and Absorption of X-rays
There are principally two interaction processes that give rise to the x-ray
attenuation (variation in photon transmission) through the patient which is
the basis of X-ray imaging. These are photoelectric absorption and scatteringprocesses.
A photon which has experienced an interaction process has either been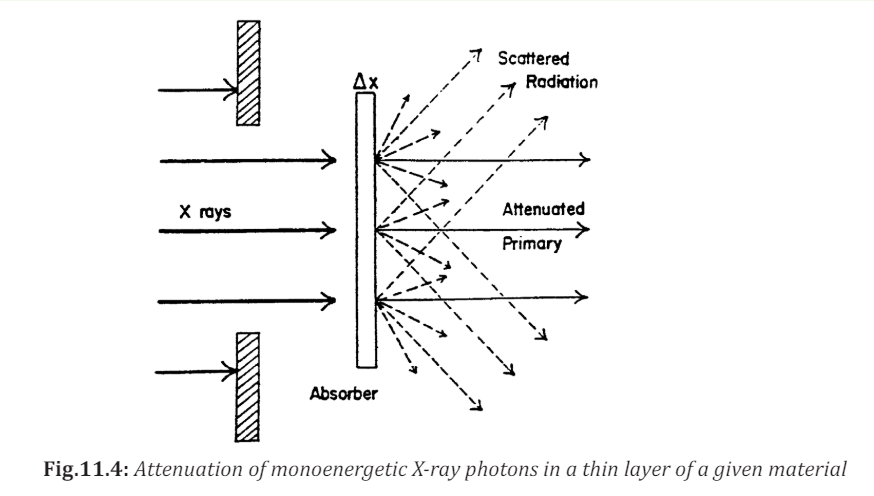
absorbed or has changed its energy and/or direction of motion. A photon that
changes its direction of motion is called a scattered photon. For monoenergetic
x-ray photons, the number of photons that experience such interactions and
therefore removed from the primary beam when this is incident on a thin
layer of material is proportional to the number of incident photons (N) and thethickness of the layer (dx) following the expression :
where µ is a constant of proportionality called the linear attenuation coefficient.
Integrating the above equation will result in

where
It can be seen that the incident beam photons (or the beam energy) is attenuated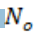 is the initial number of photons in the incident beam.
is the initial number of photons in the incident beam.
exponentially as the X-rays travel through the material. The different interaction
processes involved, that are absorption, coherent and incoherent scattering
and pair production, add their contributions to the total linear attenuationcoefficient
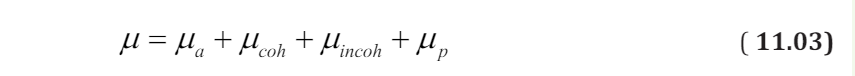
where µa, µcoh, µincoh, and µp are the contributions to the attenuation from photoelectric absorption, coherent scattering, incoherent scattering and pair production.
C. Contrast
The contrast is a measure of the difference in radiation transmission or
other parameters between two adjacent areas in a radiographic image.
Contrast plays an important role in the ability of a radiologist to perceiveimage detail.
where ε1and ε2 are energies of the monoenergetic X-ray photons per unit area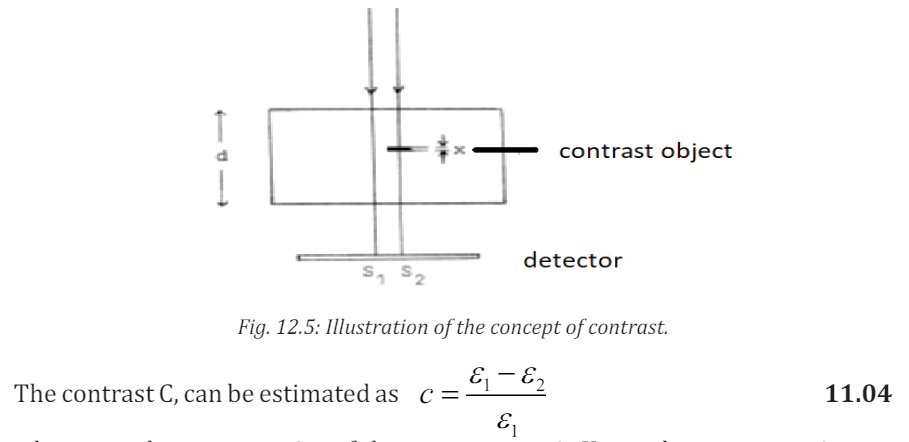
reaching the detector and therefore absorbed in the detector without and
with the contrasting detail respectively. In the case where the film is used as
image receptor, the signal is obtained in terms of the optical density. The image
contrast is then usually defined as the optical density difference beside andbehind the contrasting detail.
In such situation where monoenergetic photons are considered and no scattered
radiation is reaching the detector, the absorbed energy in the detector can bewritten as
Where d, is the thickness of the object with linear attenuation coefficient
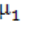
The energy through the contrasting detail can be expressed as
where ε0 is the energy absorbed in the detector with no object present, x is the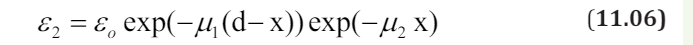
thickness of the contrasting detail with its linear attenuation coefficient µ2
Replacing equation 12.05 and 12.06 into equation 12.04 we obtain the contrast
The contrast is then proportional to the difference in the linear attenuation
coefficients and the thickness of the contrasting detail. Therefore, when
scattered radiation is neglected in the process, the contrast is independent of
the thickness d of the object but also it does not depend on where in the object
the contrasting detail is situated.
The ability of conventional radiography to display a range of organs and
structures may be enhanced by the use of various contrast materials, also
known as contrast media. The most common contrast materials are based on
barium or iodine. Barium and iodine are high atomic number materials thatstrongly absorb X-rays and are therefore seen as dense white on radiography.
11.1.2 X-rays Imaging Techniques
a. Conventional Radiography
X-rays are able to pass through the human body and produce an image of internal
structures. The resulting image is called a radiograph, more commonly known
as an ‘X-ray’ or ‘plain film’. The common terms ‘chest X-ray’ and ‘abdomen X-ray’are widely accepted and abbreviated to CXR and AXR.
As a beam of X-rays passes through the human body, some of the X-rays photons
are absorbed or scattered producing reduction or attenuation of the beam withthe internal human structure acting as contrasting details.
Therefore tissues of high density and/or high atomic number cause more X-ray
beam attenuation and are shown as lighter grey or white on radiographs. Less
dense tissues and structures cause less attenuation of the X-ray beam, and
appear darker on radiographs than tissues of higher density. The figure belowshows the typical conventional radiograph of a human body
Fig.11.6: The five principal radiographic densities. This radiograph of a benign
lipoma (arrows) in a child’s thigh demonstrates the five basic radiographic
densities: (1) air; (2) fat; (3) soft tissue; (4) bone; (5) metal. (David A Disle
(2012) Imaging for students. Fourth Edition. (Page 1))
Five principal densities are easily recognized on this plain radiograph due to
the increase in their densities:
1. Air/gas appears as black, e.g. lungs, bowel and stomach
2. Fat is shown by dark grey, e.g. subcutaneous tissue layer, retroperitoneal
fat
3. Soft tissues/water appears as light grey, e.g. solid organs, heart, blood
vessels, muscle and fluid-filled organs such as bladder
4. Bone appears as off-white
5. Contrast material/metal: bright white.
In the past, X-ray films were processed in a darkroom or in freestanding
daylight processors. In modern practice, radiographic images are produced
digitally using one of two processes, computed radiography (CR) and digital
radiography (DR).
DR uses a detector screen containing silicon detectors that produce an electrical
signal when exposed to X-rays. This signal is analyzed to produce a digital
image. Digital images obtained by CR and DR are sent to viewing workstations
for interpretation. Images may also be recorded on X-ray film for portability
and remote viewing.
The image given by a computer radiography may be reviewed and reported
on a computer workstation. This allows various manipulations of images as
well as application of functions such as measurements of length and angles
measurements.
The relative variance of the shadows depends upon the density of the materials
within the object or body part. Dense, calcium – rich bone absorbs X-rays to a
higher degree than soft tissues that permit more X-rays to pass through them,
making X-rays very useful for capturing images of bone.
In projection radiography, there is much room for adjusting the energy level of
the X-rays depending on the relative densities of the tissues being imaged and
also how deep through a body the waves must travel in order to achieve theimaging.
• Images of bones (for instance, to examine a fracture or for diagnosticb. Mammography
measures related to bone conditions like osteoarthritis or certain
cancers) require high-energy X-rays because of the high density of
bone.
• Images of soft tissues like lungs, heart and breasts (both chest X-rays
and mammography are very common diagnostic applications of X-rays)
require relatively less energy from the X-rays in order to penetrate
properly and achieve excellent images.
• In order to achieve these different energies, technologists use X-ray
generators of different voltages and equipped with anodes made ofdifferent metals.
ACTIVITY 11.2
One day a girl suffering from the breast tells her mother about the
problem. And her mother advises her to go to the hospital to consult a
doctor.a. Think of the problem that girl may have.Mammography is a specialized medical imaging that uses low-dose X-rays to
b. Try to explain what may be the cause of that problem.
c. If you are a doctor how can you detect such problem?
d. Which advise can you give to other people who are not suffering
froe. m that problem in order to prevent it?
investigate the internal structure of the breast. A mammography exam, called
a mammogram, helps in the early detection and diagnosis of women’s breast
diseases such as breast cancer before even experiencing any symptom. Below
is a typical mammography test showing the presence of abnormal areas ofdensity, mass, or calcification that may indicate the presence of cancer.
Mammography
a) the breast is pressed between two plates x-rays are used
to takes pictures of breast tissues,(b)photographic image of breast tissues, (c)
Breast with cancer. A mammography unit is a rectangular box that houses the
tube in which X-rays are produced. The unit is used exclusively for X-ray exams
of the breast, with special accessories that allow only the breast to be exposed
to the X-rays. Attached to the unit is a device that holds and compresses thebreast and positions it so images can be obtained at different angles.
In conventional film and digital mammography, a stationery X-ray tube
captures an image from the side and an image from above the compressed breast.
Breast tomosynthesis, also called three-dimensional (3-D) mammography
and digital breast tomosynthesis (DBT), is an advanced form of breast imaging
where multiple images of the breast from different angles are captured and
reconstructed (“synthesized”) into a three-dimensional image set. In this way,
3-D breast imaging is similar to computed tomography (CT) imaging in which
a series of thin “slices” are assembled together to create a 3-D reconstructionof the body.
c. Computer tomography scan (ct scan)
CT terminology
In 1970s, a revolutionary new X-ray technique was developed called Computer
tomography (CT), which produce an image of a slice through the body. Theword tomography comes from the Greek: tomos =slice, graph= picture.)
A computed tomography scan also known as CT scan, makes use of computer
processed combinations of many X-ray measurements taken from different
angles to produce cross-sectional (tomographic) images (virtual “slices”) of
specific areas of a scanned object, allowing the user to see inside the object
without cutting it. Other terms include computed axial tomography (CAT scan)
and computer aided tomography. The term “computed tomography” (CT) is
often used to refer to X-ray CT, because it is the most commonly known formbut many other types of CT exist.
CT is an imaging technique whereby cross-sectional images are obtained with
the use of X-rays. In CT scanning, the patient is passed through a rotating
gantry that has an X-ray tube on one side and a set of detectors on the other.
Information from the detectors is analysed by computer and displayed as a
grey-scale image. Owing to the use of computer analysis, a much greater arrayof densities can be displayed than on conventional X-ray films.
This allows accurate display of cross-sectional anatomy, differentiation of
organs and pathology, and sensitivity to the presence of specific materials such
as fat or calcium. As with plain radiography, high- density objects cause more
attenuation of the X-ray beam and are therefore displayed as lighter grey thanobjects of lower density.
Principle behind of computer tomography scan (CT scan).
Computer Tomography is shown in below figure: a thin collimated beam of
X- ray(“ to collimate” means to “make straight”) passes through the body to adetector that measures the transmitted intensity. Measurements are made at
a large number of points as the source and detector are moved past the body
together. The apparatus is rotated slightly about the body axis and again scanned;
this is repeated at 1 intervals for 180 . The intensity of the transmitted beam for
the many points of each scan, and for each angles, are sent to a computer that
reconstructs the image of the slice. Note that the imaged slice is perpendicular
to the long axis of the body. For this reason, CT is sometimes called computerizeaxial tomography.
The use of single detector would require a few minutes for many scans needed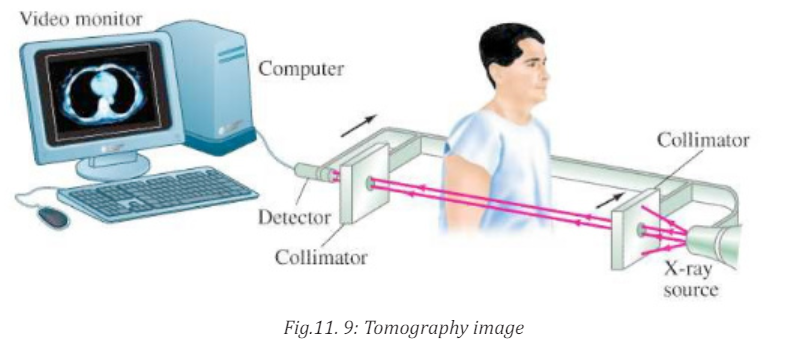
to form a complete image. Much faster scanner use a fan beam in which passing
through the entire cross section of the body are detected simultaneously by
many detectors. The x-ray source and the detectors are rotated about the
patient and an image requires only few seconds to be seen. This means that
rays transmitted through the entire body are measured simultaneously at each
angle where the source and detector rotate to take measurements at differentangles.
CT images of internal organs, bones, soft tissue, and blood vessels provide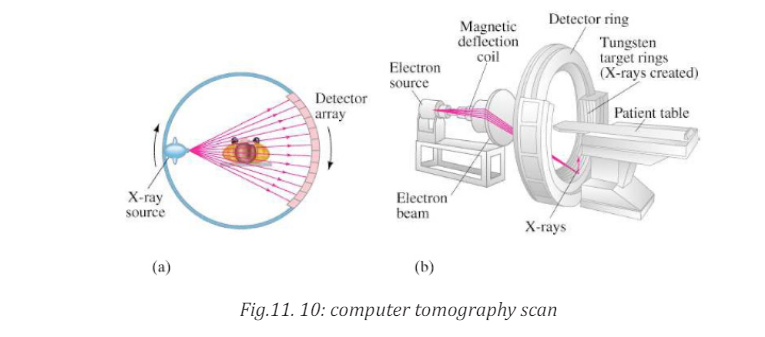
greater clarity and more details than conventional X-ray images, such as a chestX-Ray
Function of CT scan• A motorized table moves the patient through a circular opening in theNote that, it is advisable to avoid unnecessary radiation exposure; a medically
CT imaging system.
• While the patient is inside the opening, an X-ray source and a detector
assembly within the system rotate around the patient. A single rotation
typically takes a second or less. During rotation the X-ray source
produces a narrow, fan-shaped beam of X-rays that passes through a
section of the patient’s body.
• Detectors in rows opposite the X-ray source register the X-rays that
pass through the patient’s body as a snapshot in the process of creating
an image. Many different “snapshots” (at many angles through the
patient) are collected during one complete rotation.
• For each rotation of the X-ray source and detector assembly, the
image data are sent to a computer to reconstruct all of the individual
“snapshots” into one or multiple cross-sectional images (slices) of theinternal organs and tissues.
needed CT scan obtained with appropriate acquisition parameter has benefitsthat outweigh the radiation risks.
11.1.3 Checking my progress1. Outline the advantage and disadvantages CT scan11.2 ULTRASONIC IMAGING
2. Explain the types of x-ray imaging used in mammography.
3. In mammography exams, is the breast compression necessary? Why
4. A beam of X-rays passes through the human body of tissues with
different densities; explain the production of X-rays on less densetissues?
11.2.1 Basics of Ultra sound and its production
ACTIVITY 11.3
1. Distinguish ultrasound from infrasound?
2. Where do you think ultrasound may be applied in daily life?3. Advise on how ultrasound be used in medicine?
Sound can refer to either an auditory sensation in the brain or the disturbance
in a medium that causes this sensation. Hearing is the process by which the ear
transforms sound vibrations into nerve impulses that are delivered to the brain
and interpreted as sounds. Sound waves are produced when vibrating objects
produce pressure pulses of vibrating air. The auditory system can distinguishdifferent subjective aspects of a sound, such as its loudness and pitch.
Pitch is the subjective perception of the frequency, which in turn is measured in
cycles per second, or Hertz (Hz). The normal human audible range extends from
about 20 Hz to 20 000 Hz, but the human ear is most sensitive to frequencies
of 1 000 Hz to 4 000 Hz. Loudness is the perception of the intensity of sound,
related to the pressure produced by sound waves on the tympanic membrane.
The pressure level of sound is measured in decibels (dB), a unit for comparing
the intensity of a given sound with a sound that is just perceptible to the normal
human ear at a frequency in the range to which the ear is most sensitive. On the
decibel scale, the range of human hearing extends from 0 dB, which represents
the auditory threshold, to about 130 dB, the level at which sound becomespainful.
11.2.2 Interaction of sound waves with different structure inside the body
a. Introduction
Ultrasound imaging uses ultra-high-frequency sound waves to produce cross sectional
images of the body. Ultrasound is actually sound with a frequency in
excess of 20 kHz, which is the upper limit of human hearing. Typical ultrasoundfrequencies used for clinical purposes are in the 2 MHz to 10 MHz range.
Different tissues in a human or animal body alter the ultra sound waves in
different ways. Some waves are reflected directly while others scatter the
waves before they return to the transducer as echoes. The reflected ultrasound
pulses detected by the transducer need to be amplified in the scanner or
ultrasonic probe. The echoes that come from deep within the body are more
attenuated than those from the more superficial parts and therefore requiredmore amplification.
When echoes return to the transducer, it is possible to reconstruct a two
dimensional map of all the tissues that have been in the beams. The information
is stored in a computer and displayed on a video (television) monitor. Strong
echoes are said to be of the high intensity and appear as brighter dots on thescreen.
b. Reflection of ultrasound
When the pulse of ultrasound is sent into the body and meets a boundary between
two media, of different specific acoustic impedance Z, the sound wave needs to
change gear in order to continue. If the difference in Z across the boundary is
large the wave cannot easily adjust: there is an “acoustic mismatch”. Most of
the wave is reflected and a strong echo is recorded. The fraction of intensity
reflected back to that incident at the normal incidence, is known as theintensity of reflection coefficient
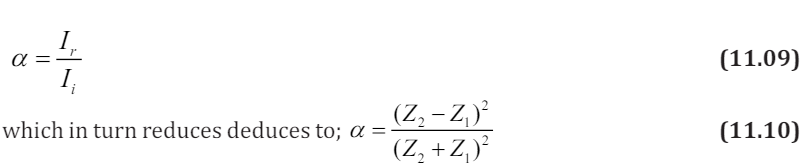
where Z is acoustic impedance
Note that large difference in Z give rise to large values for α, producing strongechoes
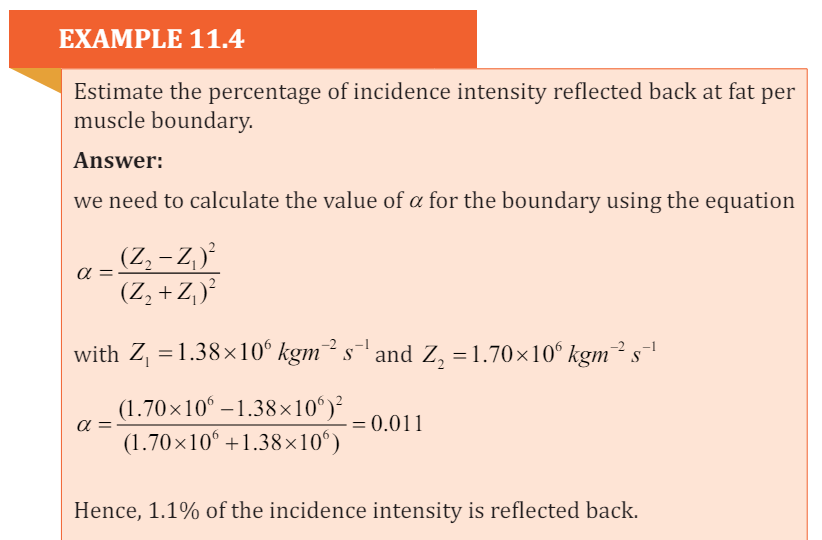
Ultrasounds are high-frequency sound waves above the human ear’s audible
range: that is with frequency sound waves greater than 20 kHz. In fact, the
frequencies used in medicine are much higher than this, typically between
1 MHz and 15 MHz. like all sound waves, ultrasound consists of longitudinal,
elastic or pressure waves, capable of traveling through solids, liquids and gases.This makes them ideal for penetrating the body, unlike transverse mechanical
waves, which cannot travel to any great extent through fluids.
c. Attenuation of ultrasound
The attenuation of the waves describes the reduction in its intensity as they
travel through a medium. This loss is due to a number of factors:• The wave simply “spreads out” and suffers an “inverse square law type”The amount of absorption of ultrasound beam in a medium is described by the
reduction in intensity.
• The wave is scattered away from its original direction
• The wave is absorbed in the medium.
absorption coefficientα , which is intensity level per unit length. It is expressed
in decibels per cm and it firstly depends on the type of medium the wave is
propagating into. As example whilst water absorbs very little ultrasound,
bone is a strong absorber, putting it at risk, for example, during high- powerultrasound therapy.
Secondly, higher frequencies suffer greater absorption. In fact if the frequency is
doubled, the absorption increases by the factor of four. This has very important
consequences when choosing the best frequency at which to image the body. If
the selected frequency is too high, the ultrasound will not be able to penetrateto the regions under investigation.
11.2.3 Ultrasonic imaging techniques
The basic component of the ultrasound probe is the piezoelectric crystal.
Excitation of this crystal by electrical signals causes it to emit ultra-high frequency
sound waves; this is the piezoelectric effect. The emitted ultrasound
waves are reflected back to the crystal by the various tissues of the body. These
reflected sound waves also called the “echoes” act on the piezoelectric crystal
in the ultrasound probe to produce an electric signal, again by the piezoelectriceffect. It is this electric signal which is analysed by a computer produces a cross sectional image.
The process of imaging is the same as the echo-locating sonar of a submarine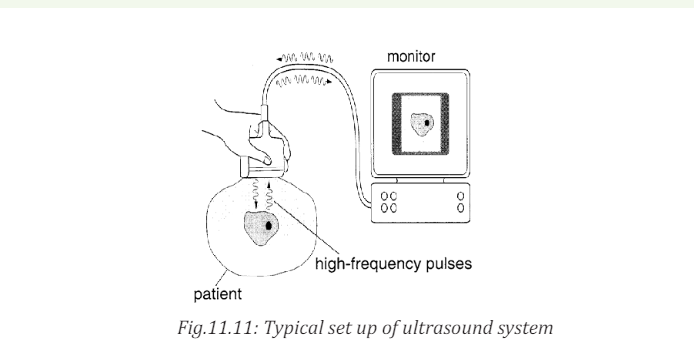
or a bat. The observer sends out a brief pulse of ultrasound and waits for an
echo. The pulse travels out, reflects off the target and returns. The ultrasound
machine uses pulses because the same device acts as both transmitter and
receiver. If it continually sent out sounds, then the receiver would not hear themuch softer echo over the louder transmission.
The duty cycle of the ultrasound imager is the amount of time spent transmittingcompared to the total time of transmitting and listening.
Sonar is an acronym for Sound Navigation and Ranging. It relies on the
reflection of ultrasound pulses. A short pulse of ultrasound is directed towards
the object interest, which then reflects it back as an echo. The total time
between transmission of pulse and reception of an echo is measured, often
using a cathode ray oscilloscope (CRO). The sonar principle is used to estimatethe depth of a structure, using
Where t is the time taken to go and back and v is the velocity of ultrasound in
the medium.
The factor of 2 is necessary because the pulse must travel “there and back”
An ultrasound beam structure is directly into the body. The reflection or
echoes from different body structure are then detected and analyzed, yielding
information about the locations. For example if the time delays between the
reception of echo pulse1 and 2 (Fig.11.12 below) is t , then the diameter of thebaby’s head can be found using the above formula.
During an investigation using ultrasound, the time delay for an echo to return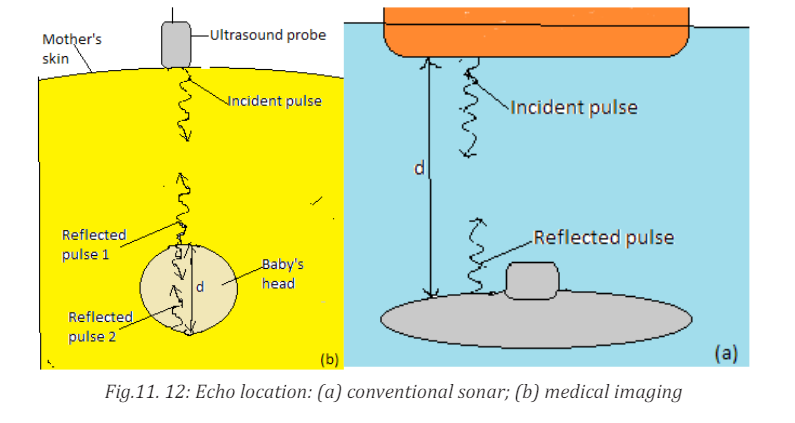
from a structure is 10.5µs If the average velocity of ultrasound in the eye is 1510m/s. Calculate the depth of the structure.
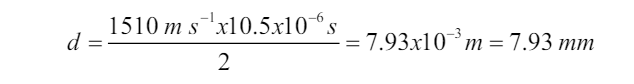
a. Doppler ultrasonic
An object travelling towards the listener causes sound waves to be compressed
giving a higher frequency; an object travelling away from the listener gives a
lower frequency. The Doppler effect has been applied to ultrasound imaging.
Flowing blood causes an alteration to the frequency of sound waves returning
to the ultra sound probe. This frequency change or shift is calculated allowing
quantization of blood flow. The combination of conventional two-dimensionalultra sound imaging with Doppler ultra sound is known as Duplex ultra sound.
The Doppler sample gate is positioned in the artery (arrow) and the frequency
shifts displayed as a graph. Peak systolic and end diastolic velocities arecalculated and also displayed on the image in centimeters per second.
As ultrasound imaging uses sound waves to produce pictures of inside of the
body. It is used to help diagnose the cause of pain, swelling and infection in
the body’s internal organs and to examine a baby in pregnant woman and the
brain and hips in infants. It is also used to help guide biopsies, diagnose heartconditions and assess damage after a heart attack.
Ultrasound examinations do not use ionizing radiation (x-rays), there is no
radiation exposure to the patient. Because ultrasound image are captured in
real time, they can show the structure and movement of the body’s internalorgans, as well as blood flowing through blood vessels.
b. Advantages and Disadvantages of ultrasounds
The advantages of ultrasound over other imaging modalities include:• Lack of ionizing radiation.Some disadvantages of ultrasound include:
• Relatively low cost
• It is noninvasive (of medicine procedures not involving the introduction
of instruments into the body)
• Quick procedure
• Good for examining soft tissues.• Portability of equipment.
• It is highly operator dependent as it relies on the operator to produce11.2.4 Checking my progress
and interpret images at the time of examination
• Not as much details as X-rays and MRI
• It cannot be used in areas that contain gas (such as lungs)
• Doesn’t pass through bones.• Can be wrong in detecting physical abnormalities.
1. Explain how ultrasound imaging is used?11.3 SCINTIGRAPHY (NUCLEAR MEDICINE)
2. Who take the decision to scan or not to scan in normal pregnancy?
3. What are the risks and side effects to the mother or baby during
ultrasound?
4. If an ultrasound is done at 6 to 7 weeks and a heartbeat is not detected,does
ACTIVITY 11.4a. What do you understand by ‘radionuclide imaging’?11.3.1 Physics of scintigraphy and terminology
b. What is a radionuclide scan used for?c. Compare radionuclide scan with mammography scan?
Scintigraphy refers to the use of gamma radiation to form images following the
injection of various radiopharmaceuticals. The key word to understanding of
scintigraphy is radiopharmaceutical. ‘Radio’ refers to the radionuclide, i.e. theemitter of gamma rays.
The most commonly used radionuclide in clinical practice is technetium,
written in this text as mTc 9 , where 99 is the atomic mass, and the ‘m’ stands for
metastable. Metastable means that the technetium atom has two basic energy
states: high and low energy states. As the technetium transforms from the highenergy state to the low-energy state, it emits a quantum of energy in the form of
a gamma ray, which has energy of 140 keV. Other commonly used radionuclidesinclude gallium citrate (67Ga), thallium (201TI), indium (111In) and iodine ( 131 I).
11.3.2 Basic functioning of radionuclide scan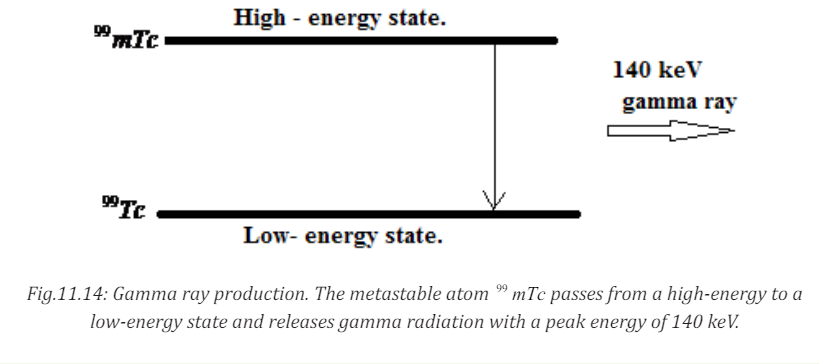
ACTIVITY 11.5
For radionuclide imaging, it is advisable for the patient to consume a
small quantity of radionuclide, or it is injected into a vein in your arm.a. How long does it take?A radionuclide scan is a way of imaging bones, organs and other parts of the
b. What is the purpose of those radionuclide chemicals?
c. Assuming the patient has already consumed the radionuclide for
him/her to be scanned and wants to take another scan on another
part of the body, will the patient be required to take another dose
of the nuclide?
d. You as a student, what advice can you give to a patient whodevelops allergies after taking the radio nuclear chemical?
body by using a small dose of a radioactive chemical. There are different types
of radionuclide chemical. The one used depends on which organ or part of thebody need to be scanned.
A radionuclide (sometimes called a radioisotope or isotope) is a chemical which
emits a type of radioactivity called gamma rays. A tiny amount of radionuclide
is put into the body, usually by an injection into a vein. Sometimes it is breathedin, or swallowed, or given as eye drops, depending on the test.
Gamma rays are similar to X-rays and are detected by a device called a gamma
camera. The gamma rays which are emitted from inside the body are detected
by the gamma camera, are converted into an electrical signal and sent to a
computer. The computer builds a picture by converting the differing intensitiesof radioactivity emitted into different colors or shades of grey.
However, radionuclide imaging techniques do not depict structural anatomy like
ultrasound, X-ray computed tomography (XCT) or conventional radiographs.
It is the only established noninvasive technique available to investigate organ
physiology, although recently Nuclear magnetic resonance (NMR) imaging
technique has shown its capability to probe organ physiology and anatomywithout ionizing radiation.
Radionuclide scans do not generally cause any after effects. Through the natural
process of radioactive decay, the small amount of radioactive chemical in your
body will lose its radioactivity over time. Although the levels of radiation usedin the scan are small, patients may be advised to observe special precautions.
11.3.3 Limitations and disadvantages of scintigraphy
The main advantages of scintigraphy are its high sensitivity and the fact that the
functional information is provided as well as anatomical information. However
it has some disadvantages that are listed below:i. Generally poor resolution compared with other imaging techniques.11.3.4 Checking my progress
ii. Radiation risks due to the administered radionuclide
iii. Can be invasive, sometimes requiring an injection into the bloodstream
iv. Disposal for radioactive waste, including that from patients, requires
special procedures.
v. Relatively high costs associated with radiotracer production andadministration.
Choose the correct answers
1. Scintigraphy refers to the use of:a. Gamma radiation to form images2. The radionuclide in clinical practice are
b. X- ray radiation to form images
c. X- rays and gamma radiations to form images
d. None of radiation to form images.a. Technetium11.4 MAGNETIC RESONANCE IMAGING (MRI)
b. Thallium
c. Galliumd. ALL of them
ACTIVITY 11.6:Principles of MRI1. What does MRI mean?11.4.1. MRI physics and terminology.
2. What is it used for?
3. What makes MRI to be powerful compared to other imaging
techniques?
4. is it advisable for a pregnant woman to be placed in MRI Scanner?Explain your view?
Magnetic resonance (MR) imaging has become the dominant clinical imaging
modality with widespread, primarily noninvasive, applicability throughout the
body and across many disease processes. The progress of MR imaging has been
rapid compared with other imaging technologies and it can be attributed in part
to physics and in part to the timing of the development of MR imaging, whichcorresponded to an important period of advances in computing technology.
Initially let us described how magnetic resonance can be demonstrated with
a pair of magnets and a compass. If a compass happens to find itself near a
powerful magnet, the compass needle will align with the field. In a normal
pocket compass, the needle is embedded in liquid to dampen its oscillations.
Without liquid, the needle will vibrate through the north direction for a period
before coming to rest. The frequency of the oscillations depends on the magneticfield and of the strength of the magnetic needle.
Let us focus on what made the needle oscillate. It was the small movements of
the magnet, back and forth, or more precisely the oscillation of a weak magnetic
field perpendicular to the powerful stationary magnetic field caused by the
movement of the magnet. But oscillating magnetic field is what we understand
by “radio waves”, which means that in reality, we could replace the weak magnet
with other types of radio wave emitters.
This could, for example, be a small coil subject to an alternating current, as shown
in figure above. Such a coil will create a magnetic field perpendicular to the
magnetic needle. The field changes direction in synchrony with the oscillation
of the alternating current, so if the frequency of the current is adjusted to the
resonance frequency of the magnetic needle, the current will set the needle
in motion. This is also applied in an MR scanner. In summary, the needle can
be set in motion from a distance by either waving a magnet or by applying an
alternating current to a coil. In both situations, magnetic resonance is achieved
when the magnetic field that motion or alternating currents produce, oscillates
at the resonance frequency. When the waving or the alternating current is
stopped, the radio waves that are subsequently produced by the oscillatingneedle will induce a voltage over the coil.
MRI uses the magnetic properties of spinning hydrogen atoms to produce
images. The first step in MRI is the application of a strong, external magnetic
field. For this purpose, the patient is placed within a large powerful magnet.
Most current medical MRI machines have field strengths of 1.5 or 3.0 Tesla.
The hydrogen atoms within the patient align in a direction either parallel orantiparallel to the strong external field.
A greater proportion aligns in the parallel direction so that the net vector of
their alignment, and therefore the net magnetic vector, will be in the direction
of the external field. This is known as longitudinal magnetization. A second
magnetic field is applied at right angles to the original external field. This second
magnetic field is known as the radiofrequency pulse (RF pulse), because it is
applied at a frequency in the same part of the electromagnetic spectrum asradio waves. A magnetic coil, known as the RF coil, applies the RF pulse.
The RF pulse causes the net magnetization vector of the hydrogen atoms to
turn towards the transverse plane, i.e. a plane at right angles to the direction
of the original, strong external field. The component of the net magnetization
vector in the transverse plane induces an electrical current in the RF coil. This
current is known as the MR signal and is the basis for formation of an image.
Computer analysis of the complex MR signal from the RF receiver coils is used
to produce an MR image.
11.4.2. The magnetism of the body
Let‘s see how magnet needles with and without spin are affected by radio
waves, we now turn to the “compass needles” in our own bodies.a. Most frequently, the MR signal is derived from hydrogen nuclei (meaning
the atomic nuclei in the hydrogen atoms). Most of the body’s hydrogen is
found in the water molecules. Few other nuclei are used for MR.
b. Hydrogen nuclei (also called protons) behave as small compass needles
that align themselves parallel to the field.
c. The compass needles (the spins) are aligned in the field, but due to
movements and nuclear interactions in the soup, the alignment only
happens partially.
d. The nuclei in the body move among each other (thermal motion) and the11.4.3. Magnetic Resonance Imaging (MRI).net magnetization in equilibrium is thus temperature dependent.
e. Due to the number of hydrogen nuclei (about 1027 ) found in the body,
the net magnetization still becomes measurable. It is proportional to the
field: A large field produces a high degree of alignment and thus a largemagnetization and better signal to noise ratio.
In MRI, a particular type of nucleus is selected and its distribution in the body
is monitored. Hydrogen is the most commonly imaged element, not only due to
its abundance in the body but also because it gives the strongest MRI signals.
The technique uses a very powerful magnet to align the nuclei of atoms inside
the body, and a variable magnetic field that causes the atoms to resonate, a
phenomenon called nuclear magnetic resonance. The nuclei produce their own
rotating magnetic fields that a scanner detects and uses to create an image.
MRI is used to diagnose a variety of disorders, such as strokes, tumors,
aneurysms, spinal cord injuries, multiple sclerosis and eye or inner ear
problems. It is also widely used in research to measure brain structure andfunction, among other things.

An MRI scan can be used to examine almost any part of the body, including the:• Brain and spinal cord
• Bones and joints
• Breasts
• Heart and blood vessels
• Internal organs, such as the liver, womb or prostate gland ,etcThe results of an MRI scan can be used to help diagnose conditions, plantreatments and assess how effective previous treatment has been.
11.4.4. Functional of MRI Scan
ACTIVITY 11.7a. Explain the function of MRI Scan.There are many forms of MRI, some of them are:
b. What are the advantages and disadvantages of MRI Scan?c. What are the hazards associated with MRI?
a. Diffusion-weighted imaging.
Diffusion-weighted imaging (DWI) is sensitive to the random Brownian
motion (diffusion) of water molecules within tissue. The greater the amount of
diffusion, the greater the signal loss on DWI. Areas of reduced water molecule
diffusion show on DWI as relatively high signal. Diffusion-weighted imaging
is the most sensitive imaging test available for the diagnosis of acute
cerebral infarction. With the onset of acute ischaemia and cell death there
is increased intracellular water (cytotoxicoedema) with restricted diffusion
of water molecules. An acute infarct therefore shows on DWI as an area ofrelatively high signal.
b. Perfusion-weighted imaging
In perfusion-weighted imaging (PWI) the brain is rapidly scanned following
injection of a bolus of contrast material (gadolinium). The data obtained may
be represented in a number of ways including maps of regional cerebral blood
volume, cerebral blood flow, and mean transit time of the contrast bolus. PWI
may be used in patients with cerebral infarct to map out areas of brain at risk of
ischaemia that may be salvageable with thrombolysis.c. Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) uses different frequencies to identify
certain molecules in a selected volume of tissue, known as a voxel. Following data
analysis, a spectrographic graph of certain metabolites is drawn. Metabolites
of interest include lipid, lactate, NAA (N-acetylaspartate), choline, creatinine,citrate and myoinositol.
Uses of MRS include characterization of metabolic brain disorders in children,
imaging of dementias, differentiation of recurrent cerebral tumour fromradiation necrosis, and diagnosis of prostatic carcinoma.
d. Blood oxygen level-dependent imaging
Blood oxygen level-dependent (BOLD) imaging is a non-invasive functional MRI
(fMRI) technique used for localizing regional brain signal intensity changes in
response to task performance. BOLD imaging depends on regional changes
in concentration of deoxyhemoglobin, and is therefore a tool to investigate
regional cerebral physiology in response to a variety of stimuli. BOLD fMRI may
be used prior to surgery for brain tumor or arteriovenous malformation (AVM),as a prognostic indicator of the degree of postsurgical deficit.
11.4.5 Advantage and disadvantages of MRI.
Advantages of MRI in clinical practice include:
1. Excellent soft tissue contrast and characterization
2. Lack of ionizing radiation.
3. Noninvasive machine.
4. Lack of artefact from adjacent bones, e.g. pituitary fossa
Disadvantages of MRI:
1. High capital and running costs.
2. Image selected and interpretation is complex.
3. Examination can be difficult for some people who are claustrophobic
4. The examination is noisy and takes long.
5. Hazards with implants, particularly pacemakers.6. Practical problems associated with large superconducting magnets.
11.4.7. Checking my progress
1. What is meant by relaxation in the context of MRI?
2. Give the reasons why the hydrogen nucleus is most used in MRI.
3. What does NMR stand for? Explain carefully the role of the three terms
involved4. Draw the basic steps in the formation of MRI image
11.5 ENDOSCOPY
ACTIVITY 11.81. How can we examine inside the stomach by using light rays?11.3.1 Description2. How is endoscope performed?
Endoscopy is a nonsurgical procedure used to examine a person’s digestive tract.
Using an endoscope, which is a flexible tube with a light and camera attached to
it, the specialist can view pictures of your digestive tract on a monitor.
During an upper endoscopy, an endoscope is easily passed through the mouth
and throat and into the esophagus, allowing the specialist to view the esophagus,
stomach, and upper part of the small intestine. Similarly, endoscopes can be
passed into the large intestine (colon) through the rectum to examine this area
of the intestine.
11.3.2 Upper endoscopy
Upper Endoscopy (also known as gastroscopy, EGD, or
esophagogastroduodenoscopy) is a procedure that enables your surgeon
to examine the lining of the esophagus (swallowing tube), stomach and
duodenum (first portion of the small intestine). A bendable, lighted tube about
the thickness of your little finger is placed through your mouth and into thestomach and duodenum.
11.3.3. How is the upper endoscopy performed?
Upper endoscopy is performed to evaluate symptoms of persistent upper
abdominal pain, nausea, vomiting, difficulty swallowing or heartburn. It is an
excellent method for finding the cause of bleeding from the upper gastrointestinaltract. It can be used to evaluate the esophagus or stomach after major surgery.
It is more accurate than X-rays for detecting inflammation, ulcers or tumors
of the esophagus, stomach and duodenum. Upper endoscopy can detect early
cancer and can distinguish between cancerous and noncancerous conditionsby performing biopsies of suspicious areas.
A variety of instruments can be passed through the endoscope that allows
the surgeon to treat many abnormalities with little or no discomfort, remove
swallowed objects, or treat upper gastrointestinal bleeding. Safe and effective
control of bleeding has reduced the need for transfusions and surgery in manypatients.
11.3.4. Advantages and disadvantages of endoscopy
Advantages• Complete visualization of the entire stomach or digestive tract.Disadvantages:
• It is very safe and effective tool in diagnosis
• Does not leave any scar because it uses natural body openings.
• It is cost effective and has low risk
• They are generally painless.
• Can do therapeutic interventions
• Allows for sampling/biopsying of small bowel mucosa• Allows for resection of polyps.
• Although the endoscope is very safe; however, the procedure has a few
potential complications which may include:
• Bleeding
• Perforation (tear in the gut wall)
• Infection
• Reaction to sedation (action of administering a sedative drug to
produce a state of calm or sleep.
• Technically difficult procedure
• Very time consuming (Procedure can take > 3 hours)• Patient may need to be admitted to the hospital
• Higher risk of small bowel perforation11.3.5 Checking my progress
• Case reports of pancreatitis and intestinal necrosis• Reported incidents of aspiration and pneumonia
1. What are instruments used to view the esophagus, stomach and upper11.3.6. hazards associated with medical imaging
small intestine of human body?
2. Explain the function of endoscope.3. Compare and contrast colonoscopy and gastroscopy
The following are some hazards associated with medical imaging:1. Exposure to ionizing radiation1. Exposure to ionizing radiation
2. Anaphylactoid reactions to iodinated contrast media
3. Contrast-induced nephropathy (CIN)
4. MRI safety issues5. Nephrogenic systemic sclerosis (NSF) due to Gd-containing contrast media
Radiation effects and effective dose Radiography, scintigraphy and CT use
ionizing radiation. Numerous studies have shown that ionizing radiation in
large doses is harmful. The risks of harm from medical radiation are low, and
are usually expressed as the increased risk of developing cancer as a result of
exposure. Radiation effects occur as a result of damage to cells, including cell
death and genetic damage. Actively dividing cells, such as are found in the bonemarrow, lymph glands and gonads are particularly sensitive to radiation effects.
2. Anaphylactoid contrast media reactions
Most patients injected intravenously with iodinated contrast media experience
normal transient phenomena, including a mild warm feeling plus an odd taste
in the mouth. With modern iodinated contrast media, vomiting at the time of
injection is uncommon. More significant adverse reactions to contrast mediamay be classified as mild, intermediate or severe anaphylactoid reactions:
• Mild anaphylactoid reactions: mild urticaria and pruritis3. Contrast-induced nephropathy
• Intermediate reactions: more severe urticaria, hypotension and mild
bronchospasm
• Severe reactions: more severe bronchospasm, laryngeal oedema,
pulmonary oedema, unconsciousness, convulsions, pulmonarycollapse and cardiac arrest.
Contrast-induced nephropathy (CIN) refers to a reduction of renal function
(defined as greater than 25 per cent increase in serum creatinine) occurring
within 3 days of contrast medium injection. Risk factors for the developmentof CIN include:
Pre-existing impaired renal function, particularly diabetic nephropathy,
Dehydration, Sepsis, Age>60 years, Recent organ transplant , Multiple myeloma.
The risk of developing CIN may be reduced by the following measures:Risk factors should be identified by risk assessment questionnaire.
Use of other imaging modalities in patients at risk including US or noncontrast-enhanced CT.
• Use of minimum possible dose where contrast medium injection is4. MRI safety issues
required.
• Adequate hydration before and after contrast medium injection.
• Various pretreatments have been described, such as oral acetylcysteine;
however, there is currently no convincing evidence that anything otherthan hydration is beneficial.
Potential hazards associated with MRI predominantly relate to the interaction
of the magnetic fields with metallic materials and electronic devices.
Ferromagnetic materials within the patient could possibly be moved by the
magnetic field causing tissue damage. Common potential problems include
metal fragments in the eye and various medical devices such as intracerebral
aneurysm clips. Patients with a past history of penetrating eye injury are at risk
for having metal fragments in the eye and should be screened prior to entering
the MRI room with radiographs of the orbits. The presence of electrically active
implants, such as cardiac pacemakers, cochlear implants and neurostimulators,
is generally a contraindication to MRI unless the safety of an individual deviceis proven.
5. Nephrogenic systemic sclerosis
Nephrogenic systemic sclerosis (NSF) is a rare complication of some Gd-based
contrast media in patients with renal failure. Onset of symptoms may occur
from one day to three months following injection. Initial symptoms consist
of pain, pruritis and erythema, usually in the legs. As NSF progresses there is
thickening of skin and subcutaneous tissues, and fibrosis of internal organs
including heart, liver and kidneys. Identifying patients at risk, including patients
with known renal disease, diabetes, hypertension and recent organ transplant,
may reduce the risk of developing NSF following injection of Gd- based contrast
media.
Risk reduction in MRI
A standard questionnaire to be completed by the patient prior to MRI should
cover relevant factors such as:• Previous surgical historyEND UNIT ASSESSMENT 11
• Presence of metal foreign bodies including aneurysm clips, etc.
• Presence of cochlear implants and cardiac pacemakers
• Possible occupational exposure to metal fragments and history of
penetrating eye injury
• Previous allergic reaction to Gd-based contrast media• Known renal disease or other risk factors relevant to NSF.
Part I: Copy the following in your notebook and chose the correct answer
1. Which are included in the system components of gamma rays camera for
producing image of the body?a. Collimator2. Which of the following modalities does not use a form of ionizing
b. Scintillation
c. Attenuation
d. All of the above
radiation?a. Radiography.
b. Computed tomography.
c. Sonography.d. Magnetic resonance imaging3. Hazards not associated with modern medical imaging include:a. Anaphylactoid reactions to iodinated contrast media
b. Complication of some Gd-based contrast media in patients with
renal failure.
c. Imaging of the breast improves a physician’s ability to detect small
tumorsd. Radiation effects and effective dose Radiography.4. Medical imaging systems are often evaluated the characteristics which
are directly related to:a. Image noise.5. Risks associated with radionuclide imaging are:
b. Image blurring.
c. Image unsharpness.
d. Visibility of anatomical detail.a. Generally poor resolution compared with other imaging modalities.
b. Rarely receiving an overdose of chemical injected in the vein of the
body.
c. High capital and running costsd. None of them.
Part II: Structured questions
6. Write the missing word or words on the space before each number.
The term ………………….. is often used to refer to X-ray CT.a. Gastroscopy is a procedure that enables your surgeon to examine the
lining of the ………….
b. The most sensitive imaging test available for the diagnosis of acute
cerebral infarction is …………...
c. Array of …………………. to transform the flashes into amplified electrical
pulses inside the body.
d. Transducers used are different depending on ………. of a patient, one
has 5 MHz and other 3.5 MHz.
e. Hydrogen nuclei (also called protons) behave as small …………… that
align themselves parallel to the field.
f. In ………………….. there are appearance three words: nuclear, magnetic
and resonance.g. Examination can be ………………… is one of the disadvantages of MRI.7. Answer by True if it is True and by False if it is Falsea. The use of gamma radiation to form images following the injection of8. Compare endoscopy imaging and radionuclide imaging
various radiopharmaceuticals is known as Scintigraphy.
b. This decision to scan or not to scan a normal pregnancy must be made
only by the photographer. There are universally accepted guidelines at
present.
c. Tissue in the body absorbs and scatters ultrasound in the same ways.
Lower frequencies are more rapidly absorbed (attenuated) than higher
frequencies.
d. Upper endoscopy uses light and camera to view the esophagus, stomach,
and upper part of the small intestine.
e. Ultrasound is both generated and detected through high frequency
oscillations in piezoelectric crystals so there is ionizing radiation
exposure associated with ultrasound imaging.
9. What are the advantages of MRI in clinical practice?
10. Is ultrasound safe? explain.
11. What areas of the body can be imaged by ultrasound?
12. Why is ultrasound used in pregnancy?
13. Explain the advantage of CT scan
14. In mammography exams, is the breast compression necessary? Why
Essay question
Historically, MRI began in the central nervous system, but it is now extended to
all regions of the human body. The excellent resolution and contrast available in
any chosen plane in the body, makes the MRI an invaluable diagnostic tool with
which to study body structure, function and chemistry, as well as disease. Discuss
the application of MRI.
UNIT 12: RADIATIONS AND MEDICINE
Key unit Competence: Analyze the use of radiation in medicine.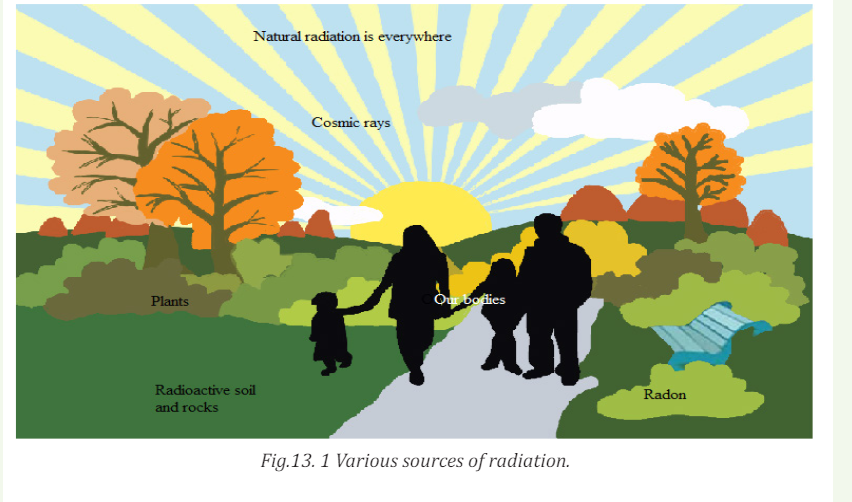
My goalsINTRODUCTORY ACTIVITY
• Explain radiation dosimetry.• Differentiate the terms exposure, absorbed dose, quality factor (relative
to biological effectiveness) and dose equivalent as used in radiation
dosimetry.
• Differentiate physical half-life, biological half-life and effective half-life
• Solve radiation dosimetry problems
• Analyse the basics of radiation therapy for cancer.
• Explain safety precautions when handling radiations• Describe the concept of balanced risk.
Radiation has always been present and is all around us. Life has evolved in
a world containing significant levels of ionizing radiation. Our bodies are
adapted to it.
People are constantly exposed to small amounts of ionizing radiation from
the environment as they carry out their normal daily activities; this is
known as background radiation. We are also exposed through some medical
treatments and through activities involving radioactive material.
Fig 13.1 above identifies four major sources of public exposure to natural
radiation: cosmic radiation, terrestrial radiation, inhalation and ingestion.
Brainstorm and try to answer the following questions:
a. Distinguish artificial source of radiation and natural source of radiation?
b. Explain briefly each major source of public exposure to natural
radiation stated above.
c. Which kind of sources of radiation are mostly preferred to be used in
medicine? Explain why
d. Does exposure to heavy ions at the level that would occur during deep space
missions of long duration pose a risk to the integrity and function
of the central nervous system? Explain to support your idea.
12.1 RADIATION DOSE
12.1.1 Ionization and non-ionization radiations
ACTIVITY 12.1:Types of radiation
Radiation is the emission of particles or electromagnetic waves from a
source. Radiation from radioactive materials has the ability to interact
with atoms and molecules of living objects.
a. With the help of the diagram below, distinguish the forms of
radiation?
b. Which type do you think is mostly used in medical treatment?
Explain your answer with supporting arguments?
c. Suggest the possible side effects of using radiations in medicine?
Which of the two forms of radiation induces more side effects
when exposed to human body? Explain to support your choice.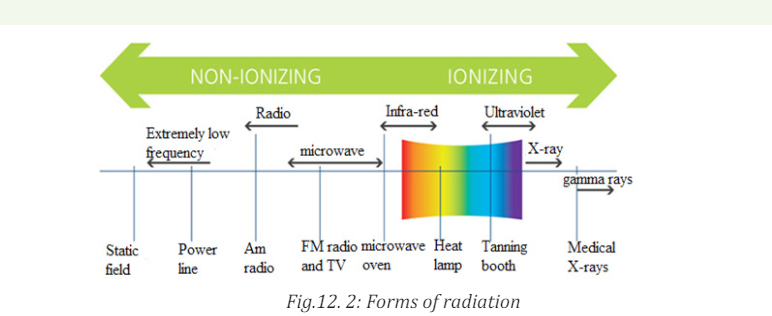
In a neutral atom, the positive charge of the nucleus is equal and opposite to the
total negative charge of the orbital electrons. If such an atom loses or gains an
electron, it becomes an ion. The atom will now have a net positive or negative
charge and is called an ion. This process is called ionization, and the radiation
responsible for it is called ionising radiation. When discussing the interaction
of radiations with matter in particularly in relation to health, two basic types ofradiation can be considered:
a. Ionizing radiation.
This is a radiation that carries enough energy to liberate electrons from atoms
or molecules, thereby ionizing them. As the more powerful form of radiation,
ionizing radiation is more likely to damage tissue than non-ionizing radiation.
The main source of exposure to ionizing radiation is the radiation used duringmedical exams such as X-ray radiography or computed tomography scans.
However, the amounts of radiation used are so small that the risk of any
damaging effects is minimal. Even when radiotherapy is used to treat cancer,
the amount of ionizing radiation used is so carefully controlled that the risk
of problems associated with exposure is tiny. All forms of living things emit a
certain amount of radiation, with humans, plants and animals accumulating
radioisotopes as they ingest food, air and water. Some forms of radiation such as
potassium-40 emit high-energy rays that can be detected using measurement
systems. Together with the background radiation, these sources of internalradiation add to a person’s total radiation dose.
Background radiation is emitted from both naturally occurring and man-made
sources. Natural sources include cosmic radiation, radon radiation in the body,
solar radiation and external terrestrial radiation. Man-made forms of radiation
are used in cancer treatment, nuclear facilities and nuclear weapons. Globally, the
average exposure to ionizing radiation per year is around 3 milliSieverts (mSv),
with the main sources being natural (around 80%). The remaining exposureis due to man-made forms such as those used in medical imaging techniques.
Exposure to man-made forms of ionizing radiations is generally much higher
in developed countries where the use of nuclear imaging techniques is muchmore common than in developing countries.
b. Non-ionizing radiations
Non-ionizing radiation refers to any type of electromagnetic radiation that
does not carry enough energy to ionize atoms or molecules. Examples of
non-ionizing radiations include visible light, microwaves, ultraviolet (UV)
radiation, infrared radiation, radio waves, radar waves, mobile phone signals
and wireless internet connections. Although UV has been classified as a nonionizing
radiation but it has been proven that high levels of UV-radiation cancause sunburn and increase the risk of skin cancer developing.
Scientific investigations suggest that the use of telecommunications devices
such as mobile phones may be damaging, but no risk associated with the use
of these devices has yet been identified in any scientific studies. This energyis emitted both inside the body and externally, through both natural and manmade processes.
12.1.2 Radiation penetration in body tissue
ACTIVITY 12.2
The figure below shows the penetrating power of radiation representedby A, B and C. Use the figure to answer the following questions.
Questions: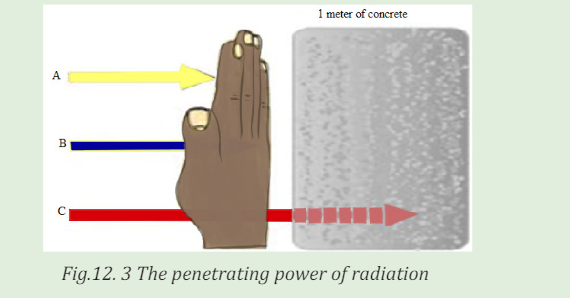
a. Interpret the figure and write the names of letters A, B and C
labeled on the figure above?
b. Which of the three types of radiation has high penetrating power?
Explain to support your idea.
c. Outline four uses of the man-made sources of radiation?
d. How does radiation affect me? Explain clearly with scientificreasoning.
An important characteristic of the various ionising radiations is how deeply
they can penetrate the body tissues. X-rays, gamma rays, and neutrons of
sufficient energy described below can reach all tissues of the body from anexternal source.
Alpha Radiation
Alpha radiation occurs when an atom undergoes radioactive decay, giving
off an α- particle consisting of two protons and two neutrons (essentially thenucleus of a helium-4 atom) following the equation
Due to their charge and mass, alpha particles interact strongly with matter,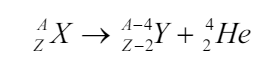
and can only travel a few centimeters in air. A thin sheet of paper, on the other
hand, stops alpha particles. They are also stopped by the superficial dead layer
of skin that is only 70 µm thick. Therefore, radionuclides that emit only alpha
particles are harmless unless you take them into the body. This you might do byinhalation (breathing in) or ingestion (eating and drinking).
Beta Radiation
Beta radiation takes the form of either an electron or a positron (a particle
with the size and mass of an electron, but with a positive charge) being emitted
from an atom. Due to their smaller mass, they are able to travel further in air,
up to a few meters, and can be stopped by a thick piece of plastic, or even a
stack of paper. Such radiation can penetrate the skin a few centimeters, posing
somewhat of an external health risk. The depth to which beta particles canpenetrate the body depends on their energy.
High-energy beta particles (several MeV) may penetrate a cm of a tissue,
although most are absorbed in the first few mm. As a result, beta emitters
outside the body are hazardous only to surface tissues such as the skin or the
lenses of the eye. When you take beta emitters into the body, they will irradiateinternal tissues and then become a much more serious hazard.
Gamma Radiation
Gamma radiation, unlike alpha or beta, does not consist of any particles, instead
consisting of a photon of energy being emitted from an unstable nucleus. Having
no mass or charge, gamma radiation can travel much farther through air than
alpha or beta, losing (on average) half its energy. Gamma waves can be stopped
by a thick or dense enough layer material, with high atomic number. Materialssuch as lead can be used as the most effective form of shielding.
X-Rays
X-rays are similar to gamma radiation, with the primary difference being that
they originate from the electron cloud. This is generally caused by energy
changes in an electron, such as moving from a higher energy level to a lower
one, causing the excess energy to be released. X-Rays are longer-wavelengthand (usually) lower energy than gamma radiation, as well.
Neutron Radiation
Neutron radiation consists of a free neutron, usually emitted as a result of
spontaneous or induced nuclear fission. They are able to travel hundreds or
even thousands of meters in air, they are however able to be effectively stopped
if blocked by a hydrogen material, such as concrete or water.
Neutron radiation occurs when neutrons are ejected from the nucleus by
nuclear fission and other processes. The nuclear chain reaction is an example
of nuclear fission, where a neutron being ejected from one fission atom will
cause another atom to fission, ejecting more neutrons. Unlike other radiations,
neutron radiation is absorbed by materials with lots of hydrogen atoms, likeparaffin wax and plastics.
12.1.3 Radiation dosimetry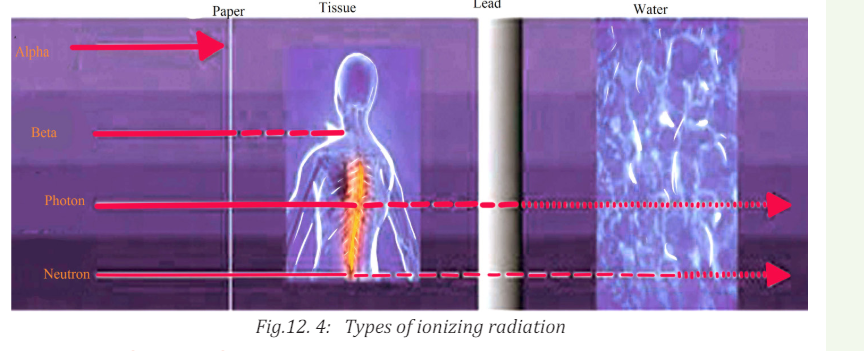
ACTIVITY 12.3:
a. What does the term Dosimeter in radiation dosimetry mean?
b. Who Should Wear a Dosimeter? Suggest reasons why it is very important
to wear a dosimeter?
Just as for drugs, the effect of radiation depends on the amount a person has
received. Therefore, amounts of radiation received are referred to as doses,and the measurement of such doses is known as dosimetry.
Dosimeters are used to monitor your occupational dose from radioactive
material or radiation-producing equipments. Most individuals working with
X-ray producing equipment in the hospital will be issued with a dosimeter. For
those individuals working in the research laboratory setting, dosimeters will
be issued based on the nuclide and total activity that will be used. Dosimeters
are integrating detectors; that is, they accumulate the radiation dose and giveoff an amount of light which is proportional to that dose.
The energy absorption properties of dosimeters are designed to be very similar
to tissue, so they are very effective as personnel dosimeters. These devices are
used to measure exposures from x-ray, gamma ray and high energy beta
particles. Dosimeters are not suitable for measuring exposures to low energybeta particles or alpha particles.
12.1.4 Radiation exposure
ACTIVITY 12.4:
a. What are the symptoms of radiation exposure?
b. Explain briefly the effects of radiation exposure to the human body?
c. It is possible that side effects can happen when a person undergoes
radiation treatment for cancer. Suggest the common side effects of
radiation exposure to the human body?
d. Does radiation exposure to the human body induce risks? Support yourdecision with clear explanations.
Long-term exposure to small amounts of radiation can lead to gene mutations
and increase the risk of cancer, while exposure to a large amount over a brief
period can lead to radiation sickness.
Exposure is a measure of the ionization produced in air by X-rays or γ rays,
and it is defined in the following manner. A beam of X-rays or γ rays is sent
through a mass m of dry air at standard temperature and pressure ( stp:0 0C ,
1 atm). In passing through the air, the beam produces positive ions whose total
charge is q. Exposure is defined the total charge per unit mass of air.The SI unitfor exposure is coulomb per unit mass (/ ) C kg .
The commonly used unit for exposure E is the roentgen(R). 1R is the amount
of electromagnetic radiation which produces in one gram of air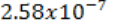
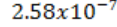 C at normal temperature (22 and pressure (760mmHg) conditions
C at normal temperature (22 and pressure (760mmHg) conditions
Since the concept of exposure is defined in terms of the ionizing abilities of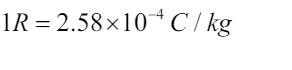
X-rays and γ rays in air, it does not specify the effect of radiation on living tissue.
For biological purposes, the absorbed dose is more suitable quantity, because it
is the energy absorbed from the radiation per unit mass of absorbing material.12.1.5 Absorbed radiation dose
ACTIVITY 12.5
a. What does the term absorbed dose mean in medical treatment?
b. In the application of radiation in medicine, we use the statement “A measure of
the risk of biological harm”. Brainstorm and explain clearly what the statement
means.
c. Explain why doses of alpha and gamma radiation produce unequal biologicaleffects?
What is important when we analyze the effect of radiation on human being is
not so much the total dose to the whole system but the dose per kg. That’s why
a doctor will prescribe smaller doses of medicine for children than for adults. A
similar approach is used in radiation protection measurements, where the unit
of absorbed dose is specified in terms of the amount of energy deposited byradiation in 1 kg of material. This unit is the Gray, abbreviated Gy.
It was named in honor of Louis Gray, who was a very big name in the early days
of radiation dosimetry. An absorbed radiation dose of 1 Gray corresponds to
the deposition of 1 joule of energy in 1 kg of material. The gray is a measure of
energy absorbed by 1 kg of any material, be it air, water, tissue or whatever. A
person who has absorbed a whole body dose of 1 Gy has absorbed one joule ofenergy in each kg of its body tissue.
As we shall see later, the gray is a fairly hefty dose, so for normal practical purposeswe use the milligray (abbreviated mGy) and the microgray (abbreviated µGy).
The gray is a physical unit. It describes the physical effect of the incident
radiation (i.e., the amount of energy deposited per kg), but it tells us nothing
about the biological consequences of such energy deposition in tissue. Studies
have shown that alpha and neutron radiation cause greater biological damagefor a given energy deposition per kg of tissue than gamma radiation does.
In other words, equal doses of, say, alpha and gamma radiation produce unequal
biological effects. This is because the body can more easily repair damage from
radiation that is spread over a large area than that which is concentrated in a
small area. Because more biological damage is caused for the same physicaldose.
12.1.6 Quality factors
Quality factors are used to compare the biological effects from different types
of radiation. For example, fast neutron radiation is considered to be 20 times
as damaging as X-rays or gamma radiation. You can also think of fast neutron
radiation as being of “higher quality”, since you need less absorbed dose to
produce equivalent biological effects. This quality is expressed in terms of the
Quality Factor (Q). The quality factor of a radiation type is defined as the ratio
of the biological damage produced by the absorption of 1 Gy of that radiation tothe biological damage produced by 1 Gy of X or gamma radiation.
The Q of a certain type of radiation is related to the density of the ion tracks itleaves behind it in tissue; the closer together the ion pairs, the higher the Q.
12.1.7 Equivalent dose
The absorbed radiation dose, when multiplied by the Q of the radiation
delivering the dose, will give us a measure of the biological effect of the dose.
This is known as the equivalent dose. The unit of equivalent dose H is the Sievert
(Sv). An equivalent dose of one Sievert represents that quantity of radiation
dose that is equivalent, in terms of specified biological damage, to one gray ofX or gamma rays.
In practice, we use the millisievert (mSv) and microsievert (µSv). The sievert is
the unit that we use all the time, because it is the only one that is meaningful in
terms of biological harm. In calculating the equivalent dose from several types
of radiation (we call this “mixed radiation”), all measurements are converted to
Sv, mSv or µSv and added. Most of the radiation instruments we use to measure
doses or dose rates read in mSv or µSv. Few other instruments can read in mGyor µGy, but they measure only gamma radiation.
The table 13.1 lists some typical relative biological effectiveness ( RBE ) values for
different kinds of radiation, assuming that an average biological tissue is being
irradiated. The values of 1 RBE = indicate that γ rays and particles produce
the same biological damage as do 200 keV X-rays. The large RBE values indicatethat protons, α -particles, and fast neutrons cause substantially more damage.
12.1.8 Radiation protection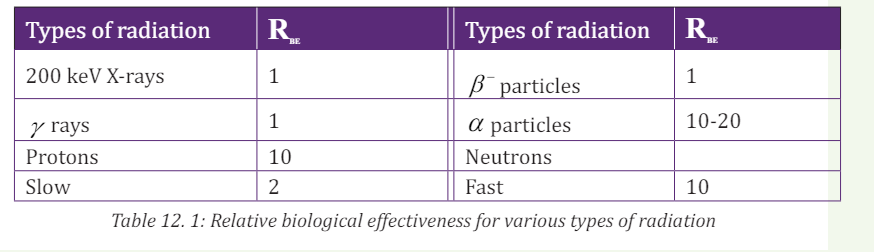
The effects of radiation at high doses and dose rates are reasonably well
documented. A very large dose delivered to the whole body over a short time
will result in the death of the exposed person within days.
We know from these that some of the health effects of exposure to radiation
do not appear unless a certain quite large dose is absorbed. However, many
other effects, especially cancers are readily detectable and occur more often in
those with moderate doses. At lower doses and dose rates, there is a degree of
recovery in cells and in tissues. Radiation protection sets examples for other
safety disciplines in two unique respects:
• First, there is the assumption that any increased level of radiation
above natural background will carry some risk of harm to health.
• Second, it aims to protect future generations from activities conductedtoday
The use of radiation and nuclear techniques in medicine, industry, agriculture,
energy and other scientific and technological fields has brought tremendous
benefits to society. The benefits in medicine for diagnosis and treatment in
terms of human lives saved are large in size. No human activity or practice
is totally devoid of associated risks. Radiation should be viewed from the
perspective that the benefit from it to mankind is less harmful than from manyother agents.
Quick check 12.2:At what level is radiation harmful? Explain your idea\
Note: The optimization of patients’ protection is based on a principle that the
dose to the irradiated target (tumor) must be as high as it is necessary for
effective treatment while protecting the healthy tissues to the maximum extentpossible.
12.1.9 Checking my progress
1. Does receiving external-beam radiation make a person radioactive or
able to expose others to radiation? Explain to support idea
2. How can I be sure that the external-beam radiating machine isn’t
damaging normal, healthy tissue in my body? Explain clearly with
scientific reasoning.
3. I am having an imaging test using radioactive materials. Will I be
radioactive after the test? Comment to support your decision.
4. All my radioactive material is secured properly and I have empty waste
containers in the lab. Do I have to lock the room? Explain clearly tojustify your decision.
12.2 BIOLOGICAL EFFECTS OF RADIATION EXPOSURE
12.2.1 Deterministic and stochastic effects
ACTIVITY 12.6
Is the use of ionizing radiation in medicine beneficial to human health?
Explain to support your point.
1. Are there risks to the use of ionizing radiation in medicine? Explain
your answer.
2. How do we quantify the amount of radiation?
3. What do we know about the nature (mechanism) of radiation induced biological effects?
4. How are effects of radiation classified?
Effects of radiations due to cell killing have a practical threshold dose below
which the effect is not evident but in general when the effect is present its
severity increases with the radiation dose.
The threshold doses are not an absolute number and vary somewhat by
individual. Effects due to mutations (such as cancer) have a probability of
occurrence that increases with dose.
a. Deterministic effects:
These effects are observed after large absorbed doses of radiation and are
mainly a consequence of radiation induced cellular death. They occur only if a
large proportion of cells in an irradiated tissue have been killed by radiation,
and the loss can be compensated by increasing cellular proliferation.
b. Stochastic effects:
They are associated with long term, low level (chronic) exposure to radiation.
They have no apparent threshold. The risk from exposure increases with
increasing dose, but the severity of the effect is independent of the dose.
Irradiated and surviving cells may become modified by induced mutations
(somatic, hereditary). These modifications may lead to two clinically significant
effects: malignant neoplasm (cancer) and hereditary mutations.
The frequency or intensity of biological effects is dependent upon the total
energy of radiation absorbed (in joules) per unit mass (in kg) of a sensitive
tissues or organs. This quantity is called absorbed dose and is expressed ingray (Gy).
In evaluating biological effects of radiation after partial exposure of the body
further factors such as the varying sensitivity of different tissues and absorbed
doses to different organs have to be taken into consideration.
To compare risks of partial and whole body irradiation at doses experienced
in diagnostic radiology and nuclear medicine a quantity called equivalent or
effective dose is used. A cancer caused by a small amount of radiation can bejust as malignant as one caused by a high dose.
ACTIVITY 12.7
1. What is magnitude of the risk for cancer and hereditary effects?
2. Is ionizing radiation from medical sources the only one to which
people is exposed?
3. What are typical doses from medical diagnostic procedures?
4. Can radiation doses in diagnosis be managed without affecting the
diagnostic benefit? Explain to support your decision.
5. Are there situations when diagnostic radiological investigations
should be avoided? Explain to support your decision.
The lifetime value for the average person is roughly a 5% increase in fatal cancer
after a whole body dose of 1 Sv. It appears that the risk in fetal life, in children
and adolescents exceeds somewhat this average level (by a factor of 2 or 3) and
in persons above the age of 60 it should be lower roughly by a factor of ~ 5.
Animal models and knowledge of human genetics, the risk of hereditary
deleterious effects have been estimated to not be greater than 10% of the
radiation induced carcinogenic risk.
All living organisms on this planet, including humans, are exposed to radiation
from natural sources. An average yearly effective dose from natural background
amounts to about 2.5 mSv. This exposure varies substantially geographically
(from 1.5 to several tens of mSv in limited geographical areas).
Various diagnostic radiology and nuclear medicine procedures cover a wide
dose range based upon the procedure. Doses can be expressed either as
absorbed dose to a single tissue or as effective dose to the entire body which
facilitates comparison of doses to other radiation sources (such as natural
background radiation.
There are several ways to reduce the risks to very, very low levels whileobtaining very beneficial health effects of radiological procedures.
Quality assurance and quality control in diagnostic radiology and nuclear
medicine play also a fundamental role in the provision of appropriate, soundradiological protection of the patient.
There are several ways that will minimize the risk without sacrificing the
valuable information that can be obtained for patients’ benefit. Among the
possible measures it is necessary to justify the examination before referring apatient to the radiologist or nuclear medicine physician.
Failure to provide adequate clinical information at referral may result in a
wrong procedure or technique being chosen by radiologist or nuclear medicinespecialist.
An investigation may be seen as a useful one if its outcome - positive or negative
influences management of the patient. Another factor, which potentially adds
to usefulness of the investigation, is strengthening confidence in the diagnosis.
Irradiation for legal reasons and for purposes of insurance should be carefullylimited or excluded.
ACTIVITY 12.8
1. Are there special diagnostic procedures that should have special
justification? Explain to support your decision.
2. Do children and pregnant women require special consideration in
diagnostic procedures?
3. What can be done to reduce radiation risk during the performanceof a diagnostic procedure?
While all medical uses of radiation should be justified, it stands to reason that
the higher the dose and risk of a procedure, the more the medical practitioner
should consider whether there is a greater benefit to be obtained.
Among these special position is occupied by computed tomography (CT), and
particularly its most advanced variants like spiral or multi slice CT.
Both the fetus and children are thought to be more radiosensitive than adults.
Diagnostic radiology and diagnostic nuclear medicine procedures (even in
combination) are extremely unlikely to result in doses that cause malformations
or a decrease in intellectual function. The main issue following in childhood
exposure at typical diagnostic levels (<50 mGy) is cancer induction.
Medically indicated diagnostic studies remote from the fetus (e.g. radiographs
of the chest or extremities, ventilation/perfusion lung scan) can be safely done
at any time of pregnancy if the equipment is in proper working order. Commonly
the risk of not making the diagnosis is greater than the radiation risk.
If an examination is typically at the high end of the diagnostic dose range and
the fetus is in or near the radiation beam or source, care should be taken to
minimize the dose to the fetus while still making the diagnosis. This can be
done by tailoring the examination and examining each radiograph as it is taken
until the diagnosis is achieved and then terminating the procedure
For children, dose reduction in achieved by using technical factors specific for
children and not using routine adult factors. In diagnostic radiology care should
be taken to minimize the radiation beam to only the area of interest. Because
children are small, in nuclear medicine the use of administered activity lower
than that used for an adult will still result in acceptable images and reduced
dose to the child. The most powerful tool for minimizing the risk is appropriate
performance of the test and optimization of radiological protection of the
patient. These are the responsibility of the radiologist or nuclear medicine
physician and medical physicist.
The basic principle of patients’ protection in radiological X-ray investigations
and nuclear medicine diagnostics is that necessary diagnostic information of
clinically satisfactory quality should be obtained at the expense of a dose as low
as reasonably achievable, taking into account social and financial factors.
12.2.2 Effects of radiation exposure
Quick check13.1:
Will small radiation doses hurt me?
Some effects may occur immediately (days or months) while others might take
tens of years or even get passed to the next generation. Effects of interest for
the person being exposed to radiation are called somatic effects and effects ofinterest that affect our children are called genetic effects.
I. Radiation Health Effects
Ionizing radiation has sufficient energy to cause chemical changes in cells and
damage them. Some cells may die or become abnormal, either temporarily or
permanently. By damaging the genetic material (DNA) contained in the body’s
cells, radiation can cause cancer.
Fortunately, our bodies are extremely efficient at repairing cell damage. The
extent of the damage to the cells depends upon the amount and duration of theexposure, as well as the organs exposed.
Exposure to an amount of radiation all at once or from multiple exposures in a
short period of time. In most cases, a large acute exposure to radiation causes
both immediate ( radiation sickness) and delayed effects (cancer or death), can
cause sickness or even death within hours or days. Such acute exposures areextremely rare.
II. Chronic Exposure
With chronic exposure, there is a delay between the exposure and the observed
health effect. These effects can include cancer and other health outcomes such
as benign tumors, cataracts, and potentially harmful genetic changes.
Some radiation effects may occur immediately (days or months) while others
might take years or even get passed to the next generation. Effects of interest
for the person being exposed to radiation are called somatic effects and effectsof interest that affect our children are called genetic effects.
ACTIVITY 12.9:Low levels of radiation exposure
What is the safe level of radiation exposure? Explain your answer.
What is the annual radiation exposure limit? Explain your answer
Radiation risks refer to all excess cancers caused by radiation exposure
(incidence risk) or only excess fatal cancers (mortality risk). Risk may be
expressed as a percent, a fraction, or a decimal value.
For example, a 1% excess risk of cancer incidence is the same as a 1 in a
hundred (1/100) risk or a risk of 0.01. However, it is very hard to tell whether
a particular cancer was caused by very low doses of radiation or by something
else. While experts disagree over the exact definition and effects of “low dose”.
Radiation protection standards are based on the premise that any radiation
dose carries some risk, and that risk increases directly with dose.
Note:
• The risk of cancer from radiation also depends on age, sex, and factors
such as tobacco use.
• Doubling the dose doubles the risk.
Acute health effects occur when large parts of the body are exposed to a
large amount of radiation. The large exposure can occur all at once or from
multiple exposures in a short period of time. Instances of acute effects fromenvironmental sources are very rare.
12.2.3 Safety precautions for handling radiations
ACTIVITY 12.10: Safety precautions to be recognized when
handling radiation
a. Who is involved in planning my radiation treatment?
b. How is the treatment plan checked to make sure it is best for me?
c. What procedures do I have in place so that the treatment team is
able to treat me safely?
d. How can I be assured that my treatment is being done correctly
every day?
e. What is the difference between a medical error and a side effect?
f. Outline the measures taken to reduce doses from externalexposure
Shortening the time of exposure, increasing distance from a radiation source
and shielding are the basic countermeasures (or protective measures) to reducedoses from external exposure.
Note: Time: The less time that people are exposed to a radiation source, the less
the absorbed dose Distance: The farther away that people are from a radiationsource, the less the absorbed dose.
Note: Shielding: Barriers of lead, concrete or water can stop radiation or reduce
radiation intensity.
There are four main factors that contribute to how much radiation a person
absorbs from a source. The following factors can be controlled to minimize
exposure to radiation:
I. The distance from the source of radiation
The intensity of radiation falls sharply with greater distance, as per the inverse
square law. Increasing the distance of an individual from the source of radiation
can therefore reduce the dose of radiation they are exposed to.
For example, such distance increases can be achieved simply by using forceps
to make contact with a radioactive source, rather than the fingers.
II. Duration of exposure
The time spent exposed to radiation should be limited as much as possible.
The longer an individual is subjected to radiation, the larger the dose from the
source will be.
One example of how the time exposed to radiation and therefore radiation
dose may be reduced is through improving training so that any operators whoneed to handle a radioactive source only do so for the minimum possible time.
III. Reducing incorporation into the human body
Potassium iodide can be given orally immediately after exposure to radiation.
This helps protect the thyroid from the effects of ingesting radioactive iodine if
an accident occurs at a nuclear power plant. Taking Potassium iodide in such anevent can reduce the risk of thyroid cancer developing.
IV. Shielding
Shielding refers to the use of absorbent material to cover the source of
radiation, so that less radiation is emitted in the environment where humans
may be exposed to it. These biological shields vary in effectiveness, dependingon the material’s cross-section for scattering and absorption.
The thickness (shielding strength) of the material is measured in g/cm2. Any
amount of radiation that does penetrate the material falls exponentially withincreasing thickness of the shield.
Some examples of the steps taken to minimize the effects of radiation exposure
are described below;
• The exposed individual is removed from the source of radiation.
• If radiation exposure has led to destruction of the bone marrow, the
number of healthy white blood cells produced in the bone marrow will
be depleted.
• If only part of the body has been exposed to radiation rather than the
whole body, treatment may be easier because humans can withstandradiation exposure in large amounts to non-vital body parts.
In every medicine there is a little poison. If we use radiation safely, there are
benefits and if we use radiation carelessly and high doses result, there areconsequences.
Ionizing radiation can change the structure of the cells, sometimes creating
potentially harmful effects that are more likely to cause changes in tissue.
These changes can interfere with cellular processes so cells might not be ableto divide or they might divide too much.
Radioactive rays are penetrating and emit ionizing radiation in the form of
electromagnetic waves or energetic particles and can therefore destroy living
cells. Small doses of radiation over an extended period may cause cancer and
eventually death. Strong doses can kill instantly. Marie Curie and Enrico Fermidied due to exposure to radiation.
Several precautions should be observed while handling radioisotopes. Some of
these are listed in the following:
• No radioactive substance should be handled with bare hands. Alpha
and beta emitters can be handled using thick gloves. Gamma ray
emitters must be handled only by remote control that is by mechanical
means. Gamma rays are the most dangerous and over exposure can
lead to serious biological damage.
• Radioactive materials must be stored in thick lead containers.
• Reactor and laboratories dealing with and conducting experiments
with radioactive metals must be surrounded with thick concrete lined
with lead.
• People working with radioactive isotopes must wear protective
clothing which is left in the laboratory. The workers must be checked
regularly with dosimeters, and appropriate measures should be taken
in cases of overdose.• Radioactive waste must be sealed and buried deep in the ground.
12.2.3 Checking my progress
1.
a. What does the term background radiation mean?
b. Hat is radiation – am I exposed to background radiation each day
even if I do not have an X-ray examination?
2. What are the risks associated with radiation from diagnostic X-ray
imaging and nuclear medicine procedures?
3. How do I decide whether the risks are outweighed by the benefits of
exposure to X-radiation when I have a radiology test or procedure?
4. Are there alternatives to procedures that involve ionizing radiation that
would answer my doctor’s question? Justify your answer with clear
facts.
5. What kinds of safety checks do you perform each day?
6. How often does the medical physicist check the various machines
involved during my treatment are working properly?
7. If I have side effects after my treatment, who can I call?
a. My best friend
b. My primary care doctor
8. I have a question about a radiation treatment I had many years ago.Who should I call?
12.3 CONCEPT OF BALANCED RISK.
12.3.1 Risks of ionizing radiation in medical treatment
ACTIVITY 12.11:balanced risk
Brainstorm and write briefly how balance risks in medical treatment
occur?
Risk in the area of radiation medicine has several dimensions that are less
common in other areas of medicine. First, there may be risks
from overexposure that do not cause immediate injury. For example, the causal connection,
if any, may be difficult or impossible to verify for a malignancy that surfaces
several years after an inappropriate exposure. Second, the risks associated
with the medical use of ionizing radiation extend beyond the patient and can
affect health care workers and the public.
In amplifying these and other aspects of the risks that attend medical uses ofionizing radiation, the discussion addresses the following issues: human error
and unintended events; rates of misadministration in radiation medicine;
inappropriate and unnecessary care; and efforts that reduce misadministrationand inappropriate care.
12.3.2 Human Error and Unintended Events
Errors occur throughout health care: A pharmacist fills a prescription with the
wrong medicine; an x-ray technician takes a film of the wrong leg; a surgeon
replaces the wrong hip. The advent of complex medical technology has
increased the opportunity for error even as it has increased the opportunity for
effecting cures.
By educating health care workers, and by circumscribing their actions, human
error may be minimized. However, some number of mistakes will always,
unavoidably, be made, and no amount of training or double-checking can erasethat fact.
12.3.3 Comparison of risks in the use of ionizing radiation
The comparison of relative risks of misadministration in by-product radiation
medicine to error rates and events in other medical practice settings, as well
as the comparison of disease and death rates with the risks of the therapeutic
administration itself, help to some extent to place ionizing radiation use in a
broader context.
To achieve this success requires the highest standards of performance (accuracy
of delivered dose), both when planning irradiation for an individual patient and
in actual delivery of the dose.
In a large number of cases, decreasing the dose to the target volume is not
possible since it would unacceptably decrease the cure rate. In these cases
present technological developments aim at optimizing the patients’ protection,
keeping the absorbed tumor dose as high as is necessary for effective treatment
while protecting nearby healthy tissues.
It should be remembered that successful eradication of a malignant tumor by
radiation therapy requires high-absorbed doses and there is a delayed (and
usually low) risk of late complication. The above mention techniques are used
to provide the best benefit/risk ratio.
A malignant tumor in a pregnant woman may require radiotherapy in attempt
to save life of the patient. If a tumor is located in a distant part of the body, the
therapy with individually tailored protection of the abdomen (screening) - may
proceed.
When thyroid cancer with metastases is diagnosed in a pregnant woman,
treatment with 131I is not compatible with continuation of the pregnancy. The
treatment should then be delayed until delivery if doing so wouldn’t put the
mother’s life in danger.
Medical radiation can be delivered to the patient from a radiation source
outside the patient. Regardless of how much dose the patient received, they do
not become radioactive or emit radiation.• Balancing risks are often summarized in the following:12.3.4 Checking progress
• The demand for imaging, especially computed tomography, that has
increased vastly over the past 20 years
• An estimated 30% of computed tomography tests that may be
unnecessary
• Ionizing radiation that may be associated with cancer.
• The risks of radiation exposure that is often overlooked and patients
are seldom made aware of these risks
• The requesting doctor who must balance the risks and benefits
of any high radiation dose imaging test, adhering to guideline
recommendations if possible
• Difficult cases that should be discussed with a radiologist, ideally at aclinic radiological or multidisciplinary team meeting.
1. When patients are intentionally exposed to ionizing radiation for12.4 THE HALF-LIVES: PHYSICAL, BIOLOGICAL, AND FFECTIVE
medical purposes, do they suffer unintentional exposures as a result of
error or accident? Comment to support your idea.
2. What can be done to reduce radiation risk during conduct of radiation
therapy?
3. Can pregnant women receive radiotherapy? Explain to support your
decision.
4. Can patients’ treatment with radiation affect other people? Explain tosupport your decision.
ACTIVITY 12.12
Distinguish between physical half-life, biological half-life and effectivehalf-life.
Brainstorm and write the distinction between physical half-life, biological halflife and effective half-life in your note books.
The half-life is a characteristic property of each radioactive species and is
independent of its amount or condition. The effective half-life of a given isotope
is the time in which the quantity in the body will decrease to half as a result ofboth radioactive decay and biological elimination.
There are three half-lives that are important when considering the use of
radioactive drugs for both diagnostic and therapeutic purposes. While both
the physical and biological half-lives are important since they relate directly
to the disappearance of radioactivity from the body by two separate pathways
(radioactive decay, biological clearance), there is no half-life as important inhumans as the effective half-life.
The half-life takes into account not only elimination from the body but also
radioactive decay. If there is ever a question about residual activity in the body,
the calculation uses the effective half-life; in radiation dosimetry calculations,the only half-life that is included in the equation is the effective half-life.
12.4.1 Physical half Lives
Physical half-life is defined as the period of time required to reduce the
radioactivity level of a source to exactly one half its original value due solely toradioactive decay. The physical half-life is designated Tp or more commonly
By default, the term T12 refers to the physical half-life and Tp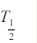
is used when either or both of the other two half-lives are
included in the discussion. Where λ is the radioactive constant of the radio substance
Where λ is the radioactive constant of the radio substance
There are a few things to note about the Tp :• The Tp can be measured directly by counting a sample at 2 different
points in time and then calculating what the half-life is.• For example, if activity decreases from 100% to 25% in 24 hours, then
the half-life is 12 hours since a decrease from 100% to 50% to 25%
implies that 2 half-lives have elapsed.
The physical half-life is unaffected by anything that humans can do to the
isotope. High or low pressure or high or low temperature has no effect on thedecay rate of a radioisotope.
12.4.2 Biological half lives
Biological Half-life is defined as the period of time required to reduce the
amount of a drug in an organ or the body to exactly one half its original value
due solely to biological elimination. It is typically designated Tb . There are afew things to note about the Tb :
• For radioactive compounds, we have to calculate the Tb because
the mass of the isotope is usually on the nanogram scale and, when
distributed throughout the body, and especially in the target organ,
concentrations are in the pictogram/ml range, much too small to
measure directly.
• For non-radioactive compounds, we can measure the Tb directly. For
example, assuming that a person is not allergic to penicillin, we could
give 1 000 mg of the drug and then measure the amount present in the
blood pool and in the urine since we administered such a large amount
of the drug• Tb is affected by many external factors. Perhaps the two most important12.4.3 Effective half lives
are hepatic and renal function. If kidneys are not working well, wewould expect to see a high background activity on our scans.
• Each individual organ in the body has its own Tb and the whole body
also has a Tb representing the weighted average of the Tb of all internal
organs and the blood pool. It is therefore very important to have a frame
of reference. For example, do you need to know the Tb of the drug in theliver or in the whole body?
• All drugs have aTb , not just radioactive ones. Drug package inserts
often refer to the half-time of clearance of a drug from the blood pool
or through the kidneys.
• Since the whole body has a Tb representing the weighted average
of the Tb of all internal organs, it will almost never equal that of aninternal organ.
Effective half-life is defined as the period of time required to reduce the
radioactivity level of an internal organ or of the whole body to exactly one halfits original value due to both elimination and decay.
It is designated Te can be measured directly. For example, one can hold a
detection device 1 m from the patient’s chest and count the patient multipletimes until the reading decreases to half of the initial reading.
The patient is permitted to use the rest room between readings as needed, so
both elimination and decay are taking place. The half-life being measured in
this case is the Te and Te is affected by the same external factors that affect Tbsince Te is dependent upon Tb .
Where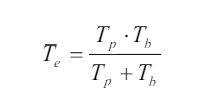
Tp: physical half-lifeTb : biological half-life

END UNIT ASSESSMENT 12
A. Multiple choices.
1. Which of the following would reduce the cell damage due to
radiation for a lab technician who works with radioactive isotopes
in a hospital or lab?a. Increase the worker’s distance from the radiation source.
b. Decrease the time the worker is exposed to the radiation.
c. Use shielding to reduce the amount of radiation that strikes
the worker.
d. Have the worker wear a radiation badge when working with
the radioactive isotopes.e. All of the above.2. If the same dose of each type of radiation was provided over the
same amount of time, which type would be most harmful?
a. X-rays. c. γ rays.
b. α Rays. d. β particles.
3. Which of the following is true?a. Any amount of radiation is harmful to living tissue.4. Which radiation induces the most biological damage for a given
b. Radiation is a natural part of the environment.
c. All forms of radiation will penetrate deep into living tissue.
d. None of the above is true.
amount of energy deposited in tissue?a. Alpha particules.5. Which would produce the most energy in a single reaction?
b. Gamma radiation.
c. C. Beta radiation.
d. D. All do the same damage for the same deposited energy.a. The fission reaction associated with uranium-235.6. The fuel necessary for fusion-produced energy could be derived from
b. The fusion reaction of the Sun (two hydrogen nuclei fused to
one helium nucleus).
c. Both (A) and (B) are about the same.
d. Need more information.
a. Water. d. Superconductors.
b. Uranium. e. Helium.
c. Sunlight.B. Structured questions
7. If the equipment isn’t working and my treatment is delayed or
postponed, who checks that it is safe to use again? And will this delay
affect my cancer?
8. Do you have weekly chart rounds where you review patient-related
information in peer review?
9. Will you take imaging scans regularly during my treatment to verify
position of my treatment? Who reviews those scans?
10. People who work around metals that emit alpha particles are trained
that there is little danger from proximity or touching the material, but
they must take extreme precautions against ingesting it. Why? (Eating
and drinking while working are forbidden.)
11. What is the difference between absorbed dose and effective dose? What
are the SI units for each?
12. Radiation is sometimes used to sterilize medical supplies and even food.
Explain how it works.
13. How might radioactive tracers be used to find a leak in a pipe?
14. Explain that there are situations in which we may or may not have
control over our exposure to ionizing radiation.a. When do we not have control over our exposure to radiation?15. Does exposure to heavy ions at the level that would occur during
b. When do we have control over our exposure to radiation?
c. Why might we want to limit our exposure to radiation when
possible?
deep space missions of long duration pose a risk to the integrity and function
of the central nervous system?
16. Radiation protection of ionizing radiation from radiation sources is
particularly difficult. Give a reason for this difficulty.
C. Essay questions17. I always lock my radioactive material-use rooms. However, renovators
came in during the weekend, worked, and left the door open while they
were on their lunch break. Am I responsible and how can I prevent this
from happening? Debate on the situation above to support your answer.
18. How can I ensure that personnel who work in my lab, but do not use
radioactive material, do not violate the security requirements? Debate
to support your idea.
19. A Housekeeping staff member opens my radioactive material-use room
after working hours and does not lock it when they leave. What should
I do? Explain clearly to support your idea.
20. Make a research and predict what steps that can or might be taken to
reduce the exposure to radiation (consider if living near a radioactive
area like an abandoned uranium mine, if finding a radioactive source, orin the event of a nuclear explosion or accident).
BIBLIOGRAPHY
eschooltoday. (2008-2018). Retrieved February 19, 2018, from natural diseasters: http
eschooltoday. (2008-2018). Retrieved February 19, 2018, from Climate change: http
http://www.threastafrican.co.ke. (2017). Retrieved from rwanda/Business/kigali.
Abbot, A. F., & Cockcroft, J. (1989). Physics (5 ed.). Heinemann: Educational Publishers.
Atkins, K. R. ( 1972). Physics-Once over Lightly. New York : New York.
Avison, J. (1989). The world of PHYSICS. Cheltenham: Thomas Nelson and Sons Ltd.
AVISON, J. (1989). The world of PHYSICS. Cheltenham: Thomas Nelson and Sons Ltd.
BIPM. (2006). The International System of Units (SI). (8 ed.). Sevres, France: International
Bureau of Weights and Measures.
Breithaupt, J. (2000). Understanding Physics For Advanced Level. (4 ed.). Ellenborough
House, Italy: Stanley Thorners.
Chand, S., & S.N., G. S. (2003). Atomic Physics (Modern Physics) (1 ed.). India.
CPMD. (2015). Advanced Level Physics Sylabus. Kigali: REB.
Cunningham, & William, P. (2000). Environmental science (6 ed.). Mc Graw-Hill.
Cutnell, J. D., & Johnson, K. W. (2006). Essentials of Physics. USA: John Wlley &Sons, Inc.
Cutnell, J. D., & Johnson, K. W. (2007). Physics. (7 ed.). USA: John Wiley; Sons, Inc.
Cuttnell, J. D., & kennety, W. J. (2007). Physics (7 ed.). United State of America: John
Willey & Sons . Inc.
Douglass, C. G. (2014). PHYSICS, Principles with applications. (7 ed.). Pearson Education.
Douglass, C. G. (2014). PHYSICS, Principles with applications. (8 ed.). Pearson Education.
Duncan, T., & Kennett, H. (2000). Advanced Physics (5 ed.). London, UK: Holder
Education.
Giancoli, D. (2005). PHYSICS: Principles with applications. New Jersey: Pearson
Education, Inc.
Giancoli, D. C. (2005). Physics principals with application. Upper Saddle River, NJ 07458:
Pearson Education, Inc.
Giancoli, D. C. (2005). Physics: principals with application. Upper Saddle River, NJ
07458: Pearson Education, Inc.
Giancoli, D. C. (2005). Physics: Principles with applications. New Jersey: Pearson
Education, Inc.
Glencoe. (2005). Physics - Principles and Problems [textbook]. McGraw.
Haber-Schaim, U., Cutting, R., Kirkesey, H. G., & Pratt, H. A. (2002). Force, Motion, and
Energy. USA: Science Curriculum, Inc.
Halliday, D., Resneck, R., & Walker, J. (2014). Fundamentals of Physics. (10 ed.). USA:
John Wiley; Sons,Inc.
Halliday, Resneck, & Walker. (2007). Fundamentals of Physics. (8 ed.). Wiley.
Hewitt, P. G., SUCH0CKI, J., & Hewitt, L. A. (1999). Conceptual Physical Science. (2 ed.).
Addison Wesley Longman.
Hirsch, A. S. (2002). Nelson Physics 12. Toronto: University Preparation.
Hugh, D. Y., & Roger, A. F. (2012). University Physics with Modern Physics (13 ed.). San
Francisco, USA: Pearson Education, Inc.
IPCC. (1996). Economics of Greenhouse Gas limitation, Main report “Methodological
Guidelines.
John, M. (2009). Optical Fiber Communications, Principals and Practice (3rd Ed.).
London: Pearsnon Prentice Hall.
Jones, E. R., & Childers, R. L. (1992). Contemporary College Physics. (2 ed.). USA:
Addison-Wesley Publishing Company.
Kansiime, J. K. (2004). Coumpound Physical Geography: Morphology, Climatology, Soils
and Vegetation. uganda.
Linda, W. (2004). Earth Sceience demystified a self-teaching guide. USA: McGraw-Hill
Campanies, inc.
Michael, E. B. (1999). Schaum’s outline of Theory and Problems of Physics for Engineering
and Science. USA: McGRAW-HILL Companies, Inc.
Michael, J. P., Loannis, M., & Martha, C. (2006). Science Explorer, Florida Comprehensive
Science. Boston: Pearson Prentice Hall.
MIDIMAR. (2012). Disaster High Risk Zones on Floods and Landslide. Kigali: MIDMAR.
Nagashima, Y. (2013). Elementary Particle Physics. Osaka University: Deutsche
Nationalbibliothek.
Nelkon, M., & Parker, P. (1997). Advanced level Physics. (7 ed.). Edinburgh: Heinemann.
Nelkon, M., & Parker, P. (2001). Advanced Level Physics (7 ed.). Edinburgh gate:
Heinemann.
Office, U. M. (2011). Warming: A guide to climate change. U.K.: Met Office Hadley Centre.
Orazio, S. (2010). Principles of Lasers (5 ed.). Milan, Italy: Springer.
Patrick, T. (2004). Mathematics standard level. (3 ed.). Victoria: Ibid Press.
R.B., B. (1984). Physical Geography in diagrams for Africa. Malaysia: Longmann Group
Limited.
Randall, D., & Knight. (2004). Physics for scientists and engineers: Stategic approach
(Vol. 2). San Fransisco: Pearson Education.
Randall, D., & Knight. (2004). Physics for scientists and engineers: Stategic approach.
(Vol. 3). San Fransisco: Pearson Education, Inc.
Randall, D., & Knight. (2008). Physics for scientists and engineers: Stategic approach. (2
ed., Vol. 3). San Fransisco: Pearson Education, Inc.
Randall, D., & Knight. (2008). Physics for scientists and engineers: Stategic approach. (2
ed., Vol. 3). San Fransisco: Pearson Education, Inc.
REMA. (n.d.). Rwanda Second National Communication Under the UNFCCC.
KIGALI: MINISTRY OF NATURAL RESOURCES,RWANDA.
Science, G. (2006). Florida Physical Science with Earth Science. USA: Mc Graw
Hill Glencoe Companies, Inc.
Serway, R. A. (1986). Physics for Scientists and Engineers (2 ed.). Saunders
College Publishing.
Serway, R. A. (1992). Principles of Physics. Orlando, Florida: Saunders College Publishing.
Serway, R. A., & Jewett, J. J. (2008). Physics for Scientists and Engineers. (7 ed.). USA:
Thomson Learning, Inc.
Serway, R. A., & Jewett, J. J. (2010). Physics for Scientists and Engineers with Modern
Physics. (8 ed.).
Silver, B., & Ginn, I. (1990). Physical Science. Unit States of America.
Stephen, P., & Whitehead, P. (1996). Physics. (2 ed.). School Edition.
Stephen, P., & Whitehead, P. (1996). Physics. (2 ed.). School Edition.
Strassler, M. (2011, September 25). What’s a Proton, Anyway? Retrieved March 05,
2018, from www.profmattstrassler.com: https://profmattstrassler.com/articles-andposts/largehadroncolliderfaq/whats-a-proton-anyway/
Subranya, K. (1993). Theory and applications of fluid mechanics. Tata McGraw: Hill
Companies.
Taylor, E., & Wheeler, J. A. (1992). Spacetime Physics: Introduction to Special Relativity.
(2 ed.). San Francisco: W.H.Freeman & Company, Publishers.
Taylor, E., & Wheeler, J. A. (1992). Spacetime Physics: Introduction to Special Relativity.
(2 ed.). San Francisco: W.H.Freeman & Company, Publishers.
Tipler, P. A. (1991). Physics for Scientists and Enginners. (3 ed., Vol. 2). USA: Worth
Publishers, Inc.
Tipler, P. A. (1991). Physics for Scientists and Enginners. (3 ed., Vol. 1). USA: Worth
Publishers, Inc.
Tom, D. (2000). Advanced Physics (5 ed.). H. Kennett.
Toyal, D. C. (2008). Nuclear Physics (5 ed.). Himalaya Publishing House.
Uichiro, M. (2001). Introduction to the electron theory of metals . Cambridge University
Press .
Weseda, Y., Mastubara, E., & Shinoda, K. (2011). X-rays properties-google search.
Retrieved 03 06, 2018, from www.springer.com: http://www.springe.com/978-3-
642-26634-1
Wysession, M., Frank, D., & Yancopoulos, S. (2004). Physical Science. Boston,
Massachusetts, Upper Saddle River, New Jersey: Pearson Prentice Hall.
