UNIT 4:ATOMIC NUCLEI AND RADIOACTIVE DECAY
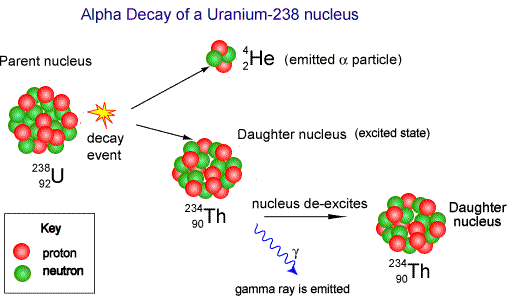
Fig.4. 1: Sign of radiation precaution
Key unit competence: Analyse atomic nuclei and radioactivity decay
My goals
• Define atomic mass and atomic number
• Identify the constituents of a nucleus
• Explain Einstein’s mass-energy relation.
• Define nuclear fusion and fission.
• Analyze determinations of a mass of nuclei by using Bainbridge mass
spectrometer.
• Derive the relationship between decay constant and half-life.
• Determine the stability of a nuclei.
• Describe properties of different radiations.
• Describe creation of artificial isotopes.
• Identify the application of radioactivity in life.
• Plot a graph of binding energy against nucleon and explain its features.
• Calculate the decay rate of unstable isotopes.
• Appreciate the safety precautions to be taken when handling radioactive
materials.
• Appreciate that the nucleus of an atom and quantum system has discreteenergy levels.
INTRODUCTORY ACTIVITY
In different places like industries, hospitals, and other sensitive places, there
are different posts that caution someone about dangerous substances one
may encounter if care is not taken. Among the reasons why these places bare
such instruction is because of chemicals and radiations that are used in such
places which may be harmful if not handled with care.
1. Discuss some of the safety signs you have ever seen in any hospitals or
industry if you have ever visited one.
2. Why do you think there is a need to put those signs in such places?
3. It is believed that there are some of diseases that are treated using
radioactive substances. Can you state some of the radiations used to
treat some diseases.
4. There are natural men made radioactive substances. All of these are
used for different purposes. What are some of negative effects of these
radiations to (i) man , (ii) environment
5. Some countries like Iran are affected by these radiations. Imagine you
were a resident of that country, what would you do to protect yourselffrom such effects of radioactive substances.
4.1 ATOMIC NUCLEI-NUCLIDE
4.1.1 Standard representation of the atomic nucleusACTIVITY 4.1: Investigating the stable and unstable nicleus
Fig.4. 2 The standard representation of an atom nucleus
Observe the Fig.4.1 above of an atom and answer to the questions that
follow:
1. What do numbers A and Z stand for?
2. Describe the relation between the two numbers and their meanings.
3. When do we say that an atom is stable or unstable?
4. Explain clearly the meaning of isotopes. Give an example of isotopesyou know.
A nucleus is composed of two types of particles: protons and neutrons. The
only exception is the ordinary hydrogen nucleus, which is a single proton. We
describe the atomic nucleus by the number of protons and neutrons it contains,
using the following quantities:
a. The atomic number or the number of protons Z in the nucleus (sometimes
called the charge number).
b. The neutron number or the number of neutrons N in the nucleus.
c. The mass number or the number of nucleons in the nucleus,
A = Z + N. (4.01)
In representing nuclei, it is convenient to use the symbol AZX
to show how many
protons and neutrons are present in the nucleus. X represents the chemical
symbol of the element. For example, 5626 Fe nucleus has mass number 56 and
atomic number 26. It therefore, contains 26 protons and 30 neutrons.
When no confusion is likely to arise, we omit the subscript Z because the
chemical symbol can always be used to determine Z. Therefore, 5626 Fe is the same
as 56 Fe and can also be expressed as “iron-56.” Each type of atom that containsa unique combination of protons and neutrons is called nuclide.
4.1.2 Classification
Depending on the combinations of protons and neutrons in the nucleus,
nuclides can be classified in the following 3 categories:
a. Isotopes: These are nuclei of a particular element that contain the same
number of protons but different numbers of neutrons. Most elements
have a few stable isotopes and several unstable, radioactive isotopes.Example of isotopes:
Therefore, the chemical properties of different isotopes of an element are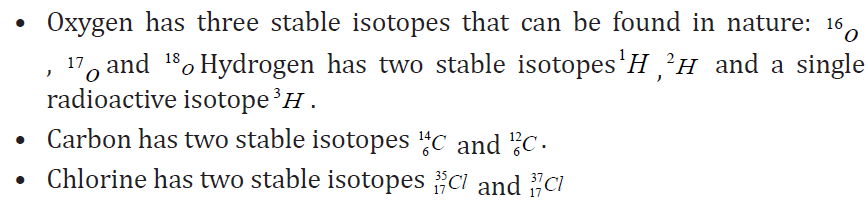
identical but they will often have great differences in nuclear stability. For
stable isotopes of light elements, the number of protons will be almost equal to
the number of neutrons. Physical properties of different isotopes of the same
element are different and therefore they cannot be separated by chemical
methods i.e. only physics methods such as the centrifugation method can be
used to separate different isotopes of an element.
b. Isobars: these are nuclei which have the same mass number but differentnumber of protons Z or neutrons N.
c. Isotones: these are nuclei in which the number of neutrons is the same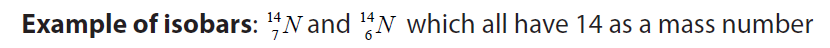
but the mass number A and the atomic number Z differ
4.1.3 Units and dimensions in nuclear physics
The standard SI units used to measure length, mass, energy etc. are too large
to use conveniently on an atomic scale. Instead appropriate units are chosen.
• The length: The unit of length in nuclear physics is the femtometer.
1 fm =10−15 m
This unit is called Fermi in the honor of the Italian Americano physicists whodid a lot of pioneering work in nuclear physics.
• The mass: The unit used to measure the mass of an atom is called the
atomic mass unit, abbreviated “amu or u” and is defined as a1⁄12 the
mass of an atom of carbon-12. Since mass in grams of one carbon-12atom is its atomic mass (12) divided by Avogadro’s number gives.
• Nuclear masses can be specified in unified atomic mass units (u).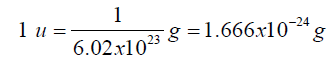
On this scale:
• A neutral 126 C atom is given the exact value 12.000000 u.
• A neutron then has a measured mass of 1.008665 u,
• A proton 1.007276 u,
• A neutral hydrogen 11H
atom (proton plus electron) 1.007825 u
Energy: the SI unit used for energy that is Joule is too large. In nuclear physics
the appropriate unit used for energy is an electronvolt (eV). An electron volt
(eV) isdefined as the energy transferred to a free electron when it is accelerated
trough a potential difference of one volt. This means that
1eV =1.6022×10−19 C×1V =1.6022×10−19 J
It is also a common practice in nuclear physics to quote the rest mass energy
calculated using ,
E = mc2 (4.02)
Since the mass of a proton is mp =1.67262 ×10 −27kg =1.007276 u, then 1 u is equal toThis is equivalent to energy in MeV of
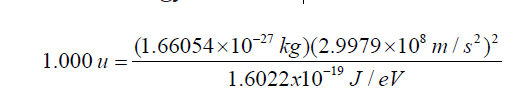
• The time: the time involved in nuclear phenomena is of the order of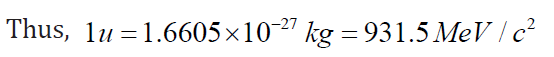
10-20s to million or billion years.
• Nuclear radius: various types of scattering experiments suggest that
nuclei are roughly spherical and appear to have the same density. Thedata are summarized in the expression called Fermi model.
Where r0 =12 fm =1.2 × 10−15 m and A is the mass number of the nucleus
The assumption of a constant density leads to the estimate of the mass densitywhich is obtained by considering.
This high density can explain why ordinary particles cannot go through the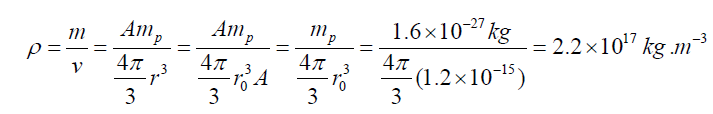
nucleus as highlighted by Rutherford experiments. The same density was only
observed in neutron stars. The nuclear mass can be determined using a mass
spectrometer.
4.1.4 Working principle a mass spectrometer
The figure below highlights the working principle of a typical mass spectrometer
used to separate charges of different masses. It can be used to differentiateisotopes of a certain element.
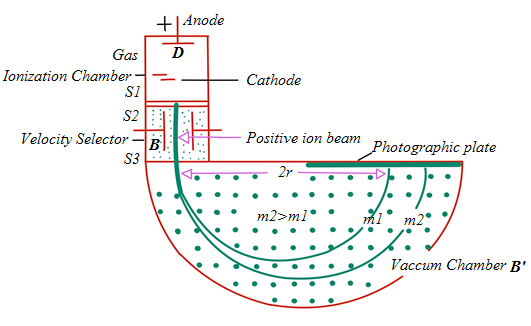
Fig.4. 3: Bainbridge mass spectrometer
Ions are formed in ionization chamber and accelerated towards the cathode.
The beam passes through the cathode and is focused by the collimating slits S1
and S2. The beam is then passed through a velocity selector in which electric
and magnetic fields are applied perpendicular to each other. The ion moves in
straight line path for which both the forces acting on it are equal
qE = qvB
The velocity of ion which passes un-deflected through the velocity selector isthen given by
The ions then reach the vacuum chamber where they are affected by the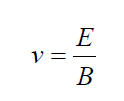
magnetic field alone and then move in circular paths; the lighter ions
alone and then move in circular paths; the lighter ions
having the larger path radius. If the mass of an ion is m, its charge q and itsvelocity v then
The radius of the path in the deflection chamber is directly proportional to the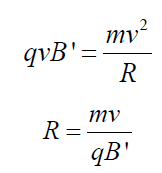
mass of the ion. The detection is done by photographic plate when the ions fall
on it. The fig. 4.5 shows the recorded mass spectrum for a gas containing three
isotopes. Note the wider line for the mass m1, showing its relatively greaterabundance.
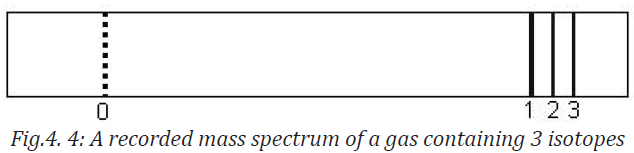
4.1.5 Checking my progress
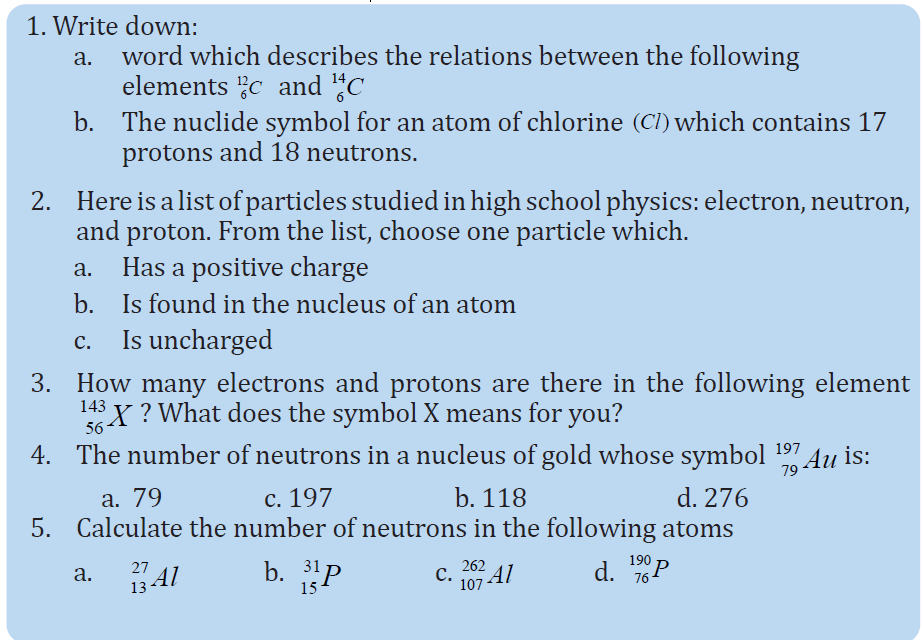
4.2 MASS DEFECT AND BINDING ENERGY
4.2.1 Mass defect
ACTIVITY 5.2: Select the words in the following puzzle
Observe the puzzle below:
1. Discover 8 different words related to particle Physics hidden in the
puzzle below, and write them in your notebook.2. Use them to formulate a meaningful sentence
3. Complete the sentences below using the words you discovered in
the puzzle
a. An …….is the SI unit of energy
c. The ………..of nucleons is greater than the mass of a nucleus.
d. The atom releases ………when its nucleus is formed from its
constituent particles
e. The binding energy per nucleon gives an indication of the …………
of the nucleus.
f. The surprising suggestion that energy and mass are equivalent
was made by ……in 1905.
4. Discuss and explain the meaning of the following expression as used in
physics
a. Mass defect c. Electronvoltb. Biding energy d. Stable nuclides
The nucleus is composed of protons that are positively charged and neutrons
that are neutral. The question is what is holding these particles together in
this tiny space?
Experiences have demonstrated that the mass of a nucleus as a whole is always
less than the sum of the individual masses of protons and neutrons composing
that nucleus.
The difference between the two measurements is called mass defect Δm . For anucleus
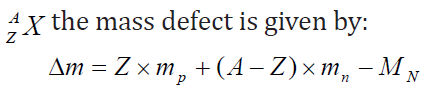
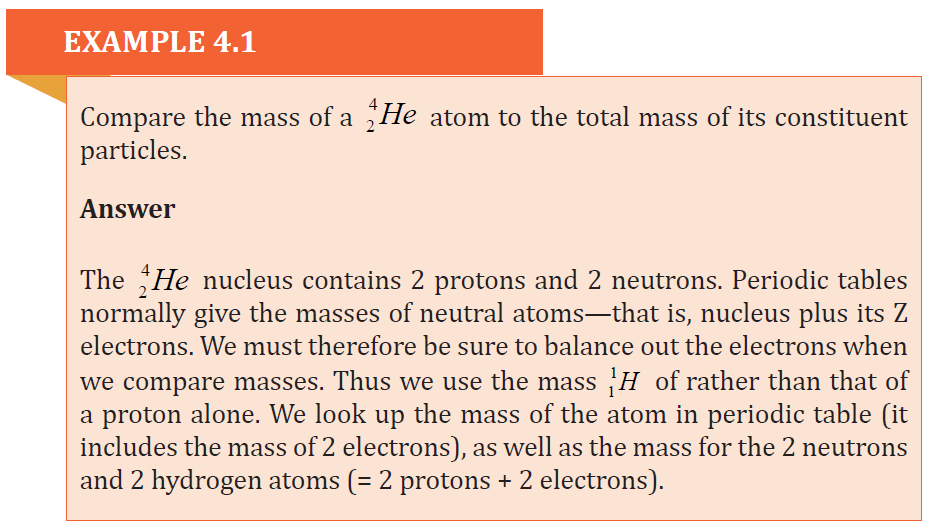
4.2.2 Einstein mass-energy relation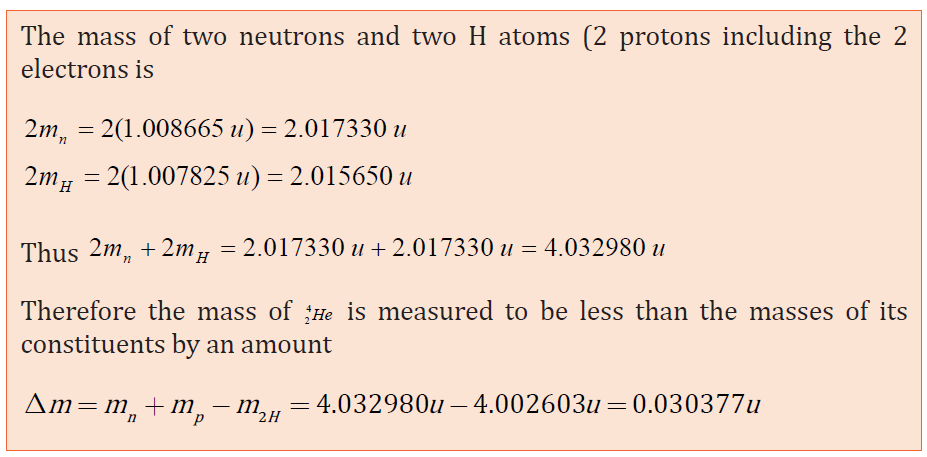
In 1905, while developing his special theory of relativity, Einstein made the
surprising suggestion that energy and mass are equivalent. He predicted that if
the energy of a body changes by an amount of energy E, its mass changes by an
amount m given by the equation
E = mc2 (4.09)
Where c is the speed of light and m mass of a body
Everyday examples of energy gain are much too small to produce detectable
changes of mass.
4.2.3 Binding energy
The mass of a nucleus is less than the combined mass of its protons and
neutrons (nucleons). The missing mass is called the mass defect. This observed
mass defect represent a certain amount of energy in the nucleus known as the
binding energy b E and calculated using the Einstein formula as:
ΔE = Δmc2 (4.10)
where c is the speed of light and Δm the mass defect.The binding energy for a nucleus containing Z protons and N neutrons is defined as
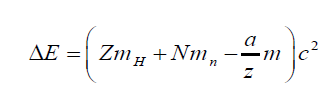
The binding energy is the energy released when a nucleus is formed from its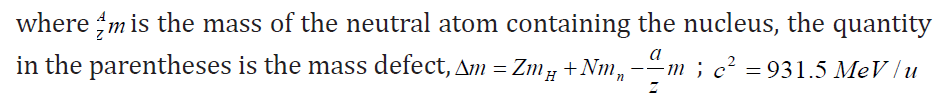
constituent particles or the energy required to break up (to split) the nucleus
into protons and neutrons. Protons and electrons are held together in the
nucleus of an atom by the strong nuclear force. So if we imagine splitting a
nucleus up into its separate protons and neutrons, it would require energy,
because we would need to overcome the strong nuclear force.
4.2.4 Binding energy per nucleon and stability
Instead of looking at the total binding energy of a nucleus, it is often more useful
to consider the binding energy per nucleon. This is the total biding energydivided by the total number of nucleons.
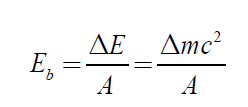
A plot of binding energy per nucleon Eb/A as a function of mass number A for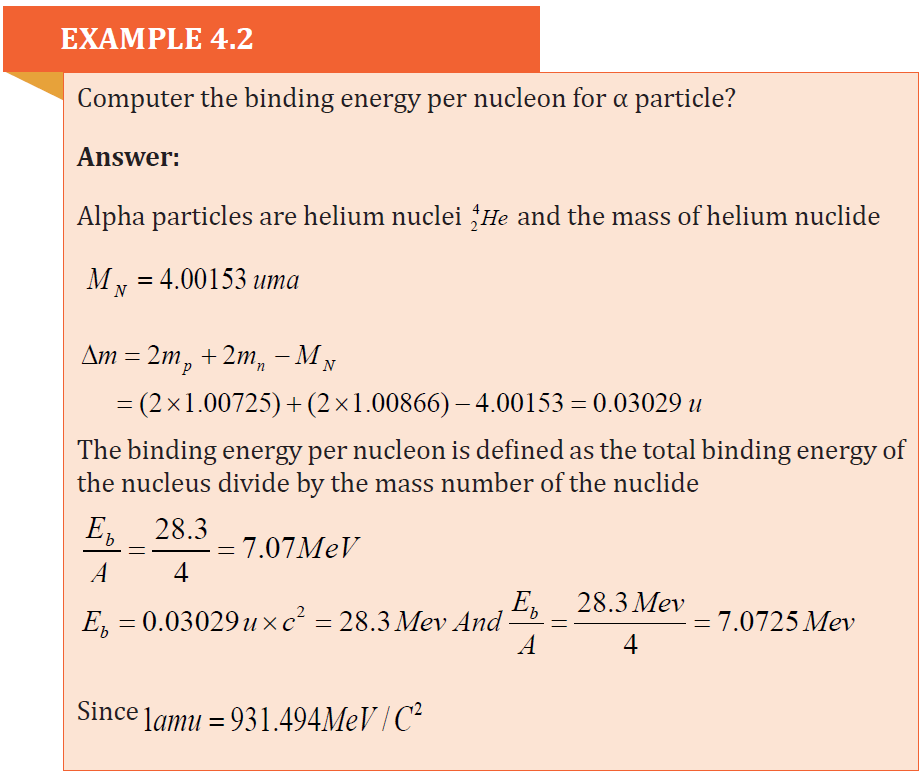
various stable nuclei is shown on Fig. 5.6.

Fig.4. 5: The graph of binding energy per nucleon of the known elements (Giancoli D. C., 2005)
The nuclear binding energy per nucleon for light element increases with the
mass number until a certain maximum is reached at around A = 56 and then
after it almost saturate. The fact that there is a peak in the binding energy per
nucleon curve means that either the breaking of heavier nucleus (fission) or
the combination of lighter nuclei (fusion) will yield the product nuclei with
greater binding energy per nucleon and therefore more stable.
As an example if a nucleus like is 23892U split into two fragments of nearly equal
masses, the two fragments will have higher binding energy per nucleon than
the original. The excess energy is released as useful energy and this process
called fission is the basis of electricity production in a nucleus plant.
If two light elements combine their nuclei in one nucleus, the formed nucleuswill have a greater binding energy per nucleon than the originals.
This process is called nuclear fusion and can only take place at a very high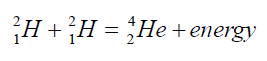
temperature. It is the source of energy in the sun and other stars. The fusion is
more energetic than the fission.
The binding energy per nucleon therefore gives an indication of the stability
of the nucleus. A high binding energy per nucleon indicates a high degree ofstability – it would require a lot of energy to take these nucleons apart.
4.2.5 Checking my Progress
4.3 RADIOACTIVITY AND NUCLEAR STABILITY
ACTIVITY 4.3: Investigating radioactivity
During the World War II, its final stage was marked by the atomic bombing
on Nagasaki and Hiroshima towns in Japan (Fig.5.6). Observe the image andread the text provided below before answering the following questions.

Fig.4. 6: The atomic bomb in Nagasaki (Japan in 1945)
In August 1945, after four years of world war, united States B-29 bomber,
dropped the atomic bomb over the cities of Hiroshima on August 6th 1945.
70.000 people died in 9 seconds, and the city of Hiroshima was leveled. 3 days
after as second bomb was dropped in Nagasaki, Japan with the same devastating
results. The bombing killed over 129.000 people.
The bomb released cataclysmic load of energy. The ones who were close enough
to see the blast lost their eyes. It was the last thing they ever saw. The bright
light of what the blinded them. The black of their eyes, the retina, melted away.
The radiation received by the body is equivalent today’s thousands of x-rays.
The human body can’t absorb unlimited radiation. It falls apart because the
cells are dying of radiation poisoning, if the radiation is intense enough, it looks
like a urn. Layers of the skin begin to fall off. The body vital functioning began
to slow down until it stops.
1. Describe and discuss the phenomena happening on two images.
2. From the text, show that the atomic bomb of Hiroshima was very harmful
to human body.
3. What are the types of radiations should be there?
4. Stable isotopes do not emit radiations. What is the name of materials which
emit radiations? Describe them.
5. What are the possible main radioisotopes used to produce energy in figure
above?
6. Which processes are used to generate such heavy energy? Describe any one
of your choice
Radioactivity is one of the dynamic properties of nuclei, in this process the
system makes a transition from a high energy state to a low energy by emitting
α and β-particles or γ-rays. This process happens naturally and is not affected by
any external agent such as pressure, temperature or electric and magnetic fields.
The α-particles are Helium nuclei and can be stopped by a piece of paper while
β-particles are either electron or positron. There are high energetic particles
and can pass through one cm thick aluminum sheet. γ-rays are electromagnetic
radiations and can be stopped by several inches of lead.
4.3.1 Radioactive decay of a single parent
Nucleus decay is a random process and the rate of disintegration is proportional
to the number of available radioactive nuclides. Let us analyses the simple case
where the first daughter nuclide is stable. Suppose that at time t, there are N
radioactive nuclide and dN is the number of nuclide disintegrating within a
time dt. As the rate of disintegration is proportional to the number of nuclides
present in the radioactive substance, we get
where λ, the proportionality constant, is called the radioactive constant.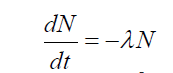
This constant depends on the nature of the radioactive substance. The negative
sign shows that an increase in disintegration rate will decrease the number of
radioactive nuclides which are present. From this we can establish the formulaof radioactive decay:
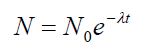
where it assumed that the initial number of radioactive nuclide is equal to N0.
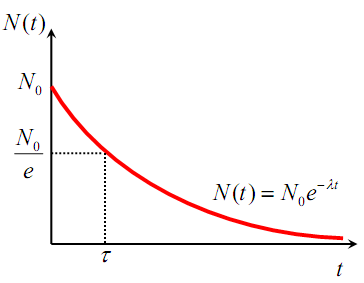
Fig.4. 7: Illustration of the radioactive decay law
If we consider the activity A of a radioactive sample which is the number of
decay events in a unit time we obtain a similar expression for the radioactivedecay law but expressed in terms of activity of the radioactive substance:
where A0 is the initial activity of the radioactive source. Another parameter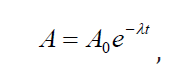
useful in characterizing nuclear decay is the half-life T1⁄2 .
The half-life of a radioactive substance is the time interval during which halfof a given number of radioactive nuclei decay. Therefore the half time period is
Finally,one shows that the mean-life of a nuclide or the average life period of a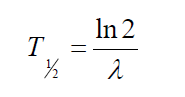
nuclide is related to the radioactive constant by
In general, after n half-lives, the number of un-decayed radioactive nuclei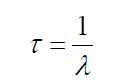
remaining is
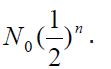
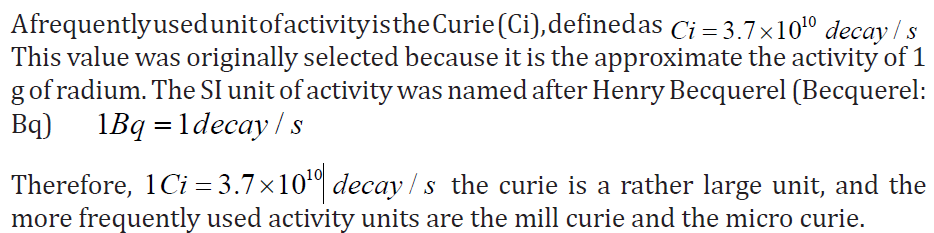
4.3.2 Characteristics of radioactive substances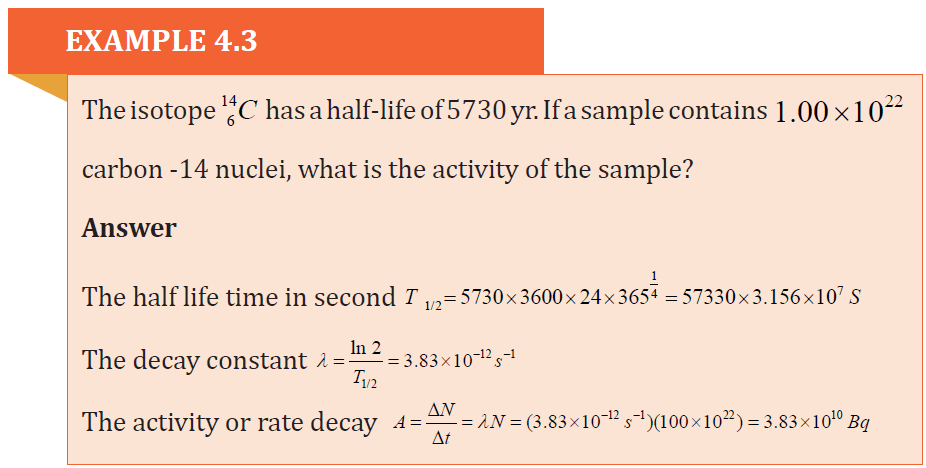
Radioactive substances (nuclides) present one or more of the following features
• The atom of radioactive elements are continually decaying into simpler
atoms as a result of emitting radiation
• The radiations from radioactive elements produce bright flashes of
light when they strike certain compounds. The compound fluoresce.
For example, rays from radium cause zinc sulphide to give off light in
the dark. For this reason, a mixture of radium and zinc sulphide is used
to make luminous paints.
• They cause ionization of air molecules. The radiations from radioactive
substances knock out electrons from molecules of air. This leaves the
gas molecules with a positive charge.
• Radiations from radioactive substances can penetrate the heavy black
wrapping around a photographic film. When the film is developed, it
appears black where the radiations struck the film.
• Radiations from radioactive substances can destroy the germinating
power of plants seeds, kill bacteria or burn or kill animals and plants.
Radiations can also kill cancers.
A. Properties of emitted radiationsSome of their properties are summarized and shown in the table below:
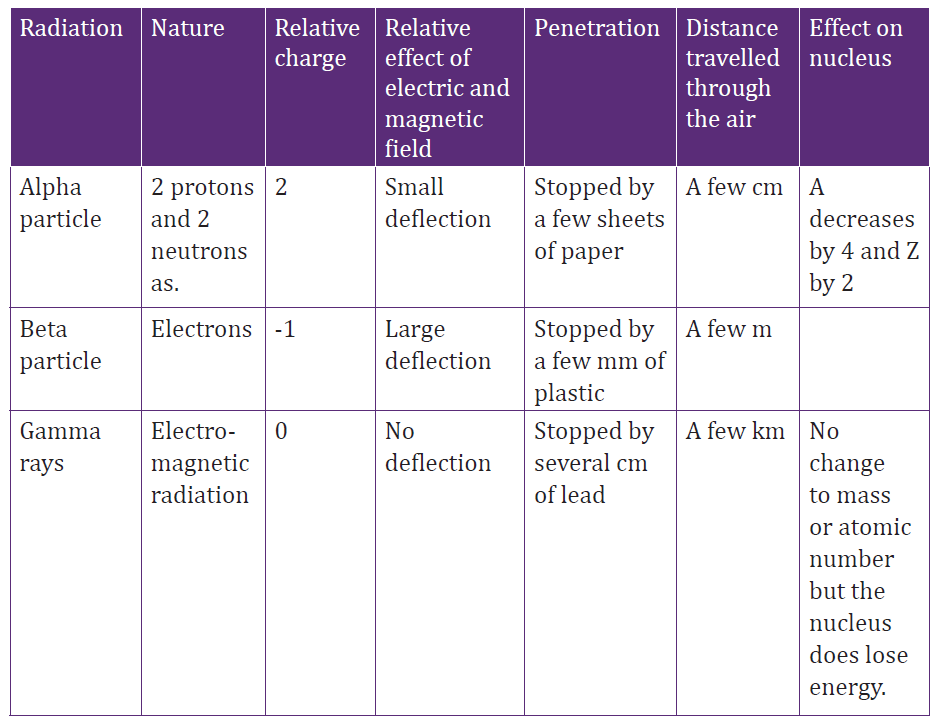
Table 4. 3 Properties of different types of radiations
a. Alpha decay ( 42He )
If one element changes into another by alpha decay, the process is called
transmutation. For alpha emission to occur, the mass of the parent must be
greater than the combined mass of the daughter and the alpha particle.
In the decay process, this excess of mass is converted into energy of other forms
and appears in the form of kinetic energy of both the daughter nucleus and
the alpha particle. Most of kinetic energy is carried away by the alpha particle
because it is much less massive than the daughter nucleus. The momentum is
conserved in this process.
The isotope whose natural radioactive decay involves the emission of alpha
particles usually have a relative atomic mass greater than 210 (Ar>210). They
have too much mass to be stable and give out alpha particles to form smallerand more stable atoms.
b. Beta decay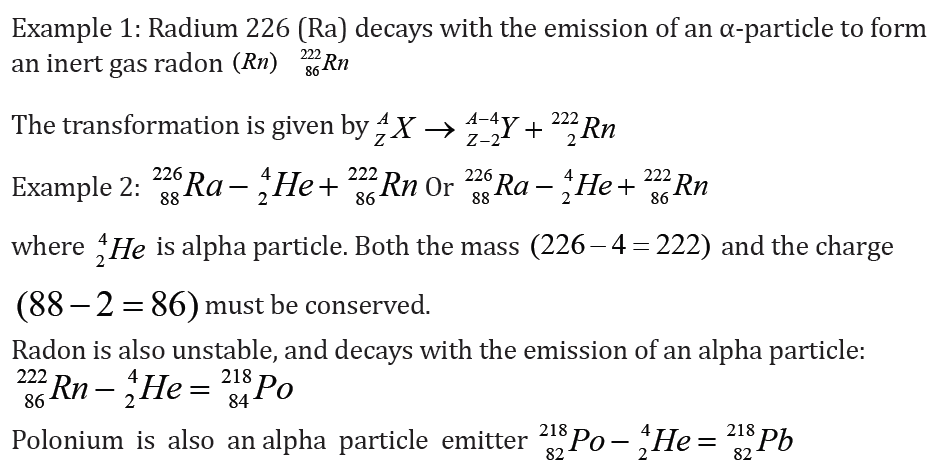
The isotopes whose radioactive decay involves the emission of beta particles
often have a relative atomic mass less than 210 (Ar < 210). Beta particles are
usually emitted from heavier nuclei that have too many neutrons compared
with the number of protons.
I. Negative β-decayIn this process of negative β-decay an electron and an antineutrino are emitted.
The emitted electron results from the following reaction where a neutron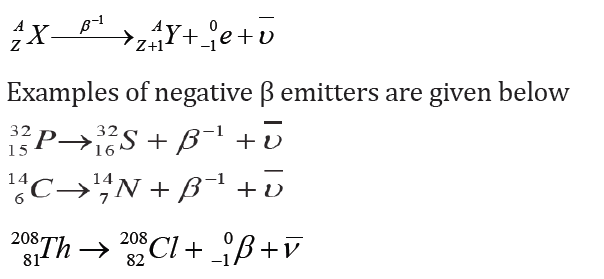
changes into a proton and an electron is emitted from the nucleus as a betaparticle:
The conservation of charges and mass number is maintained. The daughter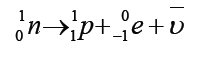
nuclide may be in an excited state and will become stable after emitting a γ-ray.
II. Positive β-decayIn this process the positron and the neutrino are emitted.
This positive decay is different from an electron capture which takes place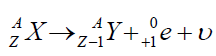
when an electron that is close to the nucleus recombines with a proton in thenucleus producing a neutron and a neutrino:
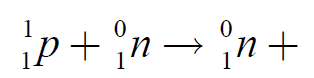
The equation of decay of the electron capture is:
The daughter nuclide is seem to be the same as that we could have been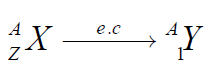
produced if a positron has been emitted. The rearrangement of the remaining
Z-1electrons will lead to emission of characteristic x-ray of the daughter
nucleus. In few case both positron and electron capture may happen.
C. Gamma decay (Y)
Very often a nucleus that undergoes radioactive decay is left in an excited
energy state. The nucleus can then undergo a second decay to a lower energy
state by emitting one or more photons. Unlike α and β decay, γ decay results
in the production of photons that have zero mass and no electric charge. The
photons, emitted in such process, are called gamma rays and they have very
high energy.
If an atom of a material Y emits a γ ray (γ photon), then the nuclear reaction canbe represented symbolically as
Gamma emission does not result in any change in either Z or A.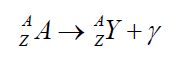
Notes:
• A transmutation does not occur in gamma decay. When an alpha
particles and beta particles are emitted, gamma rays are often emitted
at the same time. When a radioisotope emits gamma rays, it become
more stable because it loses energy.
• In both alpha and beta decay, the new element formed is called thedaughter isotope.
• Gamma rays are like X-rays. Typical gamma rays are of a higher
frequency and thus higher energy than X-rays.
• Deviations of alpha, beta and gamma radiations due to electric field and
magnetic field ( See Fig.4.9). It can be seen unlike gamma-rays, alpha
and -particles are affected by the presence of electric and magnetic
fields since these particles are charged. Gamma-rays are not affectedby these fields.
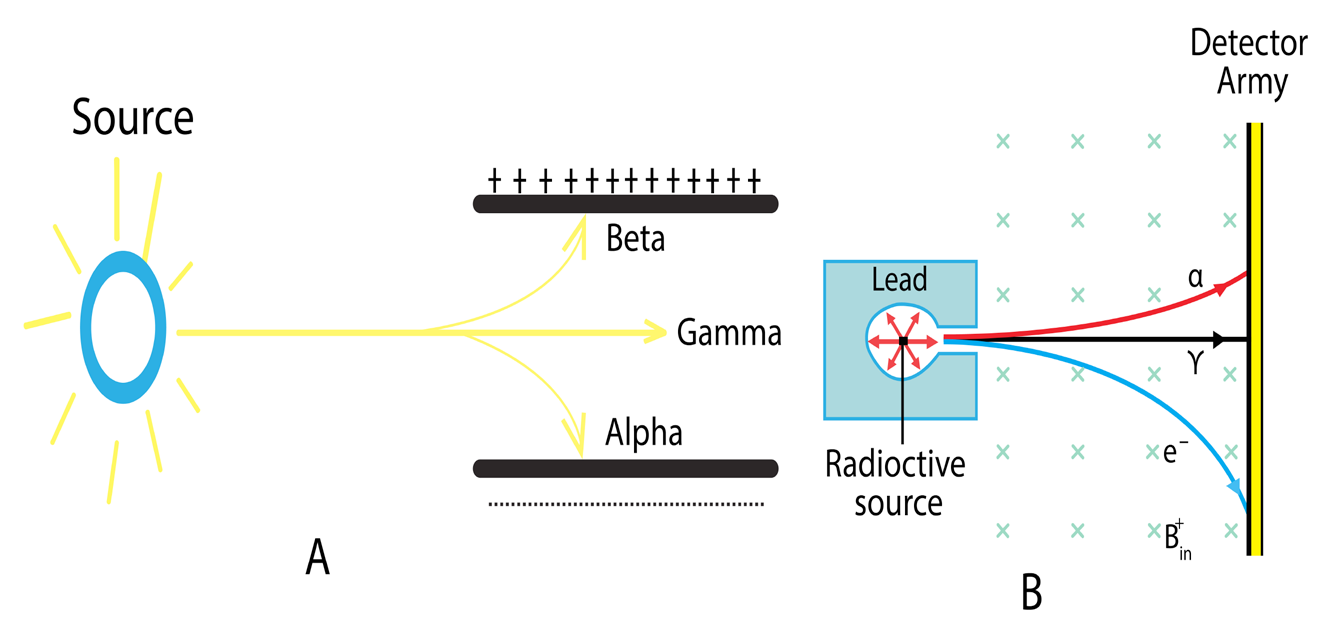
Fig.4. 8: Deviation of radiation particles in an electric field
4.3.3 Nuclear fission and Fusion
a. Nuclear fission
Heavy unstable nuclides can be broken up to produce energy in a process
called nuclear fission. When uranium decays naturally, alpha and beta particles
are emitted. However, when uranium-235 is bombarded by neutrons it forms
uranium-236. Uranium-236 is unstable and breaks down, splitting into two
large particles and emitting three neutrons. The fission of 235U by thermalneutrons can be represented by the reaction
where 236U∗ is an intermediate excited state that lasts for approximately10-12s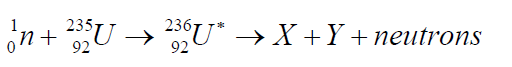
before splitting into medium-mass nuclei X and Y, and these are called fissionfragments.
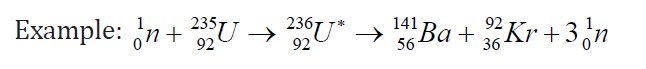
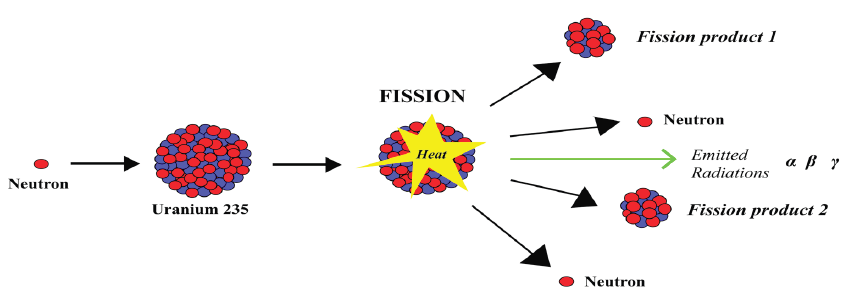
Fig.4. 9: Fission diagram illustration
When the exact masses of the final products are added together, the sum is
found to be appreciably less than the exact masses of the uranium-235 and the
original neutron. This difference in mass Δm appears as energy given by
Δm = Δmc2
Another important point arises! The three neutrons released may collide with
other nuclides and split them also resulting in cascade reactions. In this way,
chain reactions occur and as a result, the quantity of energy released can be
very large. A few kilogram of uranium can produce as much heat energy as
thousands of tons of coal.
Advantages and disadvantages of nuclear fission
The nuclear fission produces a huge amount of energy. This energy can
be released in a controlled manner in nuclear power station and be used in
driving steam turbines that produce electric power. However, when produced
in uncontrolled manner it will result in the fabrication of atomic bomb that may
release a large amount of heat energy and damaging radiations. The emitted
radiations have both short term and long term effect on the living things.
b. Nuclear fusion
When lighter nuclides merge together in a process called fusion, energy isproduced and mass is lost. For example:
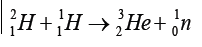
These reactions occur in the core of a star and are responsible for the outpouring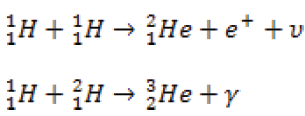
of energy from the star.
The sum of the exact masses of the helium atom is less than the sum of exact
masses of the four hydrogen atoms. This lost mass is released as energy. It is
thought that the sun’s energy is produced by nuclear fusion.
4.3.4 Radiation detectors
ACTIVITY 4.4: Smoke detector bellow
Observe the diagram of a smoke detector bellow then answer to thequestions that follow:
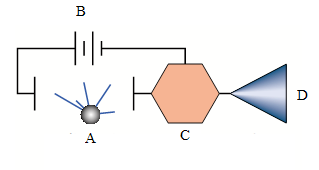
Fig.4. 10: illustration diagram of a smoke detector
1. Name the components labeled A, B, C and D on the smoke detector
above?
2. What is meant by smoke detector?
3. Describe a functioning of a smoke detector.
4. Design an inventory of other radiation detectors you know
Experiments in Nuclear and Particle Physics depend upon the detection of
primary radiation/particle and that of the product particles if any. The detection
is made possible by the interaction of nuclear radiation with atomic electrons
directly or indirectly.
a. Classification of radiation detectors
There are a variety of other radioactive detectors that we may convenientlyclassify into two classes: Electrical and Optical detectors.
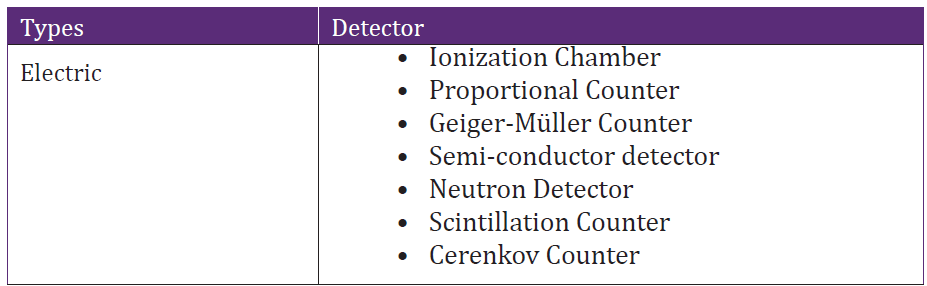

Table4. 1 Classification of radiation detectors
b. Working principle of an ionization chamber
Conventionally, the term “ionization chamber” is used exclusively to describe
those detectors which collect all the charges created by direct ionization within
the gas through the application of an electric field. Ionization chamber is filled
with inert gases at low pressure. In the chamber there are two electrodes,
namely, the cathode and the anode which are maintained at a high potentialdifference as shown on the figure below
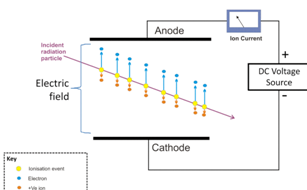
Fig,4.11: visualization of ion chamber operation
When radiation enters the chamber, it ionizes the gas atoms creating negative
and positive charges. The negative charges or electrons are attracted by the
anode while positive ions are attracted by the cathode; this produces the current
in the outside circuit depending on the strength and the type of radiation. The
current produced is quite small and dc amplified electrometers are used tomeasure such small currents.
4.3.5 Checking my progress
In the following exercises (1 to 4), choose the best answer and explain your
choice
1. Which of the following is an electron?
a. Neutrino c. Photon
b. Gamma particle d. Beta particle
2..Which of the following most accurately describe radioactive decay?
a. Molecules spontaneously break apart to produce energy
b. Atoms spontaneously break apart to produce energy beta decay, alpha
decay and positron emission are all forms of radioactive decay. Energy is
released because the atoms are converted to a more stable energy
c. Protons and neutrons spontaneously break apart to produce energy
d. Electrons spontaneously break apart to produce energy
3. Which of the following is true concerning the ratio neutrons to protons in
stable atoms?
a. The ratio for all stable atoms is 1:1.
b. The ratio for small stable atoms is 1:1, and the ratio for large stable atom is
greater than 1:1. As atomic weight goes up, the ratio of neutrons to protons
for stable atoms increases up to as much as 1.8:1 ratio.
c. The ratio for large stable atom is 1:1, and the ratio for small stable atoms
is greater than 1:1.
d. There is no correlation between the stability of the atom and its neutron
to proton ratio.
4. Polonium-218 undergoes one alpha decay and two beta decays to make
a. Polonium-214 c. Bismuth-214
b. Plomb-214 d. Plomb-210
5. a) Compare (i) the charge possessed by alpha, beta and gamma radiations
(ii) The penetrating power of these radiations
6. a.What is meant by the term (i) radioactive decay? (ii) Half-life of a radioactive
substance?
b. A 32 g sample of radioactive material was reduced to 2 g in 96 days. What is its
half-life? How much of it will remain after another 96 days?
7. 212Be Decays to 208Th by α-emission in 34% of the disintegration and to212Ra
by β-emission in 66% of the disintegration. If the total half value period is 60.5
minutes, find the decay constants for alpha and beta and the total emission.
8. If a radioactive material initially contains 3.0milligrams of Uranium234U , how
much it will contain after 150,000 years? What will be its activity at the end ofthis time?
4.4 APPLICATION OF RADIOACTIVITY
ACTIVITY 5.5: Use of nuclear energy to generate electricity
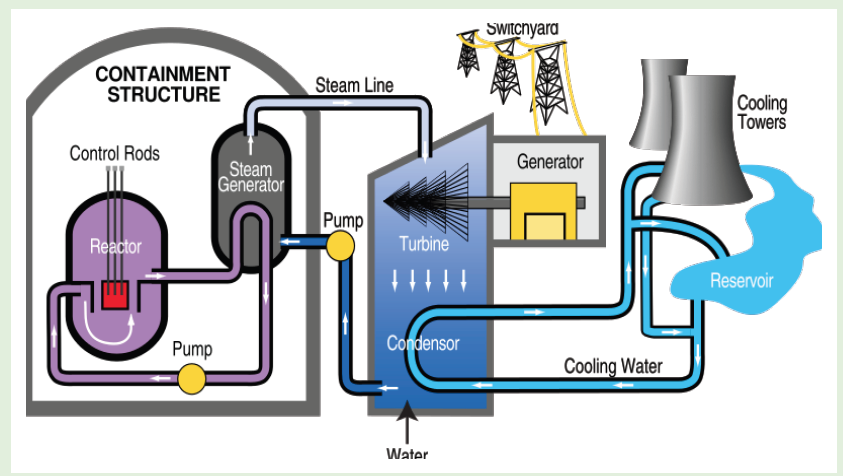
Fig.4. 12: Nuclear power plant functioning mechanism diagram.
Many people disagree to the use of nuclear power to generate our
electricity, even though the safety record of nuclear industry is extremely
good. Observe clearly the image diagram of the nuclear power plant
(Fig.4.12) and answer to the questions that follow
1. Why do you think people disagree to the use of nuclear power
station?
2. What are the main parts of the power plant station observed in
Fig.5.12?
3. Analyze and explain the steps of energy transformation from reactor
to generator
4. Write a brief explanation on the advantages and disadvantages of
using nuclear energy as a source of electricity if any.
5. Use internet and your library or any other resources to find out
about the other application of radionuclides in our daily life
People would not have fear of radiations when controlled in certain manner.
Radioisotopes and nuclear power process have been used and produced
improvement in various sectors. These includes: consumer products, food and
agriculture, industry, medicine and scientific research, transport, water
resources and the environment. The following are some descriptive examples
among others.
4.4.1 Industry
Different materials we use at home are manufactured in industry and made of
different radioactive materials. The dosage of use of radioactive substance is
thus controlled so that they are not harmful to human body.
Gamma radiation and beta radiation from radio-isotopes can be used to
monitor the level of the material inside the container. The penetrating power
of gamma rays is used to detect hidden flow in metal castings. Beta rays are
used to measure the thickness of various flat objects (the mass absorbed by the
object is proportional to its thickness).
In the textile industries, irradiation with beta radiations fixes various chemicals
onto cotton fibers. This produces for instance permanent press clothing. Again,
radioactive materials can be used as tracers to investigate the flow of liquids inchemical factories.
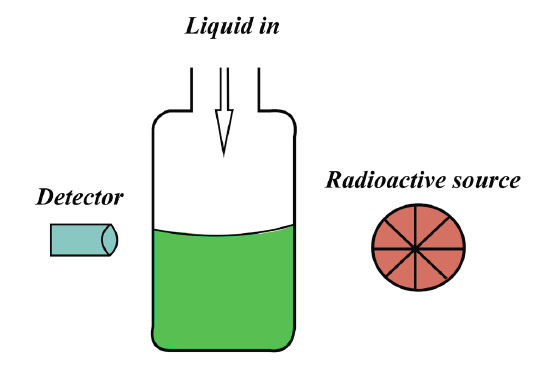
Fig.4. 13 Testing the level of a liquid in a container using radiations
If there is a sudden decrease in the amount of radiation reaching the detector,
which will happen when the container is full, then this can be used as a signal
to switch off the flow of substance into the container. A similar method is used
to monitor the thickness of sheets of plastic, metal and paper in production.
4.4.2 Tracer studies
Tracer techniques can be used to track where substances go to and where leaks
may have occurred. Leaks in gas pipes or oil pipes can be detected by using this
technique. Tracer techniques are also used in medicine to treat thyroid glands
which can be underactive or over active. The activity of the thyroid gland can
be monitored by the patient being injected with or asked to drink radioactive
iodine. The radioactivity in the vicinity of the thyroid gland is then checked to
see how much of the radioactive iodine has settled in the area around the gland.
4.4.3 Nuclear power stations
Nuclear power stations control a large amounts of energy released when
Uranium-235undergoes nuclear fission. The energy released by this controlled
chain reaction, is then used to produce electricity.
4.4.4 Nuclear fusion
In nuclear fusion, the nuclei of elements with a very low atomic number are fused
together to make heavier elements. When this takes place, it is accompanied by
a very large release of energy. In the sun, hydrogen nuclei are fusing together
all the time to make helium nuclei. This is also the process by which a hydrogen
bomb works.
4.4.5 Medicine
ACTIVITY 4.6: Radionuclides in MedicineObserve the figure below and suggest answers to the question below
Fig.4. 14: A gamma camera assembly. The photons emitted in the patients are detected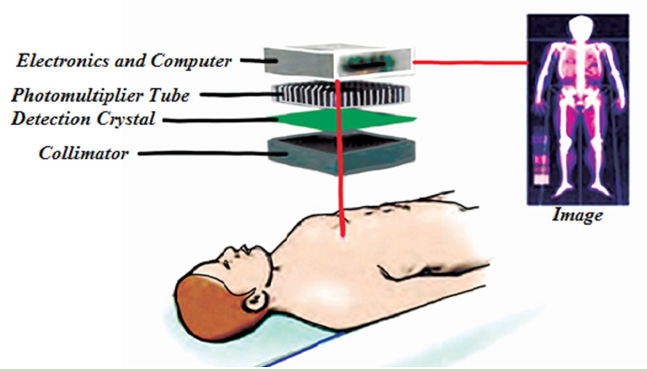
by the photomultiplier tubes. A computer monitor displays the image computed fromthe photomultiplier signals.
1. Who is this person (a man or a woman)?
2. Where is he?
3. What does the image on the right represent?
4. What do you think the patient is suffering from?
5. Using the knowledge acquired in optics, what kind of light
propagation observed there?
6. Does the imaging use reflection or refraction? Why?7. What should be the name of radiation being used in this imaging?
Nuclear medicine has revolved treatment of different disease of the century. It
consists on the use of nuclear properties of radioactive substances in diagnosis,
therapy and research to evaluate metabolic, physiologic and pathologic
conditions of human body. Today, nuclear medicine is currently used in the
diagnosis, treatment and prevention of many serious sicknesses.
Cancer cells are more easily killed by radiation than healthy cells. In medicine,
penetrating gamma rays of cobalt-60 sources are used for this purpose. Other
cancers such as skin cancers are treated by less penetrating beta radiation from
strontium-90 source. Surgical instruments can also be sterilized using gamma
radiation.
4.4.6 Food preservation
The preservation of food uses gamma rays is spreading worldwide. Treating
food with gamma rays can:
• Slow down the ripening of some fruits and the sprouting of potatoes.
In both cases, this helps storage and increases the half-life of the food.
• Kill highly dangerous micro-organisms such as salmonella
• Kill micro-organisms that spoil food
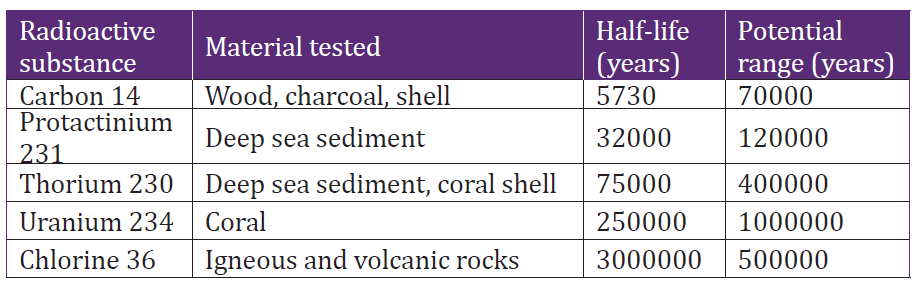
4.4.7 Radiocarbon dating
There are three isotopes of carbon: carbon-12, carbon-13 and carbon-14. The
isotope carbon-14 is radioactive and has half-life of 5760 years. To estimate
the age of a biological sample, radiocarbon146C is used as a radioactive
nuclide. Nucleus decay is independent of the physical or chemical condition
imposed on the elements. This can be used to measure the ages of biological
samples by considering the ratio of 146Cwhich is radioactive and 126C in dead
species. Radioactive carbon is produced when cosmic rays interact withair atoms to produce neutrons and these neutrons interact with nitrogen
The half-life period of 146Cis equal to 5760 years. The carbon reacts with oxygen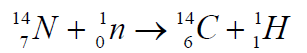
to produce CO2 and plants combine this CO2 with water in the process of
photosynthesis to manufacture their food. Therefore plants and animals are
radioactive. When plants or animals die, the 146Cin them keeps on decaying
without any new intake. The ratio of 146C and 126C will therefore be different in
dead and leaving plants and animals. The age of the dead plant or animal can beestimated by measuring this ratio.
4.4.8 Agricultural uses
In agriculture, radionuclides are used as tracers for studying plants, insect
and animals. For example, phosphorus-32 can be added to plant fertilizer.
Phosphorus is absorbed by plants and its distribution can be measured.
Radiation has been used in South American to detect and control the screw
worm fly pest. A large number of the male of the species were exposed to
gamma radiation. When the males were released back into the wild and mated
with wild females, sterile eggs resulted and no new flies were born.
The points of photosynthesis in a leaf are revealed by growing it in air containing
carbon-14. The presence of this radioactive nuclide in the leaf is the revealed
by putting the leaf onto a photographic plate and letting it take its own picture.4.4.9 Checking my progress
1. Suggest different uses of radionuclides in (i) Medicine (ii) food and
agriculture
2. In our daily life, we are exposed to radiations of different types mainly
in materials we use.
a. Make an inventory of all of the devices in your home that may have
(contain) a radioactive substance.
b. What is the origin of these radiations in the materials highlighted
above?
c. Explain the purpose of radioactive material in the device.
d. Then make research to find out how the objects shown in Fig.5.15 useradiation in their manufacture.

Fig.4. 15
4.5 HAZARDS AND SAFETY PRECAUTIONS OF WHEN
HANDLING RADIATIONSACTIVITY 4.7: Investigating the safety in a place with radiations
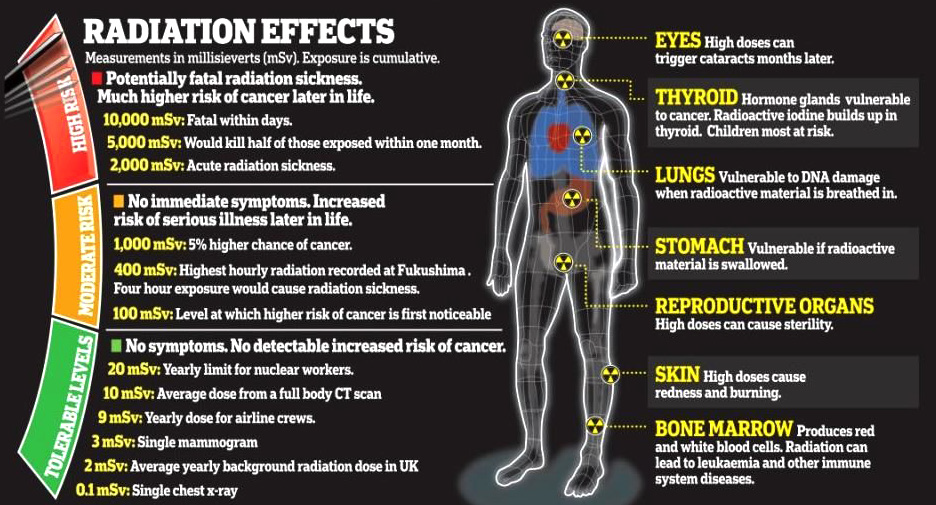
Fig.4. 16: Radiation effects on human body according to the exposure.
The image above (Fig.4.16) shows different side effects of radiation on
human body according to the exposure time taken. With reference to
section 4.3 and activity 4.3, answer to the following questions:
1. What are the dangers of radiations you may observe?
2. Analyze measures should be taken for radiation users?
4.5.1 Dangers of radioactivity
• Both beta particles and gamma rays can pass easily in the skin and can
easily destroy or even kill cells, causing illness.
• They can cause mutations in a cell’s DNA, which means that it cannot
reproduce properly, which may lead to diseases such as cancer.
• Alpha particles cannot pass through the skin. However, they are
extremely dangerous when they get inside your body. This can happen
if you inhale radioactive material.
4.5.2 Safety precautions when Handling Radiations
The precautions taken by workers who deal with radioactive materials are:
• Wearing protective suits
• Wearing radiation level badges
• Checking the radiation level regularly
• Using thick lead-walled containers for transporting radioactive
materials
• Using remote control equipment from behind thick glass or lead walls
to handle radioactive material
• They should be held with forceps and never touched with hands.
• No eating, drinking or smoking where radioactive materials are in use
• Wash your hands thoroughly after exposure of to any radioactive
materials
• Any cuts in the body should be covered before using radioactive sources
• Arrange the source during experiments such that the radiation window
points away from your body• There are ten golden rules for working safely with radioactivity.


END UNIT ASSESSMENT 4
A. Multiple choice questions
Instructions: Write number 1 to 5 in your notebook. Beside each
number, write the letter corresponding to the best choice
1. Radionuclides
a. Are those nuclides having more neutrons than protons
b. May emit X-rays.
c. Decay exponentially
d. May be produced in a cyclotron
2. Concerning Compton Effect:
a. There is interaction between a photon and a free electron.
b. The larger the angle through which the photon is scatted, the
more energy it loses.
c. The wavelength change produced depends upon the
scattering material.
d. High energy radiation is scatted more than lower energy
radiations.
e. The amount of scattering that occurs depends on the electron
density of the scattering material.
3. Classical physics offered a satisfactory explanation for
a. The diffraction of electrons by crystals
b. The deflection of charged particles in an electric field
c. The intensity spectrum of black body radiation
d. The photoelectric effect
e. Matter waves
4. When investigating β decay, the neutrino was postulated to explain
a. Conservation of the number of nucleons
b. Counteracting the ionizing effect of radiation
c. Conservation of energy and momentum
d. The production of antiparticles
e. The energy to carry away the β particles.
5. Gamma radiations differ from α and β emissions in that
a. It consist in photons rather than particles having nonzero
rest mass
b. It has almost no penetrating ability
c. Energy is not conserved in the nuclear decays producing it
d. Momentum is not conserved in the nuclear decays producing it
e. It is not produced in the nucleus
6. The process represented by the nuclear equation is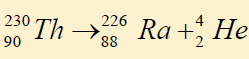
a. Annihilation c. β decay e. γ decay
b. α decay d. pair production
7. Write number (i) to (iii) in your note book. Indicate beside each number
whether the corresponding statement is true (T) or false (F). If it is false,
write a corrected version.
I. An alpha particle is also called a hydrogen nucleus
II. The neutrino was suggested to resolve the problem of conserving
energy and momentum in β decay.
III. The amount of energy released in a particular α or β decay is found
by determining the mass difference between the products and the
parent. A mass-energy equivalence calculation then gives the energy.
IV. The average biding energy per nucleon decreases with the increasing
atomic mass number
8. A radioactive source emits radiations alpha, beta and gamma a shown
below: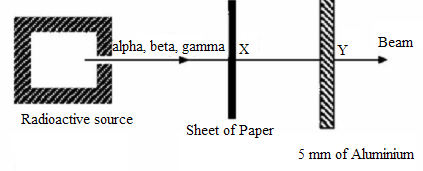
Fig.4. 17 Absorption of radiation
The main radiation(s) in the beam at X and Y are
9. The energy released by the nuclear bomb that destroyed Hiroshima was
equivalent to 12.4 kilotons of TNT. This is equivalent to 9.1 × 1026 MeV.
The mass that was Converted into energy in this explosion was (ConvertMeV in Joules, use E=mc2)
10. In the decay scheme (Conserve charge and electron lepton number) the
blanks should contain
11. Complete the following sentences by using a word, number and an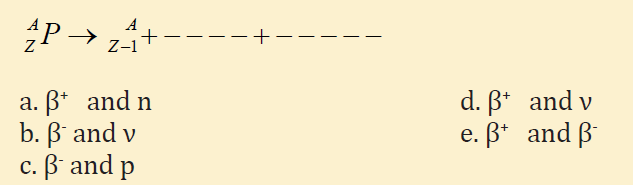
equation where necessarya. The half-life in years of the decay represented by the graph in fig.4.18
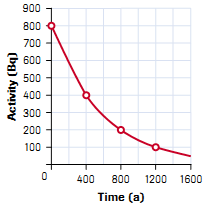
Fig.4. 18 Half life carve
b. When an animal or plant dies, no more _______________is taken in and
that which is present undergoes radioactive decay. If we measure the
amount of carbon-14 left, it is possible to determine the________ of the
sample.
c. If an atom of material Y emits a gamma ray (gamma photon), then thenuclear reaction can be represented symbolically as_________________
B. Structured questions
12. Prepare a table summarizing the three types of radioactive emission.
Classify each type under the following headings: Type of Emission,
Mass, Charge, penetrating Power and Ionization Ability.
13. Copy the following table in your notebook and answer the questionsthat follow
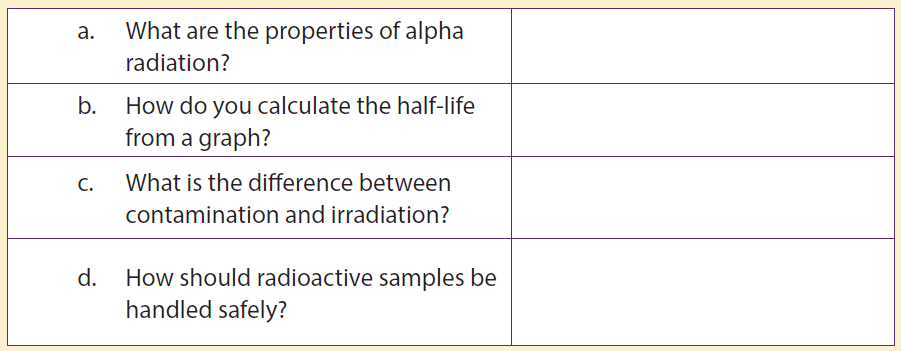
14. Give the value of x and y in each of the following equations
15. Give the value of x and y in each of the reaction classify each as α , β ,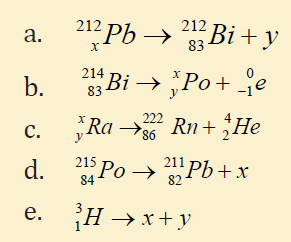
or γ decay
16. The half-life of carbon14 is 5730 years the mass of certain sample of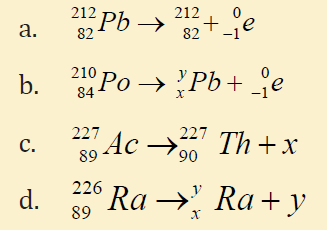
this isotope is800 μg . Graph the activity for the first 5 half-lives.
17. Beams of a, b- and g radiation of approximately the same energy passthrough electric and magnetic fields as shown below.
a. Show the path taken by each particle in the two fields. Why do
they follow these paths?
b. Which particle is the most penetrating? Explain your answer.
c. Which has the highest ionizing power?
d. How are β − ,β + and electrons different?
e. How are x-rays, y rays and photons different?
18. We are exposed to radiation all the time, indoors and outdoors. This is
called background radiation.
a. Give two examples of sources of this background radiation.
b. Which organ generally receives the most background radiation, and
why?
c. There is some concern at the moment that pilots and flight attendants
may have significantly higher exposures to radiation than the normal
exposure rates for the general public.
d. Why do pilots have a higher exposure to radiation than most other
people?
19. Nuclei can decay by emitting particles which can change the energy, mass
and charge of the nucleus.
a. How is α decay possible when the α particle must pass an energy
barrier which is greater than the energy of the particle? Describe the
process involved.
b. If isotope A emits α particles with greater energy than isotope B (of
the same element), which will have the longer half-life?
c. How can a nucleus change its charge without emitting a chargedparticle?
C. Question of research
21. Using the information in radioactivity and making an internet search
or/ and using other sources of information, consolidate your skills in
other hazardous materials you may meet in your area. Then completethe table below (not exhaustive):
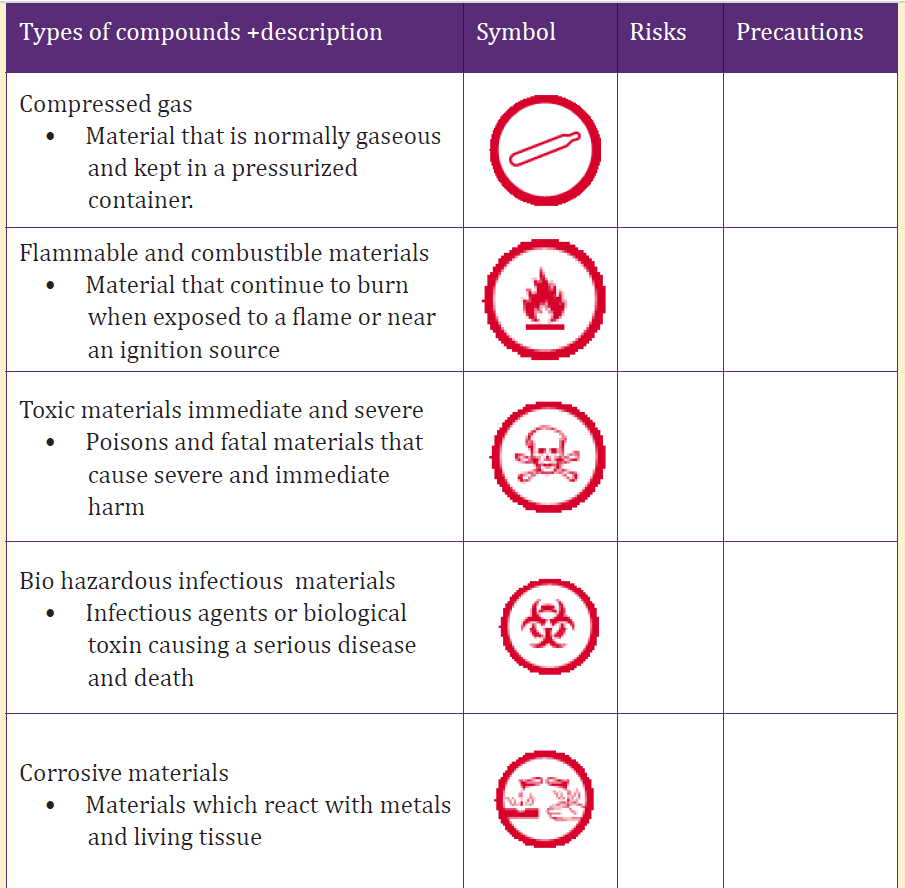
Table 4. 7 Precaution signs
