UNIT 14: ENTHALPY CHANGE OF REACTIONS
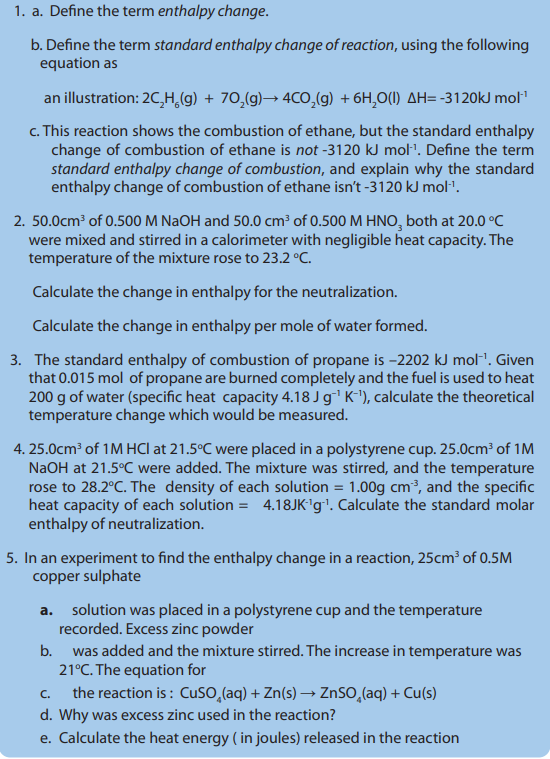
Key unit competency:
To be able to design an experimental procedure to verify the enthalpy
changes in a chemical reactionLearning objectives
• Define heat of reaction, standard enthalpy change of combustion, enthalpy
of neutralisation, enthalpy of solution, enthalpy of hydration and lattice
enthalpy
• Describe an experimental procedure in determination of heat of combustion
• Explain the relationship between quantity of heat produced and mass of
substance in combustion reaction
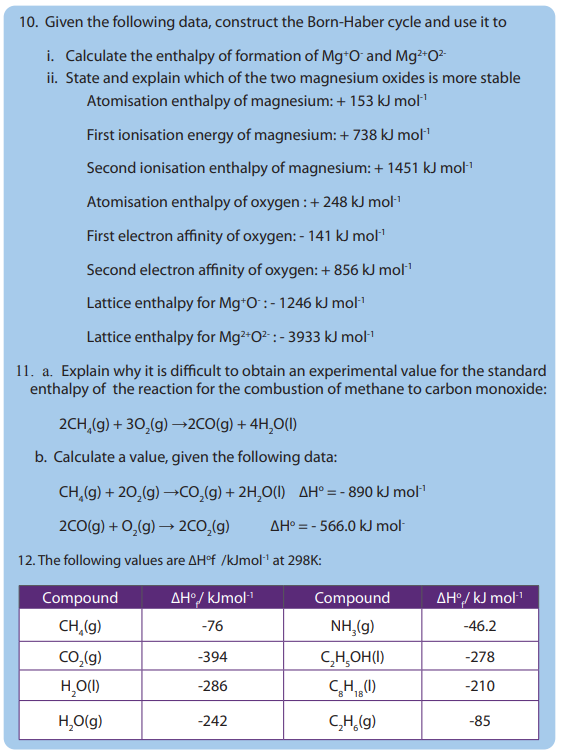
• State Hess’s law of constant heat summation
• State and explain the factors that affect the magnitude of lattice energy
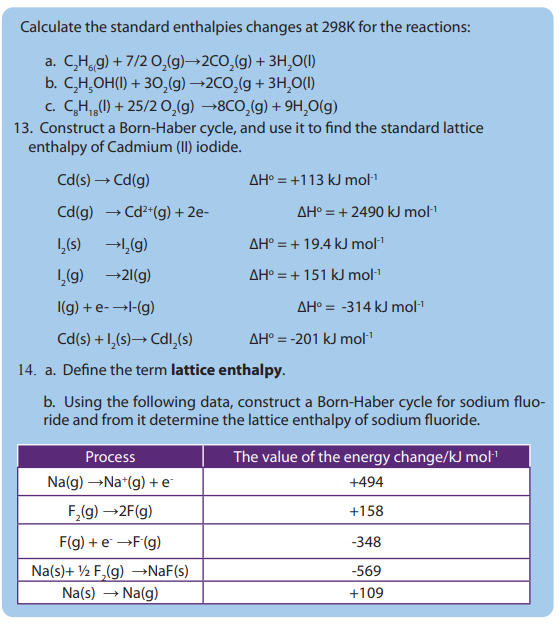
• Describe bond breaking as endothermic and bond making as exothermic
• Develop practical experimental skills about enthalpy changes of reactions,
interpreting results and drawing valid conclusions.
• Carry out practical activities to determine enthalpy change of reactions
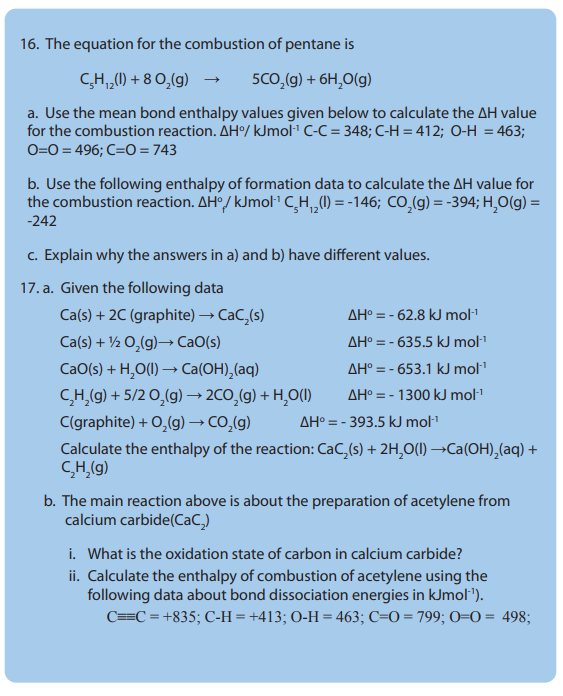
(enthalpy change of combustion of ethanol, enthalpy change of
neutralization).
• Calculate the enthalpy change of combustion, neutralization and dissolution
from experimental data
• Deduce how Hess’s law is applied to Born-Haber cycle.
• Construct Hess’s energy cycles and Born-Haber cycles from data obtained
experimentally or provided.
• Calculate the enthalpy changes of reactions using Hess’s law.
• Use the standard bond energy to determine the standard enthalpy of
reaction.
• Relate the heat of hydration and lattice energy to heat of solution.
• Respect of procedure during experiments of combustion and neutralization.
• Appreciate the contributions of other scientists such as Hess, Born andHaber’s work.
Introductory activity
Observe the pictures below, analyze them and answer the questions thatfollow.

Functioning of a vehicle’s engine Spacecraft launching Bunsen burner
1. What is the origin of the energy used for the flight of airplanes, the
functioning of vehicle’s engines or some machines used in factories,
launching of spacecrafts, energy used by our bodies, the energy
released by a burning wood, Bunsen burner or a burning match or an
exploding dynamite, etc?
2. What are the chemical reactions that are involved in the processes
above?3. How the energy used may be determined?
14.1. Definition of standard enthalpy of different reactions
Activity 14.1
Referring to the concept of energy changes and energy profile diagrams for
chemical reactions:
1. Recall the definition of:
a. enthalpy change
b. thermochemical equation
2. State the rules governing thermochemical equations.
3.By conducting your own research, differentiate the types of enthalpychange of reactions.
In thermodynamics, it is shown how energy, work, and heat are related. Every
chemical reaction occurs with a concurrent change in energy. Before to embark
the explanation of these chemical changes, some key terms have to be defined asfollows.
(i) Enthalpy change (ΔH)
In thermodynamics, the heat of reaction also known as enthalpy of reaction is
the change in the enthalpy (H) of a chemical reaction that occurs at a constant
pressure. Enthalpy, H is a state function used to describe the heat changes that
occur in a reaction under constant pressure. It is a state function as it is derived from
pressure, volume, and internal energy, all of which are state functions. The enthalpy
is a measurement of the amount of energy per mole either released or absorbed in
a reaction.
When a reaction is taking place in an open container, a quantity of heat which is
proportional to the quantity of the matter present, will be released or absorbed.
The flow of heat is the enthalpy change noted ΔH. The units of ΔH are kJ/mol or
kcal/mol.
(ii) Thermochemical equation
A thermochemical equation is a balanced equation that includes the amount ofheat exchanged (produced or absorbed).
Examples

The rules of enthalpy change of reaction:
a) The enthalpy change of a reaction is proportional to the amount of reactantsthat are involved in the reaction.
Examples:
b) In a chemical reaction, reversing a reaction automatically changes the sign of
∆H.
Example:

If the reaction is reversed, we have:
c) The enthalpy change of a reaction depends on the physical states of the
reactants and the products.
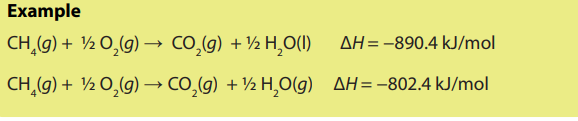
(iii) Types of enthalpy changes
There are various types of enthalpy change. Some examples of the types ofenthalpy changes are given below.
a) The enthalpy of formation of a substance is the heat change (heat released
of a substance is the heat change (heat released
or absorbed) for the chemical reaction in which one mole of the substance is formed
from its constituent elements under given conditions of temperature T and pressure
P.
The standard enthalpy of formation of a substance is the change in enthalpy
of a substance is the change in enthalpy
for the reaction that forms one mole of the substance from its elements in their most
stable form with all reactants and products at the pressure of 1 atm and usually atthe temperature of 298 K.
The standard enthalpy change of a reaction is the enthalpy change that occurs in
a system when matter is transformed by a given chemical reaction when all reactants
and products are taken in their standard states (at 1 atm and 298 K).
Consider a general reaction:
The standard enthalpy change for any reaction can be calculated from the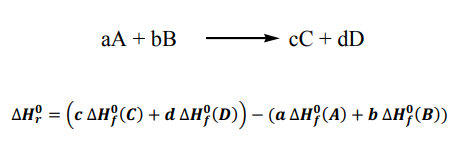
standard enthalpies of formation of the reactants and the products in the reaction:


Answer:

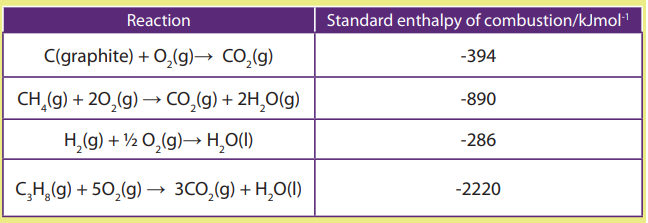
b) Enthalpy of combustion
The enthalpy of the combustion of a substance (element or compound) ΔHoc, is the
enthalpy change which occurs when one mole of a substance undergoes completecombustion with oxygen in excess at 298 K and 1 atm.
Examples:
c) Enthalpy of neutralization
The standard enthalpy of neutralization, is the enthalpy change which occurs
is the enthalpy change which occurs
when one gram equivalent of an acid is neutralized by one gram equivalent of a
base to produce a salt and water under the standard conditions of temperature andpressure.
The equation of the neutralization reaction is:
Example:
Enthalpy change for the neutralisation of sodium hydroxide by hydrochloricacid is
The enthalpy of neutralization is the heat evolved for the reaction between the
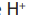
ions given by the acid with the OH→ ions given by the base to form one mole of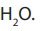
d) Enthalpy of displacement
The displacement enthalpy is the enthalpy change of a reaction in which an
element displaces another in a chemical reaction.
For example, zinc is more reactive than copper, so when zinc is added to copper (II)sulphate solution, copper is displaced.
The reaction is associated with an enthalpy change converted into the heat energy
equals to

e) Enthalpy of solution
The enthalpy of solution of a compound is the heat energy change at constant
pressure when one mole of a compound is completely dissolved in a specific
amount of water as solvent.
However, if a large volume of the solvent is used till further addition of the solvent
does not produce any more heat change it is called enthalpy of solution at infinitedilution. The symbol (aq) is used to represent the solvent at large dilution.
Examples:
The enthalpy of solution of sodium chloride solid:

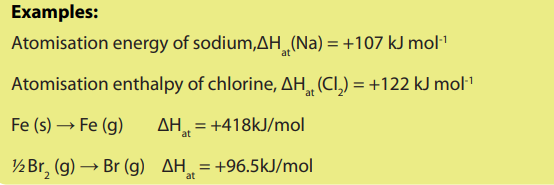
f) Enthalpy of atomization
The atomization enthalpy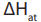 is the enthalpy change when one mole of gaseous
is the enthalpy change when one mole of gaseous
conditions. The enthalpy change of atomization is always positive.
For a diatomic molecule, the atomization enthalpy is equal to a half of the bonddissociation energy.
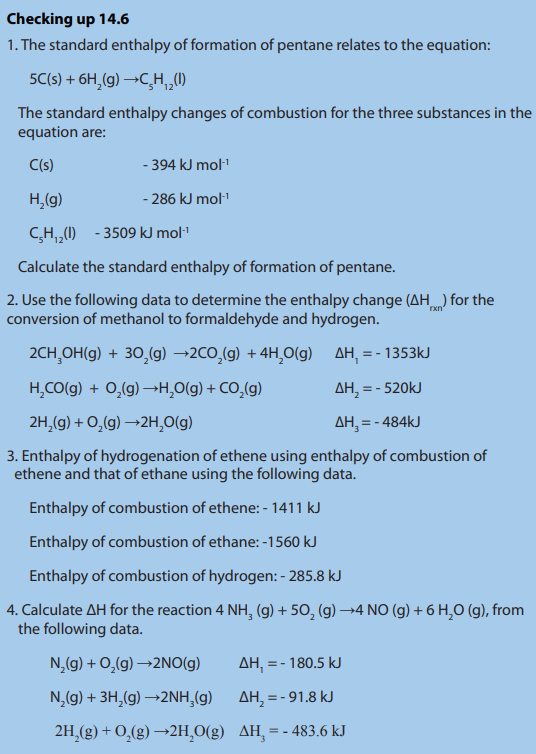
g) Lattice enthalpy
Lattice enthalpy is the enthalpychange that occurs when one mole of an ionic crystalisformed from its gaseous constituents.
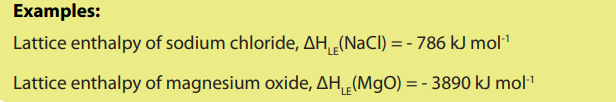
14.2. Relationship between temperature and heat
h) Hydration enthalpy
Hydration enthalpy is the enthalpy change when one mole of gaseous ions dissolvesin sufficient water to give an infinitely dilute solution.

i) Bond dissociation enthalpy
Bond dissociation enthalpy is the needed energy to break a single bond such as N-Hin an ammonia molecule.

Checking up 14.1
1. Write an equation representing the formation of each of the following
compounds from its constituent elements.
a. hexane
b. nitric acid
c. methanol
d. potassium bromide
e. butanoic acid
2. Which of the following reactions do not represent the standard enthalpyof formation?
3. Write the equations that represent the standard enthalpy of combustion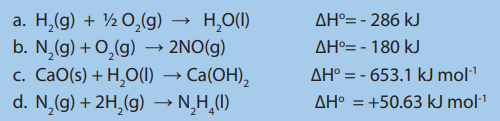
of:
a. hydrogen
b. methane
c. sulphur
d. propanol
4.Write equations for which the enthalpy of atomization is measured.
a. potassium
b. nitrogen
c. iodineActivity 14.2
To investigate the relationship between heat and temperature
Requirements
• Weighing balance
• Thermometer
• Insulated plastic beaker(calorimeter)
• Measuring cylinder
• Sodium hydroxide pellets• Distilled water
Procedure
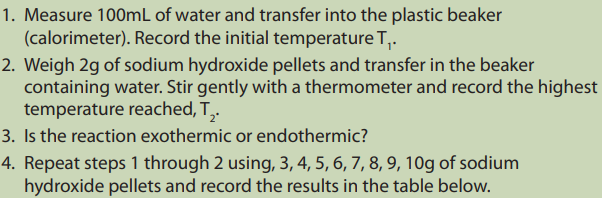

Interpretation
Heat is the exchange of thermal energy between the system and the surroundings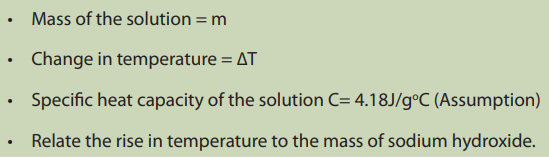
that results in the temperature difference. Heat flows from matter with high
temperature to matter with low temperature until both objects reach the same
temperature. The quantity of heat is symbolized by “q”.
When a system absorbs heat its temperature increases. The increase in temperatureis directly proportional to the amount of heat absorbed.
The heat capacity, C of a substance is the amount of heat required to raise the
temperature of a substance by 1°C. The heat capacity is expressed in J/°C or J/K.
The molar heat capacity is the amount of heat energy required to raise the
temperature of one mole of a substance by 1°C. It is expressed in Joules per mole
per degrees Celsius (or Kelvin), (J/mol.°C or J/mol. K).
For example, the molar heat capacity of lead is 26.65J/mol.°C, which means that it
takes 26.65 Joules of heat to raise 1 mole of lead by 1°C.
The specific heat capacity is the amount of heat needed to increase the temperature
of one gram of a substance by one degree. It is expressed in Joules per gram per
degree Celsius (J/g.°C).Water has a very high specific heat, 4.18J/g.°C.
The high specific heat of water allows it to absorb a lot of heat energy without large
increase in temperature keeping ocean coasts and beaches cool during hot seasons.
It allows it to be used as an effective coolant to absorb heat.The relationship between heat and temperature is given by:
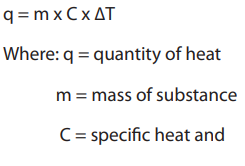
ΔT = change in temperature
Note: Heat capacity, C, can never be negative for a mass or a substance, and similarly
the specific heat of a substance can never be negative. Therefore, if the change in
temperature is negative, the initial temperature is higher than the final temperature.
The heat capacity of an object depends on its mass: 200 g of water requires twice as
much heat to raise its temperature by 1°C than 100 g of water. It also depends on the
type of material: 1000 J of heat energy will raise the temperature of 100 g of sand by12°C, but only raise the temperature of 100 g of water by 2.4°C.
14.3. Experimental methods for finding the standard enthalpy
of combustion reactions
Activity 14.3
To investigate the enthalpy of combustion of ethanol
Requirements
• spirit burner (containing ethanol)
• thermometer
• copper can
• measuring cylinder
• retort stand and accessories
• balance
• breeze shield
Safety precautions
• Ethanol is highly flammable and the main risk is from burns.
• Since a small amount is burned the build-up of any products of incomplete
combustion is negligible.
• Wear eye protection.
• Ensure the spirit burner is always sitting in a stable position.
• If you have to re-fill the spirit burner, allow it to cool and then fill it awayfrom sources of ignition.
Procedure
1. Weigh the spirit burner (already containing ethanol) with its cap on
and record its mass. (The cap should be kept on to cut down the loss of
ethanol through evaporation)
2. Using the measuring cylinder, measure out 100 cm3
of water into the copper can.
3. Set up the apparatus as directed by your teacher.
4. Measure and record the temperature of the water.
5. Remove the cap from the spirit burner and immediately light the
burner.
6. Slowly and continuously stir the water with the thermometer.
7. When the temperature has risen by about 10 °C, recap the spirit burner,
measure and record the maximum temperature of the water.8. Reweigh the spirit burner and record its mass.
Calculations
a. The heat energy gained by the water (q) can be calculated using the
formula: q = c m ∆T
b. The difference in the initial and final masses of the spirit burner gives us the
mass of ethanol burned (say x g) and so the heat energy we calculat is equal
to that released by burning x g of ethanol. It is assumed that all the heat
energy released by the burning ethanol is absorbed only by the water.
c. We can work out the mass of one mole of ethanol and knowing how much
heat energy is released when x g of ethanol is burned we can calculate the
heat energy released when one mole of ethanol is burned. This will be equalto the enthalpy of combustion of ethanol.
A calorimeter is a device used to measure the amount of heat energy exchanged
(released or absorbed) in a reaction. If a calorimetry experiment is carried out under
a constant pressure, the heat transferred provides a direct measure of the enthalpy
change of the reaction.
Simple determination of enthalpy of combustion can usea simple calorimeter described by the Figure 14.1below.
The energy produced by the combustion of the fuel is used to heat a known mass
(m) of water. Therefore, the heat provided by the fuel is equal to the heat receivedby water.
Its amount may be calculated using the relation:
Where: m is the mass of water
Cs is the specific heat capacity of water
∆T is the temperature changeKnowing the mass of the fuel used, its enthalpy of combustion may be calculated.
Example:

Answer:
A more accurate method of determining the enthalpy of combustion is the use of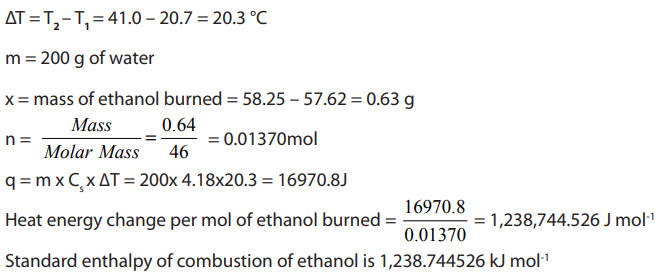
a bomb calorimeter (Figure 14.2). It is based on the same principle as the simple
experiment involving fuel burners, but it is more accurate because the heat loss isreduced to zero.
Figure 14.2 Schematic diagram of a bomb calorimeter
Source : https://www.chem.fsu.edu/chemlab/chm1045/energy.html
In a bomb calorimeter, a sample of a compound is electrically ignited and the heat
energy obtained by combustion heats the water in the calorimeter.
Checking up 14.3
14.4. Experimental methods for finding the standard enthalpy
of neutralisation reactions
Activity 14.4
To investigate the enthalpy of neutralization of hydrochloric acid by
sodium hydroxide.
Requirements:
Each group will be provided with:
2 plastic beakers
50 mL of 1 M HCl
50 mL of 1 M NaOH
Thermometer
ScaleWeigh boats
Procedure:

Discussion questions
Calorimetry is also used to determine the enthalpy change of a reaction taking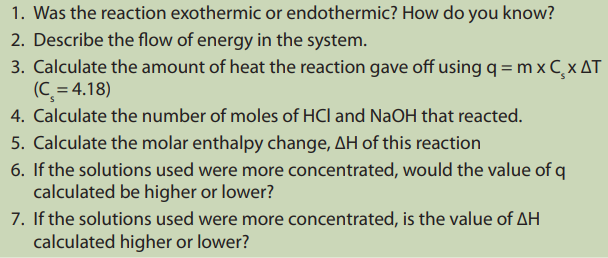
place in solution. For an exothermic reaction, the heat energy released increases the
temperature of the water in solution while for an endothermic reaction, the heat
energy absorbed is derived from water in the solution and the temperature of the
solution falls.
When hydrochloric acid reacts with sodium hydroxide, the temperature of the mixturerises and the heat is transferred to the plastic beaker, the process is exothermic.
Example:Consider the results from an experiment similar to the one described above:

Enthalpy change = mass of the solution x specific heat capacity x temperature rise

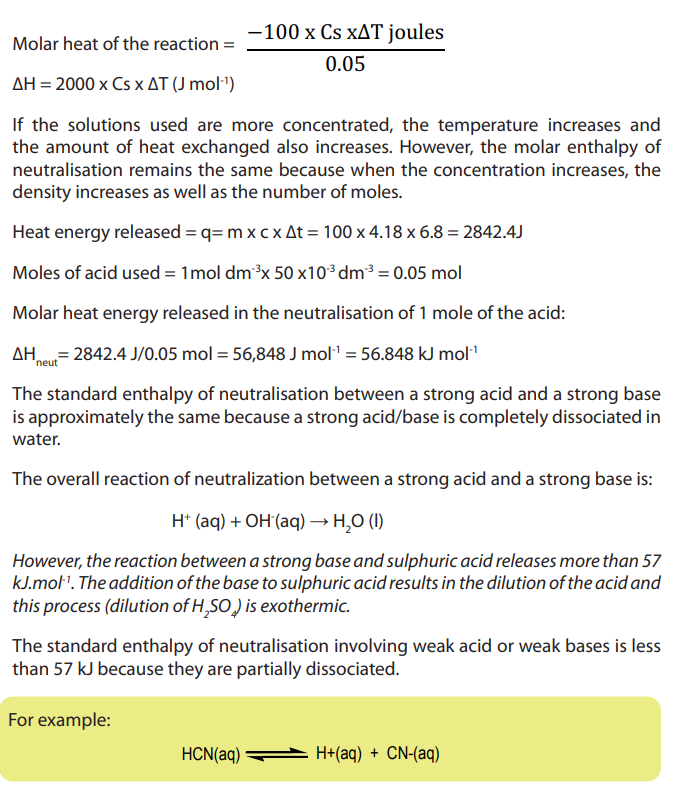

Checking up 14.4

14.5. Hess’s law or Law of constant heat summation
Activity 14.5
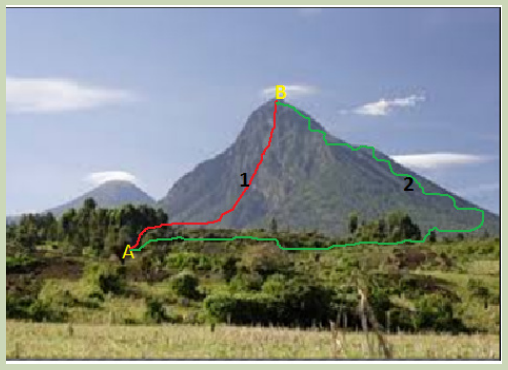
Observe the above image and answer the following questions.
1. In which case a person climbing by foot, Karisimbi volcano from point A to
point B:
a) Travels a greater distance?
b) Has more difficulties to reach point B?
2. In which case a person who climbs Karisimbi volcano from A to B, the gain in
gravitational energy is the highest?
3. Can you give a different example to illustrate the same phenomenon?
4. What conclusion to draw from the two examples?
5. Is any relation between the conclusion and the heat exchanged in a chemical
reaction? Illustrate your opinion with examples and come up with a general
conclusion.6. Compare your ideas to the first law of thermodynamics.
The increase in gravitational potential energy that occurs when a person climbs
from the base to the top of a volcano like Karisimbi or someone who is elevated
from the first to the fourth floor of a building is independent of the pathway taken.
That gain in gravitational potential is a state function that is analogous to a
thermodynamic state function.
Remember that the enthalpy (heat of reaction) is a state function. This means that a
change in enthalpy does not depend on how the change was made, but only on the
initial state and final state of the system; it is independent of the pathway.
In 1840, the Russian chemist Germain Henri Hess, a professor at the University of St.
Petersburg, discovered from his thermochemical studies that the enthalpy change is
a state function. The result from his experiment was known as Hess’s law or Law of
Constant Heat Summation. This law state that “the heat evolved or absorbed in a
chemical process is the same whether the process takes place in one or in several steps”
In other words, no matter how you go from a given set of reactants to a set of
products, the enthalpy change for the overall chemical reaction is the same whether
the reaction takes place in one step or in a series of steps.
The enthalpy change is independent of the pathway of the process and the number of
intermediate steps in the process.Hess’s law can be illustrated by the following reaction:
The reaction can be decomposed into two steps. The two steps and the overall
process are represented by the following thermochemical equations.
The two processes can be represented in a thermochemical cycle. This diagram is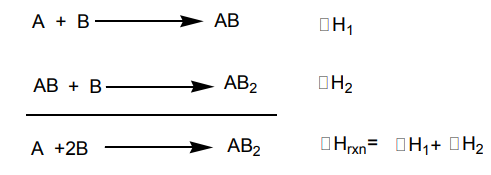
known as the Hess’s principle (Figure 14.3).
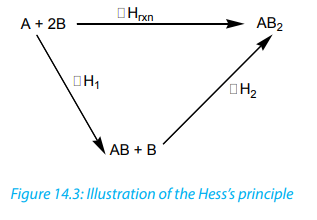
Examples:
1. Determination of the enthalpy change for the complete combustion of
carbon.
The direct reaction of carbon with oxygen yields carbon dioxide. However,
in the first step carbon gives carbon monoxide which is then oxidised
to carbon dioxide during the second step. The two-step reactions
corresponding to Hess’s law diagram for the formation of carbon dioxideare shown as follows.
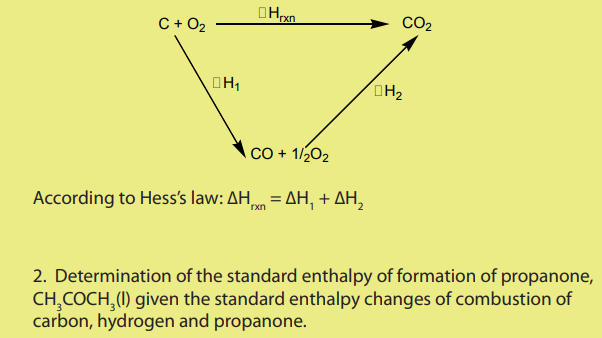
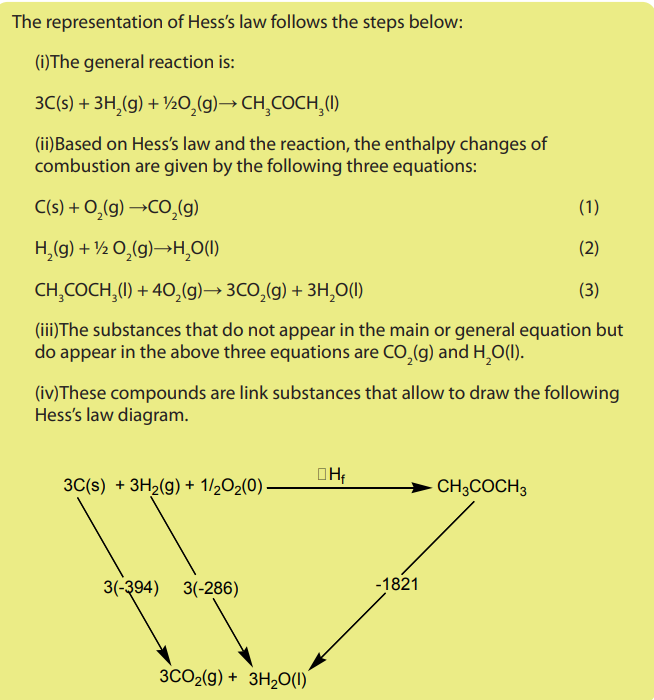
Note: The enthalpy change can be calculated by adding equations corresponding
to the different combustion to obtain the main equation. Then, the enthalpy change
is obtained by addition of the corresponding enthalpy change values multiplied byappropriate coefficient and if necessary, some of them are reversed.
Main equation:
Equation corresponding to the different combustions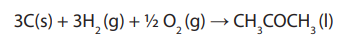
3 moles of C(s) react in the main equation so the first equation is multiplied by 3,
1 mole of propanone is formed in the main equation so the third equation should
be reversed.
Checking up 14.5
14.6. Application of Hess’s law
Activity 14.6
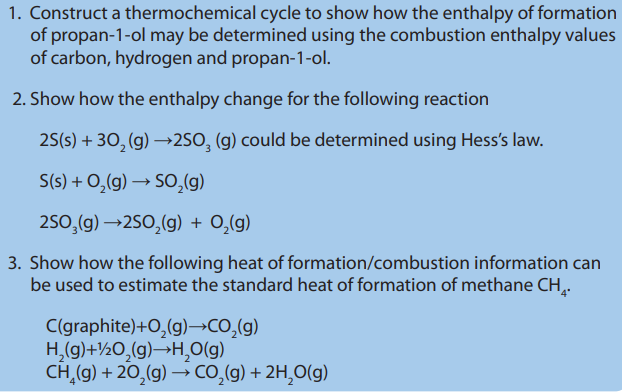
1. State the Hess’s law?
2. Describe how enthalpy of formation of propan-1-ol, hexane, sulphuricacid, can be determined.
The Hess’s law can be applied to calculate enthalpies of reactions that are difficultor impossible to measure.
Hess’s law states that if a reaction is carried out in a series of steps, ∆H of the
reaction will be equal to the sum of enthalpy changes for the steps provided
that the initial and the final conditions are the same. (Or the total enthalpy changeis independent of the route).
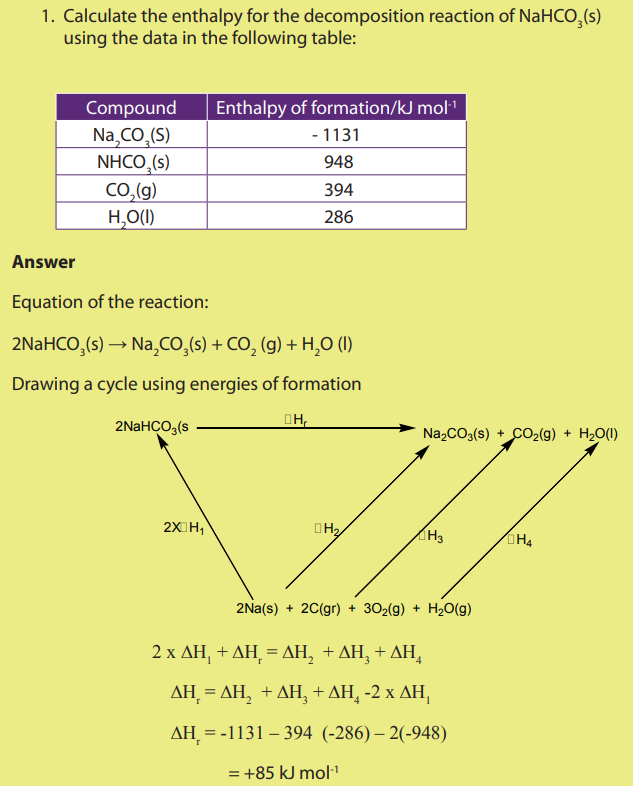
Examples:


14.7. Born-Haber cycle
Activity 14.8
1. Refer to the trends in physical properties of chemical elements and
chemical bonding, explain each of the following terms.
a. Ionization enthalpy
b. Electron affinity
c. Atomization enthalpy
d. Dissociation enthalpy
e. Sublimation energy
f. Enthalpy of formation
2. State the Hess’s law.
3. Can the Hess’s law apply or not to the formation of an ionic compound?Justify your answer using an example.
In the previous sections, the Hess’s law and Lattice energy were discussed. Recall
that the Hess’s law of Constant Heat Summation states that the enthalpy change is
independent of the pathway of the process and the number of intermediate steps in theprocess.
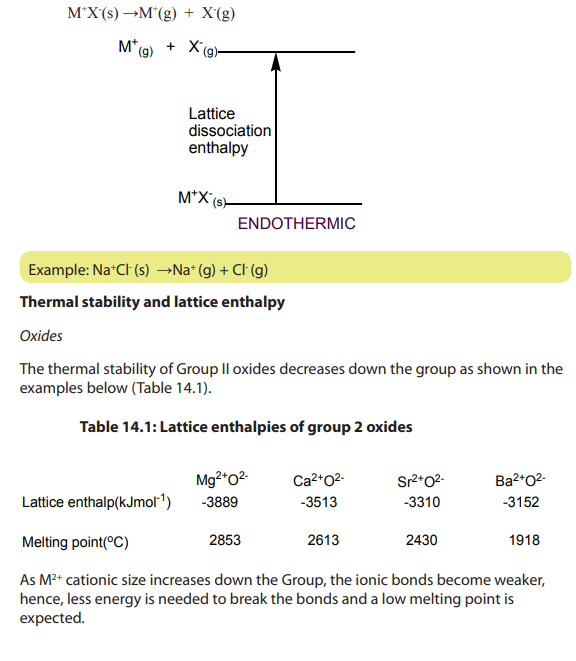
Lattice formation enthalpy is the enthalpy change when one mole of an ionic
compound is formed from its gaseous ions at the standard temperature and pressure.
Because all the bonds in the ionic lattice are broken, it is an endothermic process.
To determine directly the lattice energy of an ionic solid experimentally is not
easier.
However, an indirect process known as Born–Haber cycle can be used based onHess’s law.
A Born-Haber cycle is a thermodynamic cycle which relates the lattice energy of
an ionic compound to its enthalpy of formation and other measurable quantities.
Lattice enthalpies of ionic compounds give a good indication as to the strength of
the ionic bonding in the lattice. Born-Haber cycle provides a useful way to account
for the relative stabilities of the chemical compounds and the relative stability of
the compound is determined by the lattice enthalpy of a compound. The lattice
enthalpy of an ionic compound is determined by breaking up the formation of an
ionic compound into a series of steps and then, all the steps will be added for the
overall reaction. In general, Born-Haber cycles are enthalpy cycles that show howionic compounds are formed from their elements.

14.8. Lattice enthalpy
Activity 14.7
Referring to what you have learned so far in chemistry, attempt the following questions.
1. What is meant by ionic bond?
2. Explain the main steps in the formation of an ionic bond showing clearlythe energy change involved in each step.
A lot of energy is released as the ionic bond is formed. The reaction is highly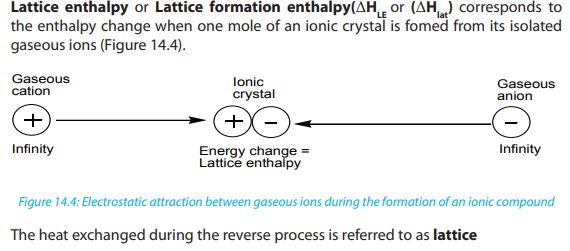
exothermic because there are strong electrostatic attractions between ions of
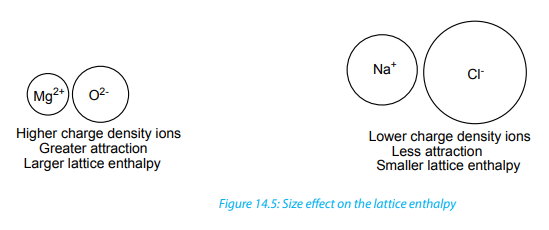
opposite charges. The relative values of lattice enthalpy are governed by the chargedenssity of the ions.
The value of lattice formation enthalpy cannot be measured directly; it is calculated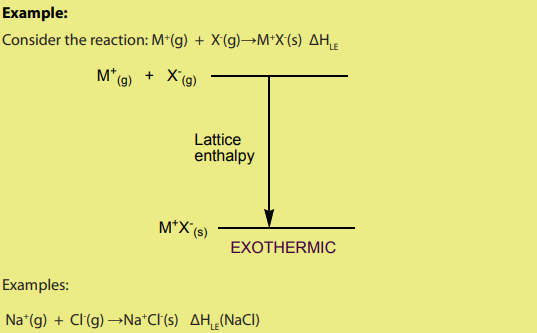
using the Born-Haber cycle. The greater the charge densities of the ions, the morethey attract each other and the larger the lattice enthalpy.
The more exothermic the lattice enthalpy, the higher is the melting point.
dissociation enthalpy.
Lattice dissociation enthalpy is the enthalpy change when one mole of an ionic
lattice dissociates into isolated gaseous ions. Since there is a strong electrostatic
attraction between ions of opposite charge, a lot of energy must be supplied toovercome the attraction.

Examples of enthalpy changes that are commonly used in Born-Haber cycles are
the following:
i. Enthalpy change of formation
ii. Atomisation enthalpies
iii. Ionisation enthalpyiv. Electron affinity
The following diagram represents a general illustration of the Born-Haber cycle.
The application of Hess’s law to this cycle made possible the calculation of the
lattice enthalpy.
The arrows pointing upwards represent the endothermic changes while those
pointing downwards show the exothermic changes.
From the Born-Haber Cycle, the enthalpy change associated with the route depicted
by the red arrow is equal to the enthalpy change of the route shown by the bluearrow.



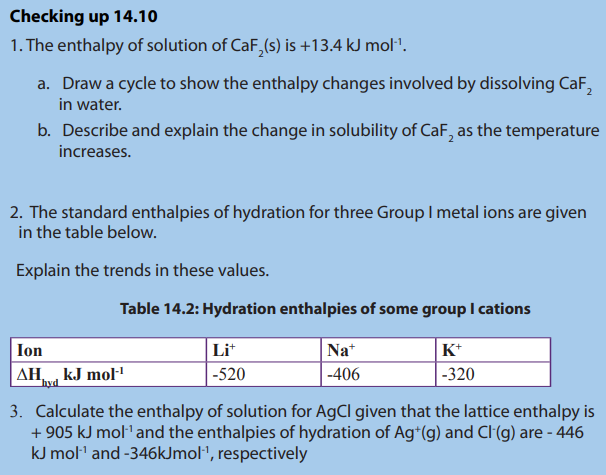
Checking up 14.8
1. Construct and interpret a Born-Haber cycle for calcium fluoride showing
clearly the enthalpy changes involved.
2. a. Write an equation for the standard enthalpy of formation of aluminium
oxide.
b. Construct and interpret a Born-Haber cycle for aluminium oxide and
explain how it would be used to calculate the lattice enthalpy of aluminiumoxide.
14.9. Born-Haber cycle: Calculations of the lattice enthalpy
Activity 14.9
1. Construct a Born-Haber cycle for the formation of an ionic compound MX.
2. Explain how the cycle can be used to calculate the lattice enthalpy ofthe compound mentioned in 1)
Examples:
1. Determining the lattice enthalpy of lithium fluoride using the Born-Haber
cycle
Using the Born-Haber cycle constructed previously for lithium fluoride, itslattice enthalpy can be calculated as follow

2. Determination of the first electron affinity of oxygen
A Born-Haber cycle for the formation of calcium oxide is shown below.
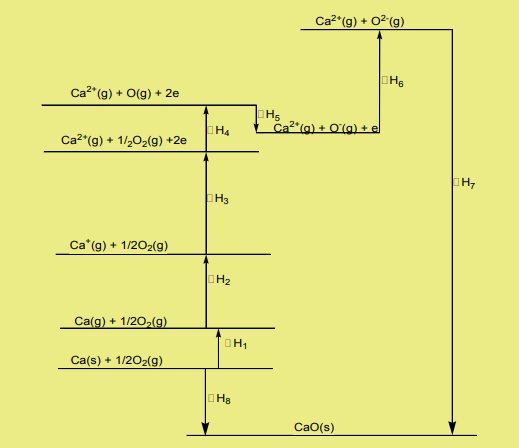
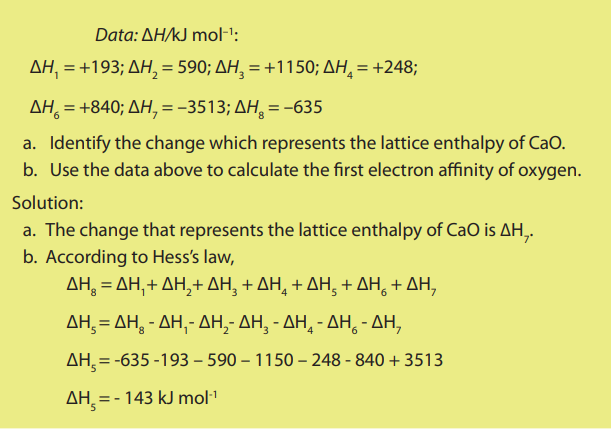

14.10. Hydration energy
Activity 14.10
Experiment on solubility of ionic compounds
Requirements:
Test tubes
Spatula
Distilled water
Sodium chloride
Sodium hydroxide
Potassium bromide
Silver chloride
Sodium carbonate
Potassium hydroxide
Magnesium hydroxide
Magnesium sulphate
Barium hydroxide
Barium sulphate
Procedure
1. Put 10mL of distilled water in a test tube
2. Add a half spatula end full of sodium chloride and shake
3. Record all your observations
4. Repeat steps 1-3 using the different salts5. Record your findings in the table below.
6. Compare the lattice enthalpy of compounds of soluble compounds to
those of sparingly soluble or insoluble compounds and deduce the general
trends.7. What term is given to the interactions between solute and solvent?
Some ionic compounds such as sodium hydroxide and sodium chloride are very
soluble while others like magnesium carbonate, are sparingly soluble or insoluble
(calcium carbonate, magnesium hydroxide, barium sulphate). The dissolution of
some compounds such as sodium hydroxide release the heat energy and the processis exothermic.
If a pair of oppositely charged gaseous ions are placed together, they are attracted
to each other. The energy change (lattice enthalpy) is highly exothermic. If the ions
were placed in water, they would be attracted to the polar water molecules leading
to the energy change (hydration enthalpy) which is highly exothermic. In both cases,the greater the charge density of the ions, the more exothermic will be the process.
The enthalpy change when one mole of a gaseous ion dissolves in water (excess) to
give an infinitely dilute solution is called enthalpy change of hydration
The solvent-solute interactions are referred as “solvation”.
When an ionic compound dissolves in water, the process can either be exothermic
or endothermic.
The enthalpy of solution of a compound is the heat energy change at constant pressure
when one mole of a compound dissolves completely in water.
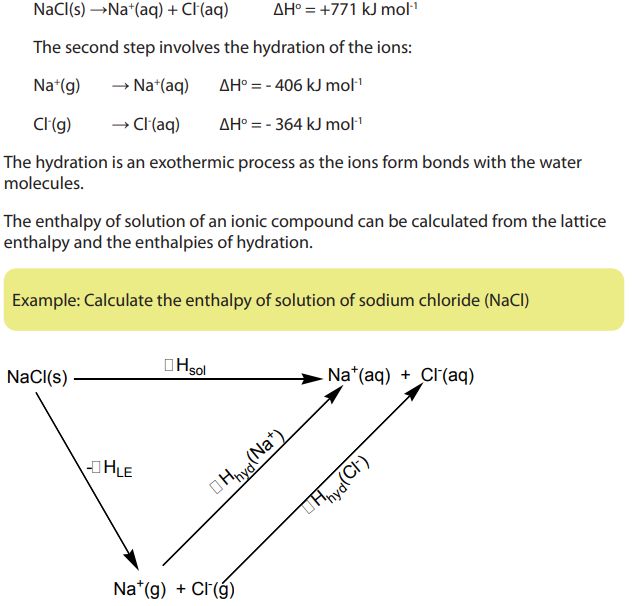
For example when sodium chloride dissolves in water, the overall process isrepresented as:
The first step is to separate the ions in the crystal. This requires energy to overcome
the attractive forces between oppositely charged ions. The corresponding latticedissociation energy according to the dissociation of the compound is:

14.11. Average standard bond enthalpy
Activity 14.11
Using internet or textbooks do research and answer the following questions.
1. Explain the term covalent bond and describe the formation of a covalent
bond.
2. What are the factors that affect the strength of a covalent bond?3. Relate the bond strength to the reactivity of a covalent compound
1. Formation of a covalent bond
The covalent bond is a bond formed when atoms share a pair of electrons to
complete the octet. The covalent bonds mostly occur between non-metals or
between molecules formed by the same (or similar) elements. Two atoms with
similar electronegativity do not exchange an electron from their outermost shell;the atoms instead share electrons and their valence electron shell is filled.
The build-up of electron density between two nuclei occurs when a valence atomic
orbital of one atom overlaps with that of another atom, each orbital containing a
single electron.
The orbitals share a region of space, i.e. they overlap. The overlap of orbitals allows
two electrons of opposite spin to share the common space between the nuclei,forming a covalent bond.
2. Factors influencing the strength of a covalent bond
i. Size of the atoms
Small atoms have shorter bond length and thus have better overlap of
orbitals while
larger atoms tend to have more diffused orbitals, resulting in less effective
overlap
ii. Number of bonds between atoms
Bond strength: triple bond > double bond > single bond
“>”: “stronger than”
Species with more electrons to share form more bonds between atoms,
resulting in atoms held closer together.Polarity of bon
iii) Polar bond is generally stronger than non-polar bond; the extra
electrostatic attraction between partial charges gives rise to a stronger bond.
iv. Presence of neighbouring lone pair electrons
Atoms which are very small in size and have lone pairs in close proximity will
result in excessive repulsion that weakens the covalent bond.
3. Bond enthalpy
Bond enthalpy (energy) is the amount of energy required to break one mole
of gaseous bonds to form gaseous atoms. It is known as the bond dissociation
enthalpy.
Bond energy may also be defined as the amount of energy released when two
atoms are linked together by a covalent bond. It is expressed in kJ mol-1 or Kcalmol-1.
The bond energy is a measure of the strength of a chemical bond. The smaller
the bond enthalpy the weaker the bond and the easier is to break the bond.
The process of breaking a bond is endothermic while the bond-formation is anexothermic process.
Note: For diatomic gas molecules, the bond enthalpy is equal to two times the
enthalpy of atomization.
The exact bond enthalpy of a particular chemical bond depends on the environment
of the bond in the compound. Therefore, the bond enthalpy values given areaveraged values (Table 14.3).
Table 14.3: Examples of some values of average bond enthalpy.
The bond enthalpy values are used to:
• compare the strengths of bonds
• estimate the enthalpy change of a reaction
• explain the mechanisms of reaction• explain the structure and bonding

14.12. Calculating enthalpy change of reaction using average
bond enthalpies
Activity 14.12
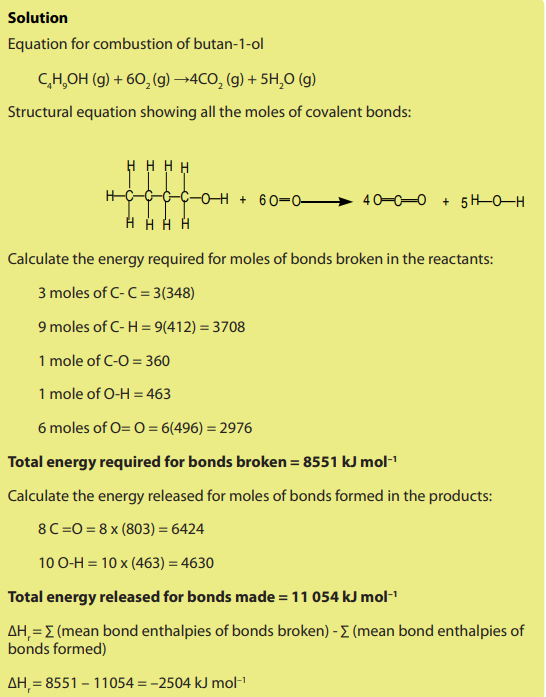
1. Define the term average bond enthalpy.2. Consider the following reactions:

a. Explain how the enthalpy change of each of them may be determined.
b. During chemical reactions, bonds are broken in the reactants and
new bonds are formed in the products. When bonds are broken, there
is a release of heat energy known as the bond dissociation energy.
When bonds are formed energy is released. Do research and show the
relationship between average bond dissociation enthalpy values and the
heat exchanged during a chemical process.
Breaking chemical bonds requires energy (endothermic), and forming chemical
bonds releases energy (exothermic). To determine if a chemical reaction is
endothermic or exothermic depends on whether or not breaking the old chemical
bonds requires more energy than the energy released from the new chemical bondsformed.
When the standard enthalpy change for a reaction cannot be measured, an
approximate value is obtained by using average standard bond enthalpies. During
a chemical reaction, the energy is provided to break bonds of the reactants, and
energy released when the new bonds of the products are formed.
The standard enthalpy of reaction is the difference between the sum of average
bonds enthalpies of the products and the sum of average standard bond enthalpiesof the reactants.




14.13. End unit assessment