UNIT 5: ALCOHOLS AND ETHERS
Key unit competency:
To be able to compare the physical and chemical properties of alcohols and ethers totheir preparation methods, reactivity and uses.
Learning objectives:
• Distinguish between alcohols from other organic compounds by
representing the functional group of alcohols
• Classify primary, secondary and tertiary alcohols by carrying out the method
of identification
• Write the name of alcohols by using IUPAC system
• Describe the physical properties of alcohols to other series of organic
compounds
• Carry out the method of preparation of alcohols
• Describe the local process of making alcohol by fermentation.
• Explain the effect of oxidation on urwagwa when it overstays
• Compare the physical, chemical and the method of preparation of alcohols to
ethers• State the use of ethers
Introductory activity
The following represent two pictures or pictures A and B. Observe each picturecarefully and answer to the related questions.

1. Discuss the meaning of each picture.
2. What objects do you observe in the above pictures?
3. Explain the consequences that can arise from the picture B
5.1. Definition and nomenclature
Activity 5.1
1. Look at the following compounds and classify them in theirhomologous series.
2. By doing your own research, distinguish the rules used to name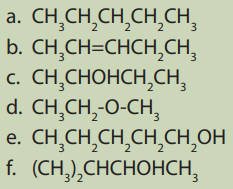
alcohol compounds.
5.1.1. Definition
Alcohols are organic compounds that are derivatives of hydrocarbons where one
or more hydrogen atoms of hydrocarbon is or are replaced by hydroxyl (-OH) group.They are represented by the general formula:
where R is a radical: alkyl group made by a chain of carbon atoms
Alcohols are called monohydric if only one hydroxyl group is present
 Dihydric alcohols are those with two hydroxyl group
Dihydric alcohols are those with two hydroxyl group
(diol: vicinal and gem), trihydric (triols) and polyhydric are those with many – C-OH groups.
The functional group attached is –OH group to any atom of carbon.5.1.2. Nomenclature
According to IUPAC system, alcohols are named by replacing the final ‘‘e’’ of the
parent hydrocarbon with ‘‘ol’’, then specify the position of -OH group before endingby ol.

When there are more than one hydroxyl group present, prefixes, di, tri, tetra,... are used.

 1. Write the structural formulas of all organic compounds containing
1. Write the structural formulas of all organic compounds containingNotice: -OH group takes priority over alkyls substituents, double or triple bonds and
even halides.

Checking up 5.1 According to IUPAC system, name each of the following compounds:
5.2. Classification and isomerism
Activity 5.2
C-OH group and fit the molecular formula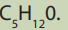
2. Based on their structures and your knowledge about classes of
halogenoalkanes, classify the compounds identified in 1) above.
3. Classify them as chain and position isomers.4. Which of them can exhibit optical isomerism?
Alcohols are classified as:
Primary alcohols: These have only one alkyl group attached to the carboncarrying the –OH.
Examples
Secondary alcohols: they are alcohols in which the OH group is attached to carbon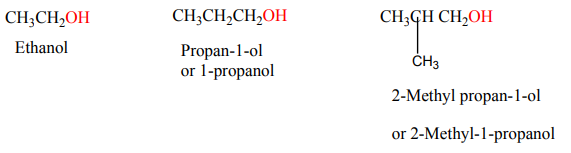
atom bonded to two other carbon atoms.
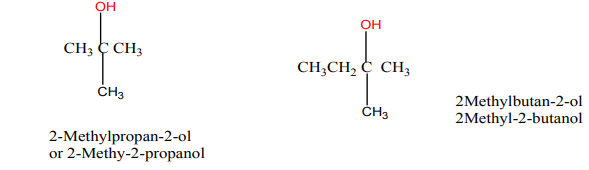
Tertiary alcohols: they are alcohols in which the OH group is attached to carbon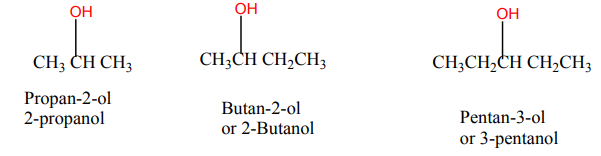
atom bonded to three other carbon atoms.
Alcohols containing at least three carbon atoms exhibit different types of isomerism:
• Chain isomerism:
This is due to the difference in the size of the chain.

• Position isomerism:
This is due to different positions taken by the –OH in the same carbon chain.

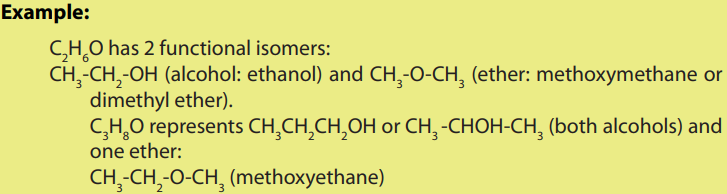
• Functional isomers:
Except methanol which has one carbon, other alcohols are isomers with ethers
another chemical function of general formula R-O-R’
where R and R’ are alkyl groupsor aryl groups but not hydrogen.
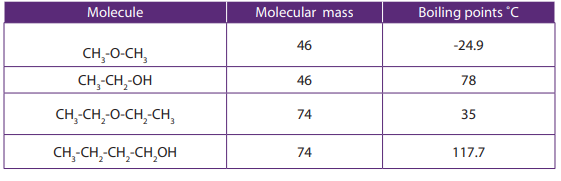
5.3. Physical properties
Activity 5.3.
Analyze the following data and answer to the question
Explain the trends in the boiling point of the molecules given in the table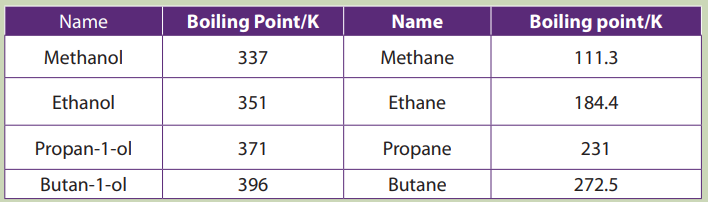
Compare and explain the differences in the boiling point of alkanes and alcohols
a. Boiling points
The chart shows the boiling points of some simple primary alcohols and alkaneswith up to 4 carbon atoms.
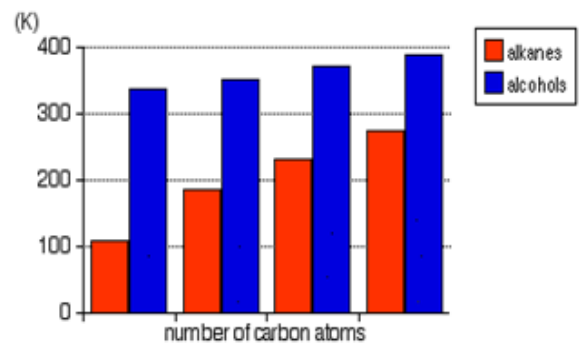
Figure 5.1: boiling points of alcohols and alkanes
• The boiling point of an alcohol is always much higher than that of the alkane
with the same number of carbon atoms.
• The boiling points of the alcohols increase as the number of carbon atoms
increases.
• The boiling point of alcohols with branches is lower than that of unbranched
alcohols with the same number of carbon atoms. This is because increased
branching gives molecules a nearly spherical shape and the surface area
of contact between molecules in the liquid. This results in weakened
intermolecular forces and therefore in lower boiling points.
• Tertiary alcohols exhibit the lowest boiling point than secondary and primary
alcohols:
• Primary alcohol > Secondary alcohol > Tertiary alcohol
Highest boiling point lowest boiling point
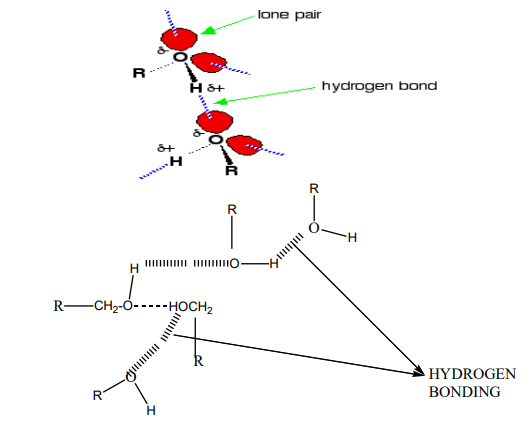
The patterns in boiling point reflect the patterns in intermolecular attractions:
In the case of alcohols, there are hydrogen bonds set up between the slightly positivehydrogen atoms and lone pairs on oxygen in other molecules.
b. Solubility of alcohols in water
The lower members of alcohols are completely soluble in water because mixed
hydrogen bonds between water and alcohol molecules are formed. As the length ofhydrocarbon group of the alcohol increases, the solubility decreases.
c. Volatility
Alcohols are volatile and the volatility decreases as the molecular mass increases.
Compared to alkyl halides, alcohols are less volatile. Polyalcohol are viscous or solids.
Example: propane-1, 2, 3-triol (glycerine). This is due to stronger intermolecularforces than those of mono alcohols.
Checking up 5.3
1. Comment on the solubility of alcohols compared to alkanes in water.
2. Ethanol with a molecular mass of 46 and butane with a molecular mass
of 58 have the boiling point of
 respectively.
respectively. Explain these differences.
3. Are alcohols electric conductors? Justify your answer.
5.4. Alcohol preparations
Activity 5.4
Complete the following chemical equations. For each, show the mechanism ofthe reaction
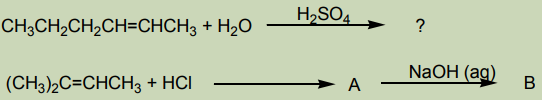
Alcohols are prepared with different methods
a. From alkyl halides
Alkyl halides when refluxed with aqueous alkali (NaOH or KOH) or moist silver
oxide (Ag OH) produce alcohols. The hydrolysis occurs by a nucleophile substitutionreaction.
Note: During the reaction of these preparations of alcohols, you have to use the
dilute NaOH, KOH and warm in order to increase the rate of
for primary alcohol while tertiary alcohols undergo
b. From alkenes
Alkenes react with water in the presence concentrated sulphuric acid to yieldsalcohols
Notice: Alkenes in the presence of Aluminum oxide reacts with water to form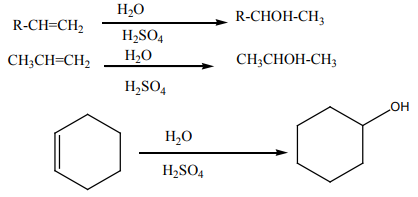
alcohols in vapour phase then condense to give liquid alcohols.
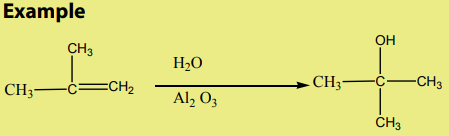
c. From carbonyl compounds
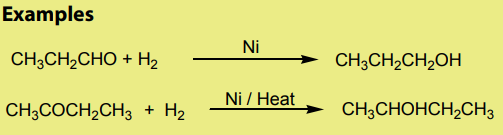
When aldehydes and ketones are reduced by hydrogen in the presence of a suitablecatalyst like Pt, Ni or Pd, they form primary and secondary alcohols respectively.
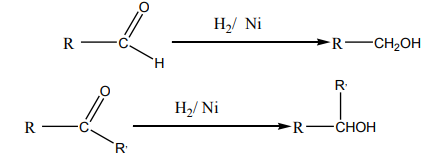
Note: Lithium tetrahydridoaluminate (LiAlH4 ) can also be used as a reducing agent.
Lithium tetrahydridoaluminate is not a stronger enough as reducing agent to reduce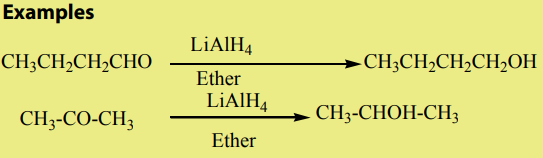
a double bond unlike
which can reduce both the double bond and the carbonyl group.
Activity 5.5
Process of alcoholic fermentation
d. From esters
Esters on hydrolysis in the presence of mineral acid or alkalis produce alcohols andcarboxylic acids.

Note:
group followed by hydrolysis.e. From Grignard reagents
The reaction between carbonyl compound and Grignard reagent (alkyl magnesium
halides) produces an alcohol with more carbon atoms. The reaction is a nucleophilicaddition on a carbonyl compound.
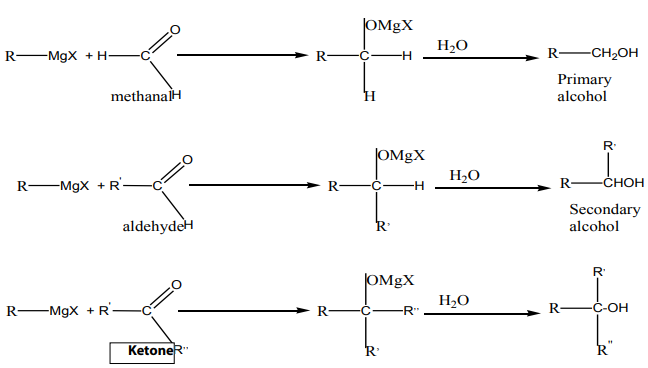

f. From primary amine to give primary alcohol
Primary amines react with nitrous acid to produce primary alcohols.
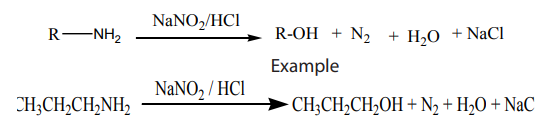
Checking up 5.4
1. Using chemical equations, explain how 3-methylbutan-2-ol could be
prepared:
a. from an alkene
b. using a Grignard reagent
c. from a halogenoalkanes
d. from an aminee. by reduction of a carbonyl compound
5.5. Preparation of ethanol by fermentation
Activity 5.5Process of alcoholic fermentation
6. Observe the above pictures and then interpret each picture.

7. Describe the process followed to produce alcohol refer to the above
pictures.
a. What are the raw materials used in the process?
b. What is the main component of the final products?
c. Give the name of the process illustrated by the pictured. Propose another process that can be used to yield the product in b.
This method is mainly used to prepare ethanol industrially. Ethanol is prepared from
starch (e.g. maize, cassava, millet, sorghum) and sugar (e.g. banana juice, molasses)
by fermentation process.
Fermentation can be defined as any of many anaerobic biochemical reactions
in which enzymes produced by microorganisms catalyse the conversion of onesubstance into another.
Alcoholic fermentation is the process in which enzymes act on carbohydrates to
give simpler compounds like ethanol (alcohol) and carbon dioxide
a. From starch
malt obtained either from maize grain, millet, or cassava contains an enzyme calleddiastase which catalyzes the hydrolysis of starch to maltose.
At room temperature, yeast is added and one of its enzymes called maltase catalyzes
the hydrolysis of maltose to simple sugar so called glucose.
Finally another enzyme of yeast called zymase catalyzes the decomposition of
glucose to ethanol.
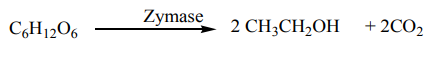
b. From sugar
Molasses containing sugars are mixed with water and yeast and then allowed to
ferment for several days after which ethanol are obtained during fermentation
process.
One enzyme of the yeast called sucrase catalyzes the hydrolysis of sucrose presentin the molasses to glucose and fructose.
Thus, another enzyme of yeast called zymase catalyzes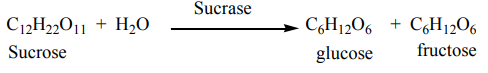
the decomposition of glucose to ethanol.
The ethanol obtained by fermentation process is only about 11%. This is made
concentrated by distillation which converts it to about 95% ethanol. This on further
distillation yields a constant boiling mixture whose composition does not change
(an azeotropic mixture).
Therefore, 100% ethanol is obtained by either:
i. Adding quick lime which removes water
ii. Distilling with of benzene as a third component
Note: Methanol can be prepared industrially by the reaction of carbon monoxide
and hydrogen at 300 °C and a pressure of 200 atmospheres.

Checking up 5.5
1. Briefly, describe the preparation of ethanol by alcoholic fermentation.
2. Compare and contrast the preparation of ethanol by hydration of etheneand by alcoholic fermentation.
Project work 5.1
The task is about the fermentation of glucose
Part A
In this project, you will investigate the fermentation of different types of
substances containing starch.
Requirements
• Conical flask,
• some yeast,
• some boiled potatoes,
• some bread,
• some boiled cassava,
• boiling tube,
• cork with delivery tubes,
• stands and cramps,
• glucose,• weighing balance
Why boiled potatoes or cassava is preferred?
Procedure
Weigh 25g of each starch including glucose and place them in separate conical
flasks. Add to each one spatula of dried yeast followed by 100 cm3
of water.
Cork the conical flask and connect it to boiling tube containing lime water as
shown in the figure below. Label each flask clearly. Leave all the flasks in a warm
environment. Record your observation for seven days.
c. The conical flask
d. The boiling tubeFor each type of starch
Note: use the same quantity and concentration of lime water in each boiling
tube.
Part B: Comparison of yield of alcohol obtained
Filter the mixture from each flask separately and collect the filtrate in measuring
cylinder. Record the volume in each case. Perform fractional distillation on each
filtrate, collecting the fraction between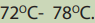 Record the volume of
Record the volume of
distillate collected from each starch.
Take of each on watch glass, ignite and note the time it takes to burn completely.
of each on watch glass, ignite and note the time it takes to burn completely.
Observe the amount of water left on watch glass.
5.6. Chemical properties of alcohols
Activity 5.6.1.
To investigate the oxidation reaction of an alcohol.
Requirements: methanol, ethanol, 2M sulphuric acid, potassium dichromate
solution, test tubes, burner, droppers, propan-2-ol and 2-methylpropan-2-ol.
Procedure:
• Place 5 drops of methanol in test tube
• Add 10 drops of dilute sulphuric acid followed by 5 drops of potassium
dichromate solution.
• Warm the mixture gently
• Repeat the experiment with ethanol, propan-2-ol and 2-methylpropan-2-ol.
1. What happens to the colour of the solution?
2. Explain the observation.3. Write an equation for the reaction taking place.
5.6.1. Oxidation
Primary and secondary alcohols are oxidized to aldehydes and ketones respectivelyby use of acidified.
 nitric acid once concentrated.
nitric acid once concentrated.
Aldehydes formed by oxidation of primary alcohols tend to undergo further
oxidation to carboxylic acid.
Ketones formed by oxidation of secondary alcohols are not further oxidized, unless
if the oxidizing agent is hot and concentrated in which case bonds around the –
CO_ group are broken and two smaller carboxylic acids are formed.

Tertiary alcohols resist oxidation because they have no hydrogen atom attached on
the functional carbon atom. Oxidation also occurs when the alcohol is in gaseous
phase by used of silver or copper catalyst under 500 °C and 300 °C respectively;
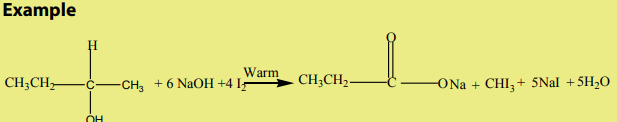
and the vapour of the alcohol is passed with air (oxygen) over heated silver.Example
These reactions help to distinguish between primary, secondary and tertiary
alcohols because primary and secondary alcohols decolourise the purplesolution of
An acidified potassium dichromate solution is turned from orange to green when it
reacts with primary and secondary alcohols.
Secondary alcohols having the following structure only undergo
only undergo
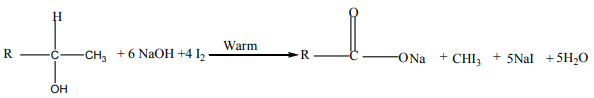
oxidation, on treatment with iodine solution in the presence of sodium hydroxide togive yellow precipitate of tri-iodomethane.
Note: This is a reaction which is characteristic of methyl ketones,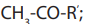 but
but
iodine here acting as an oxidizing agent first oxidizes the
then the methyl ketone formed then gives the yellow precipitate of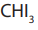 (Iodoform).
(Iodoform).From the reaction involved we have the Iodoform test.
5.6.2. Reaction with sulphuric acid
Activity 5.6.2
Show the product of the reaction referring to the preparation of alkenes andshow the mechanism of reaction.
Alcohols react with concentrated acid to give products depending on the nature of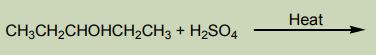
the alcohol and conditions of reactions.
a. At about 0 °C alcohols react with sulphuric acid to produce alkyl hydrogensulphates.
This reaction is a substitution reaction where the OH group has been replaced by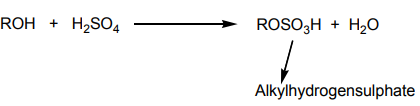

b. At about 140°C in the presence of excess primary alcohol and concentratedsulphuric acid, ether is formed.
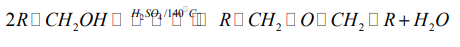
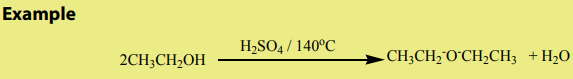
Mechanism
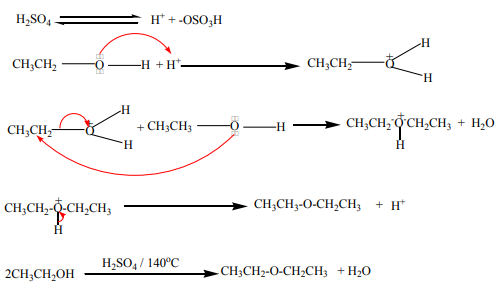
This reaction is an intermolecular dehydration.
c. Elimination reaction
Alcohols are dehydrated by heating with concentrated sulphuric acid or phosphoric
acid to alkenes. The ease of dehydration is in the order tertiary>secondary>primary’,this reaction is the intramolecular dehydration of water.
Notice: For primary alcohols any temperatures between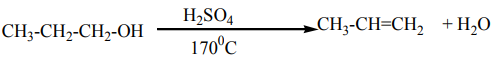
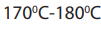
is sufficient and the acid should be sufficiently concentrated.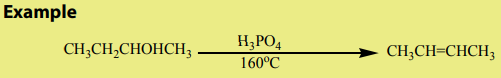
This dehydration respects Zaïtsev’s elimination law (see alkenes) reason why thehydration of butan-1-ol and butan-2-ol gives the same products which is but-2-ene via
Elimination always competes with nucleophilic substitution reaction. Substitution
leading to formation of ether is favoured by use of excess primary alcohols while
higher temperatures favour elimination. Therefore, dehydration of ethanol may
produce both alkenes by elimination and diethylether by substitution reaction. Therelative proportion of two products depends on the condition of the reaction.
Dehydration of alcohols also occurs when the vapours of the alcohols are passed
over heat aluminium oxide at about 300 °C.

5.6.3. Esterification
Alcohols react with organic acids in the presence of mineral acids lsuch as sulphuric
acid (catalyst) with elimination of water under 100 °C to produce an ester with givenoff a perfume smell.
This reaction is called “esterification”.
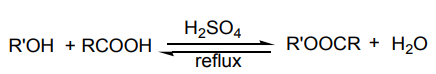
The mechanism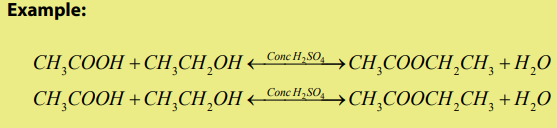
Step 1
In the first step, the ethanoic acid takes a proton (a hydrogen ion) from the
concentrated sulphuric acid. The proton becomes attached to one of the lone pairson the oxygen which is double-bonded to the carbon.
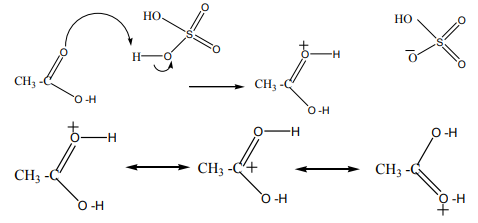
Step 2
The positive charge on the carbon atom is attacked by one of the lone pairs on theoxygen of the ethanol molecule.
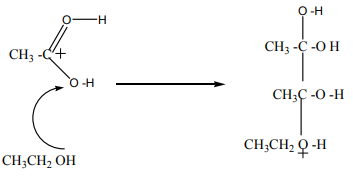
Step 3
What happens next is that a proton (a hydrogen ion) gets transferred from the
bottom oxygen atom to one of the others. It gets picked off by one of the other
substances in the mixture (for example, by attaching to a lone pair on an unreacted
ethanol molecule), and then dumped back onto one of the oxygens more or less atrandom.
The net effect is:
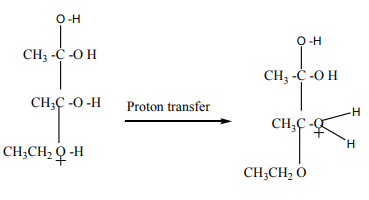
Step 4
Now a molecule of water is lost from the ion.
The product ion has been drawn in a shape to reflect the product which we are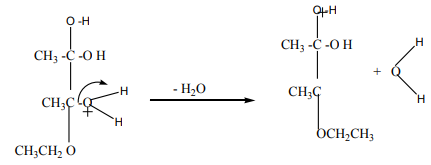
finally getting quite close to!
The structure for the latest ion is just like the one we discussed at length back in step
1. The positive charge is actually delocalised all over that end of the ion, and there
will also be contributions from structures where the charge is on the either of theoxygen atoms:
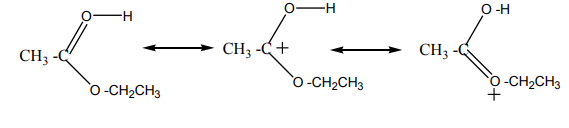
Step 5
The hydrogen is removed from the oxygen by reaction with the hydrogen
sulphate ion which was formed way back in the first step.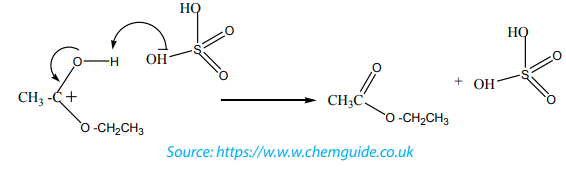
5.6.4. Reaction with strong electropositive metals and metal hydroxides
Electropositive metals like Na, K, react with alcohols forming alkoxide withevolution of hydrogen gas.

Note: Alcohols are not enough acidic to react with metal hydroxides such as
sodium hydroxide or potassium hydroxide.

5.6.5 Action of hydrohalic acids (HX)
Activity 5.6.5Referring to preparation of alkyl halides, complete the following reactions:
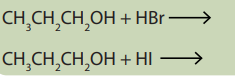
Alcohols react with hydrohalicacids to give alkyl halides.
Examples

Notice:
i. Reaction with concentrated hydrochloric acid is catalyzed by anhydrous
zinc chloride.
ii. This reaction is called LUCAS test and is used to distinguish between
simple primary, secondary or tertiary alcohols. In this reaction, the alcoholis shaken with a solution of zinc chloride in concentrated hydrochloric acid.
Observations: Immediate cloudiness indicates presence of a tertiary alcohol. If the
solution becomes cloudy within 5 minutes then the alcohol is a secondary one.
Primary alcohol would show no cloudiness at room temperature since the reactionis very slow.
For example all alcohols which are isomers of can be distinguished by the
can be distinguished by the LUCAS test.
Alcohols are also transformed into halogenoalkanes using phosphorus halides andthionyl chloride.
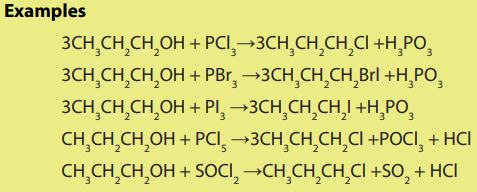
Checking up 5.6
1. An organic compound A possesses 87.6 % composition by mass and the
rest is hydrogen. If the same molecule possesses the molecular mass of
56 g/mol, deduce the molecular formula of A.
a) The reaction of A with water produces the compound B.
B can be represented in different forms called isomers. Represent theisomers of B.
When B reacts with it produces different compounds
it produces different compounds
depending on the reaction conditions. Write the structural formulae of
those compounds and state the conditions of their formation.
b) When B reacts with three products are obtained depending on
three products are obtained depending on the temperature used. Write structural formulae of those products.
2. Explain why tertiary alcohols are not oxidized.3. Complete the following chemical reactions and name the products obtained:

5.7. Uses of alcohols
Activity 5.7
In Rwnda, different types of alcoholic drinks are produced. However, some of
the them produced locally including “Kanyanga” are prohibited.
a. Explain why this alcohols is prohibited?
b. Discuss the possible effects of using non certified alcoholic drinks.
c. How would you differentiate alcoholic products from non-alcoholicones?
Ethanol is the alcohol found in alcoholic drinks. Alcoholic fermentation converts
starch sugar into ethanol. For example grapes are used to produce wine, ripe banana
to produce urwagwa, honey for spirits are obtained by distilling the ethanol –water
product obtained when sugar is fermented.
Drinking alcohol, i.e. the ethylic alcohol also called ethanol, is a normal social activity;
but excess of it is dangerous for our health. Hence excess of alcoholic consumption
must be avoided. For non-adult youth, consumption of alcohol in any form is illegalin Rwanda and many other countries.
There are some alcoholic drinks produced in Rwanda and in the Region that are
prohibited to be sold in Rwanda. However, alcohols have many other applications indaily life as indicated in the Table 5.1.
Table 5.1. Application of some alcohols

Ethanol produced by sugar cane fermentation has been used as alternative fuel to
gasoline (petrol). It has been mixed with gasoline to produce gasohol.
Project 5.2. USE OF ALCOHOLS
Consult leader to your community religious, political, professionals, e.g.
doctors, nurses, parents, teachers, and elders and find out from them thefollowing:
• What are the true recommended users of alcohols
• How should alcohol be used
• Real life examples of the effect of alcohol abuse on:
i. Social life
ii. Spiritual life
iii. Physical life of an individual
a. Come up with your own resolution and statements concerning alcohol
abuse. Write it out on a card and share it with trusted friends and your
parents/guardian and mentor.
b. Discuss with your peers show how you would help one of your members
of your family who is addicted to alcohol to come out of itc. Find out the good economic uses of alcohol.
5.8. Ethers
5.8.1 Structure and isomerism
Activity 5.8.1.
1. Represent the possible isomers of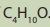 Which of them are:
Which of them are:
a. Structural isomers?
b. Functional isomers2. What homologous series do those isomers belong to?
Ethers are organic compounds in which two carbon groups are connected to a
single oxygen. The general formula of ether is R-O-R’. Based on the general structureof ether, they are classified as symmetrical, unsymmetrical and epoxide.
- For symmetrical esthers, R and R’ are identical
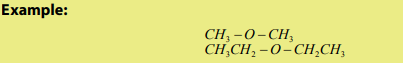
- For unsymmetrical esthers, R and R’ are different (R#R’)
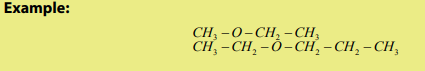
- Cyclic ethers
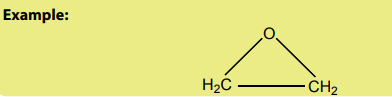
Checking up 5.8.
Classify the isomers of the molecules identified in activity 5.8.1 above intosymmetric, asymmetric and cyclic.
5.8.2. Physical properties
1. Ethers are sparingly soluble in water but are soluble in organic solvents.
2. The polar nature of the C-O bond (due to the electronegativity difference of
the atoms) results in intermolecular dipole-dipole interactions.
3. An ether cannot form hydrogen bonds with other ether molecules since
there is no H to be donated (no -OH group).
4. Their melting and boiling points increase with the increase in molecular
mass because of increasing the magnitude of Van der Waal’s forces with size.
5. The boiling points of ethers are much lower than those of alcohols of similar
molecular mass. This is because of the intermolecular hydrogen bondingwhich are present in alcohols but are not possible in ethers.
5.8.3 Preparation of ethers
Activity 5.8.2
1. With reference to alcohols, define intermolecular dehydration reaction.
2. State the reagent and conditions used in that reaction and give oneexample
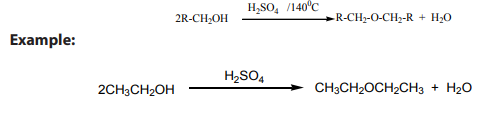
1) Intermolecular dehydration of alcohols
This is done by heating excess primary alcohol with concentrated sulphuric acid orphosphoric acid at about 140˚C.
2) From halogenoalkanes
(a) In this method halogenoalkanes are heated together with sodium or potassiumalkoxides.
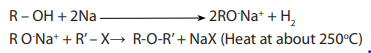
This is the Williamson’s synthesis
(b) In the second method, the halogenoalkane is heated with dry silver oxide.


5.9. Chemical properties of ethers
Activity 5.9
A compound with molecular formula has three isomers. One of them
has three isomers. One of themdoes not react with sodium metal. Identify that isomer.
Since they are saturated compounds and non-polar, they are relatively chemicallyinert reason why their chemical reactions are very few.
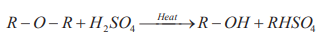
5.9.1. Reactions in which the carbon – oxygen bond is broken
a. Ethers react with hot concentrated sulphuric acid to form alcohols accordingto the following reaction.
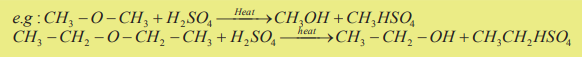
b. Reaction with hydrohalic acids
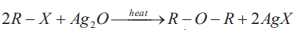
Ethers react with cold hydrohalic acids to form alkyl halides and alcohols.
R-O-R’ + HX→ ROH +R’X (cold)
Note: For unsymmetrical ethers, the halogen is attached to the smaller of the two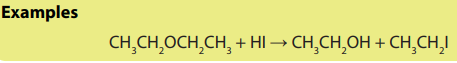
alkyl groups.

Ethers react with hot hydrohalic acids to form only alkyl halides.
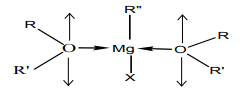
a. Ethers can act as the Lewis base due to the two non-bonded electron pair on
oxygen to form coordinative bonds with Grignard reagent. This explains clearly why
organ magnesium compounds are manipulated in ether solvent but not in watersince in water, there is a reaction which generate alkanes.
b. Combustion of ethers gives carbon dioxide and water:
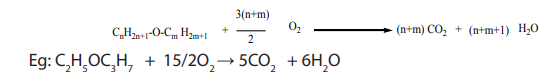
5.9.2 Oxidation reaction
Ethers react with oxygen of air to form peroxides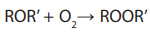
(less volatile than the parent ether)
In concentrated or solid form, these peroxides are dangerous because they are
highly explosive. The presence of peroxides contaminates the ether. This type of
contamination is purified by treatment with a reducing agent such as alkalineferrous sulphate.
5.10. Uses of ethers
Activity 5.10
A nurse is injecting anaesthesia to a patient as seen by the image below.
1. What product that form the anaesthesia.
2. Explain the effects of anaesthesia.3. In which case a patient is injected anaesthesia?
Lower ethers are used as anesthesia since they produce inert local cooling when
sprayed on a skin, ether are also used as local anesthesia for minor surgery operation.
Lower ethers are volatile liquid which on evaporation produce low temperature theyare therefore used as refrigerants.
Ether itself is one of the most important organic solvents for fats, oils, resins, andalkaloids.
Checking up 5.10:
Make a research and establish at least four uses of ethers
5.11. End unit assessment
I. Multiple choice questions
1. What is the correct name of the molecule with the skeletal formula shownbelow?
A . 1,2, 2-trimethylbutan-3-ol
B. 2-ethyl-2-methylbutan-2-ol
C. 3,3-dimethylpentan-2-olD. 4-hydroxy-3,3-dimethylpentane
2. Compound X, C4H8O2, has an unbranched carbon chain. An aqueous
solution of X has an approximate pH of 3. Compound Y, C3H8O, is a
secondary alcohol. X and Y are reacted together in the presence of
a little concentrated sulphuric acid to form Z as the major organicproduct.
What is the structural formula of Z?

3. The ester CH3CH2CH2CO2CH2CH (CH3)2 was hydrolyzed under
acidic conditions.
What are the organic products of this hydrolysis?
A. butanoic acid and 2-methylpropan-1-ol
B. butanoic acid and 2-methylpropan-2-ol
C. butan-1-ol and 2-methylpropanoic acid
D. propanoic acid and 2-methylpropan-1-ol
4. An unknown organic compound reacts with sodium to give a
combustible gas as one product but does not give a yellow precipitate
with alkaline aqueous iodine. What is a possible identity of the
unknown organic compound?
A. Propanol
B. Propan-1-olC. propan-2-ol
II. Open questions
5. A compound A is found to be soluble in sulphuric acid, A doesn’t react
is found to be soluble in sulphuric acid, A doesn’t react
with sodium or potassium permanganate. When A is heated with hydroiodic
acid, it is converted into single alkyl iodide suggest the structure of A
6. An organic compound Y possesses the centesimal composition by mass
of 87.6% carbon and the rest is hydrogen. The molecular mass of it is 56 g/
mol. Water molecule in presence of sulphuric acid was added to the same
molecule to produce M, the molecule M was subjected to sulphuric acid and
the temperatures of 140 °C, and produce N. Y possess many isomers including
cycles molecules.
Establish the structure of Y, and all isomers, M and all isomers and N. Show the
mechanism where is possible.
7. An organic liquid M contains carbon, hydrogen and oxygen. When 0.25 g of M
is combusted, 0.592g of carbon dioxide and 0.30 g of water was formed
a. i. calculate the empirical formula
ii. Molecular formula if the molecular mass is 74 g/mol
b. Write the structural formula and name of all isomers of M
c. M gives a yellow precipitate with solution of iodine in sodium hydroxide
i. Identify
ii. Describe briefly how the functional group in M may be determined
iii. Give a reaction scheme of how M can be converted into but-2-yne
8. Compare and contrast the preparation of ethanol by hydration of ethanol and
by fermentation by putting an emphasis on the advantages and disadvantagesof each process.
