UNIT 2: STRUCTURE ELECTRONIC OF CONFIGURATION AN ATOM AND

The ancient Greek philosophers Leucippus and Democritus believed that
atoms existed, but they had no idea as to their nature. Centuries later, in
1803, the English chemist John Dalton, guided by the experimental fact that
chemical elements can’t be decomposed chemically, was led to formulate
his atomic theory.
Dalton’s atomic theory was based on the assumption that atoms are
tiny indivisible entities, with each chemical element consisting of its own
characteristic atoms.
1) Dalton’s Atomic Theory
a. Each element is made up of tiny particles called atoms.
b. The atoms of a given element are identical; the atoms of different elements are different in some fundamental way(s).
c. Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has the same relative numbers and types of atoms.
d. Chemical reactions involve reorganization of the atoms—changes in the way they are bound together. The atoms themselves are not changed in a chemical reaction.
e. Dalton’s atomic theory successfully explained the following laws –
conservation of mass, constant composition and multiple proportions.
However, it failed to explain certain other observations like the
generation of electricity on rubbing glass or ebonite with silk or fur.
These observations propelled the discovery of sub-atomic particles
in the 20th century. Let’s learn about the discovery of the first sub-atomic particle – Electron.
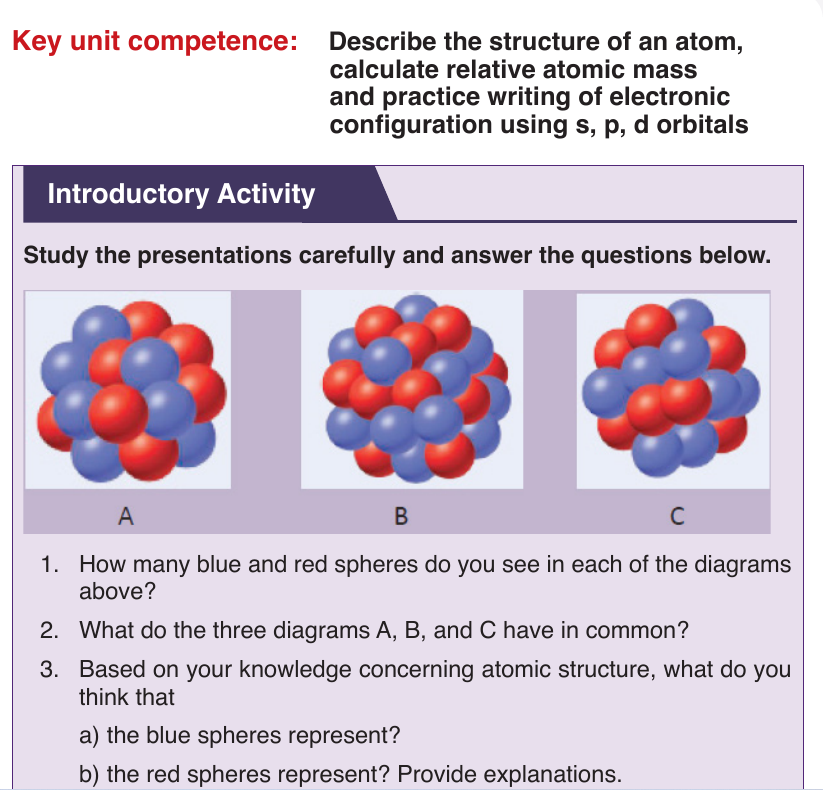
The atom is now known to consist of three primary particles: protons,
neutrons, and electrons, which make up the atoms of all matter.
A series of experimental facts established the validity of the model.
Radioactivity played an important part. Marie Curie suggested, in 1899,
that when atoms disintegrate, they contradict Dalton’s idea that atoms are
indivisible. There must then be something smaller than the atom (subatomic
particles) of which atoms were composed.
Long before that, Michael Faraday’s electrolysis experiments and laws
suggested that, just as an atom is the fundamental particle of an element, a
fundamental particle for electricity must exist. The “particle” of electricity was given the name electron.
a. Discovery of the electron
Experiments conducted by the British physicist Joseph John Thomson, in
1897 proved the existence of the electron and obtained the charge-to- mass
ratio for it.
Conclusions from the Study of the Electron:
–– All elements must contain identically charged electrons. Concluded that electron was part of an atom.
–– Atoms are neutral, so there must be positive particles in the atom to balance the negative charge of the electrons
–– Electrons have so little mass that atoms must contain other particles that account for most of the mass
Thomson believed that the electrons were like plums embedded in a
positively charged “pudding,” and thus his atomic model was called the
“plum pudding” model.
Efforts were then turned to measuring the charge on the electron, and these
were eventually successful and in 1916 – Robert Millikan determines the
mass of the electron: 1/1840 the mass of a hydrogen atom. The electron has
a mass of 9.11 x 10-28 g and has one unit of negative charge
b. Discovery of the nucleus, 1911
In 1911, Ernest Rutherford (1871-1937) and his co-workers discovered the
nucleus and their main conclusions were the following.
–– The nucleus is small
–– The nucleus is dense
–– The nucleus is positively charged and electrons are distributed around the nucleus and occupy the most of the volume.
The positively charged particles in the nucleus were called protons. The
Rutherford Atomic Model was called a “nuclear model”
Neils Bohr worked under Rutherford but found problems with his theory.
He ultimately determined that electrons are in circular orbits with increasingenergy levels.
c. Discovery of the neutrons, 1932
In spite of the success of Rutherford and his co-workers in explaining atomic
structure, one major problem remains unsolved.
If the hydrogen contains one proton and the helium atom contains two
protons, the relative atomic mass of helium should be twice that of hydrogen.
However, the relative atomic mass of helium is four and not two.
James Chadwick, English physicist (1891-1974), showed that the origin of
the extra mass of helium was due to uncharged particles present in the
nucleus that they call neutrons.
Bohr’s theory said that the protons are in the middle and the electrons travel
in specific energy levels and orbits around the nucleus
The modern model is basically the same except the nucleus contains protons
and neutrons
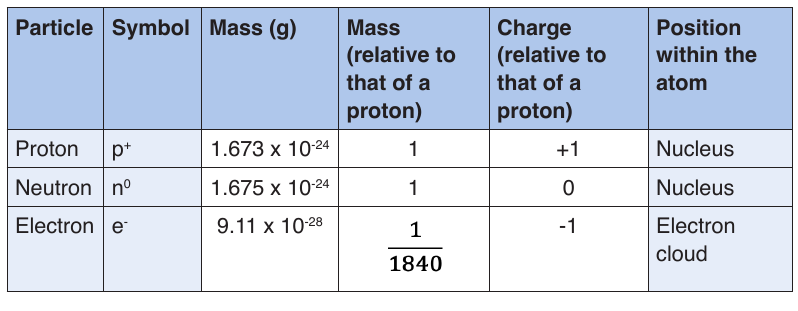
2) Properties of sub-atomic particles
The following table summarizes the relative masses, the relative chargesand the position within the atom of these sub-atomic particles.
a. Atomic number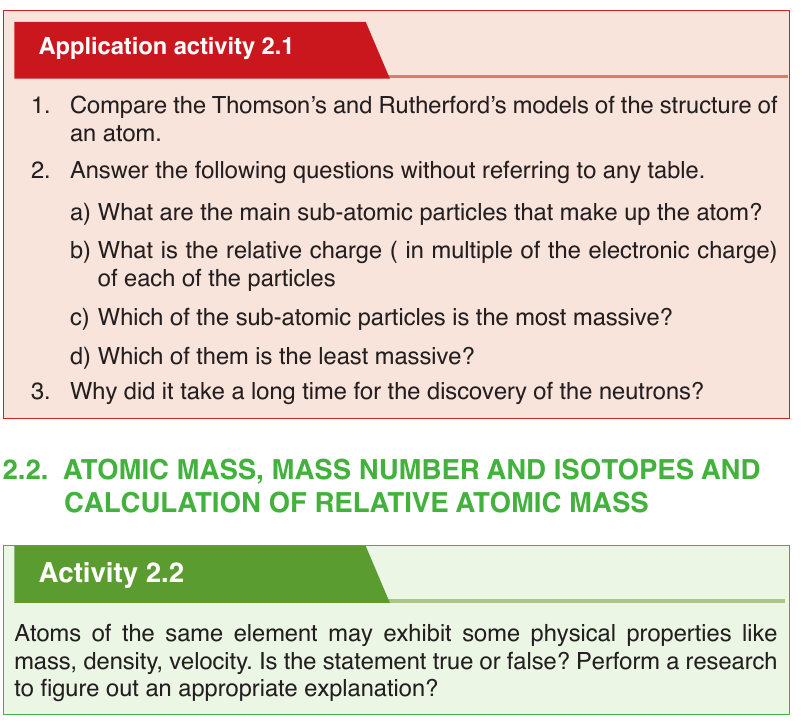
The atomic number (Z) or proton number is the number of protons in the
nucleus of an atom. It corresponds to the order of the element in the periodic
table.
The number of the protons in the nucleus of an atom determines the element
to which the atom belongs. If an atom has an atomic number of 7, the atom
must be a nitrogen atom. All nitrogen atoms have 7 protons in the nucleus.
Atoms carry no overall charge. The number of protons must therefore be the
same as the number of electrons.
b. Mass number
The mass number (A) or nucleon number is the sum of the number of
protons and the number of neutrons in the nucleus of an atom.
The number of neutrons can be obtained by subtracting the atomic number
from the mass number.
Chemists use the following shorthand to represent an atom. The mass
number is shown as a superscript (top number) and the atomic number is
shown as a subscript (bottom number) beside the symbol of the element.Example:
Each fluorine atom contains: 9 protons, 9 electrons and 10 neutrons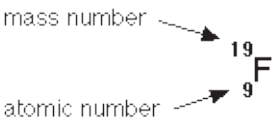
The term nuclide is used to describe any atomic species of which the
proton number and the nucleon number are specified. The species and are
nuclides.
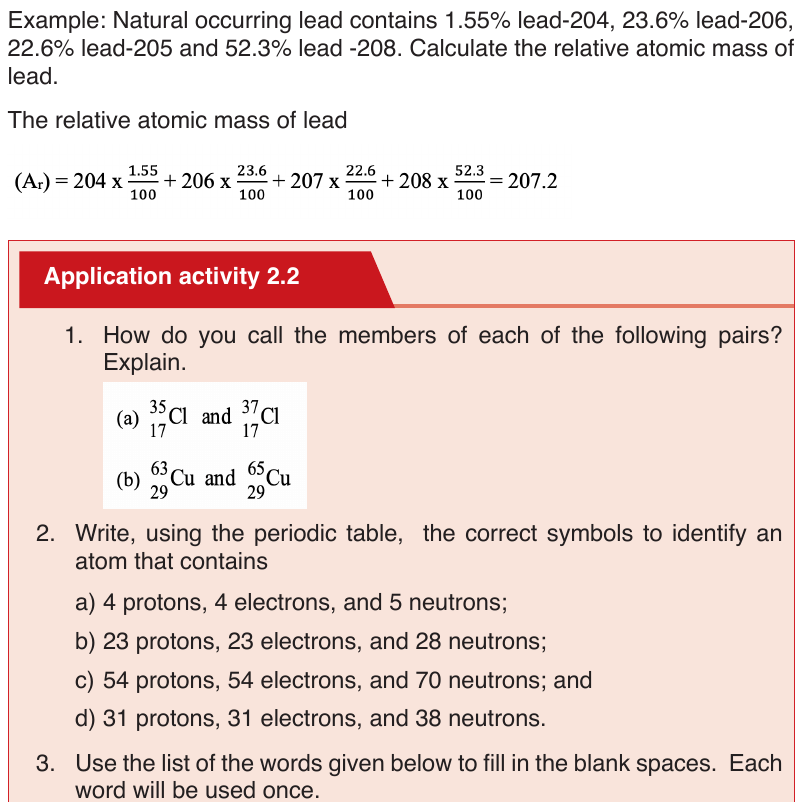
c. Isotopes
Isotopes are atoms of the same element with the same atomic number but
different mass numbers. They have different numbers of neutrons. They are
nuclides of the same element.
Example: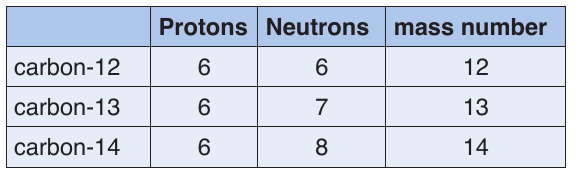
Isotopes of an element have the same chemical properties because they
have the same number of electrons.
When elements react, it is the electrons that are involved in the reactions.
This means that the isotopes of an element cannot be differentiated by
chemical reactions.
Because isotopes of an element have different numbers of neutrons,
they have different masses, and isotopes have slightly different physical
properties.
Isotopes and their abundance are estimated using an apparatus called massspectrometer (See figure 2.1)
The relative isotopic masses of all others atoms are obtained by comparison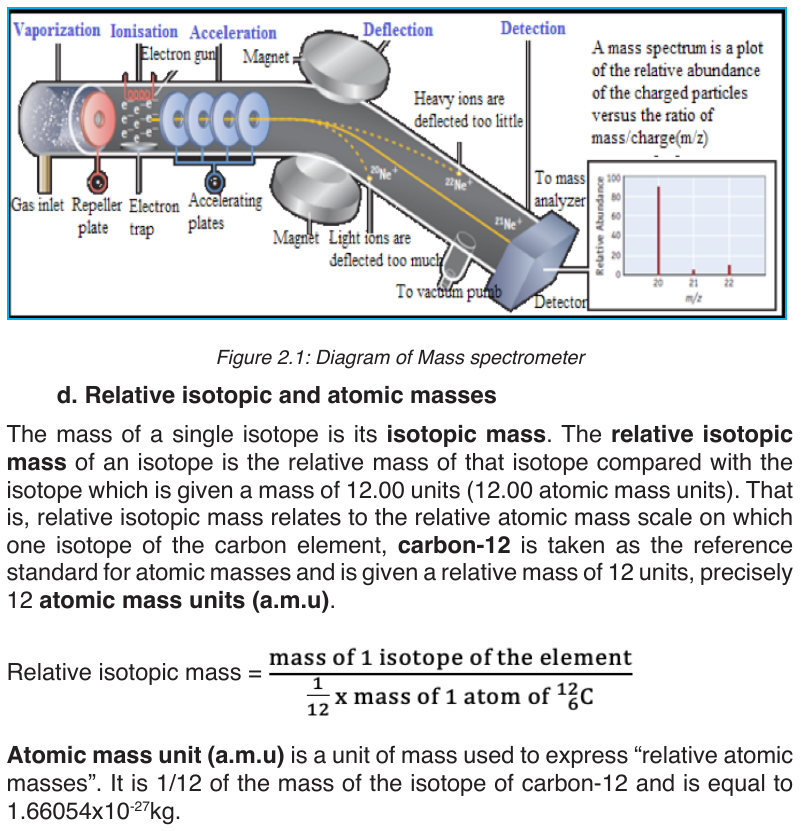
with the mass of a carbon-12 atom.
On that scale, the relative atomic mass of a proton and that of a neutron are
both very close to one unit (1.0074 and 1.0089 units respectively). Since
the relative mass of an electron is negligible (0.0005units), it follows that all
isotopic masses will be close to whole numbers.
However relative atomic masses of elements are not close to whole numbers
because natural occurring elements are often mixtures of isotopes.
The relative atomic mass (RAM) of an element, Ar , is the average of the
relative isotopic masses of the different isotopes weighted in the proportionsin which they occur.
The Electron Configuration is the way electrons are arranged around the
nucleus. Electrons occupy shells starting with the one closer to the nucleus,
i.e by increasing energy level. Energy levels are numbered 1, 2, 3, 4, 5, 6, 7
starting with K. Each of these numbers is called energy quantum number or
principal quantum numbers.
a. Quantum numbers
Energy levels or shells are subdivided into sub-shells known as s, p, d, f.
Each sub-level is split into orbitals. Orbitals of a given sub-shell have the same
name. Each electron is associated with a set of four quantum numbers s
The principal quantum number, n, can have positive integral values 1, 2,
3, 4,.... It governs the energy of the electron and also its probable distance
from the nucleus. The most stable electronic state of an atom is called its
ground state. Any higher energy state is called excited state.
The angular momentum quantum number or (azimuthal quantum
number), l, can have an integral values from zero to (n-1) for each value
of n. It determines the shape of the volume of space that an electron canoccupy. It also indicates the number of sub-levels for each level.
The values of l is generally designated by the letters:
If an electron has a principal quantum number n=2 and an angular momentum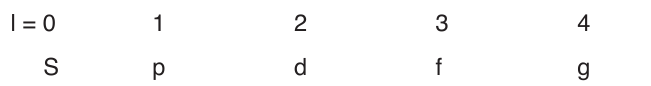
quantum number l=0 it is said to be a 2s electron.
–– The magnetic quantum number, ml, has values ranging from –l to +l.
Within a sub- shell, the value of ml depends on the value of the angular
momentum quantum number, l. For a certain value of l, there are (2l +
1) integral values of ml as follows: -l, (-l +1), . . . 0, . . . (+l-1), +l.
It determines the spatial orientation of an orbital.
–– The (Electron) Spin Quantum Number, ms, may have values of - 1⁄2
or + 1⁄2 only. The value of ms does not depend on the value of any other
quantum number. It represents the spin of an electron that occupies a
given orbital. Electrons will spin opposite each other in the same orbitalTable 2.1: Relationship among values of n, l, ml through n=4
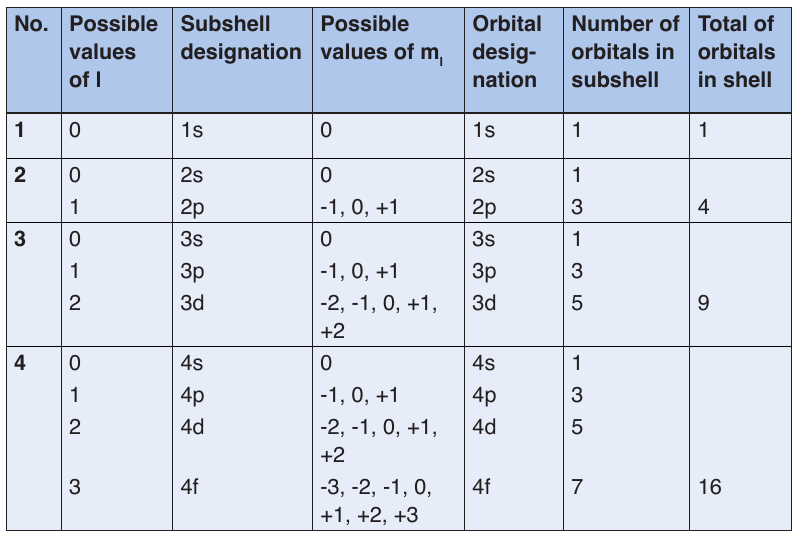
–– Atoms of the various elements differ from each other in their values of Z and electrons.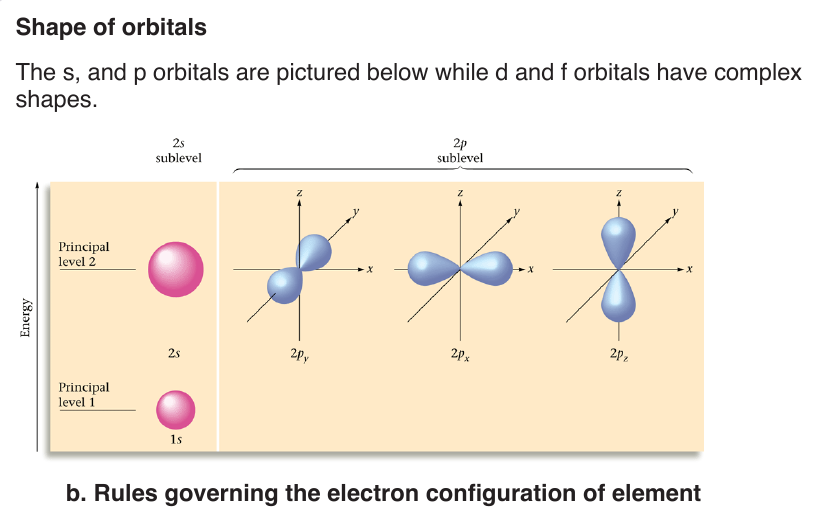
–– Electrons in atoms are arranged in orbitals and shells.
–– Orbitals are characterized by the quantum numbers n, l and ml.
–– Orbitals having the same value of n are said to be in the same shell. Orbitals having the same values of n and l are said to be in the same subshell.
–– Electrons are distributed in orbitals following the rules below.
b. 1 Pauli Exclusion Principle
No two electrons in the same atom can have the same set of the four quantum
numbers. If two electrons have the same values of n, l, ml, they must have
different values of ms. Then, since only two values of ms are allowed, an
orbital can hold only two electrons, and they must have opposite spins.
b. 2 Hunds’ rule
Electrons occupy all the orbitals of a given sublevel singly before pairing
begins.
Spins of electrons in different incomplete orbitals are parallel in the ground
state. The most stable arrangement of electrons in the subshells is the onewith the greatest number of parallel spins.
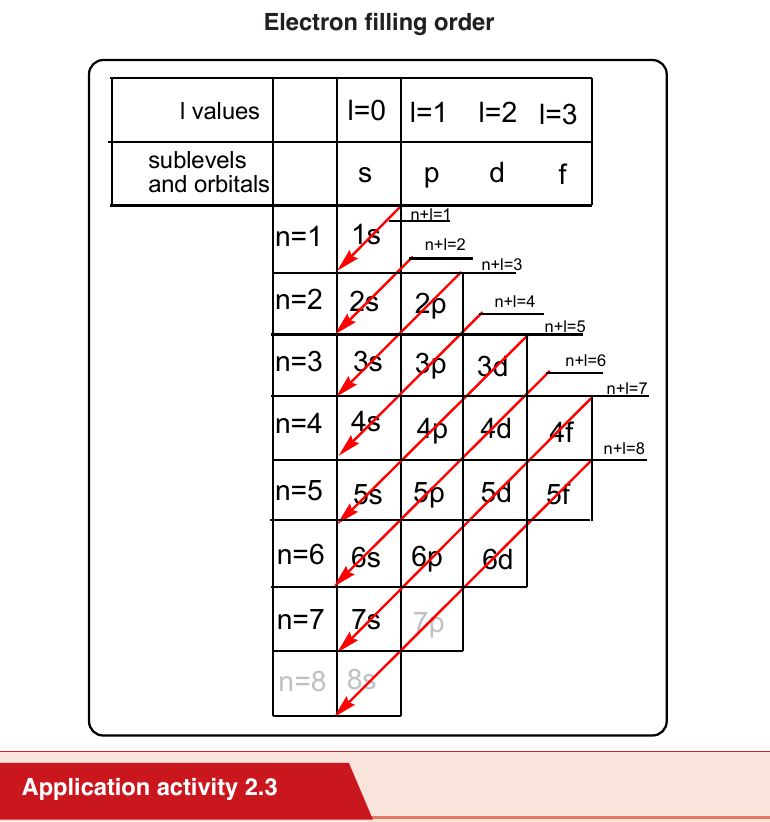
b. 3 Aufbau principle or build up principle or construction principle
The Aufbau principle or build up principle or construction principle state that
“Electrons fill lower energy orbitals (closer to the nucleus) before they fillhigher energy ones”.


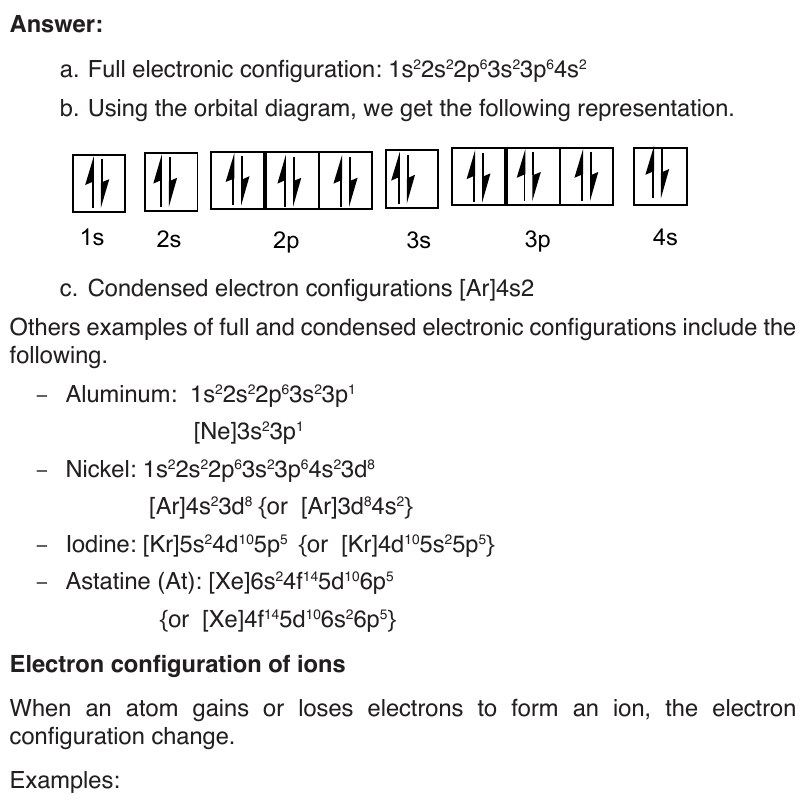
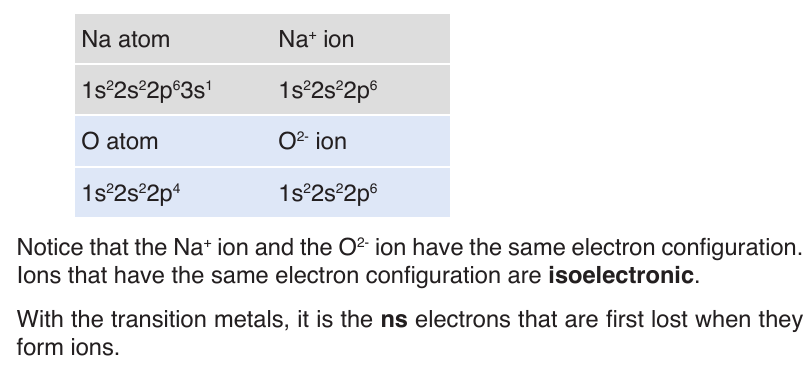
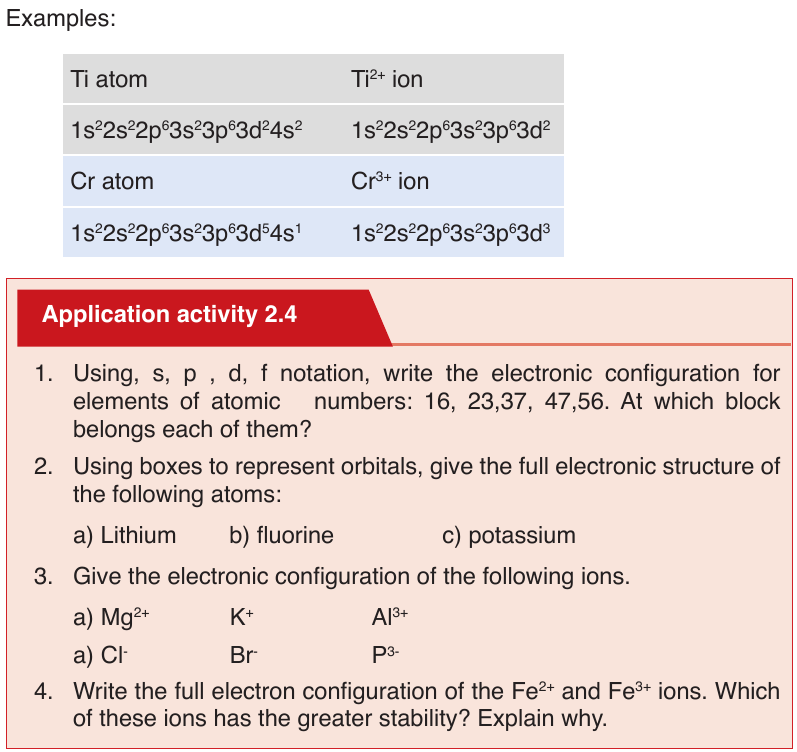
End unit assessment
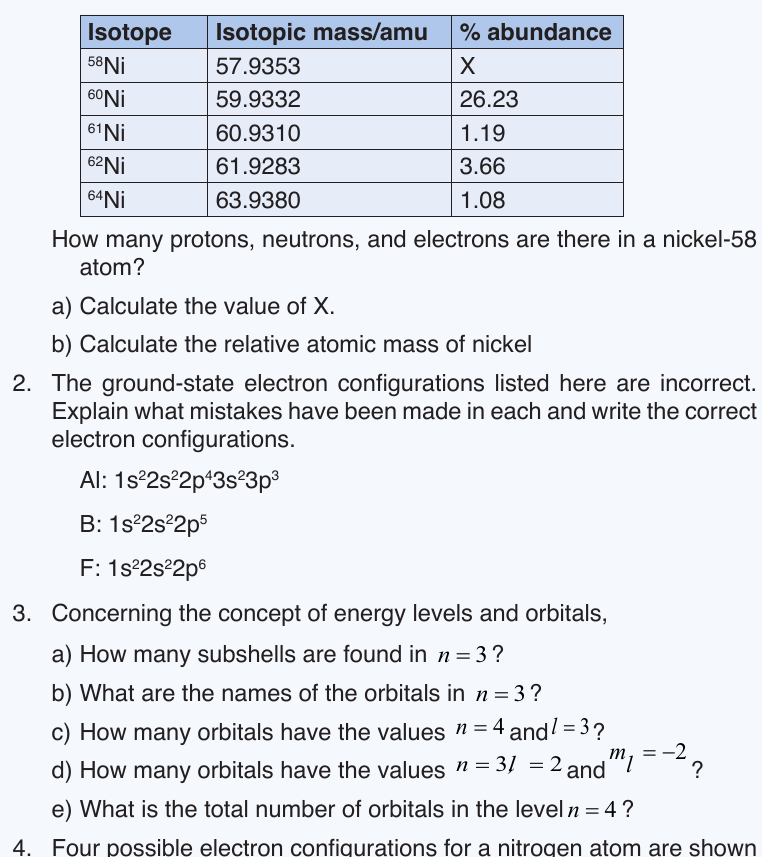
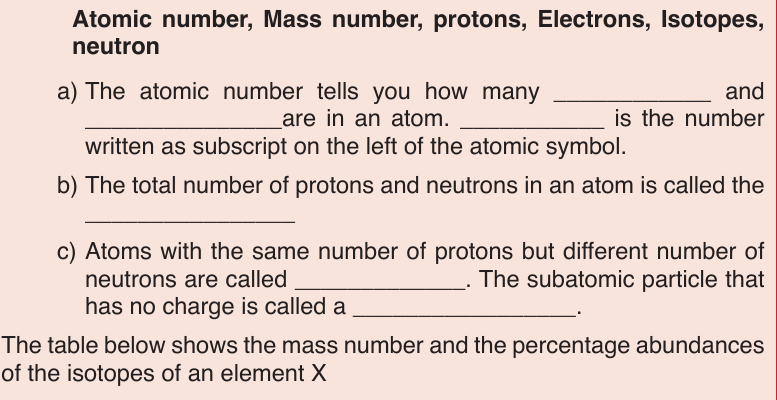
1. Given the following data concerning isotopes of nickel: