UNIT 8: ESTERS, ACID ANHYDRIDES, AMIDES AND NITRILES.
Key unit competency:
To be able to relate the functional groups of esters, acid anhydrides,
amides and nitriles to their reactivity, preparation methods and uses.
• Describe the chemical properties of esters, acid anhydrides, amides, and
nitriles.
• Describe the process of urea manufacture and its uses.• Describe the formation of the detergents.
Apply IUPAC rules to name esters, acid anhydrides, amides, and nitriles.
• Compare the physical properties of esters to those of alcohols and carboxylic
acids.
• Make a soap and compare its properties with those of soapless detergents.
• Compare the reactivity of acid anhydrides with those of acyl chlorides.
• Prepare aspirin from appropriate reagents.
• Appreciate the importance of esters, acid anhydrides, amides and nitriles intextile industry and pharmacy.
Introductory activity
The development of organic chemistry has led scientists to the production of
new substances and materials that are necessary in our everyday life which
could not be provided by our natural environment. Others were produced to
satisfy the high demand of consumers which cannot be assured by natural
products only. Analyze the items presented below and answer the questionslisted down.

1. What kind of textile is used to make umbrellas? How did scientists make
this kind of textiles?
2. One of the substances used to improve soil fertility so as to ensure food
security is urea? How is it synthesized?
3. Why is it possible to make artificial drinks with flavors of natural fruits?
4. How are pain killer drugs manufactured?5. What kinds of substances provide perfumes with their fragrances?
8.1. Structure and nomenclature of esters
Activity 8.1
3. The compounds listed below contain acid derivatives and other organicmolecules. Classify them in the following table.
2.Draw all possible isomers with molecular formula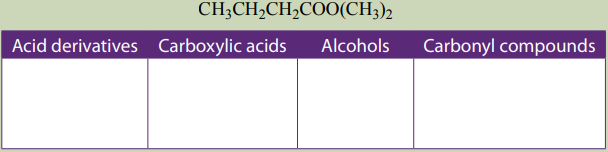
 and label esters
and label esters with letter A and acids with letter B.
8.1.1. Structure of esters
In unit 7, the reactions of carboxylic acids were discussed. The reactions of carboxylic
acids produce the derivatives of acids such as esters, acid halides, acid anhydridesand amides.
The general molecular formula of esters is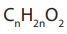 and their general structural
and their general structural formula is: RCOOR’ or
Where R may be a hydrogen atom or an alkyl group and R may be an alkyl group
or an aryl group but not a hydrogen atom. In case that R is the hydrogen atom, the
compound is no longer an ester but it is a carboxylic acid.
The following Figures, 8.1 and 8.2 show models for two common esters where green
spheres = Hydrogen atoms, red spheres = oxygen atoms; blue spheres = carbonatoms.
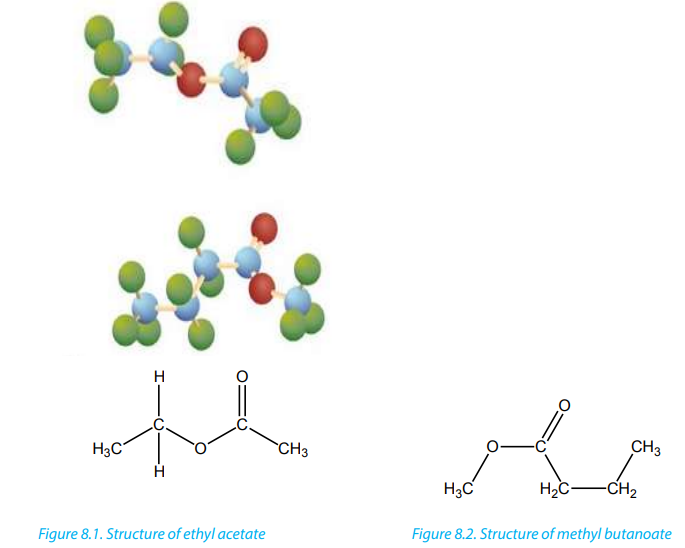
The functional group of esters is
Esters are compounds produced by the reaction involving an acid and an alcohol
with the elimination of water molecule.
For example, the reaction between acetic acid and ethanol yields an esterwith water.
Esters are known for their distinctive odor and they are commonly responsible for
the characteristic of food (fruits) aroma, flowers and fragrances. Esters are found in
nature but they can be also synthesized. Both natural and synthetic esters are usedin perfumes and as flavoring agents.
8.1.2. Nomenclature of esters
The nomenclature of esters follows some steps. When naming esters the alkyl
group R’ is named followed by the name of RCOO- group.
The group name of the alkyl or aryl portion is written first and is followed by the
name of the acid portion. In both common and International Union of Pure and
Applied Chemistry (IUPAC) nomenclature, the -ic ending of the corresponding acid
is replaced by the suffix –ate. Some examples of names of esters are given in Table8.1.
Examples:
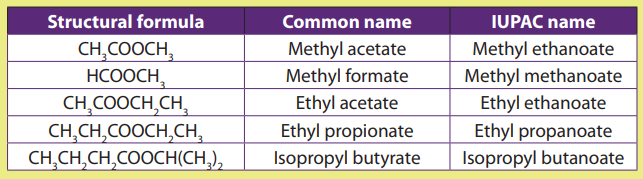
Table 8.1. Examples of structural formulae of some esters and their name
8.1.3. Physical properties and uses of Esters
Experiment
A. Analyzing the solubility of esters ( fats and oils)
Materials and Chemicals
Cooking oil, margarine, water, ethanol, stirring rods and test tubes labeled A
and B.
Procedure
1. Pour water in a test tube A and ethanol in test tube B and add some
cooking oil in each test tube. Shake well to mix and record your
observation.
2. Pour water in a test tube A and ethanol in test tube B and add a small
piece of margarine in each test tube. Use a stirring rod to mix and record
your observation.
Conclusion: Esters are soluble in organic solvents such as ethanol and insolublein water.
B. Comparing boiling points of alcohols, carboxylic acids and esters
Materials and Chemicals
Propan-1-ol, propanoic acid and methyl ethanoate, test tubes, test tube holders
(lacks), heaters, and thermometers.
Procedure
1. Put 10 mL of each substance in a labeled test tube.
2. Boil carefully substances are volatile and flammable
3. Use a thermometer to measure the boiling point of each substance.
4. Record the results and compare them. Suggest a reason for the difference
in boiling points of the three substances.
Conclusion: Esters have lower boiling points than alcohols and carboxylic
acids because they lack hydrogen bonds. A compound having hydrogen bonds
has a high boiling point because, to break that bond requires higher energy.
Other physical properties of esters
i. Lower esters have sweet fruity smells
ii. Melting and boiling points of esters increase as the molecular mass
increases.
iii. Small esters are fairly soluble in water but the solubility decreases as the
length of the chain increases
8.1.4. Uses of Esters
Esters find various uses:
i. They are used as organic solvent
ii. Due to their aroma, they are used as constituent of fragrance, essential oils,
food flavoring and cosmetics.
iii. They are used to manufacture soaps, detergents and glycerol.
iv. They are used to provide energy in the bodyv. Polyesters are used to produce plastics etc
Checking up 8.1
1. Name the following compounds by using the common and IUPACnames.
2. Draw the structural formulae corresponding to each of the following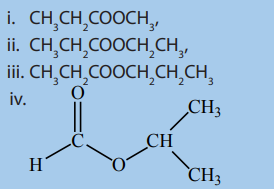
names.
i. Propyl methanoate
ii. octyl ethanoate
3. Discuss the solubility of esters
4.State one industrial and one biological use of esters.
5.Two compounds A and B of molecular formula were analyzed
were analyzed
to compare their relative boiling points. Compound A had lower boiling
point than compound B. Draw the structural formula of each compound.
6.Identify the relationship that exists between compounds A and B. Explainyour reasoning.
8.2. Preparation and chemical properties of esters
3. Using your research on internet and reading books, make a summary of
each of the following terms:
e. Reduction of esters
f. Hydrolysis of esters
g. Alkaline hydrolysis of ester
h. Trans-Esterification
i. Comparison of the reactivity of esters, acid chlorides and acid
anhydrides
4.Give an example of an equation for each of the processes in (1).5. Express the technical name given to the process in 3 (c)
8.2.1. Preparation of Esters
The preparation of esters involves different types of reaction such as esterification,
reaction of an acid chloride with an alcohol and the reaction of acid anhydrides
with alcohols.
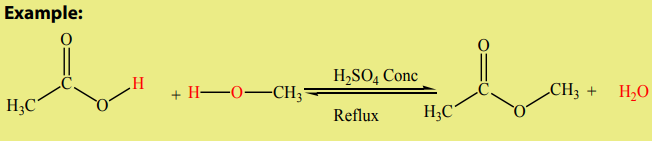
1.Esterification reaction
In units five and seven, it is mentioned that esters can be produced by a reaction
between alcohols and carboxylic acids in strong acidic medium acting as a catalyst.
The acid is commonly a concentrated sulphuric acid, under reflux (Figure 8.3). The
reaction is generally called “Esterification” (a condensation reaction which involves
the addition of the alcohol and acid molecules followed by an elimination of awater molecule).

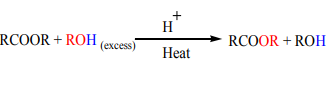
3. Reaction of acid anhydrides with alcohols ( Trans-esterification)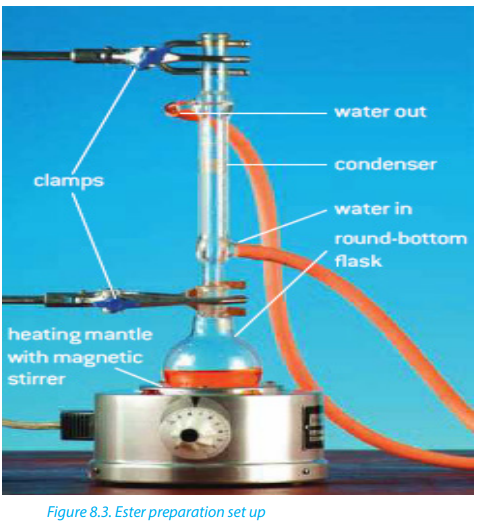
Alcohols react with esters to undergo an exchange of the alkoxide segment. The
reaction is acid catalyzed and the used alcohol must be in excess. This is a verycommon technique of producing new esters from available esters.


Reaction mechanism

2. Reaction of an acid chloride with an alcohol
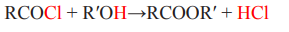

8.2.2. Chemical properties of esters
Chemical properties of esters involve their reactivity with other compounds.
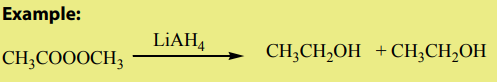
a. Reduction of esters
Compared to ketones and aldehydes, esters are relatively resistant to reduction.
Esters are reduced by giving two alcohols,
giving two alcohols,
one from the acyl segment (RC=O) and one from the alkoxide segment (R-O) asshown by the reaction below.
When a less reactive reducing agent such as diisobutylaluminium hydride (DIBAH) is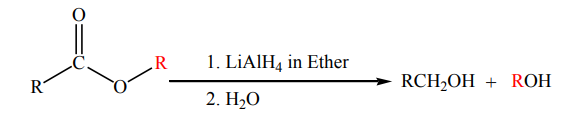
used the acyl segment is converted into an aldehyde and the alkoxide group is still
converted into an alcohol. Exactly one equivalent of the hydride must be used, andthe reaction must be carried out at -78 °C
b. Hydrolysis of esters.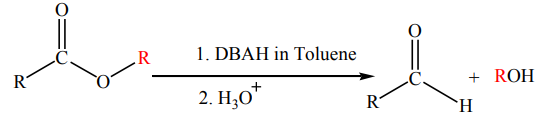
The reaction of an ester with water is called hydrolysis. This reaction is very slowunless catalyzed by a base or an acid.

Mechanism of basic hydrolysis ofeEsters
The base catalyzed hydrolysis reaction is called saponification (derived from Latin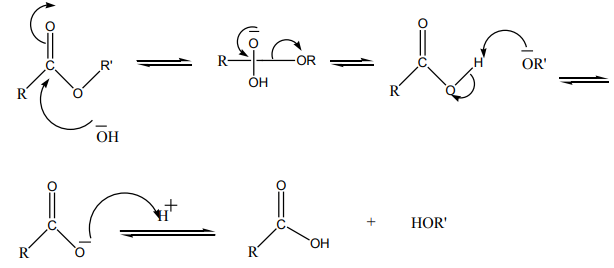
word, “sapo”, which means soap). Soaps are sodium or potassium salts made by
hydrolyzing the vegetable oil which contain higher molecular weight esters in thepresence of sodium or potassium hydroxides.
c. Trans-esterification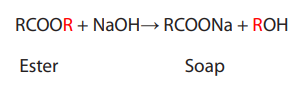
Alcohols react with esters to undergo an exchange of the alkoxide segment. The
reaction is acid catalyzed and the used alcohol must be in excess. This is a verycommon way of producing new esters from readily available esters.
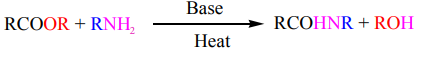
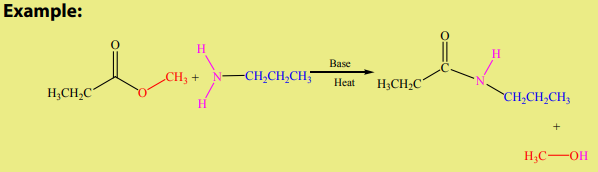
d. Reaction with amines: Aminolysis
Esters react with ammonia, primary or secondary amines to produce amides. The
reaction is carried out at high temperature in basic medium. However, this reaction isnot often used because higher yields are normally obtained by using acyl chlorides.
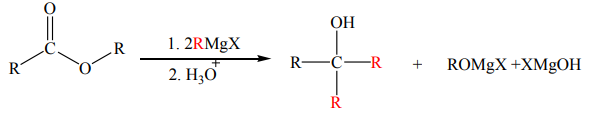
e. Reaction with Grignard reagents
Esters react with two equivalents of a Grignard reagent to form tertiary alcohols. This
reaction produces ketone intermediates which undergo a fast conversion into thealcohol because of being more reactive than esters.
Checking Up 8.2.
1. Write a balanced equation for the reaction between propanoyl
chloride and butan-1-ol and name the product.
2. Investigate how to carry out the following conversions by using a
non-organic compounds other than the one cited. Use any inorganic
substances you need.
a. Propan-1-ol to propyl propanoate
b. Ethanal to ethyl ethanoate
3. Ethanoic acid reacts with an alcohol of molecular formula C4H10O to
produce an ester which is optically active.
a. Identify the structure of the alcohol.b. Sketch the structure of the ester formed.
4. Complete the equations below:
5. For a reaction to take place, some conditions may be required
depending on the type of reaction. Discuss the conditions to be usedin order to carry out the reaction 4.a.
6. Reactions of amines with esters are not common
Explain briefly this statement.
7. You are provided with ethyl ethanoate and asked to prepare isobutyl
ethanoate.
Describe how you can proceed to prepare that compound. In your
explanations, include reagents, conditions and equation(s) for the reaction(s)
that take place.You are allowed to use any other organic compound you need.
8.3. Saponification and Detergents

Observe the above picture and answer the following questions
1. Explain the properties that these products have which make them
suitable for their use as you have stated in (1).
2. Explain how these products are manufactured?
3. Propose the differences and similarities of these products?
4. Using NaOH and cooking oil, how can you prepare a solid soap inlaboratory?
Surfactants like soaps and detergents are important cleaning products which play
an essential role in our daily life. By safely and effectively removing soils, germs and
other contaminants, they help us to stay healthy, care for our homes and possessions,
and make our surroundings more pleasant.
Soaps
Soaps are water-soluble sodium or potassium salts of fatty acids. Soaps are made
from fats and oils, or their fatty acids, by reacting them with a strong alkali. The
process is known as “saponification”.
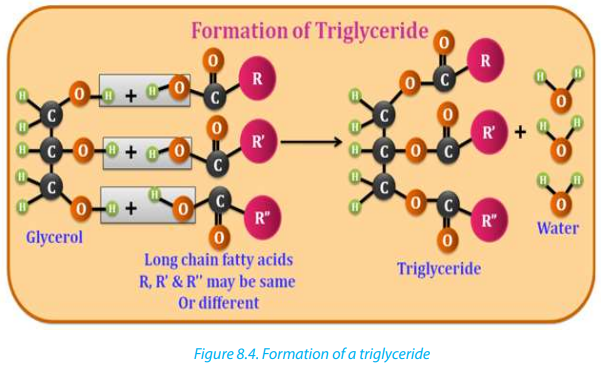
Fats and oils
The fats (solid lipids at room temperature and pressure) and oils (liquid lipids at
room temperature and pressure) used to produce soaps find their sources from
animal or plant. Each fat or oil is made up of a distinctive mixture of several different
triglycerides.
In the formation of a triglyceride molecule, three fatty acid molecules reacted withone molecule of propane-1,2,3-triol or glycerol as shown in Figure 8. 4 below.

Saponification reaction
The reaction of saponification involves the collision between triglycerides in fat/oiland aqueous NaOH or KOH. The result is the formation of soap and glycerol (Figure 8.5).
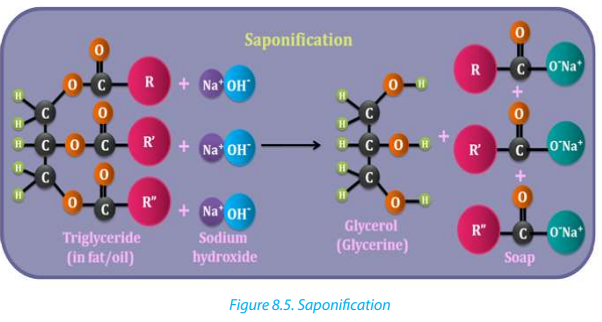
The reaction of saponification is exothermic because there is liberation of heat and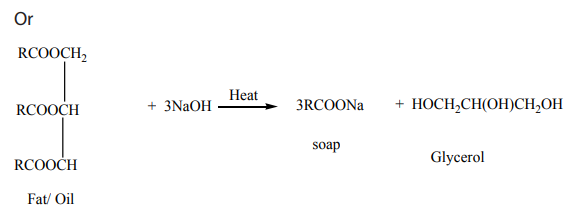
the soap formed remains in suspension form in the mixture. Soap is precipitated asa solid from the suspension by adding common salt to the suspension.
Note: Sodium soaps are “hard” soaps whereas potassium soaps are soft.
DETERGENTS
Detergents are organic liquid or water-soluble solid cleaning substances that, unlike
soap, are not prepared from fats and oils.
The chemical composition of detergents is different from that of soaps but they
have the same cleaning mechanism and are not adversely affected by hard minerals
in the water and this makes them more effective than soaps. However, they are less
environmental friendly because of a reduced biodegradability.
Detergents may be used for household cleaning, laundry or for body and hand
washing. They exist in the powder or liquid form.
How do soaps and detergents work?
When a soap or detergent is added to water, a polar solvent, the molecules form
clusters, known as micelles(Figure 8.6), in which the polar ends of the molecules areon the outside of the cluster and the non-polar ends are in the middle.
The carboxylate end of the soap molecule is attracted to water. It is called the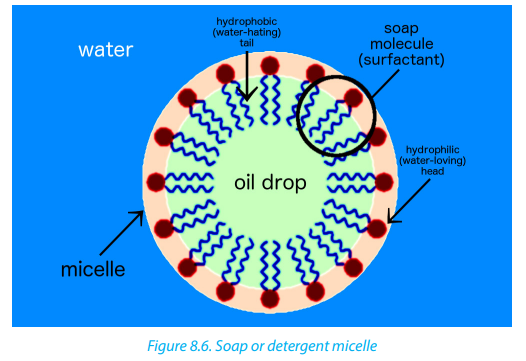
hydrophilic (water-loving) end. The hydrocarbon chain is attracted to oil and grease
and repelled by water. It is known as the hydrophobic (water-hating) end. When
washing, the hydrophobic part of the soap molecule (Figure 8.7) dissolves oil or
grease the main source of dirt and it gets washed away by water as it is insoluble init.
The other major soap-making process is the neutralization of pure fatty acids withan alkali.
The cleaning property of both soaps and detergents results from their capacity
to emulsify water-insoluble materials (dirt, oil, grease, etc.) and hold them in
suspension in water. This ability originates from the molecular structure of soaps
and detergents. When a soap or detergent adds on to water that contains oil or
other water-insoluble materials, the soap or detergent molecules surround the oil
droplets. The oil or grease is “dissolved” in the alkyl groups of the soap molecules
while the ionic end allows the micelle to dissolve in water. As a result, the oil droplets
are dispersed throughout the water (this is referred to as emulsification) and can berinsed away.

Difference Between Soap and Detergent

Checking Up 8.3.
1. Propyl tristearate reacts with sodium hydroxide to form soap.
a. Write a balanced equation for the reaction which takes place.
b. Calculate the mass of sodium hydroxide needed to react exactly with
4kg of this oil and the mass of the produced soap.
2. Describe the chemical difference of solid and liquid soaps.
3. Distinguish soaps from detergents.
4. Why are detergents more effective than soaps?
5. Describe briefly in your own words how soaps and detergents work.6. Discuss the importance of soaps and detergents in our everyday life.
8.4. Structure and nomenclature of acid anhydrides
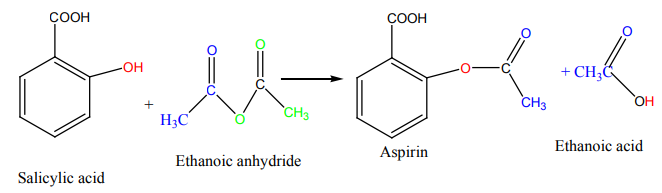
One of the most used pain killers is aspirin. This is a medical drug which can be
prepared using salicylic acid and ethanoic acid. However, ethanoic acid is not
used. Instead, one of its derivatives is used. Search from internet or the school
library and answer the questions below:
1. Propose a derivative of acetic acid is used in this preparation?
2. Explain why is it used in preference to acetic acid?
3. Write down its molecular formula and structure.4. Suggest how it is produced from acetic acid.
8.4.1. Structure of acid anhydrides
The acid anhydrides are derivatives of carboxylic acids.
The general structure of acid anhydrides is RCOOOCR, or
general structure of acid anhydrides is RCOOOCR, or
When the two R groups are identical, the acid anhydride is symmetric and when thetwo R groups are different, the acid anhydride is asymmetric. The general molecular
formula of acid anhydride is
The functional group of acid anhydrides consists of two acyl groups held togetherby an oxygen atom.
When the two R groups are identical, the acid anhydride is symmetric and when the
two R groups are different, the acid anhydride is asymmetric. The general molecularformula of acid anhydride is
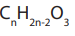
8.4.2. Nomenclature of acid anhydrides
The nomenclature of acid anhydride is based on whether they are symmetrical or
unsymmetrical. Symmetrical acid anhydrides are named as parent acid followed bythe term anhydride

Unsymmetrical acid anhydrides are named by writing alphabetically the names of
parent acids followed by the term anhydride.

Checking up 8.4.
1. Write the molecular formula of an acid anhydride which has 6 carbon
atoms
2. Draw the structure of one straight and one branched isomers of the
molecular formula in (1) above.3. Name the isomers from (2).
8.5. Preparation, chemical properties and uses of acid anhydrides
Activity 8.5.
1. Two carboxylic acids can react to form an acid anhydride and a water
molecule. However, this method is not suitable when preparing mixed
(unsymmetrical) acid anhydrides.
2. Suggest a reason why this method is not suitable.
3. Using your knowledge in organic chemistry so far, suggest a method
which may be suitable to prepare ethanoic propanoic anhydride. Write
the equation for the reaction. (Hint: you may refer to the preparation of
ethers).
4. Prepare ethanoic anhydride using ethanoic acid and phosphorous
pentoxide.
5. Aspirin is synthesized using ethanoic anhydride and salicylic acid. Suggestan equation for the reaction that occurs.
8.5.1. Preparation
Anhydride means “without water”. Two carboxylic acids can react, eliminating awater molecule to yield an acid anhydride.
The commonly used dehydrating agent is phosphorous pentoxide,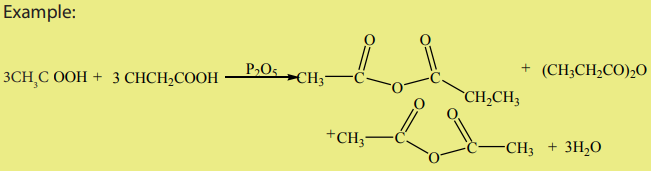
 If two
If two
different acids are used, a mixed anhydride is produced. The method is less efficient
however, as one obtains the two symmetrical anhydrides in addition to the desired
mixed anhydride.
A better method of making mixed anhydrides is to react an acid halide with a saltof a carboxylic acid. This method can be used to make symmetrical anhydrides too.
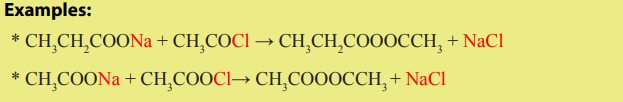
8.5.2. Chemical properties of acid anhydrides and their uses
The term “chemical properties” indicates the reactivity between two or more
compounds. In the case of acid anhydrides, their reactivity involves the electron deficient carbonyl-carbon which is attacked by nucleophiles. This reaction occurs slowly.
There are mainly four types of reactivity of acid anhydrides such as hydrolysis, reaction
with alcohols, reaction with ammonia and amines and the reduction reaction.
1. Hydrolysis
This reaction of acid anhydride in water leads to the formation of parent carboxylic
acids which were used to prepare the anhydride. The reaction is carried out in acidicmedium under reflux.

2. Reaction with alcohols
Anhydrides react readily with primary, secondary, tertiary alcohols to form estersand carboxylic acid.

Aspirin synthesis is an application of this reaction
This reaction is very important in pharmaceutical industries and it indicates the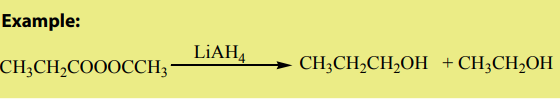
main use of acid anhydrides as it is the basis of aspirin manufacture as shown below.
3. Reaction with ammonia and amines
Anhydrides react with ammonia, primary and secondary amines to produce
amides.
The reaction with amide:

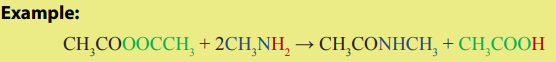
Reaction with ammonia: (RCO)2 O + NH3 → RCONH2 + RCOOH
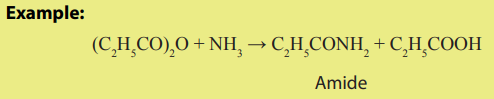
4. Reduction of acid anhydrides
Anhydrides are reduced by Lithium tetrahydridoaluminate,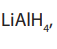
to yield two moles of primary alcohols.
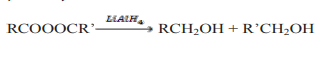
Where R and R can be hydrogen atoms (for primary amides), alkyl groups (fortertiary amides). For secondary amides only one R is a hydrogen atom. Their general
molecular formula is Cn
H2n+1ON. Examples of some amides are given in the Table 8.2.
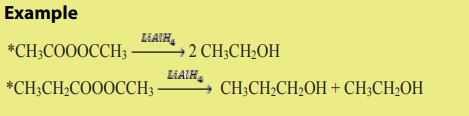
8.5.3. Uses of acid anhydrides
1. Ethanoic anhydride is used in the synthesis of acetate esters.
2. Examples: aspirin, cellulose acetate,…
3. Maleic anhydride is used in the synthesis of various resins when
copolymerized with styrene.4. They are used to synthesize polyesters and polyamides.
Checking up 8.5
1. Write the equations that can be used to synthesize the following acid
anhydrides from ethanol.
a. Ethanoic anhydride
b. Propanoic anhydride
c. Ethanoic propanoic anhydride
2. Students of senior five MCB were asked to prepare butanoic propanoic
anhydride and group A used a method similar to Williamson’s method of
synthesizing ethers whereas group B decided to use a dehydrating agent.
Which group chose a better method? Explain your reasoning3. Complete the equations below
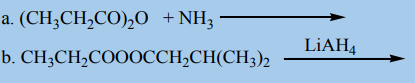
4. Propose the products from the reaction below:
5.Draw the structures of products formed when propanoic anhydride reacts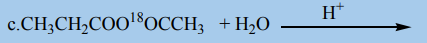
with 2-methylpropan-2-ol
6. State the necessary chemicals and conditions to prepare aspirin.
7.Chemists prefer using acid anhydrides than acyl chlorides when preparingesters. Discuss this statement.
8.6. Structure and nomenclature of amides
Activity 8.6.
In the previous unit, it has been mentioned that carboxylic acids react with
ammonia and amines to produce new organic compounds.
1. Draw and name their functional group.
2. Draw their general structure and determine their general molecular
formula.
3. What natural and artificial polymers contain the same functional group?
4. Suggest how their boiling points would be relative to those of esters.Provide an explanation for your suggestion.
8.6.1. Structure of amides
Amides are acid derivatives in which the –OH group is replaced by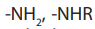 or
or
. The functional group comprises nitrogen atom which is attached to the
carbonyl carbon atom. The carbonyl group linked to nitrogen atom is called anamide linkage. The general structure of amides is:
Where R and R can be hydrogen atoms (for primary amides), alkyl groups (for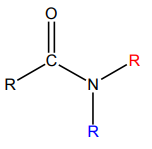
tertiary amides). For secondary amides only one R is a hydrogen atom. Their general
molecular formula is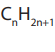 ON. Examples of some amides are given in the Table 8.2.
ON. Examples of some amides are given in the Table 8.2.Table 8.2. Examples of some amides

8.6.2. Nomenclature of amides
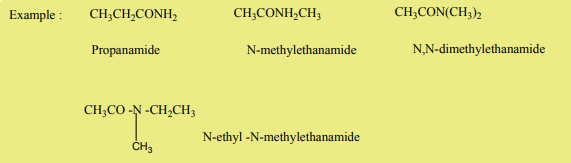
The nomenclature of amides is derived from the name of corresponding acid. The
–oic acid suffix or –ic acid is replaced by –amide.
As for other organic compounds, the first step is to consider the number of carbon
atoms forming the chain.
The alkyl group bonded to nitrogen atom is indicated by a capital N preceding thealkyl name.
8.6.3. Physical properties and uses of amides
Physical properties of amides
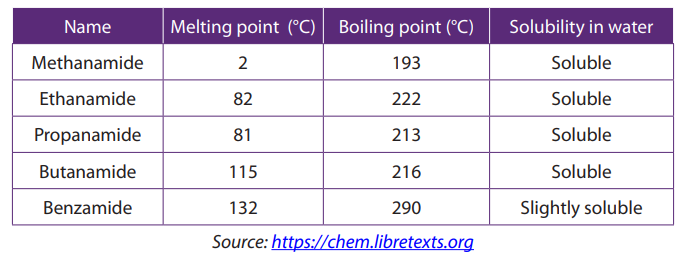
Except formamide, all the amides are crystalline solids at room temperature. Amides
have higher melting and boiling points than corresponding esters due to hydrogenbonding as shown below.
The melting and boiling points increase as molecular mass increases. Lower members
are soluble in water but this solubility decreases as the molecular mass increases. All
the amides are soluble in organic solvents. The Table 8.3 shows the comparison ofmelting and boiling points of some amides.
Table 8.3. Some physical properties of lower amides
8.6.4. Uses of Amides
Amides are used in the production of many useful chemicals and materials such asfertilizers (urea), nylon textiles and skin care substances.
Urea manufacture
Urea can be prepared in three ways:a. Reaction of phosgene and ammonia

The diagram below shows the representation of Urea.
b. From calcium cyanamide,
Calcium carbide reacts with nitrogen to produce calcium cyanamide and carbon.
 produced
produced  is then treated with a mixture of water
is then treated with a mixture of water and carbon dioxide to produce urea.

c. Reaction of carbon dioxide and ammonia
Urea is also naturally present in animal urines.
It is widely used in agriculture as a source of nitrogen, chemical fertilizer. It also finds
use in animal feeding and in resins manufacture.
Nylon manufacture
Nylon-6,6 is a synthetic textile produced when hexane-1,6-dioic acid(adipic acid)
reacts with hexane-1,6-diamine. Nylon is a polyamide. Materials and clothes aremade from nylo-6,6.
Medical use of urea
Urea containing creams are used in skin treatment to promote its rehydration. It softens theskin.
Checking up 8.6.
1. Write the molecular formula of amides with 4 carbon atoms
2. Draw all possible structural formulae of primary, secondary and tertiary
amides with molecular formula in (1) above and name them
3. Compare the solubilities of butanamide and N,N-dimethylethanamide
in water
4. The solubility of amides decreases with the increase in molecular mass.
Suggest a reason for this observation.
5. Which one between ethanol and ethanamide do you expect to have a
higher boiling point? Explain your answer.
6. Discuss the benefits and dangers of using animal urine as a source ofnitrogen for plants.
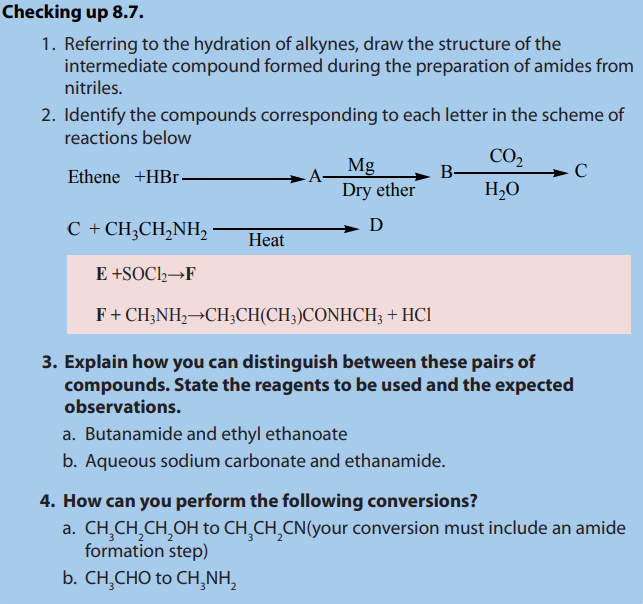
8.7. Preparation and chemical properties of amides
Activity 8.7.
1. Draw the structure of propanamide.
2. Suggest how this compound can be prepared from propanoic acid.
Include an equation in your answer and state working conditions.
3. Draw the structure of ethanoyl chloride and write an equation for its
reaction with
4. Suggest other possible reactions that can be used to prepare amides in
general5. What reagents and conditions which can be used to reduce amides?
8.7.1. Preparation of amides
Amides can be prepared from all of the other acid derivatives when they react with
ammonia and primary or secondary amines. Their production of amides involvesthe following reactions.
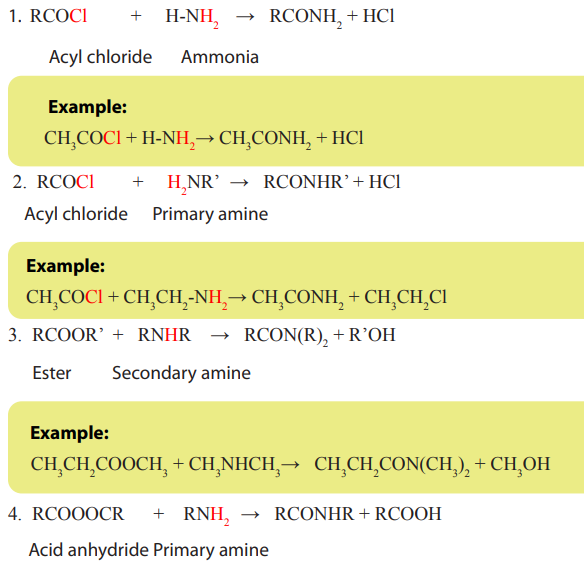

8.7.2. Chemical properties of amides
The reactivity of amides involves different types of reaction to form various organic
compounds.
1. Reduction reaction
Amides are reduced with sodium and ethyl alcohol or with lithium aluminium
hydride
 to yield primary amines.
to yield primary amines.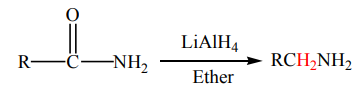

2. Hydrolysis
Amides react with water in acidic medium (dilute) at high temperatures to formacids.


3. Hoffman degradation
Amides react with a mixture of sodium hydroxide and bromine or sodium
hypobromite to produce amines. The reaction is called degradation as the carbonchain is reduced by one carbon.


This equation can be simplified as follows:
Note: Hoffman degradation reaction is used to test the presence of the amide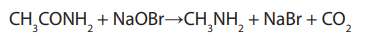
functional group. When an amide is treated with sodium hypobromite, acolorless gas which turns milky lime water is evolved,
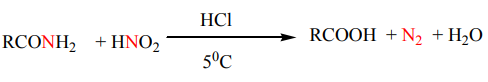
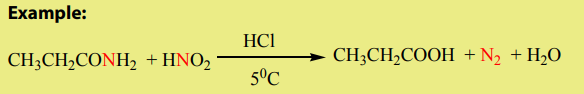
4. Reaction with nitrous acid
Amides react with nitrous acid to produce an acid, water and nitrogen gas.
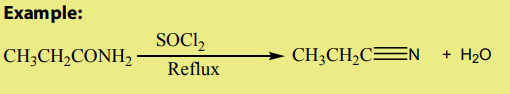
5. Dehydration reaction
Dehydrating reagents, like thionyl chloride remove one molecule of water
remove one molecule of water
from amides to give nitriles. Phosphorous pentoxide can also be used. The reactionis done under reflux.

8.8. Structure and nomenclature of nitriles
Activity 8. 8.
In the previous lesson of this unit you learnt different reactions of amides. Fromyour knowledge and understanding, answer the questions that follow:
1. Draw the structure of compound A:
2. To what homologous series does product A belong?
3. Write the molecular formula of A.
4. Suggest the general structure and the general molecular formula for all
compounds belonging to the same homologous series as A.
5. At room temperature, these compounds are liquids or solids depending
on the molecular mass and yet they lack hydrogen bonding. Suggest abrief explanation for this specialty.
8.8.1. Structure of nitriles
Nitriles are organic compounds with the general structure is its
is its
functional group. The nitrile compounds include a nitrogen atom attached to a
carbon atom by a triple covalent bond. Their general molecular formula is
Unlike other acid derivatives they do not contain an acyl group.
8.8.2. Nomenclature of nitriles
The nitriles are named using the name of the alkane parent followed by the term
–nitrile. The carbon attached to the nitrogen atom is given the location positionnumber 1.
Structure and name of some nitriles are shown in the Table 8.4.

8.8.3. Physical properties and uses of nitriles
Physical properties
The physical properties of nitriles are summarized below.
1. The nitrile compounds are present as colorless solids and liquids having a
characteristic odor.
2. Nitriles have boiling points ranging between 82 and 118 °C. The high
boiling points are due to strong dipole-dipole moments caused by the
polarity of the C N bond.
3. Nitriles compounds exhibit high polar and electronegativity
4. Lower nitriles are highly soluble in water but this solubility decreaseswith the increase in molecular mass as the non-polar part becomes lager.
Uses of nitrile compounds
Nitriles find many uses:
• Nitriles are used in the manufacture of nitrile gloves, seals, and pipes or tubes
as they exhibit resistance to chemicals.
• They are used as an antidiabetic drug which is used in the treatment of breast
cancers.
• This compound is found in many plant and animal sources.
• They are utilized in the applications of oil resistant substances and also for
low-temperature uses
They are also employed in automotive systems, hydraulic tubes and also in aircraftsystems.
Checking up 8. 8
1. Draw the structure of each of the compound below:
a. Butanenitrile
b. 3-methylpentanenitrile2. Name these compounds:

3. Draw all possible isomers of molecular formula
 and name them.
and name them.8.9. Preparation and chemical properties of nitriles
Activity 8. 9
One method of preparing nitriles is to dehydrate an amide.
1. Use your knowledge about chemistry of alkyl halides and suggest
another preparation method.
2. Name the reaction mechanism involved in that method
3. Write an equation of the preparation of propanenitrile using the methodyou have suggested.
8.9.1. Preparation of nitriles
Nitriles are prepared by dehydration of amides under reflux in the presence
of phosphorous (V) oxide,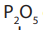 or sulphur dichloride oxide,
or sulphur dichloride oxide, 
and there is elimination of water molecule.
1.Dehydration of amides

2.Nucleophilic substitution of halogenoalkanes
The halogenoalkane is heated under reflux with a solution of sodium or potassium
cyanide in ethanol. The halogen is replaced by a -CN group and a nitrile isproduced.
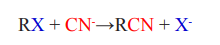
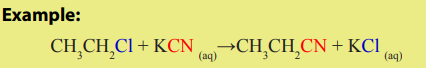
8.9.2. Chemical properties of Nitriles
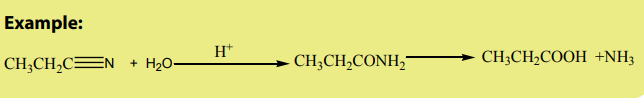
Nitrile compounds undergo various reactions. Nitriles are hydrolyzed in the
presence of an acid or a base to form carboxamides and carboxylic acids. This is
the reason why they are considered to be acid derivatives while they have no acylgroup.
1. Hydrolysis

2. Reduction reaction.
Nitriles can be reduced by to produce primary amines in the presence of
to produce primary amines in the presence of catalysts such as


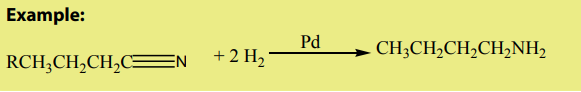
Checking up 8. 9.
1. An aldehyde of molecular formula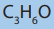 reacts with hydrogen cyanide
reacts with hydrogen cyanide
in strong basic medium to give compound A. compound A undergoes a
reduction to give compound B.
a. Suggest a reducing agent which can be used to reduce A.
b. Draw the structure of the product formed when compound A is treated with hot acidified water
2. What is meant by the term hydrolysis?
3. Nitriles are considered to be acid derivatives even though they do not
have the acyl group. Search from the internet or library a reason for thisconsideration.
8.10. End Unit assessment
Part I: Objective questions
1. The esters are ……. acyl chlorides
d. more reactive than
e. equal in reactivity
f. less reactive than
2. Secondary amines react with acid chloride to give
a. amines
b. carboxylic acids
c. amides
d. imines
3. A mixture of acetic acid and propanoic acid undergo dehydration to give
a. acetic anhydride
b. propanoic anhydride
c. acetic and propanoic anhydrides
d. acetic, propanoic and acetic propanoic anhydrides
4. Ethanoyl bromide reacts with sodium propanoate to give
c. ethanoic anhydride
d. propanoic anhydride
e. ethanoic propanoic anhydride
f. all of the above
5. Esters are made from the reaction between
a. carboxylic acid molecules
b. alcohol molecules
c. alcohol and carboxylic acid molecules
d. acid anhydride and water molecules
6. Ethyl acetate is hydrolyzed by water to give a/an
a. lactone
b. ester
c. acid anhydride
d. carboxylic acid and an alcohol
7. The reaction between ethyl ethanoate and dimethylamine gives an
a. amide
b. imide
c. acid anhydride
8.
reduces Ethanamide to give a/an
a. carboxylic acid
b. amide
c. alcohol
d. amine
9. Nitriles can be hydrolyzed with water to give
a. alcohols
b. aldehydes
c. acidsd. acids and amides
10. Reduction of nitriles gives
e. amide
f. amine
g. imine
h. carboxylic acids
Part II: Structured questions
1. Use equations to show how you could prepare the following compounds,
using the
organic compounds cited as the only organic substances and any inorganicsubstance you need:
5. Draw the structural formula of:
a. 2-chloropropanamide
b. Methylpentanoate
c. Butanoic anhydride
d. Propanoyl chloride
e. N-ethyl-N-propylbutanamide
6. Give the organic products of the following reactions:
a. Propanoic acid and ammonia.
b. Ethanoyl chloride plus methanol.
c. Butanoic anhydride plus water.
d. Propanamide plus sodium hypobromite
e. Ethanol plus propanoyl chloride
7. Give reagents, essential conditions and equations for the conversion of
ethanoic acid into:
a. Ethanoic anhydride
b. Ethanamide
c. Ethyl ethanoate
8. Ethanoic anhydride is a liquid at room temperature but Ethanamide is a
solid. Comment briefly on this.9. Discuss the uses of esters.
10. a. Write an equation for the formation of ethyl ethaonate from ethanoyl
chloride and ethanol. Name and outline the mechanism for the reaction
taking place.
Explain why dilute sodium hydroxide will cause holes in clothing made from
polymers such as terylene while polythene containers can store caustic soda.11. Ethyl oleate is an ester with the molecular structure below:
It is possible the body could synthesize this compound from the ethanol present
in alcoholic drinks and the natural fatty acid, oleic acid.
a. Write the structural formula of oleic acid
b. Construct a balanced equation for the production of ethyl oleate from
ethanol and oleic acid.c. Suggest how oleic acid can be obtained from the triglyceride below
12.This question is about the reactions of carboxylic acids and their derivatives.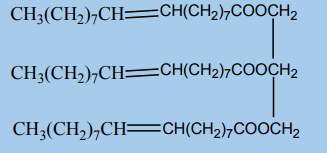
a. A carboxylic derivative X was found to contain C, H, N and O. analysis
gave the following percentage composition by mass: 49.4%, 9.6% and
19.1% for carbon, hydrogen and nitrogen respectively. Compound X had
a relative molecular of 73.
i. Calculate the empirical and molecular formulae of X.
ii. Suggest three possible structures of X.
b. Acyl chlorides such as ethanoyl chloride undergo several reactions due
to their high reactivity. What could be produced when ethanoyl chloride
reacts with:
c. A and B are two isomeric amides which can be hydrolyzed in acidic medium.
i. Water
ii. Propan-2-ol
iii. Ammoniaiv. Sodium acetate
i. Draw the structures of the products formed from hydrolyzing A and B.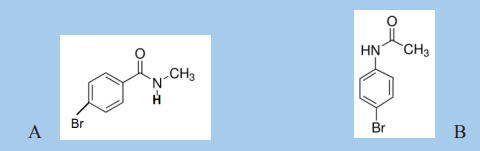
ii. What is the structure of the compound produced when A reacts with
sodium hypobromite?iii. Write an equation for the reaction of B with ethanoyl chloride.
