UNIT 1: BASIC BIOCHEMISTRY OF LIFE
Key Unit Competence:Explain the cellular respiration and photosynthesisIntroductory activity 1Observe the person in the picture below who is making physical exerciseand attempt the following questions:
 i) Where is the energy used by the person in the picture come from?ii) Why do all living organisms need a continuous supply of energy?iii) Identify the process exhibited by the person on the picture thatconsumes too much energy if compared with another one who is atrest.iv) How is the energy produced in our body? Does energy being produced
i) Where is the energy used by the person in the picture come from?ii) Why do all living organisms need a continuous supply of energy?iii) Identify the process exhibited by the person on the picture thatconsumes too much energy if compared with another one who is atrest.iv) How is the energy produced in our body? Does energy being producedin our body serve for our various activities? If yes, how?
All living organisms require a continuous supply of energy to stay alive, eitherfrom the absorption of light energy or from chemical potential energy (energystored in nutrient molecules). The process of photosynthesis transfers lightenergy to chemical potential energy, and so almost all life on Earth dependson photosynthesis, either directly or indirectly. Photosynthesis supplies livingorganisms with two essential requirements: an energy supply and usable carboncompounds.
All biological macromolecules such as carbohydrates, lipids, proteins andnucleic acids contain carbon. All living organisms therefore need a source ofcarbon. Organisms that can use an inorganic carbon source in the form ofcarbon dioxide are called autotrophs. Those needing a ready-made organicsupply of carbon are heterotrophs.
Organic molecules can be used by living organisms in two ways. They canserve as ‘building bricks’ for making other organic molecules that are essentialto the organism, and they can represent chemical potential energy that canbe released by breaking down the molecules in respiration. This energy canthen be used for all forms of work. Heterotrophs depend on autotrophs forboth materials and energy.
1.1. Cellular respiration
Activity 1.1
1. When an ocelot breathes, it acquires oxygen, and when it feeds on a
lizard, it acquires glucose. Both molecules enter its bloodstream and are
carried to the body’s cells, where there is a specific biological processwhich uses both oxygen and glucose.
2. Which biological process is represented in the figure above? Where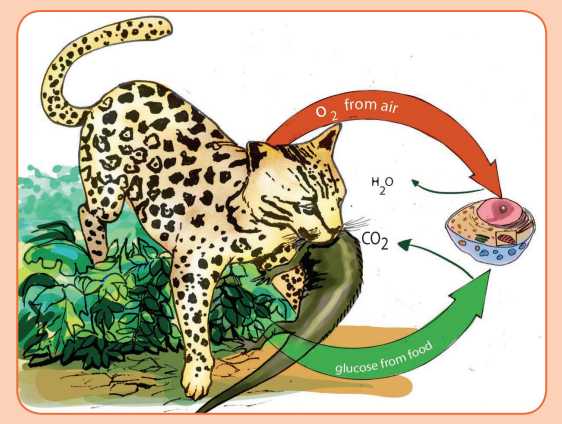
does that process take place in the organism? In a eukaryotic cell?i) Where does the biological process mentioned above take place in
the cell?
ii) Write the chemical equation of that biological process.
iii) The equation written in (iii) above is described in four steps. Conduct
research from the library textbooks or search engine to explain:– How is pyruvate formed from sugar/glucose?2. Yeast, sugar, water and flour are the main components in bread making
– What is the role of Coenzyme A in the link reaction?
– What is the role of reduced NAD+ and FAD in the Krebs cycle?
– What is the final acceptor of protons and electrons in the respiratory
chain?a) Why do bakers add yeast to flour and water when making bread?Cellular respiration is a set of metabolic reactions and processes that take
b) When yeast is added to grape juice at room temperature, vigorous
bubbling occurs. What gas produces the bubbles?
c) What type of beverage is produced by this process?d) What is the name of this process?
place in the cells of organisms to convert biochemical energy from nutrients
into adenosine triphosphate (ATP), and then release waste products.
Cellular respiration is the complex process in which cells make adenosine
triphosphate (ATP) by breaking down organic molecules. The energy stored
in ATP can then be used to drive processes requiring energy, including
biosynthesis, locomotion or transportation of molecules across cell membranes.
The main fuel for most cells is carbohydrate, usually glucose which is used by
most of the cells as respiratory substrate. Some other cells are able to breakdown fatty acids, glycerol and amino acids.
There are two types of cellular respiration, aerobic and anaerobic. Aerobic
respiration is more efficient and can be utilized in the presence of oxygen, while
anaerobic respiration does not require oxygen. Many organisms (or cells) will
use aerobic respiration primarily, however, if there is a limited oxygen supply,they can utilize anaerobic respiration for survival.
The breakdown of glucose can be divided into four stages: glycolysis, the link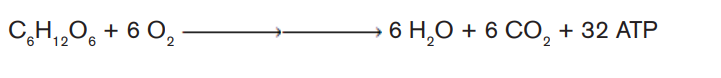
reaction, the Krebs cycle and oxidative phosphorylation.
1.1.1. Glycolysis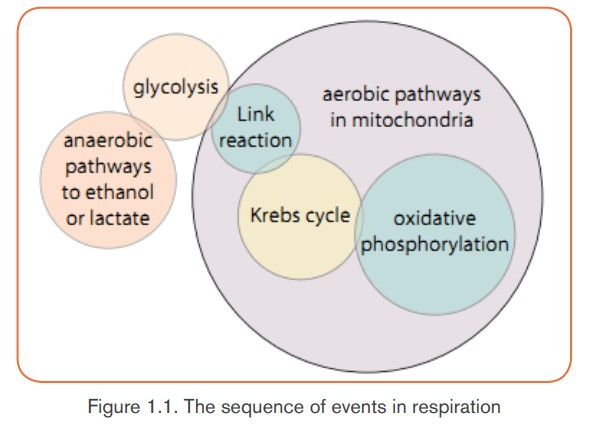
Glycolysis is the splitting, or lysis, of glucose. It is a multi-step process in which
a glucose molecule with six carbon atoms is eventually split into two molecules
of pyruvate, each with three carbon atoms. Energy from ATP is needed in the
first steps, but energy is released in later steps, when it can be used to make
ATP. There is a net gain of two ATP molecules per molecule of glucose brokendown. Glycolysis takes place in the cytoplasm of a cell.
In the first stage, phosphorylation, glucose is phosphorylated using ATP. Glucose
is energy-rich but does not react easily. To tap the bond energy of glucose,
energy must first be used to make the reaction easier. Two ATP molecules
are used for each molecule of glucose to make first glucose phosphate, then
fructose phosphate, then fructose bisphosphate, which breaks down to producetwo molecules of triose phosphate.
Hydrogen is then removed from triose phosphate and transferred to the carrier
molecule NAD (nicotinamide adenine dinucleotide). Two molecules of reduced
NAD are produced for each molecule of glucose entering glycolysis.
The hydrogens carried by reduced NAD can easily be transferred to other
molecules and are used in oxidative phosphorylation to generate ATP.
The end-product of glycolysis, pyruvate, still contains a great deal of chemical
potential energy. When free oxygen is available, some of this energy can be
released via the Krebs cycle and oxidative phosphorylation. However, the
pyruvate first enters the link reaction, which takes place in the mitochondria.
1.1.2. Link reaction
Pyruvate passes by active transport from the cytoplasm, through the outer and
inner membranes of a mitochondrion and into the mitochondrial matrix. Here it
is decarboxylated (this means that carbon dioxide is removed), dehydrogenated
(hydrogen is removed) and combined with coenzyme A (CoA) to give acetylcoenzyme A. This is known as the link reaction
Coenzyme A is a complex molecule composed of a nucleoside (adenine plus
ribose) with a vitamin (pantothenic acid), and acts as a carrier of acetyl groups
to the Krebs cycle. The hydrogen removed from pyruvate is transferred to NAD.
Fatty acids from fat metabolism may also be used to produce acetyl coenzyme
A. Fatty acids are broken down in the mitochondrion in a cycle of reactions in
which each turn of the cycle shortens the fatty acid chain by a two-carbon acetyl
unit. Each of these can react with coenzyme A to produce acetyl coenzyme A,which, like that produced from pyruvate, now enters the Krebs cycle.
1.1.3. The Krebs cycle
The Krebs cycle (also known as the citric acid cycle or tricarboxylic acid cycle)
was discovered in 1937 by Hans Krebs.
The Krebs cycle is a closed pathway of enzyme-controlled reactions.- Acetyl coenzyme A combines with a four-carbon compound (oxaloacetate)For each turn of the cycle, two carbon dioxide molecules are produced,
to form a six-carbon compound (citrate).
- The citrate is decarboxylated and dehydrogenated in a series of steps,
to yield carbon dioxide, which is given off as a waste gas and hydrogens
which are accepted by the carriers NAD and FAD.
- Oxaloacetate is regenerated to combine with another acetyl coenzyme A.
one FAD and three NAD molecules are reduced, and one ATP molecule isgenerated via an intermediate compound.
Although part of aerobic respiration, the reactions of the Krebs cycle make no
use of molecular oxygen.
However, oxygen is necessary for the final stage of aerobic respiration, which is
called oxidative phosphorylation. The most important contribution of the Krebs
cycle to the cell’s energetics is the release of hydrogens, which can be used inoxidative phosphorylation to provide energy to make ATP.
1.1.4. Oxidative phosphorylation and the electron transport
chain
In the final stage of aerobic respiration, oxidative phosphorylation, the
energy for the phosphorylation of ADP to ATP comes from the activity of the
electron transport chain. Oxidative phosphorylation takes place in the innermitochondrial membrane.
Reduced NAD and reduced FAD are passed to the electron transport chain.
Here, the hydrogens are removed from the two hydrogen carriers and each is
split into its constituent proton (H+) and electron (e−).
The energetic electron is transferred to the first of a series of electron carriers.
Most of the carriers are associated with membrane proteins, of which there are
four types. A functional unit, called a respiratory complex, consists of one of
each of these proteins, arranged in such a way that electrons can be passedfrom one to another down an energy gradient.
As an electron moves from one carrier at a higher energy level to another
one at a lower level, energy is released. Some of this energy is used to move
protons from the matrix of the mitochondrion into the space between the inner
and outer membranes of the mitochondrial envelope. This produces a higher
concentration of protons in the intermembrane space than in the matrix, setting
up a concentration gradient.
Now, protons pass back into the mitochondrial matrix through protein channels
in the inner membrane, moving down their concentration gradient. Associated
with each channel is the enzyme ATP synthase. As the protons pass through
the channel, their electrical potential energy is used to synthesise ATP in theprocess called chemiosmosis.
Finally, oxygen has a role to play as the final electron acceptor. In the mitochondrial
matrix, an electron and a proton are transferred to oxygen, reducing it to water.The process of aerobic respiration is complete.
The sequence of events in respiration and their sites are shown in Figure 1.1.
The balance sheet of ATP used and synthesised for each molecule of glucoseentering the respiration pathway is shown in Table 1.1.
Theoretically, three molecules of ATP can be produced from each molecule of
reduced NAD, and two molecules of ATP from each molecule of reduced FAD.
However, this yield cannot be achieved unless ADP and Pi are available inside
the mitochondrion. About 25% of the total energy yield of electron transfer isused to transport ADP into the mitochondrion and ATP into the cytoplasm.
Hence, each reduced NAD molecule entering the chain produces on average
two and a half molecules of ATP, and each reduced FAD produces one and a
half molecules of ATP. The number of ATP molecules actually produced varies in
different tissues and different circumstances, largely dependent on how muchenergy is used to move substances into and out of the mitochondria.
Table1.1: Balance sheet of ATP used and synthesized for each moleculeof glucose entering in respiration
Efficiency of aerobic and anaerobic respiration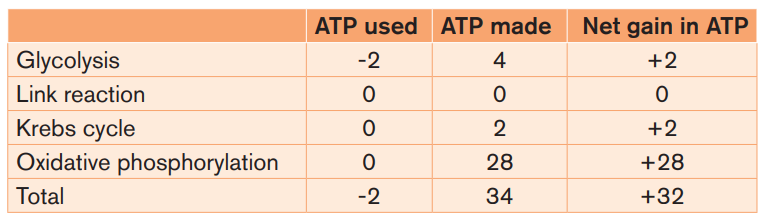
Without oxygen, pyruvate (pyruvic acid) is not metabolized by cellular respiration
but undergoes a process of fermentation. The pyruvate is not transported into
the mitochondrion, but remains in the cytoplasm, where it is converted to waste
products that may be removed from the cell. This serves the purpose of oxidizing
the electron carriers so that they can perform glycolysis again and removing the
excess pyruvate. Fermentation oxidizes NADH to NAD+ so it can be re-used in
glycolysis.
In the absence of oxygen, fermentation prevents the build-up of NADH in
the cytoplasm and provides NAD+ for glycolysis. This waste product varies
depending on the organism. In skeletal muscles, the waste product is lactic acid.
This type of fermentation is called lactic acid fermentation. In yeast and plants,
the waste products are ethanol and carbon dioxide. This type of fermentation is
known as alcoholic or ethanol fermentation. The ATP generated in this process
is made by substrate-level phosphorylation, which does not require oxygen.
Fermentation is less efficient at using the energy from glucose since only 2 ATP
are produced per glucose, compared to the 38 ATP per glucose produced by
aerobic respiration. This is because the waste products of fermentation stillcontain plenty of energy. Glycolytic ATP, however, is created more quickly.
Applications of anaerobic respiration
Some food products and drinks are produced by using anaerobic microorganisms:
- Production of beer
- Production of wine
- Production of yoghurt
- Production of cheese- Production of bread
In the bread-making process, it is the yeast that undergoes cellular respiration.
Anaerobic respiration also known as fermentation helps to produce beer and
wine and happens without the presence of oxygen. During bread production,
yeast starts off respiring aerobically, creating carbon dioxide and water and
helping the dough rise. After the oxygen runs out, anaerobic respiration begins,
although the alcohol produced during this process, ethanol, is lost through
evaporation when the bread is exposed to high temperatures during baking.
Yeast is crucial to making those soft, puffy loaves of bread and creating the
deep, craggy holes popular to traditional European breads, such as baguettes.
Yeast works as a leavening agent in bread, changing the sugars in dough into
gas, which creates the bubbles in the loaves. The longer the yeast is allowed
to work in the bread, this is the rising period of bread making and the more
flavorful the bread. However, because yeast will eventually switch from aerobic
to anaerobic respiration, the yeast will run out of nutrition of oxygen. When thebread is left to rise too long, the dough will slowly start to deflate.
To speed up the rising process, increase the sugar content, as well as add in
small amounts of vinegar, which encourages cellular respiration or fermentation
in the yeast. When you bake the bread after it has risen sufficiently, the cellular
respiration process stops, and the bubbles produced during the process arepreserved, making the holes in the bread.
The complete oxidation of glucose produces the energy estimated at 686 Kcal.
Under the condition that exists inside most of the cells, the production of a
standard amount of ATP from ADP absorbs about 7.3 Kcal. Glucose molecule
can theoretically generate up to 38 ATP molecules in aerobic respiration. Theefficiency of aerobic respiration (EAER) is calculated as follows:

This result indicates that the efficiency of aerobic respiration equals 40%. The
remainder of the energy (around 60%) is lost from the cell as heat.
Due to the fact that anaerobic respiration produces only 2 ATP, the efficiency ofanaerobic respiration is less than that of aerobic respiration.
It is calculated as follows:
Oxygen debt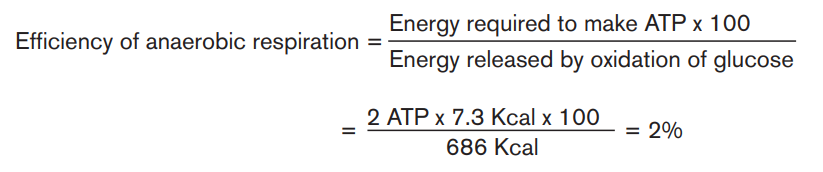
Standing still, the person absorbs oxygen at the resting rate of 0.2 dm3min−1.
(This is a measure of the person’s metabolic rate). When exercise begins,
more oxygen is needed to support aerobic respiration in the person’s muscles,
increasing the overall demand to 2.5 dm3min−1. However, it takes four minutes for
the heart and lungs to meet this demand, and during this time lactic fermentation
occurs in the muscles. Thus the person builds up an oxygen deficit. For the next
three minutes, enough oxygen is supplied. When exercise stops, the person
continues to breathe deeply and absorb oxygen at a higher rate than when
at rest. This post-exercise uptake of extra oxygen, which is ‘paying back’ the
oxygen deficit, is called the oxygen debt. The oxygen is needed for:- Conversion of lactate to glycogen in the liverThe presence of the lactic acid is sometimes described as an ‘ oxygen debt’.
- Re oxygenation of haemoglobin in the blood
- A high metabolic rate, as many organs are operating at above restinglevels.
This is because significant quantities of lactic acid can only be removed
reasonably quickly by combining with oxygen. However, the lactic acid was
only formed due to lack of sufficient oxygen to release the required energy to
the muscle tissue via aerobic respiration. Lactic acid can accumulate in muscle
tissue that continues to be over-worked. Eventually, so much lactic acid can
build-up that the muscle ceases working until the oxygen supply that it needs
has been replenished. To repay such an oxygen debt, the body must take in
more oxygen in order to get rid of the additional unwanted waste product lacticacid.
Muscle cramps
A muscle cramp is an involuntarily and forcibly contracted muscle that does not
relax. Muscle cramps can occur in any muscle; cramps of the leg muscles and
feet are particularly common.
Almost everyone experiences a muscle cramp at some time in their life. There
are a variety of types and causes of muscle cramps. Muscle cramps may occurduring exercise, at rest, or at night, depending upon the exact cause.
Overuse of a muscle, dehydration, muscle strain or simply holding a position
for a prolonged period can cause a muscle cramp. In many cases, however, the
cause isn’t known.
Although most muscle cramps are harmless, some may be related to an
underlying medical condition, such as:- Inadequate blood supply. Narrowing of the arteries that deliver blood to
your legs (arteriosclerosis of the extremities) can produce cramp-like pain
in your legs and feet while you’re exercising. These cramps usually go
away soon after you stop exercising.
- Nerve compression. Compression of nerves in your spine (lumbar stenosis)
also can produce cramp-like pain in your legs. The pain usually worsens
the longer you walk. Walking in a slightly flexed position such as you would
use when pushing a shopping cart ahead of you may improve or delay the
onset of your symptoms.
- Mineral depletion. Too little potassium, calcium or magnesium in your diet
can contribute to leg cramps. Diuretics or medications often prescribed
for high blood pressure also can deplete these minerals.The figure below accounts the energy yield per glucose molecule breakdownApplication activity 1.1
during cellular respiration in different cell organelles. Study it carefully and
answer questions that follow: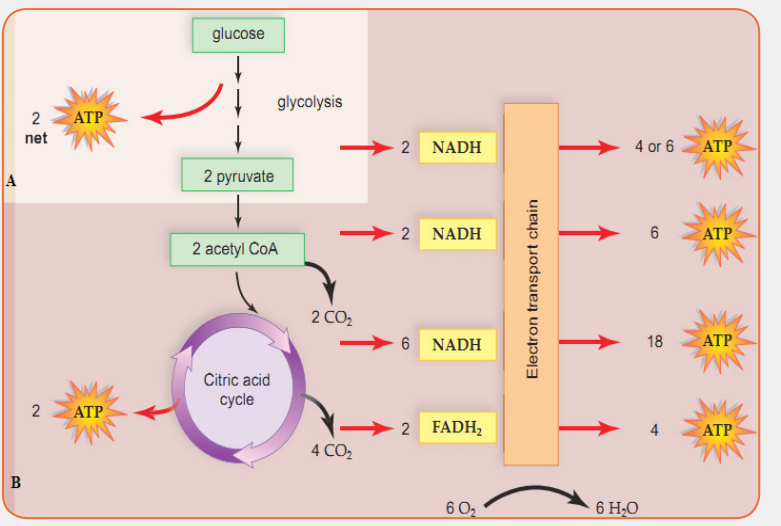 i) Where do the process labeled A and B take place in the cell?1.2. Photosynthesis
i) Where do the process labeled A and B take place in the cell?1.2. Photosynthesis
ii) Account for the total energy yield (ATP) per glucose molecule
breakdown during cellular respiration.
iii) What are the uses of energy produced during cellular respiration?
iv) What would happen to the total energy yield if glucose molecule
increases or decrease?
v) A student-teacher regularly runs 3 km each afternoon at a slow,
leisurely pace. One day, student–teacher runs 1 km as fast as she/he
can. Afterward, student-teacher is winded and feels pain in her chestand leg muscles. What is responsible for her symptoms?
Activity 1.2
Leaves and photosynthesis
Materials: test tubes, 500-mL beaker, potted houseplant with runners,
such as a spider plant, or a water plant (e.g., Elodea); wood splint; securedBunsen burner.
Procedure / protocol:
– Fill a 500-mL beaker with 400 mL of water.
– Fill a test tube with water and, without spilling, turn it upsidedown into
the water in the beaker. If an air bubble remains in the test tube, repeat
the procedure until there is no bubble or until the bubble is as small as
possible.
– Place the other test tube in the beaker repeating the steps above.
– Carefully place a spider-plant runner or sprig of a water plant into one
of the test tubes, as shown in the setup below and leave the other testtube filled with water only.
Leave the apparatus in bright sunlight or under a spotlight until there is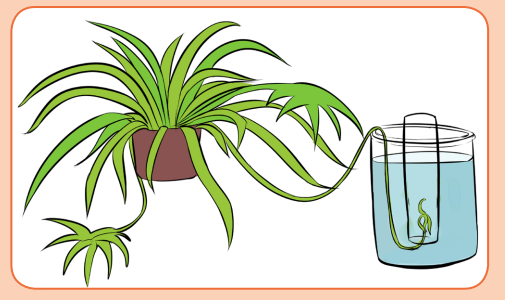
almost no water left in the tube containing the plant. Observe the test tubes
every 15 min over several hours.i) What happened to the glowing splint when it was lowered into the1.2.1. Autotrophic nutrition
test tube? Write the observation.
ii) What gas collected in the test tube?
iii) How do you know that the gas came from the plant?
iv) In which step does the gas mentioned above is produced?
Autotrophic nutrition is a process by which living organisms make their own
food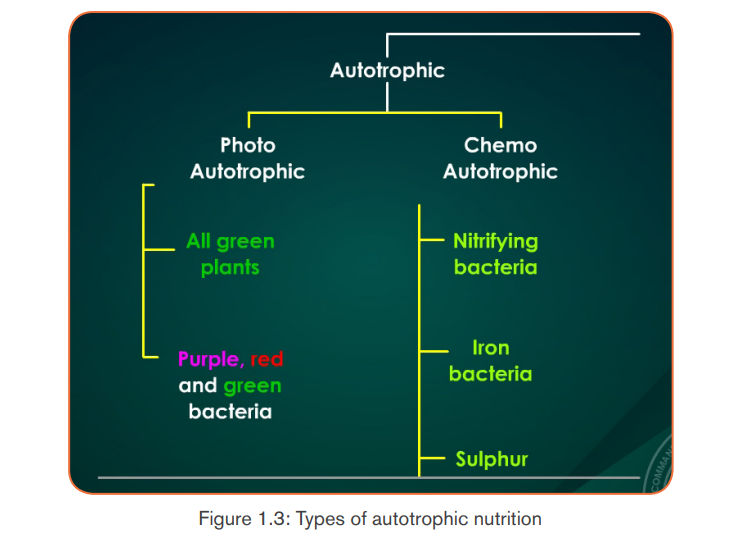 . This process is carried out by photoautotrophs like green plants, green
. This process is carried out by photoautotrophs like green plants, green
algae and green bacteria; and chemoautotrophs. Living organisms which make
their own food are called autotrophs, while others, including humans, whichcannot make their own food but depend on autotrophs, are called heterotrophs.
a. Types of autotrophic nutrition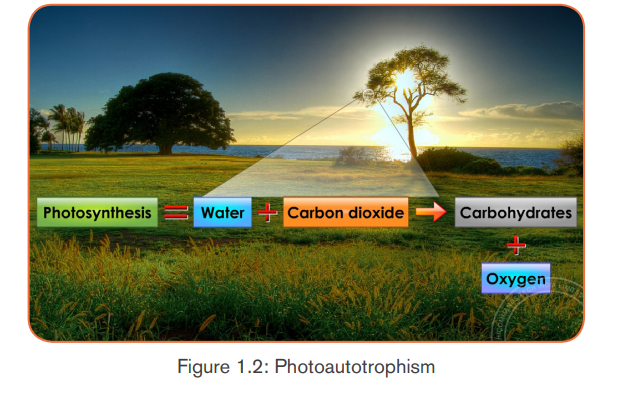
There are two types of autotrophic nutrition such as chemoautotrophic andphotoautotrophic nutrition.
a. 1 Chemoautotrophic nutrition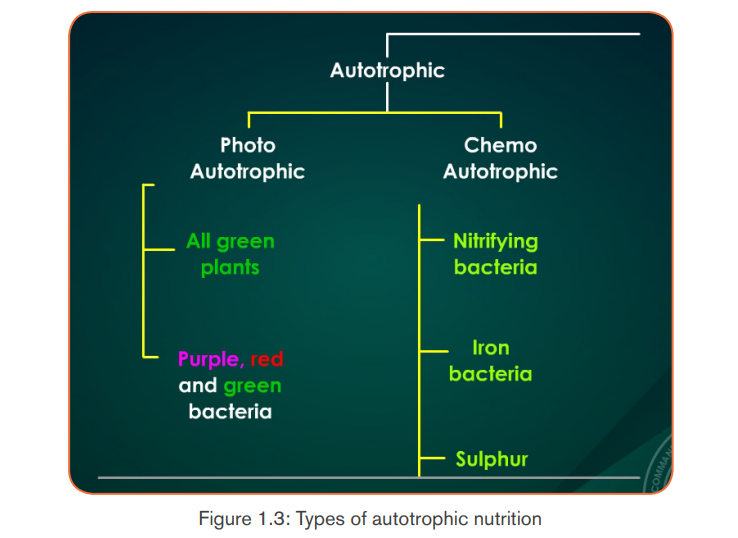
It is an autotrophic nutrition where organisms (mainly bacteria) get energy from
oxidation of chemicals, mainly inorganic substances like hydrogen sulphide andammonia.
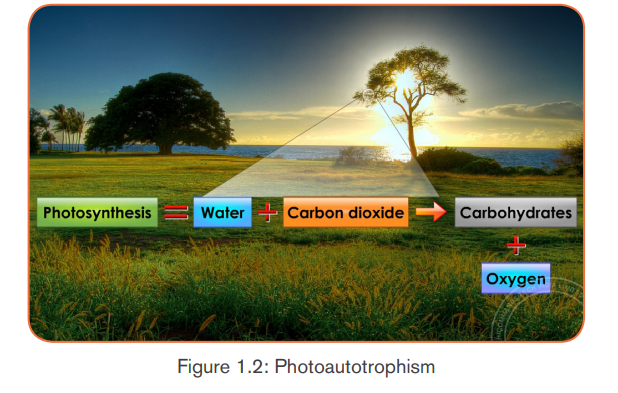
a.2 Photoautotrophic nutrition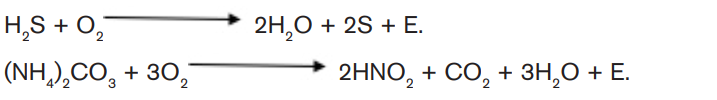
It is an autotrophic nutrition where organisms get energy from sunlight and
convert it into sugars. Green plants and some bacteria like green Sulphur
bacteria can make their own food from simple inorganic substances by a process
called photosynthesis. Photosynthesis is a process by which, autotrophs make
their own food by using inorganic substances in presence of light energy and
chlorophyll.
The function of thylakoids is to hold the chlorophyll molecules in a suitable
position for trapping the maximum amount of light. A typical chloroplast contains
approximatively 60 grana, each consisting of about 50 thylakoids. The space
outside the thylakoid membranes are made by watery matrix called stroma. Thestroma contains enzymes responsible for photosynthesis.
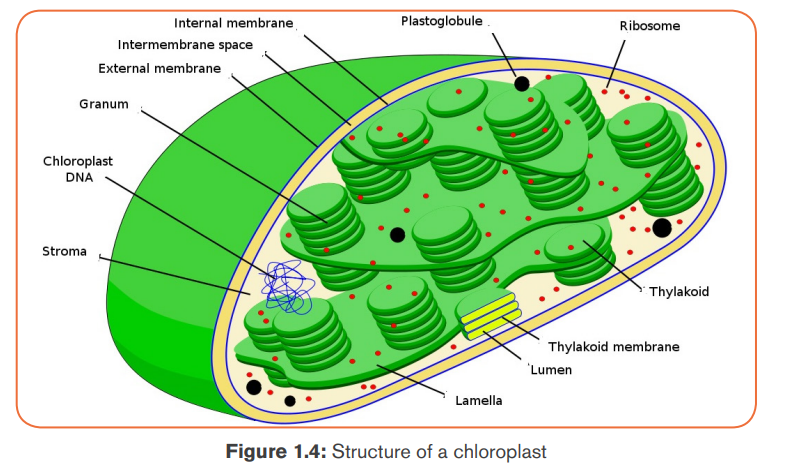
Note: Photosynthetic prokaryotes have no chloroplasts, but thylakoids often
occur as extensions of the plasma membrane and are arranged around theperiphery of the prokaryotic cell.
b. Chlorophyll
It is a sunlight- absorbing pigment, and it actually gets its green color because
it absorbs bleue and red wavelengths of light.
Structure of chlorophyll
The chlorophyll molecule is made of atoms of Carbon and Nitrogen joined in
a complex porphyrin ring containing an atom of Magnesium in the center of
the ring. The chlorophyll also has long hydrophobic carbon tail of 20 carbon
atoms (phytol) which hold it in the thylakoid membrane. In short, the chlorophyllconsists of a porphyrin ring and a phytol tail.
The chlorophyll a differs from the chlorophyll b in that: the porphyrin of the
chlorophyll a has the methyl group (-CH3) as a functional group, which is
replaced by an aldehyde group (-CHO) for chlorophyll b.
The difference between the chlorophyll a and the chlorophyll b shifts the
wavelength of light absorbed and reflected by chlorophyll b, so that thechlorophyll b is yellow-green, whereas the chlorophyll a is bright-green.
Adaptations for photosynthesis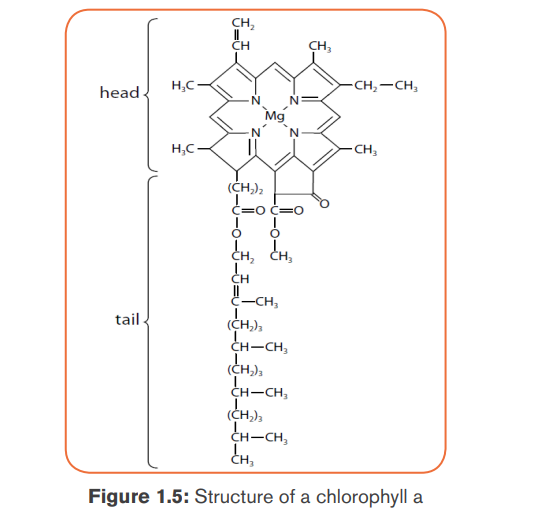
By considering both external and internal structures of the leaf, we can recognizeseveral adaptations for photosynthesis.
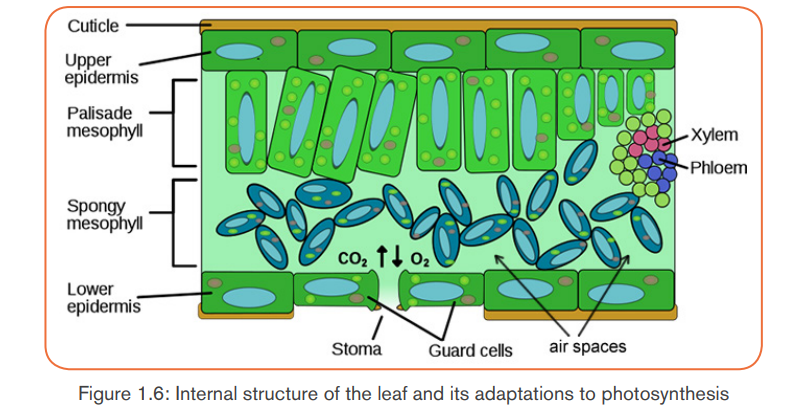
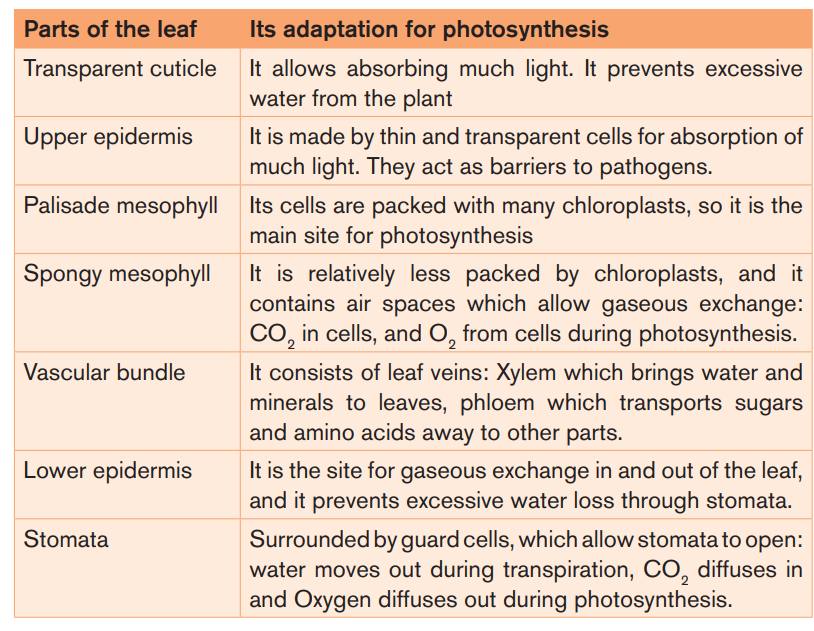
Note: when stomata are opened, the rate of photosynthesis may be 10 to 20
times as fast as the maximum rate of respiration. If the stomata are closed,
photosynthesis still can continue, using CO2 produced during cell respiration.The equilibrium can be reached between photosynthesis and cell respiration.
Photosynthesis uses CO2 from respiration, and respiration uses Oxygen from
photosynthesis. However, the rate of photosynthesis under these circumstances
will be much slower than when an external source of CO2 is available. The
stomata cannot remain closed indefinitely, they have to be open in order tomaintain transpiration of the plant.
1.2.2. Mechanism of photosynthesis
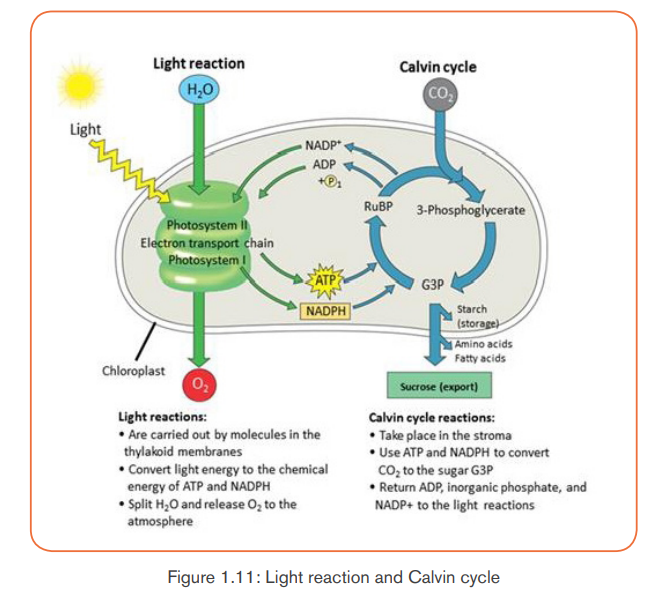
The process of photosynthesis occurs through two main stages such as:- The light-dependent reactions: which take place in thylakoids, andA. The light-dependent reactions- The light-independent reactions (Calvin cycle): which take place in stroma.
They require light energy and occur in thylakoids. They produce Oxygen gasand convert ADP and NADP+ into ATP and NADPH.
The light-dependent reactions involve the following steps:
a. Absorption and action spectra
In addition to water and CO2, photosynthesis requires light and chlorophyll.
The chlorophyll pigment is found in the chloroplasts. The light that our eyes
perceive as white light is a mixture of different wavelengths. Most of them are
visible to our eyes and make up the visible spectrum. Our eyes see different
wavelengths of visible spectrum as different colors (violet, blue, green, yellow,
orange and red) except indigo which is not visible to our eyes. Plants absorb
the light energy by using molecules called pigments such as: chlorophyll a,
chlorophyll b, carotene (orange) and xanthophyll (yellow) but chlorophyll a
is the principle pigment in photosynthesis.
The chlorophyll absorbs light very well in blue-violet and red regions of visible
spectrum. However, chlorophyll does not absorb well the green light; instead it
allows the green light to be reflected. That is why young leaves and other partsof the plants containing large amount of chlorophyll appear green.
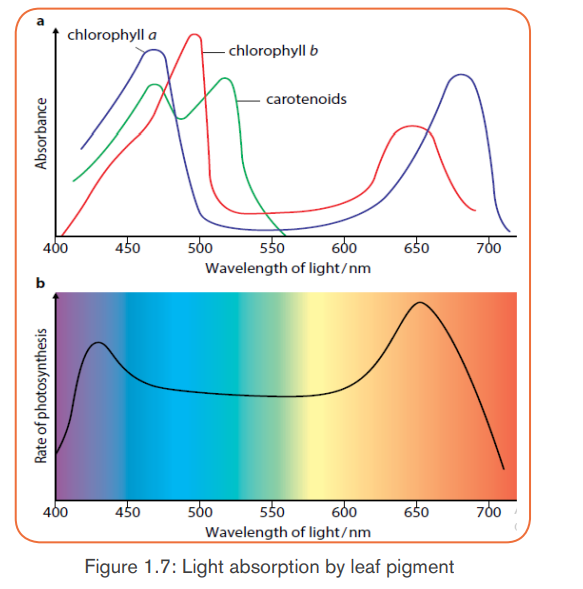
The chlorophyll a as a principle and abundant pigment, it is directly involved
in light reactions of photosynthesis. Other pigments (chlorophyll b, carotene,
xanthophyll and phaeophytin) are accessory pigments. They absorb light
colours that chlorophyll a cannot absorb, and this enables plants to capturemore energy from light.
The amount of energy that the pigment can absorb from the light depends on
its intensity and its wavelengths. So, the greater the intensity of light, the greater
amount of energy will be absorbed by the pigment in a given time.
Photosynthesis begins when the chlorophyll a in photosystem II absorbs light at
different wavelengths of light.- When the light energy hits the chlorophyll a, the light energy is absorbed
by its electrons, by raising their energy level.
- These electrons with high potential energy (electrons with sufficient
quantum energy) are passed to the electron-transport chain.
- Excited electrons are taken up by an electron acceptor (NADP+: oxidized
Nicotinamide Adenine Dinucleotide Phosphate), and pass along electron
transfer chain from photosystem II to the photosystem I. (Note: The
photosystems are the light-collecting units of the chloroplast).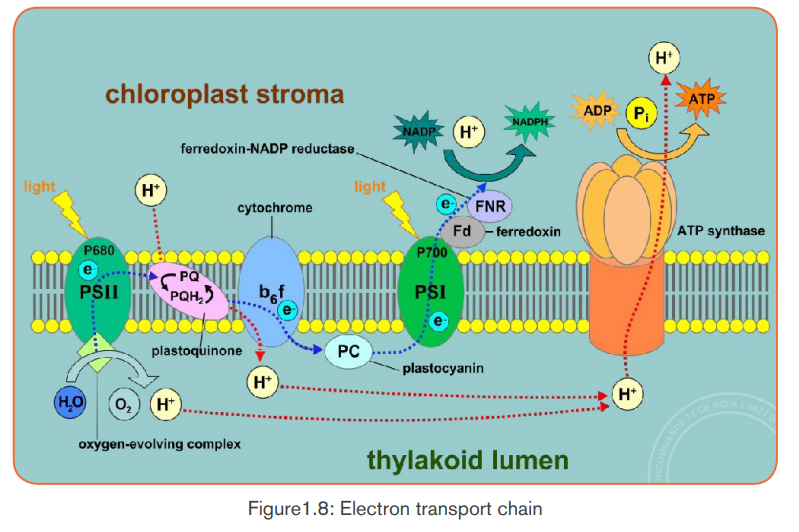
b. Enzymes in thylakoids and light absorbed by photosystem II
Enzymes in thylakoids and light absorbed by photosystem II are used to breakdown a water molecule into energized electrons, hydrogen ions H+, and Oxygen.
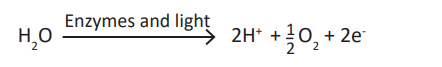 - Oxygen produced is released to be used by living things in respiration.- The light-dependent reactions also allow generation of ATP (Adenosine
- Oxygen produced is released to be used by living things in respiration.- The light-dependent reactions also allow generation of ATP (Adenosine
- Electrons and H+ from photolysis of water are used to reduce NADP+ toNADPH (Reduced Nicotinamide Adenine Dinucleotide Phosphate).
Triphosphate) by adding inorganic phosphate to ADP+ (AdenosineDiphosphate):
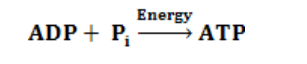
Generally, the light-dependent reactions use light energy, ADP, Pi, NADP+ andwater to produce ATP, NADPH and Oxygen. Or simply:
Both ATP and NADPH are energy carriers which provide energy to sugars
(energy containing sugars) in Light-independent reactions.
c. Photophosphorylation
The fixation of Pi to ADP+ to form ATP is called photophosphorylation.
Photophosphorylation can be done into two processes: cyclic
photophosphorylation, and non-cyclic photophosphorylation.
Cyclic photophosphorylation
It involves only photosystem I and not photosystem II. There is no production of
NADPH and no release of Oxygen. When the light hits the chlorophyll in PSI,the light-excited electron leaves the molecule.
This light-excited electron is taken up by an electron acceptor which passes
it along an electron transfer chain (a series of electron carriers) until it returns
to the chlorophyll molecule that it left (cyclic process). As an excited electron
moves along an electron transfer chain, it loses energy which will be used
for the synthesis of ATP from ADP+ and inorganic phosphate in the process
called chemiosmosis. Electron carriers can vary, but the principle includes thecytochromes.
Non-cyclic photophosphorylation
It is the main route of ATP synthesis. It is done in the following steps:- When the photosystem II (in chlorophyll) absorbs light, an electron is
excited to a higher energy level and captured by the primary electron
acceptor.
- Enzymes extract electrons from a water molecule replacing each electron
that the chlorophyll molecule lost when absorbed light energy. This reaction
dissociates a water molecule into hydrogen ions (2H+) and Oxygen which
is released for animals’ respiration.
- Excited electron moves from the primary electron acceptor of photosystemII to photosystem I, via an electron transport chain.
- When excited electron moves from the primary electron acceptor of
photosystem II to photosystem I, via an electron transport chain its energy
level lowers. The energy removed is used to synthesize ATP from ADP and
Pi in a process called: Non-cyclic phosphorylation.
- The hydrogen ions (2H+) produced from dissociation of water molecule
combines with NADP+ to form NADPH2.
- Both ATP and NADPH2 will be used in the light-independent reactions
(Calvin cycle) for synthesis of sugars.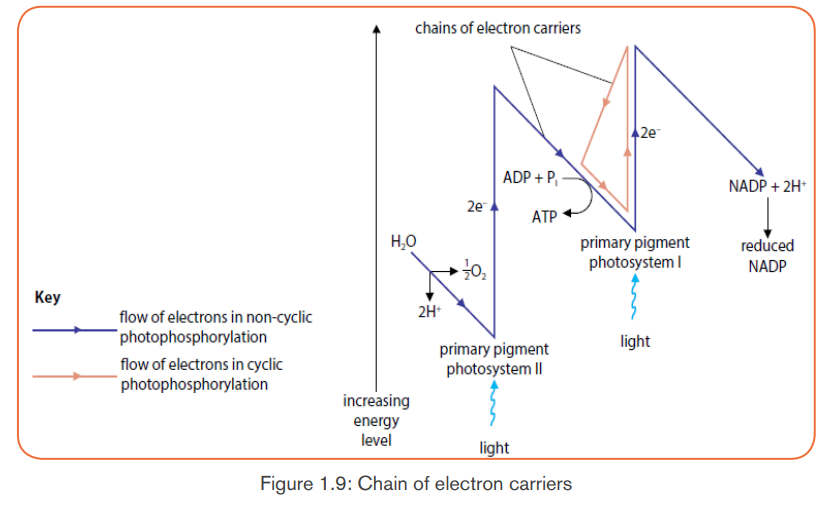
The significance of the cyclic phosphorylation
Non-cyclic photophosphorylation produces ATP and NADPH in equal quantities,
but the Calvin cycle consumes more ATP than NADPH. The concentration of
NADPH in a chloroplast may determine which pathway (cyclic versus noncyclic)
electrons pass through.
If a chloroplast runs low on ATP for the Calvin cycle, NADPH will accumulate as
the cycle slows down. The rise of NADPH may stimulate a shift from non-cyclic
(which produces ATP only) to cyclic electron pathway until ATP supply catcheswith the demand.
Table 1.2: Comparison between Non-cyclic and cyclicphotophosphorylation
B. The light-independent reactions (Calvin cycle)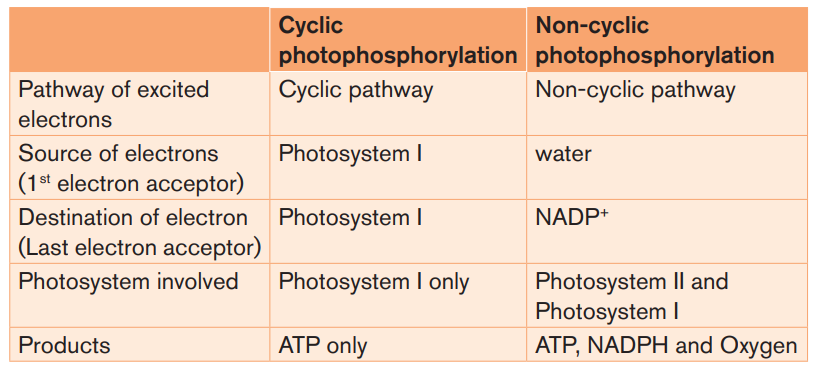
The light-independent reactions occur in stroma, and consist of reducing CO2
into sugars by using ATP and NADPH both coming from light-dependent
reactions in thylakoids. The Calvin cycle involves three main stages such as:
- Carbon fixation in form of CO2.
- Carbon reduction from CO2 to glucose.- Regeneration of RuBP.
a) Carbon fixation (Carboxylation) in form of CO2
The Calvin cycle begins with a 5-Carbon sugar phosphate called Riburose-1,
5 biphosphate (RuBP) which fixes the CO2 from air. This reaction is catalyzed
by an enzyme called RuBP carboxylase-oxygenase (RUBISCO), which makes
up about 30% of the total protein of the leaf, so it is probably one of the mostcommon proteins on the Earth.
The combination of RuBP and CO2 results in a theoretic 6-carbon compound
which is highly unstable. It immediately splits into two molecules of 3-carbon
known as phosphoglyceric acid (PGA) or glycerate 3-phosphate, or3-phosphoglycelate.
b) Carbon reduction from CO2 to glucose
With energy from ATP and reducing power from NADPH, the phosphoglyceric
acid is reduced into 3carbon molecules known as glyceraldehyde-3-phosphateor phosphoglyceraldehyde (PGAL).
Each molecule of PGA receives an additional phosphate group from ATP,
becoming 1, 3-biphosphoglycerate, and a pair of electrons and H+ from NADPH
reduces the carboxyl group of 3-phosphoglycerate to the aldehyde group ofPGAL which stores more potential energy.
ATP gives one phosphate group becoming ADP+, and NADPH gives H+ and
electrons to become NADP+. Both ADP+ and NADP+ will be used again in light
dependent reactions.
With 6 turns of Calvin cycle, the plant cell fixes 6CO2 molecules which are used
to synthesize 2 molecules of PGAL which leave the cycle and combine to makeone molecule of glucose or fructose. This glucose can be converted into:
Sucrose: when Oxygen combined with fructose. It is a form by which
carbohydrates are transported in plants.- Polysaccharides like starch for energy storage, and cellulose for structural
support.
- Amino acids when combined with nitrates,
- Nucleic acids when Oxygen combined with phosphates, and- Lipids.
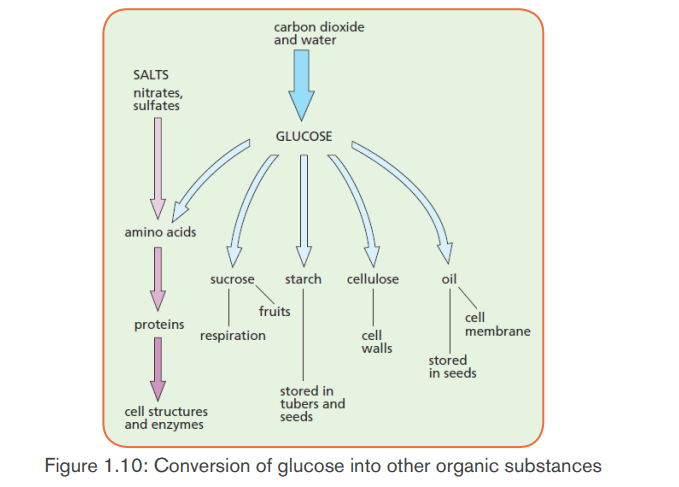
c) Regeneration of RuBP
The remaining ten 3-carbon molecules (PGAL) are converted back into six
5-carbon molecules, ready to fix other CO2 molecules for the next cycle. Thelight-independent reactions can be summarized as:
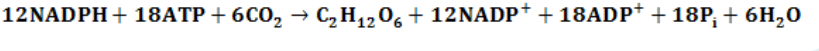
Other carbon dioxide fixation pathways (C4 CAM)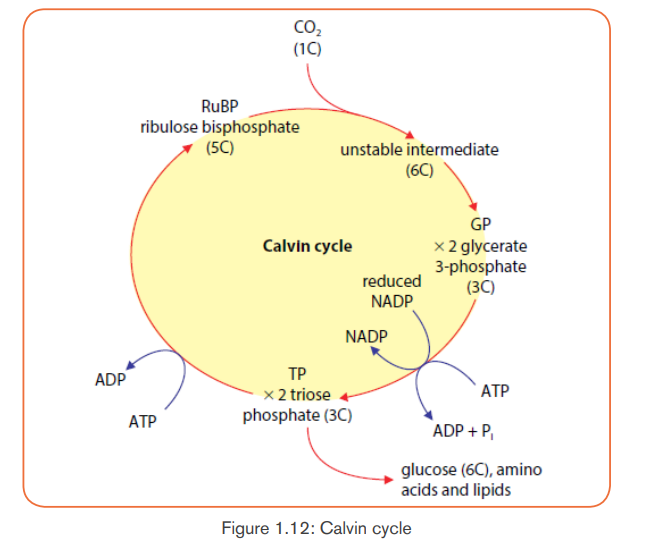 The most common pathway combines one molecule of CO2 with a 5-carbonsugar called ribulose biphosphate (RuBP). The enzyme which catalyzes this
The most common pathway combines one molecule of CO2 with a 5-carbonsugar called ribulose biphosphate (RuBP). The enzyme which catalyzes this
reaction (nicknamed “Rubisco”) is the most abundant enzyme on earth! The
resulting 6-carbon molecule is unstable, so it immediately splits into two
3-carbon molecules. The 3 carbon compound which is the first stable moleculeof this pathway gives this largest group of plants the name “C-3 plants”
Dry air, hot temperatures, and bright sunlight slow the C-3 pathway for carbon
fixation. This is because stomata, which normally allow CO2 to enter and O2
to leave, must close to prevent loss of water vapor. Closed stomata lead to
a shortage of CO2. Two alternative pathways for carbon fixation demonstrate
biochemical adaptations to differing environments. Plants such as corn solvethe problem by using a separate compartment to fix CO2.
Here CO2 combines with a 3-carbon molecule, resulting in a 4-carbon molecule.
Because the first stable organic molecule has four carbons, this adaptation has
the name C-4. Shuttled away from the initial fixation site, the 4-carbon molecule
is actually broken back down into CO2, and when enough accumulates, Rubiscofixes it a second time!
In some temperate plants such as wheat, rice, potato and bean only Calvin cycle
occurs. Such plants are called C-3 plants. While in some other plants dual
carboxylation takes place: (1) carboxylation of phosphoenol pyruvate (PEP) and
(2) carboxylation of RuBP. Such plants are called C-4 plants e.g. maize, sugar
cane and sorghum. In these, the first product formed during carbon dioxide
fixation is a four carbon compound oxalo acetic acid (OAA). C-4 plants have
special type of leaf anatomy called Kranz Anatomy. They have special large cells
around vascular bundles called bundle sheath cells. These are characterized
by having large number of chloroplasts, thick walls and no intercellular spaces.
The shape, size and arrangement of thylakoids in chloroplasts are also different
in bundle sheath cell as compared to mesophyll cell chloroplasts.
The pathway followed by C-4 plants is called C-4 cycle or Hatch and Slack
pathway. This was discovered by Hatch and Slack in sugar cane. The primary
CO2 acceptor is a 3-carbon molecule phosphoenol pyruvate (PEP). The reaction
is catalyzed by PEP carboxylase or PEP case in mesophyll cell chloroplast.
It forms 4-carbon compounds like OAA, malic acid or aspartate, which are
transported to the bundle sheath cells. In bundle sheath cells, these acids are
broken down to release CO2 and 3-carbon molecule. The 3-carbon molecule
is transported back to mesophyll cells and converted to PEP again, while CO2
enters into C-3 cycle to form sugars. C-4 plants are more efficient than C-3
plants as in C-4 plants, photosynthesis can occur at low concentration CO2
and photorespiration is negligible or absent.
Cacti and succulent (water-storing) plants such as the jade plant avoid water
loss by fixing CO2 only at night. These plants close their stomata during the
day and open them only in the cooler and more humid nighttime hours. Leaf
structure differs slightly from that of C-4 plants, but the fixation pathways are
similar. The family of plants in which this pathway was discovered gives the
pathway its name, Crassulacean Acid Metabolism, or CAM. All carbon
fixation pathways lead to the Calvin cycle to build sugar.
The CAM pathway is similar to the C4 pathway in that carbon dioxide is first
incorporated into organic intermediates before it enters the Calvin cycle. The
difference is that in C4 plants, the initial steps of carbon fixation are
separated structurally from the Calvin cycle whereas in CAM plants, the
two steps occur at separate times.
The CAM pathway and the C4 pathway compared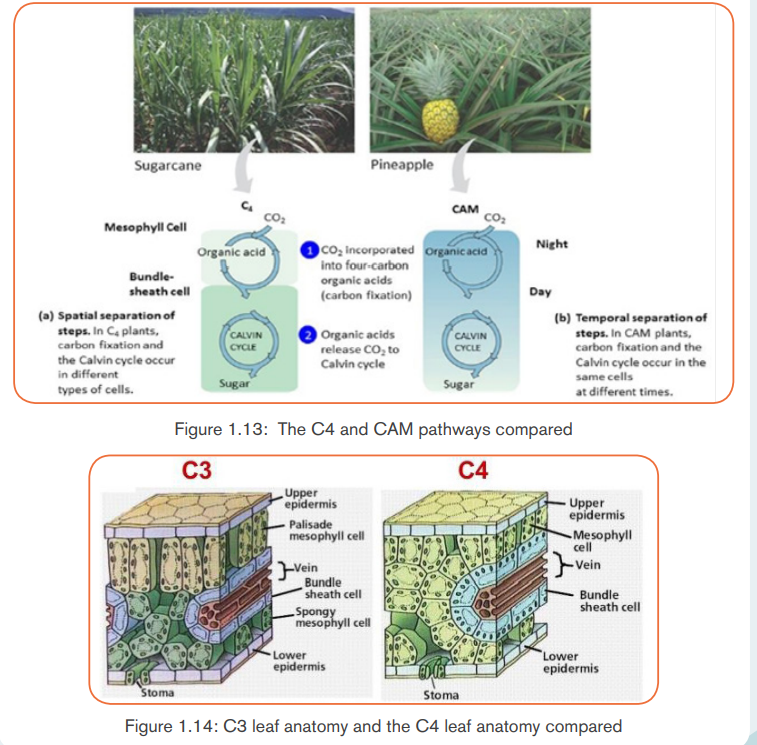
The table 1.3: Comparison between C3 and C4 plants
Photorespiration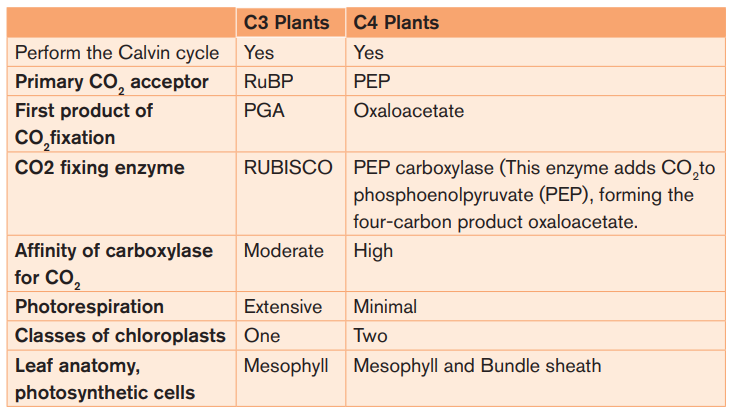
In most plants, initial fixation of carbon occurs via Rubisco, the Calvin cycle
enzyme that adds CO2 to ribulose biphosphate. Such plants are called C3
plants because the first organic product is a three carbon organic compound,
PGA. These plants produce less food when their stomata close on hot and dry
days.
The declining level of CO2 in the leaf starves the Calvin cycle. Making matter
worse, Rubisco can accept O2 in place of CO2. As O2 concentration overtakes
CO2 concentration within the air space, Rubisco adds O2 instead of CO2. The
product splits and one piece, a two-carbon compound is exported from thechloroplast. Mitochondria then break the two-carbon molecule into CO2.
The process is called photorespiration because it occurs in presence of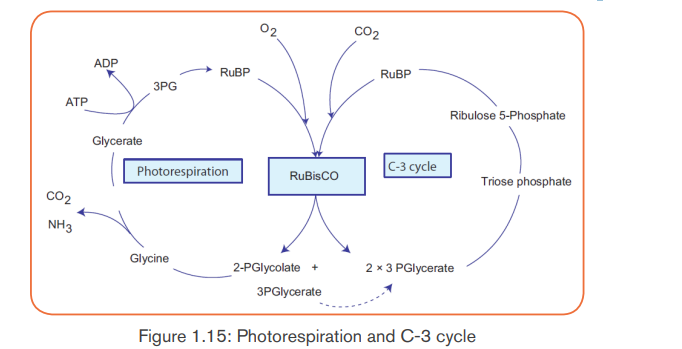
light (photo) and consumes O2 (respiration). However, unlike normal cellular
respiration, photorespiration generates no ATP, and unlike photosynthesis,
photorespiration generates no food. In fact, photorespiration decreasesphotosynthetic output by using material from the Calvin cycle.
Application activity 1.2
The diagram below illustrates photosynthesis process. Write each of the
following terms on the correct numbered line. Then answer the questions
that follow.
Carbon Dioxide Glucose Oxygen Water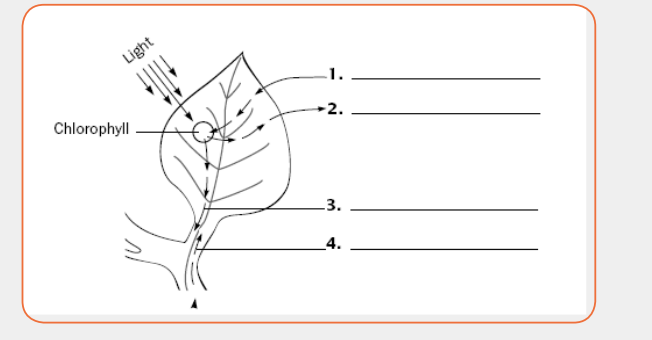 i) In photosynthesis, establish an equation of substrate with substances
i) In photosynthesis, establish an equation of substrate with substances
produced
ii) What would happen if substance labeled in 1 and 4 are absent? Justify
your answer.iii) Explain how photosynthesis and respiration are interdependent?
1.3. Factors affecting the rate of photosynthesis
Activity 1.3
1. When beans are grown under banana trees, the farmers record poor
harvest. Explain why?
2. Make a research to find out how each of the following factors can
affect the rate of photosynthesis: Temperature – Light intensity –Concentration of CO2 – Amount of water
The photosynthesis rate varies with the species but also varies within individuals
for a same species; this varies under the influence of certain external factors
which are: the temperature, CO2 concentration in the atmosphere, light intensityand soil humidity.
a. Temperature
Photosynthesis is an enzyme-controlled process. At very low temperatures the
rate of photosynthesis is slow because the enzymes are inactive. As temperature
increases, the rate of photosynthesis increases because the enzymes become
more active. Rate of photosynthesis is optimum at (35-40) °C. Beyond 40°C
the rate of photosynthesis decreases and eventually stops since the enzymesbecome denatured.
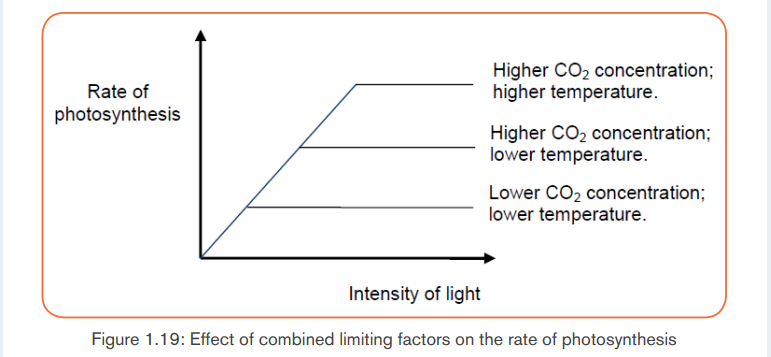
b. CO2 concentration in the atmosphere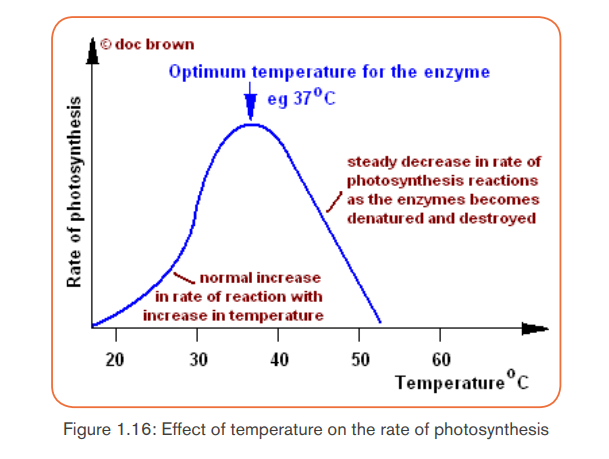
While the concentration of carbon (IV) oxide in the atmosphere is fairly constant
at 0.03%, an increase in carbon (IV) oxide concentration translates into an
increase in the rate of photosynthesis upto a certain point when the rate of
photosynthesis becomes constant. At this point, other factors such as lightintensity, water and temperature become limiting factors.
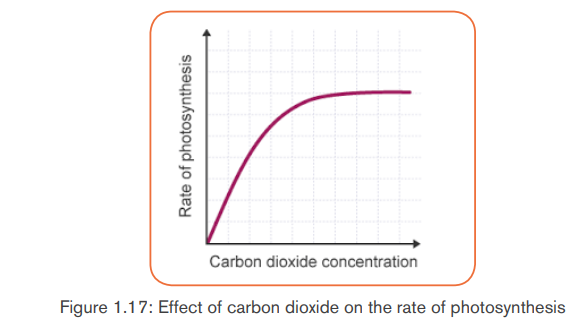
The photosynthetic rate is zero in place lacking CO2, it increases with the
increase concentration of CO2 in the atmosphere and reaches an optimumranging between 5 and 8%CO2 concentration.
c. Light intensity
The rate at of photosynthesis increases with an increase in light intensity up to
a certain level. Beyond the optimum light intensity, the rate of photosynthesis
becomes constant. To this effect, plants photosynthesize faster on bright and
sunny days than on dull cloudy days.
Light quality/wavelength also affects the rate of photosynthesis. Most plants
require red and blue wavelengths of light for photosynthesis. Light duration alsoaffects photosynthesis rate
The photosynthesis rate is low during night, it increases when the light intensity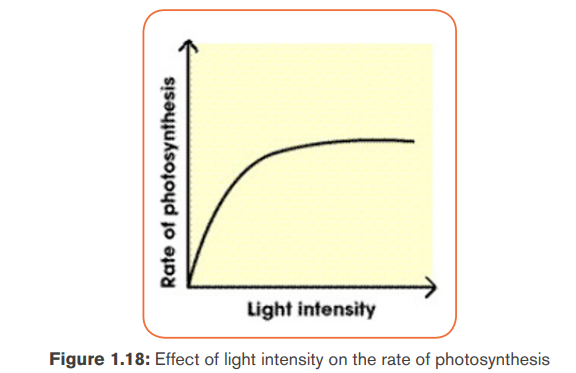
increases but the optimum varies according to the plants.
d. Availability of water for the plant
The photosynthesis rate is low when the soil is dry, it increases when the content
of water increases for the terrestrial plants, and for the aquatic plants it remains
constant as long as they are fixed in water.Note: The limiting factors work together to influence the rate of photosynthesis
Application activity 1.3
Factors affecting rate of photosynthesis.
Materials Required:Elodea plant, glass rod, sodium bicarbonate.
Procedure:
Take a fresh, healthy twig of Elodea plant with one end intact and tie it
gently to a glass rod. Put the glass rod with plant in a boiling tube containing
water and add 1mg/mL sodium bicarbonate and keep it in moderate light
condition. Note the numbers of bubbles escaping from cut end per minute.
Again add same amount of sodium bicarbonate and note the number of
bubbles escaping from cut end per minute. Do you find number of bubbles
increasing? Repeat this step until bubbles escaping per minute do not
increase. Then take set up under high light intensity and note the numbersof bubbles.
1. What is the observation made when the mass of sodium bicarbonate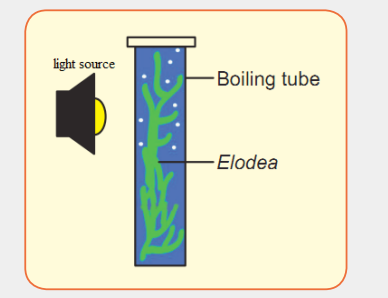
was increased?
2. What is your observation if the set up is under high light intensity?1.4. Importance of photosynthesis
Activity 1.4
The following diagram shows the link that exists between plant and animals.Observe the diagram and use it to answer the related questions.
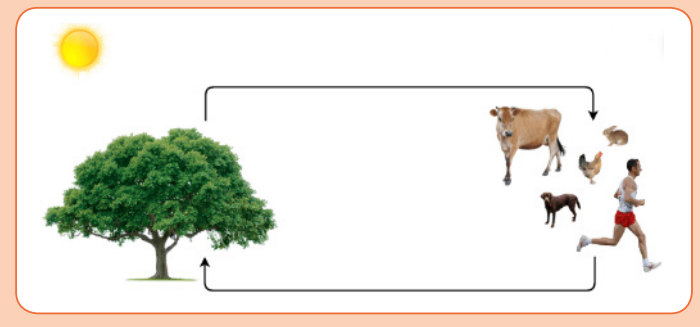 i) What do the animals receive from the plant and what do the plantsAutotrophic nutrition is a process by which living organisms (autotrophs:
i) What do the animals receive from the plant and what do the plantsAutotrophic nutrition is a process by which living organisms (autotrophs:
receive from the animal on the diagram above?
ii) Discuss how the relation between plants and animals are
interdependent?
iii) Suggest the role of aquatic plants to aquatic life of animals.
photoautotrophs and chemoautotrophs) make their own food. The autotrophism
is very essential as it allows production of Oxygen and food for not only
themselves but also for heterotrophs. The roles of autotrophic nutrition include:
a. Independence of green plants from other living organisms
This importance relates to their capacity for synthesizing organic molecules from
glucose produced by CO2 and water, this completely make them independentsof the other living organisms to the nutrition point of view.
b. Energy storage
The autotrophs like green plants, by the process of photosynthesis synthesize
certain substances like the glucose, cellulose, starch… which are variablessources of energy.
b. Energy storage
The autotrophs like green plants, by the process of photosynthesis synthesize
certain substances like the glucose, cellulose, starch… which are variables
sources of energy.
c. Production of O2 for the living organisms’ respiration
The oxygen produced by the photosynthesis is necessary for the living organisms’
respiration. Thus without photosynthesis, no oxygen can be produced; withoutoxygen no respiration; without respiration no life on Earth.
d. Cleaning the atmosphere
Photoautotrophs absorb carbon dioxide from surrounding air, and release
Oxygen (produced by photosynthesis) in atmosphere.
e. Formation of Ozone layer
Ozone layer is a thick layer in the atmosphere which is formed Ozone
molecule (O3). Oxygen atoms which make ozone molecule are produced by
photosynthesis. Ozone layer protects the Earth from high solar radiations, and
this allows the existence of the life on the Earth.
Synthesis of the organic substances: food for the heterotrophs (animal and
mushrooms). The organic substances produced by photosynthesis are the food
for the heterotrophs which are unable to synthesize these substances by their
own means.
Application activity 1.4
(a) A well watered potted bean plant was destarched by putting it in the dark
for 36 hours. Three of its leaves were smeared with Vaseline as follows: leaf
I on both sides; leaf II on the lower surface only; leaf III on the upper surface
only. All the other leaves were left untreated. The plant was then placed in
sunlight for eight hours after which an iodine test for starch was carried out.The observation was as follows:
Leaf I-brown colour; Leaf II-slight blue-black stain; Leaf III-intense blueblack stain;
Leaf IV (untreated leaf)-very intense blue-black stain. Explainthese observations.
(b) A student carried out an investigation to show how light intensity affected
the rate of photosynthesis for a water plant, Elodea. The student used a test
containing water plant immersed in water at different light intensity. After 5
minutes of each experiment, the student counted the number of bubbles.The results are shown in the following table.
Plot a suitable graph for the result of experiment.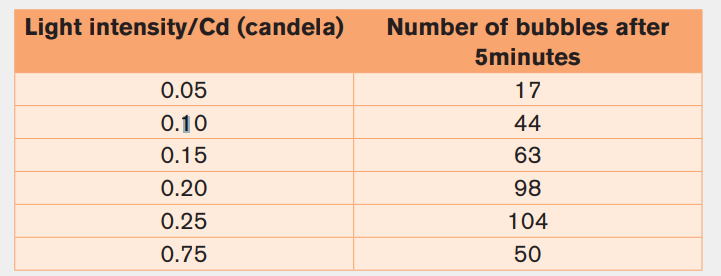
Explain the results recorded between 0.25 Cd and 0.75 Cd of light intensity
Skills Lab 1
Automobiles and machines must be supplied with gasoline or electricity
as a source of energy before they can move. Your muscles require energy
in the form of ATP to contract. Muscles can produce ATP by using oxygen
(aerobic respiration) or not using it (anaerobic respiration). Anaerobic
respiration in muscle cells produces lactic acid. When muscles do a lot
of work quickly, the buildup of lactic acid reduces their ability to contract
until exhaustion eventually sets in and contraction stops altogether. This iscalled muscle fatigue.
Materials: clothespin, timer
Procedure:- Hold a clothespin in the thumb and index finger of your dominant- Repeat this process for nine more 20-s periods, recording theresult
hand.
- Count the number of times you can open and close the clothespin in
a 20-s period while holding the other fingers of the hand straight out.
Make sure to squeeze quickly and completely to get the maximum
number of squeezes for each trial.
for each trial in a suitable table. Do not rest your fingers between
trials.
- Repeat the procedure for the nondominant hand.a) What happened to your strength as you progressed through eachEnd Unit Assessment 1
trial?
b) Describe how your hand and fingers felt during the end of your trials.
c) What factors might cause you to get more squeezes (to have less
fatigue)?
d) Were your results different for the dominant and then on dominant
hand? Explain why they would be different.
e) Your muscles would probably recover after 10 min of rest to operate
at the original squeeze rate. Explain why.f) Prepare a suitable graph of anaerobic respiration your muscle.
1. What are the products of the light dependent reactions of
photosynthesis?
a) ATP, RuBP and reduced NAD
b) ATP, oxygen and reduced NADP
c) GP, oxygen and reduced NAD
d) GP, reduced NADP and RuBP
2. Before the Krebs cycle can proceed,
pyruvic acid must be converted
intoa) Citric acid3. The net number of ATP made directly by glycolysis is
b) Glucose
c) Acetyl-CoA
d) Glucose
e) NADHa) 24. Cellular respiration is similar to photosynthesis in that they both
b) 4
c) 32
d) 38a) Produce ATP5. By accepting electrons and protons, the oxygen used in aerobic
b) Involve chemiosmosis
c) Make phosphoglyceraldehyde (PGAL)
d) All of the above
respiration turns intoa) CO26. The Krebs cycle occurs in the
b) H2O
c) C6H12O6
d) ATPa) Cytosold) Space between the inner and outer mitochondrial membrane
b) Outer mitochondrial membrane
c) Mitochondrial matrix
7. During each turn of the Krebs cycle,a) Two CO2 molecules are produced8. Most of the ATP synthesized in aerobic respiration is made
b) Two ATP molecules are consumed
c) Pyruvic acid combines with oxaloacetic acid
d) Glucose combines with a four-carbon molecule.a) During glycolysis9. Where does each stage of aerobic respiration occur in a eukaryotic
b) Through fermentation
c) In the cytosold) Through chemiosmosis
cell?
10. The diagram summarises how glucose can be used to produce ATP,
without the use of oxygen.
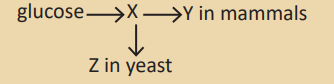
Which compounds are represented by the letters X, Y and Z ?
11. a. Copy and complete the table to show the differences between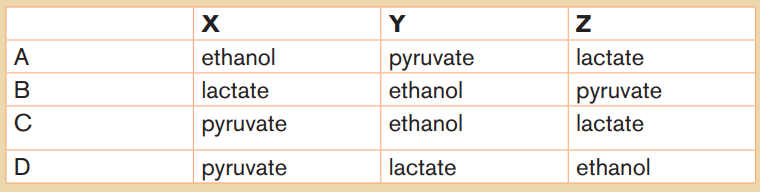
mesophyll and bundle sheath cells in C4 plants. Insert a tick (x) whenan item is present in the cell and a cross (√) when it is not.
 b. Explain what is meant by photorespiration.12. a. Explain what is meant by a limiting factor.b. List four factors that may be rate-limiting in photosynthesis.
b. Explain what is meant by photorespiration.12. a. Explain what is meant by a limiting factor.b. List four factors that may be rate-limiting in photosynthesis.
c. At low light intensities, increasing the temperature has little effect
on the rate of photosynthesis. At high light intensities, increasing
the temperature increases the rate of photosynthesis. Explain theseobservations.
