UNIT 20: BIOTECHNOLOGY AND ITS APPLICATION
UNIT 20: BIOTECHNOLOGY AND ITS APPLICATION
Key Unit Competence
Explain the biotechnology involved in the production of ethanol, biogas and bread
making.
Learning Objectives
By the end of this unit, I should be able to:– State that bacteria are useful in biotechnology and genetic engineering dueIntroductory activity:
to their rapid reproduction rate and their ability to make complex molecules.
– Discuss why bacteria are useful in biotechnology and genetic engineering.
Focus on: lack of ethical concerns over their manipulation and growth, genetic
code shared with all other organisms, and presence of plasmids.
– Show concern for the role of bacteria in genetic engineering.
– Investigate and describe the use of pectinase in fruit juice production and
lactase to produce lactose-free milk.
– Describe the role of anaerobic respiration in yeast during bread-making.
– Compare leavened and unleavened bread.
– Appreciate the role of anaerobic respiration in the production of ethanol and
in yeast during bread-making.
– Explain how fermenters are used in the production of penicillin.
– Describe the role of the fungus Penicillium in the production of the antibiotic
penicillin.
– Interpret and explain graphs showing how the pH and the concentration of
penicillin in a culture changes over time when the pH is controlled and not
controlled.
– Defend the role played by antibiotics in treatment of bacterial diseases.
– Describe the three stages of biogas production and the role of bioreactors in
economically poor rural communities
– Apply the knowledge of bioreactors, using cow dung, agricultural waste and
domestic waste to prepare and produce biogas.
– Appreciate the role of biogas production in reducing the environmentaldegradation.
Biotechnology is a broad discipline in which biological processes, organisms, cells
or cellular components are exploited to develop new technologies, remember
that biotechnology is useful and applied in our daily life activities such as in
beverages and food industries, agricultures, medicines.Brainstorm on the role
of microorganisms in biotechnology and genetic engineering.Can you think on
your own understanding on howbread, juice and beer are made? Why do bacteriabecome resistant to antibiotics? Make discuss on the biogas production.
20.1. Role of bacteria in Biotechnology and genetic
engineering
Activity 20.1
Using addition resources to your textbook available in your school such as the
books from the school library and search further information from the internet.
Discuss the role of bacteria in biotechnology and genetic engineering.
Biotechnology is a broad discipline in which biological processes, organisms, cells
or cellular components are exploited to develop new technologies. New tools
and products developed by biotechnologists are useful in research, agriculture,
industry and the clinic. For example, the use of living cells, bacteria, etc., to make
useful products (such as crops that insects are less likely to destroy or new kinds of
medicine).
The wide concept of “biotech” or “biotechnology” encompasses a wide range of
procedures for modifying living organisms according to human purposes, going
back to domestication of animals, cultivation of the plants, and “improvements” to
these through breeding programs that employ artificial selection and hybridization.
Modern usage also includes genetic engineering as well as cell and tissue culture
technologies.
The bacteria have an economic importance which derives from the fact that bacteria
are exploited by humans in a number of beneficial ways. Despite the fact that some
bacteria play harmful roles, such as causing diseases and spoiling food, the economicimportance of bacteria includes both their useful and harmful aspects.
20.1.1. Useful Bacteria in Biotechnology
Biotechnology or Industrial microbiology is defined as the use of microorganism
such as bacteria, fungi and algae for the manufacturing and services industries.
These include:– Fermentation processes, such as brewing, baking, cheese and butter
manufacturing, Bacteria, often Lactobacillus bulgaricusin combination with
yeasts and fungi, is used to make yoghurt and cheese have been used for
thousands of years in the preparation of fermented foods such as cheese,
pickles, soy sauce, sauerkraut, vinegar, and wine.
– In the chemical industry, bacteria are most important in the production of
pure chemicals for use as pharmaceuticals or agrochemicals.
– Bacteria are also used in chemical manufacturing such as ethanol, acetone,
organic acid, enzymes, and perfumes.
– Bacteria can also be used in the place of pesticides in Biological Pest Control.
This commonly uses Bacillus thuringiensis (also called BT), a Gram-positive,
soil-dwelling bacterium.– Saprophytic bacteria help in breaking of complex organic substance to simpler
forms. Thus, in this process, they help to convert farm refuse, dung and other
wastes to manure.– Number of anti-bacterial and anti-fungal antibiotics such as Hamycin,20.1.2. Useful Bacteria in Genetic engineering
Polymyxin, and Trichomycin are obtained from fungal mycelia and bacteria
(like Streptomyces). Similarly, Bacillus is used for production of antibiotics such
as Bacitracin and Gramicidin.
– Different kinds of vitamins are produced from bacteria like Riboflavin from
Clostridium butylicum, Vitamin B12 from Bacillus megatherium and Vitamin Kand B-complex from Escherichia coli.
Genetic engineering is the manipulation of genes. It is also called recombinant DNA
technology. In genetic engineering, the genetic information for many biological
products and biological processes can be introduced into microbes in order to
genetically engineer them to produce a substance or conduct a process. The
genes can come from any biological source: human, animal, plant or microbes. This
opens the possibility for microbial production of foods, fuels, enzymes, hormones,
diagnostic agents, medicines, antibiotics, vaccines, antibodies, natural insecticides
and fertilizers, and all sorts of substances useful in our civilization and society.
The pieces of DNA (genes) are introduced into a host by means of a carrier (vector)
system. The foreign DNA becomes a permanent feature of the host, being replicated
and passed on to daughter cells along with the rest of its DNA. Microorganisms
especially bacteria play a central role in recombinant DNA technology and genetic
engineering. Important tools of biotechnology are microbial cells (bacteria, fungi),microbial genes and microbial enzymes.
Bacterial cells are transformed genetically and used in production of commercially
important products. For example, bio medical technology bacteria can be
bioengineered for the production of therapeutic proteins like: Human Insulin (used
against diabetes), Human Growth Hormone (somatotropins used to treat pituitary
dwarfism), and others which can be used to fight against viral diseases. Antibiotics are
produced in nature by molds such as Penicillium and bacteria such as Streptomycesand Bacillus.
Self-assessment 20.11. What is biotechnology?
2. What do you understand by genetic engineering?20.2. Immobilization of enzymes3. Discuss on the role of bacteria in Biotechnology and genetic engineering.
Activity 20.2
Carry out research on the action of enzymes with reference to pectinase in fruit
juice production and lactase to produce lactose-free milk.
Enzymes catalyze biological reactions in our body, but they can also be used to
catalyze industrial reactions outside the body. These enzymes are often bound toa support (‘immobilized’) and can be used for a wide range of purposes.
20.2.1. The advantages of immobilized enzymes
Enzymes have an enormous range of commercial applications for example: in
medicine, food technology and industrial processing. Enzymes are expensive. No
company wants to have to keep buying them over and over again if it can recycle
them in some way. One of the best ways of keeping costs down is to use immobilized
enzymes. Using immobilized enzymes means that you can keep and re-use the
enzymes, and that the product is enzyme-free. Another advantage of this process
is that the immobilized enzymes are more tolerant of temperature changes and
pH changes than enzymes in solution. This may be partly because their molecules
are held firmly in shape by the alginate in which they are embedded, and so do
not denature as easily. It may also be because the parts of the molecules that areembedded in the beads are not fully exposed to the temperature or pH changes.
Using enzymes instead of other molecules in reactions is useful because enzymes
catalyze specific reactions and work at much lower temperatures than chemical
catalysts.
The molecule that an enzyme acts on is called a substrate. Enzymes can either be
mixed freely with the substrate in solution or immobilized to a solid support so they
do not mix freely. There are many advantages of immobilization, one of which is that
the enzymes can be reused catalyzing the same reaction many times. Binding the
enzymes to a surface also makes those more stable and less likely to denature (lose
their shape). In addition, there will be no enzyme left in the product at the end, sopurification is not necessary
20.2.2. The disadvantages of immobilized enzymes
There are some disadvantages: immobilization requires extra time, equipment and
work; there may be a reduction in reaction rates if enzymes cannot mix freely with
the substrate; and immobilized enzymes cannot be used if one of the substrates is
insoluble.
20.2.3. Advantages of Using Immobilized Enzymes
The advantages of using immobilized enzymes are: (i) reuse (ii) continuous use (iii)
less labor intensive (iv) saving in capital cost (v) minimum reaction time (vi) less
chance of contamination in products, (vii) more stability (viii) improved process
control and (ix) high enzyme: substrate ratio. The first immobilized enzymes to be
scaled up to pilot plant level and industrial manufacture were immobilized aminoacid acylase, penicillin G-acylase and glucose isomerase.
20.2.4. Methods of Enzyme Immobilization
There are five different techniques of immobilizing enzymes: (i) adsorption, (ii)
covalent bonding, (iii) entrapment, (iv) copolymerization or cross-linking, and (v)
encapsulation. For the purpose of immobilization of enzymes carriers i.e. the supportmaterials such as matrix system, a membrane or a solid surface are used.
i. Adsorption
An enzyme may be immobilized by bonding to either external or internal surface of
a carrier or support such as mineral support (aluminum oxide, clay), organic support
(starch), and modified sapharose and ion exchange resins. Bonds of low energy are
involved e.g. ionic interactions, hydrogen bonds, van der Waals forces, etc. If the
enzyme is immobilized externally, the carrier particle size must be very small in
order to achieve appreciable surface of bonding. These particles may have diameter
ranging from 500 A to about 1 mm. Due to immobilization of enzymes on externalsurface, no pore diffusion limitations are encountered.
In addition, the enzyme immobilized on an internal surface is protected from
abrasion, inhibitory bulk solutions and microbial attack, and a more stable and active
enzyme system may be achieved. There are four procedures for immobilization by
adsorption : (i)static process (enzyme is immobilized on the carrier simply by allowing
the solution containing the enzyme to contact the carrier without stirring (ii)the
dynamic batch process (carrier is placed into the enzyme solution and mixed by
stirring or agitated continuously in a shaker), (iii) the reactor loading process (carrier
is placed into the reactor that will be subsequently employed for processing, then
the enzyme solution is transferred to the reactor and carrier is loaded in a dynamic
environment by agitating the carrier and enzyme solution), and (iv) the electrode
position process (carrier is placed proximal to one of the electrodes in an enzyme
bath, the current put on, the enzyme migrates to the carrier and deposited on thesurface).
ii. Covalent bonding
Covalent bond is formed between the chemical groups of enzyme and chemical
groups on surface of carrier. Covalent bonding is thus utilized under a broad range
of pH, ionic strength and other variable conditions. Immobilization steps are
attachment of coupling agent followed by an activation process, or attachment of a
functional group and finally attachment of the enzyme. Different types of carriers are
used in immobilization such as carbohydrates proteins and amine-bearing carriers,
inorganic carriers, etc. Covalent attachment may be directed to a specific group (e.g.
amine, hydroxyl, tyrosyl, etc.) on the surface of the enzyme. Hydroxyl and amino
groups are the main groups of the enzymes with which it forms bonds, whereassulfhydryl group least involved.
There are different methods of covalent bonding such as: (i)diazoation (bonding
between the amino group of the support e.g. aminobenzyle cellulose, aminosilanised
porous glass, aminoderivatives and a tyrosyl or histidyl group of the enzyme), (ii)
formation of peptide bond (bond formation between the amino or carboxyl group
of the support and amino or carboxy group of the enzyme), (iii) group activation
(use of cyanogen bromide to a support containing glycol group i.e. cellulose,
syphadex, sepharose, etc.), and (iv) poly functional reagents (use of a bifunctional
or multifunctional reagent e.g. glutaraldehyde which forms bonding between the
amino group of the support and amino group of the enzyme). The major problem
with covalent bonding is that the enzyme may be inactivated by bringing about
changes in conformation when undergoes reactions at active sites. However, this
problem can be overcome through immobilization in the presence of enzyme’s
substrate or a competitive inhibitors or protease. The most common activatedpolymers are celluloses or polyacrylamides
iii. Entrapment
Enzymes can be physically entrapped inside a matrix (support) of a water soluble
polymer such as polyacrylamide type gels and naturally derived gels e.g. cellulose
triacetate, agar, gelatin, carrageenan, alginate, etc. The form and nature of matrix
vary. Pore size of matrix should be adjusted to prevent the loss of enzyme from the
matrix due to excessive diffusion. There is possibility of leakage of low molecular
weight enzymes from the gel. There are several methods for enzyme entrapment:
(i)inclusion in gels (enzyme entrapped in gels), (ii) inclusion in fibers (enzyme
entrapped in fiber format), and (iii)inclusion in microcapsules (enzymes entrapped
in microcapsules formed monomer mixtures such as polyamine and polybasic
chloride, polyphenol and polyisocyanate). The entrapment of enzymes has been
widely used for sensing application, but not much success has been achieved with
industrial process.
iv. Cross - linking or Co-polymerization
Cross-linking is characterized by covalent bonding between the various molecules
of an enzyme via a polyfunctional reagent such as glutaraldehyde, diazonium salt,
hexamethylenedisocyanate, and ethylene bismaleimide. The demerit of using
polyfunctional reagents is that they can denature the enzyme. This technique is
cheap and simple but not often used with pure proteins because it produces very
little of immobilized enzyme that has very high intrinsic activity. It is widely used incommercial preparation.
v. Encapsulation
The encapsulation is the enclosing of a droplet of solution-of enzyme in a
semipermeable membrane capsule. The capsule is made up of cellulose nitrate and
nylon. The method of encapsulation is cheap and simple but its effectiveness largely
depends on the stability of enzyme although the catalyst is very effectively retained
within the capsule. This technique is restricted to medical sciences only. In this
method a large quantity of enzyme is immobilized but the biggest disadvantage isthat only small substrate molecule is utilized with the intact membrane.
20.2.5. How are immobilized enzymes used in food?
i. Immobilization of enzymes use of pectinase in fruit juice production
Pectinases find commercial application in fruit juice, wine, oil, tea, coffee, textile and
paper‐making industries using a wide variety of carriers and methods. One of the
vital applications is the clarification and depectinization of fruit juices. The raw fruit
juice obtained after pressing is very turbid viscous and contains a significant amount
of colloidal compounds, mainly pectin that causes cloudiness; therefore, clarification
of fruit juices involves the removal of juice haze by enzyme hydrolysis with pectolytic
enzymes. Although pectinases enhance the clarification of juices, immobilization of
these enzymes proves to be beneficial for industrial use. Immobilization of pectinase
on celite through adsorption is a simple, cheap and effective method. For the
clarification of pineapple juice, excellent results were observed using immobilizedpolygalacturonase in comparison with free enzyme.
Fruits contain pectin, carbohydrates found in the cell wall that holds the plant cells
together. Immobilized pectinase can be used to break down this pectin, loosening
the connections between cells. This increases the amount of juice you can get fromthe fruit, makes the juice runnier and gets rid of the cloudiness that pectin can cause.
ii. Making lactose-free milk
The enzyme lactase breaks down the sugar lactose, which is found in milk, into the
sugars glucose and galactose. Most people produce this enzyme in their bodies,
but some people (and most cats) don’t, meaning that they are lactose intolerant.
Because they can’t break down lactose, it builds up in their digestive system where
bacteria feed on it, causing digestive problems.
Immobilized lactase can be used to produce lactose-free milk: normal milk is poured
down a column containing the immobilized lactase enzymes, which break down
the lactose. After the milk has passed through this system, it will only contain the
products of the reaction (glucose and galactose), so lactose-intolerant people (and
cats) can drink it. The enzyme lactase can be immobilized using alginate beads.
The figures below show one way in which enzymes can be immobilized. The enzyme
is mixed with a solution of sodium alginate. Little droplets of this mixture are then
added to a solution of calcium chloride. The sodium alginate and calcium chloride
instantly react to form jelly, which turns each droplet into a little bead. The jelly bead
contains the enzyme.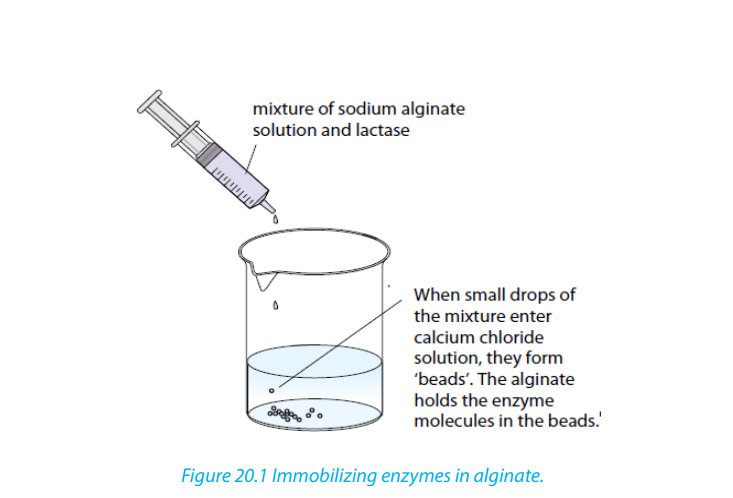
The enzyme is held in the bead, or immobilized. These beads can be packed gently
into a column. Milk is then allowed to run through the column of lactase-containing
beads. The lactase hydrolyses the lactose in the milk to glucose and galactose. The
milk is therefore lactose-free, and can be used to make lactose free dairy products
for people who cannot digest lactose. The product continues to trickle down the
column, emerging from the bottom, as illustrated in the diagram below, where it
can be collected and purified. Not only would you lose the lactase, but also you
would have milk contaminated with the enzyme.
iii. Biological washing powders containing enzymes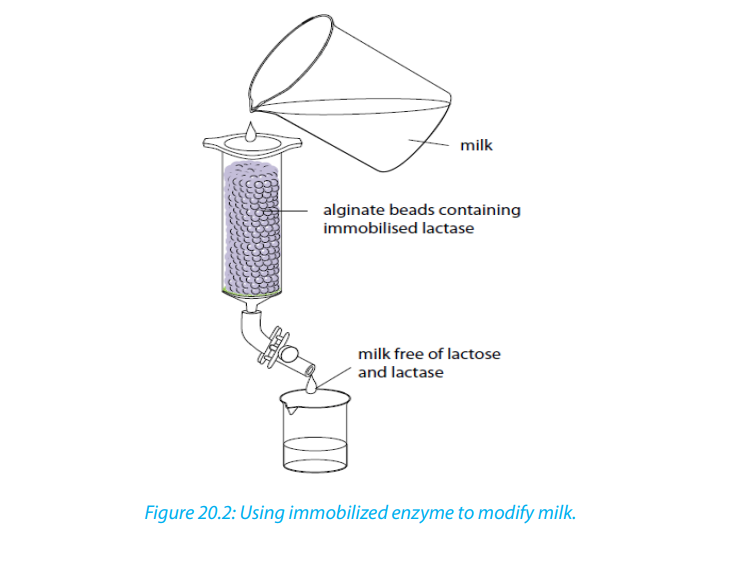
The biological washing powders contain enzymes like protease and lipase to remove
protein stains and fat/grease from clothes. The enzymes break down proteins or fats
on the fabric, forming water-soluble substances that can be washed away. Because
stains are made of different types of molecules, a range of enzymes are needed to
break them down. Proteases break down proteins, so are good for blood, egg, gravy,
and other protein stains. Amylases break down starches, and lipases break down fats
and grease.
For example: Blood contain the red protein Haemoglobin (Hb). The Proteases in
biological washing powder break Hb molecules into smaller molecules, which
are not colored and which dissolve in water and can be washed away. This makes
the washing powder more effective than detergent alone, especially at lower
temperatures. This save energy (no need to boil water), but if the temperature istoo high, the enzyme will be denatured.
iv. Fruit juices
Fruits contain pectin, carbohydrates found in the cell wall that holds the plant
together. Immobilized pectinase enzyme can be used to break down this pectin,
loosening the connections between cells. This increases the amount of juice you can
get from the fruit, makes the juice runnier and gets rid of the cloudiness that pectincan cause.
20.2.6. How are immobilized enzymes used in biosensors?
The specificity of enzymes means that they can be used to test for a unique substance,
which is exactly what a biosensor does.
Glucose test strips
People with type 1 diabetes lack the hormone insulin, so they have to test their
blood sugar levels regularly to ensure they stay within a healthy range. They do this
by measuring the amount of glucose in their blood with a glucose test strip. On
the test strip is the immobilized enzyme glucose oxidase; when glucose is present,
the enzyme catalyzes a reaction that changes glucose into hydrogen peroxide and
gluconic acid.
There is also another mediator molecule on the test strip, which catalyses a reaction
involving the products of the enzyme reaction. In the early test strips, this second
reaction caused a color change, with the color indicating the amount of glucose
present. In most modern tests, this second reaction produces electrical current,
which can be measured by a meter to give the exact concentration of glucose in theblood.
Self-assessment 20.2
1. Discuss the advantages and disadvantages of immobilized enzymes.
2. Write on the use of pectinase in fruit juice production.
3. Explain the role of lactase in making lactose-free milk.4. How are immobilized enzymes used in biosensors?
20.3. Application of enzyme in technology.
Activity 20.3
Visit a nearby bakery and verify how bread is prepared. Write a short report on theraw materials and procedures used in making bread up to the final product.
20.3.1. Enzymes in Brewing
Enzymes increase processing capacity and improve economy in the fruit juice
and wine industries. The most commonly used enzymes in these industries are
pectinase. Pectinase increases juice yields and accelerate juice clarification. They
produce clear and stable single-strength juices, juice concentrates and wines, from
not only core-fruits such as apples and pears, but also stone fruits, berries, grapes,
citrus-fruits, tropical fruits and vegetables like carrots, beets and green peppers.
Future aspects focus on a wider application of enzymes to brew with high amounts
of inexpensive raw materials like barley. Barley contains starch that has to be broken
down to fermentable sugars before the yeast can make alcohol. Therefore, traditional
brewing contains an extra step compared with wine-making, namely malting in
which enzymes needed for the degradation of starch into fermentable sugars areproduced.
20.3.2. Enzymes perform many functions in beverages
The most important field of application for enzymes in the beverage industry is the
extraction of fruit juice and vegetable juice. Pectinases, in particular, are employed
for apple and pear juice and for juices made from berries and tropical fruits. They
break down pectins found in the plant cell walls as supporting substances. This
increases the quality of juice extracted and reduces fruit waste. Enzymes can be
used in winemaking to increase the preliminary juice extraction and to obtain more
high-quality wine. Pectinase not only increase juice yields, but also increase the
colour and health-promoting antioxidants in fruit and vegetable juices. They also
increase colour extraction and juice volume by reducing fruit and vegetable mash
viscosity and improving solid/liquid separation, Pectinase and Amylase enzyme
solutions speed up filtration and prevent storage or post-packaging haze formationby depectinizing and reducing starch in raw juices.
20.3.3. Medical applications of enzymes
Development of medical applications for enzymes has been at least as extensive
as those for industrial applications, reflecting the magnitude of the potential
rewards: for example, pancreatic enzymes have been in use since the nineteenth
century for the treatment of digestive disorders. The variety of enzymes and their
potential therapeutic applications are considerable. At present, the most successful
applications are extracellular: purely topical uses, the removal of toxic substances
and the treatment of life-threatening disorders within the blood circulation.
20.3.4. Applications of enzymes in baking
For decades, enzymes such as malt and fungal alpha-amylases have been used in
bread-making. Rapid advances in biotechnology have made a number of exciting
new enzymes available for the baking industry. The importance of enzymes is likely
to increase as consumers’ demand more natural products free of chemical additives.
For example, enzymes can be used to replace potassium bromate, a chemical
additive that has been banned in a number of countries.
20.3.5. Application of enzymes in cheese
The most obvious use of enzyme action in the dairy industry is the coagulation of milk
by chymosin. Yet there are many other examples of the involvement of enzymes in
determining the quality of milk and milk products that, when the role of the enzyme
is properly understood, could be used by the industry to improve the profitability,
quality and safety of milk production, and product manufacture. Compared with
sectors such as starch hydrolysis, the volume of enzyme use in the dairy sector is low,
yet there are many opportunities for specialized applications in product ripening,
quality control, preservation and genetic improvements to fermentation cultures.
20.3.6. Application of enzymes in yoghurt
Like cheese, yoghurt is produced from milk by the action of lactate producing
bacteria, especially lactobacillus bulgaricus and streptococcus thermophiles. These
bacteria are commonly used in yoghurt starter cultures. Fermentation produces
lactate which brings the pH down to about 4.0. Fermentation-by products, including
ethanal and methanoic acid, give yoghurt its characteristics flavor. Sometimes fruit
pulp, coloring and flavors are added before packaging. Some yoghurt is heat-treated
before or after packaging to kill any bacteria, but most yoghurt contain live bacteria.
20.3.7. Application of enzymes in breads making.
Bread production involves harvesting the wheat, separating the grain from the husk,
crushing the grain to make flour, mixing the flour with water and then finally baking
it. The main difference between unleavened and leavened bread is that leavened or
risen bread uses leavened dough, and unleavened bread does not. If the leavened
bread is desired, then one adds yeast and allowing the bread to sit for a specificamount of time, depending on the type of bread being made.
Types of Unleavened Bread
1. Chapatti: Our staple chapatti is widely consumed across India and is a great
example of unleavened bread. It is made using atta flour although there are
variations that replace atta with wheat, gram, corn flour, or a combination of all
three.
2. Matzah: Jews only consume matzah during the Jewish Passover, which is
unleavened bread. This bread is consumed in remembrance of the Jewish exodus
of Egypt, during which the Jews fled in such haste that there was no time to allow
their breads to rise up. Matzah is made according to strict interpretations of the
Torah using kosher flour whole grain wheat flour.
3. Tortilla: Commonly eaten in Mexico and Spain, tortillas are made from corn flour
or wheat flour and are similar in appearance to the chapati. Tortillas are flattened
and browned over a skillet.
4. Pancakes: Pancakes without yeast are considered to be unleavened. Most
pancakes are cooked on a griddle and flipped over once the first side has beencooked.
Types of Leavened Breads
Yeast is commonly used to leaven bread and is typically added with sugar or honey
to catalyse and activate the yeast in order for the bread to rise. Breads made with
yeast is normally allowed to rest for an hour so that it can rise and double in size. It
is then punched down and allowed to rise once again before baking. Most types of
yeast breads include standard sandwich bread, pizza crust, donuts, and loaf breads
and so on.
While yeast is a commonly used leavening agent, it is not the only ingredient that
can be used for leavening. Quick breads are any type of breads that are made with
an ingredient other than eggs or yeast as a leavening agent. Baking soda and baking
powder are common leavening agents and both usually have salt added to the
recipe to activate the leavening agent. Quick breads, unlike yeasted breads, are not
let to rest before baking. Common types of quick breads include biscuits, muffins,
scones, banana bread and cornbread. There are also loaf breads like soda breads
which are a type of quick breads. Some donut and pizza recipes are made in thequick bread version.
Steps involved in bread making
The dough that we make in our bakeries follows all of these 10 steps from start to
finish. This ensures we produce the best quality bread without compromising taste,
texture, nutrition or our artisan craft. As a home baker, if you follow these 10 steps
when making breads at home, you will be on the right path to creating superb loaves.
1. Ingredients used to make breads
Using good quality ingredients is crucial to making good bread. The main ingredients
include: bread-flour, dry yeast (‘rapid rise’), levain (sourdough), salt, water, sugar, and
eggs.
2. Mixing
There are two stages to the mixing process: the first is to incorporate ingredients,
the second is to develop the structure of the dough, otherwise known as the gluten
network. Dough can be kneaded by hand, or mixed in a table top mixer. When using
a table top mixer, keep it to the lower speeds to avoid damaging the motor.
3. Primary Fermentation
Also referred to as rising, or proofing, this is where the yeast starts to do its work,
converting sugars into carbon dioxide, alcohol and organic acids. Every dough has
a different primary fermentation time, depending on its formulation. We work with
time as well as our senses to determine when the dough is properly fermented.
4. Divide and Pre-Shape
When the dough is properly fermented, it is time to divide it to the desired size and
give the divided pieces a preshape. A preshape is an intermediate shape a loose
suggestion to the dough of where it’s headed that will make final shaping easier.
5. Bench Rest
After the dough has been preshaped, it needs to rest for a short time before final
shaping. Bench rest is typically 15-20 minutes long and during that time, the gluten
network, which has been made more elastic through handling, will relax and become
more extensible.
6. Final Shaping
There are four basic shapes in bread making: the baguette (stick), the boule (round),
the bâtard (a football-like shape) and the pan loaf. After shaping, the dough must
be set somewhere to rest during its final fermentation. For baguettes and bâtards,
we use baker’s linen and wooden boards; for boules, we often use wooden proofing
baskets. The linen and the baskets help to hold the shape of the dough during the
final fermentation.
7. Final Fermentation
After shaping, the dough must rest and continue to ferment. The length of the final
fermentation varies from dough to dough; it could be anywhere from 15 minutes to
12 or more hours. Again, we work with time and with our “dough sense” to determinewhen the dough is properly fermented.
8. Scoring
Most loaves will be scored, or cut, just before they are baked. Scoring has a decorative
function, and it allows the dough to spring properly as the carbon dioxide gas that
has accumulated during fermentation expands in the heat of the oven. Scoring istypically done with a razor blade or a small serrated blade.
9. Baking
Lean dough (those like baguettes and levain breads made without fats, sugars, eggs,
etc.) are typically baked at a very high temperature, around 450-475°F. Enriched
breads (brioche, challah, sweet breads) are typically baked around 350-400°F. In
most cases, a smaller loaf should be baked at a higher temperature than a larger
one, so that it will take on the right amount of color in its baking time. There are
a few different ways to determine that a loaf is properly baked by color, by the
hollow sound you hear when you knock on the bottom of the loaf, and by internal
temperature (at least 190°F for lean breads, 165°F for enriched breads).
10. Cooling
Although it is tempting to eat hot bread right of the oven, that’s not the best way to
really taste its subtle flavors. When bread first comes out of the oven, it is still filled
with excess moisture and carbon dioxide. The bread needs time to cool so that the
moisture and gas will dissipate. After cooling, the texture, flavor and aroma of the
bread will have developed into what they should be and you will have a flavorful,palate-pleasing loaf.
Self-assessment 20.3
1. Explain the application of enzymes in brewing.
2. Explain the application of enzymes in cheese and yoghurt.
3. Discuss the steps involved in bread making.
20.4. Fermentation and fermenters and production of
penicillin
Activity 20.4
Use charts, internet, text books and illustrations to explain how fermentation isinvolved in production of penicillin.
20.4.1. Fermentation and fermenters
Fermentation is anaerobic breakdown of organic compounds by living cells
(microorganisms) that produces ethanol and carbon dioxide or lactate (lactic acid).
It occurs in yeast and bacteria, but also in oxygen-starved muscle cells, as in the case
of lactic acid. Fermentation is also used more broadly to refer to the bulk growth of
microorganisms on a growth medium, often with the goal of producing a specific
chemical product. French microbiologist Louis Pasteur is often remembered for his
insights into fermentation and its microbial causes. The science of fermentation is
known as zymology. To many people, fermentation simply means the production
of alcohol: grains and fruits are fermented to produce beer and wine. If a foodsoured, one might say it was ‘off’ or fermented. Fermentation react NADH with an
endogenous, organic electron acceptor. Usually this is pyruvate formed from the
sugar during the glycolysis step. During fermentation, pyruvate is metabolized to
various compounds through several processes:
a. Ethanol fermentation, alcoholic fermentation, is the production of ethanol and
carbon dioxide.
b. Lactic acid fermentation refers to two means of producing lactic acid:
Homolactic fermentation is the production of lactic acid exclusively.
Heterolactic fermentation is the production of lactic acid as well as other acids and
alcohols.
Sugars are the most common substrate of fermentation, and typical examples of
fermentation products are ethanol, lactic acid, Carbon dioxide, and hydrogen gas
(H2). However, more exotic compounds can be produced by fermentation, such
as butyric acid and acetone. Yeast carries out fermentation in the production of
ethanol in beers, wines, and other alcoholic drinks, along with the production of
large quantities of Carbon dioxide. Fermentation occurs in mammalian muscle
during periods of intense exercise where oxygen supply becomes limited, resultingin the creation of lactic acid.
A fermenter also known as bioreactors are an apparatus that maintains optimal
conditions for culture and growth of microorganisms (on liquid or solid media) to
be used in large-scale fermentation and in the commercial production of antibiotics
and hormones. The processes that take place in fermenters refers as fermentationwhich includes aerobic and anaerobic processes.
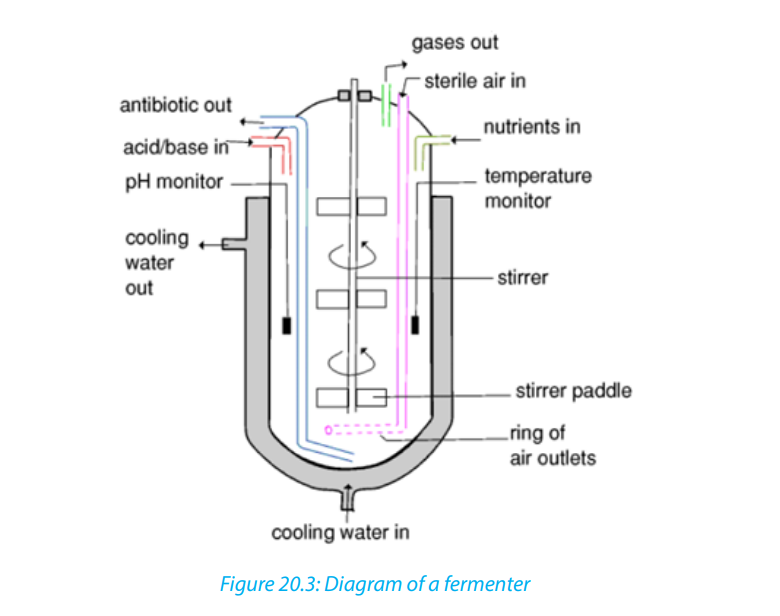
20.4.2. Production of penicillin: Antibiotic
Penicillin, an important part of our anti-microbial armament, had a significant impact
on the second half of the twentieth century. Deep-fermentation methods, which
were primarily developed for the production of penicillin during the war, gave rise
to the development of antibiotics and contributed to the nascent biotechnology
industry which appeared in the 1970s.
Penicillin production
In laboratory, it is relatively easy to grow microbes on a small scale in petri dishes,
test tubes and flasks, given a suitable nutrient medium, and good environmental
conditions. Producing chemicals like penicillin antibiotic from microbes on an
industrial scale becomes more complicated as a big number of organisms have to
be grown for the venture to be commercially viable. Laboratory procedure should
be modified so that it can be used on an industrial scale. This is called scaling up.
With scaling up, microorganisms are grown in very large vessels called fermenters
or bioreactors. Scaling up to be effective, it requires specialized biologists andengineers to deal with the following problems:
– Avoiding risks of contamination. Only desired organisms must be allowed to
grow in the vessel. Others are excluded.
– Big fermenters are built to very strict and specific design.
– Microorganisms should be kept in conditions that allow the optimum
production of required substances. This requires installing highly sensitive
equipment that maintains PH, temperature and fluid volume within very strict
limits.
– To keep nutrients at optimum levels as microbial population grows.
– Removing large amount of heat generated by high levels of microbial activity
via a heat exchanger, so that a constant temperature can be maintained.
– Minimizing the build-up of end-products (inhibitors) which may reduce
production.– Monitoring and controlling formation of the foam (unavoidable consequenceTypes of culture (of fermentation): there are two main types of culture used in
of carbon dioxide production in a nutrient-rich solution).
– Providing adequate amount of Oxygen to cultures of aerobic organisms by
aeration with small bubbles of sterile air which have a large surface area tovolume ratio.
industrial processes such as batch culture and continuous cultures.
In Batch cultures or batch fermentation (closed system), cells are grown in a
fixes volume of liquid medium in a closed vessel The conditions are set up and not
changed from outside once fermentation starts; for example: no microorganisms,
nutrients, or fluid are added or removed from the culture during the incubation
period. That is why the process is described as a closed system. The process is
stopped once sufficient products have been formed. The contents of fermenter are
then removed, isolated, microorganisms discarded and fermenter is cleaned, and
set up for a fresh batch.
Batch cultivation is used to produce secondary metabolites such as penicillin and
other antibiotics which are relatively unstable, and not essential for growth of the
culture. These secondary metabolites can be extracted economically only when
they reach a high concentration in the culture suspension.
In continuous cultures (open system), nutrients are added and cells harvested at
a constant rate, so that the volume of suspension is also kept constant. This means
that fermenters does not have to be emptied, cleaned and refilled very often. The
production is almost continuous. Continuous cultures are very expensive because
they need high equipment to maintain constant conditions, and highly skilled staffto operate the equipment.
Table 20.1: Advantages and disadvantages of batch and continuous culture
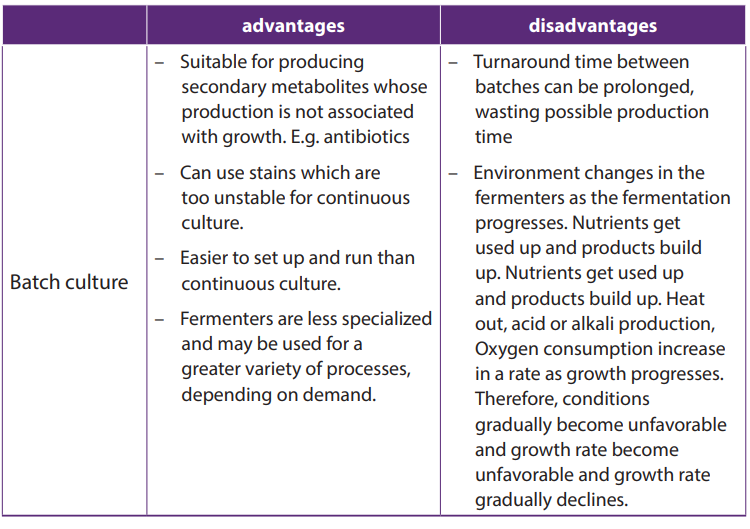
The industrial production of penicillin was generally classified into two processes: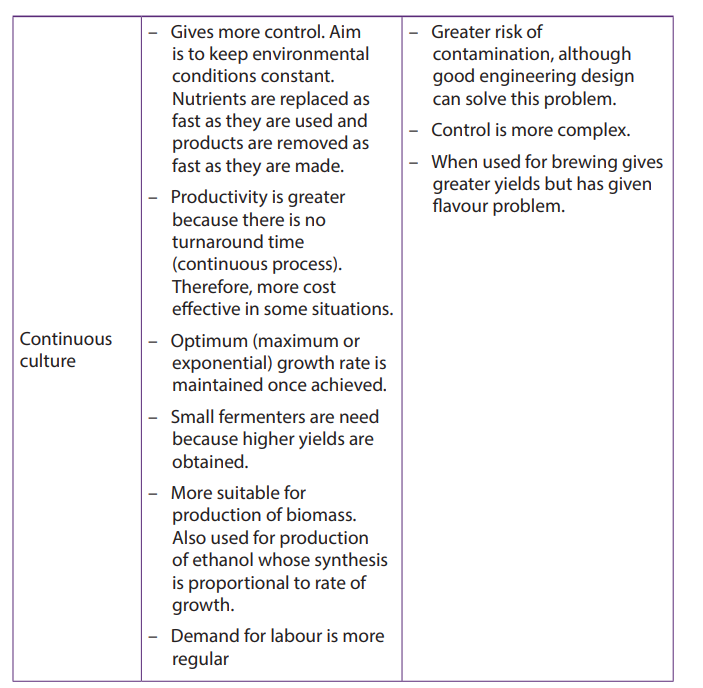
Upstream processing and downstream processing. Upstream processing
encompasses any technology that leads to the synthesis of a product and includes
the exploration, development and production. Downstream processing refers as the
extraction and purification of a biotechnological product from fermentation or at
the end of culture process. Usually the contents of fermenter are first separated into
liquid component and a solid component which contain the cells. This is usually
done by filtration or centrifugation. The liquid may contain the desired product in
solution or it may be the cells or some products inside the cells that it needs.
Penicillin is produced commercially by growing the fungus Penicillium chrysogenumin
large stirred fermenters. A solution of essential salts and a nitrogen source are put
into the fermenter together with an inoculum of the fungus. All procedures areperformed aseptically. The PH of the medium is regulated with ammonium salts
at 6.5 to 7.0. Lactose (a slowly hydrolysed disaccharide) is added to promote cell
growth and reproduction and minimize penicillin production. On completion of
fermentation (usually 6-7 days) the broth is separated from the fungal mycelium and
penicillin extracted. This penicillin can then be modified by chemical procedures toyield a variety of semisynthetic penicillins.
Modern Production Methods
Significant improvements in modern production methods have increased
production and decreased cost. Today, commercial producing strains of Penicillium
chrysogenum are grown using submerged culture in constantly agitating and
aerated 50,000- gallon stainless steel tanks. These industrial strains can now produce
40-50 grams of penicillin per liter of culture with a 90% recovery yield. This is an
overwhelming improvement from the earliest Peoria farmer’s market strain that
only produced 0.15 grams per liter with very low recovery rates. In order to achieve
these production rates, modern Penicillium strains display a host of genetic and
cellular modifications that result in increased production, including amplification of
the penicillin biosynthesis gene cluster, an increased number of peroxisomes, and
elevated levels of transporter proteins that secrete newly produced penicillin out of
the peroxisomes and the cell.
Temperature and pH are normally controlled in the fermenter. Temperature is kept
constant, while pH is held at a value of 5.5 for the first stage of the fermentation andthen raised to 6.8 and kept constant for the remainder of the fermentation period.
Self-assessment 20.4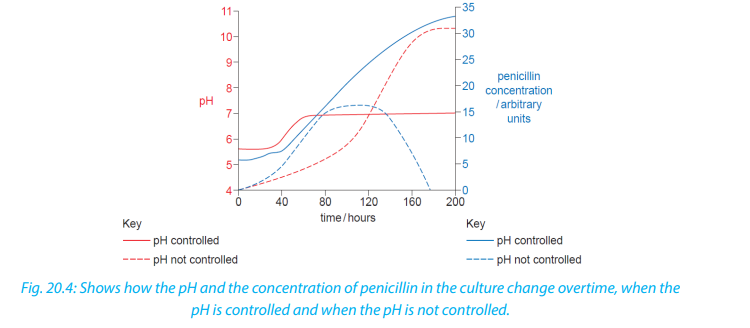 1. What is fermenter?20. 5. Antibiotics
1. What is fermenter?20. 5. Antibiotics
2. Write on upstream processing and downstream processing
3. Write on your own word penicillin
4. Contrast commercial-scale production from laboratory-scale production
of penicillin.
5. Explain why the continuous culture is described as open system.
6. Explain why the batch culture is described as closed system.
7. Discuss advantages and disadvantages of batch culture?
8. Explain why continuous culture is very expensive.
9. What are Advantages and disadvantages of continuous culture?
Activity 20.5
Using addition resources to your textbook available in your school such as the
books from the school library and search further information from the internet:
Brainstorm on the antibiotic resistance and implications of antibiotic use.
Antibiotics are powerful medicines that fight certain infections by either stopping
bacteria from reproducing or by destroying them. Before bacteria can multiply
and cause symptoms, the body’s immune system can usually kill them. The word
antibiotic means “against life.” Any drug that kills germs in your body is technicallyan antibiotic.
How do antibiotics work?
Antibiotics are used to treat bacterial infections. Some are highly specialized and are
only effective against certain bacteria. Others, known as broad-spectrum antibiotics,
attack a wide range of bacteria, including ones that are beneficial to us.
There are two main ways in which antibiotics target bacteria. They either prevent
the reproduction of bacteria, or they kill the bacteria, for example by stopping
the mechanism responsible for building their cell walls. There are now hundreds
of different types of antibiotics, but most of them can be broadly classified into six
groups. These are outlined below.
Penicillin – widely used to treat a variety of infections, including skin infections,
chest infections and urinary tract infections.
Cephalosporins – can be used to treat a wide range of infections, but are also
effective for treating more serious infections, such as septicaemia and meningitis.
Aminoglycosides – tend to only be used to treat very serious illnesses such as
septicaemia, as they can cause serious side effects, including hearing loss and kidney
damage; they break down quickly inside the digestive system, so they have to be
given by injection, but are also used as drops for some ear or eye infections.
Tetracyclines – can be used to treat a wide range of infections; commonly used to
treat moderate to severe acne and rosacea, which causes flushing of the skin andspots.
Macrolides – can be particularly useful for treating lung and chest infections; can
also be a useful alternative for people with a penicillin allergy or to treat penicillinresistant strains of bacteria.
Fluoroquinolones – broad-spectrum antibiotics that can be used to treat a wide
range of infections. They include: Hypocholesterolemic agents, Lipopeptide,
Macrolides, Monobactams, Nitrofurans, Oxazolidinones, Polypeptides, Quinolones,
Sulfonamides, Tetracyclines, Lincosamides, Glycopeptides, Immunosuppressive
agents, Anti-migraine agents, Anti-bacterials, Antifungals, Penicillins,
Aminoglycosides, Ansamycins, Carbapenems, Cephalosporins (1,2, 3, 4, 5generations), and Fluoroquinolones.
20.5.1. Antibiotic resistance
Antibiotic resistance occurs when an antibiotic has lost its ability to effectively control
or kill bacterial growth; in other words, the bacteria are “resistant” and continue tomultiply in the presence of therapeutic levels of an antibiotic.
Why do bacteria become resistant to antibiotics?
Antibiotic resistance is a natural phenomenon. When an antibiotic is used, bacteria
that can resist that antibiotic have a greater chance of survival than those that are
“susceptible.” Susceptible bacteria are killed or inhibited by an antibiotic, resulting in
a selective pressure for the survival of resistant strains of bacteria.
Some resistance occurs without human action, as bacteria can produce and use
antibiotics against other bacteria, leading to a low-level of natural selection for
resistance to antibiotics. However, the current higher-levels of antibiotic-resistant
bacteria are attributed to the overuse and abuse of antibiotics. In some countries
and over the Internet, antibiotics can be purchased without a doctor’s prescription.
Patients sometimes take antibiotics unnecessarily, to treat viral illnesses like thecommon cold.
How do bacteria become resistant?
Some bacteria are naturally resistant to certain types of antibiotics. However, bacteria
may also become resistant in two ways: by a genetic mutation or by acquiring
resistance from another bacterium.
Mutations, rare spontaneous changes of the bacteria’s genetic material, are thought
to occur in about one in one million to one in ten million cells. Different genetic
mutations yield different types of resistance. Some mutations enable the bacteria
to produce potent chemicals (enzymes) that inactivate antibiotics, while other
mutations eliminate the cell target that the antibiotic attacks. Still others close up
the entry ports that allow antibiotics into the cell, and others manufacture pumping
mechanisms that export the antibiotic back outside so it never reaches its target.Bacteria can acquire antibiotic resistance genes from other bacteria in several ways.
By undergoing a simple mating process called “conjugation,” bacteria can transfer
genetic material, including genes encoding resistance to antibiotics (found on
plasmids and transposons) from one bacterium to another. Viruses are another
mechanism for passing resistance traits between bacteria. The resistance traits from
one bacterium are packaged into the head portion of the virus. The virus then injects
the resistance traits into any new bacteria it attacks. Bacteria also have the ability
to acquire naked, “free” DNA from their environment. Any bacteria that acquire
resistance genes, whether by spontaneous mutation or genetic exchange with
other bacteria, have the ability to resist one or more antibiotics. Because bacteria
can collect multiple resistance traits over time, they can become resistant to manydifferent families of antibiotics.
How does antibiotic resistance spread?
Genetically, antibiotic resistance spreads through bacteria populations both
“vertically,” when new generations inherit antibiotic resistance genes, and
“horizontally,” when bacteria share or exchange sections of genetic material with
other bacteria. Horizontal gene transfer can even occur between different bacterial
species. Environmentally, antibiotic resistance spreads as bacteria themselves move
from place to place; bacteria can travel via airplane, water and wind.
People can pass the resistant bacteria to others; for example, by coughing or
contact with unwashed hands.
Can bacteria lose their antibiotic resistance?
Yes, antibiotic resistance traits can be lost, but this reverse process occurs more
slowly. If the selective pressure that is applied by the presence of an antibiotic is
removed, the bacterial population can potentially revert to a population of bacteria
that responds to antibiotics.
20.5.2. Implications of antibiotic use
Antibiotics are considered the keystone of modern medicine, but their excessive
use continues to generate unwanted side effects. While specialists are making
strides to preserve the effectiveness of antibiotics and to slow potential infections
through better policy, the overuse of antibiotics continues to have severe healthconsequences around the world.
Self-assessment 20.51. What do you understand by antibiotic resistance?20.6. Biogas production
2. Explain how bacteria become resistant.
3. Discuss on how bacteria lose their antibiotic resistance.
4. Write on implications of antibiotic use.5. Talk on how antibiotic resistance spreads
Activity 20.6
Use diagrams or illustrations and visiting a biogas plants in your region, describe
the stages of biogas production and its significance in your area (a simple biogas
generator can also be made in schools).
Biogas typically refers to a mixture of different gases produced by the breakdown of
organic matter (methanogens or archaebacterial) in the absence of oxygen. Biogas
is produced by anaerobic fermentation of organic wastes such as agricultural waste,
manure, municipal waste, plant material, sewage, green waste, or food waste. It is
a renewable energy source and in many cases exerts a very small carbon footprint.
Biogas is primarily methane (CH4) and carbon dioxide (CO2) and may have small
amounts of hydrogen sulphide (H2S), moisture and siloxanes. The gases methane,
hydrogen, and carbon monoxide (CO) can be combusted or oxidized with oxygen.
This energy released allows biogas to be used as a fuel; it can be used for any heatingpurpose, such as cooking.
It can also be used in a gas engine to convert the energy in the gas into electricity
and heat. Biogas can be compressed, the same way the natural gas is compressed
to compressed natural gas (CNG), and used to power motor vehicles. In the UK, for
example, biogas is estimated to have the potential to replace around 17% of vehicle
fuel. It qualifies for renewable energy subsidies in some parts of the world. Biogas can
be cleaned and upgraded to natural gas standards, when it becomes bio methane.Production
Biogas is produced as landfill gas (LFG), which is produced by the breakdown of
biodegradable wastes inside a landfill due to chemical reactions and microbes,
or as digested gas, produced inside an anaerobic digester. A biogas plant is the
name often given to an anaerobic digester that treats farm wastes or energy
crops. It can be produced using anaerobic digesters (air-tight tanks with different
configurations). These plants can be fed with energy crops such as maize silage or
biodegradable wastes including sewage sludge and food waste. During the process,
the microorganisms transform biomass waste into biogas (mainly methane and
carbon dioxide) and digestate (remaining organic matter not transformed into
biogas).
The biogas is a renewable energy that can be used for heating, electricity, and
many other operations that use a reciprocating internal combustion engine, such
as a General Electrical (GE) Jenbacher or Caterpillar gas engines. Other internal
combustion engines such as gas turbines are suitable for the conversion of biogas
into both electricity and heat. The remaining organic matter that was not transformedinto biogas. It can be used as an agricultural fertilizer.
There are two key processes: mesophilic (A mesophyll is an organism that grows
best in moderate temperature, neither too hot nor too cold, typically between 20
and 45oC) and thermophilic (A thermophile is an organism, a type of extremophile,
that thrives at relatively high temperatures, between 41 and 122 °C) digestion which
is dependent on temperature. The production of biogas involves three stages and
three communities of microorganisms namely
1. Anaerobic fermentation by eubacteria including lactobacillus, which
converts the organic waste into a mixture of organic acids and alcohol, with
some Hydrogen, Carbon dioxide, and acetate.
2. Acetogenic (acetate-producing) reaction by bacteria such as
acetobacterium which, in addition to acetate, produce hydrogen and
Carbon dioxide from the organic acid and alcohol.
3. Methanogenic (methane-producing) reactions by archaebacteria,
including Methanobacterium, Metanococcus, and Methanospirillum. Thearchaebacteria generate methane either:
– By reducing the carbon dioxide:

– By converting acetate: CH3 COOH :
Composition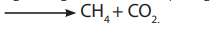
The composition of biogas varies depending upon the origin of the anaerobicdigestion process. Landfill gas typically has methane concentrations around 50%.
Table 20.2: Typical composition of biogas
In some cases, biogas contains siloxanes. They are formed from the anaerobic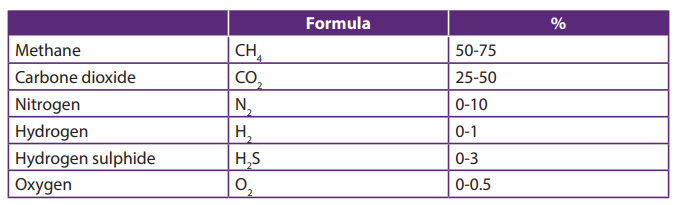
decomposition of materials commonly found in soaps and detergents. During
combustion of biogas containing siloxanes, silicon is released and can combine with
free oxygen or other elements in the combustion gas.
Applications
Biogas can be used for electricity production on sewage works, in a combined heat
and power (CHP) gas engine, where the waste heat from the engine is conveniently
used for heating the digester; cooking; space heating; water heating; and process
heating. If compressed, it can replace compressed natural gas for use in vehicles,
where it can fuel an internal combustion engine or fuel cells and is a much moreeffective displacer of carbon dioxide than the normal use in on-site CHP plants.
Self-assessment 20.6
1. What part do acetogenic reactions play in the production of biogas?
2. The archaebacteria generate methane either by reducing the carbon
dioxide, or by converting acetate: write chemical equations for the two
processes.
End of unit assessment 20
Multiple choice questions
1. During penicillin production, temperature is maintained ata. room temperature2. In penicillin production, pH of culture medium is maintained between
b. 26 °C
c. 36 °C
d. 46 °Ca. 5 and 63. To produce penicillin, main fermentable source in culture is
b. 4 and 6
c. 6 and 7
d. 4 and 5a. glucose4. Penicillin production is optimum in
b. lactose
c. sulphate
d. sugarsa. batch operation systems5. What is fermentation?
b. continuous operation systems
c. discontinuous operation system
d. unique operation system
6. The senior four Biology teacher said: “the biogas can contribute to the economic
development of Rwanda” defend his idea.
7. Explain how are immobilized enzymes made?
8. Explain the medical applications of enzymes.
9. Explain the importance of using yeast in bread making.
10. What Are the Main Ingredients of Bread?
11. Summarize the advantages of using immobilized enzymes rather than enzyme
solutions.
12. Describe the composition of biogas.
13. Describe three stages that are involved in production of biogas.
REFERENCES
Becket, B.S. (1986). Biology: A Modern introduction. GCSE Education, Oxford University
press.
Biggs, A., Kaicka, C., and Lundgren, L. (1995). Biology: The dynamics of life. McGrawHill, Westerville, USA.
Brainard, J. (2015). CK-12 Biology Foundation textbook next generation.
GoR [Government of Rwanda] (2016). National biodiversity and action plan, Kigali,
Rwada.
Government, W. (2005). Guidance for teaching. England: Charles Darwin House.
Hocking, S., Kennedy, P. and Sochacki, F. (2008). Biology. OCR and Heinemann, UK.
Ibrahim, S. (2006). Solutions to examination paper: UCE Biology paper 1and 2. PEAK
Publisher Ltd, Kampala, Uganda.
Jones, M., Fosbery, R., Gregory, J., and Taylor, D. (2014). Cambridge International AS
and A Level Biology coursebook fourth Edution. United Kingdom: Cambridge University
Press.
Jovanovich, H. B. (1986). Biology. Orlando, Harcourt Brace Jovanovich.
Juliet, M., and Magondu.J., M. V. (2017). Biology for Rwanda. Kigali: Easter African
Educational publishers
Karen, A., Camp, S., Pamela, Jenner V. J., and Zalisko J. E. (1994). Biology: A Journal into
life. 3rd Ed. Saunders College Publishing
Kay, I. (1998). Introduction to Animal Physiology, MMU, Manchester, UK.
Kennedy, P., and Sochck, F. (2008). OCR Biolgy. British: OCR and Heinemann.
Kent, M. (2000). Advanced Biology: A new Mainsream text for the new specifications.
Oxford University Press. New York, USA.
Kent, M. (2000). Advanced Biology: A new Mainsream text for the new specifications.
Oxford University Press. New York, USA.
Kinyua, S. and Oyugi, O. (1998). Secondary Biology, A practical approach; KLB, Nairobi,
Kenya.
Lee Ching and Arnasalam, J. (2008). Biology.Vol.1, Pre-U Text STPM, Pearson-Longman
Malaysia.
Level Biology coursebook fourth Edution. United Kingdom: Cambridge University
Press.
Mackean.D.G, and Hayward, D. (2014). Cambridge IGSCE biology 3rd edition. London:
Hadder education
Mader, S. S., Baldwin, A., Roush, R., & Stephanie Songer, M. T. (2010). Biology 10th
Edition. McGraw-Hill companies, Boston, UK.
Martin, E. and Hine, S.R. (2008). Oxford Dictionary of Biology, 6th edition, Oxford
University Press, Oxford, UK.
Mary, J., & Forcebery, R. (2004). Cambridge International AS and A Level. United
Kingdom: CAMBRIDGE University press.
Miller L. (2006). Biology: Florida students’ and teachers’edution. Prentice Hall Biology,
Boston. UK.
Morgan, S. (2000). Practice in Biology: Progressive questions for AS and A level.
Cambridge
Neil A. Campbell, Reece J.B., Urry L.A., Cain M.L., Wasserman S.A., Minorsky P.V., and
Jackson R.B. (2008). Biology. Pearson Benjamin Cummings. 8th Ed. San Francisco, US.
Ones, M., Fosbery, R., Gregory, J., & Taylor, D. (2014). Cambridge International AS and A
Owaka M., and Kavita P. (2006). Test it and Fix it. KCSE Revision Biology.Oxford
University Press, Nairobi, Kenya.
Peter K; Frank S. (2008). OCR Biology. British: British Library.
Roberts, M.B.B (1986). Biology for life. 2nd edition, Thomas Nilsson and sons Ltd,
London UK
Sequeira, L. (2010). Certificate Biology, form 4, Pupils’book. EAEP, Nairobi, Kenya.
Sochacki, F., and Kennedy, P. (2008). OCR Biology. Pearson Education Limited, China
The Perfect Guide for Students of Biology at School or University (2008). A Dictionary
of Biology, Oxford University Press.
Verrgilio, O.K. (2013). Senior secondary certificate biology for Rwanda, East African
Publishers Limited, Kigali, Rwanda.
Wilf Stout and Nigel Green (1990). A-level Biology. Macmillan work out series,
Macmillan.
Electronic links
https://study.com/academy/practice/quiz-worksheet-louis-pasteur-germ-thioryof-disease.html
http://printerfriendly.adam.com/content.aspx?productId=117&pid=1&gid=001439
&c-custid=758
https://www.cdc.gov/parasites/hookworm/gen_info/faqs.html
http://www.parasitesinhumans.org/hookworms.html
https://en.wikipedia.org/wiki/Tinea_corpori

