UNIT 6: TESTING FOR BIOLOGICAL MOLECULES
UNIT 6: TESTING FOR BIOLOGICAL MOLECULES
Key unit competence
Test for biological molecules in a variety of contexts, such as identifying the contentsof mixtures of molecules and to follow the activity of digestive enzymes
Learning objectives
By the end of this unit, I should be able to:– Write out procedures in the identification of biological moleculesIntroductory activity
– Explain the importance of the reagents used in the identification of biological
molecules.
– Carry out tests for the identification of biological molecules
– Compare reducing and non-reducing sugars
– Appreciate the importance of identification of food values in the food industry
and in processing and packaging.– Show resilience making observations on color changes during food tests
You are given solutions containing different food stuffs including maize flour,
vegetable cooking oil, and egg white sugar cane liquid and passion fruit. Using
prior knowledge of biological molecules to suggest the type of biological
molecule in each one of them. Suggest the chemical tests used to identify eachof the molecules.
6.1. Test for carbohydrates
Activity 6.1
Materials required:
Starch powder, Irish potatoes juice, prepared porridge, Iodine solution, beakers,
droppers, source of heat and test tubes
a. Test for starch
Procedure– Mix 1g of starch powder with 100ml of waterb. Test for reducing sugar
– Boil the mixture while stirring; then cool the solution
– Boil the mixture while stirring; then cool the solution
– Put 2ml of starch solution in a test tube labeled 1, 2ml of Irish potato juice in
a test tube labeled 2 and 2ml of prepared porridge in a test tube labeled 3
– In each test tube put 2 drops of Iodine solution and shake– Record your observation and draw a conclusion
Requirements
Glucose powder, beaker and test tube, Benedict solution, Bunsen burner, droppers
Procedures– In the beaker mix 1cm3 of water and 1g of glucose powder.Biological molecules are grouped into organic molecules including carbohydrates,
– Pour the prepared solution of glucose in a test tube and
– Add 2ml of benedict’s solution and heat– Record your observation.
proteins, lipids, nucleic acids and vitamins. They also contain inorganic molecules such
as minerals and water. The first four organic molecules are called macromolecules
because they are required in organism in large quantity. Carbohydrates including
starch, reducing and non-reducing sugars appear in this category and are the main
energy producers in the organisms. Others, including lipids and proteins are needed
for building organisms while vitamins protect the organisms against diseases. Weneed to ensure that what we take from diet have all required biological molecules.
a. Test for starch.
Carbohydrates such as starch are tested by mixing a sample with 2-4 drops of iodine
or Lugol’s solution. If the sample contains starch the solution will turn from a yellow-brown
color to a dark purple/dark blue (Figure 6.1). The color change is due to a
chemical reaction between the large carbohydrate molecule and the iodine ions. Ifthe sample does not contain starch the solution remains yellow-brown.
b. Testing for reducing and non-reducing sugar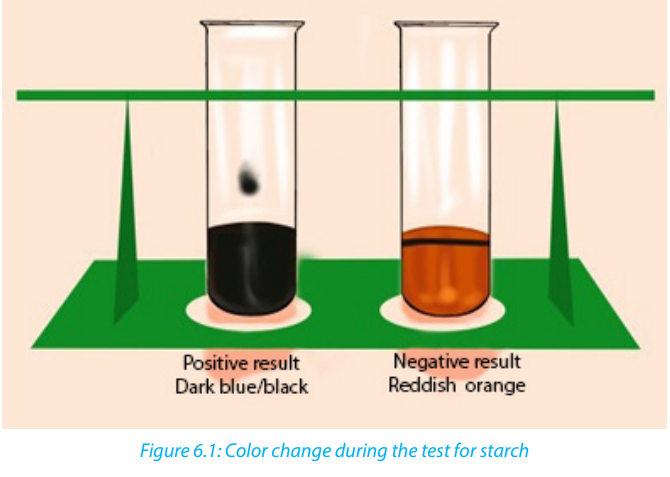
The presence of reducing sugar can be tested by using benedict reagent. Benedict
solution has copper ions that have a light blue color. When this solution is heated
in the presence of simple reducing sugars such as glucose, the blue color of copperions changes from a light green color to rusty orange-brown color (Figure 6.2).
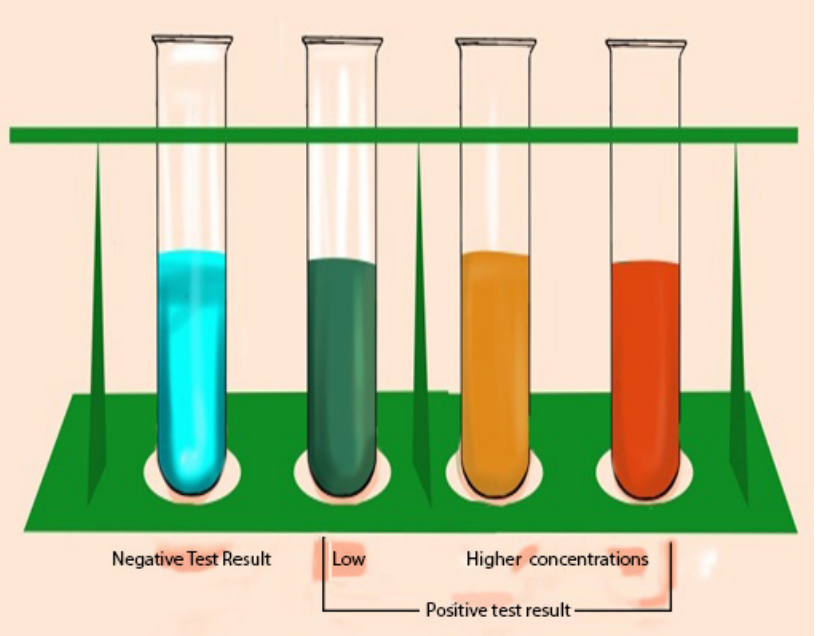
If the color of Benedict reagent persists, the sugar tested is not a reducing sugar.
Note that there is no special reagent to test for non-reducing sugar, but by the
addition of HCl, non-reducing sugars can be hydrolyzed to reducing sugars. To
test the presence of reducing sugars, a solution of sodium hydroxide is needed toneutralize the acidity because Benedict reagent works better in neutral solution
Self-assessment 6.1
A student prepared carbohydrate solution labeled C1. Perform the following
experiment to confirm whether C1 is starch, reducing sugar, or non-reducingsugar.
6.2. Test for proteins
Activity 6.2
Materials required
Milk, eggs, soybeans, test tubes, beakers, mortar for crushing beans, 1% NaOH or
1% KOH solution, 0.1M of CuSO4 solution and Millon’s reagent.
Procedure– Extract the white fluid from an eggThe Biuret reagent is used to test for the presence of proteins. It contains copper ions
– Prepare an extra of soya bean and 10ml of fresh milk
– Put 2ml of albumen solution in a test tube labelled A1 and 2ml in A2
– Put 2ml of milk solution in a test tube labelled B1 and 2ml in B2
– Put 2ml of soya bean solution in a test tube labelled C1 and 2ml in C2
– Put 1ml of KOH or NaOH solution in each of the test tubes A1, B1, and C1.
Shake the mixture and add 1ml of CuSO4 solution in each (A1, B1, and C1)
test tube
– Put 1ml of Millon’s reagent in each of test tubes (A2, B2, and C2). Shake themixture and thereafter boil the three test tubes (A2, B2, and C2).
with blue characteristic color. During the copper ions react with protein molecules
and causes the biuret solution to turn from a light blue color to purple if proteins arepresent.
The test can also be done by using Millon’s reagent, which in the presence of proteins,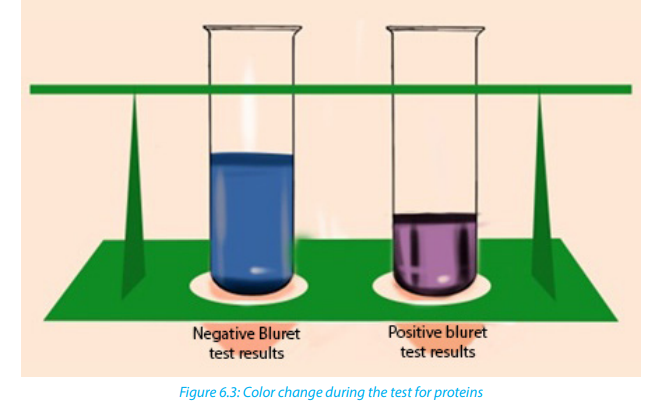
the Millon reagent changes from colorless to pink.
Self-assessment 6.2
1. You are provided with the sample of the substance M and A. Carry out thefollowing experiments and complete the table below.
2. Carry out the same experiment using the substance A and compare your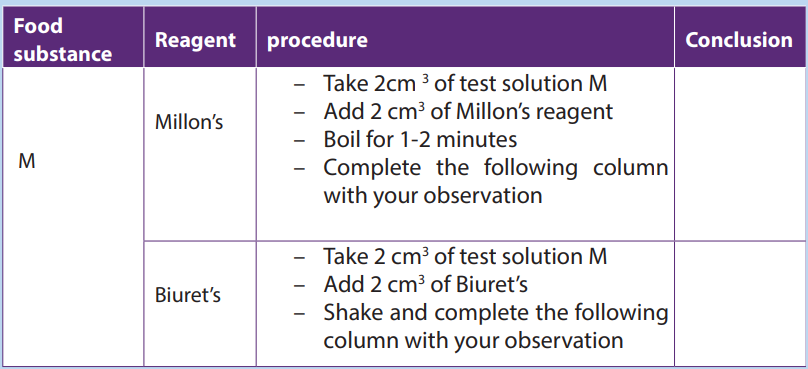
findings with M.3. Which of the substance A and M contain proteins?
6.3. Test for lipids
Activity 6.3
Materials required - Olive oil, test tubes, ethanol, water, Sudan III solution
Procedure:
Use olive oil to carry out the following experiments
Add 2 cm3
of olive oil in the test tube:– Add 5 cm3 of ethanol followed by 5 drops of water.The presence of lipids can be determined by using Sudan III indicators, which are fat-loving
– Shake the mixture and record your observation.
– To another test tube containing 2 cm3 of olive oil:
– add 5 drops of Sudan III solution
– Shake thoroughly and examine the mixture in the test tube after few minuteand record your observations
molecules that are colored. During the test for a solution containing lipids, two results are
likely to be found: there is either the separation of layers indicating the levels of water and
lipid, or the dye migrates toward one of the layers. If the mixtureis composed of water, the
conclusion is that the lipids are not present. In this case, the Sudan III indicator will form
small micelles/droplets and disperse throughout the solution. A positive result indicates
the lipid layers sitting on top of the water layer with a red-orange color. When using ethanolfor testing lipids the presence of the color changes from colorless to milky (emulsion test).
Self-assessment 6.3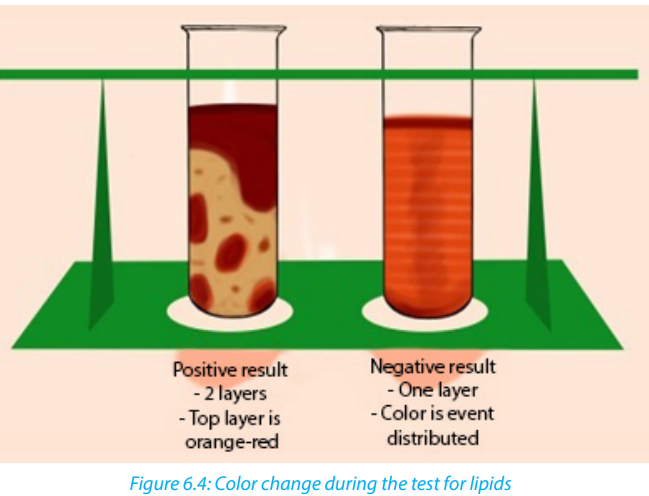
You are provided with a solution X. Use Sudan III indicator to test the presence oflipids in the solution X.
6.4. Test for vitamin C (Ascorbic Acid).
Activity 6.4Squeeze the orange fruits to extract the juice and carry out the following test.
Vitamin C is tested by using DCPIP (Dichlophenol Indophenol). Its positive (presence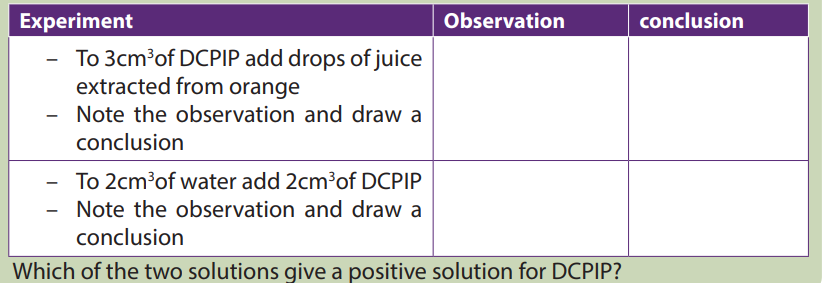
of vitamin C) test decolorizes DCPIP, while the negative (absence of vitamin C) test isindicated by the persistence blue color of DCPIP.
Self-assessment 6.4
In this experiment you are to press a tomato fruit (s) to get juice out of it. Use the
juice to carry out the test for vitamin C Draw a table of results that includes theprocedure, observation and conclusion.
End of unit assessment 6
1. Biological molecules are divided into:a. Organic molecules and inorganic molecules2. Name the reagents that are used to test for the following food substances
b. Carbohydrates and starch
c. Lipids, carbohydrates and water
d. Carbohydrates, food and potatoesa. Lipids3. You are provided with the following specimen:
b. Starch
c. Reducing sugarSpecimen A: Sorghum4. Some drops of fresh pineapple juice are added drop by drop to DCPIP solution.
Specimen B: Irish potatoes
Specimen C: Oranges
Specimen D: Sunflower seeds
a. Carry out chemical tests to determine the composition of the above seed to
tell whether they are composed of proteins, fats, starch or vitamin C.
b. Draw the table of used reagent, procedure and observation in (a)
The deep blue color of the DCPIP quickly fades.a. Explain why the blue colour disappeared?5. The result of food tests on unknown sample are shown below. Copy and
b. What is the importance of this food substance to the human body?
complete the table to show the conclusions which could be drawn from thesetests.
6. This is a practical question to be conducted using provided materials and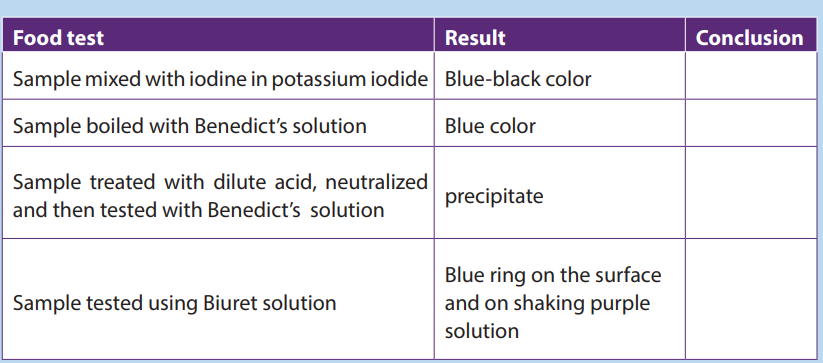
reagents to determine the food nutrients in each solution: You are provided with
the following solutions, A (sucrose 0.5%), B (1%starch), C (dilute hydrochloric
acid) and D (sodium hydroxide) and 6 test tubes labeled 1 to 6. Use the reagents
provided to determine the chemical nature of the substance present in thesolutions. Indicate your observations and conclusions in the table below:
a. Why was it necessary to boil solutions A and B with solution C in test (3)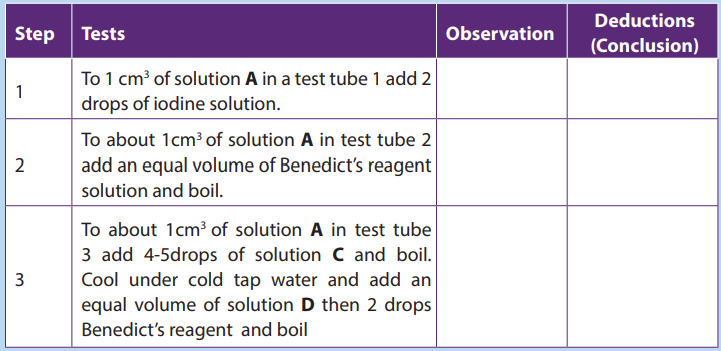
and (6)?b. Why was solution D added to test tubes 3 and 6?
