UNIT 6 CELLULAR RESPIRATION
UNIT 6: CELLULAR RESPIRATION
Key Unit Competence
To be able to describe the process of cellular respiration
Learning objectives
By the end of this unit, I should be able to:
– Outline the four stages in aerobic respiration (glycolysis, link reaction, TCA cycle
and oxidative phosphorylation) and state where each occurs in the eukaryotic
cells.
– Explain that when oxygen is available, pyruvate is converted into acetyl
coenzyme A, which then combines with oxaloacetate (4C) to form citrate (6C).
– Explain that reactions in the TCA cycle involve decarboxylation and
dehydrogenation and the reduction of NAD and FAD.
– Outline the process of oxidative phosphorylation including the role of oxygen
(details of the carriers are not required).
– Describe the relationship between the structure and function of the
mitochondrion.
– Explain the production of a small yield of ATP from anaerobic respiration in
yeast and mammalian muscle tissue, including the concept of oxygen debt.– Explain how other substrates are involved in glycolysis and the TCA cycle.
Introductory activity
Use of books from your library and search further information on the internet
and answer the following questions. The person in the picture below is usingenergy.
1. Where is the energy used by the person in the picture coming from?
2. All living organisms need a continuous supply of energy. Explain why.
3. Identify the processes exhibited by the person on the picture that
consume too much energy if compared with another one who is at rest.4. How is the energy produced in our body?
6.1 Overview of respiration process
6.1.1 Respiration
Activity 6.1.1
With the help of textbooks and simulations of the process of respiration,
answer the questions that follow:
1. Differentiate between glucose and pyruvate.2. What is the role of glycolysis?
Cellular respiration is the complex process in which cells make adenosine
triphosphate (ATP) by breaking down organic molecules. The energy stored in
ATP can then be used to drive processes requiring energy, including biosynthesis,
locomotion or transportation of molecules across cell membranes. The main fuel
for most cells is carbohydrate, usually glucose which is used by most of the cells as
respiratory substrate. Some other cells are able to break down fatty acids, glycerol
and amino acids.
Glucose breakdown can be divided into four stages: glycolysis, the link reaction, theKrebs cycle and oxidative phosphorylation.
6.1.2 Glycolysis
Activity 6.1.2
With the help of textbooks and simulations from internet / YouTube observe
the process of respiration, answer the questions that follow:
1. Observe and note the stages of the process of respiration.2. Draw the structure of a glucose molecule.
Glycolysis is the splitting or lysis of a glucose molecule. It is a multi-step process
in which a glucose molecule with six carbon atoms is eventually split into two
molecules of pyruvate, each with three carbon atoms. Energy from ATP is needed in
the first steps, and it is released in the later steps to synthesize ATP. There is a net gain
of two ATP molecules per molecule of glucose broken down.
Glycolysis takes place in the cytoplasm of a cell. Glucose enters the cell and is
phosphorylated by the enzyme called hexokinase, which transfers a phosphate
group from ATP to the sugar. The ATP used in this process has 2 advantages: the
charge of the phosphate group traps the sugar in the cell because the plasma
membrane is impermeable to large ions. Phosphorylation also makes glucose morechemically reactive. Even though glycolysis consumes two ATP molecules,
It produces a gross of four ATP molecules (4 ATP), and a net gain of two ATP (2 ATP)
molecules for each glucose molecule that is oxidized. Glycolysis results in a net gainof two ATP, two NADH and two pyruvate molecules.
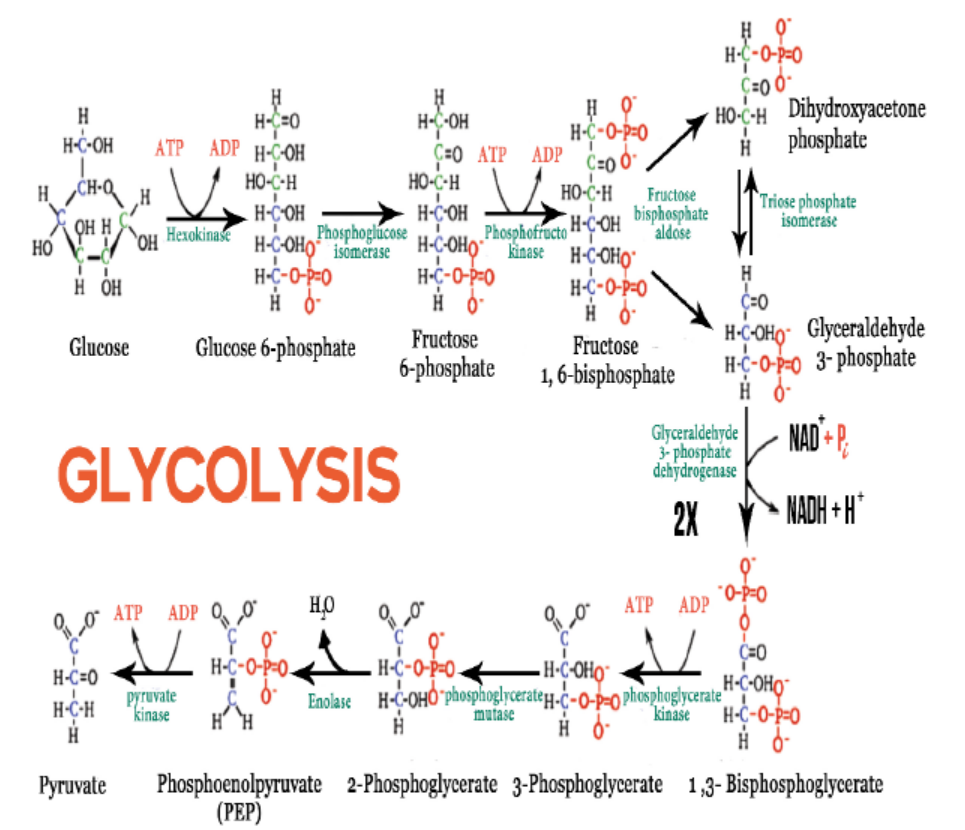
Figure 6.1: Reactions of glycolysis
Applicatioin 6.1
1. Why is ATP needed for glycolysis?
2. How many gross ATP molecules are produced during glycolysis of one
glucose molecule?3. How many NADH are made during glycolysis?
6.2 Link reaction and the Krebs cycle
Activity 6.2
Use the books from the school library and search further information on the
internet. Then:
1. Observe and write the number of carbon atoms in an acetyl-coA molecule.
2. Use the chemical equation to show the conversion of pyruvate into acetyl-
coA.
3. Observe and note the main products of the Krebs cycle from one glucosemolecule
6.2.1 Link reaction
Pyruvate, the end product of glycolysis is oxidized to Acetyl-CoA by enzymes located
in the mitochondrion of eukaryotic cells as well as in the cytoplasm of prokaryotes.
In the conversion of pyruvate to Acetyl-CoA, one molecule of NADH and one
molecule of CO2 are formed (Figure 6.2). This step is also known as the link reactionor transition step, as it links glycolysis to the Krebs cycle.
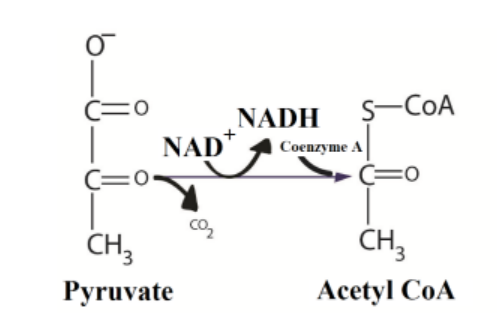
Figure 6.2: Link reaction between glycolysis and Krebs cycle
6.2.2 The Krebs cycle (Citric acid cycle)
The coenzyme has a sulphur atom, which attaches the acetyl fragment by an
unstable bond. This activates the acetyl group for the first reaction of the Krebs cycle
also called citric acid cycle or Tricarboxylic Acid Cycle (TCA). It is also known as the
citric acid cycle, because the first molecule formed when an acetyl group joins the
cycle. When oxygen is present, the mitochondria will undergo aerobic respirationwhich leads to the Krebs cycle.
In the presence of oxygen, when acetyl-CoA is produced, the molecule then enters
the citric acid cycle inside the mitochondrial matrix, and gets oxidized to CO2 while
at the same time reducing NAD+ to NADH. NADH can then be used by the electron
transport chain to create more ATP as part of oxidative phosphorylation. For the
complete oxidation of one glucose molecule, two Acetyl-CoA must be metabolized
by the Krebs cycle. Two waste products namely H2O and CO2, are released duringthis cycle.
The citric acid cycle is an 8-step process involving different enzymes and co-enzymes.
Throughout the entire cycle, Acetyl-CoA (2 carbons) combines with oxaloacetate (4
carbons) to produce citrate. Citrate (6 carbons) is rearranged to a more reactive form
called iso citrate (6 carbons). Iso citrate (6 carbons) is modified to α-Ketoglutarate (5
carbons), Succinyl-CoA, Succinate, Fumarate, Malate, and finally to Oxaloacetate. The
net energy gain from one cycle is 3 NADH, 1 FADH2, and 1 Guanosine Triphosphate
(GTP). The GTP may subsequently be used to produce ATP. Thus, the total energy
yield from one whole glucose molecule (2 pyruvate molecules) is 6 NADH, 2 FADH2,
and 2 ATP. 2 molecules of carbon dioxide are also produced in one cycle (for a totalof 4 molecules of carbon dioxide from one glucose molecule).
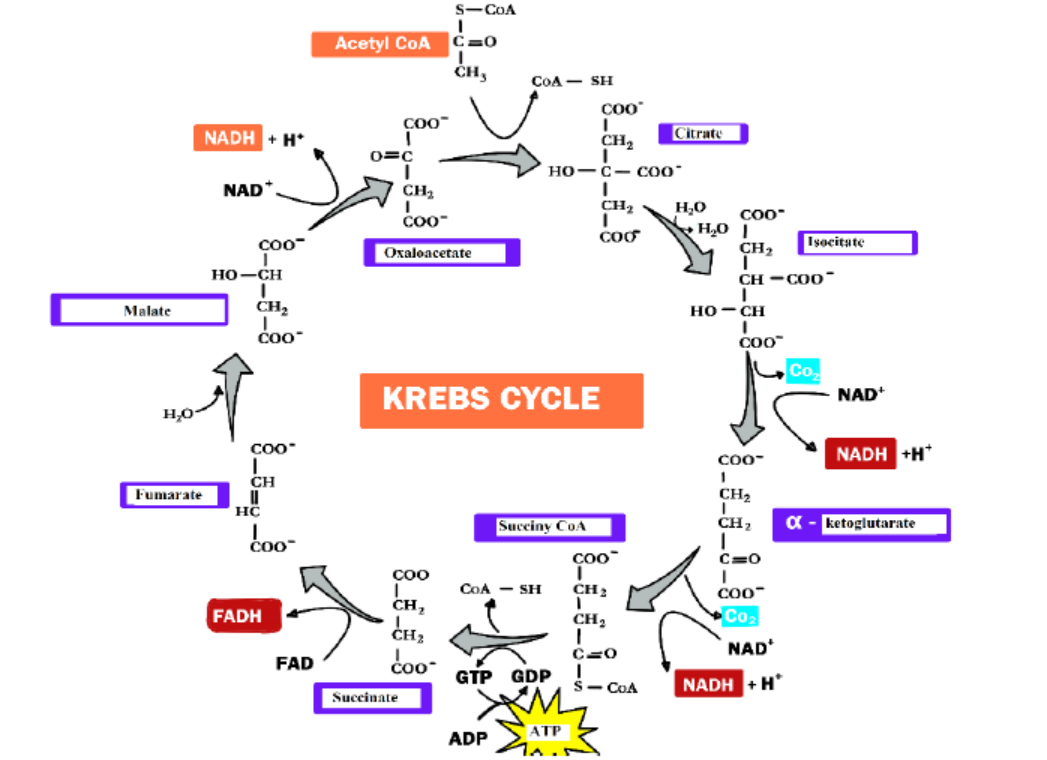
Figure 6.3: The Krebs cycle
Application 6.2
1. In which part of the cell does the Krebs cycle take place?
2. How many ATP molecules are generated by each revolution of the Krebs
cycle?3. Which six carbon sugar is formed in the first reaction of the Krebs cycle?
6.3 Oxidative phosphorylation and electron transport chain
Activity 6.3
Download and watch a movie of the electron transport chain from internet /
you tube. Make a simulation of it in the following way.
– In a line, move warm stones from one area to another.
– Take the first stone and passes it to the second up to the last one.
– The last one will have a bucket where the last stone is thrown.
– Compare what we’re doing to what you watched in the movie (carriers of
electrons)
Write short notes and share information on how the electron transport chaintakes place.
In the final stage of aerobic respiration known as the oxidative phosphorylation,
the energy for the phosphorylation of ADP to ATP comes from the activity of the
electron transport chain. Oxidative Phosphorylation is the production of ATP using
energy derived from the redox reactions of an electron transport chain.
In eukaryotes, oxidative phosphorylation occurs in the mitochondrial cristae. It
comprises the electron transport chain that establishes a proton gradient across
the inner membrane by oxidizing the NADH produced from the Krebs cycle. ATP is
synthesized by the ATP synthase enzyme when the chemiosmotic gradient is used to
drive the phosphorylation of ADP. Chemiosmosis is the production of ATP from ADP
using the energy of hydrogen ion gradients. The electrons are finally transferred to
oxygen and, with the addition of two protons, water is formed. The average ATP yield
per NADH is probably 3 and for FADH2 of this electron carrier is worth a maximum ofonly two molecules of ATP.
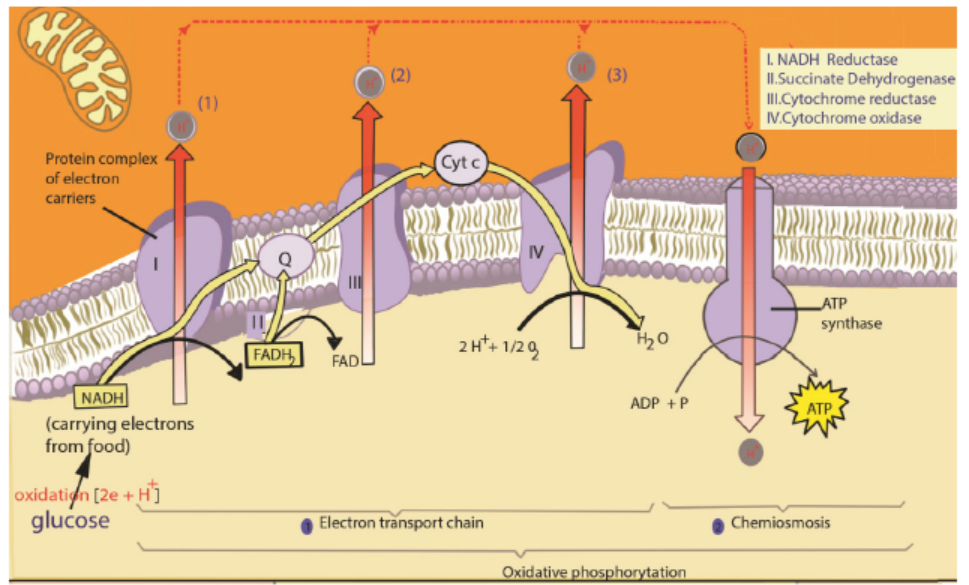
Figure 6.4: The electron transport chain
The role of oxygen in chemiosmosis
ATP can be synthesized by chemiosmosis only if electrons continue to move from
molecule to molecule in the electron transport chain. Oxygen serves as the final
acceptor of electrons. By accepting electrons from the last molecule in the electron
transport chain, and allows additional electrons to pass along the chain. As a result,
ATP can continue to be synthesized. Oxygen also accepts the protons that were once
part of the hydrogen atoms supplied by NADH and FAD2. By combining with bothelectrons and protons, oxygen forms water as shown in the following equation:
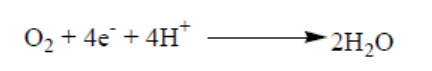
Overview of cellular respiration
A considearable number of ATP is produced during oxidative phosphorylmation
and it is estimated between 32 and 34 ATPs. These are added to 2 ATP produced
during glycolysis and 2 ATP produced during citric cycle. The total number of ATP
produced during a complete respiration process for one molecule of glucose is thenestimated between 36 and 38 ATPs.
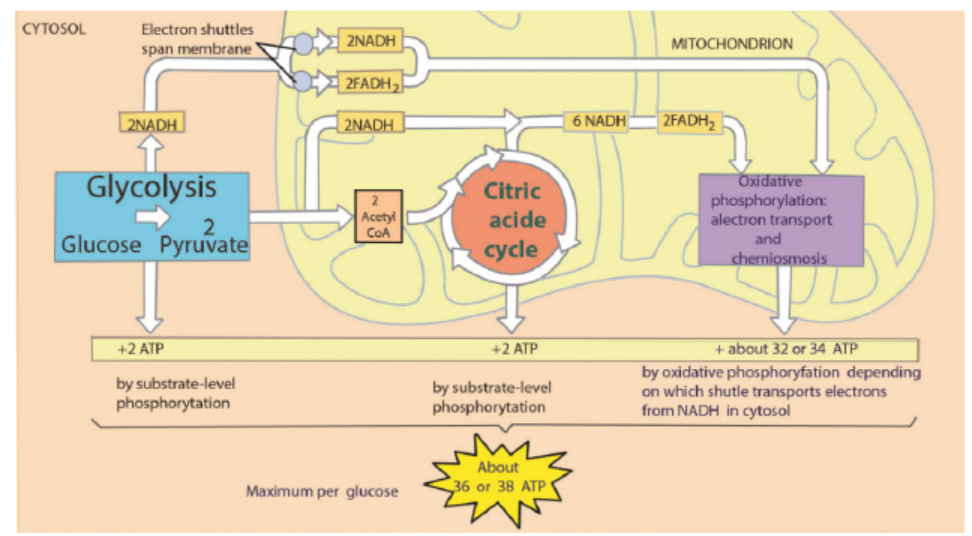
Figure 6.5: Overview of cellular respiration
Note that the amount of ATP produced from glucose is usually less than 38 ATP for
the following reasons: some ATP is used to transport pyruvate from the cytoplasm
into the mitochondria and some energy is used to transport NADH produced inglycolysis from the cytoplasm into the cristae of mitochondria.
Application 6.3
1. What is the importance of NADH and FADH?
2. How many ATP are formed from 1 NADH?
3. How many ATP are formed from 1 FADH?
4. How many ATP are formed after a complete oxidation of one glucosemolecule?
6.4 Efficiency of aerobic and anaerobic respiration
Activity 6.4
Visit a nearby bakery and observe how bread is made and answer to the
following questions. Use also books, internet and prior knowledge from
chemistry.
1. On a sheet of paper write down the ingredients used to manufacture
bread
2. Which ingredients make the bread rise?
3. What do you understand by anaerobic respiration?
4. State the examples of the applications of anaerobic respiration in
everyday life?
5. Give a table comparing aerobic to anaerobic respiration
6. How can the efficiency of anaerobic and aerobic respiration be
calculated from one glucose molecule?
7. Between aerobic and anaerobic respiration, which one do you think ismore efficient? and why?
Without oxygen, pyruvate (pyruvic acid) is not metabolized by cellular respiration
but undergoes a process of fermentation. The pyruvate is not transported into
the mitochondrion, but remains in the cytoplasm, where it is converted to waste
products that may be removed from the cell. This serves the purpose of oxidizing the
electron carriers so that they can perform glycolysis again and removing the excess
pyruvate. Fermentation oxidizes NADH to NAD+ so it can be re-used in glycolysis.
In the absence of oxygen, fermentation prevents the build-up of NADH in the
cytoplasm and provides NAD+ for glycolysis. This waste product varies depending
on the organism. In skeletal muscles, the waste product is lactic acid. This type
of fermentation is called lactic acid fermentation. In yeast and plants, the waste
products are ethanol and carbon dioxide. This type of fermentation is known as
alcoholic or ethanol fermentation. The ATP generated in this process is made bysubstrate-level phosphorylation, which does not require oxygen.
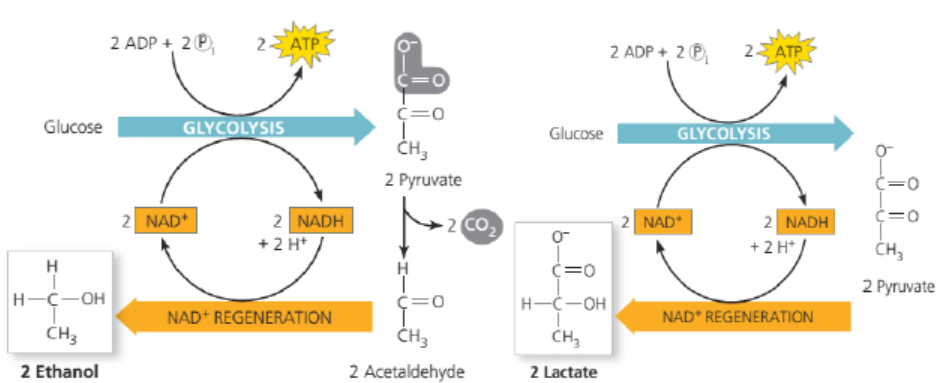
Figure 6.6: Alcoholic and lactic fermentation
Fermentation is less efficient at using the energy from glucose since only 2 ATP are
produced per glucose, compared to the 38 ATP per glucose produced by aerobic
respiration. This is because the waste products of fermentation still contain plentyof energy. Glycolytic ATP, however, is created more quickly.
a. Applications of anaerobic respiration
Some food products and drinks are produced by using anaerobic microorganisms:
– Production of beer
– Production of wine
– Production of yoghurt
– Production of cheese– Production of bread
b. Efficiency of aerobic and anaerobic respiration
The complete oxidation of glucose produces the energy estimated at 686 Kcal.
Under the condition that exists inside most of the cells, the production of a
standard amount of ATP from ADP absorbs about 7.3 Kcal. Glucose molecule can
generate up to 38 ATP molecules in aerobic respiration. The efficiency of aerobicrespiration (EAER) is calculated as follows:
This result indicates that the efficiency of aerobic respiration equals 40%. The remain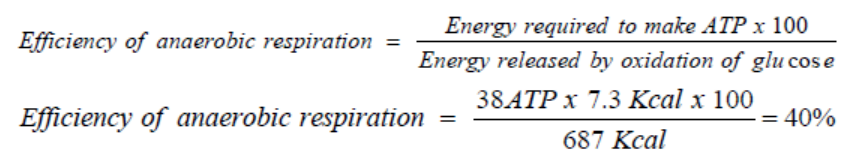
of the energy (around 60%) is lost from the cell as heat.
Due to the fact that anaerobic respiration produces only 2 ATP, the efficiency ofanaerobic respiration is less than that of aerobic respiration. It is calculated as follows:
c. Oxygen debt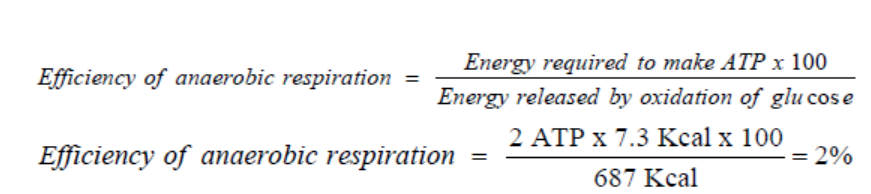
Standing still, the person absorbs oxygen at the resting rate of 0.2 dm3 min−1. (This
is a measure of the person’s metabolic rate.) When exercise begins, more oxygen is
needed to support aerobic respiration in the person’s muscles, increasing the overall
demand to 2.5 dm3 min−1. However, it takes four minutes for the heart and lungs to
meet this demand, and during this time lactic fermentation occurs in the muscles.
Thus the person b-uilds up an oxygen deficit. For the next three minutes, enough
oxygen is supplied. When exercise stops, the person continues to breathe deeply
and absorb oxygen at a higher rate than when at rest. This post-exercise uptake of
extra oxygen, which is ‘paying back’ the oxygen deficit, is called the oxygen debt.
The oxygen is needed for:
– Conversion of lactate to glycogen in the liver
– Re oxygenation of haemoglobin in the blood
– A high metabolic rate, as many organs are operating at above resting levels.
The presence of the lactic acid is sometimes described as an ‘ oxygen debt’. This is
because significant quantities of lactic acid can only be removed reasonably quickly
by combining with oxygen. However, the lactic acid was only formed due to lack
of sufficient oxygen to release the required energy to the muscle tissue via aerobic
respiration. Lactic acid can accumulate in muscle tissue that continues to be overworked.
Eventually, so much lactic acid can build-up that the muscle ceases working
until the oxygen supply that it needs has been replenished.
To repay such an oxygen debt, the body must take in more oxygen in order to get ridof the additional unwanted waste product lactic acid.
d. Muscle cramps
A muscle cramp is an involuntarily and forcibly contracted muscle that does not
relax. Muscle cramps can occur in any muscle; cramps of the leg muscles and feet
are particularly common.
Almost everyone experiences a muscle cramp at some time in their life. There are
a variety of types and causes of muscle cramps. Muscle cramps may occur during
exercise, at rest, or at night, depending upon the exact cause.
Overuse of a muscle, dehydration, muscle strain or simply holding a position for a
prolonged period can cause a muscle cramp. In many cases, however, the cause isn’t
known.
Although most muscle cramps are harmless, some may be related to an underlying
medical condition, such as:
– Inadequate blood supply. Narrowing of the arteries that deliver blood to your
legs (arteriosclerosis of the extremities) can produce cramp-like pain in your
legs and feet while you’re exercising. These cramps usually go away soon after
you stop exercising.
– Nerve compression. Compression of nerves in your spine (lumbar stenosis) also
can produce cramp-like pain in your legs. The pain usually worsens the longer
you walk. Walking in a slightly flexed position such as you would use when
pushing a shopping cart ahead of you may improve or delay the onset of your
symptoms.
– Mineral depletion. Too little potassium, calcium or magnesium in your diet can
contribute to leg cramps. Diuretics or medications often prescribed for highblood pressure also can deplete these minerals.
Application 6.4
1. What is the product of anaerobic respiration in animal cells?
2. Under which conditions can anaerobic respiration take place in animal
cells?
3. Calculate the efficiency of anaerobic and aerobic respiration, when
a complete oxidation of glucose produce the energy estimated at
500Kcal under a production of a standard amount of ATP from ADPabsorbed is about 7.3 Kcal.
6.5 Factors affecting the rate of respiration
Activity 6.5
Observe carefully the pictures below and answer the questions that follow;
1. Make a short report on the respiration rate of the person on the picture A
and that of the person on the picture B.
2. Which one between person A and that of person B has a high respiration
rate?
3. What are factors could show that the respiration rate has increased in theperson on the picture A above?
Cellular respiration is the process of conversion of chemical energy stored in the food
to ATP or higher energy compounds. The factors that affect the cellular respirationare:
a. Amount of nutrients
If the amount of nutrients is high, then the energy is high in the cellular respiration.
The nutrients which can go through cellular respiration and transform into energy
are fat, proteins and carbohydrates. The amount of nutrients available to transform
into energy depend upon the diet of the person.
b. Temperature
The rate of the cellular respiration increases if the body temperature is warmer. The
lower the temperature, the slower the rate of cellular respiration. The reason for
this is enzymes which are present in cellular respiration process. Enzyme reactions
require optimum temperatures.
c. State of the cell
Metabolically active cells such as neurons, root of human hair have higher
respiration rate than the dormant cells such as skin cells and bone cells. This is
because metabolically active cells can store energy in the body because of the many
metabolic reactions that take place in them.
d. Water
It is the medium where the reaction happens. When a cell is dehydrated the
respiration and other metabolism decreases.
e. Cellular activity
Some cells need more energy than others. For example, growing cells or very active
cells such as neurons need a lot of energy.
f. O2 /CO2 content
Higher O2 and lower CO2 make higher respiration rates.
g. ATP/ADP range
When there is more ATP than ADP, respiration rate slows down to avoid excess ofATP
Application 6.5
1. Which cells in the human body have a high respiration rate?2. Explain how the temperature affects the rate of respiration.
6.6 Use of other substrates in respiration
Activity 6.6
When one has eaten carbohydrates such as cassava and sweet potatoes you
do not feel hungry in the same time as another one who has consumed milk
or cheese.
1. Can you suggest the reason for this?2. Which one can take a short time for digestion and why?
Carbohydrates are the first nutrients that most organisms can catabolise for energy.
In some cases, living things must be able to metabolize other energy-rich nutrients
to obtain energy in times of starvation. Most organisms possess metabolic pathways
that, when necessary, metabolize proteins, lipids. In each case, the larger molecules
are first digested into their component parts, which the cell may reassemble into
macromolecules for its own use. Otherwise, they may be metabolized for energy byfeeding into various parts of glycolysis or the Krebs cycle.
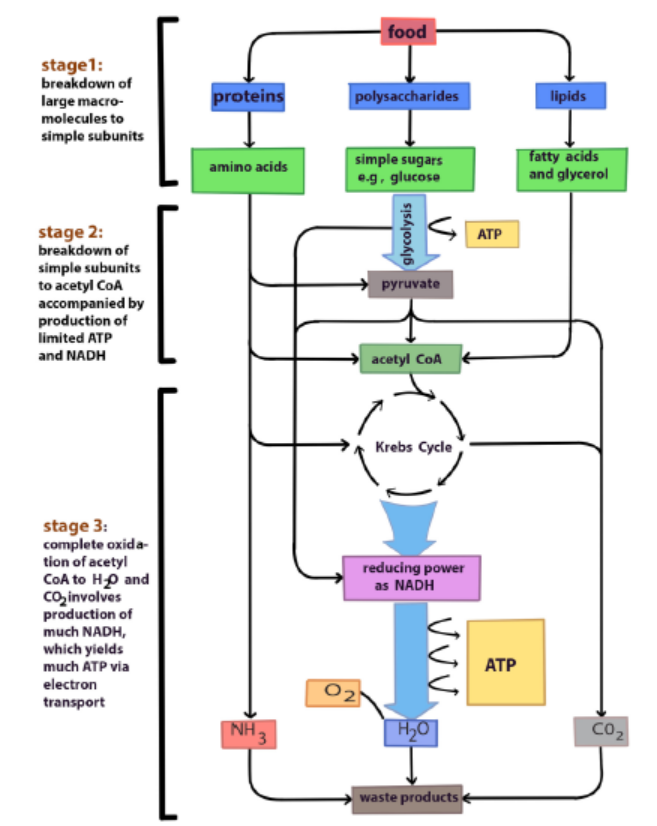
Figure 6.8: Oxidation of different organic substrates
Carbohydrates, fats and proteins can all be used for cellular respiration. Monomers
of these foods enter glycolysis or the Krebs cycle at various points. Glycolysis and the
Krebs cycle are catabolic pathways through which all kinds of food molecules arechannelled to oxygen as their final acceptor of electrons.
Application 6.6
1. Explain how proteins and lipids are metabolized for energy during
respiration
2. Explain why the body does not use fats to produce energy ascarbohydrates given that they produce much energy than carbohydrates.
End of unit assessment 6
Multiple choice questions: from question 1 to 7, choose the letter corresponding
to the best answer.
1. Before the Krebs cycle can proceed, pyruvic acid must be converted into
a. Citric acid
b. Glucose
c. Acetyl-CoA
d. Glucose
e. NADH
2. The net number of ATP made directly by glycolysis is
a. 2
b. 4
c. 32
d. 38
3. Cellular respiration is similar to photosynthesis in that they both
a. Produce ATP
b. Involve chemiosmosis
c. Make phosphoglyceraldehyde (PGAL)
d. All of the above
4. By accepting electrons and protons, the oxygen used in aerobic respiration
turns into
a. CO2
b. H2O
c. C6H12O6
d. ATP
5. The Krebs cycle occurs in the
a. Cytosol
b. Outer mitochondrial membrane
c. Mitochondrial matrix
d. Space between the inner and outer mitochondrial membrane
6. During each turn of the Krebs cycle,
a. Two CO2 molecules are produced
b. Two ATP molecules are consumed
c. Pyruvic acid combines with oxaloacetic acid
d. Glucose combines with a four-carbon molecule.
7. Most of the ATP synthesized in aerobic respiration is made
a. During glycolysis
b. Through fermentation
c. In the cytosold. Through chemiosmosis
Structured answer questions
8. What are the major differences between cellular respiration and
photosynthesis?
9. Compare aerobic respiration with anaerobic respiration or fermentation.
10. A student set up an experiment using germinating seeds and boiled seedsas shown in the diagram below:
a. State the objective of this experiment and the observation made after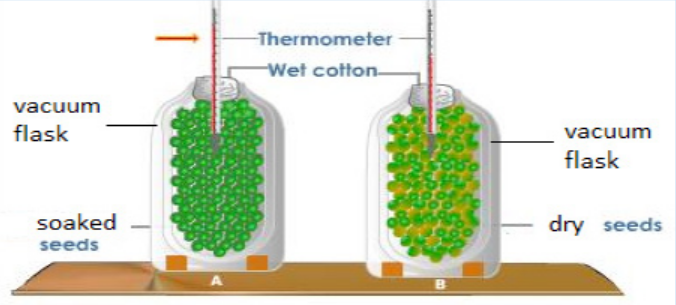
24 hours?
b. Account for the observation made in (a) above?
c. Suggest why vacuum flasks were used in the experiment?d. What was the purpose of the set-up B?
