UNIT 3 FOSSIL AND NON-FOSSIL FUEL AND POWER PRODUCTION
Key unit competence: By the end of this chapter, I should be able to evaluate
fossil and non-fossil fuel for power production.
Unit Objectives:
By the end of this unit I will be able to;
• explain the concept of fossil and no-fossil fuels and their use in power
production properly.
• explain the differences between fossil and no-fossil fuels properly.
• explain Nuclear fuel and nuclear fission and their use in energy
production and associated dangers properly.
• explain the environmental problems of fossil fuels and suggest theirsolution clearly.

3.0 INTRODUCTORY ACTIVITY
Fossil fuel is a source of conventional or non-renewable energy. There are
many examples of fossil fuels which we use in our daily lives. In fact, most
of the energy that we consume comes from fossil fuels. Coal, petroleum
and natural gas are called fossil fuels. Millions of years ago, during the
carboniferous age, due to the change in atmospheric conditions and other
changes, the forests were destroyed and they were fossilized. With the action
of bacteria and other microorganisms on the surface of the earth, these trees
and other vegetations were decayed and disintegrated. Years after these
trees were available in solid, liquid and gaseous state. The solid form is coal.It is the most widely used form of fossil fuel for domestic purposes.
ACTIVITY 3.1: The Atmosphere
Crossword puzzle: Fill the missing words in the crossword puzzle given
below.
Down
1. __________ _________ refers to the rise in the world’s average
temperature due to air pollution.
2. _________ _______ are gases in the atmosphere that absorb and
emit radiation, causing the greenhouse effect.
3. ______________ is a mixture of smoke and fog in the atmosphere.
4. ____________ ________is a non-renewable source of energy
formed from the remains of dead plants and animals.
Across
5. ______ _____ is the reduction of the amount of ozone
6. The water sources and the land are polluted by ______ ________when exhaust gases dissolve in the rain.
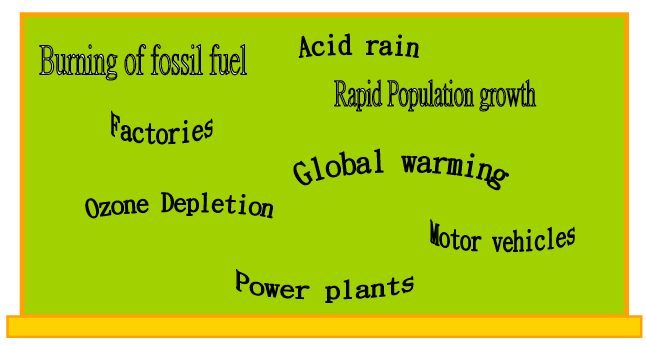
ACTIVITY 3-2: Pollution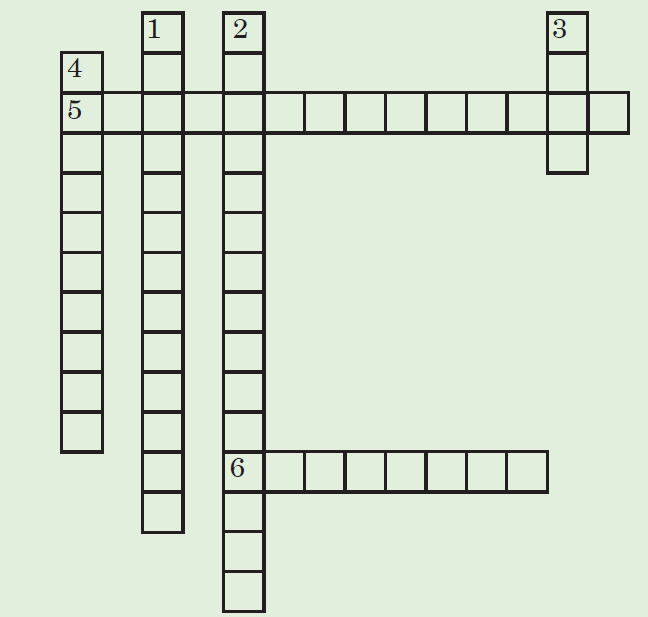
Word splashThe following are the key words we learn about air pollution.
3.1. FOSSIL FUELS AND NON-FOSSIL FUELS
3.1.1 Fossil Fuels
Fossil fuels are hydrocarbons, primarily coal, fuel oil or natural gas, formed
from the remains of dead plants and animals. In common dialogue, the term
‘fossil fuel’ also includes hydrocarbon-containing natural resources that are notderived from animal or plant sources.

Fig. 3.1. Fossil fuels in nature
Coal, oil and natural gas are called ‘fossil fuels’ because they have been formed
from the fossilized remains of prehistoric plants and animals. Fossil fuels are
non-renewable energy source since they take millions of years to form. They
ultimately get their energy from the sun.
Types of Fossil Fuels
Coal
Coal is a hard, black coloured rock-like substance formed when dead plants
were subjected to extreme heat and pressure for millions of years. It is made up
of carbon, hydrogen, oxygen, nitrogen and varying amounts of sulphur. There
are two ways to mine coal: surface mining and underground mining.
Natural Gas
Natural gas is formed from the remains of tiny sea animals and plants that
died millions of years ago. The gas then became trapped in layers of rock-like
water in a wet sponge. Raw natural gas is a mixture of different gases. Its main
ingredient is methane. The strange smell of natural gas (like rotten eggs) comes
from a chemical added by the companies. It is called mercaptan. This is added
to detect the gas leakage.
Oil (Petroleum)
Oil is formed from the remains of animals and plants that died millions of years
ago. The organic material was then broken down into hydrogen and carbon
atoms and a sponge-like rock was formed, full of oil.
Oil cannot be used as it is when it is drawn from the ground. Oil refineries clean
and separate the oil into various fuels and byproducts. The most important of
these is gasoline.
Fossil fuels are used to generate electrical energy in a series of energytransformations as shown in Fig.6.2.
3.1.2 Non-fossil fuels
Non-fossil fuels are alternative sources of energy or renewable sources of
energy that do not rely on burning up limited supplies of coal, oil or natural
gas. Examples of these fuels include: nuclear energy, wind or water generated
energy and solar power. These tend to be renewable energy sources, or means
of generating power that can be utilized indefinitely.
Non-fossil fuels are considered to be extremely important for power creation.
This is because they are usually renewable energy sources that could be tapped
for hundreds of years and not run out. In addition, energy production using
nonfossil-based fuels usually generates much less pollution than fossil-based
energy sources.
3.2 Storage and transportation of different types of fossil
fuels
3.2.1 Coal
Types of coal
• Peat
• Lignite
• Semi bituminous
• Bituminous
• Anthracite
Means of transporting coal
• Transportation by rail
• Transportation by ropeways
• Transportation by sea or river
• Road transport
• Transport by pipeline
Coal storage
Storage of coal is undesirable because it costs more as there is:
• Risk of spontaneous combustion,
• Weathering,
• Possibility of loss and deterioration during storage,
• Interest on capital cost of coal lying dormant,
• Cost of protecting the stored coal from deterioration.
Types of coal storage
1. Dead storage:
This storage supplies the coal to places where there is a shortage of coal in
plant due to failure of normal supply of coal. This is a long-term storage and
comprises 10% of annual consumption, so, it requires protection against
weathering and spontaneous combustion.
2. Living storage:
It supplies coal to plant for day-to-day usage. The capacity of live storage is less
than that of dead storage. It is usually stored in vertical cylindrical bunkers or
coal basins or silos, e.g. coal is transferred to boiler grate. Bunkers are normally
diamond-shaped cross-section storage areas made up of steel or reinforced
concrete.
Purpose of dead coal storage of coal
• To prevent shutdown of power plant in case of failure of normal
supplies of coal due to coal strike, failure of the transport system, etc.
• To permit choice of purchase allowing management to take advantage
of seasonal market conditions.
Means of coal storage
1. Storage in coal heaps
It is required to:
• Keep coal at low temperature (>70oC).
• Prevention of air circulation from bottom of coal piles.
• Proper drainage of rainy water to prevent weathering–drainage should
not be rapid to prevent washing of coal.
Hence, ground used for stocking should be dry and levelled for proper drainage.
It should have concrete floor to prevent flow of air from bottom. Coal is piled up
to a height of about 10 m to 12 m in layers of 15 cm to 30 cm.
In dead storage, coal pile is sealed by asphalt, fine coal dust, bituminous or
other coating materials.
2. Underwater storage
Possibility of slow oxidation and spontaneous combustion can be completelyeliminated by storing coal under water.

Fig. 3.3. Coal dead storage
Site selection for coal dead storage
• The storage should be free from standing water
• If well drainage is not available, artificial drainage should be provided.
• It should be free from all foreign materials like wood, paper rags, waste
oil or materials having low ignition temperature.
• Handling cost should be minimum.
• Pile should build up in successive layers and be compact.
• Pile should be dressed to prevent entry of rainy water.
• Alternative drying and wetting should be avoided.
• Stoker size coal should be oil treated to prevent absorption of water
and oxygen.
• Side of pile should not be steep.
• Air may circulate freely through pile for proper ventilation to keep
temperatures low.
• Hot surfaces or boiler blow down or hot water or steam pipe and tanks
should be kept far from coal storage
• Hot bright days should be avoided.
• There should be provision for temperature measurement at different
points.
• Conical piling should be avoided.
• Fire fighting equipment should be easily available.
Coal Transfer
Equipments used in coal transfer are:
A: Belt conveyor
It can transfer large quantities of coal over large distance economically. It haslow initial cost and ensures low power consumption.
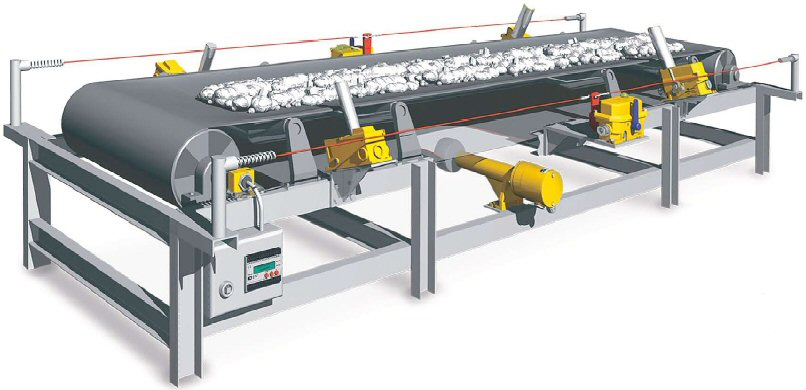
Fig. 3.4. Belt conveyor
Advantages:
• Economical, low power consumption
• Large capacity
• Rate of coal transfer rapidly change
• Low maintenance cost
Disadvantages
• Not suitable for shorter distance and inclination > 200.
• Not suitable for dust particles and slurry.
B: Flight conveyor
It is used when coal is discharged at different points in bins situated below the
conveyor. All parts are made of steel and iron, so it can handle hot materials. It
is totally enclosed, so dust of coal can get transferred. It can transfer coal at high
inclination
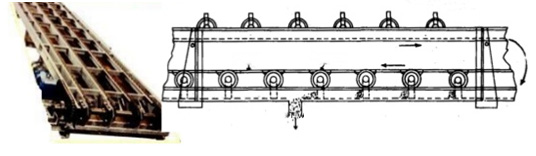
Fig. 3.5. Flight conveyor
Advantages
• It requires small head room.
• Speed and material transfer rate can easily change.
• It can handle hot materials also.
Disadvantages
• High wear and tear, so, it has short life.
• High maintenance required.
• Speed is limited up to 300 m/min due to abrasive action of material.
• High power consumption per unit of material transfer.C: Screw conveyor

Fig. 3.6. Screw conveyor
• It is used for shorter distance.
• It is totally enclosed from atmosphere.
• Coal dust can also be transferred easily.
• It is generally used in metering of coal.
• Driving mechanism is attached at the end of the shaft.
• Diameter: 15 cm to 50 cm.
• Speed: 70 rpm to 120 rpm.
• Capacity: 125 tones/h (max)
Advantage
• Cheap initial cost.
• Simple and compact.
• Dust tight.
• It can transfer coal at high inclination also.
• Most suitable for short distance.
Disadvantages
• High power consumption.
• Length is limited up to 30 m.
• High maintenance due to high wear and tear.
D: Bucket elevator
It is used for vertical lifts. Buckets are fixed on chain which moves on two wheelsor sprockets. Buckets are loaded at bottom and discharged at top.

Fig. 3.7. Bucket elevator
E: Grab bucket elevator
• It is used for lifting as well as transfer material.
• It can be used with crane or tower.
• Initial cost is high but operating cost is less.
• It is used when another arrangement is not possible.
• Bucket capacity: 2 to 3 m3
• Distance: 60 m• Capacity: 100 tonnes/h.

Fig. 3.8. Grab bucket elevator
3.2.2 Transporting Natural Gas and Crude Oil
Transporting natural gas and crude oil thousands of miles through pipelines is
the safest method of transportation. The transportation system for natural gas
consists of a complex network of pipelines, designed to transport natural gas
from its origin to the areas of high natural gas demand quickly and efficiently.
In general, pipelines can be classified in three categories depending on the
purpose:
Gathering pipelines
These are smaller interconnected pipelines forming complex networks with
the purpose of bringing crude oil or natural gas from several nearby wells to
a treatment plant or processing facility. In this group, pipelines are usually
short — a couple of hundred metres — and with small diameters. Also subsea
pipelines for collecting product from deep water production platforms areconsidered gathering systems.

Fig. 3.9. Gathering pipelines
Transportation pipelines
These are long pipes with large diameters, moving products (oil, gas, refined
products) between cities, countries and even continents. These transportation
networks include several compressor stations in gas lines or pump stations forcrude and multi-products pipelines.

Fig. 3.10. Transportation pipelines
Distribution pipelines
These are composed of several interconnected pipelines with small diameters,
used to take the products to the final consumer. Feeder lines to distribute gas
to homes and business downstream, and pipelines at terminals for distributingproducts to tanks and storage facilities, are included in this group.

Fig. 3.11. Distribution pipelines
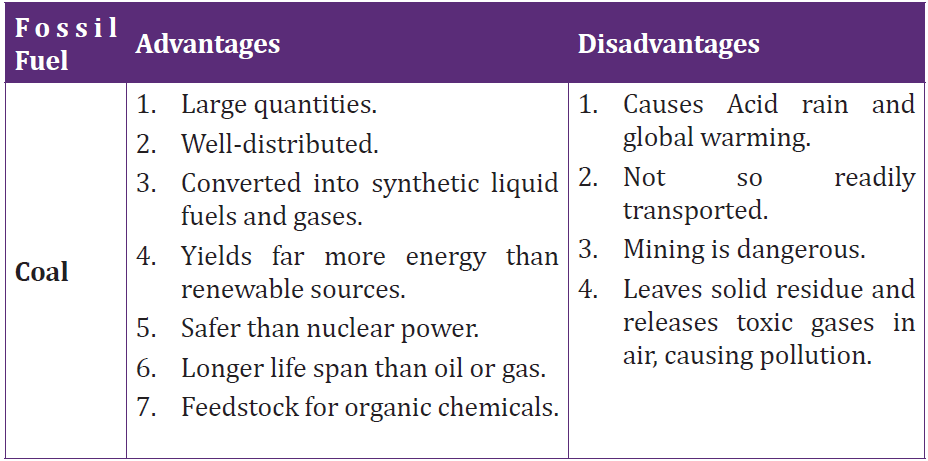
3.3 Advantages and disadvantages of fossil fuels
3.4 Energy production using fossil fuels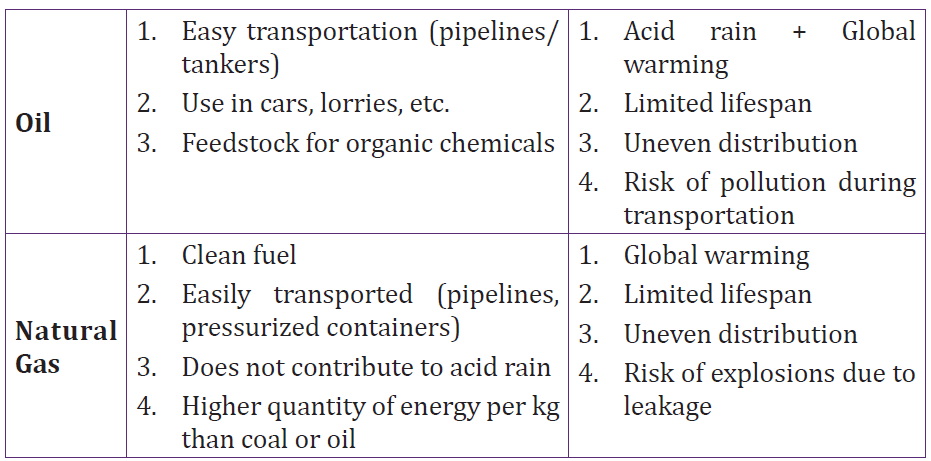
A fossil-fuel power station is a power station which burns fossil fuels, such as
coal, natural gas or petroleum to produce electricity. Central station fossil-fuelpower plants are designed on a large scale for continuous operation.
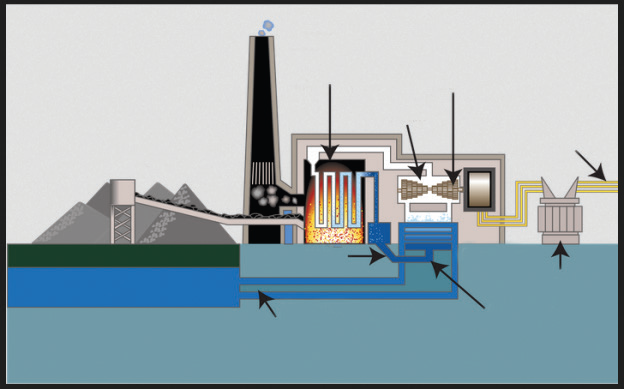
Fig. 3.12. Fossil fuel power plant
There are two main cycles in a power plant; the steam cycle and the gas turbine
cycle. The steam cycle relies on the Rankine cycle in which high pressure and
high temperature steam raised in a boiler is expanded through a steam turbine
that drives an electric generator. The generator then transforms mechanical
energy into electrical energy which is distributed for local use.
The steam gives up its heat of condensation to a heat sink, such as water from
a river or a lake and the condensate can then be pumped back into the boiler
to repeat the cycle. The heat taken up by the cooling water in the condenser is
dissipated mostly through cooling towers into the atmosphere.
3.5 Nuclear fuel and nuclear fission
Nuclear fuel is any material that can be consumed to derive nuclear energy.
The nuclear fuel can be made to undergo nuclear fission chain reactions in a
nuclear reactor. The most common nuclear fuels are 235U (uranium 235) and
239Pu (plutonium 239). Not all nuclear fuels are used in fission chain reactions.
Nuclear fission is a process, by which a heavy nucleus splits into two or
more simpler pieces. This process releases a lot of energy.
When a neutron strikes an atom of uranium, the uranium nucleus splits into
two lighter atoms and releases heat simultaneously. Fission of heavy elements
is an exothermic reaction which can release large amounts of energy both aselectromagnetic radiation and as kinetic energy of the fragments.
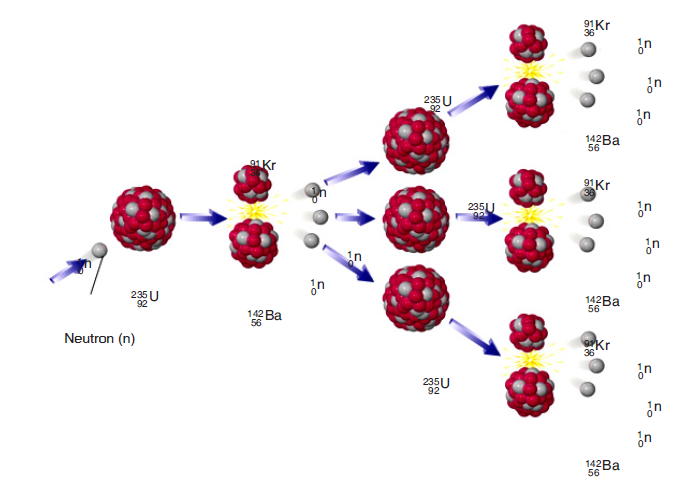
Fig. 3.13. Fission of Uranium 235
A chain reaction refers to a process in which neutrons released in fission
produce an additional fission in at least one further nucleus. This nucleus in
turn produces neutrons, and the process continues. If the process is controlled
it is used for nuclear power or if uncontrolled it is used for nuclear weapons.
Fig.3.13 illustrates a chain reaction of uranium 235.The equation of reaction is:
3.6 Controlled fission (power production)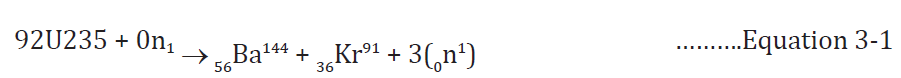
Of the three neutrons, liberated during a fission reaction, only one triggers a
new reaction and the others are simply captured. The system is in equilibrium.
One fission reaction leads to one new fission reaction, which leads to onemore, and so on. This is known as controlled fission.
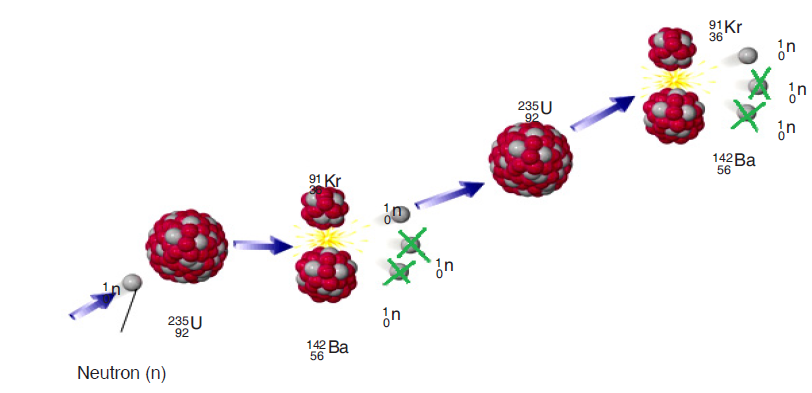
Fig. 3.14. Controlled fission reaction
In a nuclear power station, the uranium is first formed into pellets and then into
long rods. The uranium rods are kept cool by submerging them in water. When
they are removed from the water, a nuclear reaction takes place causing heat
production. The amount of heat required is controlled by raising and lowering
the rods. If more heat is required, the rods are raised further out of the water
and if less heat is needed, they are lowered further into it.
3.7 Uncontrolled fission (nuclear weapons)
A fission reaction which is allowed to proceed without any moderation (by
removal of neutrons) is called an uncontrolled fission reaction. Here more and
more neutrons are given out and cause more fission reactions, thus, releasing
large amounts of energy. An uncontrolled fission reaction is used for nuclearbombs.
Using the energy released from the nuclear fission of uranium-235, an explosive
device can be made by simply positioning two masses of U-235 so that they can
be forced together quickly enough to form a critical mass and result in a rapid,
uncontrolled fission chain reaction.
This is not an easy task to accomplish. First, you must obtain enough uranium
which is highly enriched to over 90% U-235, since natural uranium is only
0.7% U-235. This enrichment is an exceptionally difficult task, a fact that has
helped control the proliferation of nuclear weapons. Once the required mass is
obtained, it must be kept in two or more pieces until the moment of detonation.
Then the pieces must be forced together quickly and in such a geometry that
the generation time for fission is extremely short. This leads to an almost
instantaneous build up of the chain reaction, creating a powerful explosion
before the pieces can fly apart. Two hemispheres which are explosively forced
into contact, can produce a bomb, such as the one detonated at Hiroshima in1945.
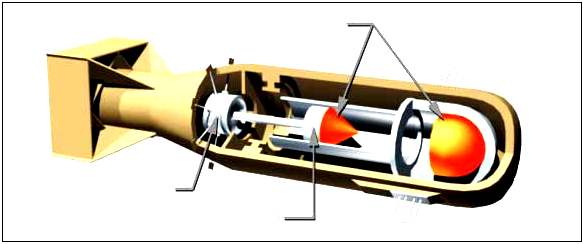
Fig. 3.15. Nuclear atomic bomb of Uranium 235.
3.8 Impacts of nuclear weapons
There are five immediate destructive effects from a nuclear explosion:
1. The initial radiation, mainly gamma rays;
2. An electromagnetic pulse, which in a high altitude explosion can knock out
electrical equipment over a very large area;
3. A thermal pulse, which consists of bright light (even many miles away) and
intense heat equal to that at the centre of the sun);
4. A blast wave that can flatten buildings; and
5. Radioactive fallout, mainly in dirt and debris that is sucked up into the
mushroom cloud and then falls to earth.
There are three long-term effects of a nuclear explosion:
1. Delayed radioactive fallout, which gradually fall over months and even years
to the ground, ofen in rain;
2. A change in the climate (possibly by lowering of the earth’s temperature
over the whole hemisphere which could ruin agricultural crops and cause
widespread famine);
3. A partial destruction of the ozone layer, which protects the earth from
the sun’s ultraviolent rays. If ozone layer is depleted, unprotected Caucasians
would get an incapacitating sunburn within 10 minutes, and people would
suffer a type of snow blindness from the rays which, if repeated, would lead
to permanent blindness. Many animals would suffer the same fate.
3.9 Energy transformations in a nuclear power station
In a nuclear power plant, Nuclear Steam Supply System (NSSS) consists of a
nuclear reactor and all of the components necessary to produce high pressuresteam, which will be used to turn the turbine for the electrical generator.
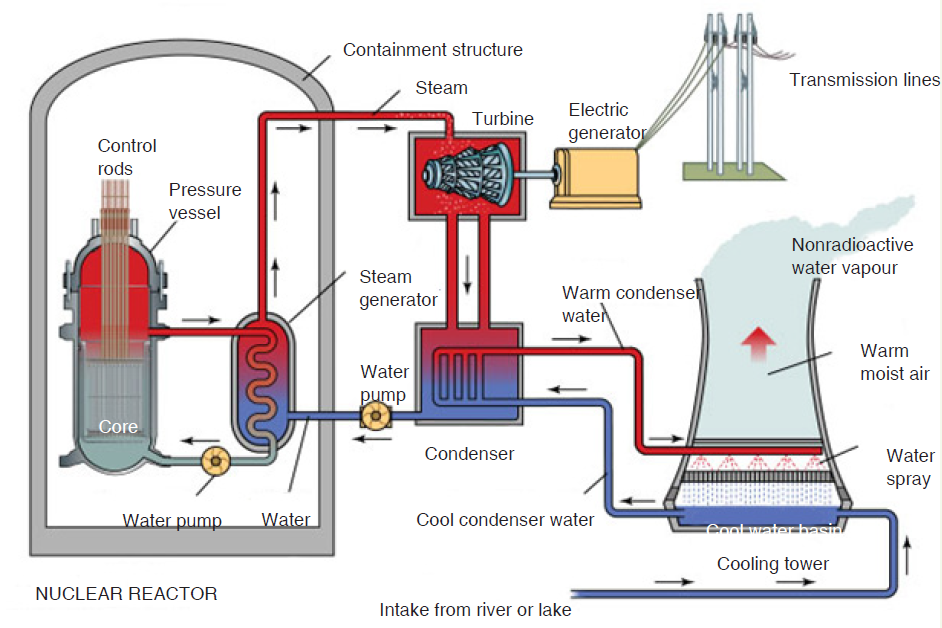
Fig. 3.16. Nuclear power plant
The nuclear reactor contains some radioactive isotopes like uranium which
undergo fission reaction when bombarded with some neutrons and a large
amount of heat energy is evolved. This heat energy converts water into steam,
which is piped to the turbine. In the turbine, the steam passes through the
blades, which spins the electrical generator, resulting in a flow of electricity.
After leaving the turbine, the steam is converted (condensed) back into water
in the condenser. The water is then pumped back to the nuclear reactor to be
reheated and converted back into steam.
3.10 Problems associated with the production of nuclear
power
• The problem of radioactive waste is still unsolved. The waste
from nuclear energy is extremely dangerous and it has to be carefully
looked after for several thousand years (10,000 years according to
United States Environmental Protection Agency standards).
• High risks: Despite a generally high security standard, accidents
can still happen. It is technically impossible to build a plant with
100% security. A small probability of failure will always last. The
consequences of an accident would be absolutely devastating both for
human beings and the nature. The more nuclear power plants (and
nuclear waste storage shelters) are built, the higher is the probability
of a disastrous failure somewhere in the world.
• Nuclear power plants as well as nuclear waste could be preferred
targets for terrorist attacks. Such a terrorist act would have
catastrophic effects for the whole world.
• During the operation of nuclear power plants, radioactive waste is
produced, which, in turn, can be used for the production of nuclear
weapons. In addition, the same is used to design nuclear power plants
can to a certain extent be used to build nuclear weapons (nuclear
proliferation).
• The energy source for nuclear energy is Uranium. Uranium is a
scarce resource; its supply is estimated to last only for the next 30 to
60 years depending on the actual demand.
• The timeframe needed for formalities, planning and building of a new
nuclear power generation plant, is in the range of 20 to 30 years in the
western democracies. In other words, it is an illusion to build new
nuclear power plants in a short time.
3.11 Environmental problems of fossil fuels
Climate Change/Global Warming and Greenhouse Effect
The earth’s atmosphere allows a lot of sunlight to reach the earth’s surface
but reflects much of that light back into space. Some gases trap more sunlight,
therefore, less light is reflected back into space. These gases are called
Greenhouse Gases, because the effect is like being in a plant glasshouse,
or in a car with the windows wound up. The result is a gradual increase in
the earth’s temperature or Global Warming. The major greenhouse gases are
carbon dioxide, methane, nitrous oxide and chlorofluorocarbons (CFCs).
The main man made causes are thought to be carbon dioxide and methane from
factory, power station and car emissions, the waste products of respiration, the
mining of fossil fuels and the breakdown of plant matter in swamps. The longterm
effects may include melting of glaciers and a rise in sea level and a global
change in climate and type of vegetation.
‘Hole’ in the Ozone Layer
Ozone is a gas in the earth’s upper atmosphere whose chemical formula is O3.
Ozone acts to block out much of the sun’s ultraviolet radiation which causes
skin cancer and contributes to the fluctuations of global climatic conditions
that affect the environment. Above Antarctica, there is a thinner layer of ozone
caused by the destruction of ozone gas by emissions of chlorofluorocarbons
and hydrochlorofluorocarbons which are propellants in pressure-pack spray
cans and refrigerants in refrigerators and air-conditioning units.
Acid Rain
When gases, such as sulphur dioxide and nitrogen oxides react with water in
the atmosphere to form sulphuric acid and nitric acid, they form an acidic ‘rain’
which can destroy vegetation. Some of these gases are from natural sources,
such as lightning, decomposing plants and volcanoes. However, much of these
gases are the result of emissions from cars, power stations, smelters and
factories.
Air Pollution
Air pollution is the release of excessive amounts of harmful gases (e.g. methane,
carbon dioxide, sulphur dioxide, nitrogen oxides) as well as particles (e.g.
dust of tyre, rubber, lead from car exhausts) into the atmosphere. To reduce
emissions, the Australian government has legislated that all new cars should
use unleaded petrol and have catalytic converters fitted to the exhausts.
Water Pollution
1. Sewage is the household waste water. Many detergents contain phosphates
which act as plant fertilisers. When these phosphates and the sewerage
reach rivers, they help water plants to grow in abundance, reducing the
dissolved oxygen in the river water. The result is death of aquatic animals
due to suffocation by the algal blooms. This harmful effect is called
eutrophication. Eutrophication is also caused by excessive use of fertilizers
in agricultural fields and subsequent surface run-off.
2. Biodegradable detergents are more environment-friendly because they are
readily broken down to harmless substances by decomposing bacteria.
3. Suspended solids in water, such as silt reduce the amount of light that
reaches the depths of the water in lakes and rivers. This reduces the ability
of aquatic plants to photosynthesise and reduce the plant and animal life.
Turbidity is the measure of ‘cloudiness’ or the depth to which light can
reach in water.
Introduced Species
They are species of plants or animals that have migrated or been brought to
Australia. Many fit into the natural ecosystems and are kept in control by natural
predators and parasites. However, some become pests as they are well-adapted
to that environment, readily obtain nutrients and lack of natural predators or
parasites. Examples include rabbits, foxes, carp and prickly pear cactus plant.
Biological Control
It is an environment-friendly method to control these pests by the introduction
of species-specific, living organisms to control their numbers. Successful
examples include the myxoma virus and the calici virus for rabbits, and the
cactoblastis moth feeding on the prickly pear. Unsuccessful examples include
the introduction of the cane toad to reduce the numbers of natural cane beetles.
Biological Magnification
It is the accumulation in body tissues of certain chemicals, such as DDT,
pesticides and mercury. The higher it moves along the food chain, the greater
is the accumulation, sometimes to such toxic levels, which causes birth defects
and even death.
Soil Salinity
Soil salinity has increased greatly since the widespread logging of trees by
farmers. Deep tree roots normally draw water from the underground water
table. However, when logging of trees occurs, the water table rises close to
the surface bringing with it, salt from rocks. This makes the soil salty so that
vegetation cannot grow effectively. The result is loss of vegetation and erosion.
Population Explosion
It is the rapid increase in population in developing countries causing famine,
and also in developed countries causing more demand for energy and with
that, it increases pollution and destruction of the environment.
ACTIVITY3-3: Sources of Pollution
Aim: the aim of this activity is to find out the causes of pollution.
Procedure: analyse the figure below and answer the questions thatfollow
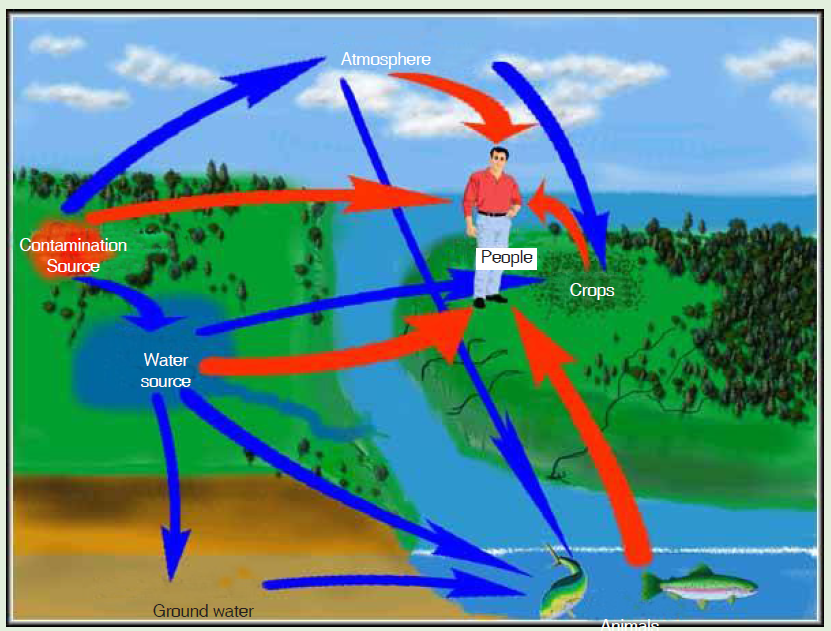
Fig. 3.17. Effects of poorly deposited nuclear wastes.
a. Outline some sources of water and air pollution shown on the figure.
b. Explain how each of the cause in (a) affect the environment.
c. Give and explain any other sources of air and/ or water pollution you
know.
d. Explain how air and water pollutions can be reduced.
ACTIVITY 3-4: Wate Pollution
Aim: to investigate the effect of water contamination
Source: internet and textbooks or journals.
Background Information
1. Scientists have studied the influence of chlorine on organic
materials in water supplies. Some of the chlorine reacts with this
organic material to form chloroform and other chlorine-containing
chemicals. Research has shown that some chlorine-containing
chemicals can increase the risk of cancer.
2. Working with your group, find out more about the benefits and
costs of using chlorine in the water supply. Have each member of
your group research information on one of the following:
a. The risk to health of not treating water supplies with chlorine
b. The risk to health of using chlorine in water treatment
c. Alternatives to using chlorine for water treatment
d. Scientific research underway on chlorine use
e. What (if anything) is used to treat your local water supply
Support Your Opinion
3. When you have finished your research, share your information
with your group. Design a presentation to summarize your group’s
findings. Be prepared to share your group’s findings with the rest
of the class.
4. Do you think that the amount of chlorine in our water should be
increased at certain times of the year? Give reasons to support youropinion
3.12 Safety issues and risks associated
with nuclear power
3.12.1 Nuclear Meltdown
A nuclear meltdown is an informal term for a severe nuclear reactor accidentthat results in core damage from overheating.

Fig. 3.18. Reactor meltdowns at Fukushima Daiichi.
A nuclear meltdown occurs when a nuclear power plant system or component
fails so the reactor core becomes overheat and melts. Usually, this occurs due to
the lack of coolant that decreases the temperature of the reactor. The commonly
used coolant is water but sometimes a liquid metal, which is circulated past the
reactor core to absorb the heat, is also used. In another case, a sudden power
surge that exceeds the coolant’s cooling capabilities causes an extreme increase
in temperature which leads to a meltdown. A meltdown releases the core’s
highly radioactive and toxic elements into the atmosphere and environment.
The causes of a meltdown occur due to:
A: A loss of pressure control
The loss of pressure control of the confined coolant may be caused by the failure
of the pump or having resistance or blockage within the pipes. This causes the
coolant to cease flow or insufficient flow rate to the reactor; thus, the heat
transfer efficiency decreases.
B: A loss of coolant
A physical loss of coolant, due to leakage or insufficient provision, causes a
deficit of coolant to decrease the heat of the reactor. A physical loss of coolant
can be caused by leakages. In some cases, the loss of pressure control and
the loss of coolant are similar because of the systematic failure of the coolant
system.
C: An uncontrolled power excursion
A sudden power surge in the reactor is a sudden increase in reactor reactivity.
It is caused by an uncontrolled power excursion due to the failure of the
moderator or the control that slows down the neutron during chain reaction.
A sudden power surge will create a high and abrupt increase of the reactor’s
temperature, and will continue to increase due to system failure. Hence, the
uncontrollable increase of the reactor’s temperature will ultimately lead to a
meltdown.
3.12.2 Nuclear (Radioactive) Wastes
Nuclear wastes are radioactive materials that are produced after the nuclear
reaction. Nuclear reactors produce high-level radioactive (having high levels
of radioactivity per mass or volume) and low-level (having low levels of
radioactivity) wastes. The wastes must be isolated from human contact for avery long time in order to prevent radiation.

Fig. 3.19. High level waste being stored in underground repository.
The ‘high-level wastes’ will be converted to a rock-like form and placed in a
natural habitat of rocks, deep underground. The ‘low-level wastes’, on the other
hand, will be buried in shallow depths (typically 20 feet) in soil.
A number of incidents have occurred when radioactive material was disposed
improperly, where the shielding during transport was defective, or when the
waste was simply abandoned or even stolen from a waste store.
The principal risks associated with nuclear power arise from health effects
of radiation, which can be caused due to contact with nuclear wastes. This
radiation consists of sub-atomic particles travelling at or near the velocity of
light (186,000 miles per second). They can penetrate deep inside the human
body where they can damage biological cells and thereby initiate a cancer. Ifthey strike sex cells, they can cause genetic diseases in progeny
END UNIT ASSESSMENT 3
1. Why should solar energy be harnessed to take care of our electric
power needs?
2. How do we confirm that the ‘greenhouse effect’ is real?
3. How does acid rain destroy forests and fish?
4. Is it possible to eliminate the air pollution from coal burning?
5. Radioactivity can harm us by radiating from sources outside our
bodies, by being taken in with food or water or by being inhaled
into our lungs. But we consider only one of these pathways. Why is
it so?
6. Cancers from radiation may take up to 50 years to develop, and
genetic effects may not show up for a hundred years or more. How,
then, can we say that there will be essentially no health effects from
the Three Mile Island accident?
7. Air pollution may kill people now, but radiation induces genetic
effects that will damage future generations. How can we justify our
enjoying the benefits of nuclear energy while future generations
bear the suffering from it?
8. Can the genetic effects of low-level radiation destroy the human
race?
9. Isn’t the artificial radioactivity created by the nuclear industry,
more dangerous than the natural radiation which has always been
present?
10. Can radiation exposure to parents cause children to be born with two
heads or other such deformities?
11. Can a reactor explode like a nuclear bomb?
12. If reactors are so safe, why don’t home owners’ insurance policies cover
reactor accidents? Does this mean that insurance companies have no
confidence in them?
13. How is radioactive waste disposed off?
14. How long will the radioactive waste be hazardous?
15. How will we get rid of reactors when their useful life is over?
Fossil fuels are hydrocarbons, primarily coal, fuel oil or natural gas, formed
from the remains of dead plants and animals.
Types of Fossil Fuels
• Coal
• Natural Gas
• Oil (Petroleum)
Types of coal storage
• Dead storage
• Living storage
Means of coal storage
• Storage in coal heaps
• Underwater storage
Energy production using fossil fuels
A fossil-fuel power station is a power station which burns fossil fuel, such as
coal, natural gas or petroleum to produce electricity.
Nuclear fuel and nuclear fission
Nuclear fuel is any material that can be consumed to derive nuclear energy.
Controlled fission (power production)
When a fission reaction leads to a new fission reaction, which leads to another
one and so on, it is called controlled fission. The amount of heat required is
controlled by raising and lowering the rods in the reactor.
Uncontrolled fission (nuclear weapons)
A fission reaction whereby the reaction is allowed to proceed without any
moderation (by removal of neutrons) is called an uncontrolled fission reaction.
An uncontrolled fission reaction is used for nuclear bombs.
Problems associated with the production of nuclear power
• problem of radioactive waste.
• high risks.
• targets for terrorist attacks.
• nuclear weapons.
• uranium is a scarce resource.
• illusion to build new nuclear power plants.
Environmental problems of fossil fuels
Climate Change / Global Warming and Greenhouse Effect
The earth’s atmosphere allows a lot of sunlight to reach the earth’s surface, but
reflects much of that light back into space.
The result is a gradual increase in the earth’s temperature or Global Warming.
‘Hole’ in the Ozone Layer
Ozone acts to block out much of the sun’s ultraviolet radiation which causes
skin cancer and contributes to the fluctuations of global climatic conditions
that affect the environment.
Acid Rain
When gases, such as sulphur dioxide and nitrogen oxides react with water in
the atmosphere to form sulphuric acid and nitric acid, they form an acidic ‘rain’
which can destroy vegetation.
Air Pollution
Air pollution is the release into the atmosphere of excessive amounts of harmful
gases as well as particles.
Other environmental problems of fossil fuels include:
• Biological Control
• Biological Magnification
• Introduced Species
• Soil Salinity• Population Explosion
