UNIT 9: AMINES AND AMINO ACIDS
Key unit competency:
The learner should be able to relate the chemical nature of the amines andaminoacids to their properties, uses and reactivity.
Learning objectives
At the end of this unit, the students will be able to:
• Explain the zwitterion forms in the solution of different pH.
• Explain the isoelectric point in amino acids.
• Describe the physical properties and uses of amines.
• Describe the preparation methods of the amines.
• Describe the reactions of amino acids and amines with other substances.
• Classify amines as primary, secondary and tertiary amines.
• Compare and contrast the physical properties of the amino acids to those of
carboxylic acids and amines.• Test the presence of amines and amino acids in the solution.
Introductory activity
Read the text below, observe the accompanying images and answer the
questions.
The cell is the basic structural, functional, and biological unit of all known living
organisms. A cell is the smallest unit of life. All cells are made up of Inorganic
compounds and Organic compounds. Inorganic compounds are compounds
that do not contain carbon atoms except carbonates ,carbides, carbon oxides
,carbonic acid while organic compounds are compounds that mainly contain
carbon (C) and hydrogen (H) atoms, and eventually other elements such as
oxygen (O) and nitrogen (N) in biological macromolecules that include thecarbohydrates, lipids, proteins and nucleic acids.
Proteins are macromolecules consisting of one or more long chains of amino
acid residues. Proteins differ from one another primarily in their sequence of
amino acids which is dictated by the nucleotide sequence of their genes,
and which usually results in protein folding into a specific three-dimensional
structure.
That determines its activity. Nucleic acids [Deoxyribonucleic acid (DNA) and
ribonucleic acid (RNA)] alongside proteins, lipids and complex carbohydrates
(polysaccharides), are one of the four major types of macromolecules that are
essential for all known forms of life. They are a thread-like chain of nucleotides
carrying the genetic instructions used in the growth, development, functioning
and reproduction of all known living organisms and many viruses.
The image below shows the partial molecular structure of DNA (A) and ofproteins (B, C).
1. Identify the common point between DNA and proteins. Explain your
answer.
2. The terminology “amino acid” is found in the text and image above.
According to you, what is it, and what is its role in living organisms?
3. If protein molecules are made essentially of Carbon, Hydrogen, Oxygen,
Nitrogen and amino acid side chains (figure C),
a. What kind of bonds do you expect to see in those structures?b. What kind of reactions do you expect in those molecules?
9.1. Nomenclature and classification of amines
Activity 9.1
Pentan-2-ol, butan-1-ol and 2-methylpropan-2-ol are alcohols.
1. For each one:
a. give its molecular formula
b. give its structural formula
c. give its displayed formula
d. give its skeletal formula
e. State whether it is a primary, secondary or tertiary alcohol.
2. Give the general formula that is used to represent alcohols.
3. Two of the alcohols in this question are isomers of each other. Identifywhich two and identify the type of isomerism they show.
4. Name the alcohol whose structural formula is

Amines are one of organic compounds containing nitrogen. They are one of the
most important classes of organic compounds which are obtained by replacing
one or more hydrogen atoms by an alkyl or aryl group in a molecule of ammonia They are present in vitamins, proteins, hormones, etc. They are extensively
They are present in vitamins, proteins, hormones, etc. They are extensively used in the manufacturing of many drugs and detergents.
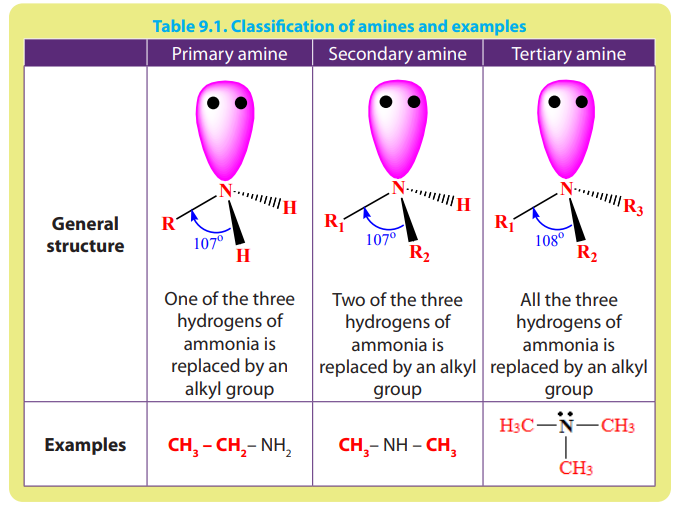
9.1.1 Classification of amines
As per VSEPR theory, nitrogen present in amines is sp3
hybridized and due to the
presence of lone pair, it is pyramidal in shape instead of tetrahedral shape which is a
general structure for most hybridized molecules. Each of the three
hybridized molecules. Each of the three 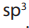
hybridized orbitals of nitrogen overlap with orbitals of hydrogen or carbon depending
upon the configuration of amines. Due to the presence of lone pair, the C-N-H angle in amines
is less than 109 degrees which is characteristic angle of tetrahedral geometry. Theangle in amines is near about 108 degrees.
The functional group of amines is Depending upon the number of
Depending upon the number of
hydrogen atoms that are replaced by an alkyl or aryl group in ammonia, amines areclassified as primary, secondary and tertiary (Table 9.1).
9.1.2 Nomenclature of amines
In organic chemistry, the names of the compounds are given according to the
guidelines provided by IUPAC. In this regards, amines are named by ending with –
amine. The IUPAC system names amine functions as substituents on the largest alkylgroup.
If the amine is secondary and has two alkyl groups attached to the nitrogen, then
each chain is named and the smaller alkyl group is preceded by an –N which playsthe same role as a number in positioning a side alkyl chain.
N-methylpropylamine is the common name and N-methyl-1-aminopropane
is the IUPAC name.
If in the common naming the lengths of the chain are the same, an –N is not usedas shown in the following example:
 (Diethylamine is the common name which does not
(Diethylamine is the common name which does not
contain N because the chains have the same length and N-ethylethanamine isthe IUPAC.
case of a tertiary amine, the use of N is applied at each alkyl side group.
The following are examples of primary, secondary and tertiary amines (Table 9.2)
with their corresponding names using IUPAC of common name.
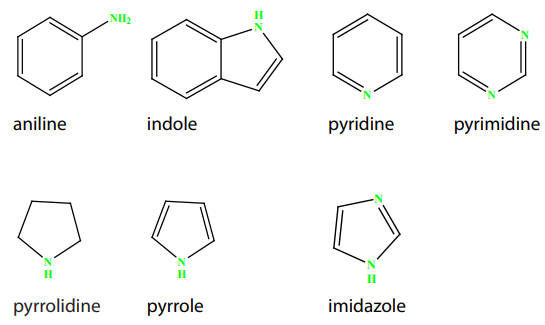
Aliphatic and Aromatic and heterocyclic amines are named after the groups
surrounding the nitrogen + amine.
In case of heterocyclic amines, there is a systematic nomenclature of these
compounds as indicated in the following examples.
Checking up 9.1
1. Classify the following as primary, secondary and tertiary amines.
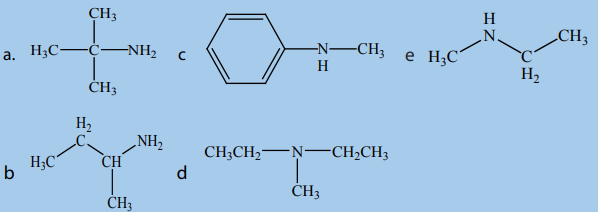
2. For each compound below, provide the respective IUPAC name.
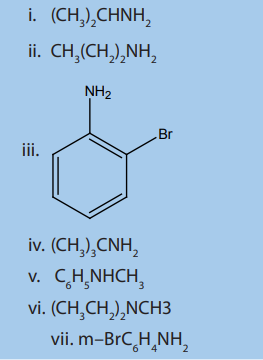
3. Write IUPAC names of the following compounds and classify them into
primary, secondary and tertiary amines.
9.2 Physical properties, natural occurrences and uses of amines.
Activity 9.2
Calculate the molecular mass of the given products and justify the differencebetween boiling points of amines, alkanes and alcohols

9.2.1 Physical properties of amines
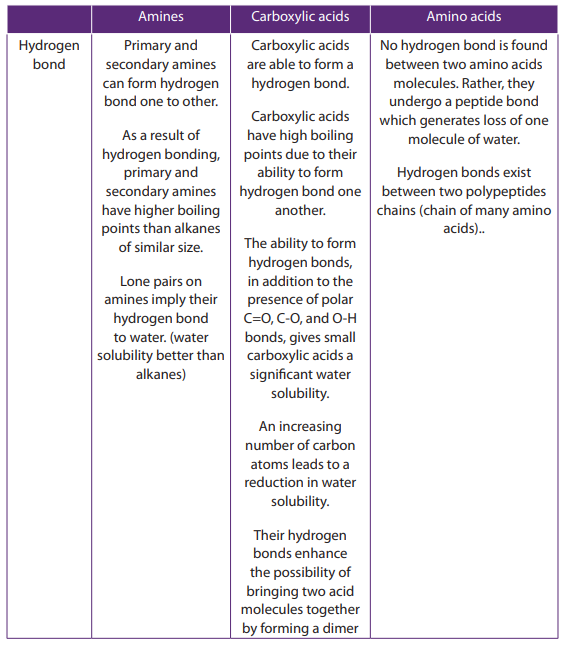
Primary and secondary amines can form a hydrogen bond to each other (as shown
in the Figure 9.1 below where two molecules of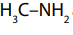 are bonded together).
are bonded together).
Because Nitrogen is less electronegative than oxygen, the N—H bond is not quite
as polar as the O—H bond reason why hydrogen bonds in amines are not strong
than these alcohol molecules. Therefore primary and secondary amines have lowerboiling points than alcohols of similar molecular weight.
Tertiary amines do not bond to each other by hydrogen bond and they have boiling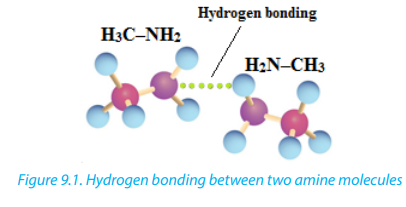
points similar to those of hydrocarbons of the same molecular weight. However,
primary, secondary and tertiary amines form hydrogen bond with water and amineswith low-molecular weight are generally soluble in water.
Generally the boiling point of amines increases as the molecular weight increase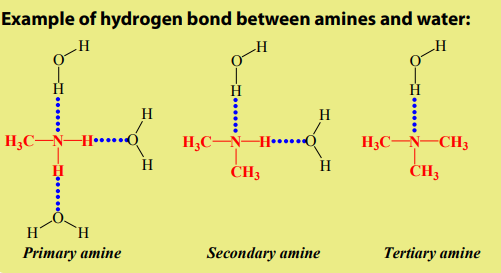
and they boil at higher temperatures than alkanes but at lower temperatures than
alcohols of comparable molar mass.
The amines are soluble in organic solvent and the solubility decreases as the
molecular weight increases. The Table 9.3 summarizes some physical properties ofsome amines.
Table 9.3: Physical pproperties of some amines compared to someoxygen containing compounds.
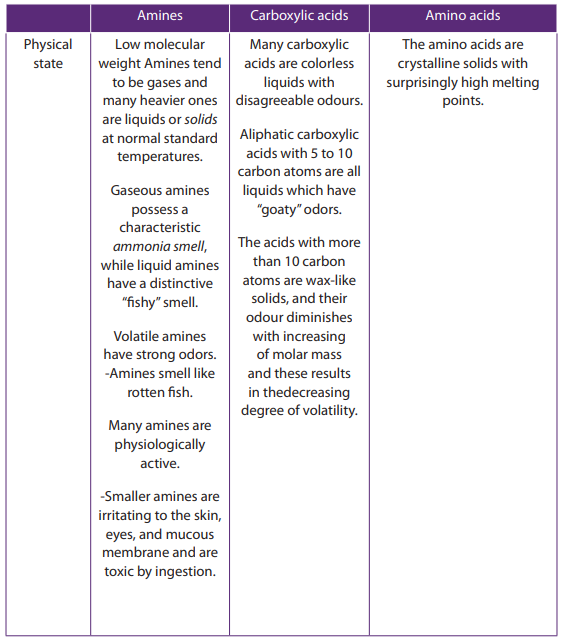
Amines tend to be gases at low molecular weight (e.g. up to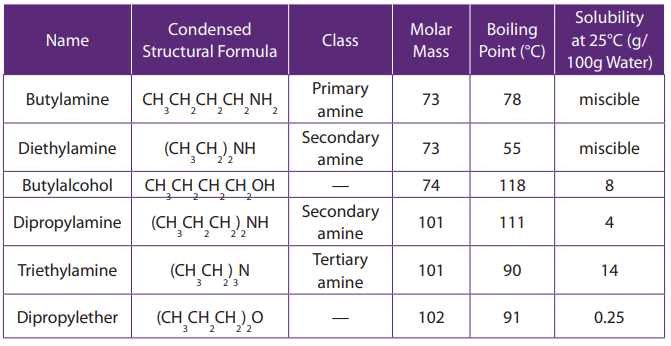
 trimethylamine)
trimethylamine)
and the heavier ones are liquids at room temperature. In fact, methyl, dimethyl,
trimethyl, and ethyl -amines are gases under standard conditions. Most common
alkyl amines are liquids, and high molecular weight amines are, quite naturally, solids
at standard temperatures. Additionally, gaseous amines possess a characteristic
ammonia smell, while liquid amines have a distinctive “fishy” smell: higher molecular
weight amines often smell like rotting fish, and are often found in decaying
animal tissues. Cadaverine and putrescine
and putrescine  are some
are some
of the examples. The lower molecular- weight amines with up to about five carbon
atoms are soluble in water. The higher-molecular-weight amines that are insolublein water will dissolve in acid to form ionic amine salts.
9.2.2 Natural occurrence of amines and their usage
Natural amines occur in proteins, vitamins, hormones, etc. and they are
also prepared synthetically to make polymers, drugs and dyes.
Amines can be used as dyes (colorants) or as drugs: Primary aromatic amines are
used as a starting material for the manufacture of azo dyes. They react with nitrous
(II) acid to form diazonium salt which can undergo a coupling reaction in order to
form an azo compound. As azo compounds are highly coloured, they are widelyused in dyeing industries. Examples include Methyl orange and Direct brown 138.
In medicine, amines can be used as drugs.
• Chlorpheniramine is an antihistamine that helps to relief allergic disorders
due to cold, hay fever, itchy skin, insect bites and stings.
• Diphenhydramine is the common antihistamine.
• Chlorpromazine is a tranquillizer that anaesthetizes without inducing sleep.
It is used to relieve anxiety, excitement, restlessness or even mental disorder.
• Acetaminophen is also known as paracetamol or p-acetaminophenol, it is
an analgesic that relieves pains such as headaches. It is believed to be less
corrosive to the stomach and is an alternative to aspirin.
Amines are widely encountered in biological and pharmacological studies. Some
important examples are the 2-phenylethylamines, some vitamins, antihistamines,
tranquilizers, and neurotransmitters (noradrenaline, dopamine and serotonin)which act at neuromuscular synapses.
Checking up 9.2
1. Which compound of each pair has the higher boiling point? Explain.a. butylamine or pentane
2. Between the two compounds
 explain which
explain which
is more soluble in water.
9.3 Preparation of amines.
Activity 9.3
Ammonia molecules react with water molecules. Write the detailed
molecules react with water molecules. Write the detailed chemical equation of that reaction.
The amines can be prepared based on the following reactions:
9.3.1 Alkylation of ammonia
Primary amines can be synthesized by alkylation of ammonia. The reaction involves
nucleophilic substitution of an alkyl halide when ammonia is used as nucleophilicagent. The reaction is carried out in a sealed tube at 100 °C or 373 K.

9.3.2. Gabriel phthalimide synthesis
This procedure is used for the preparation of primary amines. Phthalimide on
treatment with ethanolic potassium hydroxide forms potassium salt of phthalimide
which on heating with alkyl halide followed by alkaline hydrolysis produces the
corresponding primary amine. However, primary aromatic amines cannot be
prepared by Gabriel phthalimide synthesis because aryl halides do not undergonucleophilic substitution with the anion formed by phthalimide.

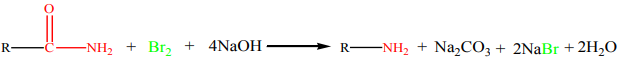
9.3.3. Hoffmann bromamide degradation reaction
Hoffmann developed a method for the preparation of primary amines by treating an
amide with bromine in an aqueous or ethanolic solution of sodium hydroxide. This
is a degradation reaction with migration of an alkyl or aryl group taking place from
carbonyl carbon of the amide to the nitrogen atom.
The reaction is valid for the preparation of primary amines only, and it yieldsuncontaminated compound with other amines.
9.3.4 Reduction of amides
Similarly to reduction of amides, lithium aluminium hydride reduces
reduces amides to amines.
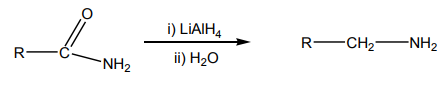
9.3.5 Reduction of nitriles
Nitriles are reduced to amines using hydrogen in the presence of a nickel catalyst,
although acidic or alkaline conditions should not be used to avoid the possible
hydrolysis of the -CN group. is more commonly employed for the reduction of
is more commonly employed for the reduction of nitriles on the laboratory scale.

9.3.6 Reduction of nitro compounds
Nitro compounds are reduced to amines by passing hydrogen gas in the presence
of finely divided nickel, palladium or platinum and also by reduction with metals
in acidic medium. Nitroalkanes can also be similarly reduced to the correspondingalkanamines.
Reduction with iron scrap and hydrochloric acid is preferred because
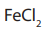
formed gets hydrolysed to release hydrochloric acid during the reaction.
Thus, only a small amount of hydrochloric acid is required to initiate the reaction.Checking up 9.3
1. Give two reagents that could be used to synthesize the followingreaction.
2.Discuss the conditions for using each of the reagents of your choice.
3.Write chemical equations for the following reactions:
a. Reaction of ethanolic
b. Ammonolysis of benzoyl chloride and reaction of amine so formed withtwo moles of
9.4. Chemical properties of amines
Activity 9.4
Experiment: In a solution of ethylamine at room temperature (A), add dilute
hydrochloric acid (B). After a while (C), add excess of sodium hydroxide in the
solution (D) to obtain the solution E.
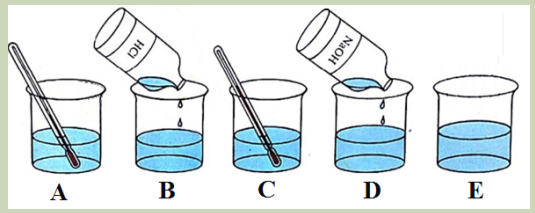
Explain:
1. What evidence is there for a chemical reaction between ethylamine and
hydrochloric acid?
2. Why does the smell of ethylamine disappear when hydrochloric acid is added?
3. Why does the smell reappear when sodium hydroxide is added?Difference in electronegativity between nitrogen and hydrogen atoms and the
presence of unshared pair of electrons over the nitrogen atom makes amines
reactive. The number of hydrogen atoms attached to nitrogen atom also is involved
in the reaction of amines; that is why the reactivity of amines differ in many reactions.
Amines behave as nucleophiles due to the presence of unshared electron pair.
The chemical properties of amines are summarized in the reactions below.
9.4.1 Reactions of amines diluted with acids
Amines, like ammonia, are bases. Being basic in nature, they react with acids toform salts.
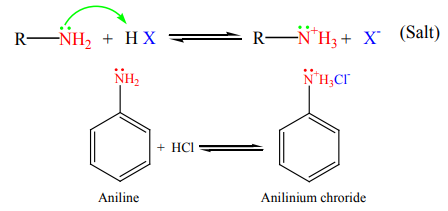
Amine salts on treatment with a base like NaOH, regenerate the parent amine.

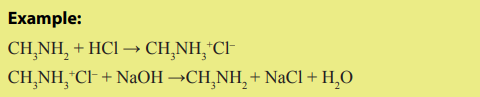
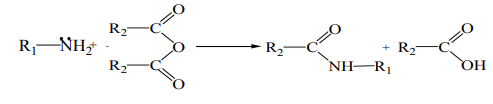
9.4.2 Reactions of amines (alkylation, acylation, and sulfonation)
Acyl chlorides and acid anhydrides react with primary and secondary amines to
form amides. Tertiary amines cannot be acylated due to the absence of a replaceablehydrogen atom.

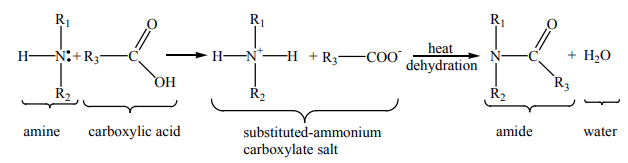
9.4.3. Reaction with carboxylic acid
Because amines are basic, they neutralize carboxylic acids to form the corresponding
ammonium carboxylate salts. Upon heating at 200°C, the primary and secondaryamine salts dehydrate to form the corresponding amides.
9.4.4. Reaction with nitrous acid
Nitrous acid,
 is unstable. It is produced indirectly using a mixture of
is unstable. It is produced indirectly using a mixture of 
and a strong acid such as
 in diluted solution. Primary aliphatic amines react
in diluted solution. Primary aliphatic amines react with nitrous acid to produce a very unstable diazonium salts which spontaneously
decomposes by losing
 to form a carbenium ion. Further, the carbonium ion is
to form a carbenium ion. Further, the carbonium ion is used to produce a mixture of alkenes, alkanols or alkyl halides, with alkanols as
major product said above.

Primary aromatic amines, such as aniline (phenylamine) forms a more stable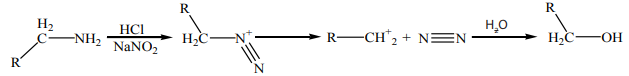
diazonium ion at Above 5°C, it will decompose to give phenol and
Above 5°C, it will decompose to give phenol and 
Diazonium salts can be isolated in the crystalline form but are usually used in solutionand immediately after preparation, due to its rapid decomposition.

9.4.5. Reactions with ketones and aldehydes
Primary amines react with carbonyl compounds to form imines. Specifically,
aldehydes become aldimines, and ketones become ketimines. In the case offormaldehyde (R’ = H), the imine products are typically cyclic trimers.
Secondary amines react with ketones and aldehydes to form enamines. An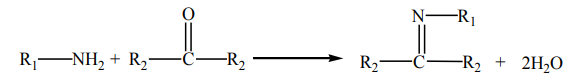
enamine contains a C=C double bond, where the second C is singly bonded to N aspart of an amine ligand.
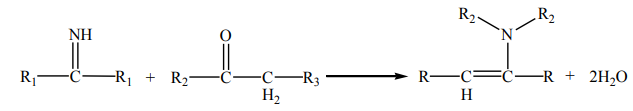
9.4.6. Neutralization reactions
Tertiary amines react with strong acids such as hydroiodic acid (HI), hydrobromic
react with strong acids such as hydroiodic acid (HI), hydrobromic
acid (HBr) and hydrochloric acid (HCl) to give ammonium salts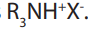
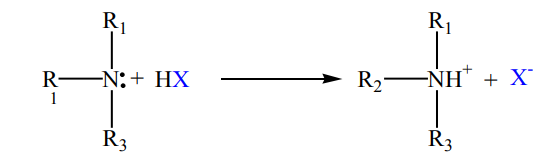
Checking up 9.4
1. Draw the structures of all amines of molecular formula . Classify
. Classify
them as primary, secondary and tertiary amines
2. a. Write the equation for the reaction which happens when dimethylamine,
 reacts with water.
reacts with water.
b. Write the formula of the product of the reaction between trimethylamine
gas, and hydrogen chloride gas, showing the essential details of
and hydrogen chloride gas, showing the essential details of
its structure.
3.Nitrous acid is unstable and has to be produced in the same test tube as the
reaction is happening in. If you were to test an amine with nitrous acid, howwould you do it?
4.This question is about the reactions between amines and halogenoalkanes
(alkylhalides).
The reaction between bromoethane and ethylamine produces a complicated
mixture of products, but the first to be formed are given by the equationsbelow:

Describe in words what is happening.
These next three questions are deliberately made uncooperative so that you
can only do them by understanding what you have read, and not just learning
it. Take your time over them.
5. Write the formulae for the corresponding products of the reaction if you started
with methylamine rather than ethylamine, but still reacted it with bromoethane.
6. If you started with a secondary amine such as dimethylamine, you would
initially get a tertiary amine and its salt in the mixture. Write the formulae of
these products if you reacted dimethylamine with bromoethane.
7. Draw the structure of the product that you would get if you reacted the tertiaryamine trimethylamine with bromoethane.
9.5. General structure of amino acids and some commonexamples
Activity 9.5
Amines are molecules that have as general formula, while carboxylic
while carboxylic
acids have as general formula, R–COOH. Predict a general structure (skeletal
formula) of a molecule that contains an amino group and a carboxyl group onan aliphatic chain.
9.5.1. General structure of amino acids
Amino acids are organic compounds containing amine and carboxyl (-COOH)
and carboxyl (-COOH)
functional groups, along with a side chain (R group) specific to each amino acid.
The key elements of an amino acid are carbon (C), hydrogen (H), oxygen (O), and
nitrogen (N). About 500 naturally occurring amino acids are known.
The general structure of amino acid is shown by the functional group and
a carboxylic acid group (-COOH) attached to the same carbon and they are calledα-amino acids.
The R group is the part of the amino acid that can vary in different amino acids. It
can be a hydrogen (in that case, the amino acid is called Glycine) or a
group (Alanine) or other radicals.
9.5.2. Common Amino Acids
Among the 500 known amino acids, there are 20 important α-amino acids, as shown
in the Table 9.4 below. Each amino acid has a common name. You will notice that
the names in common used for amino acids are not descriptive of their structural
formulas; but at least they have the advantage of being shorter than the systematic
names. The abbreviations (Gly, Glu, …) that are listed in table below, are particularly
useful in designating the sequences of amino acids in proteins and peptides.
Table 9.4: Common amino acids and their formulas [adapted from (Chang, 2005) and(Schmitz, 2018)]




The first amino acid to be isolated was asparagine in 1806. It was obtained from
protein found in asparagus juice (hence the name). Glycine, the major amino acid
found in gelatin, was named for its sweet taste (Greek glykys, meaning “sweet”). In
some cases an amino acid found in a protein is actually a derivative of one of thecommon 20 amino acids.
Checking up 9.5
1. Write the side chain of each amino acid.
a. serine
b. argininec. phenylalanine
2. Draw the structure for each amino acid.
a. alanine
b. cysteine
c. histidine
3. Identify an amino acid whose side chain contains:
a. amide functional group.
b. aromatic ring.c. carboxyl group.
9.6. Comparison of physical properties of amino acids tothose of carboxylic acids and amines
Activity 9.6
By using examples, distinguish amines, carboxylic acids and amino acids (indifferent states: gas, liquid, solid) based on their characteristic odour.
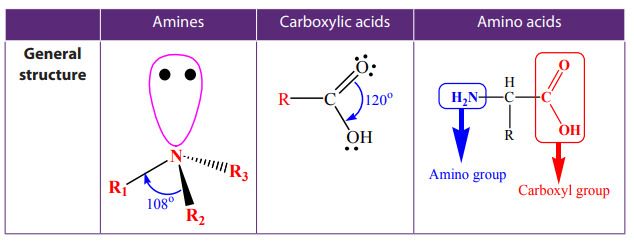
The amino acids, carboxylic acids and amines have different functional groups;
this is the base of their different physical properties as shown in the Table 9.5.Table 9.5. Comparison of physical properties of amines, carboxylic acids and amino
acids.

Checking up 9.6
Discuss the solubility of amino acids, referring on the solubility of amines andcarboxylic acids
9.7. Chemical properties of amino acids
Activity 9
A and B are organic products formed from the reactions in a) and b)respectively, propose a mechanism of those reactions and identify A and B.
The reactivity of amino acids involves the reactions of both amines and carboxylic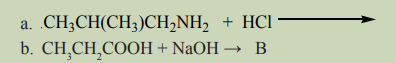
acids. Some of these reactions are given below.
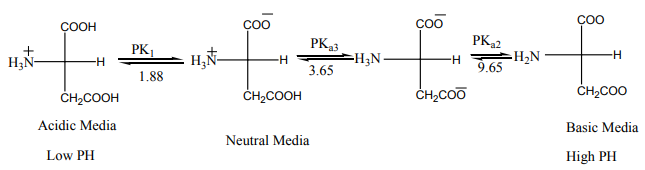
9.7.1. Acid–base properties of amino acids
As the name suggests, amino acids are organic compounds that contain both a
carboxylic acid group and an amine group. Amino acids are crystalline, high melting
point (>200°C) solids. Such high melting points are unusual for a substance with
molecules of this size — they are a result of internal ionisation. Even in the solid
state, amino acids exist as zwitterions in which a proton has been lost from thecarboxyl group and accepted by the nitrogen of the amine group:
So instead of hydrogen bonds between the amino acid molecules there are stronger
ionic (electrovalent) bonds. This is reflected in the relative lack of solubility of amino
acids in non- aqueous solvents compared with their solubility in water.
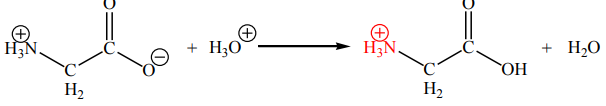
Zwitterions exhibit acid–base behaviour because they can accept and donate
protons. In acids a proton is accepted by the carboxylic acid anion, forming a unitwith an overall positive charge:
In alkalis the reverse occurs with the loss of a proton from the nitrogen atom:
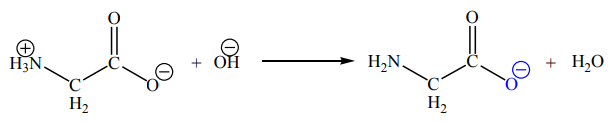
The species present in a given solution depends on the pH of the solution.
Carboxylic acids have acidic properties and react with bases. Amines have basic
properties and react with acids. It therefore follows that amino acids have bothacidic and basic properties.
9.7.2. Isoelectric point in aminoacids (pI)
The isoelectric point (pI), is the pH at which a particular molecule carries no net
electrical charge in the statistical mean. This means it is the pH at which the amino
acid is neutral, i.e. the zwitterion form is dominant. The pI is given by the average ofthe pKa that involve the zwitterion, i.e. that give the boundaries to its existence
The table below shows the pKa values and the isoelectronic point, pI, are givenbelow for the 20 α-amino acids (Table 9.6).

Table 9.6. pKa and pI values for the 20 α-amino acids


There are 3 cases to consider:
1. Neutral side chains
These amino acids are characterised by two pKa values: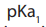 and
and  for the
for the
carboxylic acid and the amine respectively. The isoelectronic point will be halfway
between, or the average of, these two pKa values . This is most readily appreciated
when you realise that at very acidic pH (below pka1) the amino acid will have an
overall positive charge and at very basic pH (above ) the amino acid will have
) the amino acid will have an overall negative charge.
The other two cases introduce other ionisable groups in the side chain “R” described
by a third acid dissociation constant,

2. Acidic side chains
The pI will be at a lower pH because the acidic side chain introduces an “extra”
negative charge. So the neutral form exists under more acidic conditions when the
extra -ve has been neutralised. For example, for aspartic acid shown below, the
neutral form is dominant between pH 1.88 and 3.65, pI is halfway between thesetwo values
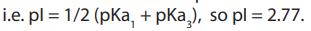
3. Basic side chains
The pI will be at a higher pH because the basic side chain introduces an “extra”
positive charge. So the neutral form exists under more basic conditions when the
extra positive has been neutralised. For example, for histidine, which has three acidic
groups of pKa’s 1.82 (carboxylic acid), 6.04 (pyrrole NH) and 9.17 (ammonium NH),
the neutral form is dominant between pH 6.04 and 9.17; pI is halfway between thesetwo values, i.e. , so pI = 7.60.
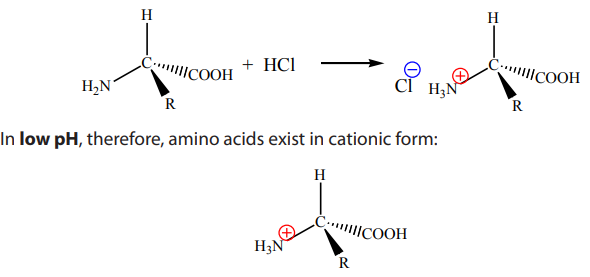
9.7.3. Reaction with strong acids
In the following reaction, amino acids react with strong acids such as hydrochloricacid:
9.7.4. Reaction with nitrous acid (deamination)
The amine function of α-amino acids and esters reacts with nitrous acid in a similar
manner to that described for primary amines. The diazonium ion intermediate loses
molecular nitrogen in the case of the acid, but the diazonium ester loses a protonand forms a relatively stable diazo compound known as ethyl diazo ethanoate:
The diazo ester is formed because of the loss of
 from the diazonium ion which
from the diazonium ion which results in the formation of a quite unfavourable carbocation.
9.7.5. Reaction with sodium hydroxide
Amino acids react with strong bases such as sodium hydroxide:
9.7.5. Reaction with sodium hydroxideAmino acids react with strong bases such as sodium hydroxide:

In high pH, therefore, amino acids exist in anionic form:

9.7.6. Reaction of amino acids with sodium carbonate
Amino acids are instantly dissolved by strong hydrochloric acid but are in part
recovered unchanged on dilution and evaporation. They are not decomposed by
sodium carbonate but are easily decomposed by sodium hydroxide. (Dakin & West,1928).
Checking up 9.7
1. Draw the structure for the anion formed when glycine (at neutral pH)
reacts with a base.
2. Draw the structure for the cation formed when glycine (at neutral pH)reacts with an acid
3. Calculate the Isoelectric point of Glycine?

4. Calculate the Isoelectric point of Lysine?
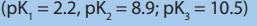
9.8. Optical isomers of amino acids
Activity 9.8
The molecule CHBrClF exhibits optical isomerism. Draw the 3D displayedformulae of both optical isomers.
Simple substances which show optical isomerism exist as two isomers known as
enantiomers. Where the atoms making up the various isomers are joined up in a
different order, this is known as structural isomerism. Structural isomerism is not
a form of stereoisomerism, which involve the atoms of the complex bonded in the
same order, but in different spatial arrangements. Optical isomerism is one form ofstereoisomerism; geometric isomers are a second type.
The general formula for an amino acid (apart from glycine, 2-aminoethanoic acid)
is shown below.The carbon at the centre of the structure has four different groupsattached. In glycine, the “R” group is another hydrogen atom.
The lack of a plane of symmetry means that there will be two stereoisomers of an
amino acid (apart from glycine) - one the non-superimposable mirror image of theother.
For a general 2-amino acid, the isomers are:
The R group, usually referred to as a side chain, determines the properties of each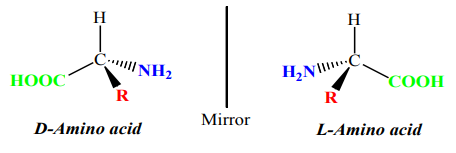
amino acid. Scientists classify amino acids into different categories based on the
nature of the side chain. A tetrahedral carbon atom with four distinct groups is
called chiral. The ability of a molecule to rotate plane polarized light to the left,
L (levorotary) or right, D (dextrorotary) gives it its optical and stereo chemicalfingerprint.
All the naturally occurring amino acids have the right-hand structure in the diagram
above. This is known as the “L-” configuration. The other one is known as the “D-”
configuration.
When asymmetric carbon atoms are present in a molecular compound, there are
two ways in which the groups attached to that carbon can be arranged in the three
dimensions, as we have just shown with the two models above. Chemically, optical
isomers behave in the same way. Biologically, they do not. One will react properly,but the other will not.
Checking up 9.8
1. Write the optical isomers of 2-aminopropanoic and2. Using diagrams, explain why glycine is not chiral.
9.9. Peptides and polypeptides
Activity 9.9
Ethyne is a compound which can undergo a polymerisation reaction.
is a compound which can undergo a polymerisation reaction.
Deduce the reaction of polymerisation of ethyne, showing the mechanism ofreaction.
9.9.1. Formation of peptide bonds
Amino acid molecules can also react with each other; the acidic –COOH group in one
molecule reacts with the basic group in another molecule.
group in another molecule.
When two amino acids react together, the resulting molecule is called a dipeptide,
forming an amide linkage (peptide bond), with the elimination of a water molecule.
Each amino acid possesses a carboxylic acid group and an amine group. The
possibilities for constructing polypeptides and proteins are enormous. Let us
consider two simple amino acids, glycine (2-aminoethanoic acid) and alanine(2-aminopropanoic acid).
The figures below show that these can be joined in two ways:
Note: the amide link between the two amino acids. An amide link between two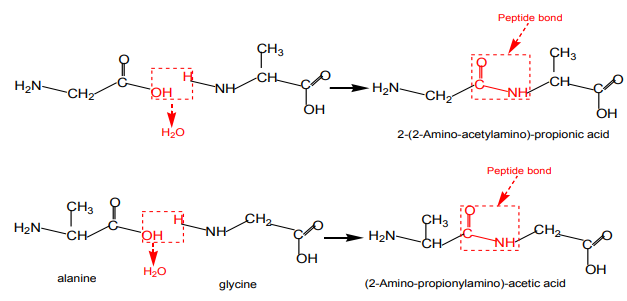
amino acid molecules is also called a peptide link. The reaction is a condensation
reaction as a small molecule. The dipeptide product still has an
group at one end and a –COOH group at the other end.Therefore the reaction can continue,
to form a tripeptide initially, and then ever-longer chains of amino acids.
The longer molecules become known as polypeptides, and then proteins as they get even
longer sequences of amino acids. A typical protein is formed from between 50 and200 amino acids joined in a variety of sequences.
9.9.2. Structure of peptides and polypetides
A series of amino acids joined by peptide bonds form a polypeptide chain, and each
amino acid unit in a polypeptide is called a residue. A polypeptide chain has polarity
because its ends are different, with α-amino group at one end and α-carboxyl
group at the other. By convention, the amino end is taken to be the beginning of
a polypeptide chain, and so the sequence of amino acids in a polypeptide chain
is written starting with the aminoterminal residue. Thus, in the pentapeptide
TyrGly-Gly-Phe-Leu (YGGFL), tyrosine is the amino-terminal (N-terminal) residue and
leucine is the carboxyl-terminal (C-terminal)residue Leu-Phe-Gly-Gly-Tyr (LFGGY) isa different pentapeptide, with different chemical properties.
This above illustration of the pentapeptide Tyr-Gly-Gly-Phe-Leu (YGGFL) shows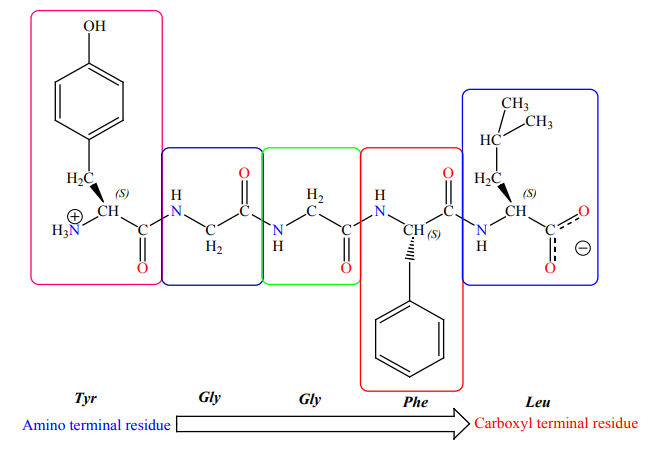
the sequence from the amino terminus to the carboxyl terminus. This pentapeptide,
Leu-enkephalin, is an opioid peptide that modulates the perception of pain. The
reverse pentapeptide, Leu-Phe-Gly-Gly-Tyr (LFGGY), is a different molecule andshows no such effects.
A polypeptide chain consists of a regularly repeating part, called the main chain
or backbone, and a variable part, comprising the distinctive side chains. The
polypeptide backbone is rich in hydrogen-bonding potential. Each residue contains
a carbonyl group, which is a good hydrogen-bond acceptor and, with the exception
of proline, an NH group, which is a good hydrogen-bond donor. These groups
interact with each other and with functional groups from side chains to stabilizeparticular structures, as will be discussed later.
A polypeptide chain consists of a constant backbone (shown in blue) and variable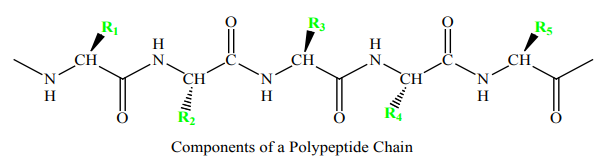
side chains (shown in green).
9.9.3. Uses of amino acids as building blocks of proteins
Like carbohydrates and lipids, proteins contain the elements carbon (C), hydrogen
(H) and oxygen (O), but in addition they also always contain nitrogen (N).sulphur(S)
is often present as well as iron (Fe) and phosphorus (P). Before understanding howproteins are constructed, the structure of amino acids should be noted.
The process of construction of proteins begins by amino acids bonding together, as
seen earlier, through peptide bonds. When many amino acids join together a long
chain polypeptide is produced. The linking of amino acids in this way takes place
during protein synthesis.
The simplest level of protein structure, primary structure, is simply the sequence of
amino acids in a polypeptide chain. The primary structure (Figure 9.2) of a protein
refers to its linear sequence of amino de (–S–S–) bridges. One of those sequences is:–Gly–Ile–Val–Cyst–Glu–Gln–Ala–Ser–Leu–Asp–Arg–Asp–Arg–Cys–Val–Pro–
The primary structure is held together by peptide bonds that are made during the
process of protein biosynthesis. The two ends of the polypeptide chain are referred
to as the carboxyl terminus (C-terminus) and the amino terminus (N-terminus) basedon the nature of the free group on each extremity.
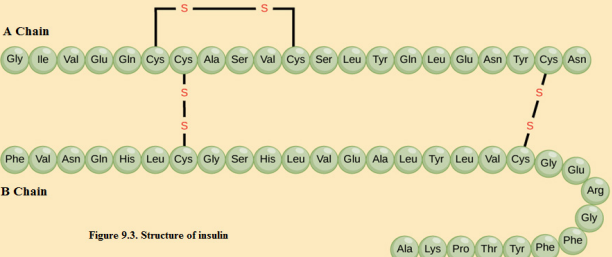
For example, the hormone insulin (Figure 9.3) has two polypeptide chains, A and B,
shown in diagram below. Each chain has its own set of amino acids, assembled in a
particular order. For instance, the sequence of the A chain starts with glycine at the
N-terminus and ends with asparagine at the C-terminus, and is different from the
sequence of the B chain. You may notice that the insulin chains are linked togetherby sulfur-containing bonds between cysteines.
Checking Up 9.9
1. Distinguish between the N– terminal amino acid and the C– terminal
amino acid of a peptide or protein.
2. Describe the difference between an amino acid and a peptide.
3. Amino acid units in a protein are connected by peptide bonds. What is
another name for the functional group linking the amino acids?
4. Draw the structure for each peptide.
a. gly-val
b. val-gly5. Identify the C– and N– terminal amino acids for the peptide lys-val-phegly-arg-cys.
9.10. End Unit Assessment
1. Ethylamine and phenylamine are two organic compounds and both are basic.
a. Draw the displayed formula of each compound, including lone pairs.
b. Write a balanced symbol equation for the reaction between one of these
compounds and an acid to form a salt.
c. Which structural feature of each compound accounts for the basicity?
2. The formulae of two amino acids, glycine (Gly) and alanine (Ala), aregiven:
a. i. Give the systematic names of both amino acids.
ii. Draw their skeletal formulae.
b. Alanine can exist as two stereoisomers.
i. Draw these two stereoisomers, showing how they differ in their spatial
arrangements.ii. Explain why glycine does not have stereoisomers.
3. The structure of a certain tripeptide is shown here:
a. i. Draw the displayed formulae of the three amino acids that make up the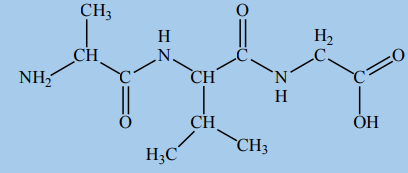
tripeptide.
ii. Which of these amino acids has two chiral carbon atoms?
b. This tripeptide can be split up into the three amino acids by refluxing with
aqueous hydrochloric acid.
i. Which bond is broken in this reaction?
ii. The reaction can be described as hydrolysis. Explain why, using adiagram.
