Topic outline
UNIT 1: PRINCIPLES OF PHARMACOLOGY
Key Unit Competence
Apply fundamental principles of pharmacology during patient care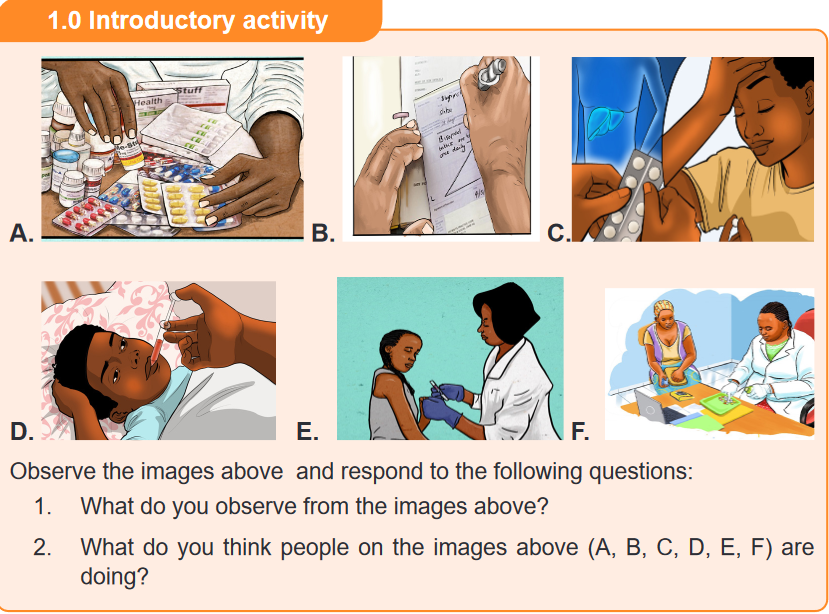
1.1. History of pharmacology
Learning activity 1.1
Read the case study below and answer the questions related to it:
A student in O’ level is concerned about different ways of managing illness.
Therefore, he asked different ways, sources and reasons of using medications.
As a student who has chosen the associate nursing program, you know that a
medicine is linked to the pharmacology science and you expect to use medicines
to help persons who have diseases.
1. What do you think are the sources of medicines?
2. What is the purpose of using drug substances in human kind?CONTENT SUMMARY
The story of pharmacology is rich and exciting, filled with accidental or unplanned
discoveries and landmark events. Its history likely began when a human first used
a plant to relieve symptoms of disease. One of the oldest forms of health care,
herbal medicine has been practiced in virtually every culture dating to antiquity /
ancient times.The Babylonians recorded the earliest surviving prescriptions on clay tablets
in 3000 Before Christ (BC), although magic and the art of reading omens were
probably considered as legitimate to healing as the use of drug remedies.At about the same time, the Chinese recorded the Pen Tsao (Great Herbal), a
40-volume compendium of plant remedies dating to 2700 BC. The Egyptians
followed in 1500 BC by archiving their remedies on a document known as the Eber
s papyrus, which contains over 700 magical formulas and remedies. Galen, the
famous Greek physician, described over 1,000 healing preparations using plant
products before his death in Dark Ages (AD) 201.Pharmacology as a distinct discipline was officially recognized when the first
Department of Pharmacology was established in Estonia in 1847. John Jacob
Abel, was considered as the father of American pharmacology due to his many
contributions to the field, founded the first pharmacology department in the United
States at the University of Michigan in 1890.Drugs are substances that are used in the diagnosis, prevention, treatment or
cure of diseases. In early times, these substances were derived from natural
sources, of which plants took up the major share. With the introduction of technology,
most drugs today are manufactured synthetically in the laboratory.The major sources of drugs can be grouped into the following: Plant, animal, mineral,
marine, synthetic/chemical derivative, Semi-synthetic, Microbiological and
Recombinant DNA technology/ Biosynthetic sources.1. Plant source
It is the oldest source of drugs. Most of the drugs in ancient times were derived
from plants. A number of plants have medicinal qualities and have been used for
centuries as drugs or drug sources. Although the earliest plant source for drugs
was the leaf, other parts of plants (e.g., barks, fruits, roots, stem, wood, seeds,
blossoms, bulb etc.) Almost all parts of the plants are used i.e. leaves, stem, bark,
fruits and roots.Leaves: The leaves of Digitalis Purpurea are the source of Digitoxin and Digoxin,
which are cardiac glycosides; used to treat HF (heart failure).
Leaves of Eucalyptus give oil of Eucalyptus, which is important component of cold
& cough syrup.
Flowers: Poppy papaver somniferous gives morphine (opoid), Vinca rosea gives
vincristine and vinblastine and Rose gives rose water used as tonic.Fruits: Senna pod gives anthracine, which is a purgative and Calabar beans Give
physostigmine, which is cholinomimetic agent.Seeds: Seeds of Nux Vomica give strychnine, which is a CNS stimulant and Castor
seeds give castor oil.Roots: Ipecacuanha root gives Emetine, used to induce vomiting as in accidental
poisoning, it also has amoebicidal properties.
Ipecacuanha root gives Emetine, used to induce vomiting as in accidental poising,
it also has ameobicidal properties.Rauwolfia serpentina gives reserpine, a hypotensive agent. Reserpine was used
for hypertension treatment.
Bark: Cinchona bark gives quinine and quinidine, which are antimalarial drugs.
Quinidine also has antiarrhythmic properties.
Cinchona hark gives quinine and quinidine, which are antimalarial drugs
Atropa belladonna gives atropine, which is anticholinergic. Hyoscyamus Niger
gives Hyosine, which is also anticholinergic.
Stem: Chondrodendron tomentosum gives tuboqurarine, which is skeletal muscle
relaxant used in general anaesthesia.2. Animal sources
Many important drugs are derived from animal source. In most instances, these
medicinal substances are derived from the animal’s body secretions, fluid or glands.
Insulin, heparin, adrenaline, thyroxin, cod liver oil, musk, beeswax, enzymes, and
antitoxins sera are some examples of drugs obtained from animal sources. Like
plant products, drugs from animal sources may be crude (unrefined) or refined
material.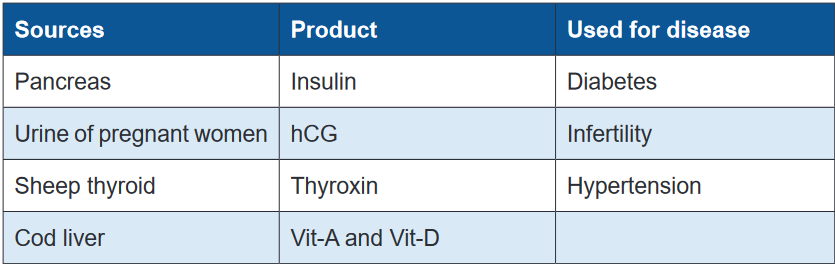
3. Mineral sources
Minerals (both metallic and non-metallic minerals) have been used as drugs
since ancient times. Our body requires trace elements of minerals in order to
maintain homeostasis. Patients lacking an adequate level of these materials may
take specific mineral-based drugs to raise the level of minerals.Examples include ferrous sulfate in iron deficiency anemia; magnesium sulfate as
purgative; magnesium trisilicate, aluminum hydroxide and sodium bicarbonate as
antacids for hyperacidity and peptic ulcer; zinc oxide ointment as skin protectant,
in wounds and eczema; gold salts as anti-inflammatory and in rheumatoid arthritis;
selenium as anti-dandruff.• Metallic and non-metallic sources: Iron is used in treatment of iron
deficiency anemia, Mercurial salts are used in Syphilis (bacterial infection),
Zinc is used as zinc supplement, Zinc oxide paste is used in wounds and in
eczema, Iodine is antiseptic and Iodine supplements are also used and Gold
salts are used in the treatment of rheumatoid arthritis• Miscellaneous sources: Fluorine has antiseptic properties, Borax has
antiseptic properties as well, Selenium as selenium sulphide is used in
antidandruff shampoos and Petroleum is used in preparation of liquid paraffin.4. Marine source (water source)
Bioactive compounds from marine flora and fauna have extensive past and
present use in the prevention, treatment or cure of many diseases. Fish andmarine microorganisms produce biologically potent chemicals with interesting anti-
inflammatory, anti-viral, and anticancer activity.5. Synthetic /chemical derivativeA synthetic drug is produced using chemical synthesis, which rearranges chemical
derivatives to form a new compound. The synthetic sources of drugs evolved with
human skills in the laboratory and advanced knowledge and understanding of
phytochemical investigation. When the nucleus of the drug from natural source
as well as its chemical structure is altered, we call it synthetic. Examples include
Emetine Bismuth Iodide. At present, majority of drugs used in clinical practice are
exclusively prepared synthetically in pharmaceutical and chemical laboratory.6. Semi-synthetic SourcesSemi-synthetic drugs are neither completely natural nor completely synthetic. They
are a hybrid and are generally made by chemically modifying substances that are
available from natural source to improve its potency, efficacy and/or reduce side
effects. Sometimes, semi-synthetic processes are used to prepare drugs when
the natural sources may yield impure compounds or when the synthesis of drugs
(complex molecules) may be difficult, expensive, and commercially unviable. When
the nucleus of drug obtained from natural source is retained but the chemical
structure is altered, we call it semi - synthetic. E.g. Apomorphine, Diacetyl morphine,
Ethinyl Estradiol, Homatropine, Ampicillin and Methyl testosterone7. Microbiological sourcesSeveral life-saving drugs have been historically derived from microorganisms.
Examples include penicillin produced by Penicillium chrysogenum,
streptomycin from Streptomyces griseus, chloramphenicol from Streptomyces
venezuelae, neomycin from Streptomyces fradiae, bacitracin from Bacillus
subtilis etc.
PenicilliumNotatum is a fungus which gives penicillin, Actinobacteria gives
Streptomycin, and Aminoglycosides such as gentamicin and tobramycin are
obtained from streptomycis and micromonosporas.8. Recombinant DNA technology/ Biosynthetic sources (genetically
engineered drugs)This is relatively a new field which is being developed by mixing discoveries fromDNA by enzyme restriction endonucleases. The desired gene is coupled to rapidly
molecular biology, recombinant DNA technology, DNA alteration, gene splicing,
immunology, and immune pharmacology. Drugs developed using living organisms
with the help of biotechnology or genetic engineering are known as biologics,
biopharmaceuticals, recombinant DNA expressed products, bioengineered, or
genetically engineered drugs Examples include recombinant Hepatitis B vaccine,
recombinant insulin and others. Recombinant DNA technology involves cleavage of
replicating DNA (viral, bacterial or plasmid). The new genetic combination is inserted
into the bacterial cultures which allow production of vast amount of genetic material.
Advantages: Huge amounts of drugs can be produced, Drug can be obtained in
pure form, and It is less antigenic (induce immune system). Disadvantages: Well-
equipped lab is required, highly trained staff is required and it is a complex and
complicated technique.Self- assessment 1.11. The use of the drug started when?2. What are the sources of drug?
3. Who first isolated morphine from opium in 1805?
4. Who is considered as the father of American pharmacology?1.2 Definition of key termsLearning activity 1.2As the new student admitted in Associate nurse program in senior 4, read the
book of pharmacology and define the following common key terms used in
pharmacology:
1. Pharmacology
2. Clinical pharmacology
3. Drugs
4. Adverse drug reaction and
5. Therapeutic effectCONTENT SUMMARYPharmacology: The word pharmacology is derived from two Greek words,
“pharmakon”, which means medicine or drug, and logos, which means study. It
is the study of medicines. It includes the study of how drugs are administered and
how the body responds (Adams et all 2014).It can be also defined as the study of drugs and their interactions with living systems.Clinical pharmacology: is defined as the study of drugs in humans.Drugs: chemicals that are introduced into the body to bring about some sort ofchange.Adverse drug reaction: Any unexpected, unintended, undesired, or excessive
response to a medication given at therapeutic dosages (Linder et al 2014).Drug actions: The processes involved in the interaction between a drug and body
cells (e.g., the action of a drug on a receptor protein); also called mechanism of
action.Drug classification: A method of grouping drugs; may be based on structure or
therapeutic use.Drug effects: The physiologic reactions of the body to a drug. They can be
therapeutic or toxic and describe how the body is affected as a whole by the drug.
The terms onset, peak, and duration are used to describe drug effects (most often
referring to therapeutic effects).Pharmacognosy The study of drugs that are obtained from natural plant and animal
sources.Therapeutic effect: The desired or intended effect of a particular medication.
Therapeutic index: The ratio between the toxic and therapeutic concentrations of
a drug.Tolerance: Reduced response to a drug after prolonged use.Toxic: The quality of being poisonous (i.e., injurious to health or dangerous to life).Toxicity: The condition of producing adverse bodily effects due to poisonous
qualities.Food and Drug Administration (FDA): federal agency responsible for the
regulation and enforcement of drug evaluation and distribution policiesSelf- assessment 1.2Define the following terms:1. Pharmacognosy
2. Therapeutic index
3. Tolerance1.3 Chemical drug nameLearning activity 1.31. Read the book of pharmacology and explain chemical drug name (using
library textbook)CONTENT SUMMARYDrugs are chemicals that are introduced into the body to bring about some sort of
change. The drugs have several names, which may cause confusion. Each drug
has three names: a chemical name, a generic name, and a brand name. The
health care professionals have to study pharmacology which is the study of drugs
and their interactions with living systems to know the exact medication to be used
and to control the complication associated.The chemical names are the scientific names, based on the molecular structure of
the drug. There are various systems of chemical nomenclature and thus various
chemical names for any one substance. The most important is the International
Union of Pure and applied Chemistry (IUPAC) name. A drug has only one
chemical name. Chemical names are typically very long and too complex to
be commonly used in referring to a drug in speech or in prose documents. For
example, “1-(isopropylamino)-3-(1-naphthyloxy) propan-2-ol” is a chemical name
for propranolol. Sometimes, a company that is developing a drug might give the
drug a company code, which is used to identify the drug while it is in development.
This chemical name is sometimes helpful in predicting a drug’s physical and
chemical properties. Examples of chemical names of common drugs include
lithium carbonate, calcium gluconate, and sodium chloride.Self- assessment 1.31. A drug can have different name. Which one among the following drug
name is chemical name?
a. N-acetyl-p-aminophenl
b. paracetamol
c. Tylenol2. A drug has how many chemical name?3. Give 3 examples of easy chemical names to remember of common drugs1.4 Generic drug nameLearning activity 1.4Your neighbour sent her child to the pharmacy to buy the Paracetamol tablets.
The pharmacist gives the child the firm coated tablet labelled as PANADOL®.
The neighbour becomes confused and returns to the pharmacy for clarification
before taking the drug. The pharmacist tells the neighbour that, it is the same drug.
One is generic name (Paracetamol) and the other is brand name (Panadol®)1. Give the difference between generic name and brand name.CONTENT SUMMARY
The generic name is simpler name, less complicated and easier to remember
than chemical names. It may be used in any country and by any manufacturer. The
first letter of the generic name is not capitalized. Students are strongly encouraged
to learn and refer to drugs by their generic names because formularies (i.e., lists of
medicines available through a pharmacy) are maintained by generic names. When
a therapeutically equivalent drug becomes available in generic form, the generic
medicine is routinely substituted for the brand-name medicine. Generic names are
provided by the United States Adopted Names Council, which is an organization
sponsored by the United States Pharmacopeial Convention, the American Medical
Association, and the American Pharmacists Association. The official name, which
is virtually always the generic name in the United States, is the name under which
the drug is listed by the US Food and Drug Administration (FDA). The FDA is
empowered by federal law to generically name the drugs for human use in the
United States.Food and Drug Administration (FDA) is federal agency responsible for the
regulation and enforcement of drug evaluation and distribution policies. Because
there is only one generic name for each drug, health care providers often
use this name and they must memorize it. Generic drugs are less expensive
than brand-name drugs, but they may differ in bioavailability. Bioavailability
is defined by the Federal Food, Drug and Cosmetic Act as the rate and extent to
which the active ingredient is absorbed from a drug product and becomes available
at the site of drug action to produce its effect. Bioavailability may be affected by
many factors, including inert ingredients and tablet compression. Anything
that affects the absorption of a drug or its travel to the target cells can certainly
affect drug action. Measuring how long a drug takes to exert its effect (onset time)
gives pharmacologists a crude measure of bioavailability. If the trade and generic
products have the same rate of absorption and have the same onset of therapeutic
action, they are said to be bioequivalent.Self- assessment 1.41. A patient/client tells to the nurse that is taking aspirin. Which type of drug
name is this?2. A drug can have different name. Which one among the following drug
name is generic name:
a. (RS)-2-(4-(2methylpropyl)phenyl) propanoic acid
b. ibuprofen
c. Motrin3. _____ means that the amount of active ingredient that reaches the
patient’s bloodstream for a generic drug must be equivalent to that of the
branded drug.
a. Bioequivalence
b. Route of administration
c. Monitoring of adverse events
d. Biohazard labels4. What does the term “bioavailability” mean?
a. Plasma protein binding degree of substance
b. Permeability through the brain-blood barrier
c. The rate and extent to which the active ingredient is absorbed
d. Amount of a substance in urine relative to the initial doze1.5 Trade drug nameLearning activity 1.5Read the book of pharmacology and explain trade drug name (using library
textbook)A drugs trade name, sometimes called the proprietary, product, or brand name,
is assigned by the pharmaceutical company marketing the drug and it is followed by
the symbol ®. This symbol indicates that the name is registered and that the use of
the name is restricted to the owner of the drug, which is usually the manufacturer.
The trade name is intentionally selected to be short and easy to remember so that
patients will remember it (and ask for it by name).Drugs with more than one active generic ingredient are called combination drugs.
Acetaminophen and aspirin are examples of agents that appear in many combinationdrugs with dozens of different trade names. To avoid this confusion, generic
names should be used when naming the active ingredients in a combination
drug. When referring to a drug, it is conventional to write the generic name in lower
case first, followed by the trade name in parentheses with the first letter capitalized.
Examples include alprazolam (Xanax) and acetaminophen (Tylenol). (Cyton et al
2017).The difference between trade name and trademark name is that a trade name
refers to the company’s official name, while a trademark provides a company’s
brand with legal protection.The key to comparing brand-name drugs and their generic equivalents lies in
measuring the bioavailability of the two agents. Bioavailability is defined by the
Federal Food, Drug and Cosmetic Act as the rate and extent to which the active
ingredient is absorbed from a drug product and becomes available at the site of
drug action to produce its effect. Bioavailability may be affected by many factors,
including inert ingredients and tablet compression. Anything that affects the
absorption of a drug or its travel to the target cells can certainly affect drug action.
Measuring how long a drug takes to exert its effect (onset time) gives pharmacologists
a crude measure of bioavailability. If the trade and generic products have the same
rate of absorption and have the same onset of therapeutic action, they are said to
be bioequivalent.The importance of bioavailability differences between a trade name drug and its
generic equivalent depend on the specific circumstances of pharmacotherapy. For
example, if a patient is in circulatory shock and the generic equivalent drug takes
5 minutes longer to produce its effect that may indeed be significant. However, if a
generic medication for arthritis pain relief takes 45minutes to act, compared to the
brand-name drug that takes 40 minutes, it probably does not matter which drug is
used, and the inexpensive product should be prescribed to provide cost savings to
the consumer.As a general rule, bioavailability is of most concern when using critical care
drugs and those with a narrow safety margin. In these cases, the patient should
continue taking the brand name drug and not switch to a generic equivalent, unless
approved by the health care provider. For most other drugs, the generic equivalent
may be safely substituted for the trade name drug.In the age of Internet pharmacies, the issue of exclusive marketing rights has
drastically changed. In some cases, they even sell the drug to consumers without
a prescription. Other countries do not have the same quality control standards as
the United States, and the patient may be purchasing a useless or even harmful
product. Furthermore, although Internet sites may appear to be based in the United
States, they may instead be obtaining their medications from unreliable sources.Nurses must strongly urge their patients not to purchase drugs from overseas
pharmacies because there is no assurance that the drugs are safe or effective.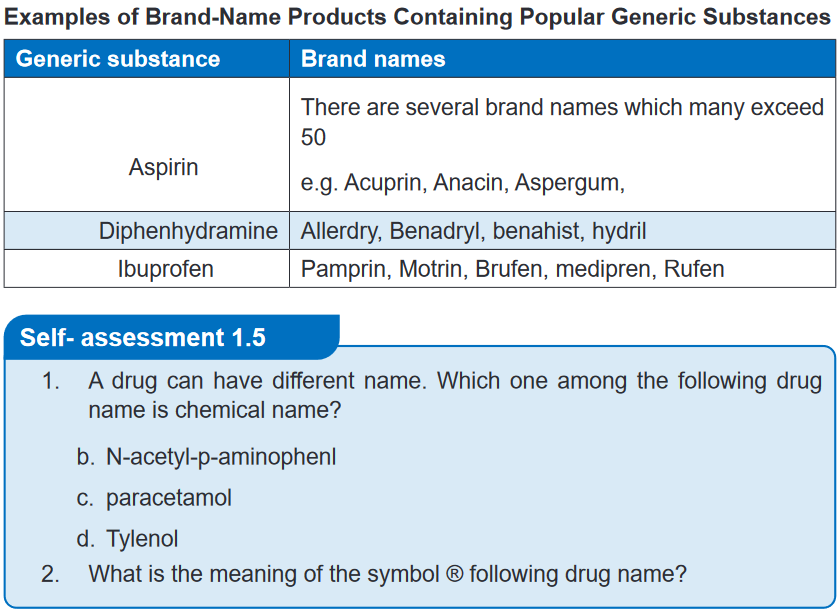 1.6. Label of drugs containerLearning activity 1.6
1.6. Label of drugs containerLearning activity 1.6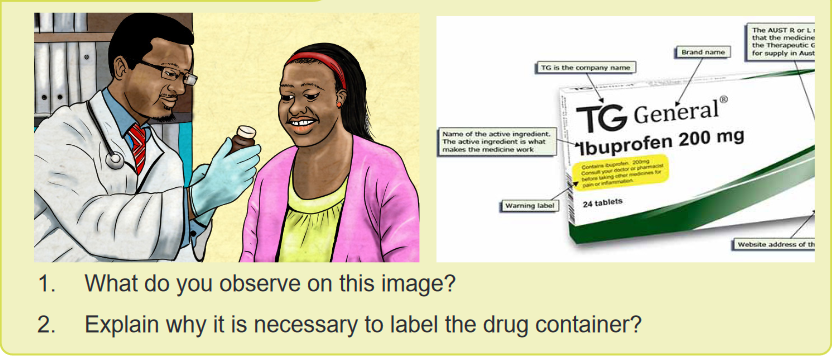 The Food and Drug Administration have specific information that identifies a specific
The Food and Drug Administration have specific information that identifies a specific
drug. It is important to obtain a thorough and accurate information from the drug
containers regarding their labelling, as they can often provide valuable information.The Drug label is a standardized label that appears on all over-the-counter
(OTC) medicines approved by the Food and Drug Administration, it have specific
information that identifies a specific drug. It is designed to tell the purpose of the
medicine, who should take the medicine and how to take it safely. For example, a
drug label identifies the brand and generic names for the drug, the drug dosage,
the expiration date, and special drug warnings. Some labels also indicate the route
and dose for administration. It’s very important to read all the information on the
label and current and approved references every time someone want to administer
a medicine because labels change regularly. Nurses need to become familiar with
each aspect of the label.A lack of information on drug labelling can result in serious mistakes in the
preparation of drugs which can place patients at risk. In all drug packaging of
the chosen drug should contain; what drug is to be used, how the drug is taken,
when the drug is to be administered, the importance of taking the drug (patient
compliance) and information about what happens if it is not taken as prescribed
(patient noncompliance), how long the drug is to be used, what adverse effects can
be expected and the alternatives available. The term compliance is the extent to
which patients follow instruction.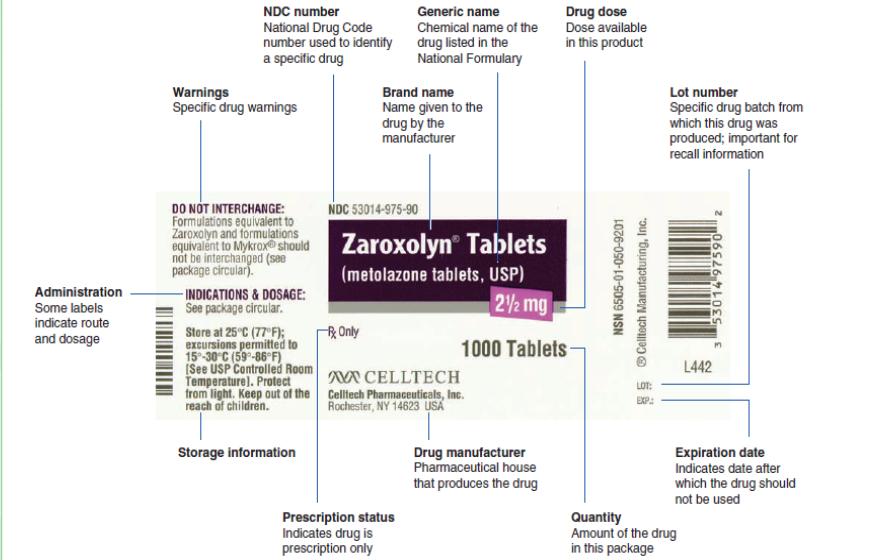 Numerous drug labels are used in the drug calculation problems to familiarize the
Numerous drug labels are used in the drug calculation problems to familiarize the
nurse with important information on a drug label. This information is then used in
correctly calculating the drug dose.Self- assessment 1.61. Enumerate the necessary information which must be on the drug
containers?2. What is drug label?3. During the drug administration the nurse found a Drug container on which
the label information is not clear?1.7. Solid drug dosage formsLearning activity 1.7 1. What do you observe on this image?
1. What do you observe on this image?
2. Are there any other solid forms of medication which are not on this image?
3. Why is it important for nurses to know different type of solid drug dosage
form?CONTENT SUMMARYSolid dosage forms include tablets, capsules, Caplets, Lozenges/ troches,
Powders and granules. The Tablets are available in variety of sizes, shapes,
colors, and thicknesses, usually obtained by single or multiple compressions of
powders or granules.Most tablets and caplets are designed to be swallowed whole and dissolve in the
gastrointestinal tract, but some are also made to be administered sublingually,
buccally, or vaginally.Tablets are normally right circular solid cylinders, the end surfaces of which are flat
or convex and the edges of which may be beveled. They may have lines or break-
marks (scoring), symbols or other markings.4. Uncoated tablets: compressed tablet or core tablet formed by compression
and contain no special coating. They are made from active ingredient in
combination with excipients such as binders, diluents, etc. Example: Analgin,
Paracetamol, Bactrim, etc 5. Sugar-coated tablets: are smooth, round or oval compressed tablets
5. Sugar-coated tablets: are smooth, round or oval compressed tablets
containing sugar coatings. Sugar coating provides both protection and sweet
taste but the coating operations take a long time. Example: Neocodion,
Paderyl, Aphatyl, Spasfon etc. 6. Film-coated tablets: are compressed tablets which are covered with a thin
6. Film-coated tablets: are compressed tablets which are covered with a thin
layer or a film of polymeric substances to protect their contents from moisture
or to mask the taste of the ingredients. Example: Ibuprofen 7. Modified release tablet: Modified-release tablets are coated, uncoated
7. Modified release tablet: Modified-release tablets are coated, uncoated
or matrix tablets containing excipients or prepared by procedures which,
separately or together, are designed to modify the rate, the place or the time
of release of the active ingredient(s) in the gastrointestinal tract. Sustained-
release tablets (Extended/Controlled/Prolonged-release): Sustained-release
tablets are designed to slow the rate of release of the active ingredient(s) in
the gastrointestinal tract.8. Example: Nifedipine. Delayed-release tablets (Entered-coated/Gastro-
resistant tablets): are coated with substances that resist solution in gastric
fluid but disintegrate in the alkaline contents of the intestine. Enteric coating
is used for medicines with a gastric irritant action, for medicines which areunstable in the acid medium of the gastric contents or if the medicine should
act on the intestine. Example: Aspirin 81mg.9. Effervescent tablets: Effervescent tablets are uncoated tablets generally
containing acid substances and carbonates or hydrogen carbonates that
react rapidly in the presence of water to release carbon dioxide. They
are intended to be dissolved or dispersed in water before administration.
Example: Efferalgan Vitamin C, Berroca Suppradine, etc 10. Chewable tablets: Chewable tablets are usually uncoated. They are intended
10. Chewable tablets: Chewable tablets are usually uncoated. They are intended
to be chewed before being swallowed; however, where indicated on the
label, they may be swallowed whole instead. They should be hard and large
which difficult to swallow. Example : Maalox, Amoxicillin chewable zentel,
etc 11. Lozenge tablets (Troche): Tablets containing palatable flavoring, indicated
11. Lozenge tablets (Troche): Tablets containing palatable flavoring, indicated
for a local (often soothing) effect on the throat and mouth. They are placed
in the mouth where they slowly dissolve, liberating the active ingredient. The
drug involved can be antiseptic, local anesthetic, antibiotic, or antitussive.
Example: Lysopaine, Horf, Strepsil, Wood, Zecuf, etc. Patient is advised not
to swallow a lozenge; it should be allowed to slowly dissolve in the mouth.
Patient is also advised not to drink liquids for approximately 15 minutes after
administration, to prevent washing of the lozenge contents from throat or
mouth. 12. Sublingual tablets: Medicine is placed under the tongue and allows dissolving.
12. Sublingual tablets: Medicine is placed under the tongue and allows dissolving.
It is absorbed into the circulation and provides the systemic effects. This
medication form is suitable for the active ingredients which is destroyed or
unstable in the gastrointestinal fluids. Example: NitroglycerinCapsules are solid dosage forms in which the drug substance is enclosed in either
a hard or soft soluble container of suitable form of gelatin. They are intended to
mask the smell and taste of the drug substances. Capsules are tasteless, easily
administered and some patients prefer them to the tablets. They are of various
shapes and sizes and contain a single dose of one or more active ingredients.
Capsules may be Hard Gelatin Capsules, Soft Gelatin Capsules and Modified-
Release Capsules:1. Hard Gelatin Capsules: Hard capsules have shells consisting of two
prefabricated cylindrical sections that fit together. One end of each section
is shorter, larger rounded, and closed (cap) and the other is open, longer
and smaller (body). The contents of hard capsules are usually in solid form
(powder or granules). Example: Amoxicillin, Ampicillin, Cephalexin, etc.2. Gelatin Capsules: Soft capsules have thicker shells than hard capsules and
antimicrobial preservatives are usually added. The shells are of one piece
and various shapes. They may be round, oval and oblong. The contents of
soft capsules are usually solutions or suspensions of the active ingredient(s)
in non-aqueous liquids. Example: Vitamin E, Vitamin A, Eugica, etc3. Modified-Release Capsules: Modified-release capsules are hard or soft
capsules in which the contents or the shell or both contain excipients or
are prepared by special procedures such as micro-encapsulation which,
separately or together, are designed to modify the rate, place or time of
release of the active ingredient(s) in the gastrointestinal tract. Sustained-
release capsules are designed to slow the rate of release of the active
ingredient(s) in the gastrointestinal tract. Example: Cardene SR (nicardipine).
Delayed-release capsules are hard or soft capsules prepared in such
a manner that either the shell or the contents resist the action of gastric
fluid but release the active ingredient(s) in the presence of intestinal fluid.
Examplse: Casprin, Esomeprazole4. The caplets/ Pills are small, round dosage forms for oral administration
which are prepared by the pharmacist. They are rarely prescribed today.
The powdered ingredients are mixed together with binding agents. The pill
mass is rolled into spheres and coated with talc, gelatin, or sugar.Example: Oral contraceptive pills.5. Oral Powder: oral powders are preparations consisting of solid, loose, dry
particles of varying degrees of fineness. They contain one or more active
ingredients, with or without excipients and, if necessary, authorized colouringmatter and flavouring substances. They are generally administered in or
with water or another suitable liquid. They may also be swallowed directly.
They are presented as single-dose or multidose preparations. Each dose of
a single-dose powder is enclosed in an individual container, for example a
packet, a sachet or a vial. Multidose oral powders require the provision of a
measuring device capable of delivering the quantity prescribed.Example: Clamoxyl 250mg, Dolipran, powder, Smecta, etc.6. Granules are dosage forms related to powders. They are particularly suitable
for the preparation of solutions or mixtures of medicines. Example: Montiget,
Biorrhee, etc.Self- assessment 1.71. The associate nurse students are reviewing principles of pharmacology,
and are reading about different forms of drugs. Enumerate 6 solid drug
dosage forms which can be used orally?2. Some tablets to treat a headache must first be dissolved in water before
swallowing. Which one of the following best describes this type of tablet?
a. Modified release
b. Oral disintegrating
c. Effervescent
d. Buccal3. Capsules in which powders are enclosed are made up of …..
a. Gelatine
b. Rice flour
c. Fructose
d. Dextrose
1.8. Semisolid drug dosage forms 1. What do you observe on this image?
1. What do you observe on this image?
2. Are there any other semisolid forms of medication which is not on this
image?
3. Why is it important for nurses to know different type of semisolid drug
dosage form?CONTENT SUMMARY
Semisolid dosage forms are normally presented in the form of creams, gels,
ointments, pastes, suppository or patch. They contain one or more active ingredients
dissolved or uniformly dispersed in a suitable base and any suitable excipients
such as emulsifiers, viscosity-increasing agents, antimicrobial agents, antioxidants,
or stabilizing agents. The choice of a base for semi-solid dosage forms depends on
many factors: the therapeutic effect desired the nature of the active ingredient to be
incorporated, the availability of the active ingredient at the site of action, the shelf-
life of the finished product, and the environmental conditions in which the product
is intended to be administered.It should be smooth, inert, odorless, physically and chemically stable, and
compatible with both the skin and the active ingredient(s) to be incorporated. It
should normally be of such a consistency that it spreads and softens easily when
stress is applied. It may be necessary for a topical semi-solid dosage form to be
sterile, for example, when it is intended for use on large open wounds or severely
injured skin.Creams are homogenous, semisolid preparation that is usually white and no
greasy and has a water base. Creams are intended for application to the skin
or certain mucous membranes for therapeutic or protective purposes. The term
“cream” is most frequently used to describe soft, cosmetically acceptable types of
preparations.Example: Hydrocortisone cream, Ketoconazole cream, etc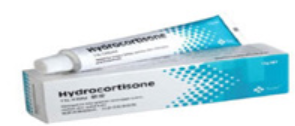 Ointments are homogeneous, semi-solid and greasy preparations intended for
Ointments are homogeneous, semi-solid and greasy preparations intended for
external application to the skin or mucous membranes for therapeutic or protective
purposes.Example: Tetracycline ointment.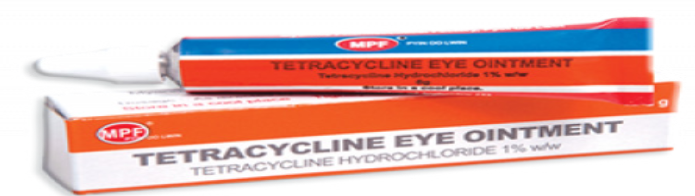 Gels are usually homogeneous, clear, semi-solid, jelly- like preparations that may
Gels are usually homogeneous, clear, semi-solid, jelly- like preparations that may
be used for topical medication. Gels are applied to the skin or certain mucous
membranes for therapeutic, or protective purposes.Example: Erythrogel, fastum gel, etc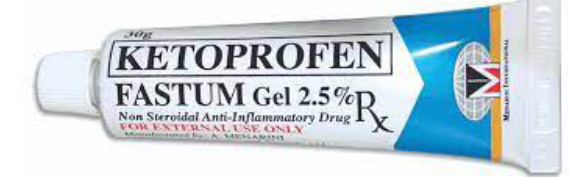 Pastes are homogeneous, semi-solid preparations containing high concentrations
Pastes are homogeneous, semi-solid preparations containing high concentrations
of insoluble powdered substances (usually not less than 20%) dispersed in a
suitable base. The pastes are usually less greasy, more absorptive, and stiffer inconsistency than ointments because of the large quantity of powdered ingredients
present. Pastes adhere reasonably well to the skin and they are suited for application
on and around moist lesions.Example: Orrepaste, Anagelsic and anti-inflammatory containing dental paste, etc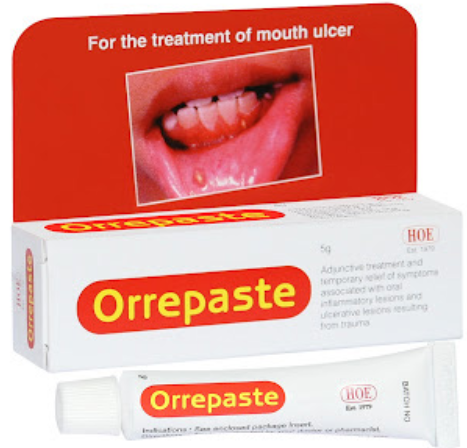 Patched/Plasters are substances intended for external application made of such
Patched/Plasters are substances intended for external application made of such
materials and of such consistency as to adhere to the skin. Inner surface of the
patch contacts skin and allows transdermal absorption of lipid-soluble medicines.
The total amount of medicine on the patch is very large, but typically only a small
fraction is absorbed. Patch are convenient because they can be applied easily and
minimize stomach upset. They can also improve compliance because there is no
need for more frequent dosing like oral dosage forms.Example: Dermal patches Suppository are semisolid dosage forms to be inserted into body cavity-rectum or
Suppository are semisolid dosage forms to be inserted into body cavity-rectum or
vagina, where medication is melt at the body temperature which provides local or
systemic effects.Example: paracetamol suppository, Flagyl suppository, etc Advantage of semisolid dosage form are: It is used externally, the probability of side
Advantage of semisolid dosage form are: It is used externally, the probability of side
effects can be reduced, first-pass gut and hepatic metabolism is avoided, local action
and site-specific action of the drug on the affected area, convenient for unconscious
patients or patients to have difficulty in oral administration, suitable dosage form for
bitter drugs and more stable than a liquid dosage form. The disadvantage of using
semisolid drug forms are: The accuracy can’t be measured, for the semisolid dosage
form, may cause staining, they are bulky to handle, application with a finger may
cause contamination, physico-chemical is less stable than a solid dosage form and
may cause irritation or allergy to some patients. The ideal properties of semisolid
dosage forms are smooth texture, elegant in appearance, non-dehydrating, non-
gritty and non-greasy and non-staining and Non-hygroscopic.Self- assessment 1.81. Enumerate the semisolid dosage forms.
2. Which of the following is not a semisolid dosage form
a. Paste
b. Cream
c. Ointments
d. Suspension
3. A semi-solid preparations containing high concentrations of insoluble
powdered substances (usually not less than 20%) dispersed in a suitable
base is known as:
a. Paste
b. Suppository
c. Ointments
d. Gels CONTENT SUMMARY
CONTENT SUMMARY
Liquid dosage forms are prepared by dissolving the active ingredient(s) in an
aqueous or nonaqueous solvent, by suspending the drug in appropriate medium
or by incorporating the drug substance, into one or two phases of an oil and water
system. These forms can be formulated for different routes of administration: oral
use, introduction into body cavities, or applied externally. Liquid drugs may also be
administered systemically by mouth or by injection throughout the body.The oral liquid forms can be readily administered to children or people unable to
swallow tablets or capsules.Syrup is a medicine dosage form that consists of a high concentration of a sugar
in water. Flavors may be added to mask unpleasant taste of certain medication.
Cherry, grape, strawberry syrup drug preparations are common for children.
Example: Sara syrup, Ibuprofen syrup, Dalfagan syrup, etc. Suspension is liquid form of medication that must be shaken well before
Suspension is liquid form of medication that must be shaken well before
administration because the medicine particles settle at the bottom of the bottle. The
medicine is not evenly dissolved in the liquid (hydrophobic agents). Example: Cotrim
suspension, Diaryl suspension, Amoxicillin suspension, Cefixim suspension, etc Elixir is liquid medicine form for oral use that contain primarily water, alcohol and
Elixir is liquid medicine form for oral use that contain primarily water, alcohol and
sugar. Their alcohol content makes elixir convenient liquid dosage form for many
drugs that are only slightly soluble in water. Example: Hosolvan elixir, Terpin hydrate
elixir, etc. Emulsion is a pharmaceutical preparation in which two agents of oil and water that
Emulsion is a pharmaceutical preparation in which two agents of oil and water that
cannot ordinarily be combined are mixed. These forms can be administered orally,
topically, or parenterally (intramuscularly). In order to prepare suitable emulsions
and to have them remain stable for a suitable period of time, a number of emulsifying
agents are used in their preparation. Example: Propofol (Diprivan), Metronidazole
topical emulsion, etc. Tincture is alcoholic or water- alcohol solution of medicines. It differs from elixir
Tincture is alcoholic or water- alcohol solution of medicines. It differs from elixir
in that it is not sweeten. Tincture can be used orally or externally. Example: Iodine
tincture Eye, Ear and Nose Drops are medicines in sterile water (purified water-deionized,
Eye, Ear and Nose Drops are medicines in sterile water (purified water-deionized,
demineralized water) to be applied by drops.Example: Ciprofloxacin eye/ear drop, New V-rotho, Tear Natural II, Pyinchin, etc.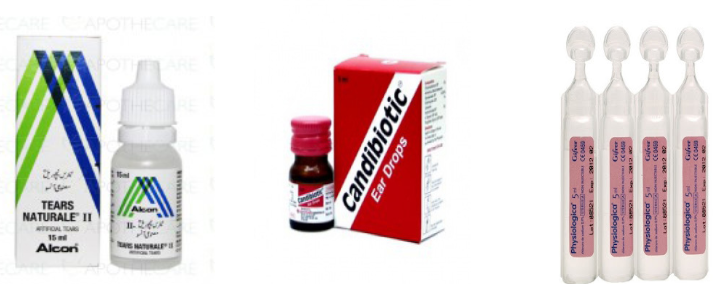 Mouth washes solution is aqueous solutions which are most often used for their
Mouth washes solution is aqueous solutions which are most often used for their
deodorant, refreshing or antiseptic effect.Example: Eludril, Septil, etc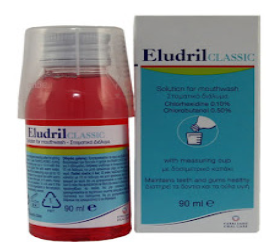 Enema is a fluid injected into the lower bowel by way of the rectum and most
Enema is a fluid injected into the lower bowel by way of the rectum and most
frequent used as a cleansing enema which is given to relieve constipation or for
bowel cleansing before a medical examination or procedure.Example: Pata enema, etc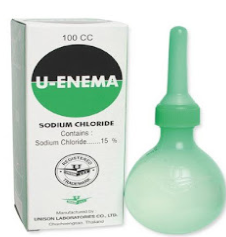 Douche solution is sterile solution, often a cleansing or antiseptic agent for part of
Douche solution is sterile solution, often a cleansing or antiseptic agent for part of
the body or body cavity.Example: Povidone iodine, H2O2 Liniment is the preparation for external use that is rubbed on the skin as a
Liniment is the preparation for external use that is rubbed on the skin as a
counterirritant. As such, the liniment creates a different sensation (e.g. tingling or
burning) to mask pain in the skin, muscle or joint.Example: Camphor liniment Medications for injection: solution have a sterile water base and are thus referred
Medications for injection: solution have a sterile water base and are thus referred
to as aqueous solution. Some solutions have an oil base, which tends to cause a
more prolonged absorption time. The oily nature of these solutions makes them
thick, thus they are referred to viscous solution.Example: Becozyme injection, Glucose 50% injection, Lactate ringer, NaCl 0.9%,
etc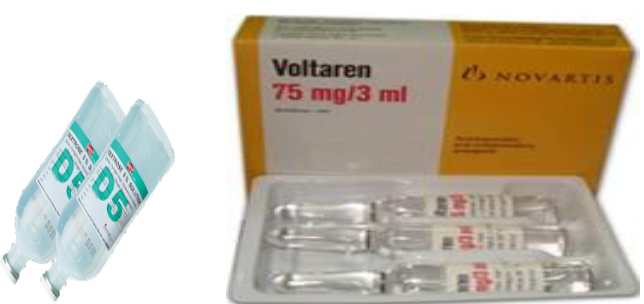 Powder are dry particle of medicines. The powder itself cannot be injected. It must
Powder are dry particle of medicines. The powder itself cannot be injected. It must
be mixed with a sterile diluting solution-solvent (sterile water or saline solution) to
render an injectable solution. This is termed reconstitution of medicine. Medicines
are supplied undiluted in powder form because of the short period of time they
remain stable after dilution.Example: Ampicillin, Ceftriaxone, etcThese products are packaged in ampoules, vials, bottles, plastic bags, and prefilled
disposable syringes. Self- assessment 1.91. Give the difference between suspension and emulsion2. Which of the following formulations would not be applicable to ocular
Self- assessment 1.91. Give the difference between suspension and emulsion2. Which of the following formulations would not be applicable to ocular
administration?
A. Solution
B. Liniment
C. Suspension
D. Ointment3. The component present in solution in small quantity is known as…..
A. Solvent
B. Solution
C. Solute
D. Liquid4. The component present in solution in large quantity is known as.
A. Solvent
B. Solution
C. Solute
D. Liquid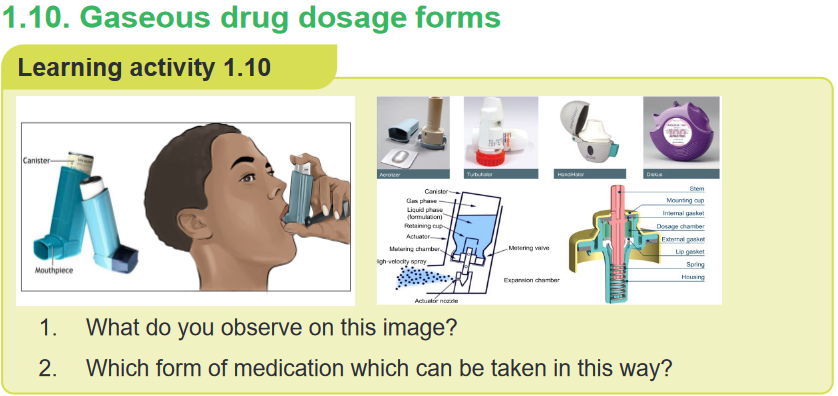 In gaseous dosage forms, the active pharmaceutical ingredients (API) are given
In gaseous dosage forms, the active pharmaceutical ingredients (API) are given
in the form of gas, are packed in a special container which gets released upon
applying pressure. It is used in the nose and mouth for local application or on the
skin. This allows medicines to be delivered to and absorbed in the lungs, which
provides the ability for targeted medical treatment to this specific region of the body,
as well as a reduction in the side effects of oral medications.E.g. Inhalers, aerosols, vaporizers, sprays, and nebulizers or atomizersAerosols are suspension of fine solid or liquid particles with gas used to apply drug
to respiratory tract having atomizer with in device. Inhalations are internal liquid
preparations containing medicaments dissolved in suitable solvent or if insoluble
suspended in the propellantSprays are Gaseous preparations of drugs containing alcohol applied to mucous
membrane of nose or throat with atomizer or nebulizer. Self- assessment 1.101. Enumerate the routes of administration of Gaseous dosage forms
Self- assessment 1.101. Enumerate the routes of administration of Gaseous dosage forms
2. What are the difference between aerosols and sprays?1.11 Dose and drug regimenLearning activity 1.10 1. What do you observe on this image?
1. What do you observe on this image?
2. Explain the importance of taking the medication as prescribed?
3. What is the effective dose?
4. Based on this image what do thing you will learn in this unit?
Learning activity 1.10The patient diagnosed with disease has to take the medications as prescribed by
the authorised health professional. To avoid and to achieve desirable therapeutic
effect, the patient has to take the correct dose. A dose refers to a specified amount
of medication taken at one time while the dosage is the prescribed administration
of a specific amount, number, and frequency of doses over a specific period of time.
A dosage guides a drug regimen.A drug regimen is a prescribed systematic form of treatment for a course of
drug(s). Regimen is a treatment plan that specifies the dosage, the schedule, and
the duration of treatment. Dose regimen includes the loading dose, maintenance
dose, dose frequency, dose duration, and dose adjustments for special populations
and for coadministration with other drugs.The drugs dose can be given as single dose, continuous administration and
irregular or several doses administration. The Single dose: After an intravenous
injection, the drug enters the bloodstream directly and the concentration rises to its
peak level almost immediately. Elimination and distribution will start immediately.With intramuscular injection, the drug is absorbed over a longer period, and following
oral administration, absorption takes even longer. The effect of a drug is usually
fastest if the route of administration that leads most rapidly to a high concentration
in the target organ is used.Continuous administration – intravenous infusion if a drug is administered by
a continuous intravenous infusion, the absorption phase will last as long as the
infusion continues.Irregular administration – several doses per day If a drug is administered in
‘portions’, or by several doses per day, the absorption and subsequent concentration
of the drug in the blood will vary between each dose. Initially, the concentration
increases for each new dose, if the time interval between the doses is so short that
the drug is not totally eliminated before the next dose is taken.This increase in concentration gradually diminishes, and steady state is eventually
achieved, as the rate of elimination of drug increases with increased concentration
of the drug. Once steady state is achieved, the concentration of the drug will only
vary between doses. The concentration rises immediately after intake, reaches a
peak level, and drops gradually until the next dose is taken.Even though many people receive the same dose of a drug, not all of them will
achieve the same effect. Some may have effect with a low dose, while others require
a higher dose. Likewise, some notice adverse effects at lower doses than others.The Effective dose is the dose that produces the desired effect. Based on the
amount the client received the dose can be effective, toxic and lethal dose. The
toxic dose is the dose that produces a toxic effect. The lethal dose is the dose
that results in death. This is an experimental term that can only be determined
in animal experiments and estimated in humans taking high doses in attempting
suicide.Drug dosage errors can occur at any time from when the drug is prescribed to its
administration and mistakes can place patients at risk; at worst, they can be fatal.
The cause of drug dosage errors can be attributed to both the health professional
and the patient. When using drugs with potent effects, it is even more important to
have a raised awareness, to avoid potential dosage errors. The same applies when
administering drugs to small children, the elderly and unconscious patients.A loading dose is a higher amount of drug, often given only once or twice, that is
administered to “prime” the blood-stream with a level sufficient to quickly induce
a therapeutic response. Before plasma levels drop back toward zero intermittent
maintenance doses are given to keep the plasma drug concentration in the
therapeutic range. Although blood levels of the drug fluctuate with this approach,
the equilibrium state can be reached almost as rapidly as with a continuous infusion.When immediate drug response is desired, a large initial dose, known as the loading
dose, of drug is given to achieve a rapid minimum effective concentration in the
plasma. After a large initial dose, a prescribed dosage per day is ordered.Loading doses are particularly important for drugs with prolonged half-lives and
for situations in which it is critical to raise drug plasma levels quickly, as might be
the case when administering an antibiotic for a severe infection. It took almost five
doses (48hours) to reach a therapeutic level using a routine dosing schedule. With
a loading dose, a therapeutic level can be reached within 12 hours.Maintenance doses are the dose taken to maintain the plasma concentration.
During the long-term use of some drugs, it is customary to prescribe fixed doses
with virtually identical long intervals between doses. With a dosage of 1 ×1, there
will be 24 h between each dose. With a dosage of 1 ×3, there will be 8 h between
each dose. With dosages that are more frequent than twice a day, the dosage
intervals will, in practice, often vary during the course of the day. Maintenance dose
can be also administered after loading dose to maintain the plasma concentration
of the drug.Self- assessment 1.111. Give the difference between the loading dose and maintenance dose
2. What are the difference between dose and dosage?
3. A client was diagnosed with malaria and is taking quinine by oral route.
The medical prescription indicate that the patient will take 10mg per Kg
per day in 3 times (every 8hours). Explain why the patient have to take
the medication every 8 hours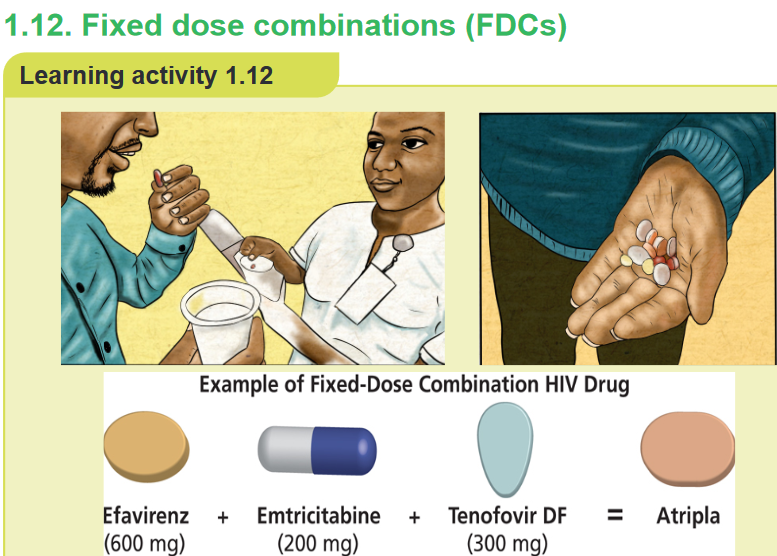 1. What do you observe on this image?
1. What do you observe on this image?
2. Atripla is combination of which drug?CONTENT SUMMARY
Good adherence to medication is one of the cornerstones of successful management
of chronic diseases. Unfortunately, such adherence is often difficult to achieve and
estimates suggest that only 50% of all chronic disease patients are able to adhere
to treatment. Fixed dose combinations (FDCs) are defined as a combination of two
or more active ingredients within a single form of pharmaceutical administration.They have been shown to appreciably reduce the risk of medication non adherence,
which is particularly important in patients with chronic diseases. An example of
a fixed-dose combination (FDC) HIV drug is Atripla (a combination of efavirenz,
emtricitabine, and tenofovir disoproxil fumarate) and Bactrim (sulfamethoxazole +
trimethoprim). By reducing the number of pills a person must take each day, fixed-
dose combination drugs can help improve adherence to treatment regimen.It is widely accepted that most drugs should be formulated as single compounds.
Fixed ratio combination products are acceptable only when the dosage of each
ingredient meets the requirement of a defined population group and when the
combination has a proven advantage over single compounds administered
separately in therapeutic effect, safety or complianceThe rationality of FDCs should be based on certain aspects such as: The drugs in
the combination should act by different mechanisms, the pharmacokinetics must
not be widely different and the combination should not have supra-additive toxicity
of the ingredients.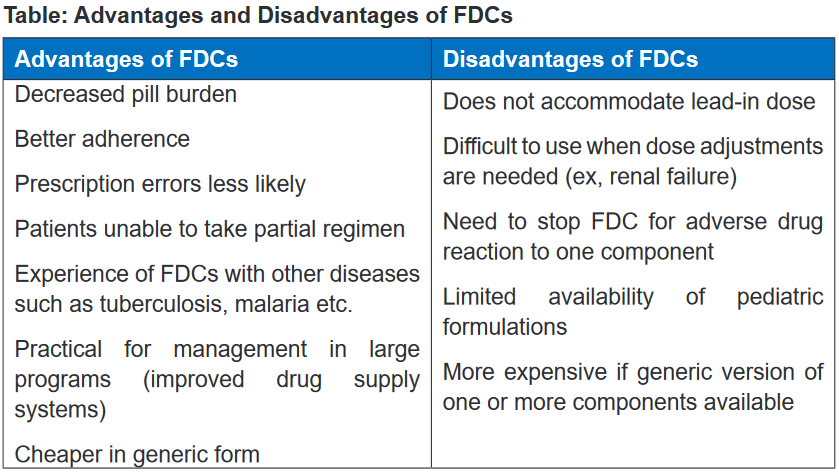 1.13 Directly observed therapy (DOT)Learning activity 1.13
1.13 Directly observed therapy (DOT)Learning activity 1.13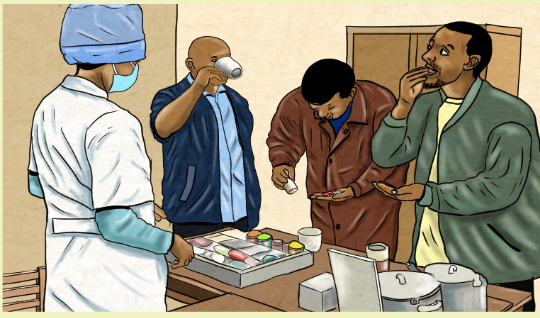 1. What do you observe on this image?
1. What do you observe on this image?
2. Explain why it is necessary to take drug while the nurse is observingCONTENT SUMMARY
Directly observed therapy (DOT) is used to ensure that the person receives
and takes all medications as prescribed and to monitor response to treatment.
DOT is widely used to manage tuberculosis (TB) disease. In HIV treatment, DOT is
sometimes called directly administered antiretroviral therapy (DAART).The World Health Organization (WHO) and the Centers for Disease Control and
Prevention (CDC) recommend directly observed therapy (DOT) for TB treatment
to monitor and provide treatment support for affected people whenever feasible.
When implemented properly, DOT fosters high levels of treatment adherence and
early detection of adherence problems, adverse drug reactions, and worsening TB
symptoms.Even if a proposed drug treatment is the optimal choice for a disease, it will not be
effective without patient compliance (the extent to which patients follow instructions).There are causes and many possible reasons for patient noncompliance: the
patient suffers adverse effects, the patient does not think the drug is effective, the
patient forgets to take the drug, the patient believes the disease is cured because
the symptoms have abated, the patient has misunderstood the user instructions,
the patient has run out of the drug, the patient does not master the administration
technique, e.g. inhalation, the drug formulation is unsuitable, the drug is unacceptable,
e.g. unpleasant taste, the patient uses many drugs simultaneously (polypharmacy),
frequent dosages and the patient has other objections towards the use of a certain
drug. In relation to drug therapy, the patient is compliant if he or she cooperates
fully in taking a prescribed medication following medical recommendations.Self- assessment 1.131. Why WHO recommended Direct Observed Therapy (DOT) for patient
taking anti-tuberculosis drugs?1.14. Therapeutic effectLearning activity 1.14 1. What do you observe on this image?
1. What do you observe on this image?
2. Discus the importance of taking the medication as prescribed?CONTENT SUMMARY
The main purpose of taking the medication is to achieve the therapeutic effect.
Therapeutic effect refers to the response after a treatment of any kind, the results
of which are judged to be desirable and beneficial. Therapeutic effects vary with the
nature of the medication, the length of time drugs was received and also vary with
client physical condition and interaction other drugs.The effect of a drug can be described at several levels: on the whole body, the
organ system(s), targets cell or at molecular target within cells.A single drug may have many effects other than its main therapeutic effect, and
in some instances these secondary effects and the responses they produce may
not be known in detail. Ideally, it is desirable that drugs should be as specific as
possible. This means that they should produce effects in as few organ systems as
possible – other than those in which an effect is required. The treatment can then
be controlled to achieve the desired effect.Drug response can be impacted by several factors including diet, comorbidities,
age, weight, drug–drug interactions, and genetics. Individual genetic variation in
key genes involved in the metabolism, transport, or drug target can contribute to
risk of adverse events or treatment failure.Self- assessment 1.141. Explain therapeutic effect?
2. Enumerate the factors affecting therapeutic effect of drug?textbook)1.15. Side effectsLearning activity 1.151. Read the book of pharmacology and explain side effects (using libraryCONTENT SUMMARYAn undesirable secondary effect which occurs in addition to the desired therapeutic
effect of a drug or medication is called side effect. It may vary for each individual
depending on the person’s disease state, age, weight, gender, ethnicity and general
health. All drugs have desirable or undesirable side effects. Even with a correct
drug dosage, side effects occur and are predicted.The terms side effects and adverse reactions are sometimes used interchangeably
in the literature and in speaking, but they are different. Some side effects are
expected as part of drug therapy. The occurrence of these expected but undesirableside effects is not a reason to discontinue therapy.
Side effects can occur when commencing, decreasing/increasing dosages, or
ending a drug or medication regimen. Side effects may also lead to non-compliance
with prescribed treatment. When side effects of a drug or medication are severe,
the dosage may be adjusted or a second medication may be prescribed. Lifestyle
or dietary changes may also help to minimize side effects.Self- assessment 1.151. What a nurse can do if the patient develops the side effect after
administrating the drug?2. Give an example where side effects may be desirable?1.16 Adverse reactionsLearning activity 1.16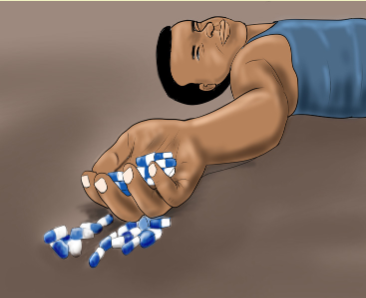 1. What do you observe on this image?
1. What do you observe on this image?
2. What do you thing happened to this person?
3. What can you do in this situation?CONTENT SUMMARYWhen the patient is taking the medications he/she can develop some effect which is
not desirable and severe which is called adverse effects. The adverse effects can
be classified into dose-related and non-dose-related effects.Dose-related adverse effects are associated with the drug’s known pharmacological
effects and occur when drugs are used in therapeutic doses. In principle, they are
predictable. All users will experience these adverse effects if the dose is high enough.
Often, an increase in the concentration of the drug due to reduced elimination, or
drug interactions which potentiate the effect, can be responsible for such adverse
effects. Toxic effects are included in this group.Non-Dose-Related Adverse Effects: In principle, all effects of drugs depend on
the dose that is taken (with a zero dose, there are no effects or adverse effects).
When adverse effects are classified as nondose- related, this means that such
effects occur at doses or concentrations that are considerably lower than the
standard dose known to produce a therapeutic effect. Such adverse effects are
not predictable, unless a patient has experienced them before. Typically, only a
few individuals experience non-dose-related adverse effects. Allergic reactions are
included in this group.Adverse reactions are more severe than side effects. The adverse reactions are
classified into type A (augmented) or type B (bizarre), C, D and E.Types A adverse reactions are therefore dose-dependent and predictable, but type
B Adverse reactions are neither predictable nor dose-dependent. Type A adverse
reactions are a kind of side effect and are normally associated with high morbidity
and low mortality. In contrast, type B adverse reactions are frequently severe or
bizarre reactions and are associated with low rates of morbidity but potentially high
rates of mortality. The immune system is commonly involved in type B adverse
reactions.Type A adverse reactions are more common than Type B reactions and account
for 80–90% of all reactions. They are a range of untoward effects (unintended and
occurring at normal doses) of drugs that cause mild to severe side effects, including
anaphylaxis (cardiovascular collapse). Adverse reactions are always undesirable.
Adverse effects must always be reported and documented because they represent
variances from planned therapy.Toxic effects, or toxicity is also an adverse drug reaction caused by excessive
dosing. However, for drugs that have a wide therapeutic index, the therapeutic
ranges are seldom given. For drugs with a narrow therapeutic index, such as
aminoglycoside antibiotics and anticonvulsants, the therapeutic ranges are closely
monitored. When the drug level exceeds the therapeutic range, toxic effects are
likely to occur from overdosing or drug accumulation.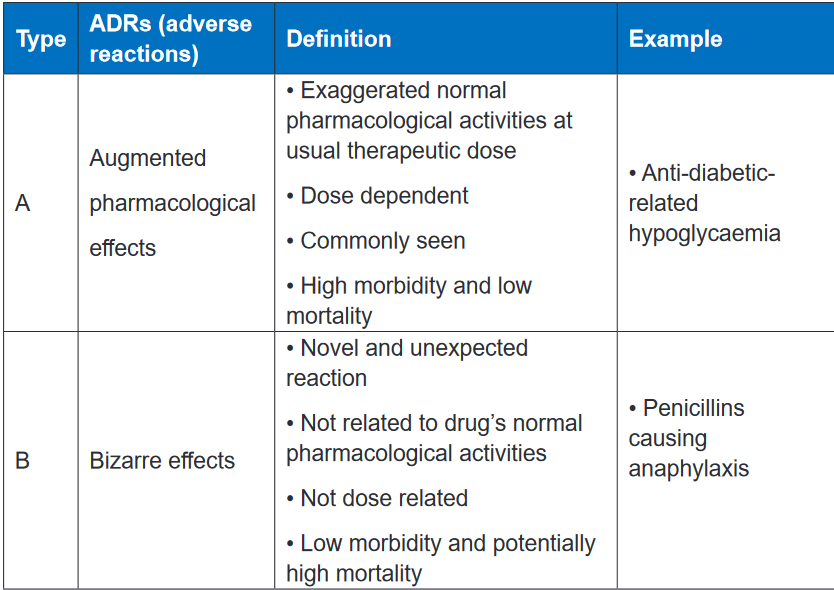 CONTENT SUMMARY
CONTENT SUMMARY
Effects most often manifest as changes in organ function. These may be changes in
the appearance of the skin, changes in the function of the respiratory, cardiovascular
and nervous systems, or changes in bone marrow function and the GI tract. The
liver and kidneys are particularly vulnerable, since the concentration of drugs and
their metabolites is usually high during drug elimination via these organs.The responsibility for reducing adverse reactions lies with everyone associated with
drug production and use. The pharmaceutical industry must strive to produce the
safest medicines possible; the prescriber must select the least harmful medicine
for a particular patient; the nurse must evaluate patients for adverse reactions and
educate patients in ways to avoid or minimize harm; and patients and their families
must watch for signs that an adverse reaction may be developing and should seek
medical attention if one appears.Anticipation of adverse reactions can help minimize them. Both the nurse and the
patient should know the major adverse reactions that a drug can produce. This
knowledge allows early identification of adverse effects, thereby permitting timely
implementation of measures to minimize harm. As noted, certain drugs are toxic to
specific organs. When patients are using these drugs, function of the target organ
should be monitored.The liver, kidneys, and bone marrow are important sites of drug toxicity. For drugs
that are toxic to the liver, the patient should be monitored for signs and symptoms of
liver damage (jaundice, dark urine, light-colored stools, nausea, vomiting, malaise,
abdominal discomfort, loss of appetite), and periodic Live Function Tests should
be performed. For drugs that are toxic to the kidneys, the patient should undergo
routine urinalysis and measurement of serum creatinine. In addition, periodic tests
of creatinine clearance should be performed. For drugs that are toxic to bone
marrow, periodic blood cell counts are required. Adverse effects can be reduced by
individualizing therapy.When choosing a drug for a particular patient, the prescriber must balance potential
risks of that drug versus its probable benefits. Drugs that are likely to harm a specific
patient should be avoided. For example, if a patient has a history of penicillin
allergy, we can avoid a potentially severe reaction by withholding penicillin and
administering a suitable substitute. Similarly, when treating pregnant patients, we
must withhold drugs that can injure the fetus.Lastly, we must be aware that patients with chronic disorders are especially
vulnerable to adverse reactions. In this group are patients with hypertension,
epilepsy, heart disease, and psychoses. When drugs must be used long term, the
patient should be informed about the adverse effects that may develop over time
and should be monitored for their appearance.Allergic Reaction is an immune response. For an allergic reaction to occur there
must be prior sensitization of the immune system. Once the immune system has
been sensitized to a drug, re-exposure to that drug can trigger an allergic response.
The intensity of allergic reactions can range from mild itching to severe rash to
anaphylaxis. Estimates suggest that less than 10% of adverse reactions are of the
allergic type.The intensity of an allergic reaction is determined primarily by the degree of
sensitization of the immune system not by drug dosage. Furthermore, since
a patient’s sensitivity to a drug can change over time, a dose that elicits a mild
reaction early in treatment may produce an intense reaction later on. In fact, most
serious reactions are caused by just one drug family, example the penicillins.The others drugs effect may include Paradoxical Effect, Iatrogenic Disease,
Carcinogenic Effect, Carcinogenic Effect, Teratogenic Effect, Organ-Specific
Toxicity and Hepatotoxic Drugs. Paradoxical Effect is the opposite of the intended
drug response. A common example is the insomnia and excitement that may occur
when some children and older adults are given benzodiazepines for sedation.
Iatrogenic Disease is a disease that occurs as the result of medical care or treatment.
The term iatrogenic disease is also used to denote a disease produced by drugs.Iatrogenic diseases are nearly identical to idiopathic (naturally occurring) diseases.
For example, patients taking certain antipsychotic drugs may develop a syndrome
whose symptoms closely resemble those of Parkinson disease. Carcinogenic Effect
refers to the ability of certain medications and environmental chemicals to cause
cancers.Teratogenic Effect is a drug and other chemicals capable of causing birth defects.
Organ-Specific Toxicity Many drugs are toxic to specific organs. Common examples
include injury to the kidneys caused by amphotericin B (an antifungal drug), injury
to the heart caused by doxorubicin (an anticancer drug), injury to the lungs caused
by amiodarone (an antidysrhythmic drug), and injury to the inner ear caused by
aminoglycoside antibiotics (e.g., gentamicin). Hepatotoxic Drugs as some drugs
undergo metabolism by the liver, they are converted to toxic products that can
injure liver cells. These drugs are called hepatotoxic drugs.However, many of these incidents are avoidable. Prescribers have to consider
the risk benefit ratio before prescribing a particular drug, and be aware that any
patient taking regular medication may develop an adverse reaction. Healthcare
professionals should know how to monitor, recognize and manage adverse drug
reactions or side effects, and this may involve stopping or changing the drug before
harm is done to patients.Self- assessment 1.161. What does the nurse have to do when the client develops allergic reaction
to the drugs?2. Discusses the concept of adverse drug reactions and drug side effects3. 30-year-old women client came to the health post where you work. She
is complaining hearing problem (tinnitus) 3 day ago after taking quinine.
How will you explain to the client about the symptom she developed after
taking quinine?4. Adverse drug reactions are mainly classified into reactions related to
the main pharmacological action of the drug (type A) and reactions that
are unpredictable and are not dose-related (Type B). Complete the table
below, using the key words and phrases provided in the box.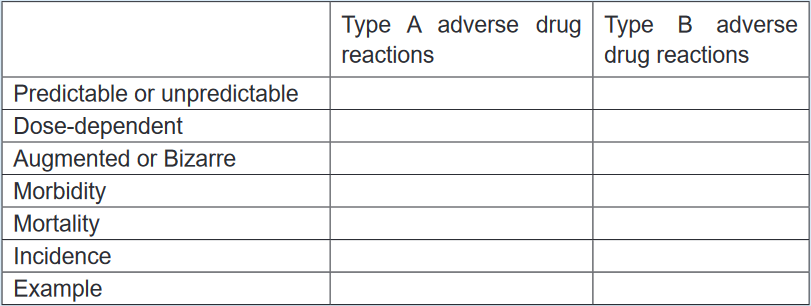 1.17 AntidotesLearning activity 1.171. Read the book of pharmacology and explain antidote (using library booksCONTENT SUMMARYDuring the drugs administration the patient can experience any unusual reaction
1.17 AntidotesLearning activity 1.171. Read the book of pharmacology and explain antidote (using library booksCONTENT SUMMARYDuring the drugs administration the patient can experience any unusual reaction
which can be reversed by administering another drug that acts as an antidote.
For example, when too much opiate is taken, the drug naloxone may be given to
counteract the effect. It is very important to monitor the drugs closely to detect or to
avoid any unusual reaction and have different antidotes at health facility which can
be used in case of overdose to prevent the complication associated. An antidote
is a drug, chelating substance, or a chemical that counteracts (neutralizes)
the effects of another drug or a poison.There are dozens of different antidotes; however, some may only counteract one
particular drug, whereas others (such as charcoal) may help reduce the toxicity of
numerous drugs. Antidotes mediate its effect either by preventing the absorption of
the toxin, by binding and neutralizing the poison, antagonizing its end-organ effect,
or by inhibition of conversion of the toxin to more toxic metabolites.Drug toxicity can be reversible or irreversible, depending on the organs involved.
Damage to the liver may be reversible, because liver cells can regenerate. However,
hearing loss from damage to the eighth cranial nerve caused by toxic reaction to the
anti-infective drug streptomycin may be permanent. Sometimes drug toxicity can
be reversed by administering another drug that acts as an antidote. For example,
when too much opiate is taken, naloxone may be given to counteract the effect.The FDA encourages nurses and other health care providers to report medication
errors to its database, which is used to assist other professionals in avoiding similar
mistakes. Poisoning occurs when an overdose of a drug damages multiple body
systems, leading to the potential for fatal reactions. Antidotes for drugs that can
cause potentially dangerous or fatal reactions must always be readily available.
Assessment parameters vary with the particular drug.Treatment of drug poisoning also varies, depending on the drug. Emergency and
life support measures often are needed in severe cases. Although some medication
errors go unreported, it is always the nurse’s legal and ethical responsibility to
document all occurrences. In severe cases, adverse reactions caused by medication
errors may require the initiation of lifesaving interventions for the patient, including
available antidotes. After such an incident, the patient may require close supervision,
and additional medical treatments may be warranted.According to mode of action the antidote can be classified as physical, chemical
and physiological and pharmacological. Physical antidote the agent use to interfere
with poison through physical properties by adsorbing. Chemical antidote interacts
specifically with a toxicant, or neutralize the toxicant. Physiological antidote act by
producing opposite effect to that of poison. Pharmacological antidote counteracts
the effects of a poison by producing the opposite pharmacological effects. They
may neutralize or antagonize the effects of a toxicant.According to site of action the antidote may act by preventing the formation of toxic
metabolites, by facilitation of more rapid or complete elimination of toxicant, and by
competing with the toxicant’s action at a receptor site. For preventing the formation
of toxic metabolites: more effective when given immediately before toxic metabolic
activation. For facilitation of more rapid or complete elimination of toxicant: change
the physiochemical nature of toxin, allowing better glomerular filtration and prohibit
tubular reabsorption.Self- assessment 1.171. Explain the mechanism of action of the antidote?
2. Give example of drug toxicity which can be reversible or irreversible,
depending on the organs involved1.18 Responsibilities of nurses regarding safe drug
administrationLearning activity 1.181. What is the role of nurse and responsibilities in medication administration?CONTENT SUMMARYNurses have a unique role and responsibility in medication administration, in that
they are frequently the final person to check to see that the medication is correctly
prescribed and dispensed before administration. It is standard during nursing
education to receive instruction on a guide to clinical medication administration
and upholding patient safety known as the ‘Nine rights’ or ‘Nine R’s’ of medication
administration (Right Patient, Right Reason or Indication, Right drug, Right dose,
Right Route and form, Right Time, Right Documentation, Right Response and Right
to Refuse).Right Patient, when administering a drug, it is important to use two methods
(visual as well as verbal methods) to identify the patient before administering the
medication. Nurse must be certain that the patient receiving the drug is the patient
for whom the drug has been ordered by reading properly the physician’s order. Call
the patient by name and ask him to repeat his name aloud. Be very careful if the
patient is deaf or otherwise does not understand the language.A visual identifier may include checking the patient’s name on his or her wristband,
on the patient’s card and on the medicine card for matching name and ID number
as on a chart. It is advisable not to address patients by first name or surname
alone, in the event, there are two or more patients with identical or similar names
in a unit. Depending on the unit that a patient may be in, some patients, such as
psychiatric patients, may not wear wristbands or may have altered mentation to the
point where they are unable to identify themselves correctly. In these instances,
nurses are advised to confirm a patient’s identity through alternative means with
appropriate due diligence.If there is no written identification verifying the patient’s name, nurse should obtain
a wristband or other form of identification before administering the drug. Nursemay also ask the patient to identify him- or herself and request another unique
identifier such as date of birth. However, do not ask, “Are you Mr or Mrs A?” Some
patients, particularly those who are confused or have difficulty hearing, may respond
by answering “yes” even though that is not their name. Some long-term care or
rehabilitation care facilities have pictures of the patient available, which allow the
nurse to verify the correct patient. If pictures are used to identify patients, it is critical
that they are recent and bear a good likeness of the individual.Right Reason or Indication addresses the appropriateness in use of the medication
to the patient. Confirm the rationale for use through researching the patient’s history
while also asking the patient the reason he or she is taking the drug. Always revisit
the rationale for long-term medication use. Knowledge of the drug’s indication
allows the nurse, prescriber, members of the health care team, patient and/or family
members to understand what is being treated. Understanding the indication helps
pharmacists and nurses to catch potential errors, provide thorough explanations to
the patient/family, and decrease challenges to medication reconciliation.The nurse has the responsibility to verify the reason that the patient is receiving
the medication. It is important to understand the indication, which is related to the
medical diagnosis. If in doubt about the reason for the order, the nurse must verify
the medication order with the prescriber before administration.Right medication or drug, some brand names or generic names may have very
similar spelling or sound very similar due to prefix, suffix, or starting with the same
first letter. Poor handwriting and abbreviations account for many medical errors
due to misreading letters or numerals that appear differently to different individuals.
Right Drug names can be confused, especially when the names sound similar, or
the spellings are similar.Quickly preparing a drug for administration or failing to look up questionable drugs
can put you at increased risk for administering the wrong drug. An error in drug
name or amount can be found when nurse compares the medication administration
record: with the container label, as the item is removed from the card, and before
the actual administration of the drug.The nurse must be careful of drugs whose names sound alike. When administering
medications, the nurse compares the label of the medication container with the
medication form three times: before removing the container from the drawer or
shelf, as the amount of medication ordered is removed from the container and
before returning the container to storage.The nurse must look for colour, odour, and consistency of the drug. Unusual
characteristics of the drugs should be questioned. The nurse must also administer
medicine only from clearly labelled container and remember to check other critical
information on packaging such as the expiration date.The nursing providers should also develop a routine habit of explicitly asking patients
about known allergies or history of an allergic. The conversation or anything that
distracts the mind not recommended during drug administration. The nurse must be
familiar with the trade names.If there is doubt consult the physician or at least seniors or other reliable sources.
Avoid accepting the verbal orders, only in emergencies are accepted. Always
identify the patient before giving medication. The nurse must make sure that the
drug has not been discontinued by the prescriber.The nurses administer only the medications they prepare. If an error occurs, the
nurse who administers the medication is responsible for the error. Clients who
self-administer medications should keep them in their original labelled containers,
separate from other medications, to avoid confusion.Right Route and form, a nurse must know the particulars about each medication
before administering it to ensure that the right drug, dose, route, and dosage form
are being used. A complete medication order includes the route of administration.
Confirm the appropriateness of the prescribed route while also making sure the
patient can take/receive the medication by the prescribed route. If a medication
order does not include the route, be sure to ask the prescriber to clarify it. Never
assume the route of administration.In addition, it is critical to patient safety to be aware of the right form of medication.
For example, there are various dosage forms of a commonly used medication,
acetaminophen.It is available in oral suspension, tablet, capsule, gelcap, and pediatric drops, as
well as rectal suppository dosage forms. Nurses need to give the right drug via the
right route with use of the correct dosage form.Medications can be given to patients in different many ways, all of which vary in the
time it takes to absorb the chemical, time it takes for the drug to act, and potential
side-effects based on the mode of administrations, include oral, intramuscular,
intravenous, topical, or subcutaneous injection and others. It is crucial that
nurses remain educated and up to date on newer medications or less commonly
administered medications to learn how they are safely delivered to patients before
being asked to do so in clinical practice.If a prescriber’s order does not designate a route of administration, the nurse
consults the prescriber. The nurse should alert the prescriber immediately if the
specified route is not the recommended route and he/she must report immediately
if an error occurs in the medication. The nurse must know and must be familiar with
the abbreviations used to designate the route of administration.‘Right time’, medications can be given to patients in different many ways, all of
which vary in the time it takes to absorb the chemical, time it takes for the drug
to act, and potential side-effects. Certain drugs have specific intervals or window-
periods during which another dose should be given to maintain a therapeutic effect
or level.Often, a guiding principle of this ‘right’ is that medications should be prescribed as
closely to the time as possible, and nurses should not deviate from this time by
more than half an hour to avoid consequences such as altering bioavailability or
other chemical mechanisms. Similarly, it is crucial that medications that are given
by an infusion, such as intravenous medications, are administered at the correct
rate.Failure to deliver a drug at the correct rate may lead to devastating consequences
for a patient. For example, vancomycin requires administration by slow intravenous
infusion to avoid a complication known as “red man syndrome,” a hypersensitivity
reaction that is managed by further slowing the infusion rate of vancomycin or
discontinuing the agent altogether.The administering medications at a time that was intended by the prescriber. The
nurse must Read the physician’s orders, know the hospital routines for the interval,
know the abbreviations for the time, give the medicine near the time ordered,
give the medicine as ordered in relation to the food intake and give the medicines
according to the actions expected. E.g., sleeping pills are given at bedtime.‘Right dose’, incorrect dosage, conversion of units, and incorrect substance
concentration are a prevalent modality of medication administration error. This error
type stems from nurses giving a patient an incorrect dose of medications, even if it
is the correct medication and the patient’s identity is verified, without first checking
to ensure it is the correct strength for the patient. This error type may be due to
misplaced decimals, errors in arithmetic, or incorrect conversion between two units.The nurse must have adapted observing positive behaviors to reduce medical
errors include consulting with pharmacy personnel, read physician orders to know
the correct dose, consider the age and weight of the patient, know the minimum
and maximum dose of the medicine administered, using calculators to assist in
arithmetic, or in some cases, cross-consulting with patients or their families about
usual doses they administer at home. Use ounce glasses instead of teaspoons to
measure ounces accurately, have written order before you prepare the drug, avoid
conversation or anything that distracts the mind.Right Documentation, medication error can result from inaccurate documentation.
Nurse should ensure appropriate documentations clearly reflect the client’s name,
the name of the ordered medications, the time the medication was administered, the
medication’s dosage, route, the date or the method of administration, frequency, thesignature of the physician, and Standing orders or routine medication orders. If any
of this information is missing the nurse should verify the order with the prescriber.After the administration of any drug, record the process immediately. Immediate
documentation is particularly important when drugs are given on an as-needed
(PRN) basis. For example, most analgesics require 20 to 30 minutes before the
drug begins to relieve pain.A patient may forget that he or she received a drug for pain, may not understand
that the administered drug was for pain, or may not know that pain relief is not
immediate, and may ask another nurse for the drug again. If the administration
of the analgesic was not recorded, the patient might receive a second dose of
the analgesic shortly after the first dose. This type of situation can be extremely
serious, especially when opioids or other central nervous system depressants are
administered. Immediate documentation prevents accidental administration of a
drug by another individual and it is essential to the process of administering drugs
correctly.Right Response refers to the drug and its desired response in the patient.
Continually assess and evaluate the achievement of the desired response, as
well as any undesired response. Examples of data gathering include, but are not
limited to, monitoring vital signs, weight, edema, intake and output, nutritional
intake, laboratory values, results of diagnostic testing, and auscultating heart and
lung sounds. Document any assessment, intervention, and monitoring as deemed
appropriate.Right to Refuse, the ninth right is that of the right of the patient to refuse. Patients
refuse medications for a variety of reasons. If refusal of a medication occurs, always
respect the patient’s right (to refuse), determine the reason, and take appropriate
action, including notifying the prescriber. Do not force! Document the refusal and
a concise description of the reason for refusal. Document any further actions you
take at this time, such as vital signs and/or system assessment.If a consequence to the patient’s condition and/or as hospital policy dictates, the
prescriber is to be contacted immediately. Never return unwrapped medication to a
container, and discard medication dose according to agency policy. If the wrapper
remains intact, return the medication to the automated medication-dispensing
system. Revise the nursing care plan as needed.The nurses bear the sole responsibility and accountability while administering
drug. They are responsible for their actions regardless of whether there is a written
order. If the physician’s prescription appears unreasonable or wrong, the nurses
should clarify with the doctor who prescribed the drug and get it clarified before
administering.The primary health care provider’s order must include the patient’s name, the drug
name, the dosage form and route, the dosage to be administered, the frequency
of administration and the health care provider’s signature and must follow the drug
order. In an emergency, nurse may administer a drug with a verbal order from the
primary health care provider. However, the primary health care provider must write
and sign the order as soon as the emergency is over. If a verbal order is given over
the telephone, write down the order, repeat back the information exactly as written,
and then ask for a verbal confirmation that it is correct. Any order that is unclear
should be questioned, particularly unclear directions for the administration of the
drug or a drug dose that is higher or lower than the dosages given in approved
references.Ways to prevent medication administration errors:• Read the medication labels carefully many products come in similar containers,
colors and shapes.• Be aware of medications with similar names many medication names sound
alike.• When new or unfamiliar medication is ordered, consult resource if prescriber
is also unfamiliar with drug, there is greater risk of inaccurate dosages being
ordered.• Do not administer medication ordered by nickname or unofficial abbreviation
know client with same last names.• Do not confuse equivalents.• Client should be educated regarding the self-administration of drugs while
getting discharged.Drug administration is a fundamental nursing responsibility. By understanding the
basic concepts of administering drugs safely and accurately, monitoring of the
therapeutic response (desired response) and reporting adverse reactions is critical.
Additionally, in the ambulatory setting, nurses are responsible for teaching the
patient and family members the information needed to self-administer drugs safely
in the home.Self- assessment 1.181. Describes the six right of drug administration?1.19. Food and drug administration (FDA) pregnancy risk
categoriesLearning activity 1.191. Read the book of pharmacology and explain why pregnant women cannot
take any drugs without medical prescription (using library textbook).CONTENT SUMMARYDrugs used by pregnant women may reach the fetus through the placenta and lead
to effects on the development, intellectual ability, birth defects, miscarriage and
stillbirth. The Food and Drug Administration has established five categories (A, B,
C, D &X) to indicate the potential for a systemically absorbed drug to cause birth
defects. The key differentiation among the categoriesCategory A: Adequate and well-controlled studies have failed to demonstrate
a risk to the fetus in the first trimester of pregnancy (and there is no evidence of
risk in later trimesters). Example drugs or substances: levothyroxine, folic acid,
liothyronine.Category B: Animal reproduction studies have failed to demonstrate a risk to the
fetus and there are no adequate and well-controlled studies in pregnant women.
Example drugs: metformin, hydrochlorothiazide, cyclobenzaprine, amoxicillin.Category C: Animal reproduction studies have shown an adverse effect on the
fetus and there are no adequate and well-controlled studies in humans, but potential
benefits may warrant use of the drug in pregnant women despite potential risks.
Example drugs: gabapentin, amlodipine, trazodone.Category D: There is positive evidence of human fetal risk based on adverse
reaction data from investigational or marketing experience or studies in humans,
but potential benefits may warrant use of the drug in pregnant women despite
potential risks. Example drugs: losartan.Category X: Studies in animals or humans have demonstrated fetal abnormalities
and/or there is positive evidence of human fetal risk based on adverse reaction
data from investigational or marketing experience, and the risks involved in use
of the drug in pregnant women clearly outweigh potential benefits. Example
drugs: atorvastatin, simvastatin, methotrexate, finasterideRegardless of the designated Pregnancy Category or presumed safety, no drug
should be administered during pregnancy unless it is clearly needed.Self- assessment 1.191. Describe FDA pregnancy risk categories?1.20 End unit assessmentEnd Unit assessment 11. Define pharmacology2. List 4 drugs dosage forms
3. What are the sources of drug?
4. Give the difference between loading dose and maintenance dose5. The nurse knows the importance of administering the right medication to
the patient and that drugs have many names. It is therefore important that
drugs be ordered by which name?6. Explain the importance of directly observed therapy in patient care
7. Explain the importance of fixed dose combination in patient care
8. How medication administration errors can be prevented?
9. What are the responsibilities of the nurse during drug administration?10. Drug X is given a prescription that reads as follows: “Ferrous sulfate 325
mg, PO for anemia.” When she goes to the pharmacy, the pharmacist
tells her that the prescription is incomplete. What is missing? What should
be done?11. Briefly discuss the “Nine Rights” and other “Rights” associated with safe
medication administration12. Clinical pharmacology is the study of
A. The biological effects of chemicals.B. Drugs used to treat, prevent, or diagnose disease.
C. Plant components that can be used as medicines.
D. Binders and other vehicles for delivering medication.13. The generic name of a drug is
A. The name assigned to the drug by the pharmaceutical company
developing it.
B. The chemical name of the drug based on its chemical structure.C. The original name assigned to the drug at the beginning of the evaluation
process.
D. The name that is often used in advertising campaigns.14. The Food and Drug Administration (FDA) pregnancy categories
A. Indicate a drug’s potential or actual teratogenic effects.
B. Are used for research purposes only.
C. List drugs that are more likely to have addicting properties.
D. Are tightly regulated by the Drug Enforcement Agency (DEA).15. Give the definition for a therapeutic dose:
A. The amount of a substance to produce the minimal biological effect
B. The amount of a substance to produce effects hazardous for an organism
C. The amount of a substance to produce the required effect in most
patients
D. The amount of a substance to accelerate an increase of concentration
of medicine in an organism16. Pick out the correct definition of a toxic dose:
A. The amount of substance to produce the minimal biological effect
B. The amount of substance to produce effects hazardous for an organism
C. The amount of substance to produce the necessary effect in most of
patients
D. The amount of substance to fast creation of high concentration of
medicine in an organism17. A rectal suppository is used to treat fever. This would represent what type
of drug delivery?
A. Parenteral and local
B. Parenteral and systemic
C. Enteral and local
D. Enteral and systemic18. Which of the following is not a semisolid dosage forms?
A. Solution
B. CreamC. Paste
D. Gel19. A suppository is generally intended for use in
A. Rectum
B. Ear
C. Nose
D. Mouth20. Vaginal suppositories also called as
A. Simple suppositories
B. Bougies
C. Pessaries
D. Soft tablet21. The nurse is reviewing the various forms of topical medications. Which of
these are considered topical medications?
A. Tablets for oral route
B. Eye drops for inflammation
C. Sublingual tablet for chest pain
D. Intradermal injection for tuberculosis testing22. An 82-year-old patient is admitted to the hospital after an episode of
confusion at home. The nurse is assessing the current medications he
is taking at home. Which method is the best way to assess his home
medications?
A. Ask the patient what medications he takes at home.
B. Ask the patient’s wife what medications he takes at home.
C. Ask the patient’s wife to bring his medications to the hospital in their
original containers.
D. Contact the patient’s pharmacy for a list of the patient’s current
medications.23. During the medication administration process, it is important that the
nurse remembers which guideline?
A. When in doubt about a drug, ask a colleague about it before giving the
drug.
B. Ask what the patient knows about the drug before giving it.C. When giving a new drug, be sure to read about it after giving it.
D. If a patient expresses a concern about a drug, stop, listen, and
investigate the concerns.24. A patient’s medical record includes an order that reads as follows:
“Pacetamol 500 mg once daily at 09h00.” Which action by the nurse is
correct?
A. The nurse does not give the drug.
B. The nurse gives the drug orally.
C. The nurse gives the drug intravenously.
D. The nurse contacts the prescriber to clarify the dosage route.UNIT 2: PHARMACOKINETICS AND PHARMACODYNAMICS
Key Unit Competence
Explain the application of pharmacokinetcs and pharmacodynamics during clinical
practice
1. That image above represents a patient who has ingested a drug. What
do you think the arrows in the image indicate?
2. In your daily life, what do you think happens in the body after ingestion of
medications (tablets)?2.1 Introduction to Pharmacokinetics
Learning activity 2.1
You are placed at a health post in the clinical placement, and the patient consults
for his medical condition follow up. As he has a chronic disease, you inquire about
his health status, focusing on kidney function, bearing in mind that the drug is
eliminated via the urinary system. Then your colleague says that he heard that
pharmacokinetics of each needs to be taken into consideration while prescribing
a drug. He is curious, and would like to get more explanations from you.1. How can you briefly explain the word “pharmacokinetics” to your
colleague?2. Mention 4 phases/processes of pharmacokinetics.
CONTENT SUMMARY
Pharmacokinetics, sometimes described as what the body does to a drug, refers to
the movement of drug into, through, and out of the body.Pharmacokinetics involves the study of absorption, distribution, metabolism
(biotransformation), and excretion of drugs. In clinical practice, pharmacokinetic
considerations include the onset of drug action, drug half-life, timing of the peak
effect, duration of drug effects, metabolism or biotransformation of the drug, and
the site of excretion.Critical Concentration
After a drug is administered, its molecules first must be absorbed into the body;
then they make their way to the reactive tissues. If a drug is going to work properly
on these reactive tissues, and thereby have a therapeutic effect, it must attain a
sufficiently high concentration in the body. The amount of a drug that is needed to
cause a therapeutic effect is called the critical concentration.Drug evaluation studies determine the critical concentration required to cause
a desired therapeutic effect. The recommended dose of a drug is based on the
amount that must be given to eventually reach the critical concentration. Too much
of a drug will produce toxic (poisonous) effects, and too little will not produce the
desired therapeutic effects.Loading Dose
Some drugs may take a prolonged period to reach a critical concentration. If their
effects are needed quickly, a loading dose is recommended.Dynamic Equilibrium
The actual concentration that a drug reaches in the body results from a dynamic
equilibrium involving several processes:
1. Absorption from the site of entry
2. Distribution to the active site
3. Biotransformation (metabolism) in the liver
4. Excretion from the bodyThese processes are key elements in determining the amount of drug (dose)
and the frequency of dose repetition (scheduling) required to achieve the critical
concentration for the desired length of time. When administering a drug, the nurse
needs to consider the phases of pharmacokinetics so that the drug regimen can be
made as effective as possible.Self- assessment 2.1
1. There are some drugs that may take a prolonged period to reach a critical
concentration. If their effects are needed quickly, a maintenance dose is
recommended. (TRUE or FALSE)2. A right sequence of pharmacokinetic processes for a drug given by oral
route is:
A. Absorption, Distribution, Biotransformation and Excretion
B. Distribution, Absorption, Biotransformation and Excretion
C. Biotransformation, Absorption, Distribution, and Excretion
D. Excretion, Absorption, Distribution, and MetabolismSource: Library textbooks of pharmacology (Karch, A.M. (2013). Focus
on Nursing Pharmacology): On chapter of pharmacokinetics and
pharmacodynamics.2.2 Absorption of drugs
Learning activity 2.2
A patient X was received at the health post presenting severe respiratory disease.
An associate nurse student in clinical practice suggests administering a drug via
oral route but the nurse tells him to administer injectable drug rather than oral
drug, as the injectable form can work quickly.1. Referring to drug absorption, explain why the nurse preferred injectable
drug form.
2. List at least 3 factors that can affect absorption of drugs administered by
oral route.CONTENT SUMMARY
Absorption refers to what happens to a drug from the time it is introduced to the
body until it reaches the circulating fluids and tissues. Drugs can be absorbed
from many different areas in the body: through the GI tract either orally or rectally,
through mucous membranes, through the skin, through the lung, or through muscle
or subcutaneous tissues.Drug absorption is influenced by the route of administration. Generally, drugs given
by the oral route are absorbed more slowly than those given parentally. Of the
parenteral route, IV administered drugs are absorbed the fastest.Routes of Administration
The oral route is the most frequently used drug administration route in clinical
practice. Oral administration is not invasive, and, as a rule, oral administration is
less expensive than drug administration by other routes. It is also the safest way to
deliver drugs. Patients can easily continue their drug regimen at home when they
are taking oral medications. Oral administration subjects the drug to a number of
barriers aimed at destroying ingested foreign chemicals. The acidic environment of
the stomach is one of the first barriers to foreign chemicals.The acid breaks down many compounds and inactivates others. This fact is taken
into account by pharmaceutical companies when preparing drugs in capsule or
tablet form. The binders that are used often are designed to break down in ascertain
acidity and release the active drug to be absorbed.When food is present, stomach acidity is higher and the stomach empties more
slowly, thus exposing the drug to the acidic environment for a longer period. Certain
foods that increase stomach acidity, such as milk products, alcohol, and protein,
also speed the breakdown of many drugs.Other foods may chemically bind drugs or block their absorption. To decrease the
effects of this acid barrier and the direct effects of certain foods, oral drugs ideally
are to be given 1 hour before or 2 hours after a meal.Drugs that are injected IM (intramuscularly) are absorbed directly into the capillaries
in the muscle and sent into circulation. This takes time because the drug must
be picked up by the capillary and taken into the veins. Men have more vascular
muscles than women do. As a result, drugs administered to men via the IM route
reach a peak level faster than they do in women. Subcutaneous injections deposit
the drug just under the skin, where it is slowly absorbed into circulation. Timing of
absorption varies with subcutaneous injection, depending on the fat content of the
injection site and the state of local circulation.Absorption Processes: Drugs can be absorbed into cells through various
processes, which include passive diffusion and filtration.Passive diffusion is the major process through which drugs are absorbed into the
body. Passive diffusion occurs across a concentration gradient.When there is a greater concentration of drug on one side of a cell membrane,
the drug will move through the membrane to the area of lower concentration.
This process does not require any cellular energy. Unlike passive diffusion, active
transport is a process that uses energy to actively move a molecule across a cell
membrane. The molecule may be large, or it may be moving against a concentration
gradient. This process is not very important in the absorption of most drugs, but it is
often a very important process in drug excretion in the kidney.Filtration involves movement through pores in the cell membrane, either down a
concentration gradient or as a result of the pull of plasma proteins (when pushed
by hydrostatic, blood, or osmotic pressure). Filtration is another process the body
commonly uses in drug excretion.TABLE 2.2 Factors that affect absorption of drugs
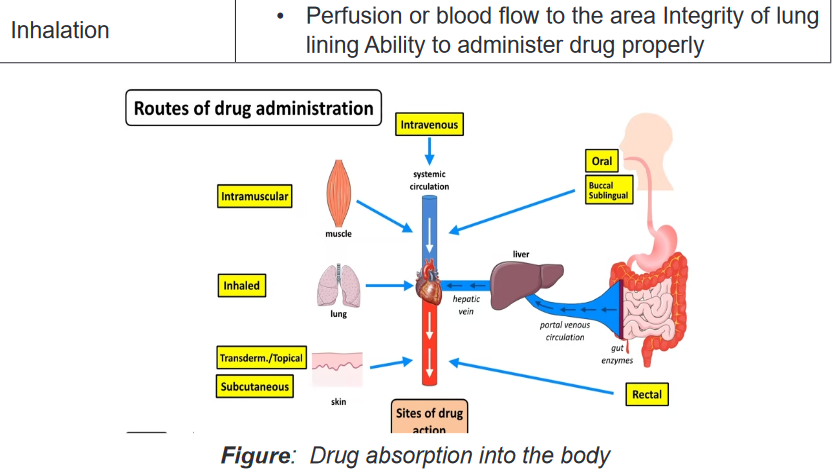
Self- assessment 2.2
Multiple choice questions
1. Which of the following drug transport ways requires energy in drug
movement in aqueous diffusion model?
A. Active transport
B. Facilitated transport
C. Passive transport
D. Filtration2. Identify the factors that can affect the absorption of the drugs administered
by the following routes:
A. IM (Intramuscularly)
B. SC (Subcutaneously)
C. IV (Intravenously)2.3 Distribution of drugs
Learning activity 2.3
Read the scenario below:
A 37-year-old female patient consults the health faciluty for her localized leg
infection. She is known as a diabetic for the last 10 years, and has developed
circulatory complications that affected different body parts including the lower
limbs. This infection can be treated by drugs that can act by either topical way or
systemic way. The nurse taking care of this patient is doubting the right mode to
use, and she wants to get advice from you as a student associate nurse carrying
out the clinical practice in her health post. You then advise him to choose the
drug that will work topically rather than the one that acts systemically.
1. Referring to the process of drug distribiution, why did you advise the
nurse to prescribe the drug that acts topically?2. Name 2 organs with high blood flow that are first to accumulate drugs
which are administered systemically?CONTENT SUMMARY
Once a drug has been absorbed from the stomach and/or intestines (GI Tract) into
the blood, it is circulated to some degree to all areas of the body to which there is
blood flow. This is the process of distribution. Organs with high blood flow, i.e.,
brain, heart, liver, etc. are the first to accumulate drugs, while connective tissue and
lesser-perfused organs are the last. The pattern of distribution of drug molecules by
different tissues after the chemical enters the circulatory system varies.Because of differences in pH, lipid content, cell membrane functions, and other
individual tissue factors, most drugs are not distributed equally in all parts of the
body. For example, the acidity of aspirin influences a distribution pattern that is
different from that of an alkaline product such as amphetamine. In same context,
tissue perfusion is a factor in treating a patient with diabetes who has a lower-leg
infection and needs antibiotics to destroy the bacteria in the area. In this case,
systemic drugs may not be effective because part of the disease process involves
changes in the vasculature and decreased blood flow to some areas, particularly
the lower limbs. If there is not adequate blood flow to the area, little antibiotic can be
delivered to the tissues, and little antibiotic effect will be seen. In addition, patients
in a cold environment may have constricted blood vessels (vasoconstriction) in the
extremities, which would prevent blood flow to those areas. The circulating blood
would be unable to deliver drugs to those areas, and the patient would receive little
therapeutic effect from drugs intended to react with those tissues.Many drugs are bound to plasma proteins such as albumin, and are not lipid soluble.
These drugs cannot be distributed to the central nervous system (CNS) because
of the effective blood–brain barrier (see later discussion), which is highly selective
in allowing lipid-soluble substances to pass into the CNS. Since only drugs that are
not bound are free to exert a pharmacologic effect, the ratio of “free” to “bound” drug
is important in determining the onset and duration of action of drugs. Highly bound
drugs are distributed less extensively throughout the body and are slower to act. By
virtue of their high binding to plasma proteins, they also stay in the body for longer
periods of time because the binding sites act as a sort of “reservoir” for the drug,
releasing drug molecules slowly.Protein Binding
Most drugs are bound to some extent to proteins in the blood to be carried into
circulation. The protein–drug complex is relatively large and cannot enter into
capillaries and then into tissues to react. The drug must be freed from the protein’s
binding site at the tissues.Some drugs are tightly bound and are released very slowly. These drugs have
a very long duration of action because they are not free to be broken down or
excreted. Therefore, they are released very slowly into the reactive tissue. Somedrugs are loosely bound; they tend to act quickly and to be excreted quickly. Some
drugs compete with each other for protein binding sites, altering effectiveness or
causing toxicity when the two drugs are given together.Blood–Brain Barrier
The blood–brain barrier is a protective system of cellular activity that keeps many
things (e.g., foreign invaders, poisons) away from the CNS. Drugs that are highly
lipid soluble are more likely to pass through the blood–brain barrier and reach the
CNS. Drugs that are not lipid soluble are not able to pass the blood–brain barrier.
This is clinically significant in treating a brain infection with antibiotics. Almost all
antibiotics are not lipid soluble and cannot cross the blood–brain barrier. Effective
antibiotic treatment can occur only when the infection is severe enough to alter the
blood–brain barrier and allow antibiotics to cross.Although many drugs can cause adverse CNS effects, these are often the result
of indirect drug effects and not the actual reaction of the drug with CNS tissue.
For example, alterations in glucose levels and electrolyte changes can interfere
with nerve functioning and produce CNS effects such as dizziness, confusion, or
changes in thinking ability.Placenta and Breast Milk
Many drugs readily pass through the placenta and affect the developing fetus in
pregnant women. As it has been approved, it is best not to administer any drugs to
pregnant women because of the possible risk to the fetus. Drugs should be given
only when the benefit clearly outweighs any risk. Many other drugs are secreted
into breast milk and therefore have the potential to affect the neonate. Because of
this possibility, the nurse must always check the ability of a drug to pass into breast
milk when giving a drug to a breast-feeding mother.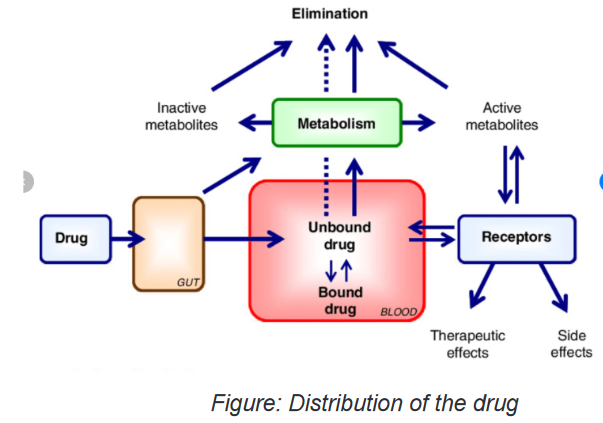
Self- assessment 2.3
1. After absorption of the drug from the stomach and/or intestines (GI Tract)
into the blood, the next pharmacokinetic step is:
A. Absorption
B. Excretion
C. Distribution
D. Metabolism2. During drug distribution, the drugs are bound to which of the following?
A. Proteins in the blood
B. Lipids in blood
C. Vitamins in blood
D. Minerals in blood2.4 Metabolism (Biotransformation) of drugs
Learning activity 2.4
Read the case study below and answer the questions related to it:
An 85-year-old male patient consults the health facility where you are placed
as an associate nurse during the clinical placement. He also suffers from a a
chronic liver disease, and he was prescribed the drugs that are metabolised in
the liver. You then advise the prescribing team to inform the patient that as he is
taking the drug, they need to advise adjust the dose and ensure that the patient
comes for follow up at the health facility.1. Referring to the metabolism of drugs, why did you advise the nurse to
adjust the dose and follow the client up?2. Name the main organ that is involved in metabolism of drugs.
CONTENT SUMMARY
Drugs in the blood and tissues must be inactivated and excreted from the body.
This process is initiated by altering the chemical structure of the drug in such a
way as to promote its excretion. The body is well prepared to deal with a myriad
of foreign chemicals. Enzymes in the liver, in many cells, in the lining of the GI
tract, and even circulating in the body detoxify foreign chemicals to protect the
fragile homeostasis that keeps the body functioning. The transformation of the
drug molecule into a chemically related substance that is more easily excretedfrom the body is called metabolism, biotransformation or detoxification. Drug
metabolism is the process by which the body breaks down and converts medication
into active chemical substances. Drugs can interact with other drugs, foods, and
beverages. Interactions can lessen or magnify the desired therapeutic effect of a
drug, or may cause unwanted or unexpected side effects.Exogenous compounds (xenobiotics) must be metabolized before they can be
excreted. The biochemical transformation of xenobiotics, such as alcohol, nicotine
and drugs is a prime activity of the liver. In addition to the liver, biotransformation
processes occur in plasma, in the lungs, in the gastrointestinal tract and in the
skin. The liver is the organ that plays a major role in metabolism, digestion,
detoxification, and elimination of substances from the body. Think of the liver as a
sewage treatment plant. Everything that is absorbed from the GI tract first enters
the liver to be “treated.” The liver detoxifies many chemicals and uses others to
produce needed enzymes and structures. Enzymes in the liver are responsible
for chemically changing drug components into substances known as metabolites.
Metabolites are then bound to other substances for excretion through the lungs, or
bodily fluids such as saliva, sweat, breast milk, and urine, or through reabsorption
by the intestines. The metabolic rate can vary significantly from person to person,
and drug dosages that work quickly and effectively in one individual may not work
well for another.First-Pass Effect
Drugs that are taken orally are usually absorbed from the small intestine directly
into the portal venous system (the blood vessels that flow through the liver on
their way back to the heart). Aspirin and alcohol are two drugs that are known to
be absorbed from the lower end of the stomach. The portal veins deliver these
absorbed molecules into the liver, which immediately transforms most of the
chemicals delivered to it by a series of liver enzymes. These enzymes break the
drug into metabolites, some of which are active and cause effects in the body,
and some of which are deactivated and can be readily excreted from the body. As
a result, a large percentage of the oral dose is destroyed at this point and never
reaches the tissues. This phenomenon is known as the first-pass effect. The portion
of the drug that gets through the first pass effect is delivered to the circulatory
system for transport throughout the body.Injected drugs and drugs absorbed from sites other than the GI tract undergo a
similar biotransformation when they pass through the liver. Because some of the
active drug already has had a chance to reach the reactive tissues before reaching
the liver, the injected drug is often more effective at a lower dose than the oral
equivalent. Thus, the recommended dose for oral drugs can be considerably higher
than the recommended dose for parenteral drugs, taking the first-pass effect into
accountFactors that influence drug metabolism
These include:
• Genetics,
• Environment,
• Nutrition, and
• Age. Infants and elderly patients may have a reduced capacity to metabolize
certain drugs, and may require adjustments in dosage.Self- assessment 2.4
1. Which of the following factors may impact negatively the drug metabolism?
A. Proper nutrition
B. Advanced age (elderly)
C. Healthy liver
D. Healthy young person2. Which of the following routes of drug administration would be more likely
to make the drug subject to first-pass effect?
A. Oral
B. Intravenous
C. Intraarterial
D. Intranasal2.5 Excretion of drugs
Learning activity 2.5
A patient known for chronic heart failure consults hospital for the appointment.
The doctor decides to test kidney function and finds the client has developed
also kidney failure. The doctor prescribes a drug eliminated by the kidneys, but
reduces the dose. The client asks the doctor why to reduce the dose.
1. Referring to the excretion of the drug, what should the doctor tell the
client?CONTENT SUMMARY
Excretion is the removal of a drug from the body. The skin, saliva, lungs, bile, and
feces are some of the routes used to excrete drugs. The kidneys, however, play the
most important role in drug excretion. Drugs that have been made water soluble
in the liver are often readily excreted from the kidney by glomerular filtration (the
passage of water and water-soluble components from the plasma into the renal
tubule).Other drugs are secreted or reabsorbed through the renal tubule by active transport
systems. The active transport systems that move the drug into the tubule often do
so by exchanging it for acid or bicarbonate molecules. Therefore, the acidity of
urine can play an important role in drug excretion.This concept is important to remember when trying to clear a drug rapidly from
the system or trying to understand why a drug is being given at the usual dose but
is reaching toxic levels in the system. One should always consider the patient’s
kidney function and urine acidity before administering a drug. Kidney dysfunction
can lead to toxic levels of a drug in the body because the drug cannot be excreted.Half-Life
The half-life of a drug is the time it takes for the amount of drug in the body to
decrease to one half of the peak level it previously achieved. For instance, if a
patient takes 20 mg of a drug with a half-life of 2 hours, 10 mg of the drug will
remain 2 hours after administration. Two hours later, 5 mg will be left (one half of
the previous level); in 2 more hours, only 2.5 mg will remain. This information is
important in determining the appropriate timing for a drug dose or determining the
duration of a drug’s effect on the body.The absorption rate, the distribution to the tissues, the speed of biotransformation,
and how fast a drug is excreted are all taken into consideration when determining
the half-life of the drug. The half-life that is indicated in any drug monograph is the
half-life for a healthy person.Using this information, one can estimate the half-life of a drug for a patient with
kidney or liver dysfunction (which could prolong the biotransformation and the time
required for excretion of a drug), allowing the prescriber to make changes in the
dosing schedule.The timing of drug administration is important to achieve the most effective drug
therapy. Nurses can use their knowledge of drug half-life to explain the importance
of following a schedule of drug administration in the hospital or at home.Self- assessment 2.5
1. The patient took 50 mg of drug with half-life of 2 hours at 8h00 AM. How
many mgs will be remaining in the body at 12h00 PM?
A. 25 mg
B. 20mg
C. 12.5mg
D. 6.25mg2. Define what half-life is.
3. In the following organs, which one plays the most important role in
excretion of a drug?
A. The skin
B. Saliva
C. Lungs
D. Kidney2.6 Factors influencing drug effects
Learning activity 2.6
A patient is brought to the health post where you are placed as a student associate
nurse, and you need to prescribe drugs for him.
It is a stunted kid who is brought by his parents, and he is aged 12 months.
1. Which factors influencing drug effects should you bear in mind for this
specific patient?2. Is it necessary to bear in mind factors that influence drug effects during
its prescription?CONTENT SUMMARY
When administering a drug to a patient, the nurse must be aware that the human
factor has a tremendous influence on what actually happens to a drug when it
enters the body. No two people react in exactly the same way to any given drug.
Even though textbooks and drug guides explain the pharmacodynamics and
pharmacokinetics of a drug, it must be remembered that such information usually is
based on studies of healthy adult males. Things may be very different in the clinical
setting. Consequently, before administering any drug, the nurse must consider a
number of factors influencing drug effects as follows:Weight
The recommended dose of a drug is based on drug evaluation studies and is
targeted at a 150-pound (around 70 kilos) person. People who are much heavier
may require larger doses to get a therapeutic effect from a drug because they have
increased tissues to perfuse and increased receptor sites in some reactive tissue.
People who weigh less than the norm may require smaller doses of a drug. Toxic
effects may occur at the recommended dose if the person is very small.
Age
Age is a factor primarily in children and older adults. Children are not just little adults.
Children metabolize many drugs differently than adults do, and they have immature
systems for handling drugs. Many drugs come with recommended pediatric doses,
and others can be converted to pediatric doses using one of several conversion
formulas.
Older adults undergo many physical changes that are a part of the aging process.
Their bodies may respond very differently in all aspects of pharmacokinetics—less
effective absorption, less efficient distribution because of fewer plasma proteins and
less efficient perfusion, altered biotransformation or metabolism of drugs because
of age-related liver changes, and less effective excretion owing to less efficient
kidneys. Many drugs now come with recommended doses for patients who are
older. The doses of other drugs also may need to be decreased for the older adult.
When administering drugs to a patient at either end of the age spectrum, one should
monitor the patient closely for the desired effects. If the effects are not what would
normally be expected, one should consider the need for a dose adjustment.Gender
Physiological differences between men and women can influence a drug’s effect.
When giving IM injections, for example, it is important to remember that men have
more vascular muscles, so the effects of the drug will be seen sooner in men than
in women. Women have more fat cells than men do, so drugs that deposit in fat
may be slowly released and cause effects for a prolonged period. For example,
gas anesthetics have an affinity for depositing in fat and can cause drowsiness
and sedation sometimes weeks after surgery. Women who are given any drug
should always be questioned about the possibility of pregnancy because, as stated
previously, the use of drugs in pregnant women is not recommended unless the
benefit clearly outweighs the potential risk to the fetus.Physiological Factors
Physiological differences such as diurnal rhythm of the nervous and endocrine
systems, acid–base balance, hydration, and electrolyte balance can affect the waythat a drug works on the body and the way that the body handles the drug. If a drug
does not produce the desired effect, one should review the patient’s acid–base and
electrolyte profiles and the timing of the drug.Pathological Factors
Drugs are usually used to treat disease or pathology. However, the disease that the
drug is intended to treat can change the functioning of the chemical reactions within
the body and thus change the response to the drug. Other pathological conditions
can change the basic pharmacokinetics of a drug. For example, GI disorders can
affect the absorption of many oral drugs. Vascular diseases and low blood pressure
alter the distribution of drug, preventing it from being delivered to the reactive tissue,
thus rendering the drug nontherapeutic. Liver or kidney diseases affect the way that
a drug is biotransformed and excreted and can lead to toxic reactions when the
usual dose is given.Genetic Factors
Genetic differences can sometimes explain patients’ varied responses to a given
drug. Some people lack certain enzyme systems necessary for metabolizing a drug,
whereas others have overactive enzyme systems that cause drugs to be broken
down more quickly. Still others have differing metabolisms or slightly different
enzymatic makeups that alter their chemical reactions and the effects of a given
drug.Immunological Factors
People can develop an allergy to a drug. After exposure to its proteins, a person
can develop antibodies to a drug. With future exposure to the same drug, that
person may experience a full-blown allergic reaction. Sensitivity to a drug can
range from mild (e.g., dermatological reactions such as a rash) to more severe
(e.g., anaphylaxis, shock, and death).Psychological Factors
The patient’s attitude about a drug has been shown to have an effect on how that
drug works. A drug is more likely to be effective if the patient thinks it will work than
if the patient believes it will not work. This is called the placebo effect.The patient’s personality also influences compliance with the drug regimen. Some
people who believe that they can influence their health actively seek health care
and willingly follow a prescribed regimen. These people usually trust the medical
system and believe that their efforts will be positive. Other people do not trust
the medical system. They may believe that they have no control over their health
and may be unwilling to comply with any prescribed therapy. Knowing a patient’s
healthseeking history and feelings about health care is important in planning aneducational program that will work for that patient. It is also important to know this
information when arranging for necessary follow-up procedures and evaluations. As
the caregiver most often involved in drug administration, the nurse is in a position
to influence the patient’s attitude about drug effectiveness. Frequently, the nurse’s
positive attitude, combined with additional comfort measures, can improve the
patient’s response to a medication.Environmental Factors
The environment can affect the success of drug therapy. Some drug effects are
enhanced by a quiet, cool, non-stimulating environment. For example, sedating
drugs are given to help a patient relax or to decrease tension. Reducing external
stimuli to decrease tension and stimulation help the drug be more effective. Other
drug effects may be influenced by temperature. For example, antihypertensives
that work well during cold, winter months may become too effective in warmer
environments, when natural vasodilation may lead to a release of heat that tends to
lower the blood pressure. If a patient’s response to a medication is not as expected,
look for possible changes in environmental conditions.Tolerance
The body may develop a tolerance to some drugs over time. Tolerance may arise
because of increased biotransformation of the drug, increased resistance to its
effects, or other pharmacokinetic factors. When tolerance occurs, the drug no long
causes the same reaction. Therefore, increasingly larger doses are needed to
achieve a therapeutic effect. An example is morphine, an opiate used for pain relief.
The longer morphine is taken, the more tolerant the body becomes to the drug, so
that larger and larger doses are needed to relieve pain. Clinically, this situation can
be avoided by giving the drug in smaller doses or in combination with other drugs
that may also relieve pain. Cross-tolerance—or resistance to drugs within the same
class—may also occur in some situations.Interactions
When two or more drugs or substances are taken together, there is a possibility
that an interaction can occur, causing unanticipated effects in the body. Alternative
therapies, such as herbal products, act as drugs in the body and can cause these
same interactions. Certain foods can interact with drugs in much the same way.
Usually this is an increase or decrease in the desired therapeutic effect of one or all
of the drugs or an increase in adverse effects.Self- assessment 2.6
1. List at least 5 factors that influence drug effects.
2. What is the ideal adult weight is considered while prescribing drugs?2.7 Drug-drug interactions
Learning activity 2.7
Read the case study below and answer the question related to it:
A 40-year-old male patient was prescribed a penicillin G injection, and he is
receiving concurrently tetracyclines taken by oral route. The symptoms of the
disease for which penicillin G was given persisted after 5 days of the treatment,
and the patient came back to the health facility where the drug was prescribed.
The prescribing personnel decide that both penicillin G and tetracyclines are
needed for this patient, and decides to increase the dose of penicillin G. After
2 days, the symptoms start to resolve until they completely disappear and the
patient improves.1. What do you think happened for this patient so that he did not improve
with the first period, and improved after increasing the dose of penicillin G?CONTENT SUMMARY
A drug-drug reaction is when there’s an interaction between two or more prescription
drugs. This can cause the medication to be less or more potent than intended or
result in unexpected side effects.Clinically significant drug-drug interactions occur with drugs that have small margins
of safety. If there is very little difference between a therapeutic dose and a toxic dose
of the drug, interference with the drug’s pharmacokinetics or pharmacodynamics
can produce serious problems. For example, drug-drug interactions can occur in
the following situations:At the site of absorption: One drug prevents or accelerates absorption of the
other drug. For example, the antibiotic tetracycline is not absorbed from the GI tract
if calcium or calcium products (milk) are present in the stomach.During distribution: One drug competes for the protein-binding site of another
drug, so the second drug cannot be transported to the reactive tissue. For example,
aspirin competes with the drug methotrexate for protein-binding sites. Because
aspirin is more competitive for the sites, the methotrexate is bumped off, resulting
in increased release of methotrexate and increased toxicity to the tissues.During biotransformation: One drug stimulates or blocks the metabolism of
the other drug. For example, warfarin (Coumadin), an oral anticoagulant, is
biotransformed more quickly if it is taken at the same time as barbiturates, rifampin,
or many other drugs. Because the warfarin is biotransformed to an inactive state
more quickly, higher doses will be needed to achieve the desired effect.During excretion: One drug competes for excretion with the other drug, leading
to accumulation and toxic effects of one of the drugs. For example, digoxin and
quinidine are both excreted from the same sites in the kidney. If they are given
together, the quinidine is more competitive for these sites and is excreted, resulting
in increased serum levels of digoxin, which cannot be excreted.At the site of action: One drug may be an antagonist of the other drug or may cause
effects that oppose those of the other drug, leading to no therapeutic effect. This is
seen, for example, when an antihypertensive drug is taken with an antiallergy drug
that also increases blood pressure. The effects on blood pressure are negated, and
there is a loss of the antihypertensive effectiveness of the drug.
If a patient is taking antidiabetic medication and also takes the herb ginseng, which
lowers blood glucose levels, he or she may experience episodes of hypoglycemia
and loss of blood glucose control.
Whenever two or more drugs are being given together, first consult a drug guide
for a listing of clinically significant drug-drug interactions. Sometimes problems
can be avoided by staggering the administration of the drugs or adjusting their
doses. For example, when penicillin G and tetracyclines are taken concurrently, the
effectiveness of penicillin G decreases. If this combination is used, the dose of the
penicillin should be increased.Drug-nonprescription treatment interaction refers the reaction between a drug
and a nonprescription treatment. These include over-the-counter (OTC) medications,
herbs, vitamins, or other supplements. An example of this type of interaction can
occur between a diuretic, a drug that attempts to rid the body of excess water and
salt taken with ibuprofen, as an non steroid anti-inflammatory drug. The ibuprofen
may reduce the diuretic’s effectiveness because ibuprofen often causes the body
to retain salt and fluid.Self- assessment 2.7
1. Referring to the lesson on drug-drug interactions, list the stages/sites at
which drug-drug interactions may happen.2. What do you understand by “drug-nonprescription treatment interaction”?
2.8 Drug- food/beverage interactions
Learning activity 2.8
our relative consulted the health post complaining of the low abdominal pain
and she has been prescribed medications. She was then told that she could not
take grapefruit juice while she is taking a drug but she does not understand why.
When she arrives home she asks you to give more explanation about why she
was requested not to take the grapefruit juice.
1. With reference to the interactions between drugs and food or beverages,
what will you tell to your sister?2. Drug-food/beverage interactions always result in decreased serum levels
of the concerned drugs. TRUE or FALSECONTENT SUMMARY
For the most part, a drug-food interaction occurs when the drug and the food are in
direct contact in the stomach. Some foods increase acid production, speeding the
breakdown of the drug molecule and preventing absorption and distribution of the
drug. Some foods chemically react with certain drugs and prevent their absorption
into the body. The antibiotic tetracycline cannot be taken with iron products for this
reason. Tetracycline also binds with calcium to some extent and should not be
taken with foods or other drugs containing calcium.Grapefruit juice has been found to affect liver enzyme systems for up to 48 hours
after it has been ingested. This can result in increased or decreased serum levels of
certain drugs. Many drugs come with the warning that they should not be combined
with grapefruit juice. This drug–food interaction does not take place in the stomach,
so the grapefruit juice needs to be avoided the entire time the drug is being used,
not just while the drug is in the stomach.In most cases, oral drugs are best taken on an empty stomach. If the patient cannot
tolerate the drug on an empty stomach, the food selected for ingestion with the drug
should be something that is known not to interact with it. Drug monographs usually
list important drug-food interactions and give guidelines for avoiding problems and
optimizing the drug’s therapeutic effects.Self- assessment 2.8
A patient consults the health post for pain during urination. He was then prescribed
antibiotic drugs. The associate nurse student in pharmacy of the health post
dispenses the medications but indicates the patient that he needs to take drugs
on the empty stomach.
1. Explain why it is better to take oral drugs on an empty stomach.2. Drug-food/beverage interactions occur for drugs administered orally only.
TRUE or FALSE2.9 Time-Response Relationships: Drug Plasma Levels
Learning activity 2.9
An associate nurse prescribed the drug to the client to be taken in equal intervals
of 4 hours a day. The first dose meant to achieve the target concentration rapidly
has to be taken at the time he consults the health post at 3h00 PM. The following
doses must then follow the first dose later on., respecting the intervals This
means that the second dose should be taken at 9.00 PM, third dose at 11 h00
PM. The client tells the associate nurse that he is going to take 2nd dose and 3
doses at the same time because he goes to bed at 8 h00’.
1. What should the associate nurse should the patient to understand the
reason of respecting the doses interval?2. Differentiate the loading dose from the maintenance dose.
CONTENT SUMMARY
Drugs are used for the treatment of diseases but the modes of administration
of drugs are different. The mode of administration is designed on the basis of
absorption, distribution, metabolism and excretion (ADME) of drugs. Drugs usually
follow two processes for their pharmacokinetic behaviour in the body. These are
first order and zero order processes.First order kinetic: This is the most common process for many drugs. The rate at
which absorption, distribution, metabolism and excretion occur are proportional to
the concentration of drugs i.e. constant fraction of this drug in the body disappears
in each equal interval of time.Zero order kinetic: It is independent of the amount of drug present at the particular
sites of drug absorption or elimination. Few drugs follow this process e.g. ethanol,
phenytoin. Here constant amount of the drug is eliminated in each equal interval of
time. On repeated administration of drug after certain stage it goes on accumulating
in the body and leads to toxic reactions.Steady state plasma concentration: When a drug dose is given repeatedly over
a given period, a steady state is eventually reached, at which point the amount of
drug absorbed is in equilibrium with that eliminated from the body. Steady state is
achieved after 4 to 5 half –lives for most of the drugs which follow first order kinetics.
For example, a drug with half-life of 6 hours will be expected to be at steady state
after more than 24 hours of administration. The pattern of drug accumulation during
repeated administration of drug at intervals equal to its elimination half-life.For some drugs, the effects are difficult to measure, toxicity and lack of efficacy
are both potential dangers, and/or the therapeutic window is narrow. In these
circumstances, doses must be adjusted carefully to a desired steady-state
concentration by giving loading and maintenance dosesLoading dose: The loading dose is one or a series of doses that may be given at
the onset of therapy with the aim of achieving the target concentration rapidly.Maintenance dose: To maintain the chosen steady-state or target concentration,
the rate of drug administration is adjusted such that the rate of input equals to rate
of loss.Self- assessment 2.9
Define the following terms
1. Steady state plasma concentration
2. Zero order kinetic
3. First order kinetic2.10 Introduction to pharmacodynamics
Learning activity 2.10
You receive a patient who consults the health post where you are placed as an
associate-nurse student. The patient consults for the difficulty swallowing and
fever. On the examination, you realize he has tonsillitis and you wish to prescribe
the drug. Before proceeding, you bear in your mind that the selective toxicity of
a drug must be considered always when the drug is being used.
1. What do you understand by the term “selective toxicity” as it is applied to
pharmacodynamics?2. How do we call the specific areas on cell membranes where many drugs
are thought to act?CONTENT SUMMARY
Pharmacodynamics is the study of the interactions between the chemical
components of living systems and the foreign chemicals, including drugs that enter
those systems. All living organisms function by a series of complicated, continual
chemical reactions. When a new chemical enters the system, multiple changes in
and interferences with cell functioning may occur. To avoid such problems, drug
development works to provide the most effective and least toxic chemicals for
therapeutic use.Drugs usually work in one of four ways:
1. To replace or act as substitutes for missing chemicals
2. To increase or stimulate certain cellular activities
3. To depress or slow cellular activities
4. To interfere with the functioning of foreign cells, such as invading
microorganisms or neoplasms (drugs that act in this way are called
chemotherapeutic agents). Drugs can act in several different ways to
achieve these results.Receptor Sites
Many drugs are thought to act at specific areas on cell membranes called receptor
sites. The receptor sites react with certain chemicals to cause an effect within the
cell. In many situations, nearby enzymes break down the reacting chemicals and
open the receptor site for further stimulation. To better understand this process,
think of how a key works in a lock. The specific chemical (the key) approaches a
cell membrane and finds a perfect f it (the lock) at a receptor site. The interaction
between the chemical and the receptor site affects enzyme systems within the cell.
The activated enzyme systems then produce certain effects, such as increased or
decreased cellular activity, changes in cell membrane permeability, or alterations
in cellular metabolism. Some drugs interact directly with receptor sites to cause
the same activity that natural chemicals would cause at that site. These drugs are
called agonists. For example, insulin reacts with specific insulin-receptor sites to
change cell membrane permeability, thus promoting the movement of glucose into
the cellOther drugs act to prevent the breakdown of naturaal chemicals that are stimulating
the receptor site. Some drugs react with receptor sites to block normal stimulation,
producing no effect.Drug-Enzyme Interactions
Drugs also can cause their effects by interfering with the enzyme systems that act as
catalysts for various chemical reactions. Enzyme systems work in a cascade fashion,with one enzyme activating another, and then that enzyme activating another, until
a cellular reaction eventually occurs. If a single step in one of the many enzyme
systems is blocked, normal cell function is disrupted. Acetazolamide (Diamox) is a
diuretic that blocks the enzyme carbonic anhydrase, which subsequently causes
alterations in the hydrogen ion and water exchange system in the kidney, as well
as in the eye.Selective Toxicity
Ideally, all chemotherapeutic agents would act only on enzyme systems that are
essential for the life of a pathogen or neoplastic cell and would not affect healthy
cells. The ability of a drug to attack only those systems found in foreign cells is
known as selective toxicity. Penicillin, an antibiotic used to treat bacterial infections,
has selective toxicity. It affects an enzyme system unique to bacteria, causing
bacterial cell death without disrupting normal human cell functioning.Unfortunately, most other chemotherapeutic agents also destroy normal human
cells, causing many of the adverse effects associated with antipathogen and
antineoplastic chemotherapy. Cells that reproduce or are replaced rapidly (e.g.,
bone marrow cells, gastrointestinal [GI] cells, hair follicles) are more easily affected
by these agents. Consequently, the goal of many chemotherapeutic regimens is to
deliver a dose that will be toxic to the invading cells yet cause the least amount of
toxicity to the host.Self- assessment 2.10
1. There are four ways through which drugs usually work. Mention these 4
ways.2. The drugs administered to humans only affect the target cells, and never
harm the human cells because they were made in a specific way. TRUE
or FALSE2.11 Agonist drugs
Learning activity 2.11
Consult the library and read agonist drug, in pharmacology and to be able to
respond to the question of the scenario below.An associate nurse is caring an old woman with diabetes at home. After receiving
insulin injection, she asks associate nurse how the insulin will work to allow the
glucose to enter the cell.1. If you were the associate nurse in the scenario. What would you explain
to the woman?CONTENT SUMMARY
Drugs that interact directly with receptor sites to cause the same activity that natural
chemicals would cause at that site are agonists drugs.An agonist medication mimics the action of the signal by binding to and
activating a receptor.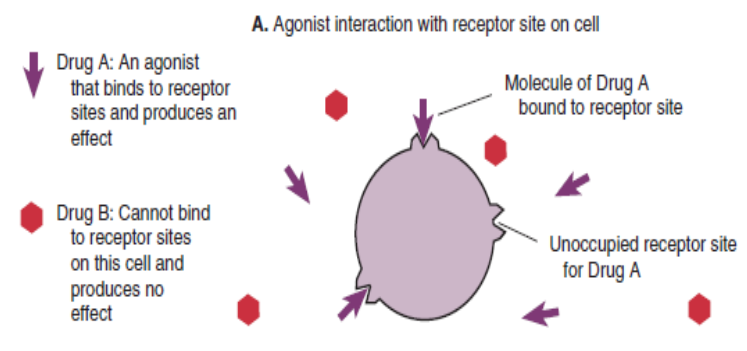
An agonist drug binds to receptor sites and produces an effect.
The insulin is an example of agonist drug as it reacts with specific insulin-receptor
sites to change cell membrane permeability, thus promoting the movement of
glucose into the cell. This is the same action as natural insulin would do in normal
human body.Full agonists are drugs when administered at concentrations sufficient to saturate
the receptor pool, can activate their receptor-effector systems to the maximum
extent of which the system is capable and this causes a shift of almost all of the
receptor pool. On the other hand, partial agonists. The term partial agonist or
agonist-antagonist drug describes a medication that produces a weaker, or less
efficacious, response than an agonist. It binds to the same receptors and activate
them in the same way but do not evoke as great a response, no matter how high
the concentration.Example of full agonist effect in clinical application is administration of bethanechol
(Urecholine). It binds to acetylcholine receptors in the autonomic nervous system
and produces the same actions as acetylcholine.Self- assessment 2.11
1. Define agonist drug
2. Differentiate full agonist from partial agonist drug2.12 Drug Antagonists
Learning activity 2.12
During your clinical placement in the hospital, you observe a nurse giving a drug
named atropine to the patient with very slow heart rate. Your colleague asks the
nurse how the atropine will increase the heart rate. The nurse explains in short
word that the atropine is antagonist of acetylcholine a neurotransmitter of the
parasympathetic nervous system that can slow the heart rate.1. Visit the library and read the content of antagonist drug and briefly
describe what the antagonist drug is.CONTENT SUMMARY
Antagonism is an interaction between two or more drugs that have opposite
effects on the body. Antagonist may block or reduce the effectiveness of one or
more of the drugs. An antagonist is a medication that typically binds to a receptor
without activating them, but instead, decreases the receptors ability to be activated
by other agonist. That drug will occupy a receptor and prevent the endogenous
chemical from acting. Antagonists often compete with agonists for the receptor
binding sitesA competitive antagonist is a drug that binds to the same receptor sites as another
drug and prevents it from binding.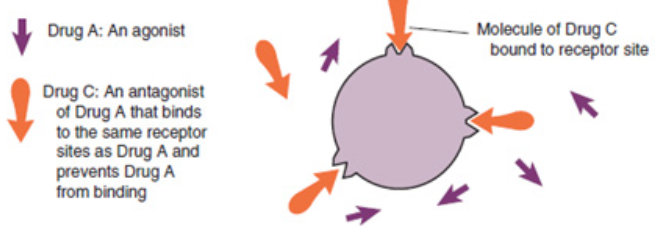
A noncompetitive antagonist is a drug that binds to different receptor sites from
another drug but still prevents that drug from binding.Not all antagonism is associated with receptors. Functional antagonists inhibit the
effects of an agonist not by competing for a receptor but by changing pharmacokinetic
factors. For example, antagonists may slow the absorption of a drug. By speeding
up metabolism or excretion, an antagonist may enhance the removal of a drug from
the body. An other example of antagonism include antidote effect on drugsThe relationships that occur between agonists and antagonists explain many of the
drug–drug and drug–food interactions that occur in the body.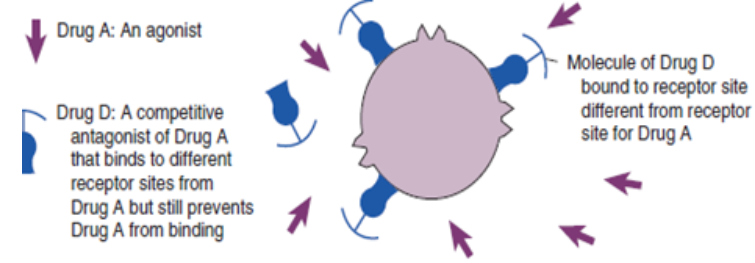
An example of antagonist effect in clinical application is the use of the drug
atropine which competes with acetylcholine for specific receptors associated with
the autonomic nervous system. If the dose is high enough, atropine will inhibit the
effects of acetylcholine, because acetylcholine cannot bind to its receptors.Self- assessment 2.12
1. Define a drug antagonist
2. Differentiate competitive from non- competitive antagonist drug2.13 Pharmacokinetics in special population
Learning activity 2.13
While you were in clinical practice in consultation room, you saw a senior nurse
played attention while prescribing the drugs to the children and old people than
other group of people between 20 to 50 years old.1. Visit the library, read the books of pharmacology on pharmacokinetics
special considerations and come up with a summary of why Children
often require different doses of drugs than adults.CONTENT SUMMARY
Pharmacokinetics are typically dependent on a variety of physiological variables
(e.g., age, ethnicity, or pregnancy) or pathological conditions (e.g., renal and hepatic
insufficiency, cardiac dysfunction, obesity, etc.).To providing safe and effective medications, pediatric drug therapy represent a
great challenge to the health professionals. Children often require different doses
of drugs than adults because children’s bodies often handle drugs very differently
from adults’ bodies. In some cases, a pediatric dose is suggested, but in many
cases it will need to be calculated based on the weight and the age of the child.Medications can affect the fetus either by interfering with some important function
in the mother which indirectly damages the fetus or by pass across the placenta or
acting directly on the fetus.Most drugs cross the placenta,30% of pregnant women take drugs and 10% take
drugs in the first trimester when the fetus is more vulnerable. It is important to
discover which drugs can produce fetal damage and which are safe to use but it is
difficult because in the period of implantation (5-15 days): Drug toxicity can result in
abortion, in Embryonic stage (15 to 55days): Embryo is changing from a group of
cells into a recognizable human being.The embryo is particularly susceptible to drug toxicity at this time and leads to fetal
malformation or teratogenesis (a process by which congenital malformations
are produced in an embryo or fetus). Fetogenic stage (55 to birth): Drug damage
is less likely but still possible, at Delivery: Drugs may interfere with labour and
modify the behaviour of neonates immediately after birth, Food drug administration
indicate the potential or actual teratogenic effects of a drug.The New-borns are unable to break down drugs as effectively older children or adults
do. Example the accumulation of chloramphenicol can cause grey syndrome due
to collapse of circulation.The period from birth to adolescent is characterised by dramatic changes in physical
growth, psychosocial development and sensitivity to drugs. Old persons are among
the most consumers of drugs. Yet their metabolism changes with age.Elder people have fewer albumins in the blood, with certain drug, less protein bound
and more are free in the blood and tissue fluids and can therefore produce a greater
pharmacological effect.With advanced age, liver enzyme decreases blood supply especially to liver
consequently the absorption decreases, as result some drugs may therefore be
more slowly broken down and their blood concentration may rise to toxic levels.Drugs are also excreted via the kidney. Old age, sometimes associated with kidney
diseases, leads to a decline in renal function, so that by the age of 80 years, renal
function is only half than at age 40. This again may cause drug accumulation in the
Body and evidence that certain systems become more sensitive to drug action with
advancing years.Self- assessment 2.13
1. What are the special considerations in case of pharmacokinetics during
drug administration?2. Why drug toxicity can rise in the people over 80 years old?
2.14 Pharmacodynamics in Special population
Learning activity 2.14
1. Visit the library and read the book of pharmacology on pharmacodynamics
special population and summarize how the medication can affect the
fetus.CONTENT SUMARY
All living organisms function by a series of complicated, continual chemical reactions.
When a new chemical enters the system, multiple changes in and interferences
with cell functioning may occur. To avoid such problems, drug development works
to provide the most effective and least toxic chemicals for therapeutic use.The reactions changes depending on many factors including receptors site age
and personal health status. Pregnant women, children and older persons are
special population for whom attention must be taken when administering them the
medication.The use of drugs in pregnancy is complicated by the potential for harmful effects
on the growing fetus, altered maternal physiology. Because experience with many
drugs in pregnancy is severely limited, it should be assumed that all drugs are
potentially harmful until sufficient data exist to indicate otherwise.Some drugs’ effect may be serious to the pregnant woman and may even be fatal
for the unborn baby. Again when administered during delivery drugs may change
neonates behaviors even lead to immediate complications. Medications can affect
the fetus either by interfering with some important function in the mother which
indirectly damages the fetus or by pass across the placenta or acting directly on
the fetus.There are drugs that have toxicity that when given during implantation period
they cause abortion. Other may cause fetal malformation or teratogenesis when
administered during the embryonic period of pregnancy. Drugs may interfere with
labor and modify the behavior of neonates immediately after birth.For every pregnant woman, it is imperative to avoid giving drugs as possible in
the first 3 months of pregnancy, give drugs at the lowest effective dose for as a
short time as possible, avoid recently introduced drugs if possible, be sure that
every female you attempt to give medication is pregnant or not, read drug
risk category before administration of any drug to a pregnant female.Most drugs pass into breast milk, but at a very low and innocuous concentration.
Generally, drugs should be avoided by nursing mothers, but if the drug is essential
the baby should feed before the mother take drugs, then when blood levels will be
low. Certain drugs should not be used by nursing mothers and, if unavoidable, will
require transfer to bottle feeding.The liver of children is immature and depending on age some are inactive this mark
the difference in drug metabolism in the body that finally may cause accumulation
and increase drug toxicity.Children have immature renal system and it is difficult to excrete drugs. This
increase risk for toxicity.Factors affecting pharmacodynamics of a drug in children are summarized
as below:
– Reduced gastric acidity some medication that require acid environment to be
broken are not well metabolized.– Small muscle mass: Drugs administered in intramuscular should be at lower
dose to allow absorption.– Thin stratum corneum: Topical application of medication can be easily
absorbed and when large amount is applied toxicity be present. Again special
attention when administered subcutaneous medication is taken as it is very
easy to reach the muscle.– High proportion of water in body: Water soluble drugs are highly absorbed
and lipid soluble drugs are poorly absorbed.– Reduced protein-binding capability this limit some drug absorption and
distribution to the whole body.– Unpredictable hepatic enzymes production
– Immature renal system
Self- assessment 2.14
1. What are the considerations a nurse might take to avoid harmful drug
effects on a pregnant woman?2.15 Dose-Response Relationships
Learning activity 2.14
An associate nurse was assigned to care for an old man on palliative care. The
man is receiving morphine as analgesic drug 5 mg Subcutaneous route (SC).
Today associated nurse visited the client and the client tells him that the dose
he is receiving do as nothing. Associated nurse called the physician, and the
physician order to increase the dose as 8mg. The client after receiving 8mg
dose, report the relief of pain.1. In your view, why 5 mg was not reducing pain and 8 mg reduce the pain?
CONTENT SUMMARY
How does a patient respond to varying doses of a drug? Common sense would
suggest that a larger dose would produce more drug effect.An antihypertensive drug would cause a greater reduction in blood pressure if the
dose was increased from 50 to 100 mg. These simple examples describe the dose–
response relationship, one of the most fundamental concepts in pharmacology.Examining and comparing dose–response curves can yield a large amount of
information about a drug. A dose–response curve plots the drug dose administered
to the patient versus the intensity or degree of response obtained.There are three distinct phases of a dose–response curve that indicate essential
pharmacodynamics principles.Phase 1 occurs at the lowest doses.
The flatness of this portion of the curve indicates that few target cells have been
affected by the drug; doses that are too small will not produce a therapeutic effect.Phase 2 is the rising, straight line portion of the curve. In this portion, there is a
linear relationship between the amount of drug administered and the degree of
response obtained from the patient. For example, if the dose is doubled, twice as
much response may be obtainedThis is the most desirable range of doses for pharmacotherapeutics, because
giving more drug results in proportionately more effect; a lower drug dose gives
less effect.In phase 3 increasing the drug dose produces no additional therapeutic response a
plateau has been reached. This may occur for a number of reasons. One possible
explanation is that all the target receptors for the drug are occupied. It could also
mean that the drug has brought 100% relief, such as when a migraine headache
has been terminated; giving higher doses produces no additional relief.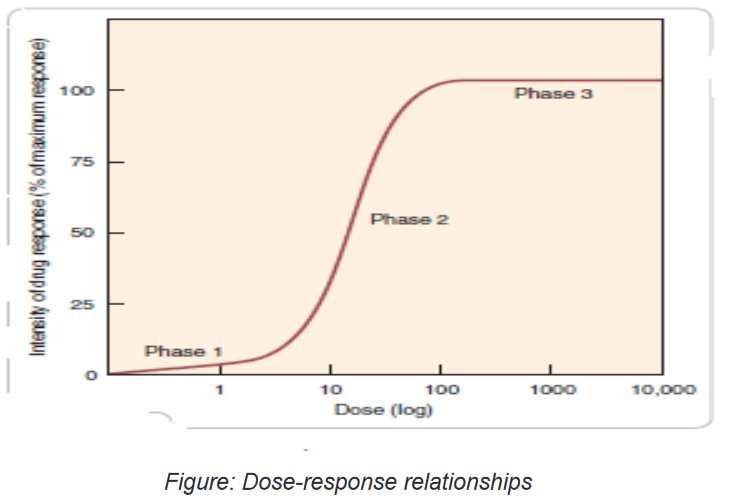
Self- assessment 2.15
1. After reading the content of the lesson, what do you understand by dose
response relationship?2.16 Potency of drug
Learning activity 2.16
1. Using library Pharmacology textbook/internet search the term Potency of
the drug and make short note on term Potency of drug.CONTENT SUMMARY
The concept of potency is first fundamental ways to compare medications
within therapeutic and pharmacologic classes. Pharmacologic potency can
largely determine the administered dose of the chosen drug. For therapeutic
purposes, the potency of a drug should be stated in dosage units, usually in terms of
a particular therapeutic end point may be used in comparing one drug with another.Potency is an index of how much drug must be administered to elicit a desired
response. A drug that is more potent will produce its therapeutic effect at a lower
dose, compared to another drug in the same class.Thus, potency is a way to compare the doses of two independently
administered drugs in terms of how much is needed to produce a particular
response.If a drug were of extremely low potency, we might need to administer that drug
in huge doses multiple times a day to achieve beneficial effects. In this case, an
alternative drug with higher potency would be desirable. Fortunately, it is rare for
a drug to be so lacking in potency that doses of inconvenient magnitude need be
given. The only consequence of having greater potency is that a drug with greater
potency can be given in smaller doses.Which is more important to the outcomes of pharmacotherapy: potency or efficacy?
Perhaps the best way to understand these important concepts is to use the specific
example of headache pain. Two common analgesic therapies are ibuprofen 200mg,
and aspirin 650mg. The fact that ibuprofen relieves pain at a lower dose indicates
that it is more potent than aspirin.In clinical practice the term potency is often misused to indicate a more
effective drug. The nurse should remember the correct definitions of the words
potency and efficacy and try to incorporate them into clinical practice.In everyday, people tend to use the word potent to express the pharmacologic
concept of effectiveness. That is, when most people say, “This drug is very potent,”
what they mean is, “This drug produces powerful effects.” They do not mean, “This
drug produces its effects at low doses.” In pharmacology, we use the words potent
and potency with the specific and appropriate terminology.Self- assessment 2.16
1. What does “DRUG POTENCY” mean?
A. A measure of how tightly a drug bind to plasma proteins
B. A measure of how tightly a drug bind to a receptor
C. A measure of inhibiting potency of a drug
D. An index of how much drug must be administered to elicit a desired
response.2.17 Efficacy of drug
Learning activity 2.17
1. Using library Pharmacology textbook/internet search the term Efficacy of
the drug and make short note on term Efficacy of drug.CONTENT SUMMARY
The concept of Efficacy is a second fundamental ways to compare medications
within therapeutic and pharmacologic classes, which is defined as the greatest
maximal response that can be produced from a particular drug or defined as the
largest effect that a drug can produce. Maximal efficacy is indicated by the height
of the dose-response curve. The maximal efficacy of a drug is obviously crucial for
making clinical decisions when a large response is needed. It may be determined
by the drug’s mode of interactions with receptors (as with partial agonists,
described above) or by characteristics of the receptor-effector system involved.
Thus, therapeutic efficacy may be affected by the characteristics of a particular
drug-receptor interaction, but it also depends on a host of other factors.The best way to understand this important concept is to use the specific example
of headache pain. Two common analgesic therapies are ibuprofen 200mg, and
aspirin 650mg. The fact that ibuprofen relieves pain at a lower dose indicates that it
is more potent than aspirin. At the given doses, however, both are equally effective
at relieving headaches; thus they have the same sufficient efficacy to bring relief.
Morphine has a greater efficacy than aspirin or ibuprofen and could effectively treat
this type of pain. From a pharmacotherapeutic perspective, efficacy is almost
always more important than potency. In the preceding example, the average
dose is unimportant to the patient, but headache relief is essential.As another comparison, the patient with cancer is much more concerned
with how many cancer cells have been killed (efficacy) than with the dose the
nurse administered (potency).
Within a pharmacologic class, not all drugs are equally effective at treating a disorder.
For example, some antineoplastic drugs kill more cancer cells than others; some
antihypertensive agents lower blood pressure to a greater extent than others; and
some analgesics are more effective at relieving severe pain than others in the same
class. Furthermore, drugs in the same class are effective at different doses: one
antibiotic may be effective at a dose of 1mg/kg, whereas another is most effective
at 100mg/kg.A drug with very high maximal efficacy is not always more desirable than a drug
with lower efficacy. Recall that we want to match the intensity of the response to
the patient’s needs. It is important to note that the potency of a drug implies nothing
about its maximal efficacy! Potency and efficacy are completely independent
qualities.Drug A can be more effective than drug B even though drug B may be more potent.
Also, drugs A and B can be equally effective even though one may be more potent.
The only consequence of having greater potency is that a drug with greater potency
can be given in smaller doses.In deciding which of two drugs to administer to a patient, the prescriber must
usually consider their relative effectiveness rather than their relative potency. It is
important to distinguish between a drug’s potency and its efficacy for clinical use. To
choose among drugs and to determine appropriate doses of a drug, the prescriber
must know the relative pharmacologic potency and maximal efficacy of the drugs
in relation to the desired therapeutic effect. The clinical effectiveness of a drug
depends not on its potency (EC50), but on its maximal efficacy and its ability to
reach the relevant receptors. This ability can depend on its route of administration,
absorption, distribution through the body, and clearance from the blood or site of
action.Self- assessment 2.17
1. The term “drug efficacy” means:
A. Two drugs combine with one another to form an inactive compound
B. Two drugs combine with one another to form a more active compound
C. The greatest maximal response that can be produced from a particular
drug or defined as the largest effect that a drug can produce
D. Two drugs combine with one another to form a more water-soluble
compound.2.18 Therapeutic index
Learning activity 2.18
1. Read the book of pharmacology, discuss on therapeutic index (using
library books) and make short notes.CONTENT SUMMARY
The therapeutic index (TI; also referred to as therapeutic ratio) is a quantitative
measurement of the relative safety of a drug. It is a comparison of the amount of
a therapeutic agent that causes the therapeutic effect to the amount that causes
toxicity. The related terms therapeutic window or safety window refer to a range
of doses which optimize between efficacy and toxicity, achieving the greatest
therapeutic benefit without resulting in unacceptable side-effects or toxicity.The larger the therapeutic index (TI), the safer the drug is. If the TI is small (the
difference between the two concentrations is very small), the drug must be dosed
carefully and the person receiving the drug should be monitored closely for any
signs of drug toxicity.In the early days of pharmaceutical toxicology, TI was frequently determined in
animals as lethal dose of a drug for 50% of the population (LD50) divided by the
minimum effective dose for 50% of the population (ED50).Self- assessment 2.18
1. What do you understand by therapeutic index?
2. What the nurse have to do before and after administrating the drug which
have small therapeutic index?
3. The term therapeutic window or safety window refer to a range of doses
which optimize between:
A. Efficacy and toxicity
B. Efficacy and Lethal
C. Loading and maintenance
D. Potency and toxicity2.19 Inter patient Variability
Learning activity 2.19
1. Read the book of pharmacology (using library books), discuss on
therapeutic index Inter patient Variability and make note.CONTENT SUMMARY
Once drugs are administered, certain patients fail to react to treatment even when
systemic exposure to the drug is within the range associated with therapeutic
response. The main reasons are many but most of the time misdiagnosis of
the disease or individual lacking the therapeutic target or inability to express a
satisfactory response.The reasons behind patient difference in responsiveness to a given dose of a
drug are many. They include genetics, disease, age, gender, body weight, drugs
given concomitantly, and various behavioral and environmental factors. Age, body
weight, disease, and concomitantly administered drugs are important because they
are measurable sources of variability that can be taken into account. Gender-
linked differences in hormonal balance, body composition, and activity of certain
enzymes manifest themselves in differences in both pharmacokinetics and
responsiveness, but overall, the effect of gender is small. Although inheritance
accounts for a substantial part of the differences in response among individuals
for many drugs, much of this variability is still largely unpredictable, particularly in
regard to pharmacodynamics.The examples of variability in drug response so far have been of the therapeutic
effect of the drug, but the situation equally applies to adverse effects. For some
relatively minor adverse effects, variability may be as great as, or even greater
than, that for the therapeutic effect, particularly when they are associated with the
inherent pharmacologic property of the drug (side effects), such as dryness of mouth
experienced with some sympathomimetic nasal decongestants. Frequent side
effects are also invariably experienced by patients undergoing chemotherapy during
cancer treatment. However, in many other therapeutic settings, moderate to severe
side effects are much less frequently experienced. Occasionally, the frequency of
an adverse effect is so low that it is only detected with any significance when tens
of thousands, if not millions, of patients have been treated with the drug. Even so,
there is still some relationship between the likelihood and severity of an adverse
effect and the exposure to the drug, although establishing it with any confidence
may be difficult. The degree and relative contribution of pharmacokinetics and
pharmacodynamics to variability in response within a patient population vary with
the drugSelf- assessment 2.19
1. hat are the reasons behind patient difference in responsiveness to a
given dose of a drug?
2. Explain how gender can affect drug response?2.20 End unit assessment
End Unit assessment 2
1. Which of the following statements is true with regard to the meaning of
selective toxicity of an antibiotic?
A. The ability of the anti-infectious agent to affect both microbial and host
cells at the same time
B. The ability of anti-infectious agent to affect the host cell with few effects
to the microbial cell
C. The ability of an anti-infectious agent to affect the bacterial cell wall
since the human cell does also have the cell wall
D. The ability of the anti-infectious agent to affect the infectious agent’s cell
without affecting the host (human) cell2. Which of the following pharmacological terms deals with Absorption,
distribution, Metabolism and Elimination (Excretion) of drugs?
A. Pharmacodynamics
B. Pharmacognosy
C. Pharmacokinetics
D. Pharmacopoeia3. Which of the following assertions describes a teratogenic drug?
A. The drug that can produce severe adverse reactions
B. The drug that can impact negatively the elderly
C. The drug that can cause congenital malformation
D. That drug that have a broad spectrum of activity4. In pharmacology, “drug tolerance” means:
A. A potential maximum therapeutic response which a drug can produce if
used at right dose
B. A decreased response to a drug, requiring an increase in dosage to
achieve the desired effect
C. An increased response to a drug, requiring an increase in dosage to
achieve the desired effect
D. A margin between the therapeutic dose and lethal dose of any given
antibiotic medication5. All of the following statements about efficacy and potency are true
EXCEPT:
A. Efficacy is usually a more important clinical consideration than potency
B. Efficacy is the maximum effect of a drug
C. Potency is a comparative measure, refers to the different doses of two
drugs that are needed to produce the same effect
D. The ED50 is a measure of drug’s efficacy6. What does “pharmacokinetics” include?
A. Complications of drug therapy
B. Drug biotransformation in the organism
C. Influence of drugs on metabolism processes
D. Influence of drugs on genes7. Pharmacodynamics involves the study of the following?
A. Mechanisms of drug action
B. Biotransformation of drugs in the organism
C. Distribution of drugs in the organism
D. Excretion of drug from the organism8. If an agonist can produce submaximal effects and has moderate efficacy
it’s called:
A. Partial agonist
B. Antagonist
C. Agonist-antagonist
D. Full agonist
UNIT 3 : PRINCIPLES OF DRUG ADMINISTRATION
Key Unit Competence
Administer safely medications to the patients
3.1 The rights of drug administration
Learning activity 3.1
You are carrying out a clinical attachment in the health centre. The patient is
prescribed the injectable medication for pain that will be injected intramuscularly.
Your colleague carrying out the clinical attachment in the same health centre
says there are key elements an associate nurse needs to consider before
administering the medication.
1. List the main 10 RIGHTs of drug administration that need to be considered
before medication administration.2. In which category of RIGHTs of drug administration would checking the
expiry date belong?CONTENT SUMMARY
It is a standard during nursing education to receive instructions on a guide to clinical
medication administration and upholding patient safety known as the ‘Ten rights’ or
‘Ten R’s’ of medication administration (Right Patient, Right Reason or Indication,
Right drug, Right dose, Right Route and form, Right Time, Right Documentation,
Right Response, Right to Refuse, and Right evaluation). These ‘rights’ came into
being during an era in medicine in which the precedent was that an error committed
by a provider was that provider’s sole responsibility and patients did not have as
much involvement in their own care.Right Patient: When administering a drug, it is important to use two methods
(visual as well as verbal methods) to identify the patient before administering the
medication. Nurse must be certain that the patient receiving the drug is the patient
for whom the drug has been ordered by reading properly the physician’s order. Call
the patient by name and ask him to repeat his name aloud. Be very careful if the
patient is deaf or otherwise does not understand the language.A visual identifier may include checking the patient’s name on his or her wristband,
on the patient’s card and on the medicine card for matching name and ID number
as on a chart. It is advisable not to address patients by first name or surname
alone, in the event, there are two or more patients with identical or similar names
in a unit. Depending on the unit that a patient may be in, some patients, such as
psychiatric patients, may not wear wristbands or may have altered mentation to the
point where they are unable to identify themselves correctly. In these instances,
nurses are advised to confirm a patient’s identity through alternative means with
appropriate due diligence.If there is no written identification verifying the patient’s name, nurse should obtain
a wristband or other form of identification before administering the drug. Nurse
may also ask the patient to identify him- or herself and request another unique
identifier such as date of birth. However, do not ask, “Are you Mr or Mrs A?” Some
patients, particularly those who are confused or have difficulty hearing, may respond
by answering “yes” even though that is not their name. Some long-term care or
rehabilitation care facilities have pictures of the patient available, which allow the
nurse to verify the correct patient. If pictures are used to identify patients, it is critical
that they are recent and bear a good likeness of the individual.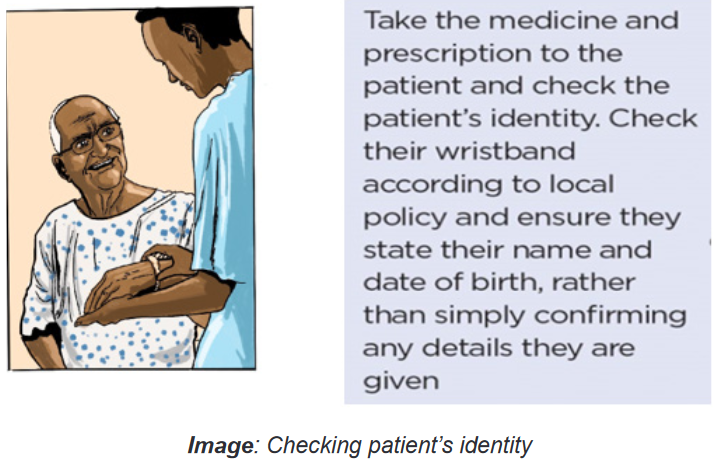
Right Reason or Indication addresses the appropriateness in use of the
medication to the patient. Confirm the rationale for use through researching the
patient’s history while also asking the patient the reason he or she is taking the
drug. Always revisit the rationale for long-term medication use. Knowledge of the
drug’s indication allows the nurse, prescriber, members of the health care team,
patient and/or family members to understand what is being treated. Understanding
the indication helps pharmacists and nurses to catch potential errors, provide
thorough explanations to the patient/family, and decrease challenges to medication
reconciliation.The nurse has the responsibility to verify the reason that the patient is receiving
the medication. It is important to understand the indication, which is related to the
medical diagnosis. If in doubt about the reason for the order, the nurse must verify
the medication order with the prescriber before administration.Right medication or drug: Some brand names or generic names may have very
similar spelling or sound very similar due to prefix, suffix, or starting with the same
first letter. Poor handwriting and abbreviations account for many medical errors
due to misreading letters or numerals that appear differently to different individuals.
Right Drug names can be confused, especially when the names sound similar, or
the spellings are similar.Quickly preparing a drug for administration or failing to look up questionable drugs
can put you at increased risk for administering the wrong drug. An error in drug
name or amount can be found when nurse compares the medication administration
record: with the container label, as the item is removed from the card, and before
the actual administration of the drug.The nurse must be careful of drugs whose names sound alike. When administering
medications, the nurse compares the label of the medication container with the
medication form three times: before removing the container from the drawer or
shelf, as the amount of medication ordered is removed from the container and
before returning the container to storage.The nurse must look for colour, odour, and consistency of the drug. Unusual
characteristics of the drugs should be questioned. The nurse must also administer
medicine only from clearly labelled container and remember to check other critical
information on packaging such as the expiration date. The nursing providers should
also develop a routine habit of explicitly asking patients about known allergies or
history of an allergic. The conversation or anything that distracts the mind not
recommended during drug administration. The nurse must be familiar with the trade
names.If there is doubt consult the physician or at least seniors or other reliable sources.
Avoid accepting the verbal orders, only in emergencies are accepted. Always
identify the patient before giving medication. The nurse must make sure that the
drug has not been discontinued by the prescriber.The nurses administer only the medications they prepare. If an error occurs, the
nurse who administers the medication is responsible for the error. Clients who
self-administer medications should keep them in their original labelled containers,
separate from other medications, to avoid confusion.Right Route and form: A nurse must know the particulars about each medication
before administering it to ensure that the right drug, dose, route, and dosage form
are being used. A complete medication order includes the route of administration.
Confirm the appropriateness of the prescribed route while also making sure the
patient can take/receive the medication by the prescribed route. If a medication
order does not include the route, be sure to ask the prescriber to clarify it. Never
assume the route of administration.In addition, it is critical to patient safety to be aware of the right form of medication.
For example, there are various dosage forms of a commonly used medication,
acetaminophen.It is available in oral suspension, tablet, capsule, gel cap, and paediatric drops, as
well as rectal suppository dosage forms. Nurses need to give the right drug via the
right route with use of the correct dosage form.Medications can be given to patients in different many ways, all of which vary in the
time it takes to absorb the chemical, time it takes for the drug to act, and potential
side-effects based on the mode of administrations, include oral, intramuscular,
intravenous, topical, or subcutaneous injection and others. It is crucial thatnurses remain educated and up to date on newer medications or less commonly
administered medications to learn how they are safely delivered to patients before
being asked to do so in clinical practice.If a prescriber’s order does not designate a route of administration, the nurse
consults the prescriber. The nurse should alert the prescriber immediately if the
specified route is not the recommended route and he/she must report immediately
if an error occurs in the medication. The nurse must know and must be familiar with
the abbreviations used to designate the route of administration.Right time: Medications can be given to patients in different many ways, all of
which vary in the time it takes to absorb the chemical, time it takes for the drug
to act, and potential side-effects. Certain drugs have specific intervals or window-
periods during which another dose should be given to maintain a therapeutic effect
or level.Often, a guiding principle of this ‘right’ is that medications should be prescribed as
closely to the time as possible, and nurses should not deviate from this time by
more than half an hour to avoid consequences such as altering bioavailability or
other chemical mechanisms. Similarly, it is crucial that medications that are given
by an infusion, such as intravenous medications, are administered at the correct
rate.Failure to deliver a drug at the correct rate may lead to devastating consequences
for a patient. For example, vancomycin requires administration by slow intravenous
infusion to avoid a complication known as “red man syndrome,” a hypersensitivity
reaction that is managed by further slowing the infusion rate of vancomycin or
discontinuing the agent altogether.The administering medications at a time that was intended by the prescriber. The
nurse must Read the physician’s orders, know the hospital routines for the interval,
know the abbreviations for the time, give the medicine near the time ordered,
give the medicine as ordered in relation to the food intake and give the medicines
according to the actions expected. E.g., sleeping pills are given at bedtime.Right dose: Incorrect dosage, conversion of units, and incorrect substance
concentration are a prevalent modality of medication administration error. This error
type stems from nurses giving a patient an incorrect dose of medications, even if it
is the correct medication and the patient’s identity is verified, without first checking
to ensure it is the correct strength for the patient. This error type may be due to
misplaced decimals, errors in arithmetic, or incorrect conversion between two units.The nurse must have adapted observing positive behaviors to reduce medical
errors include consulting with pharmacy personnel, read physician orders to know
the correct dose, consider the age and weight of the patient, know the minimumand maximum dose of the medicine administered, using calculators to assist in
arithmetic, or in some cases, cross-consulting with patients or their families about
usual doses they administer at home. Use ounce glasses instead of teaspoons to
measure ounces accurately, have written order before you prepare the drug, avoid
conversation or anything that distracts the mind.Right Documentation: Medication error can result from inaccurate documentation.
Nurse should ensure appropriate documentations clearly reflect the client’s name,
the name of the ordered medications, the time the medication was administered, the
medication’s dosage, route, the date or the method of administration, frequency, the
signature of the physician, and Standing orders or routine medication orders. If any
of this information is missing the nurse should verify the order with the prescriber.After the administration of any drug, record the process immediately. Immediate
documentation is particularly important when drugs are given on an as-needed
(PRN) basis. For example, most analgesics require 20 to 30 minutes before the
drug begins to relieve pain.A patient may forget that he or she received a drug for pain, may not understand
that the administered drug was for pain, or may not know that pain relief is not
immediate, and may ask another nurse for the drug again. If the administration
of the analgesic was not recorded, the patient might receive a second dose of
the analgesic shortly after the first dose. This type of situation can be extremely
serious, especially when opioids or other central nervous system depressants are
administered. Immediate documentation prevents accidental administration of a
drug by another individual and it is essential to the process of administering drugs
correctly.Right Response refers to the drug and its desired response in the patient.
Continually assess and evaluate the achievement of the desired response, as
well as any undesired response. Examples of data gathering include, but are not
limited to, monitoring vital signs, weight, oedema, intake and output, nutritional
intake, laboratory values, results of diagnostic testing, and auscultating heart and
lung sounds. Document any assessment, intervention, and monitoring as deemed
appropriate.Right to Refuse: The ninth right is that of the right of the patient to refuse. Patients
refuse medications for a variety of reasons. If refusal of a medication occurs,
always respect the patient’s right (to refuse), determine the reason, and take
appropriate action, including notifying the prescriber. Do not force! Document the
refusal and a concise description of the reason for refusal. Document any further
actions you take at this time, such as vital signs and/or system assessment. If
a consequence to the patient’s condition and/or as hospital policy dictates, the
prescriber is to be contacted immediately. Never return unwrapped medication to acontainer, and discard medication dose according to agency policy. If the wrapper
remains intact, return the medication to the automated medication-dispensing
system. Revise the nursing care plan as needed.Right evaluation: The health professional after administrating the medications to
the client must ensure the medication is working the way it should, ensure that the
medications are reviewed regularly and the ongoing observations if required to
detect early any sides’ effect or adverse effect associated with the taken medication.Self- assessment 3.1
1. What are the two methods a nurse can use to identify the right patient
before the drug administration?2. What does the nurse have to do if the patient refuses to take the prescribed
medications?3. Which of the following options addresses the appropriateness in use of
the medication to the patient?
A. Right indication
B. Right evaluation
C. Right documentation
D. Right to refuse4. You have been instructed to administer an oral medication (Ranitidine
150mg) to a patient. What is the minimum of times the nurse should
check the medication label before administering this drug?
A. One
B. Two
C. Three
D. Four3.2 Compliance/adherence to drug regimen
Learning activity 3.2
You are at the healthcare facility where you are carrying out a clinical attachment
as a requirement to complete your associate nursing program. A 41-year-old
female patient comes 10 days after interrupting his antiretroviral treatment.
While discussing with the patient, she reveals that she delayed to come to get
antiretroviral medications because the time of appointment coincided with the
time she had no money, and as she lives far, she could not travel to the health
facility. In your understanding, you realize that the patient was limited by the
financial constraints.
1. How can you define the word “drug adherence?”
2. What are the 5 factors (dimensions) that can lead to poor drug adherence
and compliance as stated by the World Health Organization?CONTENT SUMMARY
Adherence describes how a patient follows a medical regime recommended by
a healthcare provider. Poor treatment adherence represents a complex and
challenging problem of international healthcare systems, as it has a substantial
impact on clinical outcomes and patient safety and constitutes an important
financial burden. Since it is one of the most common causes of treatment failure, it
is extremely important for physicians to reliably distinguish between non-adherence
and non-response.Three different terms are used in the literature to describe to which extent a patient’s
behaviour corresponds with the advice given by a healthcare provider: Compliance,
adherence and concordance. These three terms are often used interchangeably,
but they reflect different philosophies of the physician-patient relationship. It can
be difficult to accurately compare studies on this topic, since the terminology used
differs amongst authors. Until around 2003, the term compliance was most widely
used in the literature. Compliance implies an authoritarian, asymmetric physician-
patient relationship, in which the doctor has the exclusive decisional power.
Physicians give instructions and patients are passive recipients and should follow
the prescribed regime without deviation.The word compliance may have negative connotations as it requests a submissive
and obedient patient. The concept of an appropriate physician-patient relationship
has substantially changed in the last years, since patients have gained more
autonomy. This paradigmatic shift is reflected by the new term adherence, which
is nowadays preferably used. The concept of adherence is based on a partnership
between physician and patient, where both parties are actively involved in findinga mutual treatment agreement. The word concordance, which originated in British
literature, goes even further and places the patient in the centre of the decision-
making process. It focuses less on compliance and more on overall success of
treatment as a shared goal.
Adherence is a multidimensional phenomenon determined by the interplay of five
sets of factors, termed “dimensions” by the World Health Organization:
1. Social/economic factors
2. Provider-patient/health care system factors
3. Condition-related factors
4. Therapy-related factors
5. Patient-related factors

Self- assessment 3.2
1. Enumerate patient-related factors affecting adherence to medications.
2. The term “Adherence to drug regimen” has negative connotations
as it requests a submissive and obedient patient. It is nowadays less
preferable, and was replace by the term “Compliance.” TRUE or FALSE3. In patient adherence to drug regimen, concordance implies an
authoritarian, asymmetric physician-patient relationship, in which the
doctor has the exclusive decisional power. TRUE or FALSE3.3 Drug storage
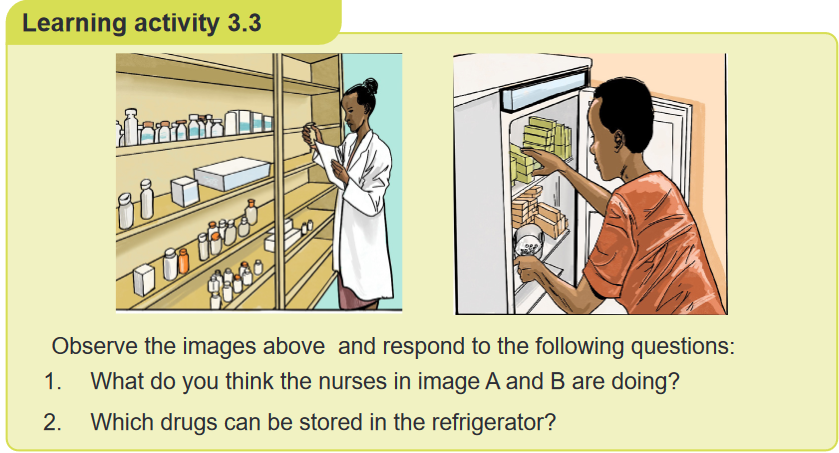
CONTENT SUMMARY
Drugs and biologicals are to be stored in a secure and orderly manner under proper
temperatures and are to be accessible only to licensed nursing and pharmacy
personnel. All medications are to be stored in the containers in which they are
received, internals separately from externals and both separately from poisons.Medications received from the Pharmacy should be stored in a secure location
that is out of reach from children. Medications that are dispensed in vials, such
as tablets and capsules, should not be placed in bathroom or kitchen cabinets
where it may be subjected to high humidity. Most medications can only be stored at
room temperature, but some medications may require refrigeration or other storage
requirements. Please consult with your pharmacist if you are unsure.The drugs which are supplied to ward are stored in drug cupboards to provide a
uniform supply of drugs to the patients. The drugs are stocked in containers, such
as boxes and on flexible racks and shelves etc. It must be ensured that drugs which
are stored remain preserved during their storage. There should not be any damage
due to high temperature or exposure to sunlight. The drugs are to be stored as per
the prescribed conditions of their storage. The drugs stored in a drug store should
be arranged in such a way that they are easily traceable when required.Drugs can be stored:
1. According to pharmacological action or
2. AlphabeticallyFactors that govern storage of drugs
Proper drug storage
Storage Environment
Arrangement of drugs on shelves
The storeroom
The dispensaryA. Proper drug storage
Drugs are stored in a specially designed secure area or space of a building in order
to:
• Avoid contamination or deterioration,
• Avoid disfiguration of labels,
• Maintain integrity of packaging and so guarantee quality and potency of drugs
during shelf life,
• Prevent or reduce pilferage (stealing things of small value), theft or losses
• Prevent infestation of pests and vermin.The storage should not hinder the cleaning and should have sufficient space for
movement of stocks and handling. Products are to be stored in a manner that
prevents damage due to excessive vertical stacking heights and not to exceed
eight stacks.Store the products as per product storage condition (As per label) to prevent
deterioration of finished product on storage. Monitor and record the temperature of
storage area on daily basis.B. The storage environment
The storage environment should possess the following:
• Adequate temperature,
• Sufficient lighting,
• Clean conditions,
• Humidity control,
• Cold storage facilities, and
• Adequate shelving to ensure integrity of the stored drugs.Drugs to be stored under condition that prevents contamination & as far as possible,
deterioration. They must be “Well closed container” precautions to be taken in
relation to the effects of the atmosphere, moisture, heat & light. “Protected from
moisture” means that the product is to be “stored in air tight container”. “Protected
from light” the product is to be stored either in a container made of material that
absorbs actinic light sufficiently to protect the contents from change induced by
such light. Temperature: In a deep freeze (-15°C), in a refrigerator 2°C-8°C, Cold
or cool 8°C-15°C and Room temperature15°C-25°C.Drugs stored in the medicines refrigerator include: vaccines; insulin; chemotherapy
drugs; topical preparations, such as some types of eye drops; and other treatments
such as glucagon, which is used to manage severe hypoglycaemia.Storage premises: The Storage area must be free from unsanitary conditions (Ex
Rodents, insects, Birds). The floor of the warehouse should be made of hard floor
(Concrete /Kota/Epoxy) and must be in a good state of repair and appearance at
all times. The floors are kept clean and free of trash, dirt, spillage water, drain water
etc. The area must be kept clean. The area used for storage of IV fluids should
have adequate space and to prevent exposure to direct sunlight. Secured area
availability for damaged, rejected and expired goods. Ensure adequate pest control
program in place and shall be carried out at a minimum frequency of a year. The
Pest control shall cover treatment for Termite and Rodents.C. Arrangement of drugs on shelves
Shelves should be made of steel or treated wood. Shelves should be strong. Drugs
are arranged in alphabetical order of generic names. Each dosage form of drug is
arranged in separate and distinct areas. Most recently received drugs are placed
behind old stock on the shelf except where new drugs have shorter expiration dates.
Always put lids properly on tins always and at the close of the day. Put drugs in a
dry place protected from light and heat. Store liquids on a pallet on the floor or on
the lowest shelf. The store must be cleaned daily and mopped at least once a week.D. The store room
A well-arranged store enables easy identification of drugs and saves time when
picking a drug from the shelves. This helps remove drugs quickly and makes for
easy inventory control. The rule of FIRST IN FIRST OUT (FIFO) should be applied
always. So, drugs that were received first should be used first, because the old
stock has shorter expiration dates than the new stock.In this regard, the principle of FIRST TO EXPIRE FIRST OUT (FEFO) should apply.
To have access to drugs with shorter expiration dates, put these in front of the
shelves. Those with longer expiration dates should be placed behind those with
shorter dates.E. The dispensaries
Clean after each use tablet counters and place within easy reach on the table.
Avoid dispensing wrong drugs by arranging drugs on the table in alphabetical
order so that the drug being dispensed is not confused with another. Always close
drug containers from which drugs are not being dispensed to prevent spillage or
dispensing the wrong drug. Medications must not be administered, and products
and equipment must not be used beyond their expiry dates. All medical equipment,
dressings and solutions used during invasive procedures must be sterile. Single-
use devices are meant for single use only and must not be re-used.Storage, maintenance and security: All drugs, including samples, should be
maintained separate from non-medications in a locked cabinet which is sufficiently
secure to deny access to unauthorized persons. Key should be available only to
authorized personnel who are assigned medication-related responsibilities. Store
medications that are “for external use only” separate from medications intended for
internal use. Store look-alike and sound-alike drugs (LASA) separately. Maintain
temperature between 59 degrees and 86 degrees Fahrenheit for non-refrigerated
medications. Where refrigeration is necessary use a “Medications Only” refrigerator
and maintain temperature between 36 degrees and 46 degrees Fahrenheit.On daily basis check, verify and document the proper temperature. All multiple-
dose injectable medications should be initialled and have the date of first entry
recorded on the label. Rotate medication stock monthly employing a “FIFO” (first
in/first out) process.Controlled drug regulation.
• Double locked container, and 2 licensed personnel count (or verify any
discrepancies) every shift (8 hours)• Witness to all discards
• Record on Control Substance Sheet all administrations and wastes
All details must be completed in the doctors own handwriting, like: Name of drug,
Dose of drug, Number of doses or length of course, Signature of prescribing doctor
and date.Storage of controlled drugs
They must be kept in a locked cabinet or cupboard. The keys to the cabinet must
be in the possession of an authorised person. Authorised person refers to ward
manager or deputy who must be a trained nurse or midwife. Students should not be
responsible for the controlled drug cupboard keys.Recording of controlled drug use: Records in the form of CONTROLLED DRUG
REGISTERS must be kept. Each drug must have its own specified page which is
headed with the drugs name and strength. The number of ampoules of a drug must
be entered and updated with every use.Must record: ü Date ü Time ü Dose of every administration ü Name of receiving
patient/client ü Number of ampoules at start and finish of administration Entry
must be signed by 2 people one of who must be registered.Self- assessment 3.3
1. What are the 5 factors that govern the storage of drugs?
2. In order to prevent damage of stored drugs, what is the number of stacks
that should not be exceeded in case of vertical stacking?
A. Two stacks
B. Twenty stacks
C. Eight stacks
D. Fifteen stacks3. How should the nurse arrange medications in the store room to ensure
the FIRST TO EXPIRE FIRST OUT (FEFO) principle?4. What are the characteristics of drug storage environment?
3.4 Enteral routes of drug administration
Learning activity 3.4
A 50-yaer-old female patient consults the health facility where you are carrying
out the clinical placement, for the follow up of her chronic disease. She used
to be taking insulin for type 2 diabetes mellitus, and her glycemia has become
stable so that she can shift to non-injectable forms. Your colleague in associate
nursing program carrying out the clinical placement at the same health facilty
wants to shift from injectable from to enteral routes, but she does not remember
what an enteral route is.A. How can you define an enteral route of drug administration to your
colleague?B. What are different types of enteral routes of drug administration would
you tell your colleague?C. Which enteral route poses a greater risk of first-pass effect (first
metabolism)?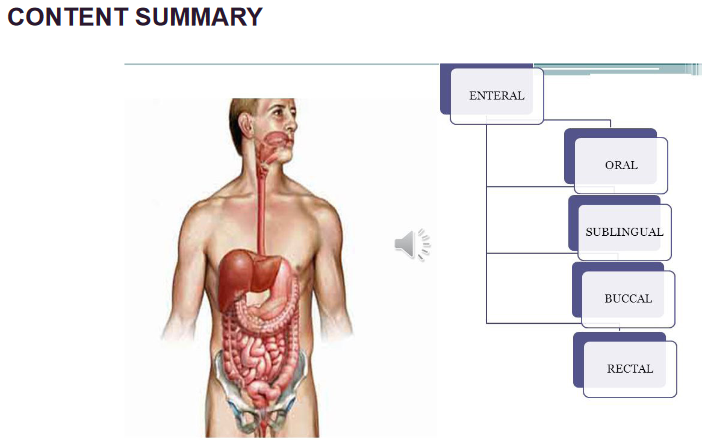
Routes of drug administration are the medium through which the drug is introduced
into the body to show its pharmacological action or for diagnosis. They are generally
classified by the location at which the substance is applied or based on the target
of action is. Route of administration and dosage form are the main aspects of drug
delivery. Enteral administration is the involvement of the gastrointestinal tract
and is further classified as follows: Oral Administration, Buccal or Sublingual
Administration, and Rectal route.i) Oral Administration
It is the first choice for the administration of drugs. It is designated as Per Os (PO),
which means to administer by mouth. The absorption of drugs administered by
this route is determined by the physiological state of the GI tract. Types of dosage
forms administered through this route include pills, tablets, capsules, solutions,
suspensions, emulsions, syrups, elixir, etc.Advantages: Most Convenient and cost-effective. Safest and painless. Self-
administered. No sterilisation required.Disadvantages: Not suitable for an emergency as the onset of action is slow. Not
suitable for unconscious patients, uncooperative and unreliable patients. For drugs
with extensive first-pass metabolism, this route is not used. Unpalatable and highly
irritant drugs are not suitable.ii) Buccal or Sublingual Administration
Sublingual administration involves placing the drug under the tongue. Buccal
administration involves placing the pill between the gums and cheek wherein both
the cases, the drug is absorbed into the blood. The types of dosage forms for this
route include tablets, troches and lozenges. Examples- Nitroglycerin.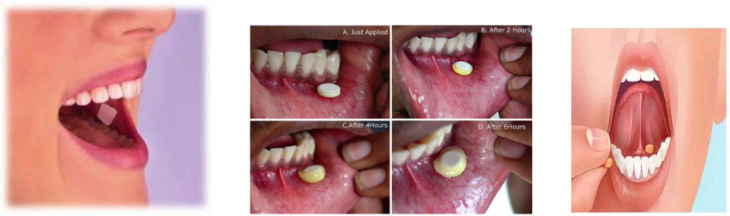
Advantages: Economic & Quick drug absorption. Bypassing the first-pass
metabolism. Quick termination-spit off. Self-administered. Increased bioavailability.Disadvantages:
Not suitable for bitter and irritating drugs. High doses can’t be taken.
Less patient compliance. Highly ionic drugs cannot be administered.iii) Rectal route
Rectal medicines are administered through the anus, into the rectum. The types
of dosage forms for this route include suppositories and enemas Ex: prednisolone
enema, indomethacin, diazepam.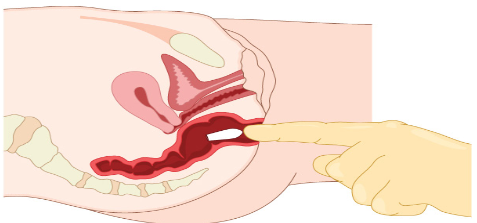
Advantages: It avoids the first-pass metabolism. Suitable for children and old age.
It is used for unconscious and vomiting patients. Irritating drugs are contraindicated.Disadvantages: Absorption is slow and erratic. Not well accepted by patients.
Inconvenient.Self- assessment 3.4
1. Which of the following is an advantage of the oral route of drug
administration?
A. It is easily self-administered method;
B. Toxicity may be overcome with antidotes;
C. Drugs avoid first-pass metabolism;
D. Drugs go directly into the systemic circulation.3. All of the following are advantages of the rectal route of drug administration,
EXCEPT:
A. Suitable for patients with nausea or vomiting
B. Suitable for the young population (children)
C. Suitable for patients with unconscious state
D. Drugs are subject to first-pass metabolism.3. Pick out the appropriate alimentary route of administration when passage
of drugs through liver is minimized:
A. Rectal
B. Sublingual
C. Oral
D. Intraduodenal4. The oral route of drug administration is suitable for an emergency situation
as the onset of action is rapid. TRUE or FALSE3.5 Parenteral routes of drug administration
Learning activity 3.5
A 20-year-old male patient is admitted in the healthcare facility for an infectious
bacterial disease. The assessment reveals that the patient must be given
the tablets to swallow twice a day with plenty of water. After 2 days of
the treatment, the nurse realizes that the patient vomits all the drugs he
takes, and the nurse needs to shift to another route that would help to
ensure that all the drug is taken into the patient’s body. The nurse then
asks you a question regarding the alternative routes she should use to
ensure that the drug is not vomited.
1. Which routes would you advise to the nurse to use?
2. Which angles of the needle would you respect while administering drugs
via the 4 main routes?CONTENT SUMMARY
During patient care, some medications can be administered by parenteral routes.
The word parenteral is derived from Greek word “para” which means outside and
“enter one” which means the intestine. These are the injection or infusion through
a needle or catheter into the body. This route helps bypass the alimentary canal.
The injection is the act of putting a liquid, especially a drug, into a person’s body
using a needle and a syringe. Injections are classified as follows:a. Subcutaneous route/injection
The drug is deposited just beneath the skin in the loose subcutaneous tissue. As
it is less vascular, absorption is slow, so prolonged action is produced. Only small
volumes can be injected. The needle is injected into the pinched skin at 90-degree
angle and do this quickly without force. If you have very little fat, then inject at a
45-degree angle. In addition to injection, it is also possible to slowly infuse fluids
subcutaneously in the form of hypodermoclysis. A subcutaneous route is used for
protein drugs because such drugs would be destroyed in the digestive tract if they
were taken orally. Certain drugs (progestins for hormonal birth control) may be
given by inserting capsules under the skin.Advantages: Onset of action is faster than oral route.
Disadvantages: Sterile technique is needed. More expensive. Some drugs can
irritate tissue and cause pain. Only small volumes must be administered.
Various forms of subcutaneous (SC) route are: Dermojet, Pellet and Sialistic (non-
biodegradable and biodegradable implants).Dermojet: It is a needleless injection system with a high-pressure jet injector. A
high velocity of drug solution is projected from a fine micro orifice using a GUN like
an implant; the solution passes through the superficial layers and gets deposited
in the subcutaneous tissue. It is nearly painless and suitable for mass inoculations.
E.g. Insulin.Pellet: Drug in the form of solid pellet is introduced with TROCHAR and CANNULA
which provides sustained release of drug for weeks and months without repeated
administration.E.g. DOCA, TESTOSTERONE.
Sialistic (non-biodegradable or biodegradable): Crystalline drug is packed in tubes
or capsules and implanted under the skin. Slow and uniform release of drug for
months with constant blood levels (non-biodegradable drug have to be removed
later). E.g. hormones and contraceptives like “NoRPLANT”.b. Intravenous route/injection
Method of administering medications directly into the vein using a needle. It is
the best way to deliver a precise dose quickly and in a well-controlled manner
throughout the body. Drugs are delivered immediately into the bloodstream and
tend to take effect more quickly than any other route. Hence it is of great value in an
emergency. A 25-gauge needle 2 cm long with 25-degree angle is inserted into the
skin. It is also used for irritable solutions which cause pain and damage to tissues if
given by subcutaneous or intramuscular injection. A solution containing a drug may
be given in a single dose, or continuous infusion from a collapsible plastic bag or
infusion pump through thin, flexible tubing inserted to the vein, usually a forearm.
Vital organs like heart, brain etc. get exposed to high concentrations of the drug.Advantages: Rapid onset of action. It bypasses the GI and first-pass metabolism.
Useful for drugs which are irritant to intramuscular route.Disadvantages: Administered by trained person. Accidental overdose can have
serious consequences. Limited to highly soluble drugs. Break of
skin barrier.c. Intramuscular route/injection
The drug is injected into one of a large skeletal muscle such as triceps and rectus
femoris among others. It is a preferred route when larger volumes of a drug product
are needed. It is more vascular; hence absorption is faster and less painful. The
angle for IM is 90 degrees. DEPOT preparations (oily solutions and aqueous
suspensions) can be injected by this route. Muscle permits the tissue to receive a
larger volume of medication (deltoid and biceps maximum of 3ml).NOTE: IM injections should be avoided in anticoagulant treatment patients as it can
produce Local haematoma.Advantages: Can administer larger volumes. Technically easier than IV. GI and
first-pass metabolism are involved.Disadvantages: Break the skin barrier, produce anxiety and painful.
d. Intradermal route/injection
The drug is delivered in the upper layer of the skin to the dermis, where the
absorption is low. The angle for ID is 5 to 15 degrees with a needle placed almost
flat to the skin. It is the common method used for allergy testing. Injections are
made with fine short needles (26 gauge) and a small barrel syringe.Advantages: Absorption is low, advantage for allergy testing.
Disadvantages: Amount of drug administered must be small.
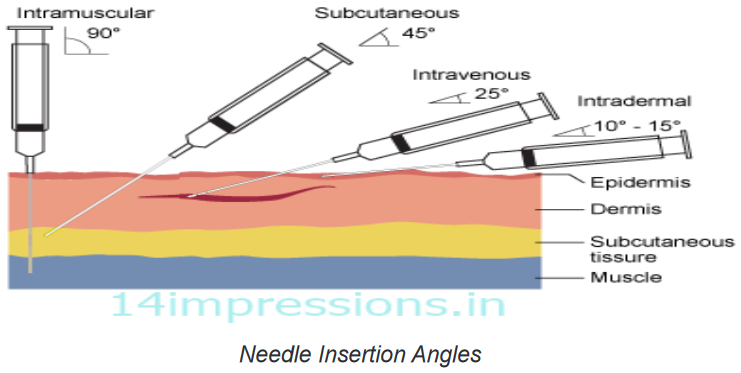
The above are the 4 main routes. The following are other parenteral routes,
less commonly used.e. Intra Arterial route/injection
Intra Arterial injection or infusion is a method of delivering a drug directly into arteries
to localise its effect to a particular organ/region while minimising the exposure of the
body to potentially toxic effects of the agent.Advantages: Used in chemotherapy to target drug organs.
Disadvantages: Drugs may be distributed to other tissues or organs.
f. Intra Articular route/injection
It is the injection which is directly delivered into the joints to relieve pain and swelling.
Most of the anti-inflammatory drugs for arthritis treatment are given by this route.Advantages: High concentration is obtained in localized areas. Rapid onset of
action.Disadvantages: Sepsis and joint damage may occur on repeated drug
administration.g. Intrathecal route/injection
Intrathecal administration is a route for drugs via an injection into the spinal canal,
or into the subarachnoid space so that it reaches the cerebrospinal fluid (CSF) and
is useful in spinal anaesthesia, chemotherapy & pain management applications.Advantages: Drugs act directly on meninges and CNS. Bypass BBB & Blood-CSF
barrier.
Disadvantages: Painful procedure. Expertise neededSelf- assessment 3.5
1. Parenteral routes of drug administration are:
A. Intravenous, intramuscular, subcutaneous
B. Intravenous, intramuscular, intranasal
C. Intravenous, sublingual, transdermal
D. Transdermal, subcutaneous, by inhalation2. All of the following are the disadvantages of intravenous drug
administration, EXCEPT:
A. A trained staff is required to administer the drug
B. Its use is limited to highly soluble drugs.
C. Accidental overdose can have serious consequences
D. Drugs undergo first-pass metabolism in the liver3. Which of the following is the correct angle to use while administering the
drugs intramuscularly?
A. 75%
B. 50%
C. 90%
D. 45%4. Which of the following statements best defines the intradermal injection?
A. The drug is delivered in the upper layer of the skin to the dermis, where
the absorption is low
B. The drug is injected into one of a large skeletal muscle such as triceps
and rectus femoris
C. Intrathecal administration is a route for drugs via an injection into the
spinal canal, or into the subarachnoid space
D. Method of administering medications directly into the vein using a
needle and a syringe3.6 Topical routes of drug administration
Observe the images above (A, B, C, D), and answer the questions below
pertaining to them:
1. What do you observe on these images (ABCD)?
2. What are the benefits of using the route of drug administration in the
images above?CONTENT SUMMARY
The topical route includes: skin, eyes, or other specific membranes, the intranasal,
inhalation, intra-vaginal. The medication is applied directly to the body surfaces,
including the skin and mucous membranes of eyes, ears, nose, vagina and rectum.
Ex: Antibiotics, hormones, narcotics and chemotherapeutics.The definition of the topical route of administration sometimes states that both the
application location and the pharmacodynamic effect thereof is local.In other cases, topical is defined as applied to a localized area of the body or to
the surface of a body part regardless of the location of the effect. By this definition,
topical administration also includes transdermal application, where the substance
is administered onto the skin but is absorbed into the body to attain systemic
distribution.BENEFITS OF THE TOPICAL ROUTE OF DRUG ADMINISTRATION
Medications delivered via the topical route offer a whole host of benefits. Here are
five benefits of using a topical drug delivery system.1. Alternative to oral administration
Many patients struggle with oral drug administration. Some risk vomiting, while
others find swallowing pills a near-impossible task. Consequently, if an orally
administered drug is rejected, this reduces a drug’s effectiveness, prolonging the
ailment. This problem is most common in infants or young children who are not
used to swallowing tablets. Parents often find it difficult to get their children to take
medication. Commonly, this results in wasted doses and slower recovery times. By
using a topical medication, parents may be able to avoid these problems and help
their children feel better more quickly.2. Fewer risks of gastrointestinal difficulties
Different individuals absorb medication at different rates. Oral medications can
cause a variety of digestive side effects. Patients who experience these often
painful side effects may opt to terminate their medication. A topical drug delivery
system overcomes this limitation, improving the patient’s recovery process.3. Fewer risks of abuse
Medication administered through tablets or injections can easily be abused. Drug
abuse by patients is far too common, especially with pain medications. Such
abuse can lead to addiction. On the other hand, administering medication through
ointments or creams greatly lowers the risk of abuse. Topical medications not only
help doctors and patients manage ailments, but also help to prevent the problem
of drug abuse.4. Easy to administer
Almost everyone has a fear of something. Some people are afraid of injections or of
swallowing tablets, but few are fearful of rubbing an ointment on their skin. For this
reason, doctors find it easier to encourage their patients to take their medication
when using a topical drug delivery system. The patient can easily manage the
medication at home.5. Reduced hospital congestion
Previously, hospitals administered many medications by injection, filling their beds
to capacity. Today, if the patient condition isn’t serious, the patient can walk into a
hospital and walk out again a short time later with topical medication. This leaves
hospital beds free to cater to more serious cases and reduces both hospital and
patient medical costs.Innovating with topical drug delivery
The increased adoption of topical medication in recent years has been impressive.
This is largely due to the fact that the medication has proven to have more
advantages than drawbacks. After all, the skin is ideal for drug administration, as it
produces both systematic and local effects.Call it a life-changing medical innovation. Topical drug delivery systems have
surely changed the way we look at medication. More and more medical institutions
and health practitioners are adopting this form of medication in an attempt to improve
their services to patients. This medical breakthrough offers a future of health care
that is definitely more effective and agreeable for patients.Some types of topical routes of drug administration
Inhalational route
Inhaled medications can be absorbed rapidly and act both systemically and locally.
A proper technique with inhaler devices is necessary to achieve the correct dose.
Total size absorbed is variable. Nasal Inhalations, Inhalation by smoking a substance
is likely the most rapid way to deliver drugs to the brain, as the substance travels
directly to the brain without being diluted in the systemic circulation. The severity of
dependence on psychoactive may increase with more rapid drug delivery.Advantages: May be used for local or systemic effects.
Disadvantages: Particle size of drugs determines anatomic placement in
the respiratory tract. May stimulate cough reflex. Some drugs may be swallowed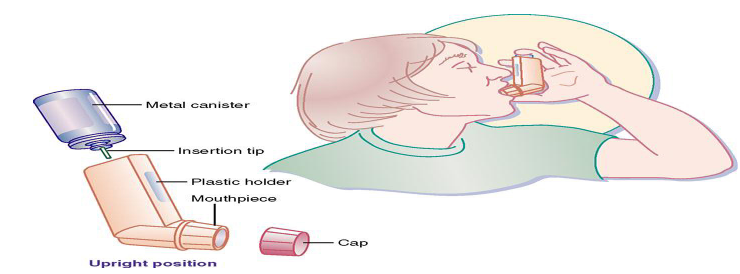
Transdermal route
Transdermal administration is a route wherein active ingredients are delivered
across the skin for systemic distribution of the drug. E.g. Transdermal patches. The
drug is administered in the form of a patch or an ointment that delivers the drug into
the circulation for systemic effect. The absorption rate may vary. It is slow. Increased
absorption with occlusive dressings. Formulations and devices for transdermally
administered substances include: Transdermal pathways are those by which drugs
can cross the skin and reach the systemic circulation. Ex: Transcellular pathway,
Intercellular pathway, Microneedles. The more direct route used is known as the
transcellular pathway.Advantages: The transdermal delivery system (patch) is easy to use and withdraw.
Continuous release of the drug is observed for a specified period of time. It is used
for lipid-soluble drugs with a low dose and low molecular weight. Low pre-systemic
metabolism.Disadvantages: Some irritation by patch or drug. Permeability of skin is variable
with the condition, anatomic site, age and gender. Type of cream or ointment base
effects the drug release and absorption.Self- assessment 3.6
1. What are the advantages of administering the drugs by the inhalational
route?2. What are the disadvantages of administering the drugs by the inhalational
route?3. Transdermal administration is a route wherein active ingredients are
delivered to the body through an injection in the upper layer of the skin.
TRUE or FALSE4. What are the advantages of administering the drugs by the transdermal
route?3.7 Introduction to medications errors and classification
of medication errorsLearning activity 3.7
A 33-year-old male patient is admitted to the hospital where he is being treated
with injectable antibiotics. In addition, the patient is receiving two tablets of pain
medication every 6 houurs. The nurse on the night shift realizes that the patient
received 2 tablets at the latest as indicated. The nurse on the night shift finally
realizes that it is the dose that was prescribed for the patient as it appears in the
treatment sheet, but after keen search, he realizes that the patient should be
taking only 1 tablet for pain medication every 12 hours, instead of 2 tablets every
6 hours. The nurse then withholds the dose, documents it and gets the view from
the working team.1. What type of medication error was committed for this patient?
2. What are other types of medication errors according to their categories
or classification?3. What is the definition of a medication error?
CONTENT SUMMARY
Medicine errors cause considerable patient morbidity, mortality and increased
healthcare cost. The most common used definition is that given by the National
Coordinating Council for Medication Error Reporting and Prevention (NCCMERP)
in the USA, which defines medication errors as: “Any preventable event that may
cause or lead to inappropriate medication use or patient harm while the medication
is in the control of the healthcare professional, patient, or consumer.” The published
studies estimated that about 5—10% of hospital admissions were due to the
medication errors. It is suspected that approximately 3% of deaths in the Swedish
population are because of the medication errors. In Canada, up to 50% of the
patient safety incidents in primary care are related to medication errors. Reporting
the medication error is one most effective strategy to improve patient safety. While,
these reports help to understand the medication errors contributing factors.Causes of Medication Errors
1. Expired Product: Usually occurs due to improper storage of preparations
resulting in deterioration or use of expired products.2. Incorrect Duration: Duration errors occur when medication is received for
a longer or shorter period of time than prescribed.3. Incorrect Preparation: This error usually occurs with compounding or
some other type of preparation before the final administration. An example
is choosing the incorrect diluent to reconstitute.4. Incorrect Strength: It may potentially occur at many points in the medication
process. It usually occurs due to human error when similar bottles or syringes
with the incorrect strength is selected.5. Incorrect Rate: Most often occurs with medications that are given as IV push
or infusions. This is particularly dangerous with many drugs and may result
in significant adverse drug reactions. Examples include tachycardia due to
rapid IV epinephrine or red man syndrome due to the rapid administration
of vancomycin.6. Incorrect Timing: In both home and institutional settings, it is challenging
to be completely accurate with scheduled doses. The concern is that some
medications absorption is significantly altered if taken with or without food.
As such, it is important to adhere to scheduled times as commonly; this may
lead to under or overdosing.7. Incorrect Dose: This error includes overdose, underdose, and an extra dose.
An incorrect dose occurs when an inappropriate or different medication dose
is given other than what was ordered, errors of omission when a scheduled
dose of medication is not given, and when a drug is given via an incorrect
route. Errors due to incorrect routes usually occur due to unclear labelling
or tubing that is adaptive to multiple connectors/lines of access. Incorrect
routes often result in result in significant morbidity and mortality.8. Incorrect Dosage Form: This occurs when a patient receives a dosage form
different than prescribed, such as immediate-release instead of extended-
release.9. Incorrect Patient Action: This occurs when a patient takes a medication
inappropriately. Patient education is the only way to prevent this type of
error.10. Known Allergen: Dispensing a drug that the patient has an allergy often
due to failure to communicate with the patient, inappropriate chart review,
inaccurate charting, or lack of technologic interface.11. Known Contraindication: This occurs when medications are not vigilantly
reviewed for drug-drug, drug-disease, or drug-nutrient interactions.Medication Errors Classification
Errors can be classified according to contextual categories; such as stage of
occurrence. So, in accordance with the medication use process, medication errors
can be classified as prescribing errors, transcription errors, dispensing errors,
administration errors or monitoring errors.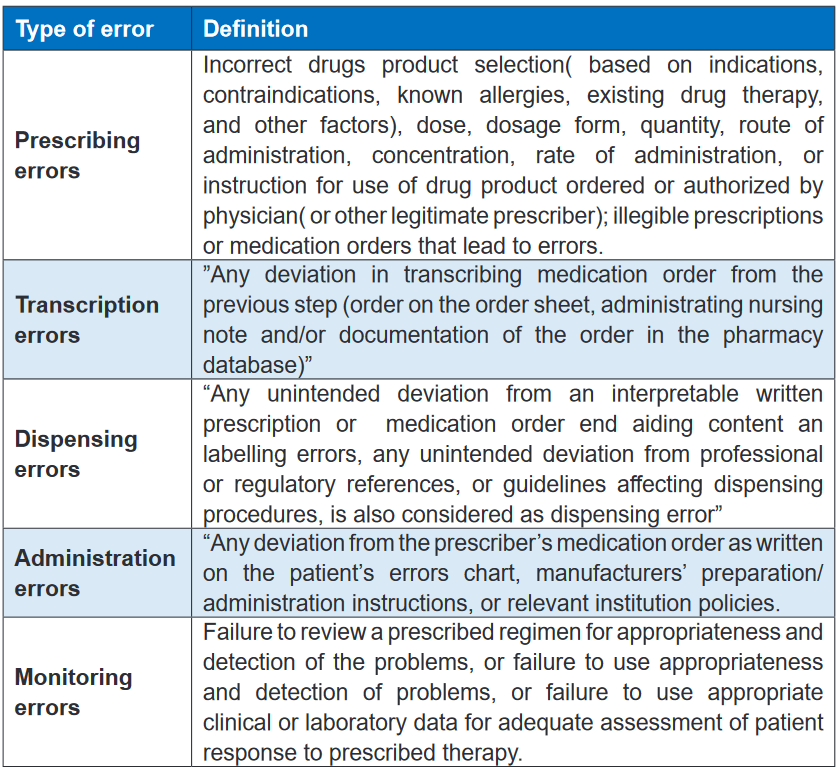
Self- assessment 3.7
1. What are the causes of medication errors?
2. In which of the following types of medication errors would a medication
error which involves incorrect drug product selection based on indications
be classified?
A. Prescribing errors
B. Transcription errors
C. Dispensing errors
D. Monitoring errors3. In which of the following types of medication errors would a medication
error which involves a failure to review a prescribed regimen for
appropriateness and detection of problems be classified?
A. Prescribing errors
B. Transcription errors
C. Dispensing errors
D. Monitoring errors3.8 Actions to take in case of medication errors, and use
of high alert medicationsLearning activity 3.8
A nurse is preparing to administer an injectable dug to patient. His colleague who
is an associate nurse says it is a high alert medication, and advises to check well
the prescription in order to avoid any risk of committing a medication error.
1. As a nurse student, how can you define a high alert medication?
2. What are the strategies to reduce errors involving High Alert Medications
on the aspect of their storage?CONTENT SUMMARY
Medication errors are a common finding in healthcare settings. The healthcare
providers need to take necessary measures in order to avoid or minimize the
medication errors. They do however often occur due to different circumstances.
When they do occur, the nurses as well as other healthcare providers must take
quick actions, and make sure they report that incident.Steps of Reporting Medication Errors
1. Any staff member who discovers a medication error whether it’s a physician,
pharmacist, or a nurse must be immediately complete the Medication Error
Report. The details include; patient name, hospital number, prescription
details, details of errors and any incorrect medicine or dose administered to
the patient2. When these details of errors are recorded on the form, the manager or
deputy need to identify those staff involved and explain the error to get them
and them write about the error causes any comments about the error. The
manager or deputy need to mention the immediate the action taken.3. Send the completed form to Pharmacy department in the hospital within
24 hours4. The Medication Safety Officer needs to complete the medication error
from such as assesses the incident severity, conduct Root Cause Analysis
if need (for all significant or potentially significant medication errors) and
suggest recommendation to reduce reoccurrence the error.5. The Medication Safety Officer needs to inform the Medication Safety
6. Medication Safety officer in the hospital needs to review all the medication
errors and to take the required action to avoid occurring similar errors in the
future.7. Forwarded to Total Quality Management (TQM) Department in the hospital.
GUIDELINES ON SAFE USE OF HIGH ALERT MEDICATIONS
High-Alert Medications are medicines that have high potential risk to the patient
when they are utilized in error. Although mistakes may or may not be common
with these medicines, the significances of an error are dearly more devastating to
patients.Examples: Adrenergic agonists. IV (E.g: Epinephrine, Norepinephrine,
Phenylephrine); Anaesthetic agents, general, inhaled and IV (e.g., Propofol,
Ketamine)• Hospitals and healthcare providers aim to provide high quality and safe medical
care to their patients, including the safe and effective use of medications.• These medications, however, can be compared to a two-edged sword: while
useful, they can also be harmful as a result of errors associated with their
use as well as from adverse events/effects especially with these medications
that have a very narrow margin of safety and can cause severe harm to the
patient.• These medications are recognized as High Alert Medications.
• The Institute for Safe Medication Practices has gathered a list of “high-alert”
medications. These medications require extra precaution because they have
highly potentially rich to the patient when used in error.Managing High Alert Medications
The pharmacy department in the hospital needs to provide general guidelines for
the proper handling of High Alert Medications including the medication list.Concentrated electrolytes (Potassium & Sodium Phosphate, Potassium Chloride,
and Sodium Chloride) are High-Alert Medications, so should not be stocked in the
patient care areas except as part of the crash cart medications. Limited quantities
of these concentrated electrolytes can be stocked in specific area such as ICU
(Intensive Care Unit) and ER (Emergency Room) and need to be kept in a separate
locker and away from the regular ward stock medications and should by monitored
frequently by nursing and pharmacy staff.Label all containers and shelves used for storing High Alert Medications as “HIGH
ALERT MEDICATIONS”High Alert Medications must be double checked before they are prepared, dispensed
and administered to the patientsThe Medication Safety officer in the hospital must be check if the staff commitment
to do the double check before they are prepared, dispensed and administered to
the patients.Strategies to reduce errors involving High Alert Medication
* Procurement
• Limit the drug strengths available in the hospital.
• Avoid frequent changes of brand or color and notify the other healthcare staff
if there are changes.
• Inform all relevant personnel regarding in the hospital about the new High
Alert Medications listed.* Storage
• Minimize High Alert Medications from clinical areas, where possible.
• High Alert Medication should be stored individually in separate labelled plastic
container.
• Label the shelves or containers used for storing Alert Medications as “HIGH
ALERT MEDICATIONS.”* Prescribing
• Avoid using abbreviations when prescribing High Alert Medications.
• Avoid ordering High Alert Medications verbally accept in case of emergency
orders.
• Prescribe oral liquid medications with the dose specified in milligrams.
• Avoid using trailing zero when prescribing (e.g. 5.0 mg can be mistaken as
50 mg)
• Reduce the total dose of High Alert Medications in continuous IV drip bags
(e.g., 12,500 Units of Heparin in 250 ml vs. 25,000 Units in 500 ml) to reduce
risk• Dispensing / Supply
All High Alert Medication containers, product packages, vials or ampoules issued
towards units need to have caution label “HIGH ALERT MEDICATIONS” except for
parenteral nutrition preparations.
Accuracy check performance must be applied for the High Alert Medications before
dispensing the medicines.GUIDE ON HANDLING LOOK-ALIKE & SOUND-ALIKE MEDICATIONS
The patient safety incidents are widely spread because the health services system
become more complex, due to new technologies, medicines and treatments
strategies.Currently, thousands of medications are available in the markets and in the hospitals.
Some of these medicines have similarity in the names or packaging. The evidences
show that Look-alike/sound-alike medicines names and packaging are one of the
most common contributed factors associated with medication errors.Look Alike Sound Alike (LASA) medications involve medicines that are visually
similar in physical appearance or packaging and names of medications that have
spelling similarities and/or similar phonetics.Contributing Factors
Several Contributing factors may lead to confusion with LASA medications,
these include:
• Illegible handwriting.
• Incomplete knowledge of drug names.
• Newly available products.
• Importantly, it has similar packaging or labelling.
• Similar strengths, dosage forms, frequency of administration.
• Finally, similar clinical useStrategies to avoid errors with LASA Medications
* Procurement
Minimize the availability of multiple medicines strengths
Whenever possible, avoid purchase of medicines with similar packaging and
appearance. As new products or packages are introduced, compare them with
existing packaging.* Storage
Use Tall Man lettering to emphasize differences in medications with sound-alike
names.Tall Man lettering (or Tallman lettering) is the practice of writing part of a medicines
name in upper case letters to help distinguish sound-alike/look-alike medications
from one another to avoid medication errors.Examples of Tall Man lettering are metFORMIN and metoPROLOL
Using caution red tag notes on shelves, in order to alert the dispenser that a
medicine has look-alike and sound-alike medicines.Using techniques such as boldface and differences to reduce the confusion
associated with the use of LASA names on labels in the medicine’s storage
containers and shelves.* Prescribing
Place LASA medications in locations separate from each other or in non- alphabetical
order.Write legibly, using both the brand and generic names for prescribing LASA
medications.Prescription should clearly specify name of medication, dosage form, dose and
complete direction for use.Write the diagnosis or medication’s indication for use. This information helps to
differentiate possible choices in illegible orders.In electronic prescribing system, using techniques such as Tall-man lettering,
boldface and color differences to reduce the confusion associated with the use of
LASA names on the computer screens and medication administration records.Communicate clearly. Take your time in pronouncing the drug name whenever an
oral order made.Ask that the recipient of the oral communication repeat the medication name and
dose.
Minimize the use of Verbal and Telephone orders.* Dispensing/Supply
Identify medicines based on its name and strength and not by its appearance or
location.Check the purpose of the medication and the dose for the medicines dispensed.
Read medication prescription and label carefully at all dispensing stages
Commitment to a final accuracy check by a qualified person, before handing over
the medicine to the patient or the patient’s representativeDouble check should be conducted at any stage during the dispensing and supply
process.Highlight changes in medication appearances to patients upon dispensing.
* Administration
Read carefully the medication labels each time during the administration process
Perform the double check to check actual medicine and compare it with the
prescription and label.
Check the purpose of the medication and the dose prior to administration.Self- assessment 3.8
1. How can you define Look Alike Sound Alike (LASA) medications?
2. How can you explain “Tall Man lettering” as a strategy to reduce the errors
associated with the use of LASA medications?
3. What are the strategies to avoid errors with LASA Medications during
their supply/dispensing?
4. What are the strategies to avoid errors with High Alert Medications during
their prescription?3.9 Systems of measurement used in pharmacology
Learning activity 3.9
You are carrying the clinical practice at a health centre. A mother brings her
24-month-old female child who has a lower respiratory infection. An oral liquid
antibiotic is prescribed, and the mother is instructed to give 5mL TDS. The
mother does not have a tool to accurately measure 5mL, and she admits to have
different materials meant for household measurement.
1. Which household measurement material equivalent to 5mL would you tell
the mother to use?
2. How many teaspoons are usually in one tablespoon?CONTENT SUMMARY
Introduction to measuring systems
One of the most essential functions of a health care professional is the ability to
perform accurate pharmaceutical measurements, calculations and conversions.
Without this ability, a health care professional is not able to apply their knowledge
of pharmacology in a practical manner during their everyday work functions. This
is important as one incorrect calculation, conversion or measurements will affect
a dosage, and can potentially harm a patient. Possessing a working knowledge
of the pharmaceutical systems of measurement will only benefit a pharmaceutical
professional.At least four different systems are currently used in drug preparation and delivery:
the metric system, the apothecary system, the household system, and the
avoirdupois system. With the growing number of drugs available and increasing
awareness of medication errors that occur in daily practice, efforts have been
made to decrease the dependence on so many different systems. In 1995, the U.S.
Pharmacopeia Convention established standards requiring that all prescriptions,
regardless of the system that was used in the drug dosing, include the metric
measure for the quantity and strength of drug. It was also established that drugs
may be dispensed only in the metric form. Prescribers are not totally converted
to this new standard, however, so the nurse must be able to convert the dose
ordered into the available dose form to ensure patient safety. It is important to be
able to perform conversions (finding the equivalent values between two types of
measure, within each system of measure, and between systems of measure).METRIC SYSTEM
The metric system is the most widely used system of measure. It is based on
the decimal system, so all units are determined as multiples of 10. This system
is used worldwide and makes the sharing of knowledge and research information
easier. The metric system uses the gram as the basic unit of solid measure and the
liter as the basic unit of liquid measure. When using the metric system to convert
from smaller to larger, a person would simply move the decimal to the appropriate
number of places to the left. When converting from larger to smaller, a person
would move the decimal the necessary number of places to the right.APOTHECARY SYSTEM
The apothecary system is a very old system of measurement that was specifically
developed for use by apothecaries or pharmacists. The apothecary system uses
the minim as the basic unit of liquid measure and the grain as the basic unit
of solid measure. It uses weight and volume as divisions of measurement, they
include measurements of ounces, gallons, pints and quarts. This system is much
harder to use than the metric system and is rarely seen in most clinical settings.
Occasionally, a prescriber will write an order in this system, and the dose will have
to be converted to an available form. An interesting feature of this system is that
it uses Roman numerals placed after the unit of measure to denote amount. For
example, 15 grains would be written “gr xv.”HOUSEHOLD SYSTEM
The household system is the measuring system that is found in recipe books. This
system uses the teaspoon as the basic unit of fluid measure and the pound
as the basic unit of solid measure. Although efforts have been made in recent
years to standardize these measuring devices, wide variations have been noted in
the capacity of some of them. Patients need to be advised that flatware teaspoonsand drinking cups vary tremendously in the volume that they contain. A flatware
teaspoon could hold up to two measuring teaspoons of quantity. When a patient
is using a liquid medication at home, it is important to clarify that the measures
indicated in the instructions refer to a standardized measuring device.AVOIRDUPOIS SYSTEM
The avoirdupois system is another older system that was very popular when
pharmacists routinely had to compound medications. This system uses ounces
and grains, but they measure differently than those of the apothecary and household
systems. The avoirdupois system is seldom used by prescribers but may be used
for bulk medications that come directly from the manufacturer. The avoirdupois
system exclusively measures weight based on 16-ounces equaling 1 lb. This
system of measurement is the everyday weight-measuring system most people
recognize. In pharmaceutical measurements, the avoirdupois system is useful
for measuring bulk quantities when buying or selling, including over-the-counter
pharmaceuticals and chemicals.OTHER SYSTEMS
Some drugs are measured in units other than those already discussed. These
measures may reflect chemical activity or biological equivalence. One of these
measures is the unit. A unit usually reflects the biological activity of the drug in
1 mL of solution. The unit is unique for the drug it measures; a unit of heparin is
not comparable to a unit of insulin. Milliequivalents (mEq) are used to measure
electrolytes (e.g., potassium, sodium, calcium, fluoride). The milliequivalent refers
to the ionic activity of the drug in question; the order is usually written for a number
of milliequivalents instead of a volume of drug. International units are sometimes
used to measure certain vitamins or enzymes. These are also unique to each drug
and cannot be converted to another measuring form.Material used for measuring liquid for metric and household
A medicine cup is a plastic container with scales (metric, household) for measuring
liquid medications. Examine the medicine cup carefully before pouring any
medication to ensure that the proper scale is being used for measurement. The
medicine cup should be placed on a hard surface when measuring liquid medication
and then read at eye level. The medicine cup is inaccurate for measuring doses
of less than 1 teaspoon, although it is reasonably accurate for larger volumes.
A syringe comparable to the volume to be measured should be used for smaller
volumes. For volumes of less than 1 mL, a tuberculin syringe should be used.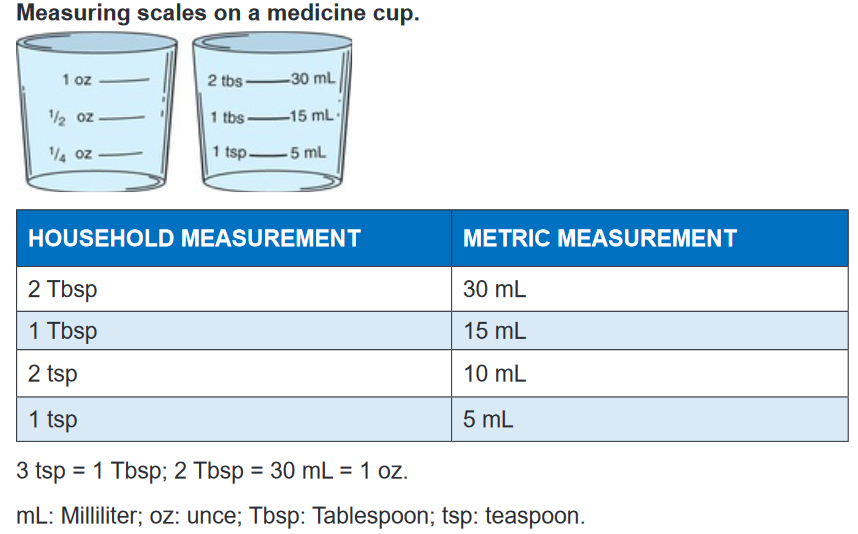
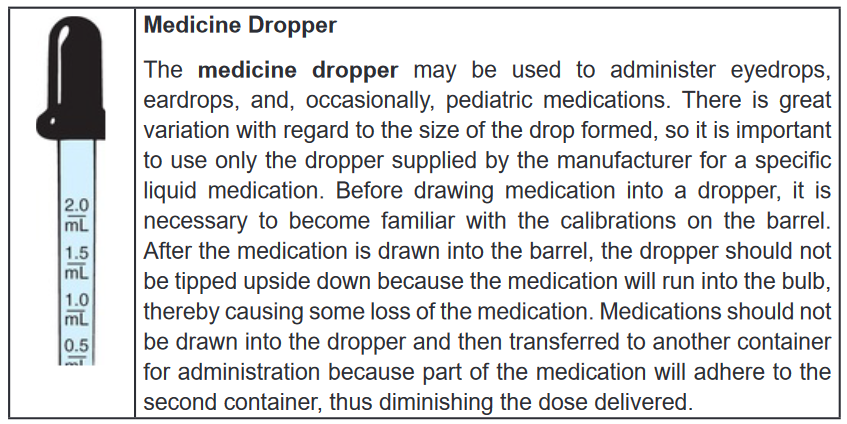
Teaspoon
Doses of most liquid medications are prescribed in terms using the teaspoon as the
unit of measure. However, there is great variation between the volumes measured
by various spoons in the home. In the hospital, 1 teaspoon is converted to 5
mL, and this is read on the metric scale of the medicine cup. For home use, an oral
syringe is recommended. If this is not available, a teaspoon that is used specifically
for baking may be used as an accurate measuring device.
Self- assessment 3.9
1. What are the 4 main measuring systems used in pharmacology?
2. What is the equivalent metric measurement (in mL) for 2 tablespoons of
household measurement?
3. What is the basic unit of liquid measure in household system?
4. What is the equivalent metric measure of 2 teaspoons?3.10 Characteristics of a well written medical prescription
Learning activity 3.10
ou are carrying out clinical practice in a health center. You colleague finds a
prescription of paracetamol 500mg PRN for a patient who is being managed
for an intermittent fever. The colleague then gets confused with the meaning of
PRN.
1. How can you explain a PRN order to your colleague?
2. What are other types of medication orders?
3. In Rwanda, who have the broadest prescriptive authority of medications?CONTENT SUMMARY
Introduction
A prescription (℞) is a health-care program implemented by a physician or other
medical practitioner in the form of instructions that govern the plan of care for an
individual patient.Prescriptions may include orders to be performed by a patient, caretaker, nurse,
pharmacist or other therapist.Commonly, the term prescription is used to mean an order to take certain
medications.Prescriptions have legal implications, as they may indicate that the prescriber takes
responsibility for the clinical care of the patient and in particular for monitoring
efficacy and safety.However, as medications have increasingly become pre–packaged manufactured
products and medical practice has become more complex, the scope of meaning
of the term “prescription” has broadened to also include clinical assessments,
laboratory tests, and imaging studies relevant to optimizing the safety or efficacy.Both pharmacists and prescribers are regulated professionals in most jurisdictions.
A prescription as a communications mechanism between them is also regulated
and is a legal document.Regulations may define what constitutes a prescription, the contents and format of
the prescription (including the size of the piece of paper and how prescriptions are
handled and stored by the pharmacist).Many jurisdictions will now allow faxed or phone prescriptions containing the same
information.Many brand name drugs have less expensive generic drug substitutes that are
therapeutically and biochemically equivalent.Prescriptions will also contain instructions on whether the prescriber will allow
the pharmacist to substitute a generic version of the drug. This instruction is
communicated in a number of ways.In some jurisdictions, the preprinted prescription contains two signature lines: one
line has “dispense as written” printed underneath the other line has “substitution
permitted” underneath.Some have a preprinted box “dispense as written” for the prescriber to check off
(but this is easily checked off by anyone with access to the prescription).Other jurisdictions the protocol is for the prescriber to handwrite one of the following
phrases: “dispense as written”, “DAW”, “brand necessary”, “do not substitute”, “no
substitution”, “medically necessary”, “do not interchange”.In other jurisdictions may they use completely different languages, never mind a
different formula of words.In some jurisdictions, it may be a legal requirement to include the age of child on
the prescription.For pediatric prescriptions, some advise the inclusion of the age of the child if the
patient is less than twelve and the age and months if less than five. In general,
including the age on the prescription is helpful. Adding the weight of the child is
also helpful.Prescriptions often have a “label” box. When checked, the pharmacist is instructed
to label the medication. When not checked, the patient only receives instructions for
taking the medication and no information about the prescription itself.Some prescribers further inform the patient and pharmacist by providing the
indicator for the medication i.e. what is being treated.This assists the pharmacist in checking for errors as many common medications
can be used for multiple medical conditions.Some prescriptions will specify whether and how many “repeats” or “refills” are
allowed, that is whether the patient may obtain more of the same medication without
getting a new prescription from the medical practitioner.Regulations may restrict some types of drugs from being refilled.
In group practices, the preprinted portion of the prescription may contain multiple
prescribers’ names.Prescribers typically circle themselves to indicate who is prescribing or there may
be a checkbox next to their name.Types of Medication Orders
The health care practitioner prescribes medications in different ways, depending on
their purpose. Medications can be prescribed as stat, single-dose, standing, and as
needed (prn) orders.STAT ORDERS
A stat order is an order for a single dose of medication to be given immediately. Stat
drugs are often prescribed in emergency situations to modify a serious physiological
response; a stat dose of nitroglycerin may be ordered for a client experiencing
chest pain.The nurse should assess and document the client’s response to all stat medications.
SINGLE-DOSE ORDERS
Single-dose orders are one-time medications or may require the administration of
drops or tablets over a short period of time.The nurse should administer single-dose orders only once, either at a time specified
by the health care practitioner or at the earliest convenient time.These drugs are often prescribed in preparation for a diagnostic or therapeutic
procedure for example, radiopaque tablets may be administered in preparation for
a gallbladder test, or a one-time order may be given for a preoperative medication.STANDING ORDERS
Standing orders are also referred to as scheduled orders because they are
administered routinely as specified until the order is canceled by another order.The standing orders stay in effect until the health care practitioner discontinues or
modifies the dosage or frequency with another order or until a prescribed number
of days has elapsed as determined by agency policy.The purpose of a standing medication order is to maintain the desired blood level
of the medication.PRN ORDERS
A drug may be ordered on a prn (as needed) basis as circumstances indicate.
The drug is administered when, in the nurse’s judgment, the client’s condition
requires it.Before administering a prn medication, the nurse must thoroughly assess the client,
using both objective and subjective data in determining the appropriateness of
administering the medication.This type of order is commonly written for analgesics, antiemetic, and laxatives.
The order written by the health care practitioner indicates how frequently a prn
medication can be given.A nurse cannot administer a prn medication more frequently than the order indicates
without consulting with the health care practitioner for a change in that order.Examples of prn orders are meperidine (a narcotic analgesic) 75 mg IM q3–4 hours
prn incisional pain and Tylenol 650 mg q4 hours prn headache.When the prn medication has been administered, the nurse documents the
assessment and the time of administration.In addition, the nurse is responsible for monitoring the effectiveness of the medication
and documenting the effect in the client’s medical record.The nurse administers the pain medication on the basis of the assessment of the
client’s pain and as specified in the order.Who can write prescriptions (that may legally be filled with prescription-only
items)?Any jurisdiction that allows freedom of written communication generally must
therefore allow anybody to write a prescription to anybody, in as much as the
prescription itself is just written advice.Therefore “who can write prescriptions” will be explained below as shorthand for
“whose prescriptions may legally be filled with items restricted to dispensing via the
order of certain persons”.National or legislation governs who can write a prescription.
In Rwanda, physicians have the broadest prescriptive authority.
Many other healthcare professions also have some form of prescriptive authority
related to their area of practice. Veterinarians, dentists, and podiatrists have
prescribing power.All the country allows registered certified Nurse practitioners prescription power
with some limitations to controlled substances.Both pharmacists and prescribers are regulated professionals in most jurisdictions.
A prescription as a communications mechanism between them is also regulated
and is a legal document.Regulations may define what constitutes a prescription, the contents and format of
the prescription including the size of the piece of paper and how prescriptions are
handled and stored by the pharmacist.Many jurisdictions will now allow faxed or phone prescriptions containing the same
information.Parts of the drug order
All orders should be written clearly and legibly, and the drug order should contain
seven main parts:
1. Identification of the client (name, age, sex, etc)
2. The date and time when the order is written
3. The name of the drug to be administered
4. The dosage5. The route by which it is to be administered and special directives about its
administration6. The time of administration and frequency
7. The signature of the person writing the order, such as the physician or
advanced practice registered nurseConventions for avoiding ambiguity
Not only the drug order and medical prescription should have the above mentioned
parts, should they also have the full information in order to give all required details
about the order or prescription.Prescribers have developed many conventions for prescription-writing, with the
goal of avoiding ambiguities or misinterpretation.These include:
• Date medication dispensed
• Sequential number
• Client full identity
• Prescriber’s direction for usage including the frequency and route of
administration
• Prescriber’s name
• Name and address of the agency dispensing
• Name and strength of the drug dispensed
Self- assessment 3.10
1. Which of the following statements best describes a STAT order?
A. The drug is administered when, in the nurse’s judgment, the client’s
condition requires it such as in case of pain management.
B. These are one-time medications or orders that require the administration
of drops or even tablets over a specified short period of time.
C. An order for a single dose of medication to be given immediately, often
in emergency situations to modify a serious physiological response
D. These are the orders for drugs that are administered routinely as
specified until the order is canceled by another order.2. What are the 7 main parts of a drug order?
3. Some prescriptions will specify whether and how many “repeats” or
“refills” are allowed for prescribed drugs. What do you understand by3.11 Drug dosage calculation
Learning activity 3.11
1. The nurse is preparing to give an oral dose of acetaminophen (Tylenol)
to a child who weighs 12 kg. The dose is 15 mg/kg. How many milligrams
will the nurse administer for this dose?2. The patient is to receive 60mg of gentamicin BID intramuscularly. The
available ampules are 80mg dissolved in 2 mL each. How many milliliters
will the associate nurse draw from the ampule at each drug administration
(dose)?3. A 20-year-old male patient is to be given tablets of erythromycin for his
respiratory infection. He has been prescribed 500mg TDS for 7 days. The
available erythromycin tablets strength is tablets of 250mg. How many
tablets will the patient receive per day?CONTENT SUMMARY
Drug Dosage Calculations
Drug dosage calculations are required when the amount of medication ordered (or
desired) is different from what is available on hand for the nurse to administer
Therefore, the nurse would administer 0.5 of a tablet.
Example 2: 1200 mg of Klor-Con is ordered. This medication is only available as
600 mg per tablet. How many tablets should the nurse give?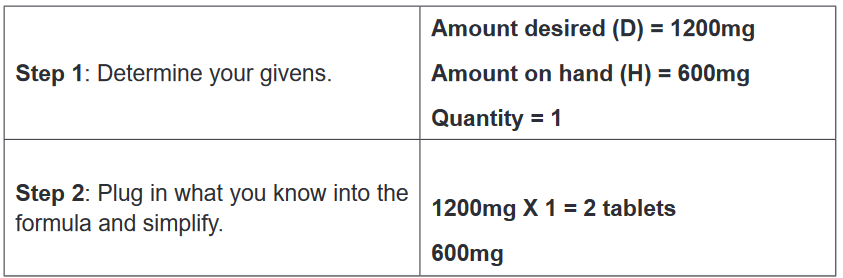
Therefore, the nurse should give 2 tablets.
The same formula can be used for dosage calculations where the medication is
available as amount per certain volume. In these types of calculations, the volume
available on hand is the QUANTITY.Example 3: Dilantin-125 is available as 125 mg/5 mL. Dilantin-125, 0.3 g PO, is
ordered. How much should the nurse administer to the patient?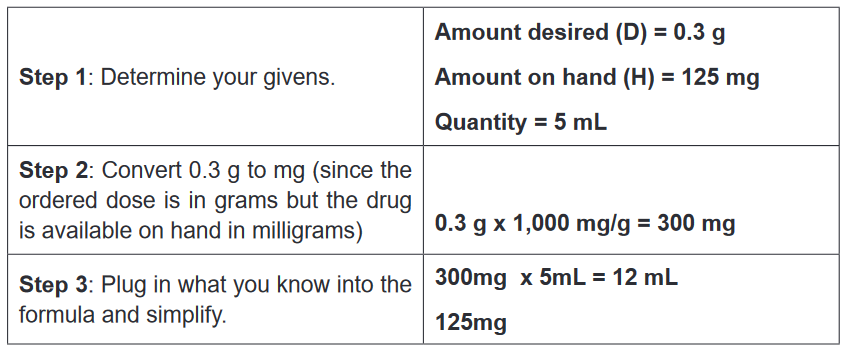
Therefore, the nurse would administer 12 mL.
Example 4: Furosemide is available as 40 mg in 1 mL. 10 mg is ordered to
be administered through an IV. What amount of furosemide should the nurse
administer?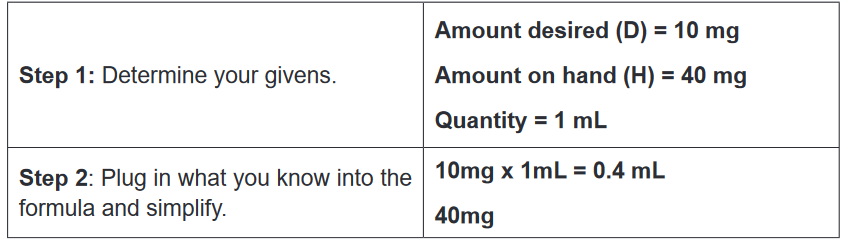
Therefore, the nurse should administer 0.4 mL of furosemide.
Dosage Calculations based on Body Weight
Dosage calculations based on body weight are required when the dosage ordered
and administered is dependent on the weight of the patient. For example, many
pediatric drugs are ordered and given per weight (usually in kg). Dosage calculations
based on body weight are calculated in two main stages.Stage 1: Using the formula below, calculate the total required dosage based on
given the body weight.
Weight (kg) x Dosage Ordered (per kg) = Y (Required Dosage)Stage 2: Apply the D/H x Q formula to calculate the actual amount of medication to
be administered.Example 1: Medrol 4 mg/kg is ordered for a child weighing 64.8 lb. Medrol is available
as 500 mg/4mL. How many milliliters of medication must the nurse administer?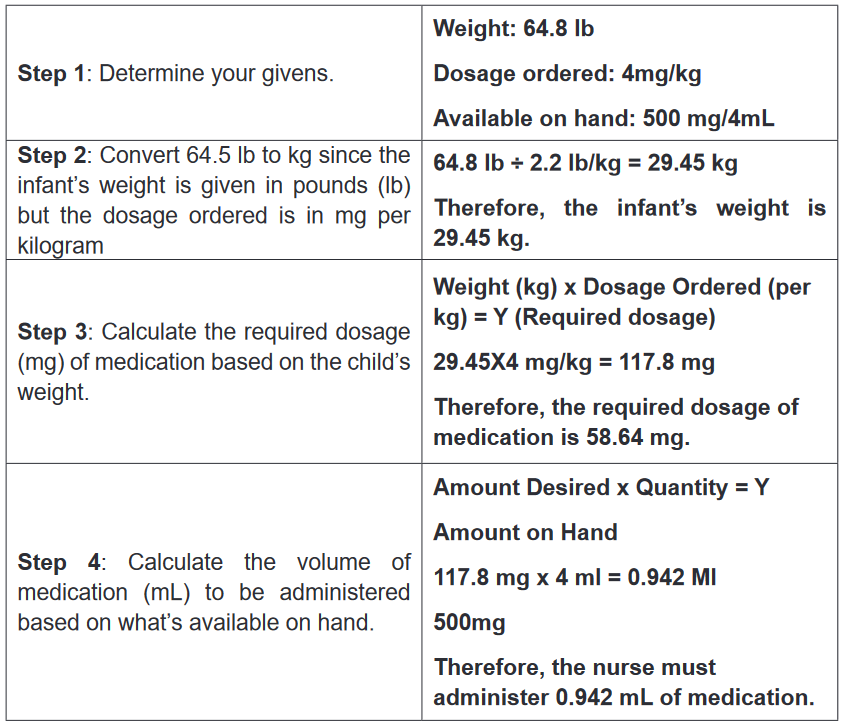
Self- assessment 3.11
1. 1000 mg of potassium chloride is ordered. This medication is only
available as 500 mg per tablet. How many tablets should the nurse give?2. Diclofenac injection is available as an ampule of 75mg/3 ml. This means
that the concentration is 25 mg/ml or 75 mg/ 3 ml. Question: How much
volume of liquid will the client receive when the prescription is to give only
50 mg?3. A syrup is available as 25mg/5ml and the patient must be given 50mg.
What volume in milliliters will be given?3.12 End unit assessment
End Unit assessment 3
1. During drug administration, the nurse needs to ensure that all the RIGHTs
of medication administration are respected. Therefore, while applying the
right time, medications should be prescribed as closely to the time as
possible, and nurses should not deviate from this time by:
A. More than two hours to avoid consequences
B. More than three hours to avoid consequences
C. More than half an hour to avoid consequences
D. More than one hour to avoid consequences2. Which of the following RIGHTs of medication administration seeks to
ensure the medication is working the way it should after its administration?
A. Right evaluation
B. Right documentation
C. Right route and form
D. Right patient3. Which of the following instructions are applied to controlled drug
regulation?
A. The controlled drugs should be in double locked container, and 1
licensed personnel counts (or verifies any discrepancies) every two
days.
B. The controlled drugs should be in double locked container, and 2
licensed personnel count (or verify any discrepancies) every shift.
C. The controlled drugs should be in double locked container, and 2
licensed personnel counts (or verifies any discrepancies) every two
days.
D. The controlled drugs should be in double locked container, and 1
licensed personnel counts (or verifies any discrepancies) four times a
day.4. Which of the following statements best defines the subcutaneous
injection?
A. The medication is deposited just beneath the skin in the loose
subcutaneous tissue.B. The drug is injected into one of a large skeletal muscle such as triceps
and rectus femoris
C. Intrathecal administration is a route for drugs via an injection into the
spinal canal, or into the subarachnoid space
D. Method of administering medications directly into the vein using a
needle and a syringe5. In which of the following types of medication errors would a medication
error which involves any deviation from the prescriber’s medication order
as written on the patient’s errors chart, manufacturers’ preparation, or
relevant institution policies be classified?
A. Prescribing errors
B. Administration errors
C. Dispensing errors
D. Monitoring errors6. How many millilitres are in one teaspoon used in the measurement of
drugs?
A. 7 millilitres
B. 10 millilitres
C. 15 millilitres
D. 5 millilitres7. Which of the following statements best describes a STANDING order?
A. The drug is administered when, in the nurse’s judgment, the client’s
condition requires it such as in case of pain management.
B. These are one-time medications or orders that require the administration
of drops or even tablets over a short period of time.
C. An order for a single dose of medication to be given immediately, often
in emergency situations to modify a serious physiological response
D. These are the orders for drugs that are administered routinely as
specified until the order is canceled by another order.8. Enumerate social and economic dimensions affecting adherence to
medications.9. Enumerate the advantages of rectal route of drug administration.
10. What are the disadvantages of administering the drugs by the transdermal
route?
