UNIT 10: ENZYMES
UNIT 10: ENZYMES
Key Unit Competence
Describe the mode of action and factors affecting enzymes and their importance for
the existence of life
Learning objectives
At the end of this unit you be able to:– Define the term enzyme.Introductory activity
– Explain the criteria of naming enzymes.
– State that enzymes function inside cells and outside cells.
– Explain that enzymes are globular proteins that catalyze metabolic reactions.
– Describe the mode of action of enzymes in terms of the lock and key and the
induced fit hypotheses.
– Explain factors affecting enzyme activity.
– Define enzyme technology and its role in industry.
– Investigate the progress of an enzyme-catalyzed reaction by measuring rates
of formation of products.
– Investigate the effects of temperature, pH, enzyme and substrate concentration,
and inhibitors on enzyme activity.
– Interpret graphs of the effects of reversible and irreversible inhibitors on the
rate of enzyme activity.
– Investigate the effect of immobilizing an enzyme in alginate as compared with
its activity when free in solution.
– Use a computer to plot graphs of the rate of enzyme controlled reaction.
Calculate Q10 of an enzyme controlled reaction.
– Acknowledge that enzymes are essential in speeding up reactions that would
be too slow to sustain life.
– Appreciate the importance of planning and carrying out experiments under
controlled conditions.– Understand the roles of enzymes in industry and medicine
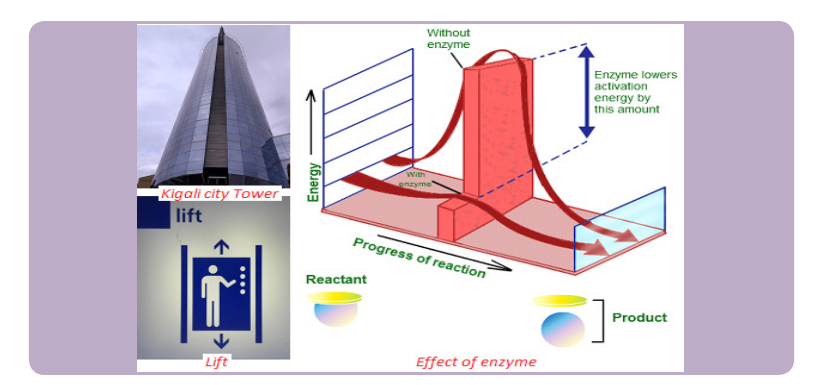
Discuss in pair the following questions and share with another pair your findings.1. What do you understand by the term enzyme?2. Two individuals want to reach the last floor of Kigali city tower. One climbs
up using the ladder but another one uses a lift. What advantage the lift
gives over the ladder?3. Why is it easy to digest hot foods than cold ones?
10.1. Criteria for naming enzymes
Activity 10.1You are provided with three groups of enzymes:
Make a research to find out:
a. specific role of each of the six enzymes mentioned aboveb. criterion followed to name enzymes of group A, B and C respectively
Enzymes are biological catalysts produced by a living organism to control the rate of
specific biochemical reactions by lowering the activation energy of reactants
First of all, individual enzymes are named by adding -ase to the name of the substrate
with which they react. The enzyme that controls urea decomposition is called urease;those that control protein hydrolyses are known as proteases.
A second way of naming enzymes refers to the enzyme commission number (EC
number) which is a numerical classification scheme for enzymes based on the
chemical reactions they catalyze. In a system of enzyme nomenclature, every EC
number is associated with a recommended name for the respective enzymecatalyzing a specific reaction. They include:
Oxidoreductases: catalyze redox reactions by the transfer of hydrogen,
oxygen or electrons from one molecule to another. Example: Oxidase catalyzes
the addition of oxygen to hydrogen to form water.
Glucose + oxygen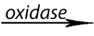 gluconic acid +water
gluconic acid +water
– Hydrolase: catalyzes the hydrolysis of a substrate by the addition of water.
Sucrose + water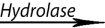 glucose+ fructose
glucose+ fructose
– Ligases: catalyze reactions in which new chemical bonds are formed and use
ATP as energy source.Amino acid + tRNA
– Transferases: catalyze group transfer reactions. The transfer occurs from one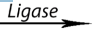 amino acid-tRNA complex
amino acid-tRNA complex
molecule that will be the donor to another molecule that will be the acceptor.
Most of the time, the donor is a cofactor that is charged with the group about
to be transferred. Example: Hexokinase used in glycolysis.
– Lyases: catalyze reactions where functional groups are added to break double
bonds in molecules or the reverse where double bonds are formed by the
removal of functional groups. For example: Fructose bisphosphate aldolase
used in converting fructose 1, 6-bisphospate to G3P and DHAP by cutting C-C
bond.
– Isomerases: catalyze reactions that transfer functional groups within a
molecule so that isomeric forms are produced. These enzymes allow for
structural or geometric changes within a compound. Sometime the inter
conversation is carried out by an intramolecular oxidoreduction. In this case,
one molecule is both the hydrogen acceptor and donor, so there’s no oxidized
product. The lack of an oxidized product is the reason this enzyme falls under
this classification. The subclasses are created under this category by the type
of isomerism. For example: phosphoglucose isomerase for converting glucose
6-phosphate to fructose 6-phosphate by moving chemical group inside thesame substrate.
A third way of naming enzymes is by their specific names e.g. trypsin and pepsin
are proteases. Pepsin, trypsin, and some other enzymes possess, in addition, the
peculiar property known as autocatalysis, which permits them to cause their ownformation from an inert precursor called zymogen.
Self-assessment 10.1
1. How to name enzymes?
2. What is the role of peptidase?
10.2. Characteristics of enzymes
Activity 10.2
Requirement: Three test tubes, match box, about 1g of liver, 1g of sands, 1% H2
O2and MnO2
Procedure:
– Label three test tubes A, B and C respectively.
– Put about 0.1 g of MnO2
powder in test tube A and 1g of liver in tube B and 0.1g
of sand in tube C.
– Pour 5 ml of H2O2 (hydrogen peroxide) in each tube. What do you observe?
– Place a glowing splint in the mouth parts of each test tube. What do you observe?
Questions
1. Explain your observations.
2. Write down the chemical equation of the reaction taking place in tube A and B3. Carry out your further research to find out the characteristics of enzymes
Enzymes speed up the rate of metabolic reactions by allowing the reaction to go
through a more stable transition state than would normally be the case. As a result,
the rate of reaction is increased. In many chemical reactions, the substrate will not be
converted to a product unless it is temporarily given some extra energy referred toas activation energy (the minimum energy required the make a reaction take place).
Enzymes speed up the rate of biochemical reactions in the cell but remain unchanged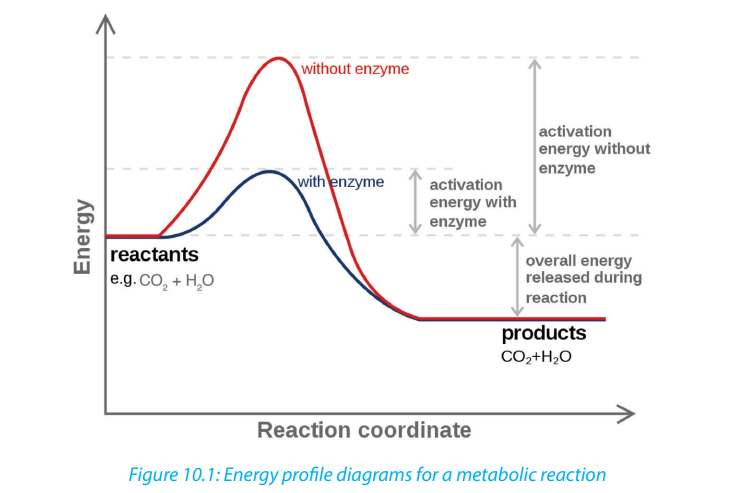
at the end of the reactions. An enzyme has no effect on the relative energy content
of products versus reactant. Chemical reactions catalyzed by enzymes are usually
reversible e.g. enzyme carbonic anhydrase catalyzes both synthesis and breakdownof carbonic acid.
An enzyme provides a reaction surface and a hydrophilic environment for a reaction
to take place. This is normally a hollow or cleft in the enzyme which is called the
active site, but it is normally hydrophobic in nature rather than hydrophilic.
A very small amount of enzymes is needed to react with a large amount of substrate.
The turnover number of an enzyme is the number or reactions an enzyme molecule
can catalyse in one second. Enzymes have a high turnover number e.g. the turnover
number of catalase is 200,000 i.e. one molecule of enzyme catalase can catalyse the
breakdown of about 200,000 molecules of hydrogen peroxide per second into waterand oxygen at body temperature.
A cofactor is the best general term to describe the non-protein substances required
by an enzyme to function properly. This term covers both organic molecules and
metal ions. A co-enzyme is an organic molecule that acts as a cofactor. A prostheticgroup is a cofactor that is covalently bound to the enzyme.
Self-Assessment 10.2
1. State any four properties of enzymes.
2. Enzymes have generally high turnover number. What is the significance ofthe high turnover of enzymes?
10.3. Mode of action of enzymes
Activity 10.3
There are two main hypotheses that explain the mode of action of an enzyme on
its substrate: the lock and key hypothesis and the induced-fit hypothesis. Carryout a research to find the relevance of each.
Enzymes do not change but substrates are converted into products. A substrate is a
molecule upon which an enzyme acts. In the case of a single substrate, the substrate
binds with the enzyme active site to form an enzyme-substrate complex. Thereafter
the substrate is transformed into one or more products, which are then released
from the active site. This process is summarized as follows: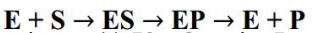
Whereby: E = enzyme, S = substrate(s), ES = Complex Enzyme-Substrate and P=
product (s). There are two main hypotheses explaining the mechanism of enzyme
action:
a. The lock and key hypothesis by Emil Fischer
In this hypothesis the substrate is the key and enzyme is the lock. The active site isexactly complementary to the shape of the substrate as shown below.
b. The induced-fit hypothesis by Daniel Koshland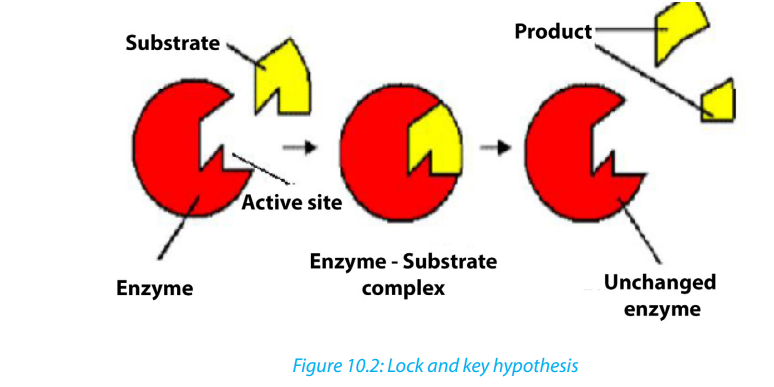
The induced-fit hypothesis is a modified version of the lock and key hypothesis and
is more widely accepted hypothesis. In this hypothesis, the active site is flexible andis not fully complementary with the shape of the substrate. An enzyme collides with
the substrate molecule and binds to the active site. This induces a slight change in
the shape of the enzyme making the substrate the fit more precisely. This reduces
the potential energy of the substrate and allows the reaction to occur. The products
formed move away from the active site and regains its original configuration readyfor the next reaction to take place.
Self-Assessment 10.3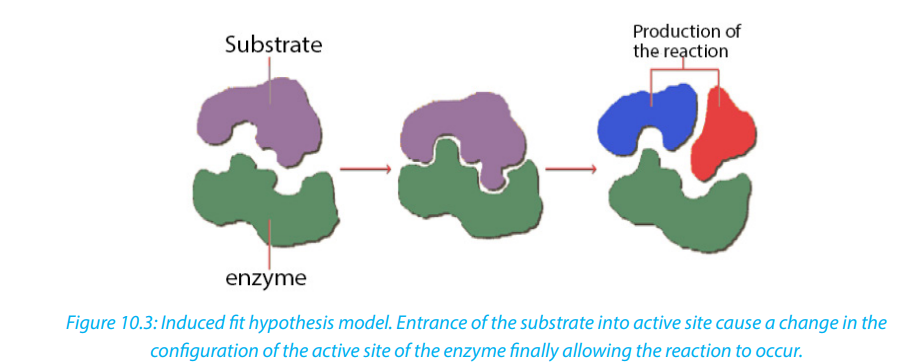
The key and lock hypothesis is a model that explain the mode of action of an
enzyme on the substrate. In the same context, analyse the diagram below andthen answer question that follow.
1. What does the lock represent?
2. What does the key represent?
3. Where is the active site?
4. Suggest another diagram that can better represent the induced fithypothesis. Write short notes to explain its functioning.
10.4 Factors affecting enzyme action
Activity 10.4
You will need
Eight test tubes containing 2 cm3
starch solution, amylase solution, cold water
(ice) water bath, iodine solution, HCl solution, and droppers
Procedure:
1. Label your test tubes A-D as follows: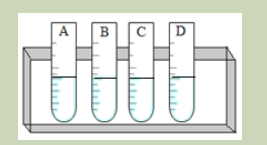
2. Add 1 cm3 of starch solution to each test tube
3. Keep tube A and B in cold (ice) and tube C and D in the water bath at 35oC
for 5 minutes.
4. Add 1 cm3 of 1M HCl on test tubes B and D, then shake the mixture to stir.
5. Add 1 cm3 of amylase solution on each test tube. Shake and therefore
keep A and B in cold and C and D in water bath for 10 minutes.
6. Take a sample from each tube and mix it with one drop of iodine. Use a
different tile for each test tube. Record and interpret your observation andthen draw a conclusion.
Enzymes activities can be limited by a number of factors such as the temperature, the pH,
the concentration of the substrate or the enzyme itself and the presence of inhibitors.i. TemperatureAt zero temperature, the enzyme cannot work because it is inactivated. At low
temperatures, an enzyme-controlled reaction occurs very slowly. The molecules
in solution move slowly and take a longer time to bind to active sites. Increasing
temperature increases the kinetic energy of the reactants. As the reactant moleculesmove faster, they increase the number of collisions of molecules to form enzyme substrate complex.
At optimum temperature, the rate of reaction is at maximum. The enzyme is in
active state. The optimum temperature varies with different enzymes. The optimum
temperature for enzymes in the human body is about 37oc.
When the temperature exceeds the optimum level, the enzyme is denatured.
The effect is irreversible. However, some species are thermophilic that is they work
better at high temperatures; others are thermophobic, that is they work better at
low temperatures. For example, some thermophilic algae and bacteria can survive
in hot springs of 60oc.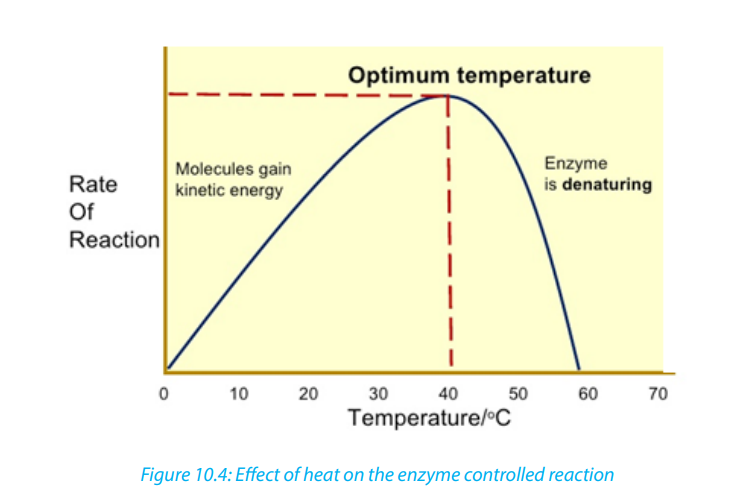
The rate doubles for each 10oC
rise in temperature between 0oC and 40oC (figure 10-
5). The temperature coefficient Q10 is the number which indicates the effect of rising
the temperature by 10oC on the enzyme-controlled reaction. The Q10 is defined as
the increase in the rate of a reaction or a physiological process for a 10°C rise in
temperature. It is calculated as the ratio between rate of reaction occurring at (X
+ l0) oC and the rate of reaction at XoC. The Q10 at a given temperature x can becalculated from:
Worked out example
The rate of an enzyme-controlled reaction has been recorded at different
temperatures as follows: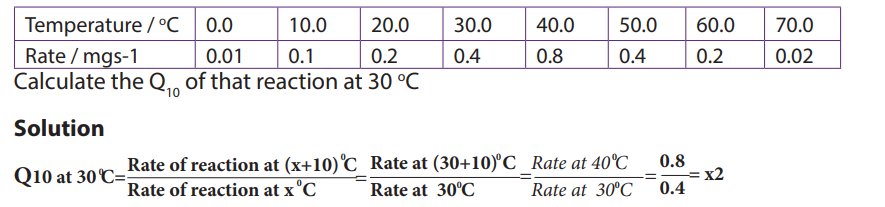
This means that the rate of the reaction doubles if the temperature is raised from
30°c to 40°c
Be aware that not all enzymes have an optimum temperature of 40°c. Some bacteria
and algae living in hot springs (e.g. Amashyuza in Rusizi) are able to tolerate very
high temperatures. Enzymes from such organisms are proving useful in variousindustrial applications because they do not denature up to 700c
ii. The pH
Most enzymes are effective only within a narrow pH range. The optimum pH is the
pH at which the maximum rate of reaction occurs. Below or above the optimum pH
the H+ or OH- ions react with functional groups of amino acids in the enzyme whichloses its tertiary structure and become natured.
Different enzymes have different pH optima (look in the table).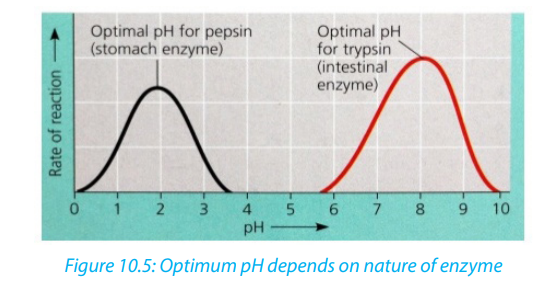 Table 10.1. Optimum pH of some digestive enzymes
Table 10.1. Optimum pH of some digestive enzymes
iii. Enzyme concentration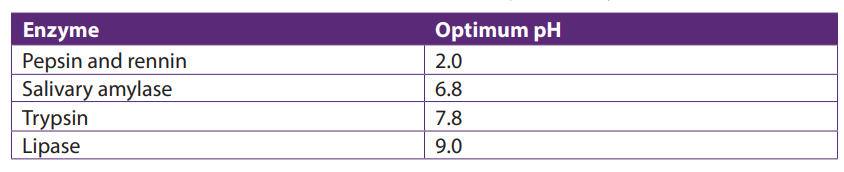
The rate of an enzyme-catalyzed reaction is directly proportional to the concentration
of the enzyme if substrates are present in excess concentration and no other factorsare limiting.
iv. Substrate concentration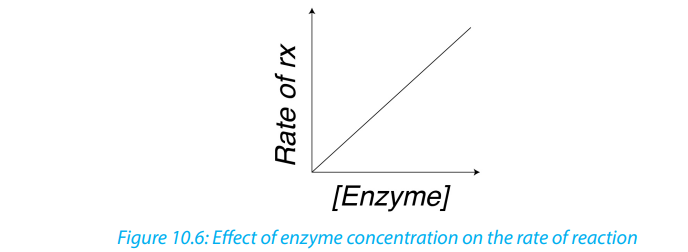
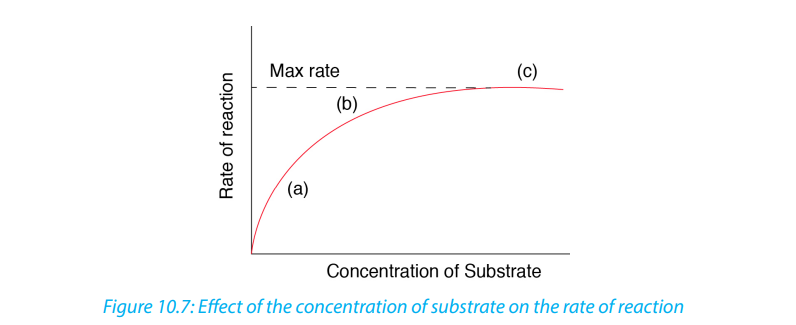
At low substrate concentration, the rate of an enzyme reaction increases with
increasing substrate concentration. The active site of an enzyme molecule can only
bind with a certain number of substrate molecules at a given time. At high substrate
concentration, there is saturation of active sites and the velocity of the reactionreaches the maximum rate.
b. Inhibitors
The inhibitors are chemicals or substances that prevent the action of an enzyme. An
inhibitor binds to an enzyme and then decreases or stops its activity. There are three
types of inhibitors:
i. Competitive inhibitors are molecules that have the similar shape as the
substrate. At high concentration, they compete with the substrate for the active
site of the enzyme e.g. O2
competes with CO2in RuBP-carboxylase.
ii. Non-competitive inhibitors are molecules that can be fixed to the other part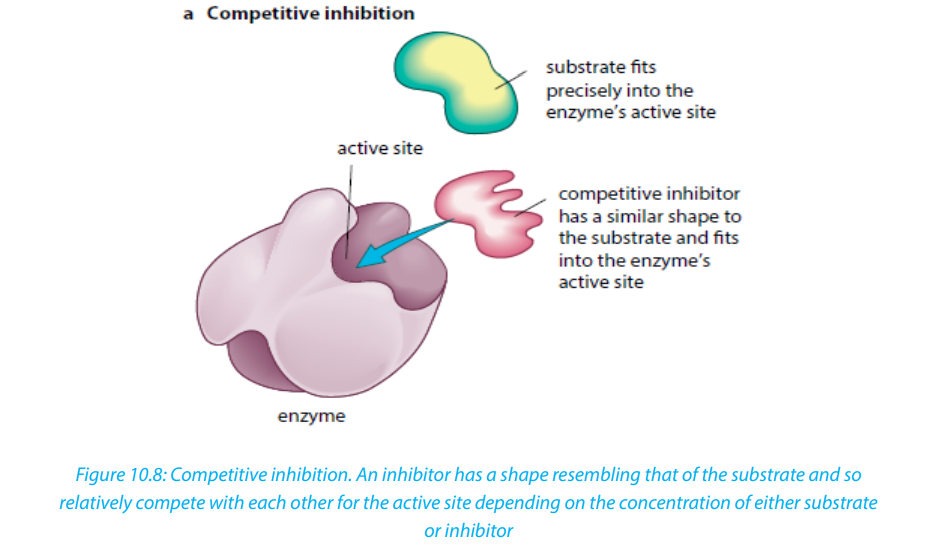
of enzyme (not to the active site) so that they change the shape of active site,due to this the substrate cannot bind to the active site of the enzyme.
iii. End product inhibitor, Allosteric inhibitor or Allostery.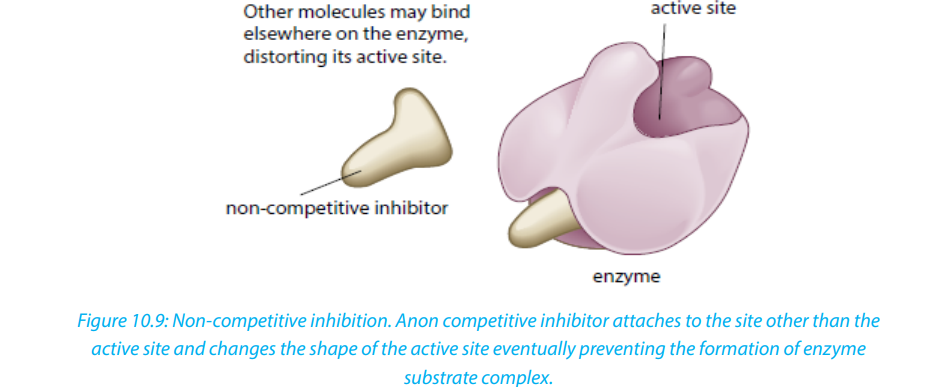
This is a chain enzymatic metabolic pathway where the final end product acts as
an allosteric reversible inhibitor for the first, the second or the third step in the
metabolic pathway. The shape of an allosteric enzyme is altered by the binding of
the end product to an allosteric site. This decreases enzymatic activity. By acting
as allosteric inhibitors of enzymes in an earlier metabolic pathway, the metabolites
can help to regulate metabolism according to the needs of organisms. This is anexample of negative feedback.
This often happens when few enzymes are working on a large number of substrate
e.g. ATP is an end-product inhibitor of the enzyme PFK (Phosphofructokinase) in
glycolysis during cell respiration. The end-product inhibitor leads to a negativefeedback.
The products of enzyme-catalyzed reactions are often involved in the feedback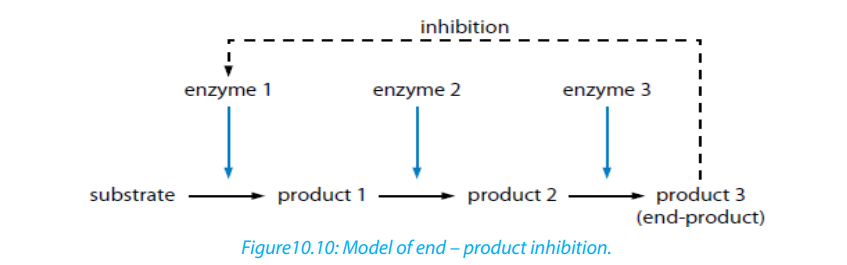
control of those enzymes. Glucose-1-phosphate is the product formed from this
enzyme-catalyzed reaction. As its concentration increases, it increasingly inhibitsthe enzyme.
Importance of reversible and irreversible inhibition
The nerve gas DIPF (DiIsopropyl Phosphor Fluoridate) is an irreversible inhibitor. It
binds permanently with enzyme acetyl cholisterase, altering its shape. The enzyme
cannot bind with and break down its substrate acetylcholine (neurotransmitter).
Acetylcholine molecules accumulate in the synaptic cleft. Nerve impulses cannot be
stopped causing continuous muscle contraction. This leads to convulsions, paralysisand eventually death.
Many pesticides such as organophosphate pesticides act as irreversible enzyme
inhibitors. Exposure to pesticides can produce harmful effects to the nervous and
muscular systems of humans. Heavy metal ions such as Pb2+, Hg2+, Ag+, As+ and
iodine-containing compounds which combine permanently with sulfhydryl
groups in the active site or other parts of the enzyme cause inactivation of enzyme.This usually disrupts disulphide bridges and cause denaturation of the enzyme.
Self-Assessment 10.4
1. What is Q10 of an enzyme controlled reaction?
2. You are provided with the table below of the rate of an enzyme controlledreaction.
Calculate the value of Q10 at:
a. 0° c
b. 10° c
c. 50° c
3. Explain why thermophile bacteria and algae are useful in some industrial
processes
4. The diagram below represents a metabolic pathway controlled by enzymes.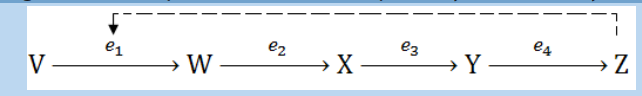 – V is a substratea. Name the type of control mechanism which regulates production of
– V is a substratea. Name the type of control mechanism which regulates production of
– W, X and Y are intermediate compounds
– Z is a product– e1, e2, e3, and e4 are enzymes
compound Zb. Explain how an excess of compound Z will inhibit its further production.
10.5. Importance of enzymes in living organisms
Activity10.5
Discuss and present your ideas about the need for different enzymes in livingorganisms.
Without enzymes, most of the biochemical reactions in living cells at body
temperature would occur very slowly or not at all. Enzyme can only catalyze reactions
in which the substrate shape fits that of its active site
There are thousands of metabolic reactions that place in the body that require
enzymes to speed up their rate of reaction, or will never happen. Enzymes are very
specific, so nearly each of these chemical reactions has its own enzyme to increase
its rate of reaction. In addition, the organism has several areas that differ from one
another by the PH. Therefore, the acid medium requires enzymes that work at low
pH while other media are alkaline and require enzymes that work at high pH. In
addition to digestion, enzymes are known to catalyze about 4,000 other chemical
reactions in your body. For example, enzymes are needed to copy genetic materialbefore your cells divide.
Enzymes are also needed to generate energy molecules called ATP, move fluid and
nutrients around the insides of cells and pump waste material out of cells. Most
enzymes work best at normal body temperature about at 370 c -- and in an alkaline
environment. As such, high fever and over-acidity reduce the effectiveness of mostenzymes. Some enzymes need co-factors or co-enzymes to work properly.
Self-Assessment 10.5
1. Fill the blank with appropriate terms:
Enzymes are biological ____________________ produced by
___________________________ cells. Enzymes reduce the amount of
____________________ energy required for reactions to occur. They consist of
globular ____________________ with _______________________ structure.
2. Answer the following questions:a. What is the main role of enzymes?10.6. Enzymes technologyb. What would happen if there are no enzymes in the cell?
Learning activity 10.6
Enzymes are needed in everyday life. At school you can use salivary amylase
to hydrolyse starch. There is industrial technique used to get large amounts of
enzyme amylase.
Read through the notes below and answer the following questions below:a. State the different processes in which enzyme technology is appliedThe market for enzymes is prosperous. The demand keeps on increasing as new
b. What is the role of thermophilic bacteria in this process?c. How is the effectiveness of an enzyme improved for used in industry?
applications of enzymes are discovered. Enzymes have been used in cheese-making,
in leather industries, and making washing powders.
Microbial cells are still the most sources of industrial enzymes because microorganisms
naturally produce enzymes inside their cells known as intracellular enzymes.
When microorganisms secrete their enzymes for an action outside their cells, the
enzymes are called extracellular enzymes. Microorganisms may have specific genes
introduced into their DNA by genetic engineering so that they produce enzymesnaturally made by other organisms.
Once enzymes are produced by the microorganisms they are isolated by
centrifugation in order to remove the large cell fragments. The enzyme is precipitated
from solution by a salt such as (NH4)2SO4 or an alcohol such as CH3-CHOH-CH3.
Thereafter the enzyme can be purified by the process known as electrophoresis or
column chromatography. The enzyme stability is a key factor in the industrial use of
enzymes. The stability of an enzyme is its ability to retain its tertiary structure undera wide range of conditions.
As many industrial processes require high temperatures and extreme pH, it is
recommended to use bacteria such as Bacillus subtilis which withstand harsh
conditions such as high temperature. Those thermophilic bacteria produce
thermostable enzymes that do not denature at high temperature because their
optimum temperature between 65 - 750c.
Some useful enzymes are not thermostable. Such enzymes should be improved by
the technique called immobilization i.e. the enzyme is attached to or located withinan unreactive support such as nylon that protects it from denaturation.
Self-Assessment 10.6
1. What is the role of alcohol or ammonium sulphate during the extraction of
enzymes?
2. Why is thermostability of enzymes so important for many industrial processes?End of unit assessment 10
1.
a. What is the meaning of the following terms related to enzyme activity?
i. Catalyst
ii. Activation energy
iii. Lock and key hypothesis
iv.Q10
b. Why are there hundreds of different enzymes in a cell?
c. How do enzymes reduce the activation energy of a reaction?
2. Enzyme activity is affected by a number of factors.
a. Explain why enzymes work faster at relatively high temperatures
b. Describe what happens to the enzyme structure if the temperature is raised
above the optimum temperature.
c. How are enzymes affected by pH?
d. Why do different enzymes have a different optimum pH?
e. What is the difference between a reversible and irreversible enzymeinhibitor?
3. Some bacteria and algae can survive in boiling water of hot springs. Enzymes
from those organisms are used in industrial processes. Why are those enzymes
useful?
4. The following set of data shows the effect of temperature on the completion
time of an enzyme reaction.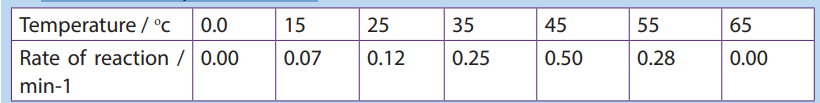
a. Plot the data on a graph
b. What is the optimum temperature of this reaction?
c. Describe the shape of the graph between 10 and 40oc
d. Calculate the rate of increase between 20 and 30oc.5. The table below shows the rate of an enzyme reaction at a range of temperature:
a. Fill that table with the values of the rate of reaction and plot a graph of rate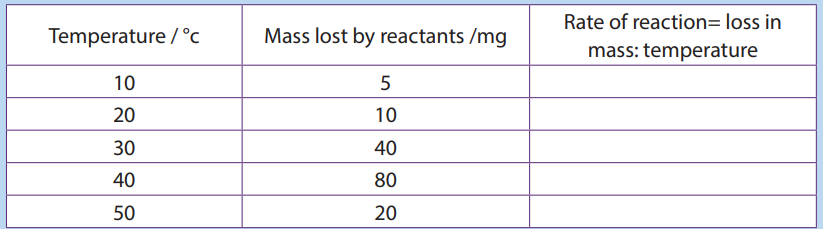
at different temperatures (use x-axis for temperature).
b. Calculate Q10 at 30°c.
c. Explain what happen between 20 and 30°c, and between 40 and 50°c.
6. The graph below shows the activity of a commercial enzyme alcalase atdifferent pH value. Alcalase is a protease enzyme.
a. What are the compounds digested by this enzyme?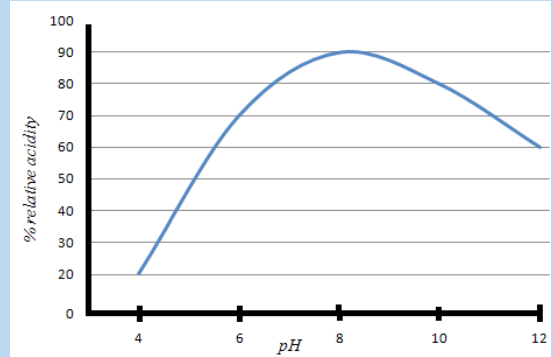
b. Describe the change in enzyme activity with PH.
c. How does this curve compare to the pH curve of a human digestive
enzyme such as pepsin?
7. Outline how a specific enzyme can be produced from bacteria.
