UNIT 11: COVALENT BONDS
Key unit competence
Demonstrate how the nature of the bonding is related to the properties of
covalent compoundsIntroductory Activity 11
As you can see from the picture above, Oxygen is the big buff creature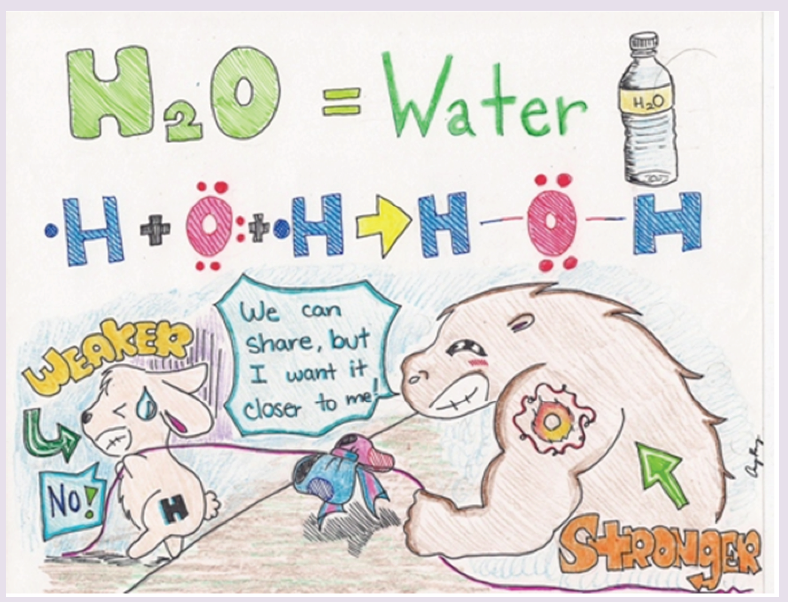
with the tattoo of “O” on its arm. The little bunny represents a Hydrogen
atom. The blue and red bow tied in the middle of the rope, pulled by the
two creatures represent the shared pair of electrons, a single bond.
Because the Hydrogen atom is weaker, the shared pair of electrons will be
pulled closer to the Oxygen atom.
1. Suggest the property used in Chemistry to describe the strength
dedicated to oxygen, the stronger?
2. Suppose that the rope being pulled represents a single covalent
bond, the electron contributed by hydrogen, the weaker will be
transferred to oxygen the stronger?
3. If not, why?
4. Suppose again that we have two oxygens. They have the same
strengths. What will happen to the pulled rope, or the shared pair
of electrons?
5. Suggest a reason why, from the figure, one oxygen needed sharing
with two hydrogens to form water.
6. Conclude about the possible types of covalent bonds.
11.1. Overlap of atomic orbitals to form covalent bonds
Activity 11.1
1. Modern research has shown that an electron moves around
the nucleus in the three dimensional space. What are these
dimensional spaces called?
2. What types of atomic orbital overlapping, what does this overlapping
lead to?
3. Using dots or crosses, give the structure of N(z=7) and Br(z=13)
showing only the electrons on the outermost shell.
Atoms have different ways of combination to achieve the stable octet
electronic structure; two of those ways of combination led to the formation of
ionic bond and metallic bond. But what happens where the two combining
atoms need electrons to complete the octet structure and no one is willing to
donate electrons? For example the combination of 2 hydrogen atoms or the
combination of 2 chlorine atoms?
When this happens, the combining atoms share a pair of electrons where
each atom brings or contributes one electron. In other words there is an
overlapping of two orbitals, one orbital from one atom, each orbital
containing one electron (see Fig.11.1): this bond is called “Covalent bond”.
The attraction between the bonding pair of electrons and the two nuclei holds
the two atoms together. This theory of covalent bond is based on the concept
that electrons are located around the atomic nucleus in orbitals. Then when
two atoms approach each other to share electrons, their two orbitals, each
containing one electron, overlap in the region between the two nuclei to form
a pair of electrons. That pair of electrons is attracted by each nucleus and
this force of attraction maintains the two atoms together; it is this force that
is called chemical bond and in this case, it is qualified as ”covalent”.
Examples:
1. Formation of H2 molecule by overlapping of two 1s orbitals of 2hydrogen atoms:
2. (2) Formation of F2 by overlapping of two p orbitals of 2 fluorine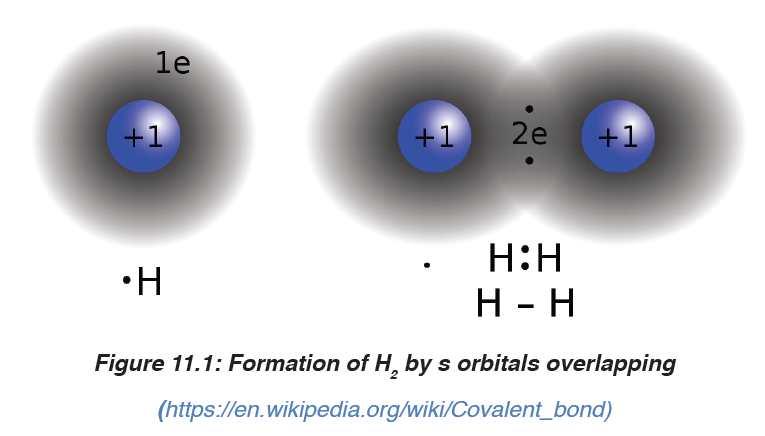
atoms:
The two examples above have in common that the concentration of the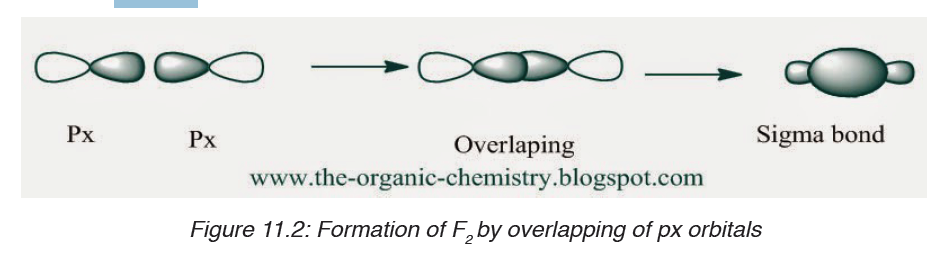
bonding electrons are on the inter-atomic (inter-nuclei) axis; such bonds are
called “sigma bond”, represented by the symbol “σ”.
As you can observe, p orbitals overlap head-to-head or axially, they form a
σ bond.
1. Formation of O2 molecule (O=O)
When O2 forms, two orbitals in the same orientation, e.g. px, overlap headto-
head to form a σ bond. The other orbitals, e.g. py, will overlap side-by-sideor laterally:
As you notice, the density of bonding electrons is not on the inter-nuclei axis,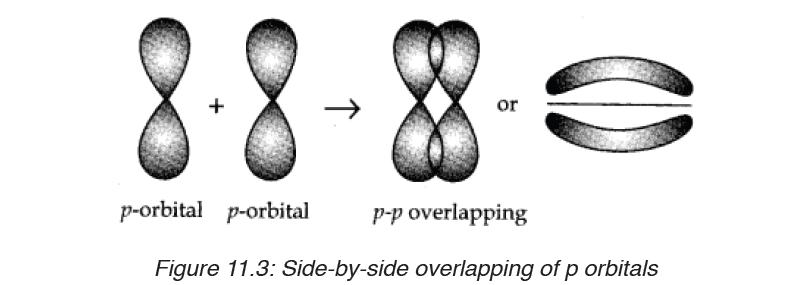
it is rather located outside the axis but surrounding it. This kind of covalent
bond is called “ Pi bond”, represented by the symbol “π”. Hence the double
bond O=O is made of two covelent bonds: a σ bond and a π bond.
Due to the position of their electrons density in relation with the two nuclei,
σ bond participates in maintaining the two nuclei together more strongly
than the π bond; that is why σ bond is stronger than π bond. In addition, π
bond cannot exist alone, it exists only where there is a double or triple bond.
Hence, in a double or triple bond, there is one σ bond and one or two π
bonds respectively.
Application activity 11.1
1. Explain the formation of sigma(σ) and pi(π) bonds in:
a) N2
b) Br2
c) NH3
2. Compare the stability of ethane CH3- CH3 and ethene CH2=CH2.
Explain your answer.
3. How many sigma and pi bonds are found in the following molecules:
CH2=CH-CH=CH2 and O2?4. Complete the table that follow by the missing data
11.2. Lewis structures using octet rule (dot and cross
structures)
Activity 11.2
Using available resources, attempt the following:
1. Draw the diagrams indicating only the valence electrons of the
following:
Chlorine molecule (Cl), Carbon atom (C), Phosphorus atom (P),
Nitrogen (N).
2. Draw the diagram to show how all electrons are shared in a
molecule of
i. NH3 indicating all unshared electrons.
ii. HCl (iii) N2
3. Identify the common feature possessed by the diagrams drawn
above in 2.
Lewis structures (also known as Dot and cross structures, Lewis dot
diagrams, Lewis dot formulas, Lewis dot structures, and electrondot
structures) are diagrams that show the bonding between atoms of
a molecule and the lone pairs of electrons that may exist in the molecule. A
Lewis structure can be drawn for any covalently bonded molecule, as well
as coordination compounds.
Lewis structures extend the concept of the electron dot diagram by adding
lines between atoms to represent shared pairs in a chemical bond.
molecule using its chemical symbol. Lines are drawn between atoms that
are bonded to one another (pairs of dots can be used instead of lines).
Excess electrons that form lone pairs are represented as pairs of dots, and
are placed next to the atoms.Examples: Lewis structure of Cl2
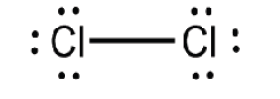
Lewis structure of NH4+
How to draw Lewis Structures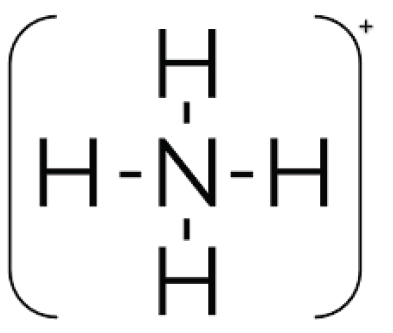
Let us use the nitrate ion (NO3-) as a typical example. An outline of how to
determine the “best” Lewis structure for NO3- is given below:1. Determine the total number of valence electrons in a molecule.
2. Draw a skeleton for the molecule which connects all atoms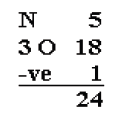
using only single bonds. In simple molecules, the atom with
the most available sites for bonding is usually placed central. The
number of bonding sites is determined by considering the number of
valence electrons and the ability of an atom to expand its octet. As
you become better, you will be able to recognize that certain groupsof atoms prefer to bond together in a certain way!
3. Of the 24 valence electrons in NO3-, 6 were required to make the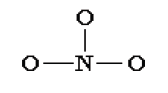
skeleton. Consider the remaining 18 electrons and place them so
as to fill the octets of as many atoms as possible (start with
the most electronegative atoms first then proceed to the moreelectropositive atoms).
4. Are the octets of all the atoms filled? If not then fill the remaining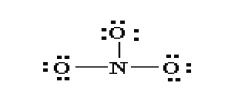
octets by making multiple bonds (make a lone pair of electrons,
located on a more electronegative atom, into a bonding pair ofelectrons that is shared with the atom that is electron deficient).
5. Check that you have the lowest formal charges (F.C.) possible for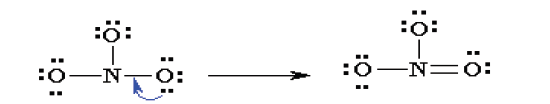
all the atoms, without violating the octet rule; F.C. = (valence e-) -(1/2 bonding e-) - (lone electrons).
6. Thus the Lewis structure of NO3- ion can be written in the following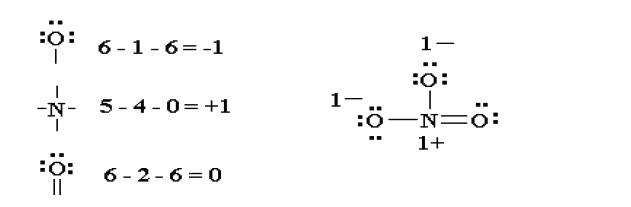
ways:
Lewis structures of unusual compounds that do not obey Octet Rule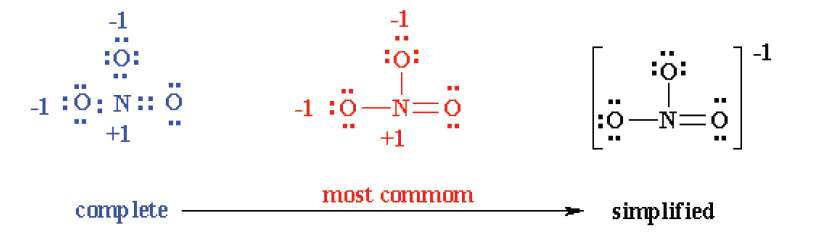
There are three general ways in which the octet rule breaks down:
• Molecules with an odd number of electrons
• Molecules in which an atom has less than an octet
• Molecules in which an atom has more than an octet
1. Odd number of electrons
Consider the example of the Lewis structure for the molecule nitrous oxide
(NO):
• Total electrons: 6 + 5 = 11• Bonding structure:
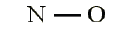
• Octet on “outer” element:
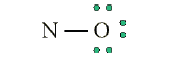
• Remainder of electrons (11-8 = 3) on “central” atom:
There are currently 5 valence electrons around the nitrogen. A double bond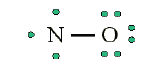
would place 7 around the nitrogen, and a triple bond would place 9 around
the nitrogen. We appear unable to get an octet around each atom.
2. Less than an octet (most often encountered with elements of Boron
and Beryllium)
Consider the example of the Lewis structure for boron trifluoride (BF3):
• Add electrons (3 x 7) + 3 = 24• Draw connectivities
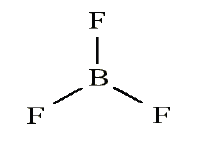
• Add octets to outer atoms:
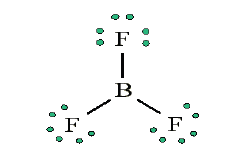
• Add extra electrons (24 – 24 = 0) to central atom:
• Does central electron have octet? No, it has 6 electrons. Add a multiple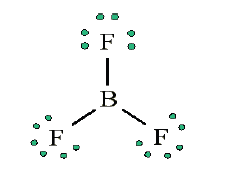
bond (double bond) to see if central atom can achieve an octet:
The central Boron now has an octet (there would be three resonance Lewis structures).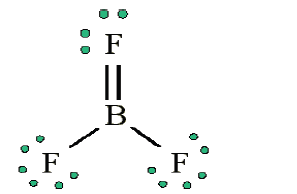
However, in this structure with a double bond the fluorine atom is
sharing extra electrons with the boron.
The fluorine would have a ‘+’ partial charge, and the boron a ‘-’ partial
charge, this is inconsistent with the electronegativities of fluorine and boron.
Thus, the structure of BF3, with single bonds, and 6 valence electrons
around the central boron is the most likely structure.
BF3 reacts strongly with compounds which have an unshared (lone) pair
of electrons which can be used to form a bond with the boron. Example:Reaction of BF3 with ammonia.
3. More than an octet (most common example of exceptions to the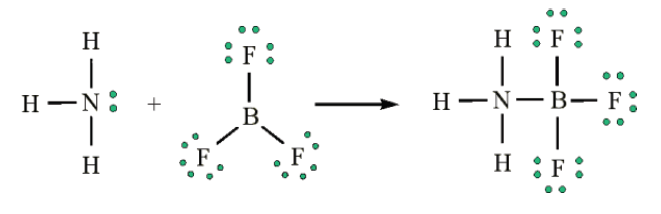
Octet Rule)PCl5 is a legitimate compound, whereas NCl5 is not.
Expanded valence shells are observed only for elements in period 3 (i.e.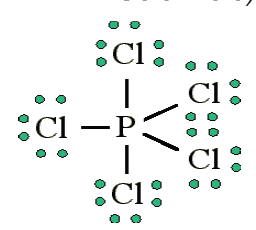
n=3) and beyond.
Size is also an important consideration: “The larger the central atom,
the larger the number of electrons which can surround it”. Expanded
valence shells occur most often when the central atom is bonded to small
electronegative atoms, such as F, Cl and O.
Example: Draw the Lewis structure for ICl 4−
• Count up the valence electrons: 7 + (4 x 7) + 1 = 36 electrons• Draw the connectivities:
The ICl4− ion thus has 12 valence electrons around the central Iodine (in the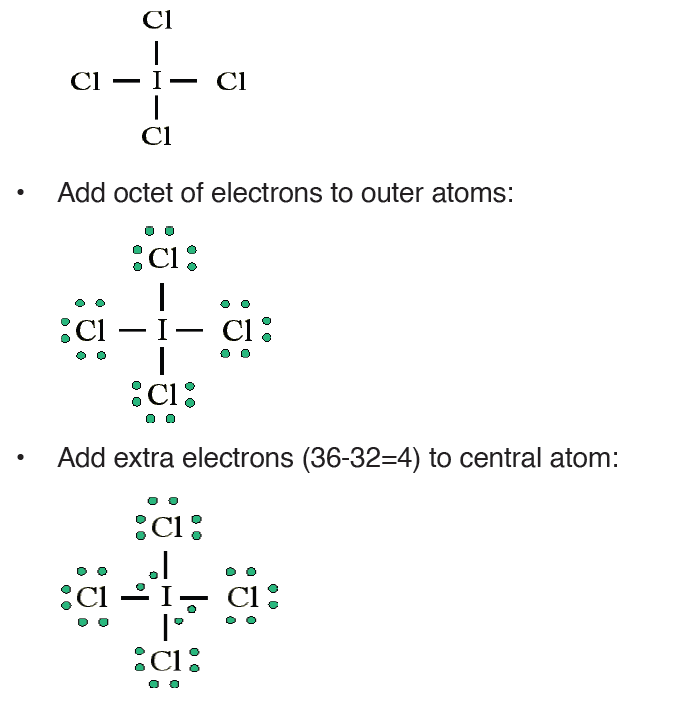
5d orbitals)
Other examples include: PCl5 and SF6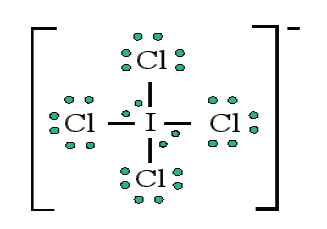
Application activity 11.2
1. Make a clear definition of the covalent bond.
2. For each of the following, write the electron –dot structures (Lewisstructures) and choose one which violates the Octet Rule?
11.3. Coordinate or dative covalent bond
Activity 11.3
Use your knowledge acquired from the previous lesson and draw the Lewis
structure of ozone (O3), NH4+, H3O+. One of the bonds in these molecules
are special. Explain how they are formed.
A dative covalent bond, or coordinate bond is a type of covalent bonding
(i.e., electron sharing) where the shared electron pair(s) are completely
provided by one of the participants in the union, and not by contributions
from the two of them.
The contributors of these shared electrons are either neutral molecules
which contain lone pair(s) of electrons on one of their atoms, or negatively
charged groups (radicals) with free electrons to donate. Examples of these
are: H2O, NH3 and CN−.
Examples of coordinate bonding: In the reaction
between ammonia and hydrogen chloride a coordinate bond takes place
forming solid ammonium chloride.
NH3 + HCl → NH4Cl
In this reaction the hydrogen ion from the hydrogen chloride leaves its
electrons and gets transferred to the lone pair of electrons on the ammonia
molecule forming ammonium ions (NH4+). This is known as a coordinate
bonding.
Seeing that the hydrogen has left its electron, the chloride will therefore have
a negative charge while the ammonium will have a positive charge. Thediagram below shows the reaction:
Note: The complete compound eventually formed comprises three types of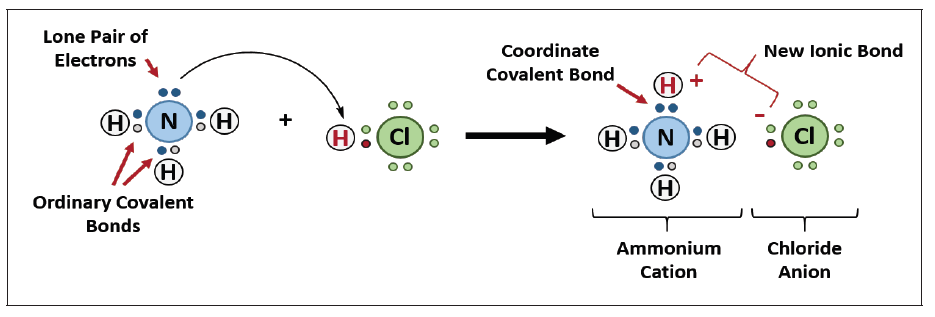
bonding, i.e., covalent, co-ordinate and electrovalent. In NH4Cl: Formation
of NH3 (covalency); formation of NH4+ (co-ordinate or dative bonding); andformation of NH4Cl (electrovalency).
Dative covalent bonds are represented on drawings as an “arrow”, which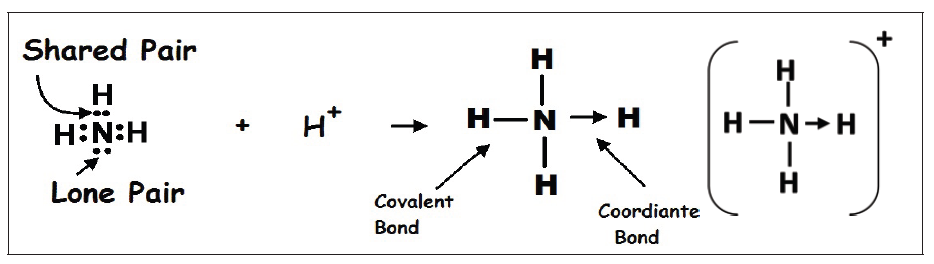
usually points from the atom donating the lone pair to the atom accepting it.
Another example would be the reaction between ammonia and boron
trifluoride. Boron trifluoride is said to be electron deficient meaning it has
3 pairs of electrons at its bonding level but it is capable of having four pairs.
In this reaction the ammonia is used to supply this extra lone pair.
A coordinate bond is formed where the lone pair from the nitrogen moves
toward the boron. The end containing the nitrogen will therefore become
more positive while the boron end will become more negative because it hasreceived electrons.
Application activity 11.3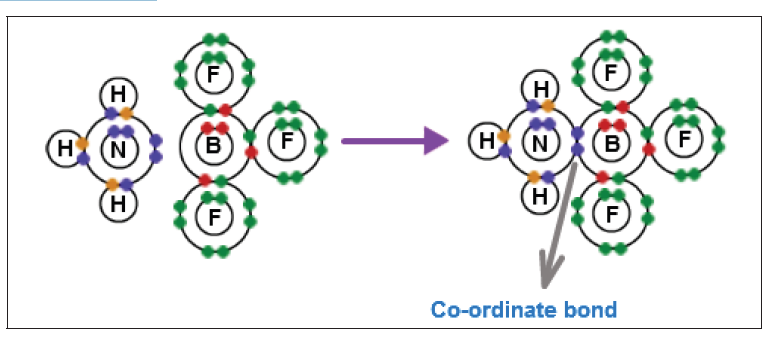
1. Give the difference and the similarity between a dative covalent
bond and the normal covalent bond.
2. An aluminium chloride molecule reacts with a chloride ion to form
the AlCl4− ion.
a) Name the type of bond formed in this reaction.
b) Explain how this type of bond is formed in the AlCl4− ion.
3. Co-ordinate bonding can be described as dative covalency.
a) In this context, what is the meaning of each of the terms covalency
and dative?
b) Write an equation for a reaction in which a co-ordinate bond is
formed.
11.4. Polarity of the covalent bond
Activity 11.4
The following figures show two types of covalent bond, namely, polar and
non-polar.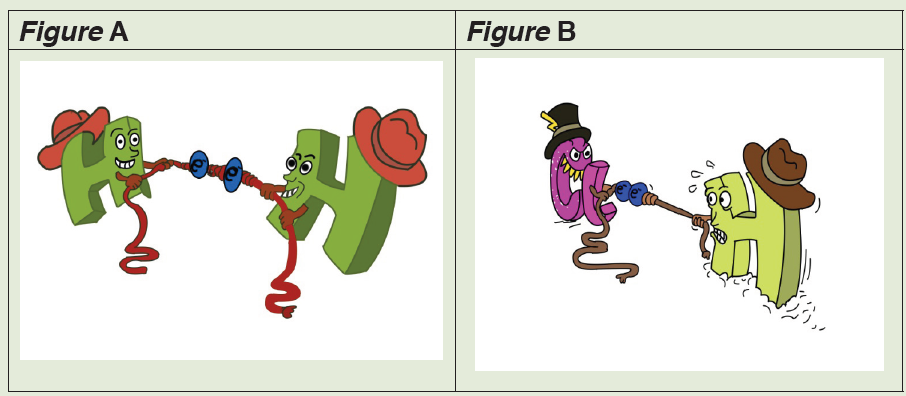
1. Covalent bond is formed between two atoms with similar or close
ability to attract electrons towards themselves, and this is the
reason why they share electrons without being transferred.
a) What is the name of the property used to compare that ability?
b) When the strengths of both atoms are equal, the covalent bond will
be non-polar. Is figure A polar of non-polar?
c) Look at the figure B. The atom, in the zone with more electrons,
will have a partial negative charge. In which zone will be more
electrons?
2. Fill a burette with water. Open the tap and bring a charged ebonite
rod close to the stream of water running from the jet. The water is
deflected from its vertical path towards the charged rod as shownin the figure. Why is this?
A quantity termed ‘electronegativity’ is used to determine the polarity of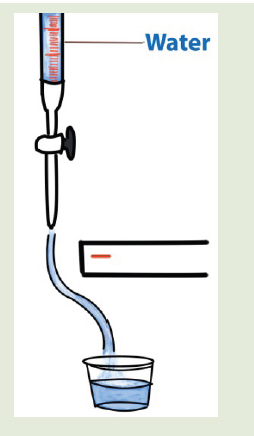
the covalent bond; whether a given bond will be non-polar covalent, polar
covalent, or ionic.
Electronegativity is defined as the ability of an atom in a particular
molecule to attract electrons to itself (the greater the value, the greater
the attractiveness for electrons).
Fluorine is the most electronegative element (electronegativity = 4.0),
the least electronegative is Caesium (electronegativity = 0.7).
The bond pair is equally shared in between two atoms when the
electronegativity difference between them is zero or nearer to zero. In
this case, neither of the atoms gets excess of electron density and hence
carry no charge. This is called non-polar covalent bond.
However, when there is a considerable difference in the electronegativity,
the bond pair is no longer shared equally between the atoms. It is shifted
slightly towards the atom with higher electronegativity by creating partial
negative charge (represented by δ-) over it, whereas, the atom with less
electronegativity gets partial positive charge (represented by δ+). This type
of bond is also referred to as polar covalent bond.
We can use the difference in electronegativity between two atoms to
gauge the polarity of the bonding between them.
• In F2 the electrons are shared equally between the atoms, the bond is
non-polar covalent.
• In HF the fluorine atom has greater electronegativity than the hydrogen
atom. The sharing of electrons in HF is unequal: the fluorine atom
attracts electron density away from the hydrogen (the bond is thus apolar covalent bond). The H-F bond can thus be represented as:
The ‘δ+’ and ‘δ-’ symbols indicate partial positive and negative charge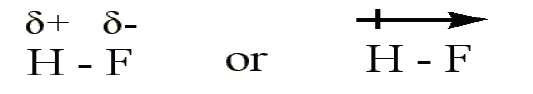
respectively.
The arrow indicates the “pull” of electrons off the hydrogen and towards the
more electronegative atom, fluorine.
• In LiF the much greater relative electronegativity of the fluorine atom
completely strips the electron from the lithium and the result is an ionic
bond (no sharing of the electron).
Note:The following is the general thumb rule for predicting the type of
bond based upon electronegativity differences:
– If the electronegativities are equal (i.e. if the electronegativity difference is 0), the bond is non-polar covalent.
– If the difference in electronegativities between the two atoms is greater
than 0, but less than 2.0, the bond is polar covalent.
– If the difference in electronegativities between the two atoms is 2.0, or
greater, the bond is ionic.Using the examples used above, we can predict the type of bond as follows:
Note: A non-polar molecule is one in which the electrons are distributed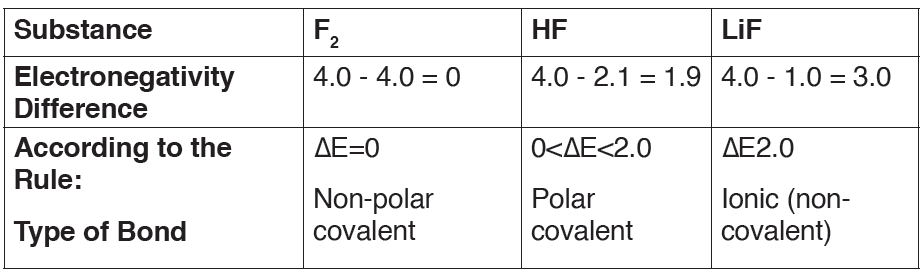
more symmetrically and thus does not have an abundance of charges
at the opposite sides. The charges all cancel out each other. Examples
of non-polar molecules include diatomic molecules, CH4, CO2, C2H4,
cyclohexene, CCl4, etc.
11.5. Physical properties of covalent compounds: simple
molecular structure
Activity 11.5
1. Carbon dioxide (CO2), Bromine (Br2) and SiO2 molecules are all
covalent substances.
a) Give the physical states of these substances at room temperature.
b) Arrange the molecules above in decreasing order of melting and
boiling points knowing their physical states at room temperature.
c) Suggest any reason for the differences in the melting points.
2. Do research and be able to explain physical properties of simple
molecular compounds
Substances composed of relatively small covalently bonded structures
are called Simple Molecular Structures. These contain only a few atoms
held together by strong covalent bonds and can be further categorised into
two types: Individual (which are usually gases like carbon dioxide) and
molecular (which are usually solids like iodine).
The Physical Properties
4. Low melting and boiling points
Simple Molecular Structures tend to have low melting and boiling
points since the forces between molecules are quite weak. Little
energy is required to separate the molecules.
5. Poor electrical conductivity
There are no charged particles (ions or electrons) delocalized
throughout the molecular crystal lattice to conduct electricity. They
cannot conduct electricity in either the solid or molten state.
6. Solubility
Polar compounds are soluble in water (polar) while non-polar
compounds are soluble in nonpolar solvents (oil, hexane…). This
means that substances with the same type of polarity will be soluble
in one another. Moreover, compounds with differing polarities will be
insoluble in one another`
Example:
Hydrogen chloride HCl, Ethanol CH2CH2OH are soluble in water because
there are all polar but they are not soluble in organic solvents which arenonpolar like hexane and heptane.
Application activity 11.5
1. Explain why:
a) Simple molecules have low melting points;
b) Simple molecules have poor conductivity of electricity;
2. Which compounds are soluble or insoluble in water?
SKILLS LAB 11
1. Using adequate materials construct any three models of molecules
of your choice. These models show shells and all electrons.
Electrons which form covalent bonds are highlighted. Molecules to
be made: HCl, CO2, H2, CH4, C2H2, NH3, BF3.
2. Plants contain many chemicals. To extract them from plants for
further studies many solvents such as water, acetone, and hexane
are used. Based on the physical properties especially solubilty do
research and find solvents that should be used to extract some
substances which are found in different plants of your environment.Solvents to be used are: water, hexane and cetone.
End unit assessment 111. Complete the table below by yes or no
2. Choose the best answer. The correct dot formulation for nitrogen
trichloride has:
a) 3 N-Cl bonds and 10 lone pairs of electrons.
b) 3 N=Cl bonds and 6 lone pairs of electrons.
c) 1 N-Cl bond, 2 N=Cl bonds and 7 lone pairs of electrons.
d) 2 N-Cl bonds, 1 N=Cl bond and 8 lone pairs of electrons.
e) 3 N-Cl bonds and 9 lone pairs of electrons.
3. Explain why the boiling point of water is much bigger than that of
methane while their masses are not very different.
4.Choose the correct answer. A (pi) bond is the result of the
a) Overlap of two s orbitals.
b) Overlap of an s and a p orbital.
c) Overlap of two p orbitals along their axes.d) Sidewise overlap of two s orbitals.
5. Show different poles δ- and δ+ in the following molecules between
O, N, Cl and other atoms bonded to them.
a) H3C-Cl b) H3C-O-H c) C2H5-NH2
6. Write the structural formula of propane and propene and compare
their reactivity on the type of bonds in their respective molecules .
7. The equation below shows the reaction between boron trifluoride
and a fluoride ion. BF3 + F− → BF4−
In terms of the electrons involved, explain how the bond between
the BF3 molecule and the F− ion is formed. Name the type of bond
formed in this reaction.
8. The table below shows the electronegativity values of someelements.
a) Define the term electronegativity.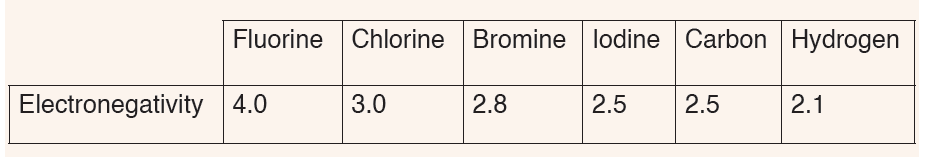
b) Write the formula of hydrogen chloride, hydrogen iodide, hydrogen
fluoride and hydrogen bromide and range them from the most polar
to the least one
9.Draw Lewis structures showing electrons in the outermost shell of
each atom in the following compounds: a) H2O2, b) HCN, c) C2H2,
d)SF6, e) Al2S3
Atomic numbers: H(z=1), O (z= 8), C (z=6), N(z= 7), S (z=16 ), F (z=
9 ), Al (z= 13)
10. How many sigma and pi electrons are contained in the following
molecule?H2C = CH- CH3
