UNIT 1:WAVE AND PARTICLE NATURE OF LIGHT
Key unit competence: Analyze the nature of light.
Unit Objectives:
By the end of this unit I will be able to;
◊ Explain the Planck’s quantum theory and apply it to other theories.
◊ Explain photoelectric effect and use it to derive and apply
Einstein’s photoelectric equation
◊ explain photoelectric effect and use it to derive and apply
Einstein’s photoelectric equation.
◊ Explain the wave theory of light and state its limitations.
◊ Evaluate properties of light as a wave.
◊ Differentiate electron microscope and Compton Effect as applied inmedecine.
1.0 INTRODUCTION
Until the late 19th century physicists used to explain the phenomena
in the physical world around them using theories such as mechanics,
electromagnetism, thermodynamics and statistical physics that are known
as classical theories.
At the turn of the 19th century, more and more experiments showed effects
that could not be explained by these classical theories. This indicated a need
for a new theory that we now know as quantum mechanics. Quantum
mechanics is the system of laws which governs the behaviour of matter on
the atomic scale. It is the most successful theory in the history of science,
having withstood thousands of experimental tests without a single verifiable
exception. So, the quantum mechanics is required to analyze the behaviour
of photons, electrons and other particles that make up the universe.
This theory is the most useful in various studies especially for Radiography
and Physiotherapy in Medicine, electrons and photons in Chemistry and
Astronomy in Geography.
Introductory Activity
Clearly observe the image shown on Fig.1-1, with kids playing on a
slide with the help of their father Mr. John and answer the questionsthat follow.
a) Sarah is climbing the ladder. How do you think her potential energy
is changing?
b) Comment on the potential energies of Jovia and Peter.c) How is the change in the potential energy of Jovia as she slides down?
What do you think is Mr. John doing on the young kid? Give your comments.
Fig.1.2 below shows how light interacts with an electron. F and B arethe terminals of the circuit (the wires of an external circuit).
The working mechanism of Fig.1.2 is used in solar cells and solar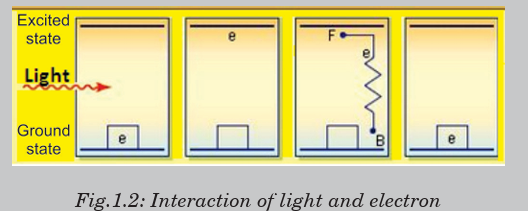
panels. Clearly analyse Fig.1.2 and compare it with the situation on
Fig.1.1, take children as electrons at different points or positions, andmake your comments.
1.1 NATURE AND PROPERTIES OF LIGHT
1.1.1 Particle theory of light
The nature and properties of light has been a subject of great interest and
speculation since ancient times. Until the time of Isaac Newton (1642
1727), the Greeks believed that light consisted of tiny particles (corpuscles)
that either were emitted by a light source or emanated from the eyes of the
viewer.
Newton the chief architect of the particle theory of light held that light
consisted of tiny particles that were emitted from a light source and that
these particles stimulated the sense of sight upon entering the eye. Using
this idea (particle theory), he was able to explain reflection and refraction
(bending) of light.
However , his derivation of the law of refraction depend on the assumption
that light travels faster in water and in glass than in air, an assumption
later shown to be false.Most scientists accepted Newton’s particle theory.
1.1.2 Wave theory and Planck’s quantum theory of light.
Does light exhibit diffraction? In the mid-seventeenth century, the Jesuit
priest Francesco Grimaldi (1618–1663) had observed that when sunlight
entered a darkened room through a tiny hole in a screen, the spot on the
opposite wall was larger than would be expected from geometric rays. He
also observed that the border of the image was not clear but was surrounded
by colored fringes. Grimaldi attributed this to the diffraction of light.
In 1678, one of Newton’s contemporaries, the Dutch physicist and astronomer Christian
Huygens (1629–1695), was able to explain many otherproperties of light by proposing that light is a wave.
According to the Huygens’ wave theory:
- Light travels in the form of longitudinal waves which travel with uni
form velocity in homogeneous medium.
- Different colours are due to the different wavelengths of light waves.
- We get the sensation of light when these waves enter our eyes.
- In order to explain the propagation of waves of light through vacuum,
Huygens suggested the existence of a hypothetical medium called alu
miniferous ether, which is present in vacuum as well as in all material objects. Since ether couldn’t be detected, it was attributed properties like:
- It is continuous and is made up of elastic particles.
- It has zero density.
- It is perfectly transparent.
- It is present everywhere
refraction of light by assuming that light travels more slowly in water and
in glass than in air.
Huygens’ Principle is particularly useful for analyzing what happens when
waves run into an obstacle. The bending of waves behind obstacles into
the “shadow region” is known as diffraction. Since diffraction occurs for
waves, but not for particles, it can serve as one means for distinguishingthe nature of light.
The Huygens’ Principle of the wave theory of light states that: “Every point
on a wavefront may be considered a source of secondary spherical wavelets
which spread out in the forward direction at the speed of light. The newwavefront is the tangential surface to all of these secondary wavelets.”
In 1801, the Englishman Thomas Young (1773–1829) provided the first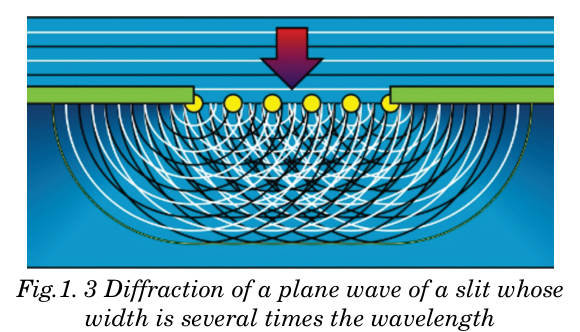
clear demonstration of the wave nature of light and showed that light beams
can interfere with one another, giving strong support to the wave theory.
Young showed that, under appropriate conditions, light rays interfere with
each other. Such behavior could not be explained at that time by a particle
theory because there was no conceivable way in which two or more particles
could come together and cancel one another.
The general acceptance of wave theory was due to the French physicist
Augustin Fresnell (1788-1827), who performed extensive experiments on
interference and diffraction and put the wave theory on a mathematical
basis. In 1850, Jean Foucault measured the speed of light in water and
showed that it is less than in air, thus ruling out Newton’s particle theory.
However, in 1900, German Physicist Max Planck (1858–1947) returned
to the particle theory of light to explain the thermal radiation emitted by
hot objects. To explain these radiations, Max Planck put forward a theory
known as Planck’s quantum theory suggests that:
1. The matter is composed of a large number of oscillating particles. These
oscillators have different frequencies.
2. The radiant energy which is emitted or absorbed by the blackbody is not
continuous but discontinuous in the form of small discrete packets of
energy and each such packet of energy is called a ‘quantum’. In case of
light, the quantum of energy is called a ‘photon’.
3. The energy of each quantum is directly proportional to the frequency (f)of the radiation, i.e.
whereas c is the speed of light, l is the wavelength and h is the Planck’s
constant (h = 6.63 × 10–34 J.s.).
4. The oscillator emits energy, when it moves from one quantized state to
the other quantized state. The oscillator does not emit energy as long as
it remains in one energy state. The total amount of energy emitted orabsorbed by a body will be some whole number quanta. Hence,

where n is an integer.
According to the Planck’s theory, the exchange of energy between quantized
states is not continuous but discrete. This quantized energy is in small
packets of bundles. The bundle of energy or the packet of energy is called
quantum (plural quanta).
1.1.3 Wave particle duality of light
Today, scientists view light as having a dual nature—that is, light exhibits
characteristics of a wave in some situations and characteristics of a particle
in other situations.
Although the wave model and the classical theory of electromagnetism
were able to explain most known properties of light, they could not explain
some subsequent experiments. The most striking of these is the photoelec
tric effect, also discovered by Hertz: When light strikes a metal surface,
electrons are sometimes ejected from the surface. As one example of the
difficulties that arose, experiments showed that the kinetic energy of an
ejected electron is independent of the light intensity. This finding contra
dicted the wave theory, which held that a more intense beam of light shouldadd more energy to the electron.
In view of these developments, light must be regarded as having a dual nature:
Wave-particle duality postulates that all particles exhibit both wave
properties and particle properties.
• Phenomena of light like interference, diffraction and polarization can
be explained by wave theory and not by particle nature of light.
• Energy distribution in perfect blackbody radiation, photo electric effect
and Compton Effect can be explained by particle nature of light and
not by wave theory. The concept of quantum mechanics is applied even
to the motion of electrons in an atom in Bohr’s atomic model.
If light waves can behave like particles, can the particles of matter behave
like waves? As we will discover, the answer is a resounding yes. Electrons
can be made to interfere and diffract just like other kinds of waves. Light
is light, to be sure. However, the question “Is light a wave or a particle?” is
inappropriate. Sometimes light acts like a wave, and at other times it acts like a particle.
1.1.4 The principle of complementarities
The principle of complementarities refers to the effects such as wave particle
duality in which different measurements made on the system reveal it to have
either particle-like or wave-like properties. Both properties are necessary to
gain the complete knowledge of the phenomena; they are complementary to
each other; but at the same time, they also exclude each other.
Within the scope of classical physics, all characteristic properties of a given
object can be ascertained by a single experimental arrangement, although
in practice various arrangements are often convenient for the study of
different aspects of the phenomena. In fact, data obtained in such a way
simply supplement each other and can be combined into a consistent picture
of the behaviour of the object under investigation. In quantum physics,
however, evidence about atomic objects obtained by different experimental
arrangements exhibits a novel kind of complementary relationship.
EXAMPLE 1.1
The laser in a compact disc player. It uses light with a wavelength of
7.8 × 102 nm. Calculate the energy of a single photon of this light.
Solution:From Equation 1.2,
EXAMPLE 1.2
What is the ratio between the energies of two radiations, one with awavelength of 200 nm and the other with 600 nm?
The energy is inversely proportional to the wavelength.
Application Activity 1.1
1. Which of the following can be thought of as either a wave or a
particle?
a. A.Light.
b. B.An electron.
c. C.A proton.
d. D.All of the above.
2. Electrons and photons of light are similar in that
a. Both have momentum given by
b. Both exhibit wave–particle duality.
c. Both are used in diffraction experiments to explore structure.
d. All of the above
e. None of the above
3. What is quantum mechanics?
4. What is Planck’s quantum theory?
5. Explain Planck’s hypothesis or what are the postulates of Planck’s
quantum theory?
6. A laser emits light energy in short pulses with frequency 4.69 ×
1014 Hz and deposits 1.3 × 10–2 J for each pulse. How many quanta
of energy does each pulse deposit?
7. A laser pointer with a power output of 5.00 mW emits red light
a. What is the magnitude of the momentum of each photon?
b. How many photons does the laser pointer emit each second?
8. a. Light of a certain orange colour has a wavelength of 589 nm.
What is the energy of one photon of this light? Speed of light
b. Show that the photons in a 1240 nm infrared light beam haveenergies of 1.00 eV.
1.2 PHOTON THEORY OF LIGHT AND
PHOTOELECTRIC EFFECT
Before Einstein, photoelectric effect had been observed by scientists, but
they were confused by the behavior because they didn’t fully understand
the nature of light. In the late 1800s, physicists James Clerk Maxwell in
Scotland and Hendrik Lorentz in the Netherlands determined that light
appear to behave as a wave. This was proven by seeing how light waves
demonstrate interference, diffraction and scattering, which are common to
all sorts of waves (including waves in water.)
So Einstein’s argument in 1905 that light can also behave as a set of
particles was revolutionary because it did not fit with the classical theory of
electromagnetic radiation. Other scientists had postulated the theory before
him, but Einstein was the first to fully elaborate on why the phenomenon
occurred – and the implications’. Einstein was awarded the Nobel Prize in
1921 for his discovery of the law of the photoelectric effect.
For example, a German physicist Heinrich Rudolf Hertz was the first
person to see the photoelectric effect, in 1887. He discovered that if he shone
ultraviolet light onto metal electrodes, he lowered the voltage needed to
make a spark move behind the electrodes, according to English astronomer
David Darling. In 1888 Hallwachs discovered that an insulated zinc plate,
negatively charged, lost its charge if exposed to ultraviolet light. So light
gives energy to the electrons in the surface atoms of the metal, and enablesthem to break through the surface. This called the photoelectric effect.
Photoelectric effect is the emission of electrons from the surface of metal
when illuminated with electromagnetic radiation of sufficient frequency.
This effect is mainly observed when charged surfaces are illuminated with
ultraviolet radiation. However, visible light can also cause photoelectric
effect on surfaces like cesium oxide. A material that exhibits photoelectriceffect is said to be Photosensitive.
An evacuated tube known as photocell contains a metal plate P connected
to a negative terminal of variable power supply and a smaller electrode C
connected at positive of variable power supply. The two electrodes are connected
to an ammeter and a source of emf, as shown in Fig.1.5.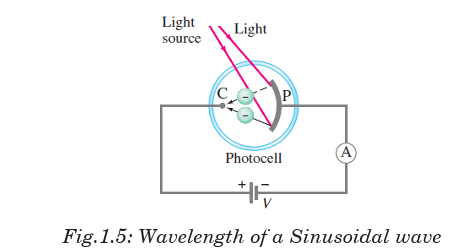
When the photocell is in the dark, the ammeter reads zero. But when light
of sufficiently high frequency illuminates the plate, the ammeter indicates
a current flowing in the circuit across the gap between P and C. This effect
is called the photoelectric effect and it occurs in many materials, but is
most easily observed with metals.
We explain completion of the circuit by imagining that electrons, ejected
from the plate by the impinging light, flow across the tube from the plate P
to a positive electrode called the “collector” C and cause a current to register
on the ammeter A as indicated in Fig. 1.5.
Photocurrent is the current that flows through a photosensitive device,
such as a photodiode, as the result of exposure to radiant power. The photo
current may occur as a result of the photoelectric, photo emissive or photovoltaic effect.
1.3 PROPERTIES OF A LIGHT WAVE
The properties of waves include the following:
The wavelength of a wave is defined as the distance over which the wave’s
shape repeats.
It is the distance between the corresponding points on successive cycles,
eg. the distance between two wave crests is known as wavelength of a
sinusoidal wave. It is measured in units of length (metres, nanometres).
The wavelength is usually represented by the symbol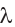 (lambda).
(lambda).
A measurement of the wavelength is made by observing the wave in spaceat a single instant of time.
Amplitude: The maximum displacement of wave quantity relative to the
undisturbed, equilibrium position of a particle is called amptitude. for
example, height of water wave, pressure of sound wave, maximum electric
field, etc.
Periodic time: This is the time between two successive wave crests or
successive wave troughs. It is measured in units of time (second). The period
is often represented by the letter T. It is measured by observing the wave
displacement at a single point in space.
Frequency: The number of cycles per second of the wave quantity, measured
in hertz (Hz) is called frequency. The frequency is usually represented by
the letter f. The observation of the frequency is made at a single point inspace.
Phase angle: The number of units of angular measure between a point on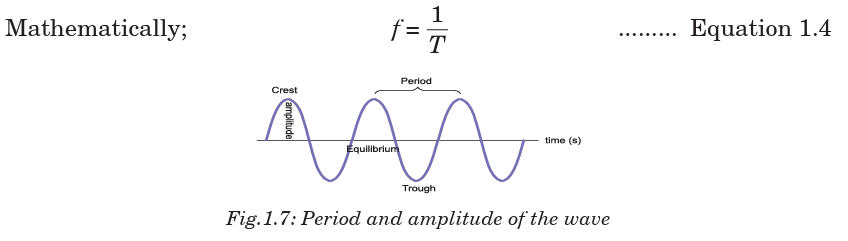
the wave and a reference point in a periodic wave is called phase angle.
The phase angle at any point is calculated using simple proportions as
shown below. Where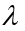 is the wavelength, x is any horizontal distance
is the wavelength, x is any horizontal distance and is the phase angle corresponding to the horizontal displacement.
ACTIVITY 1-1: Properties of waves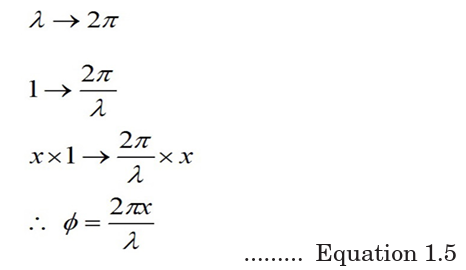
The curve of Fig.1.9 shows thevariation of height reached by
a vibrating object against the
horizontal distance it can cover.
Study the curve and answer the
questions that follow.
From the graph find;
(a) The amplitude of the wave.
(b) The wavelength of the wave.
(c) What do we call point A?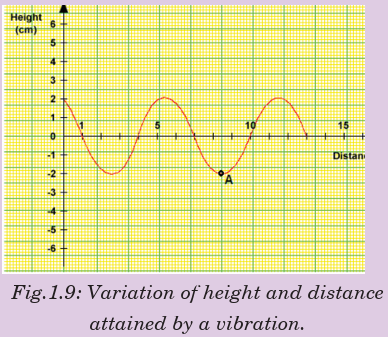
1.4 BLACKBODY RADIATION
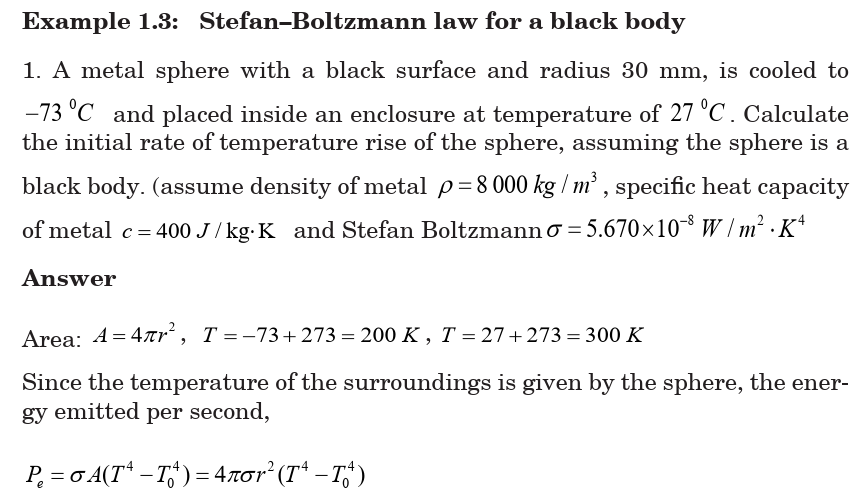
1.4.1 Stefan–Boltzmann law for a black body
By 1900 blackbody radiation had been studied extensively, and three
characteristics had been established in Stefan–Boltzmann law for a
black body:
All objects, no matter how hot or cold, emit electromagnetic radiation
(thermal radiation) whose total intensity I (the average rate of radiation
of energy per unit surface area per unit time or average power per area)
emitted from the surface of an ideal radiator is proportional to the fourthpower of the Kelvin (absolute) temperature.

1.4.2 Wien’s displacement law
Fig, 1.10 shows the measured spectral emittances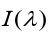 for blackbody
for blackbody
radiation at three different temperatures. Each has a peak wavelength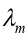
at which the emitted intensity per wavelength interval is largest.
Experiment shows that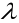 is inversely proportional to T, so their product is
is inversely proportional to T, so their product is constant. This observation is called the Wien displacement law.
The spectrum of the radiation depends on the temperature and the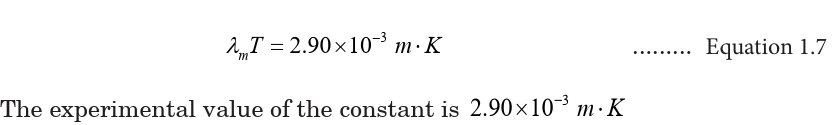
properties of the object.
At normal temperatures we are not aware of this electromagnetic radiation
because of its low intensity. At higher temperatures, there is sufficient
infrared radiation that we can feel heat if we are close to the object. At still
higher temperatures (on the order of 1000 K), objects actually glow, such
as a red-hot electric stove burner or the heating element in a toaster. At
temperatures above 2000 K, objects glow with a yellow or whitish color,such as white-hot iron and the filament of a light bulb
The spectrum of light emitted by a hot dense object is shown in Fig. 1.10 for
an idealized blackbody. The radiation such an idealized blackbody would
emit when hot and luminous, called blackbody radiation (though not
necessarily black in color), and approximates that from many real objects.
The 6000 K curve in Fig. 1.10, corresponding to the temperature of the
surface of the Sun, peaks in the visible part of the spectrum. For lower
temperatures, the total intensity drops considerably and the peak occurs
at longer wavelengths (or lower frequencies). This is why objects glow
with a red color at around 1000 K. Measured spectra of wavelengths and
frequencies emitted by a blackbody at three different temperatures.
Example 1.4: The Sun’s surface temperature and temperature
1. Estimate the temperature of the surface of our Sun, given that the Sun
emits light whose peak intensity occurs in the visible spectrum at around
500 nm.
AnswerWe assume the Sun acts as a blackbody, and use in Wien’s law (Eq. 1.08).
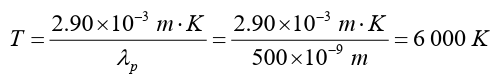
Application Activity 1.2
1. Electromagnetic radiations are emitted by which of the following?
a. Only by radio and television transmitting antennas
b. Only bodies at temperature higher than their surrounding
c. Only by red-hot bodiesd. By all bodies
2. Which of the following statements is true regarding how blackbody
radiation changes as the temperature of the radiating object
increases?
a. Both the maximum intensity and the peak wavelength
increase.
b. The maximum intensity increases, and the peak wavelength
decreases.
c. Both the maximum intensity and the peak wavelength
decrease.
d. The maximum intensity decreases, and the peak wavelength
increases.
3. Which of the following statements is true regarding how blackbody
radiation changes as the temperature of the radiating object
increases?
a. Both the maximum intensity and the peak wavelength
increase.
b. The maximum intensity increases, and the peak wavelength
decreases.
c. Both the maximum intensity and the peak wavelength
decrease.
d. The maximum intensity decreases, and the peak wavelength
increases.
4. A black body is one that
a. Transmit all incident radiations
b. Absorbs all incident radiations
c. Reflects all incident radiations
d. Absorbs, reflects and transmits all incident radiations
5. The black body spectrum of an object A has its peak intensity at
200 nm while that of another object of same shape and size has its
peak at 600 nm. Compare radiant intensities of the two bodies.
6. The sun emits mostly in the visible region. Compare the total
intensity of radiation emitted by a star of similar size as the sun
whose surface temperature is 7 200 K.
7. Estimate the radiant energy emitted by a blackbody at 6 000 K
8. The sun’s surface temperature is 5 700 K. How much power is
radiated by one square meter of the sun’s surface? Given that the
distance to earth is about 200 sun radii, what is the maximum power
possible from a one square kilometer solar energy installation?
ACTIVITY 1-2: Blackbody Rediation
Discuss blackbody radiation in group and ask questions.
1.5 ENERGY, MASS AND MOMENTUM OF A PHOTON
The famous Einstein equation of energy of the photon is E = mc2. In short,
the equation describes how energy and mass are related with speed of light.
To derive this equation, consider an X-ray photon of mass m hitting the
surface of a metal and consider if a part of its energy is gained by a surface
electron and is then emitted.
The most important laws in dynamics are those that state the conservation
of energy and the conservation of momentum. These two laws can be applied
whenever we have a closed system; that is, a system that does not interact
with its surroundings. They assert that for such systems and any process
they may undergo. Assume that; E is the energy, s is the distance, F is theforce, c is the speed, t is the time, and P is the momentum
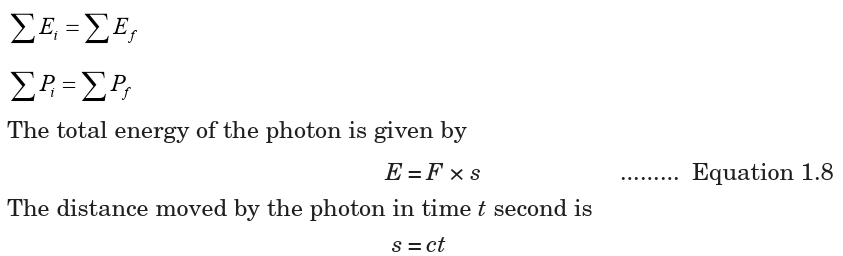

Application Activity 1.3
The mass of an electron or positron is 9.11 × 10–31 kg. The speed of light
is 3.0 × 108 m/s.
1. Show that the rest energy of an electron is 8.2 × 10–14J.
2. Use the answer to question 1, to show that the rest energy of an
electron is 0.51 MeV.
3. Write down the rest energy of a positron (antielectron).
4. An electron and a positron meet and annihilate one another. By how
much does the rest energy decrease in total? Express the answer in MeV.
5. The annihilation of an electron and a positron at rest produces a pair
of identical gamma ray photons travelling in opposite directions.
Write down (in MeV) the energy you expect each photon to have.
6. A single photon passing near a nucleus can create an electron
positron pair. Their rest energy comes from the energy of the photon.
Write down the smallest photon energy that can produce one such pair.
7. Cosmic rays can send high-energy photons through the atmosphere.
What approximately is the maximum number of electron–positronpairs that a 10 GeV photon can create?
1.6 COMPTON EFFECT AND PHOTON INTERACTIONS
1.6.1 Compton effect
The Compton Effect concerns the inelastic scattering of X-rays by electrons.
Scattering means dispersing in different directions and inelastic means
that energy is lost by the scattered object in the process. The intensity of
the scattered X-ray is measured as a function of the wavelength shift.
Photons are electromagnetic radiation with zero mass, zero charge, and a
velocity that is always equal to the speed of light. Because they are electrically
neutral, they do not steadily lose energy via Coulombic interactions with
atomic electrons, as charged particles do. Photons travel some considerable
distance before undergoing a more “catastrophic” interaction leading to
partial or total transfer of the photon energy to electron energy. These
electrons will ultimately deposit their energy in the medium. Photons are
far more penetrating than charged particles of similar energy. There are
many types of photon interactions. We will only discuss those that are
important in radiation therapy and/or diagnostic radiology.
1.6.2 Types of photon interactions
Coherent scattering
Coherent scattering is one of three forms of photon interaction which occurs
when the energy of the X-ray or gamma photon is small in relation to the
ionisation energy of the atom. It therefore occurs with low energy radiation.
Upon interacting with the attenuating medium, the photon does not have
enough energy to liberate the electron from its bound state (i.e. the photon
energy is well below the binding energy of the electron), so no energy transfer
occurs. The only change is a change of direction (scatter) of the photon,
hence it is called ‘unmodified’ scatter. Coherent scattering is not a major
interaction process encountered in radiography at the energies normally
used. There are two types of coherent scattering: Thomson scattering and
Rayleigh scattering.
• In Thomson scattering, only one electron of the atom is involved in the
interaction.
• With Rayleigh scattering, all the electrons of the atom, sometimes
called the electron cloud, are involved in a cooperative effort in the
interaction with the photon.
Photoelectric effect
The following points make this phenomena clear:
1. The photon must have an energy equal to or greater than the binding2. The incident photon must be completely absorbed by the electron.energy of electron in the atom.
3. The electron is then ejected from the atom.
4. The excess energy over the binding energy is given to the electron in
the form of kinetic energy (which is the speed of the electron).
5. The hole left in the atom is filled by an outer shell electron or a free
electron with the emission of characteristic radiation.
Compton interaction
In Compton interaction, the photon interacts with a ‘free’ or an outer shell
electron. A portion of incident energy of the photon will be transferred to
an electron in the form of kinetic energy. The incident photon, now called
a scattered photon will be deflected in a new direction with less energy.
Energy given to recoil electron is considered as the absorbed energy and the
energy retained by the photon is considered scattered.
Pair Production
The photon interacts with the nuclear field of the atom, in such a way, that
the photon transforms itself into an electron-positron pair. As the photon
interacts with the strong electric field around the nucleus, it undergoes a
change of state and is transformed into two particles (essentially creating
matter from energy).Photodisintegration
(Photo transmutation) It is a nuclear reaction in which the absorption of
high energy electromagnetic radiation (a gamma-ray photon) causes the
absorbing nucleus to change to another species by ejecting a subatomic
particle, such as a proton, neutron, or alpha particle.
ACTIVITY 1-2: Compton Effect.
Aim: In this activity you will be able to highlight the most important terms
in Compton effect
Question: highlight at least 17 important terms you may need to explain
photoelectric effect and photo interaction. Use these terms to construct at
least 5 sentences to explain this theory 1.7 THE WAVE NATURE OF MATTERBeing fully aware of the pioneering work of Einstein on the photoelectriceffect, de Broglie extended the notion of wave particle duality to matter.
1.7 THE WAVE NATURE OF MATTERBeing fully aware of the pioneering work of Einstein on the photoelectriceffect, de Broglie extended the notion of wave particle duality to matter.
All matter can exhibit wave-like behaviour. For example, a beam of electron
can be diffracted just like a beam of light or a water wave.
The concept that matter behaves like a wave is also referred to de Broglie
hypothesis.
The de Broglie wavelength is the wavelength, , associated with a massive
, associated with a massive
particle and is related to its momentum p. With p being the particle’s momentum. The particles are diffracted bypassing through an aperture in a similar manner as light waves. The waveproperties of particles mean that when you confine it in a small space its
With p being the particle’s momentum. The particles are diffracted bypassing through an aperture in a similar manner as light waves. The waveproperties of particles mean that when you confine it in a small space its
momentum and kinetic energy must increase.
 This wavelength is about the size of the interatomic spacing in solid andtherefore, leads to the observed diffraction effects.
This wavelength is about the size of the interatomic spacing in solid andtherefore, leads to the observed diffraction effects.
b) de Broglie wavelength of the baseball: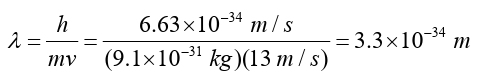 The de Broglie wavelength is very small as compared to the size of body.This why wave nature of matter is not noticeable in our diary life.
The de Broglie wavelength is very small as compared to the size of body.This why wave nature of matter is not noticeable in our diary life.
1.8 ELECTRON MICROSCOPE
A microscope can be defined as an instrument that uses one or several
lenses to form an enlarged (magnified) image. Microscopes can be classified
according to the type of electromagnetic wave employed and whether this
wave is transmitted or not through the specimen. The most common electron
microscopes are Transmission Electron Microscope (TEM) and Scanning
Electron Microscope (SEM). As it passes down through the tube the electron beam is controlled byelectromagnetic lenses formed by coils around the tube (whose effect ismoderated by adjusting the electricity flowing through the coils). These
As it passes down through the tube the electron beam is controlled byelectromagnetic lenses formed by coils around the tube (whose effect ismoderated by adjusting the electricity flowing through the coils). These
electromagnetic lenses direct the electron beam through the centre of the
tube to a very thin specimen located part-way down the tube.
Some parts of the specimen might allow electrons to pass through them
unaffected. Other regions within the specimen absorb some or all of the
electrons that reach them. If any electrons continue from that part of the
specimen further down the tube to the image formation plane with less
energy. This happens because some of their energy has been absorbed by,
or “passed to”, the part of the specimen that the electron(s) passed through.TEM Applications• TEMs provide topographical, morphological, compositional and
crystalline information.
• It is useful in the study of crystals and metals, but also has industrial
applications.
• TEMs can be used in semiconductor analysis and the manufacturing
of computer and silicon chips.
• Tech giants use TEMs to identify flaws, fractures and damages to
micro-sized objects; this data can help and fix problems and/or help to
make a more durable efficient product.
• Colleges and universities can utilize TEMs for research and studies.
1.8.2 Scanning Electron Microscope (SEM)
The SEM is designed for direct study of the surfaces of solid objects. By
scanning with an electron beam that has been generated and focussed by
the operation of the microscope, an image is formed in the same way as a TV.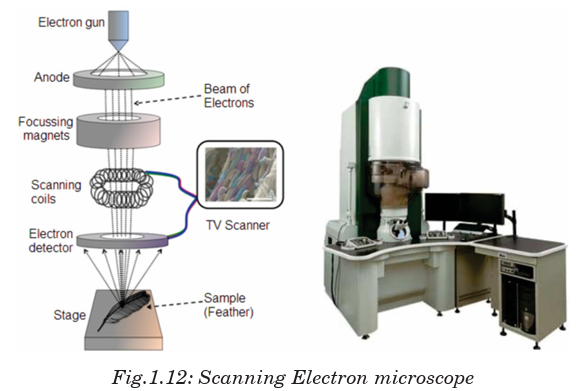 The SEM allows a greater depth of focus than the optical microscope. For this
The SEM allows a greater depth of focus than the optical microscope. For this
reason, the SEM can produce an image that is a good representation of the
three-dimensional sample.
The SEM uses electrons instead of light to form an image. A beam of electrons
is produced at the top of the microscope by heating a metallic filament. The
electron beam follows a vertical path through the column of the microscope.
It makes its way through electromagnetic lenses which focus and direct the
beam down towards the sample. Once it hits the sample, other electrons
(backscattered or secondary) are ejected from the sample. Detectors collect
the secondary or backscattered electrons, and convert them to a signal that is
sent to a viewing screen similar to the one in an ordinary television, producingThe SEM allows a greater depth of focus than the optical microscope. For thisreason, the SEM can produce an image that is a good representation of the
three-dimensional sample.
The SEM uses electrons instead of light to form an image. A beam of electrons
is produced at the top of the microscope by heating a metallic filament. The
electron beam follows a vertical path through the column of the microscope.
It makes its way through electromagnetic lenses which focus and direct the
beam down towards the sample. Once it hits the sample, other electrons
(backscattered or secondary) are ejected from the sample. Detectors collect
the secondary or backscattered electrons, and convert them to a signal that is
sent to a viewing screen similar to the one in an ordinary television, producing
an image. To produce an image on the screen, the electron beam scans over
the area to be magnified and transfers this image to the TV screen.
Applications of SEM
• Image morphology of samples (eg. view bulk material, coatings,
sectioned material, foils, even grids prepared for transmission electron
microscopy).
• Image composition and finding some bonding differences (through
contrast and using backscattered electrons).
• Image molecular probes: metals and fluorescent probes.
• Undertake micro and nano lithography: remove material from
samples; cut pieces out or remove progressive slices from samples (eg.
using a focussed ion beam).
• Heat or cool samples while viewing them (it is generally done only in
ESEM or during Cryo-scanning electron microscopy).
• Wet and dry samples while viewing them (only in an ESEM)
• View frozen material (in an SEM with a cryostage)
• Generate X-rays from samples for microanalysis (EDS; WDS) to
determine chemical composition.
• Study optoelectronic behaviour of semiconductors using
cathodoluminescence
• View/map grain orientation/crystallographic orientation and study
related information like heterogeneity and microstrain in flat samples
(Electron backscattered diffraction).
• Electron diffraction using electron backscattered diffraction. The
geometry may be different from a transmission electron microscope
but the physics of Bragg Diffraction is the same.
END OF UNIT ASSESSMENT1. Hydrogen has a red emission line at 656.3 nm, what is the energy and
frequency of photon of this light?
2. An FM radio transmitter has a power output of 100 kW and operates
at a frequency of 94 MHz. How many photons per second does the
transmitter emit?
3. State Huygens’ principle. State its application and explain the
construction of spherical wavefront.
4. Determine the de Broglie wavelength for the following:a. A moving golf ball (m = 0.05 kg, 40 / v m s ),
b. An orbiting electron in the ground state of hydrogen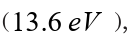
c. An electron accelerated through 100 kV in an electron microscope.
5. Determine the de Broglie wavelength of the matter wave associated with
a cricket ball of mass 0.175 kg and velocity 23.6 m/s. Use the answer
to this question to explain why we do not observe the matter waves
associated with macroscopic objects.6. Blue light of frequency 7.06 × 1014 Hz shines on sodium. Calculate the
maximum energy of the photoelectrons released.7. The range of frequency of ultraviolet rays is 7.9 × 1014 Hz to 5×1017 Hz.
What is corresponding range of energies of the photons of ultraviolet light? (Plank’s constant
8. Estimate how many visible light photons a 100 W light bulb emits per
second. Assume the bulb has a typical efficiency of about 3% (that is,
97% of the energy goes to heat).9. The following phenomena prove that light can behave like either a particle
or a wave: Reflection of light, refraction of light, interference of light,
photoelectric effect, Compton effect
a. What phenomena best prove that light is a particle instead of wave?
b. What phenomena best prove that light is a wave instead of particle?
10. One hundred years ago, Albert Einstein explained the photoelectric effect.
a. What is the photoelectric effect?
b. Write down an expression for Einstein’s photoelectric law.
c. Summarise Einstein’s explanation of the photoelectric effect
d. Give one application of the photoelectric effect.
11. Outline the advantages of Huygen’s wave theory of light.
12. If you pick up and shake a piece of metal that has free electrons, no
electrons fall out. Yet if you heat the metal, electrons can be boiled off.
Explain both of these facts and relate to the amount and distribution of
energy involved with shaking the object as compared with heating it.
13. Which formula may be used for the momentum of all particles, with or
without mass?
14. Is there any measurable difference between the momentum of a photon
and the momentum of matter?
15. Describe one type of evidence for the wave nature of matter.
16. Describe one type of evidence for the particle nature of EM radiation.
UNIT SUMMARY
Wave theory of monochromatic light: If light consists of undulations in
an elastic medium, it should diverge in every direction from each new centre
of disturbance, and so, like sound, bend round all obstacles and obliterate
all shadow.
A wave is any disturbance that results into the transfer of energy from one
point to another point.
Primary source: The geometrical centre or axis of the actual source of
light which is either a point or a line is called the primary source.
Wavelets: All points lying on small curved surfaces that receive light at the
same time from the same source (primary or secondary) are called wavelets.
Secondary source: Any point on a wavelet, acts as the source of light for
further propagation of light. It is called a secondary source.
Wavefront: The envelope of all wavelets in the same phase-receives light
from sources in the same phase at the same time is called a wavefront.
Wave normal: The normal at any point drawn outward on a wave front is
called the wave normal. Further propagation of light occurs along the wave
normal. In isotropic media the wave normal coincides with the ‘ray of light’.
A black body is a theoretical object that absorbs 100% of the radiation
that hits it and re-radiates energy which is characteristic of this radiatingsystem or body only.
The mass, energy and momentum of a photon are related according toequations;
Compton effect says that when X-rays are projected on the target, they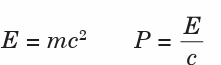
are scattered after hitting the target and change the direction in which they
were moving.
Photon interactions: because photons are electrically neutral, they do not
steadily lose energy via coulombic interactions with atomic electrons, as
do charged particles. Photon interactions include; Coherent Scattering,
Photoelectric Effect, Compton Interaction, Pair Production and
Photodisintegration.
Wave-particle duality of light: According to different experiments and
properties, light behaves as waves as well as particles.
Principle of complementarities: Both properties of light being a wave and
a particle are necessary to gaining complete knowledge of the phenomena;
they are complementary to each other but at the same time they also excludeeach other.
The wave nature of matter: The attribution of a wavelength to a massive
particle implies that it should behave as a wave under some conditions.
Electron microscope: is an instrument that uses one or several lenses to
form an enlarged (magnified) image. The most common electron microscopes
are Transmission Electron Microscopes (TEM) and Scanning ElectronMicroscope (SEM).
