UNIT 13:PROPERTIES AND USES OF GROUP 18 ELEMENTS AND THEIR COMPOUNDS
UNIT 13: PROPERTIES AND USES OF GROUP 18
ELEMENTS AND THEIR COMPOUNDS
Key unit competence: Compare and contrast the properties of the group 18
elements in relation to their position in the periodic table.
Learning objectives
By the end of this unit, students should be able to:
• State the physical properties of the Group 18 elements.
• Explain the lack of reactivity of the group 18 elements.
• Associate chemical inertia of the group 18 elements to their full valence
shell.
• Recognize the importance of noble gases or group 18 elements in the daily
life.
Introductory activity
Make a research to find out the type gas :
Inside the Bulb, in balloon, responsible for different colors dispayed by this house(or in advertising sings)

13.1. Occurrence and physical properties of noble gases
Activity 13.1
The air is composed of a mixture of gases including water vapour.
i)Make a research (with any documentation) to identify its components and arrange them according to their abundances (Component1> Component 2,
etc…)
ii)Show how these components can react each other if possible
• If not possible, justify your answer.
iii)Explain how neon lamp works
13.1.1. Occurrence
• All the noble gases except radon occur in the atmosphere. Their total
atmospheric abundance in air is 0.03%; argon is the major component.
• Helium and sometimes neon are found in minerals of radioactive origine.g., pitchblende, monazite, cleveite.


Checking up 13.2
Question: Explain why in some applications such as air balloons, helium is
preferred to hydrogen?
13.3. Uses of noble gases
Activity: 13.3
Do a research (with any documentation) to find how each noble gas has been
discovered and its uses?
Helium
• Helium is a non-inflammable and light gas. Hence, it is used in filling balloons
for meteorological observations, replacing the flammable hydrogen gas.
• It is also used in gas-cooled nuclear reactors.
• Liquid helium (B.P:-267.8o
C) finds use as cryogenic agent for carrying out
various experiments and conservation at very low temperatures.
Neon
• Neon is used in advertising signs, it glows when electricity is passed through
it. Different coloured neon lights can be made by coating the inside of the
glass tubes with colored chemicals.• Neon bulbs are more used in our daily life.
Argon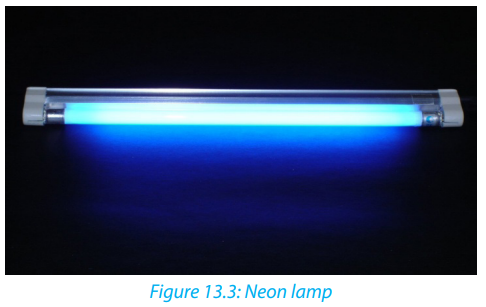
• It is used in light bulbs. The very thin metal filament inside the bulb would
react with oxygen and burn away if the bulb were filled with air instead of
argon.
• Argon is used mainly to provide an inert atmosphere in high temperature
metallurgical processes (arc welding of metals or alloys).
• It is also used in the laboratory for handling substances that are air-sensitive.
Krypton
Krypton is used in lasers. Krypton lasers are used by surgeons to treat certain eye
problems. It is used in light bulbs designed for special purposes.
Xenon
Xenon is used in fluorescent bulbs, flash bulbs and lasers. Xenon emits an instant,
intense light when present in discharge tubes. This property of xenon is utilized in
high-speed electronic flash bulbs used by photographers.
Radon
Radon is radioactive and is used in medicine as a source of gamma rays. The gas is
sealed in small capsules, which are implanted in the body to destroy malignant (e.g.,cancerous) growths.
13.4. End unit assessment
1. a) Give a reason why the first ionization energies of noble gases are very high.
b) State one use of neon and give a reason to support your answer.
c) State and explain the trend in atomic radius among noble gases.
d) Why are noble gases unreactive?
e) Explain why the value of the first ionisation energy of neon is higher than that
of sodium.
2. Explain why Group 18 elements are rare on Earth?
3. The discovery of compounds of noble gases has been done, up to date, with Xeand Kr, not with He or Ne. Can you suggest a probable reason?
