UNIT 12: CONDUCTIVITY OF SOLUTIONS
Key unity competence:
To be able to: Explain the effect of different factors on the molar conductivity ofdifferent electrolytes and the applications of conductivity measurements.
Learning objectives:
• Explain the conductivity of solutions.
• State and explain the factors that affect molar conductivity of solutions.
• State Kohlrausch’s law of individual molar conductivity.
• Use Kohlrausch’s law to calculate the molar conductivity of an electrolyte.
• Interpret a graph of molar conductivity against concentration for both weak
and strong electrolytes.
• Compare and contrast metallic conductivity and electrolytic conductivity.
• Develop a team approach and responsibility in performing experiments.
• Appreciate the contributions of other scientists like Kohlrausch’s law in
calculation of molar conductivity of solutions.• Respect the procedure in performing experiment.
12.1. Conductance of electrolytic solutions
Introductory activity

The above set up is made by
1. Solution of sodium hydroxide
2. Oranges containing citric acid
3. Solution of sugar
Carry out the three experiments as illustrated on the picture and answer to therelated questions.
1. compare the intensities of lights in set up 1,2 and 3
2. Why there is no light in set up three ?
3. What do you think are the main cause of bulbs light in set up 2 ?4. Why the light in the setup 1 and 2 are different?
Activity 12.1:
You have certainly heard about people being accidently electrocuted whenbathing at home; can you explain?
The conductance of material or solution is the property of materials due to which
a material allows the flow of ions or electrons through itself and thus conducts
electricity. It is generally defined as the reciprocal of resistance of that material. SI
unit of conductance is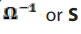 (Siemens), named after the 19th century German
(Siemens), named after the 19th century German
engineer and industrialist Ernst Werner von Siemens. It used to be called the mho
which is just ohm written backwards because the resistance is expressed in
(Ohm). The symbol for conductance is L or G. Thus G = 1/R. R = V/I soconductance is just the inverse of R: G = I/V.
The conductance of a material depends on the nature of material, number of valence
electrons for a material and temperature. Metals are good conductors of electricity
due to number and the mobility of their valence electrons. We observe that theconductance of materials decreases with increase the temperature.
Water in its pure state is known to be nonconductor because there are very little ions
The presence of electrolytes further enhances the conductivity as they supply their ions
to the solution. The conductance of electricity by ions present in the solutions is calledelectrolytic or ionic conductance.
The conductance is the inverse of resistance, therefore it is determined by calculatingthe resistance of the electrolytic solution or using the conductance cell (Figure 12.1)
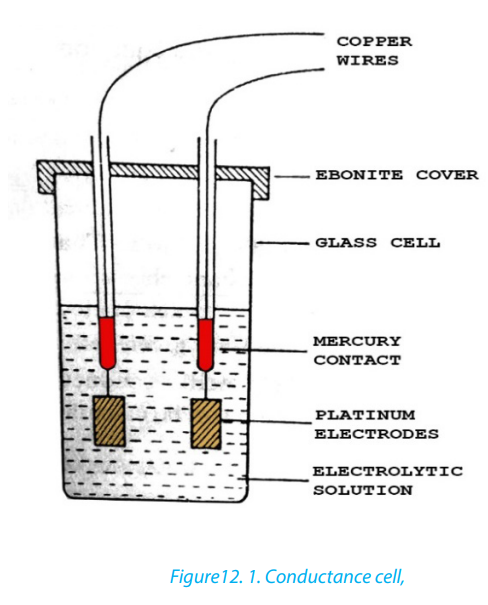
Source B.S, BAHL essentials of physical chemistry, page 476
Equivalent conductance is again called conductivity (Λ) which is the ability of a
solution to conduct electric charges, it is measured in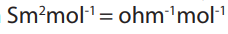
(Table 12.1)
Table 12.1: Electrochemical properties, their symbols and units
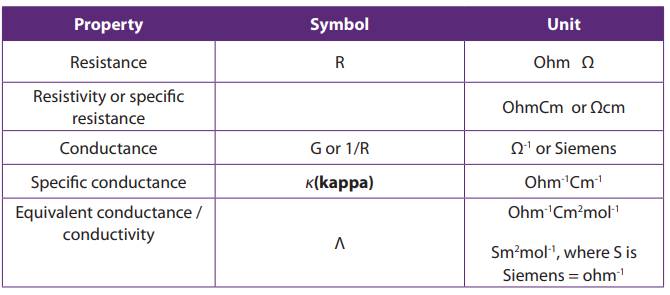
Source: B.S BAHL(2000),essentials of physical chemistry, Page 697
Checking up 12.1
a. Define conductivity
b. What is the difference between conductance and resistance?
Conductivity: Definition and description
Conductivity of a substance is defined as the ability or power to conduct or transmit
heat, electricity, or sound›. Its units are Siemens per meter [S/m] in SI and milliohmsper centimeter [m mho/cm] in U.S. customary units.
12.2. Measurement of conductivity of solutions
Activity 12.2
1. Refer to daily activity usage in electricity domain what are the objects
used to measure the voltage?
2. Refer to introductory activity above, how will you know that a solutionis conducting or not?
The conductivity is the reciprocal of the resistance (1/R) and is measured in Siemensor mhos.
Conductivity measurements are used routinely in many industrial
and environmental applications as a fast, inexpensive and reliable way of measuring
the ionic content in a solution. For example, the measurement of conductivity
is a typical way to monitor and continuously trend the performance of waterpurification systems.
Electrical conductivity meter
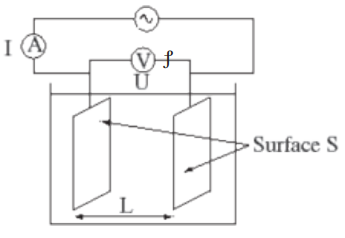
Principle of the measurement
The electrical conductivity of a solution of an electrolyte is measured by determining
the resistance of the solution between two flat or cylindrical electrodes separated
by a fixed distance. An alternating voltage is used in order to avoid electrolysis. The
resistance is measured by a conductivity meter. Typical frequencies used are in the
range 1–3kHz. The dependence on the frequency is usually small, but may become
appreciable at very high frequencies.
A wide variety of instrumentation is commercially available. There are two types of
cell, the classical type with flat or cylindrical electrodes and a second type based
on induction. Many commercial systems offer automatic temperature correction.
Tables of reference conductivities are available for many common solutions.
The conductivity of an electrolyte is the conductance of a volume of solution
containing one mole of dissolved electrolyte placed between two parallel
electrodes 1dm apart and large enough to contain between them all the solution;the conductivity is affected by temperature.
Checking up 12.2
Describe the functioning of conductivity meter and derive the formula ofcalculation of conductivity.
12.3. Specific conductivity of solutions
Activity 12.3:
1. Define resistivity
2. Establish a relation between conductivity and resistivity and among the
following substances, which ones areconductors and non-conductors,
for each you have to explain why they are or not conductors: pure
water, sugar, iron plate, clothes, plastic bags, ammonia solution, saltsolution, etc…
Specific Conductivity (better known as specific conductance) is the measure of
the ability of that material to conduct electricity. It is represented by the symbol “К”.
Hence, by definition, the specific conductance (specific conductivity), κ (kappa) is
the reciprocal of the specific resistance. The SI unit of conductivity is Siemens permeter (S/m).
Specific conductivity or conductivity of an electrolytic solution at any given
concentration is the conductance of one unit volume of solution kept between
two platinum electrodes with the unit area of cross section and at a distanceof unit length. What is the difference between Conductance and Conductivity?
. Conductance depends on the dimensions of the conductor, but conductivity does
not depend on the dimensions.. Conductance is measured in Siemens while conductivity is measured in Siemens per meter.
Checking up 12.3
12.4. Molar conductivity of solutions
Activity 12.4:
Refer to experiment done in the activity one (introductory activity) repeat the
same experiment at different concentration 1M of NaCl and 2M of NaCl, explainhow the intensity of light change with concentration
The molar conductivity of a solution at any given concentration is the
conductance of the volume of solution containing one mole of electrolyte kept
between two electrodes with the unit area of cross section and distance of unit
length. In general terms, it is defined as the ratio of specific conductivity and theconcentration of the electrolyte.
Molar conductivity of electrolytes increases with dilution. Friedrich Kohlrausch,
in 1900, experimentally found that: are the molar conductivity at a given concentration and at infinite dilution
are the molar conductivity at a given concentration and at infinite dilution
respectively, b is a constant depending on the viscosity of the solvent
and c is the concentration.Example:
Table 12.1: Variation of conductivity in terms of concentration at different temperatures
This table shows that the conductivity increases with increasing concentration and
temperature.
12.4.1. Strong electrolytes
For strong electrolyte, molar conductivity increases steadily with dilution until it
reaches the maximum value at infinite dilution (at high concentration, the lower
conductivity values are due to ionic interference. The formation of ionic pairs or
triplet and symmetrical spheres greatly reduces the mobility of ions however as
the dilution increases, there is reduced ionic interference as result of many solvent
molecules surrounding the oppositely charged ions thus an increase in molar
conductivity.
At infinite, there is independent migration of ions that is ions experience negligible
ionic interference and move independent of each other.
The molar conductivities of strong electrolytes are high. This is because, by nature,
strong electrolytes are highly dissociated when molten or when in solution into
large number of ions. These ions are mobile, hence they migrate to the electrodes,
resulting in the high conduction of electricity: the higher the number of ions arefree in solution, the higher the conductivity.
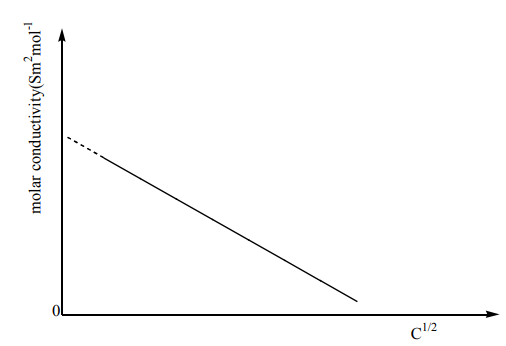
This graph can be obtained by extrapolation of the graph to zero concentration.
12.4.2. Weak electrolytes
Weak electrolytes show partial dissociation in solution, producing few ions, which
results in low conduction of electricity.
A weak electrolyte dissociates to a much lesser extent so its conductance is lowerthan that of a strong electrolyte at the same concentration.

The very large increase at infinite dilution is because the ionization increases and so
the number of ions in solution increases. The value of cannot be obtained
cannot be obtained
by extrapolation as can be seen on the graph. It is obtained by applying Kohlrausch’slaw (see later).
Summary:
• The higher the number of ions per unit volume in solution, the greater the
conductivity of the electrolytic solution. This means that the conductivity
increases with concentration of ions in solution up to an optimum level over
which it starts decreasing.
• On the other hand when the conductivity has decreased due to very high
concentration of ions, it can be increased with dilution (i.e. lower concentrations)
up to its optimum, beyond which further dilution will decrease conductivity.
• The decrease or increase of conductivity by concentrating or diluting the
solution is sharp in strong electrolytes while it is gradual in weak electrolytes.
The following graph shows.

• Λ values for strong electrolytes are larger than weak electrolytes for the same
concentration.
• Increase Λ for strong electrolyte is quite small as compared to that for weak
electrolyte towards dilution.
The table below shows the trend in conductivity with dilution for a strong and a
weak acid.
Table 12.2. Trends in conductivity

Explanation of Increase in Conductivity with Dilution:
With increase in dilution (decrease in concentration), ions become farther apart, and
inter-ionic forces (i.e. forces of attraction between unlike ions and forces of repulsion
between like ions) decrease considerably, so that greater number of ions are able to
migrate to the electrodes. In addition, due to change in equilibrium, the electrolyte
undergoes further ionization from the same mass in solution (in order to balancethe effect). Hence, more ions (conducting species) are introduced into the solution
12.5. Molar conductivity at infinite dilution
Kohlrausch’s law of independent migration of ions states that “at infinite dilution,
where ionization of all electrolytes is complete and where all interionic effects
are absent, the molar conductivity of an electrolyte is the sum of the molarconductivities of its constituent ions at constant temperature”
According to the law, the molar conductivity of KCl at infinite dilution
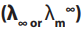 is
presented as:
is
presented as:

Some values of conductivity at infinite dilution
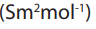
Example 1
The molar conductivity of chloride ion is What is the
What is the
molar conductivity of sodium ions given that the molar conductivity of NaCl is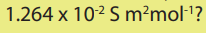
Answer:
According to Kohlrausch’s law,

Examples 2

Answer
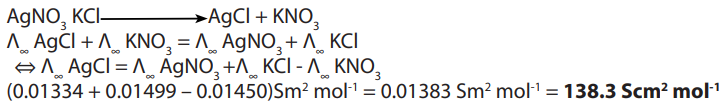
Example 3calculate the molar conductance of aqueous
 solution at infinite dilution
solution at infinite dilutionGiven
Checking up 12.4

12.6. Factors that affect molar conductivity of solutions
Activity 12.5.
Compare the conductivities of the following solutions and explain why they aredifferent

1. Temperature
The increase of temperature decreases inter-ionic attractions and increases kinetic
energy of ions and their speed. Thus,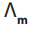 increases with temperature.
increases with temperature.
2. Concentration of the solution
The concentrated solutions of strong electrolytes have significant interionic
attractions which reduce the speed of ions and lower the value of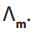 The dilution
The dilution
decreases such attractions and increases the value of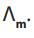
3. Nature of electrolyte
The strong electrolytes like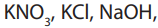 etc are completelyionized in aqueous
etc are completelyionized in aqueous
solution and have high values of molar conductivity. The weak electrolytes are ionized
to a lesser extent in aqueous solution and have lower values of molar conductivity.
Solvents of high dielectric constant yield more conducting solution. The viscosity is
inversely proportional to the conductance.
4. Ionic charge and size
Generally, the ions move at very low speeds. The velocities of hydrogen ions and
hydroxyl ions are relatively high. They contribute greatly to high conductivities ofaqueous solutions of strong acids and alkalis.
The differences in speeds of ions under similar conditions are as a result of their
difference in charge and size.
a. Ionic charge
Multiple charged ions get strongly attracted to the oppositely charged electrode.
This increases their speeds compared to singly charged ions.
b. Ionic size
Velocities of smaller ions are higher than those of larger ions of the same charge.
This is because larger ions meet many obstacles compared to small ones. However,
as ions exist in aqueous solution in a solvated form, the radius of the hydrated
ion is considerably larger than the crystal radius. Small ions get more hydrated
than larger ones due to high charge density. This reverses the expected order of
ionic velocities. Thus for group 1cations, the ionic radius increases in the order and the electric mobility increases in the same order
and the electric mobility increases in the same order  This is because of the effect of hydration.
This is because of the effect of hydration.
This explains why lithium ions have a lower molar ionic conductivity than potassium ions.
5. PressureThe molar conductance increases slightly with increase in pressure.
Checking up 12.5

Experiment
• Take two irish potatoes and wash them
• In each you have to fix the nail after you have to fix the irish potatoes on
the bench by using the glue
• Take a bulb (with two electrodes positive and negative)
• Fix also the bulb with connecting wires on the bench using also the glue
• Take the second extremity of each wire ( because the first is connected on
the bulb) and connect it on the nail fixed in the Irish potatoe• Observe the phenomenon that will happen.
12.7. Kohlrausch’s law of individual molar conductivity
Activity 12.6.
a. Given the following substances
Order those substances in their level of conductivityb. among the conductors how can you compare the conductivities

Example 1

Solution

12.7.2. Relation between molar conductivity, degree of ionization and
ionization constant
Activity 12.6.1
1. Define the following terms:
b. Degree of ionization
a. Ionization constant
2. Establish the relation of calculation of ionization constant
3. Explain why there is a relation between ionization constant and themolar conductivity
At infinite dilution, the electrolyte is completely ionized and all the ions take part
in conducting the current. At appreciable concentration, only a fraction of theelectrolyte is ionized and the degree of ionization of the electrolyte is given as

From the above equations, also the pH of the solution can be calculated.
For example, for a weak acid such as ethanoic acid,

Checking up 12.6

12.8. Use of conductivity measurement in titration and solubility product
Activity 12.7.
1. What do you understand by the term titration?
2. While titrating a solution of sodium hydroxide with hydrochloric acid,
explain how the concentration of ions change in the mixture.
3. What is solubility?
4. Define solubility product.
You have a glass of water. You add sugar or salt to dissolve. What will happen if
you continue to add sugar or salt? Can you explain?
1. What are the factors that influence solubility of a substance
2. Give an example of a substance which is insoluble or sparingly soluble
3. What is the relation between solubility of a substance and
concentration
4. Explain at which degree a sparingly soluble substance conductelectricity
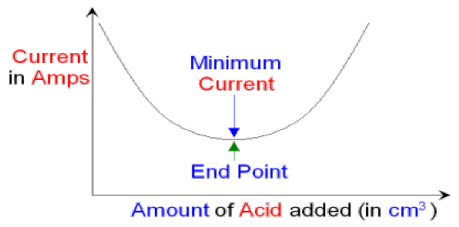
12.8.1. Using conductivity to find the end point of a titration
The end-point in titration experiment can be determined using conductivity. Theprocedure of the technique is:
At the start of this titration the conical flask contains a strong alkali that
is fully ionized in water. If electrodes are placed inside the conical flask
the ions in the water will conduct electricity and a current will flow.
The more ions there are the better the conductivity and the higher the current will
be. The current can be measured using an ammeter. As acid is added to
the alkali hydrogen ions and hydroxide ions react together to form water molecules.
The number of ions in the conical flask starts to decrease and the current flowing
through the solution will decrease. At neutralization all of the hydrogen
ions and hydroxide ions have reacted together to form water molecules.
The neutral solution contains only salt ions dissolved in water molecules. The
solution will still conduct electricity because of the salt ions but the current will be
at a minimum. As more acid is added the current will start to increase because there
will now be unreacted hydrogen ions in the solution as well as the salt ions. Thesolution is now no longer neutral but has become acidic.
If you draw a graph of current against the amount of acid added you can see wherethe minimum is. This is the end point of the titration at neutralization.
12.8.2. Determination of solubility product by conductivity measurement.
Solubility product, Ksp, is the mathematical product of its dissolved ion
concentrations raised to the power of their stoichiometric coefficients. Solubility
products are relevant when a sparingly soluble ionic compound releases ions
into solution. That is the product of the concentration of ions in the solution which
are in equilibrium with the solid ion. These concentrations can be determined viaconductivity measurements, consider the following examples :
The measurement of conductivity will depend on the value of Ksp for the sparingly
soluble substances.
The measurement of the specific conductivity, K of the saturated solution leads toa value of the concentration.


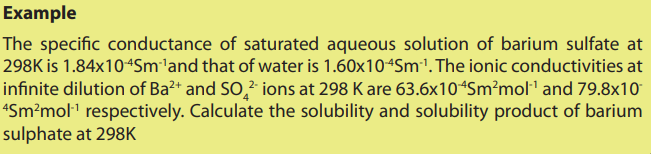

12.9. Difference between metallic conductivity and electrolytic conductivity
Activity. 12.8
Make an experiment by connecting a bulb to the batteries by using an electric
wire .After you have attempted that experience, compare the results seen
and the results you’re the introductory activity and answer to the following
questions.
a. What do you think are conductors of electricity in the two experiments
(separately)
b. Compare the reaction after 20 minutes, what is the difference betweenthe intensity of lights in the two experiments
The substances, which allow the passage of electric current, are called conductors.
The best metal conductors are such as copper, silver, tin, etc. On the other hand,
the substances, which do not allow the passage of electric current through them,
are called non-conductors or insulators. Some common examples of insulators are
rubber, wood, wax, etc.
The conductors are broadly classified into two types, Metallic and electrolyticconductors.
The electrolyte may, therefore, be defined as the substance whose aqueous solution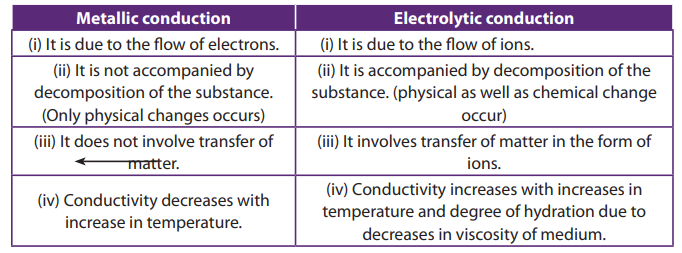
or fused state conducts electricity accompanied by chemical decomposition. The
conduction of current through electrolyte is due to the movement of ions. On the
contrary, substances, which in the form of their solutions or in their molten state donot conduct electricity, are called non-electrolytes.
Checking up 12.9
In the experiment, a student was investigating the intensity of light
In the beaker A where there was HCl solution the intensity of light was high
In beaker B where there was ethanol there was no light.
Using the plastic bag the was no light but using the copper wires there was the
intensity of light. Explain why the change in intensities of light in the aboveexperiment.




