UNIT 13 : ELECTROLYSIS
Key unit competence
Predict the products of given electrolytes during
electrolysis and work out quantitatively to determine how much is liberated at agiven electrode using Faraday’s law.
Learning Objectives:
• Define electrolysis, cathode and anode.
• Explain the electrolysis of different substances.
• State Faraday’s laws and define the Faraday’s constant.
• Develop practical experimental skills related to electrolysis, interpret results,
and draw valid conclusions.
• Carry out a practical activity to explain the phenomenon of electrolysis.
• Compare the electrolysis of dilute solutions and concentrated solutions.
• Calculate the masses and volumes of substances liberated during electrolysis.
• Relate the nature of electrode, reactivity of metal ion in solution to the
products of electrolysis.• Perform electroplating of graphite by copper metal
Introductory activity
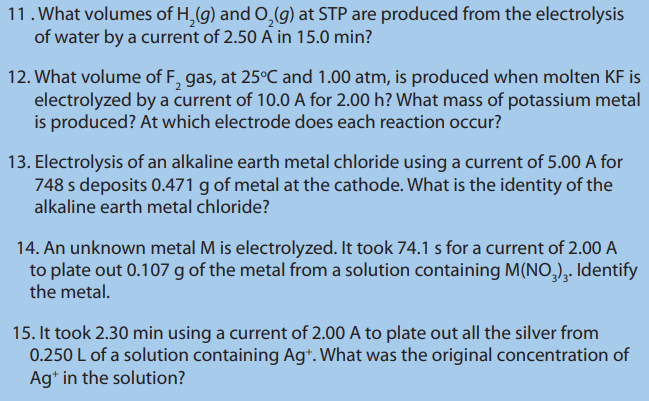
Observe carefully the figure below and answer the following question. Record youranswer and discuss them.
1. Label the set up and give the name of this Experiment.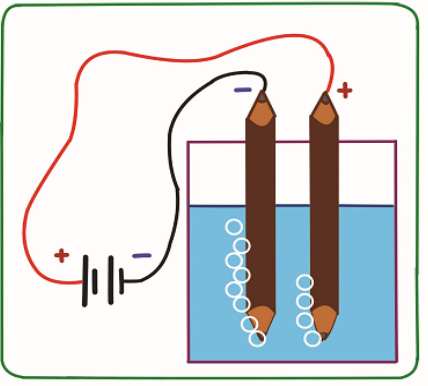
2. Suggest how water can be decomposed into hydrogen and oxygen.
13.1. Definition of electrolysis and Description of electrolyticcells.
Activity 13.1:
A.
1. In one case, you have a source of water at the top of a hill and you want
to supply water to a community in the valley down the hill
2. In another case, you have a community at the top of a hill and you
want to supply water to the community from a source located in the valley
down. Students in groups discuss how they would proceed to supply water
to the communities in the above two cases.
B. Why do we cook food by heating?
C. What is the difference between a spontaneous reaction and a non
spontaneous reaction?D. Have you heard about electrolysis? If yes, can you say what it is about?
1.Definition of electrolysis.
A spontaneous reaction is a reaction that favors the formation of products without
external energy. It is a process that will occur on its own. For example, a ball will
roll down an incline, water will flow downhill, radioisotopes will decay, and iron will
rust. No intervention is required because these processes are thermodynamically
favorable.
A non spontaneous reaction (also called an unfavorable reaction) is a chemical
reaction that necessitates external energy to occur. For example, without an
external energy source, water will remain water forever. Under the right conditions,
using electricity (direct current) will help to produce hydrogen gas and oxygen gas
from water. Cooking foods is not spontaneous reaction that is why heat is used.
Electrolyte: Sodium chloride is an ionic compound in which ions arrange themselves
in a rigid cubic lattice when in solid state. In this state, it cannot allow electric current
to pass through it. However, when it is melted, or dissolved in water, the rigid lattice
is broken, ions are free to move and electric current can pass. Therefore, it is classified
as an electrolyte.
Substances which cannot allow the flow of electric current when in molten or
in solution are referred to as non-electrolytes. When electric current (direct
current) flows through an electrolyte, it decomposes it. This phenomenon is calledelectrolysis.
Thus electrolysis is the decomposition of an electrolyte by passage of an electric
current through it. Therefore for electrolysis to take place, there must be a source
of direct current. The direct current is conveyed from its source to the electrolyte
by means of a metallic conductor and electrodes. The electrode connected to the
positive terminal of the direct current is called the anode and the one connected to
the negative terminal is the cathode. By convention, the electric current enters theelectrolyte by the anode and leaves by the cathode.
When the current passes through an electrolytic solution, ions migrate and electrons
are gained or lost by ions on the electrodes surface. Electrode that is positively
charged has deficit of electrons is called anode and the other electrode negatively
charged has excess of electrons and is called cathode. Chemical changes at theelectrodes due to the passage of electric current are called electrolysis.
2. Description of electrolytic cells.
An electrolytic cell is an electrochemical cell that drives a non-spontaneous redox
reaction through the application of external electrical energy. They are often used to
decompose chemical compounds, in a process called electrolysis . The Greek wordlysis means to break up.
Important examples of electrolysis are the decomposition of water into hydrogen
and oxygen, production of sodium metal, Na, from molten NaCl, production of
aluminium and other chemicals. Electroplating (e.g. of copper, silver, nickel or
chromium) is done using an electrolytic cell.
An electrolytic cell has three component parts: an electrolyte and two electrodes
(a cathode and an anode). The electrolyte is usually a solution of water or other
solvents in which ions are dissolved. Molten salts such as sodium chloride are also
electrolytes. When driven by an external voltage applied to the electrodes, the ions
in the electrolyte are attracted to an electrode with the opposite charge, where
charge-transferring (also called faradaic or redox) reactions can take place. Only
with an external electrical potential (i.e. voltage) of correct polarity and sufficient
magnitude can an electrolytic cell decompose a normally stable, or inert chemical
compound in the solution. The electrical energy provided can produce a chemicalreaction which would not occur spontaneously otherwise.
The main components required to achieve electrolysis are:
• An electrolyte is substance containing free ions which are the carriers of
electric current in the electrolyte. If the ions are not mobile, as in a solid salt
then electrolysis cannot occur.
• A direct current (DC) supply: provides the energy necessary to create or
discharge the ions in the electrolyte. Electric current is carried by electronds
in the external circuit.
• Electrolysis depends on controlling the voltage and current.
• Alternating current (AC) would not be appropriate for electrolysis. Because the
“cathode” and “anode” are constantly switching places, AC produces explosive
mixtures of hydrogen and oxygen.
• Two electrodes: an electrical conductor which provides the physical interfacebetween the electrical circuit providing the energy and the electrolyte.
The key process of electrolysis is the interchange of atoms and ions by the removal
or addition of electrons from the external circuit. The products of electrolysis are in
some different physical states from the electrolyte and can be removed by some
physical processes.
Electrodes of metal, graphite and semi-conductor material are widely used. Choice
of suitable electrode depends on chemical reactivity between the electrode and
electrolyte and the cost of manufacture.
Note:
The suitable electrode in electrolysis should be inert (Cu, Pt, etc.) therefore it will not
participate in the chemical reaction.
It is very easy to be confused about the names CATHODE and ANODE and what theirproperties are, both with electrochemical cells and electrolytic cells.
(To help you to remember, Cathode is the site of reduction, or, if you prefer, CCC = CathodeCollects Cations. Anode is the site of oxidation, or, AAA = Anode Attracts Anions.)
Checking up 13.1:

13.2. Electrolysis of sodium chloride
Activity 13.2
Investigating the effect of concentration on the products formed during
electrolysis of concentrated sodium chloride solution.
Materials: Carbon or graphite rods, connecting wire, U-tube, dry cell, glasssyringes, concentrated sodium chloride, cork and switch.
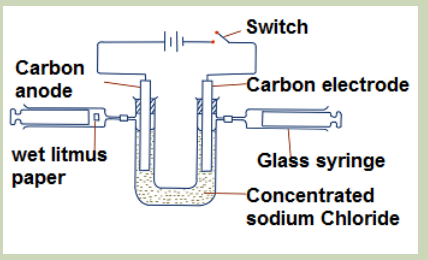
Figure 13.2.1: Electrolysis of concentrated sodium chloride solution
Procedure:
2. Add 10g of sodium chloride to 100cm3
of distilled water.
3. Warm the mixture and continue adding sodium chloride until a
saturated solution is formed.
4. Put the saturated solution in U-tube and fit it with carbon rods and glass
syringes.
5. Level the brine solution in the two arms and switch on the circuit.
Record any observations made after some time. Identify any gases
collected in the syringe.
Questions:
a. Identify the gases formed by testing them using litmus papers.
b. Using ionic equations, explain how the products are formed.
Sodium chloride may be in different forms that can be electrolytes. It may be in its
molten state, dilute solution or concentrated solution. In each case, the products of
electrolysis differ because of different factors.13.2.1. Electrolysis of molten sodium chloride
The molten salt is introduced in a container called electrolytic cell (or electrolysis
cell) in which there are two inert electrodes (platinum or graphite). Electrodes are
connected to a DC generator.
• Cations (Na+) move toward the cathode (negative electrode), where they takeelectrons and are reduced. On cathode metallic sodium is deposited:
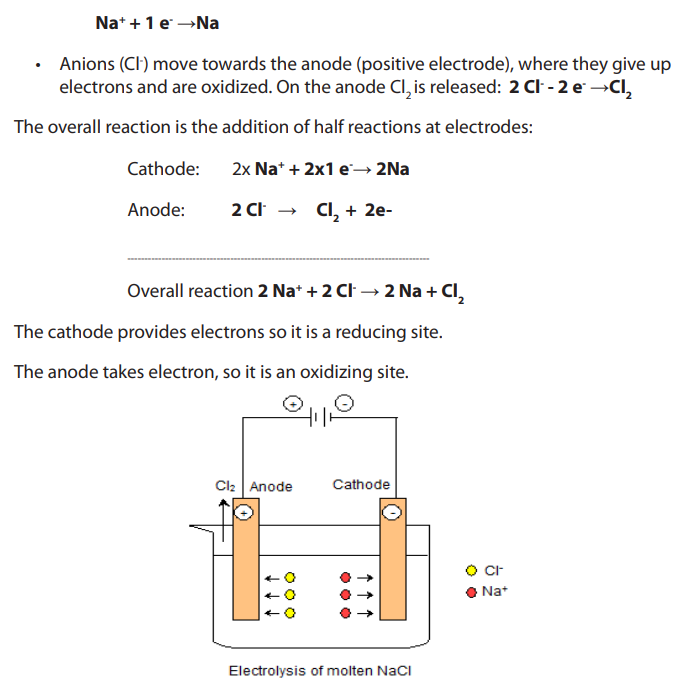
Figure 13.2.2: Electrolysis of molten sodium chloride solution
• Another important thing to note is that twice as much hydrogen is produced
as oxygen. Thus the volume of hydrogen produced is twice that of oxygen. Referto the equations above and note the number of electrons involved to help you
13.2.2. Electrolysis of Dilute Sodium Chloride Solution
An aqueous solution of sodium chloride contains four different types of ions. They
are ions from sodium chloride: Na+ (aq) and Cl-(aq)
Ions from water: H+ (aq) and OH-(aq)
When dilute sodium chloride solution is electrolysed using inert electrodes, the
Na+ and H+ ions are attracted to the cathode. The Cl- and OH- ions are attracted tothe anode.
Table 13.2: Standard reduction potentials.
The table shown below is simply a table of standard reduction potentials in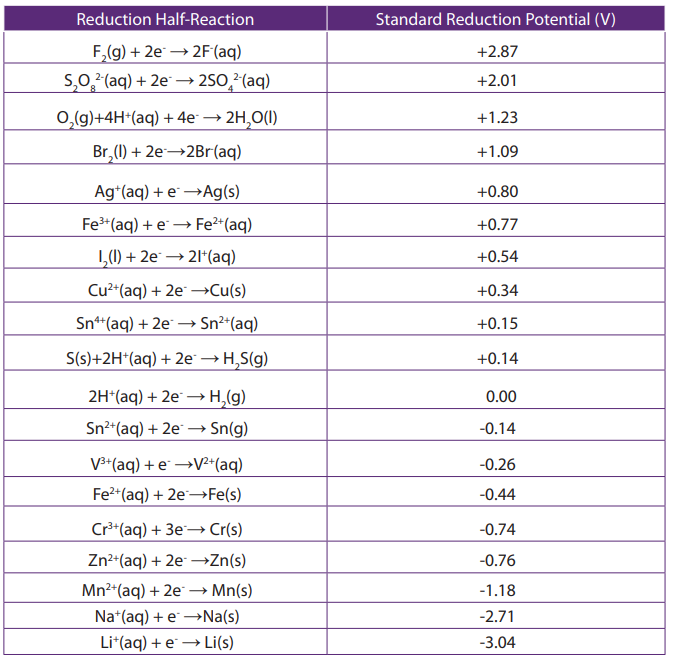
decreasing order. The species at the top have a greater likelihood of being reduced
while the ones at the bottom have a greater likelihood of being oxidized. Therefore,
when a species at the top is coupled with a species at the bottom, the one at the topwill be easily reduced while the one at the bottom will be oxidized.
• At the cathode:
The H+ and Na+ ions are attracted to the platinum cathode. H+ ions gains electrons
from the cathode to form hydrogen gas. (The hydrogen ions accept electrons more
readily than the sodium ions. As a result, H+ ions are discharged as hydrogen gas,
which bubbles off. Explanation why H+ ions are preferentially discharged will begiven later.)

• At the anode:
OH- and Cl- are attracted to the platinum anode. OH- ions give up electrons to theanode to form water and oxygen gas.
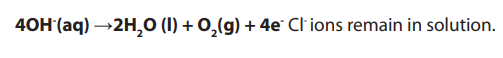
Adding the two reactions and balancing the terms:

If we remove 2 molecules of water on both sides, we get:
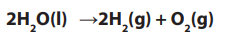
Note:
• Since water is being removed (by decomposition into hydrogen and oxygen),
the concentration of sodium chloride solution increases gradually. The overall
reaction shows that the electrolysis of dilute sodium chloride solution isequivalent to the electrolysis of water.

Figure 13.2.3: Electrolysis of dilute sodium chloride solution
13.2.3. Electrolysis of Concentrated Sodium Chloride Solution
The only difference with dilute NaCl solution is that at the anode, Cl- ions are more
numerous than OH- ions. Consequently, Cl- ions are discharged as chlorine gas, which
bubbles off.
A half-equation shows you what happens at one of the electrodes duringelectrolysis.
Sodium ions Na+ and hydroxide OH– are also present in the sodium chloride
solution. They are not discharged at the electrodes. Instead, they make sodiumhydroxide solution.
These products are reactive, so it is important to use inert (unreactive) materials forthe electrodes.
One volume of hydrogen gas is given off at the cathode and one volume of chlorine
gas is produced at the anode. The resulting solution becomes alkaline becausethere are more OH- than H+ ions left in the solution.

Checking up 13.2:
With the help of equations of reactions which occur at each
electrode, outline what happens during electrolysis of dilute aqueous sodiumchloride. What happens to the pH of the solution as electrolysis continues?
13.3. Electrolysis of water
Activity 13.3: Investigate the products formed during the electrolysis of water
Materials:
• Distilled water
• Tap water
• 2 silver-colored thumb tacks
• 9V battery
• Small, clear plastic container
• 2 test tubes
• Stopwatch
• Baking soda
• Table salt
• Lemon• Dish washing detergent

Procedure:
3. Insert the thumb tacks into the bottom of the plastic container so that
the points push up into the container. Space them so that they’re the
same distance apart as the two terminals of the 9V battery. Be careful not
to harm yourself!
4. Place the plastic container with the terminals of the battery. If the cup is
too large to balance on the battery, be sure thumb tacks are connect to
positive and negative pushpins and do no touch each other.
5. Slowly fill the container with distilled water. If the tacks move, go ahead
and use this opportunity to fix them before you proceed. Will distilled
water conduct electricity on its own? Try it!
6. Add a pinch of baking soda.
7. Hold two test tubes above each push pin to collect the gas being formed.
Record your observations. What happens? Does one tube have more
gas than the other? What gases do you think are forming?
8. Discard the solution, and repeat the procedure with a different
combination:
• Distilled water and lemon juice
• Distilled water and table salt
• Distilled water and dish detergent
• Distilled water (no additive)
• Tap water (Does tap water works? If so, why?)
Question: During the electrolysis of water, which electrolyte conductselectricity the best?
Water can be decomposed by passing an electric current through it. When this
happens, the electrons from the electric current cause an oxidation-reduction
reaction. At one electrode, called the cathode, electrons cause a reduction. At the
other electrode, called the anode, electrons leave their ions completing the circuit,
and cause an oxidation.
In order to carry out electrolysis the solution must conduct electric current.
Pure water is a very poor conductor. To make the water conduct better we can
add an electrolyte (NaCl) to the water. The electrolyte added must not be more
electrolyzable than water. Many electrolytes that we add electrolyze more easily
than water. Sulfate ions do not electrolyse as easily as water, so sulfates are often
used to enhance the conductivity of the water
Water may be electrolyzed in the apparatus shown below. Pure water is however a
very poor conductor of electricity and one has to add dilute sulphuric acid in orderto have a significant current flow.
In pure water at the negatively charged cathode, a reduction reaction takes place,
with electrons (e−) from the cathode being given to hydrogen cations to formhydrogen gas. The half reaction, balanced with acid, is:
Reduction at cathode:
At the positively charged anode, an oxidation reaction occurs, generating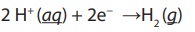
oxygen gas and giving electrons to the anode to complete the circuit:
Oxidation at anode:
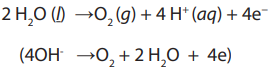
Combining either half reaction pair yields the same overall decomposition of water
into oxygen and hydrogen:
Overall reaction:
The number of hydrogen molecules produced is thus twice the number of oxygen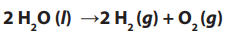
molecules. Assuming equal temperature and pressure for both gases, the producedhydrogen gas has therefore twice the volume of the produced oxygen gas.
Checking up 13.3
I understand the process of water electrolysis, that water as an electrolyte can
be decomposed into hydrogen and oxygen via an external energy source (an
electrical current). I know that the reduction of hydrogen takes place on the
cathode and the oxidation reaction takes place on the anode. I also know
that water, already partially split into H+ and OH- (though there are very few
of these ions in pure water).
1. Electric current (direct current) electrolyzes water. Discuss this
statement.2. Why alternative current are not used for the same process?
13.4. Electrolysis of concentrated copper (II) sulphate solutionusing inert electrode
Activity 13.4: Investigating what happens when a solution of copper (II)sulphate is electrolysed using carbon and copper electrodes.
Apparatus and chemicals: Glass cell, Carbon rod, 2M copper (II) sulphate
solution, connecting wires, dry cells, copper plates, propanone and litmuspaper.

Procedure:
3. Determine the mass of the graphite rods and record it.
4. Put 0.5M of copper (II) sulphate solution in a glass cell with the carbon
(graphite) rods and set up the apparatus. Carefully observe all the
changes taking place at the electrodes and the solution. Test the
resulting solution with blue litmus paper.
5. After some time, switch off the current, remove the electrodes, wash
them in propanone, dry then and then weigh them.
6. Repeat the experiment using clean strips of copper metal as electrodes.
Weigh them and then complete the circuit using freshly preparedcopper (II) sulphate solution. Record your observations.
Questions:
1. Explain the changes observed during the electrolysis of copper (II)
sulphate using :
a.Carbon electrodes
b.Copper electrodes
2. Outline the changes that occur in the solution from the beginningto the end of the experiment.
The products of electrolysing of copper sulphate solution with inert electrodes(carbon/graphite or platinum) are copper metal and oxygen gas.

Figure 13.4.1: Simple apparatus to investigate the electrolysis of aqueous solutions
Using the simple apparatus (diagram above) and inert carbon (graphite) electrodes,
you can observe the products of the electrolysis of copper sulfate solution are a
copper deposit on the negative cathode electrode and oxygen gas at the positive
anode electrode. This anode reaction differs if you use copper electrodes. You have
to fill the little test tubes with the electrolyte (dilute copper sulphate solution), hold
the liquid in with your finger and carefully invert them over the nearly full electrolysiscell. The simple apparatus (above) can be used with two inert wire electrodes.
The blue colour fades as more and more copper is deposited, depleting theconcentration of blue copper ion
Diagram for the electrolysis of copper (II) sulphate solution with carbon in solution.
in solution.electrodes.
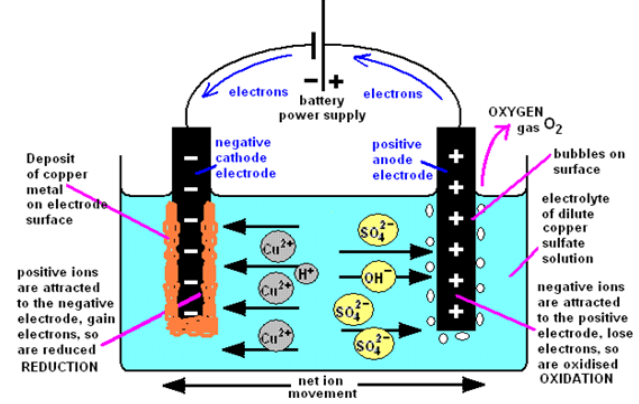
Figure 13.4.2: The electrolysis of copper (II) sulphate solution with carbon electrodes.
The electrode reactions and products of the electrolysis of the electrolyte copper
sulphate solution (with inert carbon-graphite electrodes) are illustrated by thediagram above
(a) The electrode products from the electrolysis of copper sulphate with inertgraphite (carbon) electrodes
The negative cathode electrode attracts ions (from copper sulphate) and
ions (from copper sulphate) and 
ions (from water). Only the copper ion is discharged, being reduced into copper
metal. The less reactive a metal, the more readily its ion is reduced on the electrode
surface.
A copper deposit forms as the positive copper ions are attracted to the negativeelectrode (cathode)
The traces of hydrogen ions are not discharged, so you do not see any gas collected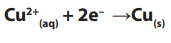 positive ion reduction by electron gain.
positive ion reduction by electron gain.
above the negative electrode. The blue colour of the copper ion will fade as the copper
ions are converted into the copper deposit on the cathode
At the positive anode reaction with graphite electrodes
Oxygen gas is formed at the positive electrode, an oxidation reaction (electronloss).
The negative sulphate ions or the traces of hydroxide ions
or the traces of hydroxide ions  are
are
attracted to the positive electrode. But the sulphate ion is too stable and nothinghappens. Instead hydroxide ions are discharged and oxidised to form oxygen.
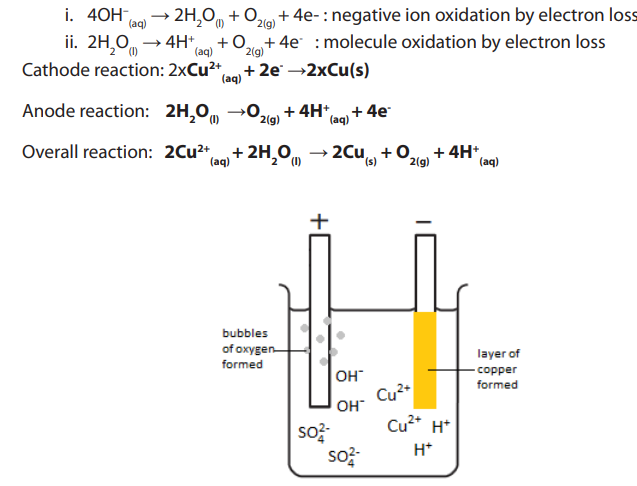
Figure 13.4.3: Electrolysis of copper(II) sulphate
Checking up 13.4
1. Name the product at the cathode and anode during electrolysis of:
a. Molten lead bromide with inert electrode.
b. Acidified copper sulphate solution with inert electrodes.
c. Acidified water with inert electrode.
d. Dilute hydrochloric acid with inert electrode.
e. Concentrated hydrochloric acid with inert electrode.
2.Predict the products formed when the following molten compounds are
electrolysed using carbon electrodes;
a. Lead(II) bromideb. Magnesium oxide
13.5. Electrolysis of concentrated copper (II) sulphate solutionusing copper electrodes
The products of electrolysis of copper sulfate solution with copper electrodes are
copper metal and copper ions (the copper anode dissolves).
The electrolysis of copper (II) sulphate solution using copper electrodes is shownbelow.

Figure 13.5.1: Electrolysis of copper (II) sulphate
Using the simple apparatus and two copper electrodes the products of the
electrolysis of copper sulphate solution are a copper deposit on the negative
cathode electrode and copper dissolves, at the positive anode electrode.
at the positive anode electrode.
This copper anode reaction differs from the one when you use an inert graphiteelectrode for the anode.
When Copper (II) sulphate is electrolysed with a copper anode electrode (thecathode can be carbon or copper), the copper deposit on the cathode (–) equals the
copper dissolves at the anode (+). Therefore the blue colour of the Cu2+ ions stays
constant because Cu deposited = Cu dissolved. Both involve a two electron transfer
so it means mass of Cu deposited = mass of Cu dissolving for the same quantity
of current flowing (flow of electrons). You can check this out by weighing the dry
electrodes before and after the electrolysis has taken place.
The experiment works with a carbon anode and you see the blackness of the graphitechange to the orange-brown colour of the copper deposit.
Figure 13.5.2: The electrolysis of copper (II) sulphate solution with a copper anode and a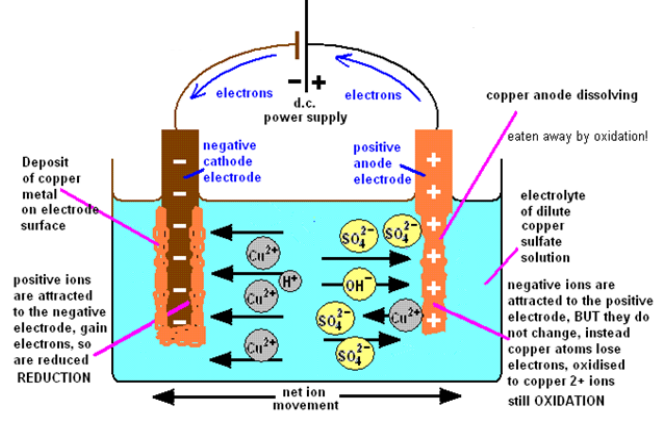
copper cathode.
The electrode reactions and products of the electrolysis of copper sulphate solution
(with a copper anode) are illustrated by the diagram above.
(a) The electrode products from the electrolysis of copper sulphate with copperelectrodes
The negative cathode electrode attracts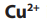 ions (from copper sulphate) and
ions (from copper sulphate) and 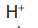
ions (from water). Only the copper ion is discharged, being reduced to copper metal.
A reduction electrode reaction at the negative cathode: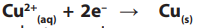
(copper deposit, reduction 2 electrons gained) reduction by electron gain.
The positive anode reaction with copper electrodes
It’s the copper anode that is the crucial difference than electrolysing copper sulphatesolution with an inert carbon (graphite) or platinum electrode.
The negative sulphate ions
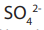 (from copper sulphate) or the traces of hydroxide
(from copper sulphate) or the traces of hydroxide
ions (from water) are attracted to the positive electrode. But both the sulphate
(from water) are attracted to the positive electrode. But both the sulphate
ion and hydroxide ion are too stable and nothing happens to them because the
copper anode is preferentially oxidised to discharge copper ions.
copper ions.
An oxidation electrode reaction at the positive anode: copper dissolves, twoelectrons are lost following the half reaction:
Copper atoms oxidised to copper (II) ions: dissolving of copper in its electrolytic
purification or electroplating (must have positive copper anode).
Copper (II) ions reduced to copper atoms: deposition of copper in its electrolytic
purification or electroplating using copper (II) sulphate solution, so the electrodecan be copper or other metal to be plated or any other conducting material.
This means for every copper atom that gets oxidised, one copper ion is reduced,therefore
when copper electrodes are used in the electrolysis of copper sulphate solution, the
mass loss of copper from the positive anode electrode should equal to the mass ofcopper gained and deposited on the negative cathode electrode.
You can show this by weighing both electrodes at the start of the experiment. After
the current has passed for some time, carefully extract the electrodes from the
solution, wash them, dry them and reweigh them. The gain in mass of the cathodeshould be the same as the loss of mass from the anode.
Checking up 13. 5
1.
a. Predict the products formed (i) at the cathode, (ii) at the anode, when
copper (II) sulphate solution is electrolysed using carbon electrodes.
b. Describe the colour changes in the electrolyte.
2. What will you observe
a. At cathode
b. At anode
c. In electrolytic, during the electrolysis of copper (II) sulphate solution
with copper electrode.
3.Write equations for the reaction taking place at cathode and at anode
during the electrolysis of:
a. Acidified copper sulphate solution with copper electrode.
b. Acidified water with inert electrode.
c. Molten lead bromide with inert electrode.
4.Using a table, highlight the similarities and differences between using
graphite electrodes and copper electrode for the electrolysis of coppersulphate.
13.6. Faraday’s Laws
Activity 13.6
Comparison of the amounts of different substances liberated by the samequantity of electricity.
Figure 13.6: Comparison of the amounts of different substances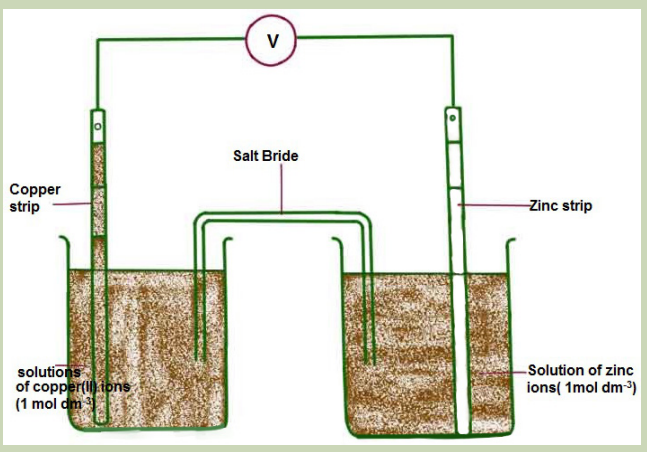
liberated by the same quantity of electricity
Set up the circuit containing a copper voltmeter and a silver voltmeter (a
voltmeter is a vessel containing two electrodes immersed in a solution of ionsthrough which a current is to be passed.)
Identify the copper and silver cathodes, clean and dry them, and after weighing
them return them in their respective voltmeters. Pass a current of about 0.5A
for 20 or 30 minutes, after which the cathodes should be removed, cleaned anddried, and reweighed.
Compare the masses of copper and silver deposited. Note that care must be
taken in removing the silver cathode from the solution as the metal does notalways adhere well to the cathode.
The relationship between the mass of product formed at an electrode and the
quantity of electricity passed through an electrolyte is given by Faraday’s laws ofelectrolysis.
Michael Faraday (1791-1867) did the first work on electrolysis and formulated what
is known today as Faraday’s laws of electrolysis.
These laws express the quantitative results of electrolysis. They assert that the
amount (expressed in moles) of an element liberated during electrolysis dependsupon:
1. The time of passing the steady current, t
2. The magnitude of the steady current passed, I3. The charge of the ions
13.6.1. Faraday’s first law
According to this law, “The amount of substance liberated at an electrode isdirectly proportional to the quantity of electricity passed”
1F = 96500 coulomb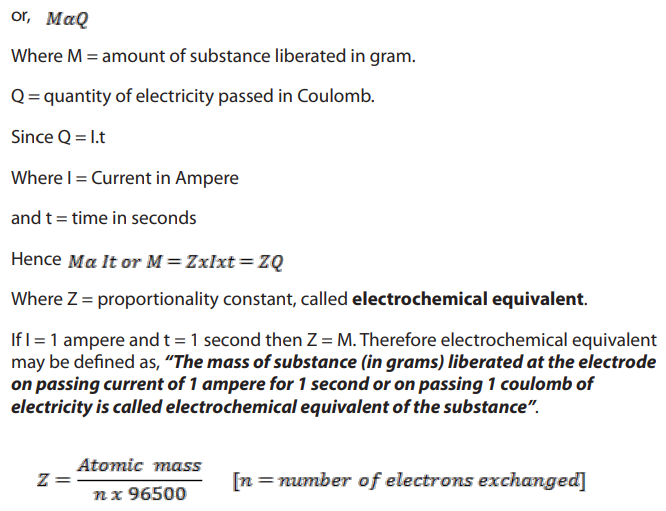
So, 1 Faraday [96500 coulomb] of electricity will produce 1 gm equivalent of Ag,
Cu and Al at cathode.
13.6.2. Faraday’s second law
According to this law, “if same quantity of electricity is passed through different
electrolytes, then the amount of substances liberated at the respective electrodesare in the ratio of their equivalent masses”.
Or
When the same quantity of electricity passes through solutions of different electrolytes,
the amounts of the substances liberated at the electrodes are directly proportional totheir chemical equivalents.
Equivalent mass is the mass of a substance especially in grams that combines with
or is chemically equivalent to eight grams of oxygen or one gram of hydrogen; theatomic or molecular Mass divided by the valence.
Example:
Calculate the amount of electric charge in coulombs which can
deposit 5.2g of aluminium when a current was passed through a solution ofaluminium sulphate for some time.
Solution:
3 moles of electrons are needed to deposit 1 mole of aluminium (24g of
aluminium).
Checking up 13.6
1. A current of 0.65A was passed through sulphate solution of metal X
for 35 minutes between platinum electrodes. If 0.449 g of the metal is
deposited at the cathode, calculate the charge on the element X (atomic
mass is 63.5)
2. When a constant current was passed through an aqueous solution
of copper (II) nitrate for one hour the mass of the copper cathode
increased by 15.24 g. Calculate the current in amperes which was used(F= 96500 Cu = 63.5)
13.7. Factors affecting Electrolysis
Activity13.7: Investigating how the nature of electrodes affects the discharge of ions during electrolysis.
Apparatus and chemicals:
• Beaker (100 cm3)
• 6V Battery
• 2 connecting wires with crocodile clips
• 2 carbon electrodes
• 2 copper electrodes
• solution
solution
Caution: Do not allow the electrodes to touch each other while the powersupply is switched on, otherwise this may damage the equipment.
A. Electrolysing of copper (II) sulphate solution using Carbon electrodes.
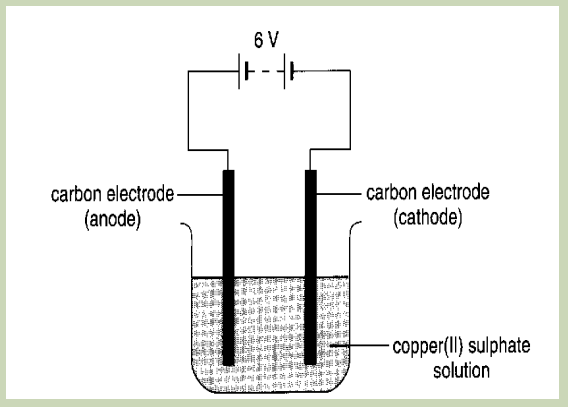
Figure 13.7.1: Electrolysis of copper (II) sulphate solution using carbon electrodes.
Procedure: let the current flow for 5 minutes
Observe what happens at each electrode
Questions:
1. What product is formed on the cathode?
2. What product is formed at the anode?
3. Has the colour of the solution changed?4. Explain the observations in 3.
B.Electrolysing of copper(II) sulphate solution using Copper electrodes
Procedure: let the current flow for 5 minutes
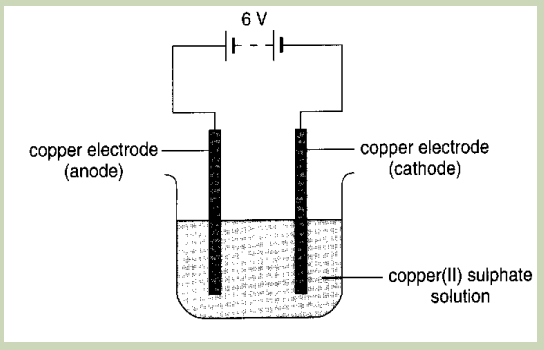
Observe what happens at each electrode
1. What product is formed on the cathode?
Questions:
2. What product is formed at the anode?
3. Has the colour of the solution changed?4. Explain the observations in 3.
In an electrolysis where there are more than one species which can be discharged
at the same electrode, only one of them is discharged at a time; for example, in an
aqueous sodium chloride solution, we have four species that is, and
and 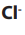
ions from sodium chloride and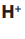 and
and  ions from water.
ions from water.
During electrolysis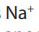 ions and
ions and 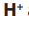 ions migrate to the cathode while
ions migrate to the cathode while  ions and
ions and
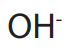 migrate to the anode.
migrate to the anode.
Now the question is, which species of ions will be discharged at the cathode and
which ones will be discharged at the anode first?The factors which decide the selective discharge of ions are:
• Nature of electrodes
• Position of the ion in electrochemical series
• Concentration• The state of the electrolyte
13.7.1. Nature of Electrodes
In the electrolysis of sodium chloride solution using a platinum cathode,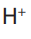 ions
ions are discharged first in aqueous solution.
However, if the cathode is mercury, sodium is discharged. The sodium atom
discharged combines with mercury cathode to form sodium amalgam.

Electrolysis of copper sulphate using copper anode
In this electrolysis, the anions, migrate to the anode but none of them
migrate to the anode but none of them is discharged. Instead the copper atoms of the anode ionise and enter the solution.
The copper (II) ions are attracted to the cathode and copper is deposited as a brown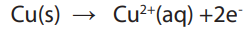
layer. The use of platinum anode gives oxygen as the product due to the reaction;
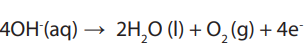
13.7.2. Position of ion in Electrochemical Series
When solving for the standard cell potential, the species oxidized and the species
reduced must be identified. This can be done using the activity series. The table 13.2
is simply a table of standard reduction potentials in decreasing order. The species
at the top have a greater likelihood of being reduced while the ones at the bottom
have a greater likelihood of being oxidized. Therefore, when a species at the top is
coupled with a species at the bottom, the one at the top will become reduced whilethe one at the bottom will become oxidized.
During electrolysis of solution containing a mixture of ions, the ion lower inelectrochemical series is discharged first in preference to the one high in the series.
Let us look at the role of water in electrolysis products.Water molecules to a small extent (degree) ionize as
Due to the above ionization, aqueous solutions of electrolytes contain two sets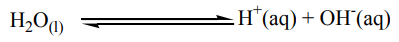
of ions that is those from the salt dissolved and the ions from water
ions from water molecules.
Example:
Electrolysis of aqueous copper (II) sulphate solution using platinum electrodes.Ions present in solution:
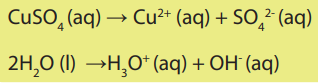
Cathode
The cathode gets coated with a brown layer of copper as the solution loses its blue
colour.
Anode

13.7.3. Concentration of electrolyte solution
Increase of concentration of an ion tends to promote its discharge, for example in
concentrated hydrochloric acid, containing
as negative ions, the highly concentrated is discharged in preference.
is discharged in preference.
However, if the acid is very dilute, some discharge of will also occur. It is important
will also occur. It is important
to know that as the acid is diluted, there will not be a point at which chlorine ceases
to be produced and oxygen replaces it. Instead a mixture of the two gases will comeoff, with the proportion of oxygen gradually increasing.
Another case in which the order of discharge according to the electrochemical series
is reversed by a concentration effect is that of sodium chloride solution.
In concentrated solution of sodium chloride called brine, the following reactions
occur.

Question 3
A university student set up three different electrolytic cells. The substances that
were electrolysed were NaCl (l), 0.05 M NaCl (aq) and 5.0 M NaCl (aq). Which of
the following statements correctly describes the results of the experiment?
a. The reactions occurring for the aqueous solutions will produce the same
products at the anode and cathode.
b. Chlorine gas is the major product when molten NaCl (aq) and 0.05 M
NaCl (aq) are electrolysed.
c. The pH at the cathode increases when solutions of NaCl are electrolysed.
d. The only means by which different products can be produced forvarying concentrations of NaCl is to alter the voltage.
13.7.4. The state of the electrolyte
The half reactions taking place at the electrode depends on whether the electrolyte
is in a molten or an aqueous state, and if in aqueous state its concentration. For
example, the electrode reactions that take place during the electrolysis of moltenpotassium iodide are:
However, if aqueous potassium iodide is used, the following electrode reactions
take place:
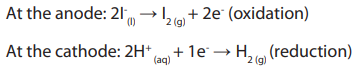
Electrode signs and reactions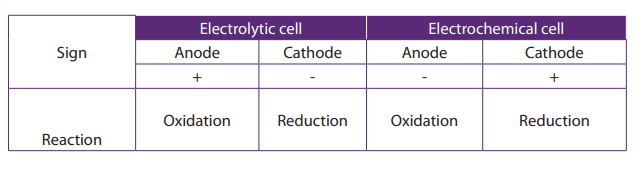
Checking up 13.7
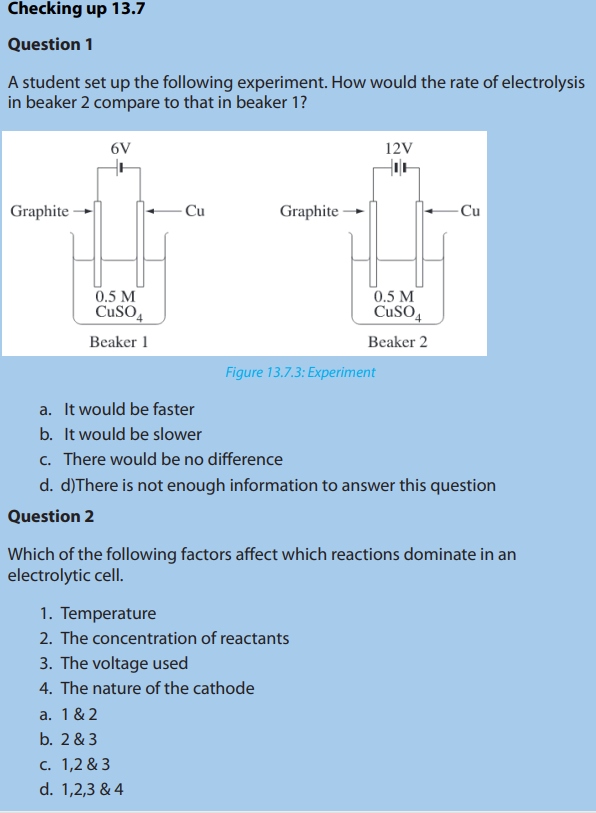
Question 1
A student set up the following experiment. How would the rate of electrolysisin beaker 2 compare to that in beaker 1?
13.8. Application of electrolysis
Activity 13.8
Copper-Plated Key
Materials:
• 1.5-volt D battery with battery holder
• Two alligator clip leads or insulated wire
• Beaker or glass
• Copper sulphate
• Copper electrode (or coil of copper wire)
• Brass key• Safety equipment
Procedure:
5. Prepare the key for copper-plating by cleaning it with toothpaste or
soap and water. Dry it off on a paper towel.
6. Stir copper sulphate into some hot water in a beaker until no more willdissolve. Your solution should be dark blue. Let it cool.
3. Use one alligator clip to attach the copper electrode to the positive terminal
of the battery (this is now the anode) and the other to attach the key to the
negative terminal (now called the cathode).
4. Partially suspend the key in the solution by wrapping the wire lead loosely
around a pencil and placing the pencil across the mouth of the beaker. The
alligator clip should not touch the solution.
5. Place the copper strip into the solution, making sure it doesn’t touch the key
and the solution level is below the alligator clip. An electrical circuit has now
formed and current is flowing.
6. Leave the circuit running for 20-30 minutes, or until you are happy with the
amount of copper on the key.
Question: Observe carefully electrolysis process and records what happenedduring the electrolysis process.
Electrolysis has a number of important industrial applications. These include
the extraction and purification of metals, electroplating and anodizing and themanufacture of other chemicals for example sodium hydroxide (NaOH).
Extraction of metals
Metals in group I and II of the periodic table cannot be reduced by chemical
reducing agents; they are extracted from their fused halides by electrolysis. Sodium
is obtained by electrolysis of molten sodium chloride in the Dawncell. Magnesium is
obtained by electrolysis of genera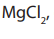 ted from dolomite and sea water.
ted from dolomite and sea water.
Extraction of aluminumThe chief ore of aluminum is bauxite,
 it contains silica and iron (III)
it contains silica and iron (III) oxide as impurities. Bauxite is dissolved in a strong solution of sodium hydroxide:
The impurities are not affected by the presence of sodium hydroxide because they
are not amphoteric and they are thus filtered off. The solution is diluted, cooled andseed by adding a few crystals of pure Al
On seeding, the aqueous tetrahydroxoaluminate is precipitated as pure Al

from the solution:

The electrolytic cell is an iron tank lined with carbon, which acts as the cathode. The
anodes are blocks of carbon dipped into the electrolyte. The electrolyte is a solution
of molten aluminum oxide in molten cryolite. Cryolite acts as a solvent to dissolve
aluminum oxide and as an impurity to lower the melting point of aluminum oxide.The electrolytic cell is maintained at around 900°C.
Aluminum ions are discharged at the cathode, forming a pool of molten aluminum
at the bottom of the tank.
At high temperature, oxygen reacts with the carbon anode to form carbon dioxide
gas. Hence, the anodes are slowly burnt away as carbon dioxide gas and needs to bereplaced frequently.
Manufacture of NaOH and extraction of
 gas in Downcell
gas in DowncellConstruction of Down’s cell
• Down’s cell consists of a rectangular container of steel.
• Inside of the tank is lined with firebricks.
• Anode is a graphite rod which projects centrally up through the base of the
cell.
• Cathode is a ring of iron, which surrounds the anode
The anode and cathode are separated from each other by a cylindrical steel
gauze diaphragm; so that and
and 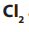 are kept apart.
are kept apart.• A bell like hood is submerged over the anode
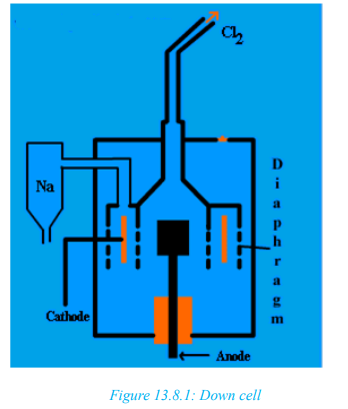

These are manufactured by electrolysis of concentrated sodium chloride, calledbrine. Hydrogen is also obtained as by products. In solution, sodium chloride ionizes:
Using carbon electrodes, the products of electrolysis are chlorine at the anode and
hydrogen at the cathode. Hydrogen is discharged in preference to sodium.
The sodium discharged at the mercury cathode forms a solution of sodium amalgam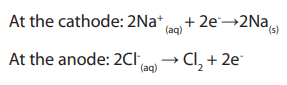
in mercury. The sodium amalgam is collected in a reservoir in which it reacts
with water to form sodium hydroxide solution and hydrogen gas. Mercury is alsorecovered and returned to the electrolytic cell to pass through the process again.
The sodium hydroxide produced is crystallized. It is used in: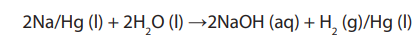
• Manufacture of soap
• The paper industry-wood contains lignin, which is a nitrogenous compound, in
addition to cellulose. Wood chips are converted into pulp by boiling the chips
with sodium hydroxide solution to remove the lignin. The digested material isbleached with chlorine.
Purification of metals
Metals such as copper, zinc and aluminium can be purified by electrolysis. The
purification of metals is known as refining. The copper obtained after the reduction
process is not very pure. It contains small amounts of impurities such as iron. This
copper is called blister copper and is refined by an electrolytic method. It is castinto bars which are used as anodes in acidified copper (II) sulphate solution.
The cathode is made of thin pure copper. During the electrolysis, ions are
ions are
transferred from the anode to the cathode where they are discharged and copper isdeposited.
The net effect is to dissolve the anode made of impure copper and thicken the
cathode (pure copper) with more pure copper.
Electroplating
This is a process of coating a metal with another of interest mainly to prevent it from
rusting, or/ and to improve its appearance, for example, in silver plating articles as
cake dishes, made of base alloy, for example cupronickel, are made the cathode in
plating bath of potassium (or sodium) dicyanoargentate(I),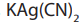 solution. This
solution. This
contains some silver ions, The anode is pure silver. When direct current passes,
The anode is pure silver. When direct current passes, the following reactions occur.
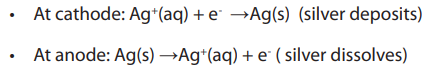
In general;
• The metal being coated is made the cathode and the metal coating is the anode
• The solution used is made of the ions of a metal that is coating, so that theanode can dissolve. Anode is the pure plating metal.
A good electroplating requires steady electric current, appropriate concentration of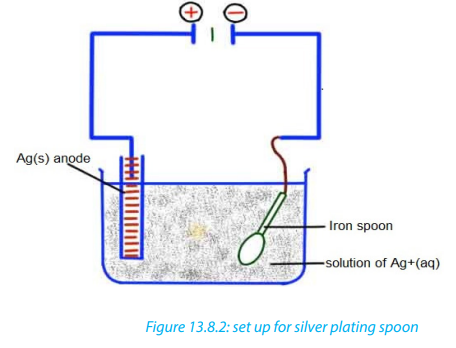
electrolyte and temperature. The metal to electroplate must be clean.
Checking up 13.8
1. a. What is the difference between electrolytic extraction of a metal and
electroplating?
b. Draw a set up used to electroplate a spoon by silver.
2.What is the material for cathode and anode during electro refining of impure
copper?
3. Electrometallurgy is the process of reduction of metals from metallic
compounds to obtain the pure form of metal. Given elements: aluminum,
lithium, sodium, potassium, magnesium, calcium, Zinc, Iron and copper
which ones can be reduced by chemical agents such as carbon and whichones are produced by electrolysis only?
13.9. End Unit Assessment
1. Choose from a list of words and fill in the missing words in the text below:
electrolysis, cathode and anode. When the current passes through an
electrolytic solution, ions migrate and electrons are gained or lost by ions
on the electrodes surface. Electrode that is positively charged has deficit
of electrons is called…………and other negatively charged has excess of
electrons is called……………. chemical changes at the electrodes due to the
passage of electric current are called ………………...
2. Answer by true or false
a. The Avogadro (1791-1867) did the first work on electrolysis and
formulated what is known today as faraday’s laws of electrolysis.
b. Electroplating is a process of coating a metal with another of interest
mainly to prevent it from rusting, and to improve its appearance.
c. A non electrolyte is a solution or molten compound which cannot be
decomposed by an electric current.
d. An ion is an atom or a group of atoms (radical) which has an electric
charge.
e. An anode is the negative electrode through which electrons leave
the electrolyte and a cathode is the positive electrode through which
electrons enter the electrolyte or leave the external circuit.
3. Which of the following involves electrolysis?
a. Photosynthesis and Respiration
b. Purification of Copper and Sea Water
c. Purification of copper and extraction of reactive metals
d. Extraction of reactive metals and Respiration
4. Which of the following is not an inert electrode?
a. Carbon
b. Copper
c. Platinum
d. Mercury
5. What ions are present in the electrolysis of aqueous Copper (II) Sulfate with
Copper electrodes?
a. Copper (II) ions, Sulfate ions
b. Hydrogen ions, Oxygen ions, Copper (II) ions
c. Copper (II)ions
d. Hydrogen ions, Hydroxide ions, Copper (II) ions, Sulfate ions
6. What happens during the electrolysis of a diluted sodium chloride solution?
a. Hydrogen ions and chlorine ions are discharged.
b. Hydrogen ions and hydroxide ions are discharged.
c. Sodium ion and chlorine ions are discharged.
d. Sodium ions and hydroxide ions are discharged.
7. State three applications of electrolysis on a large scale and describe one of
them briefly.
8. Describe how the factor of concentration affects electrolysis.
9. Calculate the amount of electricity required (in Faradays) to deposit one mole
of lead ions if a current of 2.5A was passed for 20 minutes through molten lead
(II) bromide and 3.20 g of lead metal was deposited.
10. An element X has a relative atomic mass of 88. When a current of 0.5A is
passed through the molten chloride of X for 32 minutes and 10 seconds, 0.44
g of X deposited at the electrode.
• Calculate the number of Faradays needed to liberate 1 mol of X.• Write the formula of the ion of X (1F= 96500C).