Topic outline
UNIT 1: RADIOACTIVITY
Key Unit Competence:Explain the importance and dangers of radioisotopes in everyday life.


The above photos show different ways through which radiations reach
into our body (cells). The most familiar to you is radiation from sun rays!
However, everything present in the pictures above, and many others that
are not included, emits radiation. Now, discuss on the following points.Point 1: Can you see or feel the presence of radiations?
Point 2: How are we exposed to natural ionizing radiation? (Radiation with
the ability to rip out one or several electrons from an atom or molecule is
referred to as ionizing radiation and radiation which does not have sufficient
energy to damage atoms or molecules is called non-ionizing radiation).
Point 3: What do you understand by “radioactive materials”? Do you think
all of them are natural? Do you think all of them (i.e, radioactive materials)are harmful?
All living beings have been exposed to a constant flux of natural radiations on
the surface of our friendly planet, but these radiations have no negative effect.Natural radiations are everywhere in the universe
We are constantly being bombarded by particles of cosmic radiations: several
hundred go through our bodies every second. Rocks like granite, which have
become symbols of permanence and durability (hence used in building), contain
light traces of radioactive uranium. Sitting on or walking near a block of granite
exposes you to many sources of radioactivity. Even the food we eat or the air
we breathe contains radioactive elements (such as radon) either formed by the
intervention of cosmic rays, or as old as the solar system itself.The radioactivity is an integral part of our environment.
1.1. Definition of radioactivity and radioisotopes
The discovery of the electron towards the end of the nineteenth century was
the starting point of new avenues of research in science, which were to give
physicists an insight into the structure and nature of the atoms of matter.
They discovered that there is a nuclear phenomenon in some elements that
pushes them to emit radiations. This phenomenon was called radioactivity as
proposed by Marie Curie to describe those emissions of nuclear radiation by
some of the heavy elements.
The radioactivity is a nuclear phenomenon. It is the process by which an unstable
atomic nucleus changes into another more stable atomic nucleus by emitting
energy in form of radiation. Substances which have the property of emission
of radiation are called radioactive substances. Radioactivity is also known
as radioactive disintegration or radioactive decay. Particles and rays are
emitted when a nucleus of a radioactive isotope of element breaks down. They
break down to acquire stability as all atoms want to be stable.
A radioactive decay results when an atom with one type of nucleus, called “the
parent radioactive nuclide” transforms into another atom with a different
nucleus. The new product or element is named “the daughter nuclide”, and
thus the decay process results in “transmutation”. Transmutation, in this
case, means creation of an atom of a new element. In this way, the energy or
radiation emitted may take the form of particles such as alpha (α) or beta (β)
particles.
During the transmutation process, daughter nuclides are often in metastable or
excited state; they lose energy in form of gamma (γ) ray to become de-excited.
Gamma rays, here, can be compared to the heat of reaction that accompaniesan exothermic reaction.
In nuclear chemistry, the term ‘nuclide’ is used to designate a nucleus of an
element.
‘Nucleons’ is the term used for nuclear particles such as protons and neutrons.
In radiochemistry, the nucleon number stands for mass number (sum of protons
and neutrons present in the nucleus of a given atom).
Different nuclides, which have the same proton number but different nucleon
numbers are called isotopes or isotopic nuclides.
Radioisotopes or radioactive isotopes are the atoms of an element whoseatomic nuclei undergo decay by emitting radiation(s).

1.2. Emission of alpha, beta and gamma rays and their
properties
Different forms of radiation are emitted from an unstable nucleus as it decays.
The main types of emitted particles are alpha particles, beta particles and gamma
rays. The detailed information on each particle is provided below.
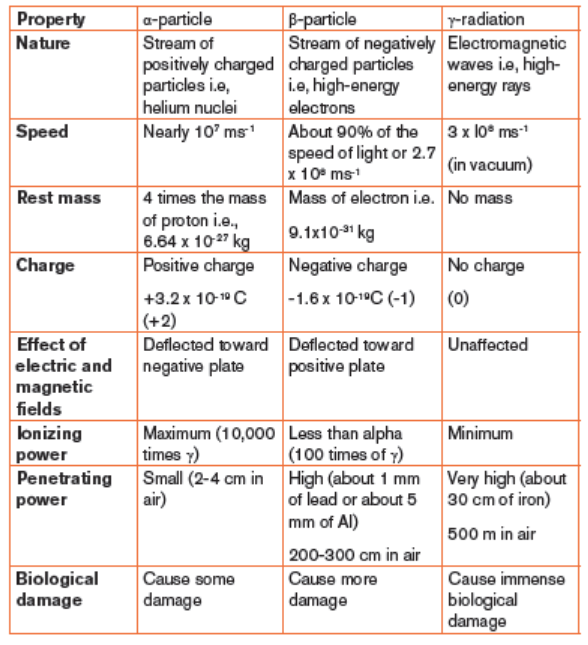
1.2.1. Alpha particles
An alpha particle contains two protons and two neutrons (so, its mass number,
A=4 and atomic number, Z=2). Because it has 2 protons, an alpha particle has
a charge of 2+ . That makes it identical to helium nucleus. In equations, it
. That makes it identical to helium nucleus. In equations, it
is written as the Greek letter “alpha (α)” or as the symbol for helium (He). The
charge of an alpha particle was found experimentally by passing it in an electric
field between two plates where it was attracted towards the negative plate.
The main properties of an alpha particle are the following:
– Alpha particle bears a positive charge of +2
– It has a mass of 4 amu
– It is deflected toward the negative pole of electric and magnetic fields.
Look at Figure 1.2 below.
– It affects a photographic plate and causes fluorescence on striking a
fluorescent material.
– It ionizes the gas through which it passes.
– Not very penetrating; a very thin sheet of aluminium foil or a sheet of
paper stops it.
– It can be shielded by paper or clothing.
– It destroys living cells and causes biological damage.
– It is strongly ionizing
When a nuclide decays by alpha emission, it loses 2 atomic number units and
4 mass units; in other words the daughter nuclide is the element located at 2places before the parent nuclide in the periodic table.
1.2.2. Beta particles
A beta particle, which is a high energy electron, has a charge of -1 and because
its mass is so much less than that of a proton, it is given a mass number of zero
(0). It is represented by the Greek letter “beta (β)” or by the symbol for electron
with the charge, -1, written on the lower left “ ”. Beta particle is deflected
”. Beta particle is deflected
toward the positive plate of an electric and magnetic field.
The main properties of a beta particle are the following.
– It bears a negative charge of -1
– It is deflected toward positive plate of an electric and magnetic fields.
The deflection is large since a beta particle is lighter than an α particle.
– It affects photographic plates.
– It is ionizing but less than alpha ray.
– It travels at speeds close to that of light (of the order of 108 m/s).
– Its penetrating power is 100 times greater than that of α particles.– It causes fluorescence on striking a fluorescent material.

1.2.3 Gamma rays
Gamma rays, γ, are high-energy radiation released as an unstable nucleus
undergoes a rearrangement of its particles to give a more stable, lower energy
nucleus.
Because gamma ray is an electromagnetic radiation, it has no mass and no
charge.
The main properties of gamma radiation (gamma ray) are the following:
– It is an electromagnetic radiation of short wavelength and higher
frequency, hence high energy
– It is not deflected by electric and magnetic fields. Refer to figure 15.4
for more understanding.
– It affects photographic plates.
– Its ionizing power is very low compared to alpha-particles and betaparticles.
– It travels at the same speed as that of light.
– It has the greatest penetrating ability, 5000-10000 times that of alpha
particles.
– It causes fluorescence when they strike a fluorescent material.
– It is diffracted by crystals.
– It can be stopped by several inches (5cm thick piece) of lead or a thick
concrete.
– It can easily pass through the human body and cause immense biological
damage.
Normally, there are very few pure gamma emitters. In radiology, one of most
commonly used gamma emitter is technetium (Tc). The excited state called
“metastable technetium” is written as technetium-99m, Tc-99m. By emittingenergy in the form of gamma rays, the excited nucleus becomes more stable.
Stable nuclides are usually in the state of least energy or ground state. But
these nuclides can be excited by particles or photon bombardment. The excitednucleus returns into the ground state by emission of excess energy as ϒ-rays.
1.2.4. Effect of electric and magnetic fields on nuclear radiations
a) Effect of electric field on nuclear radiation
Experiments involving emissions of radiations cannot be performed in an ordinary
school laboratory but can be carried out in research laboratories.
In 1899, the study of radioactivity was taken up by Ernest Rutherford. He
placed a little radium at the bottom of a small lead box and subjected the rays
that emerged from it to the action of a very strong magnetic field at right angles to
their direction. He found that the rays separated into three distinct constituentsas shown in the figure 1.2 below.
It is clear that when these radiations pass through a magnetic field or an electric
field, they are affected differently. The heavy alpha particles are slightly deflected
towards the negatively charged plates; this shows that they bear a positive
charge. The lighter beta particles are deflected more sharply towards the
positively charged plates, showing that they are bear a negative charge.
The gamma rays are not affected by the magnet, showing that they bear no
charge.
b) Effect of magnetic field on nuclear radiation
A magnetic field (only one pole is shown) affects radioactive rays differently
depending on the type of ray. Alpha rays (heavy, positively charged particles) are
deflected slightly in one direction. Beta rays (light, negatively charged electrons)
are deflected strongly in the opposite direction. Electromagnetic gamma raysare not deflected.
Summary of properties of radiations and their differences
Table 1.1. Distinction between the properties of α, β and γ radiations(summary)

1.3 Nuclear equations and radioactive decay series

When a nucleus spontaneously breaks down by emitting radiation, the process
is called natural radioactive decay. It can be shown as a nuclear equation
using the symbols for the original radioactive nucleus, the new nucleus and the
radiation emitted.
Radioactive nucleus → new nucleus + radiation (α, β, β+ or γ)
(Parent) (Daughter)
If the first daughter nuclide is stable, the activity of the radioactive source ends
there. But in many cases, the daughter nuclide is also radioactive and we obtain
a “decay series (chain)”.
A → B → C → ... Z (sterile)
More stable
A nuclear equation is balanced when the sum of the mass numbers and the sum
of the atomic numbers of the particles and atoms on one side of the equation
are equal to their counterparts on the other side.
The changes in mass and atomic numbers of an atom that emits a radioactive
particle are shown in the table below.Table 1.3: Mass number and atomic number changes due to radiation

A nuclear reaction is represented by a nuclear equation as follows:
Example 1:
Radium-226 emits an alpha particle to form a new isotope whose mass number,
atomic number and identity we must determine.
Step 1: Write the incomplete nuclear equation.

Note: Nuclear reactions involving hitting a nuclide by a particle such as proton
or neutron as in the example 2 are referred to as ‘bombardment reactions’.
Bombardment reactions are not natural; they are induced (or artificial)
nuclear reactions.
For the case of example 1, when a radioactive particle is emitted, the type of
nuclear reaction is emission reaction; those are natural radioactive isotopes.
1.3.2. Radioactive decay series
Radioactive decay Series is the series of steps by which a radioactive nucleus
decays into a non-radioactive nucleus. The element goes from radioactive to
non-radioactive.
A radioactive element disintegrates by emission of an α- or β-particle from the
nucleus to form a new “daughter element.” This again disintegrates to give
another “daughter element’. This is why the whole series of elements starting with
the parent radioactive element to the stable end-product is called radioactive
disintegration series or radioactive decay series as seen above.
Naturally radioactive nuclides disintegrate to acquire stability.
In nature, there are three radioactive decays which are Uranium, Thorium and
Actinium series. Each series starts with a radioactive element and then ends
with a reasonable stable element. Uranium series is the most important.
The three series are similar because they all involve loss of alpha and beta
particles ending with isotopes of Lead. Uranium series gives Lead-206, the
most stable isotope of Lead; Thorium gives Lead-208 and actinium series gives
Lead-207.
a) The uranium series
It starts with the parent element Uranium-238 and ends with the stable element
Lead-206. It derives its name from Uranium-238 which is the starting nuclide
of the series and has the longest half-life. In the process, 8 alpha and 6 beta
particles are emitted before Lead-206 is attained. The whole process is shownbelow:
b) The Thorium series
It begins with the parent element thorium-232 and ends with Lead-208 whichis stable.
c) The Actinium series
It starts with the radioactive element Actinium-235. The end-product is thestable element Lead-207.

1.4. Nuclear fission and fusion and their applications
While many elements undergo radioactive decay naturally, some nuclear
reactions are not spontaneous but are brought about when stable isotopes are
bombarded with high-energy particles (like neutrons, α-particles, protons ...).
Nuclear fission and fusion are good examples artificial radioactivity as they
do not take place spontaneously.
1.4.1. Nuclear fission and fusion
a) Nuclear Fission
Nuclear fission is a process in which a large atomic nucleus is split into two
smaller nuclei.
Large nuclei obviously have a large number of protons. The close proximity of so
many protons makes these nuclei unstable due to the repulsion forces between
protons. Thus, the nucleus of the unstable isotope splits to form smaller atoms
by bombardment with a suitable sub-atomic particle. Those stable isotopes that
are bombarded by a neutron to undergo fission reactions (to become fissionable)are known to be “fertile radioisotopes”.


The neutrons emitted, in this fission reaction, bombard more uranium nuclei
available to form a “reaction chain”. This chain reaction is the basis of nuclear
power. As uranium atoms continue to split, a significant amount of energy is
released, in form of heat, from the reaction. This heat released is used to produce
electricity (in a nuclear plant) or used in atomic/nuclear bombs.
The tremendous amount of energy released during nuclear fission results from
the principle of mass-energy equivalence; during the nuclear reaction, there is
loss of mass but the mass lost is transformed into energy.
Other examples: nitrogen-14 and oxygen-16 undergoing alpha and neutronbombardment respectively.
b) Nuclear Fusion
Nuclear fusion is a process that consists of joining two atomic nuclei of smaller
masses to form a single nucleus of a larger mass.
A good example is the fusion of two “heavy” isotopes of hydrogen (deuterium,
Hydrogen-2, and tritium, Hydrogen-3) into the element helium.
Similarly, when two atoms of deuterium (hydrogen-2) collide at high speed, theymight combine (fuse) to form a helium atom (He-3).

However, very high temperatures and pressures are required for the fusion to
take place because of the repulsion between the positive nuclei. Thus, for the
fusion to take place the nuclei must have enough kinetic energy to overcome
these repulsion forces between like charges.
Like fission, in the fusion process large quantity of energy is liberated in the form
of heat. This energy is also used in atomic/nuclear bombs (Hydrogen bomb).
Note that it is very difficult to carry out nuclear fusion between two large nuclei
due to the highly strong repulsion forces that are between their positively
charged nuclei. Table 15.6 provides the differences between nuclear fissionand nuclear fusion.
Table 1.4: Differences between nuclear fission and nuclear fusion
Both fission and fusion are nuclear reactions that produce energy as described 1.4.2 Application of nuclear fission and nuclear fusion
1.4.2 Application of nuclear fission and nuclear fusion
above.
Fission is used in nuclear power reactors since it can be controlled, while fusion
is not utilized to produce power since the reaction has not yet controlled up to
now. The two processes have an important role in the past, present and futurein energy creation.

Like other forms of energy, nuclear energy can be either renewable (nuclear
fusion) or non-renewable (nuclear fission).
– Nuclear fission is non-renewable because uranium or other fissile
nuclides needed for this process are not renewable.
For example: In Atomic Bomb, there are fission reactions of uranium.
The energy produced is uncontrolled and this principle is used to
manufacture the bombs and missiles. When controlled, the nuclear
fission is also useful in the production of electricity.
– Nuclear fusion energy (if mastered) could be renewable because
hydrogen needed for this process is available in nature in large amount.
For example: In Hydrogen Bomb, the nuclear reaction involves thefusion of deuterium and tritium nuclei to form helium.


1.5. Comparison between Nuclear and Chemical reactions.
2. Describe the following as pertaining to chemical reaction or nuclear
reaction:
a) Isotopes have the same chemical properties as they have the same
number of electrons
b) Hydrogen nuclei are the reactants
c) Large amounts of energy are released.
d) The mass is strictly conserved
Nuclear reaction and chemical reaction differ as shown in the following table:
Table 1.5: Differences between a chemical reaction and a nuclearreaction


1.6. Uses of some radioisotopes
Radioactive isotopes have a variety of applications. Generally, they are useful
because either we can detect their radioactivity or we can use the energy they
release.
Radioactive isotopes are effective tracers because their radioactivity is easy to
detect. A tracer is a substance that can be used to follow the pathway of that
substance through some structures. For instance, leaks in underground water
pipes can be discovered by running some tritium-containing water through
the pipes and then using a Geiger counter to locate any radioactive tritium
subsequently present in the ground around the pipes.
Tracers can also be used to follow the steps of a complex chemical reaction.
After incorporating radioactive atoms into reactant molecules, scientists can
track where the atoms go by following their radioactivity. One excellent example
of this is the use of carbon-14 to determine the steps involved in photosynthesis
in plants.
1. Radioactive Dating
Radioactive isotopes are useful for establishing the ages of various objects. The
half-life of radioactive isotopes is unaffected by any environmental factors, asseen above, so the isotope acts like an internal clock.
In another interesting example of radioactive dating, hydrogen-3 dating has
been used to verify the stated vintages of some old fine wines.
One isotope of carbon, carbon-14, is particularly useful in determining the age
of once-living artifacts. A tiny amount of carbon-14 is produced naturally in the
upper reaches of the atmosphere, and living things incorporate some of it into
their tissues, building up to a constant level. Once a living thing dies, it no longer
acquires carbon-14. As time passes the carbon-14 that was in the tissues
decays. The half-life of carbon-14 is 5,730 years. If an artifact is discovered and
analyzed many years after its death and the remaining carbon-14 is compared to
the known constant level, an approximate age of the artifact can be determined.
Radiocarbon dating is used in many fields to learn information about the pastconditions of organisms and the environments present on Earth.
2. In Medicine
Radioactive isotopes have many medical applications in diagnosing and treating
illness and diseases.
When a radiologist wants to determine the condition of an organ in the body, the
patient is given a radioisotope that is known to concentrate in that organ. After a
patient receives a radioisotope, a scanner produces an image of the organ. The
scanner moves slowly across the region of the body where the organ containing
the radioisotope is located. The gamma lays emitted from the radioisotope in
the organ are used to expose a photographic plate with a scan of the organ.
One example of a diagnostic application is using radioactive iodine-131 to test
for thyroid activity. The thyroid gland in the neck is one of the few places in thebody with a high concentration of iodine.

Iodine-131 has a half-life of only 8 days. So, it has low cell damage due to the
minimum exposure. Technetium-99 can also be used to test thyroid function.
Bones, the heart, the brain, the liver, the lungs, and many other organs can be
imaged in similar ways by using the appropriate radioactive isotope.
Other medical applications of radioisotopes include:
– Radiation from Co-60 (γ-rays) is used to irradiate the tumors (for
instance, diagnosis and treat thyroid disorders).
– Iodine-125 (I-125) is used in treatment of brain cancer and in
osteoporosis (a disease which causes bones to become weaker and
easily broken) detection.
– Iodine-131 (I-131) is used to diagnose and treat thyroid disorders, in
treatment of Graves’ disease, goiter and prostate cancer.
– Phosphorus-32 (P-32) is used in the treatment of leukemia, excess red
blood cells (tumors) and pancreatic cancer.
– Technetium-99m is used in imaging of skeleton and heart muscle, brain,
liver, heart, lungs, bones, spleen, kidney and thyroid. This is the most
widely used radioisotope in nuclear medicine.
– Cerium-141 (Ce-141) is used in gastrointestinal tract diagnosis and in
measuring blood flow to the heart.
– Sodium-24 (Na-24) in the form NaCl is used as a tracer in blood.
– Strontium-85 (Sr-85) is used in detection of bone lesions and brain
scans
– Radio Gold (Au-198) is used in Liver disease diagnosis.
– Radio iron (Fe-59) is used in Anemia diagnosis.
– In addition, radioisotopes are also used in sterilization of medical
devices.
3. In Agriculture
Obviously, we obtain food to eat and some drinks as a result of agriculture. But
contaminated food causes some diseases. Thus, there are some radioisotopes
that kill dangerous microorganisms present on food by “irradiation”.
The radiation emitted by some radioactive substances can be used to kill
microorganisms on a variety of foodstuffs thereby increasing the shelf life of
these produces. Produces such as tomatoes, mushrooms, sprouts, and berries
are irradiated with the emissions from cobalt-60 or caesium-137. This exposure
kills a lot of the bacteria that could cause spoilage and so the produce stays
longer. Eggs and some meat, such as beef, pork, and poultry, can also be
irradiated. Normally, irradiation of food does not make it radioactive.
By using known vintages (qualities of wines), oenologists (wine scientists) can
construct a detailed analysis of the cesium-137 of various wines through the
years.
The verification of a wine’s vintage requires the measurement of the activity of
cesium-137 in the wine. By measuring the current activity of cesium-137 in a
sample of wine (the gamma rays from the radioactive decay pass through glass
wine bottles easily, so there’s no need to open the bottle), comparing it to the
known amount of cesium-137 from the vintage, and taking into account the time
passed, researchers can collect evidence for or against a claimed wine vintage.
In addition in plant research, radiation is used to develop new plant types to
speed up the process of developing large amount of agricultural products.
This involves insect control, drastic reduction of pest populations and, in some
cases, elimination of insects by exposing the male ones to sterilizing doses of
radiation. Radiation pellets are used in grain elevators to kill insects and rodents.
Irradiation prolongs the shelf-life of foods by destroying bacteria, viruses, and
molds as seen above.
Other agricultural uses of radioisotopes include the following:
– Radioactive phosphorus (P-32) is used in the study of metabolism of
plants.
– Radioactive sulphur (S-35) helps to study advantages and disadvantages
of fungicides.
– Pests and insects on crops can be killed by gamma - radiations.
– Gamma - rays are used for preservation of milk, potatoes etc.
– Yield of crops like carrot, root, apples or grapes can be increased by
irradiation with radioisotopes.
4. In Industry
The applications of radioisotopes in industry are so many. Many types of thickness
gauges exploit the fact that gamma rays are attenuated when they pass through
the material. By measuring the number of gamma rays, the thickness can be
determined. This process is used in common industrial applications such as:
a) The automobile industry: to test steel quality in the manufacture of cars
and to obtain the proper thickness of tin and aluminum
b) The aircraft industry: to check for flaws in jet engines
c) Road construction: to gauge the density of road surfaces and sub
surfaces
d) Pipeline companies: to test the strength of welds and leakage
e) Oil, gas, and mining companies: to map the contours of test wells and
mine bores, andf) Cable manufacturers: to check ski lift cables for cracks.
1.7. Health hazards of radioactive substances
Radioactive materials in the environment, whether natural or artificial, do expose
people to risks.
This can happen in two ways:
– The radiation from the material can damage the cells of the person
directly. This is damage by irradiation.
– Some of the radioactive materials can be swallowed or breathed in.
While inside the body, the radiation it emits can produce damage. This
is damage by contamination.
Some health Hazards are: radiation burns, hair loss (temporary or permanent),
cancer, reproductive sterility, mutations in offspring, etc.
We are all exposed to low levels of radiation every day. Naturally occurring
radioisotopes are part of atoms of wood, brick and concrete in our homes and
the buildings of schools, hospitals, supermarkets, etc. This radioactivity is called
“background radiation” and is present in the soil, in the food we eat, in the water
we drink, and the air we breathe. For instance, one of the naturally occurring
isotopes of potassium, potassium-40, is radioactive. This one is found in the
body because it is present in any potassium-containing food. Other naturally
occurring radioisotopes in air and food are carbon-14, radon-222, strontium-90
and iodine-131.
In addition to naturally occurring radiation from construction materials in our
homes, we are constantly exposed to radiation (cosmic rays) produced in space
by the sun.
The larger the dose of radiation received at one time, the greater the effect
on the body. Exposure to low amount of radiation cannot be detected, but at
medium levels the whole-body exposure produces a temporary decrease in
number of white blood cells. If the exposure is very high, the person suffers
the symptoms of radiation sickness such as nausea, vomiting, fatigue, and a
reduction of white-cell count which can even be lowered to zero. So the victim
suffers from diarrhea, hair loss, hair loss and infection. Too much exposure is
expected to cause death.
You can ask yourself how radiation (which is in the air all around you) might
lead to lung cancer. This is explained by the presence of Radon gas (mainly
from granite rock) which is the main source of background radiation, and which
is in turn responsible for almost all the radiation we get exposed to over our
lifetime. When we breathe in; some of the radioactive atoms in the air undergo
radioactive decay and emit alpha, beta or gamma radiation. These radiations
can collide with and ionize atoms in our lung tissue, which could ultimately leadto lung cancer.







