UNIT 5 ENERGY FROM RESPIRATION
UNIT 5: ENERGY FROM RESPIRATION
Key Unity Competence
Describe the structure and importance of ATP, outline the roles of the coenzymes
NAD, FAD and coenzyme A during cellular respiration.
Learning objectives
– Discuss the need for energy in living organisms as illustrated by anabolic
reactions, active transport, and the movement and maintenance of body
temperature.
– Describe the structure of ATP as a phosphorylated nucleotide formed by
condensation reaction.
– Explain that ATP is synthesized in substrate-linked reactions in glycolysis and in
Krebs (tri-carboxylic acid [TCA] cycle.
– Explain the relative energy value of carbohydrate, lipid and protein as respiratory
substrate and explain why lipids are particularly energy-rich.
– Define the term Respiratory Quotient (RQ) as the ratio of the volume of CO2,
evolved to the volume of O2 uptake during aerobic respiration.
– Design simple experiments using respirometers to determine the RQ of
germinating seeds or small invertebrates. Example: woodlice.
– Calculate RQ values from the equations of respiration of different substrates.– Interpret graphs for varying RQ values during seed germination.
Introductory activity
From your daily experience, brainstorm the following questions.
1. What do you understand about energy used by living organisms?
2. Where is that energy obtained from?3. How is that energy obtained from the source you have mentioned?
This unit deals with the energy from respiration. It focuses on the description of the
structure and importance of adenosine triphosphate (ATP), and outline the roles of
the coenzymes including nicotinamide adenine dinucleotide (NAD), flavin adenine
dinucleotide (FAD) and coenzyme A CoA during cellular respiration. Specifically,
this unit contributes to a better understanding of the reasons why organisms need
energy, the structure of adenosine triphosphate (ATP), synthesis and breakdown of
ATP, respiratory substrates and their relative energy values, and measurement ofrespiration and respiratory quotient.
5.1 Need for energy by organisms
Activity 5.1
Use books from the school library and search further information about
metabolism reactions on the internet. Read the information and discuss thereasons why all living organisms need energy.
Chemical energy is the most important type of energy potential for life, where energy
is either released out or consumed through metabolism reactions. Metabolism
reactions constitute the sum of all chemical reactions taking place in a living cell.
The biological process by which metabolic pathways breakdown molecules into
smaller units that are either oxidized to release energy is called catabolism, while
the biological process by which a set of metabolic pathways construct molecules
from smaller units through reactions consuming energy is called anabolism. During
catabolism reactions, energy is released to the surrounding environments. These
are exergonic reactions. During anabolism reactions, energy is absorbed from thesurrounding environment. These are endergonic reactions.
All living organisms need energy to grow and reproduce, maintain their structures,
and respond to their environments. Metabolism reactions are the set of life-sustaining
chemical processes that enables organisms to transform the chemical energy stored
in molecules into energy that can be used for cellular processes. Animals consume
food to replenish energy. Their metabolism breaks down the carbohydrates, lipids,
proteins, and nucleic acids to provide chemical energy for these processes. Plants
convert light energy from the sun into chemical energy stored in molecules during
the process of photosynthesis.
Active transport of solutes such as sodium (Na+), potassium (K+) magnesium (Mg+),
calcium (Ca+) and chloride (Cl-)across the plasma membrane cannot be possible
without the use of energy. The transport proteins that move solutes against their
concentration gradients are all carrier proteins rather than channel proteins.
Active transport enables a cell to maintain internal concentrations of small solutes
that differ from concentrations in its environment. Some transport proteins act
as pumps, moving substances across a membrane against their concentration or
electrochemical gradients. Energy is usually supplied by adenosine triphosphate(ATP) hydrolysis (Figure 1).
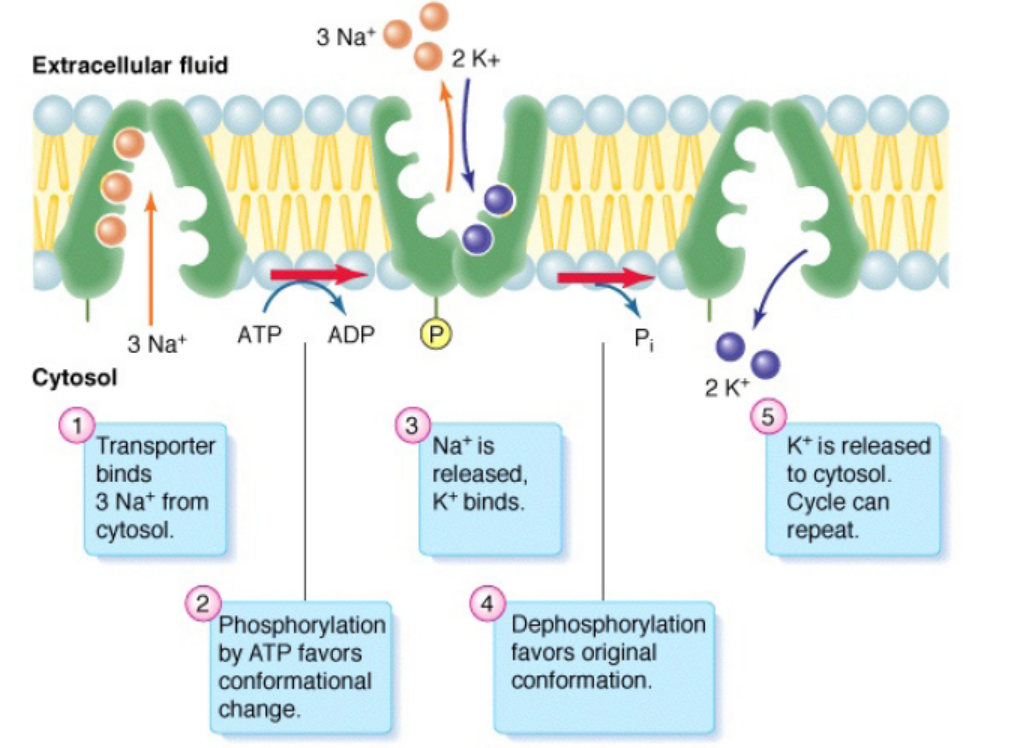
Figure 5.1: Active transport of chemical ions/anions across the cell membrane
Application 5.1
1. What is energy?
2. What is it used for?
3. What is the major source of energy for organisms?
4. What would happen to all living organisms if sunlight energy is not
available?
5. Discuss the reasons why living things need to always take food?6. Is photosynthesis an anabolic or catabolic process? Explain your answer
5.2 Structure of Adenosine Triphosphate and its importance
Activity 5.2
ATP can be describes as a nucleotide made of Ribose as pentose sugar, Adenine
as nitrogenous base and 3 phosphate groups linked by phosphodiesteric
bond.Find out the structure of ATP.
The special carrier of energy is the molecule of adenosine triphosphate (ATP). The
building blocks of ATP are carbon, nitrogen, hydrogen, oxygen, and phosphorus,
contained in the ribose sugar, a nitrogen base called adenine and a chain ofphosphate group (Figure 5.2).
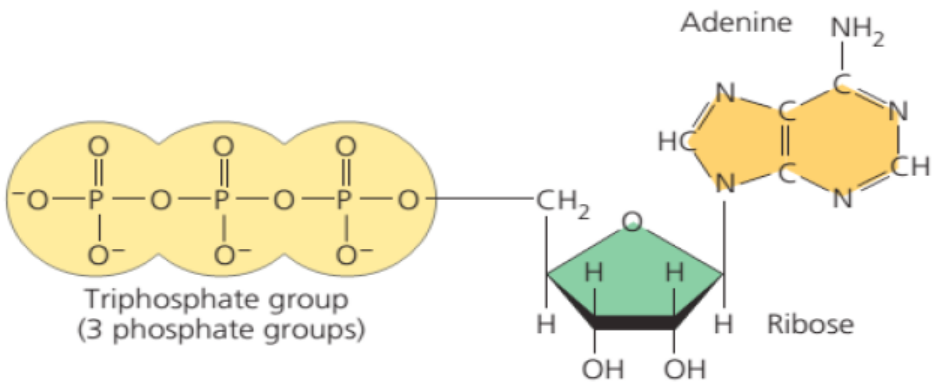
Figure 5.2: Structure of Adenosine Triphosphate (ATP
ATP has the following biological functions in the cell:
a. Active transport
ATP plays a critical role in the transport of macromolecules such as proteins and
lipids into and out of the cell membrane. It provides the required energy for active
transport mechanisms to carry such molecules against a concentration gradient.
b. Cell signaling
ATP has key functions of both intracellular and extracellular signaling. In nervous
system, adenosine triphosphate modulates the neural development, the control of
immune systems, and of neuron signaling.
c. Structural maintenance
ATP plays a very important role in preserving the structure of the cell by helping the
assembly of the cytoskeletal elements. It also supplies energy to the flagella andchromosomes to maintain their appropriate functioning.
d. Muscle contraction
ATP is critical for the contraction of muscles. It binds to myosin to provide energy
and facilitate its binding to actin to form a cross-bridge. Adenosine diphosphate
(ADP) and phosphate group (Pi) are then released and a new ATP molecule binds to
myosin. This breaks the cross-bridge between myosin and actin filaments, thereby
releasing myosin for the next contraction.
e. Synthesis of DNA and RNA
The adenosine from ATP is a building block of RNA and is directly added to RNA
molecules during RNA synthesis by RNA polymerases. The removal of pyrophosphateprovides the energy required for this reaction. It is also a component of DNA.
Application 5.2
1. Energy is contained within ATP. Explain to someone who doesn’t
have any knowledge about ATP how this biochemical compound is
important to all living organisms.2. Observe the figure and answer the following questions:
a. What does it represent?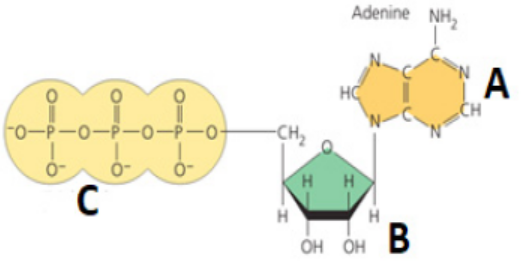
b. Give the names of the parts denoted by the letters A, B and C.
c. What might happen to a living organism if the above molecules are not
present?5.3 Synthesis and breakdown of ATP
Activity 5.3
Use books from the school library and search further information on the
internet about ATP. Read the information and discuss the synthesis andbreakdown of ATP.
Adenosine triphosphate (ATP) is the energy currency for cellular processes. It provides
the energy for both energy-consuming endergonic reactions and energy-releasing
exergonic reactions. When the chemical bonds within the phosphate group of ATP
are broken, energy is released and can be harnessed for cellular work.
a. Synthesis and hydrolysis of ATP
ATP is hydrolysed into Adenosine Diphosphate (ADP) and inorganic phosphate (Pi)in the following reaction:
ATP+H2O→ADP+Pi+free energy,

Figure 5.3: The hydrolysis of ATP: The reaction of ATP and water yields ADP and inorganic phosphate Pi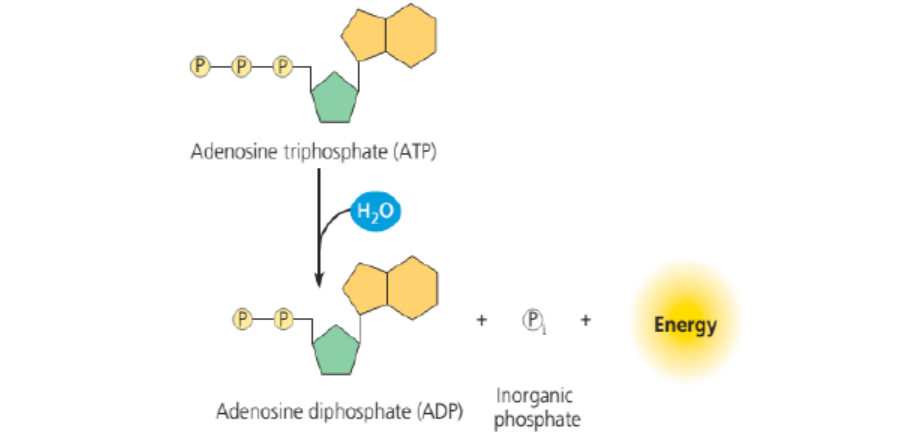
and release energy.
b. ATP and energy coupling
Now that the synthesis and breakdown of ATP is understood, the remaining
interesting question is to know exactly how much free energy denoted ΔG is
released with the hydrolysis of one mole of ATP, and how is that free energy used
to do cellular work. The calculated ΔG for the hydrolysis of one mole of ATP into
ADP and Pi is estimated at −7.3 kcal/mole equivalent to −30.5 kJ/mol. However, thisis only true under standard conditions, and the ΔG for the hydrolysis of one mole
of ATP in a living cell is almost double the value at standard conditions and equals
-14 kcal/mol or −57 kJ/mol. ATP is a highly unstable molecule. Unless quickly used
to perform work, ATP spontaneously dissociates into ADP + Pi, and the free energy
released during this process is lost as heat. To harness the energy within the bondsof ATP, cells use a strategy called energy coupling.
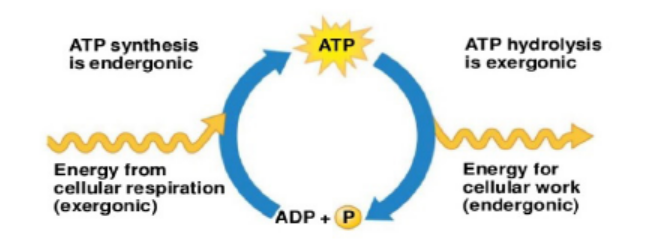
Figure 5.4: Summative processes between synthesis and hydrolysis of ATP
Application 5.3
1. Based on chemical equations explain the synthesis and the hydrolysis
of ATP in a living cell.
2. The hydrolysis and synthesis of ATP are reversible reactions. Estimate
the amount of energy for each process.
3. Calculate the amount of energy produced by 5 moles of ATP
a. Under standard conditions
b. In a living cell
4. Explain what might happen if the reaction of hydrolysis of ATP is not
reversible.5.4 Respiratory substrates and their relative energy values
Activity 5.4
Use books from the school library and search further information on respiration.
Read the information and discuss the respiratory substrates and their relative
energy values.
1. What do you understand by a respiratory substrate?
2. Give any 2 examples of respiratory substrate.
3. What is the relationship between respiratory substrate and energy
values?
A respiratory substrate refers to the substance required for cellular respiration to
derive energy through oxidation. They include carbohydrates, lipids and proteins.
Carbohydrates include any of the group of organic compounds consisting of
carbon, hydrogen and oxygen, usually in the ratio 1:2:1. Hence the general formula
of carbohydrates is . The examples of carbohydrates include sugars, starch and
cellulose. Carbohydrates are the most abundant of all classes of biomolecules,
and glucose whose chemical formula is C6H12O6 is the most known and the most
abundant. Its breakdown produces energy in the following way:
C6H12O6 +6 O2→6 CO2 +6 H2O+Energy (ATP + heat).
This breakdown is exergonic metabolic reaction, having a free-energy change of
-686 kcal (2,870 kJ) per mole of glucose decomposed.
Lipids include diverse group of compounds which are insoluble in water but
dissolved readily in other lipids and in organic solvents such as ethanol (alcohol).
Lipids mainly fats and oils contain carbon, hydrogen and oxygen, though the
proportion of oxygen is lower than in carbohydrates. Fats and oils have a higher
proportion of hydrogen than either carbohydrates or proteins. This property makes
them a more concentrated source of energy, where each gram of fat or oil yields
about 38kJ (38 kJ/g) more than twice the energy yield of a gram of carbohydrate.
Proteins are other respiratory substrate. They are large and complex biological
molecules which play many and diverse roles during respiration. They mainly work
as enzymes. Enzyme is a biological catalyst that controls biochemical reactions in
Back to glucose when it is broken down during the process called glycolysis, the
dehydrogenases enzymes transfer electrons from substrates, here glucose, to
NAD+ which in turn forms NADH. At this stage the electron transport chain accepts
electrons from NADH and passes these electrons from one molecule to another in
electron chain transfer leading to a controlled release of energy for the synthesis of
ATP. At the end of the chain, the electrons are combined with molecular oxygen and
hydrogen ions (H+) to form one molecule of water. (Figure 5). When NAD is oxidized,
its oxidized form NAD+ is converted into its reduced from NADH, and two moleculesof ATP are produced.
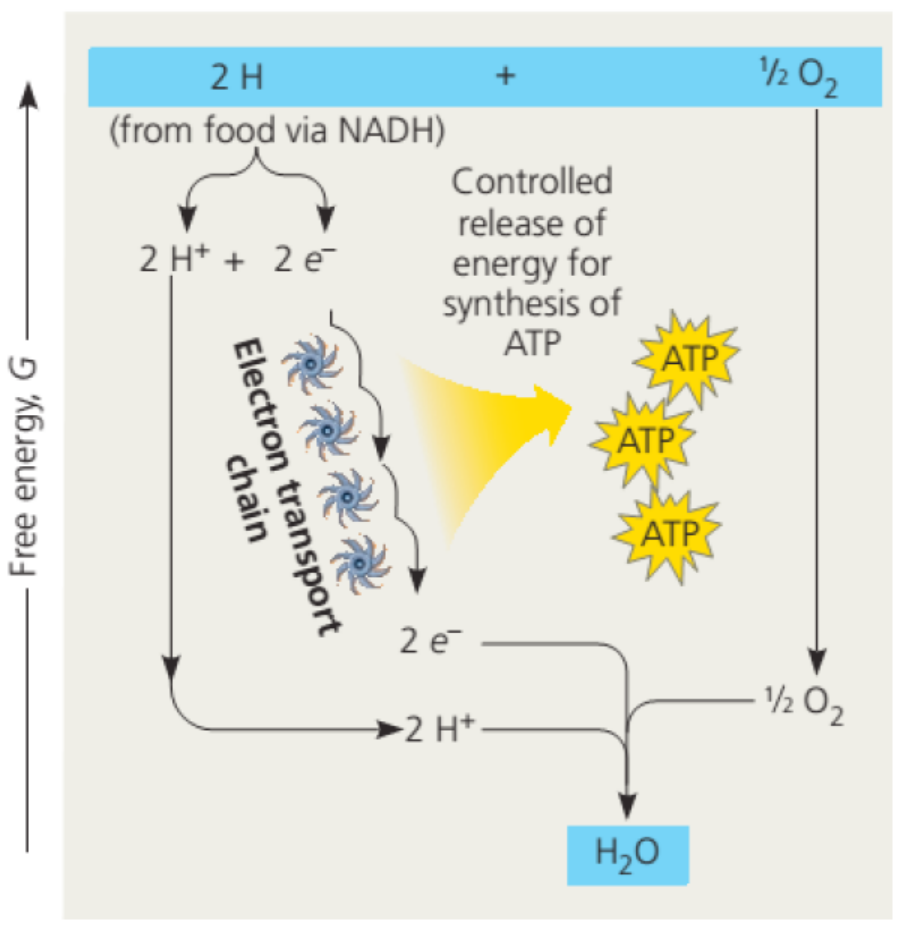
Figure 5.5: Electron transport chain from food to the formation of water
The transformation of succinate to fumarate, the sub-products of the breakdown of
glucose during glycolysis process, two hydrogens are transferred to flavin adenine
dinucleotide (FAD), forming FADH2. The reduced coenzymes NADH and FADH2
transfer higher energy electrons to the electron transport chain. Finally, another
coenzyme called coenzyme A sometimes abbreviated by CoA, a sulfur-containing
compound is attached via its sulfur atom to the two-carbon intermediate, forming
acetyl CoA. The Acetyl CoA has a high potential energy, which is used to transfer the
acetyl group to a molecule in the citric acid cycle, a reaction that is therefore highlyexergonic producing great number of energy in the form of ATP.
Application 5.4
1. What is the oxidizing agent in the following reaction?
Pyruvate + NADH + H+→ Lactate+NAD+ oxygen
a. NADH
b. Lactate
c. pyruvate
2. When electrons flow along the electron transport chains of
mitochondria, which of the following changes occurs?
a. The pH of the matrix increases.
b. ATP synthase pumps protons by active transport.
c. The electrons gain free energy.
d. NAD+ is oxidized.
3. Most CO2 from catabolism is released during which stage?
a. Glycolysis.
b. Electron transport.
4. Give the chemical equation summarizing the decomposition of glucose
and specify the amount of energy produced in kJ.
5. Calculate the amount of energy produced by moles of glucose in kcal
and kJ if one mole of glucose produce -686 kcal and 2,870 kJ per mole
of glucose.
6. Differentiate between NAD+ and NADH2? . How are they related to FAD
and FDH2?
7. Specify the number of ATP produced by glycolysis during respiration
process.5.5 Measurement of respiration and respiratory quotient
Activity 5.5
Use books from the school library and search further information on
respiration. Read the information and discuss the measurement of respiration
and respiratory quotient.
1. What do you understand by respiratory quotient?
2. Draw a well labelled figure indicating the structure of a respirometer
and specify its role in biological studies.
3. Explain how the respiratory coefficient can be calculated fromconsumed oxygen and released carbon dioxide during respiration.
The rate of respiration is measured by the use of respirometer device, typically by
measuring oxygen consumed and the carbon dioxide given out. It can also be used
to measure the depth and frequency of breathing, and allows the investigation on
how factors such as; age, or chemicals can affect the rate of respiration. Currently,
the computer technology is also used to automatically measure the volume of gases
exchanged and drawing off small samples to analyse the proportions of oxygen andcarbon dioxide in the gases.
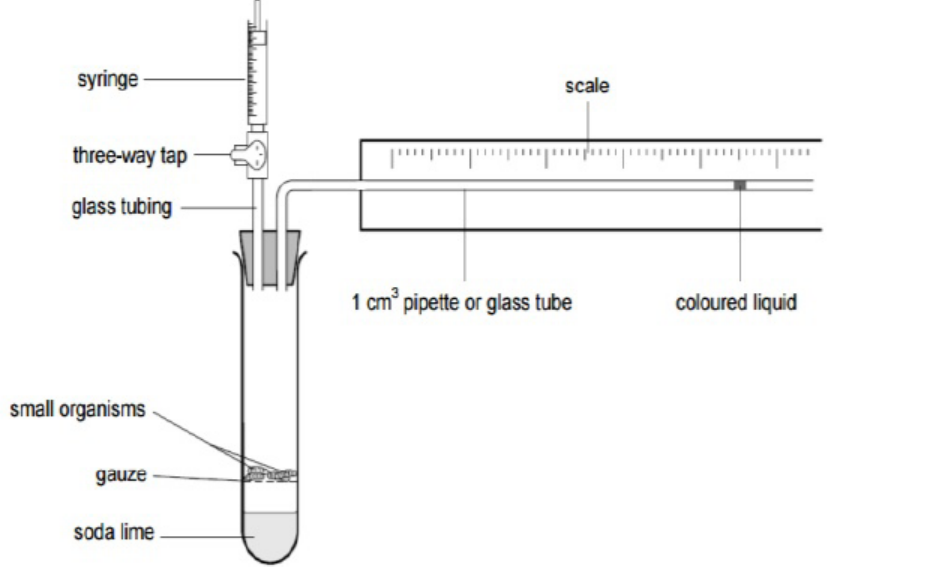
Figure 5.6: Respirometer
The respiratory quotient (RQ) is the ratio of the volume of carbon dioxide produced
to the volume of oxygen used in respiration during the same period of time. The
RQ is often assumed to equal the ratio of carbon dioxide expired: oxygen inspiredduring a given time as it is summarized in the following formula:
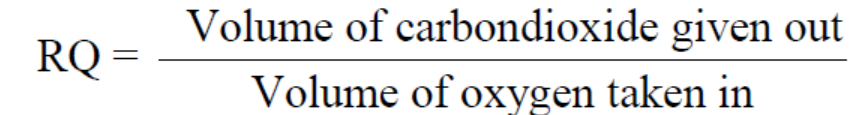
The RQ is important as it can indicate whether the respiration is aerobic or anaerobic.
C6H12O6 +6 O2→6 CO2 +6 H2O+ Energy (ATP + heat).
As each molecule of gas occupies the same volume, this would give RQ = 1.0, and this
is common for all carbohydrates. Further studies indicated the respiratory quotient
to be 0.9 for proteins and 0.7 for fats, and concluded that an, RQ greater than 1.0
indicates anaerobic respiration, while RQ equals or less than 1.0 indicates aerobicrespiration.
Note that respiration during germination, especially in early stages was also studied.
Results indicated that it is difficult for oxygen to penetrate the seed coat, so that at
this stage, the RQ is about 3 to 4. Later when the seed coat is shed, it becomes easier
for oxygen to reach respiration tissues and the levels of RQ falls. Results indicated
that eventually seeds with large carbohydrate stores have an RQ around 1.0 and
those with large lipid stores have RQs of 0.7 to 0.8.
This graph suggests that the seed begins with carbohydrate as a metabolite, changesto fat/oil then returns to mainly using carbohydrate
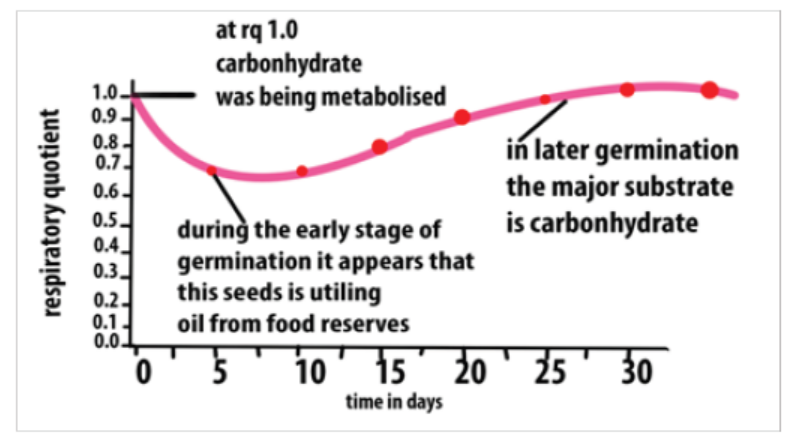
Figure 5.7: The graph showing the RQ values during seed germination
a. Measuring and obtaining the RQ values during seed germination process
During seed germination, CO2 is released. To test its presence, chemicals including
Sodium hydroxide or Potassium hydroxide are used due to their ability to absorb
CO2. As the germinating seeds use oxygen, pressure reduces in tube A so the
manometer level nearest to the seeds rises (figure 5.8). The syringe is used to return
the manometer fluid levels to normal. The volume of oxygen used is calculated by
measuring the volume of gas needed from the syringe to return the levels to the
original values. If water replaces the sodium hydroxide, then the carbon dioxideevolved can be measured.
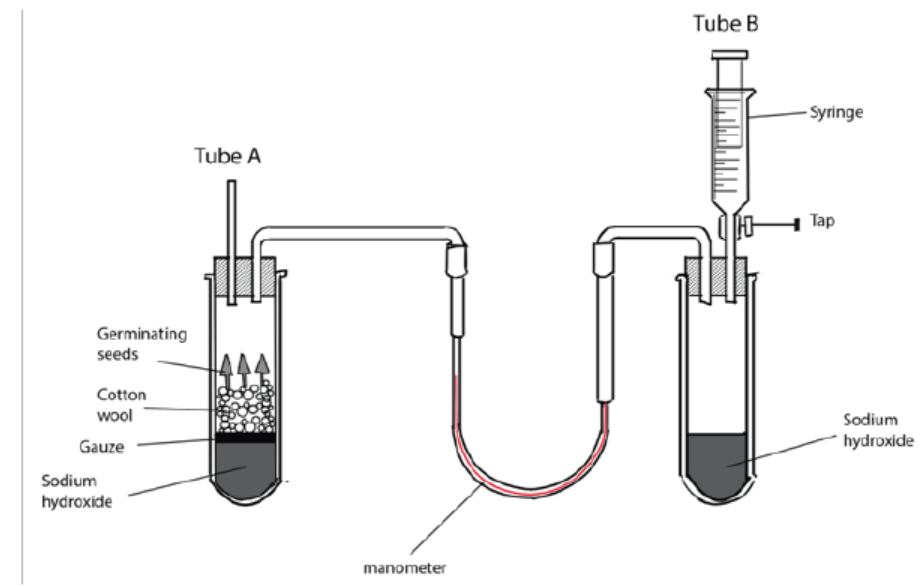
Figure 5.8: Simple experiment using respirometer to determine the RQ in germinating seeds
Measuring and obtaining the RQ values in invertebrate (e.g. woodlice)
In this particular respirometer, woodlice have been placed in a boiling tube which
is connected to a U-tube. The U-tube acts as a manometer (a device for measuring
pressure changes). The other end of the U-tube is connected to a control tube which
is treated in exactly the same way as the first tube, except that it has no woodlice but
instead glass beads which take up the same volume as the woodlice. The two boiling
tubes (but not the manometer) are kept in water bath at constant temperature. The
U-tube contains a coloured liquid which moves according to the pressure exerted
on it by the gases in the two boiling tubes. Both tubes contain potassium hydroxide
solution which absorbs any carbon dioxide produced.
When the woodlice respire aerobically, they consume oxygen, which causes
the liquid to move in the U- tube in the direction of arrows. The rate of oxygen
consumption can be estimated by timing how long it takes for the liquid to rise
through a certain height. The experiment can be repeated by replacing the potassium
hydroxide solution with water. Comparing the changes in manometer liquid level
with and without potassium hydroxide solution gives an estimate of carbon dioxide
production can be used to measure the respiratory quotient.
If the internal radius of the manometer tube is known, the volumes of gases can becalculated using the equation:
Volume of gases = π r2 h,
where π is equal to 3.14, r is the internal radius of the tube and h is the distancemoved by the liquid.
Application 5.5
1. Using the following equation of oleic acid (a fatty acid found in oliveoil):
2C18H34O2 + 51O2 →36CO2 + 34H2O.
a. Calculate the RQ for the complete aerobic respiration.
b. Based on your findings, state which substrate is being respired.
2. Suggest an explanation when RQ equals 1 for germinating maize grains.
3. Based on the values of RQ, when can you conclude that the respirationprocess is:
a. Aerobic.
b. Anaerobic.
4. Calculate the volume of gases in a manometer tube having a radius of1.7 cm, knowing that the gas was displaced about 3cm distance.
End of unit assessment 5
1. Explain the reasons why chemical energy is the most important type of
energy for living organisms.
2. Why do all organisms need energy and where does this energy come from?
3. Give the structure of ATP and specify its importance to living organisms?
4. The equation C57H104O6 + 80O2 → 57CO2 + 52H2O + Energy represents
oxidation of lipids. Calculate RQ for this equation.
5. Calculate the total amount of energy produced for:
a. 3 moles of hydrolysed ATP
b. moles of synthesized ATP
c. 5 moles of decomposed glucose
6. Active mitochondria can be isolated from liver cells. If these mitochondria are
then incubated in a buffer solution containing a substrate, such as succinate,
dissolved oxygen will be used by mitochondria. The concentration of
dissolved oxygen in the buffer solution can be measured using an electrode.
When this experiment was done, the concentration of dissolved oxygen was
measured every minute for five minutes. Sodium azide which combines with
cytochromes and prevents electron transport was added thereafter. Theresults are shown in the graph below.
a. Suggest what effect the addition of sodium azide will have on the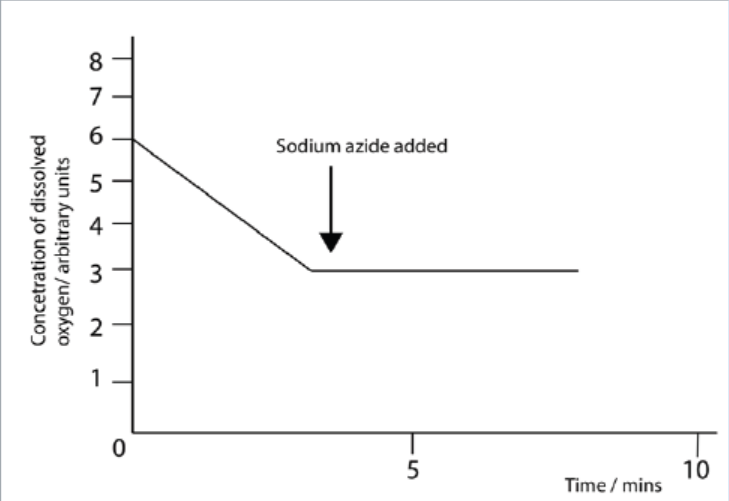
production of ATP and give an explanation for your answer.
b. Explain why the concentration of oxygen decreased during the first
five minutes.
c. Suggest what effect the addition of sodium azide will have on theproduction of ATP and give an explanation for your answer.
7. Analyse the following figure:
The graph shows the pH difference across the inner mitochondrial membrane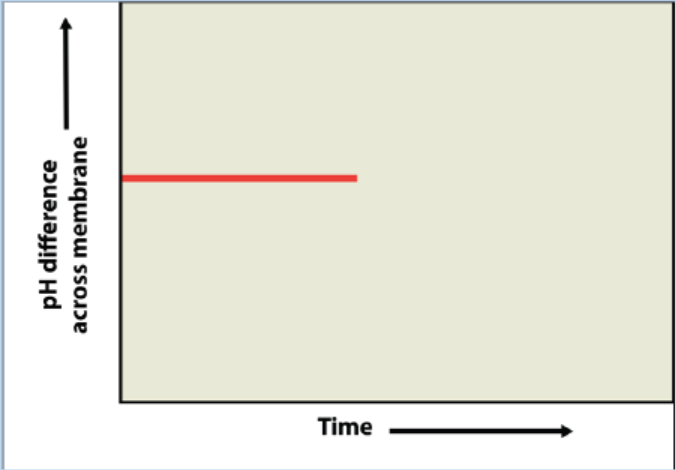
over time in an actively respiring cell. At the time indicated by the vertical
arrow, a metabolic poison is added that specifically and completely inhibits
all function of mitochondrial ATP synthase. Draw what you would expect to
see for the rest of the graphed line, and explain your graph.
8. During an experiment, the mouse was inside the bell jar. The air pipe from
the bell jar was connected to the first beaker containing lime water and filter
pump. The glass wool containing soda lime covered by a piece of paper was
connected to the second beaker by air pipe. Another air pipe was connected
from the second beaker containing lime water to the belly jar in the first step.The set of the experiment looked like the following:
a. Name the gas trapped in beaker B?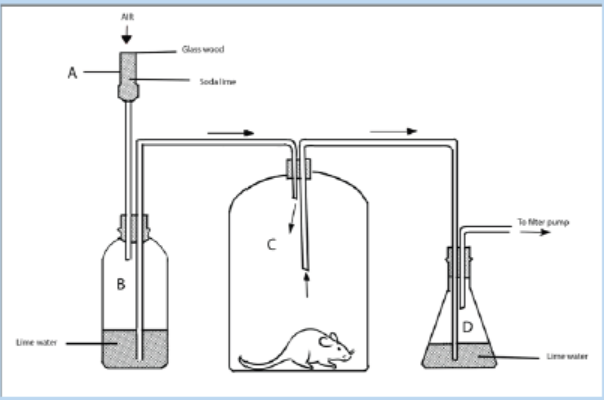
b. Why does the mouse still live since it is covered in a bell jar?
c. Why does lime water turn milky?
d. Is this experiment related to respiration and energy production or to the
respiration and energy consumption? Explain.
9. The following figure indicates the variations of RQ in function of time. Analyseit and make its interpretation
a. Observe the graph and make its interpretation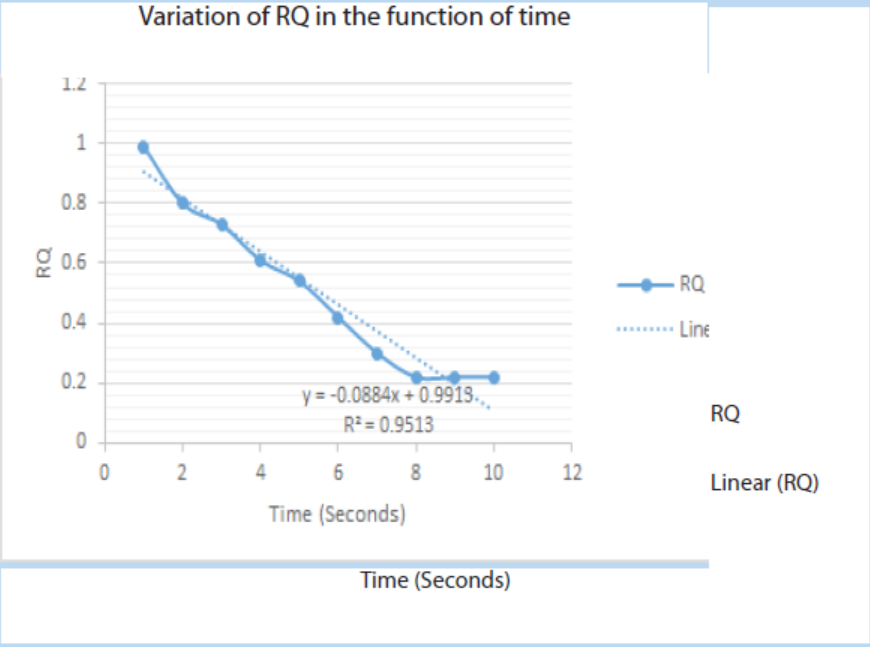
10. The following data were collected for RQ of an insect during one minute:
a. Plot the graph of RQ in function against time
b. Explain the reasons why there is no change in RQ for the last threeseconds
