Unit 10 :Applications of thermodynamics laws
Key unit Competence
Evaluate the applications of first and second laws of thermodynamics in reallife.
Unit goals
By the end of this unit, I will be able to:
* differentiate between Internal energy and total energy of a system.
* explain the work done by the expanding gas.
* state the first law of thermodynamics.
* state the second law of thermodynamics.* explain thermodynamic processes in heat engines.
Introductory activity
Mutesi is a parent of two children at a certain school. Before she takes them
to school, she first makes sure that she prepares food and drinks for them
and packs some in flasks so that her children can eat and drink during lunchtime.
She then drives them to school before she reports to her working place and
then from the school she then diverts to her working place which is about 5km away from the school.
The parking yard at her work place is a plain place without any shade but
she makes sure that her car is parked near a tree that is near the parking yard
to prevent it from different damages among which is destruction of tyres ofthe car.
a) Explain why Mutesi makes use of flasks not normal utensils like metallicbowels while parking foods and drinks for her children.
b) Is there heat exchange inside the flasks? Explain your reasoning.
c) Imagine on a certain day these two children only eat food and leaves,
the drink in the flask and by mistake they forget flask in the store and
the mother come to pick it the next day. Do you think the contents in the
flask will be at the same temperatures? Explain all scientific phenomenathat may lead to either loss or gain in energy of the contents in the flask.
d) Explain why in most cases the outer covering of a flask is always made
of a poor conductor? Explain how quality and efficiency of these flaskscan be improved by manufactures.
e) Based on statements above, Mutesi normally parks her car under a shade
to prevent her car from being exposed to sunshine. Explain how duringhot days the tyres of a car may burst.
f) Her Car uses petrol in operation. During operation of her car, the engine
draws fuel (Petrol) air mixture from the tank into the engine, explain allthe processes that take place in the engine.
Introduction
Before, you learnt that:
• Heat is a form of energy.• Heat can be changed / transformed from one form to another.
So, if in a system heat changes from one form to another, its called thermaldynamic system.
The systems to discuss in this unit include refrigerators, heat pumps, car engines.
Remember that heat is the measure of total internal energy of a body. This meansthat particles of a body vibrate because of energy they have.
Thermal energy and internal energy
Activity 1
Have you ever boiled water on a sauce pan with a cover?Describe what happens to the cover when water boils?
When water boils, the vapour pushes the cover off the sauce pan. You have
already seen in your early secondary that heat is a form of energy. Therefore,
when this saucepan is heated, the heat gained is used to boil off the water and
extra work is done to push the sauce pan cover. This total heat energy suppliedis called thermal energy.
Science in action! Discover
• Explain why an inflated bicycle tube bursts when it is left on sunshine for
a very long time.
• Similarly explain why a balloon full of air bursts as it rises in the
atmosphere.• Note down your observation in your exercise books.
You already know the characteristics of the three states of matter that is; solids,
liquids and gases. In this unit, we shall be interested in studying the behaviourof molecules in matter.
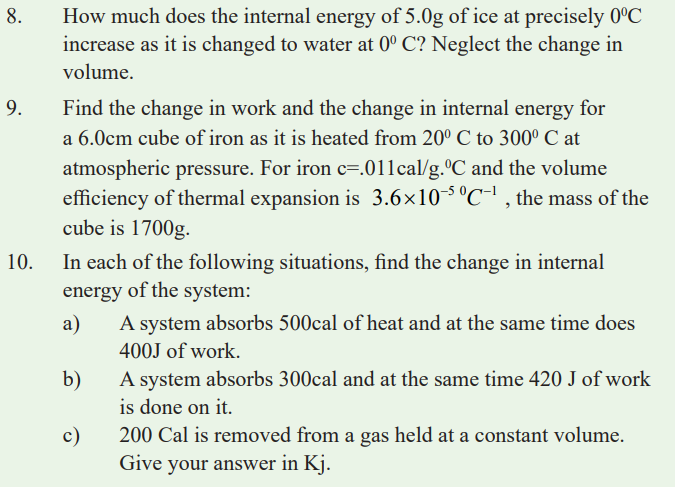
When the bicycle tube is left exposed to sunshine, it gets heated and the
molecules in the gas gain energy and hence its kinetic energy increases. As
a result, they collide frequently with the walls of the tube and therefore exerthigh pressure on the walls and the tube bursts.
The same thing happens with the balloon in air.
The energy possessed by the molecules of the gas is called internal energy
of the gas. This energy depends on the temperature of the gas. When a gas is
heated its temperature increases and hence the average speed of molecules
also increases increasing the internal energy of the gas. Further increase of
heat supplied means that extra energy is absorbed by the molecules of the gas,hence expanding and pushing the tyre. As a result the tyre bursts.
The internal energy is defined as energy associated with random disorderedmotion of particles.
Activity 2
List down three utensils used for cooking food in your homes.
Describe how these utensils are used to cook the food. Are they always leftopen while cooking?
In all the above, there exists energy exchanges and such things are called
systems. Systems can either be closed or open. When water is being boiled in
an open sauce pan, vapour is allowed to escape. It is an example of an open
system. When someone cooks meat using a closed container, no gas is allowedto escape. Its an example of a closed system.
Whenever heat flows to or from a system, or work is done on or by a system,
there is a change in the energy of this system. The study of the processes thatcause these energy changes is termed thermodynamics.
Thermodynamic systems
Heat is the energy that flows by conduction, convection or radiation from
one body to another because of a temperature difference between them. These
bodies where exchange of heat to other forms of energy occurs are calledthermodynamic systems.
A thermodynamic system consists of a fixed mass of matter, often a gas,
separated from its surroundings, perhaps by a cylinder and a piston. For example
heat engines such as a petrol engine, a steam turbine and jet engine all contain
thermodynamic systems designed to convert heat into mechanical work. Head
pumps and refrigerators are thermodynamic devices for transferring heat froma cold body to a hotter one.
A thermodynamic system is any object or set of objects that we wish to
consider. Everything else in the universe we will refer to as its environment or
the surroundings. A system is separated from the remainder of the universe bya boundary. The boundary may be at rest or in motion.
A system can be homogeneous or heterogeneous. It can be gaseous, liquid or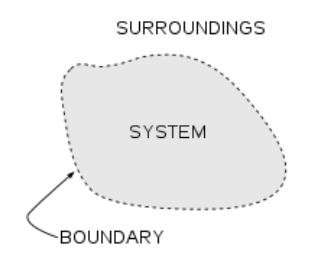
solid state. A system is in equilibrium when its properties do not change withtime.
A closed system is one for which no mass enters or leaves but may enter heat
exchanged with the environment. A closed system is sometimes referred to as
a control mass because the matter composing the system is assumed known
for all time. A closed system is said to be isolated if no energy in any formpasses across its boundaries, otherwise it is not isolated.
In an open system, mass may enter or leave as well as energy. An open system
is sometimes referred to as a control volume because the location composing
the system is assumed known for all time. The surface surrounding the control
volume is sometimes known as a control surface. The control surface can be
along a real surface of the system or it can be an imaginary surface chosen for
convenience. In general, a control mass can change shape and volume, but acontrol volume cannot.
In such devices, energy is transferred from one system to another by a forcemoving its point of application in its own direction.
The energy of a system, whether transferred to it as heat or work is termed asthe internal energy of the system.
When there is no heat transfer between two systems, that is, the two are at thesame temperature, they are said to be in thermal equilibrium.
Activity 3
Have you ever observed smoke moving in the atmosphere.
Move outside class and go towards the kitchen and observe how smoke is
moving. Describe briefly how it moves.Why does it move like that?
You have already seen in your early secondary that molecules in a gas are
more further apart and are always in constant random motion while moving at
high speed colliding with one another and the walls of the container, and whenthe gas is heated their speed increases.
Smoke particles are always in random motion and when they are moving in
air, they collide with air molecules and a zigzag pattern is seen.
Similarly, when smoke is put in a container and then closed, the particles areseen to be in a random motion. Smoke is an example of a real gas.
In thermodynamics, we are mainly interested in ideal gas. At highertemperatures, a real gas behaves like an ideal gas.
Activity 4
Have you ever heard of an ideal gas?What are the differences between a real gas and an ideal gas?
When a gas is heated, molecules move further apart and the forces of attractionsbetween them become negligible and the gas becomes ideal.
When the molecules become further apart, the gas expands and the volume of
the individual molecule becomes so small compared to the entire volume of
the gas. It therefore becomes negligible compared to the volume of the gas andthe gas becomes ideal.
When the molecules are colliding with one another, collisions are assumed to
be perfectly elastic. In this case, the gas becomes ideal because for a real gaswe expect to have time between approach and separation during collision.
Work done by an expanding gas
Activity 5: Discover
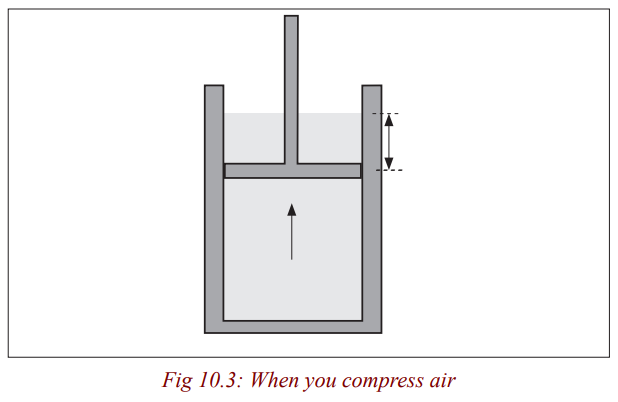
Explain why a pump gets hot when one pumps air into a tyre.
When you compress air in a bicycle pump, your muscles transfer energy
to the handle, which in turn transfers energy to the molecules of air in the
pump. This additional energy makes the molecules move faster. As they are
compressed into a smaller space, they also collide more often with the wall ofthe pump, so they transfer more energy to the metal wall and it becomes hot.
We have already seen how heat can be transferred, so you probably have a
good idea what Q means. Work is simply a force Multiplied by distance in thedirection of force.
A gas can be heated by compressing it, for example with a bicycle pump.
Hence the temperature of the gas can be raised either by doing work in
compressing it or by heating it. Likewise the temperature can be lowered byeither making the gas do work in expanding or by extracting heat from it.
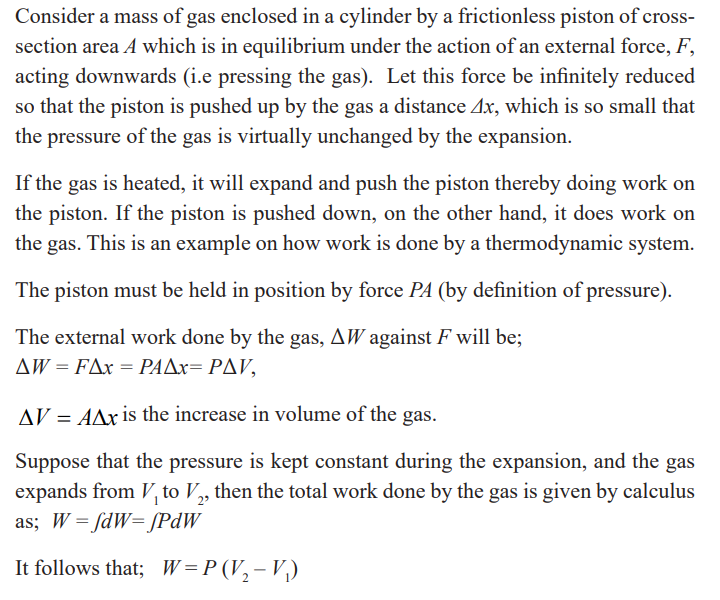
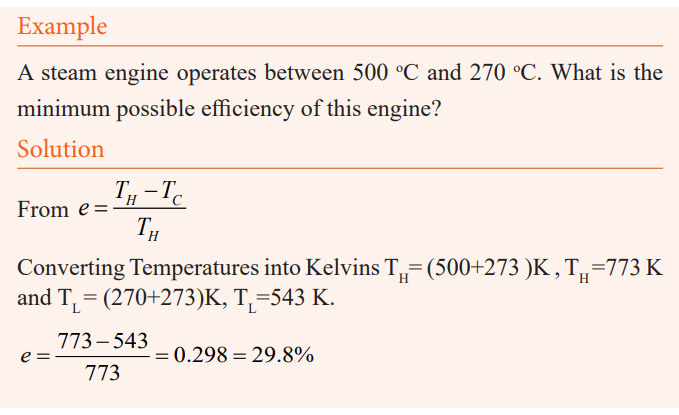
Example
Steam to drive an old-fashioned steam locomotive is supplied at a constant
gauge pressure of 1.75×106N/m2 about 250 psi) to a piston with a 0.200-m radius.
Find the work done by the steam when the piston moves 0.800 m.
Solution
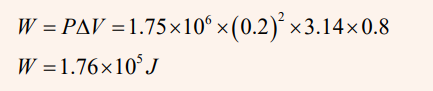
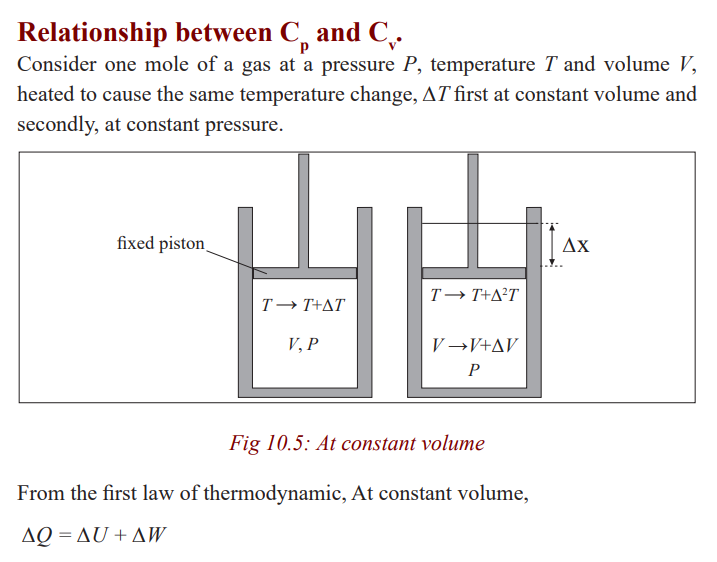
Specific heat capacities of gases
Gases are considered to have a number of specific heat capacities. A change in
temperature of a gas is likely to cause large changes in pressure and volume ofthe gas but for solids or liquids, the change in pressure is neglected.
The reason as to why this is so can be done by considering cylinders in (a) and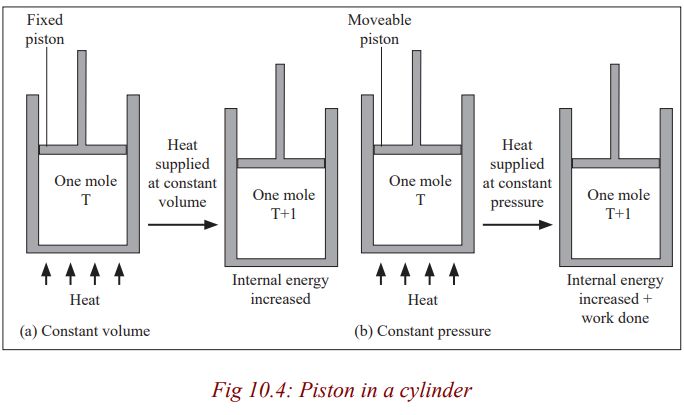
(b) each initially containing one mole of gas at temperature T and pressure,
P. The piston in (a) is fixed and that in (b) is frictionless and can move freely
but has a constant force applied to it. If heat is supplied to each until the
temperature has risen by one Kelvin, the increase of internal energy must bethe same in each case (Since the temperature rise is the same).
All the heat supplied in case (a) is used to increase the internal energy of the
gas. In (b), however, the gas expands and work is done by it on the piston; the
heat supplied in this case equals the increase of internal energy plus the workdone in the expansion of the gas.
Application activity 10.1
1. Which statement best describes the concept of a system?
A.the subject of the analysis C. a fluid-solid mixture
B.an object with fixed set of molecules D. an object that radiates heatinto its environment
2. Consider a refrigerator in a kitchen. Take the refrigerator and everything
in it to be our system. Which best describes the system's surroundings?
A.All of the air in the kitchen. C. The air inside the refrigerator
B.Any one standing in the kitchen. D. Everything in the universeexternal to the system.
3. Consider a refrigerator in a kitchen. Take the refrigerator and everything
in it to be our system. Which best describes the system's boundary?
A.All of the air in the kitchen.
B.The thin region separating the system from everything else.
C.The air inside the refrigerator.D.The outer walls of the refrigerator.
4. Which of the following statements are true?
A.A closed system is necessarily an isolated system
B.An isolated system is necessarily a closed system
C.A system cannot be both closed and isolated.
D.The concepts of closed and isolated in regards to a system are independentconcepts.
5. True or False: A system is in steady state if its properties are independentof time.
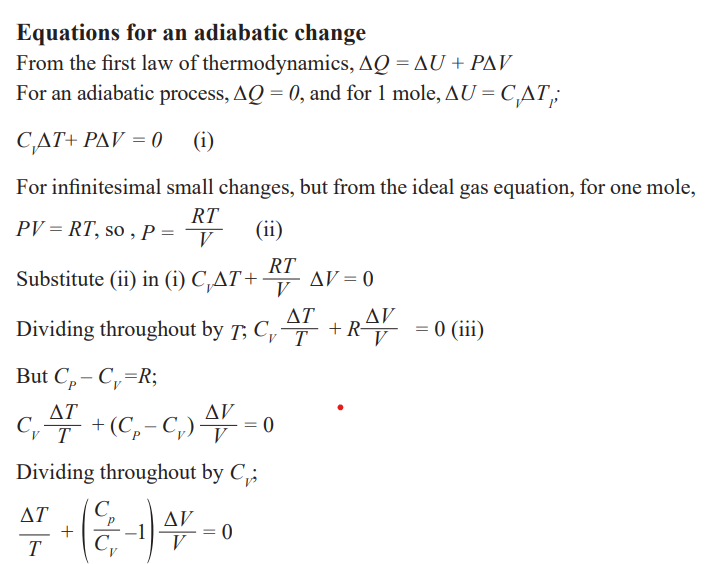
The first law of the thermodynamics
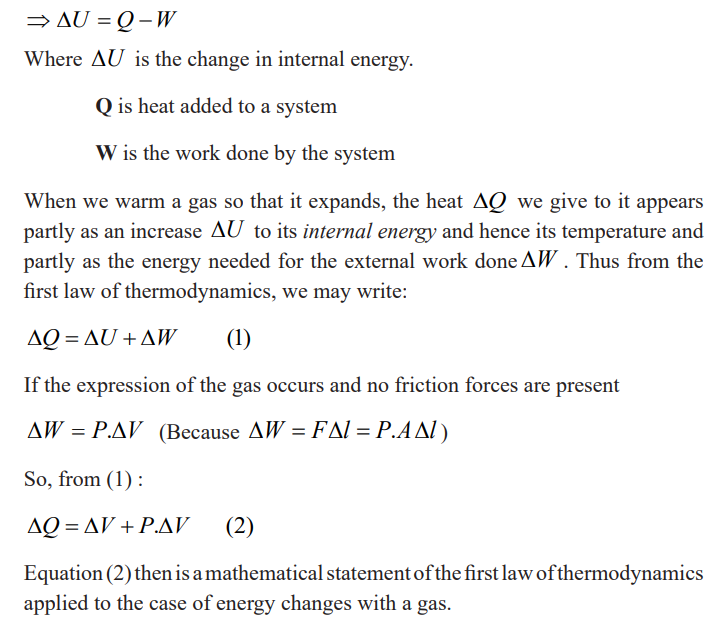
The first law of thermodynamics states that” in a closed system the energy
is conserved in any transfer of energy from one form to another. Means that
the change in internal energy of a system is equal to the heat added to the
system minus the work done by the system” this law is known as the law ofenergy conservation.

Example
Compute the internal energy change and temperature change for the twoprocesses involving 1 mole of an ideal monatomic gas.
a) 1500 J of heat are added to the gas and the gas does no work andno work is done on the gas
b) 1500 J of work are done on the gas and the gas does no work andno heat is added or taken away from the gas
Solution
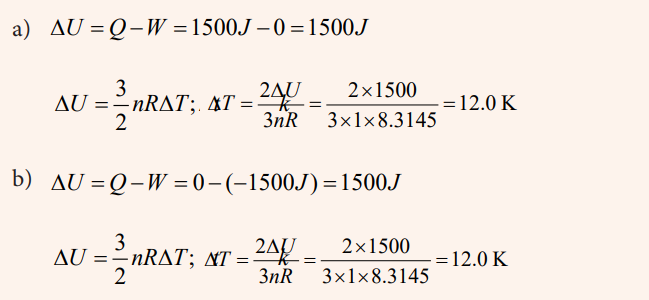
Applications of first law of thermodynamics in particular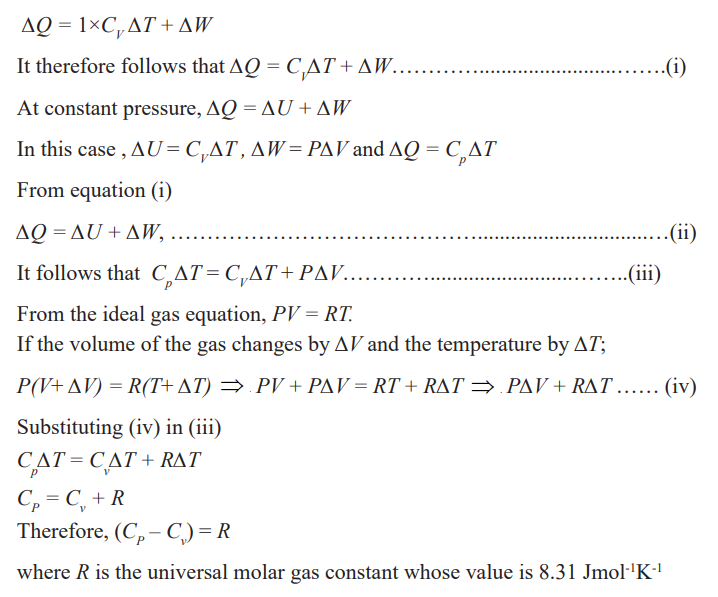
gas changes
The first law of thermodynamics that we discussed relates the changes in
internal energy of a system to transfers of energy by work or heat. In this case,
we consider applications of the first law in processes through which a gas istaken as a model.
Isovolumetric process (Isochoric process)
Activity 6
(i) Have you ever heard of an isovolumetric or isochoric process?Study the Figure and answer questions that follow;
(ii) What substance is likely to be getting cooked using the can on the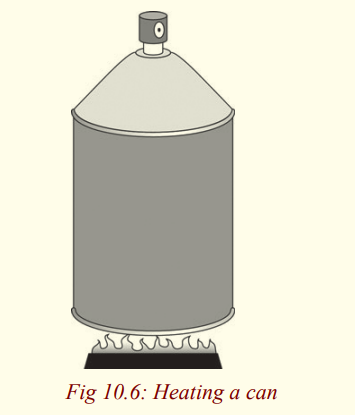
left? Give a reason for your answer.
(iii) Why is the can covered and not open?
(iv) If one tried to open it while its on fire, what do you think wouldhappen?
A process that takes place at constant volume is called an isovolumetricprocess.
From the figure 10.7, process AB takes place at a constant volume (volume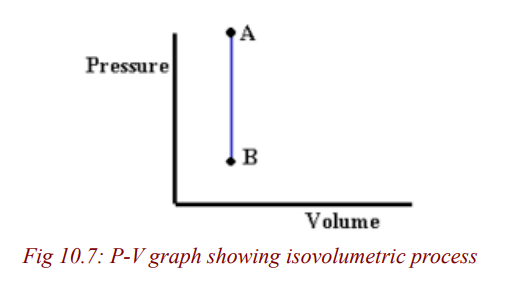
doesn’t change).
In such a process, the value of the work done is zero because the volume doesnot change. Hence, from the first law we see that in an isovolumetric process,
W = 0 and ΔU = Q (isovolumetric process)
Note:
• This expression specifies that if energy is added by heat to a system
at constant volume, then all of the transferred energy remains in thesystem as an increase in its internal energy.
• For example, when a can of spray paint is thrown into a fire, energy
enters the system (the gas in the can) by heat through the metal walls
of the can. Consequently, the temperature, and thus the pressure inthe can increases until the can possibly explodes.
Isobaric process
Activity 7
Boiling liquids in open containers is very safe for example if the container is
closed, pressure may build up in the container and force it to burst. Boiling in
open containers imply that the pressure of the substance is kept constant. This
process is called an isobaric process. An isobaric process is the one that occursat constant pressure.
Heating of water in an open vessel and the expansion of a gas in a cylinder
with a freely moving piston are typical examples of isobaric processes. In both
cases, the pressure is equal to atmospheric pressure. For example when water
is being heated, its volume increases and the pressure inside the container is
constant since the number of collisions between water molecules and the wallsof the container is constant.
The same process occurs when a gas enclosed in a cylinder with a frictionless
piston is heated such that at any time, the gas pressure equals the externalpressure.
In this process, the energy supplied is used to increase the internal energy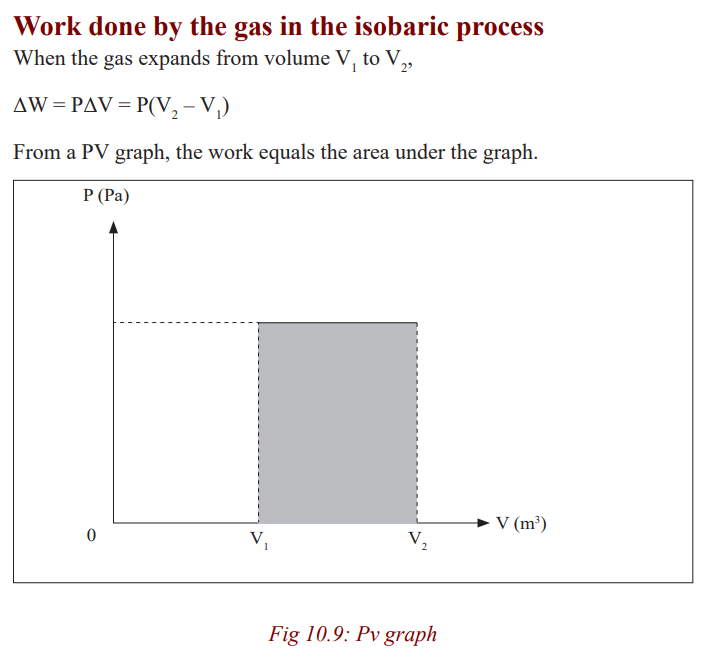
since the internal energy is independent of the volume.
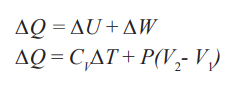
Isothermal change (constant temperature)
Activity 8
(i) Get a polythene bag and fill it with air.
(ii) Insert a thermometre in the bag and place it in the ice-water mixture.
(iii) Note what happens.
Do you notice that the gas condenses and the volume decreases?
What happens to the temperature recorded by the thermometer?
You can notice that the temperature remains constant. This change is calledCondensation and is an example of isothermal process.
Do you think this process is reversible?
An isothermal change can be reversible. An isothermal change is the change
that occurs at constant temperature. It is either a compression or expansion ofa gas at a constant temperature.
If the volume increases, the pressure must decrease and if the volumedecreases, the pressure must increase
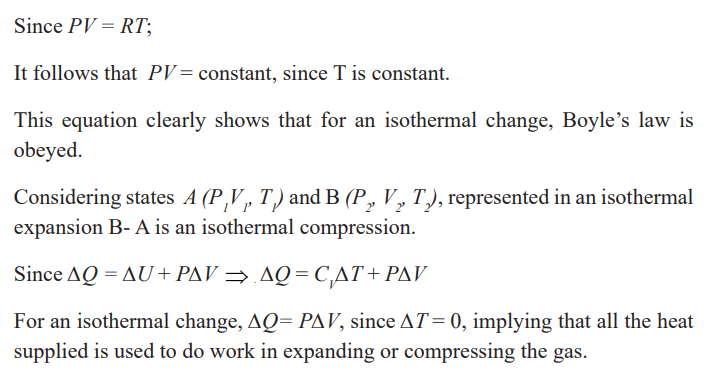
Conditions necessary for an isothermal process to occur
Activity 9: Discover
(i) On a cold day, how do you keep yourself warm?
(ii) In groups of five, describe how you can keep the temperature of thesystem constant.
For an isothermal process to take place, the gas must be contained in a thin
–walled heat conducting vessel/container in good thermal contact with aconstant temperature.
The process must be carried out slowly to allow time for heat exchange to takeplace.
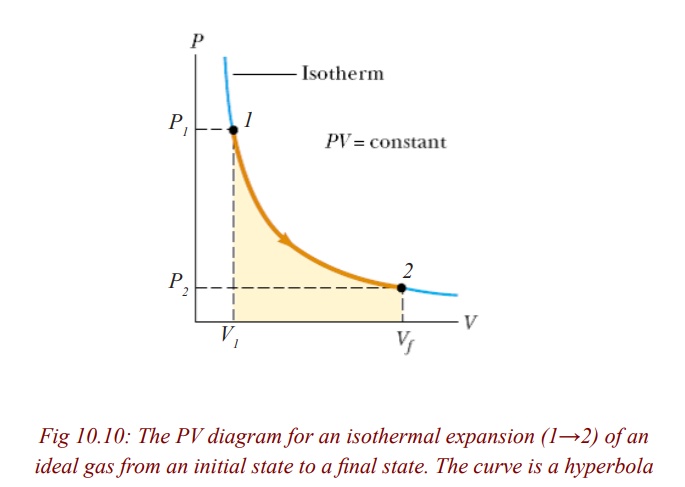
Work done in Isothermal Change
Activity 10: Science at work
(i) Have you ever tried to boil water in a closed sauce pan?
(ii) What happens to the cover when the vapour starts to come off the water?
(iii) Notice that this vapour pushes the cover off the pan.


Comment.
The answer has a negative value. This shows that the work is doneon to the gas (compressed).
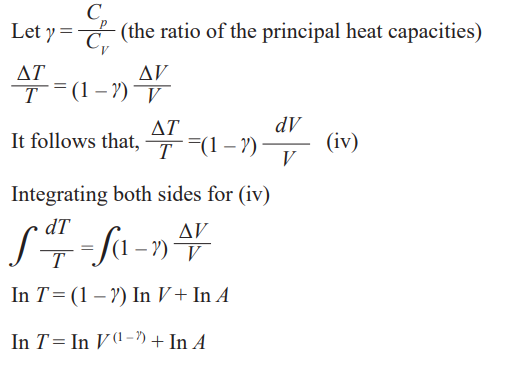
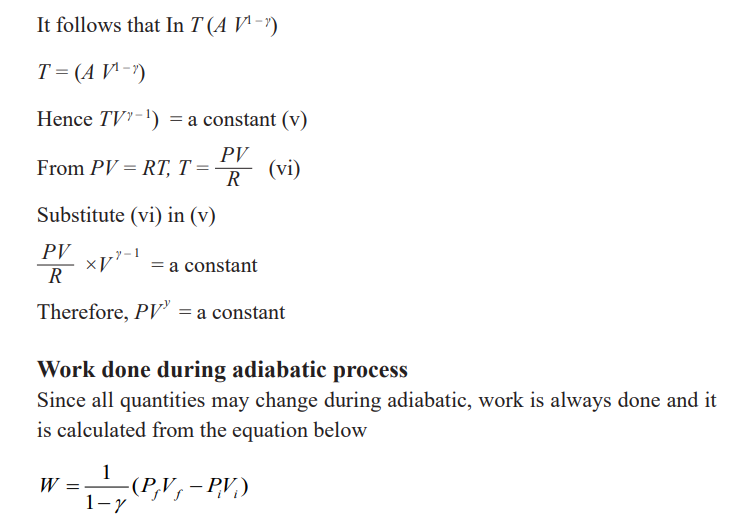
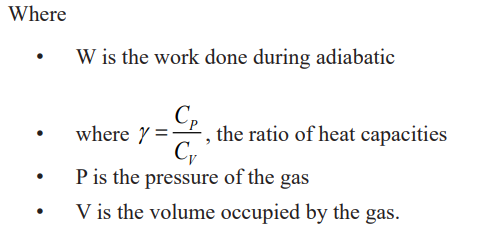
Adiabatic change
Activity 11
(i) Pump a bicycle tyre using a pump until it is full.
(ii) Open the tube slowly while placing your other hand in its path.
(iii) Do you notice that the the air coming out of the tyre is hotter thanthe surrounding air?
As one pumps, the air molecules are compressed into a smaller space. They
also collide more often with the wall of the pump, so they transfer more energy
to one another and become hot. No heat has been supplied to the system. It iscalled an adiabatic compression.
Activity:12
(i) Now pump the tyre and leave it standing for sometime.
(ii) Make sure you don’t expose it to sun shine.
(iii) Open the valve after two hours while your hand is placed in the pathof air from it.
Do you notice that the air is colder than its surrounding?
Heat has been lost but not to the surroundings. When the air is left standing,
expansion occurs. This is associated with a decrease in temperature. It is calledan adiabatic expansion.
An adiabatic change is process in which no heat enters or leaves the gassystem. It is either an expansion or a compression.
If the gas expands, it does work, its internal energy is reduced and hence the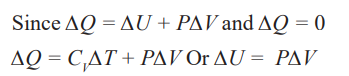
temperature is lowered.
If the gas is compressed, work is done on the gas, its internal energy willincrease and therefore its temperature rises.
Conditions that are necessary for an adiabatic change to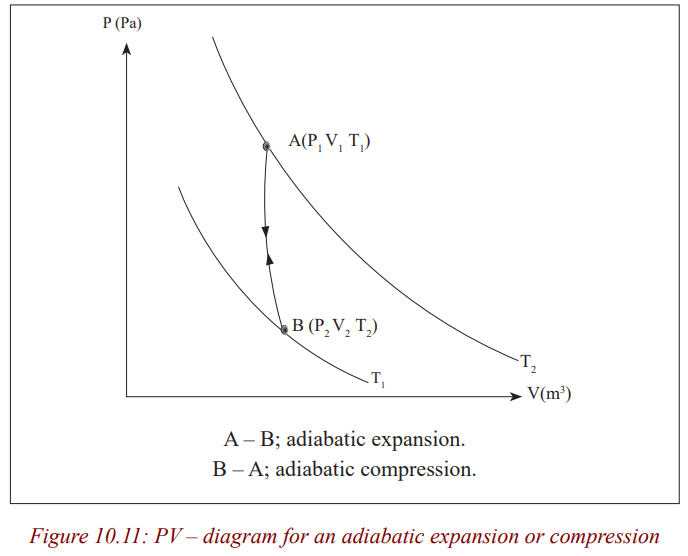
occur
Activity 13
How do you always protect yourself from a bad weather?
On a cold day, we always wear woolen jackets to protect ourselves from
coldness. Therefore no heat is either lost to the surrounding and or gained. Inthis case, an adiabatic process is achieved.
For an adiabatic process to be achieved, the gas must be contained in a thick–walled and perfectly insulated isolated container.
The process must be carried out rapidly to avoid any possible heat exchangesbetween the gas system and the surroundings.
Activity 14

Example I

Solution

Example 2
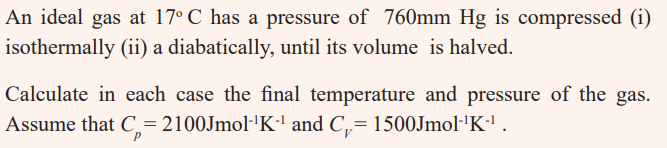
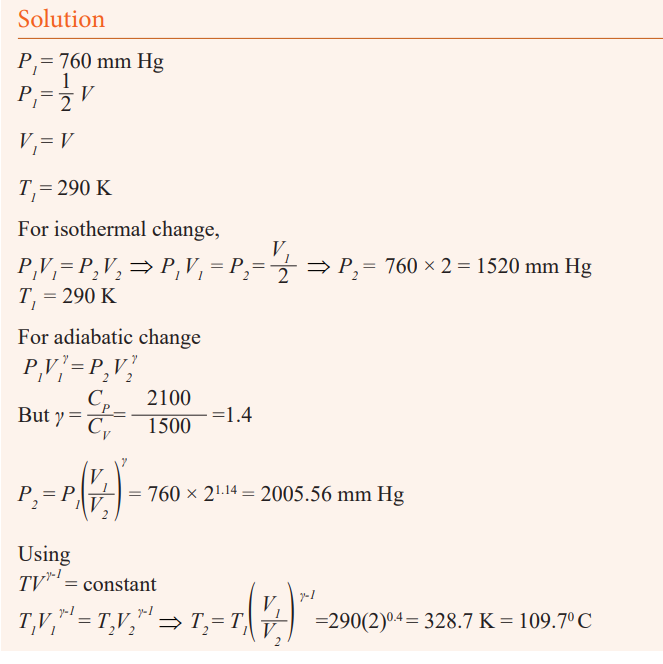
Application activity 10.2
1. A total of 135 J of work is done on a gaseous refrigerant as it undergoes
compression. If the internal energy of the gas increases by 114 J during
the process, what is the total amount of energy transferred by heat? Hasenergy been added or removed from the refrigerant by heat?
2. The internal energy of a system is initially 27 J. A total of 33 J of energy
is added to the system by heat while the system does 26 J of work. Whatis the system’s final internal energy?
3. Heat of 90 cal is supplied to a system and it is observed that no work isdone. Calculate the change in internal energy of this system.
4. One mole of an ideal gas at 250 C is allowed to expand reversibly at
constant temperature from a volume of 10 L to 20 L; calculate the workdone by the gas in joules.
5. A certain volume of dry air at N.T.P is expanded reversibly to three times
its volume
a) isothermally,b) adiabatically.
Calculate the final temperature in each case, assuming ideal behaviour.γ =1.40
Second Law of thermodynamics
Since the first law of thermodynamics states that energy is conserved. There
are, however, many processes we can imagine that conserve energy but are not
observed to occur in nature. Lets consider an example below of the first law tointroduce the second law.
For example, when a hot object is placed in contact with a cold object, heat
flows from the hotter one to the colder one, never spontaneously the reverse.
If heat were to leave the colder object and pass to the hotter one, energy couldstill be conserved. Yet it doesn’t happen spontaneously the reverse.
There are many other examples of processes that occur in nature but whose
reverse does not. To explain this lack of reversibility, scientists in the latter
half of the nineteenth century formulated a new principle known as the secondlaw of thermodynamics.
The second law of thermodynamics is a statement about which processes
occur in nature and which do not. It can be stated in a variety of ways, all of
which are equivalent. One statement is that: “Heat can flow spontaneously
from a hot object to cold object; heat will not flow spontaneously from acold object to a hot object.”
The development of a general statement of the second law of thermodynamics
was based partly on the study of heat engines. A heat engine is any device
that changes thermal energy into mechanical work, such as steam engines andautomobile engines.
Applications of the second law of thermodynamics
Heat engines
Activity 15
* Have you ever heard of an engine?
* Where exactly do we find engines?
* What do you think an engine is?
* How do you think the engine operates?
Any device that transforms heat into work or mechanical energy is called
heat engine. All heat engines absorb heat from a source at high temperature,perform some mechanical work, and discard heat at a lower temperature.
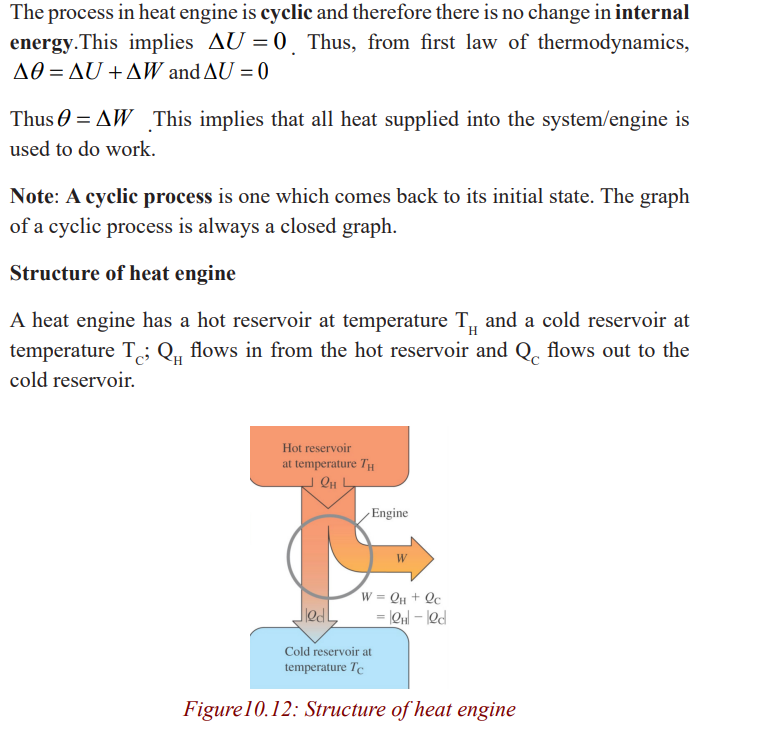
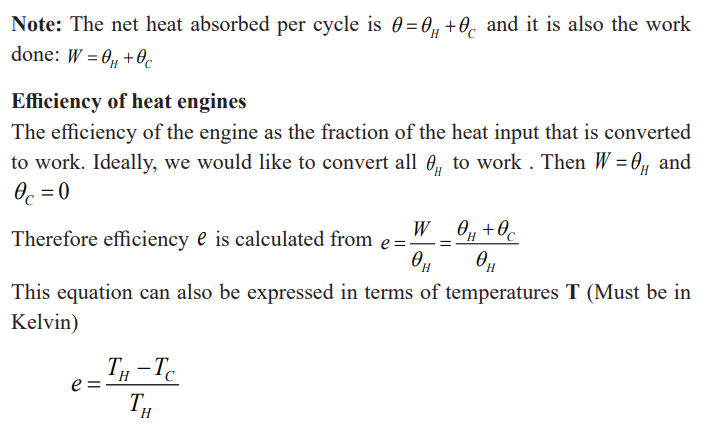
Impact of heat engines on climate
Most of air pollution is caused by the burning of fuels such as oil, natural gas
etc. The air pollution has an adverse effect on the climate. Climate change is
the greatest environmental threat of our time endangering our health. When
a heat engine is running, several different types of gases and particles areemitted that can have detrimental effects on the environment.
Of concern to the environment are carbon dioxide, a greenhouse gas; and
hydrocarbons. Engines emit greenhouse gases, such as carbon dioxide, which
contribute to global warming. Fuels used in heat engines contain carbon. Thecarbon burns in air to form carbon dioxide.
The Carbon dioxide and other global warming pollutants collect in the
atmosphere and act like a thickening blanket and destroy the ozone layer.
Therefore, the sun’s heat from the sun is received direct on the earth surfaceand causes the planet to warm up.
As a result of global warming, the vegetation is destroyed, ice melts and water
tables are reduced. Heat engines especially diesel engines produce Soot whichcontributes to global warming and its influence on climate.
The findings show that soot, also called black carbon, has a warming effect.
It contains black carbon particles which affect atmospheric temperatures in a
variety of ways. The dark particles absorb incoming and scattered heat from
the sun; they can promote the formation of clouds that can have either cooling
or warming impact. Therefore soot emissions have significant impact onclimate change.
Similarly, some engines leak, for example, old car engines and oil spills all
over. When it rains, this oil is transported by rain water to lakes and rivers.
The oils then create a layer on top of the water and prevent free evaporationof the water.
Carnot cycle and Carnot engine
In 1824 a French engineer named Sadi Carnot described a theoretical engine,
now called a Carnot engine, which is of great importance from both practical
and theoretical viewpoints. He showed that a heat engine operating in an ideal,
reversible cycle—called a Carnot cycle—between two energy reservoirs isthe most efficient engine possible.
An ideal engine establishes an upper limit on the efficiencies of all other
engines. That is, the net work done by a working substance taken through the
Carnot cycle is the greatest amount of work possible for a given amount ofenergy supplied to the substance at the higher temperature.
Carnot’s theorem can be stated that no real heat engine operating between
two energy reservoirs can be more efficient than a Carnot engine operatingbetween the same two reservoirs.
Note: No Carnot engine actually exists, but as a theoretical idea it played animportant role in the development of thermodynamics.
The idealized Carnot engine consisted of four processes done in a cycle, two
of which are adiabatic (Q = 0) and two are isothermal (ΔT = 0). This idealizedcycle is shown in figure 1.8.
Each of the processes was considered to be done reversibly. That is, each of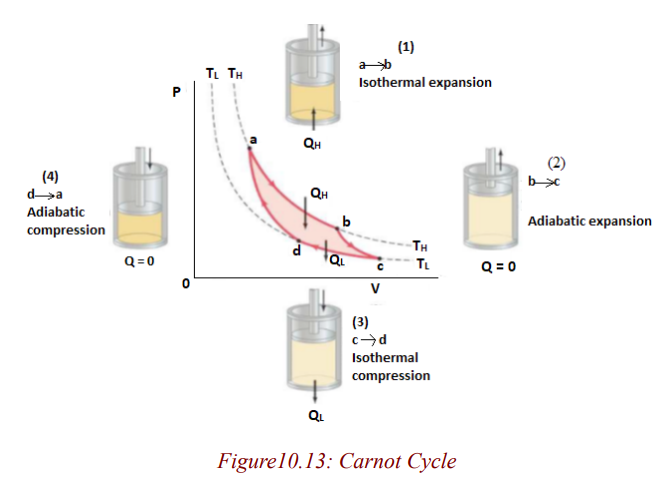
the processes (say, during expansion of the gases against a piston) was done so
slowly that the process could be considered a series of equilibrium states, and
the whole process could be done in reverse with no change in the magnitudeof work done or heat exchanged.
A real process, on the other hand, would occur more quickly; there would
be turbulence in the gas, friction would be present, and so on. Because of
these factors, a real process cannot be done precisely in reverse, the turbulence
would be different, and the heat lost to friction would not reverse itself. Thus
real processes are irreversible.
In the Carnot cycle, heat engines work in a cycle, and the cycle for the Carnotengine begins at point a on the PV diagram.
Note:
• The gas is first expanded isothermally, with addition of heat QH,along the path ab at temperature TH.
The equation above gives a Carnot (ideal) efficiency. It expresses the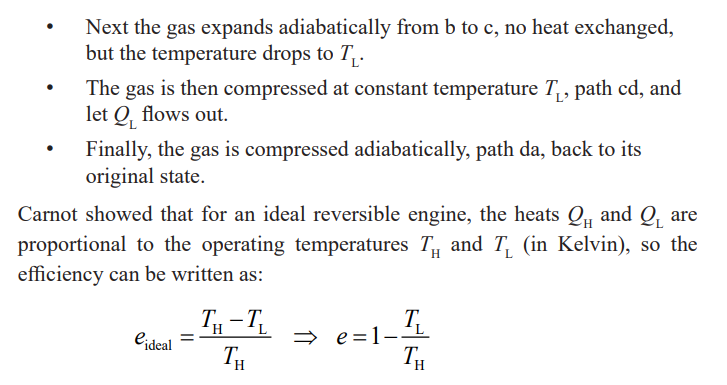
fundamental upper limit to the efficiency. Real engines always have an
efficiency lower than this because of losses due to friction. Real engines
that are well designed reach 60 to 80% of the Carnot efficiency.Otto Cycle and Diesel Cycle
Otto Cycle
An Otto cycle is an idealized thermodynamic cycle which describes the
functioning of a typical spark ignition reciprocating piston engine, thethermodynamic cycle most commonly found in automobile engine.
The Pressure Volume diagram above represents the Otto cycle which has the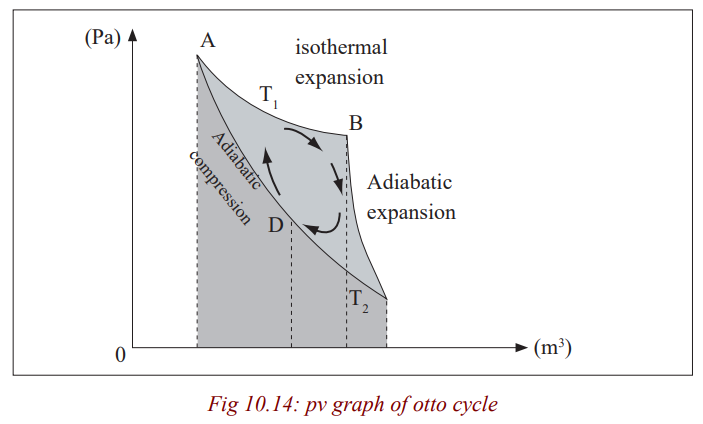
following strokes; the intake (A) stroke is performed by an isobaric expansion,
followed by an adiabatic compression (B) stroke (along 1-2). Through the
combustion of fuel, heat is added in an isovolumetric process (2-3), followed
by an adiabatic expansion process ( 3-4), characterising the power (C) stroke.
The cycle is closed by the exhaust (D) stroke, characterized by isovolumetriccooling and isobaric compression processes.
The processes are described by:
Process 1-2 is an isentropic compression of the air as the piston moves frombottom dead centre (BDC) to top dead centre (TDC).
Process 2-3 is a constant –volume heat transfer to the air from an external
source while the piston is at top dead centre. This process is
intended to represent the ignition of the fuel –air mixture andthe subsequent rapid burning.
Process 3-4 is an isentropic expansion (power stroke).
Process 4-1 completes the cycle by a constant-volume process in which heat isrejected from the air while the piston is a bottom dead centre.
The Otto cycle consists of adiabatic compression, heat addition at constant
volume, adiabatic expansion, and rejection of heat at constant volume. In the
case of a four-stroke Otto cycle, technically there are two additional processes;
one for the exhaust of waste heat and combustion products (by isobaric
compression), and one for the intake of cool oxygen –rich) air (by isobaric
expansion); however, these are often omitted in a simplified analysis. Even
though these two processes are critical to the functioning of a real engine,
wherein the details of heat transfer and combustion chemistry are relevant,
for the simplified analysis of the thermodynamic cycle, it is simpler and more
convenient to assume that all of the waste-heat is removed during a singlevolume change.
Diesel Cycle
The diesel cycle is the thermodynamic cycle, which approximates the pressure
and volume of the combustion chamber of the Diesel engine, invented by
Rudolph Diesel in 1897. It is assumed to have constant pressure during the
first part of the “combustion” phase V2 to V2
in the diagram, below). This is an idealised mathematical model;
real physical diesels do have an increase in
pressure during this period, but it is less pronounced than in the Otto cycle.
The idealized Otto cycle of a gasoline engine approximates constant volumeduring that phase, generating more of a spike in a P-V diagram.
The Idealised Diesel Cycle
From P-V diagram for the Ideal Diesel cycle, the cycle follows the numbers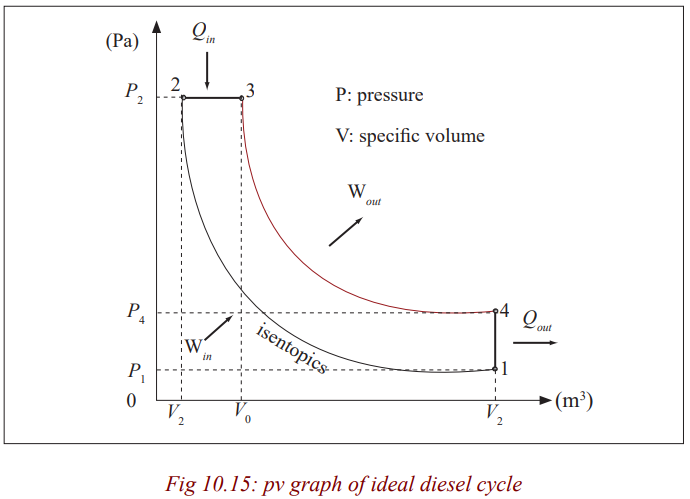
1-4 in clockwise direction.
The image on the top shows a P-V diagram for the ideal Diesel cycle; where
P is pressure and V is specific volume. The ideal Diesel cycle follows the
following four distinct processes (the colour references refers to the colour ofthe line on the diagram):
Process 1-2 is isentropic (adiabatic) compression of the fluid (blue colour).
Process 2-3 is reversible (isobaric constant pressure heating (red).
Process 3-4 is isentropic (adiabatic) expansion (yellow).
Process 4-1 is reversible constant volume cooling (green).
The Diesel is a heat engine; it converts heat into work. The isentropic processes
are impermeable to heat; heat flows into the loop through the left expanding
isobaric process and some of it flows back out through the right depressurisingprocess, and the heat that remains does the work.
Work in (Win) is done by the piston compressing the working fluid.
Heat in (Qin) is done by the combustion of the fuel.
Work out (Wout) is done by the working fluid expanding on to the piston (thisproduces usable torque).
A heat engine is a machine, which changes heat energy, obtained by burning a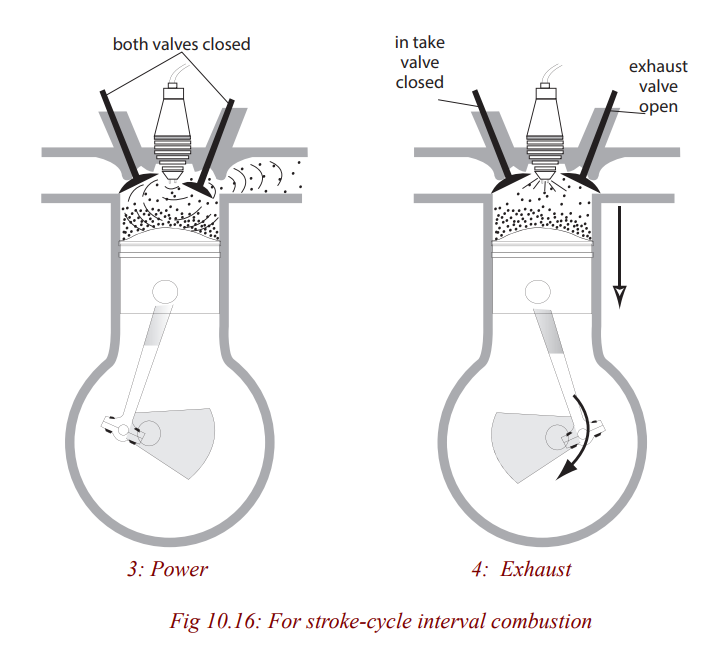
fuel, to kinetic energy. In an internal combustion engine, e.g petrol, diesel, jet
engine, the fuel is burnt in the cylinder of chamber where the energy changeoccurs. This is not so in other engines e.g steam turbine.
Petrol engine
Activity 16
(i) How many types of fuels do vehicles use to operate?
(ii) Have you ever heard of vehicles which use petrol in order to operate?
(iii) List down four vehicles which use petrol.
(iv) What type of engine do they have?
Many vehicles use petrol in order to move. Such vehicles are small cars and
motorcycles. The engine they have is called a petrol engine since it uses petrol
to operate. It operates by moving the piston. The upward and downwardmovement of the piston is called a stroke.
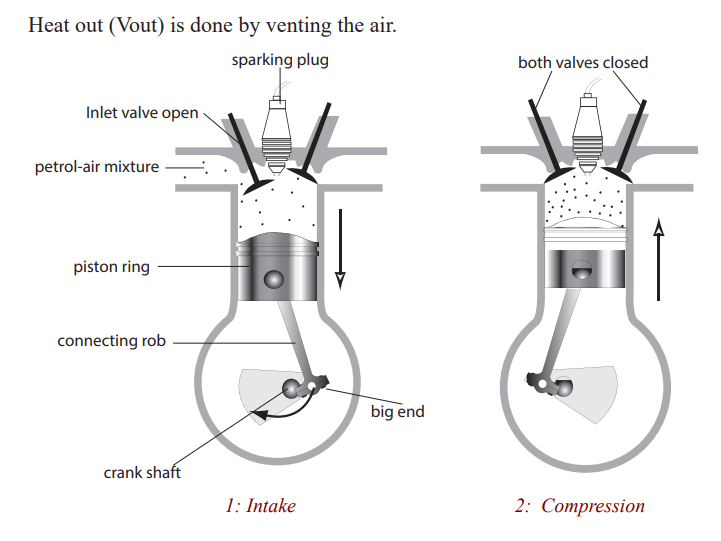
a) Four – stroke engine: On the intake stroke, the piston moves
down (due to the starter motor in a car or the kick start in a
motor cycle turning the crankshaft) so reducing the pressure
inside the cylinder. The inlet value opens and the petrol – air
mixture from the carburetor is forced into the cylinder byatmospheric pressure.
On the compression stroke, both valves are closed and the pistonmoves up, compressing the mixture.
On the power stroke, a spark jumps across the points of thesparking plug and explodes the mixture, forcing the piston down.
On the exhaust stroke, the outlet valve opens and the piston rises,pushing the exhaust gases out of the cylinder.
The crankshaft turns a flywheel (a heavy wheel) whosemomentum keeps the piston moving between power strokes.
Most cars have atleast four cylinders on the same crankshaft.
Each cylinder fires in turn in the order 1-3-4-2, giving a powerstroke every half revolution of the crankshaft. Smoother running results.
b) Two-stroke engine: This is used in mopeds, lawnmovers and
small boats. Valves are replaced by ports on the side of thecylinder which are opened and closed by the piston as it moves.
Diesel engine
Activity 17
(i) Have you ever heard of vehicles which use diesel in order to move?
(ii) What kind of vehicles are they?
(iii) What is the name of the engine in such vehicles?
The engine which uses diesel is called a diesel engine. A diesel engine canoperate by making two or more strokes.
The operation of two and four stroke diesel engines is similar to that of the
petrol varieties. However, fuel oils is used instead of petrol, there is nosparking plug and the carburetor is replaced by a fuel injector.
Air is drawn into the cylinder on the down stroke of the piston and on the
upstroke it is compressed to about one-sixteenth of its original volume (which
is twice the compression in a petrol engine). This very high compression
increases the temperature of the air considerably and when, at the end of the
compression stroke, fuel is pumped into the cylinder by the fuel injector, it
ignites automatically. The resulting explosion drives the piston down on its
power stroke. (You may have noticed that the air in a bicycle pump gets hotwhen it is squeezed. The same applies here.)
Activity: 18
State the advantages of a diesel engine over a petrol engine.
Diesel engines, sometimes called compression ignition (C.I) engines, though
heavier than petrol engines, are reliable and economical. Their efficiency of
about 40% is higher than that of any other heat engine. A disadvantage of the
diesel engine is that its higher compression ratio means that it needs to bemore robust, and is therefore more massive.
The Refrigerator
Activity: 19
* How many of you have seen a
refrigerator?
* With the help of a teacher visit any
place where there is a refrigeration
and observe it carefully.
* How useful is it to our daily lives?
* Who can describe how it works?
* Write your suggestions in thenotebook.
A refrigerator is a device used to cool substances. It cools things by evaporation
of a volatile liquid called Freon. The coiled pipe around the freezer at the top
contains Freon which evaporates and takes latent heat from the surroundings
so causing cooling. The electrically driven pump removes the vapor and
forces it into the heat exchanger (pipes with cooling fins outside the rear ofthe refrigerator).
Here the vapor is compressed and liquefies (condenses) giving out latent heat
of vaporization to the surrounding air. The liquid returns to the coils around
the freezer and the cycle is repeated. An adjustable thermostat switches the
pump on and off, controlling the rate of evaporation and so the temperature ofthe refrigerator.
The operating principle of refrigerators is just the reverse of a heat engine. Eachoperates to transfer heat out of a cool environment into warm environment.
By doing work W, heat is taken from a low-temperature region, QL (such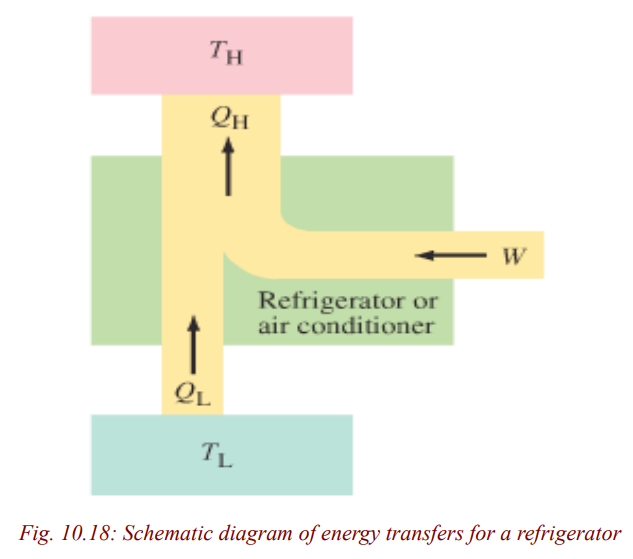
as inside a refrigerator), and a greater amount of heat is exhausted at a high
temperature, QH (the room). You can often feel this heat blowing out beneatha refrigerator.
A perfect refrigerator is the one in which no work is required to take heat from
the low-temperature region to the high temperature region is not possible.
This is Clausius statement of the second law of thermodynamics, alreadymentioned can be stated formally as:
“No device is possible whose sole effect is to transfer heat from one systemat a temperature TL into a second system at a higher temperature TH”.
To make heat flow from a low-temperature object (or system) to one at a highertemperature, work must be done. Thus, there can be no perfect refrigerator.
The coefficient of performance (COP) of a refrigerator is defined as the heat
QL removed from the low-temperature area (inside the generator) divided bythe work W done to remove the heat:
This makes sense since the more heat, QL, that can be removed from inside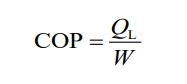
the refrigerator for a given amount of work, the better (more efficient) the
refrigerator is. Energy is conserved, so from the first law of thermodynamicswe can write
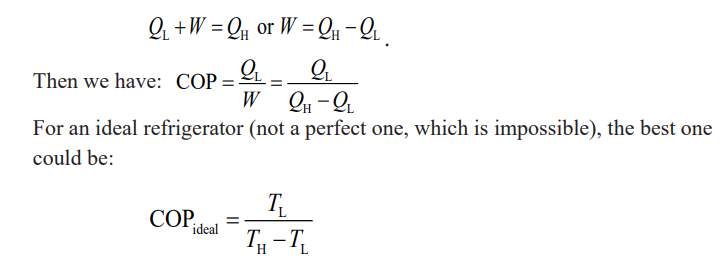
Example
An ideal refrigerator-freezer operates with a COP = 7.0 in a 24 oCroom. What is the temperature inside the freezer?
Solution

END UNIT ASSESSMENT
1. What is the heat capacity at constant volume considered to be more
important than at constant pressure?
2. Define: isothermal; isobaric; isovolumic and adiabatic processes
3. What is the relationship between the specific heat (or het capacities)
at constant pressure and at constant volume?
4. One mole of helium gas, initially at STP ( p1 latm kpa = 1.03
T1 =00c = 273.15 k) undergoes an isovolumetric process inwhich its pressure falls to half its initial value.
a) What is the work done by the gas?
b) What is the final temperature of the gas?
c) The helium gas then expands isobaric ally to twice its volume,what is the work done by the gas?
5. Find out the internal energy of a system which has constant volumeand the heat around the system is increased by 50 J?
6. In a certain process 8.0kcal of heat is furnished to the system while
the system does 6.00 KJ of work. By how much does the internalenergy of the system change during the process?
7. The specific heat of water is 4184J/kg.k. By how many joules does
the internal energy of 50g of water changes as it is heated from 210 c
to 370 c.