UNIT 1:APPLICATIONS OF THERMODYNAMICS LAWS
Mutesi is a parent of two children at a certain school. Before she takes them
to school, she first makes sure that she prepares food and drinks for them
and packs some in flasks so that her children can eat and drink during lunch
time.
She then drives them to school before she reports to her working place and
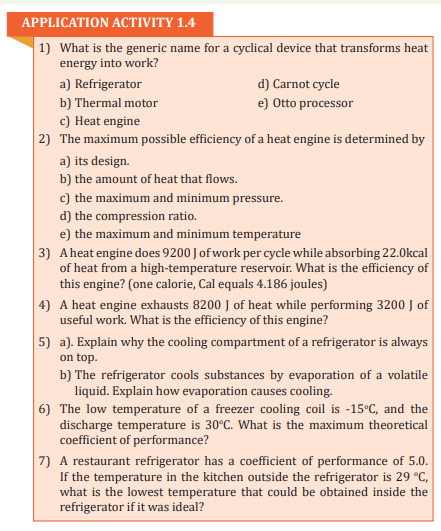
then from the school she then diverts to her working place which is about 5
km away from the school.
The parking yard at her work place is a plain place without any shade but she
makes sure that her car is parked near a tree that is near the parking yard
to prevent it from different damages among which is destruction of tyres of
the car.
a) Explain why Mutesi makes use of flasks not normal utensils like metallicbowels while parking foods and drinks for her children.
b) Is there heat exchange inside the flasks? Explain your reasoning.
c) Imagine on a certain day these two children only eat food and leaves,
the drink in the flask and by mistake they forget flask in the store and
the mother come to pick it the next day. Do you think the contents in the
flask will be at the same temperatures? Explain all scientific phenomena
that may lead to either loss or gain in energy of the contents in the flask.
d) Explain why in most cases the outer covering of a flask is always made
of a poor conductor? Explain how quality and efficiency of these flasks
can be improved by manufactures.
e) Based on statements above, Mutesi normally parks her car under a
shade to prevent her car from being exposed to sunshine. Explain how
during hot days the tyres of a car may burst.
f) Her Car uses petrol in operation. During operation of her car, the engine
draws fuel (Petrol) air mixture from the tank into the engine, explainall the processes that take place in the engine.
Thermodynamics refers to the study of heat and its transformation into
mechanical energy.
In thermodynamics, the internal energy is one of the two extremely important
state functions of the variables of a thermodynamic system. It refers to total
energy contained within the system excluding the kinetic energy of motion of
the system and the potential energy of the system due to external forces. Itkeeps account of the gains and losses of energy of the system.
The internal energy of a system may be changed by
i) heating the system
ii) doing work on it,
iii) adding or taking away matter.
The thermal energy is the portion of internal energy that changes when the
temperature of the system changes. Sometimes the term thermal energy is
used to mean internal energy. Heat is defined as the transfer of energy across
the boundary of a system due to a temperature difference between the system
and its surroundings.
When you heat a substance, you are transferring energy into it by placing it
in contact with surroundings that have a higher temperature. For example,
when you place a pan of cold water on a stove burner, the burner is at a higher
temperature than the water, and so the water gains energy.
In daily life, we recognize the difference between internal energy and heat. The
heat transfer is caused by a temperature difference between the system and its
surroundings. However, in some systems there are no temperature and pressuregradients, such systems are said to be in thermodynamic equilibrium.

Consider a gas contained in a cylinder fitted with a movable piston. At
equilibrium, the gas occupies a volume V and exerts a uniform pressure P on
the cylinder’s walls and on the piston. If the piston has a cross-sectional area
A, the force exerted by the gas on the piston is F = PA. Now let us assume that
we push the piston inward and compress slowly to allow the system to remainessentially in thermal equilibrium.
With work done by the force due to pressure, we find the same relation but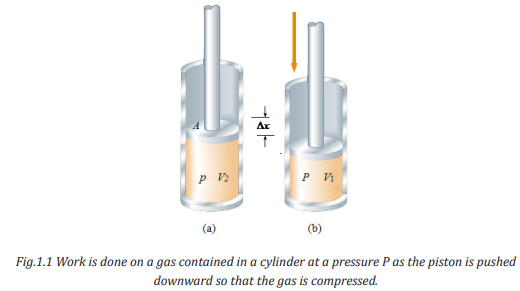
having a negative sign. The force is exerted in opposite direction and the finalvolume is less than the initial one.

The total work done on the gas as its volume changes from initial volume (Vi )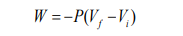
to final volume (Vf ) is given by the above equation.
If the gas is compressed, ΔV is negative and the work done on the gas is positive
(Work done by the gas is positive) and if the gas expands, ΔV is positive and
the work done on the gas is negative (Work done on the gas is negative). If
the volume remains constant, the work done on the gas is zero. Thus, no work
done. To evaluate this relation, one must know how the pressure varies with
volume during the process.
The work done on a gas in a quasi-static process that takes the gas from an
initial state to a final state is the negative of the area under the curve on a PV
diagram, evaluated between the initial and final states.
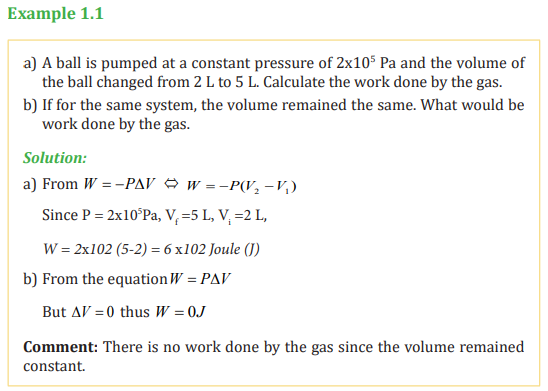
Based on the processes of compressing a gas in the cylinder indicated in figure
1.1, the work done depends on the path taken between the initial and finalstates

1.3.1. First law of Thermodynamics
It states that the change in internal energy of a system is equal to the heat added
to the system minus the work done by the system. Therefore, the law stated
gives mathematical treatment of internal energy of a system shown below.Hence the first law of thermodynamics.



Note:
- The first law of thermodynamics is a special case of the law of conservation
of energy that encompasses changes in internal energy and energy
transfer by heat and work.
- It is a law that can be applied to many processes. It is noticed that energy
can be transferred between a system and its surroundings.
- One is work done on the system, which requires that there be a macroscopic
displacement of the point of application of a force.
- The other is heat, which occurs on a molecular level whenever a
temperature difference exists across the boundary of the system.
- Both mechanisms result in a change in the internal energy of the system
and therefore usually result in measurable changes in the macroscopic
variables of the system, such as the pressure, temperature, and volume
of a gas.
- The increase in internal energy of a system is the sum of the work done on
the system and the heat supplied to the system.
- One of the important consequences of the first law of thermodynamics
is that there exists a quantity known as internal energy whose value is
determined by the state of the system. The internal energy is therefore a
state variable like pressure, volume, and temperature.
- The first law of thermodynamics is an energy conservation equation
specifying that the only type of energy that changes in the system is theinternal energy ΔU.
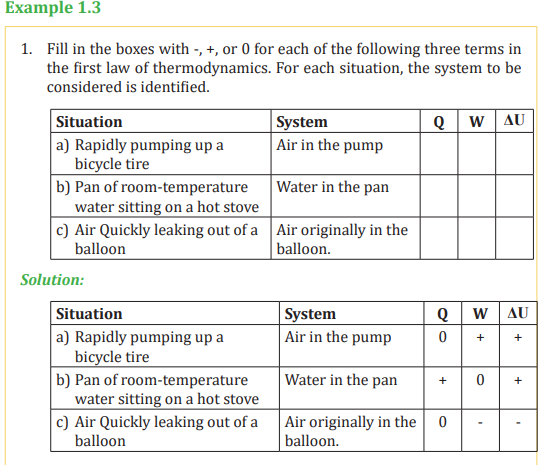

1.3.3. Applications of first law of Thermodynamics
The first law of thermodynamics that we discussed relates the changes in
internal energy of a system to transfers of energy by work or heat. In this case
, we consider applications of the first law in processes through which a gas istaken as a model.
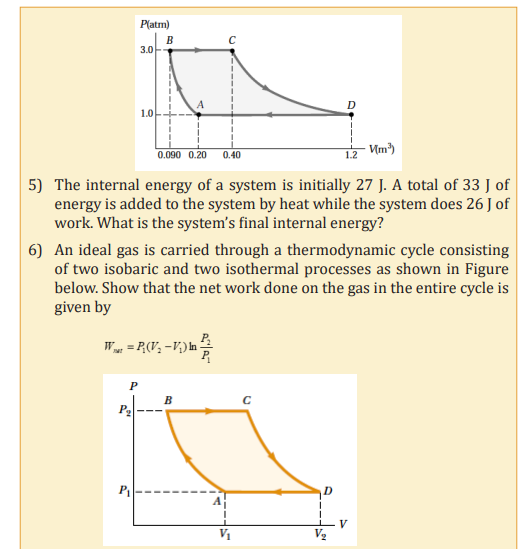
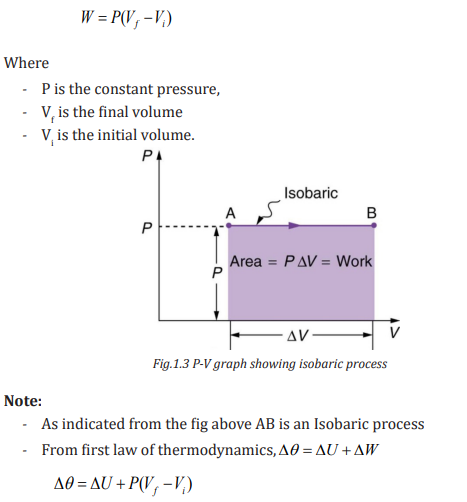
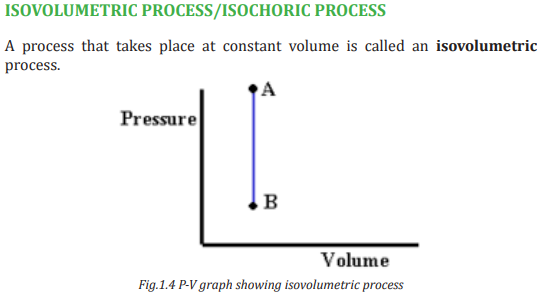
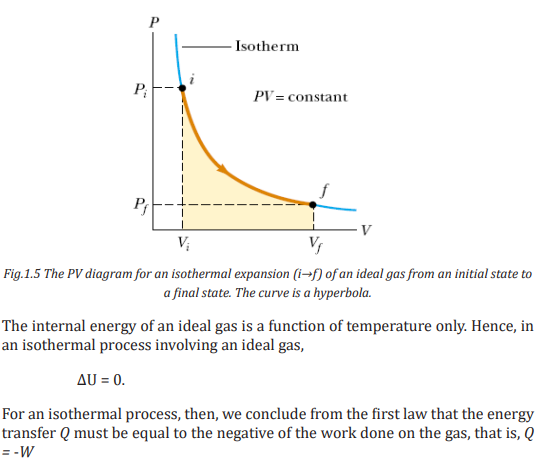
ISOBARIC PROCESS
A process that occurs at constant pressure is called an isobaric process. In such
processes, the values of the heat and the work are both usually nonzero. Thework done during isobaric process is simply



Since the first law of thermodynamics states that energy is conserved. There
are, however, many processes we can imagine that conserve energy but are not
observed to occur in nature. Lets consider an example below of the first law to
introduce the second law.
For example, when a hot object is placed in contact with a cold object, heat
flows from the hotter one to the colder one, never spontaneously the reverse.
If heat were to leave the colder object and pass to the hotter one, energy could
still be conserved. Yet it doesn’t happen spontaneously the reverse.
There are many other examples of processes that occur in nature but whose
reverse does not. To explain this lack of reversibility, scientists in the latter half
of the nineteenth century formulated a new principle known as the secondlaw of thermodynamics.
The second law of thermodynamics is a statement about which processes occur
in nature and which do not. It can be stated in a variety of ways, all of which are
equivalent. One statement is that: “Heat can flow spontaneously from a hot
object to cold object; heat will not flow spontaneously from a cold object
to a hot object”.
The development of a general statement of the second law of thermodynamics
was based partly on the study of heat engines. A heat engine is any device
that changes thermal energy into mechanical work, such as steam engines andautomobile engines.




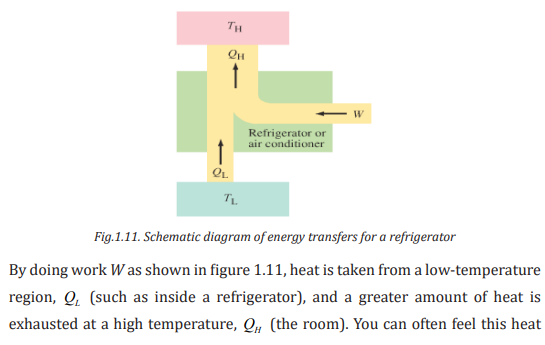
1.4.2.3. Impact of heat engines on climate
Most of air pollution is caused by the burning of fuels such as oil, natural gas
etc. The air pollution has an adverse effect on the climate. Climate change is the
greatest environmental threat of our time endangering our health. When a heat
engine is running, several different types of gases and particles are emitted that
can have detrimental effects on the environment.
Of concern to the environment are carbon dioxide, a greenhouse gas; and
hydrocarbons. Engines emit greenhouse gases, such as carbon dioxide, which
contribute to global warming. Fuels used in heat engines contain carbon. The
carbon burns in air to form carbon dioxide.
The Carbon dioxide and other global warming pollutants collect in the
atmosphere and act like a thickening blanket and destroy the ozone layer.
Therefore, the sun’s heat from the sun is received direct on the earth surface
and causes the planet to warm up.
As a result of global warming, the vegetation is destroyed, ice melts and water
tables are reduced. Heat engines especially diesel engines produce Soot which
contributes to global warming and its influence on climate.
The findings show that soot, also called black carbon, has a warming effect.
It contains black carbon particles which affect atmospheric temperatures in a
variety of ways. The dark particles absorb incoming and scattered heat from the
sun; they can promote the formation of clouds that can have either cooling or
warming impact.Therefore soot emissions have significant impact on climate
change.
Similarly, some engines leak, for example, old car engines and oil spills all over.
When it rains, this oil is transported by rain water to lakes and rivers. The oils
then create a layer on top of the water and prevent free evaporation of the water.
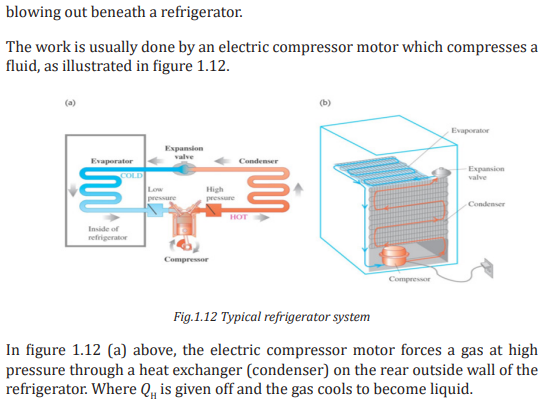
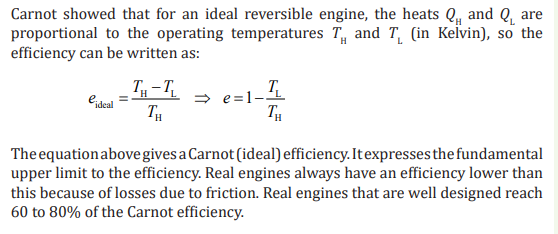
1.4.3. Carnot cycle and Carnot engine
In 1824 a French engineer named Sadi Carnot described a theoretical engine,
now called a Carnot engine, which is of great importance from both practical
and theoretical viewpoints. He showed that a heat engine operating in an ideal,
reversible cycle—called a Carnot cycle—between two energy reservoirs is the
most efficient engine possible.
An ideal engine establishes an upper limit on the efficiencies of all other engines.
That is, the net work done by a working substance taken through the Carnot
cycle is the greatest amount of work possible for a given amount of energy
supplied to the substance at the higher temperature.
Carnot’s theorem can be stated that no real heat engine operating between
two energy reservoirs can be more efficient than a Carnot engine operating
between the same two reservoirs.
Note: No Carnot engine actually exists, but as a theoretical idea it played an
important role in the development of thermodynamics.
The idealized Carnot engine consisted of four processes done in a cycle, two of
which are adiabatic (Q = 0) and two are isothermal (ΔT = 0). This idealized cycle
is shown in figure 1.8.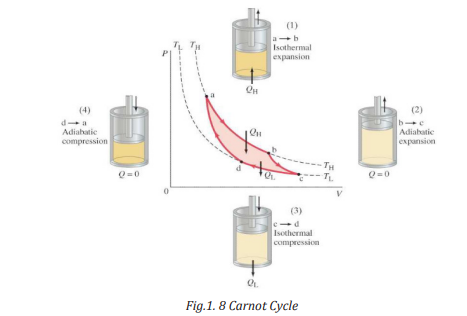



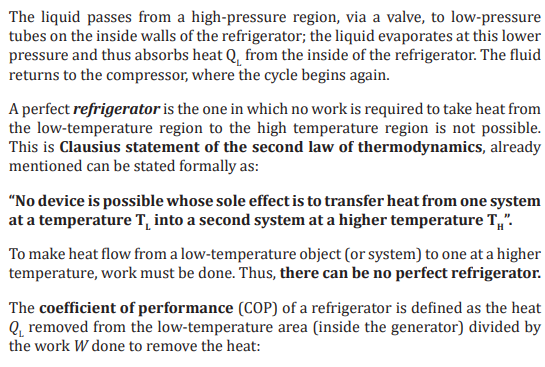
From P-V diagram for the Ideal Diesel cycle, the cycle follows the numbers 1-4
in clockwise direction. The image on the top shows a P-V diagram for the ideal
Diesel cycle; where P is pressure and V is specific volume. The ideal Diesel cycle
follows the following four distinct processes (the color references refers to the
color of the line on the diagram.
• Process 1-2 is isentropic (adiabatic) compression of the fluid (blue
color).
• Process 2-3 is reversible (isobaric constant pressure heating (red).
• Process 3-4 is isentropic (adiabatic) expansion (yellow).
• Process 4-1 is reversible constant volume cooling (green).
The Diesel is a heat engine; it converts heat into work. The isentropic processes
are impermeable to heat; heat flows into the loop through the left expanding
isobaric process and some of it flows back out through the right depressurizing
process, and the heat that remains does the work.