UNIT 15: ENTROPY AND FREE ENERGY
Key unity competence:
To be able to Predict the feasibility of chemical reactions.
Learning Objectives
By end of this unit a learner should be able to:
• Explain the term entropy
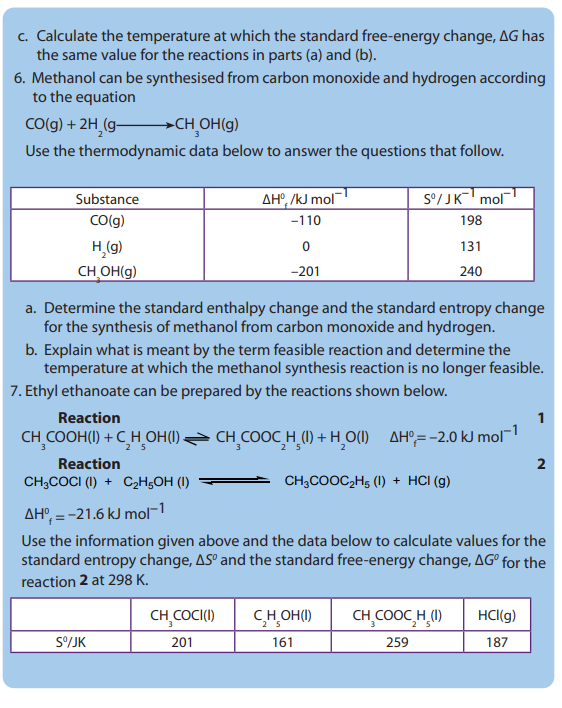
• State the second law of thermodynamics
• State if the value of free energy for a reaction will be positive or negative.
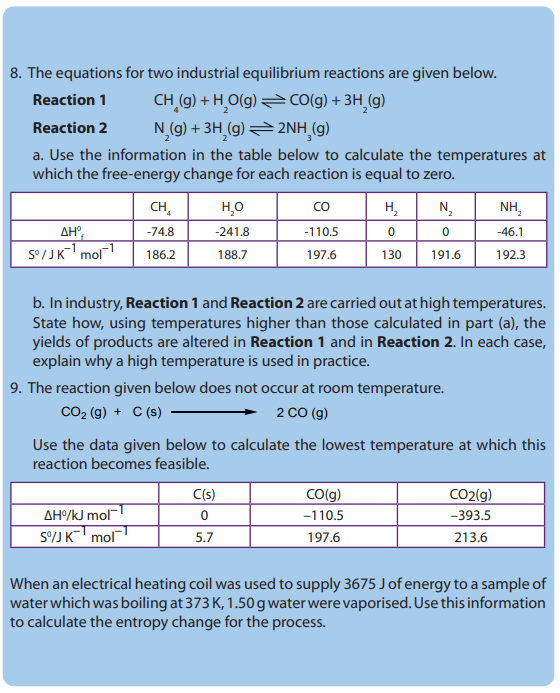
• Relate the entropy changes to the changes in degree of disorder• Predict the spontaneity of reactions using the Gibbs free energy values
INTRODUCTORY ACTIVITY
Observe the pictures above of playing cards and answer related questions.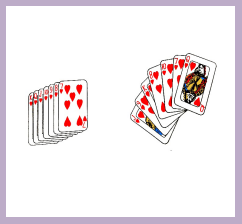
1. What is the difference between the two organizations of playing cards
2. In which conditions (arrangement) to play card is easy? Explain3. Why people toss cards before playing them
15.1. Definition of entropy and change in entropy
Activity 15.1 Investigation of entropy of a substance.
1. Take three beakers and label them by A, B, and C
2. In beaker A add 50 grams of ice
3. In beaker B add 50 mL of water
4. In beaker C add 25 mL of water and heat it up to transformation to liquid
state (vapors)
5. Pour the container of beaker A and B, then compare it to the movement
of vapors in C
a. In which beaker, A, B or C where the molecules move easily (high
speed)?
b. In which beaker A, B or C where the molecules move slowly (low
speed)?
c. Order the beakers to the order of mobility of the container.
d. Explain why the mobility of molecules in beakers A, B and C varies.
e. Which factor that make the variation of water in different states tomove differently in the beakers A, B and C?
15.1.1. Definition of entropy
Entropy is a thermodynamic function that describes the number of arrangements
(positions and/or energy levels) that are available to a system existing in a given
state. Entropy is a quantitative measure of microscopic disorder for a system. It is defined
as the degree of disorder or randomness of a system. The entropy of a system increases
as the disorder of the system increases. When we focus on the molecular motion of a
system, adding heat to this system increases the disorder because the heat increasesthe randomness of the molecular motion. So, the entropy of the system increases.
The effect of adding heat to a system increases the molecular motion, and this
results in more disorder of the system. Entropy is derived from the second law of
thermodynamics, which states that “all systems tend to reach a state of equilibrium”.
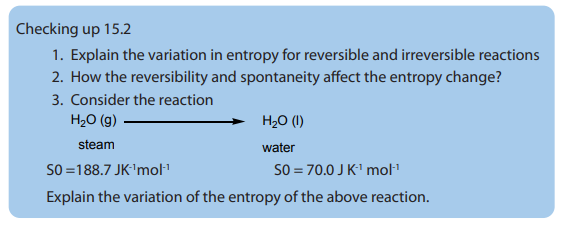
Based on the state of matter, the entropy increases in the order: Solids < liquids < gases
substances (Figure 15.1). If we consider three substances such as solid, liquid and a gas.
A mole of a substance has a much smaller volume in the solid state than it does in
the gaseous state. In the solid state, the molecules are close together, with relatively
few positions available to them; in the gaseous state, the molecules are far apart,
with many more positions available to them. The liquid state is closer to the solidstate than it is to the gaseous state in these terms.
In the above Figure, the molecules are closely packed in the solid state, in the liquid
state; the molecules are not very closed while in a gas the molecules are far apart
which increase the entropy in gas than liquid or solid.
The following Tables 15.1 and 15.2 show the relationship between the state of asubstance and its entropy:
Table 15.1. Entropy variation with state of a substance
In the above Table 15.2, the element argon has a greater entropy value than the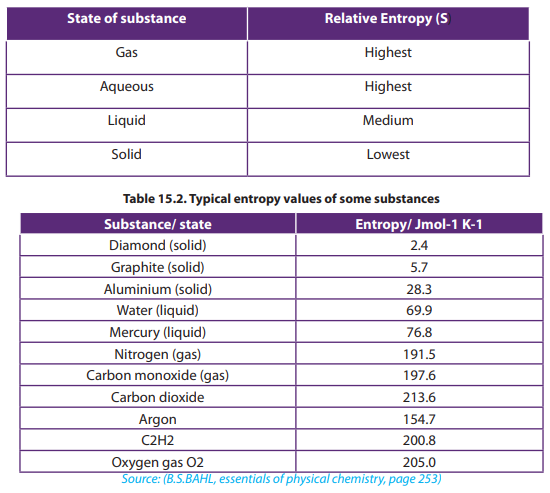
carbon element (graphite) because argon is a gas with greater disorder and random
particle movement than the solid state of carbon.
The entropy of a perfect crystal at absolute zero is exactly equal to zero. At absolute
zero (zero kelvin), the system must be in a state with the minimum possible energy.
As the temperature increases, the particles vibrate more and the disorder increases.
Melting is associated with an increase in entropy (disorder), however, boiling
is associated with a large increase in entropy: gases are associated withconsiderable random particle in movement and disorder.
15.1.2. Change in entropy
Entropy, like temperature, pressure, and enthalpy, is also a state property and is
represented in the literature by the symbol “S”. The entropy change is represented
by ΔS.
It is known that the main purpose of chemistry is the study of chemical reactions. In
this section, it is important to consider the entropy changes accompanying chemical
reactions that occur under conditions of constant temperature and pressure. The
entropy changes in the surroundings are determined by the transfer of heat that
occurs when the reaction takes place. However, the entropy changes in the system
(the reactants and products of the reaction) are determined by positional probability.For example, consider the reaction of production of ammonia in the Haber process:
It is seen that four molecules of reactants yield two molecules of ammonia product
which lead to less disorder in the system. If the number of molecules of the gaseous
products is greater than the number of molecules of the gaseous reactants, positional
entropy typically increases, and ∆S will be positive for the reaction.The calculation of the entropy change of a reaction is given by applying the formula:
For a chemical reaction which involves the reactants and the products, the change
in entropy is calculated as follows.
Any reaction that results in the formation of a gas, an increase in the number of
gaseous moles, results in the increase of the disorder. Entropy change, ∆S, relates to
increasing disorder of a process, either arising through physical change (e.g. melting,
evaporation) or chemical change (e.g. dissolution, evolution of
from hydrogen carbonates with acid) or both.

The chemical reactions are favored if they are accompanied by an increase in entropy.
2. Consider the thermal decomposition of solid calcium carbonate, predict the signof the standard entropy

In this reaction, a solid reactant produces a molecule of gas, the positional entropy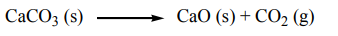
increases, and
 is positive
is positive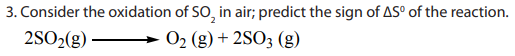
For this reaction, three molecules of gaseous reactants become two molecules of
gaseous products. Because the number of gas molecules produced decreases, the
entropy decreases also, and
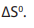 is negative.
is negative.Note:
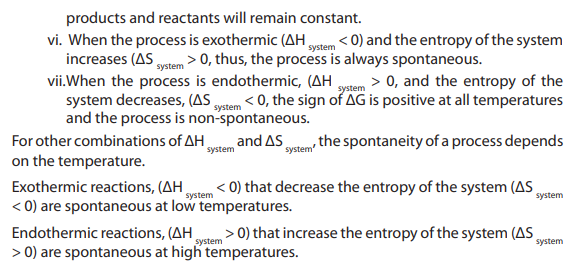
i. Many endothermic reactions proceed spontaneously under normal
conditions because there is an increase in entropy.
ii. Some exothermic reactions do not proceed spontaneously because there is
a decrease in entropy. In a system with perfect order, the entropy is equal to
zero. An example of perfect order is found in a crystalline substance at the
absolute zero of temperature, where atomic and molecular motion cease.The entropy of pure, perfect crystal can be taken to be zero at 0 K.
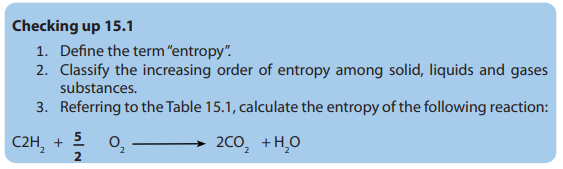
15.2. Second law of thermodynamics
Activity 15.2
1. State and explain the first law of thermodynamics
2. What is the relation between the first law of thermodynamics andthermochemistry?
In chemistry, thermodynamics deals with the energy and work of a system. There
are three laws in thermodynamics: first, second and third law. The first law establish
the relationship between the different forms of energy present in a system (kinetic
and potential), the work done by the system and the energy or heat transferred. The
second law of thermodynamics is dealing with entropy which describes the disorder
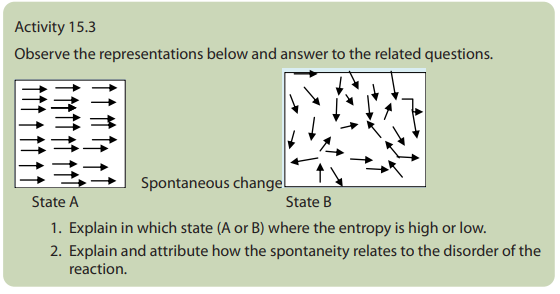
of the system. The Second law of thermodynamics states that in any spontaneous
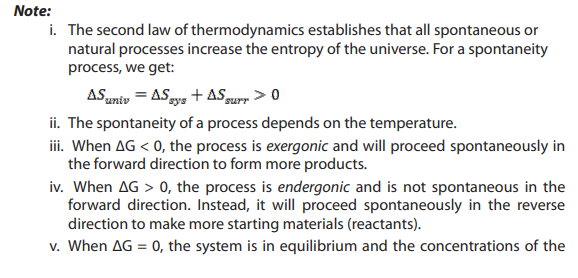
process, the state of entropy of the entire universe, as an isolated system always
increases over time. It also states that the changes in the entropy in the universe cannever be negative.
Following are the statements of second law of thermodynamics:
a. All spontaneous process are irreversible in nature.
b. The net entropy of the universe in any natural process always increases
and tends to acquire the maximum value.
c. In a reversible process, the sum of entropies of the system and surroundings
remains constant but in an irreversible process, the total entropy of the systemand surroundings increases.


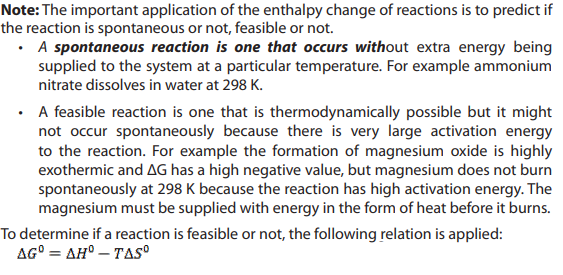
15.3. Free energy, the deciding factor
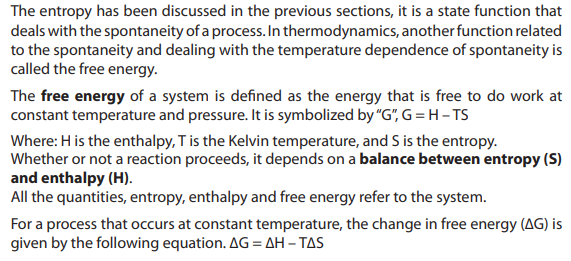
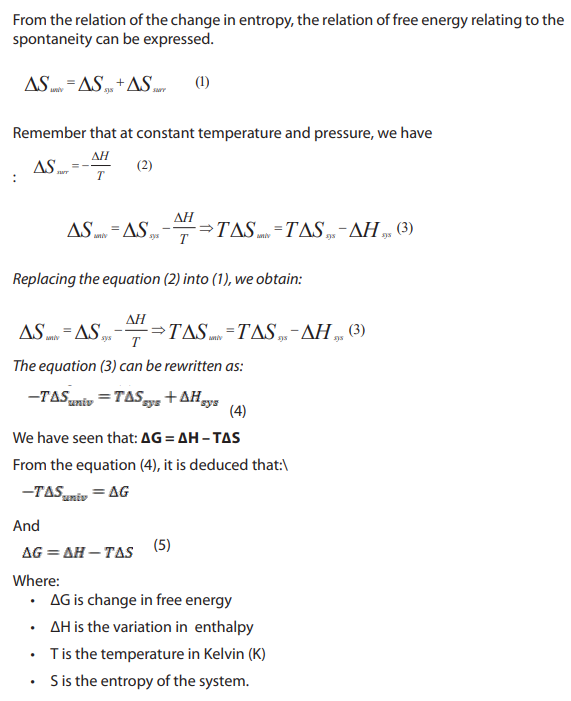

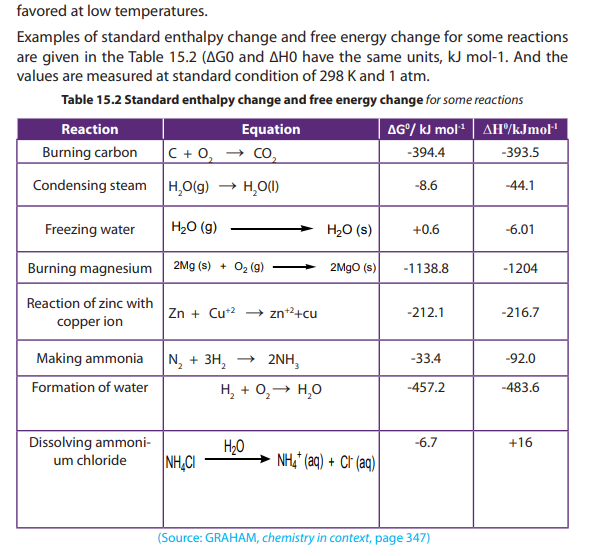
 1. Define exothermic and endothermic reaction
1. Define exothermic and endothermic reaction15.4. Feasibility of chemical reactions: relationship between free
energy, enthalpy and entropy feasibility
Activity 15.4.
2. What is the relation between enthalpy change and entropy or the
reaction?
3. Explain how a reaction is favored by the entropy.4. How the entropy and enthalpy make the reaction to be possible

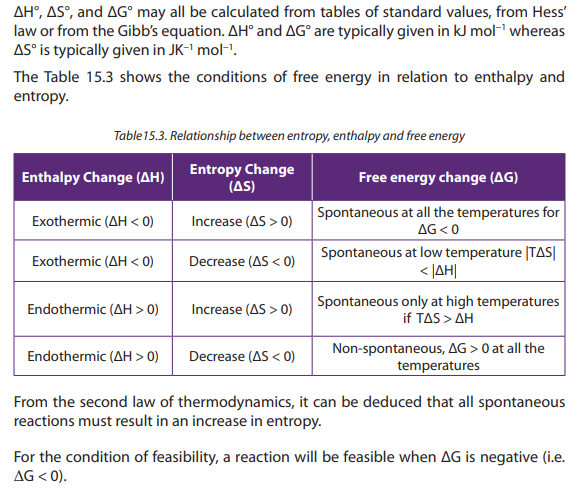
Answers: here we refer to the relation between the entropy free energy and
enthalpy
a. negative because both ∆H and (-T∆S) are negative
b. could be negative or positive because ∆H is negative and (-T∆S) is
negative
c. could be negative or positive because ∆H is negative and (-T∆S) is
positive
d. positive because both (-T∆S) and ∆H are positive
e. in (a) no, ∆H and(-T∆S) both are negative at all temperatures
f. In (b) yes at high T (-T∆S) has high negative value and may have a positive
∆H
In (c) yes, negative ∆H could be positive (-T∆S) at high temperature
In (d) no, ∆H and (-T∆S) are both positive at all temperatures


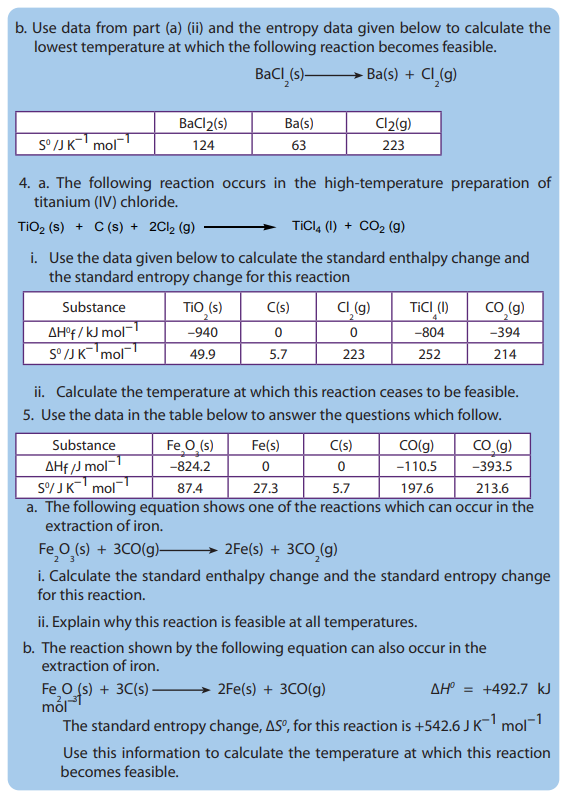
REFERENCES
Chang, R. (2005). Chemistry, Eighth Edition. New York: McGraw-Hill.
Dakin, H., & West, R. (1928). A general reaction of amino acids. II. Journal of
Biological Chemistry, 745-756. Retrieved Mars 25, 2018, from http://www.jbc.org/
content/78/3/745.citation
Schmitz, A. (2018, March 25). Introduction to Chemistry: General, Organic,
and Biological (v. 1.0). Retrieved from 18.1 Properties of Amino Acids:
https://2012books.lardbucket.org/books/introduction-to-chemistry-general
organic-and-biological/s21-01-properties-of-amino-acids.html
Kazemi, F., Kiasat, A.R. and Mombaini, B. (2007). Simple preparation of
symmetrical carboxylic acid anhydrides by means of Na2CO3/SOCl2. An
International Journal for Rapid Communication of Synthetic Organic Chemistry,
37(18): 3219-3223
E.N.Ramsden. (2000). A-level chemistry. United Kingdom: Nelson Thornes Ltd.
Holman, G. H. (1995). Chemistry in context. UK: Thomas Nelson and sons Ltd.
Petr Cann, P. H. (2002). Chemistry for advanced level. London: John Murray Ltd.
Daniel R.Bloch (2012), Organic chemistry DeMYSTiFieD, second edition,
McGrawHill.
Graham Hill, J. H. (2000). Chemistry in context,fifth edition. Nerson Thornes.
Obot, K. (2003). Organic chemistry for Advanced level.
Rwanda Education Board. (2015). Advanced level chemistry syllabus. Kigali:
PRINTEX.
Bruice, P. Y. (n.d.). Organic Chemistry fourth Edition.
COLLIN, A. (2008). Advanced organic chemistry: exercises,eighth edition.
D.Mukama, S. a. (2013). Chemistry for Rwanda Secondary Schools,Advanced
Level Senior5. Fountain.
Silver Obonyo, D. M. (2013). Chemistry for Secondary Schools. Fountain .
http://www.zygia.com/study/book ;
www.markedbyteachers.com
www.citycollegiate.com
