UNIT 12: RADIATIONS AND MEDICINE
Key unit Competence: Analyze the use of radiation in medicine.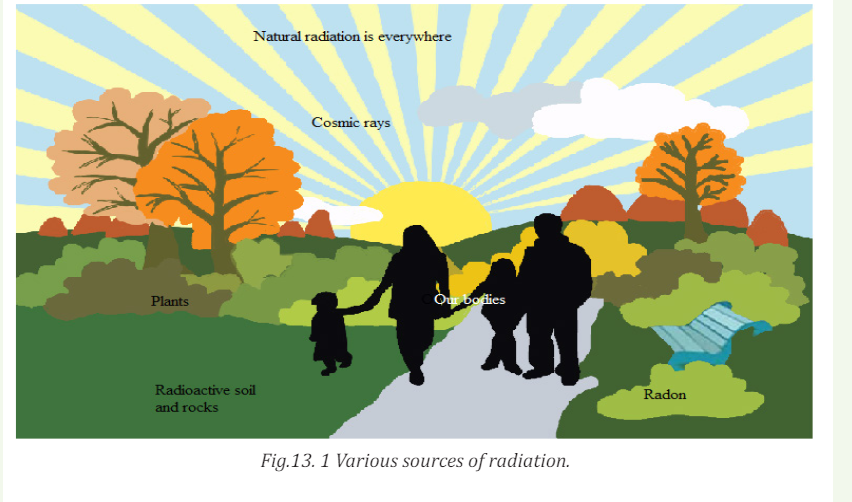
My goalsINTRODUCTORY ACTIVITY
• Explain radiation dosimetry.• Differentiate the terms exposure, absorbed dose, quality factor (relative
to biological effectiveness) and dose equivalent as used in radiation
dosimetry.
• Differentiate physical half-life, biological half-life and effective half-life
• Solve radiation dosimetry problems
• Analyse the basics of radiation therapy for cancer.
• Explain safety precautions when handling radiations• Describe the concept of balanced risk.
Radiation has always been present and is all around us. Life has evolved in
a world containing significant levels of ionizing radiation. Our bodies are
adapted to it.
People are constantly exposed to small amounts of ionizing radiation from
the environment as they carry out their normal daily activities; this is
known as background radiation. We are also exposed through some medical
treatments and through activities involving radioactive material.
Fig 13.1 above identifies four major sources of public exposure to natural
radiation: cosmic radiation, terrestrial radiation, inhalation and ingestion.
Brainstorm and try to answer the following questions:
a. Distinguish artificial source of radiation and natural source of radiation?
b. Explain briefly each major source of public exposure to natural
radiation stated above.
c. Which kind of sources of radiation are mostly preferred to be used in
medicine? Explain why
d. Does exposure to heavy ions at the level that would occur during deep space
missions of long duration pose a risk to the integrity and function
of the central nervous system? Explain to support your idea.
12.1 RADIATION DOSE
12.1.1 Ionization and non-ionization radiations
ACTIVITY 12.1:Types of radiation
Radiation is the emission of particles or electromagnetic waves from a
source. Radiation from radioactive materials has the ability to interact
with atoms and molecules of living objects.
a. With the help of the diagram below, distinguish the forms of
radiation?
b. Which type do you think is mostly used in medical treatment?
Explain your answer with supporting arguments?
c. Suggest the possible side effects of using radiations in medicine?
Which of the two forms of radiation induces more side effects
when exposed to human body? Explain to support your choice.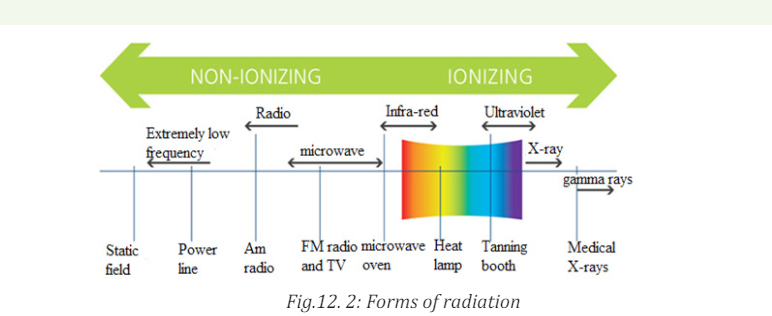
In a neutral atom, the positive charge of the nucleus is equal and opposite to the
total negative charge of the orbital electrons. If such an atom loses or gains an
electron, it becomes an ion. The atom will now have a net positive or negative
charge and is called an ion. This process is called ionization, and the radiation
responsible for it is called ionising radiation. When discussing the interaction
of radiations with matter in particularly in relation to health, two basic types ofradiation can be considered:
a. Ionizing radiation.
This is a radiation that carries enough energy to liberate electrons from atoms
or molecules, thereby ionizing them. As the more powerful form of radiation,
ionizing radiation is more likely to damage tissue than non-ionizing radiation.
The main source of exposure to ionizing radiation is the radiation used duringmedical exams such as X-ray radiography or computed tomography scans.
However, the amounts of radiation used are so small that the risk of any
damaging effects is minimal. Even when radiotherapy is used to treat cancer,
the amount of ionizing radiation used is so carefully controlled that the risk
of problems associated with exposure is tiny. All forms of living things emit a
certain amount of radiation, with humans, plants and animals accumulating
radioisotopes as they ingest food, air and water. Some forms of radiation such as
potassium-40 emit high-energy rays that can be detected using measurement
systems. Together with the background radiation, these sources of internalradiation add to a person’s total radiation dose.
Background radiation is emitted from both naturally occurring and man-made
sources. Natural sources include cosmic radiation, radon radiation in the body,
solar radiation and external terrestrial radiation. Man-made forms of radiation
are used in cancer treatment, nuclear facilities and nuclear weapons. Globally, the
average exposure to ionizing radiation per year is around 3 milliSieverts (mSv),
with the main sources being natural (around 80%). The remaining exposureis due to man-made forms such as those used in medical imaging techniques.
Exposure to man-made forms of ionizing radiations is generally much higher
in developed countries where the use of nuclear imaging techniques is muchmore common than in developing countries.
b. Non-ionizing radiations
Non-ionizing radiation refers to any type of electromagnetic radiation that
does not carry enough energy to ionize atoms or molecules. Examples of
non-ionizing radiations include visible light, microwaves, ultraviolet (UV)
radiation, infrared radiation, radio waves, radar waves, mobile phone signals
and wireless internet connections. Although UV has been classified as a nonionizing
radiation but it has been proven that high levels of UV-radiation cancause sunburn and increase the risk of skin cancer developing.
Scientific investigations suggest that the use of telecommunications devices
such as mobile phones may be damaging, but no risk associated with the use
of these devices has yet been identified in any scientific studies. This energyis emitted both inside the body and externally, through both natural and manmade processes.
12.1.2 Radiation penetration in body tissue
ACTIVITY 12.2
The figure below shows the penetrating power of radiation representedby A, B and C. Use the figure to answer the following questions.
Questions: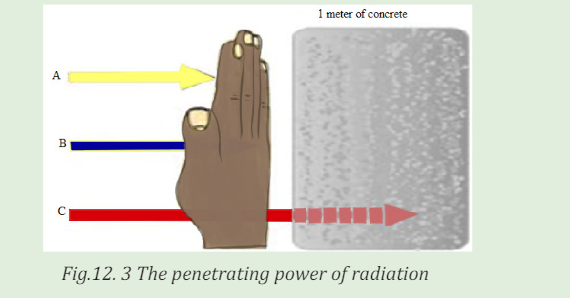
a. Interpret the figure and write the names of letters A, B and C
labeled on the figure above?
b. Which of the three types of radiation has high penetrating power?
Explain to support your idea.
c. Outline four uses of the man-made sources of radiation?
d. How does radiation affect me? Explain clearly with scientificreasoning.
An important characteristic of the various ionising radiations is how deeply
they can penetrate the body tissues. X-rays, gamma rays, and neutrons of
sufficient energy described below can reach all tissues of the body from anexternal source.
Alpha Radiation
Alpha radiation occurs when an atom undergoes radioactive decay, giving
off an α- particle consisting of two protons and two neutrons (essentially thenucleus of a helium-4 atom) following the equation
Due to their charge and mass, alpha particles interact strongly with matter,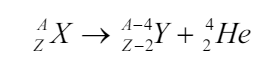
and can only travel a few centimeters in air. A thin sheet of paper, on the other
hand, stops alpha particles. They are also stopped by the superficial dead layer
of skin that is only 70 µm thick. Therefore, radionuclides that emit only alpha
particles are harmless unless you take them into the body. This you might do byinhalation (breathing in) or ingestion (eating and drinking).
Beta Radiation
Beta radiation takes the form of either an electron or a positron (a particle
with the size and mass of an electron, but with a positive charge) being emitted
from an atom. Due to their smaller mass, they are able to travel further in air,
up to a few meters, and can be stopped by a thick piece of plastic, or even a
stack of paper. Such radiation can penetrate the skin a few centimeters, posing
somewhat of an external health risk. The depth to which beta particles canpenetrate the body depends on their energy.
High-energy beta particles (several MeV) may penetrate a cm of a tissue,
although most are absorbed in the first few mm. As a result, beta emitters
outside the body are hazardous only to surface tissues such as the skin or the
lenses of the eye. When you take beta emitters into the body, they will irradiateinternal tissues and then become a much more serious hazard.
Gamma Radiation
Gamma radiation, unlike alpha or beta, does not consist of any particles, instead
consisting of a photon of energy being emitted from an unstable nucleus. Having
no mass or charge, gamma radiation can travel much farther through air than
alpha or beta, losing (on average) half its energy. Gamma waves can be stopped
by a thick or dense enough layer material, with high atomic number. Materialssuch as lead can be used as the most effective form of shielding.
X-Rays
X-rays are similar to gamma radiation, with the primary difference being that
they originate from the electron cloud. This is generally caused by energy
changes in an electron, such as moving from a higher energy level to a lower
one, causing the excess energy to be released. X-Rays are longer-wavelengthand (usually) lower energy than gamma radiation, as well.
Neutron Radiation
Neutron radiation consists of a free neutron, usually emitted as a result of
spontaneous or induced nuclear fission. They are able to travel hundreds or
even thousands of meters in air, they are however able to be effectively stopped
if blocked by a hydrogen material, such as concrete or water.
Neutron radiation occurs when neutrons are ejected from the nucleus by
nuclear fission and other processes. The nuclear chain reaction is an example
of nuclear fission, where a neutron being ejected from one fission atom will
cause another atom to fission, ejecting more neutrons. Unlike other radiations,
neutron radiation is absorbed by materials with lots of hydrogen atoms, likeparaffin wax and plastics.
12.1.3 Radiation dosimetry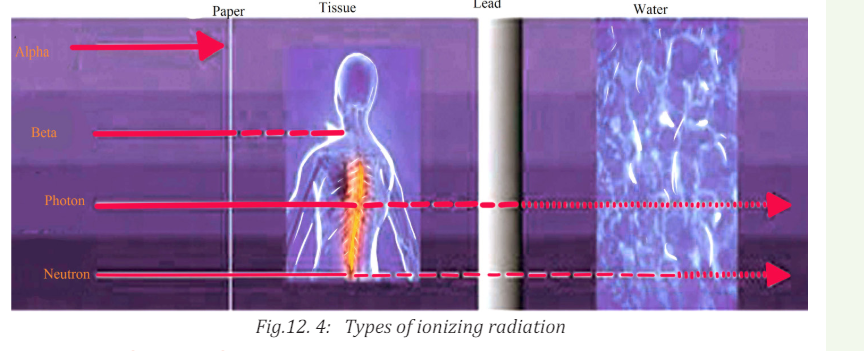
ACTIVITY 12.3:
a. What does the term Dosimeter in radiation dosimetry mean?
b. Who Should Wear a Dosimeter? Suggest reasons why it is very important
to wear a dosimeter?
Just as for drugs, the effect of radiation depends on the amount a person has
received. Therefore, amounts of radiation received are referred to as doses,and the measurement of such doses is known as dosimetry.
Dosimeters are used to monitor your occupational dose from radioactive
material or radiation-producing equipments. Most individuals working with
X-ray producing equipment in the hospital will be issued with a dosimeter. For
those individuals working in the research laboratory setting, dosimeters will
be issued based on the nuclide and total activity that will be used. Dosimeters
are integrating detectors; that is, they accumulate the radiation dose and giveoff an amount of light which is proportional to that dose.
The energy absorption properties of dosimeters are designed to be very similar
to tissue, so they are very effective as personnel dosimeters. These devices are
used to measure exposures from x-ray, gamma ray and high energy beta
particles. Dosimeters are not suitable for measuring exposures to low energybeta particles or alpha particles.
12.1.4 Radiation exposure
ACTIVITY 12.4:
a. What are the symptoms of radiation exposure?
b. Explain briefly the effects of radiation exposure to the human body?
c. It is possible that side effects can happen when a person undergoes
radiation treatment for cancer. Suggest the common side effects of
radiation exposure to the human body?
d. Does radiation exposure to the human body induce risks? Support yourdecision with clear explanations.
Long-term exposure to small amounts of radiation can lead to gene mutations
and increase the risk of cancer, while exposure to a large amount over a brief
period can lead to radiation sickness.
Exposure is a measure of the ionization produced in air by X-rays or γ rays,
and it is defined in the following manner. A beam of X-rays or γ rays is sent
through a mass m of dry air at standard temperature and pressure ( stp:0 0C ,
1 atm). In passing through the air, the beam produces positive ions whose total
charge is q. Exposure is defined the total charge per unit mass of air.The SI unitfor exposure is coulomb per unit mass (/ ) C kg .
The commonly used unit for exposure E is the roentgen(R). 1R is the amount
of electromagnetic radiation which produces in one gram of air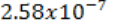
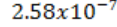 C at normal temperature (22 and pressure (760mmHg) conditions
C at normal temperature (22 and pressure (760mmHg) conditions
Since the concept of exposure is defined in terms of the ionizing abilities of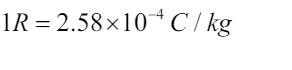
X-rays and γ rays in air, it does not specify the effect of radiation on living tissue.
For biological purposes, the absorbed dose is more suitable quantity, because it
is the energy absorbed from the radiation per unit mass of absorbing material.12.1.5 Absorbed radiation dose
ACTIVITY 12.5
a. What does the term absorbed dose mean in medical treatment?
b. In the application of radiation in medicine, we use the statement “A measure of
the risk of biological harm”. Brainstorm and explain clearly what the statement
means.
c. Explain why doses of alpha and gamma radiation produce unequal biologicaleffects?
What is important when we analyze the effect of radiation on human being is
not so much the total dose to the whole system but the dose per kg. That’s why
a doctor will prescribe smaller doses of medicine for children than for adults. A
similar approach is used in radiation protection measurements, where the unit
of absorbed dose is specified in terms of the amount of energy deposited byradiation in 1 kg of material. This unit is the Gray, abbreviated Gy.
It was named in honor of Louis Gray, who was a very big name in the early days
of radiation dosimetry. An absorbed radiation dose of 1 Gray corresponds to
the deposition of 1 joule of energy in 1 kg of material. The gray is a measure of
energy absorbed by 1 kg of any material, be it air, water, tissue or whatever. A
person who has absorbed a whole body dose of 1 Gy has absorbed one joule ofenergy in each kg of its body tissue.
As we shall see later, the gray is a fairly hefty dose, so for normal practical purposeswe use the milligray (abbreviated mGy) and the microgray (abbreviated µGy).
The gray is a physical unit. It describes the physical effect of the incident
radiation (i.e., the amount of energy deposited per kg), but it tells us nothing
about the biological consequences of such energy deposition in tissue. Studies
have shown that alpha and neutron radiation cause greater biological damagefor a given energy deposition per kg of tissue than gamma radiation does.
In other words, equal doses of, say, alpha and gamma radiation produce unequal
biological effects. This is because the body can more easily repair damage from
radiation that is spread over a large area than that which is concentrated in a
small area. Because more biological damage is caused for the same physicaldose.
12.1.6 Quality factors
Quality factors are used to compare the biological effects from different types
of radiation. For example, fast neutron radiation is considered to be 20 times
as damaging as X-rays or gamma radiation. You can also think of fast neutron
radiation as being of “higher quality”, since you need less absorbed dose to
produce equivalent biological effects. This quality is expressed in terms of the
Quality Factor (Q). The quality factor of a radiation type is defined as the ratio
of the biological damage produced by the absorption of 1 Gy of that radiation tothe biological damage produced by 1 Gy of X or gamma radiation.
The Q of a certain type of radiation is related to the density of the ion tracks itleaves behind it in tissue; the closer together the ion pairs, the higher the Q.
12.1.7 Equivalent dose
The absorbed radiation dose, when multiplied by the Q of the radiation
delivering the dose, will give us a measure of the biological effect of the dose.
This is known as the equivalent dose. The unit of equivalent dose H is the Sievert
(Sv). An equivalent dose of one Sievert represents that quantity of radiation
dose that is equivalent, in terms of specified biological damage, to one gray ofX or gamma rays.
In practice, we use the millisievert (mSv) and microsievert (µSv). The sievert is
the unit that we use all the time, because it is the only one that is meaningful in
terms of biological harm. In calculating the equivalent dose from several types
of radiation (we call this “mixed radiation”), all measurements are converted to
Sv, mSv or µSv and added. Most of the radiation instruments we use to measure
doses or dose rates read in mSv or µSv. Few other instruments can read in mGyor µGy, but they measure only gamma radiation.
The table 13.1 lists some typical relative biological effectiveness ( RBE ) values for
different kinds of radiation, assuming that an average biological tissue is being
irradiated. The values of 1 RBE = indicate that γ rays and particles produce
the same biological damage as do 200 keV X-rays. The large RBE values indicatethat protons, α -particles, and fast neutrons cause substantially more damage.
12.1.8 Radiation protection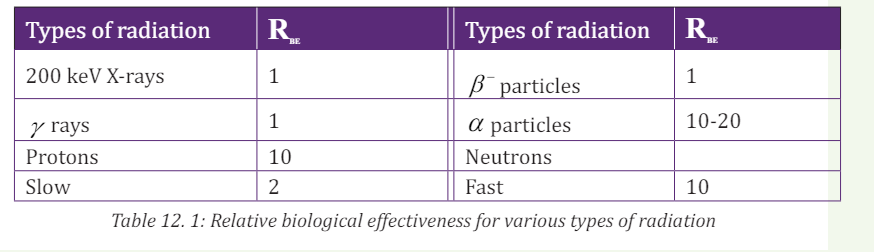
The effects of radiation at high doses and dose rates are reasonably well
documented. A very large dose delivered to the whole body over a short time
will result in the death of the exposed person within days.
We know from these that some of the health effects of exposure to radiation
do not appear unless a certain quite large dose is absorbed. However, many
other effects, especially cancers are readily detectable and occur more often in
those with moderate doses. At lower doses and dose rates, there is a degree of
recovery in cells and in tissues. Radiation protection sets examples for other
safety disciplines in two unique respects:
• First, there is the assumption that any increased level of radiation
above natural background will carry some risk of harm to health.
• Second, it aims to protect future generations from activities conductedtoday
The use of radiation and nuclear techniques in medicine, industry, agriculture,
energy and other scientific and technological fields has brought tremendous
benefits to society. The benefits in medicine for diagnosis and treatment in
terms of human lives saved are large in size. No human activity or practice
is totally devoid of associated risks. Radiation should be viewed from the
perspective that the benefit from it to mankind is less harmful than from manyother agents.
Quick check 12.2:At what level is radiation harmful? Explain your idea\
Note: The optimization of patients’ protection is based on a principle that the
dose to the irradiated target (tumor) must be as high as it is necessary for
effective treatment while protecting the healthy tissues to the maximum extentpossible.
12.1.9 Checking my progress
1. Does receiving external-beam radiation make a person radioactive or
able to expose others to radiation? Explain to support idea
2. How can I be sure that the external-beam radiating machine isn’t
damaging normal, healthy tissue in my body? Explain clearly with
scientific reasoning.
3. I am having an imaging test using radioactive materials. Will I be
radioactive after the test? Comment to support your decision.
4. All my radioactive material is secured properly and I have empty waste
containers in the lab. Do I have to lock the room? Explain clearly tojustify your decision.
12.2 BIOLOGICAL EFFECTS OF RADIATION EXPOSURE
12.2.1 Deterministic and stochastic effects
ACTIVITY 12.6
Is the use of ionizing radiation in medicine beneficial to human health?
Explain to support your point.
1. Are there risks to the use of ionizing radiation in medicine? Explain
your answer.
2. How do we quantify the amount of radiation?
3. What do we know about the nature (mechanism) of radiation induced biological effects?
4. How are effects of radiation classified?
Effects of radiations due to cell killing have a practical threshold dose below
which the effect is not evident but in general when the effect is present its
severity increases with the radiation dose.
The threshold doses are not an absolute number and vary somewhat by
individual. Effects due to mutations (such as cancer) have a probability of
occurrence that increases with dose.
a. Deterministic effects:
These effects are observed after large absorbed doses of radiation and are
mainly a consequence of radiation induced cellular death. They occur only if a
large proportion of cells in an irradiated tissue have been killed by radiation,
and the loss can be compensated by increasing cellular proliferation.
b. Stochastic effects:
They are associated with long term, low level (chronic) exposure to radiation.
They have no apparent threshold. The risk from exposure increases with
increasing dose, but the severity of the effect is independent of the dose.
Irradiated and surviving cells may become modified by induced mutations
(somatic, hereditary). These modifications may lead to two clinically significant
effects: malignant neoplasm (cancer) and hereditary mutations.
The frequency or intensity of biological effects is dependent upon the total
energy of radiation absorbed (in joules) per unit mass (in kg) of a sensitive
tissues or organs. This quantity is called absorbed dose and is expressed ingray (Gy).
In evaluating biological effects of radiation after partial exposure of the body
further factors such as the varying sensitivity of different tissues and absorbed
doses to different organs have to be taken into consideration.
To compare risks of partial and whole body irradiation at doses experienced
in diagnostic radiology and nuclear medicine a quantity called equivalent or
effective dose is used. A cancer caused by a small amount of radiation can bejust as malignant as one caused by a high dose.
ACTIVITY 12.7
1. What is magnitude of the risk for cancer and hereditary effects?
2. Is ionizing radiation from medical sources the only one to which
people is exposed?
3. What are typical doses from medical diagnostic procedures?
4. Can radiation doses in diagnosis be managed without affecting the
diagnostic benefit? Explain to support your decision.
5. Are there situations when diagnostic radiological investigations
should be avoided? Explain to support your decision.
The lifetime value for the average person is roughly a 5% increase in fatal cancer
after a whole body dose of 1 Sv. It appears that the risk in fetal life, in children
and adolescents exceeds somewhat this average level (by a factor of 2 or 3) and
in persons above the age of 60 it should be lower roughly by a factor of ~ 5.
Animal models and knowledge of human genetics, the risk of hereditary
deleterious effects have been estimated to not be greater than 10% of the
radiation induced carcinogenic risk.
All living organisms on this planet, including humans, are exposed to radiation
from natural sources. An average yearly effective dose from natural background
amounts to about 2.5 mSv. This exposure varies substantially geographically
(from 1.5 to several tens of mSv in limited geographical areas).
Various diagnostic radiology and nuclear medicine procedures cover a wide
dose range based upon the procedure. Doses can be expressed either as
absorbed dose to a single tissue or as effective dose to the entire body which
facilitates comparison of doses to other radiation sources (such as natural
background radiation.
There are several ways to reduce the risks to very, very low levels whileobtaining very beneficial health effects of radiological procedures.
Quality assurance and quality control in diagnostic radiology and nuclear
medicine play also a fundamental role in the provision of appropriate, soundradiological protection of the patient.
There are several ways that will minimize the risk without sacrificing the
valuable information that can be obtained for patients’ benefit. Among the
possible measures it is necessary to justify the examination before referring apatient to the radiologist or nuclear medicine physician.
Failure to provide adequate clinical information at referral may result in a
wrong procedure or technique being chosen by radiologist or nuclear medicinespecialist.
An investigation may be seen as a useful one if its outcome - positive or negative
influences management of the patient. Another factor, which potentially adds
to usefulness of the investigation, is strengthening confidence in the diagnosis.
Irradiation for legal reasons and for purposes of insurance should be carefullylimited or excluded.
ACTIVITY 12.8
1. Are there special diagnostic procedures that should have special
justification? Explain to support your decision.
2. Do children and pregnant women require special consideration in
diagnostic procedures?
3. What can be done to reduce radiation risk during the performanceof a diagnostic procedure?
While all medical uses of radiation should be justified, it stands to reason that
the higher the dose and risk of a procedure, the more the medical practitioner
should consider whether there is a greater benefit to be obtained.
Among these special position is occupied by computed tomography (CT), and
particularly its most advanced variants like spiral or multi slice CT.
Both the fetus and children are thought to be more radiosensitive than adults.
Diagnostic radiology and diagnostic nuclear medicine procedures (even in
combination) are extremely unlikely to result in doses that cause malformations
or a decrease in intellectual function. The main issue following in childhood
exposure at typical diagnostic levels (<50 mGy) is cancer induction.
Medically indicated diagnostic studies remote from the fetus (e.g. radiographs
of the chest or extremities, ventilation/perfusion lung scan) can be safely done
at any time of pregnancy if the equipment is in proper working order. Commonly
the risk of not making the diagnosis is greater than the radiation risk.
If an examination is typically at the high end of the diagnostic dose range and
the fetus is in or near the radiation beam or source, care should be taken to
minimize the dose to the fetus while still making the diagnosis. This can be
done by tailoring the examination and examining each radiograph as it is taken
until the diagnosis is achieved and then terminating the procedure
For children, dose reduction in achieved by using technical factors specific for
children and not using routine adult factors. In diagnostic radiology care should
be taken to minimize the radiation beam to only the area of interest. Because
children are small, in nuclear medicine the use of administered activity lower
than that used for an adult will still result in acceptable images and reduced
dose to the child. The most powerful tool for minimizing the risk is appropriate
performance of the test and optimization of radiological protection of the
patient. These are the responsibility of the radiologist or nuclear medicine
physician and medical physicist.
The basic principle of patients’ protection in radiological X-ray investigations
and nuclear medicine diagnostics is that necessary diagnostic information of
clinically satisfactory quality should be obtained at the expense of a dose as low
as reasonably achievable, taking into account social and financial factors.
12.2.2 Effects of radiation exposure
Quick check13.1:
Will small radiation doses hurt me?
Some effects may occur immediately (days or months) while others might take
tens of years or even get passed to the next generation. Effects of interest for
the person being exposed to radiation are called somatic effects and effects ofinterest that affect our children are called genetic effects.
I. Radiation Health Effects
Ionizing radiation has sufficient energy to cause chemical changes in cells and
damage them. Some cells may die or become abnormal, either temporarily or
permanently. By damaging the genetic material (DNA) contained in the body’s
cells, radiation can cause cancer.
Fortunately, our bodies are extremely efficient at repairing cell damage. The
extent of the damage to the cells depends upon the amount and duration of theexposure, as well as the organs exposed.
Exposure to an amount of radiation all at once or from multiple exposures in a
short period of time. In most cases, a large acute exposure to radiation causes
both immediate ( radiation sickness) and delayed effects (cancer or death), can
cause sickness or even death within hours or days. Such acute exposures areextremely rare.
II. Chronic Exposure
With chronic exposure, there is a delay between the exposure and the observed
health effect. These effects can include cancer and other health outcomes such
as benign tumors, cataracts, and potentially harmful genetic changes.
Some radiation effects may occur immediately (days or months) while others
might take years or even get passed to the next generation. Effects of interest
for the person being exposed to radiation are called somatic effects and effectsof interest that affect our children are called genetic effects.
ACTIVITY 12.9:Low levels of radiation exposure
What is the safe level of radiation exposure? Explain your answer.
What is the annual radiation exposure limit? Explain your answer
Radiation risks refer to all excess cancers caused by radiation exposure
(incidence risk) or only excess fatal cancers (mortality risk). Risk may be
expressed as a percent, a fraction, or a decimal value.
For example, a 1% excess risk of cancer incidence is the same as a 1 in a
hundred (1/100) risk or a risk of 0.01. However, it is very hard to tell whether
a particular cancer was caused by very low doses of radiation or by something
else. While experts disagree over the exact definition and effects of “low dose”.
Radiation protection standards are based on the premise that any radiation
dose carries some risk, and that risk increases directly with dose.
Note:
• The risk of cancer from radiation also depends on age, sex, and factors
such as tobacco use.
• Doubling the dose doubles the risk.
Acute health effects occur when large parts of the body are exposed to a
large amount of radiation. The large exposure can occur all at once or from
multiple exposures in a short period of time. Instances of acute effects fromenvironmental sources are very rare.
12.2.3 Safety precautions for handling radiations
ACTIVITY 12.10: Safety precautions to be recognized when
handling radiation
a. Who is involved in planning my radiation treatment?
b. How is the treatment plan checked to make sure it is best for me?
c. What procedures do I have in place so that the treatment team is
able to treat me safely?
d. How can I be assured that my treatment is being done correctly
every day?
e. What is the difference between a medical error and a side effect?
f. Outline the measures taken to reduce doses from externalexposure
Shortening the time of exposure, increasing distance from a radiation source
and shielding are the basic countermeasures (or protective measures) to reducedoses from external exposure.
Note: Time: The less time that people are exposed to a radiation source, the less
the absorbed dose Distance: The farther away that people are from a radiationsource, the less the absorbed dose.
Note: Shielding: Barriers of lead, concrete or water can stop radiation or reduce
radiation intensity.
There are four main factors that contribute to how much radiation a person
absorbs from a source. The following factors can be controlled to minimize
exposure to radiation:
I. The distance from the source of radiation
The intensity of radiation falls sharply with greater distance, as per the inverse
square law. Increasing the distance of an individual from the source of radiation
can therefore reduce the dose of radiation they are exposed to.
For example, such distance increases can be achieved simply by using forceps
to make contact with a radioactive source, rather than the fingers.
II. Duration of exposure
The time spent exposed to radiation should be limited as much as possible.
The longer an individual is subjected to radiation, the larger the dose from the
source will be.
One example of how the time exposed to radiation and therefore radiation
dose may be reduced is through improving training so that any operators whoneed to handle a radioactive source only do so for the minimum possible time.
III. Reducing incorporation into the human body
Potassium iodide can be given orally immediately after exposure to radiation.
This helps protect the thyroid from the effects of ingesting radioactive iodine if
an accident occurs at a nuclear power plant. Taking Potassium iodide in such anevent can reduce the risk of thyroid cancer developing.
IV. Shielding
Shielding refers to the use of absorbent material to cover the source of
radiation, so that less radiation is emitted in the environment where humans
may be exposed to it. These biological shields vary in effectiveness, dependingon the material’s cross-section for scattering and absorption.
The thickness (shielding strength) of the material is measured in g/cm2. Any
amount of radiation that does penetrate the material falls exponentially withincreasing thickness of the shield.
Some examples of the steps taken to minimize the effects of radiation exposure
are described below;
• The exposed individual is removed from the source of radiation.
• If radiation exposure has led to destruction of the bone marrow, the
number of healthy white blood cells produced in the bone marrow will
be depleted.
• If only part of the body has been exposed to radiation rather than the
whole body, treatment may be easier because humans can withstandradiation exposure in large amounts to non-vital body parts.
In every medicine there is a little poison. If we use radiation safely, there are
benefits and if we use radiation carelessly and high doses result, there areconsequences.
Ionizing radiation can change the structure of the cells, sometimes creating
potentially harmful effects that are more likely to cause changes in tissue.
These changes can interfere with cellular processes so cells might not be ableto divide or they might divide too much.
Radioactive rays are penetrating and emit ionizing radiation in the form of
electromagnetic waves or energetic particles and can therefore destroy living
cells. Small doses of radiation over an extended period may cause cancer and
eventually death. Strong doses can kill instantly. Marie Curie and Enrico Fermidied due to exposure to radiation.
Several precautions should be observed while handling radioisotopes. Some of
these are listed in the following:
• No radioactive substance should be handled with bare hands. Alpha
and beta emitters can be handled using thick gloves. Gamma ray
emitters must be handled only by remote control that is by mechanical
means. Gamma rays are the most dangerous and over exposure can
lead to serious biological damage.
• Radioactive materials must be stored in thick lead containers.
• Reactor and laboratories dealing with and conducting experiments
with radioactive metals must be surrounded with thick concrete lined
with lead.
• People working with radioactive isotopes must wear protective
clothing which is left in the laboratory. The workers must be checked
regularly with dosimeters, and appropriate measures should be taken
in cases of overdose.• Radioactive waste must be sealed and buried deep in the ground.
12.2.3 Checking my progress
1.
a. What does the term background radiation mean?
b. Hat is radiation – am I exposed to background radiation each day
even if I do not have an X-ray examination?
2. What are the risks associated with radiation from diagnostic X-ray
imaging and nuclear medicine procedures?
3. How do I decide whether the risks are outweighed by the benefits of
exposure to X-radiation when I have a radiology test or procedure?
4. Are there alternatives to procedures that involve ionizing radiation that
would answer my doctor’s question? Justify your answer with clear
facts.
5. What kinds of safety checks do you perform each day?
6. How often does the medical physicist check the various machines
involved during my treatment are working properly?
7. If I have side effects after my treatment, who can I call?
a. My best friend
b. My primary care doctor
8. I have a question about a radiation treatment I had many years ago.Who should I call?
12.3 CONCEPT OF BALANCED RISK.
12.3.1 Risks of ionizing radiation in medical treatment
ACTIVITY 12.11:balanced risk
Brainstorm and write briefly how balance risks in medical treatment
occur?
Risk in the area of radiation medicine has several dimensions that are less
common in other areas of medicine. First, there may be risks
from overexposure that do not cause immediate injury. For example, the causal connection,
if any, may be difficult or impossible to verify for a malignancy that surfaces
several years after an inappropriate exposure. Second, the risks associated
with the medical use of ionizing radiation extend beyond the patient and can
affect health care workers and the public.
In amplifying these and other aspects of the risks that attend medical uses ofionizing radiation, the discussion addresses the following issues: human error
and unintended events; rates of misadministration in radiation medicine;
inappropriate and unnecessary care; and efforts that reduce misadministrationand inappropriate care.
12.3.2 Human Error and Unintended Events
Errors occur throughout health care: A pharmacist fills a prescription with the
wrong medicine; an x-ray technician takes a film of the wrong leg; a surgeon
replaces the wrong hip. The advent of complex medical technology has
increased the opportunity for error even as it has increased the opportunity for
effecting cures.
By educating health care workers, and by circumscribing their actions, human
error may be minimized. However, some number of mistakes will always,
unavoidably, be made, and no amount of training or double-checking can erasethat fact.
12.3.3 Comparison of risks in the use of ionizing radiation
The comparison of relative risks of misadministration in by-product radiation
medicine to error rates and events in other medical practice settings, as well
as the comparison of disease and death rates with the risks of the therapeutic
administration itself, help to some extent to place ionizing radiation use in a
broader context.
To achieve this success requires the highest standards of performance (accuracy
of delivered dose), both when planning irradiation for an individual patient and
in actual delivery of the dose.
In a large number of cases, decreasing the dose to the target volume is not
possible since it would unacceptably decrease the cure rate. In these cases
present technological developments aim at optimizing the patients’ protection,
keeping the absorbed tumor dose as high as is necessary for effective treatment
while protecting nearby healthy tissues.
It should be remembered that successful eradication of a malignant tumor by
radiation therapy requires high-absorbed doses and there is a delayed (and
usually low) risk of late complication. The above mention techniques are used
to provide the best benefit/risk ratio.
A malignant tumor in a pregnant woman may require radiotherapy in attempt
to save life of the patient. If a tumor is located in a distant part of the body, the
therapy with individually tailored protection of the abdomen (screening) - may
proceed.
When thyroid cancer with metastases is diagnosed in a pregnant woman,
treatment with 131I is not compatible with continuation of the pregnancy. The
treatment should then be delayed until delivery if doing so wouldn’t put the
mother’s life in danger.
Medical radiation can be delivered to the patient from a radiation source
outside the patient. Regardless of how much dose the patient received, they do
not become radioactive or emit radiation.• Balancing risks are often summarized in the following:12.3.4 Checking progress
• The demand for imaging, especially computed tomography, that has
increased vastly over the past 20 years
• An estimated 30% of computed tomography tests that may be
unnecessary
• Ionizing radiation that may be associated with cancer.
• The risks of radiation exposure that is often overlooked and patients
are seldom made aware of these risks
• The requesting doctor who must balance the risks and benefits
of any high radiation dose imaging test, adhering to guideline
recommendations if possible
• Difficult cases that should be discussed with a radiologist, ideally at aclinic radiological or multidisciplinary team meeting.
1. When patients are intentionally exposed to ionizing radiation for12.4 THE HALF-LIVES: PHYSICAL, BIOLOGICAL, AND FFECTIVE
medical purposes, do they suffer unintentional exposures as a result of
error or accident? Comment to support your idea.
2. What can be done to reduce radiation risk during conduct of radiation
therapy?
3. Can pregnant women receive radiotherapy? Explain to support your
decision.
4. Can patients’ treatment with radiation affect other people? Explain tosupport your decision.
ACTIVITY 12.12
Distinguish between physical half-life, biological half-life and effectivehalf-life.
Brainstorm and write the distinction between physical half-life, biological halflife and effective half-life in your note books.
The half-life is a characteristic property of each radioactive species and is
independent of its amount or condition. The effective half-life of a given isotope
is the time in which the quantity in the body will decrease to half as a result ofboth radioactive decay and biological elimination.
There are three half-lives that are important when considering the use of
radioactive drugs for both diagnostic and therapeutic purposes. While both
the physical and biological half-lives are important since they relate directly
to the disappearance of radioactivity from the body by two separate pathways
(radioactive decay, biological clearance), there is no half-life as important inhumans as the effective half-life.
The half-life takes into account not only elimination from the body but also
radioactive decay. If there is ever a question about residual activity in the body,
the calculation uses the effective half-life; in radiation dosimetry calculations,the only half-life that is included in the equation is the effective half-life.
12.4.1 Physical half Lives
Physical half-life is defined as the period of time required to reduce the
radioactivity level of a source to exactly one half its original value due solely toradioactive decay. The physical half-life is designated Tp or more commonly
By default, the term T12 refers to the physical half-life and Tp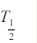
is used when either or both of the other two half-lives are
included in the discussion. Where λ is the radioactive constant of the radio substance
Where λ is the radioactive constant of the radio substance
There are a few things to note about the Tp :• The Tp can be measured directly by counting a sample at 2 different
points in time and then calculating what the half-life is.• For example, if activity decreases from 100% to 25% in 24 hours, then
the half-life is 12 hours since a decrease from 100% to 50% to 25%
implies that 2 half-lives have elapsed.
The physical half-life is unaffected by anything that humans can do to the
isotope. High or low pressure or high or low temperature has no effect on thedecay rate of a radioisotope.
12.4.2 Biological half lives
Biological Half-life is defined as the period of time required to reduce the
amount of a drug in an organ or the body to exactly one half its original value
due solely to biological elimination. It is typically designated Tb . There are afew things to note about the Tb :
• For radioactive compounds, we have to calculate the Tb because
the mass of the isotope is usually on the nanogram scale and, when
distributed throughout the body, and especially in the target organ,
concentrations are in the pictogram/ml range, much too small to
measure directly.
• For non-radioactive compounds, we can measure the Tb directly. For
example, assuming that a person is not allergic to penicillin, we could
give 1 000 mg of the drug and then measure the amount present in the
blood pool and in the urine since we administered such a large amount
of the drug• Tb is affected by many external factors. Perhaps the two most important12.4.3 Effective half lives
are hepatic and renal function. If kidneys are not working well, wewould expect to see a high background activity on our scans.
• Each individual organ in the body has its own Tb and the whole body
also has a Tb representing the weighted average of the Tb of all internal
organs and the blood pool. It is therefore very important to have a frame
of reference. For example, do you need to know the Tb of the drug in theliver or in the whole body?
• All drugs have aTb , not just radioactive ones. Drug package inserts
often refer to the half-time of clearance of a drug from the blood pool
or through the kidneys.
• Since the whole body has a Tb representing the weighted average
of the Tb of all internal organs, it will almost never equal that of aninternal organ.
Effective half-life is defined as the period of time required to reduce the
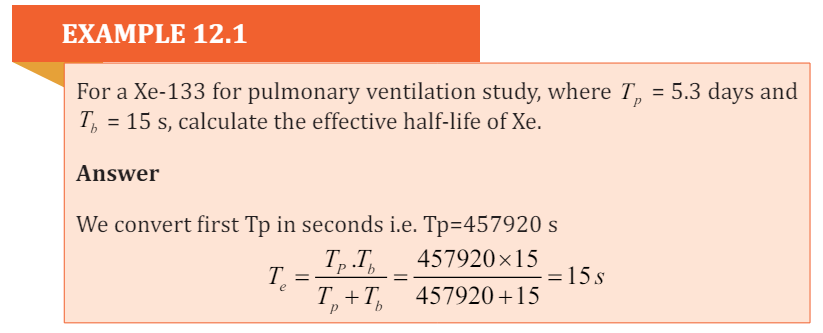
radioactivity level of an internal organ or of the whole body to exactly one halfits original value due to both elimination and decay.
It is designated Te can be measured directly. For example, one can hold a
detection device 1 m from the patient’s chest and count the patient multipletimes until the reading decreases to half of the initial reading.
The patient is permitted to use the rest room between readings as needed, so
both elimination and decay are taking place. The half-life being measured in
this case is the Te and Te is affected by the same external factors that affect Tbsince Te is dependent upon Tb .
Where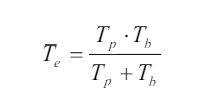
Tp: physical half-lifeTb : biological half-life

END UNIT ASSESSMENT 12
A. Multiple choices.
1. Which of the following would reduce the cell damage due to
radiation for a lab technician who works with radioactive isotopes
in a hospital or lab?a. Increase the worker’s distance from the radiation source.
b. Decrease the time the worker is exposed to the radiation.
c. Use shielding to reduce the amount of radiation that strikes
the worker.
d. Have the worker wear a radiation badge when working with
the radioactive isotopes.e. All of the above.2. If the same dose of each type of radiation was provided over the
same amount of time, which type would be most harmful?
a. X-rays. c. γ rays.
b. α Rays. d. β particles.
3. Which of the following is true?a. Any amount of radiation is harmful to living tissue.4. Which radiation induces the most biological damage for a given
b. Radiation is a natural part of the environment.
c. All forms of radiation will penetrate deep into living tissue.
d. None of the above is true.
amount of energy deposited in tissue?a. Alpha particules.5. Which would produce the most energy in a single reaction?
b. Gamma radiation.
c. C. Beta radiation.
d. D. All do the same damage for the same deposited energy.a. The fission reaction associated with uranium-235.6. The fuel necessary for fusion-produced energy could be derived from
b. The fusion reaction of the Sun (two hydrogen nuclei fused to
one helium nucleus).
c. Both (A) and (B) are about the same.
d. Need more information.
a. Water. d. Superconductors.
b. Uranium. e. Helium.
c. Sunlight.B. Structured questions
7. If the equipment isn’t working and my treatment is delayed or
postponed, who checks that it is safe to use again? And will this delay
affect my cancer?
8. Do you have weekly chart rounds where you review patient-related
information in peer review?
9. Will you take imaging scans regularly during my treatment to verify
position of my treatment? Who reviews those scans?
10. People who work around metals that emit alpha particles are trained
that there is little danger from proximity or touching the material, but
they must take extreme precautions against ingesting it. Why? (Eating
and drinking while working are forbidden.)
11. What is the difference between absorbed dose and effective dose? What
are the SI units for each?
12. Radiation is sometimes used to sterilize medical supplies and even food.
Explain how it works.
13. How might radioactive tracers be used to find a leak in a pipe?
14. Explain that there are situations in which we may or may not have
control over our exposure to ionizing radiation.a. When do we not have control over our exposure to radiation?15. Does exposure to heavy ions at the level that would occur during
b. When do we have control over our exposure to radiation?
c. Why might we want to limit our exposure to radiation when
possible?
deep space missions of long duration pose a risk to the integrity and function
of the central nervous system?
16. Radiation protection of ionizing radiation from radiation sources is
particularly difficult. Give a reason for this difficulty.
C. Essay questions17. I always lock my radioactive material-use rooms. However, renovators
came in during the weekend, worked, and left the door open while they
were on their lunch break. Am I responsible and how can I prevent this
from happening? Debate on the situation above to support your answer.
18. How can I ensure that personnel who work in my lab, but do not use
radioactive material, do not violate the security requirements? Debate
to support your idea.
19. A Housekeeping staff member opens my radioactive material-use room
after working hours and does not lock it when they leave. What should
I do? Explain clearly to support your idea.
20. Make a research and predict what steps that can or might be taken to
reduce the exposure to radiation (consider if living near a radioactive
area like an abandoned uranium mine, if finding a radioactive source, orin the event of a nuclear explosion or accident).
BIBLIOGRAPHY
eschooltoday. (2008-2018). Retrieved February 19, 2018, from natural diseasters: http
eschooltoday. (2008-2018). Retrieved February 19, 2018, from Climate change: http
http://www.threastafrican.co.ke. (2017). Retrieved from rwanda/Business/kigali.
Abbot, A. F., & Cockcroft, J. (1989). Physics (5 ed.). Heinemann: Educational Publishers.
Atkins, K. R. ( 1972). Physics-Once over Lightly. New York : New York.
Avison, J. (1989). The world of PHYSICS. Cheltenham: Thomas Nelson and Sons Ltd.
AVISON, J. (1989). The world of PHYSICS. Cheltenham: Thomas Nelson and Sons Ltd.
BIPM. (2006). The International System of Units (SI). (8 ed.). Sevres, France: International
Bureau of Weights and Measures.
Breithaupt, J. (2000). Understanding Physics For Advanced Level. (4 ed.). Ellenborough
House, Italy: Stanley Thorners.
Chand, S., & S.N., G. S. (2003). Atomic Physics (Modern Physics) (1 ed.). India.
CPMD. (2015). Advanced Level Physics Sylabus. Kigali: REB.
Cunningham, & William, P. (2000). Environmental science (6 ed.). Mc Graw-Hill.
Cutnell, J. D., & Johnson, K. W. (2006). Essentials of Physics. USA: John Wlley &Sons, Inc.
Cutnell, J. D., & Johnson, K. W. (2007). Physics. (7 ed.). USA: John Wiley; Sons, Inc.
Cuttnell, J. D., & kennety, W. J. (2007). Physics (7 ed.). United State of America: John
Willey & Sons . Inc.
Douglass, C. G. (2014). PHYSICS, Principles with applications. (7 ed.). Pearson Education.
Douglass, C. G. (2014). PHYSICS, Principles with applications. (8 ed.). Pearson Education.
Duncan, T., & Kennett, H. (2000). Advanced Physics (5 ed.). London, UK: Holder
Education.
Giancoli, D. (2005). PHYSICS: Principles with applications. New Jersey: Pearson
Education, Inc.
Giancoli, D. C. (2005). Physics principals with application. Upper Saddle River, NJ 07458:
Pearson Education, Inc.
Giancoli, D. C. (2005). Physics: principals with application. Upper Saddle River, NJ
07458: Pearson Education, Inc.
Giancoli, D. C. (2005). Physics: Principles with applications. New Jersey: Pearson
Education, Inc.
Glencoe. (2005). Physics - Principles and Problems [textbook]. McGraw.
Haber-Schaim, U., Cutting, R., Kirkesey, H. G., & Pratt, H. A. (2002). Force, Motion, and
Energy. USA: Science Curriculum, Inc.
Halliday, D., Resneck, R., & Walker, J. (2014). Fundamentals of Physics. (10 ed.). USA:
John Wiley; Sons,Inc.
Halliday, Resneck, & Walker. (2007). Fundamentals of Physics. (8 ed.). Wiley.
Hewitt, P. G., SUCH0CKI, J., & Hewitt, L. A. (1999). Conceptual Physical Science. (2 ed.).
Addison Wesley Longman.
Hirsch, A. S. (2002). Nelson Physics 12. Toronto: University Preparation.
Hugh, D. Y., & Roger, A. F. (2012). University Physics with Modern Physics (13 ed.). San
Francisco, USA: Pearson Education, Inc.
IPCC. (1996). Economics of Greenhouse Gas limitation, Main report “Methodological
Guidelines.
John, M. (2009). Optical Fiber Communications, Principals and Practice (3rd Ed.).
London: Pearsnon Prentice Hall.
Jones, E. R., & Childers, R. L. (1992). Contemporary College Physics. (2 ed.). USA:
Addison-Wesley Publishing Company.
Kansiime, J. K. (2004). Coumpound Physical Geography: Morphology, Climatology, Soils
and Vegetation. uganda.
Linda, W. (2004). Earth Sceience demystified a self-teaching guide. USA: McGraw-Hill
Campanies, inc.
Michael, E. B. (1999). Schaum’s outline of Theory and Problems of Physics for Engineering
and Science. USA: McGRAW-HILL Companies, Inc.
Michael, J. P., Loannis, M., & Martha, C. (2006). Science Explorer, Florida Comprehensive
Science. Boston: Pearson Prentice Hall.
MIDIMAR. (2012). Disaster High Risk Zones on Floods and Landslide. Kigali: MIDMAR.
Nagashima, Y. (2013). Elementary Particle Physics. Osaka University: Deutsche
Nationalbibliothek.
Nelkon, M., & Parker, P. (1997). Advanced level Physics. (7 ed.). Edinburgh: Heinemann.
Nelkon, M., & Parker, P. (2001). Advanced Level Physics (7 ed.). Edinburgh gate:
Heinemann.
Office, U. M. (2011). Warming: A guide to climate change. U.K.: Met Office Hadley Centre.
Orazio, S. (2010). Principles of Lasers (5 ed.). Milan, Italy: Springer.
Patrick, T. (2004). Mathematics standard level. (3 ed.). Victoria: Ibid Press.
R.B., B. (1984). Physical Geography in diagrams for Africa. Malaysia: Longmann Group
Limited.
Randall, D., & Knight. (2004). Physics for scientists and engineers: Stategic approach
(Vol. 2). San Fransisco: Pearson Education.
Randall, D., & Knight. (2004). Physics for scientists and engineers: Stategic approach.
(Vol. 3). San Fransisco: Pearson Education, Inc.
Randall, D., & Knight. (2008). Physics for scientists and engineers: Stategic approach. (2
ed., Vol. 3). San Fransisco: Pearson Education, Inc.
Randall, D., & Knight. (2008). Physics for scientists and engineers: Stategic approach. (2
ed., Vol. 3). San Fransisco: Pearson Education, Inc.
REMA. (n.d.). Rwanda Second National Communication Under the UNFCCC.
KIGALI: MINISTRY OF NATURAL RESOURCES,RWANDA.
Science, G. (2006). Florida Physical Science with Earth Science. USA: Mc Graw
Hill Glencoe Companies, Inc.
Serway, R. A. (1986). Physics for Scientists and Engineers (2 ed.). Saunders
College Publishing.
Serway, R. A. (1992). Principles of Physics. Orlando, Florida: Saunders College Publishing.
Serway, R. A., & Jewett, J. J. (2008). Physics for Scientists and Engineers. (7 ed.). USA:
Thomson Learning, Inc.
Serway, R. A., & Jewett, J. J. (2010). Physics for Scientists and Engineers with Modern
Physics. (8 ed.).
Silver, B., & Ginn, I. (1990). Physical Science. Unit States of America.
Stephen, P., & Whitehead, P. (1996). Physics. (2 ed.). School Edition.
Stephen, P., & Whitehead, P. (1996). Physics. (2 ed.). School Edition.
Strassler, M. (2011, September 25). What’s a Proton, Anyway? Retrieved March 05,
2018, from www.profmattstrassler.com: https://profmattstrassler.com/articles-andposts/largehadroncolliderfaq/whats-a-proton-anyway/
Subranya, K. (1993). Theory and applications of fluid mechanics. Tata McGraw: Hill
Companies.
Taylor, E., & Wheeler, J. A. (1992). Spacetime Physics: Introduction to Special Relativity.
(2 ed.). San Francisco: W.H.Freeman & Company, Publishers.
Taylor, E., & Wheeler, J. A. (1992). Spacetime Physics: Introduction to Special Relativity.
(2 ed.). San Francisco: W.H.Freeman & Company, Publishers.
Tipler, P. A. (1991). Physics for Scientists and Enginners. (3 ed., Vol. 2). USA: Worth
Publishers, Inc.
Tipler, P. A. (1991). Physics for Scientists and Enginners. (3 ed., Vol. 1). USA: Worth
Publishers, Inc.
Tom, D. (2000). Advanced Physics (5 ed.). H. Kennett.
Toyal, D. C. (2008). Nuclear Physics (5 ed.). Himalaya Publishing House.
Uichiro, M. (2001). Introduction to the electron theory of metals . Cambridge University
Press .
Weseda, Y., Mastubara, E., & Shinoda, K. (2011). X-rays properties-google search.
Retrieved 03 06, 2018, from www.springer.com: http://www.springe.com/978-3-
642-26634-1
Wysession, M., Frank, D., & Yancopoulos, S. (2004). Physical Science. Boston,
Massachusetts, Upper Saddle River, New Jersey: Pearson Prentice Hall.
