UNIT 4: HALOGENOALKANES (ALKYL HALIDES)
Key unit competency
The learner should be able to relate the physical and chemical
properties of halogenoalkanes to their reactivity and their uses
Learning objectives
• Define halogenoalkanes and homologous series.
• Explain the reactivity of halogenoalkanes.
• Explain the physical properties of halogenoalkanes.
• Describe preparation methods for halogenoalkanes.
• Explain different mechanisms in halogenoalkanes.
• Explain the uses and dangers associated with halogenoalkanes.
• Draw displayed structural formulae of halogenoalkanes and give names using
IUPAC system.
• Classify halogenoalkanes according to developed formula as primary,
secondary and tertiary.
• Write reaction mechanisms of halogenoalkanes as SN1, SN2, E1 and E2.
• Test for the presence of halogenoalkanes in a given sample organic compound.
• Appreciate the uses and dangers of halogenoalkanes in everyday life.• Develop the awareness in protecting the environment.
Introductory activity
Look at the pictures below and answer the following questions.
Record your answers and discuss them.
a. Observe carefully pictures 4.1 and 4.2 and suggest the similarity
between them.
b. Observe carefully pictures 4.1 and 4.2 and suggest the difference
between them.
Substances which are used in the pictures belong to the same homologous series.
They may be obtained from the reaction between alkanes and halogens.
What homologous series do these substances belong to?
4.1. Definition and nomenclature of halogenoalkane
Activity 4.1
1. Look at the following and answer the questions that follow.

Questions:
i. Which structures do represent halogenoalkanes?
ii. What are the similarities between the selected structures?iii. From your answers above deduce the general formula for alkanes.
1. Definition
Halogenoalkanes compounds are compounds in which the halogen atoms like
chlorine, bromine, iodine or fluorine are attached to a hydrocarbon chain. When
the halogen atom is attached to a hydrocarbon chain the compound is called ahalogenoalkane or haloalkane or an alkyl halide.
Halogenoalkanes contain halogen atom(s) attached to the hybridised carbon
hybridised carbon
atom of an alkyl group.
2. Nomenclature of halogenoalkanes
Halogenoalkanes are organic compounds that contain a halogen atom: F, Cl, Br, I.
They are named using the prefixes fluoro-, chloro-, bromo- and iodo-.
Numbers are used if necessary to indicate the position of the halogen atom in themolecule.
If the molecule contains more than one halogen atom of the same kind, the prefixes
di-, tri-, tetra-, etc… are used.
Examples:
Checking up 4.1
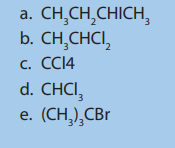
1. Name these compounds
2. Write the structural formulae for the following compounds:
a. 1,2-dibromo-3-chloropropaneb. 1,1,2-trichloro-1,2,2-trifluoroethane
4.2. Classification and isomerism
Activity 4.2
Consider the following compounds and based on the carbon atom attached tothe halogen atom, classify them.
Do research to find an appropriate name for each class
4.2.1. Classification of halogenoalkanesThere are three types of halogenoalkanes:
A primary halogenoalkane has a halogen atom attached to the ended carbon atom
of the chain. A secondary halogenoalkane has a halogen atom attached to a carbon
bonded to two other carbon atoms while a tertiary halogenoalkane has a halogenatom attached to a carbon bonded to three other carbon atoms.
4.2.2. Isomerism
Halogenoalkanes exhibit both chain and position isomerism.
Example: Molecular formula
a. Chain isomerism: This arises due to arrangement of carbon atoms in chains ofdifferent size.
b. Position isomerism: This arises due to the different positions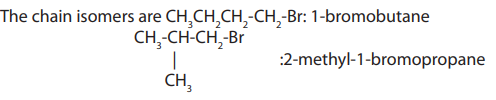
taken by the halogen atom on the same carbon chain.
The following compounds are position isomers: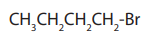 and
and
 because the atoms of bromine are on different positions
because the atoms of bromine are on different positions
of the chain.
Hence, all isomers of the compound with molecular formula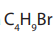
are the following.
Checking Up 4.2:
1. How many positional isomers possess the chlorobromopropane, Enumerate those able to form optical isomers.
Enumerate those able to form optical isomers. Draw their structuralformulae.
2. Illustrate the structural formulas of :a. 1,1,2-trichloropropane
a. 2-chloro-2-methylpropane
4.3. Physical properties of halogenoalkanes
Activity 4.3
1. Consider the following substances:
Sodium chloride, potassium bromide, hexane, pentane, trichlomethane,
terachloromethane.
Mix a sample of each compound (1g for solids, 2ml for liquids) with 10ml
of water.
2. Record your observations.
3. Write down your conclusions.
4. Based on the physical state and the nature of chemical bonding, predict
the increasing order in the boiling points of the compounds above.5. Write down your conclusions.
1. Volatility
Volatility is a property that shows if a substance transforms easily or not into vapour
or gaseous form. This property depends on the nature of the bonds that make up
the molecule of the substance. Generally non polar covalent compounds are more
volatile than polar covalent compounds. We know that halogens when bonded toother atoms form polar bonds because they possess high electronegativities:
F =
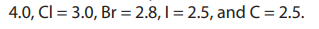
The more the difference of electronegativities of the atoms that form the bond,
the more polar is the bond. This explains the high polarity of C-F bond with an
electronegativity difference of 1.5, and the low polarity of C-Cl and C-Br bonds wherethe electronegativity differences are 0.5 and 0.3 respectively.
The presence of polarity or charge distribution results into more attraction between
polar molecules called dipole-dipole attraction forces, one type of Van der Waalsforces, as shown below:
The dashed line represents the attraction forces between the polar molecules or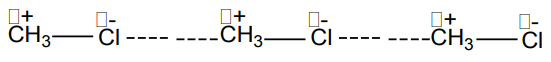
dipoles.
Therefore, more energy must be supplied to separate polar molecules and this
explains why melting and boiling temperatures of fluoroalkanes and chloroalkanes
are higher than those of alkanes of similar molecular mass.
As we have already learnt, molecules of organic halogen compounds are generally
polar. Due to the greater polarity as well as higher molecular mass as compared
to the parent hydrocarbons, the intermolecular forces of attraction (dipole-dipole
and Van der Waals) are stronger in the halogen derivatives. That is why the boiling
points of chlorides, bromides and iodides are considerably higher than those of thehydrocarbons of comparable molecular mass (Table 4.1).
Table 4.1: Comparison of boiling points of some halogenoalkanes
Chloromethane, bromomethane, chloroethane and some chlorofluoromethanes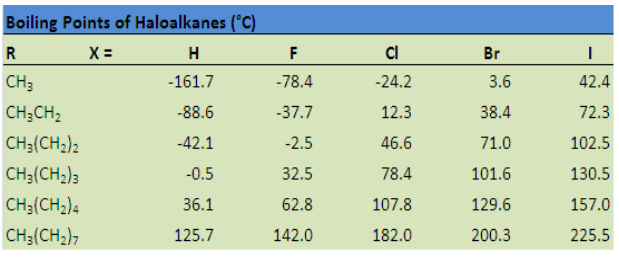
are gases at room temperature. Higher members are liquids or solids.
The attractions get stronger as the molecules get bigger in size. The pattern of
variation of boiling points of different halides is depicted in Figure 4.1. For the same
alkyl group, the boiling points of alkyl halides increase in the order: RF <RCl < RBr, <
RI This is because with the increase in size and mass of halogen atom, the magnitude
of Van der Waal forces increases.
2. Solubility
The solubility is the capacity of a substance to dissolve in a given solvent; in chemistry
the most common solvent we refer to is water. It is a result of the interaction between
the molecules of the substance, a solute, and the molecules of the solvent.
Polar molecules can interact with water molecules, but the attractive forces set
up between water molecules and molecules concerned are not as strong as the
hydrogen bonds present in water. Halogenoalkanes therefore, although they
dissolve more than alkanes, are only slightly soluble in water.
3. State
The state of matter is the physical appearance of that matter: solid, liquid and
gaseous.
Chloromethane, bromomethane, chloroethane and chloroethene are colourless
gases at room temperature and pressure. The higher members are colourless liquids
with a sweet pleasant smell.
4. Density
The density is a measure of the quantity of matter by volume unit. Cotton wool is
less dense than sand because if you compare the quantity of matter cotton wool
and sand contained in for instance you find that there more matter
you find that there more matter
in sand than in cotton wool.
The density of halogenoalkanes increases in the order RCl < RBr < RI, since the
atomic weight of halogens increases in order Cl < Br < I. Iodo, bromo and polychloroderivatives are denser than water but chloro derivatives are less dense than water.
Checking up 4.3
1. Arrange each set of compounds below in order of increasing boiling
points and explain why.
a. Bromomethane, tribromomethane, chloromethane,
dibromomethane.
b. 1-chloropropane, 2-chloro-2-methylpropane, 1-chlorobutane.
2. Explain the origin of the difference between the boiling temperatures ofthe following compounds:

4.4. Preparation methods of halogenoalkanes
1. From alkenes and alkynes
Activity 4.4.1
1. Give the product for each of the following chemical reaction.

2. Identify the class of the products of the reactions above.
Halogenoalkanes can be prepared by a reaction of alkenes or alkynes with:
i. hydrogen halides
Addition of hydrogen halide to alkenes, gives alkyl halides as the products. Theorientation in the addition reaction is described by Markovnikov’s rule (see alkenes).
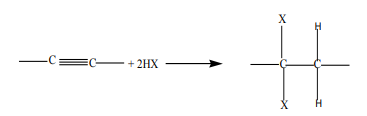
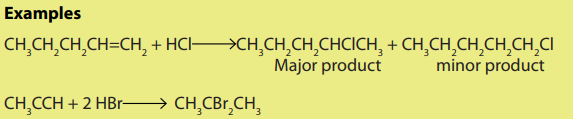
ii. Halogens

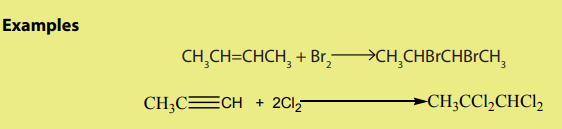
2. From alcohols
When ethanol reacts with potassium bromide in the presence of concentrated
sulphuric acid, bromoethane is formed. The reactions that took place in flask A arethe following.
In this reaction the hydroxyl group –OH is replaced with a bromine atom.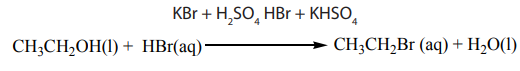
Halogenoalkanes are also obtained from alcohols using other reagents such asphosphorus halides.
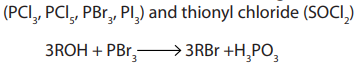

3. From alkanes
Activity 4.4.2
Observe the set up below and answer to the following questions.
Record your answers and discuss.
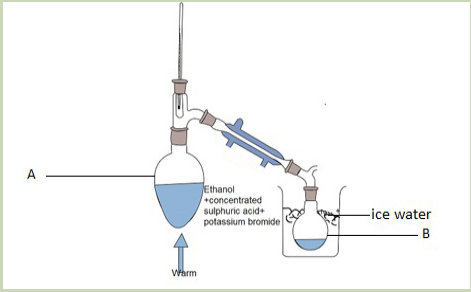
Picture 4.2: preparation of halogenoalkanes
a. Observe carefully the picture and suggest the product of the reaction in
flask A.
b. What is the role of:
i. Concentrated sulphuric acid?ii. Water in the picture above?
Direct halogenation of alkanes in the presence of ultraviolet light
gives alkyl halides and a hydrogen halide.
Example: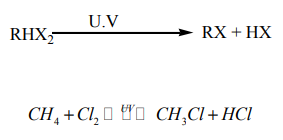
4. From aldehydes or ketones
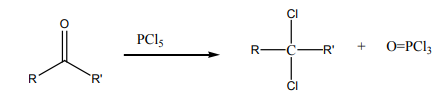

Checking up 4.4:
1. Complete the following chemical reactions :
2. Give the reagents and conditions needed to make the following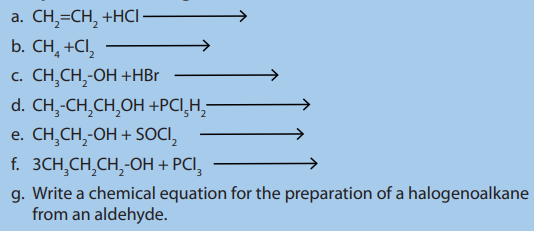
compounds from 1-bromopropane:a) propan-1-ol, b) propene.
4.5. Chemical properties
Activity 4.5.1
To investigate some reactions of halogenoalkanes
To of dilute sodium hydroxide solution in a test tube,
of dilute sodium hydroxide solution in a test tube,
add 5 drops of 1-bromobutane and gently warm the mixture.
Carefully smell the product.
Neutralize the solution with dilute nitric acid. Acidify the solution by adding 5
more drops of nitric acid.
Then add 5 drops of silver nitrate and observe. Write down your observations.
Write the equation of the reactions that take place. What is the role of sodium
hydroxide in this experiment?
When 1-bromobutane reacts with dilute sodium hydroxide solution, a product
with a sweet alcoholic smell is formed. That indicates that an alcohol is formed.
The formation of a pale yellow precipitate on addition of silver nitrate indicates
the presence of bromide ions. That means the carbon-bromine bond has been
heterolytically broken (the bromine atom takes the whole bonding electron pair). In
other words the bromine atom has been replaced by hydroxide ions. Thus, sodium
hydroxide provides the OH- ion which replaces bromine atom which leaves as a
bromide ion. As OH is a nucleophile (Lewis base) this reaction is called nucleophilicsubstitution.
The order of reactivity for the same alkyl group is such that iodides > bromides >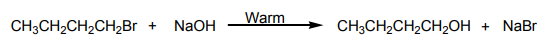
chlorides >fluorides.”>”, “are more reactive than “
The greater the electronegativity of the halogen, the greater the separation of charges
on the carbon and the halogen atoms, hence the stronger the bond. Therefore the
reaction is fastest with Iodoalkane because iodine is less electronegative compared
to bromine and Cl. Hence it will have weak C-I bond unlike that of C-Cl which will be
very strong due to the strong electronegativity of the chlorine atom. Hence bondenergies below are due to the above reason.
Because the carbon atom attached to the halogen atom is deprived of its electron, it
carries a partial positive charge . Thus when electron rich substrates
. Thus when electron rich substrates
called nucleophiles, approach the carbon atom, the halogen atom leaves as a halide
ion. Hence alkyl halides undergo nucleophilic substitution reaction, also writtenas SN.
1. Nucleophilic substitution reaction:

a. Reaction with aqueous alkali:
when alkyl halides are refluxed with aqueous alkali, or moist silver oxide, alcoholsare produced through substitution of the halogen by hydroxide ion.
This reaction is also called “hydrolysis”

Example:
Note: Tertiary alkyl halides react by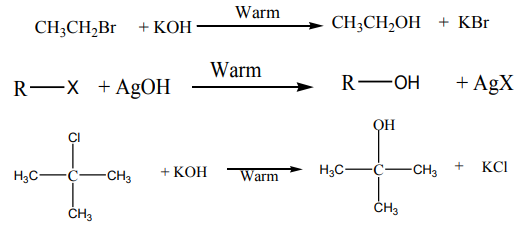
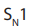 mechanism, i.e. the mechanism where
mechanism, i.e. the mechanism where
the first step is the self ionization forming a carbonium ion (carbocation), an alkyl
radical that has lost its electron and bear a positive charge on the carbon, which
immediately adds the nucleophile
Secondary alkyl halides however react by either mechanism depending
mechanism depending on the condition of the reaction while primary alkyl halides react by
 (see below).
(see below). : Unimolecular Nucleophilic Substitution that takes place in two steps; the
: Unimolecular Nucleophilic Substitution that takes place in two steps; the reaction rate is determined by the concentration of one molecule.
Example of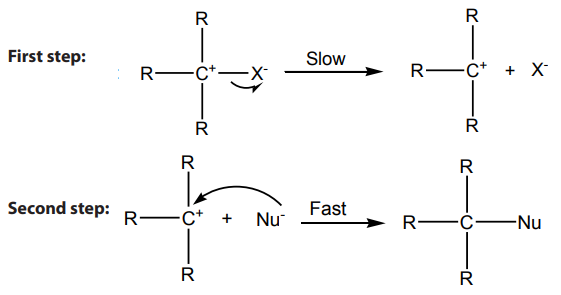
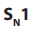 reaction: hydrolysis of tertiary alkyl halides with sodium
reaction: hydrolysis of tertiary alkyl halides with sodium hydroxide.
The hydrolysis can also take place when water alone is added to tertiary alkyl halides.
In this case water molecules act as nucleophiles.

Mechanism:
Step 1: Self ionization of the alkyl halides to a stable carbonium ion and a halide ion.This is the slowest step of the reaction hence it is the rate determining step.
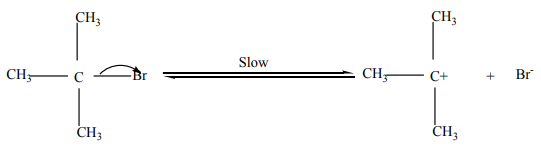
Step 2: Attack by incoming nucleophile. This is a fast reaction.
The potential energy (P.E )against reaction co-ordinate for hydrolysis of tertiary alkyl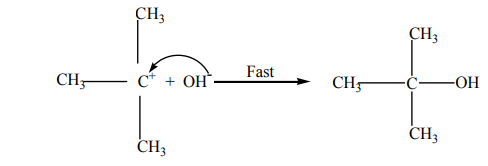
halides is as below (Fig.4.2). Potential energy is the energy stored in chemical bonds
of a substance, or the energy of an object due to its position.
The diagram below shows that the products formed have lower energy than thereactants, this indicates a favorable situation for the reaction to occur spontaneously.
Figure 4.2:
The potential energy of the system initially increases because the energy is required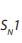 potential energy diagram
potential energy diagram
to break C-X bond; but when the stable carbocation is formed, energy is released
and the potential energy decreases a bit. As the carbocation and the nucleophile
(OH-) require a minimum energy (activation energy) to collide efficiently, the P.E
rises again until the transition state is reached, where the carbon-oxygen is being
formed. When this bond is completely formed, energy is released and the potentialenergy decreases.
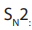 : Bimolecular Nucleophilic Substitution that takes place in one step; the
: Bimolecular Nucleophilic Substitution that takes place in one step; the
reaction rate depends on the concentration of Nu-
and the concentration of R-X.
In this mechanism, contrary to mechanism, the intermediate state also called
mechanism, the intermediate state also called
“activated complex” comprises both the leaving group and the entering group: inthe reaction below, the leaving group is X whereas the entering group is Nu.

b. Reaction with sodium alkoxides
Treatment of alkyl halides with sodium alkoxides produces ethers(Wiliamson synthesis)
c. Reactions with silver salt of carboxylic acid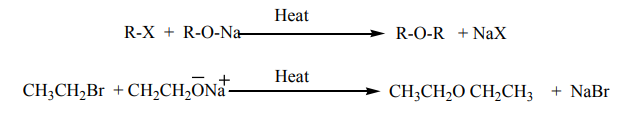
When alkyl halides are refluxed with silver salt of carboxylic acid, esters are formed:
(d) Reaction with potassium cyanide
When alkylhalides are refluxed with KCN, in presence of an alcohol, alkyl nitriles areproduced.
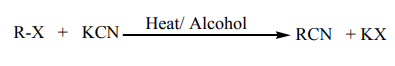
Example:
Note: This reaction is of practical importance in organic synthesis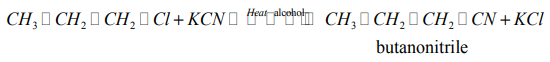
because it is used to increase the length of a carbon chain.e. Reaction with silver nitrite
When alkyl halides are refluxed with silver nitrite, a mixture of a nitro alkane and
alkyl nitrite are obtained as the products. The difference between the two products
is in the bonds between the nitrite and the alkyl: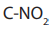
in nitro alkane and C-ONO in alkyl nitrite.
The two products can be separated by fractional distillation.
f. Reaction with ammonia and amines
Reaction of alkyl halide with concentrated ammonia produces a mixture of amines

The alkyl amine produced can then react with a molecule of alkyl iodide to producea series of substituted amines as shown in the reactions below:

Example of
 reaction: hydrolysis of primary alkyl halides with sodium hydroxide
reaction: hydrolysis of primary alkyl halides with sodium hydroxide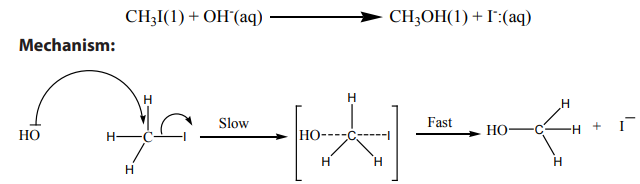
The transition state shows partial C-O bond formation and partial C-I bond cleavage.
Energy change diagram during the reaction is as below:
The P.E of the system initially increases along AB curve because energy is required
to break C-I bond; but when C-O is formed, energy is released and this is shown by
the curve BC. Since energy released by the formation of C-O bond is greater than the
energy required to break C-I, the products end up with a lower energy compared
to the reactant and this is favourable for the reaction to occur. At B a maximum
P.E is reached when C-I bond is partially broken and C-O bond is partially formed.
This state is called the transition state or activated complex. The energy barrier,
Ea, which must be overcome in order that the transition state is reached, is called
the activation energy of the reaction. The P.E of the system then falls along BCreleasing energy due to the formation of C-O bond.
Primary alkyl halides prefer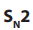 reaction because of the unstable nature of the
reaction because of the unstable nature of the
intermediate or the activated complex formed in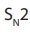 mechanism, the primary
mechanism, the primary carbonium ion,
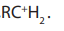
Table: 4. 1: Summary of alkyl halides reactions.

2) Elimination reactions
Activity 4.5.2
1. What is meant by elimination reaction?
2. Can halogenoalkanes undergo elimination reactions? Explain.
3. a. What are the products of an elimination reaction in halogenoalkanes?
b. What specific name is given to this reaction?
c. What are the conditions and reagent required for this type ofreaction?
An elimination reaction is where a saturated organic compound loses an atom
or group of atoms to form an unsaturated organic compound. Elimination is the
opposite of addition reaction.
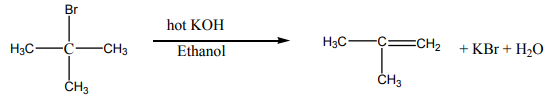
Alkyl halides when boiled with alcoholic potassium hydroxide form alkenes byelimination reaction. Hence the alkyl halide loses a molecule of the hydrogen halide.
Note: Elimination reaction usually occurs in competition with substitution reaction.
So when chloroethane is treated with a solution of potassium hydroxide two organicproducts are formed depending on the conditions of the reaction.
Ethene is formed by elimination reaction while diethyl ether is formed by substitution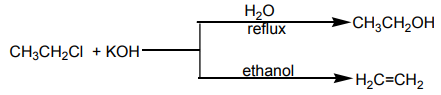
reaction.
Ether is formed by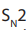 mechanism in which
mechanism in which 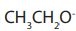 is acting as nucleophile.
is acting as nucleophile.
while Ethene is formed by elimination reaction in which is acting as base?
is acting as base?
Mechanism:

Because the two molecules are involved i.e.
and the reaction is bimolecular and since the alkyl halide loses a mole of HCl
the reaction is called elimination. Hence the reaction is a bimolemolecular elimination (E2).
In competition between and E2 in primary or secondary alkyl halides, the nature
and E2 in primary or secondary alkyl halides, the nature
of the product formed depends on the solvent, temperature, and structure of thehalide.
Elimination is favoured by use of high temperature and a strong base e.g alcoholinstead of water.
For tertiary alkyl halides, elimination occurs by E1 mechanism. In the mechanism,the tertiary alkyl halide undergoes ionization first and then later loses a proton.
3) Wurtz reaction
Alkyl halides with sodium metal to give alkanes.
These are compounds in which more than one halogen atom is present. There are
two main types of dihalides.
Gem dihalides: This is where two halogen atoms are attached to the same carbonatom.
Example
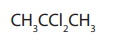
Vicinal dihalides: Here the two halogen atoms are on adjacent carbon atoms
Example
The reactions of dihalogenoalkanes are similar to those of monohalogenoalkanes
but require more reagents.
Examples
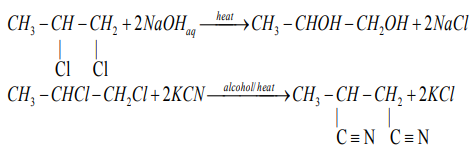
Elimination reaction with excess hot alkali produces alkynes

Checking up 4.5
1. Give the structural formula of the main product of each of the followingreactions:
2. Halogenoalkanes undergo nucleophilic substitution reaction. Discuss

this statement.
3. a. What is a nucleophile? Give two examples.
b. Why do nucleophiles attack halogenoalkanes?
c. What two types of reaction are in competition when a
halogenoalkane reacts with a nucleophile? Name two products
which can be formed from 1-bromopropane by these reactions.
4. 2- Chloro-2-methyl propane reacts with aqueous sodium hydroxide to
form 2-methylpropan-2-ol.
a. Draw what should be the energy diagram for the reaction.
b. Write the mechanism for the reaction.
c. (i) Sketch an energy diagram for the reaction of aqueous sodium
hydroxide and chloromethane.
(ii) Outline the mechanism for the reaction.
d. outline the mechanism for the reaction
4.6. Chemical test for the presence of halogenoalkanesActivity 4.6:
Put 2mL of ethanol into each of 4 test tubes labelled A-D. A is the control tube and
therefore no alkyl halides are to be added. To B, add 3-4 drops of 1-chlorobutane.
To C, add 3-4 drops of 1-bromobutane and to D, add 3-4 drops of 1-iodobutane
using Pasteur pippete. Stopper the tubes and place them in a hot water bath at
about
and leave for a few minutes to equilibrate. Working quickly add about
1mL of silver nitrate solution to each tube. Start the stopwatch and shake the
tubes to ensure complete mixing.
a. Record your observations
b. Make a comment about comparison of the reactions of the threehalogeno alkane
Halogenoalkanes can be identified due to some tests. The following Table illustratesome chemical tests of halogenoalkanes.
Table 4. 2: Chemical test for halogenoalkanes

Checking up 4.6
Given two samples A and B. You carry out the test for haloalkanes and get the
following results: A form a pale yellow precipitate and B form a white precipitate.
Which sample represents and which one represents
and which one represents 
Write chemical equations to justify your answer.
4.7. Uses of halogenoalkanes and dangers associated with CFCs
Activity 4.7
1. Do you know CFCs? If yes what do you know about them?
2. Do CFCs affect directly our health in our daily life? If yes explain how.
3. What are the dangers posed by CFCs?
4. What solutions do you propose or have been proposed to the problemof CFCs
Solvents:

Medicine:
 is used in anesthesia
is used in anesthesiaAgriculture:
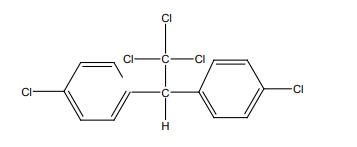
• DDT: Dichloro diphenyl trichloroethane is used as insecticide- DDT. Colorless
chemical pesticide, dichlorodiphenyltrichloroethane, used to eradicate
disease-carrying and crop-eating insects. It was first isolated in Germany
in 1874, but not until 1939 did the Swiss Nobel Prize-winning chemist Paul
Müller recognize it as a potent nerve poison on insects. The product is bannedin Rwanda. Below is the structure of DDT.
Home: Refrigeration, perfumes, etc…
Halogenoalkanes which have boiling temperatures just below room temperature
can easily be liquefied by a slight increase in pressure. Halogenoalkanes containingchlorine and fluorine and no hydrogen are Chlorofluorohydrocarbons. Examples are
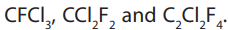 They are usually called chlorofluorocarbons or CFCs. In
They are usually called chlorofluorocarbons or CFCs. In
addition to having low boiling temperatures, they are non-flammable, odorless,stable, non-toxic and solvents.
• CFCs appeared to be ideal for use as fluids in refrigerators and as solvents
in aerosol sprays, they were developed in the 1920s as what appeared to
be ideal replacements for liquid ammonia and liquid Sulphur dioxide, which
were formerly used as fluids in refrigerators and air-conditioning units. Being
good solvents, they were also ideal as the solvents in aerosol sprays.
Aerosols were used to dispense insecticides, hairsprays, perfumes and deodorants,
window-cleaning, polishes, waxes and laundry products. As more and more
uses were found for these remarkable compounds, CFCs became big business,
with hundreds of thousands of tones being produced yearly. Now theyare being phased out. These stable, non-toxic compounds are dangerous!
•Their high stability has turned out to be a problem, during all the time that
the use of CFCs was increasing, no-one thought about what would happen to
the gases in the atmosphere. Because of their lack of reactivity and insolubility
in water, there is no natural process for removing CFCs. In fact they drift up
into the stratosphere where ultraviolet light causes photolysis, i.e. a reaction
cause by light. The chlorine radicals formed in photolysis take part in a chainreaction which converts ozone into oxygen.
As you can notice, the chain of reaction above results in the decomposition of ozone
,which does have the capacity to absorb, and stop dangerous UV from reaching the
Earth into ordinary oxygen. This can be avoided if and only if human activities sendno CFCs in the atmosphere.
• This reduce the thickness of the ozone layer. Reactions (a) to (c) form a chain.
This is why one chlorine radical from one CFC molecule can destroy thousandsof ozone molecules.
And what can be done?
• Replacements for CFCs have been found, because of concern over the
decrease in the ozone layer, many nations have agreed to cut down the use
of CFCs. Alternative compounds are already in production. Hydrohalocarbons
contain at least one hydrogen atom per molecule. The C-H bond can be
attacked by HO• radicals in the lower atmosphere and the compounds do notreach the upper atmosphere. Hydro halocarbons include
• Hydrochlorofluorocarbons,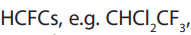 used in blowing plastics
used in blowing plastics
foam and used in air-conditioners
used in air-conditioners
• Hydrofluorocarbons, used in air-conditioners and
refrigerators.
used in air-conditioners and
refrigerators. HCFs cause no damage to the ozone layer, although they are greenhouse gases.
Checking Up 4.7
1. State four industrial uses of the halogenoalkanes. Why do fluoroalkanesfind special uses?
4.8. End Unit Assessment
1. Which of the following is NOT a halogenoalkane compound:
a. Tribromo benzene
b. 3-iodohexane
c. 2-chloro-3-methylpentaned. 2-bromopentane
2. Choose from a list of words and fill in the missing words in the text
below
Halogenoalkane, iodine, alkyl halide, haloarene, thyroxine
…………………………..compounds are compounds in which the
halogen atoms like chlorine, bromine, ………… or fluorine are
attached to a hydrocarbon chain or an aromatic ring. When the halogen
atom is attached to a hydrocarbon chain the compound is called an…………………… or ………………………..
3. Answer by True or False
a. Chloroform is employed as a solvent in paint remover.
b. Iodoform was used earlier as an antiseptic.
c. Methyl chloride, methyl bromide, ethyl chloride and some
chlorofluoromethanes are gases at room temperature.
d. The objects which are non-superimposable on their mirror image
(like a pair of hands) are said to be chiral and this property is known
as chirality. While the objects, which are superimposable on theirmirror images are called achiral.
e. (chloroform): is used as insecticide
(chloroform): is used as insecticide
f. DDT: Dichloride diphenyl trichloroethane is used as anesthesia
g. Halogenoalkanes therefore, although they dissolve more than
alkanes, are only slightly soluble in water.
h. Halogenoalkanes undergo nucleophilic substitution reactions in
which the halogen atom is replaced by a nucleophile.
i. Elimination reaction is where a saturated organic compound loses
an atom or group of atoms attached to form unsaturated organiccompound.
4. Name the following halides according to IUPAC system and classifythem as primary, secondary or tertiary halogenoalkanes
1. Write the structures of the following organic halogen compounds.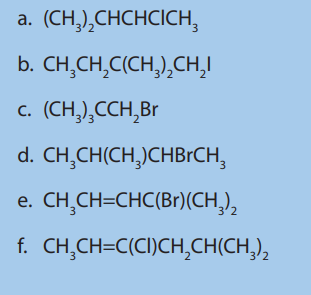
a. 2-chloro-3-methylpentane
b. 2-chloro-2-methylpropane
c. 2,3-dichlorobutane
d. 2-bromo-4-chloropentanee. 1,1,2-trichloropropane
2. Why do bromoalkanes react more readily than chloroalkanes?
3. Why does 1-bromopropane react with nucleophiles but propane doesnot?
4. Write the equations for the preparation of 1-iodobutane from
(a) 1-butanol, (b)1-chlorobutane, (c) but-1-ene
5. Write the structure of the major organic productin each of the following reactions:
6. Arrange the compound of each set in order of reactivity towards SN2
displacement:
a. 2-bromo-2-methylbutane, 1-bromopentane, 2-bromopentane
b. 1-bromo-3-methylbutane, 2-bromo-2-methylbutane, 3-bromo-2-
methyl butane
c. 1-bromobutane, 1-bromo-2,2-dimethylpropane, 1-bromo-2-methyl butane, 1-bromo-3-methylbutane.
7. a) There are four strucural isomers of molecular formula C4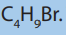 The formulae of two of these isomers are given.
The formulae of two of these isomers are given.
i. Draw the remaining two structural isomers.
ii. Give the name of isomer 2
b) All four structural isomers of
i. Give the name of the mechanism involved in these reactions.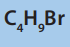 undergo similar reactions with
ammonia
undergo similar reactions with
ammonia
ii. Draw the structural formula of the product formed by the reaction
of isomer 2 with ammonia.
iii. Select the isomer of molecular formula C4
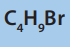 that would be most
that would be most
reactive with ammonia. State the structural feature of your chosen
isomer that makes it the most reactive of the four isomers.
iv. The elimination of HBr from Isomer 1 produces two structural
isomers, compounds A and B.
v. Give the reagents and conditions required for this elimination
reaction.
c) Ethene, C2H4, reacts with bromine to give 1,2-dibromoethane.
i. Give the name of the mechanism involved.
ii. Show the mechanism for this reaction.
vi. Give the structural formulae of the two isomers, A and B formed byelimination of HBr from isomer 1.
