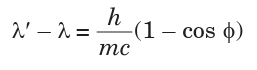UNIT 9:ATOMIC MODELS AND PHOTOELECTRIC EFFECT

Key unit competence: Evaluate the atomic models and photoelectric
effect
Unit Objectives:
By the end of this unit I will be able to;
◊ Describe different atomic models by explaining their concepts and
drawbacks.
◊ Explain the photoelectric effect and its applications in everyday life.
Introductory Activity
1. Basing on the figure above,
a. How is the structure/arrangement of balls shown in the figure
related to an atom? You can use chemistry knowledge from
O’level.
b. Relate the arrangement of electrons in an atom to how the
balls in the figure above are arranged.
c. Explain how movement of particles in an atom leads to release
or absorption of energy
4. It is important to realise that a lot of what we know about the
structure of atoms has been developed over a long period of time.
This is often how scientific knowledge develops, with one person
building on the ideas of someone else.In attempt to explain an
atom, different scientists suggest different models. An atomic model
represents what the structure of an atom could look like, based on
what we know about how atoms behave. It is not necessarily a true
picture of the exact structure of an atom.
a. Why did these scientists use the word Model not exact structure
of an atom?
b. Can you explain some of the scientific models that tried to
explain the structure of an Atom?
9.0 INTRODUCTION
An atomic theory is a model developed to explain the properties and
behaviours of atoms. An atomic theory is based on scientific evidence
available at any given time and serves to suggest future lines of research
about atoms.
The concept of an atom can be traced to debate among Greek philosophers
that took place around the sixth century B.C. One of the questions that
interested these thinkers was the nature of matter. Is matter continuous
or discontinuous? If you could break a piece of chalk as long as you wanted,
would you ever reach some ultimate particle beyond which further division
was impossible? Or could you keep up that process of division forever?
Such questions need the knowledge on the atomic structure and interaction
with photoelectric effect to be answered. This theory is helpful in Chemistry
(Atomic structure), Security (Alarm systems), Medicine, Archaeology, etc.
9.1 STRUCTURE OF THE ATOM AND THOMSON’S MODEL
structure of the atom
An atom is the smallest particle of an element that retains again the
characteristics or the properties of that element during chemical reaction.
By the early 1900s scientists were able to break apart the atoms into
particles that they called the electron and the nucleus which is made of
proton and neutrons.
• Electrons
Electrons surround the dense nucleus of an atom. It is the smallest
subatomic particle with a mass of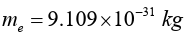
and a negative electric charge. The electron is also one of the few
elementary particles that is stable, meaning it can exist by itself for a long period of time.
Most other elementary particles can exist independently for only a fraction of a second.
Electrons have no detectable shape or structure.
The electrons revolve around the nucleus in fixed trajectory (orbits) called
energy levels or shell. These shells have the names K, L, M, N, etc…
The shell of atom just prior to the outermost shell of an atom cannot
accommodate more than 8 electrons even it has a capacity to accommodate
more electrons. The outermost shell (last shell) which contains electrons
is called the conduction shell or valence shell. On each electron shell, we
can meet N=2n2 electrons, where N is the number of the electron shell.
The valence electrons which are not very attached to the nucleus are called
free electrons. The free electrons can be easily detached from the atom
by application of a small external energy (usually thermal energy by
increasing the temperature).
• Protons
Proton is a subatomic particle with a positive charge. The charge is equal
and opposite to that of an electron. The mass of a proton is 1840 times
that of an electron. Thus the mass of an atom is mainly due to protons
and neutrons. The proton is one of the few elementary particles that are
stable—that is, it can exist by itself for a long period of time. The number of
protons is called the atomic number (Z).
In normal atom, the number of electrons is equal to the number of protons.
The atomic number (Z) of an atom is equal to the number of protons (or
electrons) contained in atom.
• Neutron
Neutron is a subatomic particle with a mass almost equal to the mass of a
proton. It has no electric charge. The neutron is about 10-13 cm in diameter
and weighs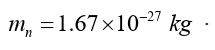 The number of protons and neutrons
The number of protons and neutrons
is called nucleons number, or, alternatively, the mass number (A). The mathematical
relationship between atomic number (Z), mass number (A) and neutron numberis


Thomson’s model
English scientist Joseph John Thomson’s cathode ray experiments (end
of the 19th century) led to the discovery of the negatively charged electron
and the first ideas of the structure of these indivisible atoms. In his model
of the atom, Sir J J Thomson (1856-1940) suggested a model of atom as
“The atom is like a volume of positive charge with electrons embeddedthroughout the volume, much like the seeds in watermelon.”Fig.9.4
Success and Failure of Thomson’s model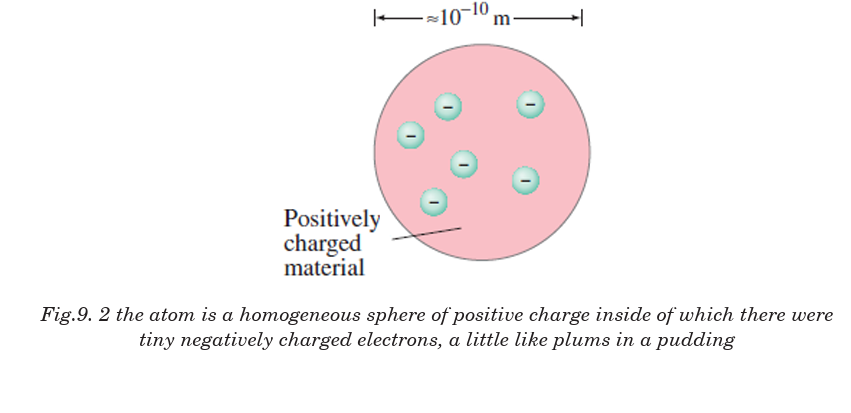
Thomson’s model explained the phenomenon of thermionic emission,
photoelectric emission and ionization. The model fails to explain the
scattering of a-particles and it is the origin of spectral lines observed in the
spectrum of hydrogen and other atoms.
9.2 RUTHERFORD’S ATOMIC MODEL
Rutherford performed experiments on the scattering of alpha particles byextremely thin gold foils and made the following observations;
Note:
• Some of a-particles are deflected through small angles.
• A few a-particles (1 in 1000) are deflected through the angle more
than 90°.
• A few a-particles (very few) returned back i.e. deflected by 180°.
• Distance of closest approach (Nuclear dimension) is the minimum
distance from the nucleus up to which the a-particle approach. It is
denoted by r0 . From figure
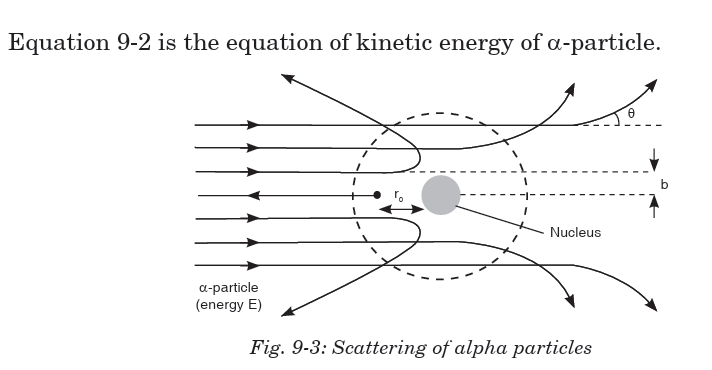
From these experiments a new model of the atom was born called
Rutherford’s planetary model of the atom. The following conclusions were
made to describe the atomic structure:
• Most of the mass and all of the charge of an atom is concentrated in a
very small region called atomic nucleus.
• Nucleus is positively charged and it’s size is of the order of 10–15 m .
• In an atom there is maximum empty space and the electrons revolve
around the nucleus in the same way as the planets revolve around the sun.
Drawbacks : Rutherford's model could not explain the following:
• Stability of atom: It could not explain the stability of atom because
according to classical electrodynamics, an accelerated charged particle
should continuously radiate energy. Thus, an electron moving in a
circular path around the nucleus should also radiate energy and thus
move into and smaller orbits of gradually decreasing radius and itshould ultimately fall into nucleus.
• According to this model, the spectrum of atom must be continuous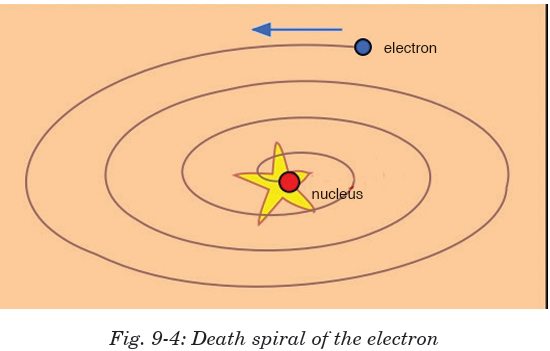
whereas practically it is a line spectrum.
• It did not explain the distribution of electrons outside the nucleus.
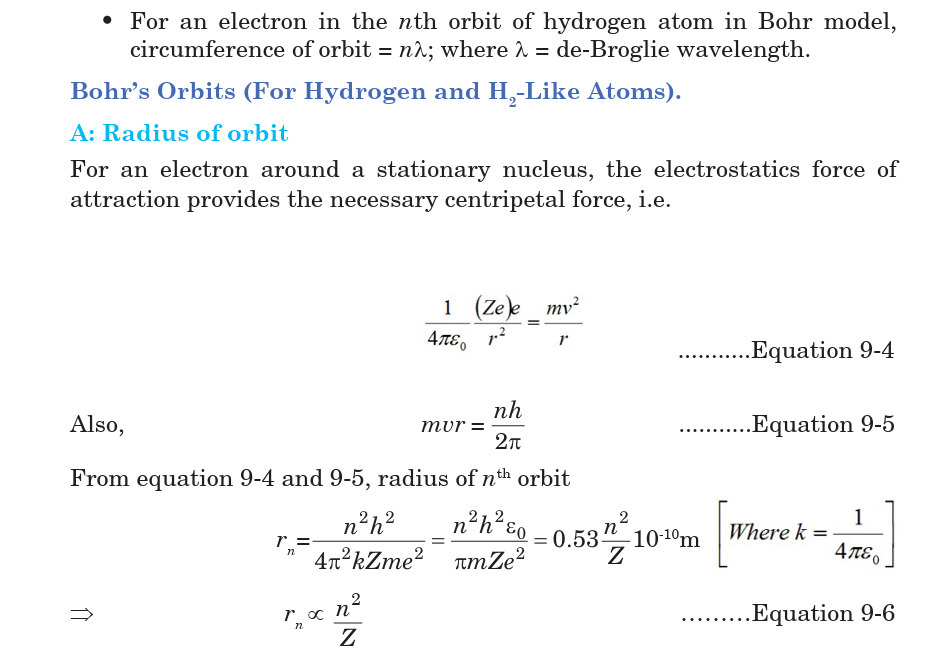
9.3 BOHR’S ATOMIC MODEL
Bohr proposed a model for hydrogen atom which is also applicable for
some lighter atoms in which a single electron revolves around a stationary
nucleus of positive charge Ze (called hydrogen like atom). Bohr’s model is
based on the following postulates:
• Each electron moves in a circular orbit centered at the nucleus.
• The centripetal force needed by the electron moving in a circle is
provided by electrostatic force of attraction between the nucleus and
electrons.• The angular momenta p of electrons are whole number multiples of


Drawbacks of Bohr’s atomic model
• It is valid only for single valency atoms, e.g. : H, He+2, Li+, Na+1 etc.
• Orbits were taken as circular but according to Sommerfield these are
elliptical.
• Intensity of spectral lines could not be explained.
• Nucleus was taken as stationary but it also rotates on its own axis.
• It could not explain the minute structure in spectral lines.
• This does not explain the Zeeman effect (splitting up of spectral lines
in magnetic field) and Stark effect (splitting up in electric field)
• This does not explain the doublets in the spectrum of some of the
atoms like sodium (5890x10-10m & 5896x 10-10m)
9.4 ENERGY LEVELS AND SPECTRAL LINES OF
HYDROGEN
When hydrogen atom is excited, it returns to its normal unexcited state (or
ground state) by emitting the energy it had absorbed earlier. This energy
is given out by the atom in the form of radiations of different wavelengths
as the electron jumps down from a higher orbit to a lower orbit. Transition
from different orbits causes different wavelengths. These constitute spectral
series which are characteristic of the atom emitting them. When observed
through a spectroscope, these radiations are imaged as sharp and straightvertical lines of a single colour.
The spectral lines arising from the transition of electron forms a spectra
series. Mainly there are five series and each series is named after its
discover as Lyman series, Balmer series, Paschen series, Brackett series
and Pfund series. First line of the series is called first member, for which
line wavelength is maximum (λmax). Last line of the series (n2= ∞) is called
series limit, for which line wavelength is minimum (λmin).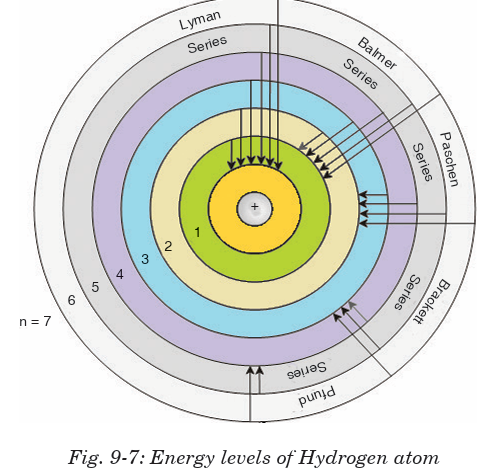
9.5 THERMIONIC EMISSION ( THERMO ELECTRONIC
EMISSION)
Thermionic emission means the discharge of electrons from heated materials.
It is widely used as a source of electrons in conventional electron tubes (e.g.,
television picture tubes) in the fields of electronics and communications. The
phenomenon was first observed (1883) by Thomas A. Edison as a passage ofelectricity from a filament to a plate of metal inside an incandescent lamp.
In thermionic emission, the heat supplies some electrons with at least the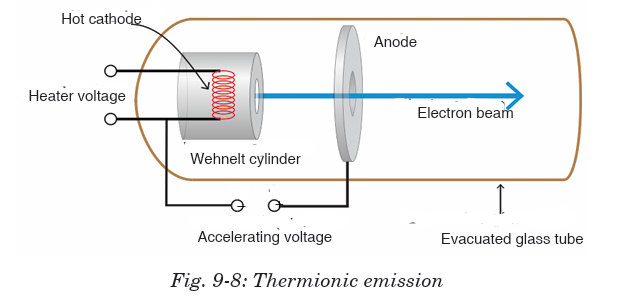
minimal energy required to overcome the attractive force holding them in
the structure of the metal. This minimal energy, called the work function,
is the characteristic of the emitting material and the state of contaminationof its surface.
9.6 APPLICATIONS OF CATHODE RAYS
9.6.1 Cathode ray oscilloscope
The cathode-ray oscilloscope (CRO) is a common laboratory instrument that
provides accurate time and amplitude measurements of voltage signals over
a wide range of frequencies. Its reliability, stability and ease of operationmakes it suitable as a general purpose laboratory instrument.
The main part of the C.R.O. is a highly evacuated glass tube housing parts
which generates a beam of electrons, accelerates them, shapes them into
a narrow beam and provides external connections to the sets of plates
changing the direction of the beam. The heart of the CRO is a cathode-raytube shown schematically in Fig.9-10;
Working of a C.R.O
• An indirectly heated cathode provides a source of electrons for the
beam by ‘boiling’ them out of the cathode.
• The anode is circular with a small central hole. The potential of anode
creates an electric field which accelerates the electrons, some of which
emerge from the hole as a fine beam. This beam lies along the central
axis of the tube.
• The grid has the main function of concentrating the beam at the
centre controlling the potential of the grid that controls the number
of electrons for the beam, and hence the intensity of the spot on the
screen where the beam hits.
• X and Y are two deflection plates. The X plates are used for deflecting
the beam from left to right (the x-direction) by means of the ‘ramp’
voltage. The Y plates are used for deflection of the beam in the vertical
direction. Voltages on the X and Y sets of plates determine where the
beam will strike the screen and cause a spot of light.
• The screen coated on the inside with a fluorescent material which
shines with green light (usually) where the electrons are striking.
9.6.2 TV tubes
The picture tube is the largest component of a television set, consisting
of four basic parts. The glass face panel is the screen on which images
appear. Suspended immediately behind the panel is a steel shadow mask,
perforated with thousands of square holes. (Connected to the mask is a
metal shield to neutralize disruptive effects of the Earth’s magnetic field.)
The panel is fused to a glass funnel, which comprises the rear of the picture
tube. The very rear of the funnel converges into a neck, to which an electrongun assembly is connected.
The inside of the panel is painted with a series of very narrow vertical
stripes, consisting of red, green and blue phosphors. These stripes are
separated by a narrow black graphite stripe guard band. When struck by an
electron beam, the phosphors will illuminate, but the graphite will not. This
prevents colour impurity by ensuring that the electron beam only strikes
the phosphor stripes it is intended to light.
The electron beam is generated by the electron gun assembly, which houses
three electron guns situated side-by-side. Each of the three guns emits an
electron beam (also called a cathode ray) into the tube, through the maskand onto the panel.
Because the three beams travel side-by-side, the holes in the mask ensure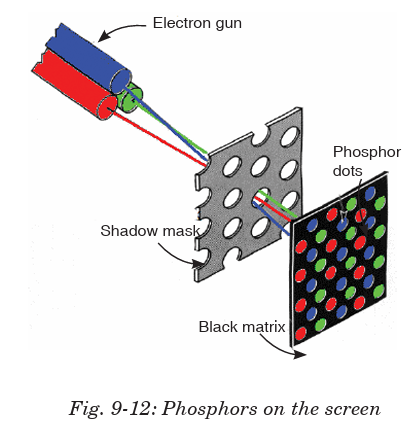
that each beam, because of its different angle of attack, will hit only a
specific phosphor stripe; red, green or blue. The three phosphors, lighted
in different combinations of intensity, can create any visible colour when
viewed from even a slight distance.
The three electron beams are directed across the screen through a series of
electromagnets, called a yoke, which draw the beams horizontally across
the screen in line at a time. Depending on the screen size, the beam draws
about 500 lines across the entire screen. Each time, the phosphors light up
to produce an image.
The electron guns and the yoke are electronically synchronized to ensure
the lines of phosphors are lighted properly to produce an accurate image. The
image lasts only for about a 1/30th of a second. For that reason, the picture
must be redrawn 30 times in a second. The succession of so many picturesproduces the illusion of movement, just like the frames on movie film.
9.7 FLUORESCENCE AND PHOSPHORESCENCE
Fluorescence is the emission of light by a substance that has absorbed light
or other electromagnetic radiation. It is a form of photoluminescence.
In most cases, the emitted light has a longer wavelength, and therefore,
lower energy than the absorbed radiation. However, when the absorbed
electromagnetic radiation is intense, it is possible for one electron to absorb
two photons; this two-photon absorption can lead to emission of radiation
having a shorter wavelength than the absorbed radiation. The emitted
radiation may also be of the same wavelength as the absorbed radiation,
termed “resonance fluorescence”.
Fluorescence occurs when an orbital electron of a molecule or atom relaxes
to its ground state by emitting a photon of light after being excited to a
higher quantum state by some type of energy. The most striking examples
of fluorescence occur when the absorbed radiation is in the ultraviolet region
of the spectrum, and thus invisible to the human eye, and the emitted light
is in the visible region.
Phosphorescence is a specific type of photoluminescence related to
fluorescence. Unlike fluorescence, a phosphorescent material does not
immediately re-emit the radiation it absorbs. Excitation of electrons to
a higher state is accompanied with the change of a spin state. Once in a
different spin state, electrons cannot relax into the ground state quickly
because the re-emission involves quantum mechanically forbidden energy
state transitions. As these transitions occur very slowly in certain materials,
absorbed radiation may be re-emitted at a lower intensity for up to severalhours after the original excitation.
9.8 PHOTOELECTRIC EMISSION LAWS
Law 1:
The photocurrent is directly proportional to the intensity of light and is
independent of frequency.
Explanation
According to quantum theory, each photon interacts only with each
electron. When the intensity is increased more photons will come and they
will interact with more electrons. This will increase the amount of photo
current.
Law 2:
The kinetic energy of the photoelectrons is directly proportional to frequency
and is independent of intensity.
Explanation
According to Einstein’s equation, hf0 is constant. Then kinetic energy is
directly proportional to frequency.
Law 3:
Photoelectric effect does not happen when the incident frequency is less
than a minimum frequency (threshold frequency).
Explanation
From Einstein’s equation, if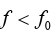 , then kinetic energy becomes negative
, then kinetic energy becomes negative
and it is impossible, in other words photoelectric effect does not happen.
Law 4:
There is no time lag between the incidence of photon and emission of
electrons. Thus, photoelectric process is instantaneous.
Explanation
According to quantum theory, each photon interacts with each electron.
So different electrons will interact with different photons at same instant.Thus there is no time lag between incidence and emission.
9.9 PHOTOELECTRIC EFFECT
The photoelectric effect is the emission of electrons from the surface of a
metal when electromagnetic radiation (such as visible or ultraviolet light)
shines on the metal. At the time of its discovery, the classical wave model
for light predicted that the energy of the emitted electrons will increase as
the intensity (brightness) of the light increased. It was discovered that it
did not behave that way. Instead of using the wave model, treating light
as a particle (photon) led to a more consistent explanation of the observed
behaviour.
From photon theory, we note that in a monochromatic beam, all photons
have the same energy (equal to hf). Increasing the intensity of the light
beam means increasing the number of photons in the beam but does not
affect the energy of each photon as long as the frequency is not changed.
From this consideration and suggestions of Einstein, the photon theory
makes the following predictions:
1. For a given metal and frequency of incident radiation, the number
of photoelectrons ejected per second is directly proportional to the
intensity of the incident light.
2. For a given metal, there exists a certain minimum frequency (f0 ) of
incident radiation below which no emission of photoelectrons takes
place. This frequency is called the threshold frequency or cutoff
frequency.
3. Above the threshold frequency, the maximum kinetic energy of
the emitted photoelectron is independent of the intensity of the
incident light but depends only upon the frequency (or wavelength) of
the incident light.
4. The time lag between the incidence of radiation and the emission of a
photoelectron is very small (less than 10-9 second).This is evidence of the particle nature of light.
9.10 FACTORS AFFECTING PHOTOELECTRICEMISSION
Photoelectric current is produced as a result of photoelectric effect. Therefore,
understanding the factors which influence the photoelectric effect is very
important. The previous studies on photoelectric effect have presented the
following factors which may have a direct impact on photoelectric effect.
Intensity of Light:
If a highly intense light of frequency equal to or greater than threshold
frequency falls on the surface of matter, the photoelectric effect is caused.
Studying the impact of this factor is the focus of this research study. One
thing which is very clear is that the emission of electrons does not depend
upon the intensity of light unless the frequency of light is greater than thethreshold frequency. The threshold frequency varies from matter to matter.
Number of Photoelectrons:
The increase in intensity of light increases the number of photoelectrons,
provided the frequency is greater than threshold frequency. In short, thenumber of photoelectrons increases the photoelectric current.
Kinetic Energy of Photoelectrons:
The kinetic energy of photoelectrons increases when light of high energy
falls on the surface of matter. When energy of light is equal to threshold
energy, then electrons are emitted from the surface, whereas when energy
is greater than threshold energy, then photoelectric current is produced.
The threshold frequency is not same for all kinds of matter and it variesfrom matter to matter.
9.11 PHOTON, WORK FUNCTION AND PLANCK'S
CONSTANT
The photon is the fundamental particle of visible light. In some ways,
visible light behaves like a wave phenomenon, but in other respects it acts
like a stream of high-speed, submicroscopic particles.
Minimum amount of energy which is necessary to start photo electric
emission is called Work Function. If the amount of energy of incident
radiation is less than the work function of metal, no photo electrons areemitted.
Planck’s constant describes the behaviour of particles and waves on the
atomic scale. The idea behind its discovery, that energy can be expressed
in discrete units, or quantized, proved fundamental for the development ofquantum mechanics.
Project 9-1: Photoelectric Effect
planck introduced the constant (h = 6.63 × 10–34 J.s) in his description of
the radiation emitted by a blackbody (a perfect absorber of radiant energy).
The constant’s significance, in this context, was that radiation (light, for
example) is emitted, transmitted and absorbed in discrete energy packets.
Aim: this project aims at gaining the deep knowledge on photoelectric
effect.
Question: Describe the observations made of the photoelectric effect and
how this supports the particle model and wave model of light studied in
unit 1.
Hypothesis: write a hypothesis on the phenomenon of photoelectric
effect.
Procedure
1. State the main principle of photoelectric effect.
2. Outline your observations on different conditions
Collecting Data
Use internet and textbooks to analyse the phenomenon of photoelectric
effect.
Report design
Write your report of at least five supporting points including the onegiven in the format below:
9.12 EINSTEIN’S EQUATION
According to Einstein’s theory, an electron is ejected from the metal by
a collision with a single photon. In the process, all the photon energy is
transferred to the electron and the photon ceases to exist. Since electrons
are held in the metal by attractive forces, a minimum energy (W0 ) is
required just to get an electron out through the surface. W0
is called the
work function, and is a few electron volts (1eV = 1.6 × 10–19 J ) for mostmetals.
Definitions
Photoelectric emission is the phenomenon of emission of electrons from
the surface of metals when the radiations of suitable frequency and suitable
wavelength fall on the surface of the metal.
Work function is the minimum energy required to set free an electron
from the binding forces on the metal surface.
The Threshold Frequency is defined as the minimum frequency of
incident light required for the photoelectric emission.
If the frequency f of the incoming light is so low that hf is less than W0
, then the photons will not have enough energy to eject any electrons at all. If
hf > W0, then electrons will be ejected and energy will be conserved in the
process.
So Einstein suggested that the energy of the incident radiation hf was
partly used to free electrons from the binding forces on the metal and the
rest of the energy appeared as kinetic energy of the emitted electrons. This
is stated in the famous Einstein’s equation of photoelectric effect as statedin equation 9-7 below.
Equation 9-8 is called the Einstein’s photoelectric equation.
Many electrons will require more energy than the bare minimum W0
to get out of the metal, and thus the kinetic energy of such electrons will be lessthan the maximum.
Application Activity 9.1Match the mathematical symbols and their descriptions
Stopping potential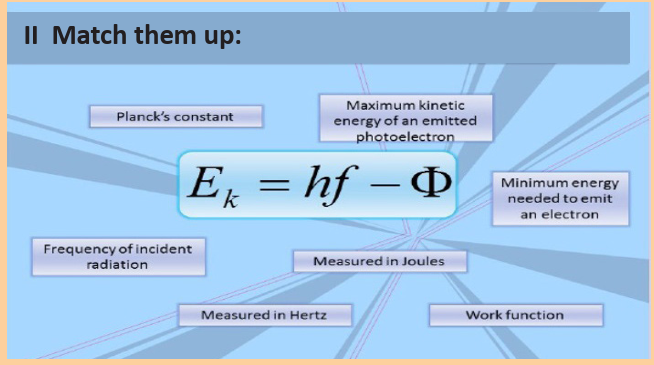
The circuit is exposed to radiations of light of frequency f and the supply of
potential difference V is connected as shown in Fig.9-15 below. The cathode
C is connected at the positive terminal of the supply and the anode P isconnected on the negative terminal of the supply.
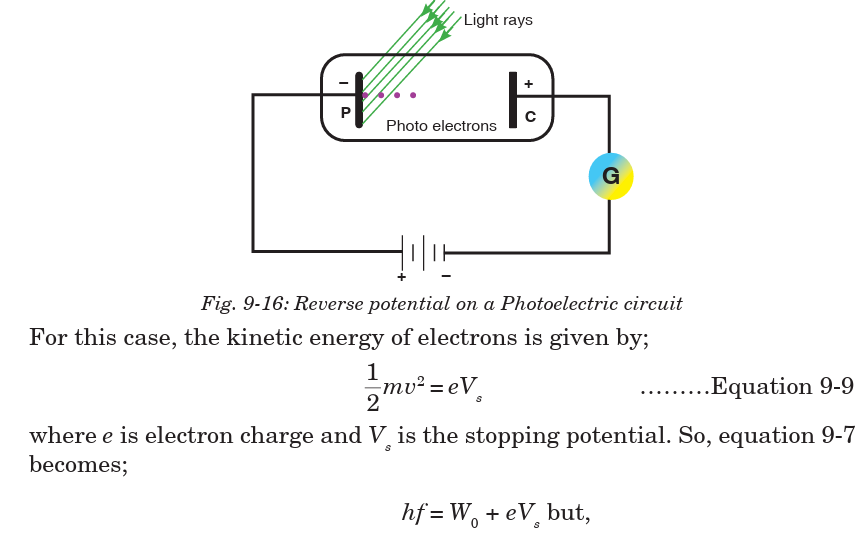
If the circuit is exposed to radiations with the battery reversed as shown in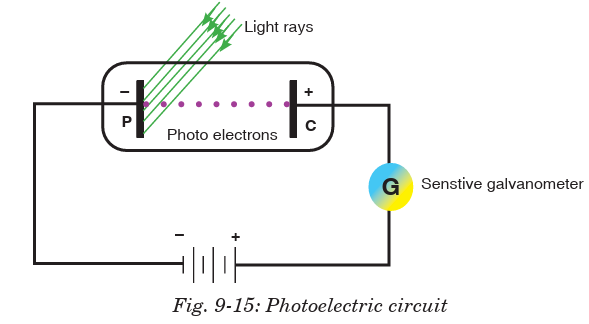
Fig. 9-16, current reduces due to the fact that all electrons emitted are not
able to reach the anode P. If this potential difference is increased until no
electron reaches the anode P, no current flows and this applied potential iscalled a stopping potential.
EXAMPLE 9-1
The work function for lithium is 4.6 × 10-19 J.
(a) Calculate the lowest frequency of light that will cause photoelectric
emission.
(b) What is the maximum energy of the electrons emitted when the light offrequency 7.3 × 1014 Hz is used?
EXAMPLE 9-2
Selenium has a work function of 5.11 eV. What frequency of light would just
eject electrons?
Solution:
When electrons are just ejected from the surface, their kinetic energy is zero.So,
Application Activity 9.2
1. Complete table 1 below.
Table 1: Applying Einstein’s photoelectric equation incalculations
2. The stopping potential when a frequency of 1.61 × 1015 Hz is
incident on a metal is 3 V.
(a) What is energy transferred by each photon?
(b) Calculate the work function of the metal.
(c) What is the maximum speed of the ejected electrons?
Aim: To know the concepts and use of photoelectric equation.
3. It is useful to observe the photoelectric effect equation represented
graphically.
(a) Express equation 9-7 in the form y = a + b, hence or otherwise,
explain how Planck’s constant can be calculated from the, graph.
(b) Express equation 9-8 in the form y = ax + b, hence or otherwise
explain how Planck’s constant can be calculated from the graph.
Aim: To graphically analyse the use of photoelectric equation.
4. In an experiment to measure the Planck’s constant, a light emitting
diode (LED) was used. Fig. 1-6 was plotted for varying energy of
the photon and frequency of the diode. Use the graph to answer thequestions that follow.
(a) Determine the slope of the line.
(b) What are the intercepts of the graph?
(c) Write down the equation of the line.
(d) What do you think is the vertical intercept?
(e) What is the value of the Planck’s constant?
(f) Write the Einstein photoelectric equation in relation to the answerof (e)
9.13 APPLICATION OF PHOTOELECTRIC EFFECT
(PHOTO EMISSIVE AND PHOTOVOLTAIC CELLS)
a) Photo electric cell
Photoelectric effect is applied in photoelectric cells or simply photocells.
These cells change light energy into electric current. Photoelectric cell
makes use of photoelectric effect and hence converts light energy into
electrical energy. The strength of the current depends on the intensity of
light falling on the cathode.
A photocell consists of an evacuated tube which is transparent to radiations
falling on it. It contains two electrodes; a semi-cylindrical cathode coated
with photosensitive material and an anode consisting of a straight wire orloop.
When radiations fall on the cathode, photoelectrons are emitted which are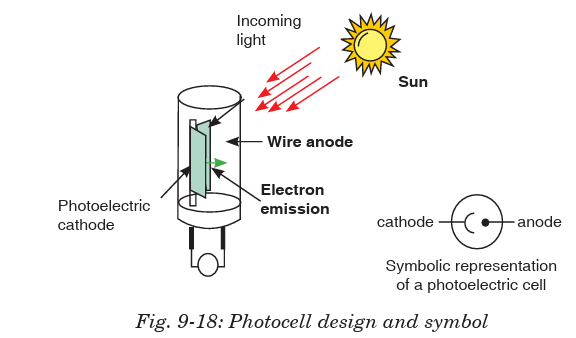
collected by the anode if it is positive with respect to the cathode. They,
then, go through the external circuit causing electric current. As intensity
of radiations increases, the number of electrons emitted by photoelectric
effect also increases. Hence current also increases.
An everyday example is a solar powered calculator and a more exoticapplication would be solar panels and others.
b) Automatic door opener
• Automatic doors operate with the help of sensors. Sensors do exactly
what they sound like they would do:
They sense things. There are many different types of sensors that cansense different types of things, such as sound, light, weight, and motion.
c) Smoke detectors
• Photoelectric Smoke Detectors. A photoelectric smoke detector is
characterized by its use of light to detect fire. The alarm detects smoke;
when smoke enters the chamber, it deflects the light-emitting diode light
from the straight path into a photo sensor in a different compartment inthe same chamber.
d) Remote control
• An Infra-Red (IR) remote (also called a transmitter) uses light to carry
signals from the remote to the device it controls. It emits pulses of
invisible infrared light that correspond to specific binary codes. Radiofrequency remotes work in a similar way.
9.14 COMPTON EFFECT
Convincing evidence that light is made up of particles (photons) and photons
have momentum can be seen when a photon with high energy hf collides
with a stationary electron.
Compton effect says that when x-rays are projected on the target, they
are scattered after hitting the target and change the direction they were
moving. This means that as a photon interacts with a free electron, the
process of photon absorption is forbidden by conservation laws, but the
photon scattering may occur. If the electron was originally at rest, then, as
a result of interaction, it acquires a certain velocity.
The energy conservation laws require that the photon energy decreases by
the value of the electron kinetic energy, which means that its frequency
must also decrease. At the same time, from the viewpoint of the wave
theory, the frequency of scattered light must coincide with the frequency ofincident light.


The photon scattering on an electron can be considered as an elastic collision
of two particles obeying the energy and momentum conservation laws
END OF UNIT ASSESSMENT
1. Describe briefly the two conflicting theories of the structure of the atom.
2. Why was the nuclear model of Rutherford accepted as correct?
3. What would have happened if neutrons had been used in Rutherford’s
experiment? Explain your answer.
4. What would have happened if aluminium had been used instead of gold
in the alpha scattering experiment? Explain your answer.
5. What three properties of the nucleus can be deduced from the Rutherford
scattering experiment? Explain your answer.
6. Monochromatic light of wavelength 560 nm incident on a metal
surface in a vacuum photocell causes a current through the cell due to
photoelectric emission from the metal cathode. The emission is stopped
by applying a positive potential of 1.30 V to the cathode with respect to
the anode. Calculate:
(a) the work function of the metal cathode in electron volts.
(b) the maximum kinetic energy of the emitted photoelectrons when
the cathode is at zero potential.
7. In a Compton scattering experiment, the wavelength of scattered
X-rays for scattering angle of 45 degree is found to be 0.024 angstrom.
(a) What is the wavelength of the incident photon?
(b) What is the percentage change in the wavelength on Compton
scattering?
8. You use 0.124-nm x-ray photons in a Compton-scattering experiment.
(a) At what angle is the wavelength of the scattered x-rays 1.0%
longer than that of the incident x-rays?
(b) At what angle is it 0.050% longer?
9. (a) What is the energy in joules and electron volts of a photon of 420
nm violet light?
(b) What is the maximum kinetic energy of electrons ejected from
calcium by 420-nm violet light, given that the binding energy (or
work function) of electrons for calcium metal is 2.71 eV?
10. An electron and a positron, initially far apart, move towards each other
with the same speed. They collide head-on, annihilating each other and
producing two photons. Find the energies, wavelengths and frequencies
of the photons if the initial kinetic energies of the electron and positron are
(a) both negligible and
(b) both 5.000 MeV. The electron rest energy is 0.511 MeV.
11. (a) Calculate the momentum of a visible photon that has a wavelength
of 500 nm.
(b) Find the velocity of an electron having the same momentum.
(c) What is the energy of the electron, and how does it compare with
the energy of the photon?
12. For an electron having a de Broglie wavelength of 0.167 nm (appropriate
for interacting with crystal lattice structures that are about this size):
(a) Calculate the electron’s velocity, assuming it is non-relativistic.
(b) Calculate the electron’s kinetic energy in eV.
UNIT SUMMARY
Structure of atom
An atom is a sphere in which positively charged particles called protons and
negatively charged particles called electrons are embedded.
Rutherford’s atomic model
Rutherford performed experiments by the scattering of alpha particles on
extremely thin gold foils. From these experiments, a new model of the atom
called Rutherford’s planetary model of the atom was born. The following
conclusions were made as regard as atomic structure:
• Most of the mass and all of the charge of an atom concentrated in a
very small region which is called atomic nucleus.
• Nucleus is positively charged and its size is of the order of 10–15 m ≈ 1
Fermi.
• In an atom, there is maximum empty space and the electrons revolve around
the nucleus in the same way as the planets revolve around the sun.
Bohr’s atomic model
Bohr’s model is based on the following postulates:
• Each electron moves in a circular orbit centered at the nucleus.
• The centripetal force needed to the electron moving in a circle is
provided by electrostatic force of attraction between the nucleus and
electrons.
• The angular momenta of electrons are whole number multiples of
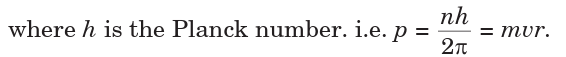
• When electron moves in its allowed orbit, it doesn’t radiate energy.
The atom is then stable, such stable orbits are called stationary orbits.
• When an electron jumps from one allowed orbit to another it radiates
energy. The energy of radiation equals energy difference between levels.
hf = Ei – Ef
Energy levels and spectral lines of Hydrogen
When hydrogen atom is excited, it returns to its normal unexcited (or ground
state) state by emitting the energy it had absorbed earlier. Transition from
different orbits cause different wavelengths. These constitute spectral
series which are characteristic of the atom emitting them.
The spectral lines arising from the transition of electron forms a spectra
series. Mainly there are five series and each series is named after its
discover as Lyman series, Balmer series, Paschen series, Brackett series
and Pfund series.
Thermionic emission
Thermionic emission or discharge of electrons from heated materials, is
widely used as a source of electrons in conventional electron tubes (e.g.,
television picture tubes) in the fields of electronics and communications.
Applications of cathode rays
• Cathode ray oscilloscope
• TV tubes
Fluorescence and phosphorescence
Fluorescence is the emission of light by a substance that has absorbed light
or other electromagnetic radiation.
Phosphorescence is a specific type of photoluminescence related to
fluorescence. Unlike fluorescence, a phosphorescent material does not
immediately re-emit the radiation it absorbs.
Photoelectric emission laws’
Law 1: The photo current is directly proportional to the intensity of light
and is independent of frequency.
Law 2: The kinetic energy of the photo electrons is directly proportional to
frequency and is independent of intensity.
Law 3: Photoelectric effect does not happen when the incident frequency is
less than a minimum frequency (threshold frequency).
Law 4: There is no time lag between the incidence of photon and emission
of electrons.
Photoelectric effect
The photoelectric effect is the emission of electrons from the surface of a
metal when electromagnetic radiation (such as visible or ultraviolet light)
shines on the metal.
Factors affecting photoelectric emission
• Intensity of Light:
• Frequency:
• Number of Photoelectrons
• Kinetic Energy of Photoelectrons
Einstein’s equation photoelectric effect
Einstein suggested that the energy of the incident radiation hf was partly
used to free electrons from the binding forces on the metal and the rest
of the energy appeared as kinetic energy of the emitted electrons and his
famous equation is;
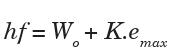
If the reverse potential difference applied on the circuit is increased until
no electron reaches the anode, no current flows and this applied potential
is called a stopping potential. This changes the Einstein’s photoelectricequation to;

Application of photoelectric effect
Photoelectric effect is applied in photoelectric cells or simply photocells.
These cells change light energy into electric current. Photoelectric cell
makes use of photoelectric effect and hence converts light energy into
electrical energy. The strength of the current depends on the intensity oflight falling on the cathode.
Compton effect
Compton effect says that when x-rays are projected on the target, they
are scattered after hitting the target and change the direction they were
moving.The Compton equation (or Compton shift) is given by;