UNIT11:TRENDS OF CHEMICAL PROPERTIES OF GROUP 16 ELEMENTS AND THEIR COMPOUNDS
UNIT 11: TRENDS OF CHEMICAL PROPERTIES OF GROUP
16 ELEMENTS AND THEIR COMPOUNDS
Key unit competence:
Compare and contrast the chemical properties of the Group 16 elements and their
compounds in relation to their position in the Periodic Table.
Learning objectives
By the end of this unit, students should be able to:
• Describe the physical properties of Group 16 elements.
• Describe the reactions between sulphur and oxygen.
• Describe the steps and conditions applied in the industrial preparations of
sulphuric acid.
• Describe the chemical properties of sulphuric acid.
• Describe the properties of oxoanions.• State uses of the Group 16 elements and compounds.
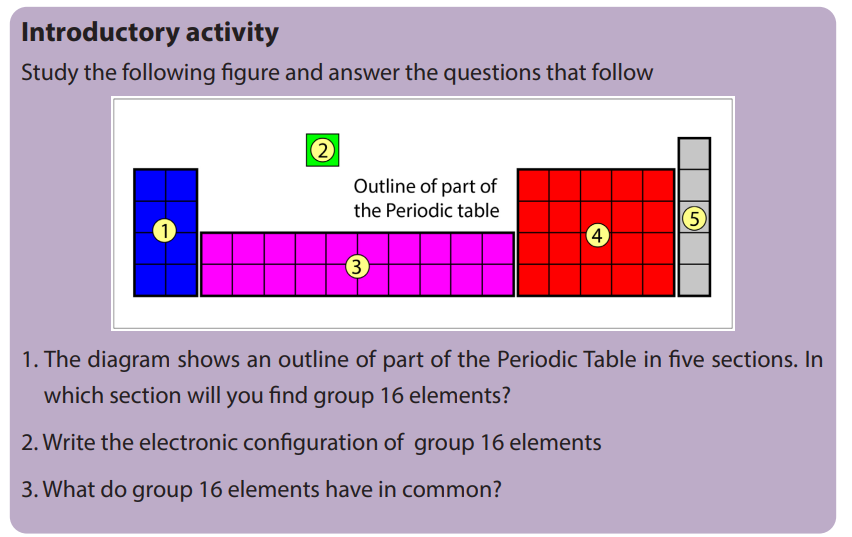
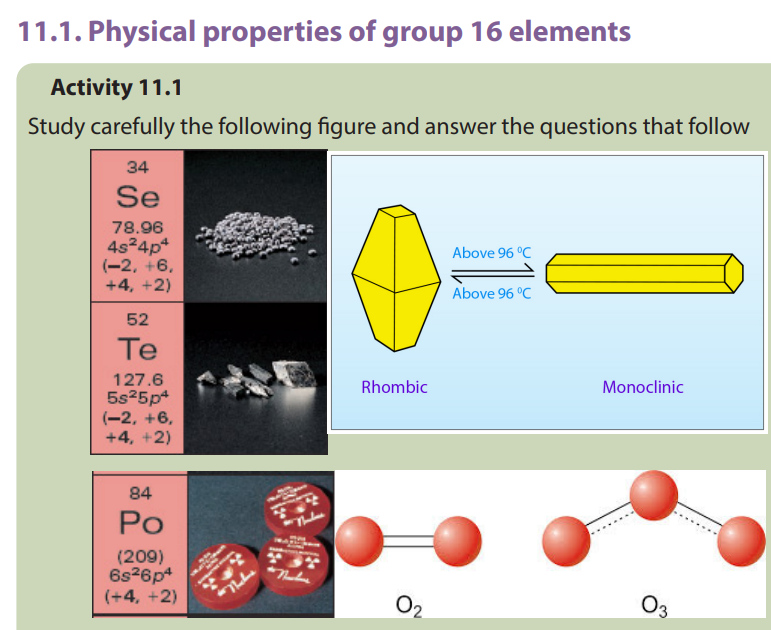


11.2. Comparison of acidity and volatility of group 16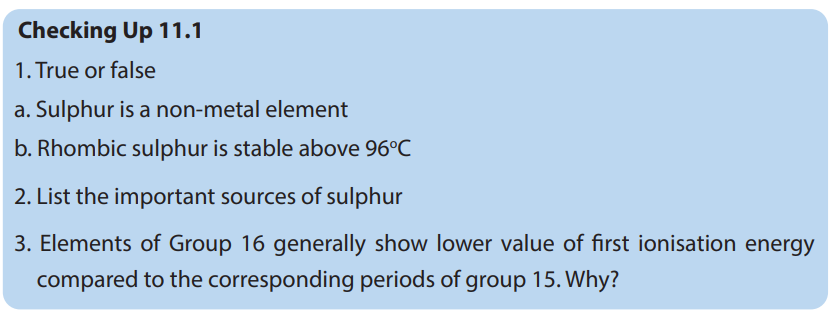
hydrides
Activity 11.2
1. In pairs, carry out research and write a note on the following terms:
a. Hydrides
b. The strength of an acid
d. A weak acid
e. A strong acid
2. With an example, explain what is meant by the term “hydrogen bond” and showhow it is formed.

Safety:
Sulfuric acid is a very strong acid and is extremely corrosive to skin. Wear gloves
and safety goggles. During the reaction, steam is generated. It is hot. It is
recommended to work in a fume cupboard.
Procedure:
Spread some paper towels on the tray.
1. Put sugar into 300 ml beaker.
2. Insert stirring rod into center of sugar.
3. Put beaker on paper towels on the tray.
4. Add 70 ml of sulfuric acid to the sugar and stir briefly.
5. Stand about 1 - 2 meters away and wait for reaction to begin and observe what
will happen.
Clean Up: You might want to incorporate part of the clean up procedure into the
demonstration.
Remove black carbon column from the beaker and put it into a liter beaker with
some sodium bicarbonate (hydrogen carbonate). With spatula, break the column
of carbon into smaller pieces. Add a little water and set back on the tray. The
foaming action is also exciting.
Neutralize any acid spills with sodium bicarbonate and wipe clean. Leave lecture
hall clean for the next class.
Rinse all glassware and carbon chunks with lots of water. Carbon can be thrown
away in trash.
Study questions
1. Record your observations2. Write an equation for a reaction that takes place in this experiment

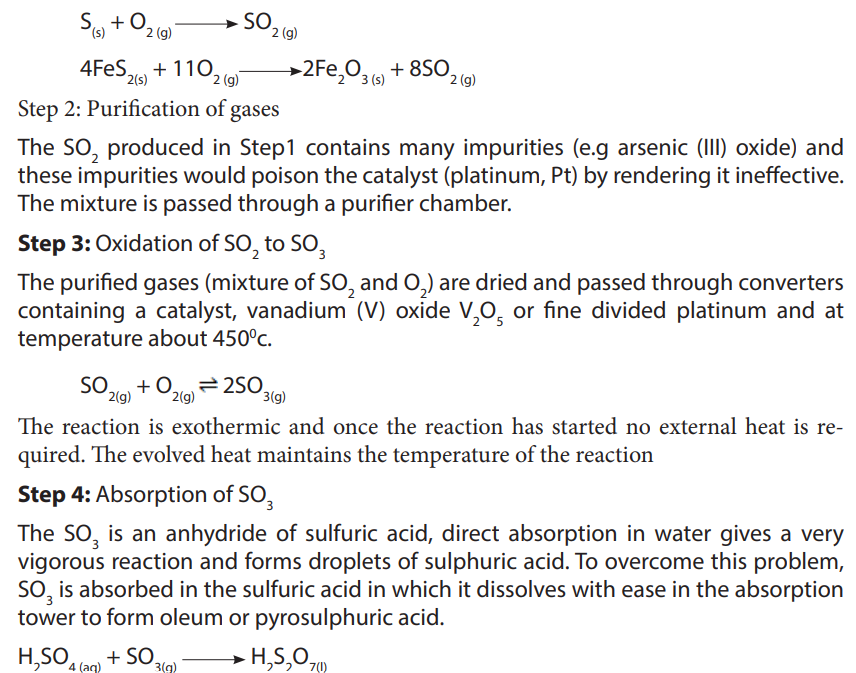
Step 1: Production of sulphur dioxide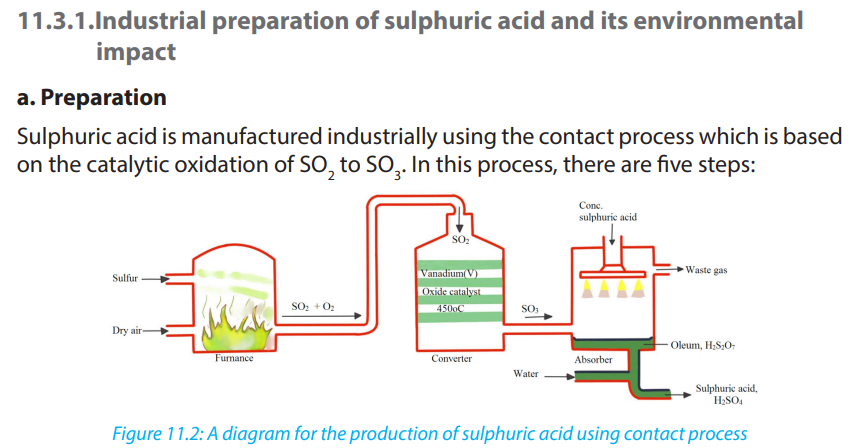
Sulphur dioxide is obtained by either burning elementary sulphur or roasting metalsulphides in air in combustion chamber.

Checking Up 11.3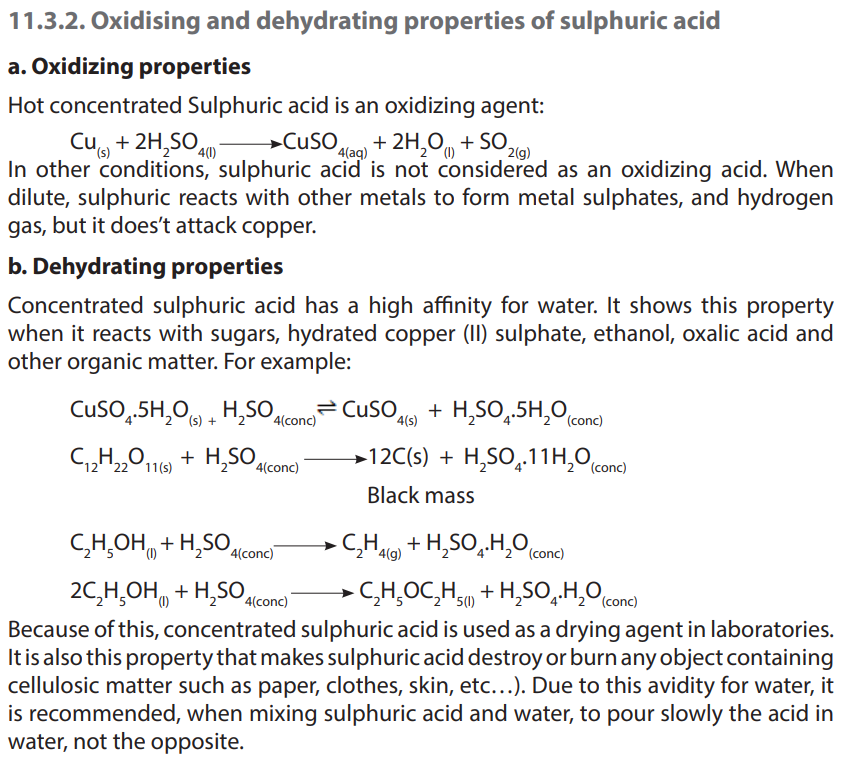
1. a) Describe the Haber or Contact process for the manufacture of sulphuric acid.
b) Why is sulphur trioxide formed in this process not absorbed directly in water?
2. Concentrated sulphuric acid acts as a dehydrating agent. What does it mean?
3. Write equations to show how concentrated sulphuric acid reacts with:
a. Zinc
b. Magnesiumc. Carbon
11.4 Properties of oxoanions of sulphur
Activity 11.4 (a)
1. Use the library and/or internet to explain the following:
a. Oxidation
i) In terms of oxidation state
ii) In terms of electron transfer
b. Reduction
i) In terms of oxidation state
ii) In terms of electron transfer
c. Oxidizing agent
d. Reducing agent
Activity 11.4 (b)
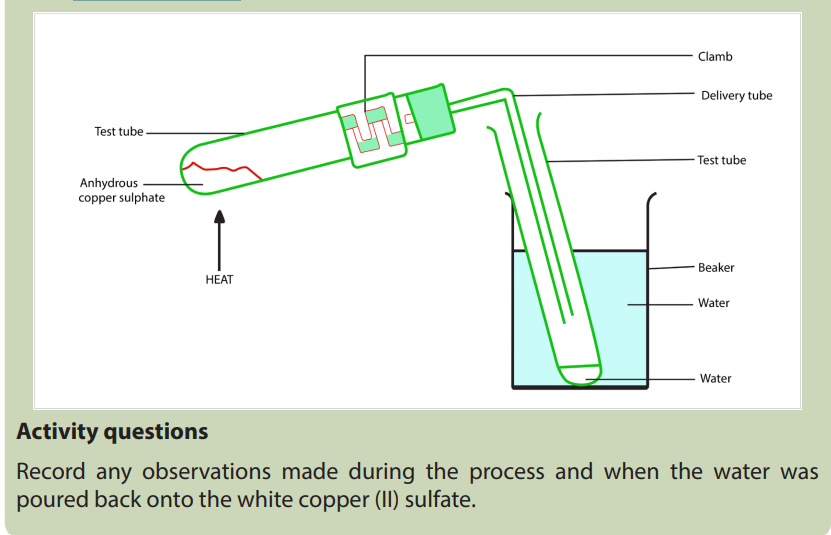
2. An experiment for Heating hydrated copper(II) sulfate
Objectives:
Students remove the water of crystallisation from hydrated copper (II) sulfate
by heating. Condensing in a test-tube collects the water. The white anhydrous
copper (II) sulfate can then be rehydrated, the blue colour returns.
Apparatus and equipment (per group)1. Two test-tubes
potassium sulphate, and calcium sulphate are not decomposed by heat.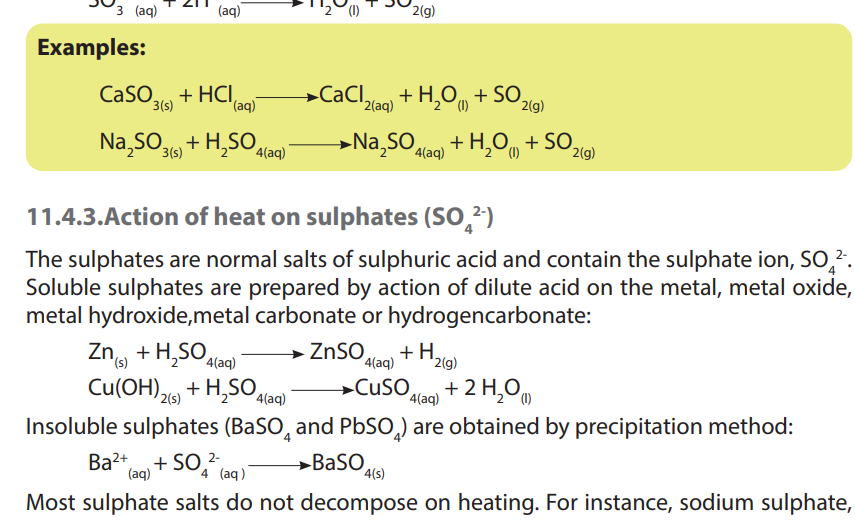
Only certain sulphate salts are decomposed by heat when heated strongly. On
heating, some sulphates decompose to give either sulphur trioxide or sulphurdioxide or both.
Checking Up 11.4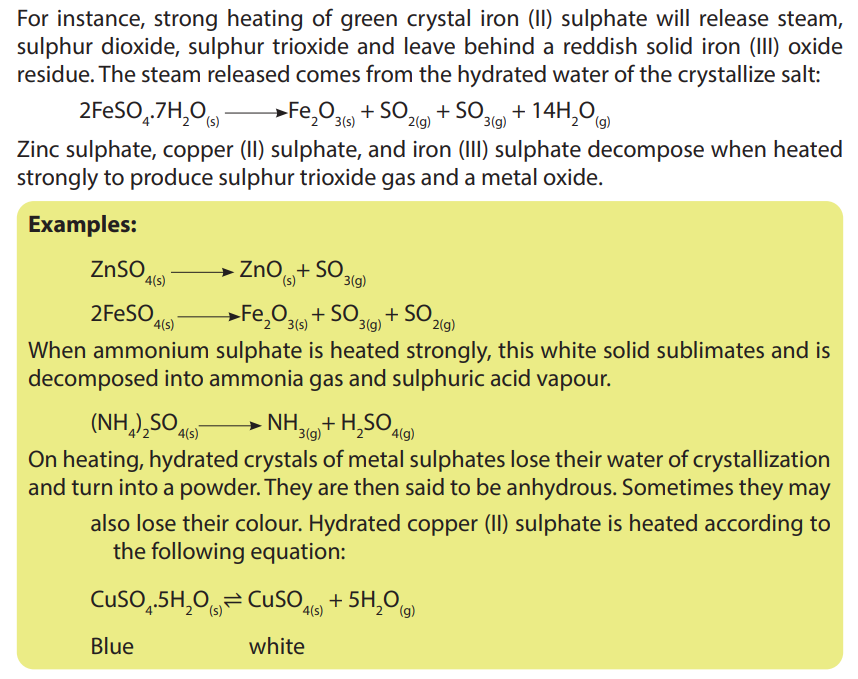
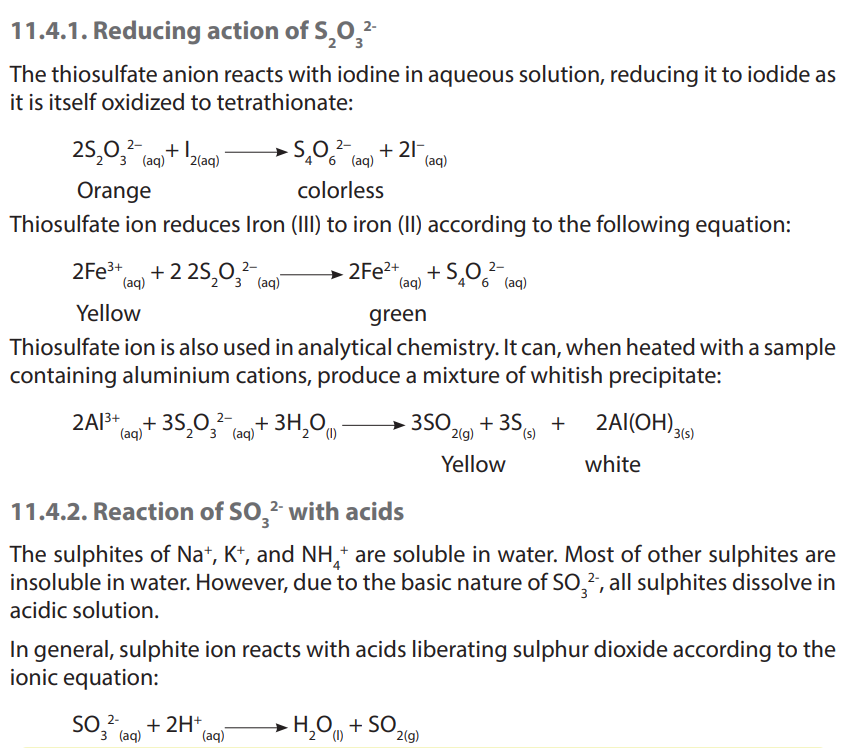
1. Write equations to show how thiosulfate ions reduce the following substances:
a. Iodine
b. Iron (III) ion
c. Aluminium ion
2. Write equations to show the action of heat on the following sulphates:
a. Zinc (II) sulphate
b. Iron (III) sulphate
c. Copper (II) sulphate
3. When hydrated copper II sulphate solid is heated in a boiling tube, a white solid
Q and droplets of a colourless liquid P are observed.
a. Identify substances; liquid P and solid Q.
b. Explain the observation above.
Explain what would be observed if water is added to white solid Q.
11.5 Identification of sulphite and sulphate ions
Activity 11.5
Given a substance Y which contains one cation and one anion, identify the cation
and the anion in Y. Carry out the following tests on Y and record your observationsand deductions in the table below.
11.6.1.Uses of oxygen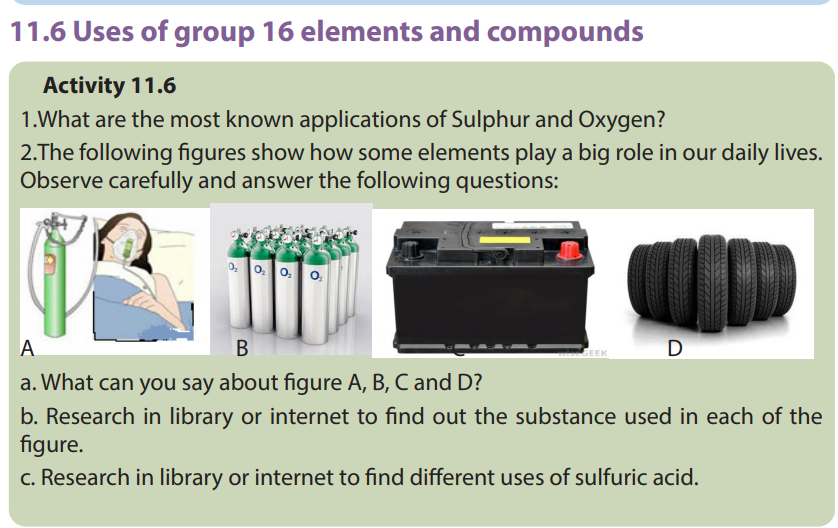
The first use of oxygen is in breathing and metabolism processes of all living
organisms.
There are many other commercial uses for oxygen gas, which is typically obtained
through fractional distillation of air. It is used in all operations involving combustion
as the active component of air.
It is used in the manufacture of iron, steel, and other chemicals. Oxygen is also used
as an oxidizer in rocket fuel, and for medicinal purposes. Mixture of oxygen and
ethyne (oxyacetylene) is used for welding and metal cutting.
11.6.2. Uses of sulphur
The main use of Sulphur is the manufacture of sulphuric acid.
Sulphur is also used in vulcanization of rubber, a chemical process for
converting natural rubber or related polymers into more durable and pressure
resisting materials by heating them with sulfur or other equivalent curatives
or accelerators. These additives modify the polymer by forming cross-links (bridges)
between individual polymer chains, making the final product very hard and resistant
to pressure and other conditions.
Sulphur is an ingredient in the manufacture of dyes, fireworks and other sulphur
compounds.
11.6.3. Uses of sulphuric acid
Sulphuric acid is a very important industrial chemical. It used to be called the giant
of chemical industry. It is used in the manufacture of hundreds of other compounds
in many industrial processes.
• The bulk of sulphuric acid produced is used in the manufacture of fertilisers
(e.g., ammonium sulphate, superphosphate).
• Sulfuric acid is also used in many other applications such as in: metallurgicalindustry, storage batteries, chemistry laboratories, etc….