UNIT 7: CARBOHYDRATES AND LIPIDS
UNIT 7: CARBOHYDRATES AND LIPIDS
Key Unit Competences
Explain the important roles of carbohydrates and lipids in the provision and storage
of energy and for a variety of other functions.
Learning objectives
By the end of this unit, I should be able to:– State the roles of carbohydrates and lipids.Introductory activity
– Recall the elements that make up carbohydrates and lipids.
– Explain the proportion of hydrogen in carbohydrates and lipids and relate this
to the amount of energy released when oxidized.
– Define the terms monomer, polymer, macromolecule, monosaccharide,
disaccharide and polysaccharide.
– Describe the ring forms of α-glucose and β-glucose structure.
– Explain the formation of glycosidic bonds.
– Describe the structure of phospholipids and relate to their functions in living
organisms.
– Describe the molecular structure and formation of triglycerides and
phospholipids, and give their functions in living organisms.
– Demonstrate that phospholipids have a hydrophilic head and hydrophobic
tails using a heterogeneous mixture made up of water and cooking oil.
– Interpret the charts and illustrations of molecular structure and the formation
of maltose and triglycerides.
– Demonstrate through a process of combustion that sugars and lipids are
biological fuel
– Differentiate between starch and cellulose.
– Appreciate the importance of carbohydrates and lipids in organisms.
– Be aware of the other roles of lipids in the formation of soap and withcarbohydrates and syrups in medicine
1. In the previous unit (test for biological molecules), we tested carbohydrates,
starch, reducing sugar, lipids, proteins, and vitamins. Where do you classify
monosaccharide, disaccharides and polysaccharides in the above tested
biochemical compounds?
2. Sometimes people say that fatty persons do not feel cold. What could be
the reasons?
7.1. Classes of monomers
Activity 7.1
Visit the internet and conduct a research the following:
1. The description of the term monomer
2. Where can we find monomers?
3. The biological importance of monomers?
A monomer is a molecule that can combine with others of the same kind to form
a polymer. A polymer is a large molecule or macromolecule composed of many
repeated sub-units (monomers). Because of their broad range of properties, both
synthetic and natural polymers play essential and ubiquitous roles in everyday life.
Polymers make up many of the materials in living organisms including proteins,
cellulose, and nucleic acids. Glucose molecules for example, are monomers that
combine to form the polymer cellulose. The examples of monomers are summarized
in the table 7.1.
Table 7.1: Biological molecules and their monomers
Carbohydrates comprise a large group of organic compounds which contain
carbon, hydrogen and oxygen. The word carbohydrate suggests that these organic
compounds are hydrates of carbon. Their general formula is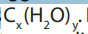 In carbohydrates
In carbohydrates
the ration hydrogen-oxygen is usually 2:1. Carbohydrates are divided into three
groups including the monosaccharide (single sugars), disaccharides (double
sugars) and polysaccharides (many sugars). The most common monosaccharide of
carbohydrates is glucose with molecular formula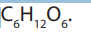
Self-assessment 7.1
1. What are some examples of polymers and monomers?
2. How are monomers, polymers and macromolecules related?
7.2. Ring form of α-glucose and β-glucose
Monosaccharides are group of sweet and soluble
Activity7.2
1. Based on the knowledge acquired during the lesson of monomers and further
information from books and internet:a. What are the examples of monosaccharide?crystalline molecules of relatively low molecular mass. They are named with the
b. Give the molecular formula of each of the monosaccharide stated above
c. Use the books to illustrate the structural formula of each of themonosaccharide stated above
suffix –ose. The general formula of a monosaccharide is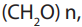 , with n the number
, with n the number
of carbon atoms. The simplest monosaccharide has n=3 and it is a triose sugar.
When n = 5, this is a pentose sugar, and when n = 6, this is a hexose sugar. The two
common pentose sugars are ribose and deoxyribose, while the most known hexose
is glucose. Its molecular formula is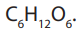 . It is the most important simple sugar in
. It is the most important simple sugar in
human metabolism called simple sugar or monosaccharide because it is one of the
smallest units which has the characteristics of this class of carbohydrates.
Monosaccharides can exist as isomers. The isomer is defined as each of two or more
compounds with the same formula but a different arrangement of atoms in the
molecule and different properties. For example, glucose, fructose and galactose
share the same molecular formula which is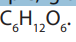 . However, they differ by their
. However, they differ by their
structural formulae as follow: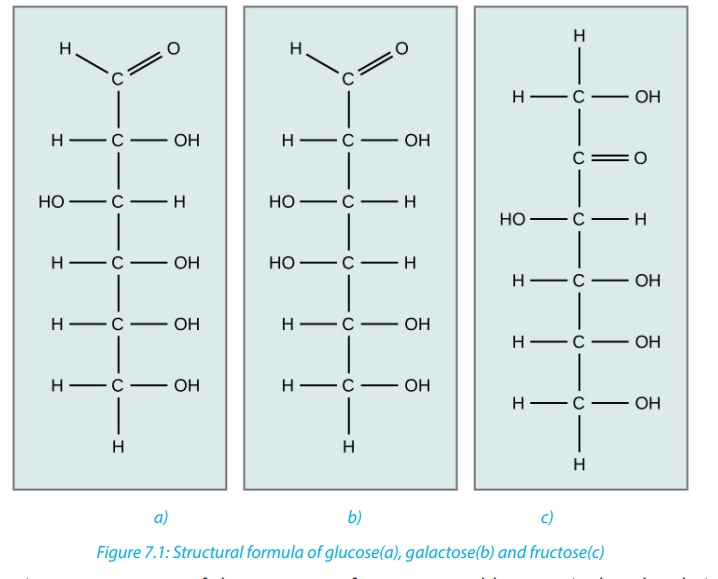
One important aspect of the structure of pentoses and hexoses is that the chain
of carbon atoms is long enough to close up on itself and form a more stable ring
structure. This can be illustrated using glucose as an example. When glucose forms
a ring, carbon atom number 1 joins to the oxygen on carbon atom number 5 (Figure
7.2)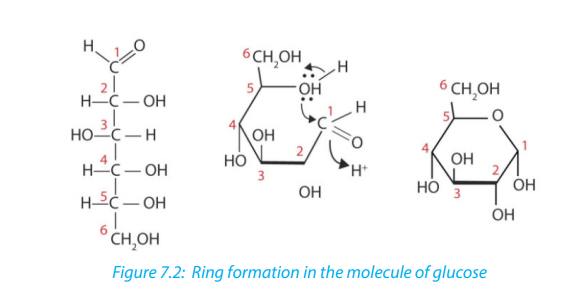
All hexoses sugars can exist as straight-chain structures but they tend to form ringstructures. Glucose, fructose, galactose can exist in ring structures (Figure 7.3).
Ring monosaccharides are said to be alpha (α) if the -OH group located on carbon 1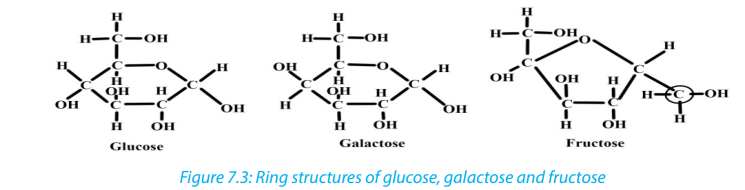
is below the ring and beta (β) when the -OH group is above the ring. The molecule
of glucose for example can exist as alpha and beta glucose denoted by α-glucoseand β-glucose (Figure 7.4)
Self-assessment 7.2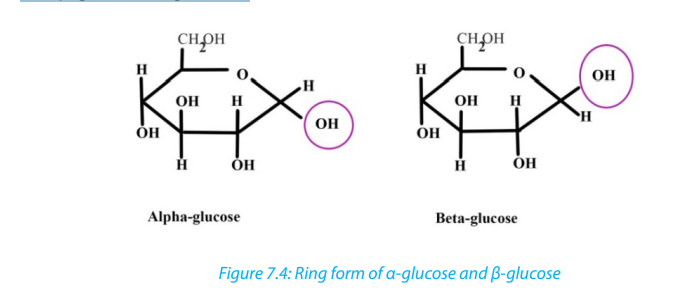
1. How do we call the monosaccharide with 3, 5 and 6 carbon atoms?
2. Differentiate between α and β glucose3. What are the properties of glucose?
7.3. Formation and breakdown of glycosidic bonds
Activity 7.3
1. Monomers are joined to form polymers, use a point as a monomer to
illustrate how a polymer can be formed
2. How do you call joining structures between atoms?
3. Use books or other sources to show how monosaccharide form adisaccharide.
7.3.1. Monosaccharides
Monosaccharides may combine together in pairs to give a disaccharide (double sugar).
The union involves the loss of a single molecule of water and is therefore a
condensation reaction. The bond which is formed is called a glycosidic bond. It is
usually formed between carbon atom1of one monosaccharide and carbon atom 4
of the other, hence it is called a -1, 4- glycosidic bond. Any two monosaccharides
may be linked together to form a disaccharide of which maltose, sucrose and lactoseare the most common.
The addition of water under suitable conditions is necessary if the disaccharide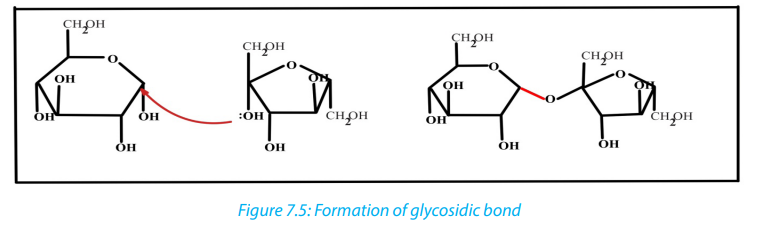
is to be split into its constituent monosaccharide. This is called hydrolysis \
water breakdown or more accurately, breakdown by water.
7.3.2. Disaccharides
These are carbohydrates made of two monosaccharides. They include maltose
(glucose + glucose), sucrose or saccharose (glucose +fructose), and lactose
(glucose+ galactose). The maltose is the sugar from the germinating seeds, sucrose
or saccharose is the common table sugar obtained from sugarcane, while lactose isthe sugar from the milk. In addition, sucrose is a non-reducing sugar.
Table 7.2: Types of disaccharides and their monomers
In maltose ring, the two ring of glucose are bonded by the -1, 4-glycosidic bond
while in sucrose the glucose and fructose are bonded by -1, 2-glycosidic bond.
All the disaccharides are non-reducing sugar, except maltose which behaves in
the same as a reducing sugar with benedict’s solution. All monosaccharides and
disaccharides have the following characteristics: sweet taste, soluble in water andlower molecular mass.
Self-assessment 7.3
1. Write the molecular structure of sucrose
2. How is the glycosidic link is formed
3. Sucrose is formed when two monosaccharide are assembled together:
a. Name those two monosaccharides.
b. Using the ring form of these monosaccharide named above to explain andshow sucrose formation?
7.4. Polysaccharides: starch, glycogen and cellulose
Activity 7.4
1. Based on the meaning of monosaccharide, what is a polysaccharide?
2. Classify the following compound into polysaccharide, monosaccharide and
disaccharidea. Glucose, fructose and galactose3. Use glucose to form any polysaccharide of your choice
b. Lactose, sucrose, and maltose
c. Starch, cellulose and glycogen
In the same way that two monosaccharides may combine in pairs to give a
disaccharide, many monosaccharides may combine by condensation reactions to
form a polysaccharide. The number of monosaccharides that combine is variable
and the chain produced may be branched or unbranched. Polysaccharide are manybut the most known are starch, glycogen and cellulose.
a. Starch
Starch is made up of two components: amylose and amylopectin. Amylose is a linear
unbranched polymer of 200 to 1500 α-glucose units in a repeated sequence of α-1,
4-glucosidic bonds. The amylose chain coils into helix held by hydrogen bonds
formed between hydroxyl groups. A more compact shape is formed. The amylose
helices are entangled in the branches of amylopectin to form a complex compact
three dimensional starch molecule.
Amylopectin is a branched polymer of 200 to 200,000 α-glucose units per starch
molecule. The linear chains of α-glucose units are held together by α-1, 4-glucosidic
bonds. Branches occur at intervals of approximately 25 to 30 where α-1, 6-glucosidic
bonds occur. Starch grains are found in chloroplast, potato tubers, cereals and
legumes. Starch is insoluble in cold water. It is digested by salivary amylase and
pancreatic amylase into maltose and the latter is hydrolyzed by maltase enzyme to
form glucose. Therefore, diabetic people should avoid tubers since they are rich instarch which in turn gives glucose (Figure 7.8).
b. Glycogen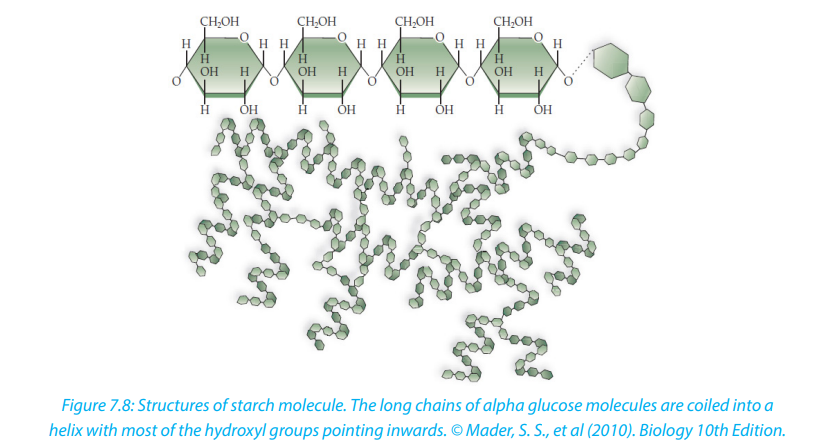
Glycogen is often called animal starch because it is a major polysaccharide storage
material in animals and fungi. The brain and other tissues require constant supply of
blood glucose for survival. Some tissues particularly the liver and skeletal muscles
store glycogen in the form that can be rapidly mobilized to form glucose. Like starch,
glycogen is made up of α-glucose and exists as granules. It is similar to amylopectin instructure but it has shorter chains (10-20 glucose unit) and is more highly branched.
c. Cellulose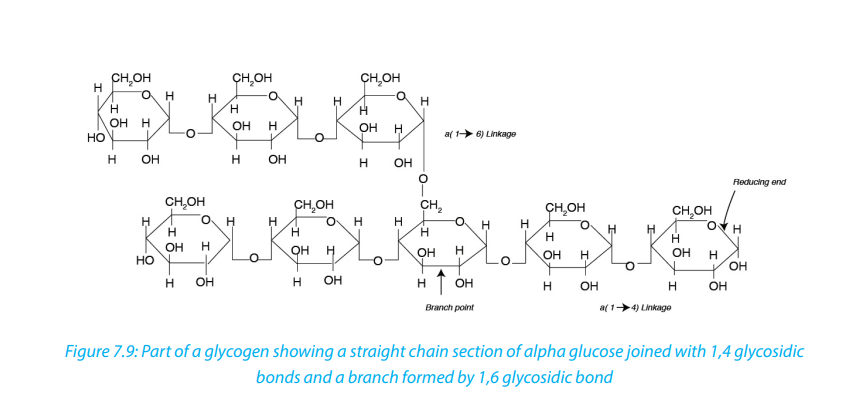
Cellulose is the structural polysaccharide in plant cell wall. It is found in vegetables
and fruits but it cannot be hydrolyzed by enzymes in the human digestive system.
Cellulose is composed of long unbranched chains of up to 10,000 β-glucose units
linked by β-1,4-glucosidic bonds. Each β-glucose unit is related to the next by a
rotation of 180 ͦ C with OH groups projecting outwards on either side of the chain.
Cellulose chains run parallel to one another. Unlike amylopectin and glycogen
molecules, there are no side chains (no branch) in the cellulose. This allows the linear
chains to lie close together. Many H-bonds are formed between the OH groups of
adjacent chains. The chains group together to form microfibrils arranged in larger
bundles of macrofibrils. The fibrils give the plant cell their high tensile strength andrigidity. The layers of fibrils are permeable to water and solutes.
Cellulose is formed from ß - glucose units linked by 1,4 glycosidic bonds. The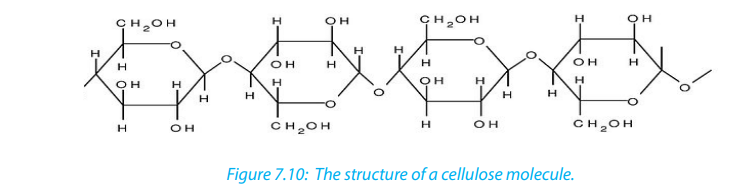
hydroxyl groups alternate on either side of the molecule forming straight chains
giving cellulose a fibrous structure. Cellulose are strengthened further by hydrogen
bonds that link adjacent chains.
d. Chitin
Chitin is one of naturally occurring Polymers. It forms a structural component of
many animals such as exoskeleton in arthropods. Chitin is a polymer of glucose
although in its structure a molecule of amino acid is added to each glucose. Thedigestion of chitin yields simple sugars and ammonia.
Self-Assessment 7.4
1. What type of reaction is involved in the formation of glucose from starch?
2. Use the type of reaction above to form glucose from sucrose molecule3. What are the 2 main components of starch? Give the difference between them
7.5. Lipids
Activity 7.5
Use textbooks and/or internet to
1. List the monomers that are present in lipids
2. Locate where can we find lipids?
3. Discuss the reasons why animals like pig do not like hot weather.
Lipids are a broad group of naturally occurring molecules which include fats,
waxes, sterols, fat soluble vitamins (such as vitamins A, D, E and K), monoglycerides,
diglycerides, Phospholipids and others. Lipids are grouped into fats which are solid
at room temperature and oils which are liquid at room temperature. Lipids are
made by carbon, hydrogen and oxygen, but the amount of oxygen in lipids is much
smaller than in carbohydrates. Lipids are made by two components namely glycerol
and fatty acids. The chemical formula for glycerol is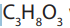 with structural formula
with structural formulaas shown in the figure 7.11
In all lipids glycerol do not show any variation while fatty acids vary. Therefore, the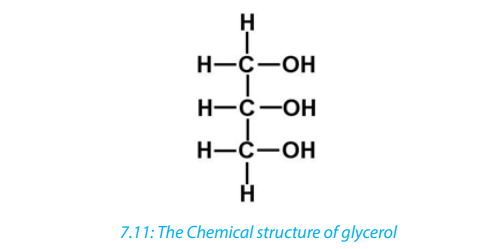
nature of lipid depends on the fatty acid it contains. There are two types of fatty
acids: unsaturated fatty acid characterized by the chain of hydrocarbon containing
one or more double and triple bonds; and saturated fatty acid characterized by the
chain of hydrocarbon without any double or triple bond.
Lipids are formed when glycerol combines with one, two, or three fatty acids to form
monoglyceride, diglyceride or Triglyceride. A bond is formed between the carboxyl
(-COOH) group of a fatty acid and one of the hydroxyl (-OH) groups of the glycerol.
This is a condensation process and water is lost. The resulting bond is known as ester
link, and the type of reaction is called esterification.
Lipids are of different types as it is summarized in the following table (Table 7.3)
Table 7.3: Types of lipids, their structure, main role and features
a. Waxes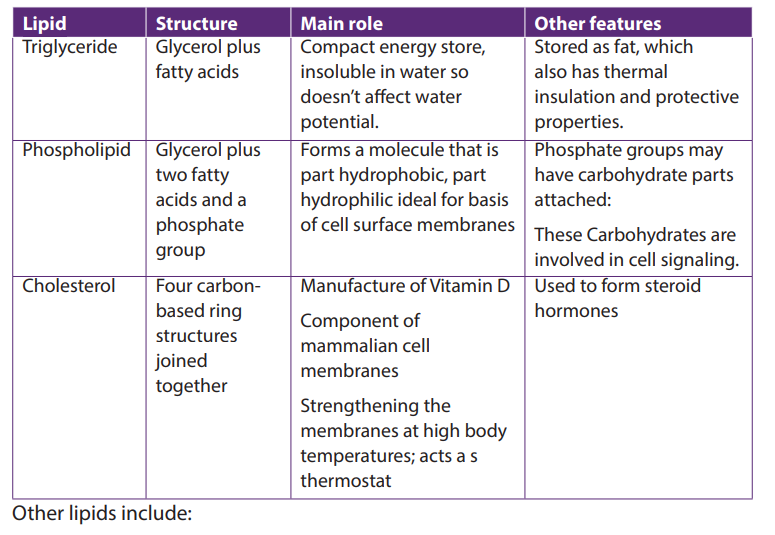
Waxes are similar to triglycerides but contain fatty acids bonded to long chain
alcohol rather than to glycerol. Waxes form the cuticle that protects the leaves andsurfaces of insects against the loss of water
b. Steroids
A steroid is an organic compound with four rings of carbon and hydrogen atoms
with various side chains. Steroids have several functions. It is a component of mostanimal hormones like estrogen, testosterone.
General functions of lipids
Lipids perform a number of functions within living organism:– Lipids are source of energy: due to the presence of C-H bond, lipids canSelf-assessment 7.5
generate more ATP compared to the carbohydrates of the same mass
– Lipids are storage of energy in adipose cells forming adipose tissue in fat of
organism
– Lipids act as insulators of the organism. For example, they reduce heat loss.
Lipids also are electrical insulators around the nerve cells, the Myelin sheath
– Lipids have a role of protection, in the cuticle of plant leaves against drying, in
exposed organ like hand and knees
– Synthesis of hormones such as steroid hormones (most of sex hormones) are
made by lipids– Lipids are used in production of soap by saponification reaction
1. Name the small units found in lipids2. Differentiate between fats and oils
End of unit assessment 7
1. Write the formula of a monosaccharide with 3 atoms of carbon
2. Compare the structure of fat(triglycerides)and the phospholipids
3. Give two examples of how carbohydrates are used in the body.
4. The formula for a hexose is What would be the formula of?a. Triose5. What type of chemical reaction would be involved in the formation of glucose
What would be the formula of?a. Triose5. What type of chemical reaction would be involved in the formation of glucose
b. Pentose
from starch or glycogen?
6. Distinguish between:a. Alpha glucose and beta glucose
b. Glycogen and cellulosec. Amylopectin and amylose
