UNIT 2: ALKANE
Key unit competency
Relate the physical and chemical properties of the alkanes to the preparation
methods, uses and isomerism.
Learning objectives
• Name straight chain alkanes up to carbon-20
• Define homologous series
• Use IUPAC system to name straight and branched alkanes
• Describe the preparation methods of the alkanes
• Prepare and collect methane gas
• Respect of procedure in experiment to carry out preparation of methane or
propane
• Describe and explain the trend in physical properties of homologous series of
alkanes
• Be aware of the dangers associated with combustion reactions of the alkanes
• Write reaction for free radical mechanism for a photochemical reaction
• State the chemical properties of the alkanes
• Develop practical skills,interpret results make appropriate deductions.
• Appreciate the importance of the alkanes in daily life
• Appreciate the dangers caused by the alkanes to the environment as major
sources of air contaminants
• State the uses of the alkanes
Introductory activity
Analyze the picture below and answer to the proposed questions

a. Explain the process observed in the above picture
b. What is the source of the gas produced as shown by the picture?
c. Analyse the environmental problems caused by gas observed in the
picture and suggest different ways to solve it.
Alkanes are the simplest class of organic compounds. They are made of carbon and
hydrogen atoms only and contain two types of bonds, carbon-hydrogen (C-H) and
carbon-carbon (C-C) single covalent bonds. They do not have functional groups.
Alkanes form a homologous series with the general formula where n is the
where n is the
number of carbon atoms in the molecule. The first member of the family has the
molecular formula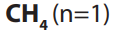 and is commonly known as methane and the second
and is commonly known as methane and the second
member with molecular formula is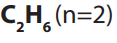 is called ethane.
is called ethane.
These compounds are also known as saturated hydrocarbons. This name is more
descriptive than the term “alkane’’ because both their composition (carbon and
hydrogen) and the fact that the four single covalent bonds of each carbon in their
molecules are fully satisfied or ‘’saturated’’.
The name alkane is the generic name for this class of compounds in the IUPAC
system of nomenclature. These hydrocarbons are relatively unreactive under
ordinary laboratory conditions, but they can be forced to undergo reactions by
drastic treatment. It is for this reason that they were named paraffins (Latin parumaffinis = little activity).
2.1. Nomenclature of alkanes
Activity 2.1
4. Discuss IUPAC rules for naming straight and branched alkanes.
5. Draw the structure of the following compounds:
a. 3-ethyl-4-propyloctane
b. 4-ethyl-2-methylhexane
c. 2,2-dimethylpentane
IUPAC Rules for the nomenclature of alkanes
a. Find and name the longest continuous carbon chain.
b. Identify and name groups attached to this chain.
c. Number the chain consecutively, starting at the end nearest a substituent
group.
d. Designate the location of each substituent group by an appropriate
number and name.
e. Assemble the name, listing groups in alphabetical order. The saturated
hydrocarbon form homologous series (series in which members have similar
chemical properties and each differs from the preceding by a methylene
group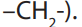
The first four members are known by their common names, from C5
and above the Roman prefixes indicating the number of carbon atoms is written
followed by the ending “ane” of the alkanes.
Note: Alkyl groups are obtained when one hydrogen atom is removed from alkanes;
therefore their names are deduced from the corresponding alkanes by replacing“ane” ending with “yl” desinence (Table 2.1).
Table 2.1. Naming straight chain alkanes

Note: n is the number of carbon atoms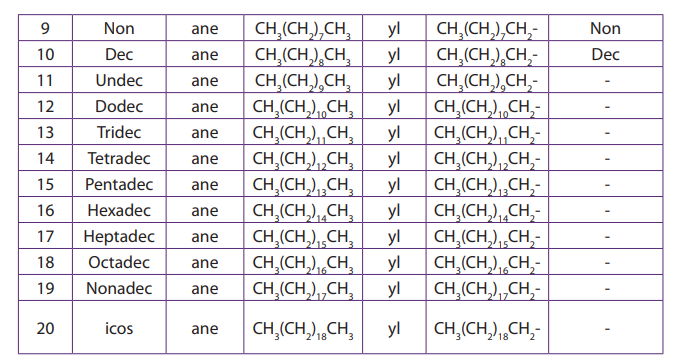
Prefixes di, tri, tetra, sec, tert, are not considered when alphabetizing.
f. In case of chains of the same length, the priority is given for part wheremany branched of alkyl groups appear.
g. For cyclanes or cycloalkanes, the prefix “cyclo” is recommended, followed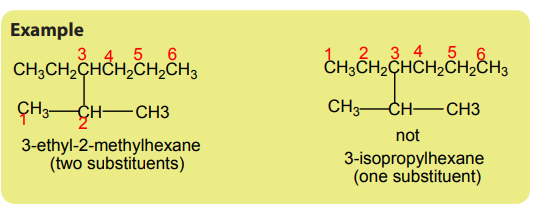
by the name of the alkanes of the same carbon number.But in case of ramified cyclanes, the priority is for the ring.
The organic compounds comprise aliphatic compounds that can be acyclic or
cyclic named respectively as alkanes and cyclanes.
Note: If there are more than one substituent, the numbering is done so that the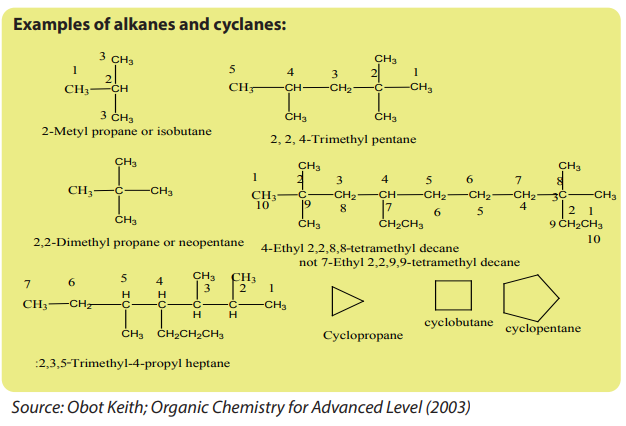
sum of the numbers used to locate the locants is minimum. This is the lowest sumrule.
The longest chain has 6 carbons, it is a hexane chain. The sum of locants
Since the sum of the locants for R-L numbering is minimum, then it is preferred.
h. The name of alkane is given by the numbers of the locants (2,3,5-) followed
by the prefixed substituent (trimethyl), followed by the name of the long chain(hexane): 2,3,5-trimethylhexane.
Checking up 2.1
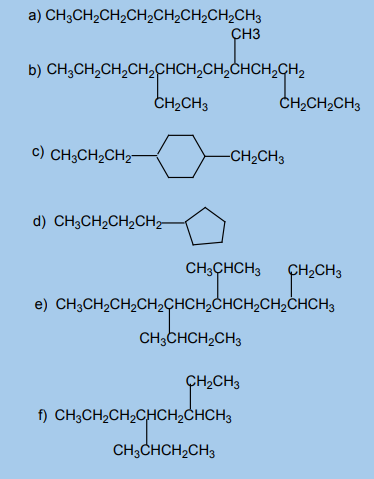
1. What are alkanes? Why are they called saturated hydrocarbons?2. Name each of the following alkanes according to the IUPAC system.
2.2. Isomerism
Activity 2.2.
Identify and write down all the structural formulas that fit the molecularformula
Alkanes show structural isomerism. The easiest way to find isomers is to draw the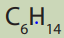 and classify them into the position and chain isomers.
and classify them into the position and chain isomers.
longest chain of carbon atoms first and then reduce it by one carbon first untilrepetition begins to occur.
-Putting the methyl group on position 1 or 5 gives you the same straight chain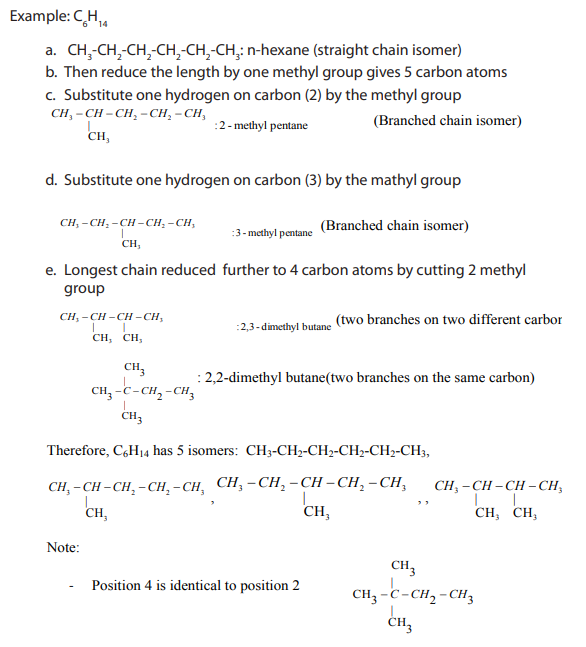
isomer.
Checking up 2.2
Write the structural formulae of all isomers which fit the molecular formula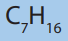
and name each of them according to the IUPAC system.
2.3 Occurrence of Alkanes
Activity 2.3:
Some organic compounds are found in living beings whereas others are
synthesised by humans. Under which category do alkanes fall? Justify youropinion.
1. The alkanes exist in nature in form of natural gases and petroleum. Natural
gas and petroleum existence are the results of decomposition of died bodies
after many years ago.
2. The most natural gas is found in lake Kivu as methane gas but in form of
traces like ethane, propane and butane.
3. Petroleum is one of the largest source of energy in the work. It is formed by
decomposition by bacteria for millions of years died marine living things
and as the last product is petroleum and natural gases which are separated
in fractional distillation of their crude oil and the results are obtainedaccording to their boiling point.
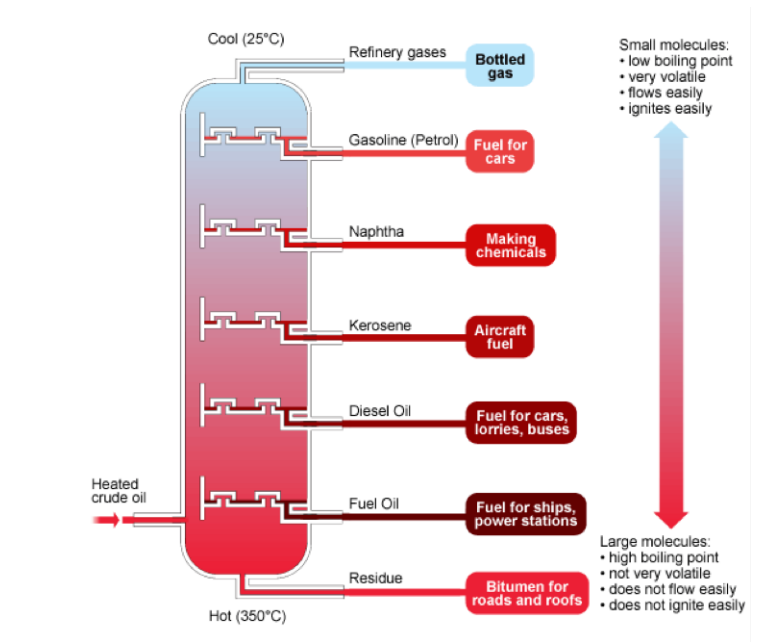
Figure 2.1. Fractional distillation of petroleum
Source:www.bbc.uk/schools/gcse/fractionaldistillationofcrudeoil ;
The fractional distillation and the different fractions are summarized in the followingtable and in the Table 2.2.
Table 2.2. Fractions of crude petroleum

Checking up 2.3:
What are the main sources of alkanes?
2.4. Preparation of alkanes
Activity 2.4
Laboratory preparation of methane gas
Requirements:
Stand and accessories
Delivery tube
NaOH(s)
Sodium acetate(s)
Calcium oxide(s)
Procedure:Set up the apparatus as shown on the diagram below
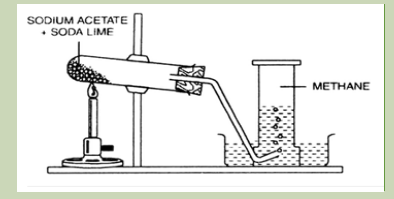
Figure 2.2 Laboratory preparation of methane
Source: https.www. zigya.com/study/book
Prepare a mixture of the reagents in ratio 1:1. Weigh about 3 grams of sodium
acetate and the same quantity as soda lime. Mix them thoroughly in a beaker.
Place about 4 grams of the mixture into a boiling tube.
Seal the boiling tube with a stopper with a gas-delivery tube. The gas-delivery
tube should look upwards.
Fix the boiling tube on a stand.
Heat the test-tube gently with the cold part of the flame. To avoid local
overheating keep the flame in motion.
After a while the gas starts liberating.
Prepare an empty test-tube. Collect some gas keeping this test-tube on top of
the gas delivery tube.
Methane is a flammable gas. To set it on fire turn the covering test tube and
hold a burning match to the end of the gas delivery tube.
The gas burns with a blue (red) fire.
Methane can be prepared by the reaction between sodium acetate and sodium
hydroxide solid according to the equation:
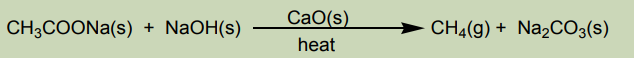
It is collected by the downward displacement of water.
Other gaseous alkanes can be prepared in the same way according to the generalequation.
Note: The reaction is practically used to reduce by one carbon the length of carbon
chain. It is referred as decarboxylation of sodium carboxylates.
Other reactions used for the preparation of alkanes are the following:
1. Addition reaction of hydrogen to alkenes and alkynes in the presence of
catalyst like Nickel, Palladium or platinum produces alkanes: this reaction
is called hydrogenation reaction of alkenes and alkynes; it is also called areduction reaction of alkenes and alkynes.
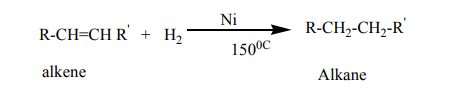
N.B: In organic chemistry, reduction reaction is the reaction that results in
increasing of hydrogen content in the new product.
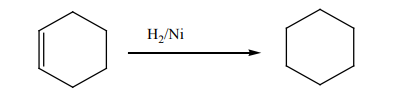
[Hydrogen content in the product
 is higher than the hydrogen content in the reactant
is higher than the hydrogen content in the reactant
Note: Reduction with Platinum and Palladium as catalyst occurs at room
temperature, while using Nickel requires a temperature of about

2. From halogenoalkanes or Alkyl halides
On reduction of alkyl halides with Zn and concentrated hydrochloric acid, alkyl
halides are converted to alkanes.
Checking up 2.4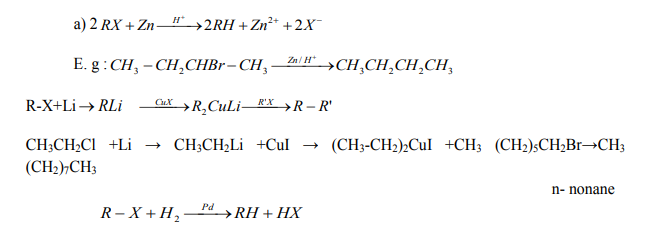
Describe the main reactions used in the preparation of alkanes.
b) Alkyl halides when heated with sodium metal in ether solution give higher
alkanes (alkanes with more carbon atoms) (Wurtz reaction).
Note: This reaction is practically useful in organic synthesis to increase the length of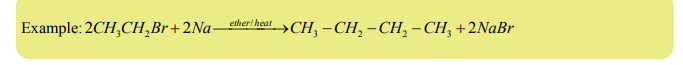
carbon chain.
c) When Alkyl halides are treated with Zn-Cu couple, in the presence of ethanol,
alkanes are formed.
Note: Zn-Cu couple is obtained by adding Zinc granules in aqueous copper (II)sulphate solution where copper is deposited on the Zn pieces.

3. From carbonyl compounds
Reduction of carbonyl compounds, with amalgamated Zinc (alloy made of zincand mercury) and HCl. This is the Clemmensen reduction).
Note: Under special conditions, reduction also is realized by use of H2 and Raney
Nickel or using hydrazine (NH2NH2) and KOH.This is calledWolf Kushner reduction
2.5. Physical properties of alkanes
Activity: 2.5
1. Put 5 ml of hexane in a test tube. Add 5ml of water and shake.
2. Record your observations
3. Repeat the above procedure using,
4. (i) cyclohexane
5. (ii) heptane
6. Repeat the steps 1-3 using carbon tetrachloride instead of water.
7. Record your observations.
8. Search about the melting and boiling point values of the alkanes used inthe experiments above and record your findings.
Melting and boiling points
The values of melting, boiling points, density and physical state of some alkanesare summarized in the table below.
Table 2.3 Physical properties of alkanes
The above Table shows that the boiling and melting points of homologue alkanes
increase with the number of carbon i.e. molecular mass.
Explanation:
The boiling and melting points depend on the magnitude of the Van Der Waal’s
forces that exist between the molecules. These forces increase in magnitude with
molecular mass.
Note: Branched chain isomers have lower boiling and melting points than their
straight chain isomers, because straight chain isomers are closely packed than thebranched chain isomers.
Boiling points decrease with increase in branching because increased branching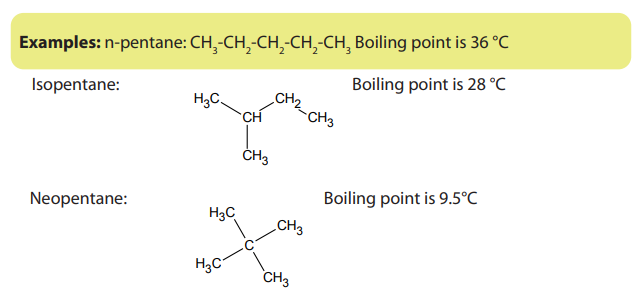
gives the molecule a more nearly spherical shape and this reduces the extent of
contact between neighboring molecules, in other words the branched isomers are
less packed than straight chain isomers, and hence the attractive force betweenthe molecules are reduced hence decrease in the boiling points.
Alkanes are not soluble in water, because of their low polarity and also because
of their inability to form hydrogen bonds. They are, however soluble in non polar
solvents, like benzene, and are miscible with one another.
benzene, and are miscible with one another.
Checking up 2.5
Using data in the Table 2.3, plot a graph of boiling and melting points against
the number of carbon atoms, explain the shapes of the graphs drawn.2.6. Chemical properties of alkanes
Activity 2.6.1
Experiment to investigate the reactivity of alkanes
1. Put 5ml of hexane in a test tube.
2. Add drop wise 5ml of potassium hydroxide and shake
3. Repeat steps 1-2 using bromine water instead of potassium hydroxide
4. Repeat steps 1-3 using octane.5. Record all your observations in the table below.
For a positive test put “yes” and “no” for a negative result.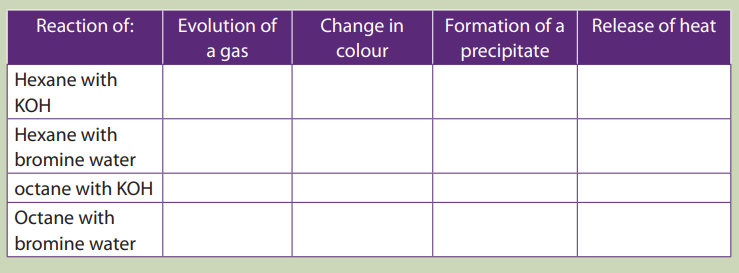
6.What do you deduce from your observations?
Generally, alkanes are quite inert towards common reagents because:
• The C-C bond and C-H bonds are strong and do not break easily.
• Carbon and hydrogen have nearly the same electronegativity valuehence
• C-H bond only slightly polarized; generally C-H bond is considered as covalent.
• They have unshared electrons to offer.They, however, undergo the following reactions.
1. Reaction with oxygen
Alkanes react with oxygen to produce carbondioxide and water. However, if oxygenis insufficient carbonmonoxide gas and water are formed.
Carbon dioxide
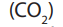 produced from the burning of alkanes or fossil fuels for
produced from the burning of alkanes or fossil fuels for
heating, transport and electricity generation is the major atmospheric pollutant that
increases the green house potential of the atmosphere. Carbon dioxide is the major
Green House Effect (GHE) gas.
Burning wood and forests produce also carbon dioxide and lead to the increase of
that gas in the atmosphere. Methane as another GHE gas is produced by humanactivities, agriculture (Rice), and cattle-rearing.
Activity 2.6.2
Carry out research and discuss different ways of avoiding or reducing theproduction of GHE gases such as carbon dioxide.
There are many natural ways of reducing atmosphere carbon dioxide:
i. Water in seas dissolves millions of tonnes of gas (but less now than it did in
the past, since the average ocean temperature has increased by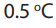
in the last 100 years, and gases are less soluble in hot than in cold water).
ii. Plankton can fix the dissolved carbon dioxide into their body mass by
photosynthesis
iii. Trees fix more atmospheric carbon dioxide than do grass and othervegetation through photosynthesis according to the equation below.
iv.
There are other ways than natural ways of reducing GHE gases and among them
there are the use of technologies that reduce the green house gas emissions, therecycling of the GHE.
Notice: (i) reacts as
reacts as  but slowly while iodine hardly reacts.
but slowly while iodine hardly reacts.
Notice: (i) reacts as
reacts as  but slowly while iodine hardly reacts , Fluorine, the most
but slowly while iodine hardly reacts , Fluorine, the most
electronegative element of the periodic table reacts with alkanes to givecoke, i.e. a decomposition reaction:
(ii) Due to radical formation involved, the main product of reaction is the one
from the most stable radical, starting with tertiary, secondary, primaryand methyl in decreasing order of stability.
A tertiary free radical is better stabilised by the electron donating methyl groups
than the secondary, primary and methyl ones where the carbon atom is attached tomore hydrogen atoms
3. Dehydrogenation of alkanes gives alkenes under heat and a catalyst like
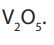
4. Cracking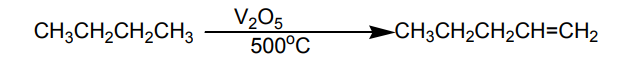
On heating or in the presence of a catalyst, large molecules of alkanes are decomposed
into smaller alkanes and alkenes. If the cracking is performed on heating, it is referredas themocracking.
If the cracking is performed using a catalyst; it is referred as catalytic cracking andmany products result from one reactant as shown below.
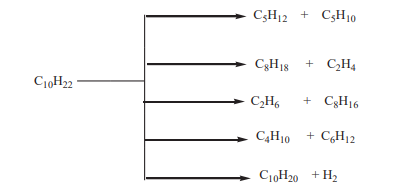
Checking up 2.6
1. Using a series of chemical equations, show how butane can be prepared
from bromoethane
2. Kerosene is an alkane obtained from fractional distillation of
is an alkane obtained from fractional distillation of
crude petroleum. Write an equation to show that kerosene can be usedas a source of energy.
2.7. Uses of alkanes
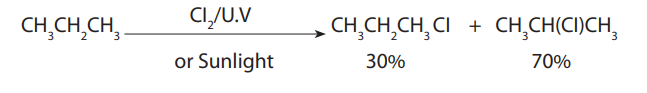
2. Reaction with halogens (halogenations)
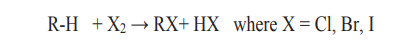
Example: Reaction of methane with bromine
Mechanism of the reaction: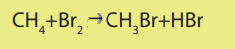
A mechanism of a reaction is a description of the course of the reaction which showssteps of the reaction and the chemical species involved in each step.
The mechanism for the reaction between methane and bromine is the following.i. Phase 1: Initiation (radical formation: formation of Br atom)
ii. Phase 3: Termination steps (Radicals combination and end of the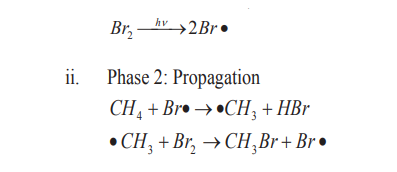
formation of radicals).
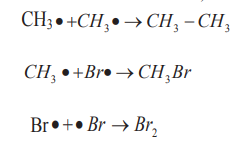
Hence, the generalized reaction

Activity 2.7.
In Rwanda, gas methane has been discovered in Lake kivu and the government
is under its exploitation.
1. Outline all possible uses of methane gas2. Discuss the economic impact of the gas to the livelyhood of Rwandans.

Picture 2.1: Kivu watt power station
3. With the help of the pictures below, deduce the uses of alkanes
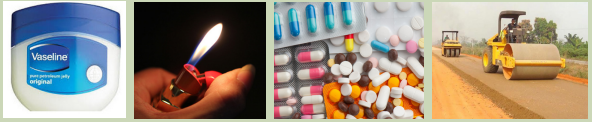
1. Methane
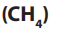
Methane finds many uses:
• It is used as a fuel at homes, ovens, water heaters, kilns and automobiles
as it combusts with oxygen to produce heat.
• Highly refined liquid methane is used as rocket fuel.
• Methane is used as fuel for electricity generation.
• It is used as a vehicle fuel in the form of liquefied natural gas (LNG).
• Methane can be used as raw material in the production of urea, afertilizer.
In general, methane is more environmental friendly than gasoline/petrol and diesel.
2. Butane
• Butane is a key ingredient of synthetic rubber.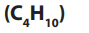
• It is used as fuel in cigarette lighters.
• When blended with propane and other hydrocarbons, it may be referred
to commercially as LPG, for liquefied petroleum gas.
• Butane gas cylinders are used in cooking.• Also used in aerosol spray cans.
3. Propane
• Propane is used as a propellant for aerosol sprays such as shaving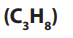
creams and air fresheners.Used as fuel for home heat and back up
electrical generation in sparsely populated areas that do not have
natural gas pipelines.
• Propane is commonly used in movies for explosions
4. Ethane
• Ethane is used in the preparation of ethene and certain heavier
hydrocarbons.
• Ethane can be used as a refrigerant in cryogenic refrigeration systems.
5. Pentane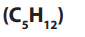
• Pentane is used in the production of polystyrene foams and other foams.
• Used in laboratories as solvents.
• It is also an active ingredients of pesticides.
• Used as solvent in liquid chromatography
6. Hexane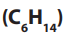
• It is used in the formulation of glues for shoes, leather products, and
roofing.
• It is also used to extract cooking oils such as canola oil or soy oil from
seeds.
• Hexane is used in extraction of pyrethrine from pyrethrum; e.g. Horizon
SOPYRWA (a pyrethrum factory in Musanze District).
• Also for cleansing and degreasing a variety of items, and in textilemanufacturing.
7. Heptane
• Heptane is used as solvent in paints and coatings.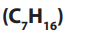
• Pure n-heptane is used for research, development and pharmaceutical
manufacturing.
• Also as a minor component of gasoline.• It is used in laboratories as a non-polar solvent.
2.8. End Unit Assessment
1. Give the general formula of alkanes
2. Answer by True or False
a. 2,2-dimethylbutane is an isomer of hexane
b. Boiling point of alkanes increases with increasing the length of the
chain. Explain why?c. Alkanes are polar molecules; justify your answer
3. Draw the structures of the following formulas:
a. 2, 3, 5-trimethyl-4-propylheptane
b. 2, 2-dimethylpropane
c. 2-methyl pentaned. 4-ethyl-2, 3-dimethyloctane
4. Explain the different steps of the chlorination reaction of methane
5. An alkane with molecular mass of 72 forms only one monochlorinated
product. Suggest the structure of the alkane.
6. a) What do you understand by the term hydrocarbon?
b) What is the relationship between the number of carbon atoms in ahydrocarbon and its boiling point?
c) The hydrocarbon C5H12 burns to form carbon dioxide and water.Write the balanced equation for the reaction.
d) Name the environmental problem that is caused by the formation ofcarbon dioxide during the combustion of hydrocarbon.
7. Consider the alkane with the formula CH3-CH2-CH2-CH2-CH2-CH3
a. Determine the percentage composition of carbon and hydrogen in
the compound,
b. Determine the empirical formula of the above compound,
c. From the results in a) calculate the molecular formula of the
compound,
d. Write down the balanced chemical equation of combustion of the
compound,
e. Name the environmental problem that is caused by the performance of
the reaction in d) and suggest different ways to solve that environmental
problem.
8. Show how each of the following conversions can be accomplished with agood yield
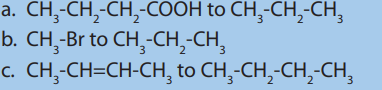
9. a. Referring to methane as an example of alkanes, discuss the importance of
alkanes in our every day life.
b. Gaz methane is extracted in Lake Kivu in Rwanda. Explain its perspectivesin Rwandan economy?
