UNIT1:STRUCTURE OF AN ATOM AND MASS SPECTRUM
Key unit competency
Interpret simple mass spectra and use them to calculate the relative atomic mass
(R.A.M) of different elements.
Learning objectives
By the end of this unit, students should be able to:
• Outline the discovery of the sub-atomic particles.
• Compare the properties of sub-atomic particles.
• Explain what is an isotope of an element.
• Assess the relationship between the number of protons and the number of
electrons.
• Calculate the mass number knowing the number of protons and the
number of neutrons.
• Understand the meaning of relative atomic mass and relative abundances
• Calculate the relative atomic mass of an element, given isotopic masses and
abundances.
• Draw and label the mass spectrometer.
• Explain the fundamental processes occurring in the functioning of a mass
spectrometer.
• Interpret different mass spectra.
• State the uses of the mass spectrometer.• Calculate the relative atomic mass of an element, from a mass spectrum.
2. What do the three diagrams A, B, and C have in common?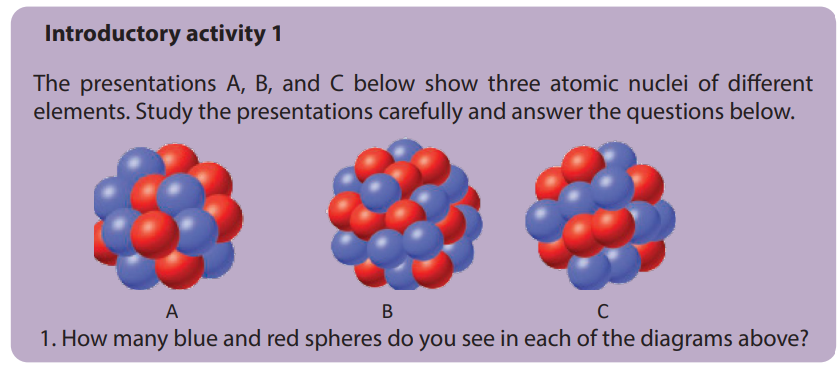
3. Based on your knowledge concerning atomic structure, what do you think
that
a) the blue spheres represent? b) the red spheres represent?
Provide explanations.
4. Using the information obtained in question (3) write the atomic symbol for
each of the diagrams.
5. Are there some other particle(s) missing from the above diagrams? If yes name
the particle(s).
6. What could you obtain if the atom is broken down?
Each country has its own culture (language, traditions and norms, attitudes and
values, etc.). Our culture defines our identity which is unique to each Rwandan
citizen and differentiates us from foreigners; if one element of our culture is rejected
or disappears, we become a different Rwandan people. When we introduce foreign
cultures to replace ours, we can lose our identity. However, some of our cultural
elements such as language can be shared with others to build the social relationship.
Similarly, in the atom, the number of protons within the nucleus defines the atomic
number, which is unique to each chemical element; the atomic number or the
number of protons of an atom defines its identity. If a proton is added or removed
from an element, it becomes a different element. Electrons around the nucleus can
be lost, gained, or shared to create bonds with other atoms in chemical reactions to
produce useful substances, but this does not change the identity of the elements
involved.
1.1. Outline of the discovery of the atom's constituents and
their properties
Activity 1.1
1. Regardless of some exceptions, all atoms are composed of the same components.
True or False? If this statement is true why do different atoms
have different chemical properties?
2. The contributions of Joseph John Thomson and Ernest Rutherford led the
way to today’s understanding of the structure of the atom. What were
their contributions?
3. Explain the modern view of the structure of the atom?
4. Using your knowledge about atom, what is the role each particle plays inan atom
1.1.1. Constituents of atoms and their properties
Atoms are the basic units of elements and compounds. In ordinary chemical
reactions, atoms retain their identity. An atom is the smallest identifiable unit of
an element. There are about 91 different naturally occurring elements. In addition,
scientists have succeeded in making over 20 synthetic elements (elements not
found in nature but produced in Laboratories of Reasearch Centers).
An element is defined as a substance that cannot be broken down by ordinary
chemical methods in simpler substances. Some examples of elements include
hydrogen (H), helium (He), potassium (K), carbon (C), and mercury (Hg). In an
element, all atoms have the same number of protons or electrons but the number
of neutrons can vary. A substance made of only one type of atom is also called an
element or elemental substance, for example: hydrogen (H2
), chlorine (Cl2
), sodium
(Na). Elements are the basic building blocks of more complex matter.
A compound is a matter or substance formed by the combination of two or more
different elements in fixed ratios. For example, Hydrogen peroxide (H2
O2
) is a
compound composed of two elements, hydrogen and oxygen, in a fixed ratio (2:2).
During the early twentieth century, scientists discovered that atoms can be divided
into more basic particles. Their findings made it clear that atoms contain a central
portion called the nucleus. The nucleus contains protons and neutrons. Protons
are positively charged, and neutrons are neutral. Whirling around the nucleus are
particles called electrons which are negatively charged. The relative masses andcharges of the three fundamental particles are shown in Table 1.1
The mass of an electron is very small compared with the mass of either a proton or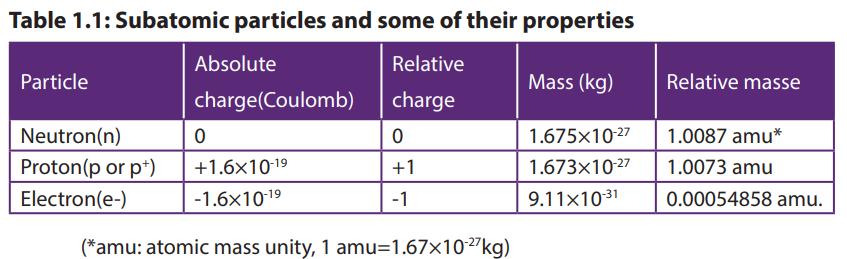
a neutron.
The charge on a proton is equal in magnitude, but opposite in sign, to the charge
on an electron.
1.1.2. Discovery of the atom constituents.
The oldest description of matter in science was advanced by the Greek philosopher
Democritus in 400 BC.
He suggested that matter can be divided into small particles up to an ultimate
particle that cannot be divided, and called that particle atom. Atoms came from the
Greek word atomos meaning indivisible.
The work of Dalton and other scientists such as Avogadro, etc., contributed more
so that chemistry was beginning to be understood. They proposed new concepts
of atom, and from that moment scientists started to think about the nature of the
atom. What are the constituents of an atom, and what are the features that make
atoms of the various elements different?
In 1808 Dalton published his book called A New System of Chemical Philosophy, in
which he presented his theory of atoms:
a) Dalton’s Atomic Theory
1. Each element is made up of tiny particles called atoms.
2. The atoms of a given element are identical; the atoms of different elements are
different in some fundamental way or ways.
3. Chemical compounds are formed when atoms of different elements combine with
each other. A given compound always has the same relative numbers and types of
atoms.
4. Chemical reactions involve reorganization of the atoms—changes in the way theyare bound together. The atoms themselves are not changed in a chemical reaction.
Figure 1.1: John Dalton’s Atomic Model
b)Discovery of Electrons and Thomson’s Atomic Model
In 1897 J. J. Thomson (1856–1940) and other scientists conducted several
experiments, and found that atoms are divisible. They conducted experiments withgas discharge tubes. A gas discharge tube is shown in Figure 1.2.
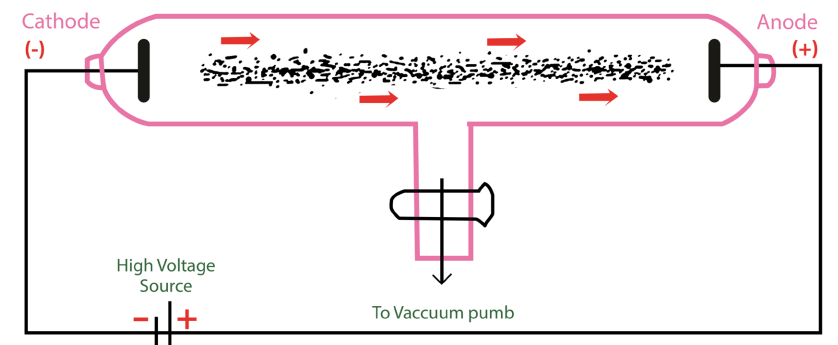
Figure 1.2: Gas discharge tube showing cathode rays originating from the cathode
The gas discharge tube is an evacuated glass tube and has two electrodes, a cathode
(negative electrode) and an anode (positive electrode). The electrodes are connected
to a high voltage source. Inside the tube, an electric discharge occurs between the
electrodes.
The discharge or ‘rays’ originate from the cathode and move toward the anode, and
hence are called cathode rays. Using luminescent techniques, the cathode rays are
made visible and it was found that these rays are deflected away from negatively
charged plates. The scientist J. J. Thomson concluded that the cathode ray consists
of negatively charged particles, and later they were called electrons.
Thomson postulated that an atom consisted of a diffuse cloud of positive charge
with the negative electrons embedded randomly in it. This model, shown in Figure
1.3, is often called the plum pudding model because the electrons are like raisinsdispersed in a pudding (the positive charge cloud), as in plum pudding.
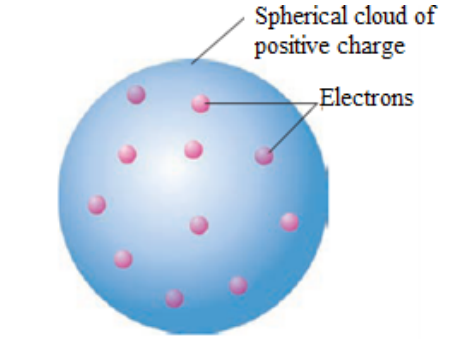
Figure 1.3.The plum pudding model of the atom
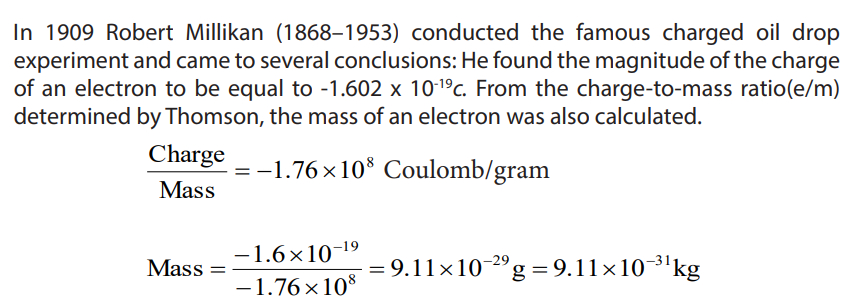
In 1909 Robert Millikan (1868–1953) conducted the famous charged oil drop
experiment and came to several conclusions: He found the magnitude of the charge
of an electron to be equal to -1.602 x 10-19c. From the charge-to-mass ratio(e/m)
determined by Thomson, the mass of an electron was also calculated.
c)Discovery of Protons and Rutherford’s Atomic Model
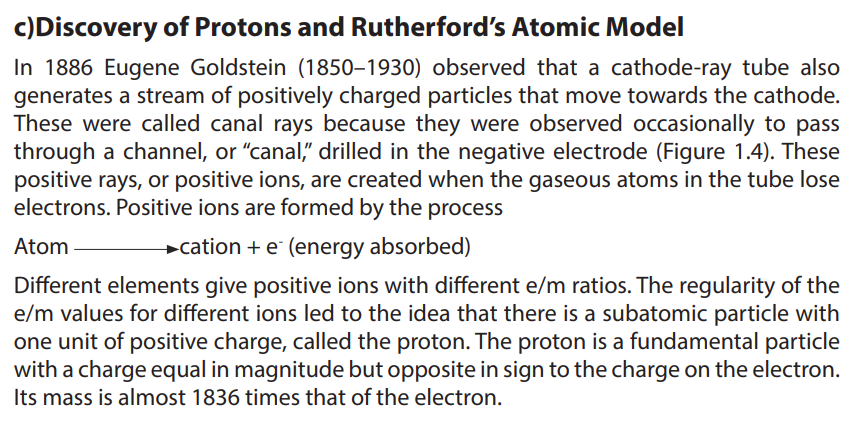
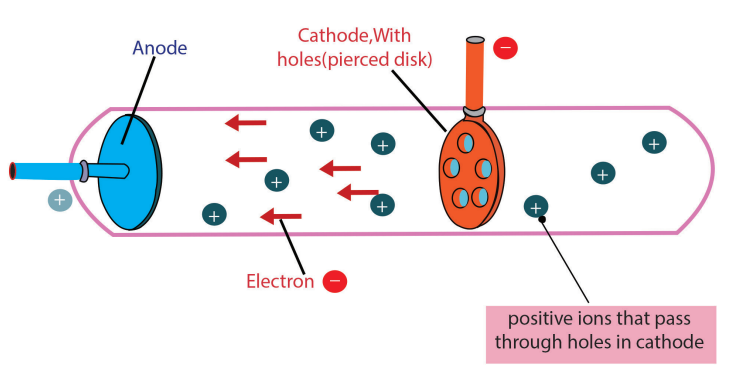
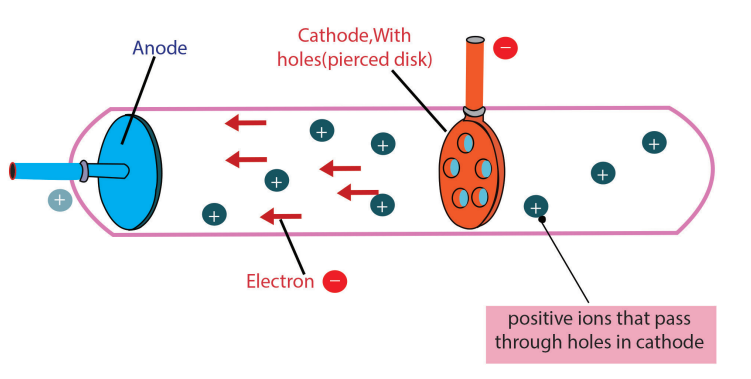
Figure 1.4: A cathode-ray tube with a different design and with a perforated cathode
The proton was observed by Ernest Rutherford and James Chadwick in 1919 as aparticle that is emitted by bombardment of certain atoms with α-particles.
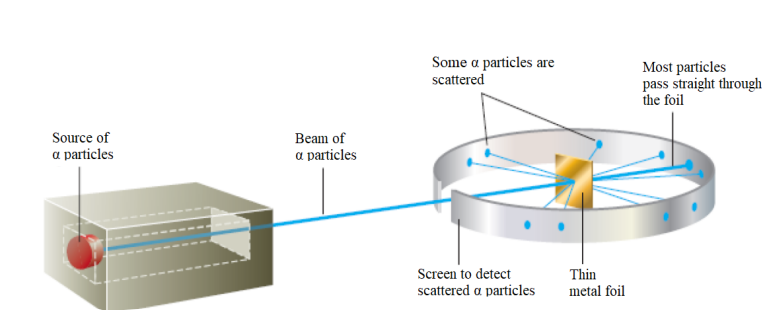
Figure 1.5: Rutherford’s experiment on α-particle bombardment of metal foil
Rutherford reasoned that if Thomson’s model were accurate, the massive α-particles
should crash through the thin foil like cannonballs through gauze, as shown in Figure
1.6(a). He expected α-particles to travel through the foil with, at the most, very minor
deflections in their paths. The results of the experiment were very different from
those Rutherford anticipated. Although most of the α- particles passed straight
through, many of the particles were deflected at large angles, as shown in Figure
1.6(b), and some were reflected, never hitting the detector. This outcome was a great
surprise to Rutherford. Rutherford knew from these results that the plum pudding
model for the atom could not be correct. The large deflections of the α-particles
could be caused only by a center of concentrated positive charge that contains most
of the atom’s mass, as illustrated in Figure 1.6(b). Most of the α-particles pass directly
through the foil because the atom is mostly an open space. The deflected α-particles
are those that had a “close encounter” with the massive positive center of the atom,
and the few reflected α-particles are those that made a “direct hit” on the much more
massive positive center.
In Rutherford’s mind these results could be explained only in terms of a nuclear
atom—an atom with a dense center of positive charge (the nucleus) with electronsmoving around the nucleus at a distance that is large relative to the nuclear radius.
d)Discovery of Neutrons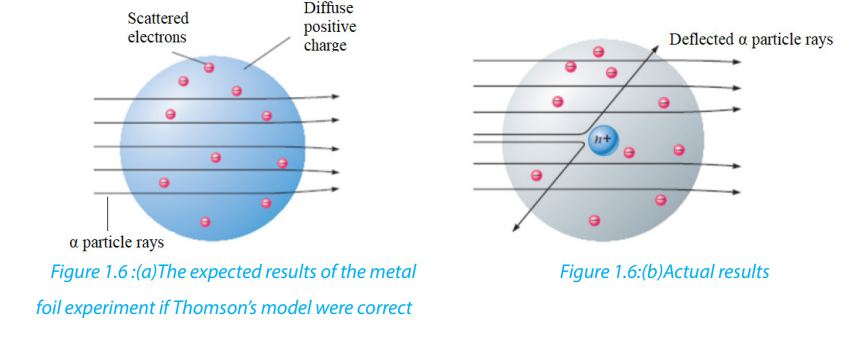
In spite of the success of Rutherford and his co-workers in explaining atomic
structure, one major problem remained unsolved.
If the hydrogen contains one proton and the helium atom contains two protons,
the relative atomic mass of helium should be twice that of hydrogen. However, the
relative atomic mass of helium is four and not two.
This question was answered by the discovery of James Chadwick, English physicist
who showed the origin of the extra mass of helium by bombarding a beryllium foilwith alpha particles. (See figure 1.7)
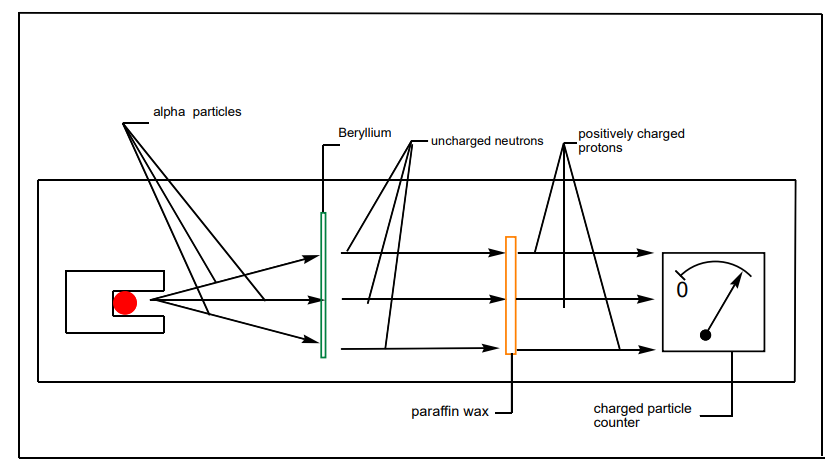
Figure 1.7. Chadwick’s experiment
In the presence of beryllium, the alpha particles are not detected; but they displace
uncharged particles from the nuclei of beryllium atoms. These uncharged particles
cannot be detected by a charged counter of particles.
However, those uncharged particles can displace positively charged particles from
another substance. They were called neutrons.The mass of the neutron is slightly
greater than that of proton.
Figure 1.8 shows the location of the elementary particles (protons, neutrons, and
electrons) in an atom. There are other subatomic particles, but the electron, the
proton, and the neutron are the three fundamental components of the atom that
are important in chemistry.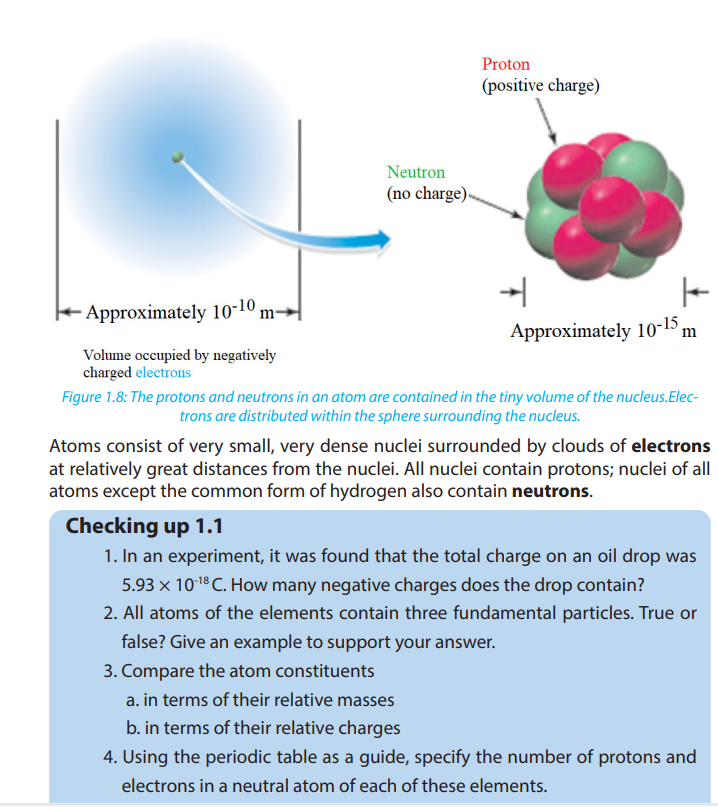
1.2. Concept of atomic number, mass number, and isotopic
mass
1. Compare the two sodium isotopes in the figures above.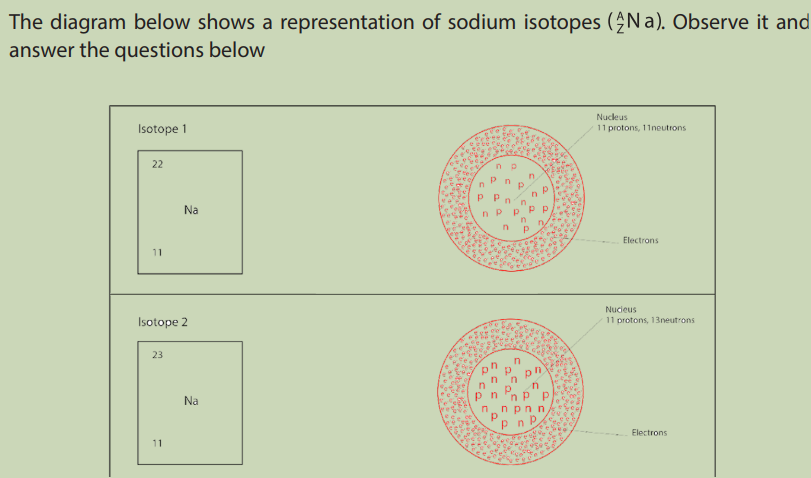
2. From your observation, how do you define the isotopes of an element?
3. How is the mass number, A, determined?
4. What information is provided by the atomic number, Z?
5. What is the relationship between the number of protons and the number of electrons
in an atom?
6. Where are the electrons, protons, and neutrons located in an atom?
7. Why is the mass of an atom concentrated in the center?
8. Sodium-24 and sodium-23 react similarly with other substances. Explain the statement
9. Say which one(s) of the following statements is(are) correct and which one(s) is(are)
wrong: (i) isotopes differ in their number of electrons, (ii) isotopes differ in their mass
numbers, (iii) isotopes differ in their number of protons, (iv) isotopes differ by their
number of neutrons, (v) all the statements are wrong.
The atomic number denotes the number of protons in an atom’s nucleus. The mass
number denotes the total number of protons and neutrons. Protons and neutrons
are often called nucleons. By convention, the atomic number is written on the left
side of the element symbol as a subscript, and the mass number on the same sidebut as a superscript.
Some atoms of the same of element have the same atomic number, but different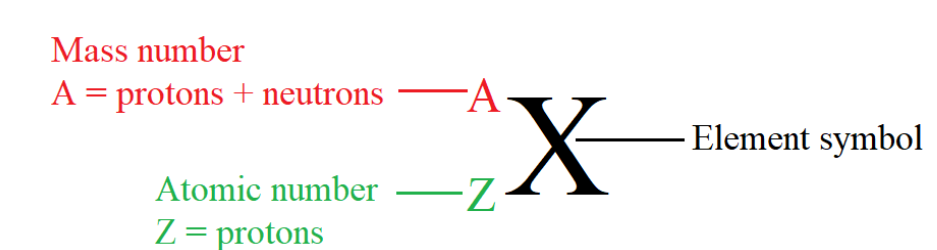
mass numbers. This means a different number of neutrons. Such atoms are called
isotopes of the element.
Isotopes are atoms of the same element with different masses; they are atoms containing
the same number of protons but different numbers of neutrons.
In a given atom, the number of protons, also called “atomic number” is equal to the
number of electrons because the atom is electrically neutral. The sum of the number
of protons and neutrons in an atom gives the mass number of that atom.
Mass number = number of protons + number of neutrons= atomic number + neutron number
Atomic number, Mass number, protons, Electrons, Isotopes,
neutron
a) The atomic number tells you how many……………………………. and
……………………………………………………. are in an atom.
......................................................is the number written as subscript on the left of
the atomic symbol.
b) The total number of protons and neutrons in an atom is called the
……………………………………………………………..
c) Atoms with the same number of protons but different number of neutrons are called ………………………………………….
d) The subatomic particle that has no charge is called a………………………………………………
1.3. Calculation of relative atomic mass of elements withisotopes
Relative atomic mass, symbolized as R.A.M or Ar, is defined as the mass of one atom
of an element relative to 1/12 of the mass of an atom of carbon-12, which has a
mass of 12.00 atomic mass units. The relative atomic mass, also known as the atomic
weight or average atomic weight, is the average of the atomic masses of all of the
element’s isotopes.
Relative isotopic mass is like relative atomic mass in that it deals with individual
isotopes. The difference is that we are dealing with different forms of the same
element but with different masses.
Thus, the different isotopic masses of the same elements and the percentage
abundance of each isotope of an element must be known in order to accurately
calculate the relative atomic mass of an element.
Notice: Remember that mass number is not the same as the relative atomic mass or
isotopic mass! The mass number is the number of protons + neutrons; while relative
atomic mass (or isotopic mass) is the mass if you were to somehow weigh it on abalance.
By applying the same formula, the relative abundance of the isotopes may be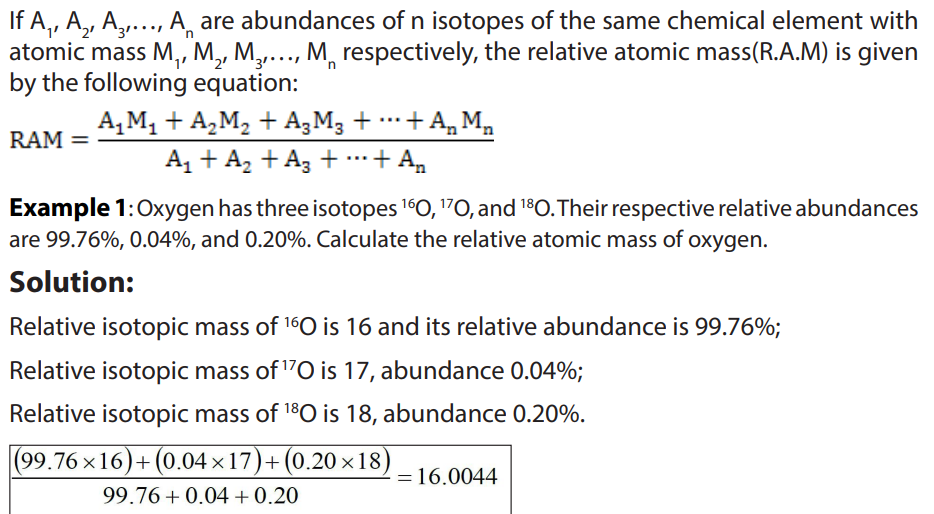
calculated knowing the relative atomic mass of the element and the atomic masses
of the respective isotopes.
Example 2: Chlorine contains two isotopes 35Cl and 37Cl, what is the relative abundance of
each isotope in a sample of chlorine if its relative atomic mass is 35.5?Solution:

3. Inlet system is also known as which of the following?
a) Initial system
b) Sample reservoir
c) Sample handling systemd) Element injection system
The mass spectrometer is an instrument that separates positive gaseous atoms
and molecules according to their mass-charge ratio and records the resulting mass
spectrum.
In the mass spectrometer, atoms and molecules are converted into ions. The ions are
separated as a result of the deflection which occurs in the magnetic field.
The basic components of a mass spectrometer are: vaporisation chamber (to
produce gaseous atoms or molecules), ionization chamber (to produce positive
ions), accelerating chamber (to accelerate the positive ions to a high and constant
velocity), magnetic field (to separate positive ions of different m/z ratios), detector
(to detect the number and m/z ratio of the positive ions) and the recorder (to plotthe mass spectrum of the sample)
A mass spectrometer works in five main stages, namely vaporization, ionization,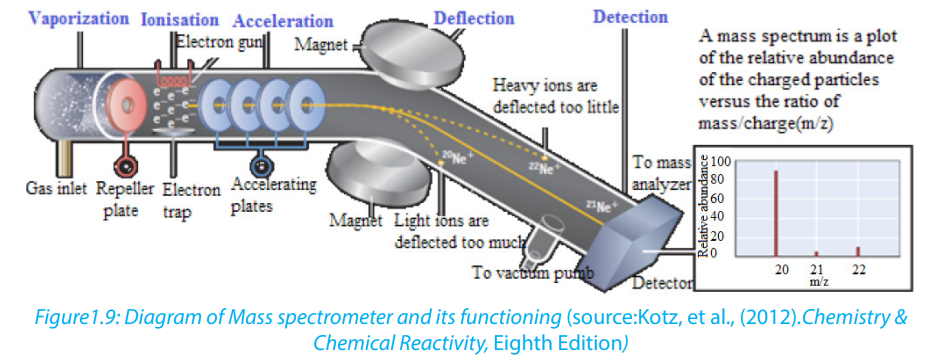
acceleration, deflection, and detection to produce the mass spectrum.
Stage 1: Vaporization
At the beginning the test sample is heated until it becomes vapour and is introduced
as a vapour into the ionization chamber. When a sample is a gas, it can directly be
introduced into the ionization chamber.
Stage 2: Ionisation
The vaporized sample passes into the ionization chamber (with a positive voltage of
about 10,000 volts). The electrically heated metal coil gives off electrons which are
attracted to the electron trap which is a positively charged plate.
The particles in the sample (atoms or molecules) are therefore bombarded with a
stream of electrons (from the electrons gun), and some of the collisions are energetic
enough to knock one or more electrons out of the sample particles to make positive
ions. Mass spectrometers always work with positive ions.
Most of the positive ions formed will carry a charge of +1 because it is much more
difficult to remove further electrons from an already positively charged ion.
Most of the sample molecules are not ionized at all but are continuously drawn off by
vacuum pumps which are connected to the ionization chamber (figure 1.9). Some of
the molecules are converted to negative ions through the absorption of electrons.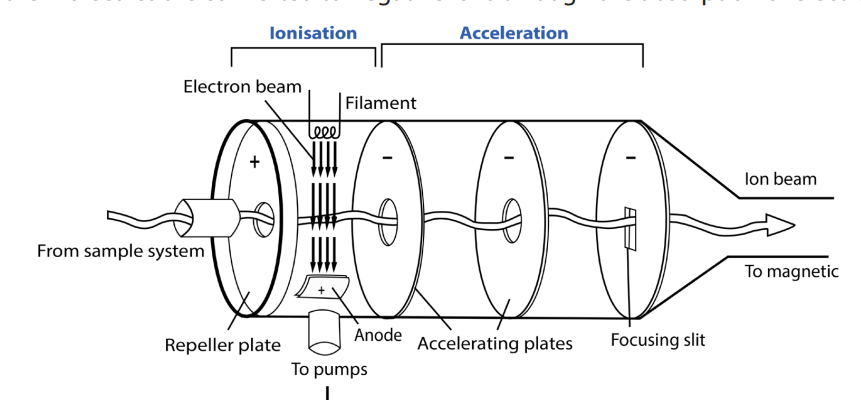
Figure 1.10: A typical ionisation chamber and the nearby accelerating plates
The repeller plate absorbs these negative ions. A small proportion of the positive
ions which are formed may have a charge greater than one (a loss of more than one
electron). These are accelerated in the same way as the singly charged positive ions.
Stage 3: Acceleration
The positive ions are accelerated by an electric field so that they move rapidly
through the machine at high and constant velocity.
Stage 4: Deflection
The ions are then deflected by a magnetic field according to their mass to charge
ratios. Different ions are deflected by the magnetic field at different extents. The
extent to which the beam of ions is deflected depends on four factors:
1. The magnitude of the accelerating voltage (electric field strength). Higher voltages
result in beams of more rapidly moving particles to be deflected less than the
beams of the more slowly moving particles produced by lower voltages.
2. Magnetic field strength. Stronger fields deflect a given beam more than weaker
fields.
3. Masses of the particles. Because of their inertia, heavier particles are deflected less
than lighter particles that carry the same charge.
4. Charges on the particles. Particles with higher charges interact more strongly with
magnetic fields and are thus deflected more than particles of equal mass with
smaller charges
The two last factors (mass of the ion and charge on the ion) are combined into the
mass/charge ratio. Mass/charge ratio is given the symbol m/z (or sometimes m/e).
For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio
would be 28. An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28.
In the figure 1.11 above, ion stream A is most deflected: it will contain ions with the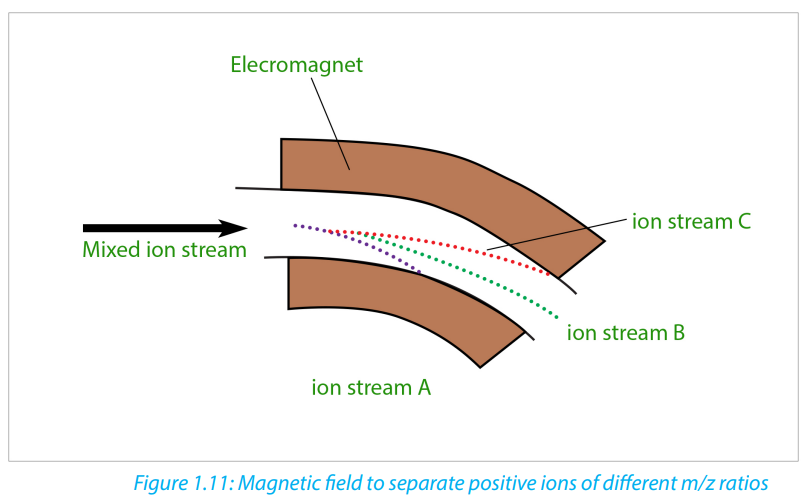
smallest mass/charge ratio. Ion stream C is the least deflected: it contains ions with
the greatest mass/charge ratio. Assuming 1+ ions, stream A has the lightest ions,
stream B the next lightest and stream C the heaviest. Lighter ions are going to be
more deflected than heavy ones.
Stage 5: Detection
The beam of ions passing through the machine is detected electrically. As they
pass out of the magnetic field, ions are detected by an ion detector which records the
position of the ions on the screen and the number of ions that hit the screen at each
position. These two pieces of information are used to produce a mass spectrum for
the sample.
A flow of electrons in the wire is detected as an electric current which can be
amplified and recorded. The more ions arriving, the greater the current.
Detecting the other ions
How might the other ions be detected (those in streams A and C which have been
lost in the machine)?
Remember that stream A was most deflected. To bring them on to the detector, you
would need to deflect them less by using a smaller magnetic field.
To bring those with a larger m/z value (the heavier ions if the charge is +1) to the
detector you would have to deflect them more by using a larger magnetic field.
If you vary the magnetic field, you can bring different ion streams, one at a time
on the detector to produce a current which is proportional to the number of ions
arriving. The mass of each ion being detected is related to the size of the magnetic
field used to bring it on to the detector. The machine can be calibrated to record
Recorder
The detector of a typical instrument consists of a counter which produces a current
that is proportional to the number of ions which strike it. Through the use of electron
multiplier circuits, this current can be measured so accurately that the current caused
by just one ion striking the detector can be measured. The signal from the detector is
fed to a recorder, which produces the mass spectrum. In modern instruments, the
output of the detector is fed through an interface to a computer. The computer can
store the data, provide the output in both tabular and graphic forms, and compare
the data to standard spectra, which are contained in spectra libraries also stored inthe computer.
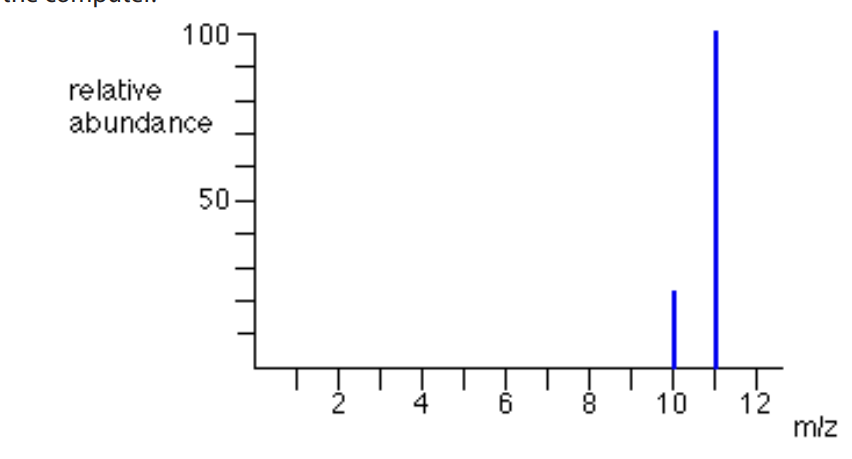
This is an example a mass spectrum of unknown element that has 2 isotopes.
Checking up 1.4
1. Use the list of the words given below to fill in the blank spaces. Each word
will be used once.
Vaporization chamber, mass spectrum, velocity, ionization, deflection,
detector, acceleration
A sample of the element is placed in the _________ where it is converted
into gaseous atoms. The gaseous atoms are ionized by bombardment of
high energy electrons emitted by a hot cathode to become positive ions (in
practice, the voltage in the ________chamber is set in such a way that only one
electron is removed from each atom). The positive ions (with different masses)
are then given a high and constant _________by two negatively charged
plates: the process is called_________. The positive ions are then deflected by
the magnet field. This process is called ____________ (ions with smaller mass
will be deflected more than the heavier ones). These ions are then detected
by the ion _________. The information is fed into a computer which prints out
the________ of the element.
2. The correct order for the basic features of a mass spectrometer is...
a. acceleration, deflection, detection, ionization
b. ionisation, acceleration, deflection, detection
c. acceleration, ionisation, deflection, detection
d. acceleration, deflection, ionisation, detection
3. Which one of the following statements about ionisation in a mass spectrometer
is incorrect?
a. gaseous atoms are ionised by bombarding them with high energy electrons
b. atoms are ionised so they can be accelerated
c. atoms are ionised so they can be deflected
d. it doesn’t matter how much energy you use to ionise the atoms
4. The path of ions after deflection depends on...
a. only the mass of the ion
b. only the charge on the ion
c. both the charge and the mass of the ion
d. neither the charge nor the mass of the ion5. Which of the following species will be deflected to the greatest extent?

6. Which of the following separates the ions according to their mass-to-charge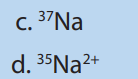
ratios?
a) Ion source
b) Detector
c) Magnetic sectord) Electric sector
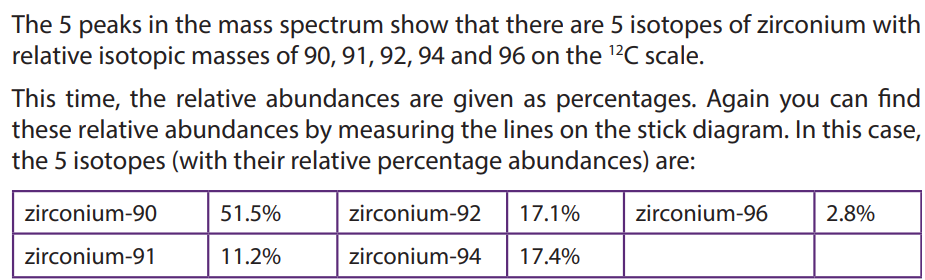
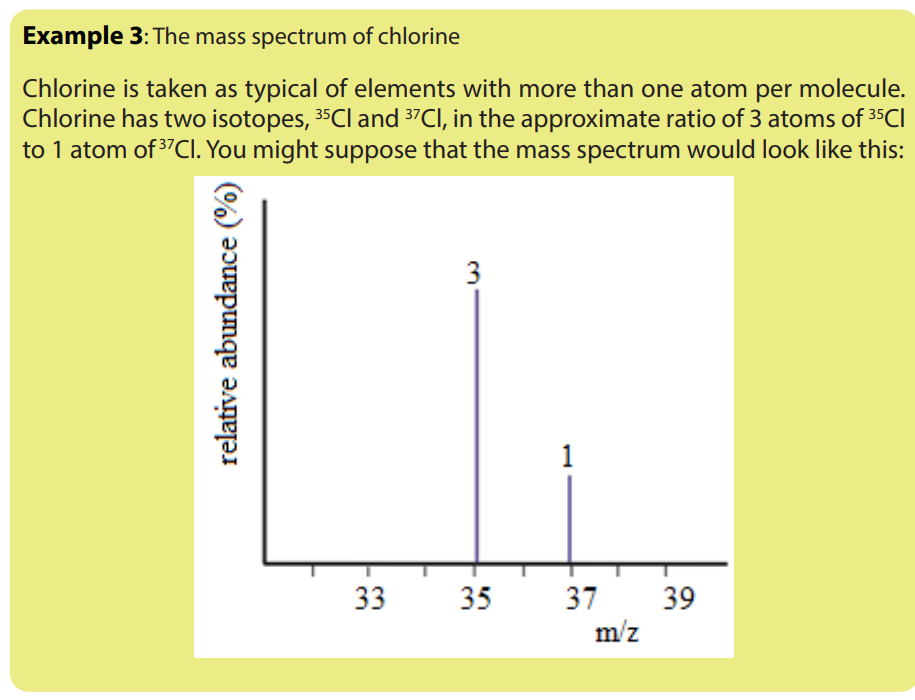
1.5. Interpretation of mass spectra.
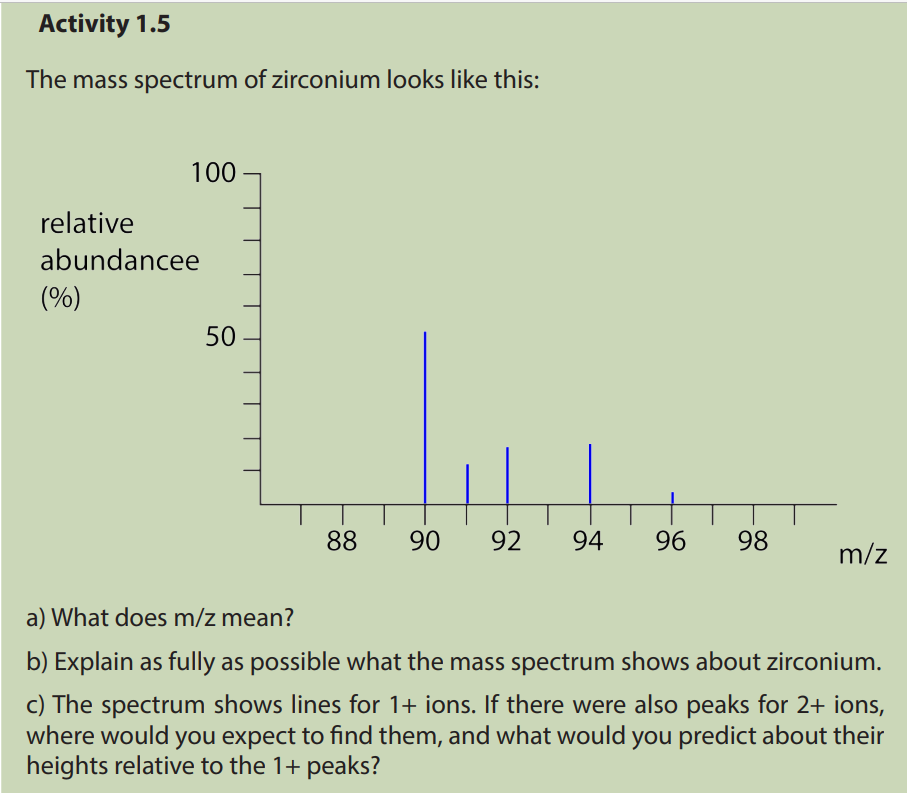
The mass spectrum of an element shows how you can find out the masses and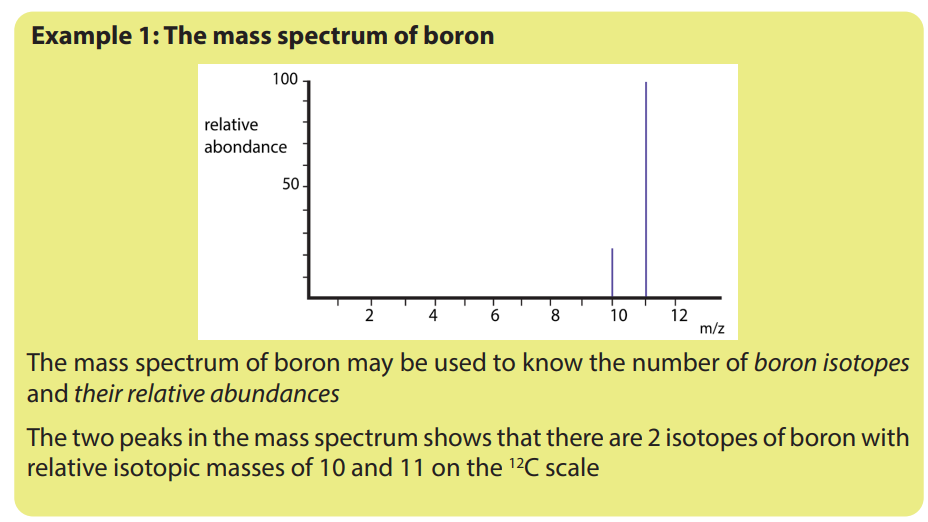
relative abundances of the various isotopes of the element and use that information
to calculate the relative atomic mass of the element.
The relative size of the peaks gives you a direct measure of the relative abundances
of the isotopes. The tallest peak is often given an arbitrary height of 100 but you
may find other scales used; it doesn’t matter. You can find the relative abundances
by measuring the lines on the stick diagram.In this case, the two isotopes (with their relative abundances) are:

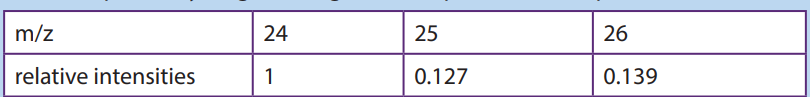
a. How many isotopes does magnesium possess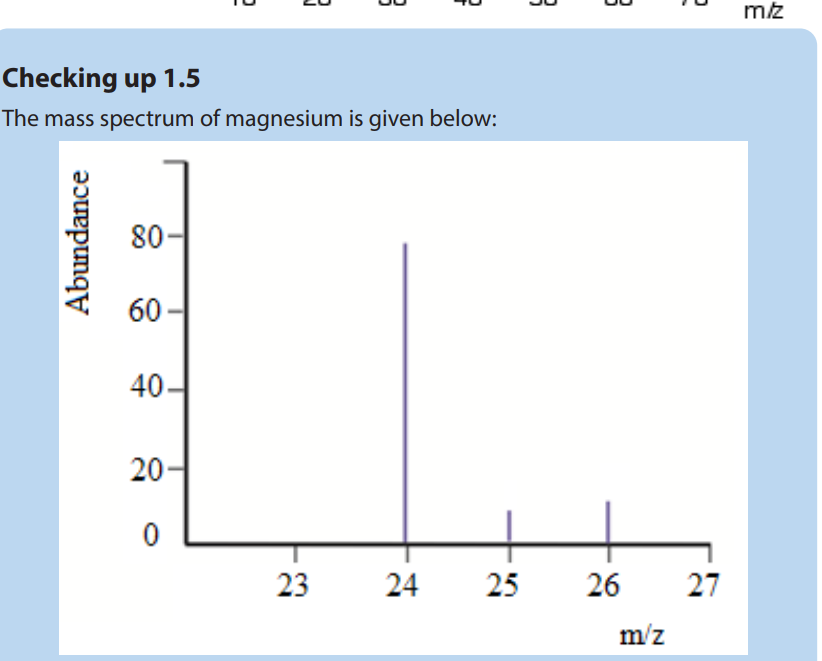
b. Estimate the isotopic mass of each of the magnesium isotopes
c. Estimate the relative abundance for each of the isotopes of magnesium
1.6. Uses of the mass spectrometer and involved calculations
Activity 1.6
1. Mass spectrometers are used to determine which of the following?
a) The atomic mass
b)Composition in sample
c) Concentration of elements in sample
2. The mass spectrum of an element, A, contained four lines at mass/charge
of 54; 56; 57 and 58 with relative intensities of 5.84; 91.68; 2.17; 0.31
respectively. Explain these data and calculate the relative atomic mass of A
1.6.1. Calculation of RAM using mass spectrum
When the mass spectrum of the element is given, you can calculate the relativeatomic mass of that element by using the information from the mass spectrum.
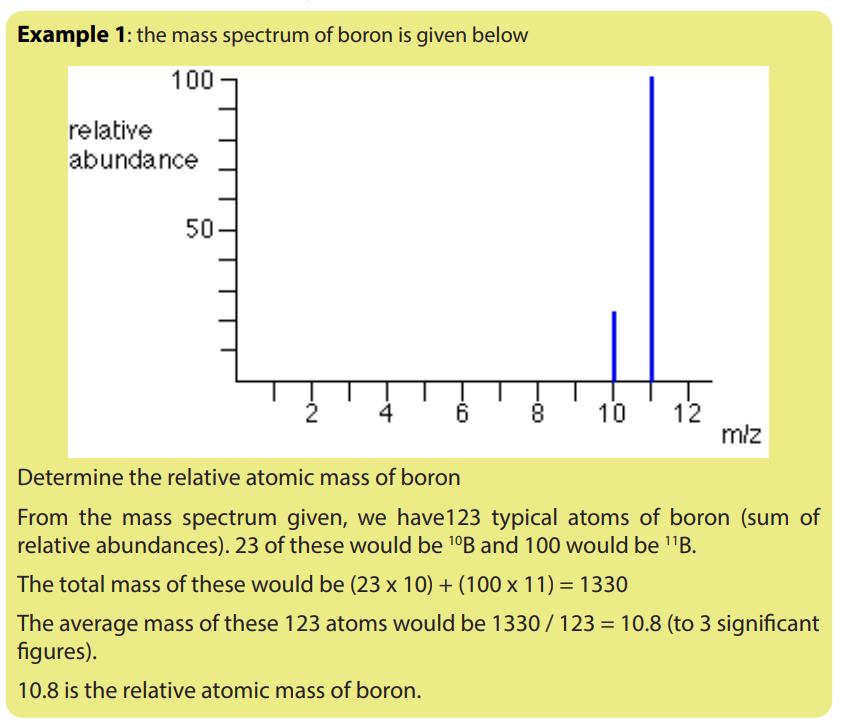
1.6.2. Uses of mass spectrometer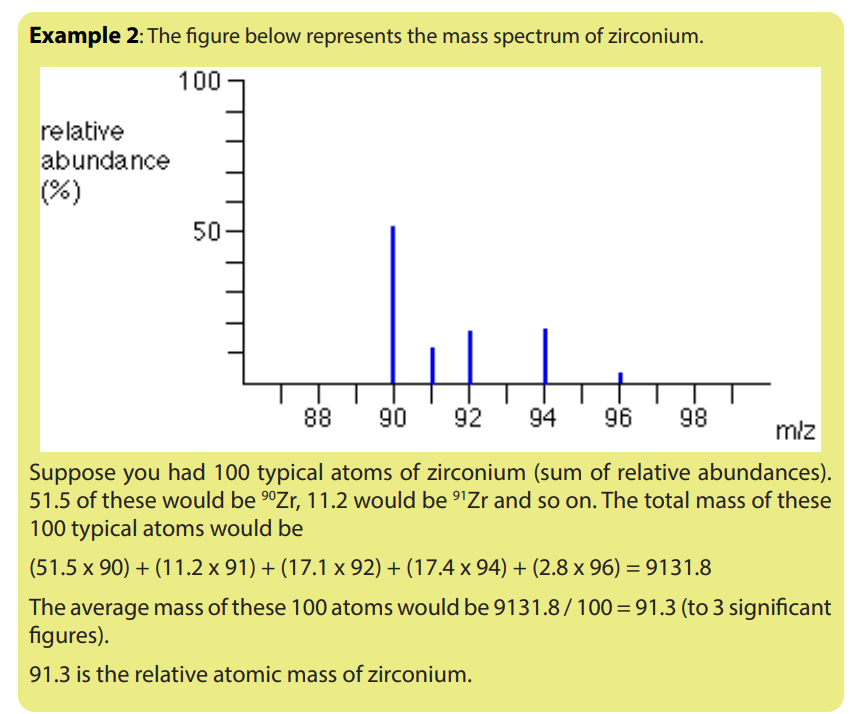
In addition to the use of mass spectrometer in the determination of isotopes of
elements and their relative abundances, other applications of mass spectrometry
are:
• Pharmaceutical: drug discovery, combinatorial chemistry, pharmacokinetics,
drug metabolism.
• Clinical: neonatal screening, haemoglobin analysis, drug testing.
• Environmental: water quality, soil and groundwater contamination, food
contamination, pesticides on foods.
• Geological: oil composition.• Biotechnological: the analysis of proteins, peptides
Checking up 1.6
1. Which of the following is not done through mass spectrometry?
a. Calculating the isotopic abundance of elements
b. Investigating the elemental composition of planets
c. Confirming the presence of O-H and C=O in organic compounds
d. Calculating the molecular mass of organic compounds
2. Mass spectra enable you to find relative abundances of the isotopes of a
particular element.
a) What are isotopes?
b) Define relative atomic mass.
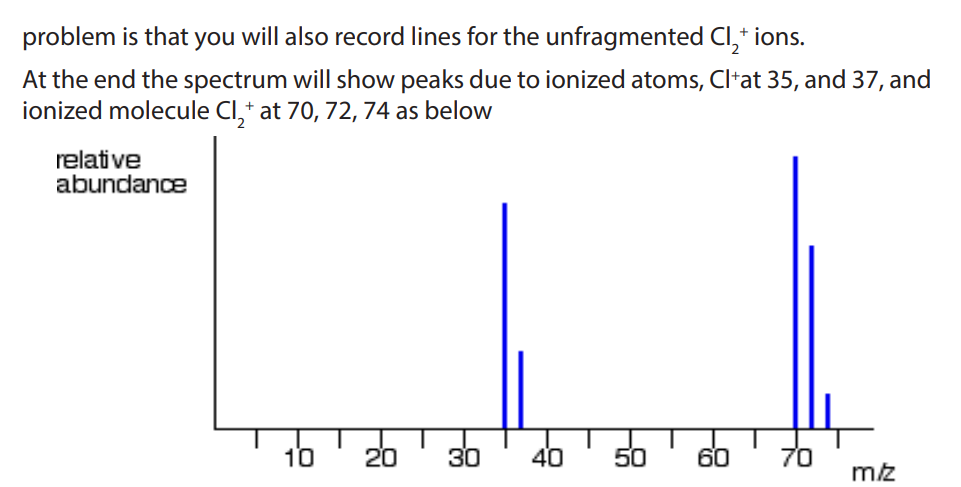
c) The mass spectrum of strontium contains the following relative abundancesfor 1+ ions:
a) Explain why there are two separate groups of peaks.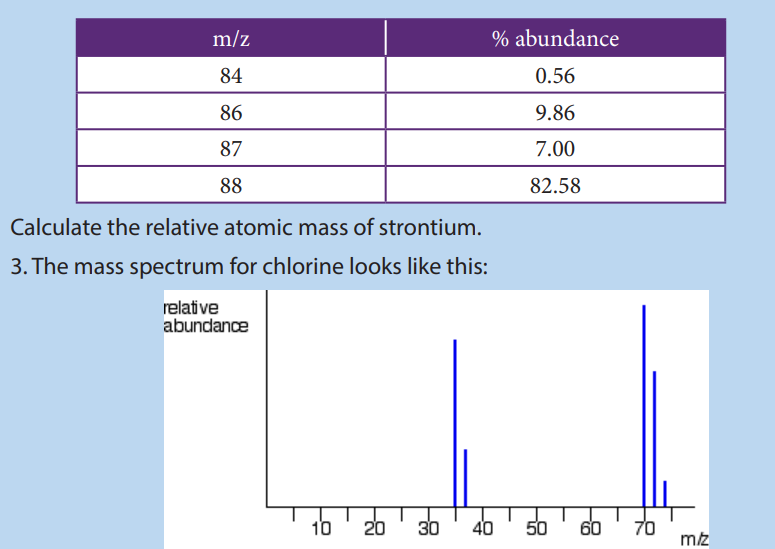
b) State what causes each of the 5 lines.
c) Explain the approximate relative heights of the lines at 35 and 37.
d) Why cannot you predict the relative heights of the two clusters of lines (35/37and 70/72/74)?
1.7. End unit assessment
I. Multiple choice questions
1.Which of the following is true regarding a typical atom?
a. Neutrons and electrons have the same mass.
b. The mass of neutrons is much less than that of electrons.
c. Neutrons and protons together make the nucleus electrically neutral.
d. Protons are more massive than electrons
2. Which of the following statements is(are) true? For the false statements,
correct them.
a. All particles in the nucleus of an atom are charged.
b. The atom is best described as a uniform sphere of matter in which
electrons are embedded.
c. The mass of the nucleus is only a very small fraction of the mass of the
entire atom.
d. The volume of the nucleus is only a very small fraction of the total
volume of the atom.
e. The number of neutrons in a neutral atom must equal the number of
electrons.
3. Each of the following statements is true, but Dalton might have had trouble
explaining some of them with his atomic theory. Give explanations for the
following statements.
a. Atoms can be broken down into smaller particles.
b. One sample of lithium hydride is 87.4% lithium by mass, while another
sample of lithium hydride is 74.9% lithium by mass. However, the two
samples have the same chemical properties
4. In mass spectrometer, the sample that has to be analysed is bombarded
with which of the following?
a. Protons
b. Electrons
c. Neutronsd. Alpha particles
5. Mass spectrometer separates ions on the basis of which of the following?
a. Mass
b. Charge
c. Molecular weight
d. Mass to charge ratio
6. In a mass spectrometer, the ions are sorted out in which of the following
ways?
a. By accelerating them through electric field
b. By accelerating them through magnetic field
c. By accelerating them through electric and magnetic field
d. By applying a high voltage
7. The procedure for mass spectroscopy starts with which of the following
processes?
a. The sample is bombarded by electron beam
b. The sample is accelerated by electric plates
c. The sample is converted into gaseous state
d. The ions are detected
8. Which of the following ions pass through the slit and reach the collecting
plate?
a. Negative ions of all masses
b. Positive ions of all masses
c. Negative ions of specific mass
d. Positive ions of specific mass
9. Which of the following statements is not true about mass spectrometry?
a. Impurities of masses different from the one being analysed interferes with
the result
b. It has great sensitivity
c. It is suitable for data storage
d. It is suitable for library retrieval
10. In a mass spectrometer, the sample gas is introduced into the highly
evacuated spectrometer tube and it is ionised by the electron beam.
a. True
b. False
II. Short and long answer questions
11. What are the three fundamental particles from which atoms are built? What
are their electric charges? Which of these particles constitute the nucleus of
an atom? Which is the least massive particle of the fundamental particles?
12. Verify that the atomic weight of lithium is 6.94, given the followinginformation:
a. Describe the different steps involved in ionizing the particles of a sample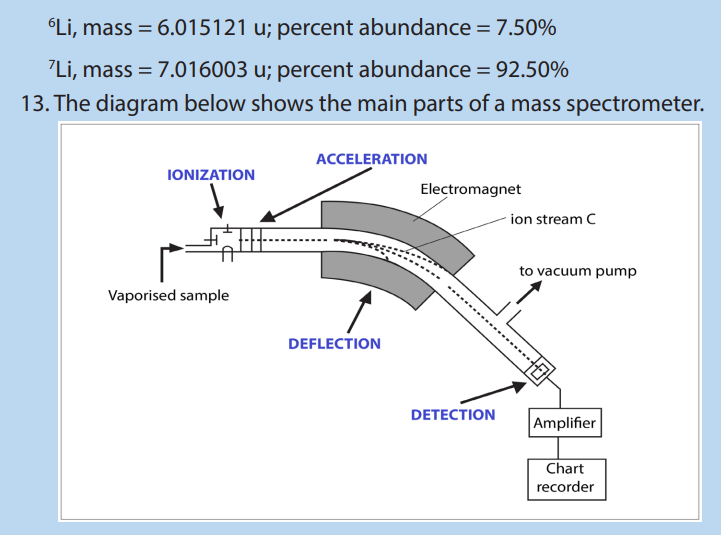
b. (i) Which two properties of the ions determine how much they are
deflected by the magnetic field? What effect does each of these
properties have on the extent of deflection?
(ii) Of the three different ion streams in the previous diagram, why is the
ion stream C least deflected?
(iii) What would you have to do to focus the ion stream C on the detector?
c. Why is it important that there is a vacuum in the instrument?
d. Describe briefly how the detector works.
14. (a) A mass spectrum of a sample of indium shows two peaks at m/z = 113
and m/z = 115. The relative atomic mass of indium is 114.5. Calculate the
relative abundances of these two isotopes.
(b)The mass spectrum of the sample of magnesium contains three peaks with
the mass-to-charge ratios and relative intensities shown belowi. Explain why magnesium gives three peaks in mass spectrum?