UNIT 3: ALKENES AND ALKYNES
Key unit competency
Relate the physical and chemical properties of alkenes and alkynes to their reactivity and uses.
Learning objectives
• Explain the reactivity of alkenes in comparison to alkanes
• Explain the existence of geometrical isomerism in alkenes
• Describe the industrial process of preparing alkenes and alkynes
• Apply IUPAC rules to name alkenes and alkynes
• Carry out an experiment to prepare and test ethene gas
• Outline the mechanisms for electrophilic addition reactions for alkenes and
alkynes
• Write the structural formulae of straight chain alkenes and alkynes
• Apply Markovnikov’s rule to predict the product of hydrohalogenation of
alkenes
• Classify alkynes as terminal and non-terminal alkynes using their different
structures
• Appreciate the combustion reaction as source of fuels.
• Appreciate the uses and dangers of addition polymers (polythene used forpolythene bags, polypropene for plastic bottles etc.)
Introductory ActivityObserve the following picture and answer the questions that follow.
1. What is the collective name of the substances used to manufacture the
items showed in the above picture?
2. a). What are the raw materials used in the manufacture of the
substances identified in 1)?
b). These raw materials may be obtained from different sources. Discuss
this statement.
c). Do you expect these raw materials be soluble or not in water? Justify
your answer.
3. Even though the items which appear in the picture above are interesting,they also present some disadvantages. Discuss this statement.
3.1. Definition, structure and nomenclature of alkenes
Activity 3.1.1
1. Describe the formation of a carbon-carbon double bond. What is the
hybridisation state of a carbon doubly bonded?2. What is the shape of the molecule around the double bond? Explain.
Alkenes are a homologous series of hydrocarbons which contain a carbon-carbon
double bond. Since their skeleton can add more hydrogen atoms, they are referredas unsaturated hydrocarbons.
The general formula of alkenes is

Example: Ethene
Alkenes are abundant in the nature and play important roles in biology. Ethene,
for example, is a plant hormone, a compound that controls the plant’s growth and
other changes in its tissues.
Ethene affects seed germination, flower maturation, and fruit ripening.
They are described as unsaturated hydrocarbons because they can undergoaddition reactions.
The double bond in alkenes is made of one sigma bond and one pi bond. This gives
rise to the resistance of rotation around the double bond.
The hybridization state in alkenes is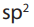
and the structure around each carbon doubly bonded is trigonal
planar with a bond angle value of
Activity 3.1.2
Refer to the IUPAC system used in the nomenclature of alkanes, name thefollowing compounds.
IUPAC names of alkenes are based on the longest continuous chain of carbon atoms
that contains the double bond.
The name given to the chain is obtained from the name of the corresponding alkane
by changing the suffix from –ane to –ene.
If the double bond is equidistant from each end, number the first substituent that
has the lowest number. If there is more than one double bond in an alkene, all of
the bonds should be numbered in the name of the molecule, even terminal double
bonds. The numbers should go from lowest to highest, and be separated from one
another by a comma.
The chain is always numbered from the end that gives the smallest number for the
location of the double bond.
In naming cycloalkenes, the carbon atoms of the double bond are numbered 1 and 2
in the direction that gives the smallest numbers for the location of the substituents.
If a compound contains two or more double bonds, its location is identified by a
prefix number. The ending is modified to show the number of double bonds:
• a diene for two double bonds,
• a triene for two three bonds• a tetraene for four double bonds
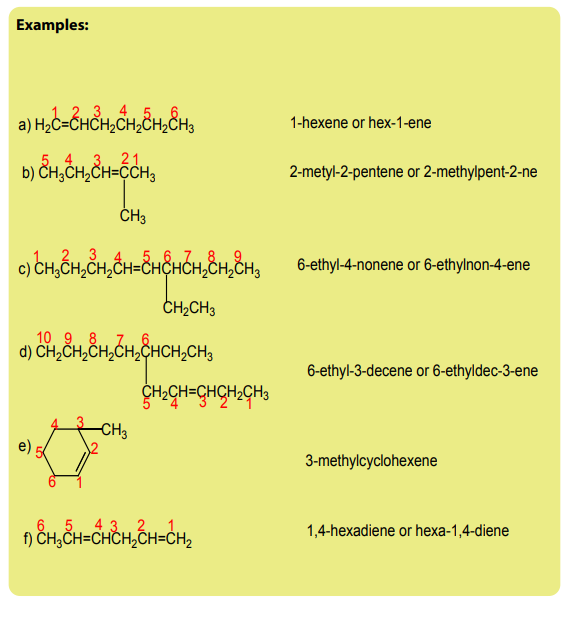
Checking up 3.1
1. Write the structural formula of:
a. 4-ethylhept-3-ene
b. 5-isopropyl-2,6-dimethylundec-3-ene
c. 3-ethyl-2,4,5-trimethyl oct-2-ene
d. 3-ethyl-2-methylcyclohexene
e. Buta-1,2-adiene2. Name each of the following compounds according to the IUPAC system.
3.2. Isomerism in alkenes
Activity 3.2
1. What is meant by isomers and what are the types of isomers?2. Which types of isomerism can be exhibited by alkenes? Give your reasons
Alkenes exhibit two types of isomerism: structural isomerisms and stereoisomerism.
1. Structural isomerism
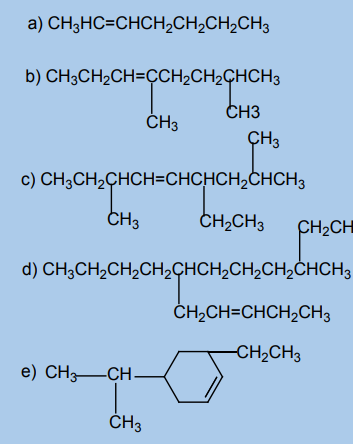
Alkenes exhibit position isomerism, chain isomerism and functional isomerism.
In position isomerism, the position of the double bond changes but the length ofthe chain remains the same.
Example:
Alkenes and cycloalkanes have the same molecular formula because they both have
two fewer hydrogen atoms than alkanes. That is why, they have the same molecular
formula. However, they belong to different homologous series. Therefore, they are
functional group isomers. This isomerism that relates open chain compounds toring chain compounds is referred to as ring isomerism.

2. Stereoisomerism
Due to the resistance of rotation around the double bond, alkenes give rise to cistransor geometrical isomerism. Sometimes known as E- and Z- Isomers
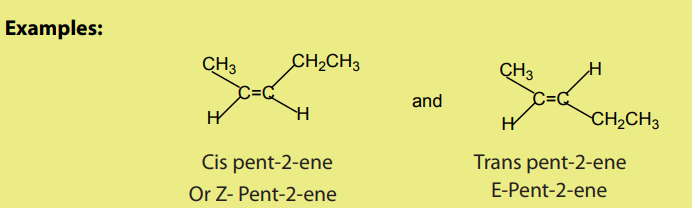
Checking up 3.2
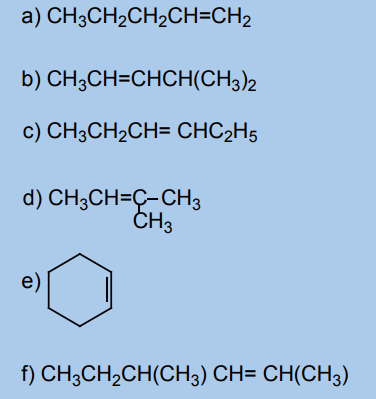
1. State the necessary condition for the existence of cis-trans isomerism in
alkenes?2. Which of the following alkenes can exhibit a cis-trans isomerism?
3.3. Preparation of alkenes
Activity 3.3
Different methods can be used to prepare alkenes. Discuss the possible
reactions which may be involved in the preparation of alkenes and propose themechanisms, where it is possible.
Different methods are used for the preparation of alkenes. Most of them areelimination reactions.
An alkene may be obtained by dehydration of an alcohol. The reaction involves1. Dehydration of alcohols
the loss of H and OH from adjacent carbons of an alcohol to form an alkene. The
dehydration is carried out by heating an alcohol with concentrated sulphuric acid
or 85% phosphoric acid.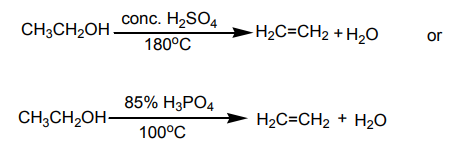
Mechanism of the reaction
The dehydration of alcohols giving alkenes occurs in three steps.
If two or more alkenes may be obtained, the one having more substituents on the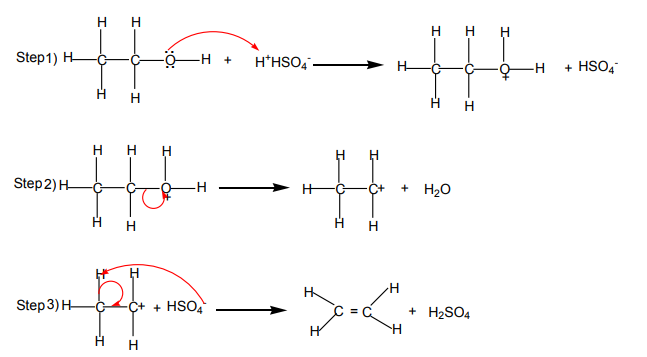
double bond generally predominates. This is the Zaitsev’s rule.This is due to the stability of the intermediate carbocation. The carbocation
produced in step 2 may undergo a transposition (rearrangement) of a hydride ion
or a methyl group giving a more stable carbocation and therefore a more stable
alkene.From the secondary carbocation, two products can be obtained and the reaction
Mechanism
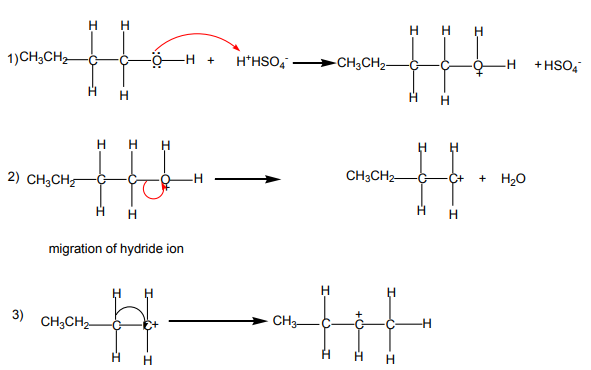
follows the Zaitsev’s rule.
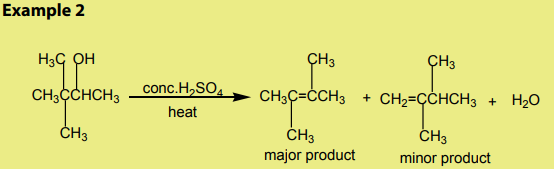
From the tertiary carbocation, two products can be obtained and the reaction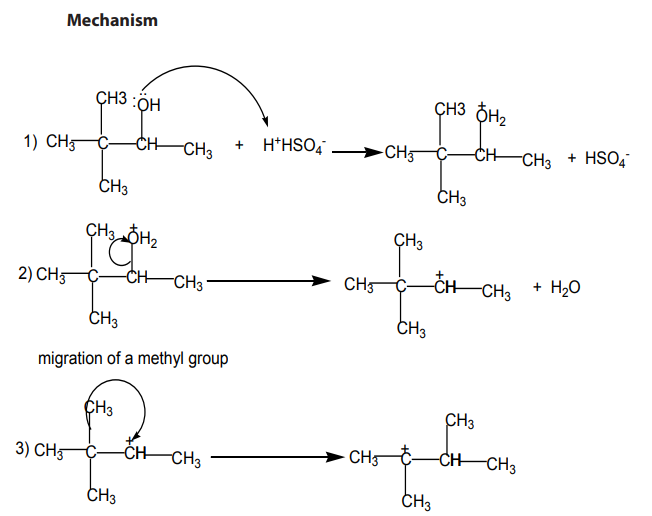
follows the Zaitsev’s rule.
The dehydration of alcohols leading to alkenes may also be effected by heatingalcohols in the presence of alumina.
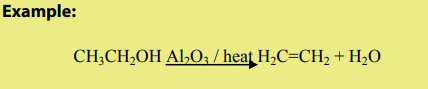
2. Dehydrohalogenation of halogenoalkanes
Halogenoalkanes react with hydroxide ions in ethanolic solution to yield alkenes.The reaction follows the Zaitsev’s rule.
Examples
When a compound containing two halogen atoms on the adjacent carbon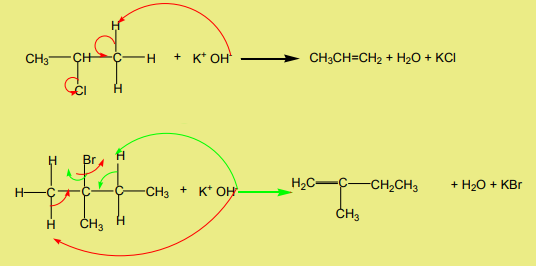
3. Dehalogenation of dihalogenoalkanes
atoms is treated with magnesium or zinc it transforms to an alkene.
Examples:
When the two halogen atoms are attached to non-adjacent carbon atoms, a cyclic
alkane is formed.

Checking Up 3.3
1. Refer to the IUPAC system, name the alkenes formed when the following
alcohols are dehydrated in the presence of sulphuric acid.
a. Pentan-2-ol
b. 2-methylpropan-1-ol
c. 2,3-dimethylbutan-2-ol
d. 2-methylcyclohexanol
e. 2-methylbutan-2-ol
2. What are the products of the dehydrohalogenation of the following
compounds? Show the major product.
f. 1-bromo-2-methylpropane
a. 2-bromo-3-methylpentane
b. 2-bromo-2,3-dimethylbutanec. 3-chloro-3-ethylpentane
3. Write the formula of the compounds formed when each of the followingdihalogenoalkanes react with magnesium.
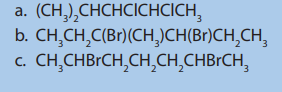
3.4. Laboratory preparation and chemical test for ethene
Activity 3.4
Preparation of ethene Set up the apparatus as shown in the Figure below (Figure
3.1) and follow the instructions to perform the experiment on the preparation ofethene.
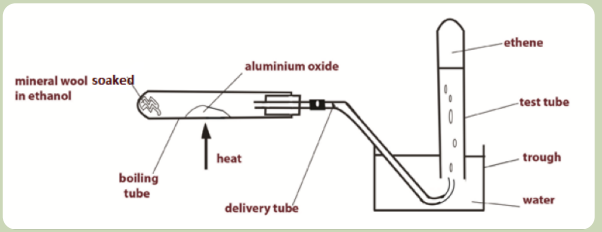
Figure 3.1: Laboratory preparation of ethene
Requirements:
Chemicals:
• Ethanol, aluminium oxide, lime water, mineral wool, bromine water, acidified
potassium permanganate solution (very dilute), water.
Additional apparatus:
• Boiling tube
• Rubber stopper with hole
• Delivery tube
• Trough
• Test- tube rack
• 5 test tubes
• 5 rubber stoppers for test tubes
• Spatula Procedure and setting
• Bunsen burner
• Glass rod
• Splint• Matches
1. Preparation of ethene:
- Pour some ethanol into the boiling tube to a 3 cm depth
- Add some glass wool to soak up the ethanol, using a glass rod to push
the wool down the tube.
- Clamp the boiling tube in a horizontal position using a retort stand.
- Put a small amount of aluminium oxide about half way along the
boiling tube. - Complete the set up of the apparatus as shown in the
diagram above.
- Light the Bunsen burner, adjust it to a blue flame and heat the
aluminium oxide. (Make sure the test tube is filled with water when
you start to collect the gas produced.)
- As the aluminium oxide gets hot the heat reaches the ethanol at the
end of the tube. The ethanol then changes to vapour, passes over the
hot aluminium oxide and is dehydrated to produce ethene gas.
- Collect 5 test tubes of the gas and put a stopper on each tube when
it is filled. - When the test tubes have all been filled, loosen the retort
stand and raise the apparatus so that the delivery tube no longer dips
into the water. This avoids suck back of water as the tube begins to
cool which could cause the boiling tube to crack. Turn off the Bunsen
burner.
2. Testing the properties of ethene
Addition of bromine:
- Taking great care, add about 1ml of the test tube of bromine water to
one of the test tubes of ethene.
- Replace the stopper and shake the tube a few times.
- Record your observations.
- Write down your conclusions
- Addition of acidified potassium permanganate:
- Add about 1ml of very dilute potassium permanganate solution to
one of the test tubes of ethene and shake the tube a few times.
- Record your observations.
- Write down your conclusions
Combustion:
- Remove the stopper of one of the tubes filled with ethene and apply a
light to the mouth of the test tube using a lighted splint.
- Allow the gas to burn and when it has stopped burning add a small
amount of lime water to the test tube, stopper it and shake the tube
a few times.- Write down your observations.
Interpretation
When ethanol is heated in the presence of aluminium oxide, a gas is produced. This
gas does not react with lime water. This means that the produced gas is not carbondioxide. The equation of the reaction is:
The gas decolourises bromine water. Bromine water is a test used to identify the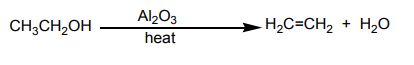
presence of a carbon-carbon double bond or triple bond. The bromine adds across
the double bond and a dibromoalkane is formed. The reaction between alkene andbromine water is shown below:
If you shake an alkene with bromine water (or bubble a gaseous alkene through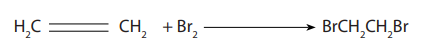
bromine water), the solution becomes colourless. Alkenes decolourise bromine
water.
The Figure 3.2 shows Bromine water added to ethene: before the reaction (left)
the color of bromine appears, and after the reaction (right) the colour of brominedisappears.

Picture 3.1: Test for unsaturation
When ethene reacts with acidified potassium manganate (VII), the purple colour of
the permanganate solution turned to colourless or light pink indicating the presenceof the carbon – carbon double bond.The reaction is the following:
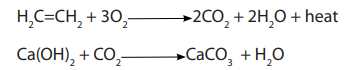
The gas burns with a smoky flame producing carbon dioxide and heat energy. The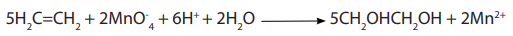
carbon dioxide produced turns into milky lime water.
Checking Up 3.4
1. Ethene is prepared by dehydration of ethanol in the presence of alumina,
explain other reactions that produce ethene.
2. Describe the chemical test used to identify the presence of a carbon-carbon
double bond in an organic compound.
3. Explain how ethene can be differentiated from carbon dioxide using achemical test?
3.5. Physical properties of alkenes
Activity 3.5
1. How does the physical state of alkenes change with molecular mass.
2. Put in a test tube 5ml of cyclohexene. Add 5ml of water and mix. Record
your observations.
3. Put in a test tube 5ml of cyclohexene. Add 5ml of tetrachloromethane
and mix. Record your observations.
4. Cis-but-1-ene and trans-but-2-ene exhibit geometric isomerism. Statewhich one of them is less volatile and why?
• Alkenes which have less than 5 carbon atoms are gaseous at ordinary
temperature, the other are liquid up to 18 while others are solids as the number
of carbon atoms increases.
• Boiling points and melting points of alkenes are less than those of alkanes but
also increase as the molecular weight increase.
• Alkenes are insoluble in water but soluble in most organic solvents.
• Cis-alkenes have a slightly higher boiling point than the trans-isomers becausethe dipole moments in trans structures cancel each others----.
Checking Up 3.5
Which one of the following compounds has higher boiling point? Explain.
a. Cis-butene and trans-butene
b. Ethene and propene
3.6. Chemical properties
3.6.1. Addition reactions3.6.1.1. Electrophilic additions
Activity 3.6.1
1. Explain the following terms and give two examples for each.
a). Addition reaction
b). Lewis acid
2. Distinguish other name given to a Lewis acid.
3. Predict if Lewis acids can react with alkanes.
4. Justify if Lewis acids react with alkenes.5. Differentiate the reactivity of alkenes and alkanes.
Alkenes are far more reactive than alkanes due to the carbon-carbon double bond.
These compounds are unsaturated and they can easily undergo addition reactions
to yield saturated products.
The double bond in alkenes is a region of high density of electrons. Therefore, this
region is readily attacked by electrophiles. An electrophile is an atom, a molecule oran ion which is electron-deficient; i.e. it is a Lewis acid or an electron pair acceptor.
Electrophilic addition reactions take place in two steps:
i. Formation of a carbocation
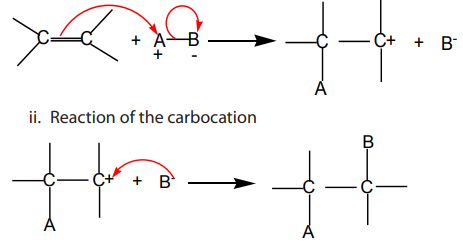
1. Addition of hydrogen halides
Hydrogen halides (HCl, HBr, HI) react with alkenes to yield halogenoalkanes. The
reaction is carried out either with reagents in the gaseous state or in inert solventsuch as tetrachloromathane.

When hydrogen halides add to unsymmetrical alkenes, the reaction leads to the
formation of two products in two steps. The first step leads to the formation oftwo different carbocations with the major product formed from the more stable
carbocation. This is the Markownikov’s rule. That is “The electrophilic addition of an
unsymmetric reagent to an unsymmetric double bond proceeds by involving the moststable carbocation.
The order of stability of the carbocations is:

In the presence of peroxide, the reaction follows a free radical mechanism and it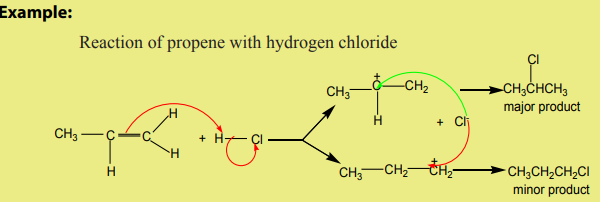
does not follow the Markonikov’s rule.


2. Addition of water
The hydration of alkenes catalysed by an acid is an electrophilic addition. Ethene
can be transformed into ethanol. The first step consists of adding concentrated
sulphuric acid. The second step consists of the hydrolysis of the product of the
first step.
In industry the reaction is carried out at approximately 300 °C in the prence ofphosphoric acid as a catalyst.
3. Addition of cold concentrated sulphuric acid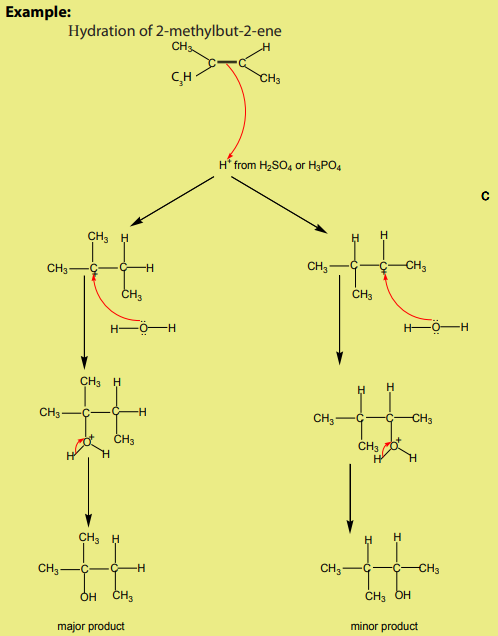
When cold concentrated sulphuric acid reacts with alkene, an alkyl hydrogen
sulphate is obtained. If the starting alkene is unsymmetrical, two different alkyl
hydrogen sulphates are obtained. If the alkyl hydrogen sulphate is warmed in thepresence of water, an alcohol is obtained.

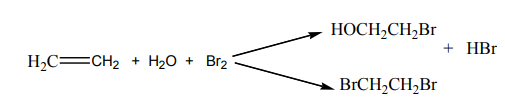
4. Addition of halogens
The addition of halogens (halogenation) on alkenes yields vicinal
dihalogenoalkanes. The reaction takes place with pure reagents or by mixing
reagents in an inert organic solvent.
When a chlorine or bromine molecule approaches an alkene, the pi electronscloud interact with the halogen molecule causing its polarisation.
Example:
Reaction of ethene with bromine in an inert organic solvent gives:

The reaction follows the mechanism below:
he reaction with bromine is a useful test for alkenes.
The brown red colour of bromine is discharged in alkenes.With bromine water, the reaction gives a mixture of organic products.
Example:
Bromine water containing sodium chloride gives a mixture of three organic products.
Example:
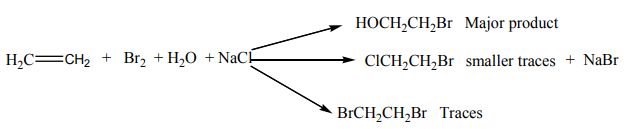
3.6.1.2. Hydrogenation
In the presence of a catalyst (Pt, Ni, Pd), alkenes react with hydrogen to give alkanes.
This reaction is very useful when transforming vegetable oils into fats such as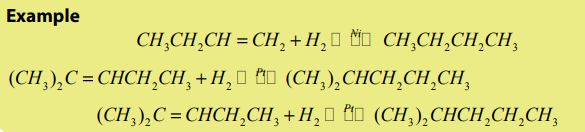
margarine by hydrogenation. The process is referred as hardening.
Checking up 3.6.1
1. Predict the products formed when alkenes (But-1-ene and
3-methylpent-2-ene react with each of the following reactants:
i. HCl
ii. Water in acidic medium
iii. Cold sulphuric acid
iv. hydrogen
2. Outline the mechanism of the reaction between 2-methylpent-2-enewith hydrogen bromide.
3.6.2. Oxidation reactions
Activity 3.6.2
1. Explain the terms oxidation, oxidising agent based on examples
2. Explain the terms reduction, reducing agent and give examples
3. The combustion of alkenes yields products, illustrate it by a reaction and
indicate the types of products generated.
4. Explain what happens when alkenes react with oxidising agents.
Alkenes are readily oxidised due to the presence of the double bond.
1. Reaction with oxygen
i. Transformation to epoxidesEthene react with oxygen in the presence of silver as a catalyst to yield epoxyethane.
Epoxyethane is a very reactive substance. It reacts with water to give 1,2-ethanediol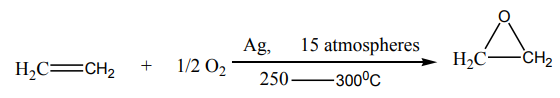
which is used in the making of polyesters, detergents, and so on.
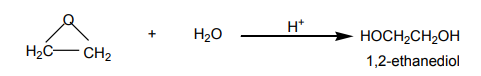
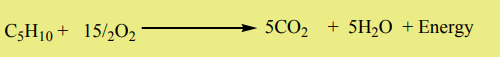
ii. Combustion
Alkenes burn in oxygen to give carbon dioxide, water and energy

Example:
2. Reaction with ozone
An alkene reacts with ozone to give an ozonide.
The reaction is carried out at low temperature (below ) in non-aquous medium.
) in non-aquous medium.
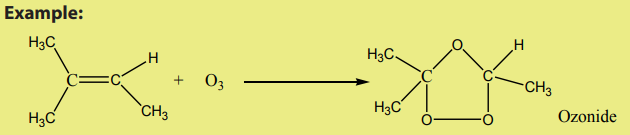
On hydrolysis, the ozonide splits into two carbonyl compounds. The reaction which
is an oxidative cleavage is referred to as ozonolysis.
Since the by-product is hydrogen peroxide, the hydrolysis is carried out in thepresence of a reducing agent.

The interest of the ozonolysis reaction is that it can help to identify the location of
the double bond in an alkene.
3. Reaction with potassium permanganate
Alkenes react with dilute potassium permanganate solution to give diols. The
reaction takes place in the cold.The colour change depends on the medium of the reaction.
This reaction also is used to test for the presence a double bond.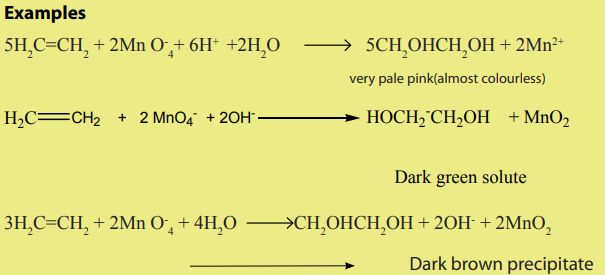
An alkane does not react with KMnO4 (left), but an alkene reacts with KMnO4producing a dark brown precipitate of MnO2 (right) (Figure 3.3).

Picture 3.2: Reaction of alkenes and KMnO
4. Hydroformylation
The hydroformylation is a process by which alkenes react with carbon monoxideand hydrogen in the presence of rhodium catalyst to give aldehydes.
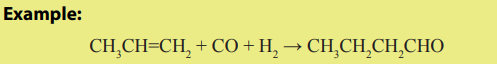
Checking up 3.6.2
1. Write the equations of the reaction between 3-methylpent-2-ene with:
a. Oxygen in the presence of silver catalyst.
b. Cold dilute potassium permanganate solution
c. Ozone
2. Describe the purpose of the reaction between alkenes and ozone.
3. Describe the observations when butane and but-2-ene react separatelywith potassium manganate (VII) solution.
3.6.3. Addition polymerisation
Activity 3.6.3
1. The students of a given class are asked to form separate couples of students.
In each couple, the students hold each other by their two hands. Now
each couple is asked to free one hand per student so that each student of
each couple can hold a hand of another student from a different couple.
What will be the result of such an arrangement compared to the first one?
2. From this example, predict what will happen in an addition reaction ofmany molecules of one or different alkenes?
Alkenes undergo addition polymerisation reaction to form long chain polymers.i.e
a polymer is a large molecule containing a repeating unit derived from small unit
called monomers. A polymerisation reaction involves joining together a large
number of small molecules to form a large molecule.
Many different addition polymers can be made from substituted ethene compounds.
Each polymer has its physical properties and therefore many polymers have wide
range of uses.Mechanism for the polymerisation of ethene.
1.Initiation
It is a free radical initiation.

2. Propagation
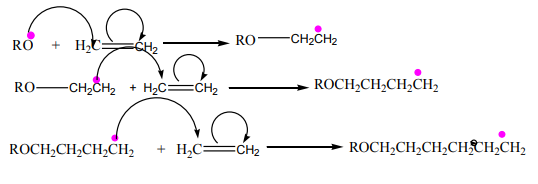
3. Termination
where the part between brackets indicates a unit of the formula of the polymer that
repeats itself in the formula; n indicates the number of the units in a formula of apolymer and is a very large number.
Summary of most alkene polymers obtained from alkenes as monomers and theiruses (Table 3.1)
Table 3.1: Polymers of alkenes and their uses

Checking Up 3.6.3
1. Explain the terms
a. addition polymerisation
b. monomer
c. polymer
2. The use of some plastic bags is banned in our country. Analyse the
scientific and environmental reasons of this prohibition and suggestalternative solutions.
Project Work
Although they have many uses, plastics have side effects and therefore some of
them are being replaced by more eco-friendly plastics.
Design a project for the making of plastics using starch from plants. In your
project you will:
1. Perform the extraction of starch
2. Make plastics using starch you will have extracted
3. Test the properties of your plastics4. Differentiate between bioplastics and biodegradable plastics.
3.7. Structure, classification and nomenclature of alkynes
Activity 3.7
1. Explain the formation of a carbon-carbon triple bond.
2. What is the hybridisation state of a carbon atom triply bonded and what
is the shape of the structure around it.3. Differentiate between the following compounds
4.
A triple bond consists of one sigma bond and two pi bonds. Each carbon of the triple
bond uses two sp orbital to form sigma bonds with other atoms. The unhybridised 2p
orbitals which are perpendicular to the axes of the two sp orbitals overlap sidewaysto form pi bonds.
According to the VSEPR model, the molecular geometry in alkynes include bond
angle of 180o
around each carbon triply bonded.Thus, the shape around the triplebond is linear.
There are two types of alkynes: terminal alkynes and non-terminal (internal) alkynes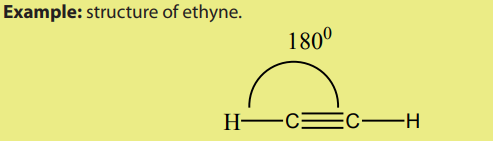
A terminal alkyne has a triple bond at the end of the chain e.g.: :
A non-terminal alkyne has a triple bond in the middle of the chain:

Examples:
Alkynes are named by identifying the longest continuous chain containing the triple
bond and changing the ending –ane from the corresponding alkane to –yne.

Checking Up 3.7
1. Name according to the IUPAC system, each of the following compounds.
5. Write structural formula for:
a. 2,5- dimethyl-3-hexyne
b. 6-isopropyl-5-propyldec-3-ynec. 5-ethyl-4-methloct-1-yne
3.8. Laboratory and industrial preparation of alkynes
1. Preparation of ethyne
Activity: 3.8Set up the apparatus as shown in the diagram below

Figure 3.3 Laboratory preparation of ethyne
Procedure:
• Place 2g of calcium carbide in a conical flask
• Using the dropping funnel, add water drop by drop.
• Collect the gas produced in the test tube.
• Remove the first tube and connect a second test tube.
• To the first test tube add two drops of bromine water. Record your
observations
• To the second tube add two drops of potassium manganate (VII). Record your observations.
Ethyne (acetylene) can be prepared from calcium carbide which is obtained byreduction of calcium oxide by coke at high temperature.
A more quick industrial production consists of heating methane alone at high
temperature for 0.01-0.05second.
When bromine water is added to acetylene, the red colour of bromine is discharged.
The solution becomes colourless. The decolourisation of bromine water is a test
for unsaturation in a compound.
When potassium manganate (VII) is added to acetylene, its purple colour isdischarged.
2. Alkylation of acetylene
The hydrogen atom of ethyne as that of other terminal alkynes is slightly acidic and
therefore it can be removed by a strong base like NaNH2 or KNH2
.The products of the reaction are acetylides. Acetylides react with halogenoalkanes
to yield higher alkynes.
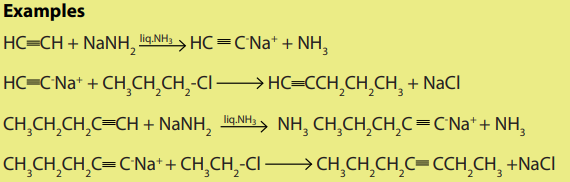
3. Dehydrohalogenation
The dehydrohalogenation of vicinal or geminal dihalogenoalkanes yields alkynes


4. Dehalogenation
The dehalogenation of a tetrahalogenoalkane yield an alkyne.

Checking Up 3.8
1. Using chemical equations, describe the preparation of ethyne(acetylene)
2. By which reactions higher members of the alkynes family are prepared?
3. Suggest a synthesis for each of the following compounds using
acetylene as the starting organic material.
a. Propyne
b. 2-butynec. 3-hexyne
3.9. Physical properties of alkynes
Activity 3.9
Alkynes have the general formula
They have two fewer hydrogen atoms
than alkenes, and four fewer H than alkanes. Do you expect alkynes to be more
or less volatile than alkenes? Explain by referring to the nature of the chemicalbonding and the structure of alkenes and alkynes.
Alkynes are non-polar compounds with physical properties similar to those of
alkenes with the same number of carbon atoms. Their linear structure gives themgreater intermolecular forces than alkenes

Alkynes are water insoluble but they dissolve in each other and in non-polar solvents.
Checking up 3.9
1. Which of 3,4,4-trimethylpent-1-yne and oct-3-yne has a high volatility?
Explain2. Table salt (NaCl) is water soluble but hex-2-yne is not. Explain why.
3.10. Chemical reactions of alkynes
Activity 3.10
Alkynes have a carbon-carbon triple bond. That is why they have a higher electron
density than alkenes. Do you expect alkynes to be more reactive than alkenes?Which types of reactions can be exhibited by alkynes?
Addition reactions
As unsaturated hydrocarbons, alkynes are very reactive. Because they are unsaturated
hydrocarbons, alkynes undergo addition reactions. Alkynes can add two moles of
reagents.
Even though they have a higher electron density than alkenes, they are in general
less reactive because the triple bond is shorter and therefore the electron cloud is
less accessible.
1. Addition of hydrogen halides
Alkynes react with hydrogen halides to yield vicinal dihalogenoalkanes, thereaction follows the Markownikov’s rule. The reaction takes place in four steps.
Example:
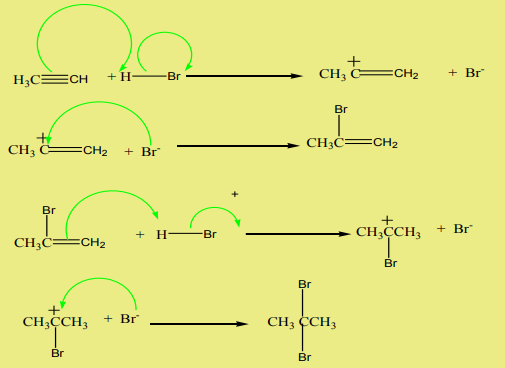
2. Addition of water
Alkynes react with water in the presence of sulphuric acid and mercury sulphate
at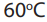 to give carbonyl compounds.
to give carbonyl compounds.
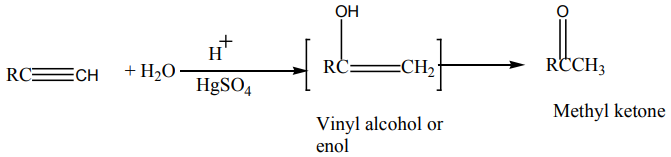
Example: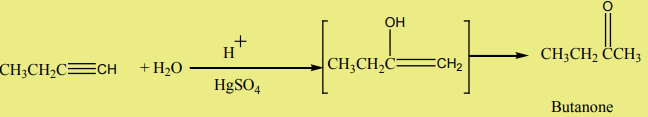
3. Hydrogenation
The hydrogenation of alkynes in the presence of palladium catalyst gives alkanesThe reaction requires two moles of hydrogen for a complete saturation.
Example:
In the presence of Lindlar catalyst, the alkynes are partially hydrogenated giving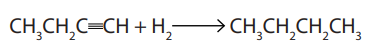
alkenes
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited
on calcium carbonate and poisoned with different lead derivatives such as lead
oxide or lead acetate.
Reaction with metals
Terminal alkynes react with active metals to yield alkynides and hydrogen gas.Internal alkynes do not react as they do not have an acidic hydrogen atom.

4. Reaction with metal salts
When a terminal alkyne is passed through a solution of ammoniacal silver nitrate, awhite precipitate of silver carbide is formed.
When a terminal alkyne is passed through a solution of ammoniacal copper(I)
chloride, a red precipitate of copper(I)carbide is formed.
The reactions above are used to:
• Differentiate between terminal and non-terminal alkynes.
• Differentiate ethene and ethyne
The reaction shows that hydrogen atoms of ethyne are slightly acidic, unlike thoseof ethene.
Checking up 3.10
1. Write the formula(s) and the name (s) of the products of the reaction of
pent-1-yne with:
a. water
b. hydrogen chloride
c. sodium metal
2. Outline the mechanism of the reaction between but-2-yne with
hydrogen bromide.
3.11. Uses of alkenes and alkynes
Activity 3.11
Look at the picture below and mention the importance of alkenes and alkynes.
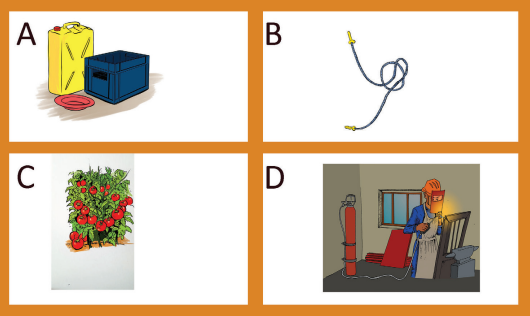
Figure 3.5: Some plastic materials (A& B), tomatoes which are ripening (C)
and a person who is welding (D)
• Alkenes are extremely important in the manufacture of plastics which have
many applications such as: packaging, wrapping, clothing, making clothes,
artificial flowers, pipes, cups, windows, ...
• Ethene is a plant hormone involved in the ripening of fruits, seedgermination, bud opening;
Picture 3.3: Ethene is a plant hormone which causes bananas to ripen.
image source m.yukie .mobi.
• Ethene derivatives are also used in the making of polymers such as
polyvinylchloride (PVC), Teflon,...
• Alkenes are used as raw materials in industry for the manufacture of
alcohols, aldehydes, ...
• Alkynes are used in the preparation of many other compounds. For example
ethyne is used in the making of ethanal, ethanoic acid, vinyl chloride,
trichloroethane, ...
• Ethyne (acetylene) is used as a fuel in welding and cutting metals.• Propyne is used as substitute for acetylene as fuel for welding.
Checking up 3.11
Alkenes, alkynes and their derivatives have many applications in our daily life.Discuss this statement.
3.12. End unit assessment
I. Multiple choice questions. Choose the best answer in the following by
noting the corresponding letter.
1. Which of the following is given off during ripening of fruits and
vegetables?
b. Ethane
c. Ethene
d. Ethyne
e. Methane
2. Loss of hydrogen halide is called:
a. Halogenation
b. Dehydration
c. Dehydrohalogenation
d. Hydrogenation
3. Alkenes can be oxidized using powerful oxidizing agent in acidified
medium.
a. Potassium manganate (VII)
b. Sodium manganate (VI)
c. Calcium manganate (VI)
d. All of them
4. The molecular formula of-------- fit the general formula
a. Alkanes
b. Alkynes
c. Alcohols
d. Alkenes
5. Example of addition reactions include all but one of the following.
Which is the odd one out?
a. Combustion of propene.
b. Reaction of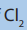 with propene.
with propene.
c. Reaction of HBr with but-2-ene.
d. Polymerization of ethene
6. Which statement is incorrect about reactions of propene?
a. Reaction with and
and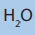 gives 1-bromo propan-2-ol as the main
gives 1-bromo propan-2-ol as the main
product.
b. Polymerization of propene gives polypropene, of which the isotactic and
syndiotactic forms are commercially valuable.
c. Reaction with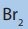 in the presence of a radical initiator yields
in the presence of a radical initiator yields
2-bromopropane as the major product.
d. No correct answer
7. Which one of the following statements is incorrect?
a. The electrophilic addition of HBr to but-2-ene involves a secondary
carbonium ion intermediate.
b. In the presence of a radical initiator, HBr reacts with but-1-ene to give
1-bromobutane as the major product.
c. In the presence of a radical initiator, HBr reacts with but-1-ene to give a
Markovnikov addition product.
d. The major product of the electrophilic addition of HBr to hex-1-ene is
2-bromohexane.
8. What type of reaction do alkynes undergo across triple bond?
a. Elimination reaction
b. Substitution reaction
c. Addition reaction
d. Halogenation
96 Chemistry Senior Five Student Book
9. Acetylene is also called:
a. Ethyne
b. Ethene
c. Ethane
d. Methane
10. What product(s) will be obtained from the acid-catalysed hydration of
pent-2-yne?
a. pentanal
b. pent-2-one and pentan-3-one
c. pentan-2-one
d. pentan-3-one
II. Open questions
11. Give all possible isomers of
12. Explain the following observations
a. When bromine in presence of dichloromethane is added to propene,
only one product is formed i.e. 1,2-dibromopropane.
b. When bromine water is added to propene, a mixture of
is added to propene, a mixture of
products namely 1,2-dibromopropane and bromopropan-2-ol are
obtained.
c. When bromine in presence of carbon tetrachloride and sodium chloride
is added to propene, a mixture of products namely, 1,2-dibromopropaneand bromo-2-chloropropane are formed.
13. Show how the following conversions may be accomplished
14. a. In an experiment it was found that 35g of pure alkene reacted
with 100g of bromine .
i. Calculate the molecular mass of the alkene
ii. Write the molecular formula of the alkene
iii. Write the structural formulae and the systematic names of one of
any two alkenes in (ii)
b. Using equations only show the mechanism for the reaction of oneof alkenes in (iii) with bromine.
15. Three hydrocarbons D, E and F, all have the molecular formula C6H12.
D decolourises an aqueous solution of bromine and shows geometric
isomerism. E also decolourises an aqueous solution of bromine but does
not show geometric isomerism. F does not decolourise an aqueous solution
of bromine. Draw one possible structure each for D, E and F.
16. Alkenes such as ethene and propene have been described as the building
blocks of the organic chemical industry. Discuss this statement, giving
examples. What particular features of the chemistry of alkenes make themsuitable for this role and why are alkanes less suitable.
