UNIT:TRENDS OF CHEMICAL PROPERTIES OF GROUP 15 ELEMENTS AND THEIR COMPOUNDS
UNIT 10: TRENDS IN CHEMICAL PROPERTIES OF GROUP
15 ELEMENTS AND THEIR COMPOUNDS
Key unit competency: Compare and contrast the properties of Group 15 elements
and their compounds, in relation to their position in the Periodic Table.
Learning objectives
By the end of this unit, students should be able to:
• Describe the physical properties of Group 15 elements.
• Describe the variation in the metallic and non-metallic character of Group
15 elements.
• Explain recall the physical properties of the allotropes of phosphorus.
• Describe the chemical reactions of nitrogen compounds.
• Describe the impact of nitrogen oxides to the environment.
• Describe the industrial preparation of ammonia and nitric acid.
• Explain the reactions of nitric acid with metals and non-metals.
• Describe the chemical properties of phosphorus compounds.• State the uses of the group 15 elements and its compounds
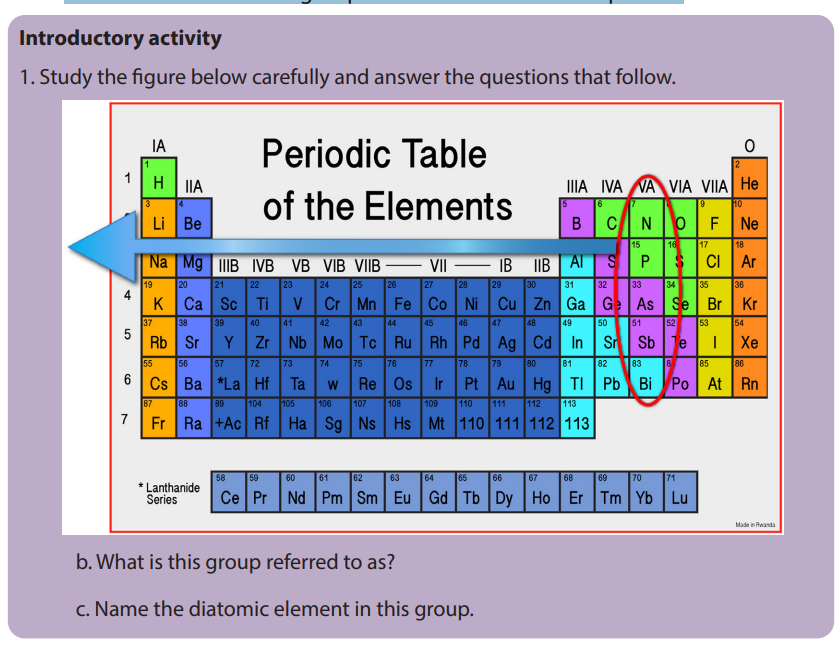
10.1. Physical properties of group 15 elements and the
relative inertness of nitrogen
Activity 10.1
In pairs:
1. Assign the physical state for each of the elements in group 15
2. Explain what is meant by the term “metallic character”.
3. Classify each element in this group as metal, non-metal or metalloid.
4. Study the following figure carefully and answer the questions that follow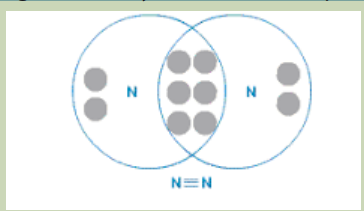
a. Identify the molecule represented in the figure.
b. What type of bond is there in the molecule?c. Suggest if the bond is strong or weak

b. Metallic character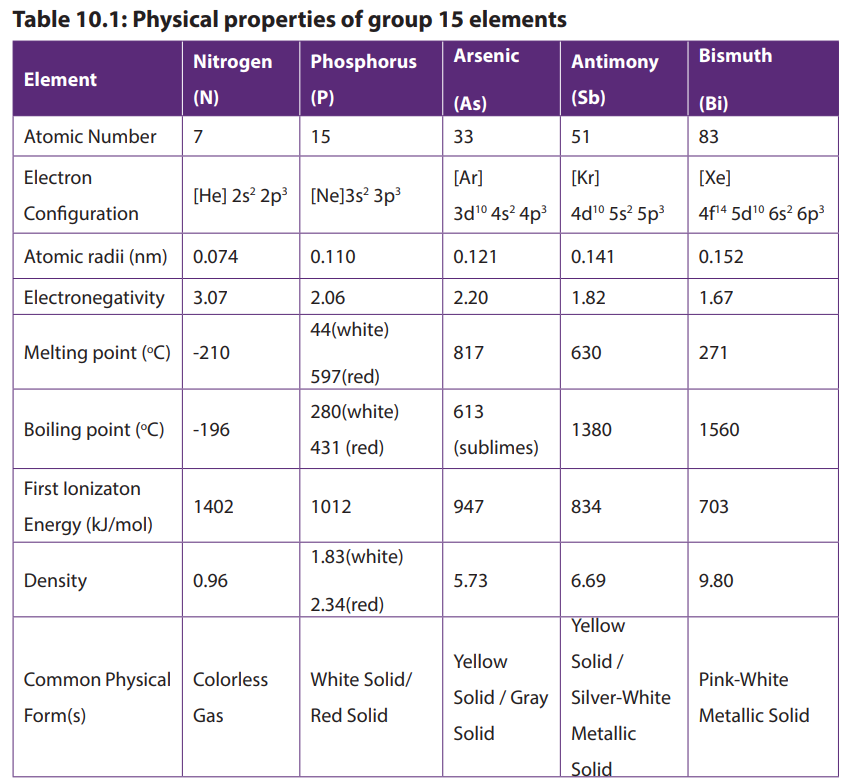
Down group 15 elements, the atomic radius increases which makes the outermost
electron to be less attracted by the nucleus as you move down the group. Therefore,
less energy is required to remove the outermost electron, which results in the
increase in the metallic character down the group. This results also in decreasing of
ionization energy down the group.
Nitrogen and phosphorous are non-metals, with the metallic properties first
appearing in arsenic and increasing down the group. Arsenic and antimony aremetalloids. Bismuth is a metal.
Checking Up 10.1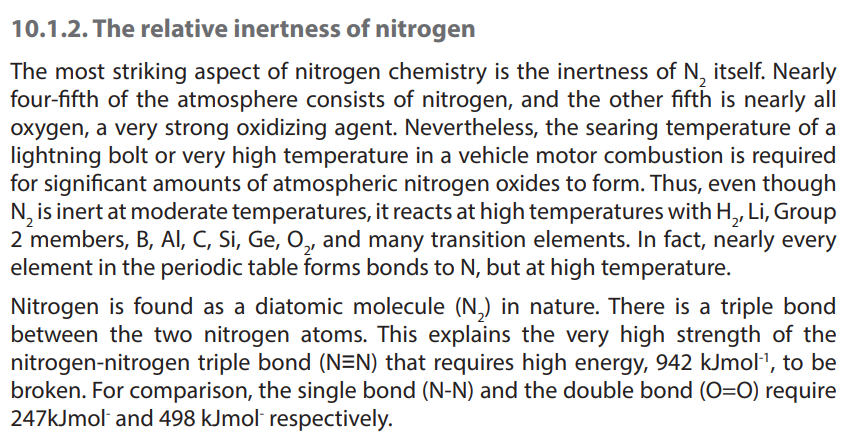
1. Briefly describe how each of the following factors varies in group 15 elements:
a) Atomic radius
b) Electron affinity
c) Melting point
d) First ionization energy
2. Explain the following observations:
a) In group 15 of the periodic table, metallic character increases as you move
down the group.
b) The atomic radii of two elements A and B from group 15 are 0.121nm and 0.141nmrespectively. Identify the element with more metallic character. Justify your answer
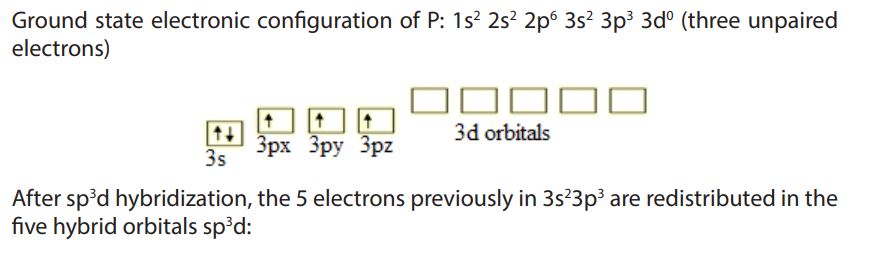
10.2. Reactions of group 15 elements
All group 15 elements exhibit a common valency of three. They can complete their
octet structure in chemical combination by gaining three electrons.
However, with the exception of nitrogen, group 15 elements have vacant d-orbitals
which they use to expand their octet to form compounds with a valency of five. For
instance phosphorous has a covalency of 5 due to availability of easily accessible
empty d orbitals which can be used for sp3
d hybridization that allows it to have 5unpaired electrons. Consider phosphorous, atomic number 15.
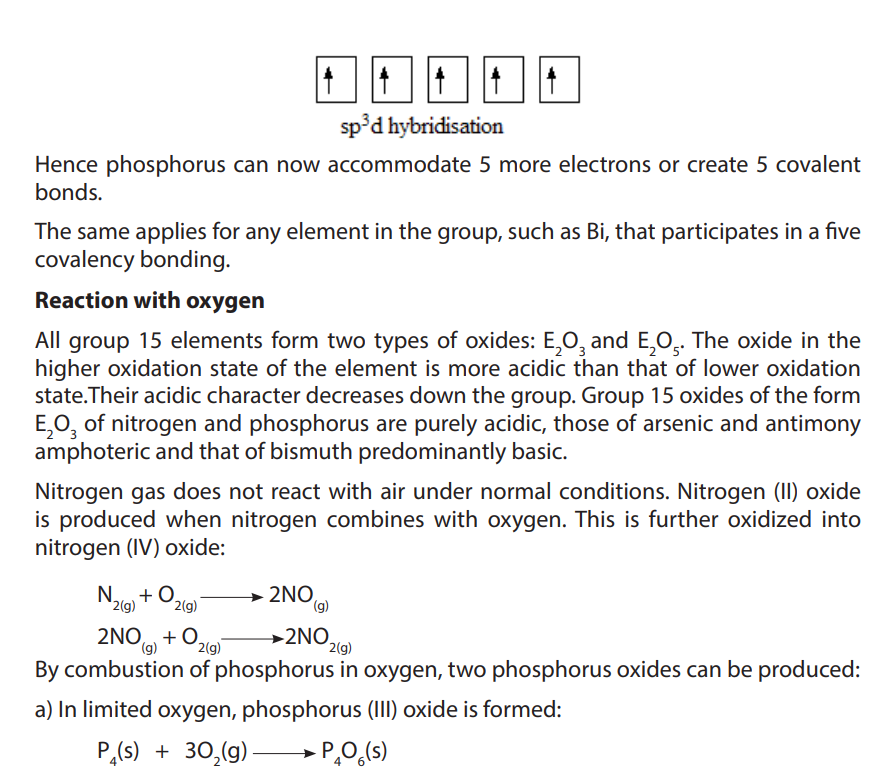
c. i) State whether each oxide of A you have given in (b) is acidic, basic, neutral, or amphoteric and justify.
ii) Write the equation of reaction to illustrate your answer.
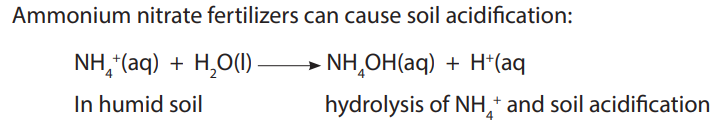
10.3. Ammonia and nitric acid
Activity 10.3 (a)
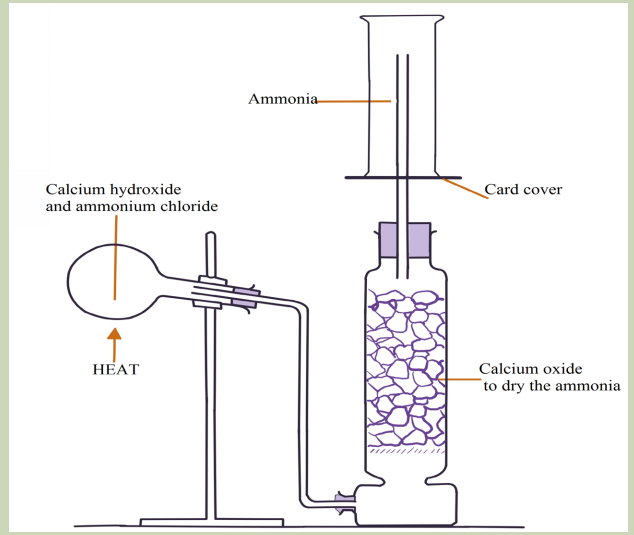
Experiment: Laboratory preparation of ammonia
Materials and chemicals
Round bottom flaskor hard glass test tube, U-tube, 3 corks, 10 grams of calcium
hydroxide, gas jars, bent delivery tube and straight delivery tube, 5 grams of
ammonium chloride on a watch glass and calcium oxide lumps.
Procedure
1. Set up the apparatus as shown in the diagram, with the chemicals indicated. Do
not start heating yet.
2. When everything is in position, heat the hard flaskandcollect several gas jarsofammonia. Cover each jar with a glass slip and keep the jars for other experiments.
Activity evaluation questions
1. Record your observations
2. Write a balanced equation of the reaction that take place.
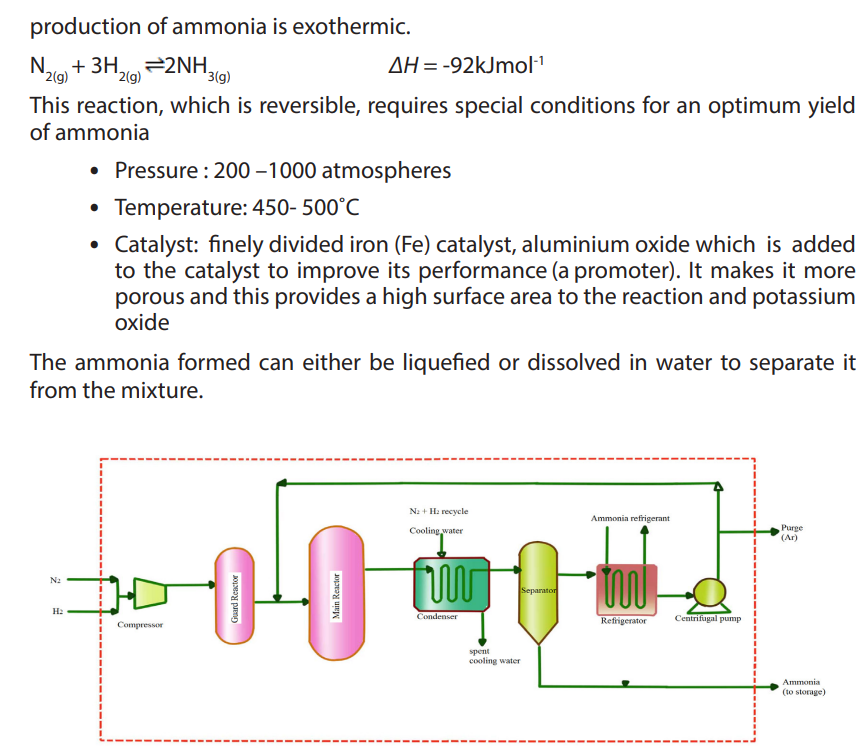
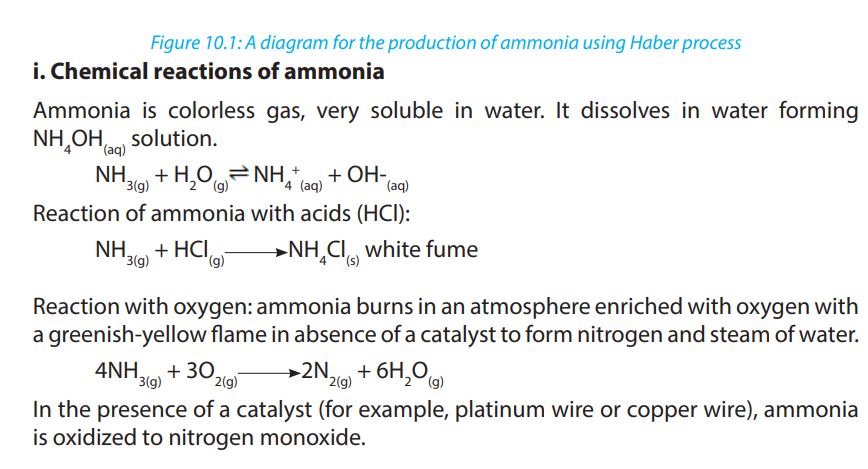
10.3.1. Laboratory preparation of ammonia and nitric acid
a. Laboratory preparation of ammonia
Ammonia is a covalent compound, consisting of nitrogen bonded to three hydrogen
atoms. It exists as a colourless gas at room temperature and it is naturally produced
during the decaying of nitrogenous organic compounds such as proteins. Ammonia
has a characteristic pungent odour.It is less dense than air and thus collected by
upward delivery method. In the laboratory it is prepared by heating a mixture of anyammonium salt and an alkali.
i. Uses of ammonia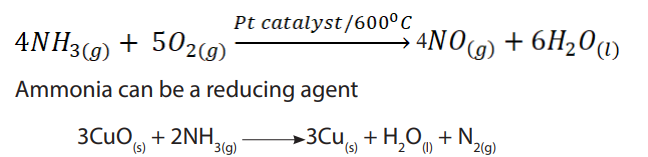
Agricultural industries are the major users of ammonia. Ammonia and urea are used
as fertilizer, as very valuable source of nitrogen that is essential for plant growth.
Ammonia and urea are used as a source of protein in livestock feeds for ruminating
animals such as cattle, sheep and goats.
Ammonia can also be used as a pre-harvest cotton defoliant, an anti-fungal agent
on certain fruits and as preservative for the storage of high-moisture corn.
The pulp and paper industry uses ammonia for pulping wood and as casein
dispersant in the coating of paper.
The food and beverage industry uses ammonia as a source of nitrogen needed for
yeast and microorganisms involved in the fermentation process.
ii. Environmental impact for industrial production of ammonia
Making ammonia using the Haber process requires a lot of energy, which usually
involves burning fossil fuels. This releases carbon dioxide which causes global
warming.
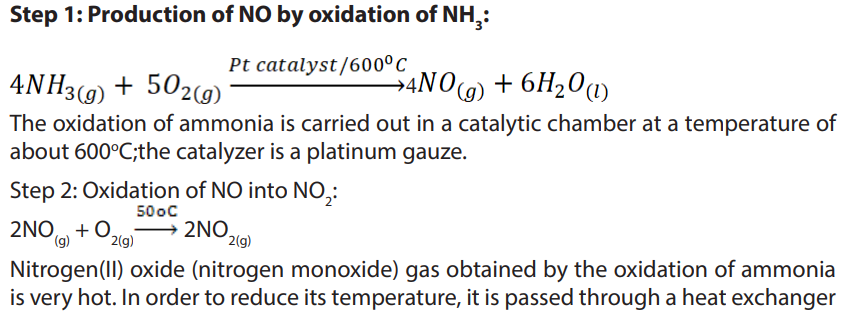
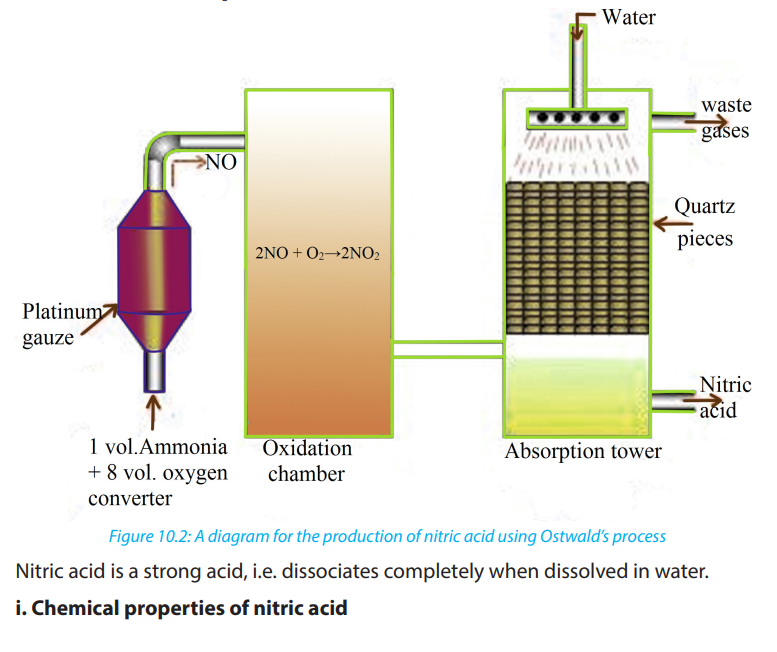
b. Production of Nitric acid (Ostwald’s process)
In the industrial manufacture of nitric acid a catalytic oxidation of ammonia to
nitrogen (II) oxide,NO, is carried out then a further oxidation of nitrogen (II) oxide
produces nitrogen (IV) oxide, NO2
. Nitrogen dioxide is passed through water sprays
in a steel absorption tower to produce nitric acid. The excess nitrogen monoxide
is recycled back for more oxidation. Platinum is used as a catalyst. There are threesteps:


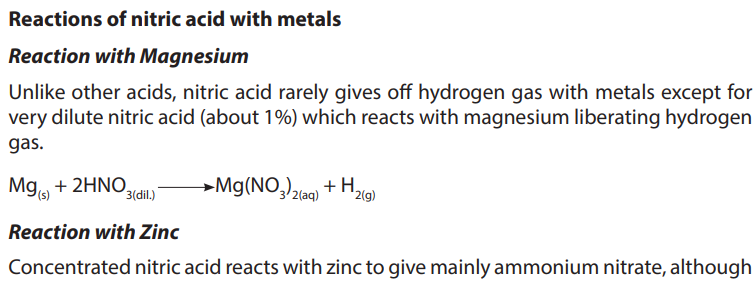


b. Oxygen in the presence of a catalyst
c. Copper (II) oxide
d. Hydrochloric acid
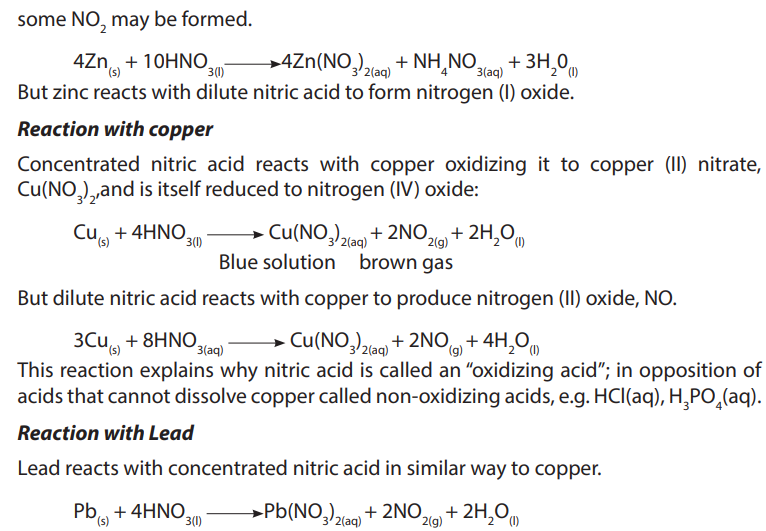
6. Write equations to show how nitric acid reacts with the following substances:
a. Copper
b. Sulphurc. Potassium hydroxide
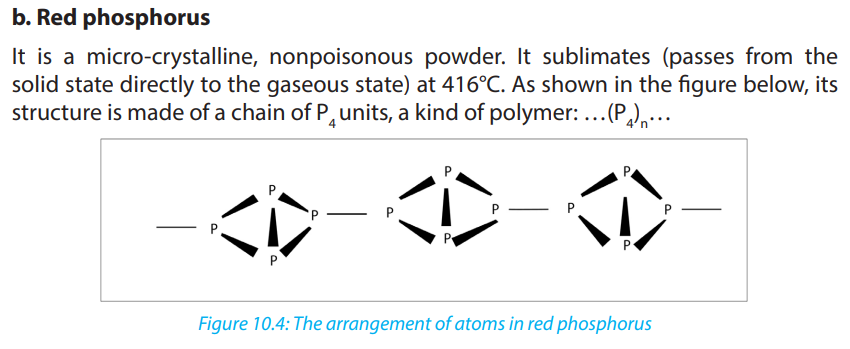
10.4.1. Allotropes of phosphorus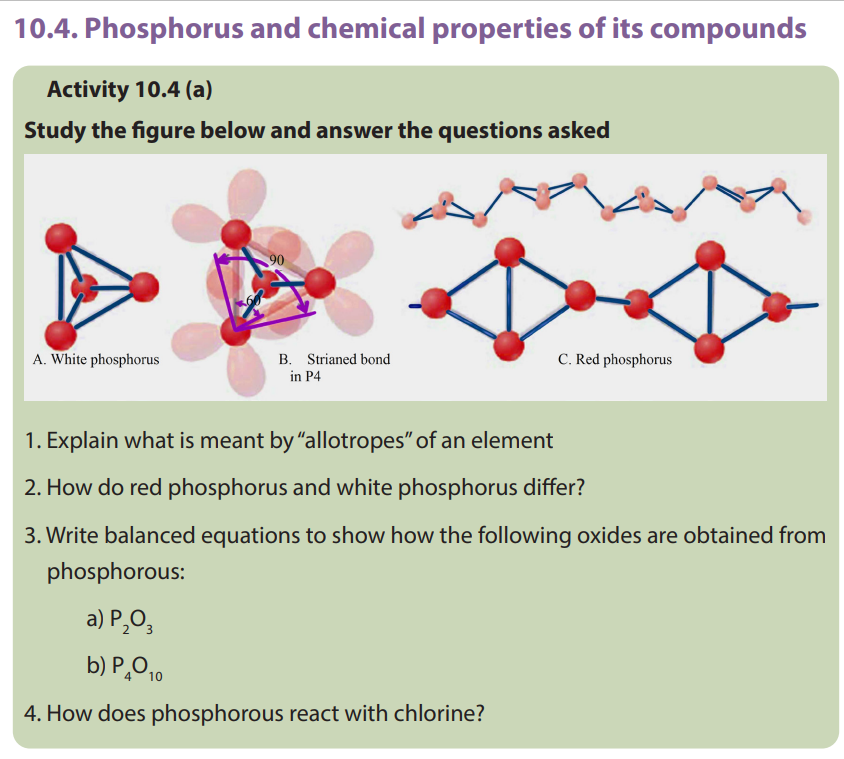
By definition, allotropy is a property exhibited by some elements to exist in multiple
forms with different crystal structures. Allotropes are any two or more physical forms
in which an element can exist. Phosphorus exists in two main allotropic forms:• White phosphorus
When prepared, ordinary phosphorus is white, but it turns light yellow when exposed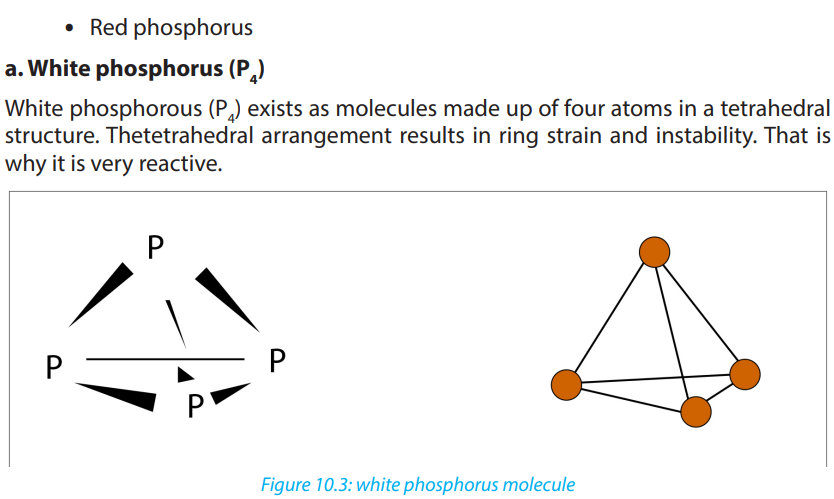
to sunlight. It is a crystalline, translucent, waxy solid, which glows faintly in moist
air and is extremely poisonous.It ignites spontaneously in air at 34°C and must be
stored under water. It is insoluble in water, slightly soluble in organic solvents, and
very soluble in carbon disulfide. White phosphorus melts at 44.1°C, boils at 280°C.
White phosphorus is prepared commercially by heating calcium phosphate with
sand (silicon dioxide) and coke in an electric furnace. When heated between 230°C
and 300°C in the absence of air, white phosphorus is converted into the red form.
White phosphorus spontaneously takes fire in contact with air. White phosphorus isconsidered and has been used as a chemical weapon.




10.1.1 Environmental problems of using chemical fertilizers of nitrates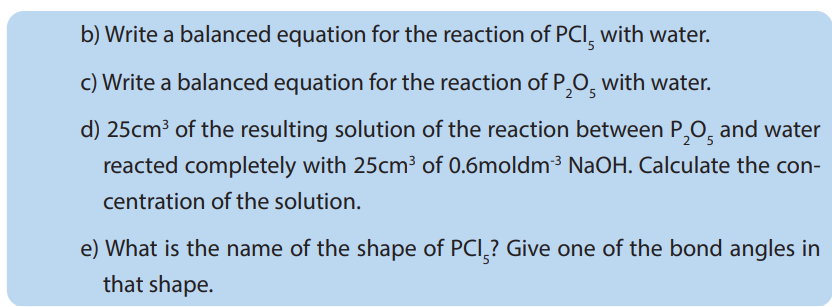
and phosphates
Nitric acid is mainly used in the manufacture of nitrates fertilizers. Excess use of
nitrates as fertilizers is responsible of one type of pollutions of lakes and rivers called
eutrophication.
Eutrophication results from the excessive richness of nutrients in a lake or a water
body which causes a dense growth of plant life. When those water plants die and
are decomposed, during the decomposition process that uses oxygen, they deplete
the oxygen of the water body and render that water incapable of sustaining living
aquatic organisms. In that case, the body of water is said to be dead (biologically).
The fraction of the nitrogen-based fertilizers which is not converted to be used
by plants accumulates in the soil or gets lost as run-off. High application rates of
nitrogen-containing fertilizers combined with the high water solubility of nitrate
leads to increased runoff into surface water as well as leaching into groundwater,thereby causing groundwater pollution.