UNIT 5:VARIATION IN TRENDS OF THE PHYSICAL PROPERTIES
UNIT 5: VARIATION IN TRENDS OF THE PHYSICAL
PROPERTIES
Key unit competence
Use atomic structure and electronic configuration to explain the trends in the
physical properties of elements.
Learning objectives
By the end of this unit, students should be able to:
• Outline the historical back ground of the Periodic Table.
• Explain the trends in the physical properties of the elements across a period
and down a group.
• Classify the elements into respective groups and periods using electronic
configuration.
• Relate trends in physical properties of the elements to their electronic
configuration.• Classify the elements into blocks (s, p, d, f-block).
Introductory activity
1. Explain how elements can be classified into a periodic table?
2. Explain on which basis elements can be classified?
3. How many groups and periods comprises a modern periodic table?
4. How does electronic configuration of elements influence the structureof
modern periodic table?
5. Discuss the basis of the location of the elements in the periodic table.
5.1. Historical Background of the Periodic Table
Activity 5.1
Who is the father of the periodic table? Explain your answers.
Differentiate the laws of triads and octaves
During the nineteenth century, many scientists contributed to the development of
the periodic table. In the beginning, a necessary prerequisite to the construction of
the periodic table was the discovery of the individual elements. Although elements
such as gold, silver, tin, copper, lead and mercury have been known since antiquity,
the first scientific discovery of an element occurred in 1649 when Hennig Brand
discovered phosphorous. The periodic table of elements is a chart created in order
to help to organize the elements that had been discovered at that time. By 1869,
a total of 63 elements had been discovered. As the number of known elements
grew, scientists began to recognize patterns in properties and began to develop
classification schemes.
Some important dates help us to understand more about how the periodic table has
been created.
• In 1669, Hennig Brand a German merchant and amateur alchemist invented
the Philosopher’s Stone; an object that supposedly could turn metals into
pure gold. He heated residues from boiled urine, and a liquid dropped out
and burst into flames. He also discovered phosphorus.
• In 1680 Robert Boyle also discovered phosphorus without knowing about
Henning Brand’ discovery.
• In 1809, curiously 47 elements were discovered and named, and scientists
began to design their atomic structures based on their characteristics.
• In 1869, Dimitri Mendeleev based on John Newlands’ ideas started
the development of elements organized into the periodic table. The
arrangement of chemical elements were done by using atomic mass as the
key characteristic to decide where each element belonged in his table. The
elements were arranged in rows and columns. He predicted the discovery
of other elements, and left spaces open in his periodic table for them. At the
same time, Lothar Meyer published his own periodic table with elements
organized by increasing atomic mass.
• In 1886, French physicist Antoine Becquerel first discovered radioactivity.
During the same period of 1886, Ernest Rutherford named three types of
radiation; alpha, beta and gamma rays.
• In 1886, Marie and Pierre Curie started working on the radioactivity and
they discovered radium and polonium. They discovered that beta particles
were negatively charged.
• In 1895, Lord Rayleigh discovered a new gaseous element named argon
which proved to be chemically inert. This element did not fit any of the
known periodic groups.
• In 1898, William Ramsay suggested that argon be placed into the periodic
table between chlorine and potassium in a family with helium, despite the
fact that argon’s atomic weight was greater than that of potassium. This
group was termed the “zero” group due to the zero valency of the elements.
Ramsey accurately predicted the future discovery and properties neon.
• In 1913, Henry Moseley worked on X-rays and determined the actual
nuclear charge (atomic number) of the elements. He has rearranged the
elements in order of increasing atomic number
• In 1897 English physicist J. J. Thomson discovered small negatively charged
particles in an atom and named them as electrons;John Sealy Townsend
and Robert A. Millikan investigated the electrons and determined their
exact charge and mass.
• In 1900, Antoine Becquerel discovered that electrons and beta particles
as identified by the Curies are the same thing.
• In 1903, Ernest Rutherford proclaimed that radioactivity is initiated by
the atoms which are broken down.
• In 1911, Ernest Rutherford and Hans Geiger discovered that electrons are
moving around the nucleus of an atom.
• In 1913, Niels Bohr suggested that electrons move around a nucleus in
discreete energy levels called orbits. He observed also that light is emitted
or absorbed when electrons transit from one orbit to another.
• In 1914, Rutherford identified protons in the atomic nucleus. He also
transformed a nitrogen atom into an oxygen atom for the first time.
English physicist Henry Moseley provided atomic numbers, based on the
number of electrons in an atom, rather than based on atomic mass.
• In 1932 James Chadwick discovered neutrons, and isotopes were
identified. This was the complete basis for the periodic table. In that same
year Englishman Cockroft and the Irishman Walton first split an atom by
bombarding lithium in a particle accelerator, changing it to two helium
nuclei.The last major changes to the periodic table give rise from Glenn
Seaborg’s work in the middle of the 20th Century. In 1940, he discovered
plutonium and all the transuranic elements from 94 to 102.
• In 1944, Glenn T. Seaborg discovered 10 new elements and moved out 14
elements of the main body of the periodic table to their current location
below the lanthanide series. These elements were known as Actinides series.
• In 1951, Seaborg was awarded the Nobel Prize in chemistry for his work.
Element 106 has been named seaborgium (Sg) in his honor.• Presently, 118 elements are in the modern Periodic Table.
5.1.1. Law of Triads
In 1817 Johann Dobereiner noticed that the atomic weight of strontium fell midway
between the weights of calcium and barium, elements possessing similar chemical
properties.
In 1829, after discovering the halogen triad (three) composed of chlorine, bromine,
and iodine and the alkali metal triad of lithium, sodium and potassium he proposed
that nature contained triads of elements the middle element had properties that
were an average of the other two members when ordered by the atomic weight (the
Law of Triads).
Between 1829 and 1858 a number of scientists (Jean Baptiste Dumas, Leopold
Gmelin, Ernst Lenssen, Max von Pettenkofer, and J.P. Cooke) found that these types
of chemical relationships extended beyond the triad. During this time fluorine was
added to the halogen group; oxygen, sulfur, selenium and tellurium were grouped
into a family while nitrogen, phosphorus, arsenic, antimony, and bismuth were
classified as another. Unfortunately, research in this area was hindered by the fact
that accurate values were not always available.
5.1.2. Law of Octaves
In 1863, John Newlands, an English chemist suggested that elements be arranged in
“octaves”. He wrote a paper in which he classified the 56 established elements into
11 groups based on similar physical properties, noting that many pairs of similar
elements existed which differed by some multiple of eight in atomic weight. This
law stated that any given element will exhibit analogous behavior to the eighth
element following it in the table. However, his law of octaves failed beyond the
element calcium.
Although Dimitri Mendeleev is often considered the “father” of the periodic
table, however the work of many scientists contributed to its present form. The
representation of a modern Periodic Table of Elements is shown below.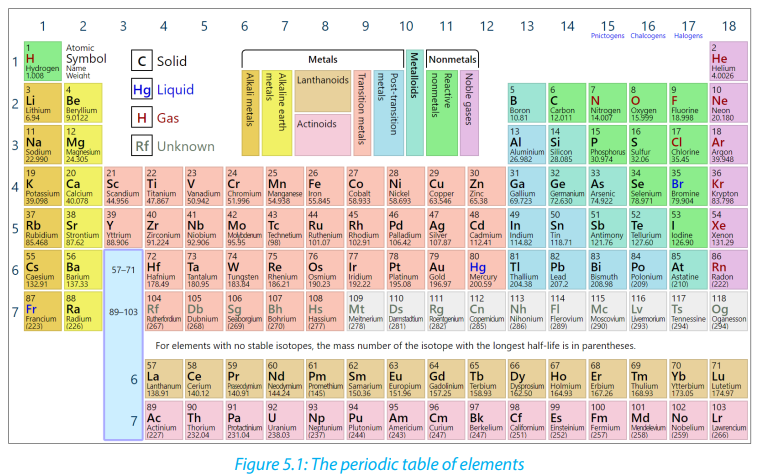
Checking up 5.1
The periodic table is an important tool used in chemistry:
1.Explain why the elements are classified in groups and periods of the periodic
table
2. Chose one element of Group 1 and one of group 17 and make their electronic
configurations using orbitals.
5.2. Comparison of Mendeleev’s Table and Modern Periodic
Table
Activity 5.2
1. Discuss the similarities and differences of Mendeleev’s table and modern
periodic Table.
2. How were the positions of cobalt and nickel resolved in the modern periodic
table?
The periodic table is the arrangement of chemical elements according to their
chemical and physical properties. The modern periodic table was created after
a series of different versions of the periodic table. The Russian Chemist/Professor
Dmitri Mendeleev was the first to come up with a structure for the periodic table with
columns and rows. This feature is the main building block for the modern periodic
table as well. The columns in the periodic table are called groups, and they group
together elements with similar properties. The rows in the periodic table are called
periods, and they represent sets of elements that get repeated due the possession of
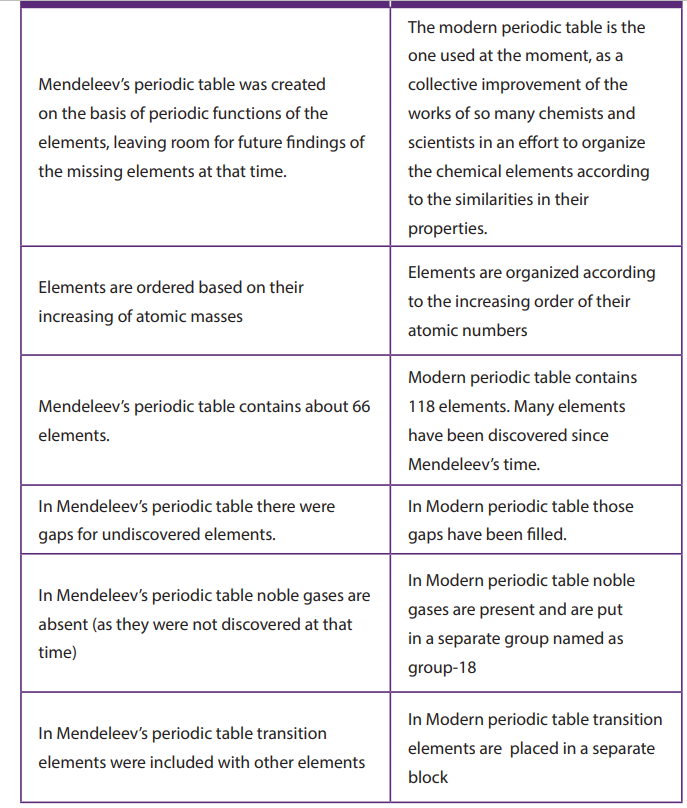
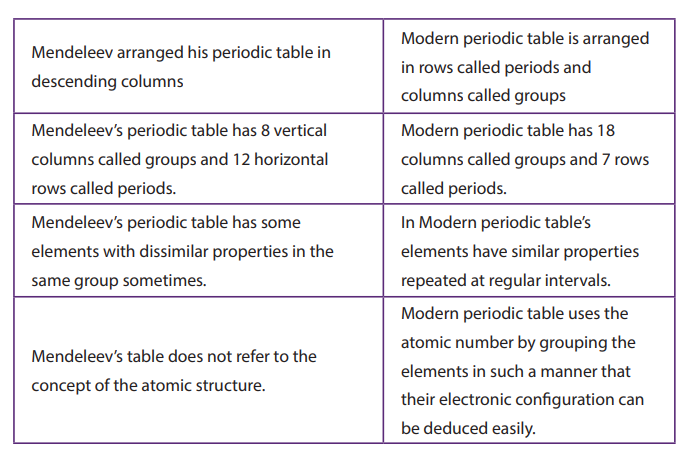
similar properties. The main difference between Mendeleev and Modern PeriodicTable are shown in the Table below (Table 5.1).
Table 5.1. Differences between Mendeleev’s table and the modern Periodic
Table
Checking up 5.2
1. The periodic table is an arrangement of elements based on their properties.
Explain the gaps found in the Mendeleev periodic table compared to the modern
one?
2. How many elements does the modern periodic table contain?
3. Look at the modern periodic table and write down four things it tells you.
5.3. Location of Elements in the Periodic Table Based On the
Electronic Configuration
Activity 5.3
1. Based on knowledge gained in the previous years:
a. Represent the electronic configuration of the elements 25X and 11Y.
b. Discuss the information given by the number of electrons in the last orbitals
of the above element about their position in the periodic table?
c. Explain the period and the group of the periodic table in which the above
elements are located.
2. Is it possible to have an element with atomic number 1.5 between hydrogen
and helium?
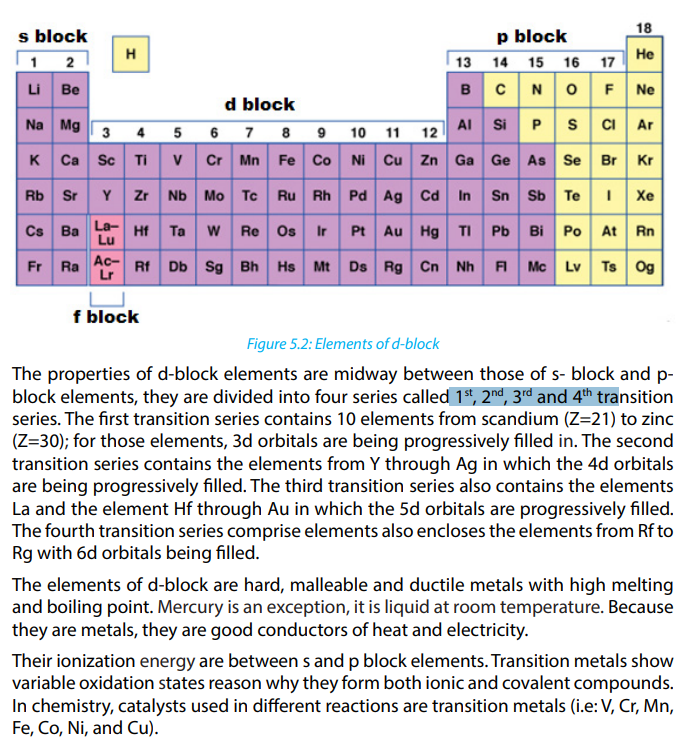
5.3.1. Major Divisions of the Periodic Table
The periodic table is a tabular of the chemical elements organized on the basis of
their atomic numbers, electron configurations, and chemical properties.
In the periodic table, the elements are organized by periods and groups. The period
relates to the principal energy level which is being filled by electrons. Elements
with the same number of valence electrons are put in the same group, such as the
halogens and the noble gases. The chemical properties of an atom relate directly to
the number of valence electrons, and the periodic table is a road map among those
properties such that chemical properties can be deduced by the position of an
element on the table. The electrons in the outermost or valence shell are especially
important because they participate in forming chemical bonds.
Elements are presented in increasing atomic number. The main body of the table is
a 18 × 7 grid. There are four distinct rectangular areas or blocks such as s, p, d and
f blocks. The f-block is usually not included in the main table, but rather is floated
below, as an inline f-block would often make the table impractically wide. Using
periodic trends, the periodic table can help predict the properties of various elements
and the relations between properties. It therefore provides a useful framework for
analyzing chemical behavior and is widely used in chemistry and other sciences
(Petrucci et al., 2007).
5.3.2. Location of elements in modern Periodic Table using examplesIn the periodic table, the elements are located based on groups and periods.
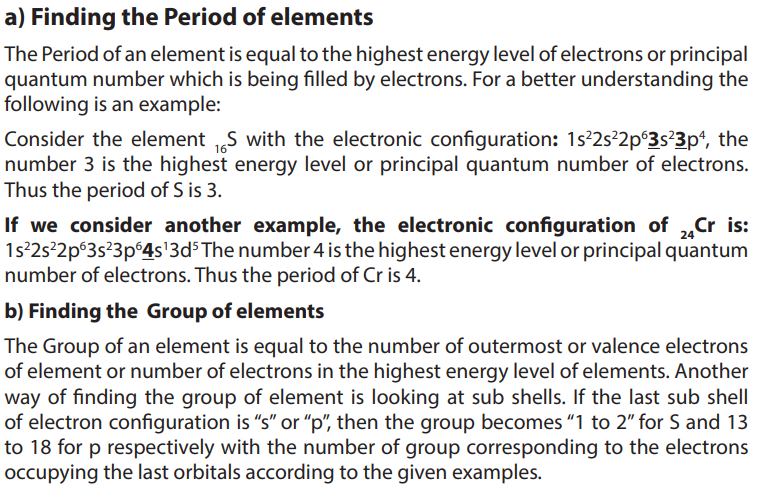
receives the last electrons. The s-block has two groups of reactive metals: Group 1
and 2.
p-block is composed of metals and nonmetals of Group 13 to 18.
d-block is made of transition metals:Group 3 to Group 12, and f-block is made of
lanthanide and actinide series or inner transition metals.
The division of elements into blocks is primarily based upon their electronic
configuration as shown in Figure 5.1. Two exceptions to this categorization can
be mentioned. Helium is placed in p-block although its valence electrons are in s
orbital because it has a completely filled valence shell (1s2
) and as a result, displays
properties representative of other noble gases. The other exception is hydrogen. It
has only one s-electron and hence can be placed in group 1 (alkali metals); but in
many modern Periodic Tables, hydrogen is left hanging above the Periodic Table and
doesn’t belong to any group. This is due to the particular properties of hydrogen:
• Hydrogen is the smallest chemical element
• Hydrogen is a gas while the other elements of group 1 are solids,
• Hydrogen is not a metal whereas the other elements of group1 are metals,
• In some compounds where hydrogen combine with non-metals, it behaves
like a metal, e.g. in the polar molecule Hδ+Clδ-, hydrogen tends to lose an
electron,
• When combined with very active metals, it behaves as a non-metal and
forms a negative ion H-
, hydride ion; e.g. Na+H-
(sodium hydride).
Elements within the same group have the same number of electrons in their
valence (outermost) shells, and they have similar valence electron configurations.
They exhibit similar chemical properties. Elements within the same period have
different numbers of electrons in their valence shells and the number of electrons is
increasing from left to right. Therefore, elements in the same period are chemically
different, changing from metals to non-metals across the period from left to right.
Checking up 5.5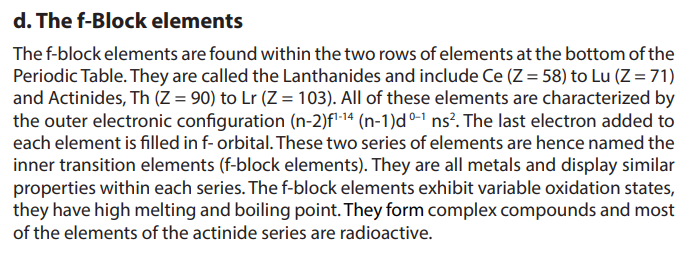
1. Among the common blocks, s, p, and d; which block has a tendency to form
complex compounds?
2. Why d-block are called transition elements?
3. Why f-block are called inner transition elements?
5.6. Variation of Physical Properties down the Groups and
across the Periods
Activity 5.6
1. The elements in the periodic table display many trends which can be used to
predict their physical properties. Explain three of the factors that you think can
influence the physical properties of elements in the periodic table.
2. Discuss the trends of the above factors across a period and down a group in the
periodic table.
The elements in the periodic table are arranged in order of increasing atomic number.
All of these elements display several other trends and we can use the periodic table
to predict their physical properties. There are many noticeable patterns in the
physical and chemical properties of elements as we descend in a group or move
across a period in the Periodic Table.
Those trends can be observed in: ionization energy, electronegativity, electropositivity,
electron affinity, melting and boiling point, density and metallic character and hereafter
are some factors which cause those trends.
5.6.1. Atomic radius
The atomic radius of an atom is defined as half the distance between the nuclei of
two atoms of the same element that are joined together by a single covalent bond.
Atomic radius of elements decreases as we move from left to right in periodic table.
This is explained by the number of outer electrons and protons which increase while
there is no change in the energy level. The results increase the attracting forces
making the radius smaller.
Increasing nuclear charge (more protons) pulls the electrons closer to the nucleus,
and the screening effect of inner electron shells will be the same for all members of
a given period. The combined effect of both factors results in the electrons being
pulled closer to the nucleus and a smaller radius.
On the other side, in the same group, as we go down, the atomic radius of elements
increases. This is due to the energy level which increases when you move down in
group of the periodic table, the attraction of external electrons by nucleus decreases
and atomic radius increases.
In general, atomic radii increase down a group because a new shell is added for
each successive member of a group, leading to a greater radius. Then an increased
screening effect of extra electron shells i.e. the nucleus has less of a pull on the outer
electrons.
5.6.2. Electronegativity
Electronegativity is a measure of the tendency of an atom to attract to itself the
shared pair of electrons making a bond. The charge in the nucleus increases from
left to right across a period.The electronegativity of atoms is affected by both the
charge of the nucleus and the size of the atom. The higher its electronegativity, the
more an element attracts electrons. In general, the electronegativity of a non-metals
is greater than that of metals. Trends are observed in the period (Figure 5.3) or in a
group of the Periodic Table (Figure 5.4).
• In a period, the electronegativity increases from left to right. This is
explained by the fact that as we go from left to right, there in an increase
of positive charge in the nucleus, since the number of protons increases;
but the electrons are being added to the same energy level. This results in
the reduction of the volume or radius of the atoms from left to right and
explains why attraction of external electrons by the nucleus increases from
left to right.
• In a group, the electronegativity decreases from top to bottom. This is due
increase of energy levels down in a group, and thus there is an increased
distance between the valence electrons and the nucleus, or a greater atomic
radius.The positive charge of the nucleus is further away from the valenceelectrons and the nucleus cannot attract efficiently external electrons.
Note: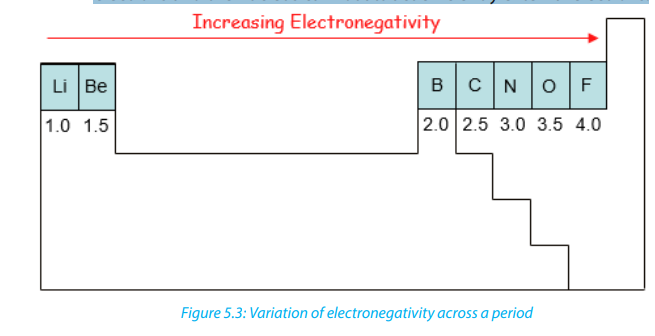
• Since noble gases do not react or do not form chemical bonds, their
electronegativity cannot be determined.
• For the transition metals, the electronegativity does not vary significantly
across the period and down a group. This is because their electronic structure
affects their ability to attract electrons easily like the other elements.
• The lanthanides and actinides possess more complicated chemistry
that does not generally follow any trend. Therefore, they do not have
electronegativity values.
According to these two general trends, the most electronegative element is fluorineand Francium is the least (Figure 5.4 and Figure 5.5).
The charge in the nucleus increases across a period. Greater is the number of protons,
greater is the attraction for bonding electrons.
5.6.3. Ionization energy (I.E)
Ionization energy: it is the amount of energy required to remove an electron from
a neutral gaseous atom. The lower this energy is, the more readily the atom loses
electron and becomes a cation. Therefore, the higher this energy is, the more
unlikely it is the atom to become a cation. We can distinguish, first, second, and third
ionization. Helium is the element with the highest ionization energy (Zumdahl and
Zumdahl, 2010). The noble gases possess very high ionization energies because of
their full valence shells compared to the elements of group 1 (Table 5.5).
The table shows that generally the IE decreases down the Group, as the size of theatoms increases down the Group.
Ionisation energy of rare gases or any species with an octet electronic structure show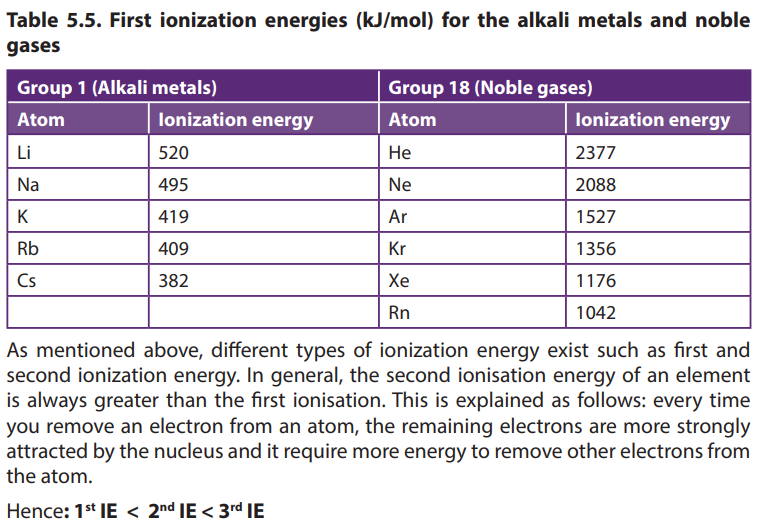
very high IE because the electron is being removed from a very stable electronic
structure.
The ionization energy varies across a period and down a group.
Across a period ionisation energies increase because the nuclear charge increases
(greater positive charge on the nucleus) and holds the outer electrons more strongly.
More energy needs to be supplied to remove the electron.
Down a group ionisation energies decrease because the outer electrons are further
away from the nucleus. The screening effect of the inner electron shells reduces the
nuclear attraction for the outer electrons, despite the increased (positive) nuclear
charge.
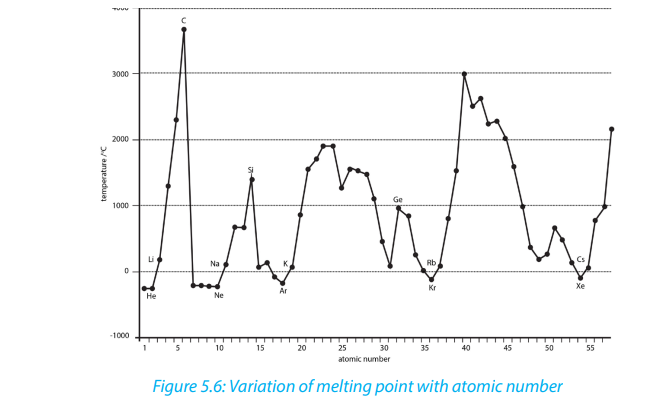
5.6.4. The melting points and boiling points
Melting points and boiling points show some trends in groups and periods of the
Periodic Table.
As you already know, the Periodic table can be subdivided into two main area or
regions:
• the left region where you find only metallic elements
• the right region where you find both metallic and non-metallic elements;
all non-metallic elements are in the extreme right part of that region.
The general trends of melting and boiling points depends on the regions:
• in the left region, melting and boiling points generally decrease down the
groups due to the decrease of strength of the metallic bond down the
groups;
• on the contrary, in the right region at the extreme right in groups 17 and
18, there is a general increase of melting and boiling points down the group
due to the increase of the molecular mass;
• from left to the middle of the periodic table, there is an increasing of melting
and boiling points from left to right in a periode due the the increasing of
the strength of the metallic bond;
• whereas from the middle of the periodic table, there is a decrease of melting
and boiling points from left to right due to the progressive increase of nonmetallic character where elements exist as simple molecules.
The melting and boiling points vary in a regular way or pattern depending on their
position in the Periodic Table. In general the forces of attraction for elements on the
left of the table are strong metallic bonds; they require higher energy to be broken,
hence higher melting and boiling points.
As we cross toward the right side of the periodic table, the non-metal character of
elements increases and elements, except few elements, form molecules that are
held together by weak intermolecular forces; hence their melting and boiling points
are generally low.
For example going down in group 1, the melting point and boiling point of the
alkali metals decrease. This is due to the weakning of metallic bond down the group.
However, going down in group 17 of the halogens the melting point increases
meaning that there is an increase in the force of attraction between the molecules.
The illustrations below show the variation of melting and boiling point for someelements of the periodic table (Figures 5.6 and 5.7).
5.6.5. The density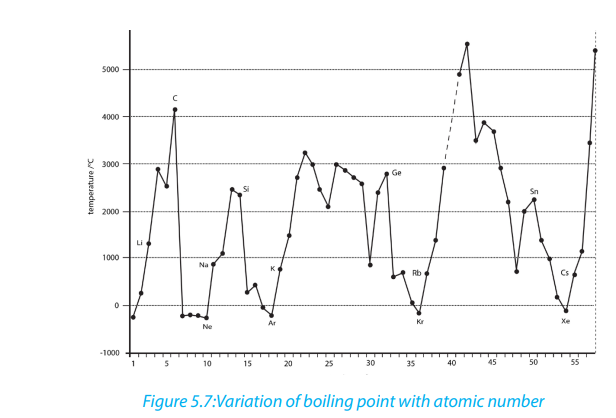
The density of a substance is its mass per unit volume, usually in g/cm3
. The density
is a basic physical property of a homogeneous substance; it is an intensive property,
which means it depends only on the substance’s composition and does not vary
with size or amount.
The trends in density of elements can be observed in groups and periods of the
periodic table. In general in any period of the table, the density first increases from
group 1 to a maximum in the centre of the period because the mass increases while
the size decreases, and then the density decreases again towards group 18 because
of the nature of bonds.
Going down a group gives an overall increase in density because even though the
volume increases down the group, the mass increases more.The variation of density with atomic number is shown in the Figure 5.8.
5.6.6. Electrical and thermal conductivity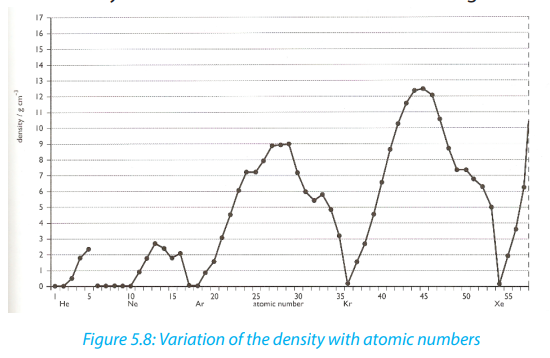
The electrical conductivity is the ability of a substance to conduct an electric current.
The electrical conductivity of elements increases from non-metals to metals. Metals
are good conductor of electricity. This is due to the presence of free electrons in
metallic lattice. The capacity of metals to conduct heat is called thermal conductivity
of metals. Electrical conductivity results from the transfer or mobility of electrons,
whereas the thermal conductivity in metal is due to heat transfer by free electrons
from one end of metal to another end.
As we move across the period from the left to the right, the electrical conductivity
increases for the metals as the number of free electrons increases and then decreases
for the non-metals because they do not have free and mobile electron.
1. Metallic character
Metallic character refers to the level of reactivity of a metal. Metals tend to lose
electrons in chemical reactions, as indicated by their low ionization energies. Within
a compound, metal atoms have relatively low attraction for electrons, as indicated
by their low electronegativities.
Metals are located in the left and lower three-quarters of the periodic table, and tend
to give electrons to nonmetals. Nonmetals are located in the upper right quarter of
the table, and tend to gain electrons from metal. Metalloids are located in the region
between the other two classes and have properties properties.
• Metallic character is strongest for the elements in the leftmost part of the
periodic table and tends to decrease as we move to the right of any period.
• Within any group of the representative elements, the metallic character
increases progressively going down.
2. The electron affinity (E.A)
The electron affinity is the ability of an isolated gaseous atom to accept an electron.
Unlike electronegativity, electron affinity is a quantitative measurement of the
energy change that occurs when an electron is added to a neutral gas atom. The more
negative the electron affinity value, the higher an atom’s affinity for electrons. In the
periodic table, the first electron affinities of elements are negative in general except
the group 18 and group 2 elements. The second electron affinities of all elements
are positive. This is because the negative ion creates a negative electric field. And
if now the other electrons enter the negative field, energy has to be applied to the
system to overcome the repulsion between the negative electric field and incoming
electron.
The more the electron affinity value is negative, the higher is the electron affinity of
an atom. Electron affinity decreases down a group of elements because each atom is
larger than the atom above it (refer to atomic radius trend).This means that an added
electron is further away from the atom’s nucleus compared with its position in the
smaller atom. With a larger distance between the negatively-charged electron and
the positively-charged nucleus, the force of attraction is relatively weaker. Therefore,
electron affinity decreases down the group.
Moving from left to right across a period, the electron affinity increases because
the electrons added to energy levels become closer to the nucleus and there is astronger attraction between the nucleus and electrons.
Checking up 5.6
1. The following table shows a part of a periodic table. Students have to answer
the following
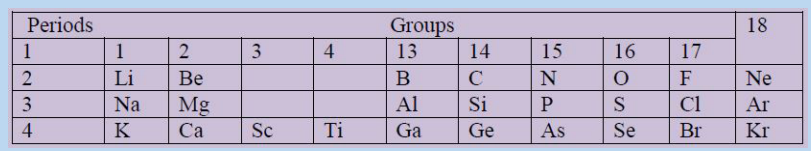
Fill in the blank space with the correct term based on the above table.
The element with the least nuclear charge is …….and the one with the highest
nuclear charge …..Nuclear charge of S is ….than the nuclear charge of Se.
As you go from Na to Cl along the period nuclear charge ….
Effective nuclear charge….from B to Ga while it….from Na to Ar. Shielding or
screening effect ….. down the group but ……along the period from left to right.
Atomic size of Li is…. than that of K. Element with the least atomic size is (10)……
and the element with the highest atomic size is …….
Atomic size of Ca is …. than atomic size of Be because number of …….increases
down the group.
Atomic size …….. from K to Kr because electrons are filled on the same shell, the
…….continuously increase and attraction force increases.
Analyze and complete the following concept map using: ionization energy, atomicsize, electron affinity, electronegativity and metallic character
5.7. End unit assessment
1. The following are coded groups/families of the representative elements of the
periodic table (first 4 periods, s, p blocks only). The groups are in number of
particular order. Use the hints below to identify the group and place of three
elements of each group in their correct location in the periodic table: AOU, BVW,CKM, DLQ, ENT, FIJ, GPY, and HRS.
Hints
A has only one electron in p subshell
B is more electronegative than V
C has a larger atomic radius than both M and W
D has electronic configuration ending in p5
E is one of the most reactive metals
F has a smaller ionization energy than J
G has only 1 energy level with any electrons
H has one more proton than O and is in the same period as O
I is the largest alkaline earth metal
J has one more proton than E
K has electron configuration ending in p3
L has more filled energy levels than D
M is larger than K
N has the largest radius in its family
O is smaller than F but in the same energy level as F
P is smaller than Y
Q is the most reactive non-metal
R has the highest electronegativity in its family
T has the lowest density in its family
U more easily loses electrons (think about ionization energy) than either A or O
V has only 4 electrons in a p-subshell
W has 3 completely filled energy levels
Y has the lowest ionization energy in its family.
2. Based on the variation of ionization energy in groups and periods, how should
you explain the variation of first and second ionization energy down a group and
across a period?
3. Justify the following statements:
a) The first ionization energy of nitrogen is higher than that of oxygen even though
nuclear charge of nitrogen is less compared to oxygen.
b) Noble gases are having high ionization energies.
4. Give reason
a. Alkali metals (group 1 elements) are not found free in nature.
b. Atomic radius of gallium is smaller than that of aluminium.(Z of Al = 13, Z of Ga= 31
