UNIT 1: INTRODUCTION TO ORGANIC CHEMISTRY
Key unit competency
Apply IUPAC rules to name organic compounds and explain types of isomers for
organic compounds
Learning objectives: Students will Should able to:
• Classify organic compounds as aliphatic, alicyclic and aromatic
• Determine different formulae for given organic compounds
• Describe the common functional groups and relate them to the homologous
series
• Use IUPAC rules to name different organic compounds• Describe the isomers of organic compounds
Introductory Activity
Consider the following substances: Sodium chloride, starch, table sugar,
magnesium carbonate, glucose, sodium hydrogen carbonate, water.
1. Heat a small sample of each ( 5g for solids, 5ml for liquids) in a crucible
2. Record your observations.
3. From the observations, classify the substances listed above.
4. What criterion do you use for that classification?5. Interpret your observations
Organic chemistry is defined as the study of the compounds mainly composed by
carbon and hydrogen atoms, and sometimes oxygen, nitrogen, phosphorus, sulphur
and halogens atoms. The study of the rest of the elements and their compounds falls
under the group of inorganic chemistry. However, there are some exceptions such
as carbonates, cyanides, carbides, carbon oxides, carbonic acid, carbon disulphide
which are considered as inorganic compounds. Since various organic compounds
contained carbon associated with hydrogen, they are considered as derived from
hydrocarbons. Thus, a more precise definition of organic chemistry is: “the study ofhydrocarbons and the compounds which could be thought of as their derivatives’’.
The organic and inorganic compounds can be differentiated based on some of theirproperties as summarised in the following table.
Table 1.1 General features of organic and inorganic compounds
Why to study organic chemistry as a separate branch?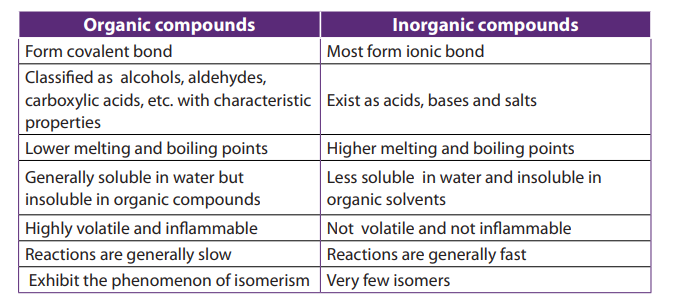
The organic chemistry involves the study of all chemical reactions that are commonly
used in industries and many other organic reactions that take place in living systems.
Materials used in everyday life, food processing and other manufacturing objects are
obtained based on organic chemistry. Some other reasons are highlighted below.
• Large number of compounds: up to now, no one knows exactly the number
of organic compounds that are present in nature.
• Built of relatively few elements: The elements frequently encountered
in organic compounds are carbon, hydrogen, oxygen, nitrogen, sulphur,
phosphorous, and halogens;
• Unique characteristic of carbon to undergo catenation: carbon atom is
unique among other elements whose atoms possess the capacity to unite
with each other by the covalent bonds resulting in a long chain of carbons ( i.e:
polysaccharides, proteins, polyesters, polyamides…).
Isomerism is the existence of compounds that have the same molecular formula
but different arrangements of atoms; these compounds are called “isomers”.
• Functional groups as basis of classification: Organic molecules contain
active atoms or groups of atoms which determine their chemical behaviour.
These are called functional groups joined in a specific manner. Therefore,
organic compounds with similar functional groups display similar propertiesand form a class.
• Combustibility: organic compounds are combustible.
• Nature of chemical reactions: organic compounds being formed by covalentbonds, they are slow and often have a low yield.
Importance of organic chemistry
The organic chemistry is a subject that plays an important role in modern life. In
general, there is no art, science or industry where knowledge of organic chemistryis not applied.
Examples where organic chemistry is applied:
1) Application in daily life.
In our day-to-day life, we find many substances or materials that are commonly used
and the later are made of organic compounds.
• Food: starch, fats, proteins, vegetables,...
• Clothes: cotton, wool, nylon, dacron, ....
• Fuels: petrol, diesel oil, and kerosene
• Dyes of all kinds
• Cosmetics (body lotion,…)
• Soaps and detergents
• Medicine: cortisone, sulphonamide, penicillin,…
• Drugs: morphine, cocaine,...
• Stationery: pencils, paper, writing ink,…• Insecticides, rodenticides, ovicides …
2) Applications in industry
The knowledge of organic chemistry is required in many industries such as
manufacture of food, pharmacy, manufacture of dyes and explosives, alcoholindustry, soil fertilisers, petroleum industry, etc.
3) Study of life processes
Organic chemistry in other words is the chemistry of life. For example the vitamins,
enzymes, proteins and hormones are important organic compounds produced inour body to ensure its proper development.
1.1. Classification of organic compounds

Organic compounds are classified as: aliphatic, alicyclic and aromatic (Figure 1.1)
(https://chemistry.tutorvista.com/organic-chemistry/hydrocarbons.html) ;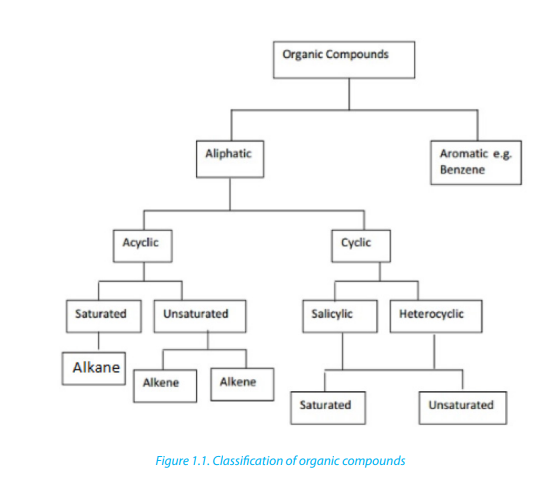
1.1.1. Aliphatic compounds
Aliphatic compounds are organic compounds in which the carbon atoms are
arranged in a straight or branched chain.Examples
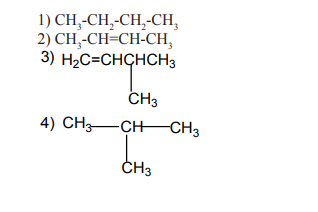
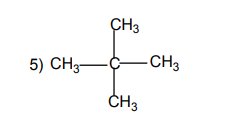
1.1.2. Alicyclic compounds
Alicyclic compounds are organic compounds that contain one or more carbon rings
that may be saturated or unsaturated.Example: 1) cyclobutane
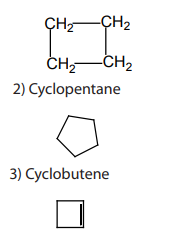
1.1.3. Aromatic compounds
Aromatic compounds are compounds that contain a closed ring that consists of
alternating single and double bonds with delocalised pi electrons.Example:
Aromatic compounds are designated as monocyclic, bicyclic and tricyclic if they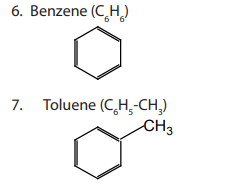
contain one, two or three rings, respectively.
Examples:
Note: Heterocyclic compounds: Are also classified as cyclic compounds which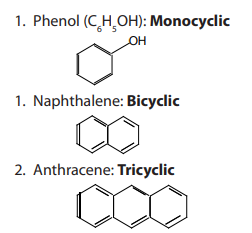
include one or two atoms other than carbon (O, N, S) in the ring.Thus furan, thiopheneand pyridine are heterocyclic compounds.
Checking up 1.1: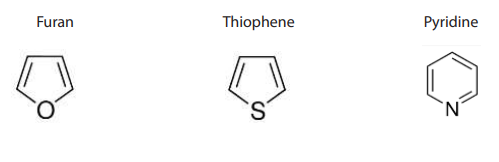
Observe the following compounds and classify them as aliphatic, alicyclic andaromatic.

1.2. Types of formulas for organic compounds
Activity 1.2
1. Explain the terms empirical, molecular and structural formulae.
2. Use examples of organic compounds to differentiate the types of theformulae above.
Atoms bond together to form molecules and each molecule has a chemical formula.
In organic chemistry, we can distinguish empirical, molecular and structural formulas.
1.2.1. Empirical formula
The empirical formula is the simplest formula which expresses the ratio of the
number of atoms of each element present in a particular compound. The empirical
formula is determined using the percentage composition according to the
following steps.
i. The percentage of each element, considered as grams of that element in
100g of the compound, is divided by its atomic mass. This gives the number
of moles of the element in 100g of the compound.
ii. The result in i. is then divided by the lowest ratio (number of moles in 100g
of the compound), seeking the smallest whole number ratio.
iii. If the atomic ratios obtained in ii. are not the whole number, they should
be multiplied by a suitable common factor to convert each of them to the
whole numbers (or approximatively equal to the whole numbers). Minorfractions are ignored by rounding up or down (ex: 7.95 = 8).
Example
An analysis of organic compound showed that it has 39.13% carbon, 52.23%
oxygen and the remaining is hydrogen. Determine the empirical formula of thecompound.
Note: 2.65 can not be adjusted to 3 and it is multiplied by 3 equals to 7.95 which
is rounded to 8.
1.1.2. Molecular formula
The molecular formula is a formula expressing the exact number of atoms of each
element present in a molecule.Molecular formula = Empirical formula x n
Note: When n = 1, the molecular formula is the same as the empirical formula.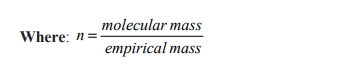
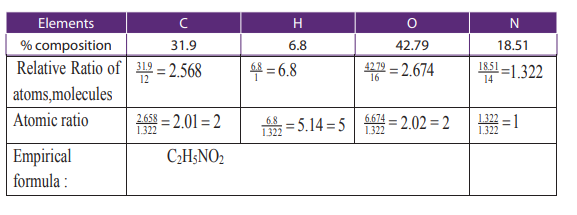
Example 1:
An organic compound contains 31.9% by mass of carbon, 6.8% hydrogen and 18.51%
nitrogen and the remaining percentage accounts for oxygen. The compound has avapour density of 37.5. Calculate the molecular formula of that compound.
Answer:
Vapour density = a half molecular mass
Molecular mass = 2 x vapour density = 2 x 37.5 = 75g/mol
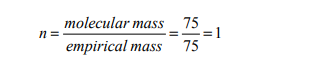
Hence the molecular formula = empirical formula

Example 2:
0.45g of an organic acid on combustion gave 0.44g of carbon dioxide and 0.09gof water. If the molecular mass of the acid is 90, deduce the molecular formula.
Answer:
• Percentage of carbon in CO2 : x x100 =26.66%
• Percentage of hydrogen in H2 O: x x100 =2.22%• Percentage of oxygen = 100 – (26.66 + 2.22) = 71.12%
Note: From the above calculations, we can extend our generalized expression:
% of Oxygen = 100 – (% hydrohen + % carbon)
Finding formulae by combustion method
the formula of a hydrocarbon can be found from the results of combustion experiment.
A hydrocarbon in vapour phase is burned more than oxygen to form carbon dioxide
and water vapour. When the mixture of gases is cooled to room temperature, water
vapour condenses to occupy a very small volume. The gaseous mixture consists of
carbon dioxide and unused oxygen. The volumes of carbon dioxide can be found by
absorbing it in an alkali. From the volumes of gases, the equation for the reactionand the formula of hydrocarbon can be found.
The combustion method can be used for other compounds also, e.g. ammonia
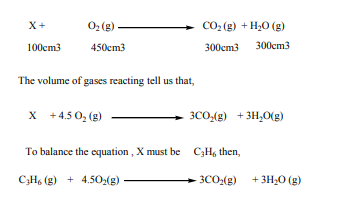
Example 1:
when 100cm3 of hydrocarbon X burn in 500cm3 of oxygen, 50cm3 of oxygen are
unused, 300cm3 of carbon dioxide are formed and 300cm3 of steam are formed.Deduce the equation for the reaction and the formula of the hydrocarbon
Answer:
Example 2:
10cm3 of a hydrocarbon, Ca Hb, are exploded with an excess of oxygen.
A contractionb of 35cm3
occurs, all volumes being measured at room temperature and pressure.
On treatment of the products with sodium hydroxide solution,
a contraction of 40cm3 occurs. Deduce the formula of the hydrocarbon

1.2.3. Structural formulas
Structural formula shows how the different atoms in a molecule are bonded
(i.e. linked or connected)
There are three types of structural formulas: displayed, condensed and skeletal(stick) formulas.
Example:
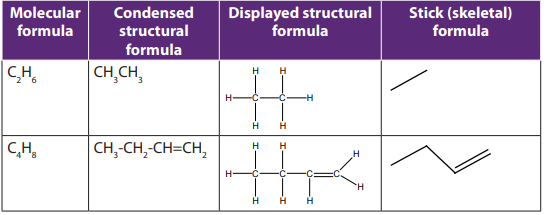
Note: Stick formula is also considered as structural formula.
1.3. Functional groups and homologous series1.3.1 Functional groups
Activity: 1.3.1
Using books or internet, explain the term functional group and point out somecommon functional groups.
A functional group is an atom or group of atoms in a molecule which determines
the characteristic properties of that molecule. Examples of some fuctionnal groupsare indicated in the Table 1.2.
Table 1.2: Name and give examples of functional groups in organic compounds

1.3.2. Homologous series
Activity 1.3.2.
By doing your own research, provide the meaning of “homologous series”. What
are the characteristics of such a series? Illustrate your answer by using examplesof alkanes, alcohols, carboxylic acids.
When members of a class of compounds having similar structures are arranged in
order of increasing molecular mass, they are said to constitute a homologous series.
Each member of such a series is referred to as a “homologous” of its immediate
neighbours. For example, the following sequence of straight chain of alcohols formsa homologous series.
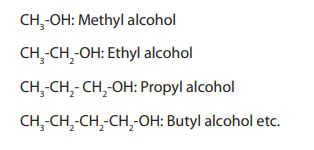
Characteristics of a homologous series
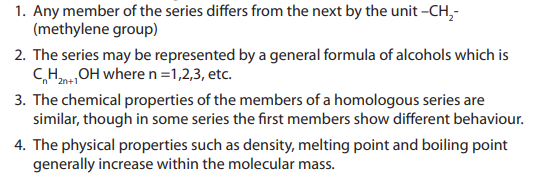
Checking Up 1.3
1.4. General rules of nomenclature of organic compounds
according to IUPAC
Activity 1.4.
By your own research, describe the rules that are applied to name the organic compounds.Your answers can be given as a form of a report.
The organic compounds are named by applying the rules set by the International
Union of Pure and Applied Chemistry (IUPAC). The purpose of the IUPAC system of
nomenclature is to establish an international standard of naming compounds tofacilitate the common understanding.
In general, an IUPAC name has three essential parts:
• A prefix that indicates the type and the position of the substituents on the
main chain.
• The base or root that indicates a major chain or ring of carbon atoms found
in the molecule’s structure. e.g. Meth- for one carbon atom, eth- for 2 carbon
atoms, prop- for 3 carbon atoms, hex- for five carbon atoms, etc.
• The suffix designates the functional group.
Example -ane for alkanes, -ene for alkenes, -ol for alcohols, -oic acid for carboxylic
acids and so on.
Steps followed for naming organic compounds:
1. Identify the parent hydrocarbon:It should have the maximum length, or the longest chain.
Example

3. Identification of the side chains.
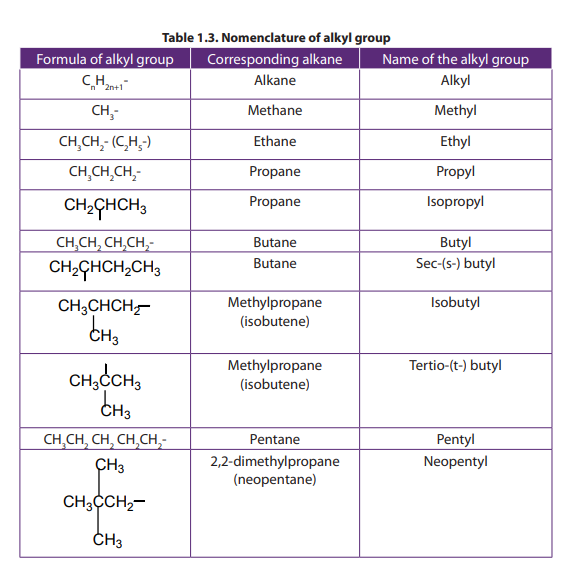
Side chains are usually alkyl groups. An alkyl group is a group obtained by a
removal of one hydrogen atom from an alkane. The name of alkyl group is obtainedby replacing -ane of the corresponding alkane by –yl (Table 1.3).
A side chain must be identified by the smallest possible numbers
4. If the same substituent occurs two or more times, the prefix di, tri,tetra, ...is
attached to substituent’s name. Its locants separate the prefix from the name ofthe substituent.
5. Identify the remaining functional groups, if any, and name them. Different side
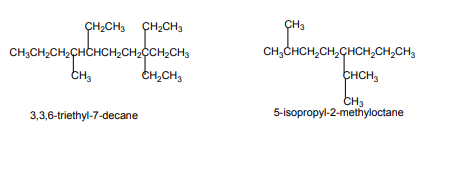
chains and functional groups will be listed in alphabetical order.
The prefixes di, tri, tetra,...are not taken into consideration when grouping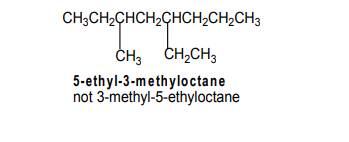
alphabetically. But prefixes such iso-, neo- are taken into account.
Example:
Identify the position of the double/triple bond.
Example:
Number the chain (left to right) or right to left).
The sum of the numbers which show the location of the substituents is the possible smallest.

The correct name will be the one which shows the substituents attached to the third
and fifth carbon, respectively and not to the fourth and the fifth carbon atom.
Numbers are separated by commas Hyphens are added between numbers andwords. Successive words are merged in one word.
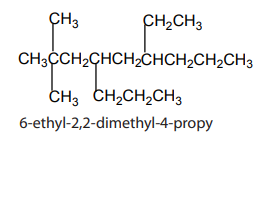
Checking up 1.4
1. Complete the sentence; the systematic nomenclature of organic
compounds follows rules established by the ……………………………
…..............................
2. What are the main parts which made up the name of an organic
compound?3. Name each of the following compounds using the IUPAC system.
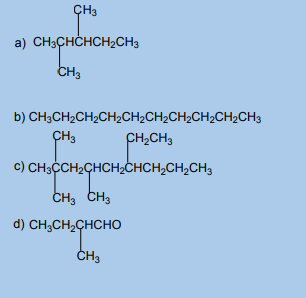
1.5. Isomerism in organic compounds
Activity 1.5:
Consider the following set of compounds:
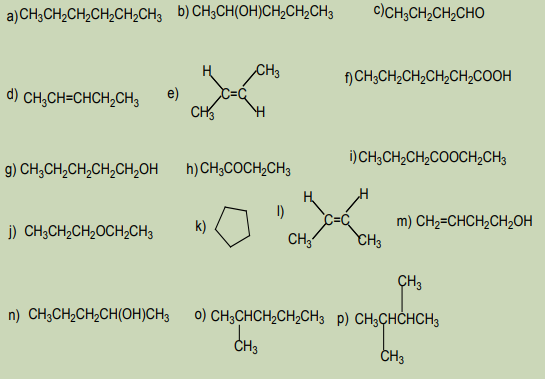
Analyze the structure of the compounds listed above and point out:
1. Compounds with the same structural formula
2. Compounds with the same molecular formula
3. Are there any compounds having the same molecular formula but
different by their structures? Explain the main differences displayed by
them? Name the relationship between them?4. Explain the behavior of the identified in 3)
Isomerism is the existence of compounds that h ave the same molecular formula
but different arrangements of atoms; these compounds are called “isomers”.
Isomers have different physical or/and chemical properties and the difference may
be great or small depending on the type of isomerism.There are two main classes of isomerism: Structural isomerism and stereoisomerism.
1.5.1. Structural isomerism
Activity 1.5.1
1. Referring to the previous activity 1.5 above, what is the relationship
between compounds: a), o and p) in the list of the activity 1.5?
2. Identify the relationship between compounds b) and g) in the activity 1.5?
3. Relate the relationship between compounds: b) and J) in the activity 1.5?
4. Identify the relationship between compounds c) and h) in the activity 1.5?
5. Investigate if there is a relationship between compounds d) and k) in theactivity 1.5?
Structural isomers are compounds with the same molecular formula but withdifferent structural formula.
1. Position isomerism
Position isomers are compounds with the same molecular formula but differentpositions of the functional group or substituent(s).
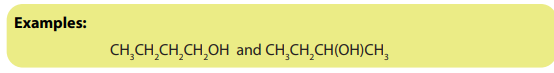
2. Chain isomerism
Chain isomers are compounds with the same molecular formula, belonging to thesame homologous series, with chain of carbon atoms of different length.
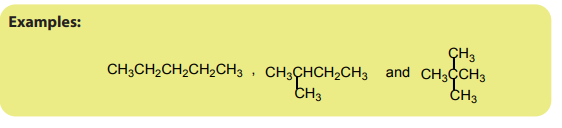
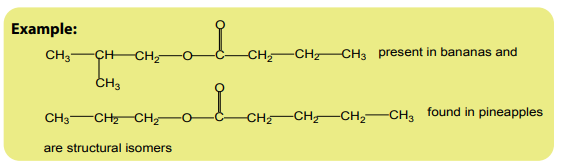
3. Functional isomerism
Functional (group) isomers are compounds which have the same molecular formulabut different functional groups.

1.5.2. Stereoisomerism
Activity 1.5.2
1. What is the relationship between compounds e) and l) in the activity 1.5. ?
2. Suggest examples of other organic compounds which have a similarrelationship.
1. Geometrical isomerism
Geometrical isomers or cis-trans isomers are compounds with the same molecular
formula, same arrangement of atoms but differ by spatial arrangements.
This type of isomers is mainly found in alkenes due to the restricted rotation around
the carbon-carbon double bond.
Note: For more information, visit the website below. (https://www.youtube.com/
watch? v=7tH8Xe5u8A0).
The necessary condition for an alkene to exhibit geometrical isomerism is that eachcarbon doubly bonded has two different groups attached to it.

2. Optical isomerism
Activity 1.5.3.
1. Look at your two hands or the Figure 1.2 and discuss the relationship
between them?
2. What are the necessary conditions for such pairs of organic compounds
to exhibit that relationship?3. What name is given to such compounds?
Optical isomers are compounds with the same molecular formula and arrangements
of atoms but have different effect on the plane polarised light.
• A compound that rotates the plane polarised light is said to have an optical
activity.
• This type of isomerism occurs in compounds containing an asymmetric
(asymmetrical) carbon atom or chiral centre1
• When a molecule has chiral centre, there are two non superimposable isomers
that are mirror images of each other.• Such compounds are called enantiomers.
In a mirror, the left hand is the image of the right hand and they are non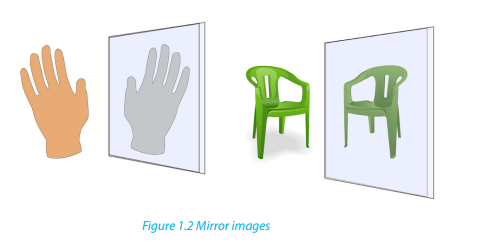
superimposable, i.e. they are enantiomers. An achiral object is the same as its mirrorimage, they are nonsuperimposable.

Checking Up 1.5
1. What is meant by “isomers”
2. Using examples, distinguish structural isomers and stereoisomers.
Describe the sub-classes of each type of isomers.
3. Explain how the nature of the C=C bond gives rise to cis-trans isomerism.
4. Identify which of the isomers of hexane exhibit geometrical isomerism.
5. Which of the following compounds can exist as optical isomers? Justifyyour answer.

6. Give examples of items which are enantiomers.
1.6. End unit assessment
1. a). An atom or group of atoms which determines the characteristic properties
of an organic compound is…………………………………………………..
b). A set of compounds that have the same functional group is referred
as ………………
c). An organic compound that rotates the plane polarized light is said to
be……………….
2. Chain isomers belong to the same class. True/False
3. Organic compounds belonging to the same class have similar physical
properties. True/False4. What is the name of the following compound?

A). 1,1-butyl-2- mthylpropane
B). 2,2,4-trimetylpentane
C). 2,2,4-methylpentane
D). 2,4,4-trimethylpentane
E). none of the previous answer
5. The compound that follows belongs to which class of organic compounds?

A). alcohols
B). alkenes
C). alkynes
D). aromatic
6. The compound that follows belongs to which class ofcompounds?
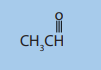
A). ethers
B). aldehydes
C). ketones
D). alcohols
7. Write the structural formula of:
a. 4-ethyl-3-methylheptane
b. 3-ethyl hexane
c. 3,3,5-trimethyloctane
d. 4-ethyl-2,2-dimethylnonane
8. Consider the following compound.
a. Determine the percentage composition of each element present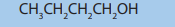
in the compound.
b. Determine the empirical formula of the above molecule
c. From the results from a) calculate the molecular formula of the
compound
d. Write all possible structural formulae of isomers of the compound.
e. Name the isomers in d) according to the IUPAC system.
f. (i) From the results in d) classify the isomers as chain, position,
functional and optical isomers.
(ii) From the results in d) show the compound that can exhibitoptical isomerism.
