UNIT 11: SOLUTIONS AND TITRATION
Key unit competency:
Be able to prepare standard solutions and use them to determine concentration ofother solutions by titration.
Learning objectives:
• Define the terms standard solution and primary standard solution.
• Explain the properties of a standard primary solution.
• Explain the titration process, emphasising the need for precise measurements.
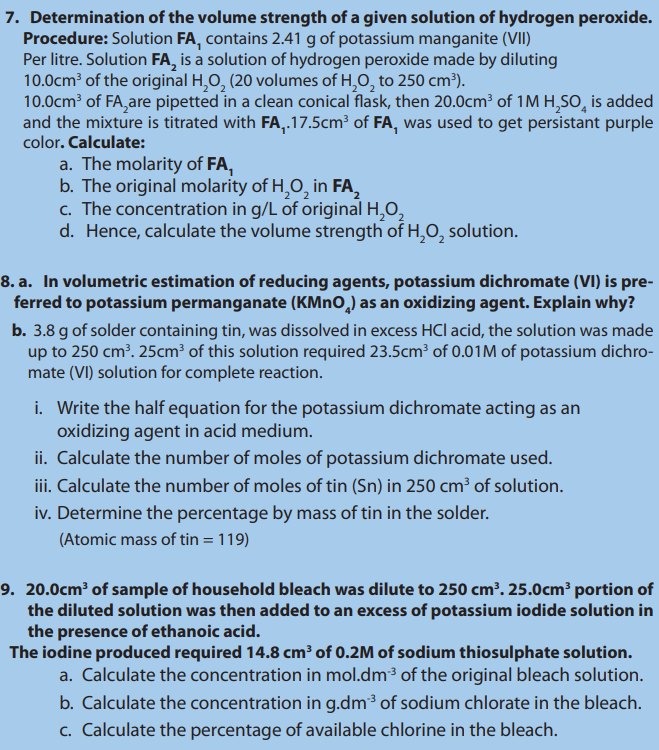
• Prepare solutions with different concentrations.
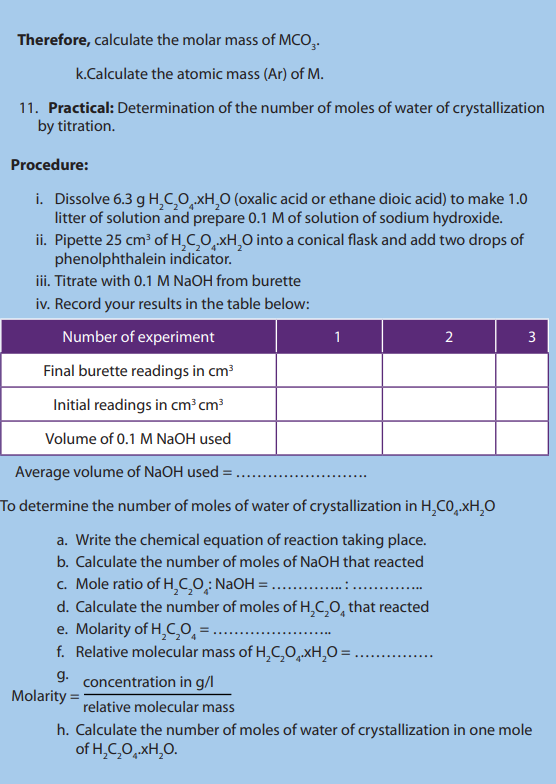
• Properly use the burettes, pipettes during titration.
• Interpret the experimental data obtained by titration and report.
• Carry out acid-base, redox titrations and do calculations involved.
• Develop a team approach and a sense of responsibility in performing the
experiments of titration.
• Respect of procedure in practical experiment.
• Develop a culture of orderliness in performing practical experiments.• Appreciate the use of appropriate measurements in daily life.
Introductory Activity

Observe the above photo and attempt the following questions:
1. For the bottle of PRIMUS, and that of MUTZIG, we find on the bottle of its
labels 5% alcohol and 5.5% alcohol respectively.
a. Explain the meaning of 5% and 5.5% alcohol.
b. 5% alcohol corresponds to which volume of alcohol of the total
volume of 72cl of the bottle of PRIMUS.
c. Calculate the volume of alcohol corresponding to 5.5% alcohol of the
bottle of MUTZIG of the total volume of 65cl.
2 . On the label of the bottle of AGASHYA JUICE we find that dilution is 1:5.Explain this ratio and explain also the purpose of diluting substances.
11.1. Definition of standard solution and primary standard solution.
Activity 11.1
1. You have a bag full of rice. How do you determine its weigth?
2. You have a jerrycan half-full of a liquid substance, how are you going to
proceed to know the exact quantity of the liquid?
3. You are given a basic solution, NaOH (aq), and you are requested todetermine its concentration. What do you need to do that?
In analytical chemistry, a standard solution is a solution containing a precisely known
concentration of an element or a substance and used to determine the unknown
concentration of other solutions. A known weight of solute is dissolved to make a
specific volume. It is prepared using a standard substance, such as a primary standard.
A primary standard is defined as a substance or compound used to prepare standard
solutions by actually weighing a known mass, dissolving it, and diluting to a definitevolume.
Or a substance, which is chemically stable in aqueous solution and its concentration
remains constant with change in time such that it can be used to standardize other
solutions.
Some important examples of primary standard are;

Standard solutions are normally used in titrations to determine unknown concentrationof another substance.
Checking up 11.1Differentiate between standard solution and primary standard solution
11.2. Properties of a primary standard solution.
Activity 11.2
Do research and find out the role and characteristics of a good primary standard.
A good primary standard meets the following criteria:
• High level of purity
• High stability
• Be readily soluble in water
• High equivalent weight (to reduce error from mass measurements)
• Not hygroscopic (to reduce changes in mass in humid versus dry environments)
• Non-toxic
• Inexpensive and readily available
• React instantaneously, stoichiometrically and irreversibly with other substances
i.e. should not have interfering products during titration.
• It should not get affected by carbon dioxide in air
Molar concentrations are the most useful in chemical reaction calculations because
they directly relate the moles of solute to the volume of solution. The formula formolarity is:
Checking up 11.2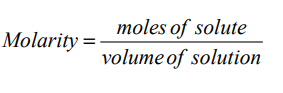
Discuss the properties of a good primary standard
11.3. Preparation of standard solutions
Activity 11.3
Describe how you would prepare 1L of a 1M solution of sodium chloride. The gramformula weight of sodium chloride is 58.5g/mol
The preparation of the solution requires a reagent that is so to say the quantity to be
weighed for mass if the reagent is in solid state ; The volume to be pipetted using
pipette if the solute is a liquid and then after dissolve it in water, So the solution can beprepared by two methods such as dissolution method and dilution method.
In the preparation of solution, glasses, volumetric flask, pipette, glass rod, measuring
cylinder, analytical balance, spatula, beakers, magnetic stirrer and other laboratorydevices are used.
11.3.1. Preparation of standard solution by dissolution of solids
Activity 11.3.1
Preparation of 250 mL of 1M NaOH solution
Materials
• Beaker - balance - sodium hydroxide solid
• Spatula - funnel - glass rod• Volumetric flask - stopper - distilled water
Procedure:
i. Calculate the mass of sodium hydroxide needed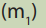
ii. Weigh a clean beaker, and record its mass with a clean spatula, add
a clean spatula, add
sodium hydroxide until the combined mass of weighing beaker and
sodium hydroxide is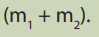
iii. Add about 50 cm3of distilled water, stir with a glass rod until all solids
have dissolved.
iv. Pour all the solution carefully through a funnel into a volumetric flask;
wash all the solution out of the beaker and off the glass rod.
v. Add distilled water until the level is about below the graduation
below the graduation
mark on the graduated flask. Add the rest of distilled water from a washing
bottle until the bottom of the meniscus is at the level of mark when viewed
at eye level.
vi. Insert the stopper of the flask and invert the flask several times to mix the
solution.
vii. Label the solution.
Scope: This method is applied for solute in solid state and you should be able to
determine the mass required from calculation to be weighed and provide distilledwater to dissolve the solute.
Examples:1. Describe in details how you can prepare the following solution: 50 mL of NaOH, 10%.
Answer:
10% means that in 100 mL of solution only 10 g are pure in NaOH, So 50 mL of NaOH
will be prepared by taking the mass of NaOH, dissolving it in water and madding up to50 ml.
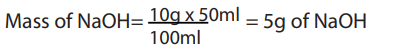
Procedure:
• Weigh 5g of NaOH accurately using glass watch, spatula and analytical balance.
• Dissolve it in a volumetric flask of 50 mL containing already little water and mix
using a baguette and shake till you get homogeneous mixture (you should take
care since it is an exothermic reaction).
• Top up using distilled water and shake again and cover your solution.• Label your solution: 10% NaOH; 50 mL and the date of preparation.
2. Describe in details how you can prepare
 solution.
solution.Answer:
Step 1: CalculationsCalculate the amount of anhydrous sodium carbonate required to be dissolved in
 of solution. i.e.:
of solution. i.e.:
Step 2: Weighing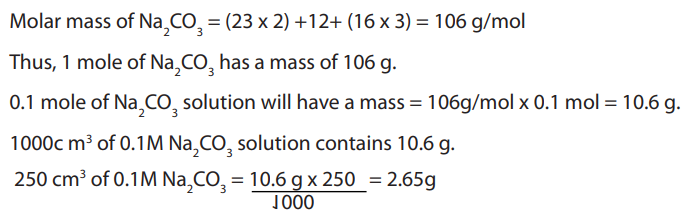
• Weigh a clean empty beaker and record its mass,
• Using a clean spatula add the mass of pure anhydrous sodium carbonate equal
to the calculated mass. Let it be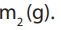
• Actual mass of the carbonate transferred into the beaker =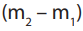 g
g
Note: Not the entire sample gets transferred into the beaker since part of it sticks onthe walls of the weighing bottle.
Step 3: Procedure
• Using a wash bottle, carefully add about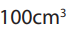 of distilled water. Stir using a
of distilled water. Stir using a
glass rod until the entire solid has dissolved.
• Carefully pour all the solution through a filter funnel into a volumetric flask. Wash
off all the solution out of the beaker and off the glass rod; ensure that all the
washings run into the volumetric flask.
• Add distilled water until the level of the volumetric is about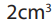
below the mark. Add the rest of the distilled water drop by drop using
mark. Add the rest of the distilled water drop by drop using
a dropping pipette until the bottom of the meniscus is level with the 250cm3
mark when viewed at eye level.
• Insert the stopper of the flask and invert the flask several times to mix the solution.
Note: Since the concentration of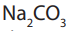 solution has been determined, thus the
solution has been determined, thus the solution prepared is a standard solution.
Checking up 11.3.1
Describe in details, how you can prepare the following solutions:
a.
b. 250 mL acidified potassium dichromate 1Nc. 100 mL oxalic acid 5g/L.
11.3.2. Preparation of standard solution by dilution
Activity 11.3.2
Materials
• Measuring cylinder
• Volumetric flask
• Stopper
• Concentrated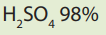
• Distilled water
Notice: Take care when mixing water and acid. When you mix acid with water, it is
extremely important to add the acid to the water rather than the other way around.
It is because acid and water react in a vigorous exothermic reaction, releasing heat,
sometimes boiling the liquid. If you add acid to water, the water is unlikely to splash
up, but even if it did, it is less likely to hurt you than if you add water to acid. Whenwater is added to acid, the water boils and the acid may splatter and splash.
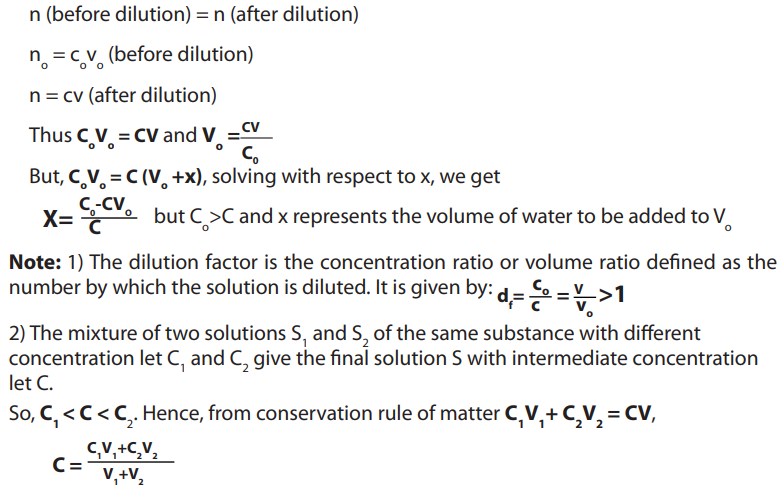
Apart from, dissolution method, we can prepare a solution by dilution from the stock
solution. This consists of reducing the concentration of a concentrated stock solution
to less concentrated solution by adding water.
The rationale is how we can prepare the desired solution of concentration C, with the
volume V, from the stock solution whose concentration is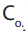 .The question consists of
.The question consists of
determination of the volume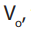 to be pipetted from stock solution and diluted using .
to be pipetted from stock solution and diluted using .
Xml of water to get V. So, from the conservation principle of matter, the quantity of thesolute before and after dilution should be the same.

Procedure: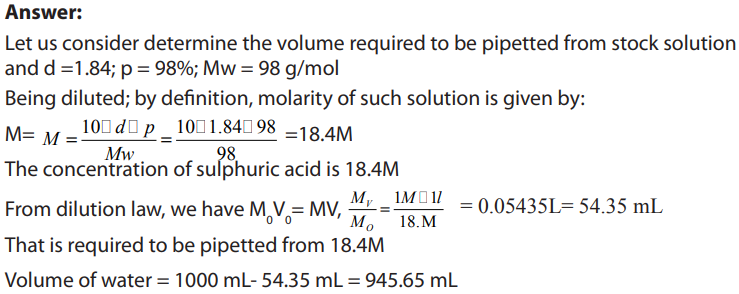
• Pipette only 54.35 ml of sulphuric acid accurately using a pipette from a stock
solution.
• Pour them gently in the flatted balloon of 1L containing already little water and
shake. Note that you should take much care since the reaction is exothermic
and sulphuric acid is harmful to skin remember that we pour acid to water
not water to acid that is A-W not W-A.
• Top up to 1L using distilled water and shake again in order to homogenize the
solution.
• Cover the solution, then label it: and date of preparation.
and date of preparation.2. Calculate the volume of
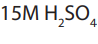 that would be required to prepare
that would be required to prepare 

Checking up 11.3.2
A 0.2N solution was diluted by addition of 200 mL of water.
Calculate the dilution factor if the solution is diluted from 0.2N to 0.05N.Determine the final volume of the solution after dilution.
11.4. Simple acid-base titrations
Activity 11.4
You are provided with

Materials
- Burette - Indicator (phenolphthalein)
- Measuring cylinder - Washing bottle
- Conical flask - Beakers- Retort stand - Funnel
Procedure
a. Using a pipette, transfer 10 mL of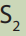 into a conical flask.
into a conical flask.
b. Add three drops of phenolphthalein indicator and titrate it with
from the burette.
c. Repeat the titration until you obtain consistent values.d. Record your readings in the table below:
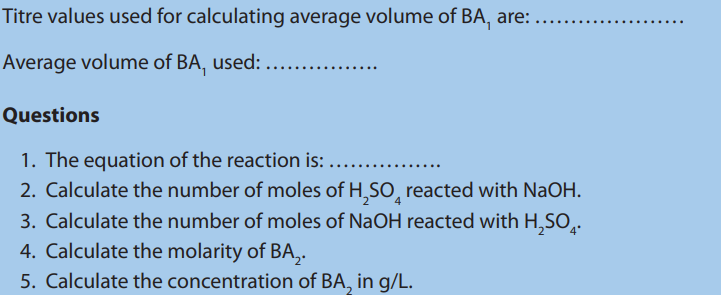
a. Calculate the average volume of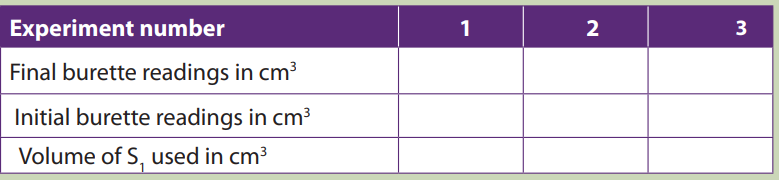
 used.
used.
b. Calculate the number of moles of HCl that react with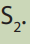
c. Calculate the molarity of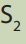 in 10 mL.
in 10 mL.
Titration is the controlled addition and measurement of the amount of a solution
of known concentration required to react completely with a measured amount of asolution of unknown concentration.
Acid-base titration
It is the determination of the concentration of an acid or base by exactly neutralizingthe acid or base of known concentration
Alkalimetry and acidimetry
• Alkalimetry is the specialized analytic use of acid-base titration to determine
the concentration of a basic substance.
• Acidimetry is the same concept of specialized analytic use of acid-base titrationto determine the concentration of an acidic substance.
The point at which the two solutions used in a titration are present in chemically
Equivalence point
equivalent amount is the equivalence point. At this point the moles of two solutionswill be equal.
Indicators and pH-meters can be used to determine the equivalence point. The
point in a titration at which an indicator changes color is called the end-point of thetitration.
Equipment's and set up (Figure 11.1) of materials for Titration
The common equipment used in a titration are:
• Burette
• Pipette
• pH-indicator/acid-base indicator
• White tile: used to see a color change in the solution(a white paper can also be
used)
• Conical flask (Erlenmeyer flask)
• Titrant: a standard solution of known concentration• Analyte: a solution of unknown concentration

How to perform titrations
Knowing the use of pipette and burettes and how to handle them, the following pointsare useful in order for a correct titration to be done:
1. The apparatus should be arranged as shown in the above Figure.
2. The burette tap is opened with the left hand and the right hand is used to
shake the conical flask.
3. The equivalence-point is reached when the indicator just changes permanently
the colour.
4. At the end point, the level of the titrant is read on the burette
5. The titration is now repeated, three more times are recommended. Towards
the end-point, the titrant is added dropwise to avoid overshooting.
Notice: Before titration, check if the tip of the burette is filled with the titrant, and doesn’t
contain bulb of air. If there is a bulb of air, a quick opening and closing of the tap will expelthe air out of the burette.
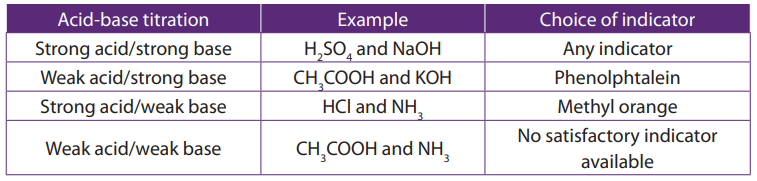
Choice of indicators in acid-base titrations
When the technique of acid-base titration is extended to a wide variety of acidic and
alkaline solution, care needs to be taken about the choice of indicator for any givenreaction.
The choice of an inappropriate indicator would lead to incorrect results, and it is
therefore extremely important that the indicator is chosen carefully.
The principle on which a choice of indicator is made concerns the strength of the acid
or base involved in the reaction. Note that the strength of an acid or base is not to be
confused with the concentration of its solution. Example of strong and weak acids andbases and choice of indicator are given in the Table below.
Table 11.1. Examples of strong/weak acids and bases
Indicators which are suitable for particular types of acid-base titrations are given in
the Table 11.2.
Table 11.2. Examples of indicators
The results are summarized in a table as shown below.
Table 11.3. Sample results
Notice: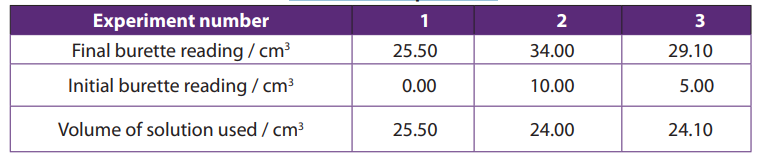
• Burette readings should be written to two decimal places (for burette havingprecision up to hundredth)
Average title should be obtained using values which differ not by more
. Consistent values :24.00 and 24.10
Average volume
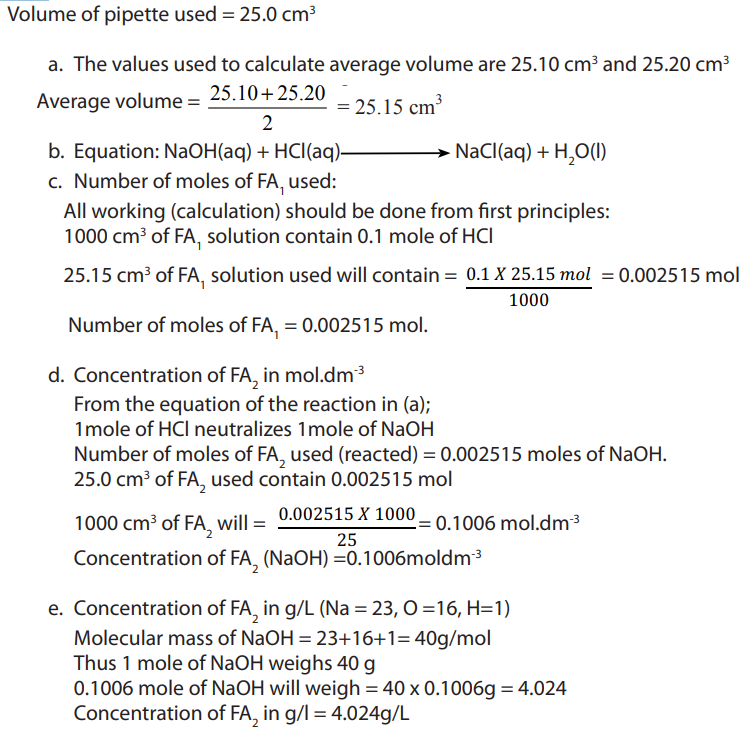
Examples: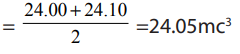
1) Suppose 20.00 mL of NaOH is required to reach the end-point in the
NaOH is required to reach the end-point in the
titration of 10.0 mL of HCl of unknown concentration. How can these titration databe used to determine the molarity of the acidic solution.
Answer:
Begin with the balanced neutralization reaction equation. From the equation,determine the chemically equivalent amount of HCl and NaOH.

Calculating the number of moles of NaOH used in the titration;
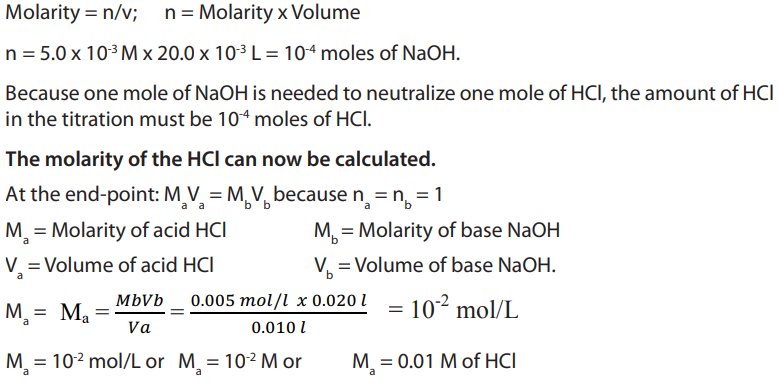

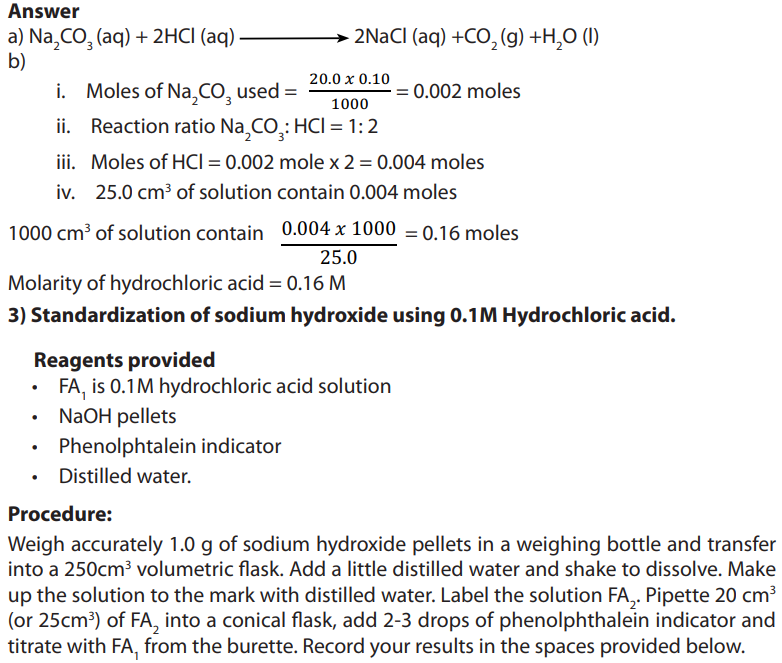
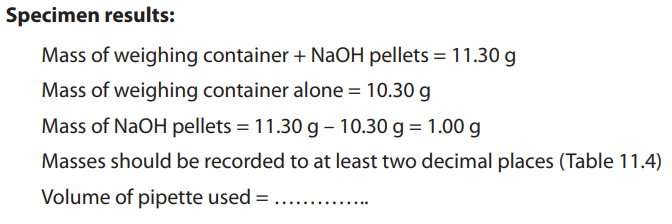
Table 11.4. Burette readings
Each value or entry in the table must be recorded or written to two decimal places.
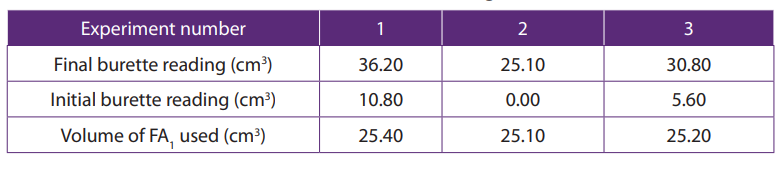
Different initial readings should be used. Initial reading in each experiment should becorrectly subtracted from the final reading.
Questions:

Answer:


11.5. Titration involving redox reactions
Activity 11.5
1. What does differentiate redox reactions from other reactions?2. Balance the following redox equations using half-equation method:
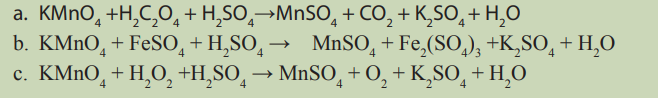
11.5.1. Titrations with potassium manganate (VII);
Potassium manganate (VII),
 is a useful oxidizing agent in a sufficiently acidic
is a useful oxidizing agent in a sufficiently acidic
medium normally used in the reactions such as:
• Oxidation of iron (II) to iron III
• Oxidation of ethanedioate (oxalates) or oxalic acid to carbon dioxide.
• Oxidation of nitrites to nitrates
• Oxidation of hydrogen peroxide to oxygen, etc.
During the reactions, the manganate (VII) ions, which is purple, is reduced to
which is purple, is reduced to
manganese(II), which is pale pink but practically colorless.
which is pale pink but practically colorless.
Thus, titrations involving potassium manganate (VII), do not require an indicator.
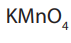 acts as its own indicator.
acts as its own indicator.
The end-point of titration is detected when the solution shows a permanent faint pink
color, which is as a result of slightly excess of potassium manganate (VII).
For reduction of the potassium manganate (VII) to be complete, sufficient acid should
be used and the solution added slowly, not rapidly. Use of insufficient acid results in
precipitation of manganese (IV) oxide, which appears as a brown precipitate.
which appears as a brown precipitate.
The suitable acid for this process is sulphuric acid. Hydrochloric acid is unsuitable
because the ion is a stronger oxidizing agent than
ion is a stronger oxidizing agent than 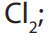 it oxidizes the chloride
it oxidizes the chloride ion in the acid to molecular chlorine.
Nitric acid is also not used to acidify the solution of potassium manganate (VII) since it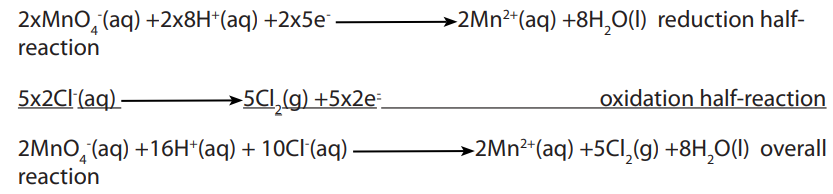
is also a powerful oxidizing agent. In potassium manganate (VII) titrations, an indicator
is used only when the reducing agent forms a colorless solution which makes it difficult
to observe the pink color.
11.5.1. Titration of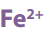 by Potassium manganate (VII),
by Potassium manganate (VII),
Ativity 11.5.1Balance the following redox equations using half-equations method:
Most redox titration labs utilize an iron (II) salt, frequently iron (II) sulphate
heptahydrate. This compound is used for two reasons. First, it has a large molar mass,
making it a good (but not the best) primary standard. Second, its solution is near
colorless, so the pink/purple endpoint is easy to detect. Color proves to be an issue
when dealing with many other compounds. Iron (II) salts are an excellent choice for atitration utilizing potassium permanganate.

Answer:
Write balanced equation:

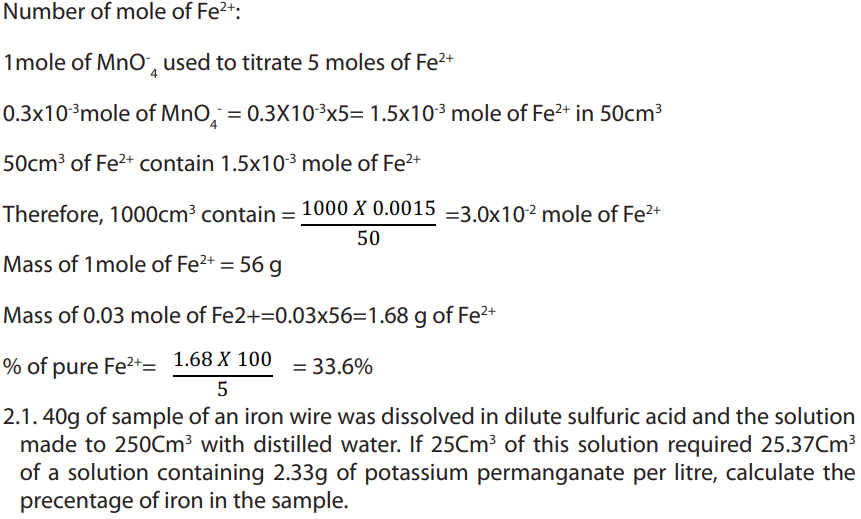
Answer
Iron dissolves in dilute sulphuric acid by the reaction equation:
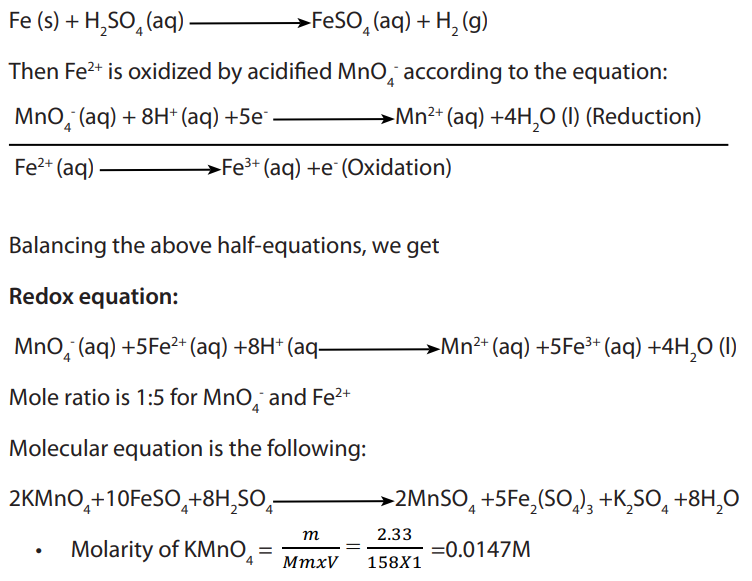


11.5.2. Titration of oxalic acid or oxalates by potassium manganate (VII)
Answer
The reducing agent is the oxalate ion,
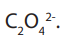 Oxalic acid is a white crystalline
Oxalic acid is a white crystalline solid of formula
The reaction between the oxalate ion and manganate (VII) ion is kinetically slow at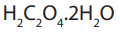
temperature below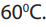 Titrations at room temperature take too long time for the
Titrations at room temperature take too long time for the
purple color of the manganate (VII) ion to disappear. In the oxalate ion, carbon has the
oxidation state of +3; when reacted with potassium permanganate the oxidation state
changes into +4. Titrations involving the oxalates are a little more involved, but the
results were very good. The reaction below describes the overall redox reaction:
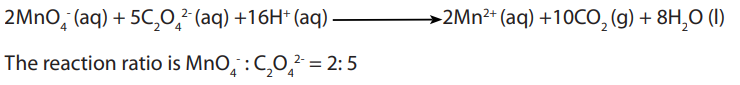

Answer:Overall equation is:
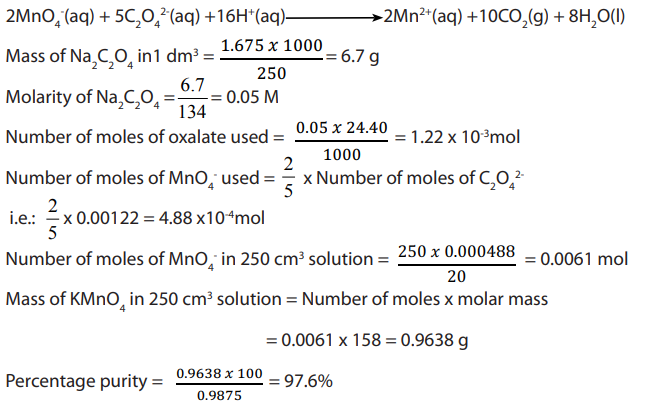
Procedure
i. A fixed mass 2.4 g of calcite is weighed and dissolved in dilute hydrochloric acid
ii. To this solution is then added ammonia solution until the solution is alkaline
and this is followed by addition of ammonium ethanedioate (oxalate) toprecipitate all calcium ions as calcium oxalate
iii. The precipitate is then filtered off, washed and then dissolved in a minimum
dilute sulphuric acid solution and then solution made up to in a
in a
volumetric flask with distilled water.
iv.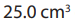 of the resultant solution in (iii) is pipetted and to it is added 20 cm3
of the resultant solution in (iii) is pipetted and to it is added 20 cm3
of diluted sulphuric acid and the mixture is heated to about 70 °C.v. The hot solution is then titrated with 0.02M potassium permanganate solution
Results: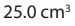 of the oxalate solution required of 0.02M
of the oxalate solution required of 0.02M 

solution for complete reaction which is detected by the intense purple colour.
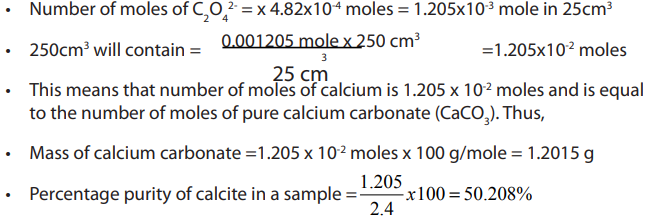
a. Write down the overall equation for the above sequence of analysisb. Calculate the percentage of purity of calcite
Answer:

Checking up 11.5.2

11.5.3. Titration of hydrogen peroxide by potassium manganate (VII),

Activity 11.5.3
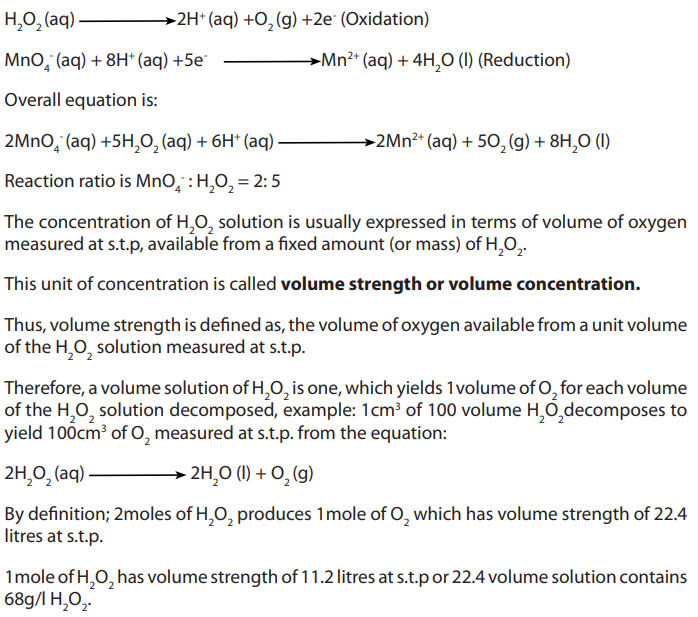
Hydrogen peroxide is a powerful reducing agent in the presence of a strong oxidizing
agent such as potassium permanganate. For this reason dilute concentrations of both
compounds must be used. A common laboratory experimentally determines the
concentration of commercially sold hydrogen peroxide (about 3%) via titration with
potassium permanganate (usually prepared to be approximately 0.02 M). Concentratedsolutions of these reactants will react explosively and must be avoided.
Example:


Answer
Write the balanced equation
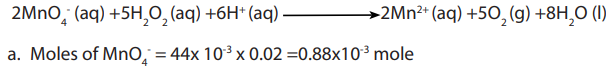
Note: The advantages of potassium permanganate as oxidizing agent are:
i. It acts as its own indicator (i.e. purple color)
ii. The crystals are obtained at high state of purity
iii. The crystals are anhydrous and not deliquescent
iv. It has a fairly high relative molecular mass
v. A wide range of substances can be oxidized by it.
However, potassium permanganate has disadvantages:
i. The crystals are not very soluble in water
ii. The compound is decomposed by the light
iii. The compound is reduced by water and organic matter from atmosphereiv. The meniscus of the solution may be difficult to see
Checking up 11.5.3

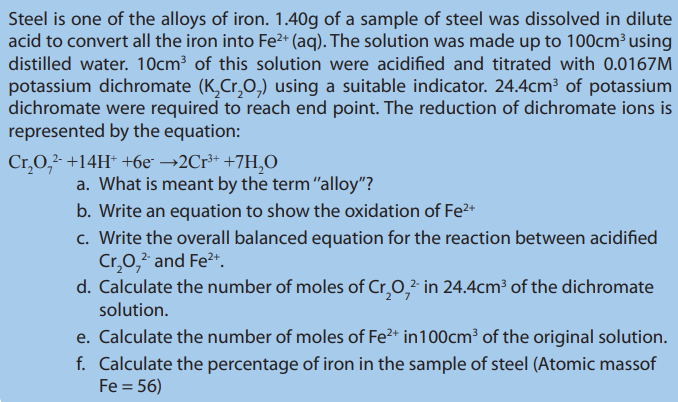
11.5.4. Titration with potassium dichromate

Activity 11.5.4
Discuss the advantages and disadvantages of using
Potassium dichromate (VI) unlike potassium manganate (VII) is a primary standard. In as an oxidizing agent in titration.
as an oxidizing agent in titration.
the titration of potassium dichromate (VI), the color change is from orange to green
and this makes it not possible to detect the sharp end-point.
Thus, an indicator is necessary for this reaction. Therefore redox indicator such as
barium diphenylamine sulphonate must be used, which changes its colour to blue atthe end point.
Potassium dichromate (VI) is a weaker oxidizing agent than potassium manganate (VII).
As such it cannot oxidize the chloride ion to chlorine, and therefore hydrochloric acid
in addition to sulphuric acid can be used to acidify solution of potassium dichromate(VI).
The reactions of potassium dichromate (VI) are similar to those of potassium manganate
(VII), however at the end-point the color is green due to the presence of chromium(III) ions that is;

a. Determination of the concentration of iodide ions.
Since iodide ions can be oxidized to molecular iodine by dichromate (VI) ion,potassium dichromate (VI) can be used as a primary standard in this reaction.
The amount of iodine liberated in the reaction is determined by titrating it against a
standard solution of sodium thiosulphate using starch indicator.
b. Analysis of iron (II) salts
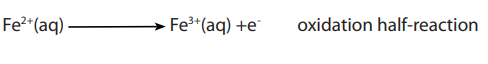
Overall equation is:

c. Analysis of nitrite salts
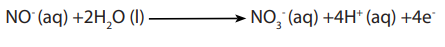
Advantages of
Unlike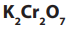 over
over 
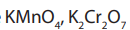 is used as a primary standard i.e. it can always be obtained pure;
is used as a primary standard i.e. it can always be obtained pure;
it is stable under ordinary conditions, its aqueous solution can be stored for long timeif sufficiently protected from evaporation.
Examples 2 :

Answer:
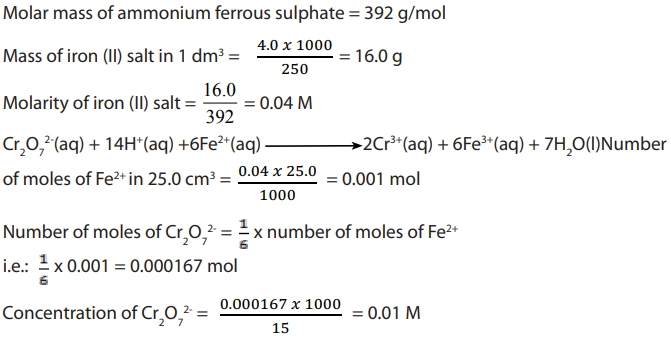
Examples 2:

Answer:
Write balanced equation
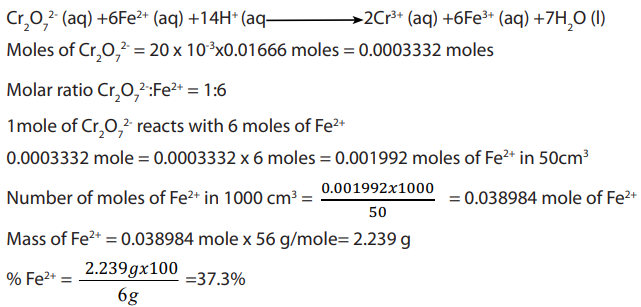
Checking up 11.5.4
11.5.5. Titrations involving sodium thiosulphate and iodine
Activity 11.5.5
Sodium thiosulphate is a white crystalline solid with the formula
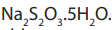
It is not a primary standard as the water content of the crystals is variable.
The solid acts as a reducing agent in a redox reaction and the reducing agent being thethiosulphate ion,

However, the thiosulphate ion is very sensitive to acid (weak or strong). In presence
of an acid (hydrogen ions), it decomposes to sulphur dioxide and elemental sulphur(which settles as a yellow precipitate).
Therefore, solutions of sodium thiosulphate should never be acidified.
The thiosulphate ions reduce iodine according to the reaction below:

Overall equation is:

a. Detection of the end point:
In titrations involving iodine and sodium thiosulphate, starch is normally used as anindicator.
Addition of the starch produces iodine-starch complex which is blue. Further drop
by drop addition of the thiosulphate solution is continued until the solution turns
colorless which marks the end point of titration.
Thiosulphate titration has important applications in the laboratory and water
treatment plants.
Potassium iodate (V), potassium dichromate (VI), iodine, potassium manganate (VII)
solutions can be used to standardize sodium thiosulphate solutions.
For example, potassium iodate (V) reacts with iodide ions in presence of an acid toliberate iodine.
The liberated iodine is then titrated against standard sodium thiosulphate using the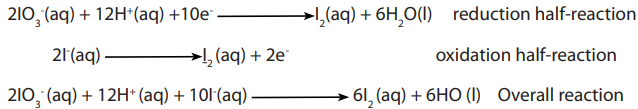
starch indicator.

b. Preparation of starch solution:
0.5g of soluble starch powder is mixed with water into a thin smooth paste which is
then poured into of boiling water. The mixture is then boiled after for about a
of boiling water. The mixture is then boiled after for about a
minute. The solution is then cooled and used when still fresh.But starch solution may be preserved by addition of few crystals of mercuric iodide.
Example 1 :

Answer:
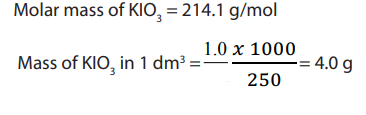
Example 2 :
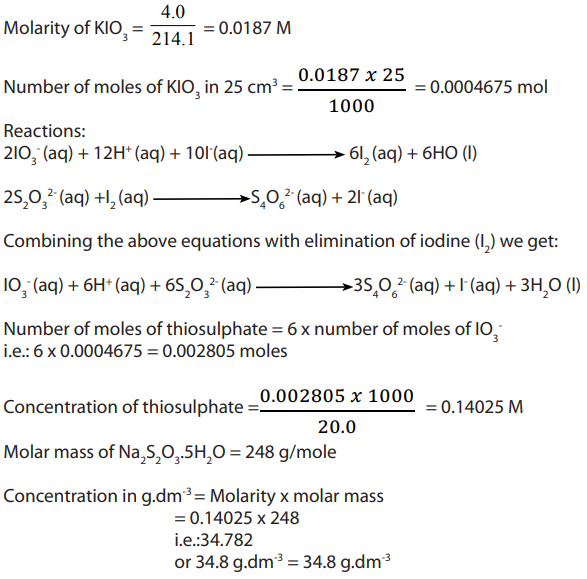
A weighed sample of impure solid potassium iodate (V) of 0.80g is dissolved in
water and made up to of solution in a volumetric flask.
of solution in a volumetric flask.  of this
of this
solution is reacted with excess potassium iodide solution, to liberate iodine.
Find the percentage of the purity of potassium iodate (V). In the titration, sodium thiosulphate were needed to react with the liberated
sodium thiosulphate were needed to react with the liberated iodine.
Answer:
Write balanced equations:


c. Finding the percentage of copper in copper ore or in its salt
There are two possible methods depending on the copper ore (or salts).
a. In the first method which is applicable to any copper salt, a solution of excess
potassium iodide is added to a solution of the copper salt. A dark brown mixture
is produced (white precipitate stained brown). The brown colour is due to iodineliberated.
The iodine liberated in the reaction is now titrated with a standard solution of sodium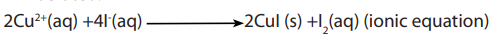
thiosulphate in the second reaction according to the following equation.
b. The second applicable method is to liberate iodine from iodate (V) ions, then titrate
it with thiosulphate ions as indicated by the following equations:
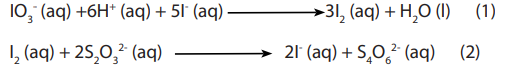
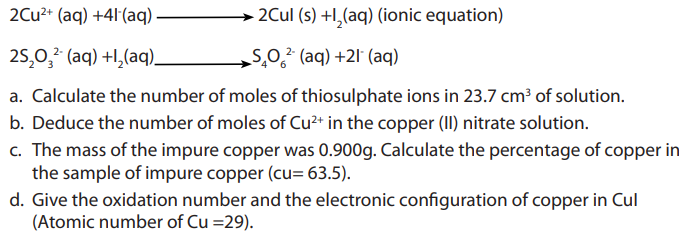
Examples:
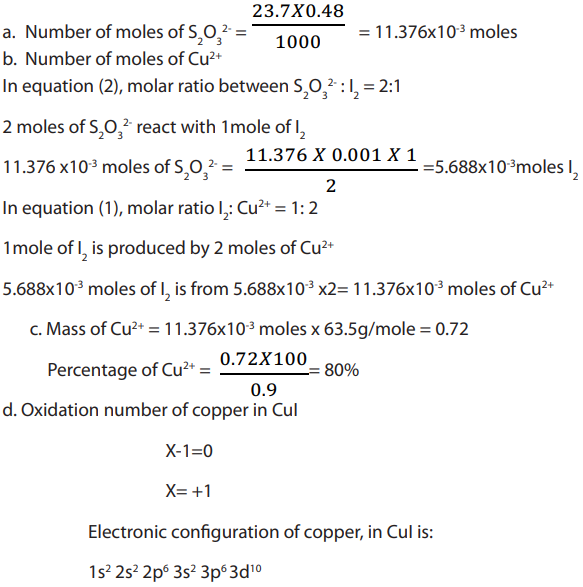
An experiment was carried out in a laboratory to determine the percentage of copper
in sample of impure copper metal. Nitric acid was added to a sample of impure
copper metal. The resulting copper (II) nitrate was reacted with excess of potassium
iodide. The iodine liberated was titrated with a solution of sodium thiosulphate of
concentration 0.480M. The volume of sodium thiosulphate required was
Use the following equations in your calculations.
Answer:
d. Determination of the percentage of available chlorine in a liquid
The method consists of measuring a fixed volume of the original liquid and then dilute
it to with distilled water. Aliquot portions of this solution is pipetted and added
with distilled water. Aliquot portions of this solution is pipetted and added to an excess of KI solution acidified with ethanoic acid.
The iodine liberated is then titrated, for example, with a standard solution of sodium
thiosulphate. This is a displacement (redox) reaction in which, aqueous potassium
iodide reacts with chlorine to form potassium chloride and iodine. The equation forthe reaction is:

Examples:
1. Concentration of chlorine in treated water for domestic use can be monitored by
testing water samples. In one such test, excess potassium iodide (KI) was added to
 sample of water.
sample of water.
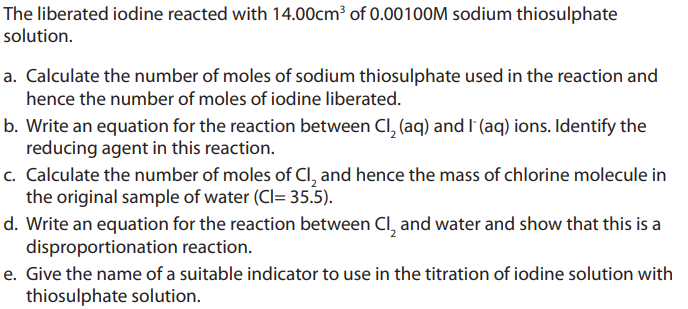
Answer:
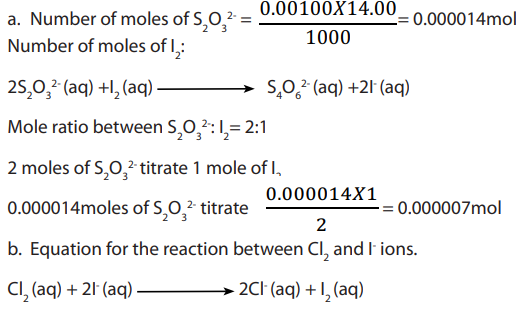
Reducing agent is I-(iodide ions)
c. Equations:
In the above reaction, chlorine underwent both oxidation and reduction, hence it is a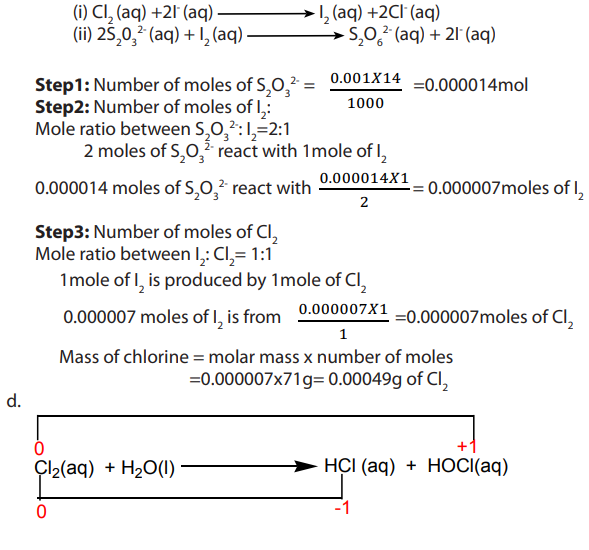
disproportionation reaction.
e. Starch solution, which changes colour from white to blue black.
Determination of the percentage of available chlorine in bleaching powder.
Procedure:
Solution was made by weighing 2.5g of bleaching powder. This is stirred in a little
was made by weighing 2.5g of bleaching powder. This is stirred in a little
water and the supernatant milk suspension poured into volumetric flask.
volumetric flask.
The solid residue is then mixed again with little water and the fine suspension decanted
into the volumetric flask.
This process is continued until most of the sample has been transferred.The solution is then made up to
The flask is then shaken vigorously and immediately mark.
mark. 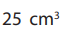
of the suspension in pipetted into a clean conical flask.
of 2M sulphulic acid or 2M ethanoic acid is added followed by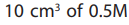
of 0.5M potassium iodide. The liberated iodine is then titrated with solution FA2which is 0.1M sodium thiosulphate solutions using starch indicator.
Specimen results:
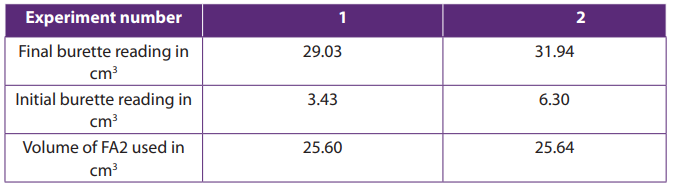
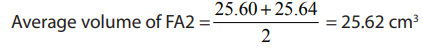
Calculations:
b. Calculate the number of moles of available chlorine in
c. Calculate the mass of the available chlorine in the sample of bleaching
powder weighed.d. Hence calculate the percentage of available chlorine bleaching powder.
Theory:
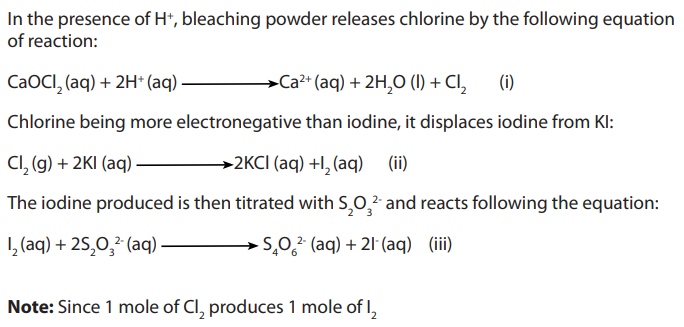
Number of moles of iodine is equal to the amount of chlorine set free.
Answers:
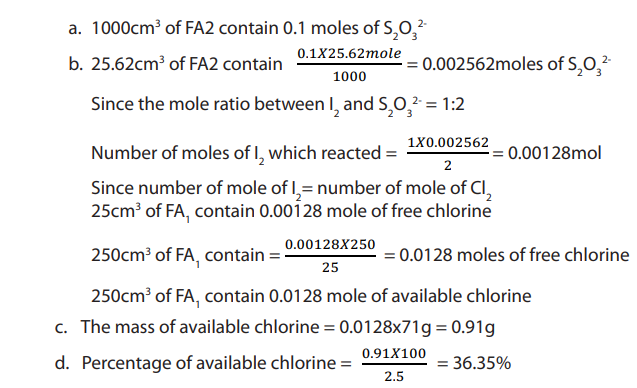
Checking up 11.5.5


11.6. Back titration
Activity 11.6
Do research and explain the term back titration and discuss the applications of backtitration.
This is a technique of determining the concentration of an unknown substance by
calculating backwards. In this case a known amount of a titratable reagent (substance)
X in excess amount is reacted with an unknown amount of substance Y to give products
and at the end of the reaction, the excess of X is titrated with a standard solution ofsubstance Z and this allows to determine the amount of Y that has reacted.
Applications of back titration:
1. Analysis of calcium carbonate materials (Examples: limestone, marble……..)
2. Determination of percentage of ammonia in an ammonium salt.3. Determination of concentration of chromates and iodides in redox titration.
Example 1 :

Answer:
Step 1: Write balanced equations

Step 2: Calculate initial number of moles of HCl

Step 3: Calculate number of HCl in excess solution
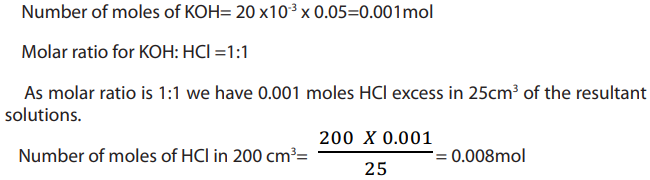
Step 4: Moles of HCl which reacts = 0.024- 0.008= 0.016mol
Initial moles of reactants = 0.024molNumber of moles in excess= 0.008mol
Step 5: Number of moles of HCl which react with


Example 2:
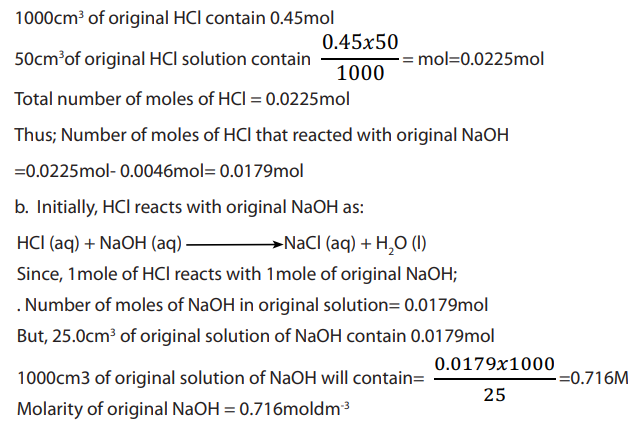
50cm3 of 0.45M hydrochloric acid solution were added to
of sodium hydroxide solution and the resultant solution on titration required
of 0.2M sodium hydroxide solution for complete reaction. Calculate the:
a. Number of moles of hydrochloric acid that reacted with original sodium
hydroxide solution.b. Molarity of the original sodium hydroxide solution.
Answer:

Total number of moles of HCl:
Example 3:

Answer:
a. Equation:

b.
 of the resultant solution contain 0.00478mole of NaOH
of the resultant solution contain 0.00478mole of NaOH 
c. NaOH reacts with
 Cl as shown below:
Cl as shown below:
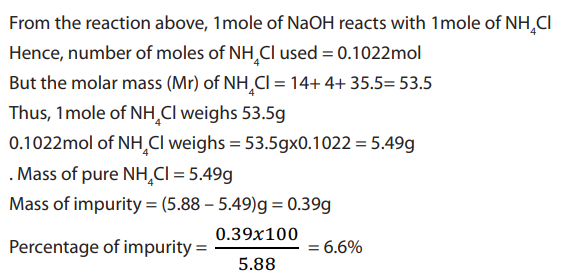
d. From the reaction in (c) above

Checking up 11.6

11.7. Applications of titration
11.7.1. Determination of the number of moles of water of crystallization
Activity 11.7.1
Do research and explain the term water of crystallization
Water of crystallization is defined as that definite amount of water
which a substance associate with on crystallizing out of an aqueous solution.
Many crystals cannot form without the presence of water i.e. water of hydration.
Examples of some hydrated substances
a. Weighing method
This method is preferably used when the crystals are neutral, and they cannot reactwith acid or basic solution.
Procedure:
• Weigh the mass of a clean dry crucible with lid.
• Two or three grams of hydrated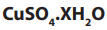 are added and the whole set up is
are added and the whole set up is
weighed.
• The crucible, lid and the content are heated on a pipe-clay triangle on a tripod
stand, gently at first and later strongly, to drive off water of crystallization.
• The set up is allowed to cool in a dessicator (to exclude moisture) and
reweighed.
• Heat again the crucible, lid and content and then cool as before and weigh
again.
• Repeat the process of heating, cooling and reweighing until a constant mass is
reached which shows that all water has been expelled.
• Using calculations, it is possible to get x moles of water of crystallization foundin one mole of substance. See the following example:
Example:
4.99g of hydrated copper (II) sulphate,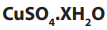 was strongly heated until
was strongly heated until
all water of crystallization was eliminated. The mass of anhydrous copper
sulphate was found to be 3.19g. Calculate the number of moles of water of
crystallization contained in one mole of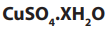 (Cu = 63.5, S = 32, O
(Cu = 63.5, S = 32, O =16, H = 1)
Answer:
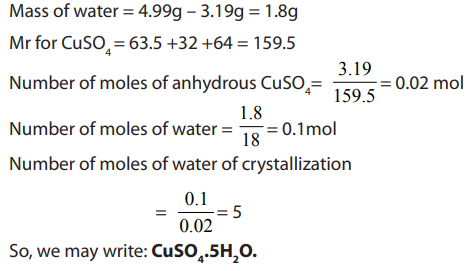
b. Using titration

Answer
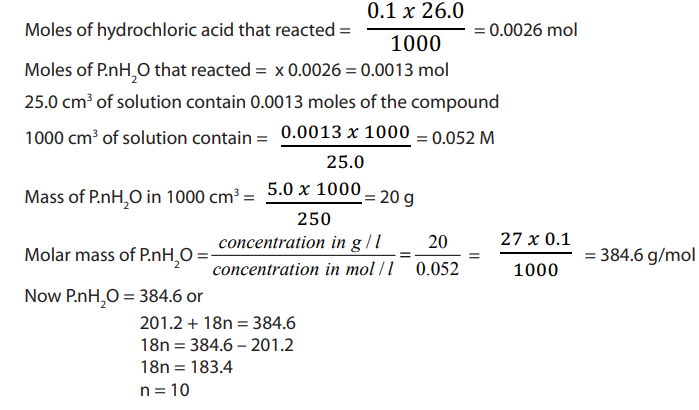
Checking up 11.7.1

11.7.2. Determination of atomic masses
Activity 11.7.2
4.99g of hydrated copper (II) sulphate, was strongly heated until
was strongly heated until
all water of crystallization was eliminated. The mass of anhydrous copper
sulphate was found to be 3.19g. Determine the number of moles of water ofcrystallization(x).
It is possible to find the atomic mass of the metal atom in titrating a basic compoundwhich reacts with acidic solution.
Example:

Answer:
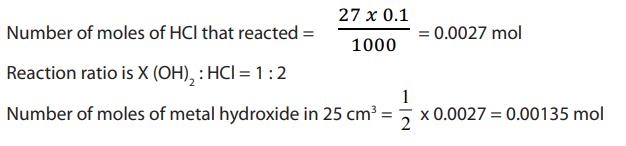

Checking up 11.7.2

11.8. End Unit Assessment
1. Explain the following terms:
a. Standard solutionb. Primary standard.
2. Describe the characteristics of a primary standard.

a. Calculate the value of x in the formula
b. Calculate the value of y in the formula
c. Calculate the value of z in the formula
d. Calculate the molecular mass of the oxalatee. Calculate the number of water of crystallization n.