UNIT 16:ACIDS AND BASES
UNIT 16: ACIDS AND BASES
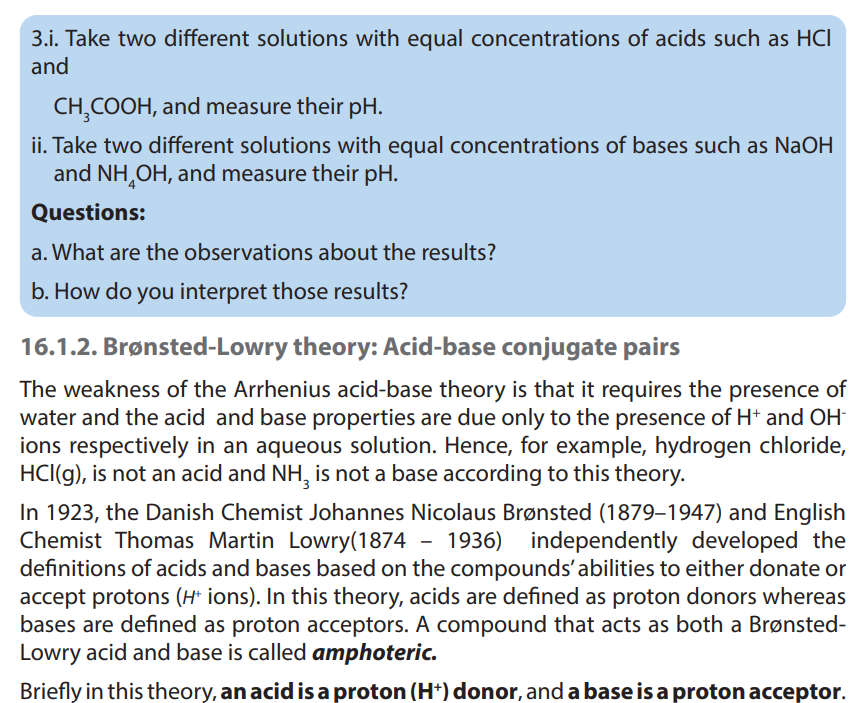
Key unit Competence: Explain the acid-base theories (Arrhenius, Bronsted–Lowry,
Lewis).
Learning Objectives:
By the end of this unit, students should be able to:
• Explain the concept of acid and base using Arrhenius, Brønsted-Lowry and
Lewis’ theory.
• Distinguish strong acids from weak acids and strong bases from weak bases
using Brønsted-Lowry theory.
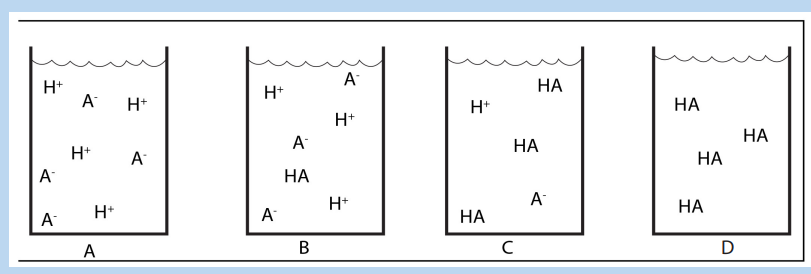
• Classify the acids and bases as strong or weak according to their dissociation
in aqueous solution.
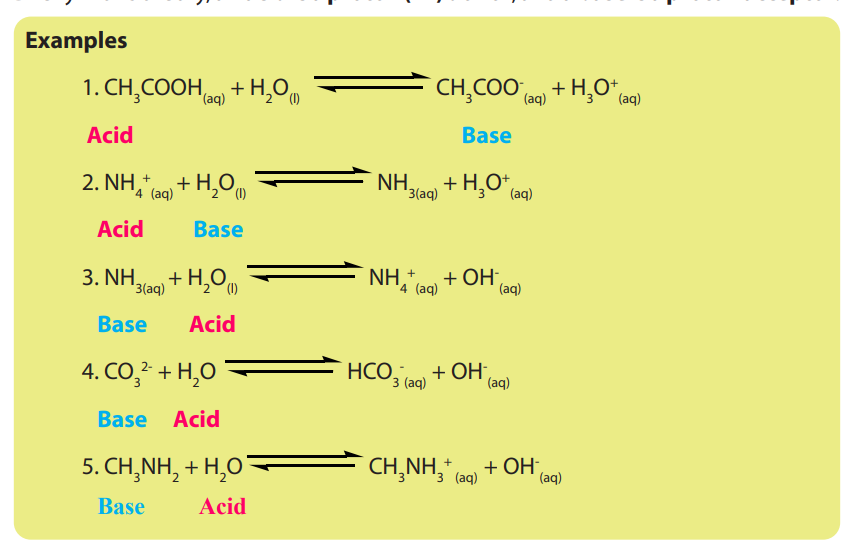
• Distinguish between Brønsted-Lowry and Lewis’ Acid-Base theories.
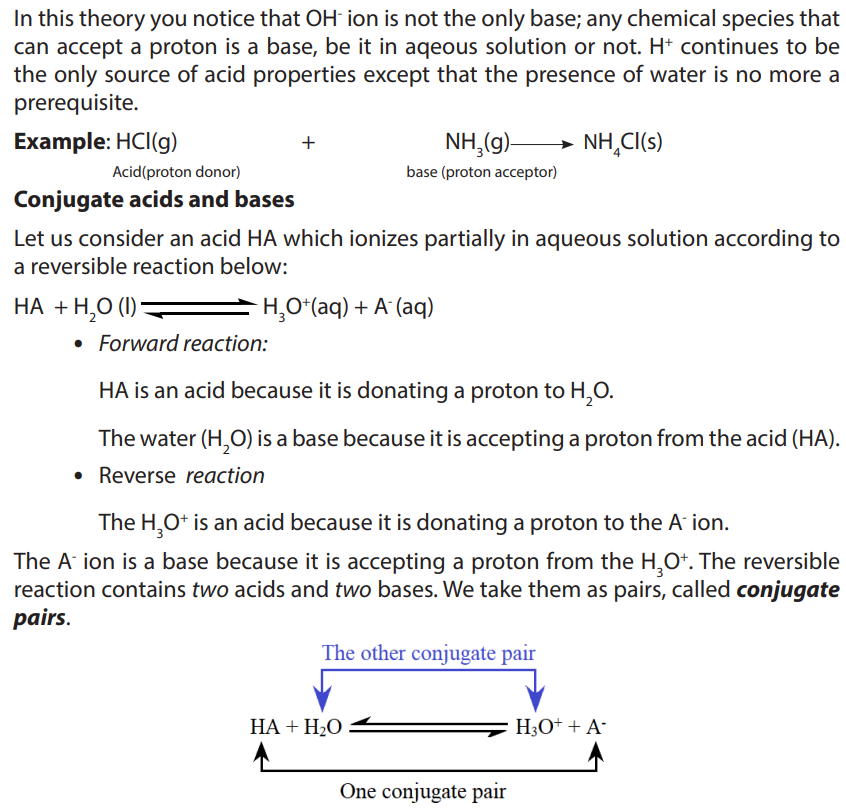
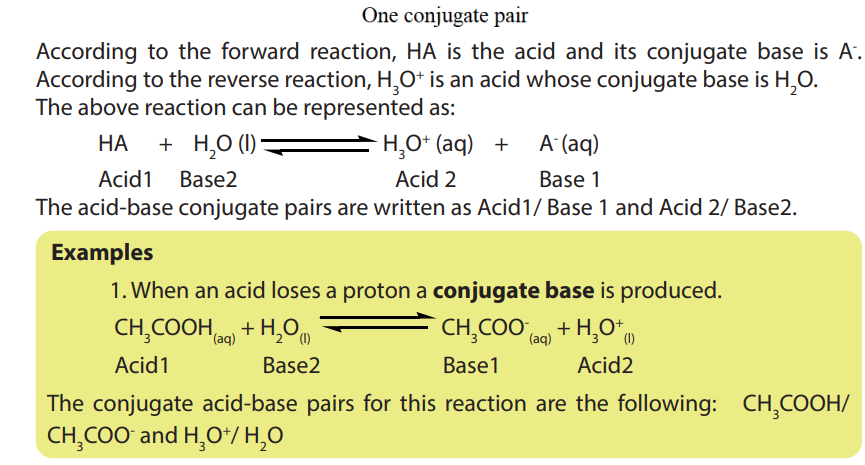
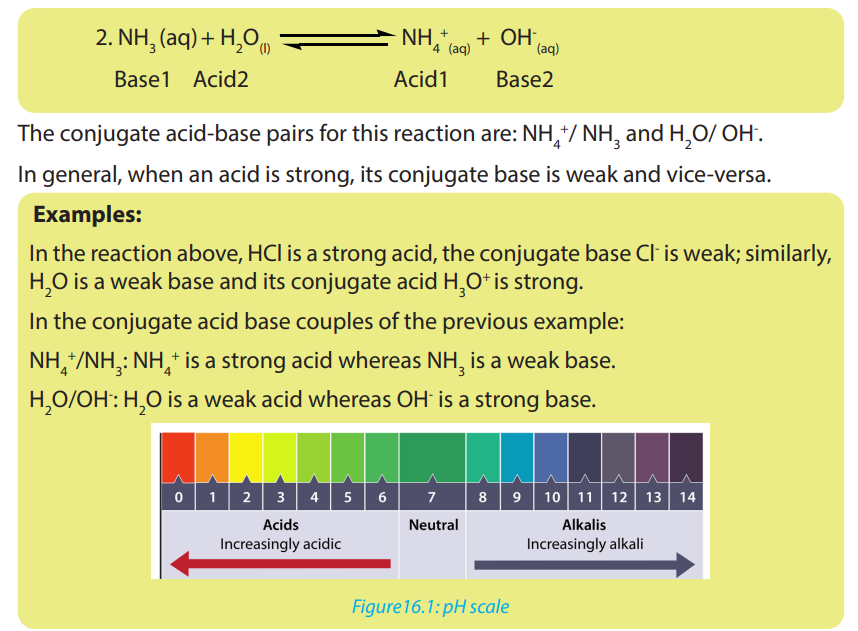
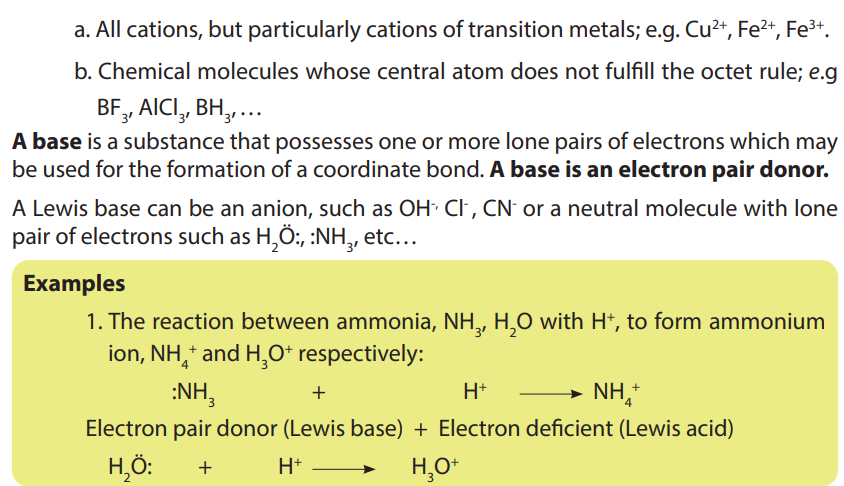
• Write the dissociation of acids and bases and identify the acid-baseconjugate pairs
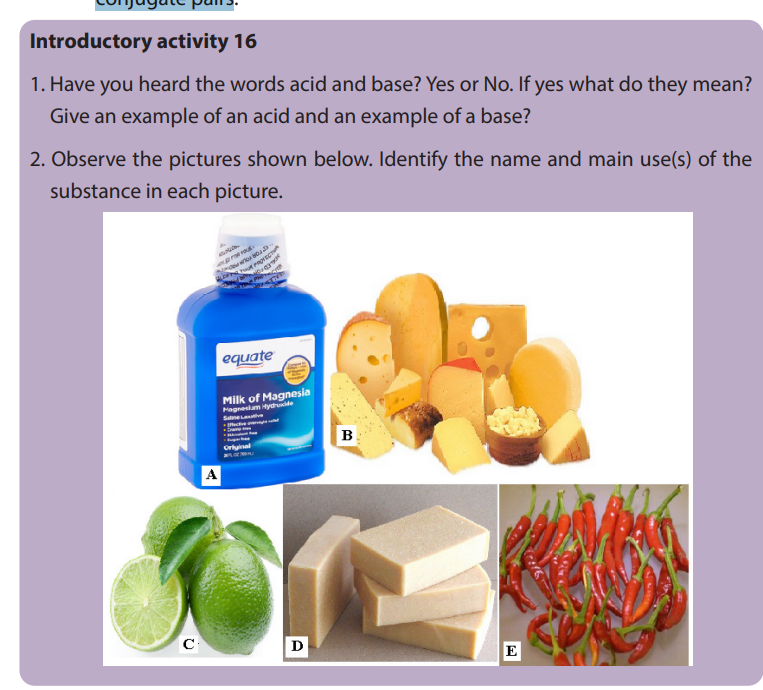

16.1.1. Arrhenius Theory of Acid-Base
The first person to recognize the essential nature of acids and bases was the Swedishscientist Svante Arrhenius (1859–1927). On the basis of his experiments with
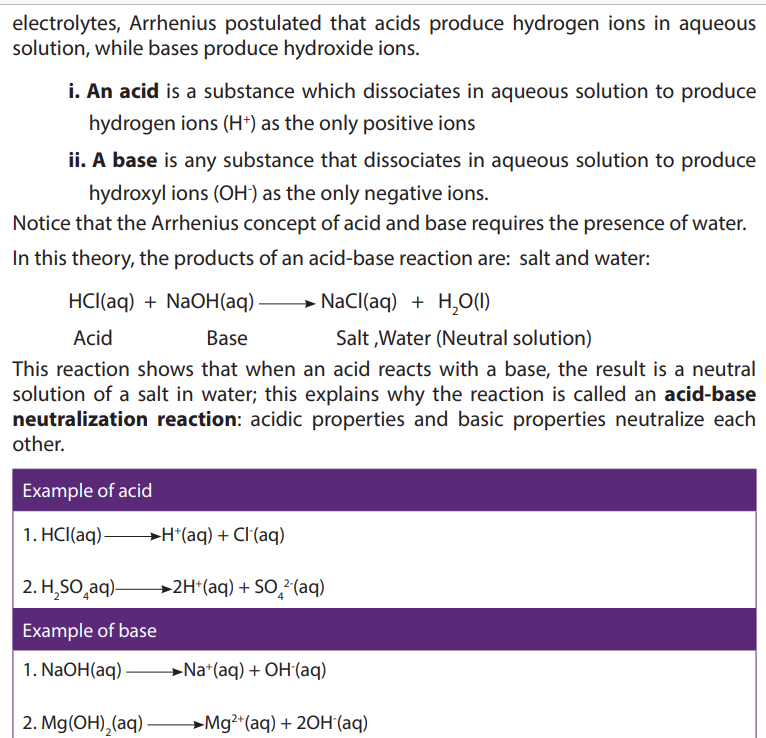

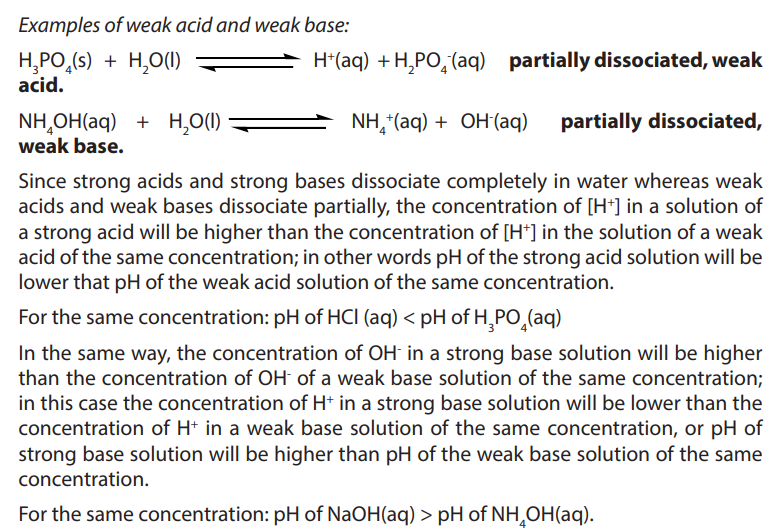
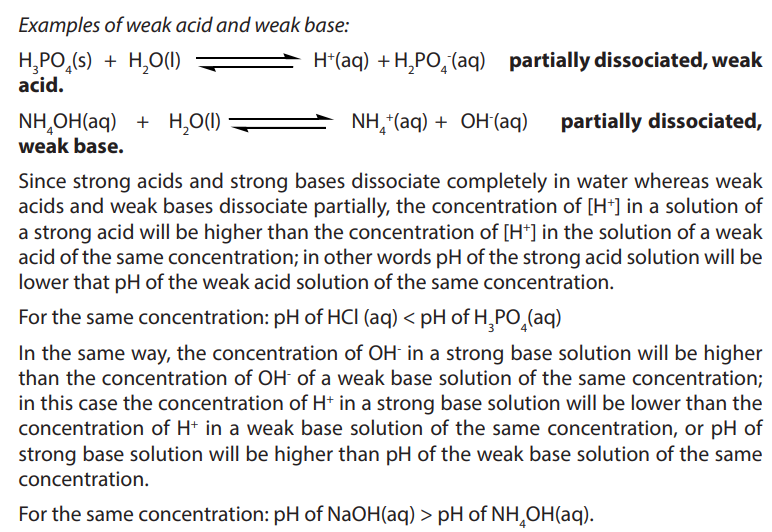
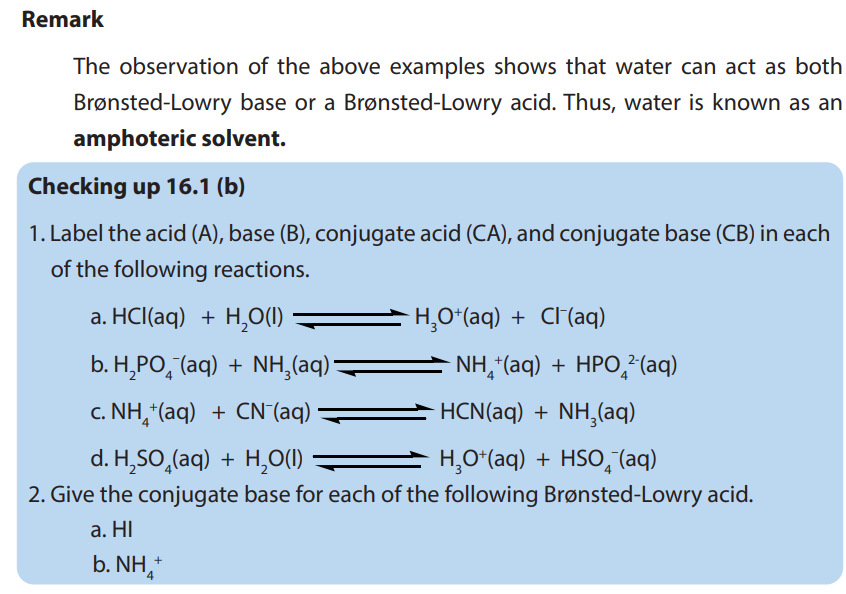
a. Two strongest acidic substances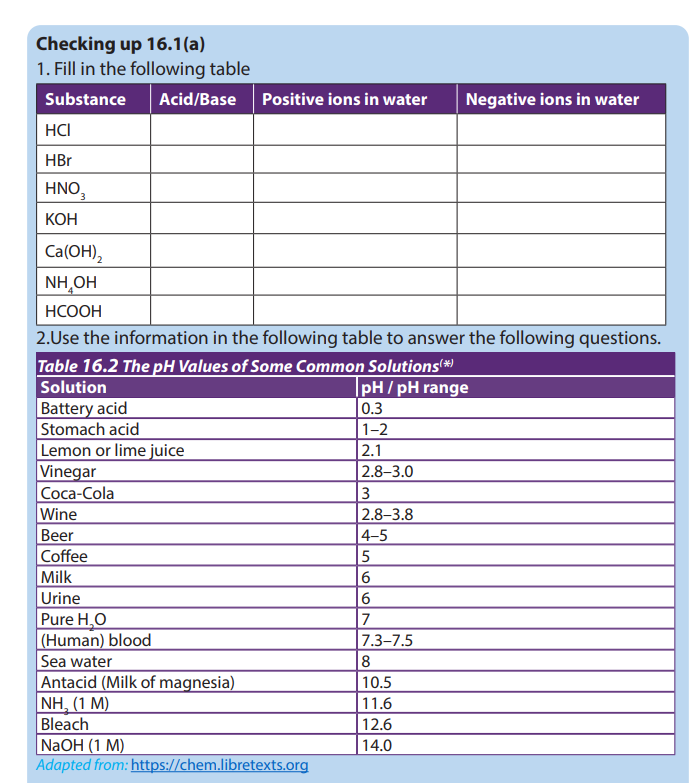
b. Two weakest acidic substances
c. Two most alkaline substances
d. Two least alkaline substancese. Neutral substance (s)


16.2. End unit Assessment