UNIT 7:ELECTRON CONFIGURATIONS OF ATOMS
Key unit competence
Relate Bohr’s model of the atom with hydrogen spectrum and energy levels,
practice writing electronic configurations using s, p, d, f orbitals.
Introductory Activity
At the beginning of this century, it was already known that atoms were
made of protons at the center (the nucleus) and electrons orbiting around
them. Niels Bohr proposed that the energy of an electron in an atom is not
continuous, but quantized.
To understand this better, think about a bookshelf as shown in the Figure
7.1 above.
1. If each of the books were electrons, what will be considered as to
be each shelf?
2. Each shelf is different from the others. What differentiate one shelf
from the other?
3. A book cannot be placed half way between consecutive shelves,
it can only be placed within one shelf. Deduce from this what is
predicted by Bohr, if you remember what shelves and books are
representing.
4. The places corresponding to each of the allowed orbit are referred
to as energy levels. Each shelf is labeled with the number n (n=1,
2, 3, etc). Suppose that this is the same for the atomic model to
be equivalent for the shelf. What is the technical term for these
numbers?
5. Formulate the simple, abbreviated way you can use to represent
the books in each shelf including the number they occupy.
7.1. Bohr’s atomic model and concept of energy levels
Activity 7.1
An atom is known to be further composed by other subatomic particles.
1. State three main subatomic particles.
2. Make a research and reveal the subatomic particles discovered
with the contribution of Rutherford.
3. Draw the structure of how you think boron atom would be looking
like, labeling each of the charged particles. Remember that the
proton number of boron is 5.
4. Suggest the reason why the electrons, in an atom, occupy different
levels.
5. Describe what would happen to the electron to change the level
it occupied.
The Bohr Model has an atom consisting of a small, positively-charged
nucleus orbited by negatively-charged electrons. Here is a closer look at the
Bohr Model, which is sometimes called the Rutherford-Bohr Model.
Overview of the Bohr Model
Niels Bohr proposed the Bohr Model of the Atom in 1915. Because the
Bohr Model is a modification of the earlier Rutherford Model, some people
call Bohr’s Model the Rutherford-Bohr Model. The modern model of the atom
is based on quantum mechanics. The Bohr Model contains some errors, but
it is important because it describes most of the accepted features of atomic
theory in a simple way and tries to answer the following questions failed to
answer by Rutherford.
• Why do atomic spectra consist of discrete (separate) lines?
• Why do atoms absorb or emit light of certain frequencies?
• Why do the spectral lines converge to form a continuum?
Unlike earlier models, the Bohr Model explains the “Rydberg formula for the
spectral emission lines of atomic hydrogen”.
The Bohr Model is a planetary model in which the negatively-charged
electrons orbit a small, positively-charged nucleus similar to the
planets orbiting the Sun(except that the orbits are not planar).
Bohr used the term energy levels (or shells) to describe these orbits of
differing energy. He said that the energy of an electron is quantized, meaning
“electrons can have one energy level or another but nothing in between”
The energy level that an electron normally occupies is called its ground state.
But it can move to a higher-energy, less-stable level, or shell, by absorbing
energy. This higher-energy, less-stable state is called the electron’s excited
state.
After it is done being excited, the electron can return to its original ground
state by releasing the energy it has absorbed, as shown in the diagram
below.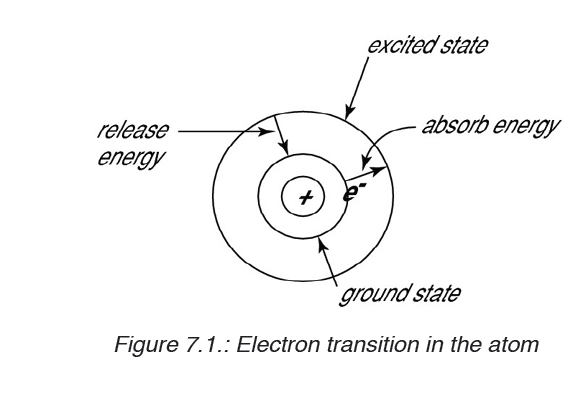
Sometimes the energy released by electrons occupies the portion of
the electromagnetic spectrum (the range of wavelengths of energy) that
humans detect as visible light. Slight variations in the amount of the energy
are seen as light of different colours.
Bohr referred to Max Planck’s recently developed quantum theory, according
to which energy can be absorbed or emitted in certain amounts, like separate
packets of energy, called quanta. The energy change is accompanied by
absorption of radiation energy of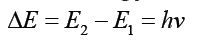 where,
where,
• h is a constant called “Planck’s constant” and
• v is the frequency of radiation absorbed or emitted.
• The value of h is 6.626 x 10-34 Js.
Each of these small “packets” of energy is called photon also called
“quantum of energy”. Energy can be gained or lost only in whole-number
multiples of the quantity hv, that is,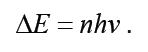
The absorption and emission of light due to electron jumps are measured by
use of spectrometers.
Bohr found that the closer an electron is to the nucleus, the less energy it
needs, but the farther away it is, the more energy it needs. So Bohr numbered
the electron’s energy levels.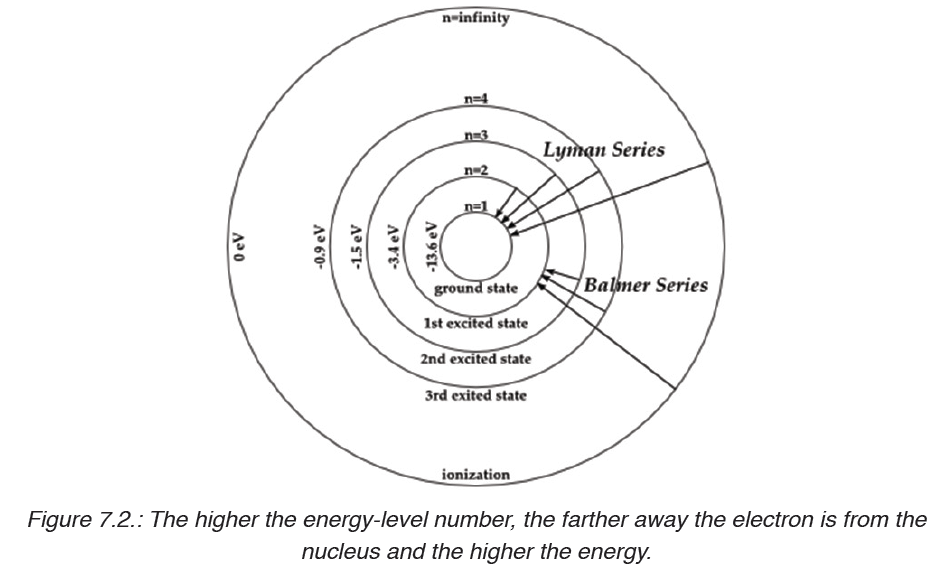
Bohr also found that the various energy levels can hold differing numbers of
electrons: Energy level1 may hold up to 2 electrons,energy level 2 may hold
up to 8 electrons, and so on.
In summary, the postulates of BohrAtomic Model are as follows:
• Electrons revolve around the nucleus in a fixed circular path termed
“orbits” or “shells” or “energy levels”.
• The orbits are termed as “stationary orbits”.
• Every circular orbit will have a certain amount of fixed energy and these
circular orbits were termed orbital shells. The electrons will not radiate
energy as long as they continue to revolve around the nucleus in the
fixed orbital shells.
• The different energy levels are denoted by integers such as n=1 or n=2
or n=3 and so on. These are called as quantum numbers. The range
of quantum number may vary and begin from the lowest energy level
(nucleus side n=1) to highest energy level. Learn the concept of an
Atomic number here.
• The different energy levels or orbits are represented in two ways such
as 1, 2, 3, 4, … or K, L, M, N, … shells. The lowest energy level of the
electron is called the ground state.
• The change in energy occurs when the electrons jump from one
energy level to other. In an atom, the electrons move from lower to
higher energy level by acquiring the required energy. However, when
an electron loses energy it moves from higher to lower energy level.
Bohr Model of Hydrogen
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1)
or for a hydrogen-like ion (Z > 1), in which one negatively-charged electron
orbits a small positively-charged nucleus.
Electromagnetic energy will be absorbed or emitted if an electron moves from
one orbit to another. Only certain electron orbits are permitted. The radius of
the possible orbits increases as n2, where n is the principal quantum number
which represents the number of energy levels in a given atom.
Main Points of the Bohr Model
• Electrons orbit the nucleus in orbits that have a set size and energy.
• The energy of the orbit is related to its size. The lowest energy is found
in the smallest orbit.
• Radiation is absorbed or emitted when an electron moves from one
orbit to another.
Weakness of Bohr’s Model
The Bohr model works well for very simple atoms such as hydrogen (which
has 1 electron) but not for more complex atoms. Although the Bohr model
is still used today, especially in elementary textbooks, a more sophisticated
and complex model (the quantum mechanical model) is used much more
frequently.
Application activity 7.1
1. State any weakness of the Bohr Model.
2. Outline three postulates of Bohr.
3. In Bohr atom, what is represented by the distance between an
orbital and the nucleus of an atom?
4. Explain why some people call Bohr’s Model the Rutherford-Bohr
Model.
5. Give the meaning of each of the following terms:
a) Electromagnetic spectrum
b) Quantized
c) Photon
6.Try to describe the atomic structure in the same way as Bohr can do.
7.2. Absorption and emission spectra and energy
associated
Activity 7.2
Observe the picture below, discuss with your colleagues and answer the
following questions.
1. What do you see on the above photo?
2. State the physical phenomenon which is related to the given
picture.
3. Think of any other means of producing the same pattern. List two
of them.
4. What property can you attribute to light with reference to the above
process?
The colours we see in a rainbow never fail to captivate us! Did you know
that even though we identify the distinct colours of a rainbow, it is actually a
continuous range of colours? A similar range of colours appears when white
light passes through a prism; this range of colours is a spectrum. A rainbow
is a multicoloured arch in the sky, produced by prismatic refraction of light
within droplets of rain in the air or any prismatic refraction of light showing a
spectrum of colours.
7.2.1. Spectrum
Ordinary white light consists of waves of all wavelengths in the visible range.
This is why, when white light passes through a prism, a series of coloured
bands are seen called spectrum. This dispersion of white light demonstrates
that white light contains all the wavelengths of colour and is thus considered
to be continuous. Each colour blends into the next with no discontinuity.
Since the colours merge into each other i.e. violet merges into blue, blue into
green and so on, we call it a “continuous spectrum”.
The interaction of electromagnetic radiation with matter causes the atoms and
molecules to absorb energy and go to a higher energy state. Since this state
is unstable, they need to emit radiations to return to their normal states. This
gives rise to emission and absorption spectra.
7.2.2. Emission and absorption spectra
1. Emission spectrum
Every substance reacts differently when it interacts with light. The material
starts off with being in the ground state, where all molecules are stable and
settled. However when heat, energy or light is applied to a substance, some
of the molecules transition into a higher energy state or an excited state.
During this state the molecules are unstable and try to emit the energy
in order to reach the state of equilibrium. The molecules emit energy in
the form of photons or light. The difference between the substance in ground
state and excited state is then used to determine the emission level of the
substance.
Each element or substance has a unique emission level or the amount of
energy it radiates; this helps the scientists identify elements in unknown
substances. The emission of an element is recorded on an emission spectrum
or atomic spectrum. The emittance of an object measures how much light is
emitted by it. The amount of emission of an object varies depending on the
spectroscopic composition of the object and temperature. The frequencies
on an emission spectrum are recorded in light frequencies, where the
colour of the light determines the frequency.
Emission can happen in the form of light and rays, such as gamma and
radio. The spectrum is a dark wavelength with bands of color on it, which is
used to determine the emission of the object.
The emission spectrum is the spectrum of radiation emitted by a
substance that has absorbed energy. Atoms, molecules, and ions that have
absorbed radiation are called ‘excited‘.
2. Absorption spectrum
The absorption spectrum is the opposite of the emission spectrum. Absorption
can be plotted in a wavelength, frequency or wave number. There are two
types of absorption: atomic absorption spectra and molecular absorption
spectra.
Absorption is used:
• To determine the presence of a particular substance in a sample, or the
quantity of the present substance in the sample.
• In molecular and atomic physics, astronomical spectroscopy and
remote sensing. Absorption is primarily determined by the atomic and
molecular composition of the material.
They can also depend on temperature, electromagnetic field, interaction
between the molecules of the sample, crystal structure in solids and
temperature.
In order to determine the absorption level of a substance, a beam of radiation
is directed at the sample and the absence of light that is reflected through
the object can be used to calculate the absorption. The absorption spectrum
is usually light colored, with dark bands that run through it. These dark bands
are used to determine the absorption of the object.
Absorption spectrum is the plotting of the energy that is absorbed by an
element or substance. It is the spectrum formed by electromagnetic radiation
that has passed through a medium, in which radiation of some frequencies is
absorbed.
Emission and absorption spectra are techniques that are used in chemistry
and physics. Spectroscopy is the study of emission and absorption spectra.
It is the interaction of radiation and matter. Using spectroscopy, a scientist
can figure out the composition of a certain matter. This is really beneficial,
of dealing with unknown substances. Emission spectra and absorption
spectra are different from each other but still related.
7.2.3. Comparison between absorption and emission spectra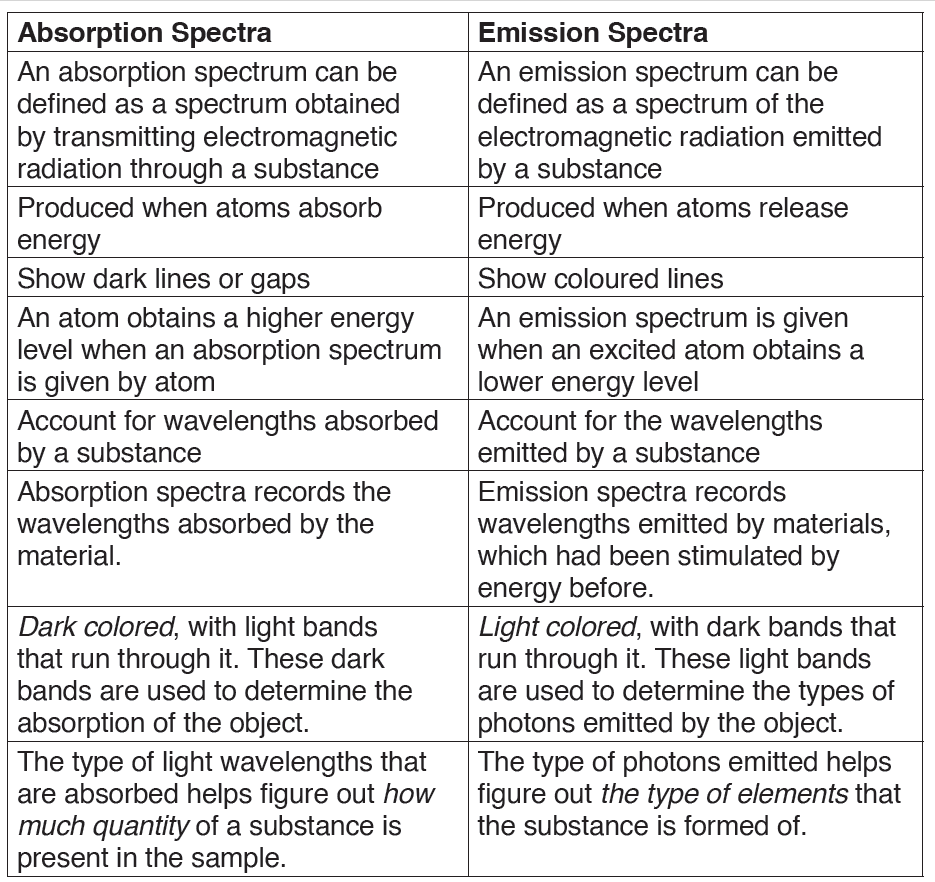
Application activity 7.2
1. State the meaning of the term “Spectrum”.
2. Why do we say that the spectrum of the white light is continuous?
3. Find out some differences between emission and absorption
spectra by filling the table below.
7.3. Hydrogen spectrum and spectral line series
Activity 7.3
Think about any spectrum you have come across with. This might be
composed of vertical lines (that form that spectrum).
1. Formulate the name that can be dedicated to such spectrum.
2. If atoms and molecules are heated to sufficiently high temperatures,
they emit light of certain wavelengths. Do you think the spectrum
drawn to be emission or absorption (spectrum)?
3. Describe the look that spectrum would have.
4. The vertical lines described in the spectrum above are different for
one element to another. How these separate lines can be used to
identify the element?
Unlike visible white light which shows a continuous spectrum of all wavelengths,
the emission spectra of atoms in the gas phase emit light only at specific
wavelengths with dark spaces between them. This is called line spectra or
atomic spectra since the emitted radiation is identified by bright lines in the
spectra. Each element has its own unique line emission spectrum.
Did you know that just the way fingerprints are used to identify people; the
characteristic lines in an atomic spectrum are used to identify unknown atoms!
Line Spectrum of Hydrogen
Hydrogen molecules dissociate when we pass electric discharge through
gaseous hydrogen. Subsequently, the energetically excited H2 atoms emit
electromagnetic radiation of discrete frequencies giving rise to a
spectrum emitted light is analysed with a spectrometer and discrete bright
lines in a dark background are observed.
The well-defined separation of lines is experimental evidence for the
existence of separate, discrete or ‘quantized’ energy levels in the atom.
No two gases give the same exact line spectrum.
The hydrogen spectrum has many series of lines. In 1885, the scientist
Balmer showed that if spectral lines are expressed as wavenumber, thenthe visible lines of the hydrogen spectrum obey the following formula:
The value 109,677 is the Rydberg constant for hydrogen.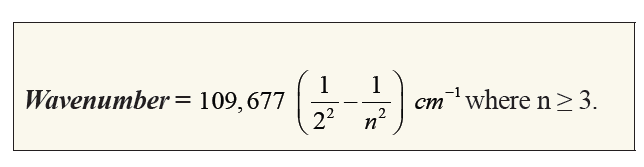
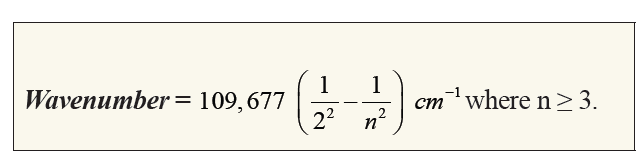
We call this series of lines, Balmer series. These lines are the only lines in
the hydrogen spectrum that appear in the visible region of electromagnetic
radiation. The 3 → 2 transition produces the first line of the Balmer series.
For hydrogen (Z = 1) this produces a photon having wavelength 656 nm (red light).
Johannes Rydberg, a Swedish spectroscopist, showed that all series of linesin the hydrogen spectrum can be described by the formula:

λ is the wavelength;
n1 is the initial energy level
n2 is the final energy level
The lines that correspond to n1 = 1, 2, 3, 4, 5 are called Lyman, Balmer,
Paschen, Brackett and Pfund series, respectively.
The hydrogen atom has the simplest line spectrum of all elements. For heavier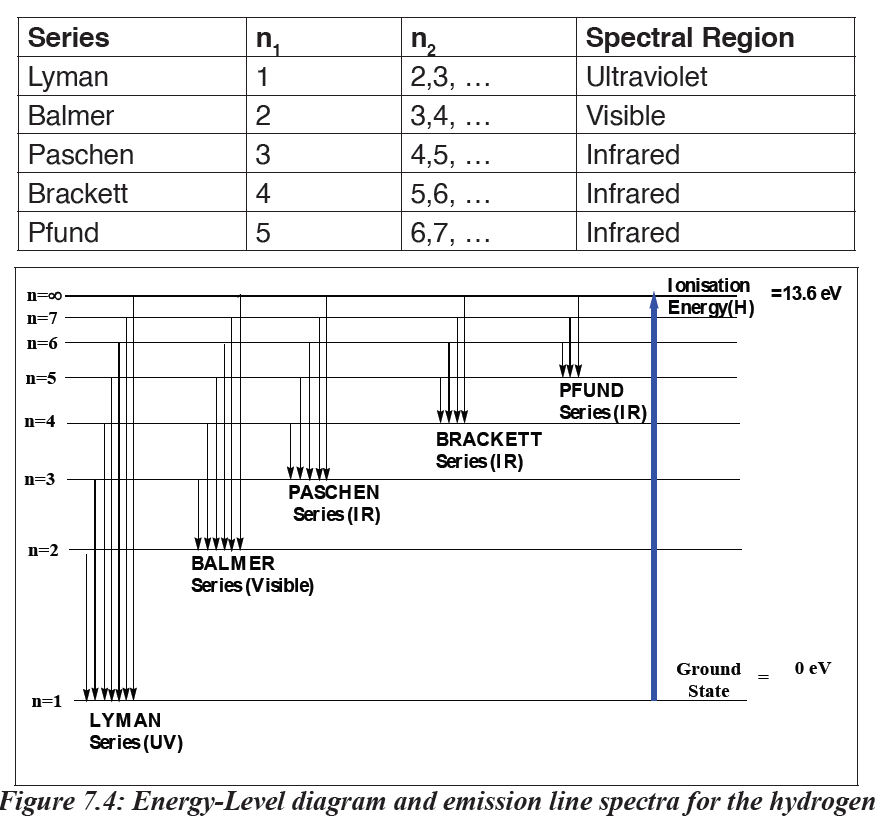
atoms, the line spectrum becomes more and more complex. However, there
are certain features that are common to all line spectra:
• Line spectrum of every element is unique.
• There is regularity in the line spectrum of each element.
Now, that we understand the line spectrum of hydrogen, let us understand the
features of the hydrogen atom, its structure, and its spectrum.
In each series, the intervals between the frequencies of the lines become
smaller and smaller towards the higher frequency end of the spectrum
until the lines run together or converge to form a continuum of light.
Explanation of Line Spectrum of Hydrogen
Bohr’s model can explain the line spectrum of the hydrogen atom. Radiation
is absorbed when an electron goes from orbit of lower energy to higherenergy; whereas radiation is emitted when it moves from higher to lower orbit.
The energy gap between the two orbits is: ΔE = Ef – Ei where:
• f is the final orbit,• i is the initial orbit
The frequency and wavenumber associated with the absorption and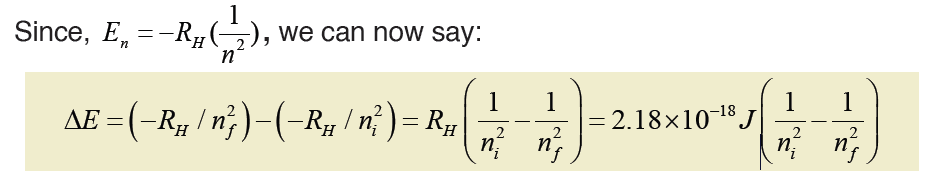
emission of the photon can also be calculated:

Example: Calculate the frequency and after the wavelength of the hydrogen
line that corresponds to the transition of the electron from the n = 4 to the n= 2 states.
Answer:
(The negative frequency or wavelength is physically meaningless, so the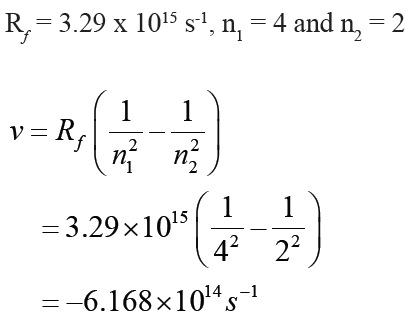
sign is ignored)
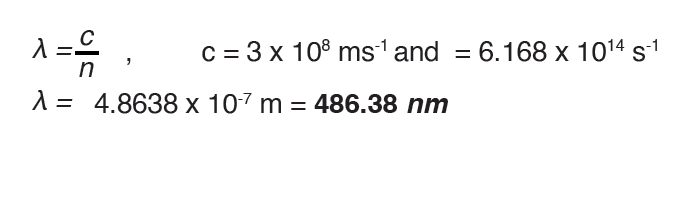
Note: The spectrum of white light ranges from violet (at 7.5 x 1014 Hz) to red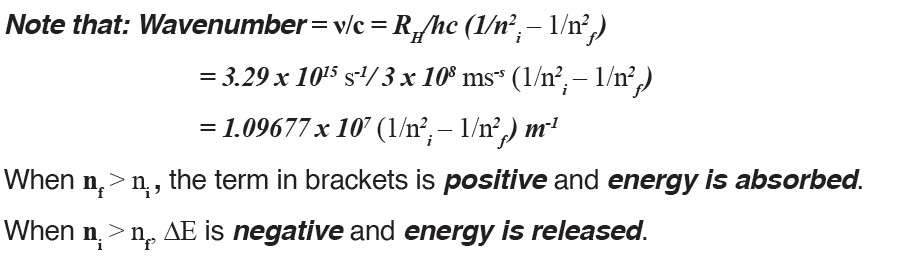
(at 4 x 1014 Hz). When this light passes through an object or medium, the wave
with the shortest wavelength (violet) deviates the more than the one with the
longest wavelength (red).
WORKED EXAMPLES
1. Find the wave length and frequency in Balmer series associated
with a drop of an electron from the fourth orbit.Answer
2. Find the wave length, frequency and energy of the third line in the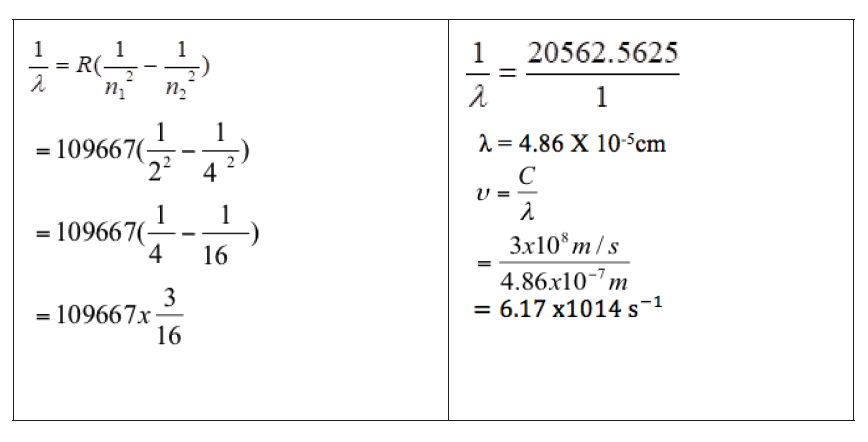
Lyman series.
Answer:
3. A certain source emits radiation of wavelength 500.0 nm. What is
the energy, in kJ, of one mole of photons of this radiation?
Solution:
Convert nm to m: 500.0 nm = 500.0 x 10-9m = 5.000 x 10-7mDetermine the frequency:
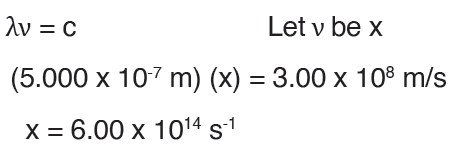
Determine the energy: E = hν
= (6.626 x 10– 34 J s) (6.00 x 1014 s-1)
= 3.9756 x 10–19 J
Important point: this is the energy for one photon.
Determine energy for one mole of photons: (3.9756 x 10–19 J) (6.022 x1023 mol–1) = 239.4 kJ/mol
Note: If you wished to do a direct calculation, you could use this equation:
E = hc / λ. Just make sure that the units for c and λ match.
Application activity 7.3
1. What is the meaning of infinity level in the hydrogen spectral lines?
2. Given a transition of an electron from n=2 to n=5. Calculate
a) Wavelength
b) Frequency
c) Energy
3. Explain how atomic emission spectra arise and how they relate to
each element on the periodic table.
4. How do the lines on the atomic spectrum relate to electron
transitions between energy levels?
5. Explain the difference between atomic absorption and emission
spectra.
6. Describe how the absorption and emission spectra of the gases in
the atmosphere give rise to the Greenhouse Effect.7. Use the figure below to answer the following questions.
a) What colour is the light emitted by hydrogen when an electron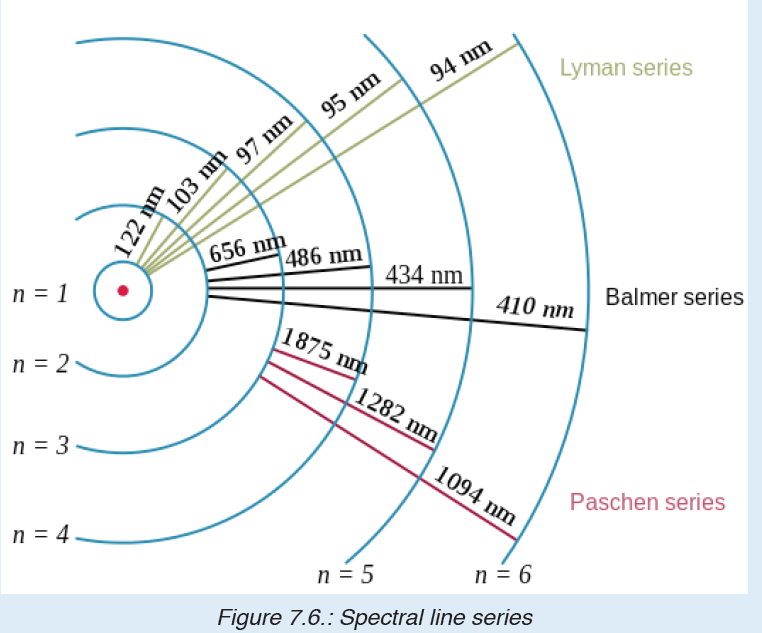
makes the transition from energy level 5 down to energy level 2?
(Use the figure above to find the energy of the released photon.)
b) I have a glass tube filled with hydrogen gas. I shine white light onto
the tube. The spectrum I then measure has an absorption line at
a wavelength of 474 nm. Between which two energy levels did the
transition occur?
8. Calculate the wavelength of a line in the Balmer series that is
associated with energy transition, E4 to E2 (E4 = -1.362×10-19 J, E2= -5.448×10-19J).
7.4. Concept of orbitals and quantum numbers
Activity 7.4
1. Recall the main weakness of the Atomic Bohr’s Model.
2. What do you understand with the term “orbit” in the atomic
structure?
3. Suppose that the orbit you talked about above is subdivided into
other sub-parts, said orbitals. Formulate a definition of an “orbital”.
4. There are numbers used to locate the orbitals. These are of four
types. One usually encountered is qualified to be “principal”.
a) What is the name given to those numbers?
b) Make a research and state them.
c) The principal one gives relevant information about the given atom.
State at least two points that are revealed when this principal
number is given.
We have seen the weakness and critics against the atomic Bohr’s model.
In order to answer the questions not answered by that model, other atomic
models were proposed. One of those models is the Quantum Model that
has been developed by the Australian physicist Erwin Schrödinger (1887-
1961). The model is based on a mathematical equation called Schrödingerequation.
7.4.1. Orbitals
This Quantum Model is based on the following assumptions or hypotheses:
• An electron is in continuous movement around the nucleus but cannot
be localized with precision; only the high probability of finding it in a
certain region around the nucleus can be known.
• The region where the probability of finding electron is high, at more
than 95%, is called “orbital”; in other words, the orbital is the volume
or the space (tridimensional) around the nucleus where there is a high
probability of finding the electron.
The orbitals are of 4 types. They are named s, p, d, f. The s, p, d, and f
stand for sharp, principal, diffuse and fundamental, respectively.1. “s” orbitals are spherically shaped.
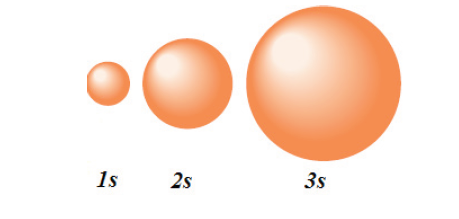
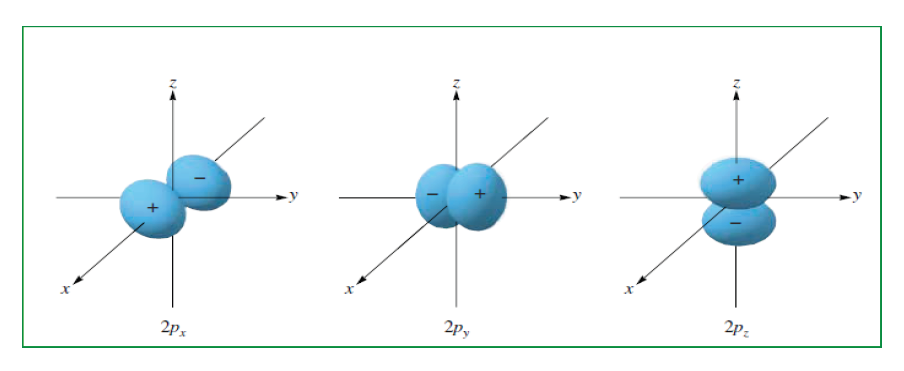
2. “p” orbitals are often described as dumb-bell shaped.
3. “d” orbitals and “f” orbitals are not easily visualized.
7.4.2. Quantum numbers
Without going into the mathematical development of the Schrödinger
equation, we can say that the energy of the electron depends on the orbital
where it is located and an atomic orbital is described by a certain number
of “quantum numbers” according to the solution of Schrodinger equation.
Quantum numbers are a set of numbers that describe the state of an
electron in an atom (and they are derived from quantum mechanical
treatment).
Four numbers, called quantum numbers, were introduced to describe the
characteristics of electrons and their orbitals:
• Principal quantum number: n
• Angular momentum quantum number: ℓ
• Magnetic quantum number: mℓ
• Spin quantum number: ms
1. The Principal Quantum Number
The principal quantum number n describes the average distance of the orbital
from the nucleus (the size of the shell) — and the energy of the electron
in an atom. It can have positive integer (whole number) values: 1, 2, 3, 4,
and so on. Thelarger the value of n, the higher the energy and the larger
the orbital. Chemists sometimes call the orbitals electron shells. The shells
(values of n) can be represented by letters K, L, M, N, O, P.
2. The Angular Momentum Quantum Number
The angular momentum quantum number ℓ is also called Secondary
Quantum number or Azimuthal Quantum Number. It describes the shape
of the orbital, and the shape is limited by the principal quantum number n:
The angular momentum quantum number ℓ can have positive integer values
from 0 to n–1. For example, if the n value is 3, three values are allowed for
ℓ: 0, 1 and 2. l=0(s), l=1(p), l=2(d), l=3(d).
The value of ℓ defines the shape of the orbital, and the value of n defines
the size. Orbitals that have the same value of n but different values of ℓ are
called subshells.
3. The Magnetic Quantum Number
The magnetic quantum number is designated as: mℓ. It describes how thevarious orbitals are oriented in space.
The value of this number depends on the value of ℓ. The values allowed are
integers from – ℓ to 0 to +ℓ. For example, if the value of l = 1 (p orbital), you
can write three values for this number: –1, 0, and +1. This means that thyou
can write three values for this number: –1, 0, and +1. This means that there
are three different p orbitals for the subshells. The orbitals have the same
energy but different orientations in space.
The three p orbitals correspond to magnetic quantum number values of –1,
0, and +1, oriented along the x, y, and z axes.
4. The Spin Quantum Number
The fourth and final quantum number is the spin quantum number, designated
as: ms This number describes the direction the electron is spinning in a
magnetic field — either clockwise or counterclockwise. Only two values are
allowed: +1/2 or –1/2. For each subshell, there can be only two electrons,
one with a spin of +1/2 and another with a spin of –1/2.
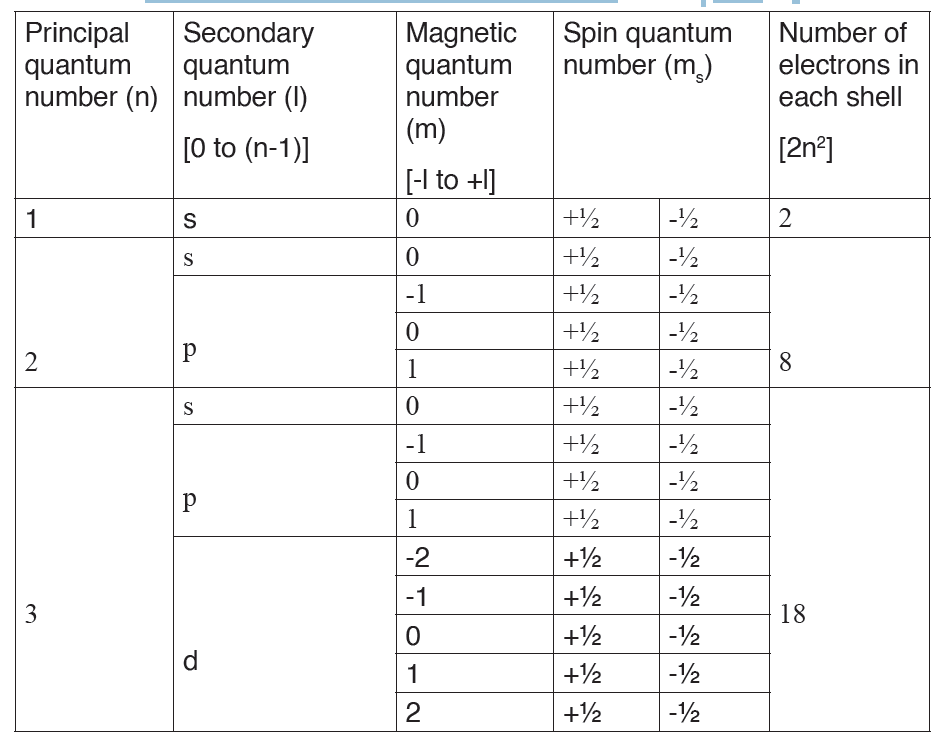
Table 7.1.: Relationship between the n, ℓ, mℓ and ms
Application activity 7.4
1. Define the following terms:
a) Orbital
b) Quantum number
2. Give the different types of orbitals stating their shapes where it is
possible.
3. We have four quantum numbers. Use the knowledge of quantum
numbers to complete the table below.
4. Which of the following sets of quantum numbers are not allowed?
For each incorrect set, state why it is incorrect.
7.5. Rules governing the electronic configurations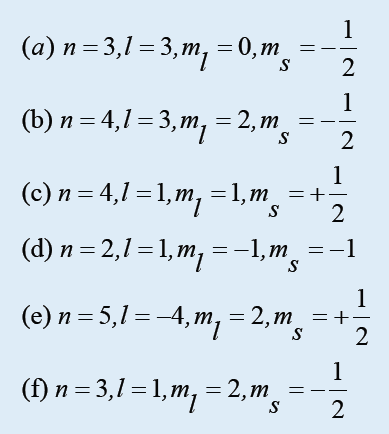
Activity 7.5
1. Write electronic configuration of the following atoms using K, L, M,
N… orbit representations: Ca (z= 20), Cl (Z= 17), Sr (Z=38)
2. Potassium contains 19 electrons while sulphur contains 16. It is
found that the potassium ion (K+) has 18 electrons like the sulphide
ion (S2-).
a) Explain why the two ions contain the same number of electrons.
b) What is the element and its group on the Periodic table which is
isoelectronic with the ions mentioned?
3. State two differences between
a) Calcium atom (Ca) and its ion (Ca2+).
b) Nitrogen (N) and its ion (N3-)
The electron configuration of an atom is the representation of the arrangement
of electrons distributed among the orbital shells and subshells. Commonly,
the electron configuration is used to describe the orbitals of an atom in its
ground state, but it can also be used to represent an atom that has ionized
into a cation or anion by compensating with the loss of or gain of electronsin their subsequent orbitals.
Many of the physical and chemical properties of elements can be correlated
to their unique electron configurations. The valence electrons, electrons
in the outermost shell, are the determining factor for the unique chemistry of
the element.
Before assigning the electrons of an atom into orbitals, one must become
familiar with the basic concepts of electron configurations. Using the
periodic table to determine the electron configurations of atoms is a key,
but also keep in mind that there are certain rules to follow when assigning
electrons to different orbitals. The periodic table is an incredibly helpful tool
in writing electron configurations.
7.5.1. Rules for assigning electron orbitals
Electrons fill orbitals in a way to minimize the energy of the atom.
Therefore, the electrons in an atom fill the principal energy levels in order of
increasing energy (the electrons are getting farther from the nucleus). Theorder of levels filled looks like this:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p
One way to remember this pattern, probably the easiest, is to refer to the
periodic table and remember where each orbital block falls to logically
deduce this pattern. Another way is to make a table like the one below anduse vertical lines to determine which sub-shells correspond with each other.
1. Pauli Exclusion Principle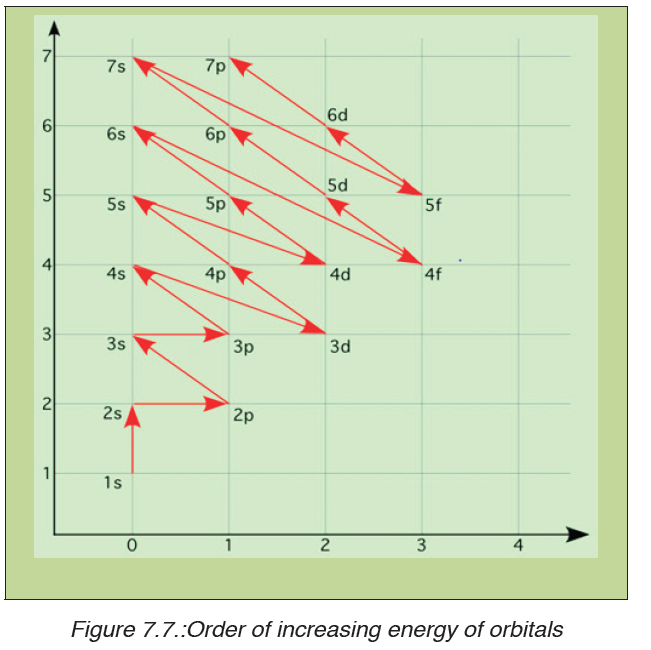
The Pauli Exclusion Principle states that no two electrons can have the same
four quantum numbers.
As said before, the first three (n, ℓ, and mℓ ) may be the same, but the
fourth quantum number must be different. A single orbital can hold a
maximum of two electrons, which must have opposing spins; otherwise
they would have the same four quantum numbers, which is forbidden. One
electron is spin up (ms = +1/2) and the other would spin down (ms = -1/2).
This tells us that each subshell has double the electrons per orbital. The s
subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3
orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold
up to 10 electrons, and the f subshell has 7 orbitals with 14 electrons.Example: Hydrogen and Helium
The first three quantum numbers of an electron are n=1, ℓ=0, mℓ=0. Only two
electrons can correspond to these, which would be either ms = -1/2 or ms =+1/2.
As we already know from our studies of quantum numbers and electron
orbitals, we can conclude that these four quantum numbers refer to the 1ssubshell. Visually, this can be represented as:
As shown, the 1s subshell can hold only two electrons and, when filled, the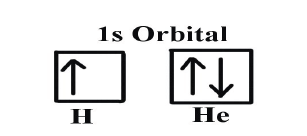
electrons have opposite spins.
If only one of the ms values are given then we would have 1s1 (denoting
hydrogen); if both are given we would have 1s2 (denoting helium).
2. Hund’s Rule
When assigning electrons in orbitals, each electron will first fill all
the orbitals with similar energy (also referred to as “degenerate”) before
pairing with another electron in a half-filled orbital. Atoms at ground states
tend to have as many unpaired electrons as possible. When visualizing this
process, think about how electrons are exhibiting the same behavior as the
same poles on a magnet would if they came into contact; as the negatively
charged electrons fill orbitals they first try to get as far as possible from each
other before having to pair up.
Example: Oxygen and Nitrogen
If we look at the correct electron configuration of the Nitrogen (Z = 7) atom,a very important element in the biology of plants: 1s2 2s2 2p3
If we look at the element after Nitrogen in the same period, Oxygen (Z = 8)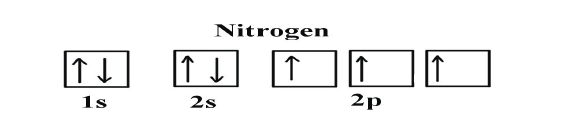
its electron configuration is: 1s2 2s22p4 (for an atom).
Oxygen has one more electron than Nitrogen and as the orbitals are all half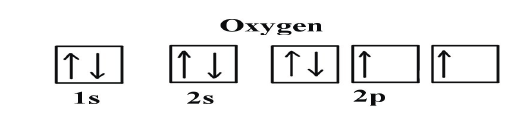
filled, the electron must pair up.
3. The Aufbau Principle
Aufbau comes from the German word “aufbauen” meaning “to build.” When
writing electron configurations, orbitals are built up from atom to atom.
Example: 3rd Row Elements
Following the pattern across a period from B (Z=5) to Ne (Z=10), the number
of electrons increases and the subshells are filled. This example focuses on
the p subshell, which fills from boron to neon.
B (Z=5) configuration: 1s2 2s2 2p1
C (Z=6) configuration: 1s2 2s2 2p2
N (Z=7) configuration: 1s2 2s2 2p3
O (Z=8) configuration: 1s2 2s2 2p4
F (Z=9) configuration: 1s2 2s2 2p5
Ne (Z=10) configuration: 1s2 2s2 2p6
According to the Aufbau Process, when writing the electron configuration
for an atom, orbitals are filled in order of increasing atomicnumber. However, there are some exceptions to this rule.
7.5.2. Writing electron configurations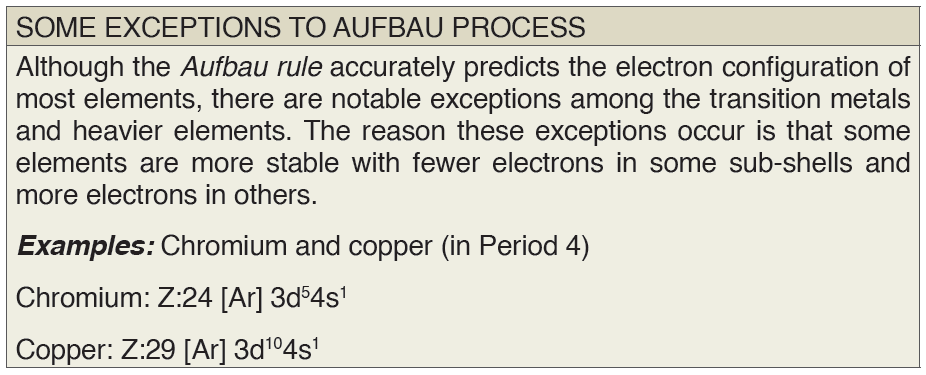
When writing an electron configuration, first write the energy level (the
period), then the subshell to be filled and the superscript, which is the
number of electrons in that sub-shell. The total number of electrons is theatomic number, Z.
The rules above allow one to write the electron configurations for all the
elements in the periodic table. Three methods are used to write electron
configurations:
• Spdf notation
• Orbital diagrams
• Noble gas notation
Each method has its own purpose and each has its own drawbacks.
4. spdf Notation
The most common way to describe electron configurations is to write
distributions in the spdf notation. Although the distributions of electrons
in each orbital are not as apparent as in the diagram, the total number of
electrons in each energy level is described by a superscript that follows the
relating energy level.
To write the electron configuration of an atom, identify the energy level
of interest and write the number of electrons in the energy level as its
superscript as: 1s2. This is the electron configuration of helium; it denotes a
full s orbital. The periodic table is used as a reference to accurately write the
electron configurations of all atoms.
Example:
Potassium has 19 electrons.
– Begin by filling up the 1s sublevel. This gives 1s2. Now the n = 1 level
is filled.
– Since we used 2 electrons, there are 19 − 2 = 17 electrons left
– Next, fill the 2s sublevel. This gives 1s22s2
– Since we used another 2 electrons, there are 17 − 2 = 15 electrons left
– Next, fill the 2p sublevel. This gives 1s22s22p6. Now the n = 2 level is
filled.
– Since we used another 6 electrons, there are 15 − 6 = 9 electrons left
– Next, fill the 3s sublevel. This gives 1s22s22p63s2
– Since we used another 2 electrons, there are 9 − 2 = 7 electrons left
– Next, fill the 3p sublevel. This gives 1s22s22p63s23p6
– Since we used another 6 electrons, there are 7 − 6 = 1 electron left
– Here’s where we have to be careful – right after 3p6!!
– Remember, 4s comes before 3d!The final electron goes into the 4s sublevel. This gives 1s22s22p63s23p64s1
5. Orbital Diagrams
An orbital diagram, like those shown above, is a visual way to reconstruct
the electron configuration by showing each of the separate orbitals and the
spins on the electrons. This is done by first determining the subshell (s,p,d or
f) then drawing in each electron according to the stated rules above.
Example: The atomic number of Iridium (Z) is 77. Write the electron
configuration of Iridium using orbital diagram method.Answer:
Although drawing out each orbital may prove to be helpful in determining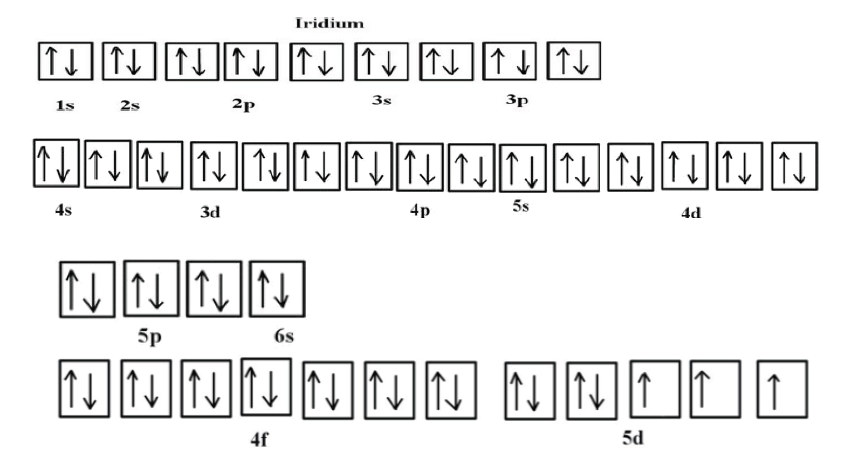
unpaired electrons, it is very time consuming and often not as practical
as the spdf notation, especially for atoms with much longer configurations.
Hund’s rule is also followed, as each electron fills up each 5d orbital before
being forced to pair with another electron.
6. Noble Gas Notation
This brings up an interesting point about elements and electron configurations.
As the p-subshell is filled in the above mentioned example of the period
from B (Z=5) to Ne (Z=10) about the Aufbau principle, it reaches the group
commonly known as the noble gases. The noble gases have the most
stable electron configurations, and are known for being relatively inert. All
noble gases have their subshells filled and can be used them as a shorthand
way of writing electron configurations for subsequent atoms.
This method of writing configurations is called the noble gas notation, in
which the noble gas in the period above the element that is being analyzed
is used to denote the subshells that element has filled and after which the valence electrons (electrons filling orbitals in the outer most shells) are written. This looks slightly different from spdf notation, as the reference noble
gas must be indicated.
Example: Vanadium (V, Z=23)
Vanadium is the transition metal in the fourth period and the fifth group.
The noble gas preceding it is argon (Ar, Z=18), and knowing that vanadium
has filled those orbitals before it, argon is used as the reference noble
gas. The noble gas in the configuration is denoted in brackets.
To find the valence electrons that follow, subtract the atomic numbers: 23 -
18 = 5. Instead of 23 electrons to distribute in orbitals, there are 5. Now there
is enough information to write the electron configuration:
Vanadium, V: [Ar] 4s2 3d3
This method streamlines the process of distributing electrons by showing
the valence electrons, which determine the chemical properties of atoms.
In addition, when determining the number of unpaired electrons in an atom,
this method allows quick visualization of the configurations of the valance
electrons. In the example above, there are one full s orbital and three half
filled d orbitals.
7.5.3. Electron configurations of ions
We already know that ions are formed when atoms gain or lose electrons.
A cation (positively charged ion) forms when one or more electrons are
removed from a parent atom. For main group elements, the electrons that
were added last are the first electrons removed. For transition metals and
inner transition metals, however, electrons in the s orbital are easier to
remove than the d or f electrons, and so the highest ns electrons are lost,
and then the (n – 1)d or (n – 2)f electrons are removed.
An anion (negatively charged ion) forms when one or more electrons are
added to a parent atom. The added electrons fill in the order predicted by
the Aufbau principle.
Example: What is the electron configuration of: Na+, P3–, Al2+, Fe2+ and Sm3+
Solution
• First, write out the electron configuration for each parent atom. We
have chosen to show the full, unabbreviated configurations to provide
more practice for students who want it, but listing the core-abbreviatedelectron configurations is also acceptable.
• Next, determine whether an electron is gained or lost. Remember
electrons are negatively charged, so ions with a positive charge
have lost an electron. For main group elements, the last orbital gains
or loses the electron. For transition metals, the last s orbital loses anelectron before the d orbitals.
Application activity7.5
1. The electron energy levels of a certain element can be represented
as 1s2, 2s2, 2p6, 3s2, 3p4
a) What is the atomic number of the element?
b) What is the name of the element?
2. The element nitrogen forms compounds with metals and nonmetals.
Nitrogen forms a nitride ion with the electron configuration
1s2 2s2 2p6.
a) Write the formula of the nitride ion.
b) An element forms an ion Q with a single negative charge that has
the same electron configuration as the nitride ion. Identify the ionQ.
3. tUsing the noble gas notation, write the electronic configuration of
the following atoms/ions.
a) Ge (Z=32)
b) S (Z=16)
c) Co2+ (Z=27)
d) Br- (Z=35)e) Sr (Z=38)
SKILLS LAB
“ELECTRON CONFIGURATION BINGO ACTIVITY”
Introduction
The wave-mechanical model of the atom states that the exact position of
an electron at any given moment cannot be determined. Instead, electrons
are located in clouds outside the nucleus. These clouds are described by
energy level and type of sublevel. An electron configuration may be written
to identify the placement of electrons in these levels and sublevels.
Objectives
1. Determine electron configurations for given elements.
2. Identify elements given their electron configurations.
Materials: (per student)
• 1 bingo card
• 25 bingo markers
• 1 periodic table
Procedure
1. Choose 25 elements from the provided list.
2. On your bingo card, fill in each box with either the symbol of the
chosen element or its electron configuration. DO NOT WRITEBOTH.
3. Your tutor will call out either the electron configuration or an element.
4. From the question, determine either the element or the electron
configuration. Mark your card appropriately. For example, if the
question is “Oxygen”, you may mark your card only if you have 1s2
2s2 2p4. If the question is “1s2 2s2 2p6”, you may mark your card
only if you have Ne.
5. The winner of the game is the first person to have 5 squares in a row marked.Element List
End unit assessment 7
1. For each of the following, choose the letter corresponding to the
best answer.
a) The principal quantum number describes the following, except
i. The size of the shell
ii. The energy of an electron in an atom
iii. The shape of the orbital
iv. The average distance of the orbital from the nucleus
b) On the following list of quantum numbers, the one which is not
correct is:
i. Principal
ii. Spin
iii. Magmatic Quantum
iv. Azimuthal
c) The electron configuration for gallium (Z=31) is:
i. [Ar] 4s24d104p1
ii. [Ar] 4s23d104p1
iii. [Ar] 4s23d103p1
iv. None of the above.
d) The four other spectral line series, in addition to the Balmer series,
are named after their discoverers. They are, except:
i. Lyman
ii. Pfund
iii. Brackettiv. Planck
2. According to the Aufbau principle, which orbital is filled
immediately before each of the following?
a) 3p
b) 4p
c) 4f
d) 5d
3. Hafnium element has 72 electrons. Write its s, p, d, f electron
configuration.
4. Why are the outer-most electrons the only ones included in the
electron dot diagram?
5. The orbital filling diagram has arrows pointing in opposite
directions when two electrons occupy the same orbital. What do
these arrows indicate?
6. The emission spectrum of hydrogen consists of several series
of lines. The series of highest energy is called the Lyman series
(see Figure below). Each line in the series is the result of anelectronic transition between energy levels.
a) State in which direction the energy increases: A to G or G to A.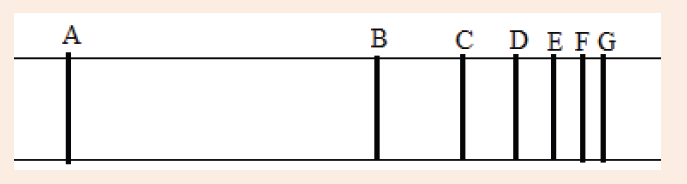
b) State in which direction the frequency increases A to G or G to A.
c) Explain why the spectrum consists of lines.
d) What do transitions in the same series all have in common?
7. a) Write the electronic configuration of the following elements/
ions:
“Sodium, magnesium ion (Mg2+), aluminium, aluminium ion (Al3+),
oxygen ion (O2-)”
b) Identify the common feature of ions in (a) and why do they have
such feature.
c) Suggest what happened to aluminium atom when it changed to
aluminium ion (Al3+).
d) Identify the group and the period of aluminium, sodium and
oxygen atom.
8. Four possible electron configurations (A, B, C and D) for a nitrogen
atom are
a) Which one is the correct electron configuration?
b) Which configurations violate the Pauli Exclusion Principle?c) Which configurations violate Hund’s rule?
9. Complete the electronic configurations for the sulphur atom, S,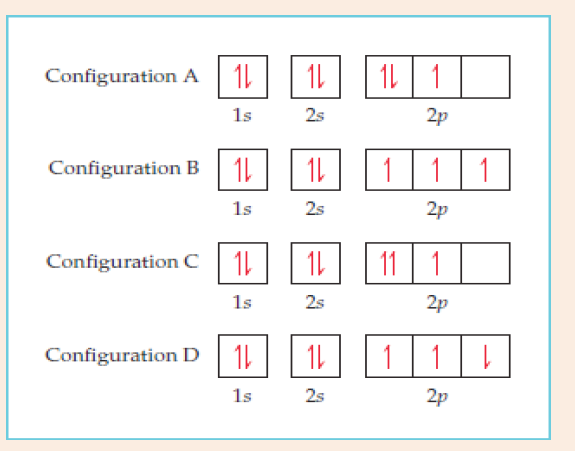
and the sulphide ion, S2-. State the block in the Periodic Table in
which sulphur is placed and explain your answer.10. The diagram below shows the electronic structure of boron.
a) The electrons are represented by arrows. What property of the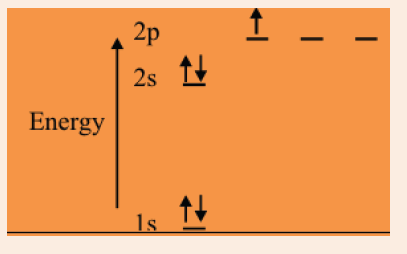
electrons do these ‘up’ and ‘down’ arrows represent?
b) Suggest why electrons which occupy the 2p sub-levels have ahigher energy than electrons in the 2s sub-level.
