UNIT 7:TRENDS IN CHEMICAL PROPERTIES OF GROUP 2 ELEMENTS AND THEIR COMPOUNDS
UNIT 7: TRENDS IN CHEMICAL PROPERTIES
OF GROUP 2 ELEMENTS AND THEIRCOMPOUNDS
Key unit competence: compare and contrast the properties of the group 2 elements
and their compounds in relation to their position in the periodic table
Learning objectives
By the end of this unit, students should be able to:
• Describe the physical properties of group 2 elements.
• Describe the properties of group 2 oxides and hydroxides.
• Explain the trends in the thermal decomposition of group 2 carbonates and
nitrates.
• Explain the trends in the solubility of group 2 compounds.
• State the uses of group 2 elements and their compounds.
• Describe the industrial manufacture of cement.
• Discuss the environmental and health issues associated with the
manufacturing of the cement.
• Perform experiments to compare and contrast the reactivity of group2
elements.
• Write balanced equations of the reactions of group 2 elements, different
elements and the compounds.
• Illustrate practically the trends in solubility and thermal decomposition of
group 2 compounds.
• Test the alkaline character of group 2 hydroxides.
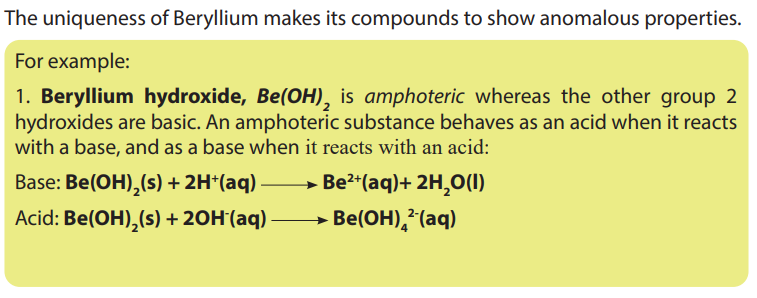
• Be aware that the compounds of beryllium are different from the compounds
of the other group elements.
• Perform chemical test for the presence of group 2 cations in solution.
• Suggest preventive measures for environmental and health issues
associated with the manufacture of the cement.
• Appreciate the logic underlying the position of elements in the periodic
table ,their electronic structure and the properties.
• Appreciate the application of the chemistry of group 2 elements and their
compounds in the social economic development.
• Develop the team work approach while performing experiment and writing
field –study reports.
• Develop the attitude of sustainable exploitation of natural resources .
• Stimulate the culture of entrepreneurship in the area of chemistry.
Introductory activity 7
Complete the table below to identify the group 2 elements in the substancesfound in our surroundings: at home and at the school.
7.1. Occurrence and physical properties of group 2 elements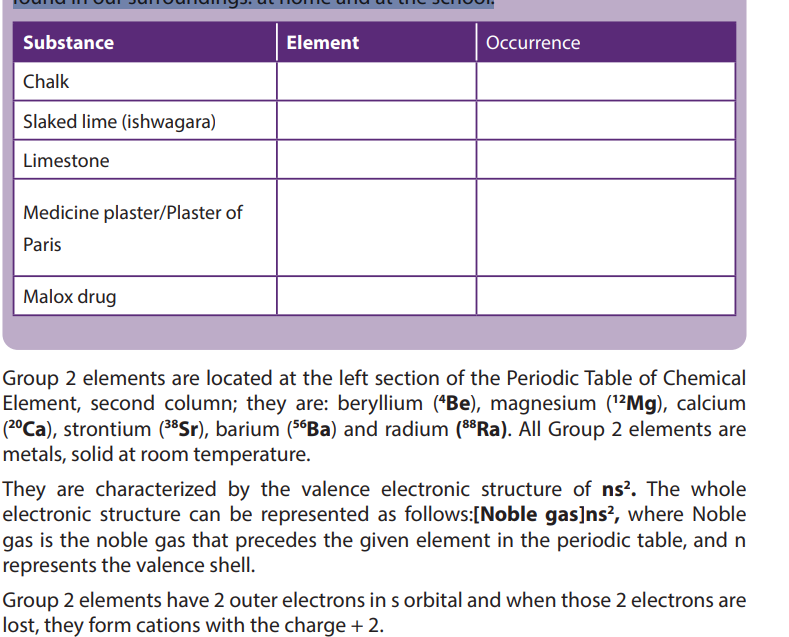
Activities
Name 1 or 2 elements of group 2 or their compounds that we commonly find inRwanda
7.1.1. Occurrence
Group 2 elements are active metals and are found in nature in form of compounds
or minerals such as: Limestone and marble for calcium, dolomite and magnesite for
magnesium etc… Hence Group 2 metals must be produced from the minerals theyare found in.
7.1.2. Physical properties
Group 2 elements are all metals, solid at room temperature; they are good conductors
of electricity. They have a silvery luster that soon disappears upon exposure to air.
They are malleable and ductile but less than alkali metals of Group 1. Their atomic
radius and their volume are smaller than those of Group 1 elements in the same
period. The Table 7.1 below shows some other physical properties of Group 2
elements.
Atomic radius increases down the group due to increasing of electronic levels.
Melting and boiling temperature decreases down the group due to increasing of
atomic radius resulting in weakening of the metallic bond.
The increasing of atomic radius explains also the decreasing of first ionization of
the elements down the group. This also explains that the metallic character of theelements increases down the group.
Checking up 7.1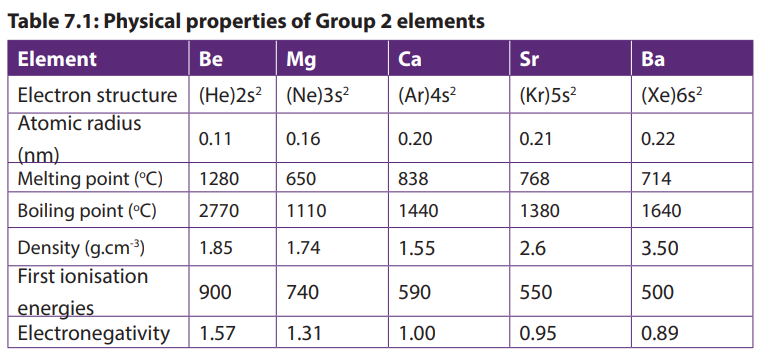
Question1: Metals are reducing agents because they lose easily electrons. You are given
3 elements of Group 2: Be, Ca and Ba. Which one are you going to choose as the best
reducing agent, and explain why?
Question 2: How does each of the following properties of the elements in Group 2
change down the group and why?
i) Atomic radius
ii) Ionisation energyiii) Electropositivity
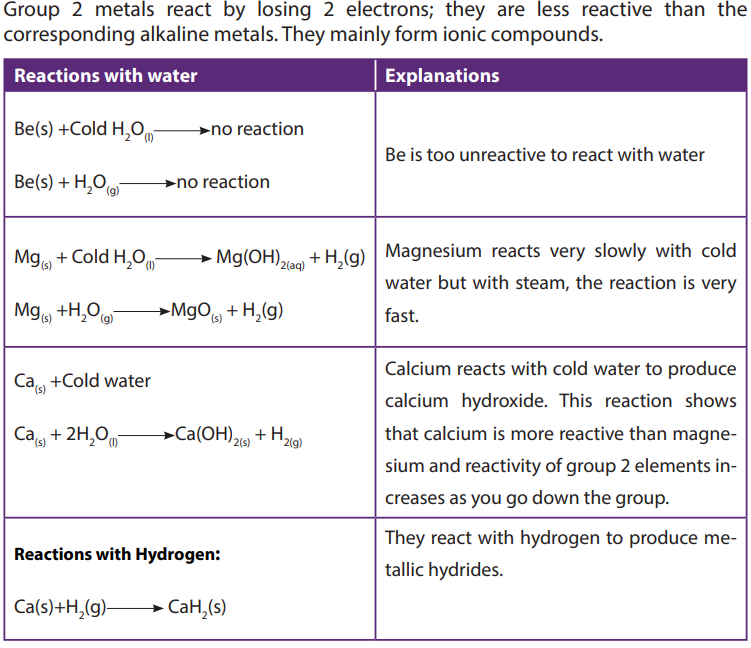
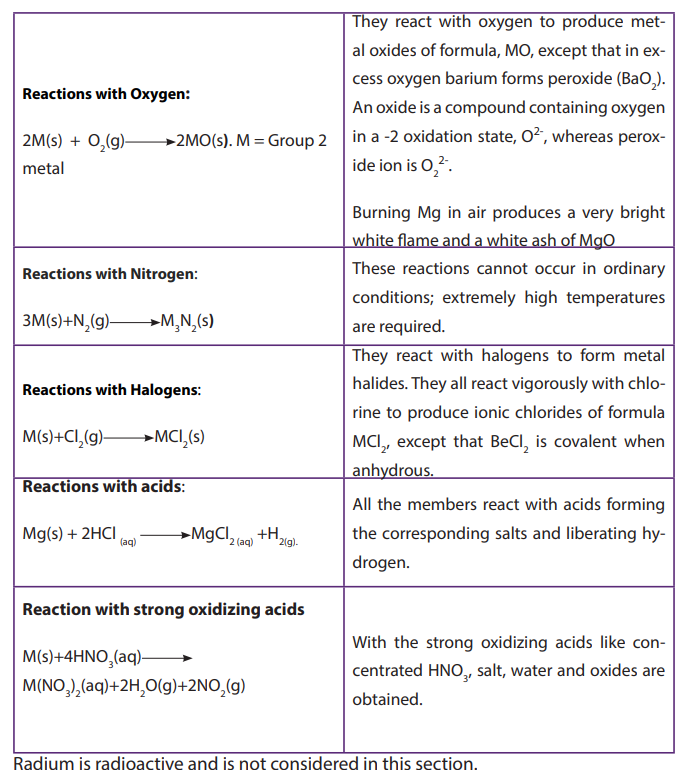
7.2. Reactivity of group 2 elements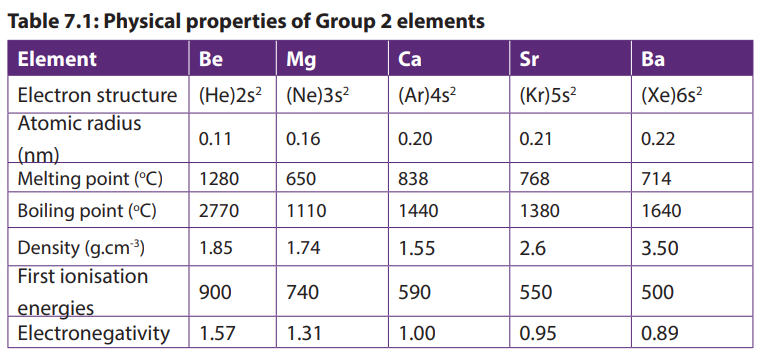
Activities 7.2 (a)
Activity 1:
• Pour 200cm3
of water in two different beakers
• To the first beaker, add a small piece of magnesium ribbon. To the second
beaker, add a very small piece of sodium.
• Record your observation.
• Put a piece of blue and red litmus paper in both beakers
• Record your observations.
Activity 2:
• Pour 200cm3 of water in pyrex beaker or borosilicate beaker
• Heat until water boils
• Using crucible tongs, hold a large piece of magnesium in the steam• Record your observations


7.3. Properties of group 2 compounds
7.3.1. Ionic and covalent character of oxides and halides
Activity 7.3.1 (a)
• Pour 50 ml of paraffin in a beaker
• Put 1g of calcium chloride and try to make a solution.
• Pour 50 ml of water in another beaker
• Put 1g of calcium chloride and try to make aqueous solution
• Write down your observations and comments.
Activity 7.3.1 (b)
• Place a beaker on a table
• Cut 15cm of magnesium ribbon
• Using crucible tongs, hold and burn the magnesium ribbon over the
beaker.
• What do you observe?
• Add some water to the ash in the beaker.
• Shake the mixture and add 2 drops of phenolphthalein or touch the
mixture with a red litmus paper• Record all your observations.


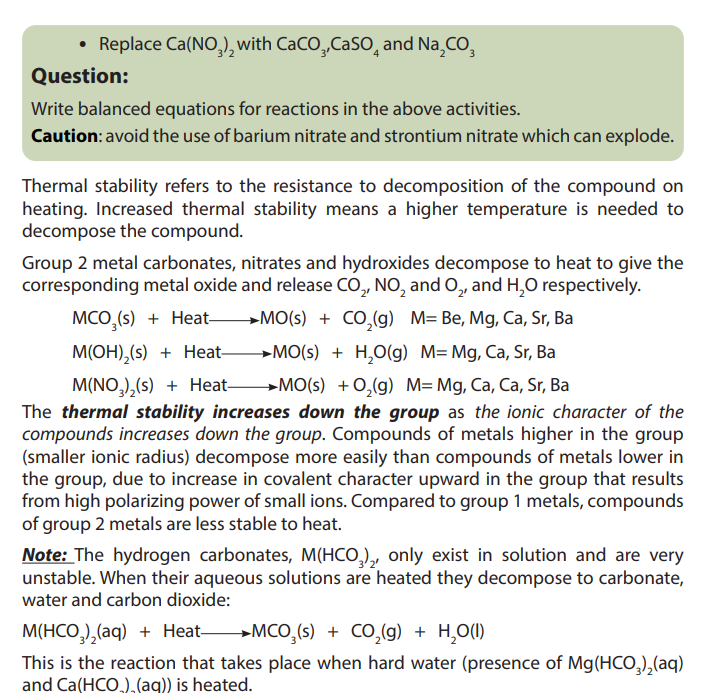
The solubility of salts depends on two main opposite factors:
The energy of dissociation of the crystal: The energy needed to dissociate the
solid crystal into its ions. This process requires energy; the process is endothermic.
The energy of hydration of the ions produced: the amount of energy released
when ions undergo hydration or are surrounded by water molecules; this process is
exothermic.
When the combination of the two processes above is in favor of the hydration of
ions, the salt is soluble; otherwise the salt is not soluble.
The solubility will increase when the hydration process predominates moreand more the dissociation process and vice-versa.

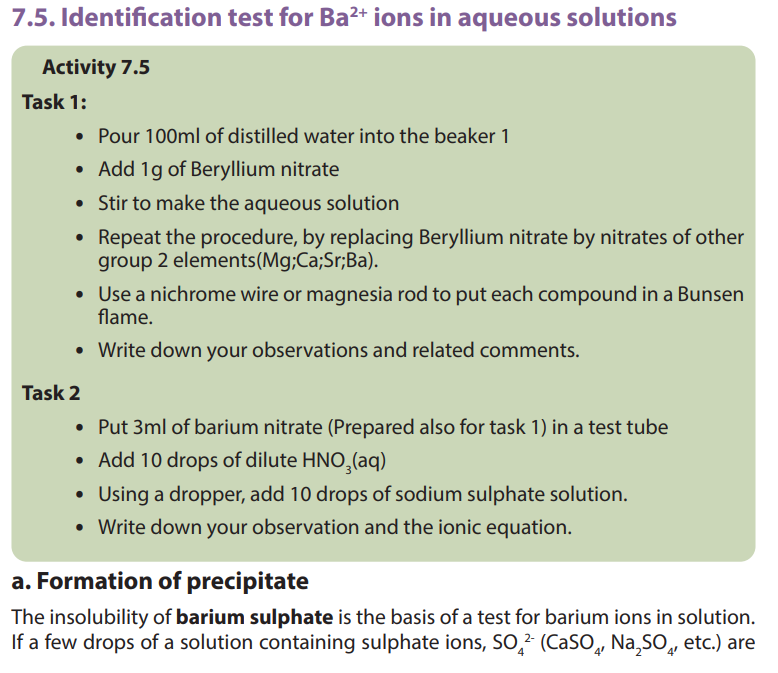

7.6. Uses of group 2 elements and their compounds
Activity 7.6
Describe the following compounds and show how each compound can be used
to prepare another if possible.
a. Limestone b) Quicklime c)Slaked lime
b. Have you heard about soil amendment in Rwanda? What is it?
c. In groups, the students do research to find out how chalk used on
blackboard is produced.
1. Beryllium
Because beryllium is relatively light and has a wide temperature range, it can be
used in the manufacture of aircrafts’components.
2. Magnesium
• Chlorophyll, the pigment that absorbs light in plants, is a complex of
magnesiumand is necessary for photosynthesis.
• Magnesium hydroxide is used as Anti-acid medicine
• Magnesium is used in making Grignard reagents, the organomagnesium
compounds.
• Magnesium is used as sacrificial anode to prevent iron sheet from rusting.
• Salts of magnesium and calcium are used in chemistry laboratory as dryingagents.