UNIT 10: THE CHEMICAL BASIS OF LIFE
Key unit competence
Explain the use of biological molecules in living organism.Introductory Activity 10
Analyze all foods in the figure and answer to the questions below:
a) Among the foods given in figure, which one do you prefer to eat
every day? Why?
b) Do you think that there are some missing foods to complete all
chemicals of life? Suggest them.
10.1. Biological molecules
Activity 10.1
Discuss chemical elements, sub-units of different types of carbohydrates,
lipids and proteins.
What do you know about the function of water to living organisms?
10.1.1 The chemical elements that make up carbohydrates,
lipids and proteins
a) Carbohydrates
Carbohydrates comprise a large group of organic compounds which contain
carbon, hydrogen and oxygen. The word carbohydrate indicates that these
organic compounds are hydrates of carbon. Their general formula is
Cx(H2O)y.
In carbohydrates the ration hydrogen-oxygen is usually 2:1.
Carbohydrates are divided into three groups including the monosaccharide
(single sugars), disaccharides (double sugars) and polysaccharides (many
sugars). The most common monosaccharide of carbohydrates is glucose
with molecular formula C6H12O6.
b) Lipids
Lipids are a broad group of naturally occurring molecules which include
waxes, sterols, fat soluble vitamins (such as vitamins A, D, E and K),
monoglycerides, diglycerides, triglycerides, Phospholipids and others.
Lipids are made by carbon, hydrogen and oxygen, but the amount of oxygen
in lipids is much smaller than in carbohydrates.
Lipids are grouped into fats which are solid at room temperature and oils
which are liquid at room temperature.
c) Proteins
Proteins are organic compounds of large molecular mass. For example,
the hemoglobin has a molecular mass of 64500. In addition to carbon,
hydrogen and oxygen, proteins always contain nitrogen, usually Sulphur and
sometimes phosphorus.
Water
Living organisms contain between 60% and 90% of water, the remaining
being the dry mass. Water is made up of only two elements, Hydrogen and
Oxygen.
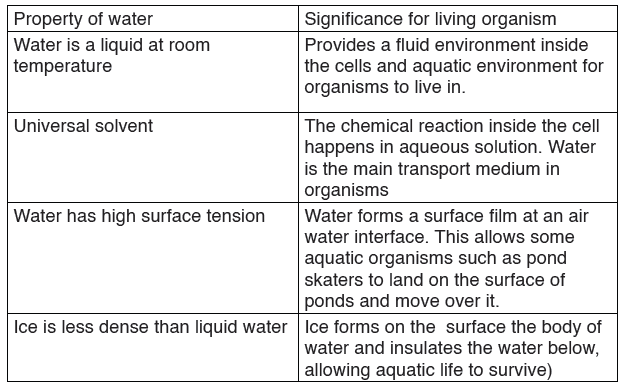
The function of water is defined by its physical and chemical properties thatdiffer from those of most liquids and make it effective in supporting life.
10.1.2. The sub-units that make up biological molecules
a) Sub-units of Carbohydrates
In carbohydrates the following three categories are identified:
Monosaccharides , disaccharides and polysaccharides.
i. Monosaccharides
Monosaccharides are the smallest subunits and are made up of single sugar
molecules. monosaccharides are the sugars like galactose, fructose and
glucose with a general formula C6H12O6, and these typically take on a ringshaped
structure.
All monosaccharides are reducing sugars capable of acting as a reducing
agents because they have a free aldehyde group or a free ketone group.
Sources of Monosaccharides:
Glucose: Fruits and vegetables are natural sources of glucose. It’s also
commonly found in syrups, candy, honey, sports drinks, and desserts.
Fructose: The primary natural dietary source of fructose is fruit, which is
why fructose is commonly referred to as fruit sugar.
Galactose: The main dietary source of galactose is lactose, the sugar in
milk and milk products, such as cheese, butter, and yogurt. https://www.
healthline.com/nutrition/simple-sugars
ii. Oligosaccharides
These are complex carbohydrate chains made up of two to twenty
simple sugars joined together with a covalent bond. The most common
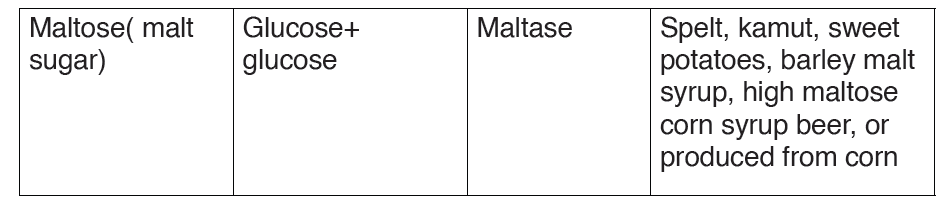
oligosaccharide is the disaccharide, and examples of this include sucrose,
maltose and lactose whose general formula is C12H22O11
A disaccharide is the sugar formed when two monosaccharides are joined
by glycosidic bond. Like monosaccharides, disaccharides are soluble in
water, have sweet taste, and they are reducing sugars because they are
able to reduce Copper II Sulfate of benedict solution directly by heating
into copper II oxide except sucrose which is non-reducing sugar which are
unable to reduce the copper ions in Benedict’s solution. This makes the
color of Benedict’s solution to persist when this sugar is boiled with it.
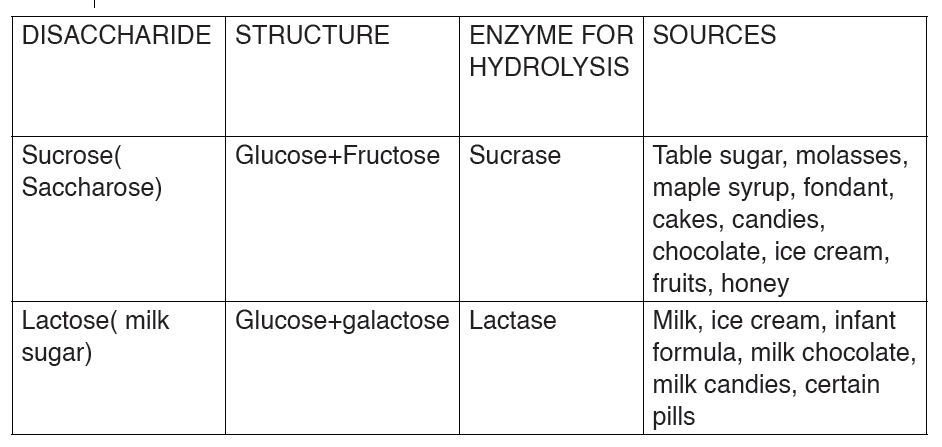
Sucrose is made up of two monosaccharides Glucose and fructose
Maltose is made up of two monosaccharides Glucose and glucose
Lactose is made up of two monosaccharides Glucose and galactose
In maltose ring, the two rings of glucose are bonded by the -1, 4-glycosidic
bond whilein sucrose the glucose and fructose are bonded by -1, 2-glycosidic bond.
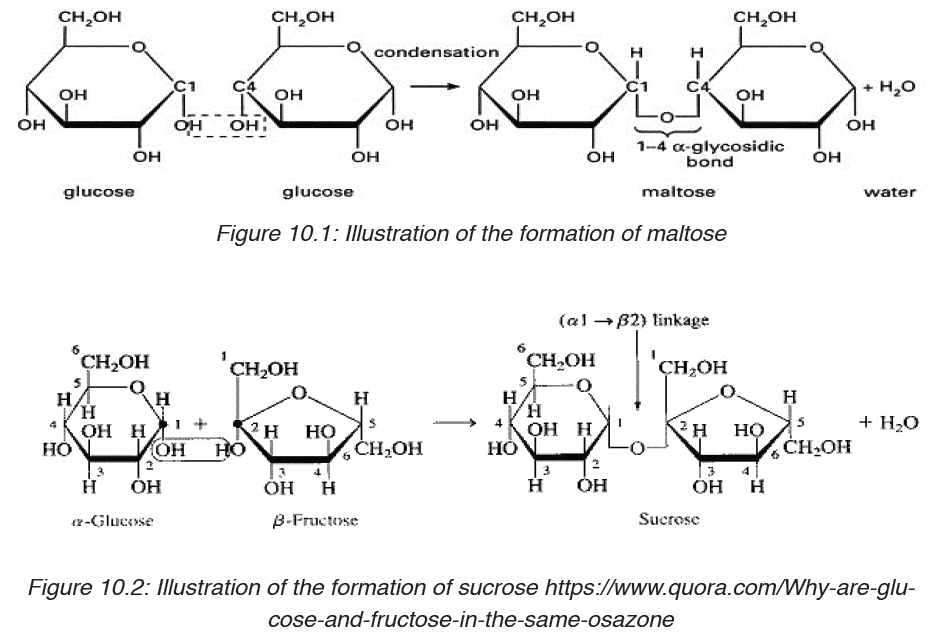
Table 10.1: different groups of Disaccharides, their structure, enzyme and source
iii. Polysaccharides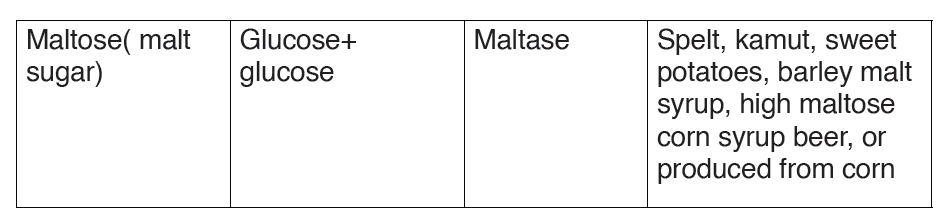
These are known for their ability to store energy and are made up of long
chains of glucose sugars. The most common polysaccharides are starch(
sugar of plant tissues), glycogen ( glucose in the human liver and muscles),
cellulose ( structural polysaccharide in plants; which acts as a dietary fiber
when consumed), chitin( sugar found in exoskeleton of arthropods) and
murein or peptidoglycan( sugar found in the bacteria cell membrane) . http://
www.nutrientsreview.com/carbs/polysaccharides.html
Table 10.2.: different groups of polysaccharides, their composition andsource
b) Sub-units of Proteins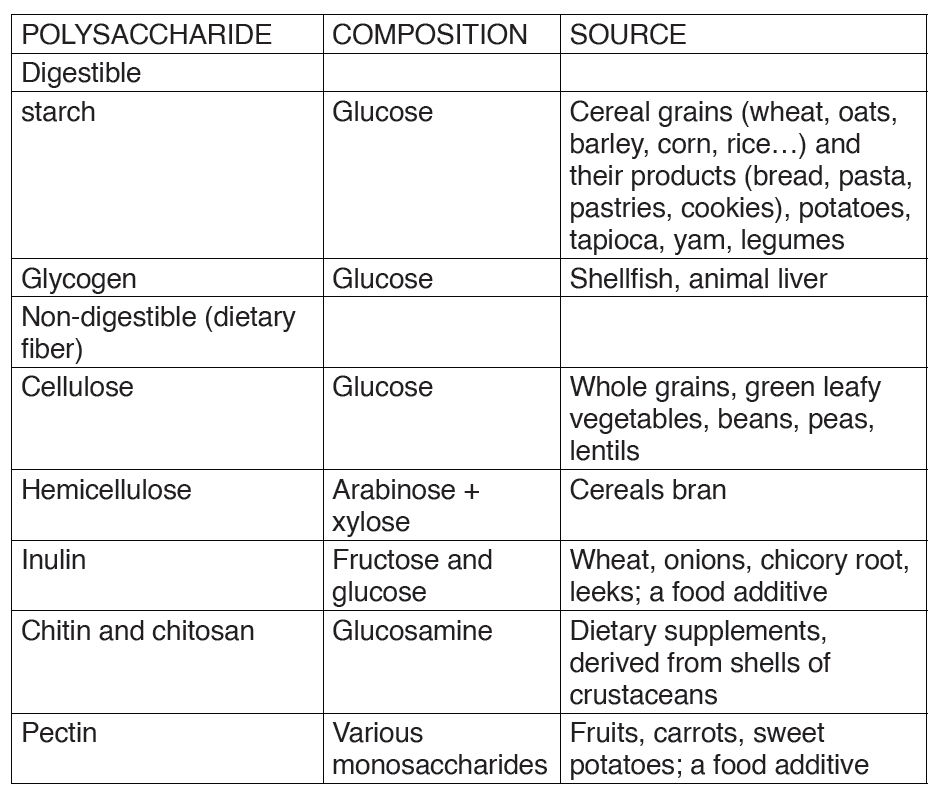
These are also referred to as macro-nutrients. The proteins are also called
body- building food.
The protein molecules are made up of small units called amino acids joinedtogether like links in a chain.
There are 21 different amino acids and each has its own chemical name.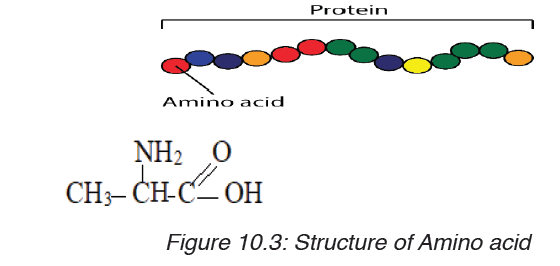
Different proteins are made when different numbers and types of amino
acids combine through a covalent peptide bond. Proteins are therefore
known as polypeptides.
Examples of proteins:
a) Collagen, myosin and elastin found in meat,
b) Caseinogen, lactalbumin, lacto globulin found in milk,
c) valbumin, mucin and liporitellin found in eggs,
d) Zein found in maize
The 21 different amino acids found in protein are
Arginine, Serine,Selenocysteine, Leusine, Histidine,Threonine, Glycine,
Methionine, Lysine, Asparagine, Proline, Phenylalanine, Aspartic acid,
Glutamine, Alanine, Tyrosine, Glutamic acid, Cysteine, Valine, Tryptophan,
Isoleucine.
They are used to repair, to build, to maintain our bodies; to make muscles
and to make breast milk during lactation period. The proteins are classified
into two categories: animal or complete proteins and plant proteins or
incomplete proteins
c) Sub-units of lipids
Lipids are made by two components namely glycerol and fatty acids. The
chemical formula for glycerol is C3H8O3.Structural formula of glycerol is
Sources and classification of lipids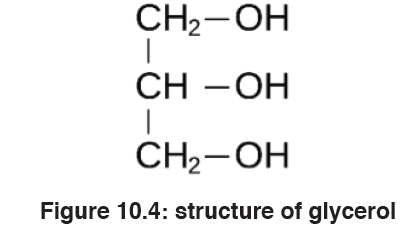
Fats and oils are obtained from both the plants and animals. And fat is
present in food either as visible fat or invisible fat.
Visible fat is the one that is easily seen or detected in food for example; fatin meat, butter, margarine, lard, suet and cooking fat and oil.
Invisible fat is the part of food that is not easily seen for example fat with
in lean meat, egg yolk, flesh of oily fish, groundnuts, soya beans, avocado
and fat found in prepared foods, for example, pastry, cakes, biscuits, Frenchfries, pancakes, croquettes.
Lipids are of different types as it is summarized in the following table
Table 10.3: Lipids, structure, main role and features
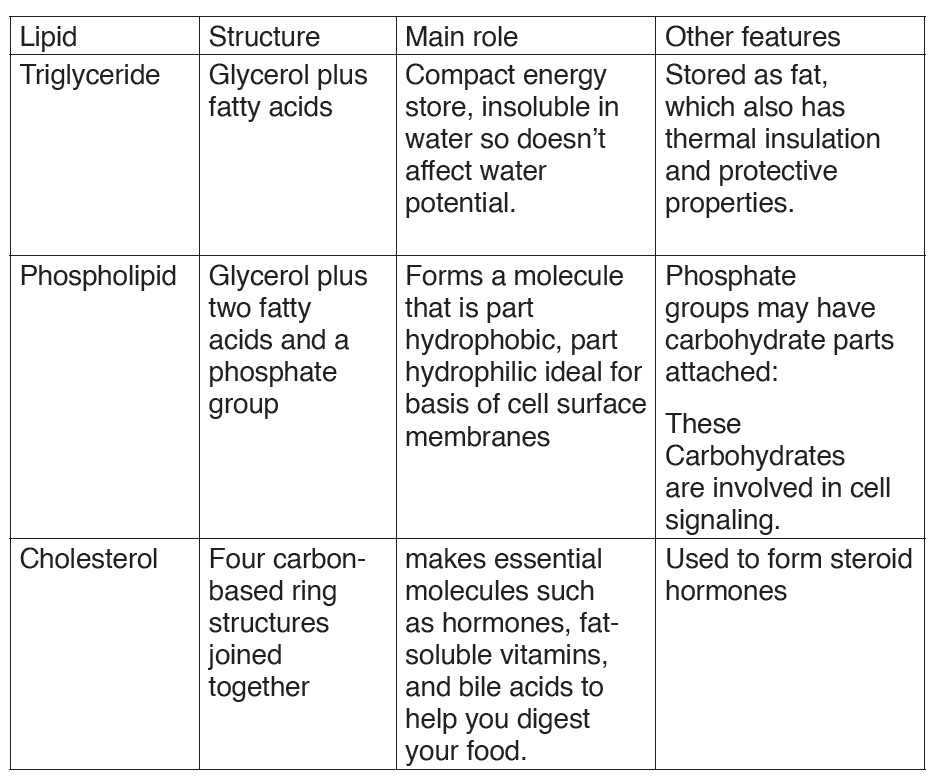
Structure of fatty acid
The following are three main types of lipids: Triglycerides, phospholipids and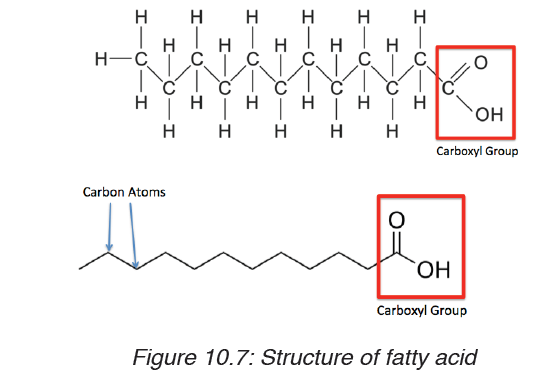
steroids
Triglycerides: these are lipids that are obtained from cooking oils, butter
and animal fat. They are made up with: one molecule of glycerol and three
molecules of fatty acids bonded together by Ester bonds. The triglycerides
play the role like storing energy they have thermal insulation and protectiveproperties
Sterols: these are lipids that include steroid hormones like testosterone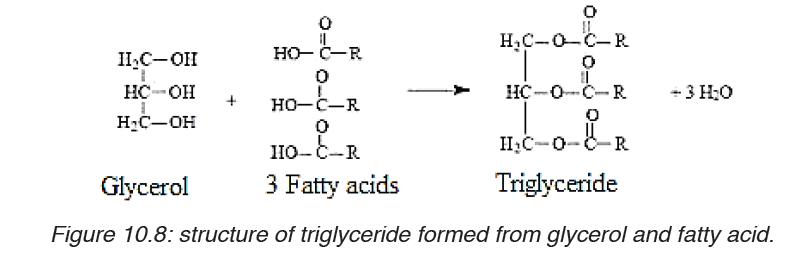
and oestrogen, cholesterol that is formed four carbon-based rings and it
helps in regulation of fluid and strength of the cell membrane.
Phospholipids: They are made up of one molecule of glycerol, two
molecules of fatty acids and one phosphate group. The phospholipids form
a molecule that is part hydrophobic, part hydrophilic, ideal for basis of cellsurface membranes
10.1.3. The proteins and their functions
Activity 1.1
1. From the books make a research on proteins and answer to the
following questions:
Differentiate globular proteins and fibrous proteins.
2. Take a plastic cord, create the bulk on it and suppose that those
are monomers of a long chain of polymer (the whole cord), heat
it using a Bunsen burner or another source of fire. Discuss the
change that takes place.
Proteins are organic compounds of large molecular mass ranging up to
40000000 for some viral proteins but more typically several thousand. For
example, the hemoglobin has a molecular mass of 64500. Proteins are
polymers of amino acids and they are not truly soluble in water, but form
colloidal suspensions. In addition to carbon, hydrogen and oxygen, proteins
always contain nitrogen, usually Sulphur and sometimes phosphorus.
Whereas there are relatively few carbohydrates and fats, the number of
proteins is limitless. Coined by a Dutch chemist Mulder the word protein
etymologically means “of the first importance” due to the fundamental role
they play in living cells.
a) Amino acids
Amino acids are group of over a hundred chemicals of which around 20
commonly occur in proteins. They always contain a basic group, the amine
group (-NH2) and an acid group (-COOH) together with -R group side chain
(Figure 10.14). All the amino acid differs one to another by the structure oftheir side chain.
Amino acids are divided into two categories including essential amino acid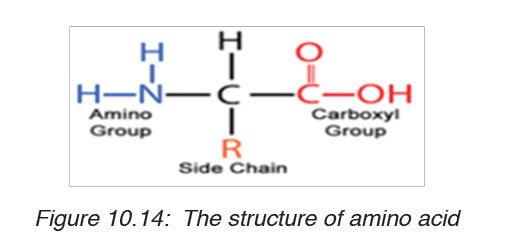
and non-essential amino acid. Essential amino acids are those amino acids
which cannot be synthesized by the body. They include isoleucine, leucine,
lysine, methionine, phenylalanine, threonine, tryptophan, valine, arginine
and histidine. Non –essential amino acids are synthesized by the organism.
They include alanine, asparagine, aspartic acid, cysteine, glutamine,
glutamic acid, glycine, proline, serine and tyrosine. The simplest amino acid
is glycine with H as -R group (Figure 10.15. a). The other one is Alanine with
–CH3 as -R group (Figure 10.15.b). All 21 amino acids can be found in diet
from animals such as meat, eggs, milk, fish…but diet from plant lack one ortwo essential amino acids such plant are beans, soy beans…
When an amino acid is exposed to basic solution, it is deprotonated (release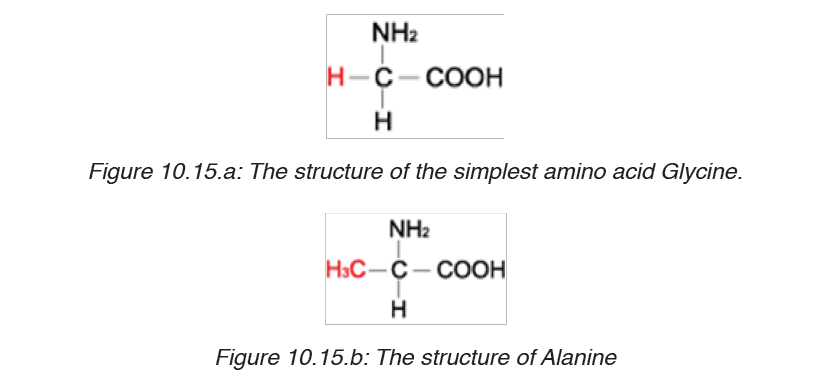
of a proton H+) to became negative carboxylate COO-while in acid solution
it is protonated (gains of a proton H+) to became ammonium positive ion
-NH3+(Figure 10.16.a and Figure 10.16.b).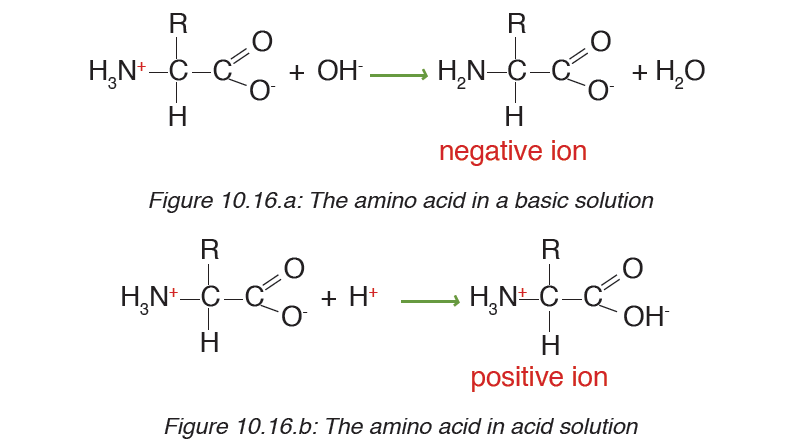
At a physiological pH, usually around 7, the amino acid exists as ZWITTERION
(from German means hermaphrodite) it is a molecule with two differentcharges (positive and negative) at the same time (Figure 10.17).
Formation and breakage of peptide bond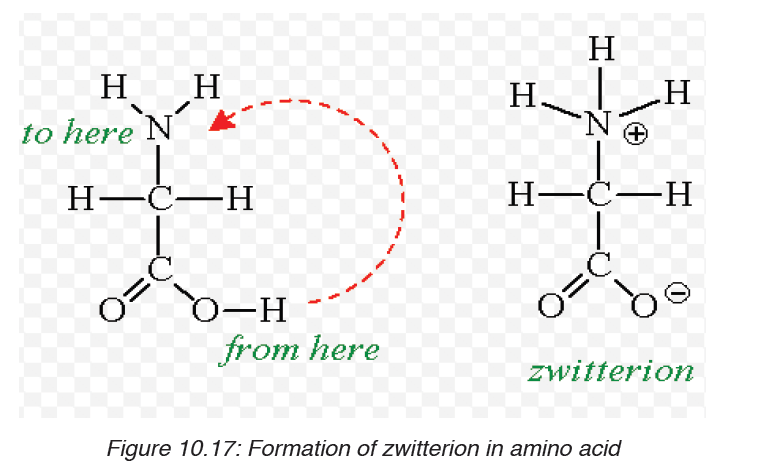
The formation of peptide bond follows the same pattern as the formation
of glycosidic bond in carbohydrates and ester bond in fats. A condensation
reaction occurs between the amino group of one amino acid and the carboxylgroup of another, to form a dipeptide (fig 10.18).
A peptide bond is formed between two amino acids to form a dipeptide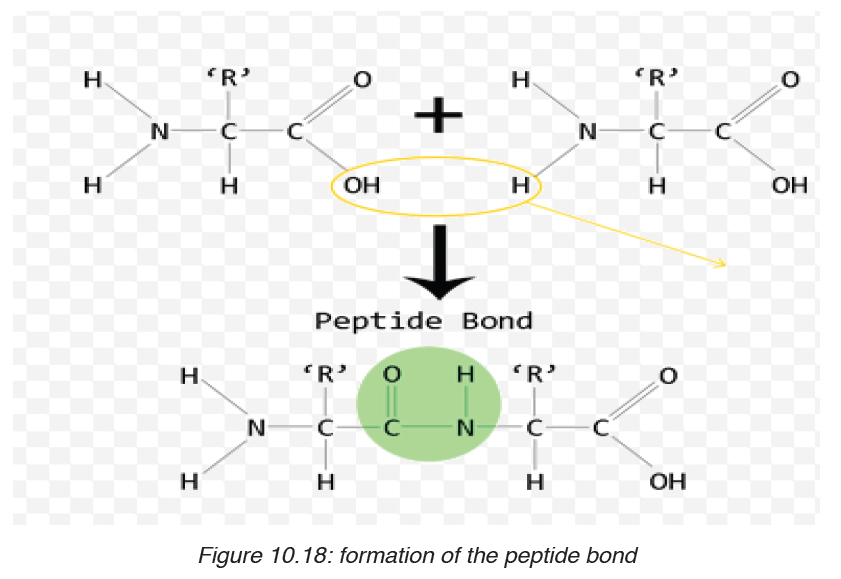
molecule, if three amino acids are assembled together it is a tripeptide, four
amino acids form a tetrapeptide and so on.
A long chain of amino acid it is called a polypeptide. The polypeptide chain
or oligopeptide comprise more than 50 amino acids joined together by a
peptide bond.
During digestion, proteins are hydrolyzed and give their monomer amino
acids small molecules that can be diffused in the wall of intestine to the
organism. In hydrolysis the peptide bond break down by the addition of awater molecule (Figure 10.19).
Functions of proteins.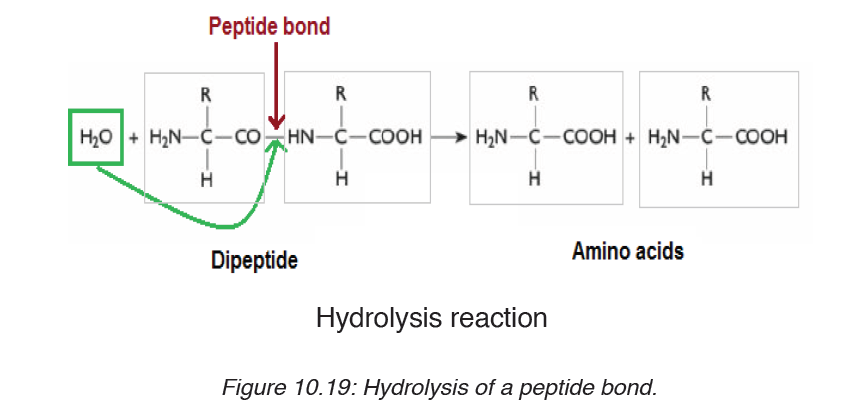
– Proteins such as lipase, pepsin and protease act as enzymes as they
play a crucial role in biochemical reaction where they act as catalysts.
– Proteins play an important role in coordination and sensitivity (hormones
and pigments).
– Proteins have a transport functions. Example: Haemoglobin transport
oxygen
– Proteins in the cell membrane facilitate the transport of substance
across the cell membrane.
– Proteins provide a mechanical support and strength.
– Proteins such as myosin and actin are involved in movement.
– Proteins play the role of defense of the organisms. Example: Antibodies
are proteins
Application activity 10.1
1. Provided with different kinds of biological molecules such as
carbohydrates, proteins, lipids, make a table to show their food
source you always take and suggest their functions.
2. Explain what is meant the essential amino acids
3. Describe the formation of a peptide bond?
4. Alanine is an amino acid with -CH3 as a side chain. Writes its
structural formulae.
5. Most of the plant lacks one or more of the essential amino acids
needed by the body explain how a vegetarian can obtain the
essential amino acids.
6. Use the type of reaction above to form glucose from sucrose
molecule
7. Describe how the glycosidic bond is formed.8. Describe the major types of starch
10.2. Water and enzymes
Activity 10.2
1. You are provided with three groups of enzymes: Group A Group B Group
C Enzymes Maltase and lactase Dehydrogenase and oxidase Pepsinand renin
Make a research to find out: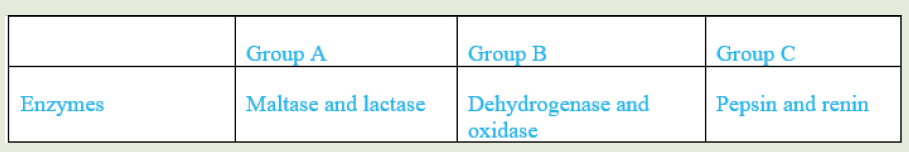
a) specific role of each of the six enzymes mentioned above
b) criterion followed to name enzymes of group A, B and C respectively
2. a. What is the medium of reaction in the organisms?
b. If two people are boiling the same quantity of cooking oil and water,
which one could evaporate first? Explain your choice.
10.2.1. Water
Living organisms contain between 60% and 90% of water, the remaining
being the dry mass. The scientist accepts that life originates from water
and most of animals live in water. The function of water is defined by its
properties mainly: Its physical properties, solvent properties, heat capacity,
surface tension and freezing points. The physical and chemical properties of
water differ from those of most other liquids but make it uniquely effective in
supporting living activities.
a) Physical properties of water
Water has the high boiling point (100°C) compared to other liquid due to the
hydrogen bond that exists among molecules of water. This help the water toexist on the surface in a liquid state otherwise it would evaporate.
Table 10.4: Biological significance of the physical properties of water
a) Solvent properties of water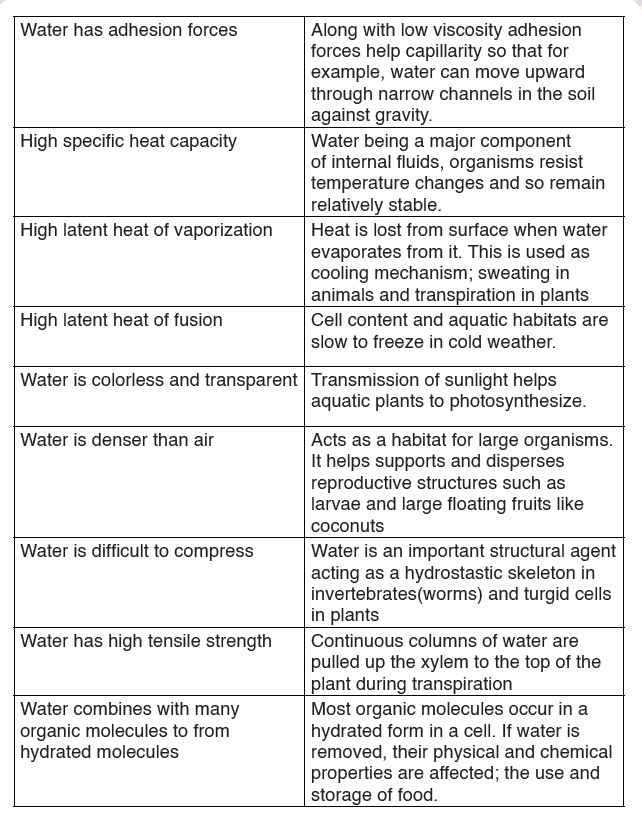
Water is a polar molecule due to its chemical arrangement of hydrogen
and oxygen atom in asymmetric shape instead of being linear. Most of the
substance that are transported in the blood is dissolved in the plasma, the
fluid part of the blood. Water occupies around 92% of the constituents of
plasma. Thus the oxygen atom has a positive charge and hydrogen atom
net positive charges. This is of great importance because all negative and
positive ions are attracted by water. Therefore, water is a good solventbecause the ionic solids and polar molecules are dissolved in it.
b) Heat capacity and latent heat of vaporization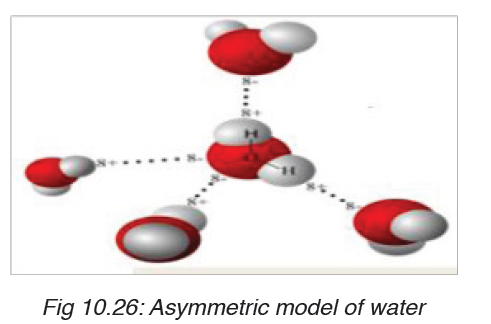
Large changes of heat results in a comparatively small rise in water
temperature this explain why water has a high heat capacity compared to
other liquid. The high heat capacity is defined as the amount of heat required
to raise the temperature of 1gram to 10C.The high thermal capacity of water
make the ideal environment for life in plant and animals because it helps in
maintaining the temperature even if there will be environmental fluctuations
in temperature. The biological importance of this is that the range of
temperatures in which biochemical processes can proceed is narrow.
The latent heat of vaporization is a measure of heat energy needed to cause
the evaporation of a liquid, which means to change from water liquid to
water vapor. During vaporization the energy transferred to water molecules
correspond to the loss of energy in the surroundings which therefore cool
down. During sweating and transpiration living organisms use vaporization
to cool down.
i. Surface tension
The surface tension of water results from its polar nature, and is defined as
the ability of the external surface of the liquid to resist to external force due
to cohesive nature of its molecules. The high surface tension of water and
the cohesion force play a vital role in capillarity thus help the transport of
substance in vessels (tracheid of plant) to the stems and to fulfill the blood in
the cardiovascular vessels. Water being the second liquid with high surface
tension after mercury its surface tension is lowered by the dissolution of ions
and molecules and tend to collect at the interface between its liquid phase
and other.
ii. Freezing points
Oppositely to other liquid water expand as it freezes, under 40 C temperatures
the hydrogen bond becomes more rigid but more open. This explains why
the solid water (ice) is less dense than the liquid water and why the ice floats
over water rather than sinking. When the bodies of water freeze the ice float
over the liquid act as an insulator and prevent water below it from freezing.This protects the aquatic organisms so that they can survive the winter.
10.2.2. Enzymes, their characteristics and actions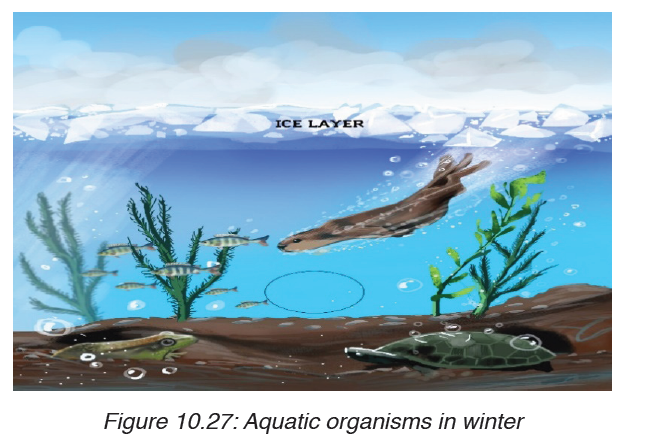
a) Criteria for naming enzymes
Activity 10.2.2.aYou are provided with three groups of enzymes:
Make a research from text book or internet to find out: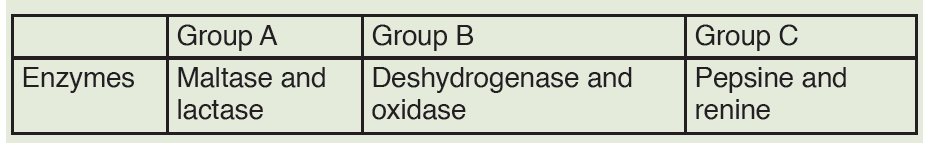
What is the specific role of each of the six enzymes mentioned above?
What criterion was followed to name enzymes of group A, B and C
respectively?
Enzymes are biological catalyst produced by a living organism to control
the speed of specific biochemical reactions (metabolism) by reducing its
activation energy.
First of all, Individual enzymes are named by adding -ase to the name
of the substrate with which they react. The enzyme that controls urea
decomposition is called urease; those that control protein hydrolyses are
known as proteases.
A second way of naming enzymes refers to the enzyme commission number
(EC number) which is a numerical classification scheme for enzymes
based on the chemical reactions they catalyse. As a system of enzyme
nomenclature, every EC number is associated with a recommended name
for the respective enzyme catalysing a specific reaction. They include:
Oxidoreductases catalyse redox reactions by the transfer of hydrogen,
oxygen or electrons from one molecule to another. Example: Oxidasecatalyses the addition of oxygen to hydrogen to form water.
– Transferases catalyze group transfer reactions. The transfer occurs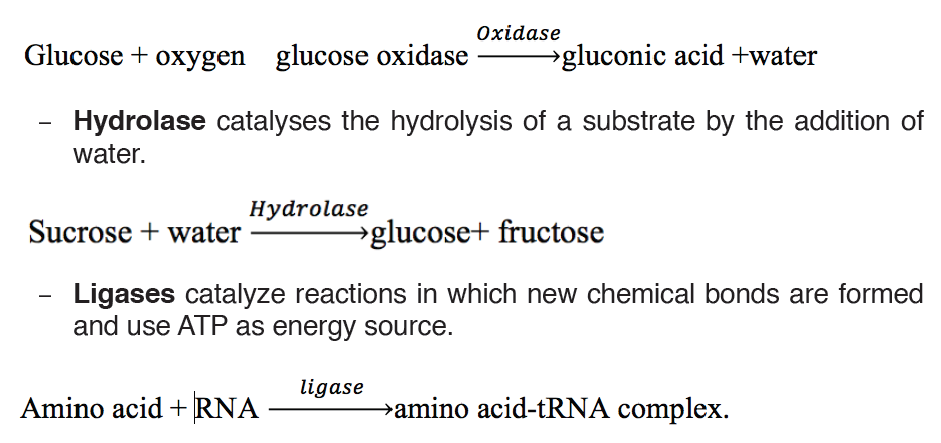
from one molecule that will be the donor to another molecule that will
be the acceptor. Most of the time, the donor is a cofactor that is charged
with the group about to be transferred. Example: Hexokinase used in
glycolysis.
– Lyases catalyze reactions where functional groups are added to break
double bonds in molecules or the reverse where double bonds are
formed by the removal of functional groups. For example: Fructose
bisphosphate aldolase used in converting fructose 1,6-bisphospate to
G3P and DHAP by cutting C-C bond.
– Isomerases catalyze reactions that transfer functional groups within a
molecule so that isomeric forms are produced. These enzymes allow
for structural or geometric changes within a compound. Sometime the
interconverstion is carried out by an intramolecular oxidoreduction.
In this case, one molecule is both the hydrogen acceptor and donor,
so there’s no oxidized product. The lack of a oxidized product is the
reason this enzyme falls under this classification. The subclasses are
created under this category by the type of isomerism. For example:
phosphoglucose isomerase for converting glucose 6-phosphate to
fructose 6-phosphate. Moving chemical group inside same substrate.
A third way of naming enzymes is by their specific names e.g. trypsin
and pepsin are proteases. Pepsin, trypsin, and some other enzymes
possess, in addition, the peculiar property known as autocatalysis,
which permits them to cause their own formation from an inert precursor
called zymogen.
b) Characteristics of enzymes.
Activity 10.2.2.b
Requirement:
Three test tubes, match box, about 1g of liver, 1g of sands, 1% H2O2 and
MnO2 powder.
Procedure:
– Label three test tubes A, B and C respectively.
– Put about 0.1 g of MnO2 powder in test tube A and 1g of liver in tube
B and 0.1g of sand in tube C.
– Pour 5 ml of H2O2 (hydrogen peroxide) in each tube. What do you
observe?
– Place a glowing splint in the mouth parts of each test tube. What do
you observe?
Questions
1. Explain your observations.
2. Write down the chemical equation of the reaction taking place in
tube A and B
3. Carry out your further research to find out the characteristics ofenzymes
The following are main characteristics of enzymes:
– Enzymes are protein in nature: all enzymes are made up of proteins.
– Enzymes are affected by temperature. They work best at specific
temperatures; for example, enzymes found in human bodies work best
at 37oC. This is called the optimum temperature.
– Very low temperatures inactivate enzymes. Therefore enzymes are
not able to catalyse reactions.
– High temperatures beyond the optimum temperature denature
enzymes. The structure of the protein molecule is destroyed by heat.
– Enzymes work best at specific pH. Different enzymes have a given
specific pH at which they act best.
This pH is called optimum pH. Some enzymes work best at low pH
(acidic medium) while others work best at high pH (alkaline medium).
Most enzymes in the human body for instance work best at neutral or
slightly alkaline pH. Examples are: lipases, peptidases and amylase.
A few enzymes like pepsin that digests proteins in the stomach works
best at an acidic pH of 2.
– Enzymes remain unchanged after catalysing a reaction. Enzymes
are catalysts and can therefore be used over and over again in small
amounts without being changed.
– Enzymes catalyse reversible reactions. This means that they can
change a substrate into products and the products back to the originalsubstrate.
– Enzymes are substrate-specific. This means that an enzyme can only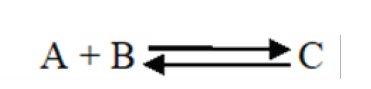
catalyse one reaction involving aparticular substrate. This is because
they have active sites which can only fit to a particular substrate whose
shape complements the active site. For example, pepsin works on
proteins but not on fats or starch.
– Enzymes work rapidly. Enzymes work very fast in converting
substrates into products. The fastest known enzyme is catalase, which
is found in both animal and plant tissues.
– Enzymes are efficient. This is best described by the fact that:
• They are required in very small amounts.
• They are not used up in a reaction and can therefore be used repeatedly.
– Enzymes are globular proteins.
– Enzymes lower the activation energy (Ea) required for reactions
to take place.
In many chemical reactions, the substrate will not be converted to a
product unless it is temporarily given some extra energy. This energy
is called activation energy i.e. the minimum energy required the makea reaction take place.
An enzyme provides a reaction surface for a reaction to take place. This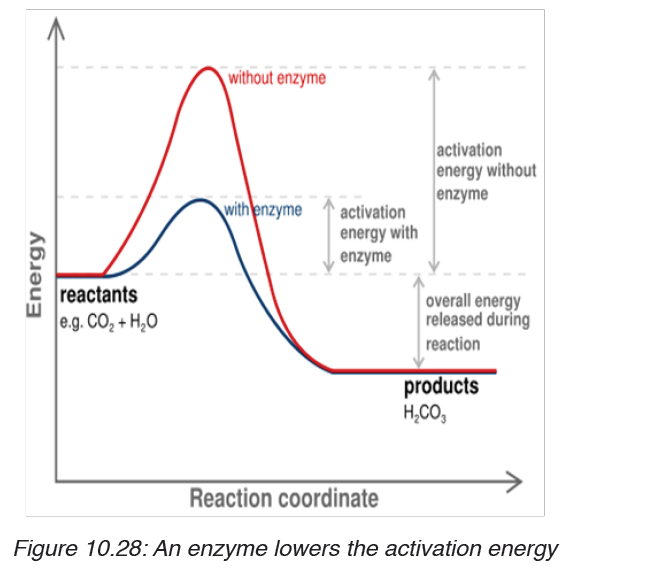
is normally a hollow or cleft in the enzyme which is called the active site,
but it is normally hydrophobic in nature rather than hydrophilic. An enzyme
provides a reaction surface and a hydrophilic environment for the reaction
to take place.
A very small amount of enzymes is needed to react with a large amount of
substrate. The turnover number of an enzyme is the number or reactions
an enzyme molecule can catalyse in one second. Enzymes have a high
turnover number e.g. the turnover number of catalase is 200,000 i.e. one
molecule of enzyme catalase can catalyse the breakdown of about 200,000
molecules of hydrogen peroxide per second into water and oxygen at body
temperature.t
A cofactor is the best general term to describe the non-protein substances
required by an enzyme to function properly. This term covers both organic
molecules and metal ions. A co-enzyme is an organic molecule that acts as
a cofactor. A prosthetic group is a cofactor that is covalently bound to the
enzyme.
Factors affecting enzyme action
Activity 10.2.2.c
You will need
Eight test tubes containing 2 cm3 starch solution, amylase solution, and
cold water (ice) water bath, iodine solution, HCl solution, and droppers
Procedure:1. Label your test tubes A-D as follows:
2. Add 1 cm3 of starch solution to each test tube
3. Keep tube A and B in cold (ice) and tube C and D in the water bath
at 35°C for 5 minutes.
4. Add 1 cm3 of 1M HCl on test tubes B and D, then shake the mixture
to stir.
5. Add 1 cm3 of amylase solution on each test tube. Shake and therefore
keep A and B in cold and C and D in water bath for 10 minutes.
6. Take a sample from each tube and mix it with one drop of iodine.
Use a different tile for each test tube. Record and interpret your
observation and then draw a conclusion.
Enzymes activities can be limited by a number of factors such as the
temperature, the pH, the concentration of the substrate or the enzyme itself
and the presence of inhibitors.
i. Temperature
At zero temperature, the enzyme cannot work because it is inactivated. At
low temperatures, an enzyme-controlled reaction occurs very slowly. The
molecules in solution move slowly and take a longer time to bind to active
sites. Increasing temperature increases the kinetic energy of the reactants.
As the reactant molecules move faster, they increase the number of collisions
of molecules to form enzyme-substrate complex.
At optimum temperature, the rate of reaction is at maximum. The enzyme is
still in active state. The optimum temperature varies with different enzymes.
The optimum temperature for enzymes in the human body is about 37oC.
When the temperature exceeds the optimum level, the enzyme is denatured.
The effect is irreversible. However, some species are thermophilic that is
they better work at high temperatures; others are thermophobic, that is they
better work at low temperatures. For example, some thermophilic algae andbacteria can survive in hot springs of 60oC.
The rate doubles for each 10oC rise in temperature between 0oC and 40oC.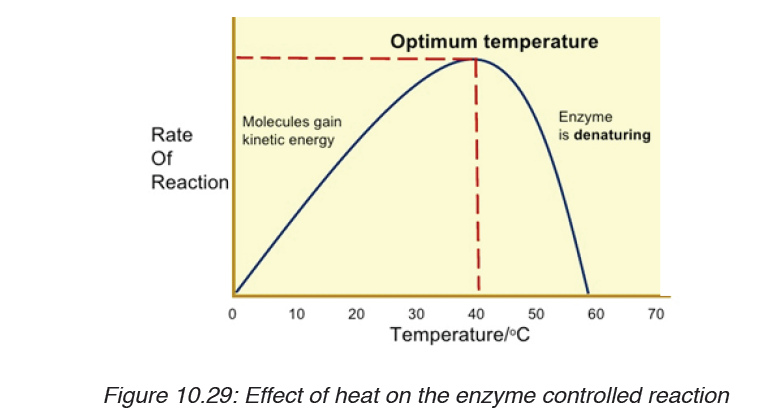
The temperature coefficient Q10 is the number which indicates the effect of
rising the temperature by 10oC on the enzyme-controlled reaction. The Q10
is defined as the increase in the rate of a reaction or a physiological process
for a 10°C rise in temperature. It is calculated as the ratio between rate of
reaction occurring at (X + l0) oC and the rate of reaction at X oC. The Q10 ata given temperature x can be calculated from:
Worked out example
The rate of an enzyme-controlled reaction has been recorded at differenttemperatures as follows:
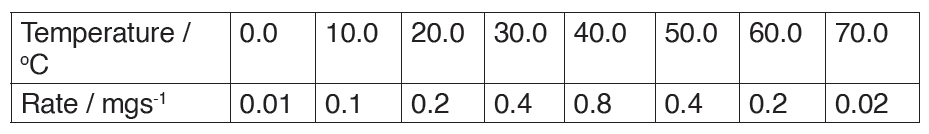
Calculate the Q10 of that reaction at 30 oC
This means that the rate of the reaction doubles if the temperature is raised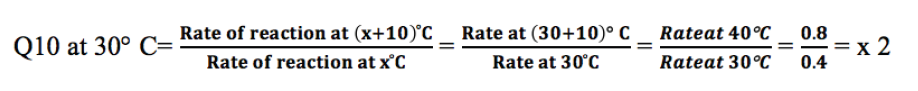
from 30°C to 40°C
Be aware that not all enzymes have an optimum temperature of 40°C. Some
bacteria and algae living in hot springs (e.g. Amashyuza in Rubavu) are
able to tolerate very high temperatures. Enzymes from such organisms are
proving useful in various industrial applications.
ii. The pH
Most enzymes are effective only within a narrow pH range. The optimum pH
is the pH at which the maximum rate of reaction occurs. Below or above the
optimum pH the H+ or OH- ions react with functional groups of amino acids inthe enzyme which loses its tertiary structure and become natured.
Different enzymes have different pH optima (look in the table).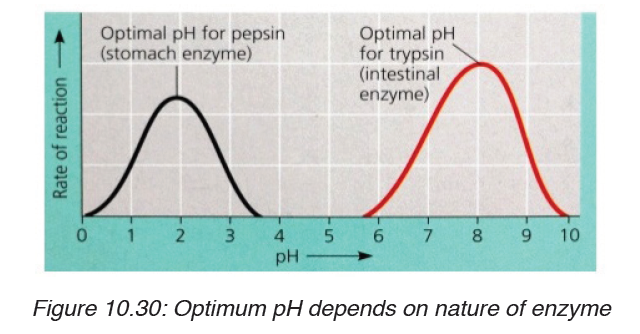
Table 10.5. Optimum pH of some digestive enzymes
Enzyme concentration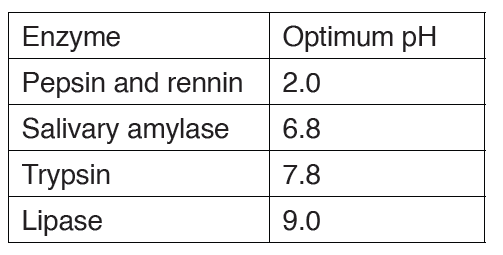
The rate of an enzyme-catalyzed reaction is directly proportional to the
concentration of the enzyme if substrates are present in excess concentrationand no other factors are limiting.
iii. Substrate concentration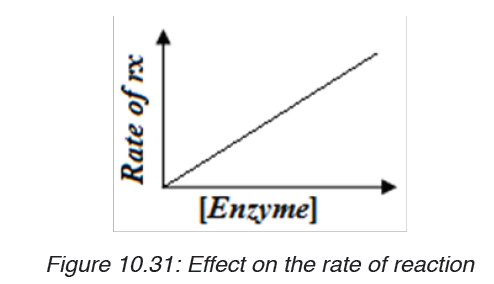
At low substrate concentration, the rate of an enzyme reaction increases with
increasing substrate concentration. The active site of an enzyme molecule
can only bind with a certain number of substrate molecules at a given time.
At high substrate concentration, there is saturation of active sites and thevelocity of the reaction reaches the maximum rate.
iv. Inhibitors
The inhibitors are chemicals or substances that prevent the action of an
enzyme. An inhibitor binds to an enzyme and then decreases or stops its
activity. There are three types of inhibitors:
i. Competitive inhibitors are molecules that have the similar shape
as the substrate. They are competing with the substrate to the activesite of the enzyme e.g. O2 compete with CO2 for the site of RuBPcarboxylase
ii. Non-competitive inhibitors are molecules that can be fixed to the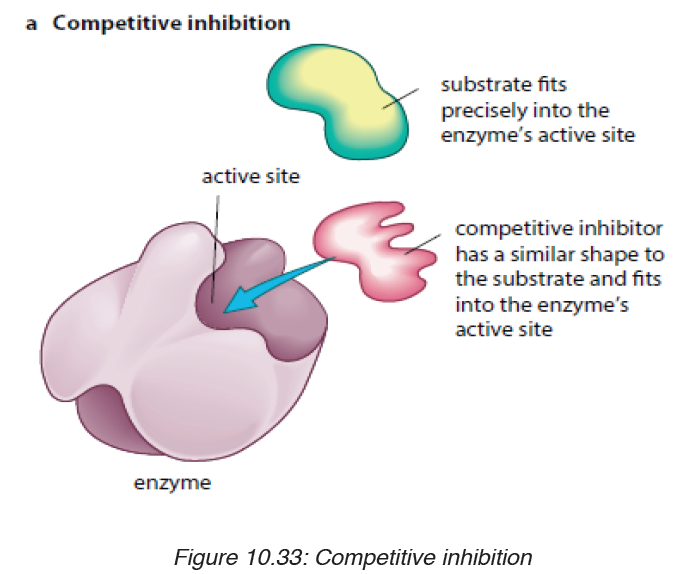
other part of enzyme (not to the active site) so that they change
the shape of active site, due to this the substrate cannot bind tothe active sit of the enzyme.
iii. End product inhibitor Allosteric inhibitor or Allostery.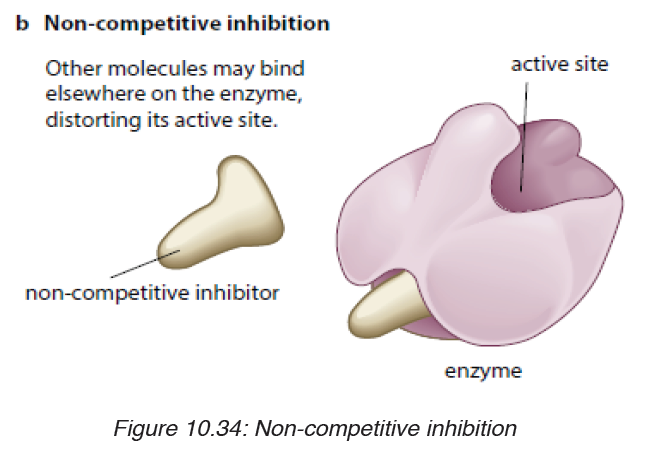
This is a chain enzymatic metabolic pathway where the final end product acts
as an allosteric reversible inhibitor for the first, the second or the third step
in the metabolic pathway. The shape of an allosteric enzyme is altered by
the binding of the end product to an allosteric site. This decreases enzymatic
activity. By acting as allosteric inhibitors of enzymes in an earlier metabolic
pathway, the metabolites can help to regulate metabolism according to the
needs of organisms. This is an example of negative feedback.
This often happen when few enzymes are working on a large number
of substrate e.g. ATP is an end-product inhibitor of the enzyme PFK
(Phosphofructokinase) in glycolysis during cell respiration. The end-productinhibitor leads to a negative feedback.
The products of enzyme-catalysed reactions are often involved in the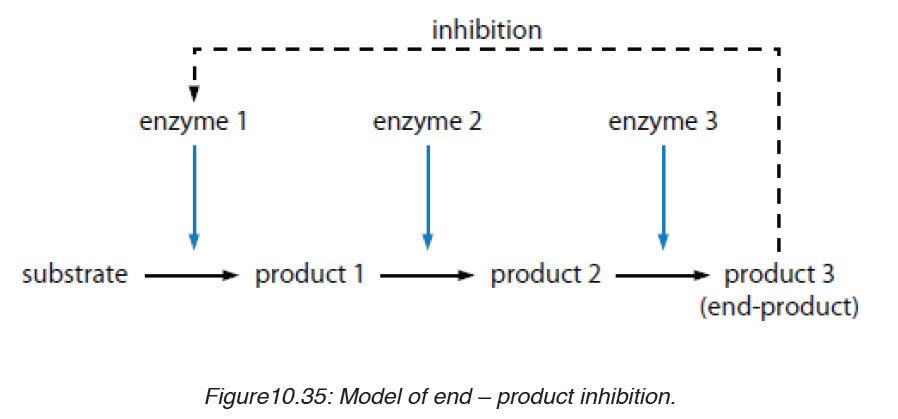
feedback control of those enzymes. Glucose-1-phosphate is the product
formed from this enzyme-catalysed reaction. As its concentration increases,
it increasingly inhibits the enzyme.
Note: Reversible and irreversible inhibition
Competitive inhibitor is reversible inhibitor as it binds temporarily to the
active site. It can be overcome by increasing the relative concentration of
the substrate. Some non-competitive inhibitors are reversible, that is, if the
inhibitor binds temporarily and loosely to the allosteric site. Some inhibitors
have very tightly, often, by forming covalent bonds with enzyme.
The nerve gas DIPF (DiIsopropylPhosphoFluoridate) is an irreversible
inhibitor. It binds permanently with enzyme acetylcholisterase, altering
its shape. The enzyme cannot bind with and break down its substrate
acetylcholine (neurotransmitter). Acetylcholine molecules accumulate in the
synaptic cleft. Nerve impulses cannot be stopped causing continuous muscle
contraction. This leads to convulsions, paralysis and eventually death.
Many pesticides such as organophosphate pesticides act as irreversible
enzyme inhibitors. Exposure to pesticides can produce harmful effects to
the nervous and muscular systems of humans. Heavy metal ions such as
Pb2+, Hg2+, Ag+, As+ and iodine-containing compounds which combine
permanently with sulphydryl groups in the active site or other parts of the
enzyme cause inactivation of enzyme. This usually disrupts disulphide
bridges and cause denaturation of the enzyme.
d) Importance of enzymes in living organisms
Activity 10.2.d
Discuss and present your ideas about the need for different enzymes in
living organisms.
Without enzymes, most of the biochemical reactions in living cells at body
temperature would occur very slowly at not at all. Enzyme can only catalyze
reactions in which the substrate shape fits that of its active site
There are thousands upon thousands of metabolic reactions that happen
in the body that require enzymes to speed up their rate of reaction, or will
never happen. Enzymes are very specific, so nearly each of these chemical
reactions has its own enzyme to increase its rate of reaction. In addition, the
organism has several areas that differ from one another by the pH. Therefore,
the acid medium requires enzymes that work at low pH while other media
are alkaline and therefore require enzymes that work at high pH. In addition
to digestion, enzymes are known to catalyze about 4,000 other chemical
reactions in your body. For example, enzymes are needed to copy genetic
material before your cells divide.
Enzymes are also needed to generate energy molecules called ATP, move
fluid and nutrients around the insides of cells and pump waste material out
of cells. Most enzymes work best at normal body temperature -- about 98
degrees Fahrenheit -- and in an alkaline environment. As such, high fevers
and over-acidity reduce the effectiveness of most enzymes. Some enzymes
need co-factors or co-enzymes to work properly.
10.2.3. Mode of action of enzymes
Activity 10.2.3
There are two main hypotheses that explain the more of action of an
enzyme on its substrate: the lock and key hypothesis and the induced-fit
hypothesis. Carry out a research to find the relevance of each.
Enzymes do not change but substrates are converted to products. A substrate
is a molecule upon which an enzyme acts. In the case of a single substrate,
the substrate binds with the enzyme active site to form an enzyme-substratecomplex. Thereafter the substrate is transformed into one or more products,
which are then released from the active site. This process is summarized asfollows:
Whereby: E = enzyme, S = substrate(s), ES = Complex Enzyme-Substrate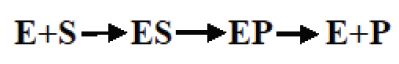
and P= product (s). There are two main hypotheses explaining the mechanism
of enzyme action:
a) The lock and key hypothesis by Emil Fischer
In this hypothesis the substrate is the key and enzyme is the lock. In otherwisethe active site is exactly complementary to the shape of the substrate.
b) The induced-fit hypothesis by Daniel Koshland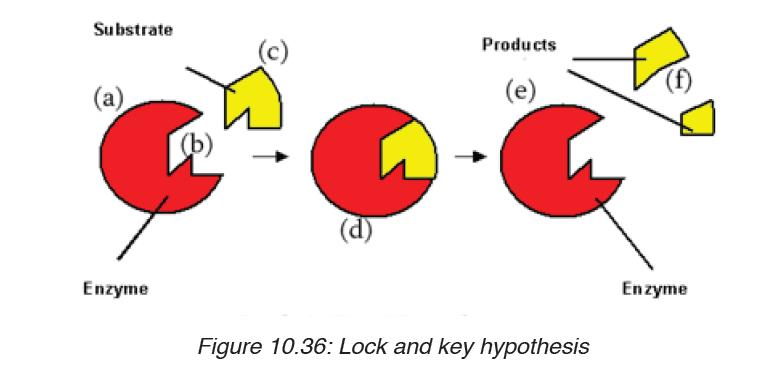
The induced-fit hypothesis is a modified version of the lock and key hypothesis
and is more widely accepted hypothesis. In this hypothesis, the active site
is flexible and is not exactly complementary to the shape of the substrate.
An enzyme collides with the substrate molecule. The substrate binds to the
active site. The bindings induce a slight change in the shape of the enzyme
to enclose the substrate making the fit more precise. The active site now
becomes fully complementary with the substrate as the substrate binds tothe enzyme.
Note that the activity of a given enzyme in vitro (outside a living body) may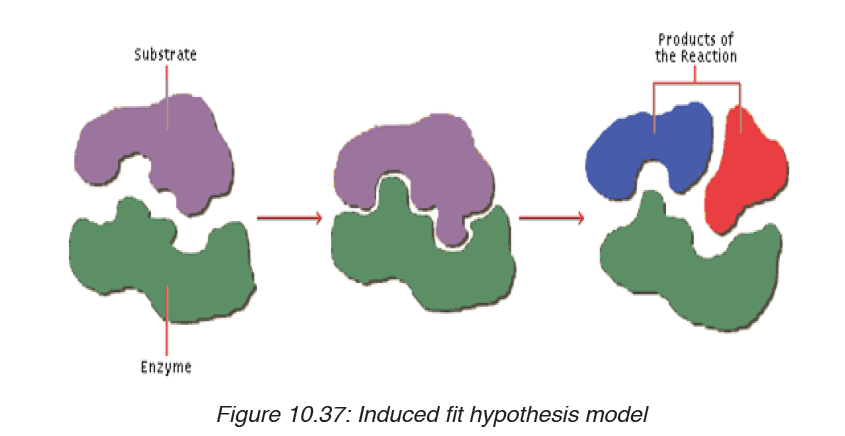
not be necessarily the same in vivo (inside a living body).
Application activity 10.2
Fill the blank with appropriate terms: Enzymes are biological
____________________ produced by ___________________________
cells. Enzymes reduce the amount of ____________________
energy required for reactions to occur. They consist of globular
____________________ with _______________________ structure.
Answer the following questions:
a) What is the main role of enzymes?
b) What would happen if there are no enzymes in the cell? State any
four properties of enzymes.
SKILLS LAB 8
Student-teachers will use the knowledge they acquired in this unit 10 by
testing the components of the food and beverages in their food producing
businesses. Here they will need the knowledge of food test techniques.
End unit assessment 10
1. Biological molecules are divided into:
a) Organic molecules and inorganic molecules
b) Carbohydrates and starch
c) Lipids, carbohydrates and water
d) Carbohydrates, food and potatoes
2. Write the formula of a monosaccharide with 3 atoms of carbon
3. Compare the structure of fat(triglycerides)and the phospholipids
4. Give two examples of how carbohydrates are used in the body.
5. The formula for a hexose is C6H12O6 or (CH2O)6. What would be
the formula of?
a) Triose
b) Pentose
c) Distinguish between:
d) Alpha glucose and beta glucose
e) Glycogen and cellulose
f) Amylopectin and amylose
7. The drug can cleave the covalent bond between two sulfur atoms
of non-adjacent amino acids. Which level of protein can be affected
the most if the drug is mixed with primary, secondary, tertiary and
quaternary structure of proteins.
8. State the property of water that allows each of the following to take
place. In each case, explain its importance:
a) The cooling of skin during sweating
b) The transport of glucose and ions in a mammal
c) Much smaller temperature fluctuations in lakes and oceans than in
terrestrial (land-based) habitats.
9. Construct the table that organize the following terms and label the
columns and rows.
Phosphodiester linkages Monosaccharide polypeptides
Peptide bonds Nucleotides Triacylglycerol
Glycosidic linkages Amino acids Polynucleotides
Ester linkages a ftty acids Polysaccharides
10. Explain what happen during protein denaturation?
11. Enzymes are biocatalysts.
a) What is the meaning of the following terms elated to enzyme
activity?
ii. Catalyst
iii. Activation energy
iv. Lock and key
v. Q10
b) Why are there hundreds of different enzymes in a cell?
c) How do enzymes reduce the activation energy of a reaction?
12. Enzyme activity is related to a number of factors.
a) Explain why enzymes work faster at high temperatures
b) Describe what happens to the enzyme structure if the temperature
is raised well above the optimum temperature.
c) How are enzymes affected by pH?
d) Why do different enzymes have a different optimum pH?
e) What is the difference between a reversible and irreversible enzyme
inhibitor?
13. Some bacteria and algae can survive in the boiling waters of hot
springs. Enzymes from these organisms are used in industrial
processes. Why are these enzymes useful?
14. The following set data show the effect of temperature on the
completion time of an enzyme reaction.
Temperature / 0C 0.0 15 25 35 45 55 65
Rate of reaction / min-1 0.00 0.07 0.12 0.25 0.50 0.28
0.00
a) Plot the data on a graph
b) What is the optimum temperature of this reaction?
c) Describe the shape of the graph between 10 and 400Cd) Calculate the rate of increase between 20 and 300C.
