UNIT 7 NATURE OF PARTICLES AND THEIR INTERACTIONS
Key unit competence: Organize the properties and basic principles of quarks.
My goals
• The key varieties of fundamental subatomic particles and how they
were discovered.
• Distinguish between fundamental particles and composite particles
• Distinguish between particles and antiparticles
• Describe how antimatter can be used as a source of energy
• State some applications for elementary particles
• Compare matter and antimatter
• The four ways in which subatomic particles interact with each other.
• Analyze the structure of protons, neutrons, and other particles can beexplained in terms of quarks
INTRODUCTORY ACTIVITY
Investigating the elementary particles discovery
In the study of matter description and energy as well as their interactions;
the fascinating thing of discovery is the structure of universe of unknown
radius but still to know the origin of matter one need to know about small
and smallest composites of matter. The smallest particle was defined to be
electron, proton, and neutron. But one can ask:
1. Are electron, proton and neutron the only particle that can define the
origin of matter?
2. What are other particles matter is composed of?3. Describe and discuss how particles interact with energy to form matter
7.1 ELEMENTARY PARTICLES.
7.1.1 Introduction
ACTIVITY 7.1: Investigate the presence of smaller particles
1. Use internet and retrieve the definition and the information about
elementary particles, and then answer to the following questions.
2. What does elementary particle physics talk about?
3. What are the elementary particles found through your research?
4. Discuss and explain the use of knowledge about the elementaryparticles.
Particle physics, also known as high-energy physics, is the field of natural
science that pursues the ultimate structure of matter.
The protons and neutrons are collectively called hadrons, were considered
as elementary particles until 1960. We now know that they are composed of
more fundamental particles, the quarks. Electrons remain elementary to
this day. Muons and τ-leptons, which were found later, are nothing but heavy
electrons, as far as the present technology can tell, and they are collectively
dubbed leptons.
Quarks and leptons are the fundamental building blocks of matter. The
microscopic size that can be explored by modern technology is nearing. The quarks and leptons are elementary at this level (Nagashima, 2013).
Particle physics is the study of the fundamental constituents of matter and
their interactions. However, which particles are regarded as fundamental have
changed with time as physicists’ knowledge has improved. Modern theory called
the standard model attempts to explain all the phenomena of particle physics
in terms of the properties and interactions of a small number of particles of
three distinct types (see Fig.7.1):
• Two families of fermions (of spin ½): leptons and quarks• One family of bosons (of spin 1)
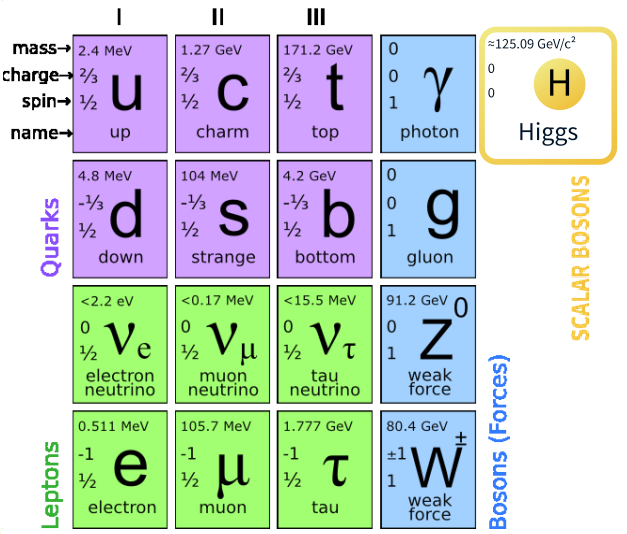
Fig.7. 1 Fundamental Standard model of elementary particle
I, II and III represent the first, second and the third generations. In addition,
at least one spin-0 particle, called the Higgs boson, is postulated to explain the
origin of mass within the theory, since without it all the particles in the model
are predicted to have zero mass (see Fig.7.1).
All the particles of the standard model are assumed to be elementary; i.e. they
are treated as point particles, without internal structure or excited states. The
most familiar example of a lepton is the electron (the superscript denotes the
electric charge), which is bound in atoms by the electromagnetic interaction,
one of the four fundamental forces of nature. A second well-known lepton is
the electron neutrino, which is a light, neutral particle observed in the decay
products of some unstable nuclei (the so-called β-decays). The force responsiblefor the β-decay of nuclei is called the weak interaction.
7.1.2 Checking my progress
1. Particles that make up the family of Hadrons are:
a. Baryons and mesons c. Protons and electrons
b. Leptons and baryons d. Muons and Leptons
2. Using the elementary particles, Complete the following sentences
I. One family of bosons of spin 1 called__________ which act as ‘force
carriers’ in the theory
II. Two fermions of spin 1/2 called_________ and ________
3. The first antiparticle found was the
a. Positron c. Quark
b. Hyperons d. baryon4. Explain what is meant by particle physics?
7.2 CLASSIFICATION OF ELEMENTARY PARTICLES.
ACTIVITY 7.1: Classes of elementary particles
Based on the previous introduction section, reread the text and the
answer to the following questions.1. What are the types of elementary particles?There are three properties that describe an elementary particle ‘’mass,’’2. What properties are based on to classify elementary particles?
‘’charge’’ and ‘’spin’’. Each property is assigned as number value. These
properties always stay the same for an elementary particle.• Mass (m): a particle has mass if it takes energy to increase its speed or
to accelerate it. The values are given in MeV/C2
. This comes from special
relativity, which tells us that energy equals mass times the square of
the speed of light. 2 E mc = × . All particles with mass are affected by
gravity even particles with no mass like photon.
• Electric charge (Q): particles may have positive, negative charge or
none. If one particle has a negative charge and another particle has a
positive, the two particles are attracted to each other. If particles have
a similar charge, they repel each other. At a short distance this force
is much stronger than the force of gravity which pulls all particles
together. An electron has a charge -1 and a proton has a charge +1. A
neutron has average charge 0. Normal quarks have charge of 2/3 or-1/3
Spin: the angular momentum or constant turning of particles has a7.2.1 Classification of particles by mass
particular value, called its spin number. Spin for elementary particle
is 0, 1 or . The spin property only donates the presence of angular
. The spin property only donates the presence of angularmomentum.
The most basic way of classifying particles is by their mass. The heaviest
particles are the hadrons and the lightest one is the leptons.
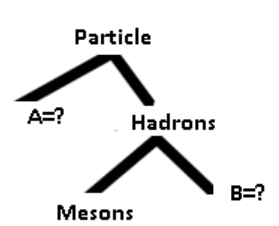
As seen the diagram above hadrons group is divided into baryons and mesons.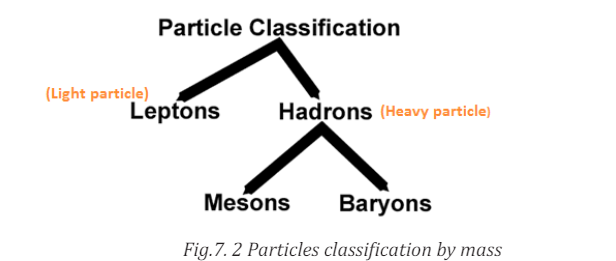 Baryons are the heaviest particles and are followed by mesons.
Baryons are the heaviest particles and are followed by mesons.
Hadrons are composite particles made of quarks held together by the strong
force in a similar way as molecules are held together by electromagnetic force.
They are subjected to the strong nuclear force and are not fundamental particlesas they are made up of quarks.
Baryons are composite sub-atomic particle made up of 3 quarksEx: Protons and neutrons.
(triquarks are distinct from mesons which are composed of one quark and
one antiquark). Baryon comes from Greek word which means “heavy”.
The protons are only stable baryons; all other baryons eventually decayinto proton.
• Mesons are hadrons sub-atomic particles made up of one quark andEach pion has quark and one anti-quark therefore is a meson.one anti-quark bound together by strong interaction. Ex: Pion and kaon
It is the lightest meson and generally the lightest hadrons. They are unstable.
Leptons do not interact via the strong force. They carry electric charge also interactvia the weak nuclear force. They include electron, muons, tau and three the types
of neutrino: the electron neutrino (νE), the muon neutrino (νμ
) and the tau neutrinovτ .
In summary, leptons are subjected to the weak nuclear force and they do not
feel the strong nuclear force.
Examples: Electron, muons and neutrino.
7.2.2 Classification of particles by spin.
The spin classification determines the nature of energy distribution in a
collection of particles. Particles of integer spin obey Bose-Einstein statistics
whereas those of half-integer spin behave according to Fermi-Dirac statistics
as shown in the following chart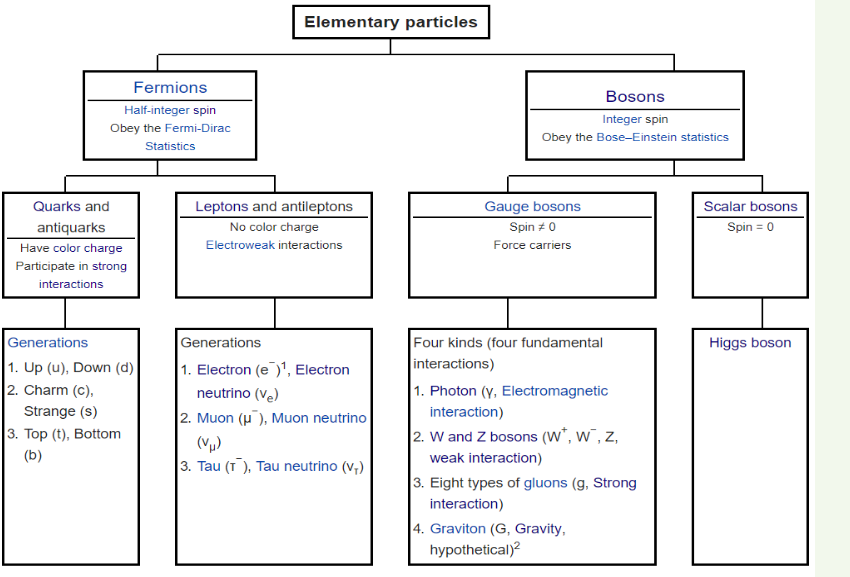
Fermions are particles which have half-integer spin and therefore are
constrained by the Pauli Exclusion Principle (see Section 7.4). It includes
electrons, protons and neutrons.
The fact that electrons are fermions is foundational to the buildup of the
periodic table of elements since there can be only one electron for each state
in an atom (only one electron for each possible set of quantum numbers).
The fermion nature of electrons also governs the behavior of electrons in a
metal where at low temperatures all the low energy states are filled up to a
level called the Fermi energy. This filling of states is described by Fermi-Dirac
statistics.
7.2.3 Checking my progress
7.3 ANTI PARTICLE AND PAULI’S EXCLUSION PRINCIPLE7.3.1 Concept of particle and antiparticle
ACTIVITY 7.3Discuss the following terms:There are two important points about pair production. The first is that you need
1. Particle
2. Antiparticle
to collect energy to produce the electron-positron pair. You need the equivalent
rest mass of energy that is the amount of energy contained in the both particle
and anti-particle when at rest. The energy converted to mass is ‘lost’ or fully
‘’bound’’ until the particle is annihilated and the energy can be recovered. The
second thing is that it needs a correct environment. The process does not occur
unless certain conditions are present.
Viewing the phenomena as a creative process we can say a threshold amountof energy is sacrificed in a correct context to manifest a pair of particle with
a physical mass. It can be said something was created out of nothing. That is
before the interaction, no particles with mass existed. After interaction, there
were two particles with mass. Hence something was created out of nothing.
But this can be said only because of the perspective taken when viewing the
process.
For every charged particle of nature, whether it is one of the elementaryparticles of the standard model, or a hadron, there is an associated particle
of the same mass, but opposite charge, called its antiparticle.
This result is a necessary consequence of combining special relativity withquantum mechanics. This important theoretical prediction was made by Dirac
and follows from the solutions of the equation he first wrote down to describe
relativistic electrons
7.3.2 Pauli’s exclusion principle,Pauli’s exclusion principle is a quantum mechanical principle which states
that:
“Two or more identical fermions (particles with half-integer spin) cannot
occupy the same quantum state simultaneously.”
In case of electrons in atoms it can be stated as follows: it is impossible for two
electrons of a poly-electron atom to have the same values of the four quantum
numbers:
The principle quantum number, the angular momentum quantum number
(l), the magnetic quantum number (ml) and the spin quantum number (ms).
For example, if two electrons reside in the same orbital and if their msmust be
different and thus like electrons must have opposite half integer spin projections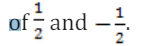
This principle was formulated by Austrian physicist Wolfgang Pauli in 1925 for
electrons, and later extended to all fermions with his spin–statistics theorem of
1940.
Particles with an integer spin, or bosons, are not subject to the Pauli Exclusion
Principle: any number of identical bosons can occupy the same quantum state,
for instance, photons produced by a laser and Bose–Einstein condensate.
The Pauli Exclusion Principle describes the behavior of all fermions (particles
with “half-integer spin”), while bosons (particles with “integer spin”) are
subject to other principles. Fermions include elementary particles such
7.3.3 Checking my progress1. What do you understand by antiparticle?
2. State Pauli’s exclusion principle?
3. Why Pauli’s exclusion Principle is known as exclusion?
7.4 FUNDAMENTAL INTERACTIONS BY PARTICLE EXCHANGE
ACTIVITY 7.4: Fundamental interaction
Using internet, discusses the fundamental interactions in terms of
exchange particles, then find the relation between the following concepts.
1. Gravitational forces
2. electroweak force,
3. Strong force and
4. Weak forces.
7.4.1 Forces and Interactions
have recognized three basic forces:• The gravitational force is an inherent attraction between two masses.In the 1860s, the Scottish physicist James Clerk Maxwell developed a theory
Gravitational force is responsible for the motion of the planets and
Stars in the Universe. It is carried by Graviton. By Newton’s law of
gravitation, the gravitational force is directly proportional to the
product of the masses and inversely proportional to the square of
the distance between them. Gravitational force is the weakest force
among the fundamental forces of nature but has the greatest large−
scale impact on the universe. Unlike the other forces, gravity works
that unified the electric andmagnetic forces into a single electromagnetic force.
Maxwell’s electromagnetic force was soon found to be the “glue” holding atoms,
molecules, and solids together. It is the force between charged particles such
as the force between two electrons, or the force between two current carrying
wires. It is attractive for unlike charges and repulsive for like charges. Theuniversally on all matter and energy, and is universally attractiveelectromagnetic force obeys inverse square law. It is very strong compared
• The electric force is a force between charges
• The magnetic force, which is a force between magnets or between
magnetic body and ferromagnetic body.
to the gravitational force. It is the combination of electrostatic and magnetic forces.
The discovery of the atomic nucleus, about 1910, presented difficulties that
could not be explained by either gravitational or electromagnetic forces.
The atomic nucleus is an unimaginably dense ball of protons and neutrons.
But what holds it together against the repulsive electric forces between the
protons? There must be an attractive force inside the nucleus that is stronger
than the repulsive electric force. This force, called the strong force, is the force
that holds the protons and neutrons together in the nucleus of an atom. It is the
strongest of all the basic forces of nature. It, however, has the shortest range,
of the order of 10−15 m. This force only acts on quarks. It binds quarks together
to form baryons and mesons such as protons and neutrons. The strong force is
mediated or carried by Gluons. Quarks carry electric charge so they experienceelectric and magnetic forces.
In the 1939, physicists found that the nuclear radioactivity called beta decay
could not be explained by either the electromagnetic or the strong force. Careful
experiments established that the decay is due to a previously undiscovered
force within the nucleus. The strength of this force is less than either the strong
force or the electromagnetic force, so this new force was named the weak
force. Weak nuclear force is important in certain types of nuclear process such
as β-decay. This force is not as weak as the gravitational force. The weak force
acts on both leptons and quarks (and hence on all hadrons). The weak force is
carried by W+, W- and Z. Leptons – the electrons, muons and tau – are chargedso they experience electric and magnetic forces.
Of these, our everyday world is controlled by gravity and electromagnetism. The
strong force binds quarks together and holds nucleons (protons & neutrons)
in nuclei. The weak force is responsible for the radioactive decay of unstablenuclei and for interactions of neutrinos and other leptons with matter.
By 1940, the recognized forces of nature (fundamental forces)were four:• Gravitational forces between masses,
• Electromagnetic forces resulting from the combination of electric and
magnetic fields,
• Strong force (nuclear force) between subatomic particles,
• Weak forces that arise in certain radioactive decay processes.By 1980, Sheldon Glashow, Abdus Salam, and Steven Berg developed a theory
that unifies electromagnetism and weak force into electroweak force. Hence,
our understanding of the forces of nature is in terms of three fundamentalforces:
• The gravitational force,
• The electroweak force,• The strong force.
The Table 7.1 below summaries the fundamental forces and force carriers.
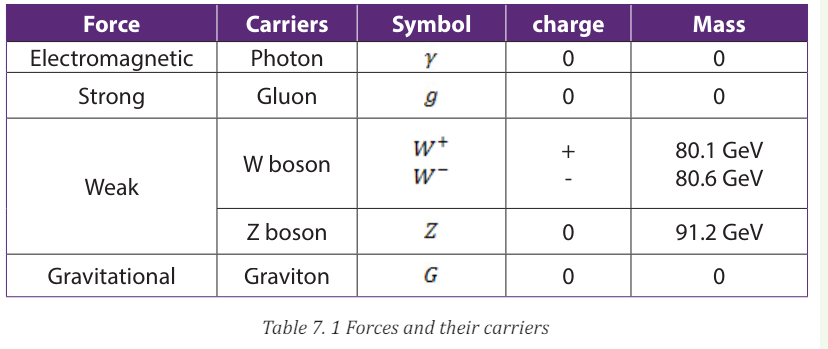
• W boson: short-lived elementary particle; one of the carriers of the
weak nuclear force
• Z boson: short-lived elementary particle; one of the carriers of the
weak nuclear force
• Graviton: the hypothetical particle predicted to carry the gravitationalforce
All the forces of nature should be capable of being described by single theory.
But only at high energies should be the behavior of the forces combines, this is
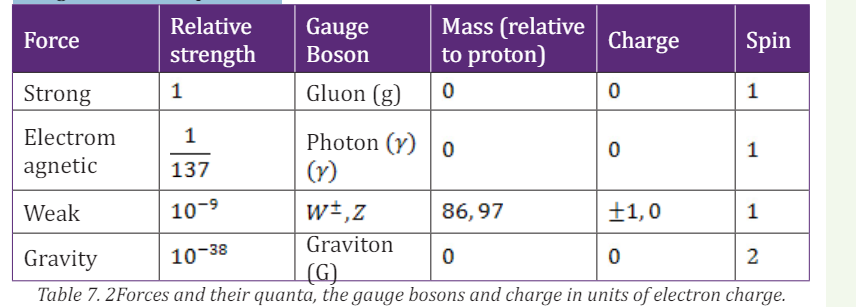
called unification. We can compare the relative strengths of the electromagnetic
repulsion and the gravitational attraction between two protons of unit chargeusing the above equations.
EXAMPLE 7.1
Thus the gravitational is the weakest of the fundamental forces. These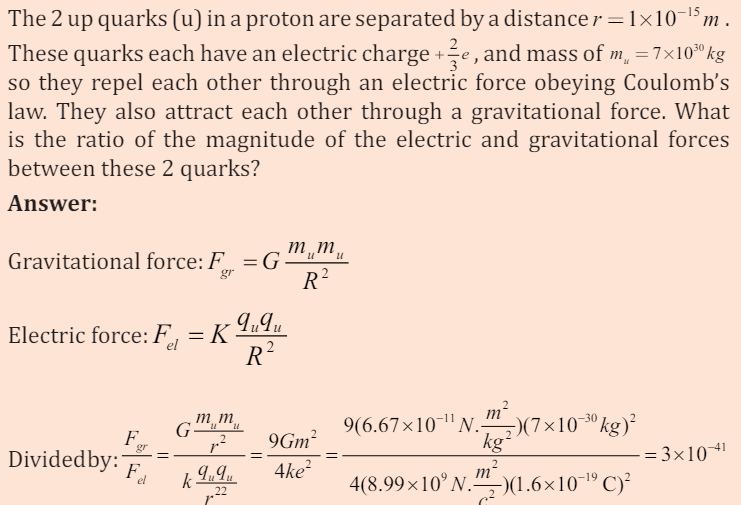
interactions and their relative strengths are summarized in Table 7.1
7.4.2 Checking my progress
1. Particles that interact by the strong force are called:
a. Leptons c. Muons
b. hadrons d. Electrons2. Name the four fundamental interaction and the particles that mediate each
7.5 UNCERTAINTY PRINCIPLE AND PARTICLE CREATION
7.5.1 The concept of uncertainty principle
ACTIVITY 7.5: Investigation of particle creation and position.
Basing on the knowledge and skills obtained from the previous sections
of this unit, use internet to find the meaning of the particle creation.
a. Is it possible to know the exact location of an elementary particle?b. Discuss and explain your findings
The discovery of the dual wave–particle nature of matter forces us to re-evaluatethe kinematic language we use to describe the position and motion of a particle.
In classical Newtonian mechanics we think of a particle as a point. We can
describe its location and state of motion at any instant with three spatial
coordinates and three components of velocity. But because matter also has a
wave aspect, when we look at the behaviour on a small enough scale comparable
to the de Broglie wavelength of the particle we can no longer use the Newtonian
description. Certainly no Newtonian particle would undergo diffraction likeelectrons do.
To demonstrate just how non Newtonian the behaviour of matter can be, let’s
look at an experiment involving the two-slit interference of electrons (Fig.7.4).
We aim an electron beam at two parallel slits, just as we did for light. (The
electron experiment has to be done in vacuum so that the electrons don’t collidewith air molecules.)
What kind of pattern appears on the detector on the other side of the slits?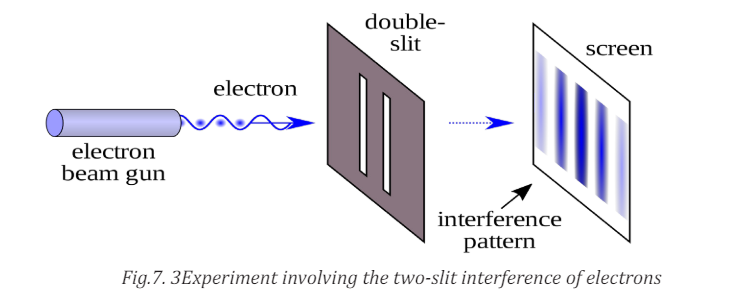
The answer is: exactly the same kind of interference pattern we saw for photons.
Moreover, the principle of complementarily, tells us that we cannot apply the
wave and particle models simultaneously to describe any single element of
this experiment. Thus we cannot predict exactly where in the pattern (a wave
phenomenon) any individual electron (a particle) will land. We can’t even ask
which slit an individual electron passes through. If we tried to look at where the
electrons were going by shining a light on them that is, by scattering photons
off them the electrons would recoil, which would modify their motions so thatthe two-slit interference pattern would not appear.
Just as electrons and photons show the same behaviour in a two-slit interference
experiment, electrons and other forms of matter obey the same Heisenberguncertainty principles as photons do:
Heisenberg uncertainty principle for position and momentum is given by
This is a mathematical statement of the Heisenberg uncertainty principle Or
it is sometimes called, the indeterminacy principle. It tells us that we cannot
measure both the position and momentum of an object precisely at the sametime.
The uncertainty principle relates energy and time, examining this as follows.
The object to be detected has an uncertainty in position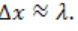 the photon that
the photon that
detects it travels with speed c, and it takes a time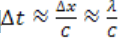 to pass through
to pass throughthe distance of uncertainty.
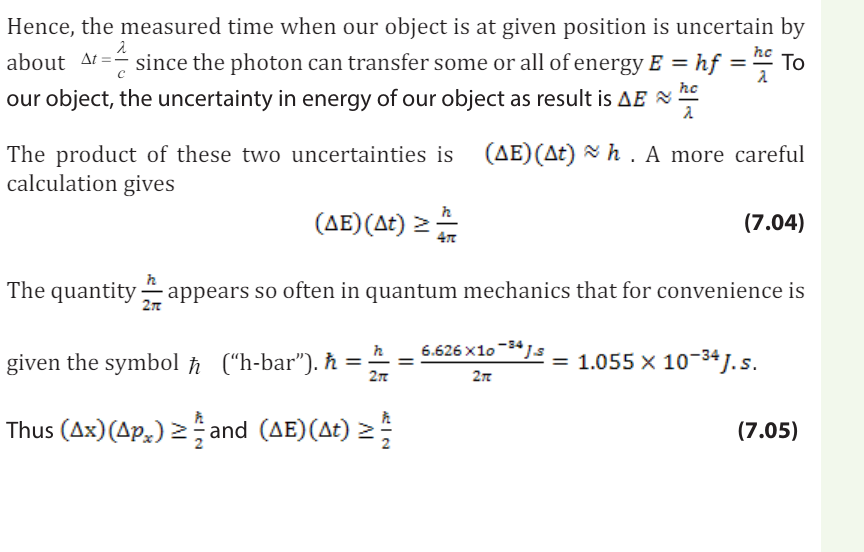
7.5.2 Checking my progress
1. The idea of uncertainty is used in many contexts; social, economic
and scientific. People often talk about uncertain times, and when you
perform a measurement you should always estimate the uncertainty
(sometimes called the error). In physics the Heisenberg Uncertainty
relation has a very specific meaning.a. Write down the Heisenberg uncertainty relation for position and2. An electron is confined within a region of width 11 5 10 m − × (Roughly
momentum.
b. Explain its physical significance.
c. Does the Heisenberg uncertainty principle need to be considered
when calculating the uncertainties in a typical first year physics
experiment? Why or why not?
d. Discuss the following statement: the uncertainty principle places a
limit on the accuracy with which a measurement can be made. Do
you agree or disagree, and why?
the Bohr radius)a. Estimate the minimum uncertainty in the component of the
electron’s momentum.
b. What is the kinetic energy of an electron with this magnitude of
momentum? Express your answer in both joules and electronvolts.
7.6 MATTER AND ANTIMATTER (PAIR PRODUCTION AND
ANNIHILATION)ACTIVITY 7.5: Describing the matter and antimatter
Use internet to describe the following concepts:
1. Matter and give examples of matter particles
2. Antimatter and give examples of antimatter particles
3. Pair production
4. Annihilation7.6. 1 Introduction
Matter is a substance that has mass and takes up a space by having a volume. This
include atoms and anything made up of these but no other energy phenomena
or wave such as light or sound. Everything around you is made up of matter
and is composed of particles including the fundamental fermions (quarks,
leptons, antiquarks and antileptons) which generally are matter particles andantimatter particles.
Antimatter is a material composed of the antiparticle to the corresponding
particle or ordinary particles. In theory a particle and its antiparticle have the
same mass as one another but opposite electric charge and other differences in
quantum numbers. Neutrons have antineutrons, electrons have positrons and
neutrons have antineutrons as their respective antimatter. It was once thought
that matter would neither be created nor destroyed. We know that energy andmass are interchangeable.
7.6.2 Pair production and annihilation
Pair production is a crucial example that photon energy can convert into
kinetic energy as well as rest mass energy. Schematic diagram about the process
of pair production is shown in Fig.7.5. The high-energy photon that has energy
hf loses its entire energy when it collides with nucleus. Then, it makes pair ofelectron and positron and gives kinetic energy to each particle.
Annihilation: When a particle collides with its antiparticle, the two annihilate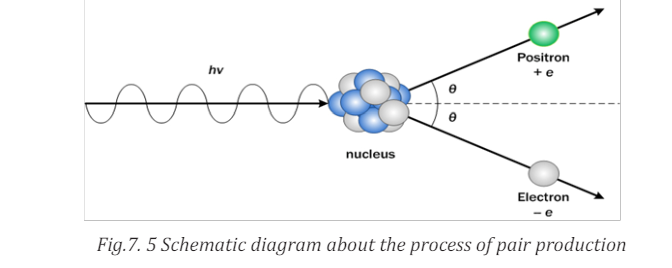
each other with their mass being entirely converted into energy by the process
called ‘’Annihilation’’
These particles and anti-particles can meet each other and annihilate one
another (See Fig.7.6). In each case the particle and its antiparticle annihilate
each other, releasing a pair of high energy gamma photons.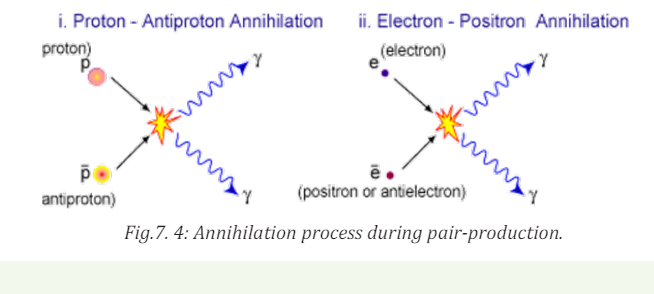
In this example, a proton and an anti-proton meet each other and annihilate,
producing high energy gamma rays in the form of photons. Rest mass, charge,
momentum and energy are conserved. They can also be produced from a highenergy photon, this is called pair production.
7.6.3 Application of antimatter
Antimatter as a form of antiparticle of sub atomic particles has a variety of
applications:• Positron emission tomography can be used to potentially treat cancer.
• Stored antimatter can be used for interplanetary and inter stellar
travel.
• Antimatter reactions have practical applications in medical energy.
• Antimatter has been considered as a trigger mechanism for nuclear
weapons because whenever antimatter meets its corresponding
matter the energy is released by annihilation.7.6.4 Checking my progress
1. Antimatter as a form of sub atomic particlesa. Electron2. The process in which a particle and antiparticle unite annihilate each
b. proton
c. matter
d. antiparticle
e. none of them is correct
other and produce one or more photons is called………
3. What happens when matter and antimatter collide?4. Compare matter and antimatter
END UNIT ASSESSMENT 7
A. Multiple choices
1. The positron is called the antiparticle of electron, because ita. Has opposite charge and Annihilates with an electron2. Beta particles are
b. Has the same mass
c. Collides with an electron
d. Annihilates with an electrone. Neutrons3. If gravity is the weakest force, why is it the one we notice most?
f. Protons
g. Electrons
h. Thermal neutronsa. Our bodies are not sensitive to the other forces.B. Structured questions
b. The other forces act only within atoms and therefore have no
effect on us.
c. Gravity may be “very weak” but always attractive, and the
Earth has enormous mass. The strong and weak nuclear
forces have very short range. The electromagnetic force has a
long range, but most matter is electrically neutral.
d. At long distances, the gravitational force is actually stronger
than the other forces.
e. The other forces act only on elementary particles, not on
objects our size.
4. According to the classification of elementary particles by mass.Complete the following figure
5.
I. State two differences between a proton and a positron.
II. A narrow beam of protons and positrons travelling at the same
speed enters a uniform magnetic field. The path of the positrons
through the field is shown in Fig.7.7. Sketch on this figure thepath you would expect the protons to take.
III. Explain why protons take a different path to that of the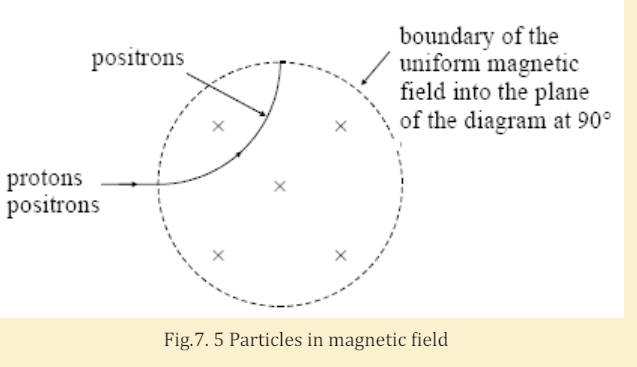
positrons.
6. A positron with kinetic energy 2.2 MeV and an electron at rest
annihilate each other. Calculate the average energy of each of the
two gamma photons produced as a result of this annihilation.
C. Essay question
7. Describe briefly the following particle-terms terms: π -meson,muon, neutrino, antiparticle, hadrons and lepton.
