UNIT 1: PROPERTIES AND USES OF TRANSITION METALS
Key Unit Competence:
The learner should be able to explain the properties and uses of transition metals.
Learning objectives
At the end of this unit , students will be able to:
• Discuss qualitatively the propertie of transition elements;
• Explain the principle of ligan exchange;
• State the rules of naming complex ions and stereoisomers;
• Describe reactions of transition metals;
• State the use of transition metals;
• Relate the electronic configurations to special properties of transition metals;
• Predict the shape of the complex compounds of transition metal cations;
• Perform the confirmatory tests for transition metal ions.
Most of the metals in the periodic table belong in the d-block of transition metals. They are hard and strong, and many of them are very familiar to us. For instance, zinc Chemistry Senior Six Student Book 3 is in brass instruments like trumpets and tubas. Have you ever heard of the element “scandium” before? But you’ve interacted with it if you have ever ridden a bicycle.
1.1. Definition and electronic configuration of transition metals.
According to IUPAC system, a transition metal is “an element whose atom has a partially filled d sub-shell, or which can give rise to cations with an incompleted-orbitrals”. Transition metals are located between groups 1& 2 (s-block) and group 13 (p-block) on the periodic table. The elements are also called d-block elements because their valence electrons are in d-orbitals.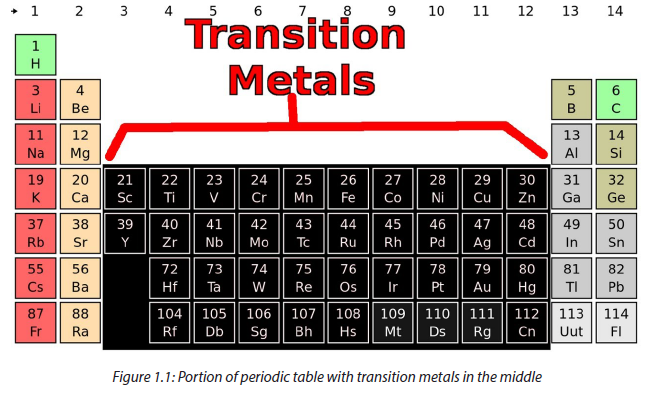
The properties of transition elements are between the highly reactive metallic elements of the s-block which generally form ionic compounds and the less reactive elements of the p-block which form covalent compounds. Transition metals form ionic compounds as well as covalent compounds.
The first 3 rows, i.e. period 4, period 5 and period 6, are called first transition series, second transition series and third transition series respectively. The metals of the first series are all hard and dense, good conductors of heat and electricity.
This block is known as the transition metals because some of their properties show a gradual change between the active metals in s-block and p-block where non-metals are found. Electronic configuration is the arrangement of electrons in orbitals around the nucleus. The electronic structure of the first transition series is shown in the table below: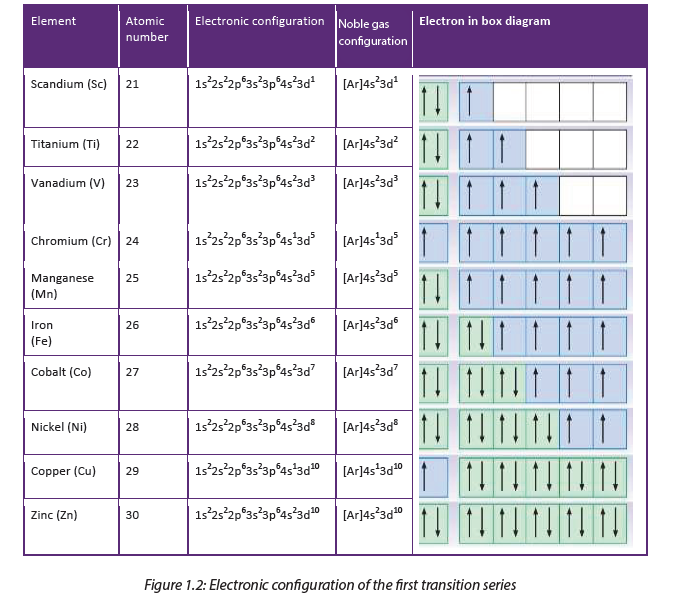
When building electronic structure of transition metals, 4s orbital is filled before 3d orbitals. The transition elements are stable when their d-orbitals are filled (d10) or when theird-orbitals are half filled (d5).This explains the electronic structure of copper, [Ar] 4s13d10 instead of [Ar] 4s23d9. The same applies for Cr: [Ar] 4s13d5 and not [Ar] 4s23d4.
In order to attain that stability an electron can jump from 4s orbital to 3d orbital because those two orbitals are close in energy.
This also explains why Fe2+ with 3d6 is easily oxidized to Fe3+ with 3d5 and Mn2+ with 3d5 is resistant to oxidation to Mn3+ with 3d4. Transition metals form ions by losing electrons first from the 4s sub-shell rather than the 3d sub-shell. Hence electronic configuration of Fe, Fe2+ and Fe3+ are the following: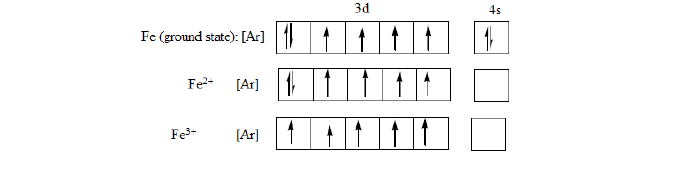
The 4s electrons are removed before 3d electrons. This is because the 3d electrons are inner while the 4s electrons are outer therefore the outer electrons (4s) have to be removed before the inner electrons.
1.2. Properties of the transition metals
1.2.1. Melting and boiling points
The melting points and the molar enthalpies of fusion of the transition metals are both high in comparison to main group elements. Most of the transition metals have melting points above 1000 oC; mercury is liquid at room temperature.
This is due to the high number of valence electrons that increases the electrostatic attraction force between those electrons and the metallic cations, hence increasing the strength of the metallic bond and the melting point.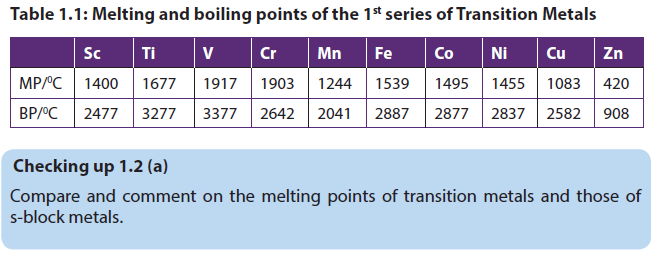
1.2.2. Densities and atomic/metallic radii
The transition elements are much denser than the s-block elements and show in general a gradual increase in density from left to right in a period as you can see below from scandium to copper. This trend in density can be explained by a decrease in metallic radii coupled with the relative increase in atomic mass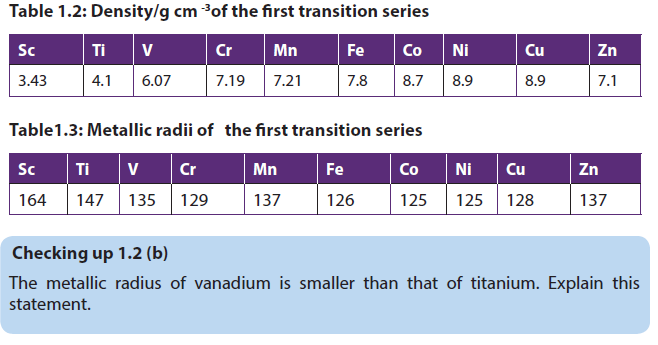
1.2.3. Ionization energies
The ionization energy of transition metals is related to the energies of its d orbitals, its ease of oxidation, and its basicity.In simplest terms, the greater the ionization energy of a metal, the harder it is to pull an electron from it.
As the number of protons increases across a period (or row) from left to right of the periodic table, the first ionization energies of the transition-metal elements are relatively the same, while that for the main-group elements increases. In moving across the series of metals from scandium to zinc, a small change in the values of the first and second ionization energies is observed. This is due to the buildup of electrons in the immediately underlying d-sub-shells that efficiently shields the 4s electrons from the nucleus and minimizing the increase in effective nuclear charge from element to element.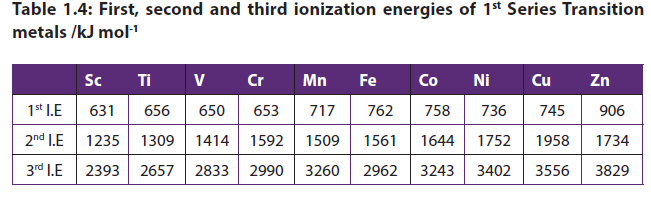
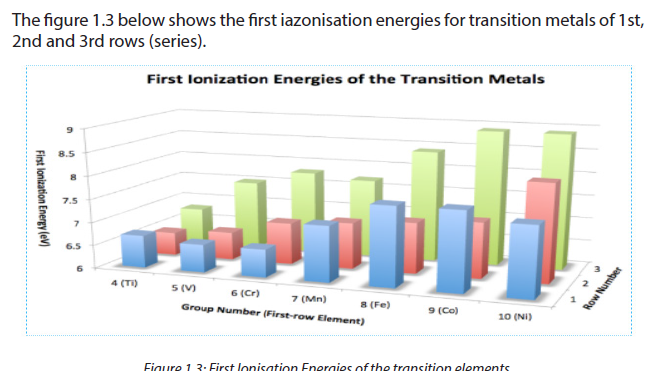
In general, ionization energy increases as we move from left to right across theperiod. Notable dips occur at row 1, group 10 (Ni) and row 3, group 7 (Re).
Oxidation state is a number assigned to an element in chemical combination which represents the number of electrons lost or gained.The transition elements from Titanium to Copper all form ions with two or more oxidation states. In most cases, this is the result of losing the two electrons of 4s orbital and electeons in 3d orbitals. The 4s electrons are lost first because they are in the highest energy level. However, because the 3d and 4s energy level are so close in energy, the 3d electrons can also be lost when an atom forms a stable ion. The common oxidation states shown by the first transition series are: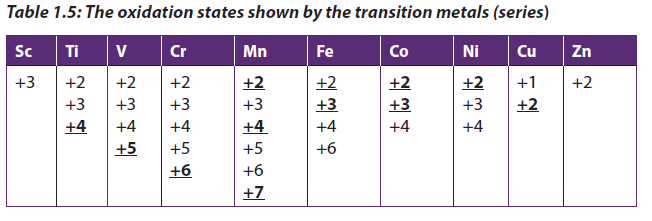
- The common stable oxidation states for those transition metals with variableoxidation states are bolded and underlined.
- The oxidation state corresponding to a full or half-filled d-orbital is energetically stable. For example, Fe3+ is more stable than Fe2+and Mn2+ is more stable than Mn3+.
- However, in most compounds and solutions, copper exist as Cu2+ ion rather than Cu+ ion. Meaning that the former is more stable than the latter. The explanation of this is beyond this level.


A catalyst is a substance that can speed up (positive catalyst) or that can slow down (negative catalyst) the rate of reaction and is found unchanged at the end of the reaction. But generally the term catalyst is used for the substance that helps in accelerating the rate of the reaction. A catalyst that speeds up the reaction provides another pathway with lower activation energy.
In some catalytic process, transition metal ions undergo changes in their oxidation states but are regenerated at the end of the reaction.
The reasons for transition metals to work as catalysts:
• Presence of empty d orbitals which enable transition metal ions (or atoms) to form temporary bonds with reactant molecules at the surface of a catalyst and weakens the bond in the reactant molecules
. Variable oxidation states which allow them to work as catalysts in the reactions involving the transfer of electrons
Table 1.6: Reactions catalysed by transition metals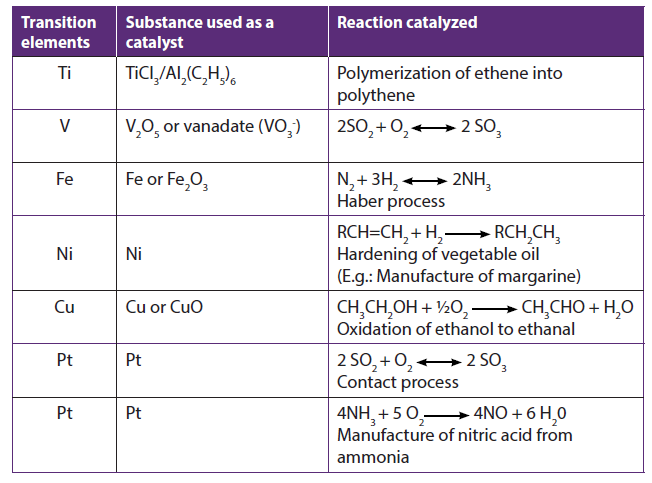

1.2.6. Most transition metal ions are paramagnetic
Paramagnetism is a property of substances to be attracted in a magnetic field. Substances which are not attracted (i.e slightly repelled) in a magnetic field are said to be diamagnetic.Transition metal ions show paramagnetism because of the presence of unpaired electrons in their 3d arbitrals.
The greater the number of unpaired electrons, the stronger the paramagnetism; that is the reason why:- Fe3+ is more paramagnetic than Fe2+ because Fe3+ has five unpaired electrons while Fe2+ has four unpaired.
- Sc3+ and Zn2+ are not paramagnetic, they are diamagnetic because they do not have unpaired electrons

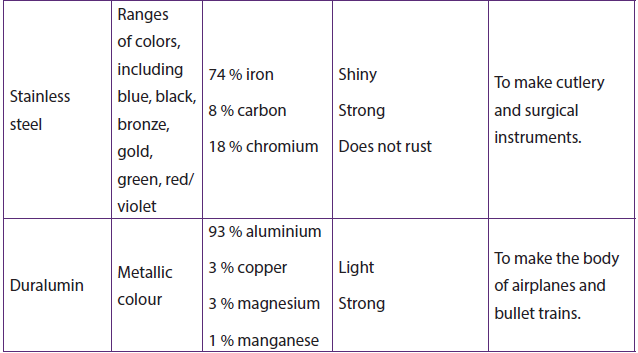
1.2.7. Formation of alloys
An alloy is a homogenious solid mixture (solid solution) made by combining two or more elements where at least one is a metal.
Importance of alloying:- Increase of the strength of a metal,
- Resistance to corrosion,
- Gives to the metal a good appearance
Table 1.7: The properties and uses of some common alloys formed by transition metals (first series)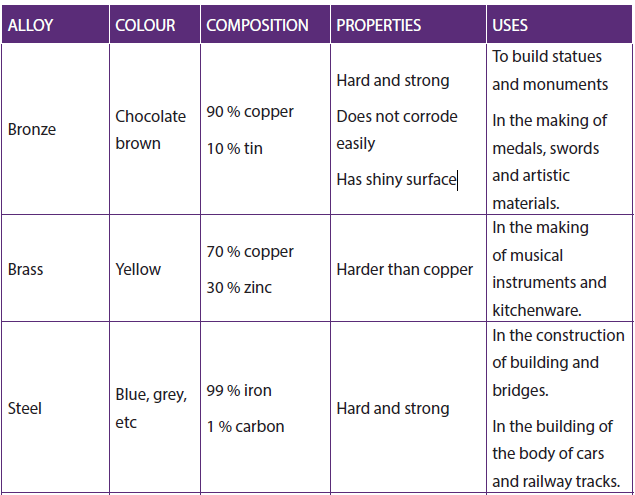


1.2.8. Formation of complex ions
A complex or coordination compound is a chemical species made of a central metal (cation or neutral) bonded to other chemical species called ligands by coordination or dative bonds. A complex may be neutral, positively or negatively charged.
Transition metal form complexes because of:- Their small and highly charged ions,
- The presence of vacant (empty) d-orbitals which can accommodate lone pair of electrons donated by other groups (ligands)
Where:- M-metal ion or atom
- L-Ligand
- n-the number of ligands surrounding the metal
- y-the charge of the complex; [MLn] indicates a neutral complex
- Ligand: It is a species (anion or a molecule) that is bonded to a central metal ion or atom in a complex. A ligand should have at least one lone pair of electrons to form a coordinate bond.
Ligands are classified depending on the number of sites at which one molecule of a ligand is coordinated to the central metallic atom; the ligands are classified as monodentate (or unidentate), ambidentate and polydentate (or multidentate) lingards.
a. Monodentate ligands
The ligands which have only one donor atom or are coordinated through one electron pair are called monodentate ligands because they have only one tooth with which to attach themselves to the central cation or atom. Such ligands are coordinated to the central metal at one site or by one metal-ligand bond only. These ligands may be neutral molecules or in anionic form.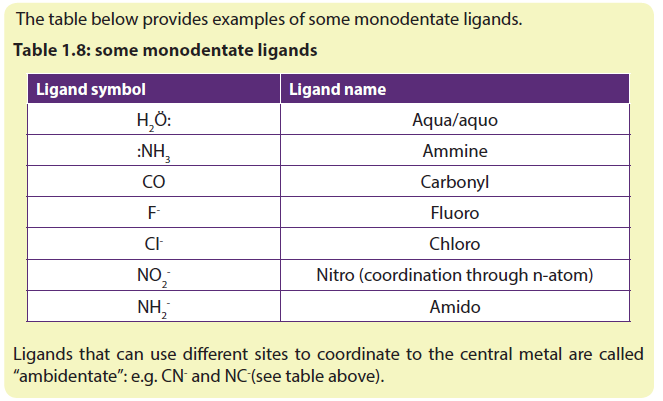
Notice that a ligand with a donor atom that possesses 2 lone pairs of electrons, such as H2Ö:, is not bidentate, since it cannot use both lone pairs simultaneously to bind to the metal because of the steric effect.
b. Polydentate ligandsThese may be bidentate, tridentate, tetradentate, pentadentate, and hexadentateligands if the number of donor atoms present in one molecule of the ligand attached with the central metallic atom is 2, 3, 4, 5, and 6 respectively. Thus one molecule of these ligands is coordinated to the central metallic atom at 2, 3, 4, 5, and 6 sites respectively. In other words, we can say that one molecule of these ligands makes 2, 3, 4, 5, and 6 metal-ligand coordinate bonds respectively.
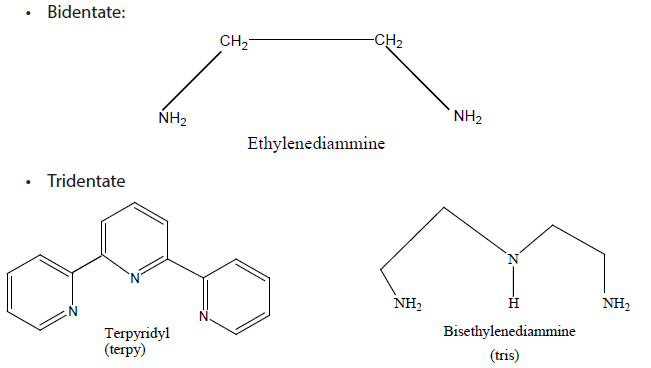
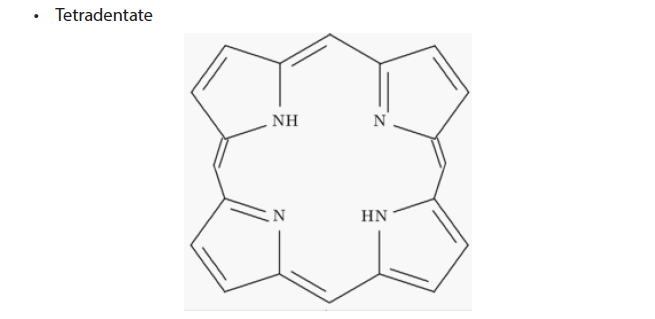
• Hexadentate
The structure shows that it has two neutral N- atoms and four negatively charged Oatoms as its donor atoms which can form coordinate bonds with a transition metal ion.
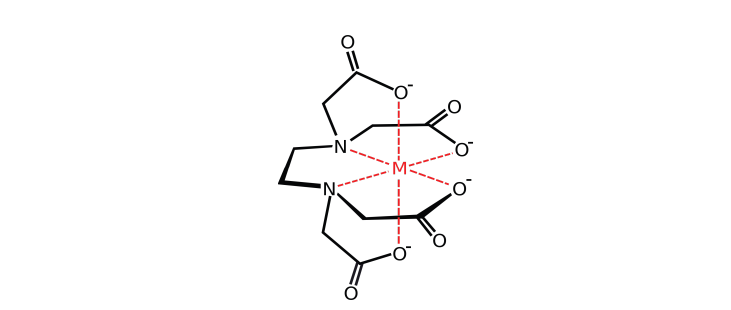
The complex ions which form between polydentate ligands and cations are known as chelates or chelated complexes.
In general, polydentate ligands form more stable complexes than monodentate ligands. The stability of complex is much enhanced by chelation. A polydentate ligand can hold the central cation more strongly.
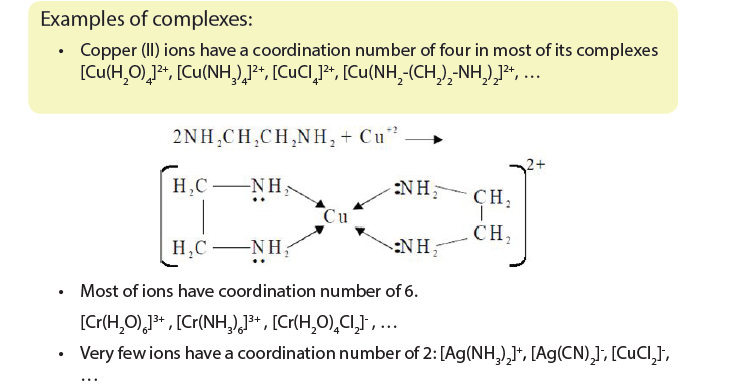
Geometry of complexes
Complexes have a variety of geometries or shapes, but the most common geometries are the following:
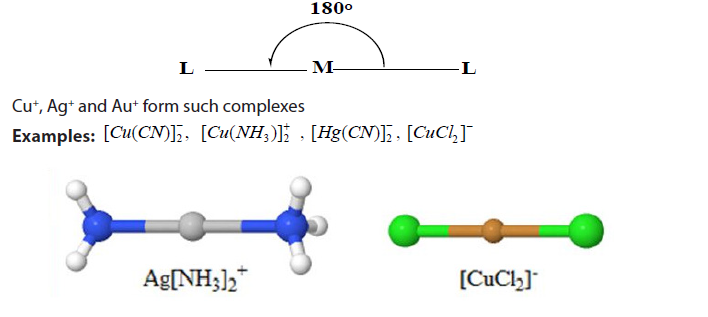
- Complexes with coordination number 2 adopt a linear shape. Example:
[Ag(NH3]2 +: [H3N-Ag-NH3]+
The complexes having coordination number of 2 are linear since minimises ligand repulsion.
- Complexes with coordination number 4 generall adopt a tetrahedral shape. But few of them can form a square planar shape.

The square plannar geometry is characteristic of transition metal ions with eight d electrons in the valence shell, such as platinum(II)and gold(III).
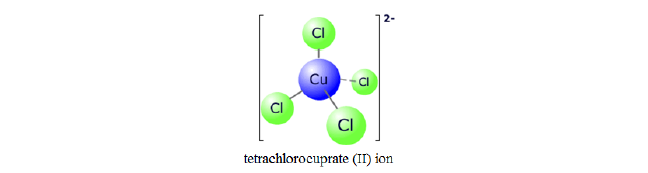
Copper (II) and cobalt (II) ions have four chloride ions bonded to them rather than six, because the chloride ions are too big to fit any more around the central metal ion.
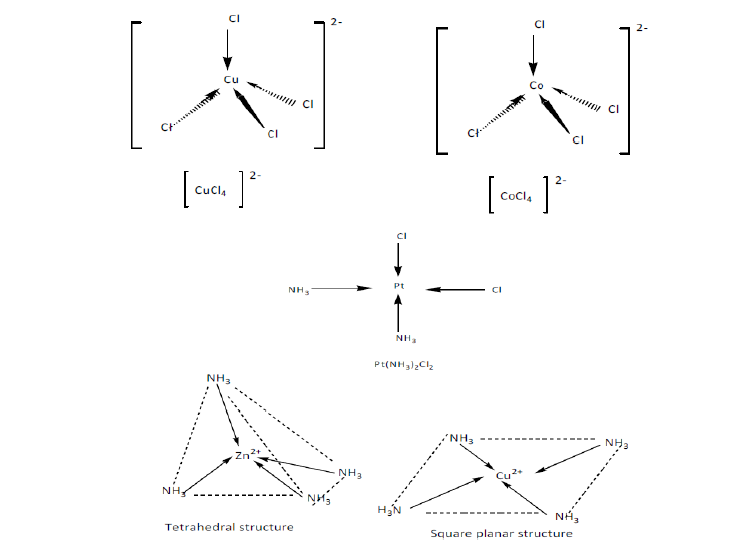
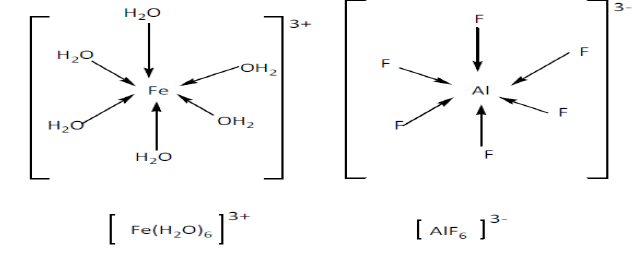
- Complexes with coordination number 6 adopt an octahedral shape. Example:[Cr(NH3)6]3+.
These ions have four of the ligands in one plane, with the fifth one above the plane, and the sixth one below the plane.

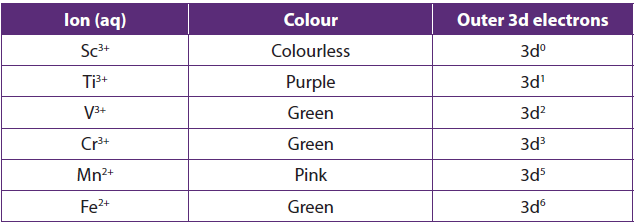
1.2.9. Many transition metal ions and their compounds are coloured

The formation of colored ions by transition elements is associated with the presence of incompletely filled 3d orbitals.

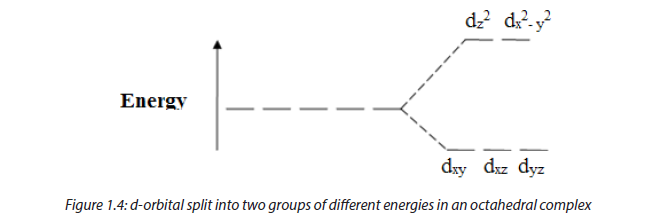
This property has its origin in the excitation of d electrons from lower energy d-orbitals to higher energy. In fact, when the central metal is surrounded by ligands, these cause d orbitals to be split into groups of higher and lower energy orbitals. When electrons fill d-orbitals, they fill first of all the lower energy orbitals; if there is free space in higher energy d-orbitals, an electron can be excited from lower energy d-orbitals to higher energy d-orbitals by absorbing a portion of light corresponding to a given colour, the remaining color light is the white light minus the absorbed colour.
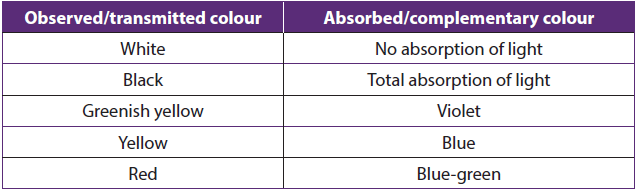
When a coloured object is hit by white light, the object absorbs some colour and the colour transmitted or reflected by the object is the colour which has not been absorbed. The observed colour is called complementary colour.
When a metal cation has full d-orbitals, such as Cu+or Zn2+ or no electron in d orbital, such as Sc3+.
Table1.9: Complementarities of colors observed and absorbed when light is emitted

The colour of a particular transition metal ion depends upon two factors:
• The nature of the ligand
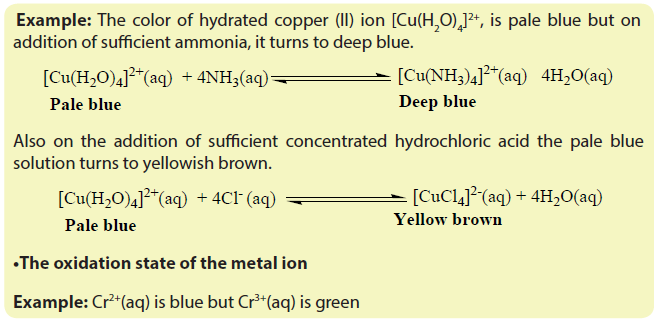
The principle of ligand exchange
Complexing reactions involve competitions between different ligands for central metal. A more powerful ligand displaces a less powerful ligand from a complex. During the process there is a change in colour.
Here below is a list of some ligands in increasing order of strength.
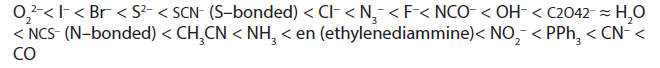
The above series are called the spectrochemical series and shows that cynide ion and carbon monoxide are very strong ligands
The stability of a complex ion is measured by its stability constant. The higher the stability constant of a complex, the more stable is the complex.

1.3. The anomalous properties of Zinc and Scandium

On the basis of the properties of transition metals, zinc and scandium are not considered as typical transition metals even though they are members of the d-block.
Zinc:
- It has a complete d-orbital.
- Zinc forms only the colourless Zn2+ ion, isoelectronic with the Ga3+ion, with 10 electrons in the 3d subshell.
- Zinc and its compounds are not paramagnetic
Scandium:
- Has one oxidation state,+3
- Sc forms only the colourless Sc3+ion, isoelectronic with the Ca2+ ion, with no electrons in the 3d subshell.
- Its compounds are diamagnetic
- It forms compounds containing ions with a completely empty 3d subshell.

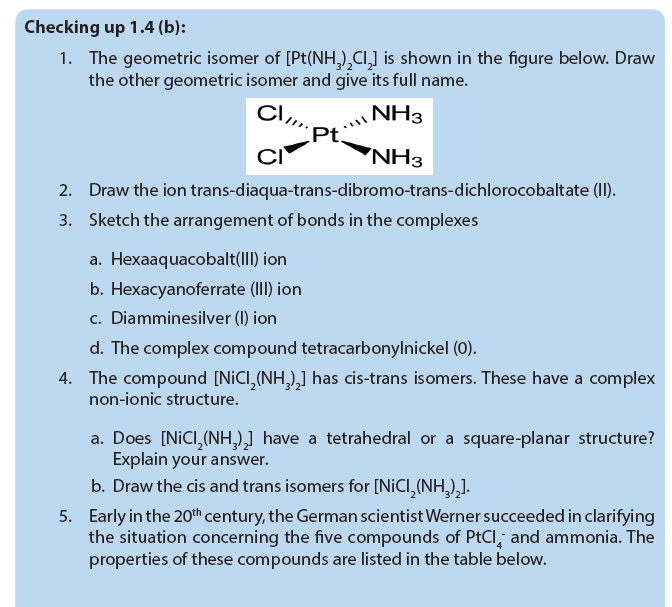
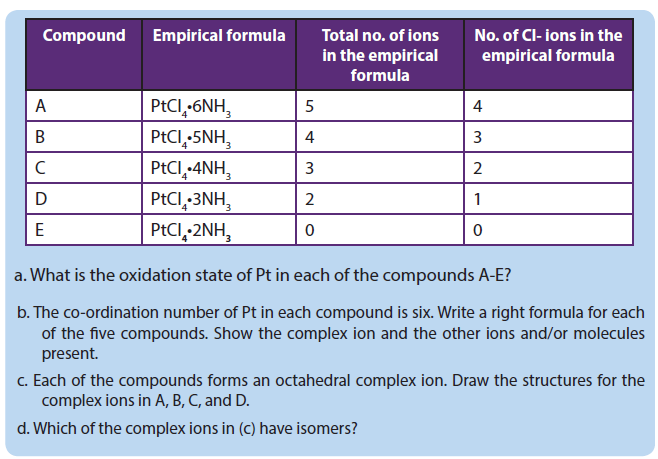
1.4. Naming of complex ions and isomerism in of transition metal complexes
1.4.1. Naming of complex ions

Naming molecules requires the knowledge of certain rules, such as how to name cations, anions, where to start from when both a cation and an anion are combined in an ionic molecule or when two non metals are combined in a covalent molecule.
Like other compounds, complex compounds/ions are named by following a set of rules. You are familiar with some of them and the new ones can be understood and applied easily.
- In simple metal compounds, the metal is named first then the anion.
Example: CaCl2: calcium chloride
2. In naming the complex:
- Name the ligands first, in alphabetical order, then the metal atom or cation, followed by its oxidation state written between brackets as Roman number, though the metal atom or cation is written before the ligands in the chemical formula.
Example: [CuBr4]2-: Tetrabromocuprate (II) ion
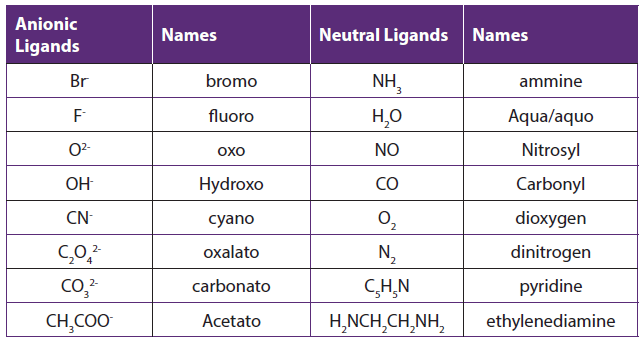
The names of some common ligands are listed in the table below:
Table 1.10: Names of common ligands
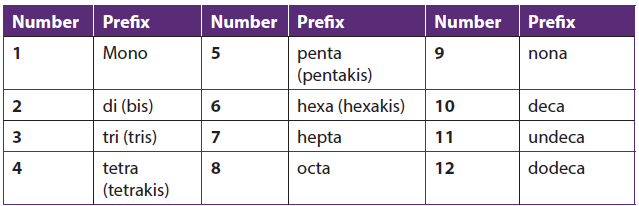
- Greek prefixes are used to indicate the number of each type of ligand in the complex:
The numerical greek prefixes are listed in the following table:
Table 1.11: Greek numerical prefixes
- After naming the ligands, name the central metal.
- If the complex bears a positive charge (cationic complex), the metal is named by its usual name.
Example: Cu: Copper Pt: Platinum
- If the complex bears a negative charge (anionic complex), the name of the metal ends with the suffix –ate
Example: Co in a complex anion is called cobaltate and Pt is called platinate.
For some metals, the Latin names are used in the complex anions e.g. Fe is called ferrate (not ironate). See table below:
Table 1.12: Latin names of some transition metals in anionic complexes
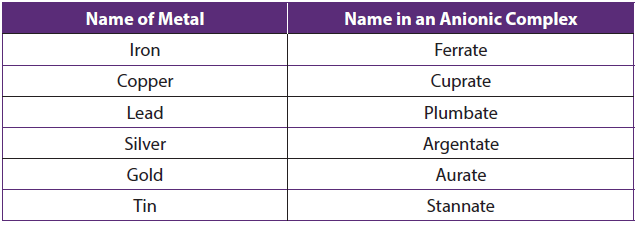
- For historic reasons, some coordination compounds are called by their common names.
Example: Fe(CN)6 3- and Fe(CN)6 4- are named ferricyanide and ferrocyanide respectively, and Fe(CO)5 is called iron carbonyl.
2. To name a neutral complex molecule, follow the rules of naming a complex cation
Example: [Cr(NH3)3Cl3]: triamminetrichlorochromium (III)
You can have a compound where both the cation and the anion are complex ions. Notice how the name of the metal differs even though they are the same metal ions. Remember: Name the cation before the anion.
Example: [Ag(NH3)2][Ag(CN)2] is diamminesilver(I)dicyanoargentate(I)
Note that:
- The names are written as a one word: Tetraamminecopper (II), not Tetraammine copper (II).
- Complex ions formula is written between square brackets and the charge of the ion as superscript outside the brackets: [Cu(NH3)4]2+. When oppositely chargedions approach the complex ion, a neutral molecule can be obtained:
[Cu(NH3)4]2+2Cl- or simply, [Cu(NH3)4]Cl2: tetraamminecopper(II)chloride.
The ions outside the square brackets are known as “counter ions”.

1.4.2. Isomerism in complexes

Isomers are chemical species that have the same molecular formulal, but different molecular structures or different arrangements of atoms or groups of atoms in space. Isomerism among transition metal complexes arises as a result of different arrangements of their constituent ligands around the metal.
The diagram below shows the different categories of isomerism in transition metal complexes.
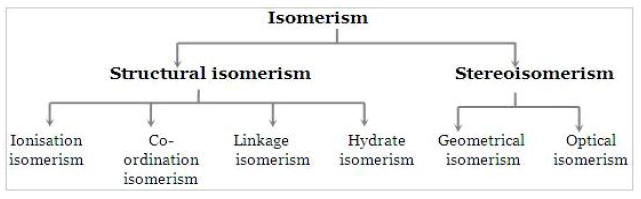
In this unit, we are specifically concerned with ‘stereoisomerism’ which gives rise to isomers known as “stereoisomers”. Stereoisomers have the same structural formulal but different arrangements of ligands in space.
They are classified in two categories: geometrical isomers and optical isomers.
1. Geometrical isomers
Coordination complexes, with two different ligands in the cis and trans positions from a ligand of interest, form isomers.
For example, the square planar, diammine dichloroplatinum (II) Pt(NH3)2Cl2),can be presented as follows:
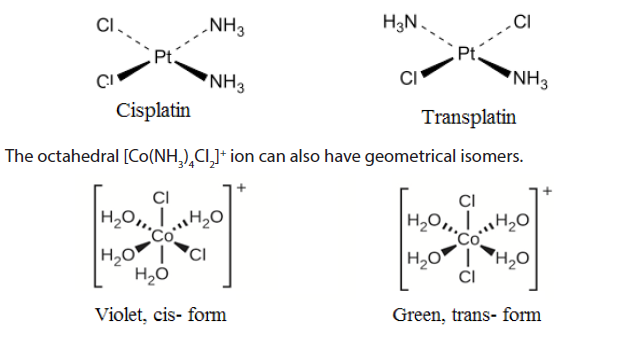
Different geometrical isomers are different chemical compounds. They exhibit different properties, even though they have the same formula. For example, the two isomers of [Co(NH3)4Cl2]NO3 differ in color; the cis form is violet, and the trans form is green. Furthermore, these isomers have different dipole moments, solubilities and reactivities.
2. Optical isomers (enantiomers)
example of this is your two hands (left and right); hold them face-to-face: one is the mirror image of the other. Now try to superimpose them one over another: they are non superimposable (only the middle fingers superimpose one over the other. Chemical compounds that behave like the hands are called “chiral”, in reference to the Greek word for hands.
Optical isomers are very important in organic and biochemistry because living systems often incorporate one specific optical isomer and not the other.
Unlike geometric isomers, optical isomers have identical physical properties (boiling point, polarity, solubility, etc.). Optical isomers differ only in the way they affect polarized light and how they react with other optical isomers.
For coordination complexes, many coordination compounds such as [M(en)3]n+ [in which Mn+ is a central metal ion such as iron(III) or cobalt(II)] form enantiomers, as shown in figure below.These two isomers will react differently with other optical isomers. For example, DNA helices are optical isomers, and the form that occurs in nature (right-handed DNA) will bind to only one isomer of [M(en)3]n+ and not the other.
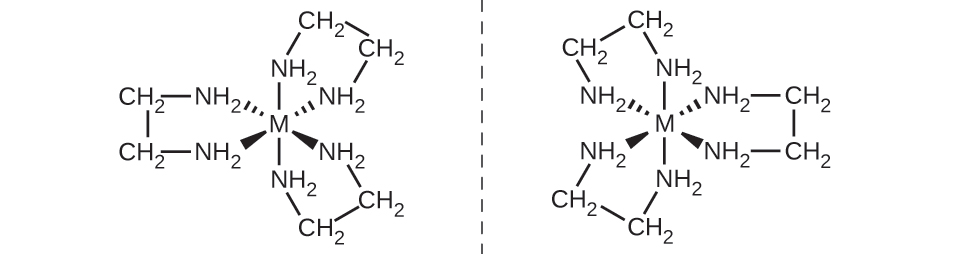
1.5. The Chemistry of individual transition metals

1.5.1. Scandium
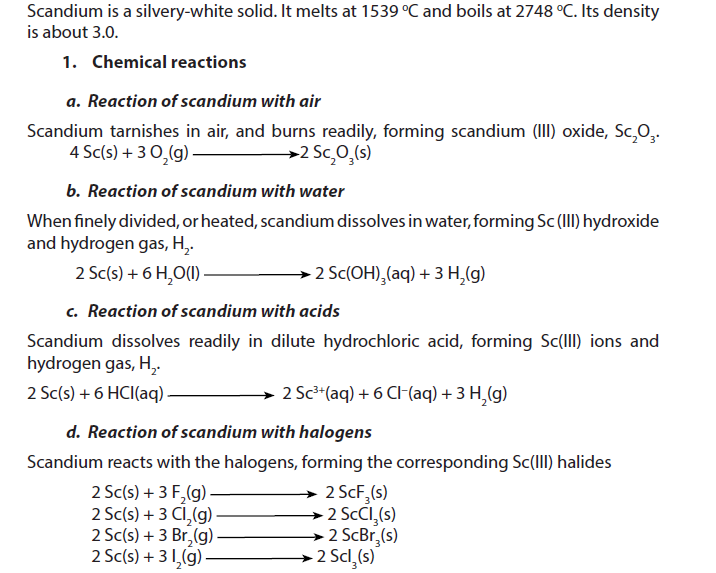
2. Uses
- Scandium has as low density (2.99 g/cm3) asaluminium (2.7 g/cm3) but a much higher melting point.
- An aluminium-scandium alloy has been used in fighter planes, high-end bicycle frames and baseball bats.
- Scandium iodide is added to mercury vapour lamps to produce a highly efficient light source resembling sunlight. These lamps help TV cameras to reproduce colour well when filming indoors or at night-time.
1.5.2. Titanium
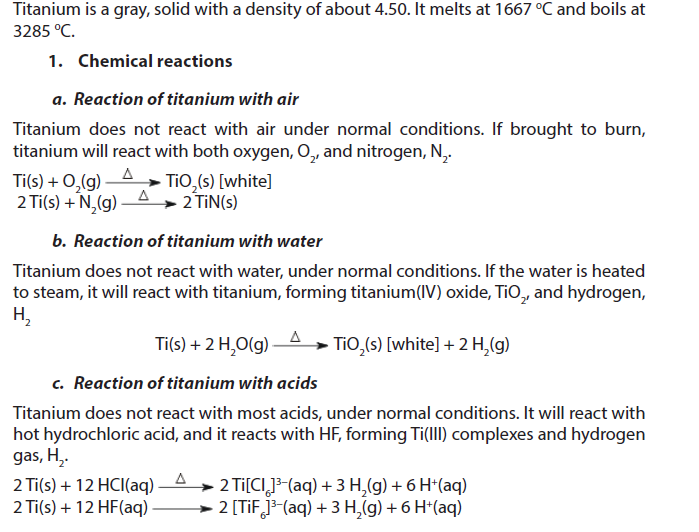

2. Uses
- Titanium is as strong as steel but much less dense. It is therefore important as an alloying agent with many metals including aluminium, molybdenum and iron. These alloys are mainly used in aircraft, spacecraft and missiles because of their low density and ability to withstand extremes of temperature. They are also used in golf clubs, laptops, bicycles and crutches.
- Power plant condensers use titanium pipes because of their resistance to corrosion. Because titanium has excellent resistance to corrosion in seawater, it is used in desalination plants and to protect the hulls of ships, submarines and other structures exposed to seawater.
- Titanium metal connects well with bone, so it has found surgical applications such as in joint replacements (especially hip joints) and tooth implants.
- The largest use of titanium is in the form of titanium (IV) oxide. It is extensively used as a pigment in house paint, artists’ paint, plastics, enamels and paper. It is a bright white pigment with excellent covering power. It is also a good reflector of infrared radiation and so is used in solar observatories where heat causes poor visibility.
1.5.3. Vanadium
Vanadium is a grey, solid with a density of about 6.11. It melts at 1915 oC and boils at 3350 oC. It is insoluble in water at room temperature.
2. Uses
- About 80% of the vanadium produced is used as a steel additive. Vanadium- steel alloys are very tough and are used for spanners, armour plate, axles, piston rods and crankshafts. Less than 1% of vanadium, and as little chromium, makes steel shock resistant and vibration resistant. Vanadium alloys are used in nuclear reactors because of vanadium’s low neutron-absorbing properties.
- Vanadium (V) oxide is used as a pigment for ceramics and glass, as a catalyst and in producing superconducting magnets.
1.5.4. Chromium
Chromium is a silver gray metal with density of about 7.14. It melts at 1900 oC and boils at 2690 oC. Chromium is insoluble in water at room temperature.
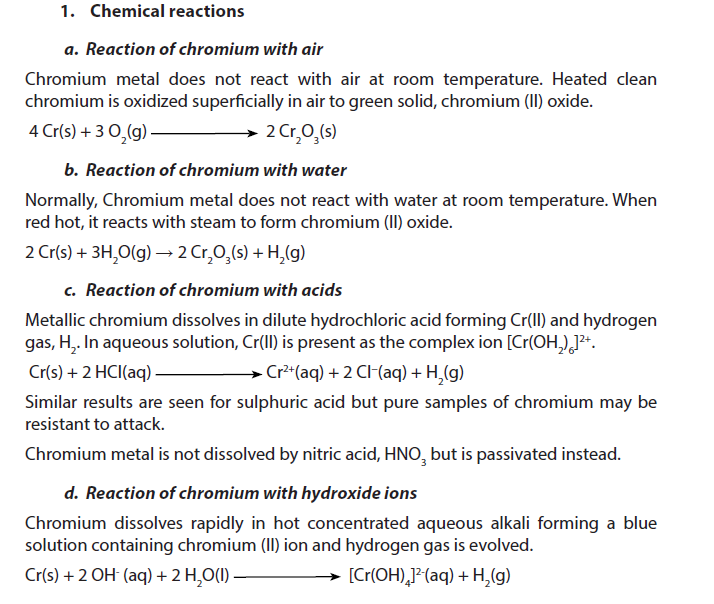
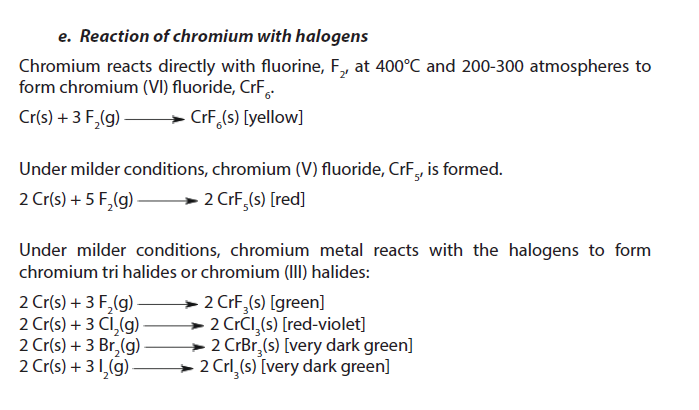
2. Uses
- Chromium is used to harden steel, to manufacture stainless steel (resists to corrosion) and to produce several alloys.
- Chromium plating can be used to give a polished mirror finish to steel. Chromium-plated car and lorry parts, such as bumpers, were once very common. It is also possible to chromium plate plastics, which are often used in bathroom fittings.
- About 90% of all leather is tanned using chromium. However, the waste effluent is toxic so alternatives are being investigated.
- Chromium compounds are used as industrial catalysts and pigments (in bright green, yellow, red and orange colours). Rubies get their red colour from chromium, and glass treated with chromium has an emerald green colour.
- Chromium (IV) oxide is used in magnetic tapes for sound/video recording.
- Chromium is used in the control of cholesterol and help insulin sugar control in blood.
1.5.5. Manganese
Manganese is a grey-white solid with a slightly red colour. Its density is about 7.44 oC. Manganese melts at 1244 oC and boils at 2060 oC. It is insoluble in water but soluble in diluted acids, at room temperature.
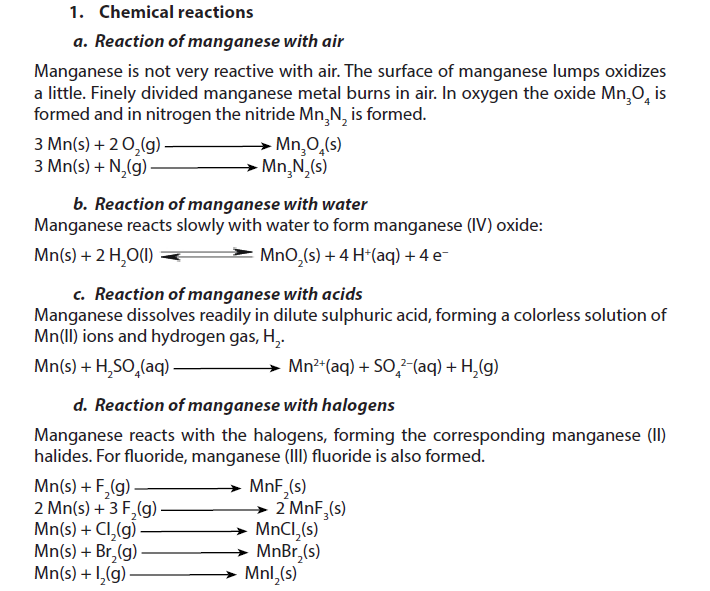
2. Uses
- Manganese is too brittle to be of much use as a pure metal. It is mainly used in alloys, such as steel. Steel contains about 1% manganese, to increase the strength and also improve workability and resistance to wear. Manganese steel contains about 13% manganese. This is extremely strong and is used for railway tracks, safes, rifle barrels and prison bars.
- Drinks cans are made of an alloy of aluminium with 1.5% manganese, to improve resistance to corrosion. With aluminium, antimony and copper it forms highly magnetic alloys.
- Manganese (IV) oxide is used as a catalyst, a rubber additive and to decolourise glass that is coloured green by iron impurities. Manganese (IV) oxide is a powerful oxidising agent and is used in quantitative analysis. It is also used to make Fertilizers and ceramics.
- Manganese sulphate is used to make a fungicide.
1.5.6. Iron
Iron is a grey to black, odourless metal with density 7.874. It melts at 1535 oC and boils at 2750 oC.
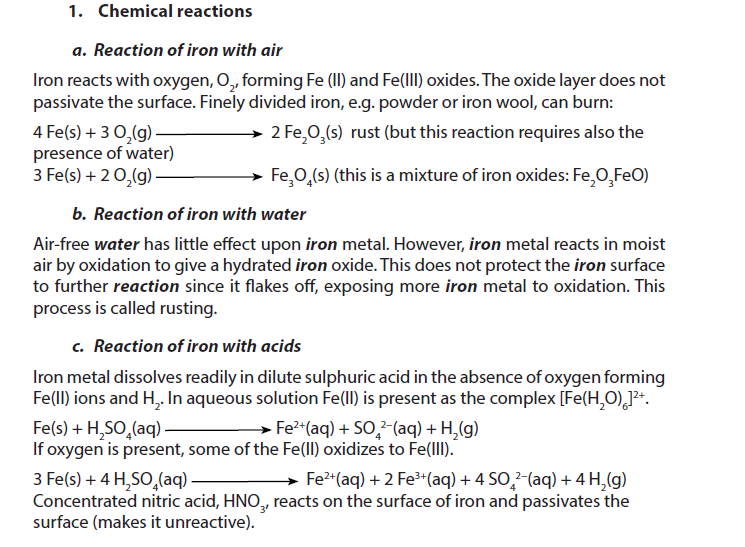

2. Uses
- Iron is an enigma – it rusts easily, yet it is the most important of all metals. 90% of all metal that is refined today is iron. Most is used to manufacture steel, used in civil engineering (reinforced concrete, girders etc) and in manufacturing.
- Alloy steels are carbon steels with other additives such as nickel, chromium, vanadium, tungsten and manganese. These are stronger and tougher than carbon steels and have a huge variety of applications including bridges, electricity pylons, bicycle chains, cutting tools and rifle barrels.
- Stainless steel is very resistant to corrosion. It contains at least 10.5% chromium. Other metals such as nickel, molybdenum, titanium and copper are added to enhance its strength and workability. It is used in architecture, bearings, cutlery, surgical instruments and jewellery.
- Cast iron contains 3–5% carbon. It is used for pipes, valves and pumps. It is not as tough as steel but it is cheaper.
- Magnets can be made of iron and its alloys and compounds.
- Iron catalysts are used in the Haber process for producing ammonia, and in the Fischer–Tropsch process for converting syngas (hydrogen and carbon monoxide) into liquid fuels.
- Iron plays an important role in the transfer of oxygen by hemoglobin. Each hemoglobin binds four iron atoms. Iron in hemoglobin binds with oxygen as it passes through the blood vessels in the lungs and releases it in the tissues.
1.5.7. Cobalt
Cobalt is a dark grey metal with a density of 8.90. It is insoluble in water at room temperature.
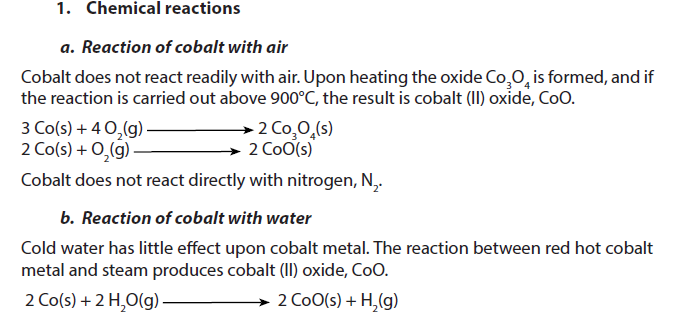
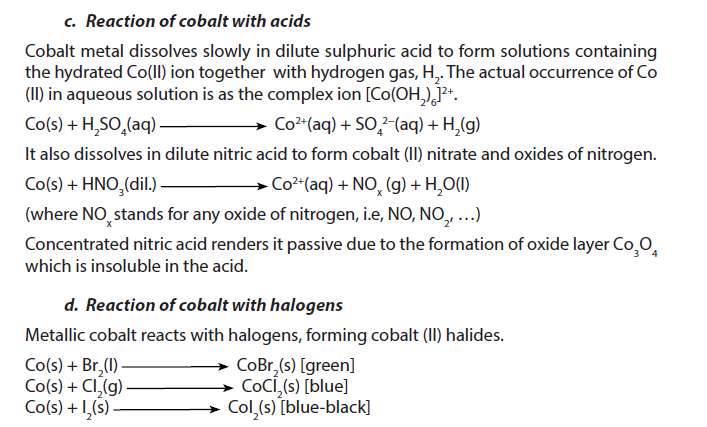
2. Uses
- Cobalt, like iron, can be magnetized and so is used to make magnets. It is alloyed with aluminium and nickel to make particularly powerful magnets.
- Other alloys of cobalt are used in jet turbines and gas turbine generators at high temperature.
- Cobalt metal is sometimes used in electroplating because of its attractive appearance, hardness and resistance to corrosion.
- Cobalt salts have been used for centuries to produce brilliant blue colours in paint, porcelain, glass, pottery and enamels.
- Cobalt is an essential trace element and found at the centre of the vitamin B12 (cobalmin, C63H88CoN14O14P). It contains a cobalt(III) ion and is necessary for the prevention of pernicious anaemia and the formation of red blood corpuscles, but it is involved many other functions too.
1.5.8. Nickel
Nickel is a grey solid metal with density of about 8.9. It melts at 1455 oC and boils at 2920 oC.
1. Chemical reactions
a. Reaction of nickel with air
Nickel does not react with oxygen, O2, at room temperature, under normal conditions. Finely divided nickel can burn in oxygen, forming nickel (II) oxide, NiO.


2. Uses
- Nickel resists corrosion and is used to plate other metals to protect them. It is, however, mainly used in making alloys such as stainless steel. Nichrome is an alloy of nickel and chromium with small amounts of silicon, manganese and iron. It resists corrosion, even when red hot, so is used in toasters and electric ovens. A copper-nickel alloy is commonly used in desalination plants, which convert seawater into fresh water. Nickel steel is used for armour plating. Other alloys of nickel are used in boat propeller shafts and turbine blades.
- Nickel is used in batteries, including rechargeable nickel-cadmium batteries and nickel-metal hydride batteries used in hybrid vehicles.
- Nickel has a long history of being used in coins. The US five-cent piece (known as a ‘nickel’) is 25% nickel and 75% copper.
- Finely divided nickel is used as a catalyst for hydrogenating vegetable oils. Adding nickel to glass gives it a green colour.
1.5.9. Copper
Copper is a light pink to red (shiny-reddish) metal of density 8.95 g/cm3. It melts at 1083 oC and boils at 2570 oC.
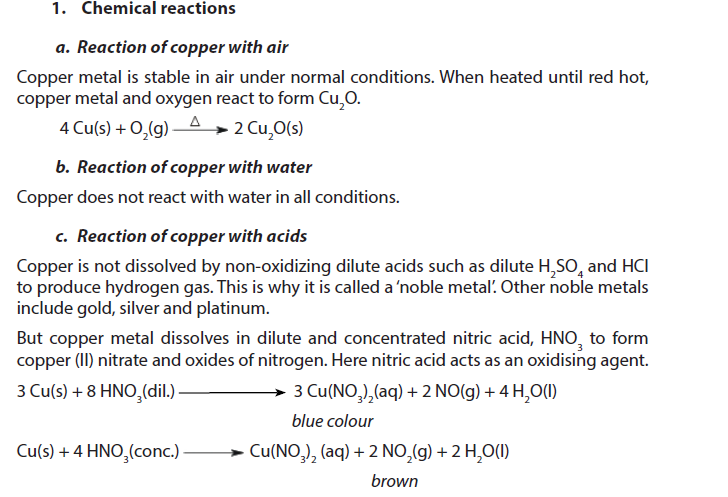
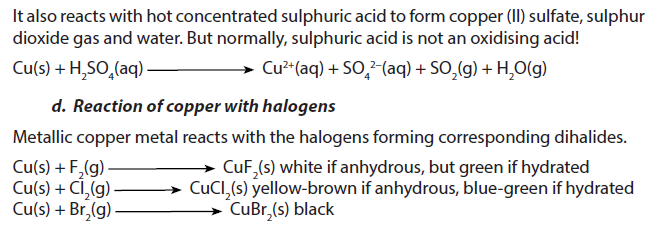
2. Uses
- Historically, copper was the first metal to be worked by people. The discovery that it could be hardened with a little tin to form the alloy bronze gave the name to the Bronze Age.
- Traditionally it has been one of the metals used to make coins, along with silver and gold. However, it is the most common of the three and therefore the least valued. All US coins are now copper alloys, and gun metals also contain copper.
- Most copper is used in electrical equipment such as wiring and motors. This is because it conducts both heat and electricity very well, and can be drawn into wires. It also has uses in construction (for example roofing and plumbing), and industrial machinery (such as heat exchangers).
- Copper sulphate is used widely as an agricultural poison and as an algaecide in water purification.
- Copper compounds, such as Fehling’s solution, are used in chemical tests for sugar detection.
- Copper helps in storing iron, is involved in production of pigments for colouring hair, skin and eyes.
1.5.10. Zinc
Zinc is a grey solid with a density of 7.14 g/cm3. It melts at 419.5 oC and boils at 907 oC.
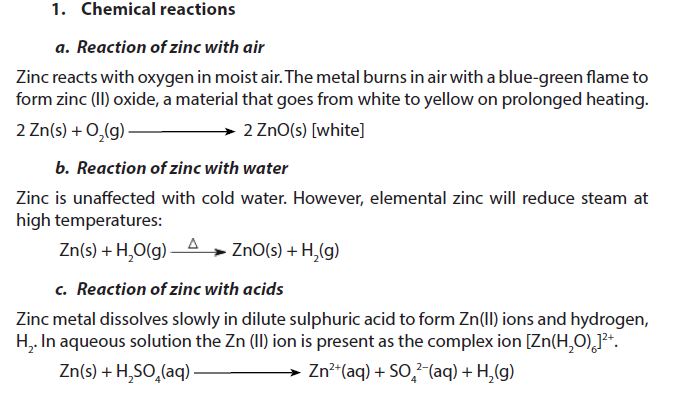
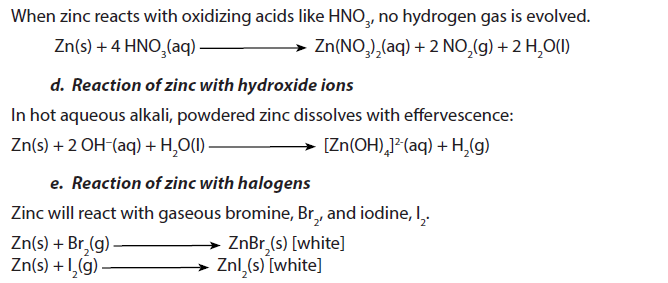
2. Uses
- Mostly, zinc is used to galvanise other metals, such as ironsheets (amabati), to prevent corrosion. Galvanised steel is used for car bodies, street lamp posts, safety barriers and suspension bridges.Many houses in Rwanda are covered by galvanized iron sheets (amabati).
- Large quantities of zinc are used to produce die-castings, which are important in the automobile, electrical and hardware industries.
- Zinc is also used in alloys such as brass, nickel silver and aluminium solder.
- Zinc oxide is widely used in the manufacture of many products such as paints, rubber, cosmetics, pharmaceuticals, plastics, inks, soaps, batteries, textiles and electrical equipment. Zinc sulphide is used in making luminous paints, fluorescent lights and x-ray screens.
- It is a component of insulin.


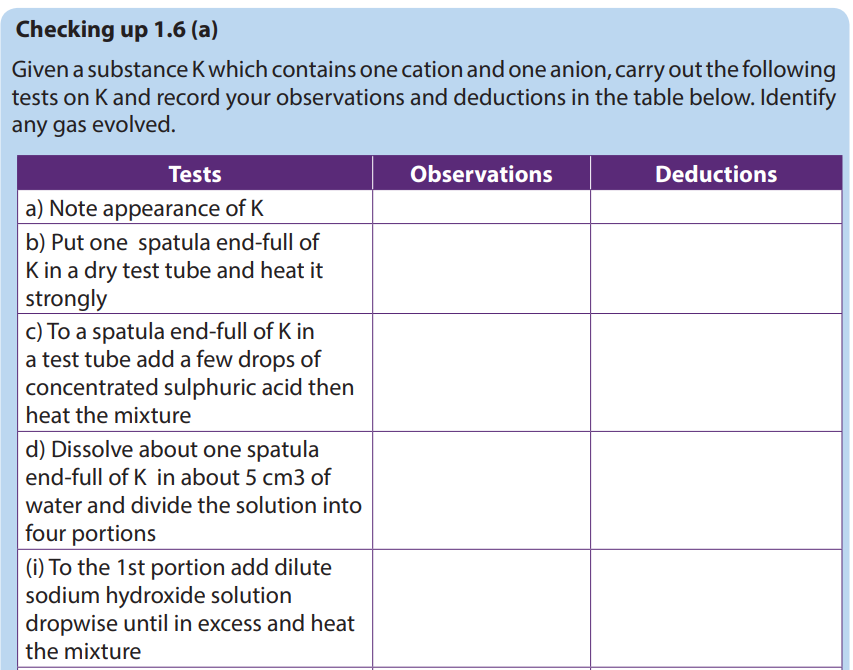
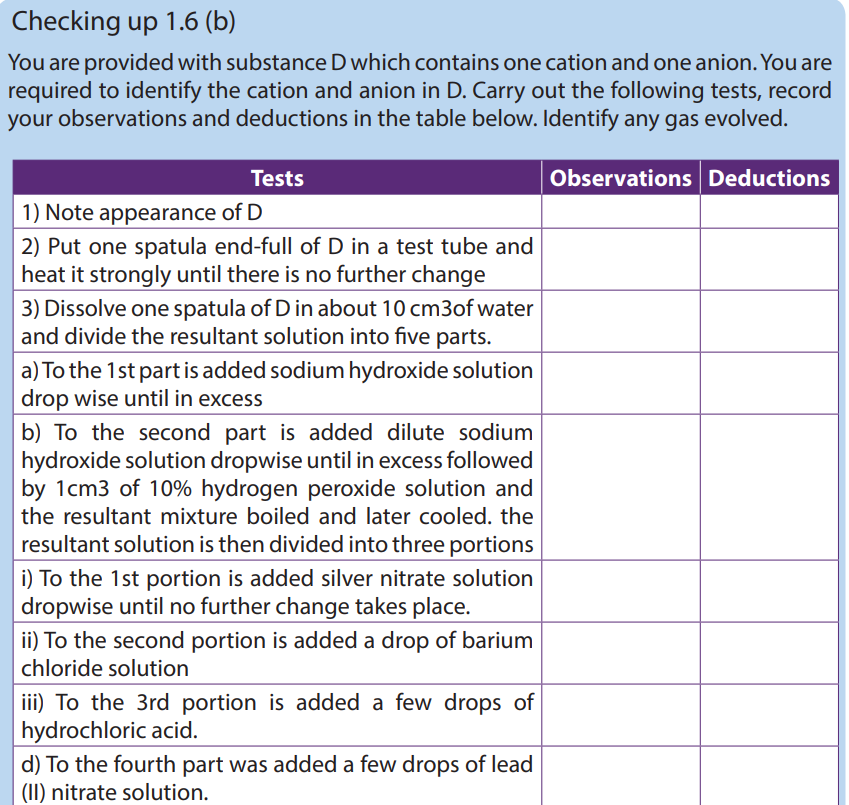
1.6. Identification of transition metal ions
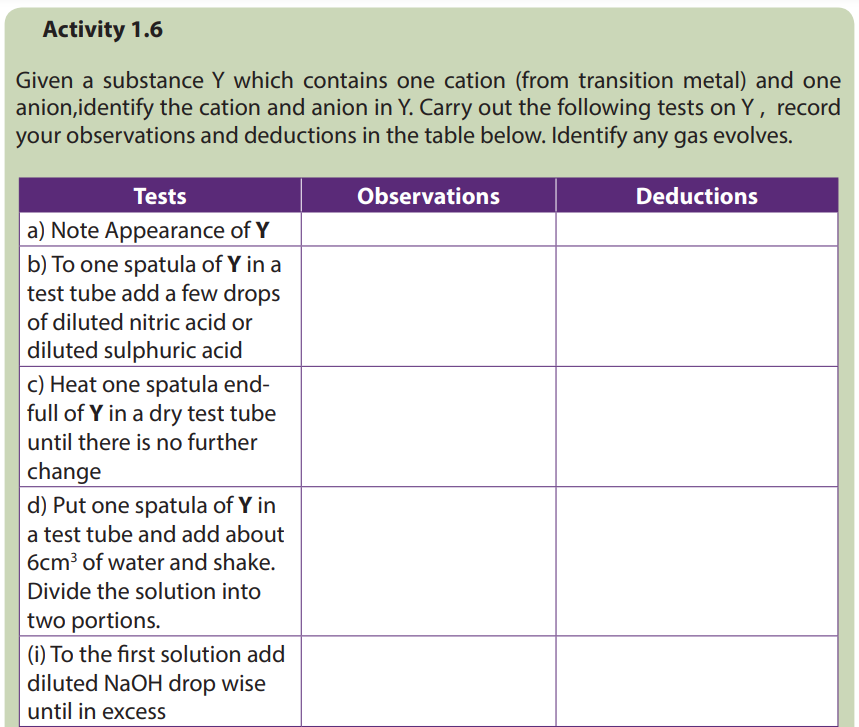

Different transition metals have different colors. Also, different charges, or cations of one transition metal can give different colors. Another factor is the natural of the ligand. The same cations of a transition metal can produce a different color depending on the ligand it binds to.
Many compounds containing transition metals have certain characteristic colours and thus, by observing a compound, we can not identify it.
- Appearance or colour of different solid compounds containing transition metals
Table 1.13: Colours of different solid compounds containing transition metals (first series)
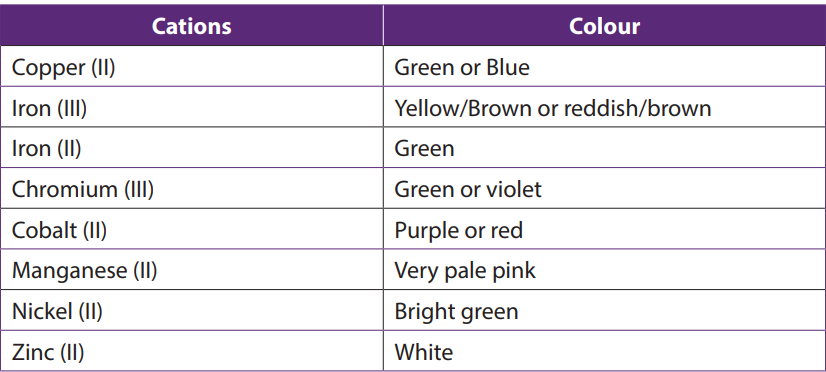
- Colours of aqueous solutions of some transition metal ions
In aqueous solutions where water molecules are the ligands, the colours of some metal ions observed are listed in the table below:
Table 1.14: Colours of different aqueous solutions containing some transition metals (first series)
- Action of heat on solid compounds containing transition metal ions
Table 1.15: Colours of different solid compounds containing transition metals (first series) due to action of heat.
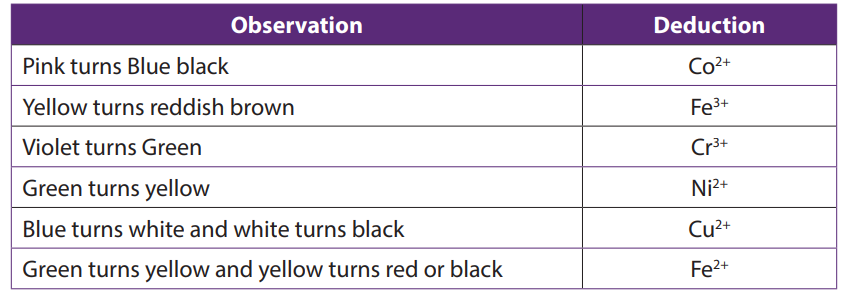
Note: On heating the following temporary colour changes may also occur:

Effect of aqueous sodium hydroxide and aqueous ammonia on solutions containing transition metal ions
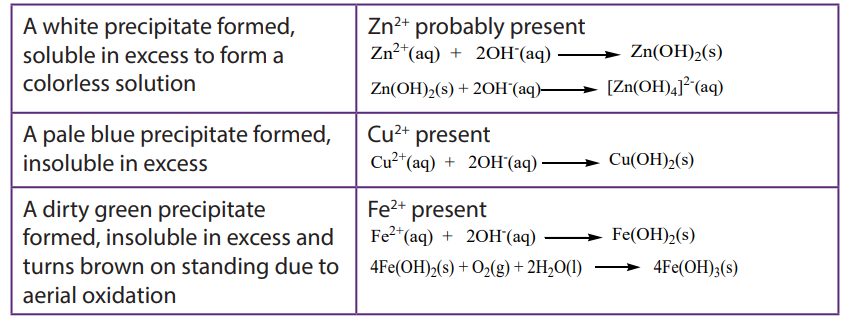
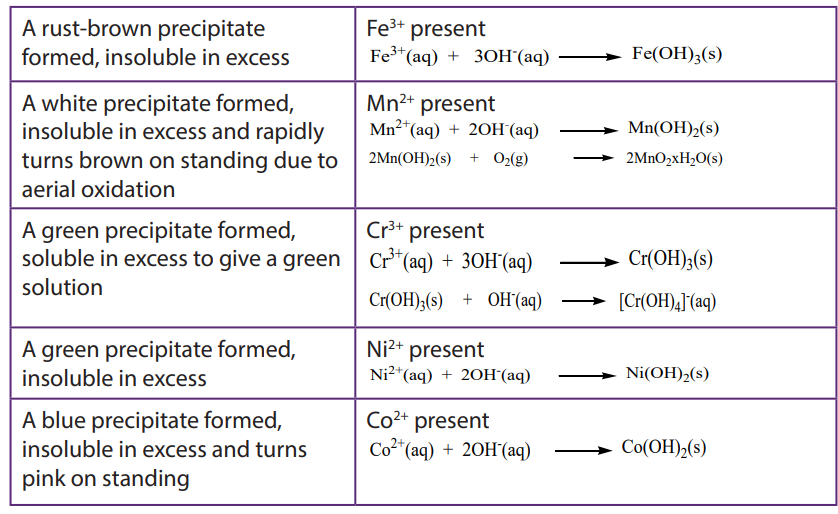
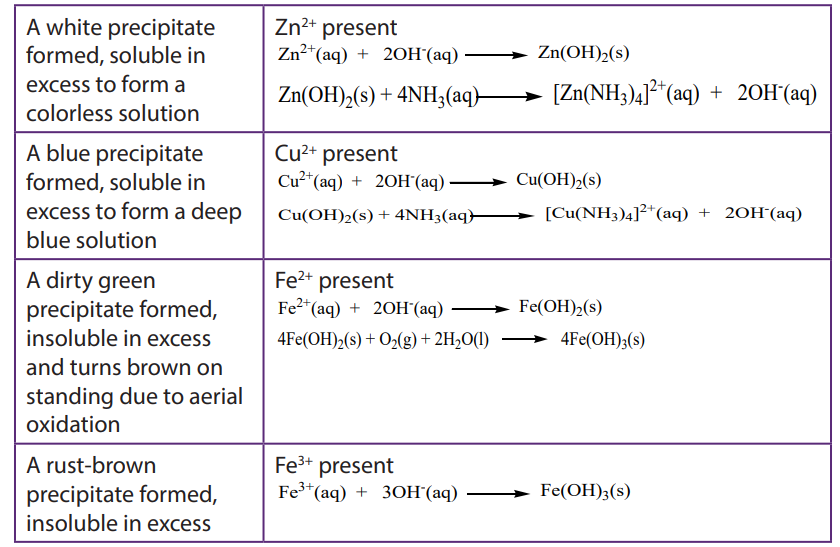
The hydroxides of transition metals are precipitated from solutions of the metal ions by the addition of hydroxide ions or ammonia. The colour of the precipitate can often be used to identify the metal present. The precipitates formed are gelatinous and often coloured and some form soluble complex ions with excess ammonia.
a. To about 1cm3 of the solution containing the positive ion (cation), add 2M aqueous sodium hydroxide dropwise until in excess
b. To about 1cm3 of the solution containing the positive ion (cation), add 2M aqueous ammonia dropwise until in excess
- Confirmatory tests for some transition metal ions
Confirmatory tests are the tests required to confirm the analysis. Generally, a confirmatory test is used after other tests have been carried out to isolate/identify the ion. In order to confirm the ion without any dought.
- Zinc ions
i. Addition of little solid ammonium chloride followed by disodium hydrogen phosphate solution to a solution of zinc ions gives a white precipitate. The precipitate dissolves in excess ammonia or dilute mineral acids
ii. Addition of potassium ferrocyanide solution to a solution of zinc ions gives a white precipitate.
Zn2+(aq) + [Zn(CN)6] - (aq) Zn2[Fe(CN)6](s)
2.Chromium ions
To a solution of chromium (III) ions, add excess aqueous sodium hydroxide followed by little hydrogen peroxide and boil the resultant mixture. A yellow solution of a chromate is formed.
2Cr3+(aq) + 10OH-(aq) + 3H2 O2 (aq) → 2CrO4 2-(aq) + 8H2 O(l)
Treatment of the yellow solution with:
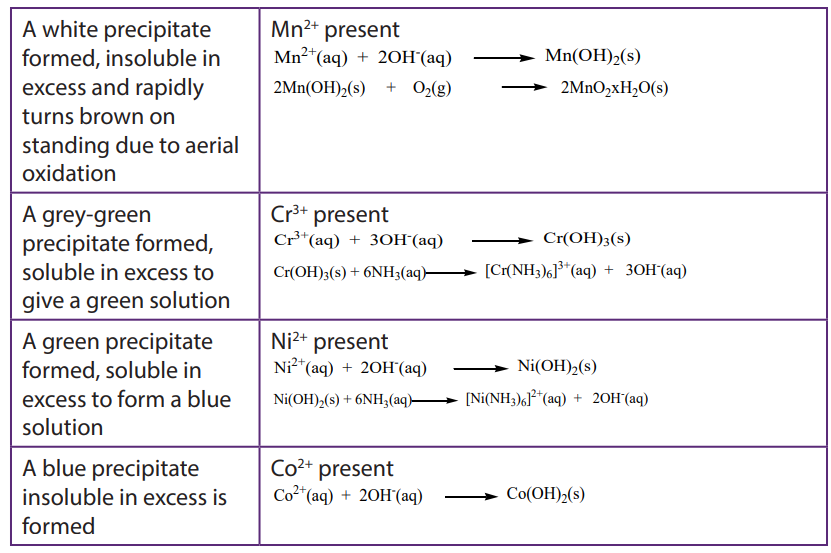
i. Lead (II) ethanoate or Lead(II)nitrate solution gives a yellow precipitate of Lead(II) chromate. Pb2+(aq) + CrO4 2-(aq) →PbCrO4(s)
ii. Barium nitrate (or chloride) solution gives a yellow precipitate of barium chromate.
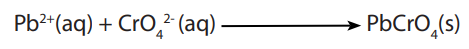
iii. Silver nitrate gives a brick red precipitate of silver chromate

iv. A little alcohol (for example, butan-1-ol) and dilute sulphuric acid , a blue color is formed in the alcohol layer. The blue color is due to unstable CrO5
c. Manganese (II) ions
To the solution of manganese (II) ions, add little concentrated nitric acid followed by little solid lead(IV) oxide or solid sodium bismuthate(V) and boil the mixture. A purple solution is formed due to MnO4 - ion
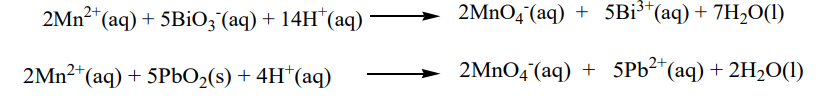
d. Iron (II) ions
i. Addition of potassium hexacyanoferrate (III) solution to a solution of iron (II) ions gives a dark blue precipitate .
ii. Addition of potassium thiocyanate or ammonium thiocyanate solution to a solution of iron (III) ions gives a blood red coloration.
f. Cobalt (II) ions
Addition of potassium thiocyanate or ammonium thiocyanate solution to a solution of cobalt(II) ions gives a blue colored product of potassium cobalt(II) tetrathiocyanate.

g. Nickel (II) ions
i. Addition of potassium cyanide solution gives a yellow-green precipitate of Nickel(II) cyanide. The precipitate dissolves in excess reagent to form a dark yellow solution tetracyanonickel (II) ion.
ii. Addition of aqueous ammonia followed by 2 to 3 drops of dimethylglyoxime solution to a solution of nickel (II) ions gives a red precipitate. The formation of this precipitate may sometimes require that the solution mixture would be warmed.
h. Copper (II) ions
In addition to use of aqueous ammonia, the copper(II) ions can be confirmed by addition of the following reagents to an aqueous solution of copper(II) ions:
i. Potassium iodide solution: A white precipitate of copper (I) iodide stained brown with free iodine.
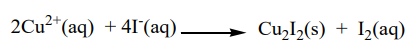
Brown color fades on addition of sodium thiosulphate solution due to the reaction below:

ii. Potassium hexacyanoferrate (II) solution: A brown precipitate is formed.
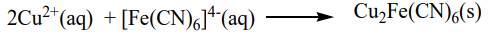
iii. Potassium (or ammonium) cyanide solution: A yellow precipitate is formed. The precipitate rapidly turns white.
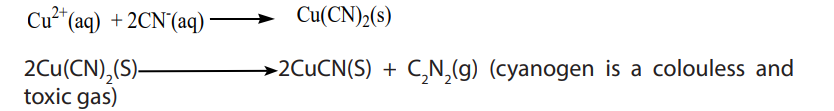
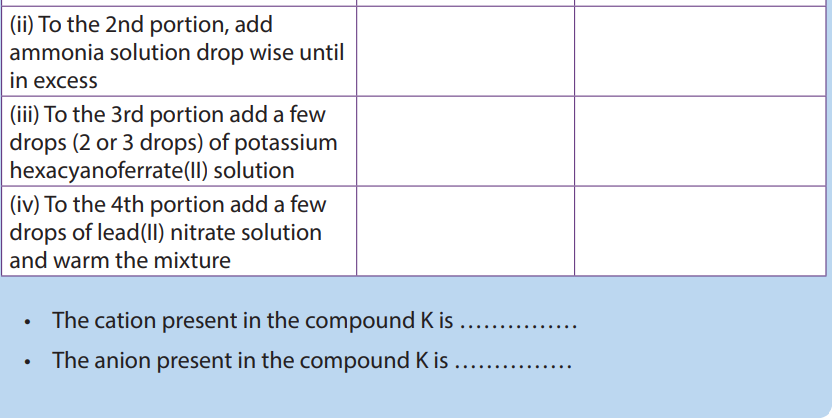

Checking up 1.6 (c)
Aqueous sodium hydroxide is added separately to solutions of salts of the transition metals A, B and C. Identify A, B and C from the following observations
A: The white precipitate which appears is soluble in an excess of aqueous sodium hydroxide and also in aqueous ammonia.
B: The blue precipitate which appears is insoluble in an excess of aqueous sodium hydroxide but dissolves in aqueous ammonia to form a deep blue solution.
C: The green precipitate which appears is insoluble in an excess of aqueous sodium hydroxide and also in aqueous ammonia.
END UNIT ASSESSMENT
a. Multiple choice questions: Write the Roman number corresponding to the correct answer.
1. Which of the following elements is not a transition metal?
i. Copper
ii. Nickel
iii. Iron

iv. Magnesium
2. Which of the following complexes is linear?
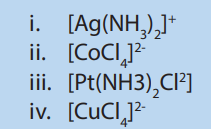
5. Which of the following complexes forms both geometric and optical isomers?
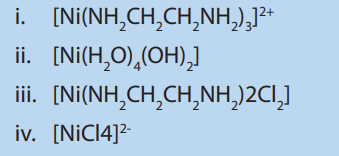
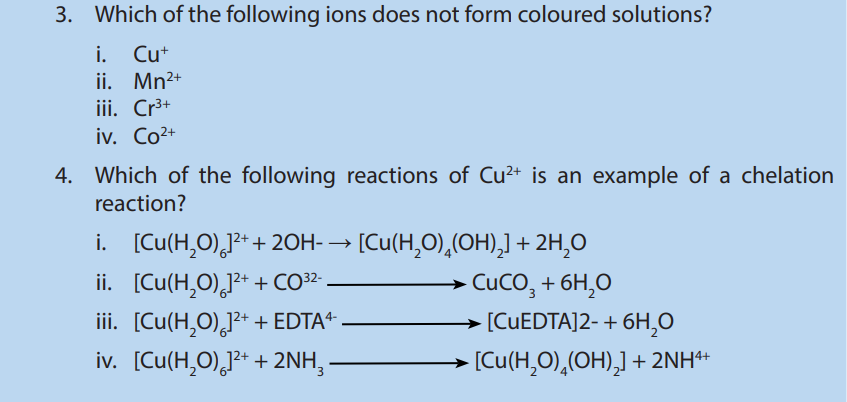
6. Which of the following properties is not a characteristic of transition metal ions
i. Variable oxidation states
ii. Form coloured solutions
iii. Act as catalysts
iv. Are diamagnetic
7. Which complex ion shows optical isomerism and geometrical isomerism?
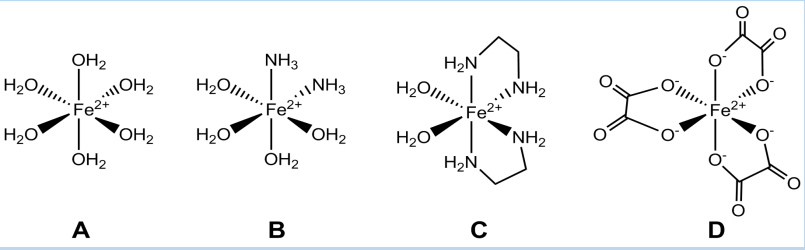
8. All the following complex ions contain metal ions. Overall charges are not shown.
Which complex ion has no overall charge? The charge of the central metal ion is given in brackets next to the formula of the complex
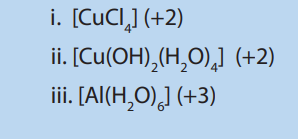
9. Which atom(s) among the following transition elements have only 1 electron in 4s orbital? Chromium
i. Cobalt
ii. Scandium
iii. Zinc
b. Open Questions 1.
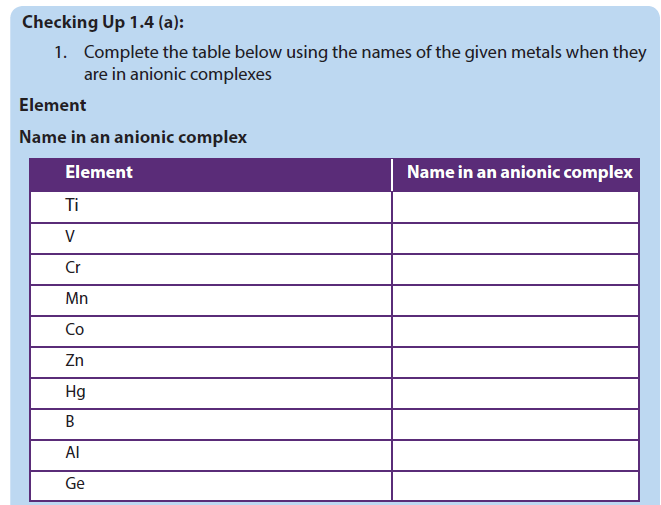
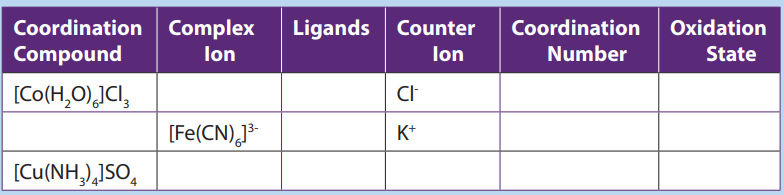
Complete the following table:
2. What is the characteristic of electron configurations of transition metals?
3. Which electrons, 3d or 4s, have the lowest ionization energies in a transition metal?
4. a. Name any three transition metals that are essential to the biological system.
b. Why do you think transition metals form coordination compounds that have covalent bonds?
5. Name the following coordination compounds using systematic nomenclature.

6. a. (i) What is meant by the term co-ordinate bond?
(ii) Explain why co-ordinate bonds can be formed between transition metal ions and water molecules.
b. What name is given to any ligand that can form two co-ordinate bonds to one metal ion? Give an example of such a ligand.
7. In order to determine the concentration of a solution of cobalt(II) chloride, a 25.0 cm3 sample was titrated with a 0.0168 M solution of EDTA4–; 36.2 cm3 were required to reach the end-point. The reaction occurring in the titration is:

a. What type of ligand is EDTA4–?
b. Calculate the molar concentration of the cobalt (II) chloride solution.
8. The ethanedioate (oxalate) ion, 2− C2O4 , acts as a bidentate ligand. This ligand forms an octahedral complex with iron (III) ions.
a. Deduce the formula of this complex and draw its structure showing all the coordinate bonds present.
b. Give the name of a naturally-occurring in human body complex compound which contains iron
c. What is theimportant function of this complex compound?
9. The compound [Co(NH3 ) 4 Cl2 ]Cl contains both chloride ions and ammonia molecules as ligands.
a. State why chloride ions and ammonia molecules can behave as ligands.
b. What is the oxidation state and the co-ordination number of cobalt in this complex compound?

b. Name and give the formula of an ammonia complex used to distinguish between aldehydes and ketones
11. Chloride ions form the tetrahedral complex ion [AlCl4 ] – but fluoride ions form the octahedral complex ion [AlF6 ] 3-. Suggest a reason for this difference
