UNIT 6: CARBONYL COMPOUNDS: ALDEHYDES AND KETONES
Key unit competency
To be able to compare the chemical nature of carbonyl compounds to theirreactivity and uses.
Learning objectives
• Describe the reactivity of carbonyl compounds
• State the physical properties of aldehydes and ketones
• Describe the preparation reactions of ketones and aldehydes
• Explain the mechanism of nucleophilic addition reactions of carbonyl
compounds
• Prepare ketones from secondary alcohols by oxidation reactions
• Compare aldehydes and ketones by using Fehling’s solution and Tollens’
reagent
• Write and name carbonyl compounds and isomers of ketones and aldehydes
• Write equations for the reactions of carbonyl compounds with other
substances
• Compare the physical properties of carbonyl compounds to those of alcohols
and alkenes
• Differentiate the methyl ketones from other ketones by using the iodoform
test
• Carry out an experiment to distinguish between carbonyl compounds and
other organic compounds
• Carry out an experiment to distinguish between ketones and aldehydes• Carry out an experiment to prepare ethanol and propan-2-one.
6.1. Definition and nomenclature of carbonyl compounds
Introductory activity
Many fruits such as mangoes and honey contained sugar. The following imagesrepresent mangoes, honey and some sugars such as fructose and glucose.
1. State the functional groups found in fructose and glucose.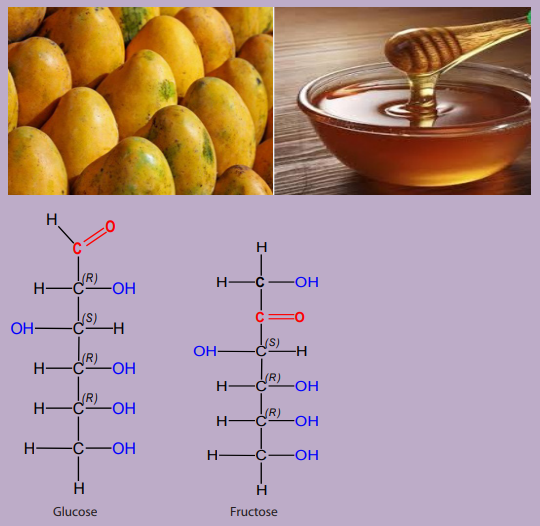
2. Enumerate other foods that contain sugars
3. Describe the similarity and difference between the two sugars in term ofstructure formulae.
6.1.1 Definition
Activity 6.1
Observe the following molecules and answer to the questions.
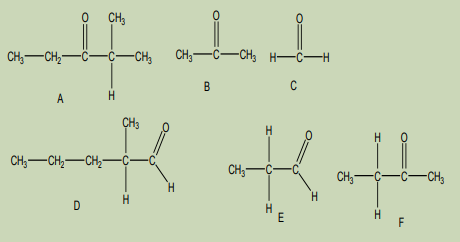
1. Categorize the above molecules
4. What criteria have you used to categorize?
5. Name those categories6. Name individual molecules
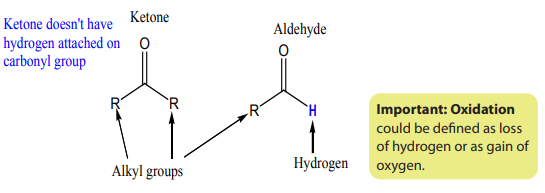
Carbonyl compounds are compounds that contain carbon-oxygen double bond
(C=O). Carbonyl compounds are classified into two general categories based on the
kinds of chemistry they undergo. In one category there are aldehydes and ketones;
in the other category there are carboxylic acids and their derivatives. This unit lookson category of aldehydes and ketones.
Aldehyde molecules
For aldehydes, the carbonyl group is attached to hydrogen atom and alkyl group as
shown in the molecule of propanal below. Methanal is the smallest aldehyde, it hastwo hydrogen atoms attached to carbonyl group.
If you are going to write this in a condensed form, you write aldehyde as –CHO,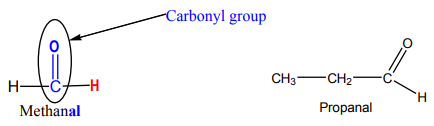
don’t write it as -COH, because that looks like an alcohol functional group.
Ketone moleculesKetone has two alkyl groups attached to the carbonyl group. Examples:

6.1.2 Nomenclature
Aldehydes
The systematic name of an aldehyde is obtained by replacing the terminal “e” from
the name of the parent hydrocarbon with “al.” In numbering the carbon chain of analdehyde, the carbonyl carbon is numbered one.

Ketones
The systematic name of a ketone is obtained by removing the terminal“e” from the
name of the parent hydrocarbon and adding “one.”The chain is numbered in the
direction that gives the carbonyl carbon the smallest number.Ketone contains
a carbon-oxygen double bond just like aldehyde, but for ketone carbonyl groupisbonded to two alkyl groups.

Checking up 6.1
7. For each of the following structures, justify whether it is an aldehyde ora ketone, and name each.
2. Draw the structural formulas derived from the following names.
i. Hexan-3-one
ii. Pentan-2-oneiii. 2-methylpropanal
6.2. Isomerism
Activity 6.2
Look at the molecules below and answer the following questions.
4. Write molecular formula of A and B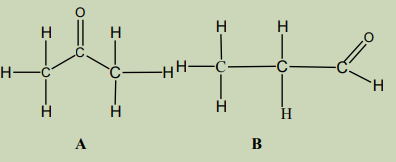
5. Compare the molecular formulae of A and B
6. State a term that can be used to describe relationship between
molecules A and B.7. Write down other three different examples which are related as A and B.
6.2.1 Functional isomerism in aldehydes and ketones
Isomers are molecules that have the same molecular formula, but have a different
arrangement of the atoms in space. Functional group isomers are molecules that
have same molecular formula but contain different functional groups, and theybelong to different homologous series of compounds.
Example1; structural formulae of this molecular formula can be either
structural formulae of this molecular formula can be either propanal or propanone, aldehyde or ketone.

You could draw others possible structural formula of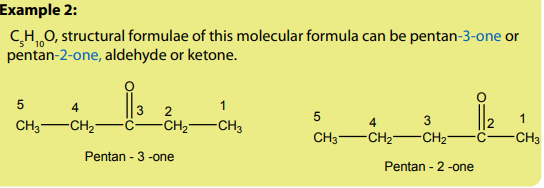

that have alkene and alcohol functional groups.
6.2.2. Position isomerism in ketones
Position isomerism is isomerism where carbon skeleton remains constant, but thefunctional group takes different positions on carbon skeleton.
6.2.3. Chain isomerism in aldehydes and ketones
In chain isomerism the same number of carbons forms different skeletons. Aldehydes
with 4 or more carbon atoms and ketones with five or more carbon atoms showchain isomerism.
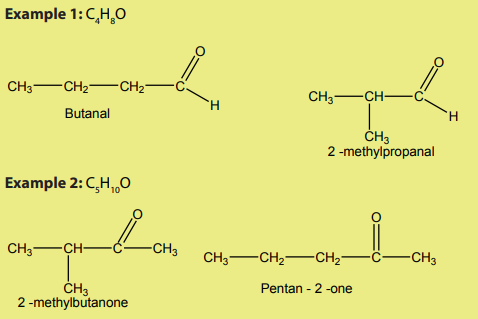
Checking up 6.2
Draw as many as possible all the structural isomers of

6.3. Physical properties of aldehydes and ketones
Activity 6.3
• Take 50 ml for each substance: ethanal, butanal and propanone.
• Mix ethanal with 50ml of water in beaker
• Mix butanal with 50ml of water in beaker
• Mix propanal with 50ml of water in beaker
viii. Compare the solubility of ethanal, butanal and propanone in water.
ix. State intermolecular forces present in each substances
x. Explain what happen in term of intermolecular forces during mixing
those above substances with water.
xi. Explain why some substances have high solubility in water than other.
xii. how the intermolecular forces present in ethanal, butanal and
propanone affect other physical properties like boiling and meltingpoint of these substances.
6.3.1. Solubility in water aldehydes and ketones
The small molecules of aldehydes and ketones are soluble in water but solubility
decreases with increase of carbon chain. Methanal, ethanal and propanone - the
common small aldehydes and ketones are soluble in water at all proportions.
Even though aldehydes and ketones don’t form hydrogen bond with themselves,they can form hydrogen bond with water molecules.
The slightly positive hydrogen atoms in a water molecule can be sufficiently attracted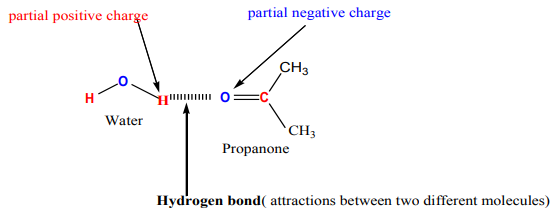
to the lone pair on the oxygen atom of an aldehyde or ketone to form a hydrogen
bond.
Other intermolecular forces present between the molecules of aldehyde or ketone
and the water are dispersion forces and dipole-dipole attractions.
Forming these attractions releases energy which helps to supply the energy needed
to separate the water molecules and aldehyde or ketone molecules from each other
before they can mix together.
Apart from the carbonyl group, hydrocarbon chains are non polar, they don’t dissolve
in water. By forcing hydrocarbon chain to mix with water molecules, they break the
relatively strong hydrogen bonds between water molecules without replacing them
by other attractions good like hydrogen bonds. This makes the process energeticallyless profitable, and so solubility decrease.
6.3.2. Boiling points of aldehydes and the ketones
Methanal is a gas and has a boiling point of
and ethanal has a boiling point of The other aldehydes and ketones
The other aldehydes and ketones
are liquids or solids, with boiling points rising with rising of molecular mass
hence rising of strength of Van der Waals force.
Comparing the physical properties of carbonyl compounds to those of alcoholsand alkanes.
Physical properties of covalent compounds depend on intermolecular forces.
Compounds that have similar molecular mass but different intermolecular forceshave different physical properties.
Example of comparison between molecules of similar mass but differentcompositions.
Alcohols have higher boiling point than aldehydes and ketones of similar lengths. In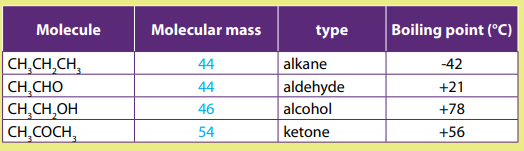
the alcohol, there is hydrogen bonding, but the molecules of aldehydes and ketones
don’t form hydrogen bonds.Aldehydes and ketones are polar molecules but alkanesare non polar molecules.
Checking up 6.3
The Table below shows the boiling points of an alkane, an aldehyde and analcohol.
m. Explain why the boiling point of an aldehyde is greater than that of the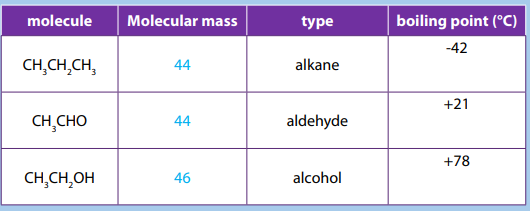
alkane?
n. Why is the boiling point of the alcohol still higher?
o. Explain why, unlike the similar-sized alkanes, the small aldehydes and
ketones are soluble in water.p. Describe the solubility variation of aldehydes and ketones.
6.4. Chemical properties of carbonyl compounds
6.4.1. Nucleophilic addition reactions
Activity 6.4.1
• KCN is a reagent used to add HCN to carbonyl compounds.
Write equation that show how KCN dissociates in polar solvent
• Observe carefully the following carbonyl functional group and answer thefollowing questions.

a. Polarity of carbonyl group
by comparing carbon-carbon double bond and carbon- oxygen double bond
the only difference between bonds C=C and C=O is distribution of electrons. The
distribution of electrons in the pi bond is heavily attracted towards the oxygen atom,because oxygen atom is much more electronegative than carbon.
During chemical reactions nucleophiles will attack carbon of the carbonyl functional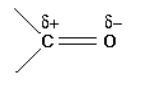
group which bears apartial positive charge. While electrophile will attack oxygen ofthe carbonyl functional group which bears a partial negative charge.
b. Reaction of HCN with aldehydes and ketones
Because hydrogen cyanide is a toxic gas, the best way to carry out this reaction is to
generate hydrogen cyanide during the reaction by adding HCl to a mixture of the
aldehyde or ketone and excess sodium cyanide. Excess sodium cyanide is used in
order to make sure that some cyanide ion is available to act as a nucleophile. The
solution will contain hydrogen cyanide (from the reaction between the sodium or
potassium cyanide and the HCl)
The pH of the solution is maintained in range 4 - 5, because this gives the fastestreaction. The reaction takes place at room temperature.
c. The mechanism of reaction between HCN and propanone
1st Step: A nucleophilic, CN-, attacks on the slightly positive charged carbon ofcarbonyl group.
2ndStep: The negative ion formed picks up a hydrogen ion from hydrogen cyanide.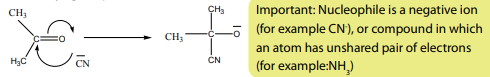
Water or the ions
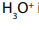 present in solution can serve as source of the hydrogen ion.
present in solution can serve as source of the hydrogen ion.
These are examples of nucleophilic addition

e. Reaction of
The aldehyde or ketone is shaken with a saturated solution of sodium hydrogen with aldehydes or ketones
with aldehydes or ketones
sulphite in water. Hydrogen sulphite with negative charge act as nucleophile,
where the product formed is separated as white crystals. Propanone react hydrogensulphite, as below:
Impure aldehyde and ketone can be purified by using this reaction. Impure
aldehyde or ketone is shaken with a saturated solution of sodium hydrogensulphite
to produce the crystals. Impurities don’t form crystals; these crystals formed are
filtered and washed to remove any impurities. Addition of dilute acid to filtered
crystals regenerates the original aldehyde. Dilute alkali also can be added insteaddilute acid.
Checking up 6.4.1
Aldehydes and ketones undergo addition reactions involving hydrogen
cyanide in which H and CN add on the carbon-oxygen double bond.
a. Why isn’t hydrogen cyanide itself normally used in these reactions?
b. Give a mixture which can be used instead of starting with hydrogen
cyanide itself.
c. Draw the structures and give the names of the products of the
reaction between hydrogen cyanide and
i. (Ethanal
ii. Propanone
d. One use of the products of these reactions (known as hydroxy nitriles)
is as a part of a sequence of reactions to make more complicated
molecules like amino acids from more simple ones. The amino acidvaline has the structure:
i. Write the structure of the hydroxy nitrile which you would have to modify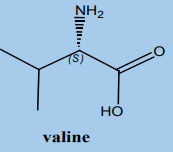
in order to make valine
ii. Write the structure of the aldehyde or ketone which you would have toreact with hydrogen cyanide in order to get that hydroxy nitrile
6.4.2. Condensation reactions
Activity.6.4.2
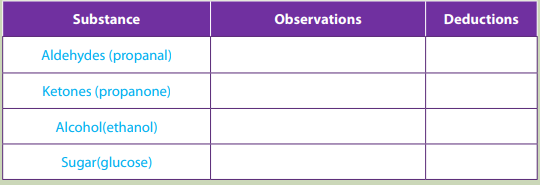
You are provided with the following: propanal, propanone, alcohol (ethanol),
glucose solution and 2,4-dinitrophenylhydrazine (Brady reagent )
Take about 2ml of each solution; propanal, propanone, alcohol (ethanol) and
glucose solution in test tubes. Add 6 drops of the 2,4-dinitrophenylhydrazine
to each of the test tubes containing: propanal, propanone, (alcohol)ethanol or
glucose solution. If no precipitate forms immediately, warm for 5 minutes in thewater bath. Record your observations in the table below.
a. Experimental reaction
The procedure of the preparation of Brady’s reagent and carbonyl compounds
changesslightly depending on the nature of the aldehyde or ketone, and the solvent
in which 2,4-dinitrophenylhydrazine is dissolved in. The Brady’s reagent for activities(6.4.1) is a solution of the 2,4-dinitrophenylhydrazine in methanol and sulphuric acid.
Add a few drops of Brady’s reagent to either aldehyde or ketone. A bright orange or
yellow precipitate indicates the presence of the carbonyl group in an aldehyde orketone.
b. Structural formula of 2,4-dinitrophenylhydrazine.
The carbon of benzene attached to hydrazine is counted as number one.
In 2,4-dinitrophenylhydrazine, there are two nitro groups,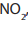
,attached to the phenyl group in the 2- and 4- positions.
2,4-dinitrophenylhydrazine is often abbreviated as 2,4-DNP or 2,4-DNPH.
c. The reaction of carbonyl compounds with 2,4-dinitrophenylhydrazine
Brady’s reagent is a solution of the 2,4-dinitrophenylhydrazine in methanol
and sulphuric acid. The overall reaction of carbonyl compounds with2,4-dinitrophenylhydrazine is:
Where R and R’ represent alkyl groups or hydrogen(s); if both or only one is hydrogens
the starting carbonyl compound is an aldehyde. If both R and R’ are alkyl groups
the carbonyl compound is a ketone. The following molecule shows clearly how theproduct is formed.
The product formed is named”2,4-dinitrophenylhydrazone”. The simple difference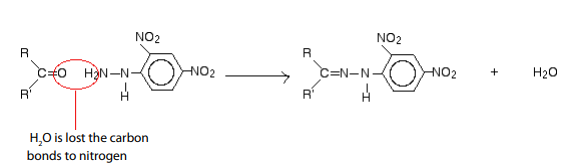
consists in replacing suffix “-ine” by “-one”.
The reaction of 2,4-dinitrophenylhydrazine with ethanal produces ethanal
2,4-dinitrophenylhydrazone; The reaction of 2,4-dinitrophenylhydrazine with
butanal produces butanal 2,4-dinitrophenylhydrazone. This is an example ofcondensation reaction.
During the chemical reaction, the change takes place only on nitrogen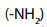 of
of
hydrazine in 2,4-dinitrophenylhydrazine. If the group is attached to other
group is attached to other groups a similar reaction as that of 2,4-dinitrophenylhydrazine will take place:
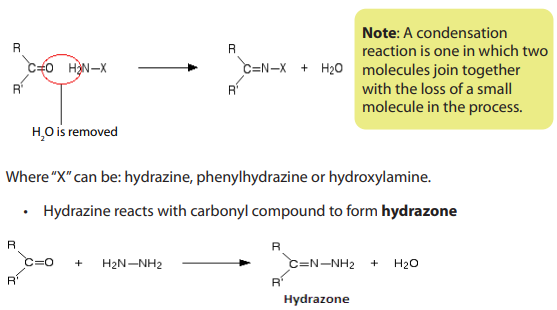

• Phenylhydrazine reacts with carbonyl compound to form“phenylhydrazone”.
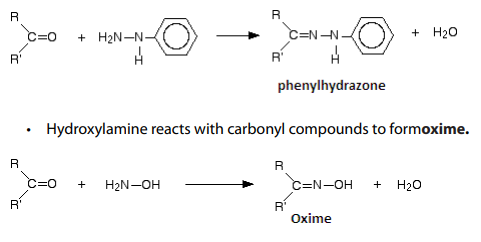
Checking up 6.4.2
a. Brady’s reagent is a solution of 2,4-dinitrophenylhydrazine in a mixture
of methanol and sulphuric acid.
i. How is Brady’s reagent used to test for an aldehyde or ketone?
b. Draw the structural formulae for
ii. Propanone hydra zone
iii. Propanone phenylhydrazone
6.4.3. Oxidation reactions using
Activity 6.4.3
Materials:
• Test tubes
• Test tubes holder
• Test tube racks
• Count droppers
• Beakers
You are provided with the following: propanal, propanone and potassium dichromate
(VI) solution acidified with dilute sulphuric acid.
Take about 2ml of each solution; propanal and propanone; add 6 drops of the
potassiumdichromate(VI) solution acidified with dilute sulphuric acid.
Record your observations in the table below.
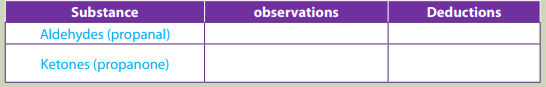
a. Difference in reactivity of ketones and aldehydes with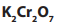
By considering the structural formulae of aldehydes and ketones, the difference is
only the presence of a hydrogen atom attached to the carbonyl functional group inthe aldehyde whereas ketones have a alkyl group instead.
During chemical reaction aldehydes react with oxidizing agent; hydrogen on
carbonyl functional group is replaced by oxygen, look on figure below. The presence
of hydrogen atom makes aldehydes very easy to oxidize, in other words aldehydesare strong reducing agents.
For ketone, absence of hydrogen on carbonyl functional group makes ketones to
resist oxidation. But very strong oxidising agents like potassium permanganate
solution oxidize ketones - and they do it in a destructive way, by breaking carbon bonds.
Aldehyde oxidation can take place in acidic or alkaline solutions. Under acidic
solutions, the aldehyde is oxidized to a carboxylic acid. Under alkaline solutions, acidformed react with base to form a salt of carboxylic acid.
Add few drops of the aldehyde or ketone to a solution of potassium dichromate
(VI) acidified with dilute sulphuric acid. If the color doesn’t change in the cold, themixture is warmed gently in a beaker containing hot water.

b. Oxidation of aldehyde by
Add few drops of the aldehyde or ketone to a solution of potassium dichromate solution
solution
(VI) acidified with dilute sulphuric acid. If the color doesn’t change in the cold, themixture is warmed gently in a beaker containing hot water.

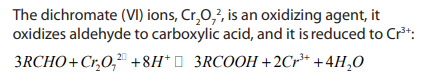
Checking up 6.4.3
1. If you react ethanal with acidified potassium dichromate (VI) solution,
what organic product would you get?
2. Write a half-equation for the formation of that product from ethanal.
3. iii.Write a half-equation for the dichromate(VI) ion acting as an oxidisingagent is
Use this equation and the one you wrote in part (ii) to work out the ionic
equation for the reaction.
6.4.4. Oxidation reactions using Tollens’ reagent
Activity 6.4.4
Materials:
• Test tubes
• Test tubes holder
• Test tube racks
• Count droppers
• Beakers
• Bunsen burner
You are provided with the following:
• propanal,
• propanone
• Tollens’ reagent.
Take about 2ml of each solution; propanal and propanone. Add 6 drops of
the Tollens’ reagent to each of the following in the test tubes; propanal or
propanone. Warm gently the mixture in a hot water bath for a few minutes.Record your observations in the table below.
a. Difference in reactivity of Ketones and Aldehydes with Tollens’ reagent
Aldehydes can also be oxidized into carboxylic ions in basic medium.Tollens’ reagent
is a solution of diamminesilver (I) ion,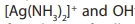 .In order to identify if a
.In order to identify if a
substance is aldehyde or ketone, add few drops of Tollens reagent to test tubes
containing aldehyde or ketone and warm gently in a hot water bath for a few
minutes. The formations of sliver mirror or grey precipitate is an indication of thepresence of aldehyde.

Refer to experimental student book:
Checking up 6.4.4
1. Tollens’ reagent is alkaline because of the sodium hydroxide solution
and ammonia solution used to make it. What organic product would you
get in this case if you reacted propanal with Tollens’ reagent?
2. Write half equation for the formation of that product from propanal.
3. Write the half-equation for the reaction of the ion when it
ion when it
forms the visible product of the reaction.
Combine these two half-equations to give an ionic equation for the reaction ofTollens’ reagent with ethanal.
6.4.5. Oxidation reactions using Fehling ;or Benedict; solution
Activity 6.4.5
Materials:
• Test tubes
• Test tubes holder
• Test tube racks
• Count droppers
• Beakers
• Bunsen burner
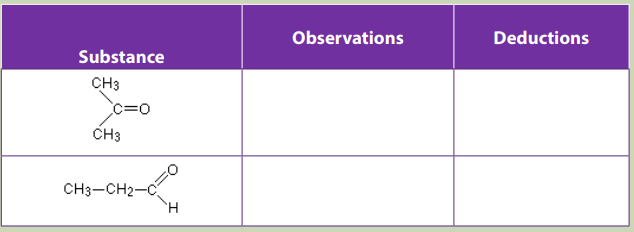
You are provided with the following: ethanal, propanone, Fehling’s solution and
Benedict’s solution.
Take about 2ml of each solution. Add 6 drops of the Fehling’s solution
or Benedict’s solution to each of the tubes containing 2ml of ethanal or
propanone to be tested. Warm gently the mixture in a hot water bath for a fewminutes. Record your observations in the table below.
a. Difference in reactivity of Ketones and Aldehydes with Fehling or Benedict
solution.
Fehling’s solution and Benedict’s solution react with aldehyde in the same way;
both solutions contain . In Fehling’s solution
. In Fehling’s solution  is complexed with tartrate
is complexed with tartrate
ligand butin Benedict’s solution is complexed with citrate ligand.
is complexed with citrate ligand.
Don’t worry about ligands, important reagents are ligands tartrate and
ligands tartrate and
citrate are used to prevent formation of precipitate copper (II) hydroxide or copper
(II) carbonate.
A few drops of Fehling’s solution or Benedict’s solution is added to the aldehyde orketone and the mixture is warmed gently in a hot water bath for a few minutes.
Fehling’s solution and Benedict’s solution are oxidizing agent, they oxidize aldehydes
to carboxylic acid. Remember that reaction takes place in basic solutions, acid
formed is neutralized by base, and hence the products area salt of carboxylic acidinstead of carboxylic acid. Equations of reaction.

Checking up 6.4.5
Fehling’s solution and Benedict’s solution both contain copper (II) complexes
in an alkaline solution. The copper (II) complex can be simplified to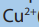 (in
(in complex), and the electron-half-equation given as
a. Write the electron-half-equation for the oxidation of propanal in an

alkaline solution.
b. Combine this with the equation above to give the ionic equation for thereaction between Fehling’s or Benedict’s solution with propanal
6.4.6. Iodoform reaction with aldehydes and ketones
Activity 6.4.6
Materials:
• Test tubes
• Test tubes holder
• Test tube racks
• Count droppers
• Beakers
• Bunsen burner
You are provided with the following: propanone, propanal, 6M NaOH solution
and solution
solution
Put 4 drops of each tested substances, propanone, propanal, into different test
tubes.
Add to this 0.5 mL distilled water to each test tube.
Add 0.25mL 6M NaOH and 0.25 mL of water to each test tube.
Add 6 drops of -s solution to each test tube.
solution to each test tube.
If no precipitate forms immediately, warm the mixture very gently.Record your observations in the table below.
a. Reagents for iodoform reaction
There are two different mixtures that can be used to do iodoform test, these
mixture are:
• Iodine and sodium hydroxide solution
• Potassium iodide and sodium chlorate (I) solutions
Don’t worry about Potassium iodide and sodium chlorate(I) solutions, Potassium
iodide and sodium chlorate(I) react to form final solution
.Both mixtures contain the same reagents.
Each of these mixtures contains important reagent which react
which react
with aldehyde or ketone. When is added to a carbonyl compound
is added to a carbonyl compound
containing the group (blue in the cycle) as shown below, pale yellow precipitate
(blue in the cycle) as shown below, pale yellow precipitate (triiodomethane) is formed.

a. Description of iodoform test
For iodine and sodium hydroxide solution
Iodine solution,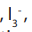 is added to aldehyde or ketone, followed by just enough sodium
is added to aldehyde or ketone, followed by just enough sodium hydroxide solution to remove the colour of the iodine. If pale yellow precipitate
doesn’t form in the cold, it may be necessary to warm the mixture very gently.
The positive result is pale yellow precipitate of
For potassium iodide and sodium chlorate (I) solutions
Potassium iodide solution is added to a small amount of aldehyde or ketone, followed
by sodium chlorate (I) solution. If pale yellow precipitate doesn’t form in the cold,warm the mixture very gently. The positive result is pale yellow precipitate of
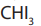
Reaction of iodoform test
The reagents of iodoform test are and OH solution. The reaction takes place into
and OH solution. The reaction takes place into
two main steps:
• Three hydroxides, OH-, remove three hydrogens from methyl group and theplace of hydrogen is taken by iodide
• group
 is a good leaving group;
is a good leaving group; 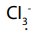 is replaced by OH-to form carboxylic
is replaced by OH-to form carboxylic
acid, because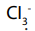 is a base according to Bronsted-Lowry, it reacts with acid to
is a base according to Bronsted-Lowry, it reacts with acid to form the following product:
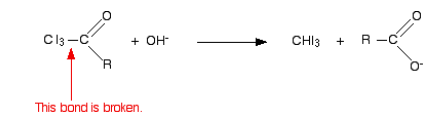
The overall equation for reaction of iodoform test:
The same reaction takes place for other halogen elements in the same way.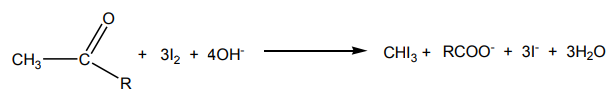
Refer to experimental student book.
When methyl ketones or methyl aldehyde, ethanal, are treated with the halogen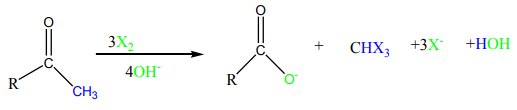
element in basic solution, hydrogens of the methyl group are replaced by halogen
element followed by cleavage of the methyl group. The products are the salt of
carboxylic acid and trihalomethane. The reaction is fast until the 3 hydrogens at themethyl group have been replaced by a halogen.
Checking up 6.4.6
A has the formula Its oxidation gives B with the formula
Its oxidation gives B with the formula 
B reacts with 2,4-dinitrophenylhydrazine to give a positive test. A is dehydrated by
concentrated . Reductive of C gives butanal.
of C gives butanal.Identify the compound A
6.5. Preparation methods of aldehydes and ketones
6.5.1. Oxidation of alcohols
Activity 6.5.1
The set up below represents the method of preparation of ethanal from ethanol.Look it carefully and answer the following questions.
Ethanol reacts with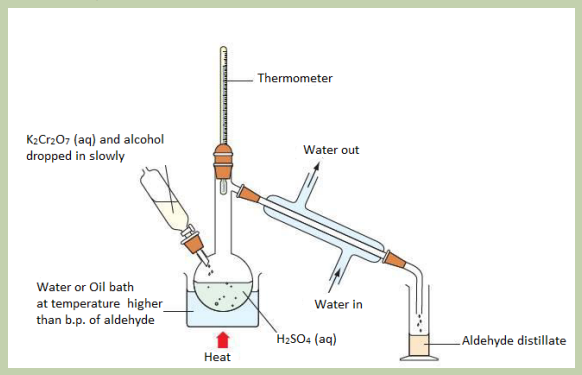
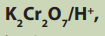 does ethanol undergoes oxidation or
does ethanol undergoes oxidation or
reduction in this reaction?
3. Write down chemical equation that takes place in this experiment
4. Explain why it is necessary to heat and explain the point of choosing
temperature at which reaction takes place.5. Write a balanced equation of the reaction between propan-2-ol and
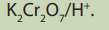
a. alcohol by
Pdichromate (VI) ions is reduced to chromium (III) ions, Cr3+ which is green.otassium dichromate (VI) acidified with dilute sulphuric acid is used as oxidizing
agent during the preparation of aldehyde or ketone. Primary alcohol is oxidized to
aldehyde, oxygen atom from the oxidising agent removes two hydrogens; one from
the -OH group of the alcohol and the other hydrogen comes from the carbon that isattached to hydroxide functional group.
• Primary alcohol undergoes oxidation to produce aldehyde

• Secondary alcohol undergoes oxidation to produce ketone
• Tertiary alcohol doesn’t undergo oxidation because the carbon bonded to
hydroxide doesn’t have hydrogen to be removed.
The solution of dichromate (VI) ions,
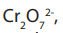 , is orange, during chemical reaction
, is orange, during chemical reactiondichromate (VI) ions is reduced to chromium (III) ions,
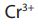 which is green.
which is green.
b. Technique of stopping oxidation of aldehyde
The aldehyde produced by oxidation of alcohol could make further oxidation to a
carboxylic acid if the acidified potassium dichromate (VI) is still present in solution
where reaction takes place. In order to prevent this further oxidation of aldehyde to
carboxylic the following technique are used.
• Use an excess of the alcohol than potassium dichromate (VI). Potassium
dichromate (VI) is limiting reactant hence there isn’t enough oxidising agent
present to carry out the second stage of oxidizing the aldehyde formed to a
carboxylic acid.
• Distil off the aldehyde as soon as it forms. Removing the aldehyde as soon as it
is formed this means that aldehyde is removed from solution where oxidizing
agent is, to prevent further oxidation. Ethanol produces ethanal as shown bythe following reaction.
To simplify the writing of the reaction, [O] represents oxygen from an oxidising
agent. Then the reaction is written as follows:
1. If you want to oxidize ethanol to ethanal without further oxidation to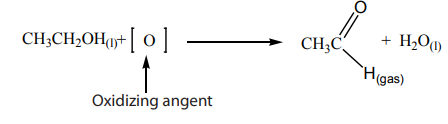
ethanoic acid, how do you proceed?
2. Which oxidising agent is used to oxidize alcohols to either aldehydes orketones, and what would you observe during the reaction?
Checking up 6.5
1. Draw the structure of the aldehyde or ketone that would be formed
if each of the following alcohols is oxidised. You can assume that
conditions are fulfilled to avoid further oxidation of the aldehyde to acarboxylic acid.
2. Draw the structure of the alcohol you would oxidize in order to obtain
each of the following compounds.
i. pentan-2-oneii. Butanal
c. Oxidation of alkene by
Oxidation of alkenes with hot concentrated acidified potassium manganate (VII)
solution produces carbonyl compounds. Consider the general formula of alkenebelow:
Where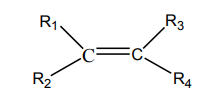
 represent alkyl groups or hydrogen atoms
represent alkyl groups or hydrogen atoms
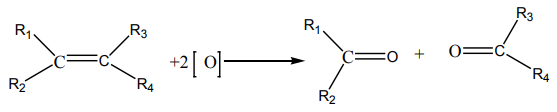
Carbon-carbon double bond of alkene is broken by acidified potassium manganate
(VII) and is replaced by two carbon-oxygen double bonds to each carbon fromdouble bond. General equation:
If acidified potassium manganate (VII) is still present in solution, aldehyde makes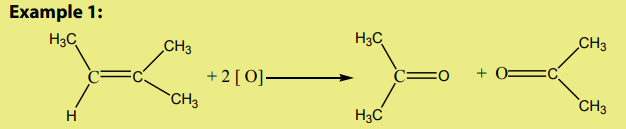
further oxidation to carboxylic acid.
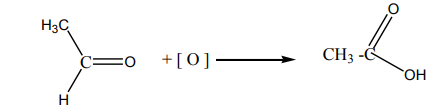
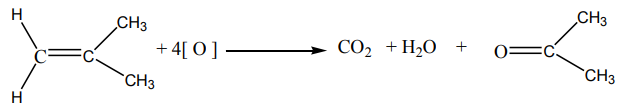
Methanoic acid has hydrogen attached on carbonyl group hence it makes further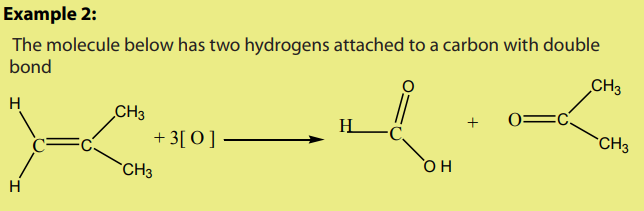
oxidation to carbon dioxide. Final equation is written as below:
6.5.2. Preparation of ketone by distillation of calcium acetate
Procedure: Transfer 15g of calcium acetate in 50ml round bottom flask fixed on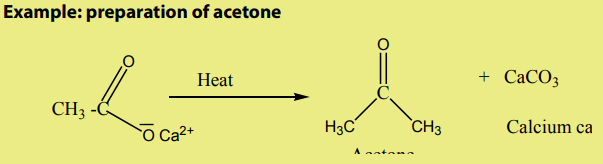
a stand, and place it on a heating mantle fitted with a condenser and a receiver
flask. Adjust the temperature until the condensation starts. Use the aluminium
foil to insulate the flask. Heat the flask and collect the acetone in receiver flask. The
obtained product is a crude acetone and needs to be purified. Set up a distillation
apparatus and distil the crude product to obtain pure acetone Do not forget
Do not forget
to use stirrer bar which must be placed in the round bottom flask containing theacetone.

6.6. Uses of aldehydes and ketones
Aldehydes and ketones have many uses for example in industries such aspharmaceutical industry and in medicine.
a. Formaldehyde:
Formaldehyde is a gas at room temperature but is sold as a 37 percent solution in water.
Formaldehyde is used as preservative and germicide, fungicide, and insecticide
for plants and vegetables. Formaldehyde is mainly used in production of certain
polymers like Bakelite (Figure 6.1). Bakelite and formaldehyde is used asmonomers in production of Bakelite.
b. Acetone as solvent:
Acetone is soluble in water at all proportions and also dissolves in many organic
compounds. Boiling point of acetone is low, 56 °C, which makes it easier to be removed by
evaporation. Acetone is an industrial solvent that is used in products such as paints,
varnishes, resins, coatings, and nail polish removers.
c. Aldehydes and ketones
Organic molecules that contain ketones or aldehydes functional group are found in
different foods such as irish potatoes, yellow bananas.
Aldehydes and ketones perform essential functions in humans and other living organisms.
For examples sugars, starch, and cellulose, which are formed from simple molecules that
have aldehyde or ketone functional group.
d. Aldehydes and ketones in human’s body

Aldehydes and ketones functional group are found in humans hormones likeprogesterone, testosterone.
6.7. End Unit Assessment
1. An aliphatic aldehyde A has the formula RCHO.
a. A reacts with 2,4-dinitrophenylhydrazine. Explain what happens and
name the type of reaction.Say how the product of reaction could be used to identify A
b. When A is treated with warm, acidified solution, B is formed.
solution, B is formed.
Give the structural formula of B.
c. When A is treated with lithium tetrahydridoaluminate (reducing agent)
in ethoxyethane solution C is formed. Give the structural formula of C.
d. A is warmed gently with ammoniacal silver nitrate. Explain what
happens, and say what is observed.
e. B and C react to form D. Write the structural formula of D.
f. From the compounds A, B, C, and D, which would you predict to
possess:
i. Highest boiling pointii. Lowest boiling point
2. Three compounds E, F, and G all have the molecular formula
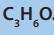
E is an alcohol, F is ketone and G is aldehyde.
i. Draw all possible structural formulae for E, F, and G.
ii. Describe tests (reagents, conditions and observations with each
compound) that would allow you to show that.
3. E is an alcohol whereas F and G aren’t
4. F and G are carbonyl compounds whereas E isn’t
5. G is aldehyde, whereas E and F aren’t.
6. Write balanced equations for all reactions that occur.
a. One of the compounds responsible for the flavor of butter is butan-2,3-
dione.
i. Give the structural formula of butan-2,3-dione.
Give the structural formula of the organic products formed when butan-2,3-dione reacts with

3. Carbonyl compounds X undergoes the following reactions
X gives an orange precipitate with 2, 4-dinitrophenylhydrazine.
X gives pale yellow precipitate with mixture of potassium iodide and sodium
iodate (I) X Doesn’t react with warm acidified
X doesn’t react with aqueous bromine.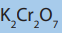 solution.
solution.
X is reduced by hydrogen in the presence of catalyst to a mixture of isomers
Y and Z of formula Identify X, and give the structural formulae of X, Y
Identify X, and give the structural formulae of X, Yand Z.
4. P has the formula . It forms a compound by reaction with hydrogen
. It forms a compound by reaction with hydrogen
cyanide which has the formula . P gives a positive iodoform test, a
. P gives a positive iodoform test, a
silver mirror with Tollens’ reagent and can be reduced to pentane. What is P?
5. a. The carbon-oxygen double bond present in aldehydes and ketones is very
polar. What does this mean and how does it arise?
b. The carbon-oxygen double bond is readily attacked by nucleophiles like
cyanide ions or ammonia.
i. What do you understand by the term nucleophile?
ii. Which part of the carbon-oxygen double bond is attractive to
nucleophiles?
6. Warfarin is an oral anticoagulant, a drug that inhibits the clotting of blood.
It prevents the formation of blood clots by reducing the production of factors
by the liver that promote clotting, factors II, VII, IX, and X, and the anticoagulantproteins C and S. The structural formula of Warfarin is:
a. Name any three different functional groups present in the Warfarin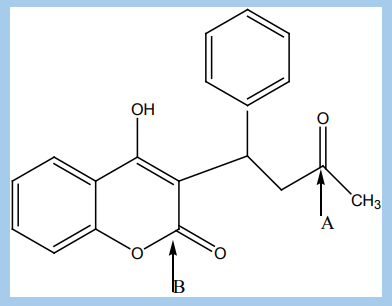
molecule.
