Topic outline
UNIT 1:THE CONCEPT OF INTEGRATED SCIENCE AND MEASUREMENTS OF PHYSICAL QUANTITIES
Key unit competence
Explain the concept of Integrated science and Use appropriate materials to measure different physical quantities.
Introductory Activity ILook carefully at the following illustrations and answer the questions below:
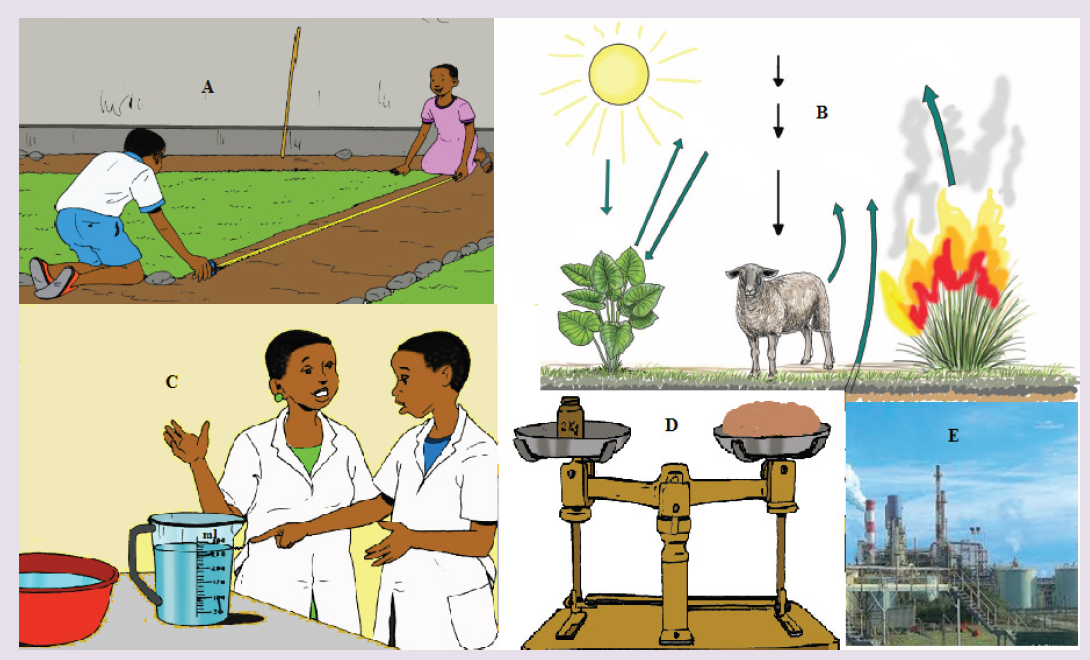
Questions:
a) Describe the illustration A, B, C, D.
b) Based on your knowledge from O-level, what are scientific concepts can you associate to each of those illustrations? Group the noted concepts in their science subject areas.
c) Is there any one illustration in which you find application of many science subjects area? Justify your answer by providing other examples found in everyday life.
d) Can you explain how and why every person should have integrated understanding of those science subject areas?
e) What kind of physical quantities that can be measured in the illustration above? Suggest the names of the tools used in the
illustration above?
f) Outline other examples of physical quantities and the corresponding measuring tools
g) What can be considered to select the best tool(s) to be used in measuring a given measurable quantity?
1.1 Introduction to integrated science
Activity 1.1
Task 1
It is known that an Integrated Science course serves the purpose of unifying sciences in a whole one subject covering both the physical and life sciences. These courses are integrated in that the fields of science are not segmented. For example, in describing the physics of light, we show how this applies to the inner workings of our eyes, which, in turn, are sensitive to visible light in great part because of the chemical composition of our atmosphere.
Use the paragraph above to answer the following questions:
a) What does the term Integrated Science mean?
b) Explain why Integrated Science is very important in finding appropriate solutions in various complex situations? Justify your
answer based on the paragraph above and other examples observed in everyday life.
Task2
Suppose you visited two industries and took the photos below and saw that distinguished science subjects are involved in the process of production. Write a paragraph about your visit identifying how Physics, Biology and Chemistry are integrated in the process.
1.1.1 Definition and rationale of integrated science
Human survival depends on knowledge through the exploration of the environment. Science provides knowledge while technology provides ways of using this knowledge. It is therefore very important to be aware of the global dimension of science needed in our lives in order to effectively deal with every day situation. The word “integrated” means “to restore the whole, to come together, to be a part of, to include.” Integrated science is a subject which incorporates the knowledge base of all the science fields, both physical and life sciences and these science fields are included in one subject as a whole “integrated science” in that the fields of science are not segmented. It is a subject which offers experiences which help people to develop an operational understanding of the structure of science that should enrich their lives and make them more responsible citizens in the society. Hence, integrated approach of learning science is appropriate as science knowledge is a tool to be used by every person to effectively deal with real world problems and life.
For examples, when you are studying digestion process of animals, you will need the knowledge of chemical processes. Another example, in describing the physics of light, we show how this applies to the inner workings of our eyes, which, in turn, are sensitive to visible light in great part because of the chemical composition of our atmosphere.
Aims and Objectives of Integrated Science subject The overall aim of the integrated science subject is to enable students
develop scientific literacy so that students can participate actively in the rapidly changing knowledge based society, prepare for further studies or careers in fields where the knowledge of science will be useful.
However, the broad aims of integrated science subject are to enable students to:
– Develop interest in and maintain a sense of wonder and curiosity about the natural and technological world;
– Acquire a broad and general understanding of key science ideas and explanatory framework of science and appreciate how the ideas were developed and why they are valued;
– Develop skills for making scientific inquiries;
– Develop the ability to think scientifically, critically and creatively and to solve problems individually or collaboratively in science related contexts;
– Use the language of science to communicate ideas and views on science – related issues;
– Make informed decisions and judgments about science related issues;
– Be aware of the social, ethnical, economic, environmental and technological implications of science and develop an attitude of
responsible citizenship; and
– Develop conceptual tools for thinking and making sense of the world.
1.1.2. Interconnection between science subjects
The purpose of science is to produce useful models of reality which are used to advance the development of technology, leading to better quality of life for human being and the environment around him or her.
There are many branches of science and various ways of classifying them.
One of the most common ways is to classify the branches into natural sciences, social sciences, and formal sciences.
Natural sciences: the study of natural phenomena (including cosmological, geological, physical, chemical, and biological factors of the universe).
Natural science can be divided into two main branches: physical science and life science (or biological science). Social sciences: the study of human behavior and societies. The social sciences include, but are not limited to: anthropology, archaeology, communication studies, economics, history, musicology, human geography, jurisprudence, linguistics, political science, psychology, public health, and sociology. Formal science is a branch of science studying formal language disciplines concerned
with formal systems, such as logic, mathematics, statistics, theoretical computer science, artificial intelligence, information theory, game theory, systems theory, decision theory, and theoretical linguistics.
Note:
– Chemistry mainly deals with the study of matter’s properties and behaviors as well as reactions between them to produce new useful products. For a physicist to understand the working mechanism of chemical cells, help is sought from a chemist. On the other hand, the reasons behind the various colours observed in most of the chemical reactions are explained by a physicist. Petroleum products are dealt with by the chemist, but the transportation of such products make use of the principles of physics.
– In Biology, the study of living cells and small insects by a biologist requires magnification. The concept of magnification using simple or compound microscope is a brain child of a physicist. A good physicist needs to have good health.
1.1.3. Relationship between science with other subjects
The concepts of science and other subjects might be expanded or explainable in broader senses than you might have been exposed to, this should then predict not only the interconnection senses already known, but should also predict much broader interconnections. This might be useful to you and our future civilization.
Science is about observation and experimentation of things in the physical and natural world. If there no creative ideas, no destructive ideas, just more ideas of the same things that exist can this be healthy. There is such a thing as inductive reasoning not just deductive reasoning.
Now, science is the practical application of scientific knowledge. So we could have science as a conservative subject, or we could have science as a creative (conservative and destructive) subject, then leading to smaller or larger sets of science.
Note:
– In Geography, weather forecast, a geographer uses a barometer, wind gauge, etc. which are instruments developed by a physicist.
– In Agriculture, the water sprinkler, insecticide sprayer, etc. make use of the principles developed by physicists.
– In History, the determination of age fossils by historians and archaeologists use the principle developed by physicists.
– In games and sports, accurate measurement of time, distance, mass, and others uses instruments developed by physicists.
Application activity 1.1
1. Write a paragraph to convince someone that science is related to other subjects. Use clear examples to support your arguments and reasoning.
2. How can you describe the interconnections between science and technology, using at least three specific examples?
1.2 Measurements of physical quantities.
Activity 1.2
Task 1:
Look around the place and identify possiple physical quantities that can be measured? Explain the meaning of the physical quantities you have identified? Mention the SI units of the identified physical quantities?
Task 2:
It is possible to determine the nature and magnitude of the physical quantities that are measurable. Which of the following situations can be determined with the guidance of measurements? Support your answer with explanations and mention the physical quantity to be measured if possible.
a) Love between a boy and girl.
b) Size of the body.
c) Size of the garden?
d) Amount occupied by water in a tank.
1.2.1 Physical quantities and their measurements.
A quantity is any observable property or process in nature with which a number may be associated.
A physical quantity is defined as a property of a material that can be quantified by measurement.
Physical quantities are classified into fundamental and derived quantities.
FUNDAMENTAL PHYSICAL QUANTITIES
A quantity may be defined as any observable property or process in nature with which a number may be associated. This number is obtained by the operation of measurements. The number may be obtained directly by a single measurement or indirectly, say for example, by multiplying together two numbers obtained in separate operations of measurement. Fundamental quantities are those quantities that are not defined in terms of other quantities.
In physics there are 7 fundamental quantities of measurements namely length, mass, time, temperature, electric current, amount of substance and luminous intensity.
• DERIVED PHYSICAL QUANTITIES
Quantities which are defined in terms of the fundamental quantities via a system of quantity equations are called derived quantities. Examples of derived quantities include area, volume, velocity, acceleration, density, weight and force.
The SI units of derived quantities are obtained from equations using mathematical expressions
Note that some derived units have been given names. For example, force is measured in kg m/s2 and has been given a named unit called a newton (N).
1.2.2 International system of units
In order to measure any quantity, a standard unit (base unit) of reference is chosen. The standard unit chosen must be unchangeable, always reproducible and not subject to either the effect of aging and deterioration or possible destruction.
In 1960, an international system of units was established. This system is called the International System of Units (SI).
The International System of Units is an internationally agreed metric system of units of measurement. SI base quantities and units: The value of a physical quantity is usually expressed as the product of a number and a unit.
Name, Symbol and factor of metric prefixes in everyday use at workplace.SI prefixes used to form decimal multiples and submultiples of S I units (table 2 below). Table 2: SI prefixes
Example for length
• 10 mm= 1cm
• 1m= 106μm
• 1m=10-9Gm
• 1m2=(1012pm)2=1024pm2
Note: Numbers in the SI system are based on the number 10. Units in the SI system can therefore be multiplied or divided by 10 to form larger or smaller units.
1.2.3 Measuring fundamental physical quantities
• MEASURING LENGTH AND DISTANCE
We use different tools for measuring length: metre rule, ruler, tape measure, vernier caliper and the micrometer screw gauge based on the kind of length to measure. Straight distances that are less than one metre in length are generally measured using metre rules. Straight distances that are more than one metre in length are generally measured using tape measure.
A tape measure or measuring tape is a flexible ruler and used to measure distance. A tape measure is in form of a strip of metal, plastic or cloth that has numbers marked on it as shown in figure below and is used for measuring.
The figure below represents examples of tape measures:
It is a common measuring tool purposely designed to allow for a measure of great length to be easily carried out and permits one to measure around curves or corners. Surveyors use tape measures in lengths of over 100 m.
Metre rules are graduated in millimetres (mm). Each division on the scale represents 1 mm unit (Fig 1.3).
The direct way to measure length is by means of the straight edge of a ruler or metre ruler. The ruler is placed alongside the object to be measured, and the number of unit intervals of the ruler equal to the length of the object is then noted.
Metre rule is used to measure lengths up to about 100 cm and has a sensitivity of 0.5 mm.
Vernier caliper is an instrument used to measure outer dimensions of objects inside dimensions and depths. The figure below shows the vernier calipers:
We can measure outer dimensions of objects (using the main jaws), inside dimensions (using the smaller jaws at the top), and depths (using the stem).
The vernier calipers have a main scale and a sliding vernier scale that can allow readings to the nearest 0.02 mm. To measure outer dimensions of an object, the object is placed between the jaws, which are then moved together until they secure the object.
The screw clamp may then be tightened to ensure that the reading does not change while the scale is being read.The first significant figures are read immediately to the left of the zero of the vernier scale and the remaining digits are taken as the vernier scale division that lines up with any main scale division.
The internal diameter of the test tube is given by MSR + (VC × LC) Whereby the main scale reading (MSR), the vernier coincidence (VC).and The smallest reading called the least count (LC) that can be read from vernier callipers is 1 mm – 0.9 mm = 0.1 mm or 0.01 cm .
The main scale called the vernier coincidence (VC) and multiplying it with the least count i.e 0.01 cm.Therefore, the external diameter of the cylindrical object is MSR + (VC × LC)
A micrometer screw gauge is an instrument for measuring very short length such as the diameters of wires, thin rods, and thickness of a paper. Figure below shows a screw gauge:
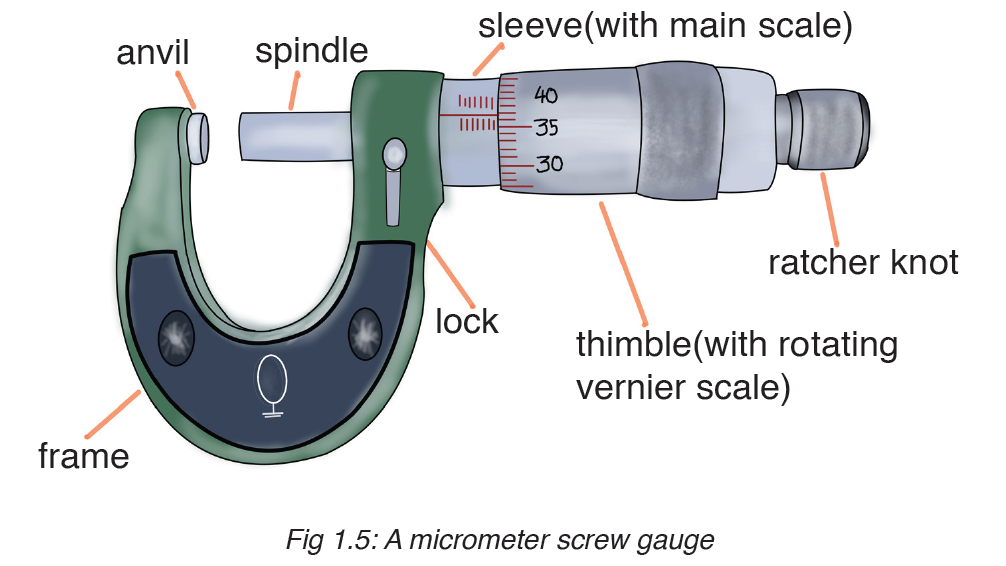
The micrometers have a pitch of 0.50 mm (two full turns are required to close the jaws by 1.00 mm). The rotating thimble is subdivided into 50 equal divisions. The thimble passes through a frame that carries a millimetre scale graduated to 0.5 mm. Thimble, which has a circular rotating scale that is calibrated from 0 to either 50 or 100 divisions. This scale is called the head
scale (thimble scale).
When the thimble is rotated, the spindle can move either forward or backwards. Ratchet which prevents the operator from exerting too much pressure on the object to be measured.The least count = 0.01 mm. The micrometer screw gauge reading = MSR + (HSC × LC). When the pitch is 1 mm, the thimble has 100 divisions called head scale divisions. In this case each division represents 0.01 mm. This is the least count (LC) of this screw gauge.
The thimble reading called the head scale coincidence(HSC) is the value of the mark on the thimble that coincides with the horizontal line on the sleeve.
Main scale reading is taken by considering the reading of a mark on the fixed scale that is immediately before the sleeve enters the rim of the head scale.
The jaws can be adjusted by rotating the thimble using the small ratchet knob. This includes a friction clutch which prevents too much tension being applied. The thimble must be rotated through two revolutions to open the jaws by 1 mm.
In order to measure an object, the object is placed between the jaws and the thimble is rotated using the ratchet until the object is secured. The ratchet knob must be used to secure the object firmly between the jaws, otherwise the instrument could be damaged or give an inconsistent reading.The lock may be used to ensure that the thimble does not rotate while you take the
reading.
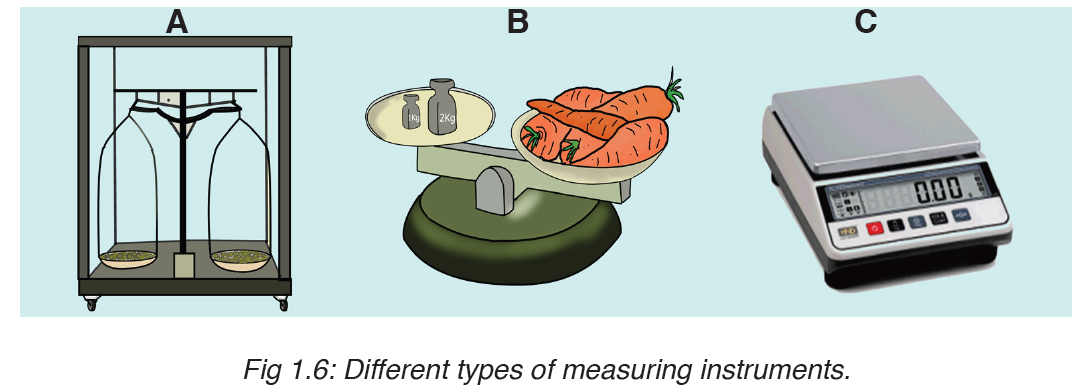
• MEASURING MASS
The mass of an object can be measured using a beam balance and a set of standard masses. It is noticed that the volume of the displaced water in measuring cylinder is equal to volume of an object lowered in the cylinder.
There are many kinds of balances used for measuring mass illustrated below:
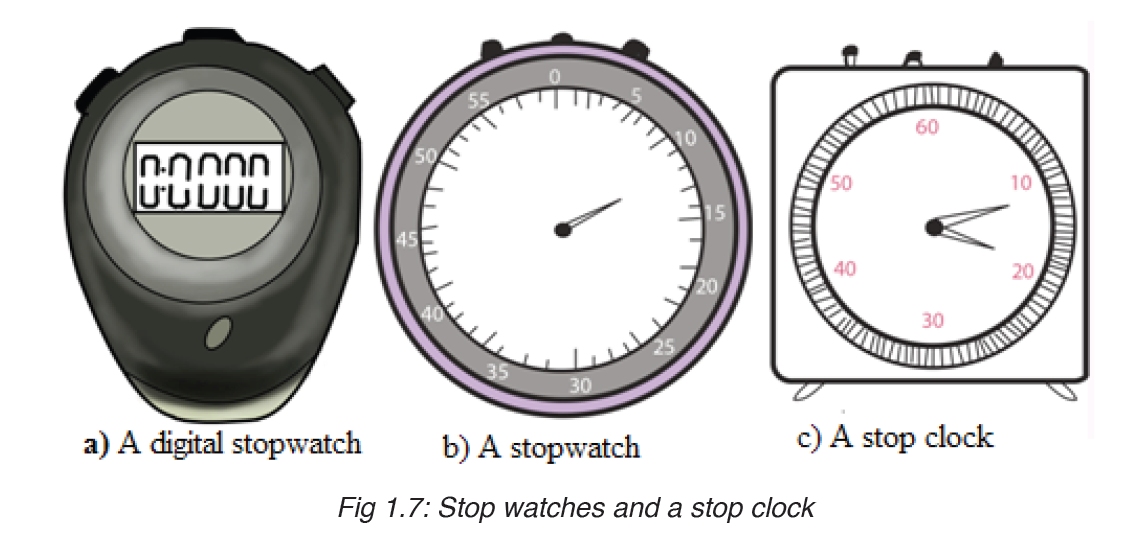
• MEASURING TIME
Time is measured using either analogue or digital watches and clocks and illustrated in figure below:
Application activity 1.2
1.Mention the appropriate instruments you would use to measure each of the following:
a) The The mass of an object.
b) The circumference of your waist.
c) The time someone uses to cover a certain length.
d) The diameter of a small ball.
2. It is possible to read and record the readings using a scale of a vernier caliper in order to measure the external diameter of the rod.
Steps followed in using vernier
a. Place the object to be measured between the outside jaws as shown in Figure below. Slide the jaw until they touch the rod.
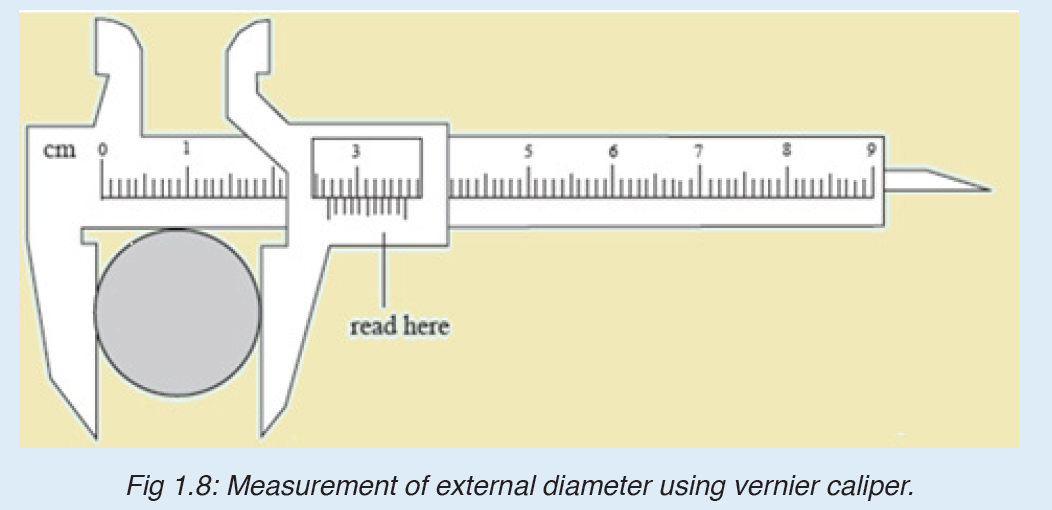
b. Record the readings on the main scale and the vernier scale. The main scale reading is the mark on the main scale that is immediately before the zero mark of the vernier scale.
c. Multiply the vernier scale reading by 0.01 cm.
d. Add the main scale reading (in cm) and the vernier scale reading (in cm) to get the diameter of the rod.
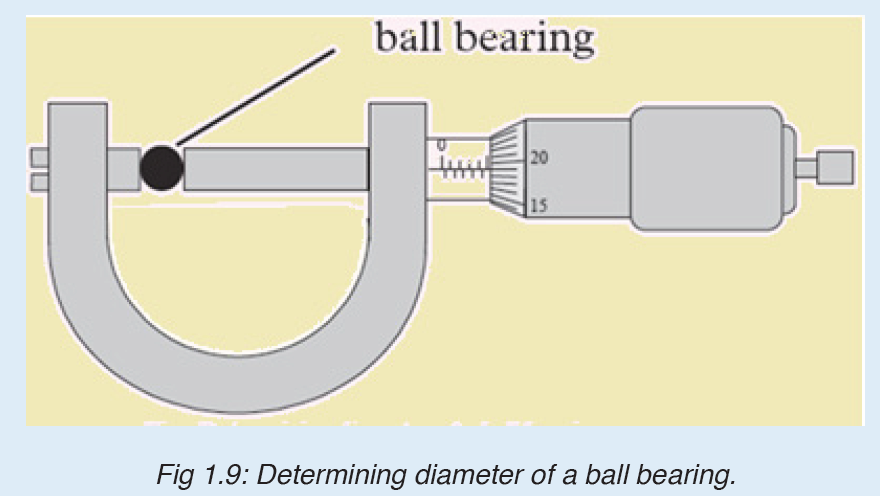
3. What is the diameter of the ball bearing shown in Figure below?
4.a) What does S I Units stands for?
b) Explain why it is correct to say that SI units are very important in measurements?
c) Suppose you wish to know the length of a big garden. How do you get the length of your garden?
d) Look at the following physical quantities: Mass, density, length, and time. Do all these quantities represent the fundamental quantities?
Justify your decision by identifying the ones included in the category mentioned above.
5. Look at the table below and try to complete it based on the skills gained in the previous activities done;
6. Choose two physical quantities with which you are familiar. Imagine that you are skilled in physical quantities and its measurements. Explain briefly how the values of these quantities can be obtained?
7. Express the following the indicated units and fill in blank spaces:
a) 250 m in …..cm.
b) 320 mg in ……g.
c) 5μg in ………g.
d) 7200 cm in …..m.
e) 3 kg in ……… g.
1.3 Dimensions of physical quantities
Activity 1.3
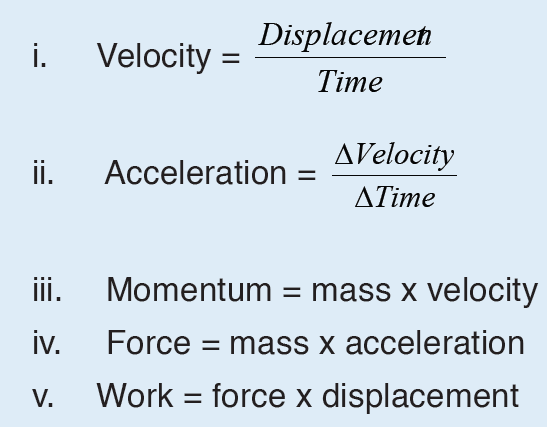
Given the formulas for the following derived quantities, try go get the dimensions of each quantity.
a) velocity = displacement/time
b) acceleration = change of velocity/time
c) momentum = mass x velocity
d) force = mass x acceleration
e) work = force x displacement
1.3.1 Introduction to dimensions of physical quantities
The nature of physical quantity is described by nature of its dimensions.
When we observe an object, the first thing we notice is the dimensions.
In fact, we are also defined or observed with respect to our dimensions that is, height, weight, the amount of flesh. The dimension of a body means how it is relatable in terms of base quantities. When we define the dimension of a quantity, we generally define its identity and existence. It becomes clear that everything in the universe has dimension, thereby it has presence.
Note: Dimensions are responsible in defining shape of an object.
1.3.2 Definition of dimensions of physical quantities
The dimension of a physical quantity is defined as the powers to which the fundamental quantities are raised in order to represent that quantity. The seven fundamental quantities are enclosed in square brackets [ ] to represent its dimensions.
• EXAMPLES OF ASSIGNING DIMENSIONS TO PHYSICAL QUANTITIES
Dimension of Length is described as [L], the dimension of time is described as [T], the dimension of mass is described as [M], the dimension of electric current is described as [A] and dimension of the amount of quantity can be described as [mol].Adding further dimension of temperature is [K] and that dimension of luminous intensity is [Cd] Consider a physical quantity Q which depends on base quantities like length, mass, time, electric current, the amount of substance and temperature, when they are raised to powers a, b, c, d, e, and f. Then dimensions of physical quantity Q can be given as:
[Q] = [LaMbTcAdmoleKf]
It is mandatory for us to use [ ] in order to write dimension of a physical quantity. In real life, everything is written in terms of dimensions of mass, length and time. Look out few examples given below:
1. The volume of a solid is given is the product of length, breadth and its height. Its dimension is given as:
Volume = Length × Breadth × Height
Volume = [L] × [L] × [L] (as length, breadth and height are lengths)
Volume = [L]3
As volume is dependent on mass and time, the powers of time and mass will be zero while expressing its dimensions i.e. [M]0 and [T]0
The final dimension of volume will be [M]0[L]3[T]0 = [M0L3T]
2. In a similar manner, dimensions of area will be [M]0[L]2[T]0
3. Speed of an object is distance covered by it in specific time and is given as:
Speed = Distance/Time
Dimension of Distance = [L]
Dimension of Time = [T]
Dimension of Speed = [L]/[T]
[Speed] = [L][T]-1 = [LT-1] = [M0LT-1]
4. Acceleration of a body is defined as rate of change of velocity with respect to time, its dimensions are given as:
Acceleration = Velocity / Time
Dimension of velocity = [LT-1]
Dimension of time = [T]
Dimension of acceleration will be = [LT-1]/[T]
[Acceleration] = [LT-2] = [M0LT-2]
5. Density of a body is defined as mass per unit volume, and its dimension is given as:
Density = Mass / Volume
Dimension of mass = [M]
Dimension of volume = [L3]
Dimension of density will be = [M] / [L3]
[Density] = [ML-3] or [ML-3T0]
6. Force applied on a body is the product of acceleration and mass of the body
Force = Mass × Acceleration
Dimension of Mass = [M]
Dimension of Acceleration = [LT-2]
Dimension of Force will be = [M] × [LT-2]
[Force] = [MLT-2]
1.3.3 Rules for writing dimensions of a physical quantity
We follow certain rules while expression a physical quantity in terms of dimensions, they are as follows:
– Dimensions are always enclosed in [ ] brackets
– If the body is independent of any fundamental quantity, we take its power to be 0
– When the dimensions are simplified we put all the fundamental quantities with their respective power in single [ ] brackets, for example as in velocity we write [L][T]-1 as [LT-1]
– We always try to get derived quantities in terms of fundamental quantities while writing a dimension.
– Laws of exponents are used while writing dimension of physical quantity so basic requirement is a must thing.
– If the dimension is written as it is we take its power to be 1, which is an understood thing.
– Plane angle and solid angle are dimensionless quantity that is they are independent of fundamental quantities.
– Therefore, some of the examples of dimensions of physical quantities include the following:
Force, [F] = [MLT-2]
Velocity, [v] = [LT-1]
Charge, (q) = [AT]
Specific heat, (s) = [L2T2K-1]
Gas constant, [R] = [ML2T-2K-1 mol-1]
• Benefits of Dimensions
Before writing dimensions of a physical quantity, it is must know a thing to understand why do we need dimensions and what are benefits of writing aphysical quantity. Benefits of describing a physical quantity are as follows:
– Describing dimensions help in understanding the relation between physical quantities and its dependence on base or fundamental quantities, that is, how dimensions of a body rely on mass, time, length, temperature and others.
– Dimensions are used in dimension analysis, where we use them to convert and interchange units.
– Dimensions are used in predicting unknown formulae by just studying how a certain body depends on base quantities and up to which extent.
– It makes measurement and study of physical quantities easier.
– We are able to identify or observe a quantity just because of its dimensions.
– Dimensions define objects and their existence.
Limitations of Dimensions
Besides being a useful quantity, there are many limitations of dimensions, which are as follows:
– Dimensions can’t be used for trigonometric and exponential functions.
– Dimensions never define exact form of a relation.
– We can’t find values of certain constants in physical relations with the help of dimensions.
– A dimensionally correct equation may not be the correct equation always.
• Dimension Table
It consumes a lot of time while deriving dimensions of quantities. So in order to save time, we learn some basic dimensions of certain quantities like velocity, acceleration, and other related derived quantities.
For Example, suppose you’re asked to find dimensions of Force and you remember dimension of acceleration is [LT-2], you can easily state that the dimension of force as [MLT-2] as force is the product of mass and acceleration of a body.
The table below depicts dimensions of several derived quantities which one can use directly in problems of dimension analysis.
Application activity 1.3
1.i. What are four uses of dimensional analysis? Explain with one example for each.
ii. What are three limitations of dimensional analysis in physics?
2. Show that 1/2 gt2 has the same dimensions of distance.
3. What are the missing words in the following statements?
a) The dimensions of velocity are ………………………………. .
b) The dimensions of force are ……………………………………. . .
4.a) What does the term dimension mean in Physical quantities?
b) Given the formulas for the following derived quantities, calculate the dimensions of each quantity.
SKILLS LAB
Conduct a survey, collect and analyze data about when, where, and why people use different measuring instruments or devices and physical laws.
To complete this project you must Develop a survey sheet about physical quantities, measuring instruments or devices, physical laws needed, appropriate SI units and metric prefixes used in everyday life.
Distribute your survey sheet to other student-teachers, family members and neighbors.
• Compile and analyze your data.
• Create a report to display your findings in your sheet.
Plan it! To get started, think about the format and content of your survey sheet. Brainstorm what kinds of questions you will ask. Develop a plan for involving student-teachers in your class or other classes to gather more data.
End unit assessment 1
1. Differentiate between a fundamental quantity and a derived quantity.
Give one example of each and its corresponding SI units.
2. Express the following in millimetres:
a) 2.7 m
b) 26.9 cm
c) 356 μm.
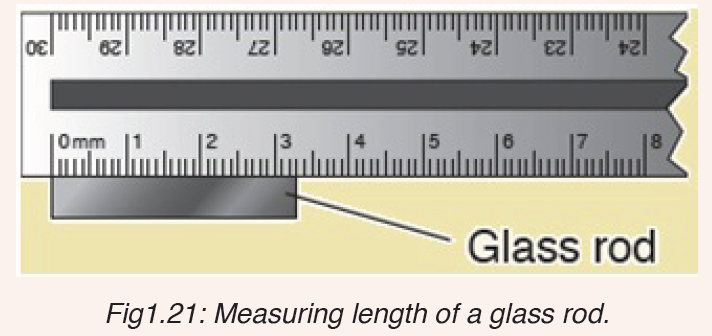
3. What is the length of the glass rod shown in Figure below?
4. Use the knowledge and skills gained from the previous concepts to complete the following sentences:
a) A quantity may be defined as any ………………………. in nature with which a number may be associated.
b) Physical quantities are classified into ……………. .and………………… quantities.
c) ……………………………are those quantities that are not defined in terms of other quantities.
d) The value of a physical quantity is usually expressed as the product of a …………………and a ……………………
e) The SI units stands for ………………………………………………….
5. Kaneza conducted an experiment on the growth of plants and recorded the results in a table. He used four plants of the same type and size and measured their growth after one month.
Fig: Table of results based on each plant type.
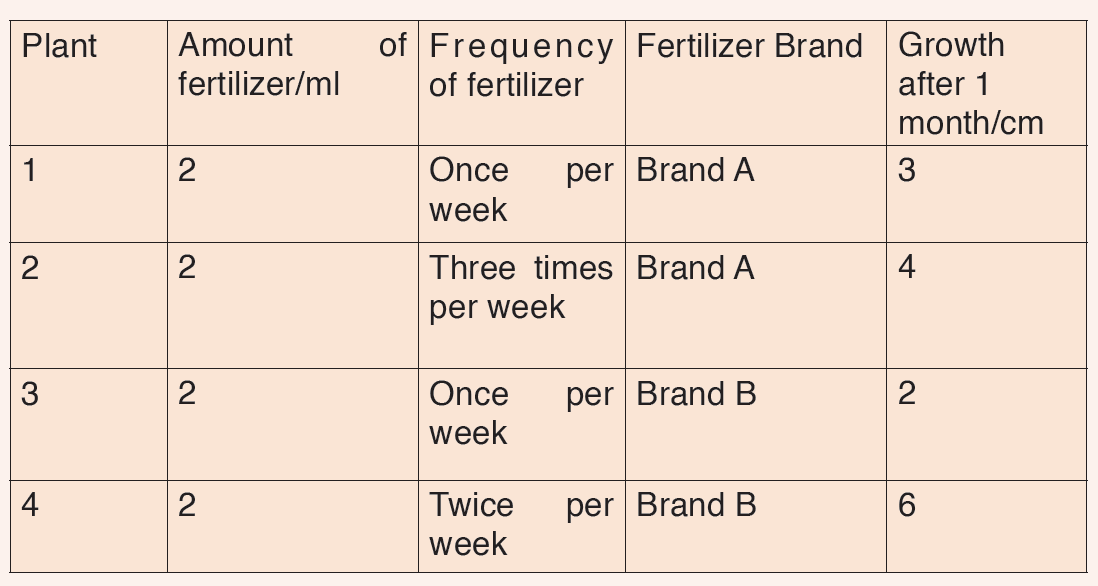
Questions on scientific report above:
a) Identify the possible data types that were considered in Kaneza’s experiment?
b) Tell whether each data type was controlled effectively?
c) Explain what is wrong with Kaneza’s experiment?
d) What could he change to allow better conclusions to be drawn in the scientific investigation above?
UNIT 2: COMMON DISEASES AND HYGIENE
Key unit competence
Implement ways of preventing and controlling common diseases and hygiene related issues.
Introductory Activity
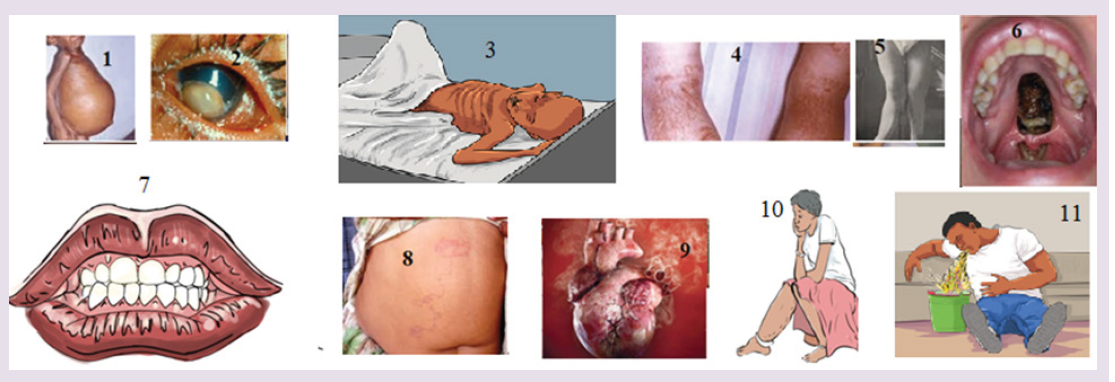
The figure 1-11 indicates different situations.
a) According to what you always observe in community you live in, suggest what happened.
b) What do you think is a cause of each case in figure?
c) What can you do in community for limiting those situations?
2.1. Common diseases
Activity 2.1.a
1) The government of Rwanda encourages people to visit hospitals and test their blood. A person has done that test and he found having HIV positive.
a. Which opportunistic information can be associated with HIV?
b. What is its causative agent?
c. How is it transmitted from one people to another?
2) Basing on the ways of transmission, suggest other diseases that can be transmitted as the disease stated in a.1
3) How can you prevent those diseases?
Infectious diseases are caused by microorganisms known as pathogens which may include viruses, bacteria, fungi and protozoa. Those diseases are called communicable diseases as they can be transmitted from one person to another. They include cholera, malaria, typhoid, HIV and AIDS...Malaria is one of the most dangerous infectious diseases, endemic in Latin America, Africa and South-East Asia.
Some infectious diseases can also be from animals to humans.
Some technical terms used when discussing about infectious diseases are:
– Aetiology: The study of the cause of disease.
– Epidemiology: The study of all the factors that contribute to the appearance of a particular disease
– Causative agent: The organism which causes the disease
– Vector: An organism which carries the causative agent of the disease from one person to another or from infected animal to human.
– Incubation period: The period of time between the original infection and the appearance of signs and symptoms.
– Infective period: The time during which a person is capable of passing the disease on to another person.
– Carrier: The person who has been infected but develop no signs and symptom, the carrier can pass the disease on to another person.
– Prevention: Measures taken to prevent diseases.
– Treatment: Measures taken to cure diseases.
– Antibody: Is a protein produced by the body’s immune system when it detects harmful substances called antigen.
– Antigen: Is any substance that causes your immune system to produce antibodies against it.
– Host: A host can be anything living organism ion which pathogens can survive
– Hygiene: Practices that help to maintain health and prevent the spread of diseases
– Immunity: Is the ability of the body to resist to infections.
Some groups of communicable diseases
• Bacterial diseases: these are diseases caused by bacteria. They include cholera, typhoid, tetanus, tuberculosis, etc.
• Viral diseases: these are diseases caused by viruses. They include AIDS, polio, measles, Ebola, etc.
• Protozoan diseases: these are diseases caused by protozoa. They include malaria, sleeping sickness, trichomoniasis, etc.
• Fungal diseases: these are diseases caused by fungi. They include candidiasis, athlete’s foot, ring worms, etc.
• Worm diseases: these are diseases caused by worms. They include elephantiasis, bilharzias, etc.
• Sexually transmitted diseases: these are diseases transmitted through sexual contact. They include HIV-AIDS, syphilis, gonorrhea, etc. c.
Transmission of infectious diseases
Pathogens can spread when you have direct contact with an infected person.
For example, if you have contact with the person’s blood, body fluids or open wounds. Pathogens can also be spread through contaminated food, water or air. Infected animals can spread pathogens to people.
The following conditions lead to the spread of an infectious disease:
• A pathogen which causes the disease.
• A source which is an infected organism.
Mode of transmission: A pathogen must be able to enter the body of the new host to cause an infection. Infectious diseases follow a pattern of development from the time of infection.
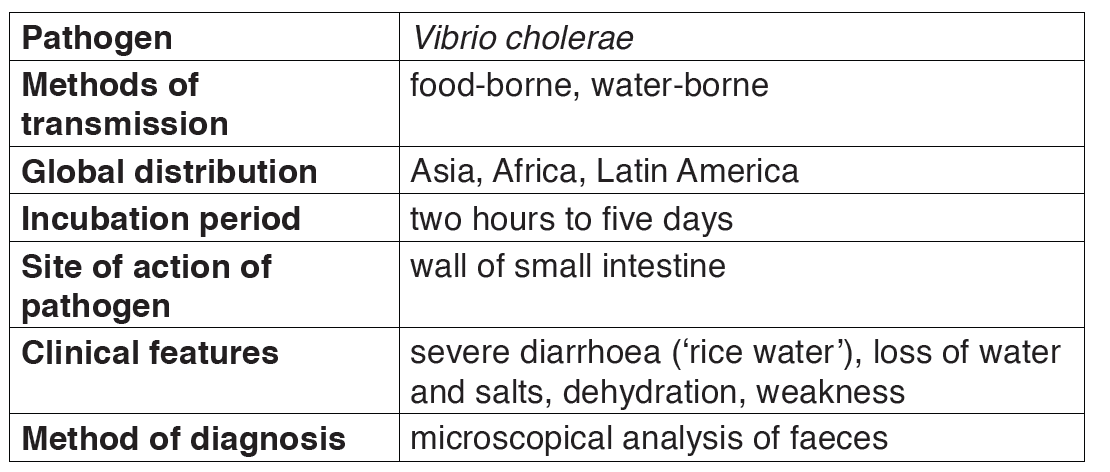
2.1.1. Cholera
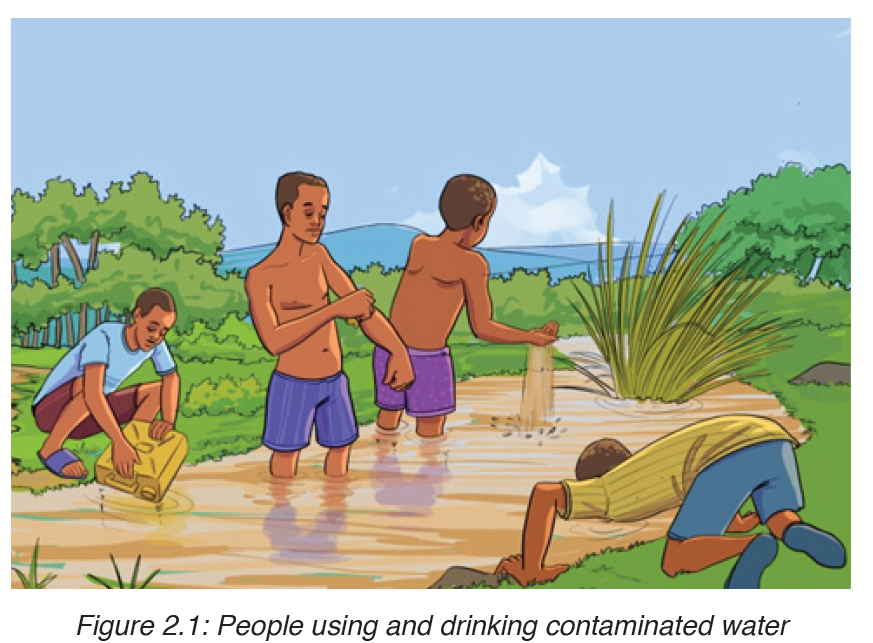
Cholera is a good example of a waterborne disease. It is endemic in parts of Asia, particularly India. The organism which causes cholera is a comma shaped motile bacterium called Vibrio cholerae.
a) Transmission and symptoms of cholera
The main source of infection is water contaminated by feces with Vibrios.
It is estimated that only about one infected person in 50 develops the disease, the rest being carriers. Drinking contaminated water, or washing food or utensils in it, is the most common means of transmission. Direct contamination of food with feces as a result of poor hygiene is also possible, house flies being the main vector in this last case.
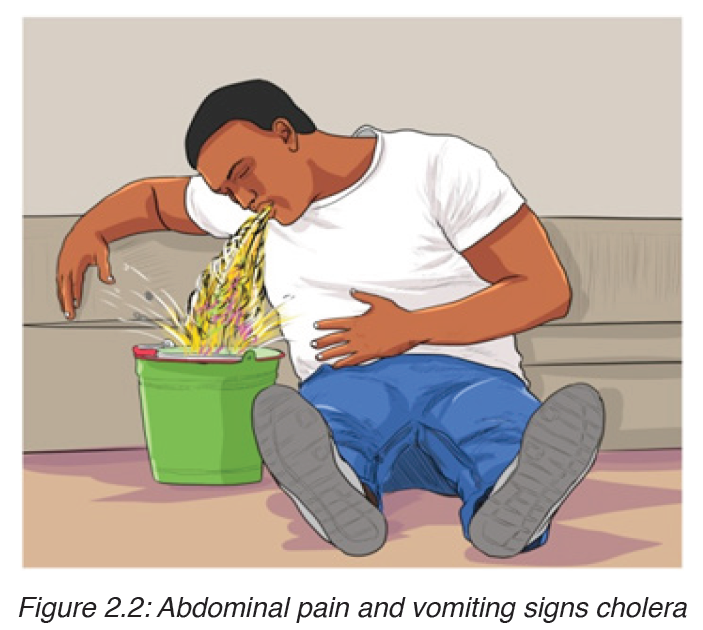
b) Signs and symptoms of cholera
Vibrio cholerae multiply in the intestine, releasing a powerful toxin which results in violent inflammation of the intestine and production of the watery diarrhea.
The main sign of the disease is severe diarrhea due to irritation of the bowel by toxins from the vibrios. The liquid of the feces is so profuse and cloudy like “rice water”.
Abdominal pain and vomiting are also common. Dehydration is rapid and quickly results in death unless rehydration treatment is given.
Fever is absent; in fact, the skin feels deathly cold and often damp.
Table 2.1: The features of cholera.
c) Treatment of cholera
The prime cause of death from cholera is dehydration i.e. loss of water with its minerals salts. For that it is obligatory to rehydrate with oral serum which contain mineral salts and sugar, The fluid lost may be replaced by administration of a drip food into a vein.
Various antibiotics, such as tetracyclines and chloramphenicol, are used to treat cholera. Chloramphenicol is effective against tetracycline-resistant vibrias.
d) Prevention of cholera
– Use clean drinking water,
– Proper treatment of sewage and sanitation
– High standards of public and personal hygiene, particularly in relation to food ( such as washing hands after defecation)
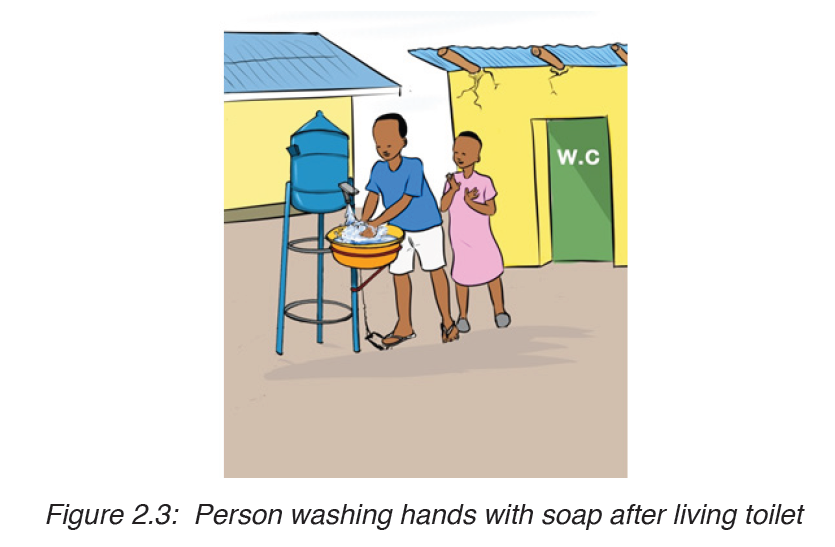
– Health education
– Vaccination is recommended for people visiting areas where cholera is endemic and for those living in such areas. But this vaccine lasts few months.
– Isolation of patients and hygienic disposal of feces and vomit from patients.
e) Failure to eradicate cholera
– Vaccination is not very effective
– It is a waterborne disease i.e. transmitted through contaminated water
– Poor sanitation condition in camps.
2.1.2. Tuberculosis
a) Causal agent of tuberculosis
Tuberculosis is caused by bacterium called Mycobacterium tuberculosis, first discovered by Robert Koch in 1882. It is sometimes referred to as the tubercle bacillus, bacilli being rod-shaped bacteria. The common form is pulmonary TB which infects the lungs, although other organs may be affected.
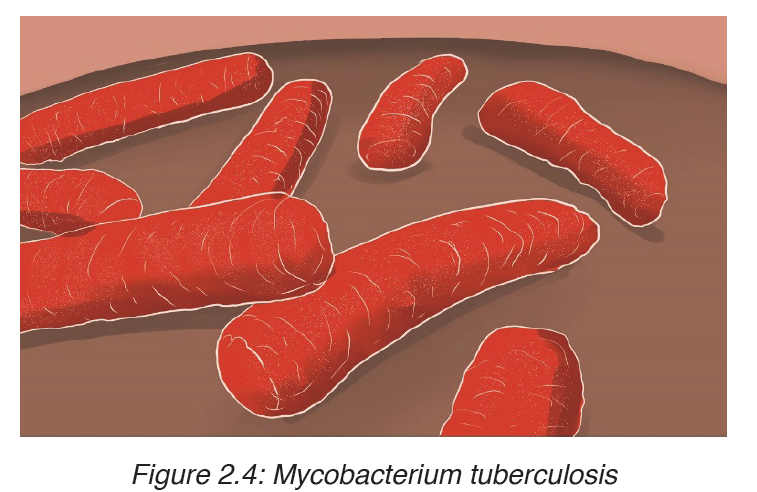
Two strains of the bacterium may cause the disease, the human and the bovine forms. The latter can be present in cattle and can enter the milk of cows. It is very resistant and can remain alive for long time in milk products as well as in durst.
Table 2.2: Features of TB.
b) Transmission of tuberculosis
Tuberculosis is mainly airborne disease. The infection is done through the droplets from the patient.
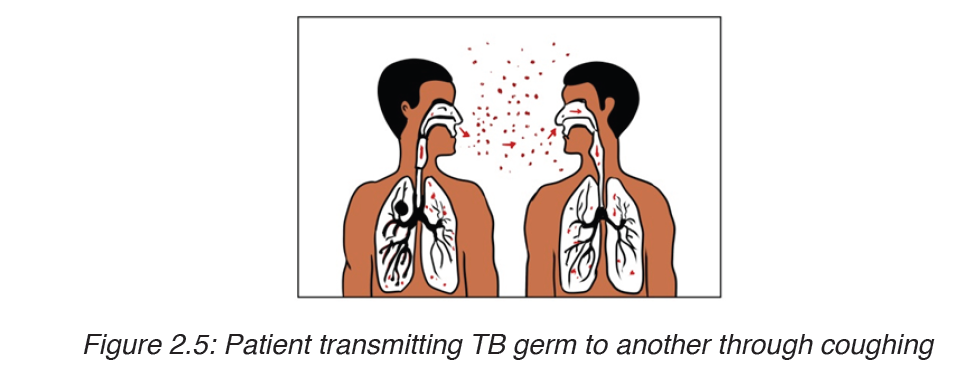
It is much less infectious than the common cold and requires prolonged contact between people, poor ventilation and overcrowded living conditions.
Other factors include poverty, bad housing, malnutrition, age, smoking and AIDS. In addition, TB is an opportunistic infection, striking many people with a depressed immunity.
c) Signs and symptoms of tuberculosis
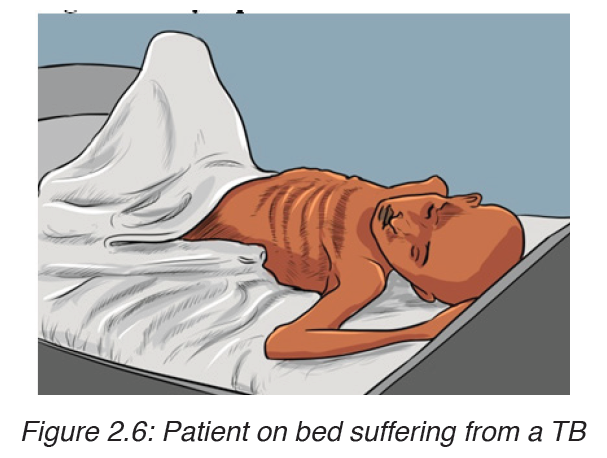
The disease frequently shows itself by vague symptoms such as: loss of appetite; loss of weight; excessive sweating; coughing, appearance of blood in the sputum, pains on the chest, shortness of breath (case of lung tuberculosis).
d) Treatment and prevention of tuberculosis
The development of an effective vaccine against the disease result of the work of Abert Calmette and Camile guérin (BCG). A cure for people already affected by T.B did not come until 1843 when the antibiotic streptomycin was discovered. The number of cases started to fall more rapidly after this and continued to decline aided by introduction of further antibiotics such as rifampicin, isoniazid and others.
e) Failure to eradicate tuberculosis
– Patients can carry pathogen and infection without showing symptoms.
Therefore they are difficult to identify and isolate / long period of incubation
– Germs of tuberculosis can survive longer in the house dust
– The disease is related to poverty where many people share the same room and have malnutrition.
– The disease is associated with AIDS that reduced the body immunity
– Long period of medication (6-8 moths), hence patients give up when not yet fully healed. The pathogens then form endospores that resists to medicines.
– The disease is also spread through milk from infected animals. Hence it is difficult to vaccinate.
– Tuberculosis is an airborne disease i.e. spread in air.
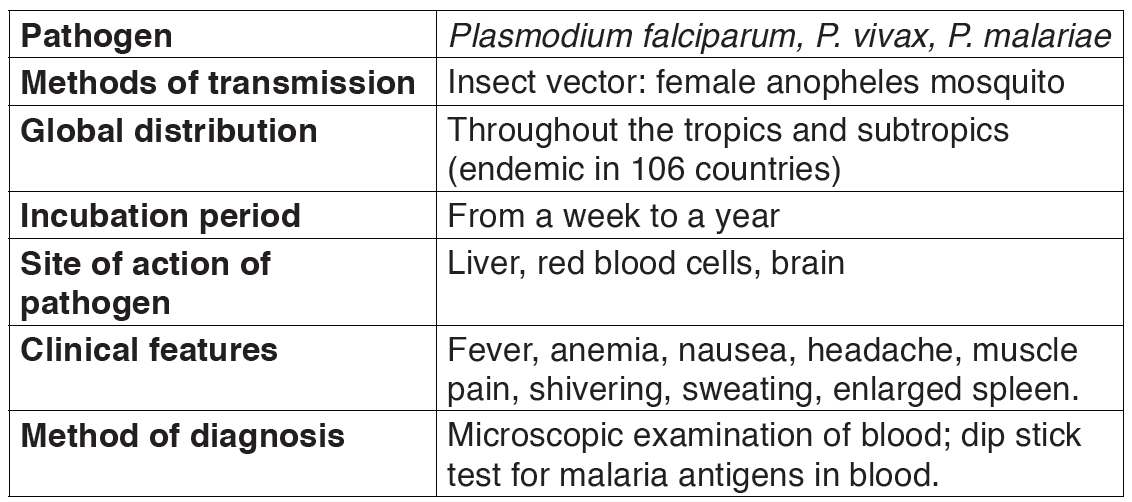
2.1.3. Malaria
a) Causal agent of Malaria
Human malaria is caused by infections from four species of plasmodium:
Plasmodium falciparum, P. vivax, P. ovale, and P. malariae, each responsible for a different form of the disease.
Malaria is characterized by chills, fever, and, in the most severe cases, coma leading to death.
The parasite, Plasmodium is transmitted by the bite of female mosquitoes (the vector) belonging to the genus Anopheles.
The life cycle of Plasmodium involves both sexual reproduction within the host mosquito, and asexual reproduction within the human being.
It is the reproductive activity of the parasite within the human bloodstream that produces the characteristic recurrent attacks of the disease.
The infection begins when a mosquito vector injects parasite particles (infectious stages) called sporozoites, present in the mosquito’s saliva, into the bloodstream when it feeds on a human blood.
b) Symptoms
Malaria is a very serious disease characterized by severe chills, fever, sweating, fatigue and great thirst. Malaria is caused by a protozoan of the genus Plasmodium.
Victims die of anemia, kidney failure or brain damage. The genus Plasmodium infects humans, and all have life cycles that involve the female anopheles mosquito.
c) Life cycle of Plasmodium
It is the reproductive activity of the parasite within the human bloodstream that produces the characteristic recurrent attacks of the disease.
The infection begins when a mosquito vector injects parasite particles (infectious stages) called sporozoites, present in the mosquito’s saliva, into the bloodstream when it feeds on a human blood.
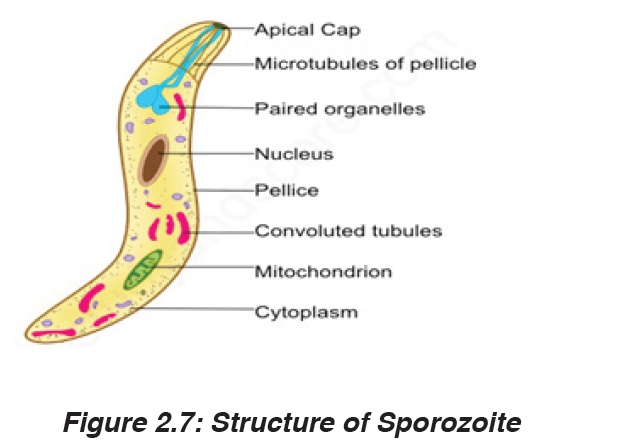
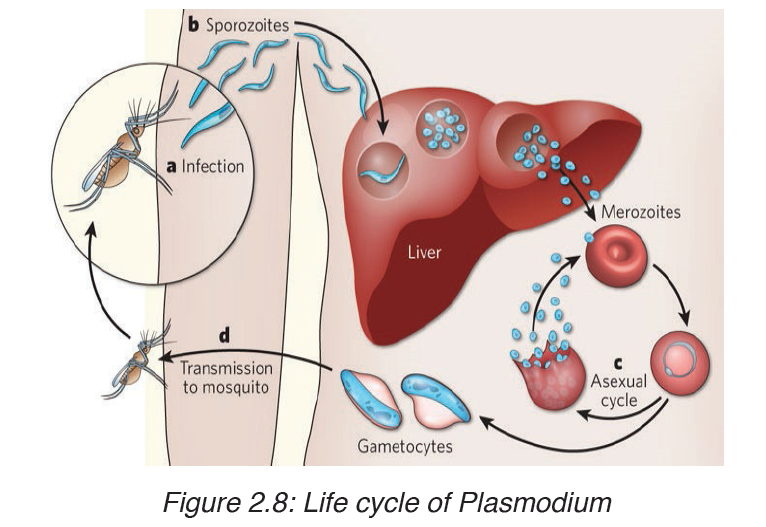
These enter liver cells where they multiply by asexual reproduction for about 7 to 14 days (the incubation period of the disease) before producing daughter cells called merozoite, which invade red blood cells. The parasites multiply in the red cells, again by asexual reproduction, to produce between 8 and 16 merozoites every 48 or 72 hours, depending on the species of Plasmodium. These merozoites are released by the bursting of the infected red blood cells and the cycle is repeated.
The bursting of the red blood cells and the release of toxic substances cause the characteristic fever of malaria. After a number of such cycles, sexual stages, male and female gametocytes, are produced, and these are taken up by a feeding mosquito, in which the Plasmodium life cycle is completed by sexual reproduction, resulting in new sporozoites.
d) Prevention of malaria
- Drainage of stagnant water: The larval stages of the mosquito live in stagnant water, so drainage removes breeding sites. This has had some success.
- Destruction of the breeding sites of the mosquito: The larvae and pupae of mosquitoes obtain their oxygen by means of small tubes which are pushed through the water surface film. Thus any method of blocking these tubes will result in the death of the intermediate life stages of the mosquito (petrol, oil….)
- Destruction of the adult mosquitoes: This is aimed at killing the mosquitoes that enter houses. Thus, the indoor surfaces are sprayed with a persistent insecticide.
Use of mosquito nets.
e) Occurrence of malaria
The disease now occurs in tropical and subtropical regions of the world, and its distribution is limited by conditions that are inimical to the development of the mosquito vector, such as temperature and altitude.
Malaria is endemic in tropics because:
– Tropical climate provides the best breeding and living conditions for the Anopheles mosquito which transmits malaria
– The Anopheles cycle requires areas of stagnant water and these are common within tropics
– In the tropical areas there is presence of bushes or abundant vegetation which makes suitable habitat for mosquitoes
Plasmodium needs temperature in excess of 20ᵒC for it to complete its cycle within the mosquito.
Table 2.3: The features of malaria
Treatment of malaria
The common drugs used in treatment of malaria include the following:
Chloroquine, Atovaquone-proguanil (Malarone), Artemether-lumefantrine (Coartem), Mefloquine, Quinine, Doxycycline (used in combination with quinine)
f) Failure to eradicate malaria
– There is no effective vaccine against malaria
– The pathogens are transmitted by mosquitoes which are not eradicated.
– The plasmodium have become resistant to different anti-malarial drugs
– Ignorance of some people toward the disease and how it is spread.
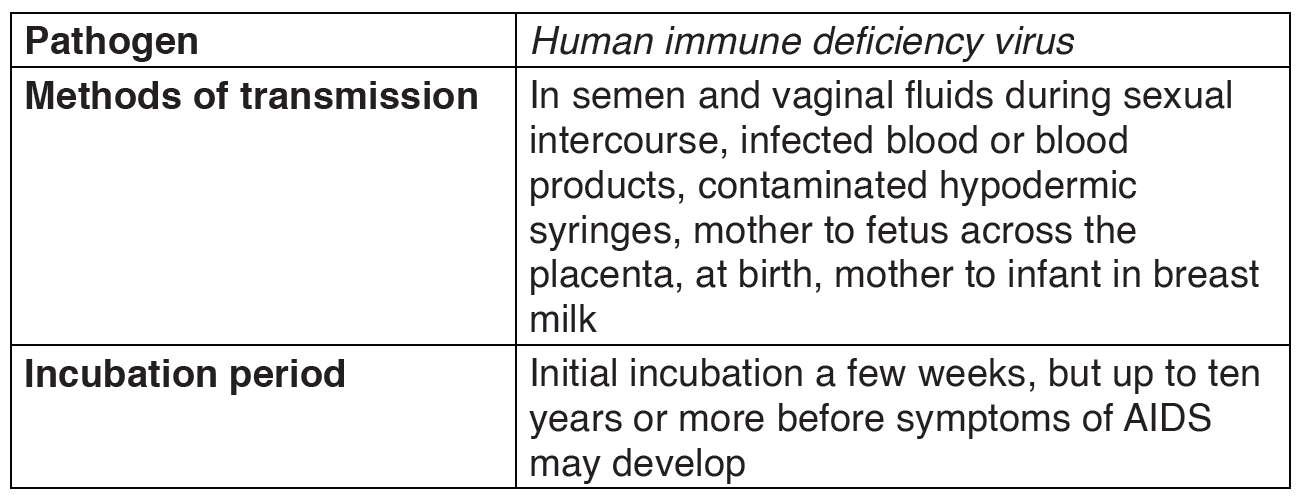
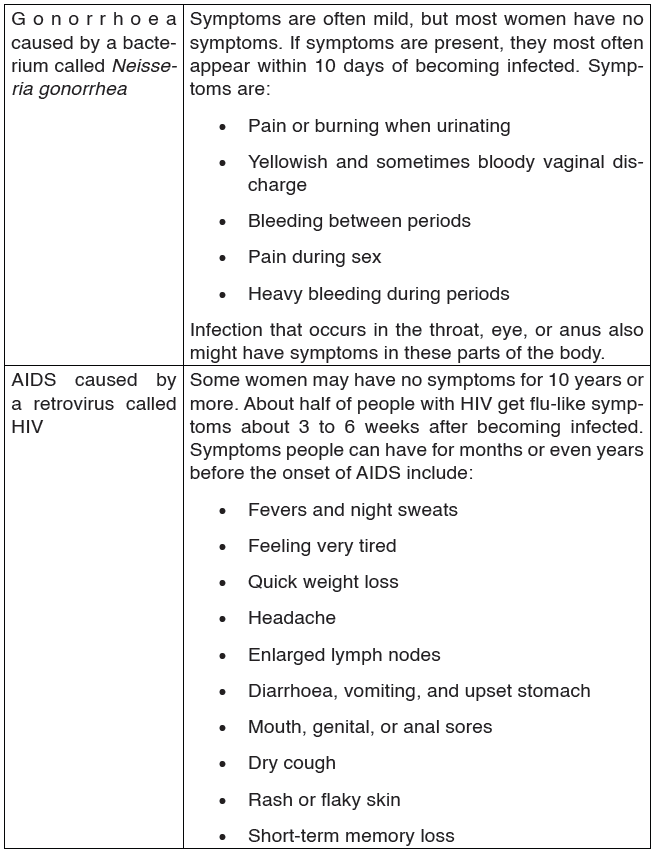
2.1.4. HIV/AIDS and other Sexual Transmission Diseases (STD)
AIDS (Acquired Immune Deficiency Syndrome) is a disorder which damages the human body’s immune system. It is caused by the HIV virus (Human Immunodeficiency Virus).
This is an RNA virus. The virus replicates inside the T4 lymphocytes or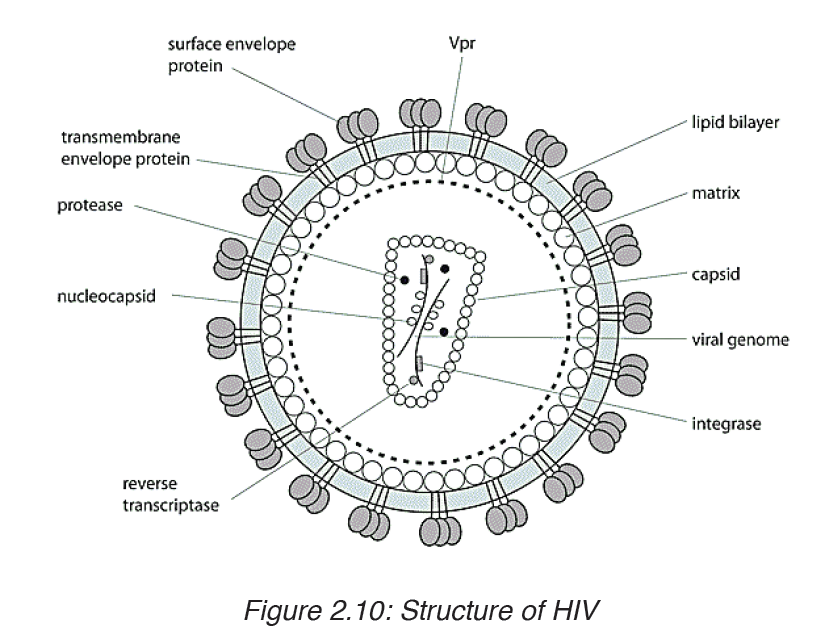
helper T cells. Thus these cells can no longer help or induce other T cells,
called killer cells, to fight invaders. The body’s immune system breaks down
leaving the patient exposed to a variety of diseases called opportunistic
infections. AIDS is not a disease; it is a collection of these opportunistic
diseases associated with immunodeficiency caused by HIV infection. It is
important to realize, however, that the infection with the HIV virus does not
necessarily result in AIDS. As with other diseases, some people remain
symptomless and are therefore called carriers.
TRANSMISSION, SIGNS AND SYMPTOMS
The HIV virus can only survive in body fluids and is transmitted by blood orsemen. In 90% of cases the transmission is achieved by sexual contact.
People can contract the disease as follows:
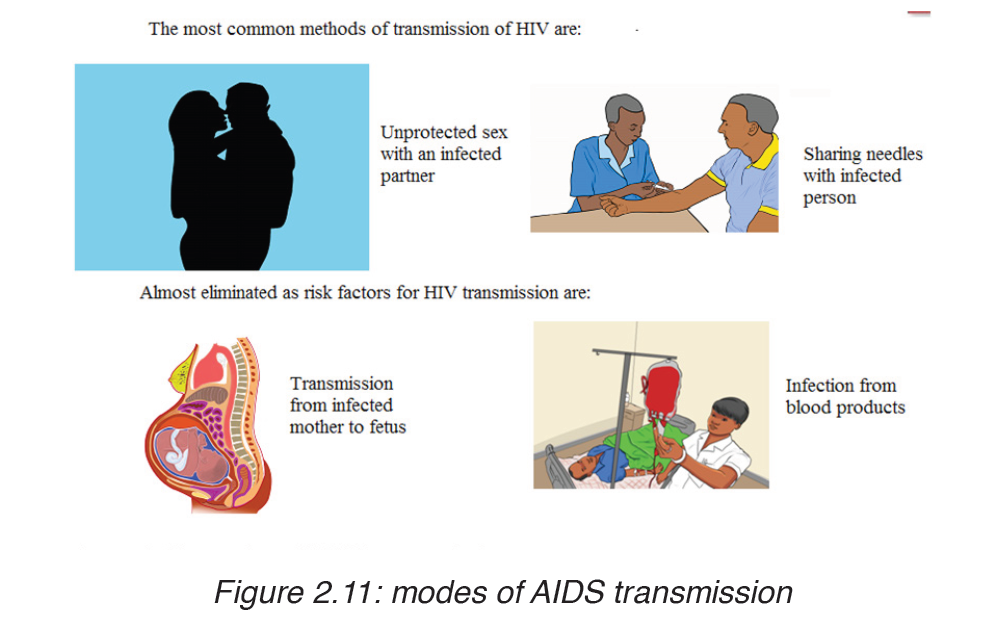
– Intimate sexual contact. The most frequent mode of transmission of HIV is through sexual contact with an infected person.
– Infected blood entering bloodstream: by means of unsterilized needles and syringes. Unfortunately the disease can be contracted after being given blood or blood products already infected with HIV.
Close contact between infected and non infected people through cuts and open wounds has also been known to pass on the virus.
– From mother to baby: An infected pregnant woman can pass on the virus to her baby through the placenta, at birth or through breast milk during suckling. The chances of infection being transmitted from the mother to her baby are currently estimated to be 25-50%.
TEST FOR THE DISEASE
A blood test is used to tell whether or not a person has been infected by the HIV virus. Under normal circumstances the immune system reacts to infection by producing antibodies and when the HIV virus enters the body, anti-HIV antibodies are produced. The blood of the person being tested is added to HIV proteins which have been commercially prepared. If there are anti-HIV antibodies in the blood sample they will bind to the viral proteins and the person is described as HIV positive. However, if the test proves negative that person may still be infected. This is because it takes up to three months or longer for HIV antibodies to be produced after infection.
PREVENTION OF THE DISEASE
There are many precautions which can be followed in trying to prevent the disease:

The use of a barrier during intercourse can prevent the virus from infecting through blood or semen. Thus the use of a sheath or condom is recommended.
This practice has been encouraged through many advertising campaigns throughout the world.
• Restriction to one sex partner and the absence of promiscuity will also clearly reduce the risk of infection.
• A reduction in the spread of HIV can be brought about by the use of clean needles and syringes by drugs addicts.
• The blood donated should be tested for the presence of antibodies to HIV which indicates whether or not the donor is infected. Blood containing these antibodies is not used.
• Educating the people about the disease.
• Taking antiretroviral during pregnancy and delivery.
• To avoid breastfeeding and to administer antiretroviral drugs to the newborn.
Table 2.4: The features of HIV/AIDS
Other sexual transmission diseases (STD)
Sexually transmitted diseases (STDs) are transmitted by infected persons to healthy persons during sexual intercourse.
Sexually transmitted diseases (STD), also referred to as sexually transmitted infections (STI) and venereal diseases (VD), are illnesses that have a significant probability of transmission between humans by means of sexual behavior, including vaginal intercourse, anal sex and oral sex. Some STIs can also be contracted by using drug needles after their use by an infected person, as well as through any incident involving the contact of a wound with contaminated blood or through childbirth or breastfeeding.
Sexually transmitted infections have been well known for hundreds of years, and venereology is the branch of medicine that studies these diseases.
While in the past, these illnesses have mostly been referred to as STDs or VD, the term sexually transmitted infections (STIs) has been preferred by many up-to-date medical sources, as it has a broader range of meaning; a person may be infected, and may potentially infect others, without having a disease.
There are 19 million new cases of sexually transmitted infections every year in the United States, and, in 2005, the World Health Organization estimated that 448 million people aged 15–49 were being infected a year with curable STIs (such as syphilis, gonorrhea and chlamydia).
Until the 1990s, STIs were commonly known as venereal diseases, the word venereal being derived from the Latin word venereus, and meaning relating to sexual intercourse or desire, ultimately derived from Venus, the Roman goddess of love. Social disease was a phrase used as a euphemism.
While many people with STDs show no signs or symptoms of their infection, when there are signs of STDs they are most likely to be in the genital area.
The genital area in women includes the vulva (the area around the vagina including the lips), vagina (the opening where menstrual blood comes out),buttocks, urethra (the opening above the vagina where urine comes out) and anus (the opening where a bowel movement comes out). The genital area in men includes the penis, scrotum (“balls”), urethra, and anus.
What Are the Symptoms of STDs?
Sometimes, there are no symptoms of STDs. If symptoms are present, they may include one or more of the following:
• Bumps, sores, or warts near the mouth, anus, penis, or vagina.
• Swelling or redness near the penis or vagina.
• Skin rash.
• Painful urination.
• Weight loss, loose stools, night sweats.
• Aches, pains, fever, and chills.
• Yellowing of the skin (jaundice).
• Discharge from the penis or vagina. (Vaginal discharge may have an odor.)
• Bleeding from the vagina other than during a monthly period.
• Painful sex.
• Severe itching near the penis or vagina.
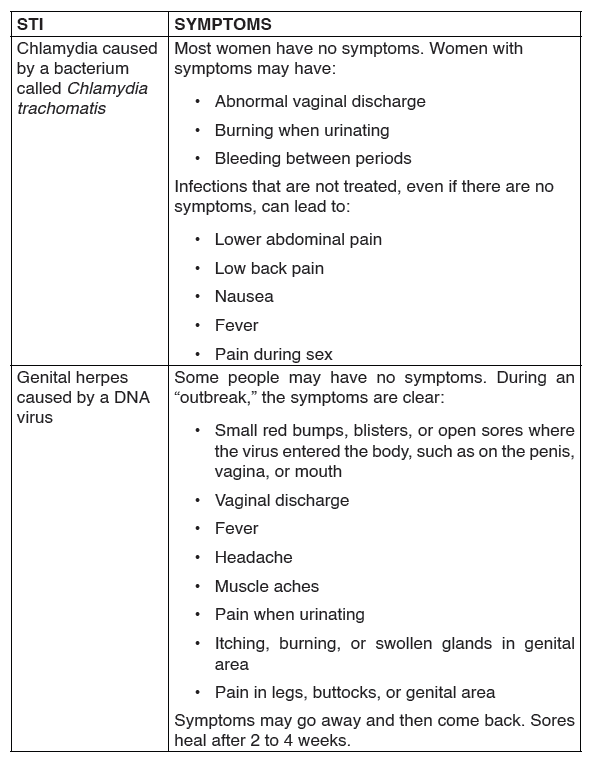
Examples of these diseases are chlamdia, gonorrhoea, syphillis and, HIV and AIDS.
1. Chlamydia
Chlamydia is caused by the bacterium Chlamydia trachomatis.
The disease is spread by oral, vaginal or anal sex, and also through touch, for example, touching the eyes with a contaminated hand, may lead to conjunctivitis. Chlamydia can also be passed to the infant during birth.
It causes inflammation of the cervix in women, urethra and rectum in both men and women. Occasionally, other parts of the body like eyelids and throat may be affected. Any sexually active person is at risk of contracting the disease. However, it is more common in young people.
The disease is known as a ‘silent’ infection because it is mainly asymptomatic, thus the symptoms can be mild or be confused with gonorrhea.
Signs and symptoms of chlamydia
In males
• Pain when passing out urine.
• White discharge from the penis.
• The testicles may be painful or swollen.
• Swelling of skin around the anus.
In females
• Painful and frequent urination.
• Smelly yellowish and abnormal vaginal discharge.
• Pain in the lower abdomen.
• Swollen skin in the vagina or around the anus.
Treatment
Chlamydia is easily treated using antibiotics usually Azithromycin or doxyclyne.
2. Gonorrhoea
Gonorrhoea is transmitted through sexual contact with the penis, vagina, mouth or anus of an infected partner. Gonorrhoea can also be spread from mother to baby during childbirth. Gonorrhoea is caused by a bacterium called Neisseria gonorrhoeae.
The bacteria attaches on the epithelial cells of the vagina or male urethra.
This results in inflammation and discharge of pus. If left untreated, the infection spreads to the other reproductive parts and may eventually block the passages resulting to infertility. Signs and symptoms of gonorrhea.
Some men with gonorrhea may have no symptoms at all. However, men who do have symptoms may have:
• A burning sensation when urinating.
• A white, yellow, or green discharge from the penis.
• Painful or swollen testicles (although this is less common).
Most women with gonorrhea do not have any symptoms. Even when a woman has symptoms, they are often mild and can be mistaken for a bladder or vaginal infection.
Women with gonorrhea are at risk of developing serious complications from the infection, even if they do not have any symptoms.
Symptoms in women can include:
• Painful or burning sensation when urinating.
• Increased vaginal discharge.
• Vaginal bleeding between periods.
Treatment
Gonorrhea can be treated using antibiotics like penicillin.
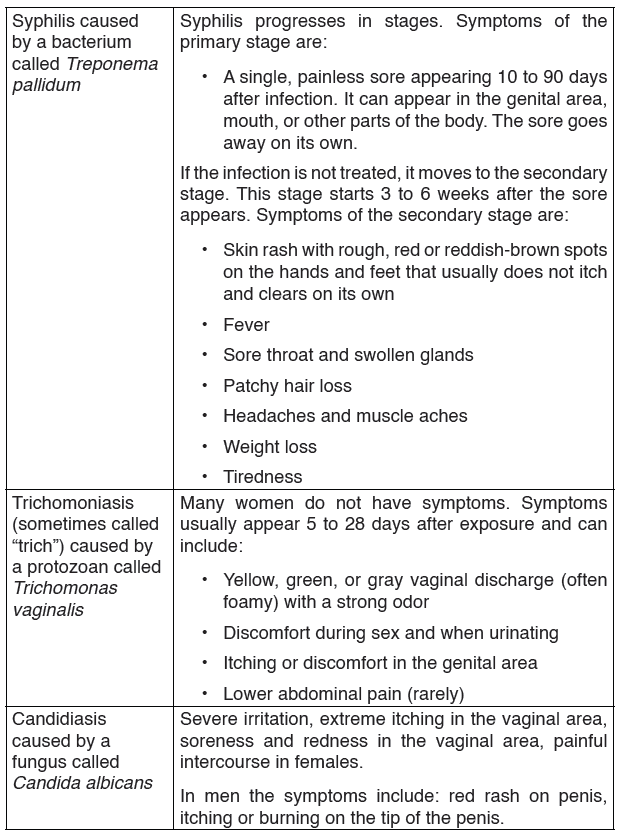
3. Syphilis
Syphilis is transmitted from person to person by direct contact with a syphilitic sore, known as a chancre. Chancres occur mainly on the external genitals, vagina, anus or in the rectum. Chancres also can occur on the lips and in the mouth.
Transmission of syphilis occurs during vaginal, anal or oral sex.
Syphilis is caused by a bacteria called Treponema pallidum.
The bacterial infection progresses through several stages:
• In the primary stage, small hard painless sores develop at the site of infection usually the penis and the vagina.
• The disease enters secondary stage several weeks later characterized by rashes on the skin and mild fever. These symptoms subside after a few weeks followed by a latent asymptomatic period.
• In the tertiary stage, lesions develop and cause extensive tissue damage that may lead to paralysis, insanity, blindness and eventually death.
Treatment
Antibiotics like penicillin, erythromycin or tetracycline are used to treat syphilis although some strains can be resistant to certain antibiotics.
The following are general ways of reducing STDs and HIV infection:
a) Abstinence is the only sure way to prevent STDs.
b) Being faithful to one trusted partner.
c) Using condoms every time when engaging in sexual intercourse.
Condoms are not 100% effective at preventing disease or pregnancy.
However, they are extremely effective if used properly.
d) Reduce the number of sexual partners.
e) Avoid sharing towels or underclothing.
f) Get a vaccination for hepatitis B.
g) Get tested for HIV.
h) Avoiding alcohol consumption and abuse of drugs. Individuals who are drunk or on drugs often fail to have safe sex.2.5 Table summarizing sexual transmitted infections

• DEFICIENCY DISEASES:
Deficiency diseases these are also known as malnutrition or macronutrient deficiencies. They are diseases that are caused by a dietary of specific nutrients, especially a vitamin or mineral, possibly coming from insufficient intake, digestion, absorption, or utilization of a nutrient. The deficiency diseases are common in Africa due to the poverty, and bad use of nutrients.
The examples of common deficiency diseases are Kwashiorkor, Marasmus, Vitamin deficiencies.
1. KWASHIORKOR
Kwashiorkor is deficiency disease known as ‘wet malnutrition’ caused by eating a food of energy giving food without adequate proteins.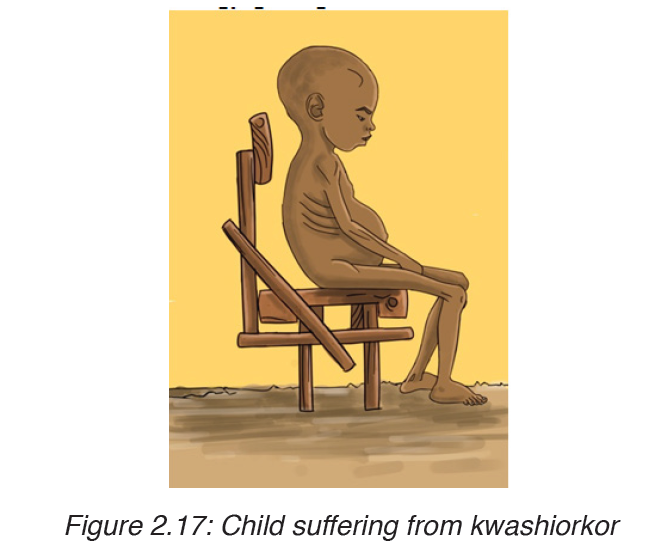
Signs and symptoms of kwashiorkor
The following are well defined symptoms and signs
– Pitting edema (swelling of the ankles and feet).
– Distended abdomen,
– An enlarged liver with fatty infiltrates,
– Thinning of hair,
– Loss of teeth,
– Skin depigmentation and dermatitis.
Children suffering from kwashiorkor often develop irritability and anorexia.
Mode of Prevention
To prevent from kwashiorkor, a person should be given small but frequent rations in every two to four hours. During the first week, he or she must be given a diet high in sugar and enriched in protein as well as essential elements: sweet milk with mineral salts and vitamins. The diet may include lactases so that children who have developed lactose intolerance can ingest
dairy products and antibiotics to compensate for immunodeficiency.
After two to three weeks, the milk is replaced by boiled cereals fortified with minerals and vitamins until the person’s mass is at least 80% of normal weight.
Treatment of the disease
– Generally, the disease can be treated by adding protein to the diet;
however, it can have a long-term impact on a child’s physical and mental development, and in severe cases may lead to death.
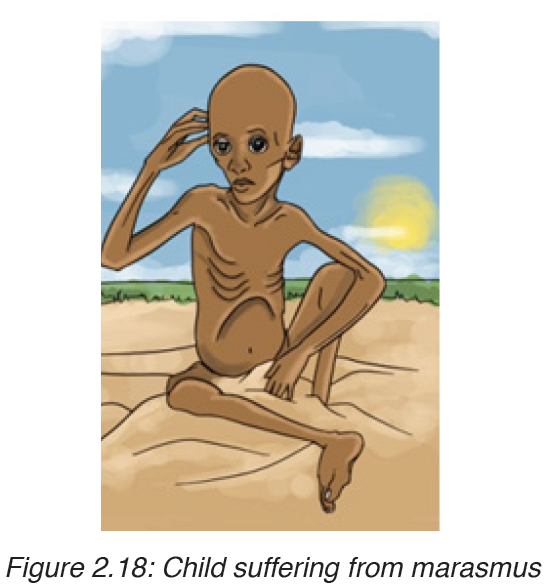
2. MARASMUS
Marasmus is also known as “dry malnutrition”. It is a form of severe malnutrition that usually occurs in children. It typically occurs in developing countries. Marasmus can be life-threatening, but you can get treatment for it.
Causes of marasmus
Nutrient deficiency is the main cause of marasmus. It occurs in children that do not eat enough protein, calories, carbohydrates, and other important nutrients. This is usually due to poverty and a scarcity of food.
Symptoms of marasmus
In children with marasmus, the following are symptoms:
• Chronic diarrhea
• Respiratory infections
• Intellectual disability
• Being underweight
• Stunted growth
• Subcutaneous fat is the layer of fat just under the skin.
• Dry skin and brittle hair
• Children may look older.• Children are short-tempered and irritable
OTHER DEFICIENCY DISEASES
Most of these diseases are micro-nutrient deficiencies like goitre, anaemia,
night blindness, rickets, obesity.
1. Goitre
The goitre is the swelling in the neck caused by lack of an adequate iodine
in the diet the pregnant mother
Prevention and treatment
– Eat food rich in iodine
– Go to doctor for treatment and the surgery is done to remove the
swelling
2. Anaemia
This disease is due to luck of an adequate Iron mineral in the diet. The iron
is essential for formation of haemoglobin pigment of blood contained by red
blood cells. If haemoglobin is not formed due to lack of iron, the oxygen will
not be transported into the body, hence the person with anaemia becomes
tired easily and has little energy to do a given work due to inadequate oxygen
in the body.
Signs and symptoms
– Shortness of breath
– Filling weak and tired quickly
– Filling one has no energy to work
– Inside the eyelids and tongue looks pale
– Short attention span
Prevention and treatment
– Regular taking lots of leafy vegetables to provide iron
– Eat food with plenty of calcium and vitamin D
• VITAMIN DEFICIENCIES
3. Scurvy
Scurvy happens when there is a lack of vitamin C, or ascorbic acid. The
deficiency leads to symptoms of weakness, anemia, gum disease, and skin
problems.
This is because vitamin C is needed for making collagen, an important
component in connective tissues. Connective tissues are essential for
structure and support in the body, including the structure of blood vessels.
A lack of vitamin C will also affect the immune system, absorption of iron,
metabolism of cholesterol and other functions.
Symptoms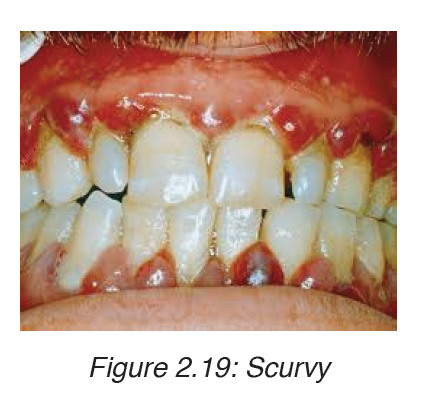
Symptoms of vitamin C deficiency can start to appear after 8 to 12 weeks.
Early signs include a loss of appetite, weight loss, fatigue, irritability, and
lethargy.
Within 1to 3months, there may be signs of:
– Anemia
– Myalgia, or pain, including bone pain
• Swelling, or edema
• Petechiae, or small red spots resulting from bleeding under the skin
• Corkscrew hairs
• Gum disease and loss of teeth
• Poor wound healing
• Shortness of breath
• Mood changes, and depression
Treatment
Treatment involves administering vitamin C supplements by mouth or by
injection.
The recommended dosage is:
• 1 to 2 grams (g) per day for 2 to 3 days
• 500 milligrams (mg) for the next 7 days
• 100 mg for 1 to 3 months
Within 24 hours, patients can expect to see an improvement in fatigue,
lethargy, pain, anorexia, and confusion. Bruising, bleeding, and weakness
start to resolve within 1 to 2 weeks.
After 3 months, a complete recoveryis possible. Long-term effects are
unlikely, except in the case of severe dental damage.
Prevention
Scurvy can be prevented by consuming enough vitamin C, preferably in the
diet, but sometimes as a supplement.
During pregnancy, women should consume 85 mg of vitamin C, rising to 120
mg while breastfeeding.Smokers need 35 mg more than nonsmokers every day.
4. Rickets
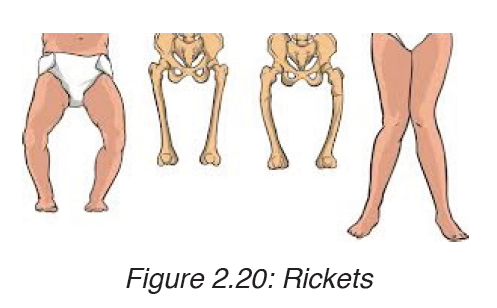
Rickets is a skeletal disorder that’s caused by a lack of Vitamin D, calcium,or phosphate which are nutrients important for the development of strong,healthy bones. The sufferer of rickets may have weak and soft bones, stunted growth, and, in severe cases, skeletal deformities.
Symptoms of rickets
Symptoms of rickets include:
• Pain or tenderness in the bones of the arms, legs, pelvis, or spine
• Stunted growth and short stature
• Bone fractures
• Muscle cramps
• Teeth deformities, such as: delayed tooth formation, holes in the enamel, abscesses, defects in the tooth structure, an increased number of cavities
• skeletal deformities, including: an oddly shaped skull, bowlegs, or legs that bow out, bumps in the ribcage, a protruding breastbone, a curved spine, pelvic deformities
Rickets treatment
Treatment for rickets focuses on replacing the missing vitamin or mineral in the body. This will eliminate most of the symptoms associated with rickets. The exposure to sunlight, if possible is recommended. To consume food products high in vitamin D, such as fish, liver, milk, and eggs is also necessary.
If skeletal deformities are present, the child may need braces to position their bones correctly as they grow. In severe cases, the child may need corrective surgery.
For hereditary rickets, a combination of phosphate supplements and high levels of a special form of vitamin D are required to treat the disease.
Prevention
– The best way to prevent rickets is to eat a diet that includes adequate amounts of calcium, phosphorous, and vitamin D. People with kidney disorders should have their calcium and phosphate levels monitored on a regular basis by their doctors.
– Rickets can also be prevented with moderate sun exposure. We need to expose the hands and face to sunlight a few times a week during the spring and summer months to prevent rickets.
5. Beriberi
Beriberi is a disease caused by a vitamin B-1 deficiency, also known as
thiamine deficiency. There are two types of the disease: wet beriberi and dry
beriberi.
Wet beriberi affects the heart and circulatory system. In extreme cases, wet
beriberi can cause heart rate
Dry beriberi damages the nerves and can lead to decreased muscle strength
and eventually,muscle paralysis. Beriberi can be life-threatening if it isn’t
treated.
Today, beriberi mostly occurs in people with an alcohol use disorder. Still,
the disease can be seen in women who have extreme nausea and vomiting
in pregnancy, in people with AIDS, and after bariatric surgery.
Symptoms of beriberi
The symptoms of beriberi vary depending on the type.
Wet beriberi symptoms include:
• Shortness of breath during physical activity
• Waking up short of breath
• Rapid heart rate
• Swollen lower legs
Dry beriberi symptoms include:
• Decreased muscle function, particularly in the lower legs
• Tingling or loss of feeling in the feet and hands
• Pain
• Mental confusion
• Difficulty speaking
• Vomiting
• Involuntary eye movement
• Paralysis
Beriberi treatment
Beriberi is easily treated with thiamine supplements. Doctor may prescribe
a thiamine shot or pill. For severe cases, a healthcare professional will
administer intravenous thiamine.
Prevention
– To prevent beriberi, eat a healthy, balanced diet that includes foods
rich in thiamine. These include: beans and legumes, seeds, meat, fish,
whole grains, nuts, dairy, certain vegetables, such as asparagus, acorn
squash, Brussels sprouts, spinach, and beet greens and breakfast
cereals that are enriched with thiamine
– Cooking or processing any of the foods listed above decreases their
thiamine content.
– Always be sure to purchase infant formula from a reliable source.
– Limiting alcohol consumption will reduce your risk of developing beriberi
6. Pellagra
Pellagra is a disease caused by low levels of niacin, also known as vitamin
B-3. It’s marked by dementia, diarrhea, and dermatitis, also known as “the
three Ds”. If left untreated, pellagra can be fatal.
Symptoms
The main symptoms of pellagra are dermatitis, dementia, and diarrhea.
Dermatitis related to pellagra usually causes a rash on the face, lips, feet, or
hands. In some people, dermatitis forms around the neck.
Additional dermatitis symptoms include:
• Red, flaky skin
• Areas of discoloration, ranging from red to brown
• Thick, crusty, scaly, or cracked skin
• Itchy, burning patches of skin
As the disease progresses, possible dementia symptoms include: apathy,
depression, confusion, irritability, or mood changes, headaches, restlessness
or anxiety, disorientation or delusions
Other possible pellagra symptoms include: sores on the lips, tongue, or
gums, decreased appetite, trouble eating and drinking, nausea and vomiting
Causes
There are two types of pellagra, known as primary pellagra and secondary
pellagra.
Primary pellagra is caused by diets low in niacin or tryptophan. Tryptophan
can be converted to niacin in the body, so not getting enough can cause
niacin deficiency.
Secondary pellagra occurs when your body can’t absorb niacin.
There are things that can prevent from absorbing niacin like: alcoholism,
eating disorders, certain medications, including anti-convulsants and
immunosuppressive drugs, gastrointestinal diseases, such as ulcerative
colitis, cirrhosis of the liver, carcinoid tumors, Hartnup disease
Treatment
Primary pellagra can be treated by dietary changes and a niacin or
nicotinamide supplement.
Nicotinamide is another form of vitamin B3. If left untreated, primary pellagra
usually causes death after four or five years.
Treating secondary pellagra usually focuses on treating the underlying
cause. However, some cases of secondary pellagra also respond well to
taking niacin or nicotinamide either orally or intravenously.
7. Night blindness
This is a disease caused by lack of Vitamin A in the diet. The main sources
of vitamin A are green leafy vegetables, liver, egg yolk and milk products.

Symptoms
– Inability of seeing during night
Prevention
– Provide diet with a plenty of vitamin A
WORM DISEASES
In our country, even in other African country many people suffer from worm
diseases. There are many worms that can infest people due to poor sanitation.
Worms causing infection in people are parasites that live and breed mostly in the intestine. Infection is caused by worms such as roundworms, hookworms and tapeworms.
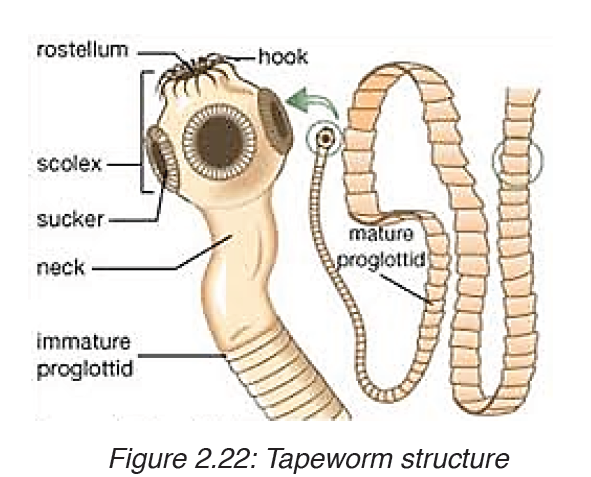
1. Ascariasis
Ascariasis is an infection of the and it is the most common roundworm
infection. Ascariasis is most common in places without modern sanitation.
People get the parasite through unsafe food and water. The infection
usually causes no symptoms, but a high number of roundworms (heavier
infestations) can lead to problems in the lungs or intestines.
Causes an ascariasis infection
You can become infected with ascariasis after accidentally ingesting the
eggs of the Ascaris lumbricoides roundworm. The eggs can be found in soil
contaminated by human feces or uncooked food contaminated by soil that
contains roundworm eggs.
Children often become infected when they put their hands in their mouths
after playing in contaminated soil, even it can also be passed directly from
person to person.
Symptoms of ascariasis?
People with ascariasis often have no symptoms. Symptoms become more
noticeable when the roundworm infestation grows.
Roundworms in your lungs can cause:
• Coughing or gagging
• Wheezing or shortness of breath
• Aspiration pneumonia (rarely)
• Blood in mucus
• Chest discomfort
• fever
Roundworms in your intestines can cause:
• Nausea
• Vomiting
• Irregular stools or diarrhea
• intestinal blockage, which causes severe pain and vomiting
• Loss of appetite
• visible worms in the stool
• Abdominal discomfort or pain
• Weight loss
• Growth impairment in children due to malabsorption.
Life cycle of the roundworm
After ingestion, the A. lumbricoides roundworm reproduces inside your
intestine. The worm goes through several stages:
• Swallowed eggs first hatch in the intestine.
• The larvae then move through the bloodstream to your lungs.
• After maturing, the roundworms leave your lungs and travel to your
throat.
• You’ll either cough up or swallow the roundworms in your throat. The
worms that are swallowed will travel back to your intestine.
• Once they’re back in your intestine, the worms will mate and lay more
eggs.
• The cycle continues. Some eggs are excreted through your feces.
Other eggs hatch and return to the lungs.
Environmental risk factors for ascariasis include:
• lack of modern hygiene and sanitation infrastructure
• use of human feces for fertilizer
• living in or visiting a tropical or subtropical climate
• exposure to an environment where dirt might be ingested
You can limit your exposure to roundworms by avoiding unsafe food and
water. Keeping your immediate environment clean also helps. This includes
laundering clothing exposed to unsanitary conditions and cleaning cooking
surfaces well.
You should make sure to take precautions if you’re visiting a remote area.
It’s important to:
• Always wash your hands with soap and water before eating or preparing
food.
• Boil or filter your water.
• Inspect food preparation facilities.
• Avoid unclean common areas for bathing.
• Peel or cook unwashed vegetables and fruit in regions that lack
sanitation infrastructure or that use human feces for fertilizer.
Complications of ascariasis
Dangerous complications, including:
• Intestinal blockage. Intestinal blockage occurs when a mass of worms
blocks your intestines, causing severe pain and vomiting. Intestinal
blockage is considered a medical emergency and requires treatment
right away.
• Duct blockage.Duct blockage occurs when the worms block the small
passageways to your liver or pancreas.
• Nutritional deficiency. Infections that lead to loss of appetite and
poor absorption of nutrients put children at risk of not getting enough
nutrients, which can affect their growth.
Children are more likely to have gastrointestinal complications because the
smaller size of their intestines increases their chances of having an intestinal
blockage.
How ascariasis is treated
Doctors usually treat roundworm with antiparasitic drugs. Medications most
commonly used include:
• Albendazole (Albenza)
• Ivermectin (Stromectol)
• Mebendazole (Vermox)
If you have an advanced case, you may need other treatment. Your doctor
may recommend surgery to control a larger infestation. You’ll need surgery
if the roundworms are completely blocking your intestines.
The best way to avoid ascariasis is by:
• Practicing good hygiene. That means always wash your hands with
soap and water before eating or handling food, and after using the
bathroom. Teach your children to do the same.
• Dining only at reputable places.
• Drinking only bottled water and avoiding raw fruits and vegetables
unless you’re able to wash and peel them yourself when you’re in
places without modern sanitation.
2. Schistosomiasis
• This disease is also known as snail fever and bilharzia, is a disease
caused by parasitic flatworms called schistosomes. The urinary tract or
the intestines may be infected.
• Symptoms include abdominal pain, diarrhea, bloody stool, or blood in
the urine. Those who have been infected for a long time may experience
liver damage, kidney failure, infertility, or bladder cancer. In children, it
may cause poor growth and learning difficulty
Modes of transmission
Infected individuals release Schistosoma eggs into water via their fecal
material or urine. After larvae hatch from these eggs, the larvae infect a very
specific type of freshwater snail (Vector of the disease). The Schistosoma
larvae undergo the next phase of their life cycles in these snails, spending
their time reproducing and developing. Once this step has been completed,
the parasite leaves the snail and enters the water column. The parasite can
live in the water for only 48 hours without a human host. Once a host has
been found, the worm enters its blood vessels. For several weeks, the worm
remains in the vessels, continuing its development into its adult phase. When
maturity is reached, mating occurs and eggs are produced. Eggs enter the
bladder/intestine and are excreted through urine and feces and the process repeats. If the eggs do not get excreted, they can become engrained in the
body tissues and cause a variety of problems such as immune reactions and
organ damage.
Humans encounter larvae of the Schistosoma parasite when they enter
contaminated water while bathing, playing, swimming, washing, fishing, or
walking through the water.
Modes of prevention
– Avoiding drinking or coming into contact with contaminated water in
areas where schistosomiasis is common.
– Increasing access to clean water and sanitation
– Snail control
– Health education.
Treatment
There are two drugs available, praziquantel and oxamniquine , for the
treatment of schistosomiasis. They are considered equivalent in relation
to efficacy against S. mansoni and safety. Because of praziquantel’s lower
cost per treatment, and oxaminiquine’s lack of efficacy against the urogenital
form of the disease caused by S. haematobium, in general praziquantel is
considered the first option for treatment. Schistosomiasis is treatable bytaking by mouth a single dose of the drug praziquantel annually.
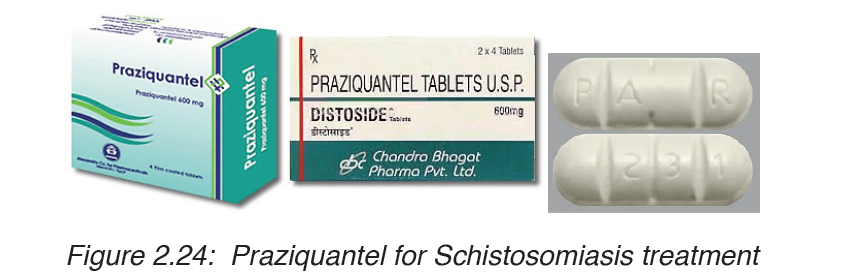
3. Elephantiasis lymphatic filariasis
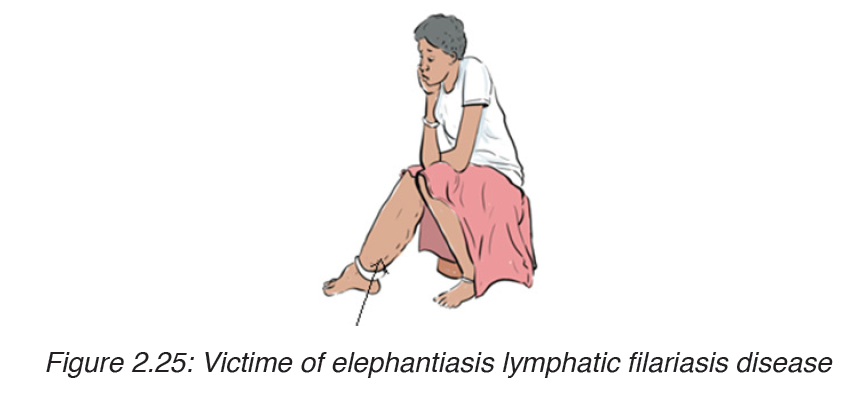
Elephantiasis, also known as Lymphatic filariasis, is a human disease
caused by parasitic worms known as filarial worms.Most cases of the disease
have no symptoms. Some people, however, develop a syndrome called
elephantiasis, which is marked by severe swelling in the arms, legs,breasts
or genitals. The skin may become thicker as well, and the condition may
become painful.
The worms are spread by the bites of infected mosquitoes. Three types of
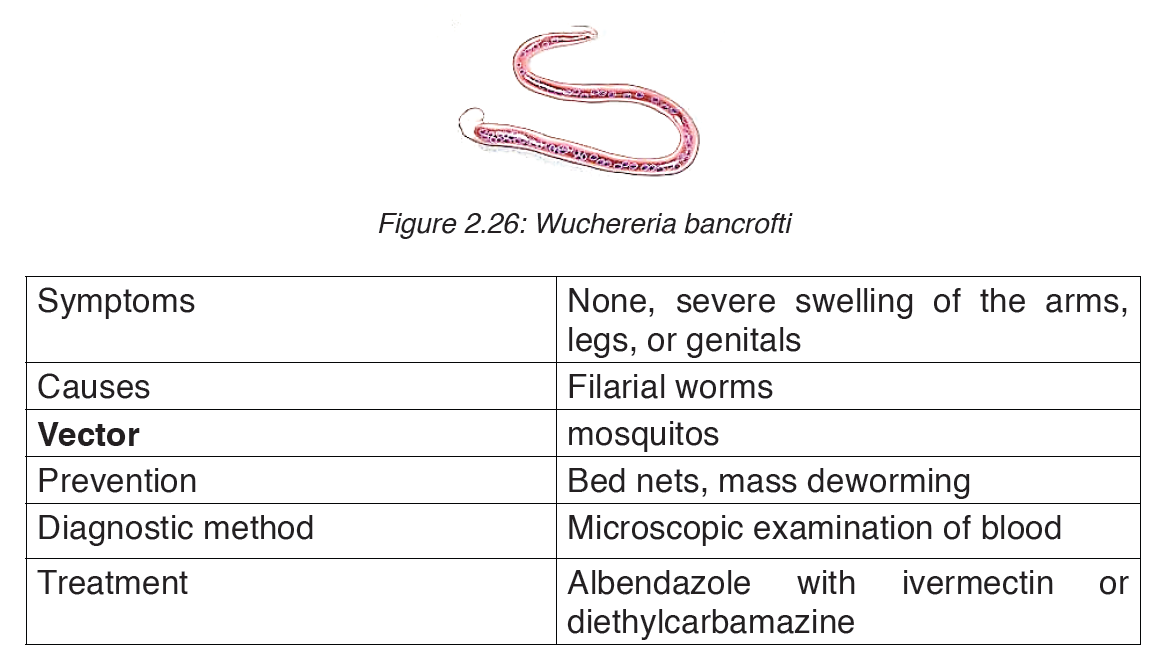
worms are known to cause the disease: Wuchereria bancrofti,Brugia malayi
and Bruggia timori with Wuchereria bancrofti being the most common.
These worms damage the lymphatic system. The disease is diagnosed by
microscopic examination of blood collected during the night. The blood is
typically examined as a smear after being stained with Giesma stain.
4. Ankilostomiasis
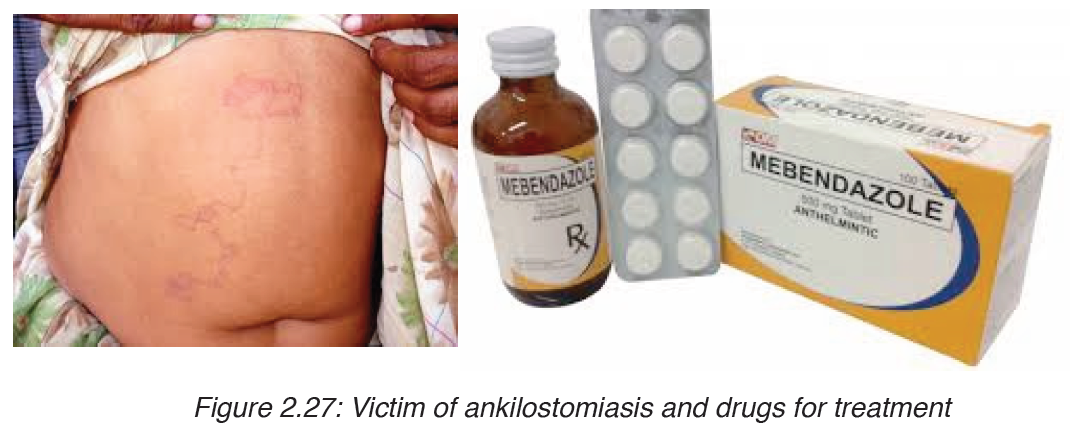
Ankilostomiasis is ahookworm disease caused by infection with Ancylostoma
hookworms. Ankilostomiasis is caused when hookworms, present in large
numbers, produce an iron deficiency anemia by sucking blood from the
host’s intestinal walls.
Signs and symptoms
Depending on the organism, the signs and symptoms vary.Ancylostoma
duodenale and Necator americanus can enter the blood stream while
Ancylostoma braziliensis cannot.
Transmission
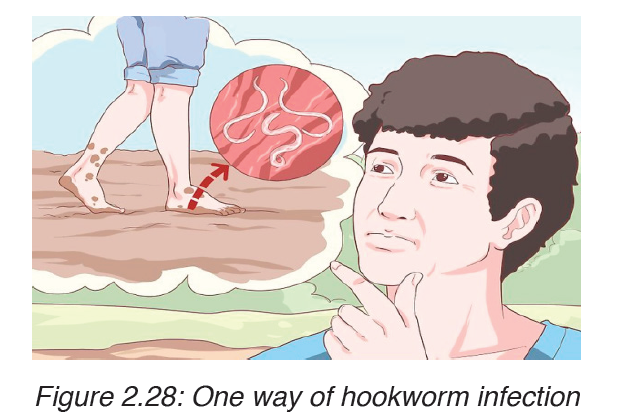
The infection is usually contracted by people walking barefoot over
contaminated soil. In penetrating the skin, the larvae may cause an allergic
reaction. It is due to the itchy patch at the site of entry that the early infection
gets its nickname “ground itch”. Once larvae have broken through the skin,
they enter the bloodstream and are carried to the lungs (however, unlike
ascaris, hookworms do not usually cause pneumonia). The larvae migrate
from the lungs up the windpipe to be swallowed and carried back down to the
intestine. If humans come into contact with larvae of the dog hookworm or
the cat hookworm, or of certain other hookworms that do not infect humans,
the larvae may penetrate the skin. Sometimes, the larvae are unable to
complete their migratory cycle in humans. Instead, the larvae migrate just
below the skin producing snake-like markings.
Prevention
– Control of this parasite should be directed against reducing the level of
environmental contamination.
– Treatment of heavily infected individuals is one way to reduce the
source of contamination.
– improve access to sanitation e.g. toilets
– Wearing shoes when you walk outdoors, especially in areas that might
have feces in the soil
– Drinking safe water
– Properly cleaning and cooking food
– Practicing proper handwashing
Treatment
The drug of choice for the treatment of hookworm disease ismebendazole
which is effective against both species, and in addition, will remove the
intestinal worm Ascaris also, if present. The drug is very efficient, requiring
only a single dose and is inexpensive.
An infection of N. americanus parasites can be treated by using
benzimidazoles, albendazole, and mebendazole.
Application activity 2.1
1. List the ways in which cholera is transmitted from person to person.
2. Explain why there is such a high risk of cholera following natural
disasters such as earthquakes, hurricanes, typhoons and floods.
3. Explain why there is a high death rate from TB in countries with a
high proportion of the population who are HIV-positive.
4. TB is an opportunistic infection. Why?
5. Describe how malaria is transmitted.
6. Describe the biological factors that make malaria a difficult disease
to control.
7. Describe the precautions that people can take to avoid catching
malaria.
8. Which of the following diseases is transmitted by an insect vector?
a) Cholera
b) HIV/AIDS
c) Malaria
d) TB
9. Tuberculosis is a serious infectious disease.
a) Name the causative agent of tuberculosis.
b) Explain how tuberculosis is transmitted.
c) State the regions of the world with the highest number of cases of
tuberculosis.
d) Suggest reasons for the high number of tuberculosis in some parts
of the world.
10. The table shows the number of cases of cholera and deaths from
the disease for the five countries with the greatest outbreaks as
reported to the WHO in 2010.
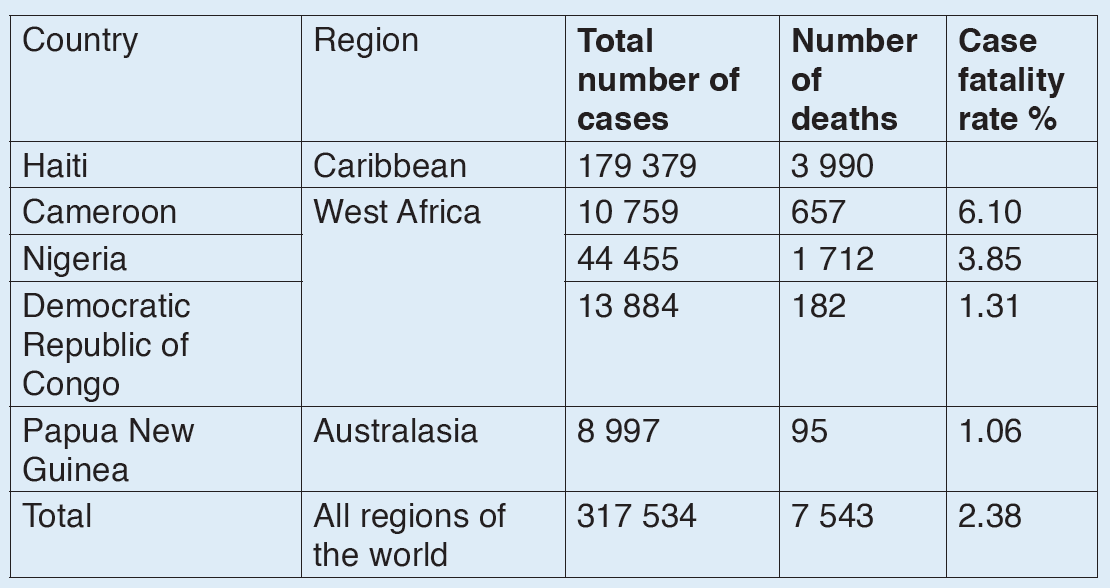
a) Describe how cholera is transmitted.
b) With reference to the table:
i. Calculate the case fatality rate for Haiti in 2010.
ii. Suggest why the case of fatality rate varies between countries.
iii. Explain why it is important that the WHO collects data on
outbreaks of cholera.
c) Explain why there no epidemics of cholera in highly economically
developed countries such as Australia, Malaysia and USA.
2.2. Hygiene practices
Activity 2.2
Discuss the possible hygiene practices you always have every
morning at your school.
It is important to understand family health issues, because poor personal
and environmental hygiene may cause hygiene related diseases. It
is therefore important to acquire knowledge and skills on personal and
environmental hygiene in order to avoid such diseases.
2.2.1. Personal hygiene
Personal hygiene and sanitation is very important because:
– It insures proper growth and development of children.
– It helps to prevent diseases especially hygiene related diseases.
– It prevents bad smell it helps to keep the environment clean, tidy and
beautiful.
– It makes the environment appealing and attractive.
The personal hygiene may be maintained through the hygiene practices
below:
– Washing the body regularly with clean water and soap.
– Wearing clean clothes.
– Living in clean environment with adequate fresh air.
– Eating adequate balanced diet. Young children should be fed between
5 to 6 times per day. Their diet should be rich in proteins.
– Having regular exercises.
To insure personal hygiene, the important aspects below are considered:
Care of body
To prevent skin diseases and bad smell one needs:
– Washing the body regularly with clean water and soap.
– Avoid sharing clothes, socks and towels.
– Wearing clean under wears.
– Stay in clean environment.
– Wear clean clothes.
Care of the feet
– Washing the feet regularly with water and soap.
– Keeping the feet dry to avoid fungi or foot rot and bad smell.
– Wearing clean socks.
– Avoid sharing socks.
– Keep nails short and clean.
– Airing your feet daily.
– Apply some oil like Vaseline to keep the feet smooth.
Care of hands
– Wash hands regularly with clean water and soap.
– Keep nails short and clean.
– Wash hand before touching and preparing the food
– Wash hand before eating.
– Wash hand after visiting the toilet.
– Apply some oil like Vaseline to keep the hands smooth.
Care of hair
– Wash your head regularly with clean water and soap.
– Keep your hair well combed.
– Oil your hair to prevent dandruff.
Care of teeth
– Brush your teeth daily with a tooth brush, clean water and tooth paste.
– Avoid eating too much sweet, biscuits and sugary foods, as they can
cause tooth decay and dental cavities.
– Eating foods that help build strong teeth like milk, eggs, vegetables,
fruits and fish.
– To visit dentist regularly for checkups.
– To avoid putting sharp dirty objects in the mouth.
– Pregnant mothers should feed on dies rich in Calcium which is used to
make teeth.
Care for eyes, ears and nose
These sensory organs are very important. We must care for them well. Injury
may result in permanent disability such as: blindness, deafness and lack
ability to detect smells.
Care of the eyes
– Wash eyes with clean water.
– Protect your eyes from foreign objects and dusts.
– Protect your eyes from too much or too low light. You always read
under adequate light.
Care for ears
– Wash ears with clean water and soap.
– Never insert hard objects in the ear, this may damage your ear and
cause deafness.
– Avoid very loud noise.
– Clean ears with cotton around a matchstick or use ear buds and use it
gently.
Care for the nose
– Never insert hard objects in the nose.
– Keep the nose clean by blowing it regularly.
– Avoid being hit in the nose.
2.2.2. Environmental hygiene
Our surrounding environment including water, air and soil should be protected
and kept clean. There should be different hygiene practices to prevent soil
pollution, water pollution and air pollution.
Application activity 2.2
1) What are possible hygiene activities are required to care for;
a) Eyes
b) Nose
c) Feet
2) Explain the general human body hygiene practices.
2.3 Human immune system: immunity, structure and role of
antibodies, immunization and vaccine
Activity 2.3
The human body is always exposed to environment full of pathogens and
it remains healthy. How is this possible?
IMMUNITY is a process by which the body of a living organism defends itself
against pathogens. The immune system is a protective system that is made
of a series of defenses that fight against diseases by: recognizing, attacking,
destroying and remembering each type of pathogen that enters the body. It
does this by producing specialized cells which inactivate pathogens.
Defense mechanisms against infections
The immune system includes two general categories of defense mechanisms
against infections: nonspecific defense and specific defense.
2.3.1 Nonspecific defenses
They include physical barriers (skin) and chemical barriers (mucus, tears and
sweat) which prevent foreign pathogens from entering the body. Nonspecific
defenses occur into two lines of defense:
a) First line of defense: its function is to keep pathogen out of the body. This
role is carried out by: the skin, sweat, tears and mucus. The most important
nonspecific defense is the skin. The skin epidermis consists of layers of dead
cells called keratinocytes (which have been keratinized: their cytoplasm is
replaced by a protein called keratin). The keratinized layers of dead cells
act as an effective barrier to pathogens and cannot normally be penetrated
by bacteria or viruses.
Likewise, the mucous that line the digestive,respiratory, nose and genitals
block the entry of harmful microbes. Beyond theirrole as a physical barrier,
the skin and mucous membranes counter pathogens with chemicaldefenses.
For example, secretions from sebaceous and sweat glands give the skin an
acidic PH ranging from3 to 5, which kills bacteria. Many secretions of the
body including: tears, mucus and saliva contain lysozyme, ananti-microbial
enzyme that breaks the walls of bacteria. Also, stomach acid and digestive
enzymes destroy many pathogens which are swallowed with the food.
b) The second line of defense: Microbes which have managed to penetrate
the first line of defense face the second line of defense which depends mainly
on phagocytosis,inflammatory response and on certain antimicrobial
proteins.
i. Phagocytosis:
When pathogens are detected in the body, the immune system produces
millions of white blood cells which fight the infection. Many of this white
blood cells are phagocytes, which engulf and destroy bacteria.
The word Phagocyte comes from Greek where: phag means: to eat and
kutos means cell. Thus phagocytes are cells that engulf and destroy
pathogens. There are two types of phagocytes:
• Neutrophils are the common phagocytes produced in bone marrow
and carried in blood to be released in tissue fluid. They will be released
in large number as a result of infection.
The phagocytic cells called neutrophils constitute about 60 % to 70%
of all white blood cells (leucocytes). The neutrophils enter the infected
tissue, engulfing and destroying microbes there.
• Macrophages are large cells manufactured in bone morrow and travel
in blood as monocytes. They tend to be settle in lymph nodes, liver
and in spleen.
How phagocytes work: when pathogens enter the body, phagocytes move
towards them, they engulf and destroy them. Once the phagocyte is bound
to the pathogen, it will envelop it by folding its membrane inwards. The
pathogen is trapped inside the vacuole called phagosome. Lysosomes
fuse with the phagosome and release enzymes called lysins into it. These
enzymes digest pathogens and the end products are harmless nutrients that
can be absorbed into the cytoplasm.
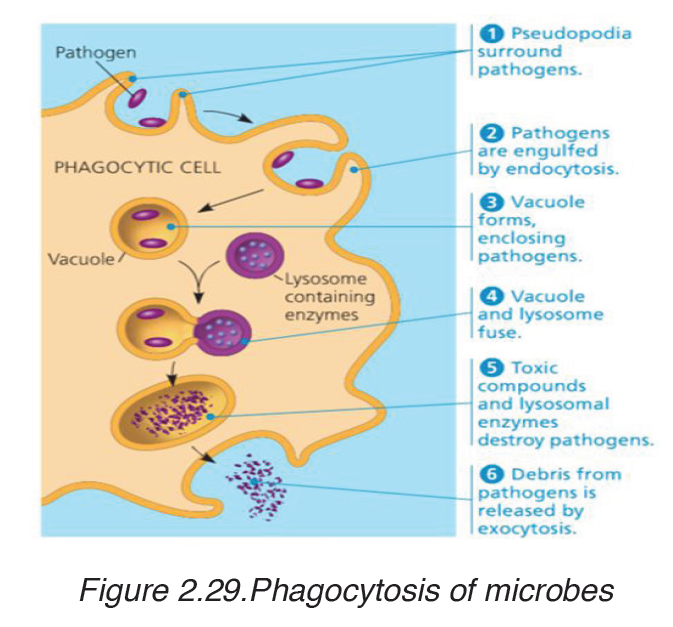
ii. Inflammatory response
One result of phagocytosis is inflammation or inflammatory response
that is characterized by: redness, hotness, swollen and painful at the site
of local infection. An inflammatory response is a nonspecific defense
reaction to tissue damage caused by injury or infection.
The tissues injured by bacteria or physical damage (like a cut) release
chemicals such as histamine and prostaglandins. These chemicals work
as signals which induce an increased heart rate and increased blood
capillary permeability (so that white blood cells get to the site of infection
faster).
Infected cells also release chemicals which attract phagocytes. When
phagocytes and macrophages arrive at the site of injury, they engulf and
destroy pathogens and the tissues heal.
The inflamed tissues contains many bacteria and phagocytes, many of which
die and form the pus. The increased body temperature slows or stops the
growth of pathogens. The fever and increased number of leucocytes are two
indications that the body is working hard at fighting infections
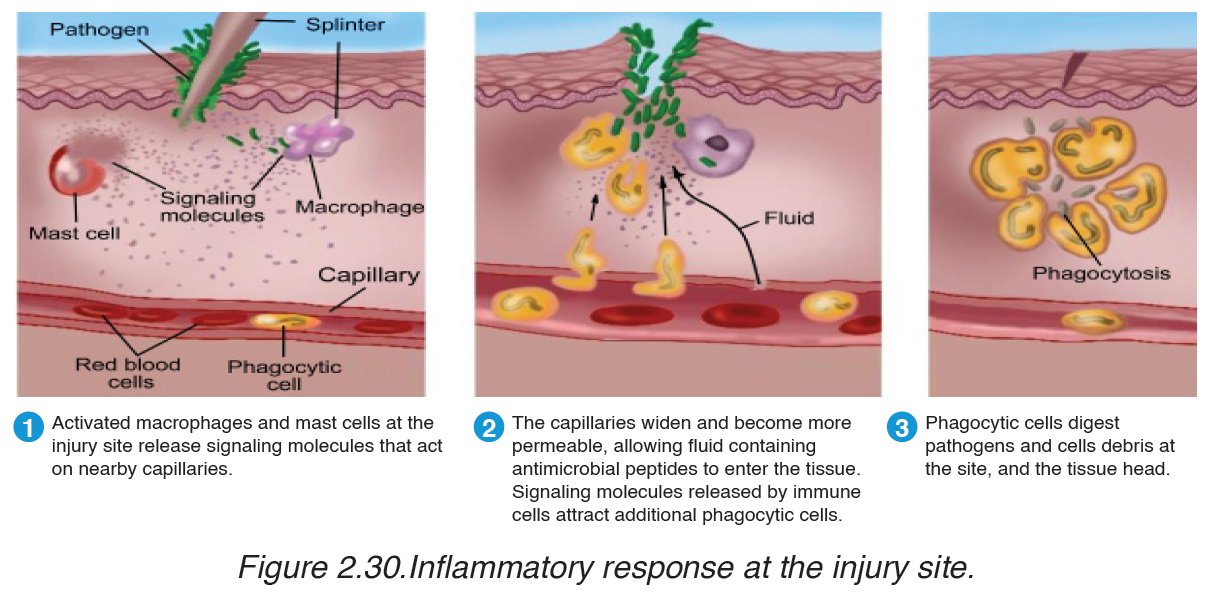
iii. Anti-microbial proteins
Two groups of proteins: interferons and the complement system provide
short-term, nonspecific defense to viral and bacterial infections. Interferons
are proteins secreted by virus-infected cells which inhibit the synthesis of
viral proteins in affected cells and help to block viral replication. They
diffuse to neighboring cells and stimulate them to produce antiviral proteins,
which prevent viruses from multiplying within them.
Interferons also activate natural killer cells and macrophages, which
destroy infected host cells before they release more viruses. Interferons can also promote destruction of cancer cells. The complement system consists of about 30 proteins in blood plasma that function together to fight
infections. These proteins circulate in an inactive state and are activated by substances on the surface of many microbes. Activation results in a cascade of biochemical reactions leading to lysis (bursting) of invading cells. The complement system also functions in inflammation.
2.3.2 Specific defense/immunity: third line of defense
If pathogens are able to get past the body’s non-specific defenses, the immune system reacts with a series of specific defenses that attack the particular disease-causing agent. These defenses are called immune response. A substance that triggers (causes) this response is known as an antigen. Viruses, bacteria and other pathogens may serve as antigens.
The cells of the immune system that recognize specific antigens are two types of lymphocytes:
a) B lymphocytes (B cells): which originate in the bone marrow and migrate to the lymph nodes where they proliferate into plasma cells which produce antibodies.
B cells provide immunity against antigens and pathogens in the body fluid.
B lymphocytes give rise to the humoral immunity or antibody-mediated immunity.
b) T lymphocytes (T cells): originate in bone marrow and migrate into the
thymus. They don’t produce antibodies, but they themselves provide
defense against antigens and pathogens inside living cells. T lymphocytes
give rise to the cell-mediated immunity.
Humoral immunity
When pathogens invade the body, their antigens are recognized by few B
cells of the body. These B cells grow and divide rapidly, producing large
number of plasma cells and memory cells. Plasma cells produce and
release antibodies. Antibodies are proteins that recognize and bind to
antigens. They are carried in the bloodstream to attack the pathogen at the
site of infection. As antibodies overcome pathogens, the plasma cells die out
and stop producing antibodies.
Once the body has been exposed to a pathogen, millions of memory B cells
remain capable of producing antibodies specific to that pathogen. These
memory B cells, greatly reduce the chance that the disease could develop a
second time. If the same antigen enters the body a second time, a second
response occurs. The memory B cells divide rapidly, forming new plasma
cells. Plasma cells produce the specific antibodies to destroy the pathogen
as faster as possible.
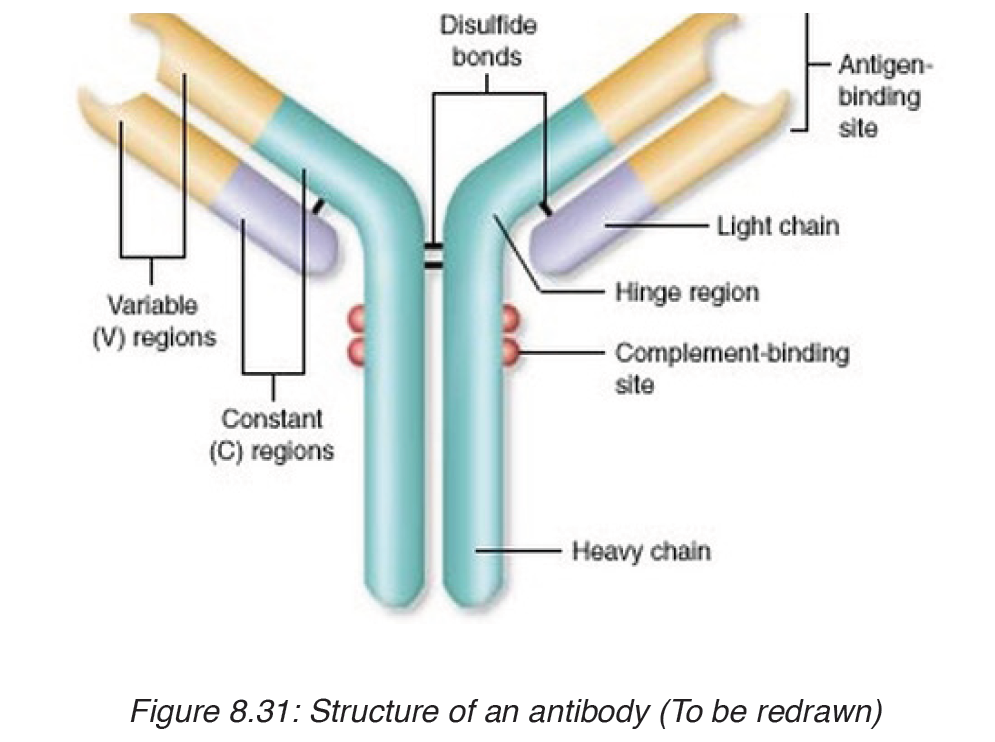
An antibody is shaped like the letter Y, and has two identical antigenbinding
sites. The shape of the binding site allows the antibody to recognize
a specific antigen with a complementary shape. The different shapes give
antibodies the ability to recognize a large variety of antigens. A healthy adult
cane produce about 100 million different types of antibodies.
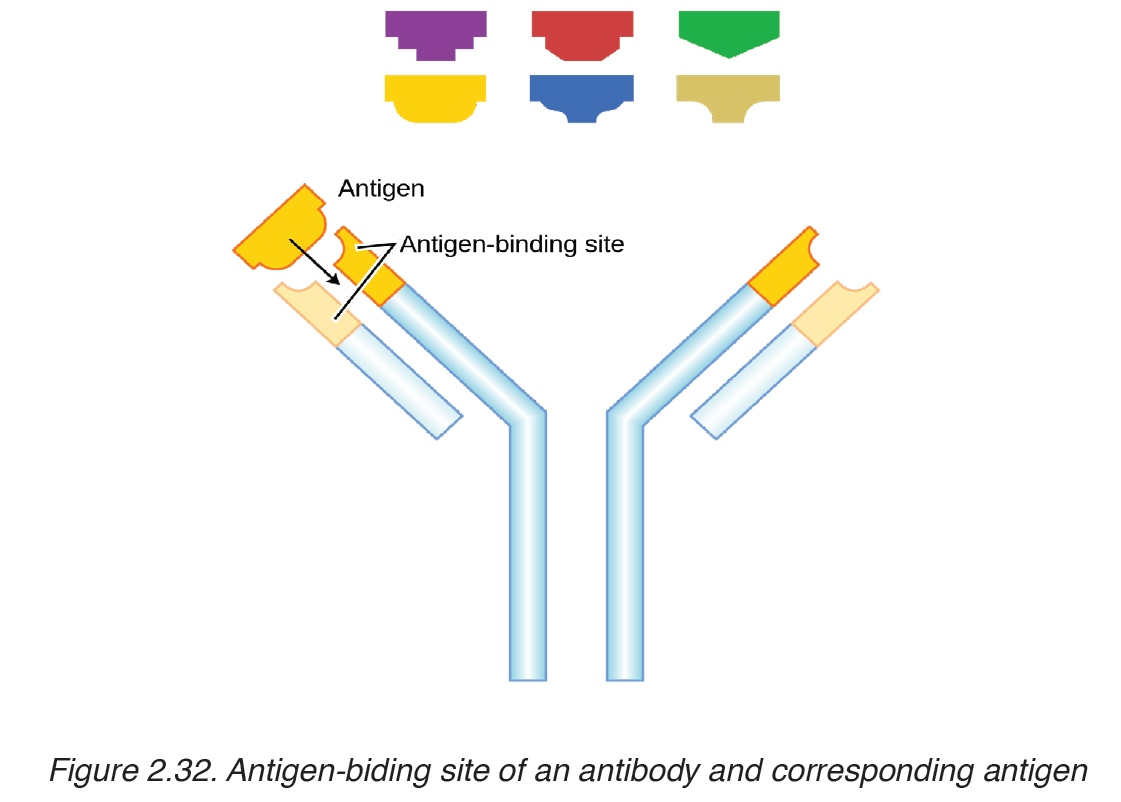
Cell-mediated immunity
It is the body’s primary defense against its own cells when they have become
cancerous or infected by viruses. The cell-mediated immunity is also
important in fighting infection caused by fungi and protists. When viruses
and other pathogens get inside living cells, antibodies alone cannot destroy
them. During cell-mediated immunity, T cells divided and differentiated into:
killer T cells (cytotoxic T cells), helper T cells, suppressor T cells and
memory T cells.
Killer T cells track down and destroy bacteria, fungi, protozoa and foreign
tissues containing antigens. Helper T cells produce memory T cells. The
memory T cells, like memory B cells, will cause a secondary response, if
the same antigen enters the body again. As pathogens are brought under
control, suppressor T cells release substances that shut down the killer T
cells.
Types of immunity
Immunity can be active or passive. In active immunity, the body makes its
own antibodies after exposure to an antigen, whereas in passive immunity,
the body acquires antibodies produced by other person or other animals.
Either active immunity or passive immunity can be naturally or artificially.
Thus, there are four types of immunity:
a) Natural active immunity
In natural active immunity, the body makes its own antibodies, in response
to an antigen.
b) Artificial active immunity
In artificial active immunity, the body makes its own antibodies, as a result
of vaccination against diseases. Vaccination (immunization) is the
production of immunity as a result of injection of weakened pathogens
which cannot cause diseases to the body, but which can induce production
of specific antibodies to those pathogens.
c) Natural passive immunity
Natural passive immunity is a temporary immunity resulting from acquiring
antibodies produced by another individual. The only natural passive immunity
is when a foetus acquires antibodies from the mother, either through the
placenta before birth, or through the breast feeding after birth. This immunity
protects the child against most infectious diseases for the first few months of
its life, or longer, if the infant is breast-fed.
d) Artificial passive immunity
It is a temporary immunity that results from the injection of the serum
containing antibodies from another organism. In this passive form of
immunity, a recipient is not induced to produce his or her own antibodies,
but is supplied with them, from an outside source. For example: the horse
serum well prepared, can then be used to protect humans against diseases.
The trouble with the protection induced by this immunity is that it is shortlived.
Nowadays, the passive immunity is normally used only in emergencies,
when is too rate for active immunization to work quickly enough.
Application activity 2.3
I. Multiple Choice Questions
1. Humoral immunity is carried out by the
a) B lymphocytes
b) T lymphocytes
c) Phagocytes
d) T lymphocytes, phagocytes and NK cells
2. Plasma cells represent
a) B lymphocytes which are actively secreting antibodies.
b) T lymphocytes which are actively secreting cytokines
c) Monocytes which have entered tissues
d) CTL which are secreting perforins.
3. Live attenuated vaccine types have the disadvantage over the inactivated
vaccine types as
a) They require booster shots
b) They do not confer life-long immunity
c) They may mutate to virulent form
d) They do not stimulate the immune system strongly.
4. Immune response is
a) The defense mechanism of our body.
b) Reaction of the cells and fluids of the body.
c) A substance that destroys or inhibits the growth of other
microorganisms
d) None of the above
II. Long Answer Type Questions
1. With an illustrative diagram, state the origin and describe the mode of
action of phagocytes.
2.4. Common addictive substances and their effects
Application activity 2.4
Carefully, Analyze the substances 1-7 in figure above and answer to the
following questions
1. Among those substances which one you use in daily life?
2. Which do you think are dangerous to life?
3. Suggest one effect of each of the dangerous substances
Tobacco, Alcohol and drugs addiction is the physical and psychological
need to continue using these substance, despite its harmful or dangerous
effects.The signs and symptoms of drug addiction drug vary according to the
individual and the substances he or she uses.
2.4.1. Tobacco smoking and its effects
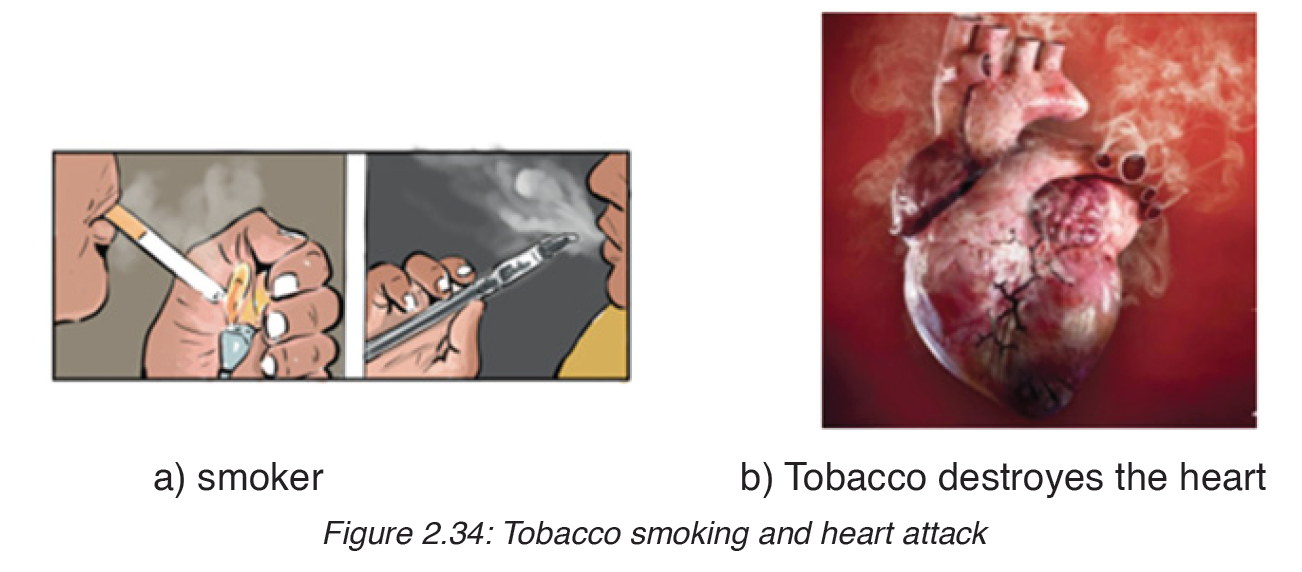
Smoking harms nearly every organ in your body. Among heavy smokers,
two in three will die from a disease caused by smoking. Tobacco smoke
is made up of thousands of chemicals and many of them are very harmful.
Around 70 of them cause cancer. Cigarette smoke contains over 4000
different chemicals. Many of them are harmful. The most harmful substances
in cigarette smoke include: nicotine, carbon monoxide and tar.
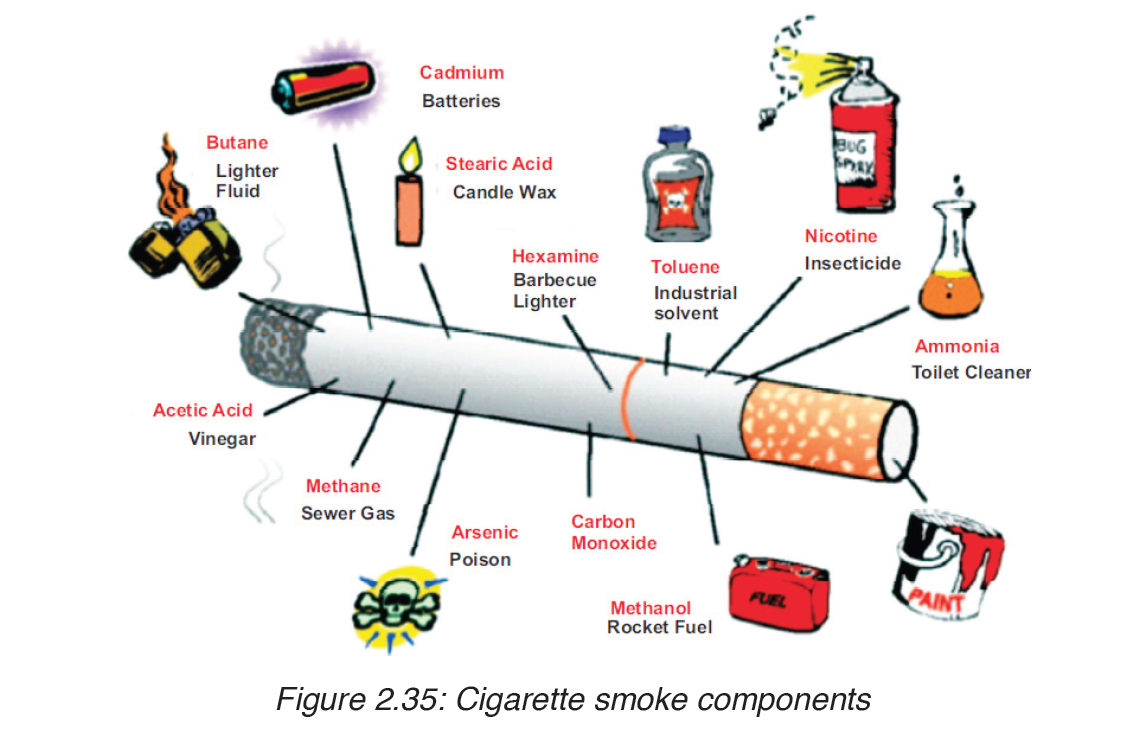
Cigarette smoke contains over 4,000 chemicals, including 43 known
cancer-causing (carcinogenic) compounds and 400 other toxins. These
cigarette ingredients include nicotine, tar, and carbon monoxide, as well as
formaldehyde, ammonia, hydrogen cyanide, arsenic, and DDT. Nicotine is
highly addictive.
a) Effects of Tar and carcinogens in tobacco smoke on the gas
exchange system
Tar is a combination of severe chemicals lying on the lining of the airways
and alveoli. This increases the diffusion distance for Oxygen entering the
blood and CO2 leaving the blood. The lumen of airways gets smaller and this
restricts the flow of air to the alveoli. The tar paralyses or destroys the cilia
on the surface the airways, so they are unable to move the mucus away.
Bacteria and viruses trapped in mucus are not removed. They can multiply
in the mucus and may block the bronchioles and affect breathing process. A
combination of bacteria, viruses and mucus in airways and alveoli causes
the lungs to be more susceptible to infections. Smokers are more likely to be
attacked by influenza and pneumonia.
Tar contains also the carcinogen compounds which cause cancer. When
the tar lies on the surfaces of airways, carcinogens enter the cells of lung
tissues. They enter the nucleus of these cells and have a direct effects on
their genetic material. Any change on genetic material is called mutation,
if mutations affect the genes that control cell division, then uncontrolled cell
division take place. This is cancer. Lung cancer often takes 20-30 years to
develop, and a cancer may grow for many years before it is discovered.
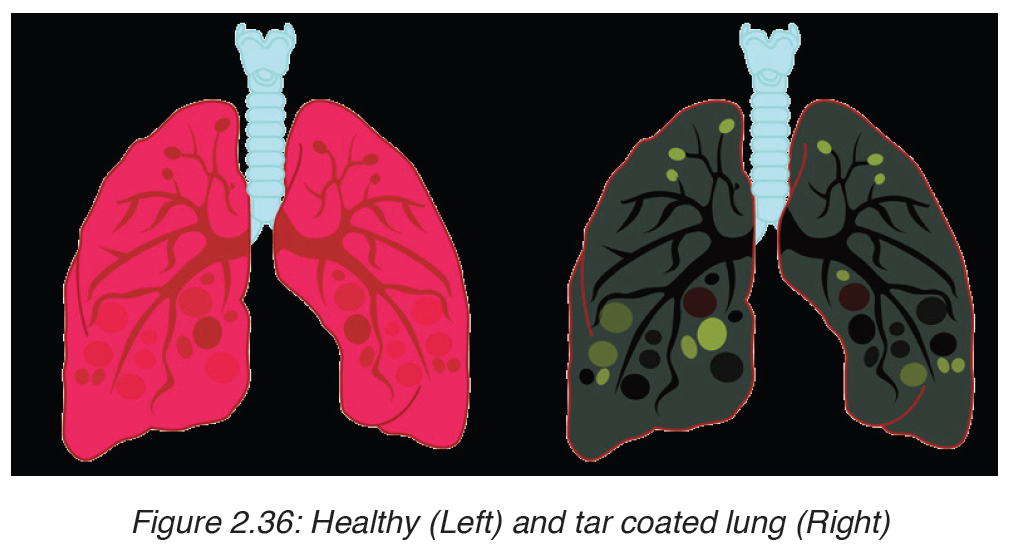
b) Effects of Nicotine and carbon monoxide on the cardiovascular
system
i. Carbon monoxide (CO): is fatal in large amount. This poisonous
gas is also found in car exhaust fumes. CO enters the red blood cells
and combines with haemoglobin more rapidly than Oxygen, and form
the carboxyhaemoglobin. This reduces the oxygen-carrying capacity
of blood, preventing your lungs, heart, and other organs from getting
oxygen they need to function properly. CO can also damage the lining
of arteries, and may rise the heart beat rate.
ii. Nicotine: is a poisonous alkaloid drug that is additive. 60 mg of
nicotine placed on the tongue would kill an individual within minutes.
It is absorbed by the body very rapidly, reaching the brain in less than
30 seconds.
It is a highly toxic chemical and its manufacture, use and sale is
controlled under the State Poisons Acts, except where it occurs in
tobacco.This exception of tobacco is for political reasons, not because
nicotine is deemed ‘safe’ in cigarettes. Nicotine, once inhaled, affects
the body very quickly. It causes changes to the structure and the
working of the brain, which lead to nicotine addiction. Nicotine also
raises heart rate, blood pressure, releases hormones (adrenaline)
affecting the central nervous system, and constricts small blood
vessels (arterioles) under the skin. In the long term, nicotine may be a factor in causing coronary disease. It is believed to be involved
in the development of gastrointestinal disorders and problems during
pregnancy, and is linked with the development of cancers.
c) Contribution of tobacco smoking to atherosclerosis and
coronary heart disease
i. Atherosclerosis is the deposition of fatty substances in the walls of
the arteries. The CO in the cigarette smoke can damage the inner
lining of arteries. This encourages the deposit of fatty substances like
cholesterol on the walls of arteries. This reduces the lumen of arteries,
which reduces blood flow, and causes high blood pressure.
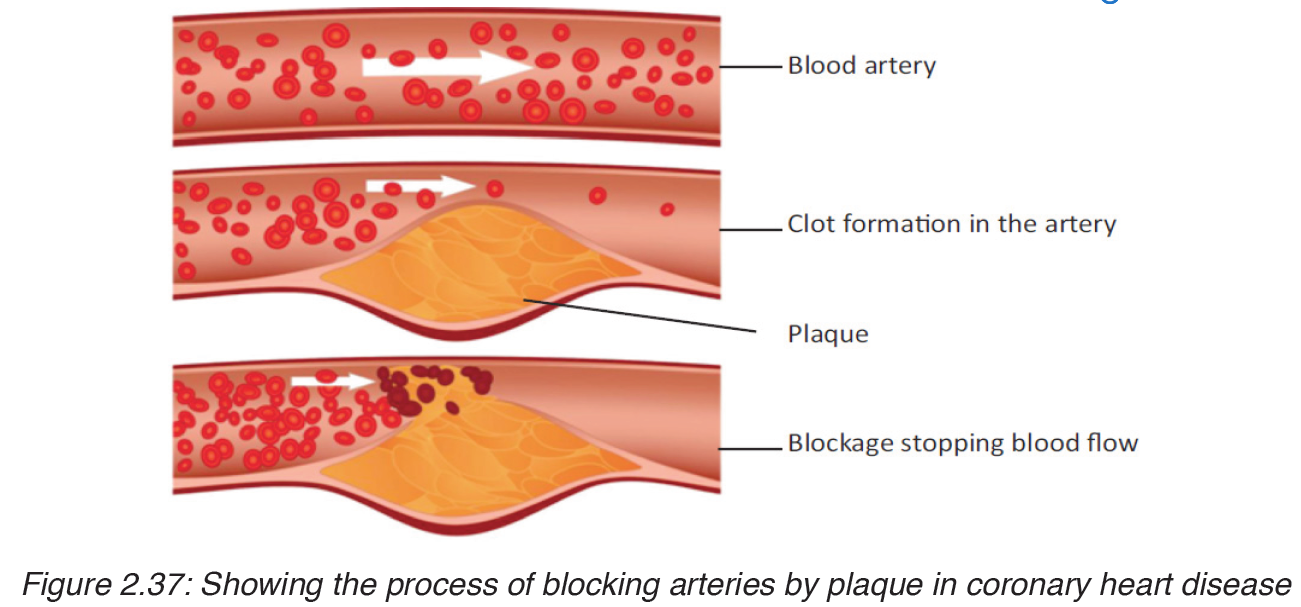
ii. Coronary heart disease (CHD) is a disease of the heart caused by
malfunction of the coronary artery. Coronary arteries are the arteries
which carry blood to the heart.
The nicotine and carbon monoxide found in cigarette enter the lungs
and diffuse into blood in blood capillaries, that conducting them into
coronary arteries. This causes the atherosclerosis in coronary arteries,
which reduces the blood flow in the heart muscles which receive less
oxygen for respiration. This can lead to coronary heart diseases, which
take three forms: In circulatory system, they cause changes which lead
to cardiovascular diseases like:
iii. Angina: a severe pain in the chest, which may extend down the left
arm or up into the neck.
iv. Heart attack or myocardial infections: caused by a clot in the
coronary arteries, blocking the flow of blood to the heart muscles.
v. Heart failure: when the heart cannot sustain its pumping action. This
can be caused by the blockage of the major coronary arteries.
d) Symptoms of lung cancer and chronic obstructive pulmonary
diseases (COPD)
– Chronic obstructive pulmonary disease: is a combination of
diseases that include chronic bronchitis, emphysema and asthma.
The symptoms of COPD are a combination of symptoms of chronic
bronchitis, and of emphysema.
– Chronic bronchitis: is the inflammation of the airways. This is
accompanied by damage to the cilia and the over production of mucus.
The symptoms are: irritation in lungs, continual coughing, and coughing
up mucus that is often filled with bacteria and white blood cells.
– Emphysema: is the loss of elasticity in alveoli, which causes the alveoli
to bust. The symptoms are: short breath, hard exhalation, the blood is
less well oxygenated and fatigue occurs.
– Lung cancer: the symptoms of lung cancer can be recognized by:
continual coughing, shortness of breath, pain in the chest and the blood
coughed up in the sputum is often the first sign of the lung cancer.
e) Evidences linking cigarette smoking to diseases and early
death.
i. Links of cigarette smoking to early death
– Regular smokers are three times more likely to die prematurely than a
non-smoker.
– 50% of regular smokers are likely to die of a smoking-related disease
– The more cigarettes a person smokes per day, the more he is likely to
die prematurely.
ii. Links of cigarette smoking to lung cancer
– A smoker is 18 times more likely than a non-smoker to develop lung
cancer.
– 25% of smokers die of lung cancer.
– A heavy smoker (more than 25 cigarettes a day) is 25 times more likely
than a non-smoker to die of lung cancer
– The chances of developing lung cancer reduce as soon as a person
stops smoking.
iii. Links to other lung diseases
– COPD is rare in non-smokers
– 98% of people with emphysema are smokers.
– 20% of smokers have emphysema
2.4.2. Alcohol
Alcohol is any organic compound in which the hydroxyl functional group (–
OH) is bound to a carbon. The term alcohol originally referred to the primary
alcohol ethanol (ethyl alcohol) which is used as a drug and is the mainalcohol
present in alcoholic beverages.
Alcohol is produced by adding yeast on a liquid that contains sugar in absence
of oxygen. This yeast ferment sugar to produce energy, alcohol and alcohol.
Most popular alcohol containing drinks include fermented drinks such as
beer and wine, other drinks such as vodka, whiskey, scotch, and gin are
made by distillation
Effects of alcohol
The effects of alcohol vary from person to person. While some people may
be able to limit their drinking, others have a difficult time controlling their
alcohol consumption.
Effects of alcohol include: Slurred speech, vision impairment, lack of
coordination, extreme shifts in mood, memory lapses and slowed breathing.
The most immediate alcohol effects are on the nervous system. The small
dose of alcohol slows down the rate of nervous system functions. So, alcohol
is a depressant.
The pregnant women who drink regularly, run the risk of” fetal alcohol
syndrome”or damage to the developing babies due to the effect of alcohol.
People who have become addicted to alcohol suffer from a disease called
alcoholism. If someone cannot work effectively without alcohol, that
indicates an alcohol abuse problem.
Taking alcohol in excess leads to damage of neurons in the brain, cells in the
liver. The damage of liver cells causes the live to become less able to deal with high amount of alcohol, then the formation of the scar tissue known as
cirrhosis in liver occurs. Finally the drinker may die from the chronic liver
failure.
Basing on the effect of alcohol on nervous system, driving can lead to suddenaccident that may kill people.
2.5.3. Drugs
Drug is any substance that cause a change in the body. Drugs affect the
body in different ways, because some are very powerful and dangerous and
their possession is not allowed. Other drugs like penicillin and codeine are
drugs that can be used under the supervision of doctor.
All drugs (legal and illegal ones) have the capacity of harming life if they are
abused. In this section, some of the most commonly abused drugs and the
way they affect the body will be considered.
Drugs affect the particular system of the body like digestive and circulatory
systems.
The following are the most powerful drugs that can also affect the nervous
system and their negative side effects.

a) MARIJUANA ( Also called Hashish or Hash)
i. Negative effects of using marijuana
– Addiction: Marijuana is physically addictive and psychologically
addictive, especially as it concerns younger.
– Memory Loss: People who always use marijuana developed a poorer
verbal memory in middle age than people who didn’t smoke.
– Social Anxiety Disorders: Regular use of marijuana can lead to mental
health issues such depression, anxiety and even schizophrenia.
– Paranoia: researches indicated that the use of marijuana can lead
users to feel a sense of paranoia as a result of the changes in their
sensory perception
– Heart Damage: Marijuana can also significantly raise a person’s heart
rate for up to three hours. Even, people who use marijuana are more
likely to have a stroke https://www.livescience.com/58210-stroke-heartfailure-
linked-to-marijuana.html at some point in their lives than people
who didn’t use it.
– Other effect are: Lung problems, low testosterone, appetite
irregularities, risk of greater potency, decrease in motor
Responses, poor decisions.
b) HALLUCINOGENS
Hallucinogens are drugs that cause hallucinations. Users see images,
hear sounds and feel sensations that seem very real but do not exist. Some
hallucinogens also produce sudden and unpredictable changes in the
mood of those who use them.
There are many types of hallucinogens but the two common types such
as LSD(Lysergic acid diethylamide ) and PCD(Phencyclidine) are the ones
which are used.
LSD is a powerful hallucinogen that interferes with the normal transmission
of nerve impulses in the brain. Its effects vary from person to person and all
people who use LSD regularly have abad trip. Some of LSD users have lost
touch with reality after only one single dose.
PCP produces fillings of strength and great power. High dose of PCP heart
attacks. The users of PCP often become extremely violent and are danger
to themselves and others.
c) STIMULANTS
These are drugs that speed up the actions of nervous system. The most
powerful stimulants are Amphetamines, these chemically resemble natural
neurotransmitters found in the body ( Compounds that pass nerve impulse
from one neuron to another). Once amphetamine enters the blood stream, it
floods the body and behaves like neurotransmitters. This causes the nervous
system to increase its activity producing a feeling of strength and energy in
the user body. Later the user suffers from fatigue and depression as the
nervous system becomes unable to handle the overstimulation produced by
amphetamines after their dose wears off.
Long-term use of amphetamines causes hallucinations, circulatory problems.
Psychological difficulties.
d) DEPRESSANTS
These are drugs that reduce the rate of nervous system activity. These drugs
are also known as downers. People become dependent on them as long as
they use them. Example of downers is barbiturates. Use of the barbiturates
with alcohol is fatal, because the nervous system becomes so depressed
and breath stops.
The barbiturates abuse is danger because it brings about serious medical
problems that must be immediately treated once the user stops supplying
the drugs to the body.
e) COCAINE
This drug is compound extracted from leaves of coca plant. Users supply it
to the body by smoking it, sniffing it or inject it into the bloodstream.
Cocaine stimulate the release of neurotransmitter called dopamine which is
normally released by the brain if person is satisfied during lunch or dinner.
So, cocaine creates feeling of pleasure and satisfaction
i. Effects of using cocaine
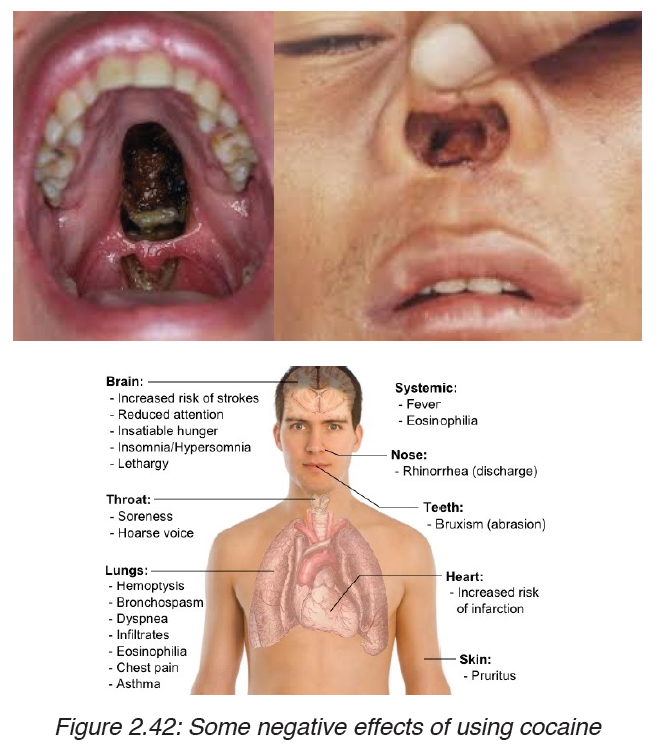
– Heavy use of cocaine leads to lung damage similar to emphysema.
Overdose stops breathing.
– Use of cocaine increases the heart rate and blood pressure leading to
risk of irregular heartbeat or even heart attack.
– Cocaine causes extreme mood changes, irritability. Long-term use can
lead to psychological problems.
– Cocaine causes the users to experience the sensation of bugs crawling
over the skin.
– Cocaine usage reduces a desire for food, leading to body weight loss.
f) OPIATES
This is a group of drugs produced from the opium poppy. The most common
opiates are opium produced directly from the opium poppy. Other examples
of opiates are morphine and heroin which are forms of opium. Apart from
heroin, other opiates can be used under supervision of doctor to reduce
severe pain as they are used as pain killers. If opiates are taken in large
dose, they can result to death.
In addition, opiates such as heroin, are example of drugs that cause a strong
physical dependence. The regular user becomes addicted and the nervous
system becomes also dependent on the supply of drugs. Any attempt of
withdrawal or stopping the use of drug will cause severe pain, nausea, chills and fever.
Application activity 2.4
I. Choose whether the following statements are True (T) or False (F)
1. Smoke from cigarettes can make non-smokers sick.
2. Smoking can affect a person’s ability to smell and taste food.
3. Secondhand smoke kills about 3,000 non-smokers each year from lung disease.
4. It takes about ten seconds for nicotine absorbed into the
bloodstream to reach the brain.
5. Smoking is a difficult habit to quit.
6. Nicotine, the chemical found in cigarettes, is an addictive drug.
7. A smoker is twice as likely to have a heart attack as a non-smoker is.
8. Cigarette brands that are heavily advertised on TV, in magazines,
on billboards, and on T-shirts are the brands more teens buy.
9. One out of every ten smokers will die of a smoking-related sickness.
10. More germs get into your lungs when you smoke.
II. Suggest the components of a tobacco smoke
III. Give the effects of alcohol on human body health
SKILLS LAB 2:
After studying this unit” Common diseases and hygiene”, and after finishing
their studies as well as working as cooperative, Student-teachers will help
society by creating a center for people workshop about nutrition, hygiene
and sanitation, so that different diseases can be eradicated from Rwanda society.
They will be helping people to fight against malnutrition by talking about
deficiency diseases and how they can prevent them. Here every house
should have its own vegetable plantation around kitchen.
Sensitization about general body hygiene, food hygiene, clothes hygiene,
home hygiene, water sanitation will be the first priority. And again, according
to what they have acquired from the school student-teacher will help people
to know all method of cooking like:
a) Boiling: this is a method involving cooking food completely covered
with water and boiling it until fully cooked
b) Steaming: this is cooking food using steam from boiling water.
c) Frying: this method involves cooking food with hot fat or oil.
d) Stewing: this involves cooking food with low quantities of water.
e) Roasting: this involves cooking food with very hot radiant heat
f) Baking: this involves cooking food using a direct heat
g) Grilling: this involves cooking food with direct heat using a grill.
These methods will be very important in preventing from being infected by
disease germs from uncooked food. Again good storage and preservation
are very significant in preventing many infectious diseases.
Conclusion: This unit will lead student-Teachers when they are still at
school or at home to protect their own life, and even the society life.
End unit assessment 2
I. Choose whether the given statements are True (T) or False (F)
1. Innate immunity is present at birth.
2. Breast milk confers protection to newborn by providing IgE type of antibodies.
3. Antibodies can work by promoting phagocytosis of microbial agents.
4. Antibiotics help a patient in mounting an effective immune response.
5. Treating tuberculosis is becoming difficult because Mycobacterium tuberculosis has become resistant to a number of antibiotics.
6. High fever is a generalized allergic reaction caused by release of active mediators from mast cells.
7. Secondary immune response appears much faster because of the presence of memory cells persisting from previous infection.
8. Vaccination against snakebite is an example of passive immunization.
9. Allergies are of two types—innate and adaptive.
10. Beta-lactam antibiotics kill bacteria by blocking synthesis of their cell walls.
II. Long answers type questions
1. Answer the following questions
a) Name the causative agent of tuberculosis.
b) Explain how tuberculosis is transmitted.
c) State the regions of the world with the highest number of cases of tuberculosis.
d) Suggest reasons for the high number of tuberculosis in some parts of the world.
2. State why vaccination programs are able to eradicate smallpox but not measles, TB, malaria or cholera.
3. Compare roles of B cells and T cells in the immune response.
4. Name organism which have the following effects on health
UNIT 3: STRUCTURE OF AN ATOM
Calculate relative atomic mass (R.A.M) of different elements.
Introductory Activity
The illustrations A, B, and C below show three atomic nuclei of different elements. Study the illustrations carefully and answer the questions below.
1. How many blue and red spheres do you see in each of the diagrams above?
2. What do the three diagrams A, B, and C have in common?
3. Based on your knowledge concerning atomic structure, what do you think that
a) the blue spheres represent?
b) the red spheres represent? Provide explanations.
4. Are there some other particle(s) missing from the above diagrams?
If yes name the particle(s).
5. What could you obtain if the atom is broken down?
Each country has its own culture (language, traditions and norms, attitudes
and values, etc.). Our culture defines our identity which is unique to each
Rwandan citizen and differentiates us from foreigners; if one element of our
culture is rejected or disappears, we become a different Rwandan people.
When we introduce foreign cultures to replace ours, we can lose our identity.
However, some of our cultural elements such as language can be shared
with others to build the social relationship.
Similarly, in the atom, the number of protons within the nucleus defines
the atomic number, which is unique to each chemical element; the atomic
number or the number of protons of an atom defines its identity. If a proton
is added or removed from an element, it becomes a different element.
Electrons around the nucleus can be lost, gained, or shared to create bonds
with other atoms in chemical reactions to produce useful substance, but this
does not change the identity of the elements involved.
3.1. The constituents of an atom, their properties and the outline of their discovery
Activity 3.1
1. Chemists study the structures, physical properties and chemical
properties of material substances. These consist of matter.
a) What is matter?
b) From the list that follows, show what is matter and what is not
matter: gold, energy peanuts, light, smoke, ideas, sounds. Provide explanations
2. List the components of matter and describe each component.
3. Regardless of some exceptions, all atoms are composed of the
same components. True or False? If this statement is true why do different atoms have different chemical properties?
4. The contributions of Joseph John Thomson and Ernest Rutherford
led the way to today’s understanding of the structure of the atom.
What were their contributions?
5. Explain the modern view of the structure of the atom?
6. Using your knowledge about atom, what is the role each particle plays in an atom?
Atoms are the basic units of elements and compounds. In ordinary chemical
reactions, atoms retain their identity. An atom is the smallest identifiable unit
of an element. There are about 91 different naturally occurring elements. In
addition, scientists have succeeded in making over 20 synthetic elements
(elements not found in nature but produced in Laboratories of Research
Centres).
An element is defined as a substance that cannot be broken down by
ordinary chemical methods in simpler substances. Some examples of
elements include hydrogen (H), helium (He), potassium (K), carbon (C) and
mercury (Hg). In an element, all atoms have the same number of protons
or electrons although the number of neutrons can vary. A substance made
of only one type of atom is also called element or elemental substance, for
example: hydrogen (H2), chlorine (Cl2), sodium (Na). Elements are the basic
building blocks of more complex matter.
A compound is a matter or substance formed by the combination of two or
more different elements in fixed ratios. Consider, Hydrogen peroxide (H2O2)
is a compound composed of two elements, hydrogen and oxygen, in a fixed ratio (2:2).
3.1.1. Discovery of the atom constituents
The oldest description of matter in science was advanced by the Greek
philosopher Democritus in 400 BC.
He suggested that matter can be divided into small particles up to an ultimate
particle that cannot any more be divided, and called that particle atom. Atoms
came from the Greek word atomos meaning indivisible.
The work of Dalton and other scientists such as Avogadro, etc., contributed
more so that chemistry was beginning to be understood. They proposed new
concept of atom, and from that moment scientists started to think about the
nature of the atom. What are the constituents of an atom, and what are the
features that make atoms of the various elements to differ?
In 1808 Dalton published A New System of Chemical Philosophy, in which he presented his theory of atoms:
1. Dalton’s Atomic Theory
a) Each element is made up of tiny particles called atoms.
b) The atoms of a given element are identical; the atoms of different elements are different in some fundamental way(s).
c) Chemical compounds are formed when atoms of different elements combine with each other. A given compound always has the same relative numbers and types of atoms.
d) Chemical reactions involve reorganization of the atoms—changes in the way they are bound together. The atoms themselves are not changed in a chemical reaction.

2. Discovery of Electrons and Thomson’s Atomic Model
In 1897 J. J. Thomson (1856–1940) and other scientists conducted several
experiments, and found that atoms are divisible. They conducted experiments
with gas discharge tubes. A gas discharge tube is shown in Figure 3.2.
The gas discharge tube is an evacuated glass tube and has two electrodes, a cathode (negative electrode) and an anode (positive electrode). The electrodes are connected to a high voltage source. Inside the tube, an electric discharge occurs between the electrodes.
The discharge or ‘rays’ originate from the cathode and move toward the
anode, and hence are called cathode rays. Using luminescent techniques,
the cathode rays are made visible and it was found that these rays are
deflected away from negatively charged plates. The scientist J. J. Thomson
concluded that the cathode rays consist of negatively charged particles, and
he called them electrons.
Thomson postulated that an atom consisted of a diffuse cloud of positive
charge with the negative electrons embedded randomly in it. This model,
shown in Figure 3.3, is often called the plum pudding model because the
electrons are like raisins dispersed in a pudding (the positive charge cloud), as in plum pudding.
In 1909 Robert Millikan (1868–1953) conducted the famous charged oil
drop experiment and came to several conclusions: He found the magnitude
of the charge of an electron equal to −1.602×10−19C .From the charge-tomass
ratio(e/m) determined by Thomson, the mass of an electron was also
calculated.
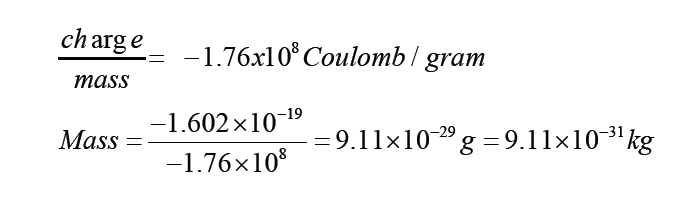
3. Discovery of Protons and Rutherford’s Atomic Model
In 1886 Eugene Goldstein (1850–1930) observed that a cathode-ray tube
also generates a stream of positively charged particles that move towards
the cathode. These were called canal rays because they were observed
occasionally to pass through a channel, or “canal,” drilled in the negative
electrode (Figure 3.4). These positive rays, or positive ions, are created
when the gaseous atoms in the tube lose electrons. Positive ions are formed by the process
Atom→ cation + e- (energy absorbed)
Different elements give positive ions with different e/m ratios. The regularity
of the e/m values for different ions led to the idea that there is a subatomic
particle with one unit of positive charge, called the proton. The proton is a
fundamental particle with a charge equal in magnitude but opposite in sign to
the charge on the electron. Its mass is almost 1836 times that of the electron.
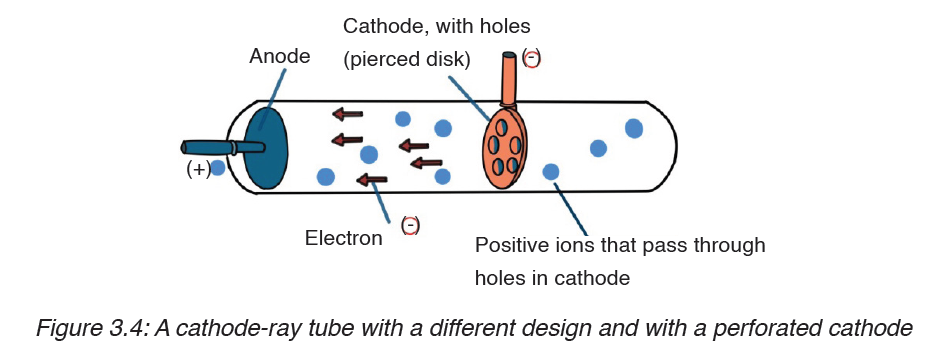
The proton was observed by Ernest Rutherford and James Chadwick in 1919 as a particle that is emitted by bombardment of certain atoms with α-particles.
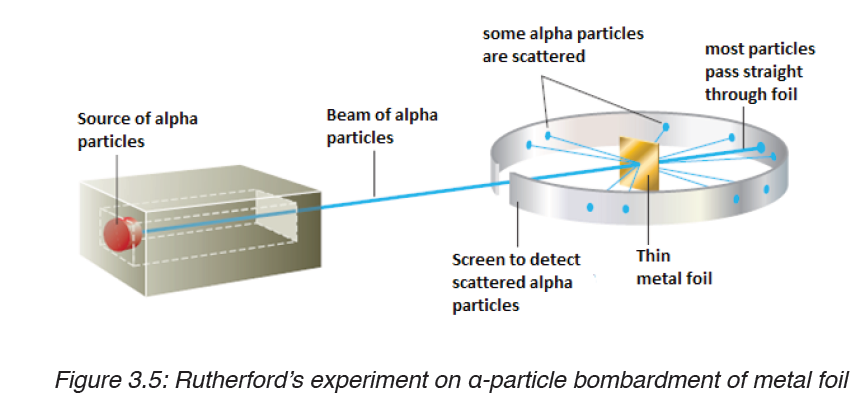
Rutherford reasoned that if Thomson’s model were accurate, the massive
α-particles should crash through the thin foil like cannonballs through gauze,
as shown in Figure 3.6(a). He expected α-particles to travel through the foil
with, at the most, very minor deflections in their paths. The results of the
experiment were very different from those Rutherford anticipated. Although
most of the α- particles passed straight through, many of the particles were
deflected at large angles, as shown in Figure 3.6(b), and some were reflected,
never hitting the detector. This outcome was a great surprise to Rutherford.
Rutherford knew from these results that the plum pudding model for the
atom could not be correct. The large deflections of the α-particles could be
caused only by a centre of concentrated positive charge that contains most
of the atom’s mass, as illustrated in Figure 3.6(b). Most of the α-particles
pass directly through the foil because the atom is mostly empty space. The
deflected α-particles are those that had a “close encounter” with the massive
positive centre of the atom, and the few reflected α-particles are those that
made a “direct hit” on the much more massive positive centre.
In Rutherford’s mind these results could be explained only in terms of a
nuclear atom—an atom with a dense centre of positive charge (the nucleus)
with electrons moving around the nucleus at a distance that is large relative
to the nuclear radius.
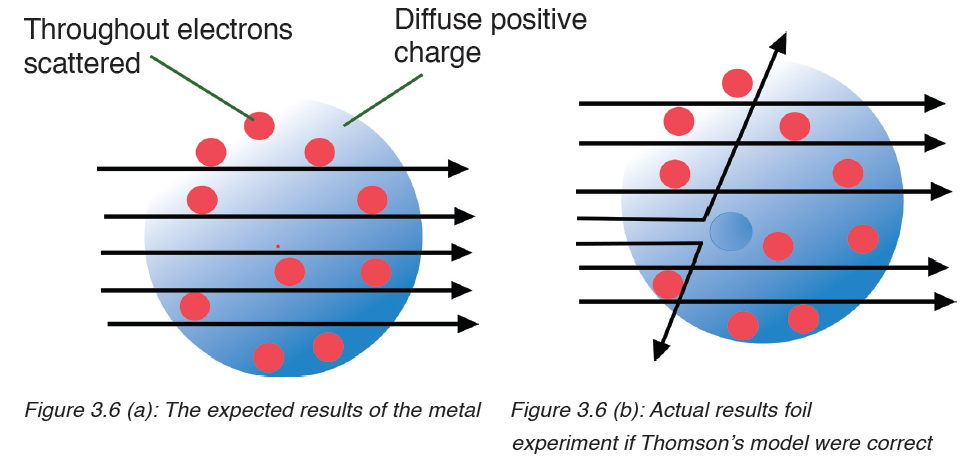
4. Discovery of Neutrons
In spite of the success of Rutherford and his co-workers in explaining atomic
structure, one major problem remained unsolved.
If the hydrogen contains one proton and the helium atom contains two
protons, the relative atomic mass of helium should be twice that of hydrogen.
However, the relative atomic mass of helium is four and not two.
This question was answered by the discovery of James Chadwick, English
physicist who showed the origin of the extra mass of helium by bombarding
a beryllium foil with alpha particles.
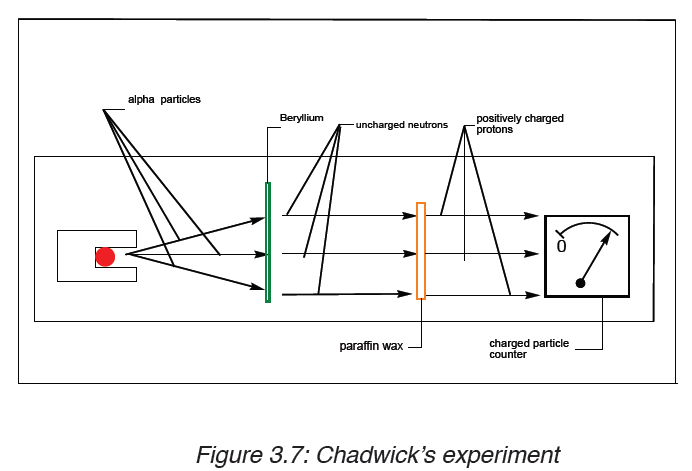
I n the presence of beryllium, the alpha particles are not detected; but they
displace uncharged particles from the nuclei of beryllium atoms. These
uncharged particles cannot be detected by a charged counter of particles.
However, those uncharged particles can displace positively charged
particles from another substance. They were called neutrons. The mass of
the neutron is slightly greater than that of proton.
Figure 3.8 shows the location of the elementary particles (protons, neutrons,
and electrons) in an atom. There are other subatomic particles, but the
electron, the proton, and the neutron are the three fundamental components
of the atom that are important in chemistry.
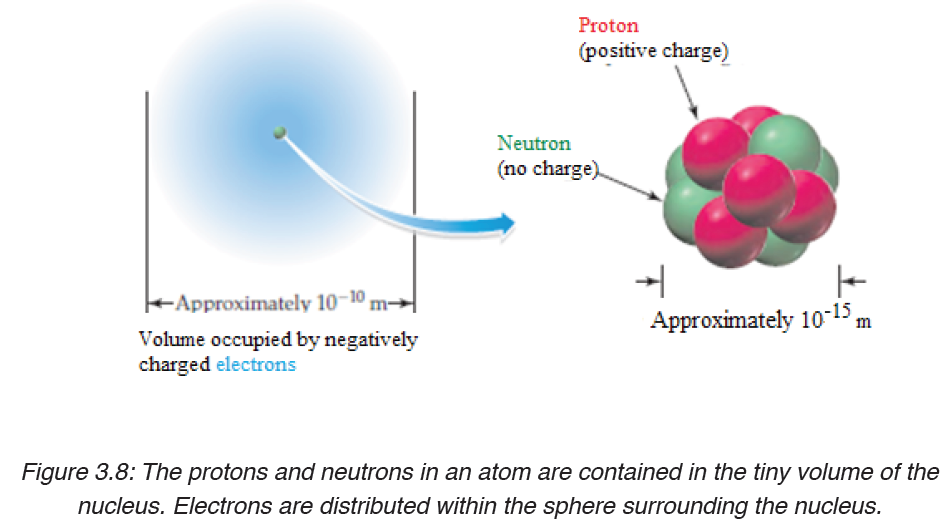
As a result of the experiments described above, it is found that atoms consist
of very small, very dense nuclei surrounded by clouds of electrons at
relatively great distances from the nuclei. All nuclei contain protons; nuclei
of all atoms except the common form of hydrogen also contain neutrons.
5. Properties of sub-atomic particles
Protons and neutrons are collectively known as nucleons. Both protons and
neutrons have a mass almost equal to that of hydrogen atom. The neutron
has no charge whereas the proton carries one positive charge. The electron
with one negative charge occupies the space outside the nucleus.
The following table summarizes the relative masses, the relative charges
and the position within the atom of these sub-atomic particles.
Table 3.1: Subatomic particles and some of their properties
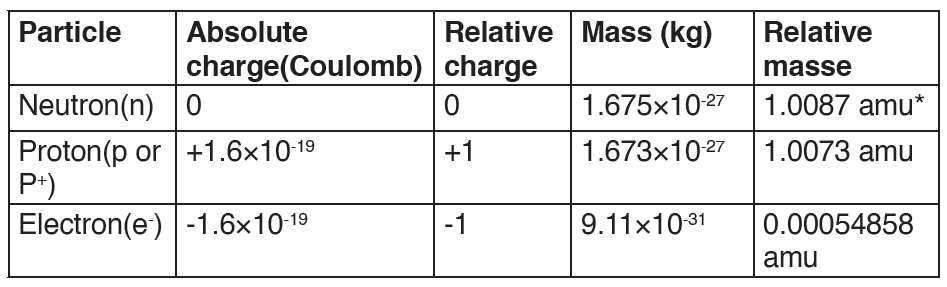
(*amu: atomic mass unity, 1 amu=1.67×10-27kg)
Application activity 3.1
1. In an experiment, it was found that the total charge on an oil drop
was 5.93 × 10-18 C. How many negative charges does the drop contain?
2. All atoms of the elements contain three fundamental particles.
True or false? Give an example to support your answer.
3. Compare the atom constituents
a) in terms of their relative masses
b) in terms of their relative charges
4. Explain why:
a) J.J Thomson concluded that all atoms have the same negatively charged particles.
b) Most alpha particles pass straight through tin metal foil.
c) Some alpha particles are scattered.
3.2 Concept of atomic number, mass number, isotopic mass and relative atomic mass
Activity 3.2
The diagram below shows a representation of sodium isotopes ( AZNa ).
Observe it and answer to the questions that follow.
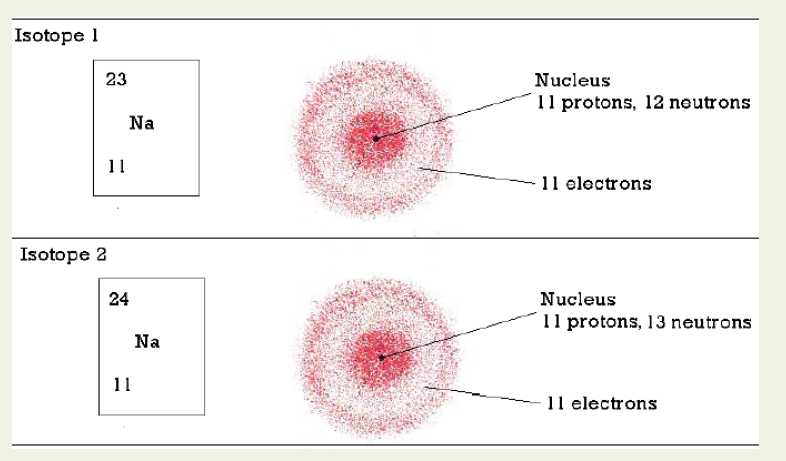
1. Find the values of A and Z for isotope 1 and isotope 2.
2. From your observation, how do you define the isotopes of an element?
3. How is A, the mass number, determined?
4. What information is provided by the atomic number, Z?
5. What is the relationship between the number of protons and the number of electrons in an atom?
6. Where are the electrons, protons, and neutrons located in an atom?
7. The mass of an atom is concentrated in the centre. Try to find an explication to this.
8. Say which one(s) of the following statements is(are) correct and
which one(s) is(are) wrong: (i) isotopes differ in their number of
electrons, (ii) isotopes differ in their mass numbers, (iii) isotopes
differ in their number of protons, (iv) isotopes differ by their number
of neutrons, (v) all the statements are wrong.
– The atomic number or proton number, Z, denotes the number of
protons in an atom’s nucleus. It corresponds to the order of the element in the periodic table.
– The mass number or nucleon number, A, denotes the total number of protons and neutrons in an atom.
Mass number = number of protons + number of neutrons
= atomic number + neutron number
– The number of neutrons can be obtained by subtracting the atomic number from the mass number.
– Chemists use the following shorthand to represent an atom. The mass number is shown as a superscript (top number) and the atomic number is shown as a subscript (bottom number) beside the symbol of the element.
By convention, the atomic number is usually written to the left subscript of the elemental notation, and the mass number to the left superscript of the elemental notation as represented by the example below, where X represents any elemental symbol.
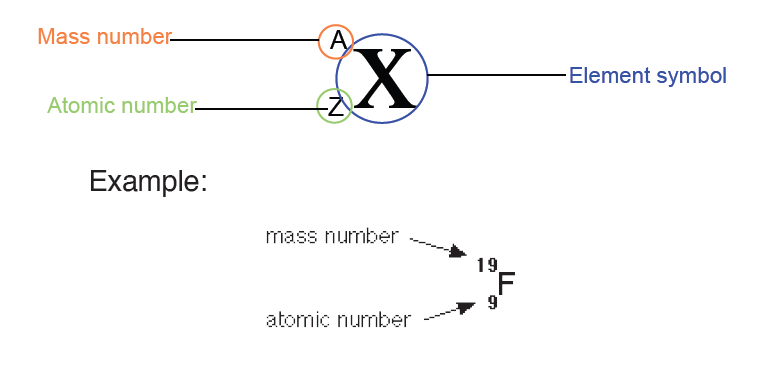
Some atoms of the same element have the same atomic number and different
mass numbers. This means a different number of neutrons. Such atoms are
called isotopes of the element. They are nuclides of the same element.
Example:
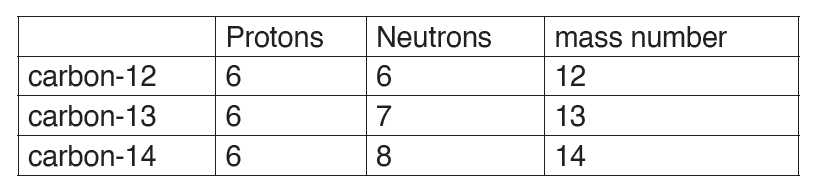
Isotopes of an element have the same chemical properties because they
have the same number of electrons.
– When elements react, it is the electrons that are involved in the reactions:
this means that the isotopes of an element cannot be differentiated by chemical reactions.
– Because isotopes of an element have different numbers of neutrons,
they have different masses, and isotopes have slightly different physical properties.
– The mass of a single isotope is its isotopic mass. The relative isotopic
mass of an isotope is the relative mass of that isotope compared with
the isotope which is given a mass of 12.00 units (12.00 atomic mass
units). That is, relative isotopic mass relates to the relative atomic mass
scale on which one isotope of the carbon element, carbon-12, is taken
as the reference standard for atomic masses and is given a relative
mass of 12 units, precisely 12 atomic mass units (a.m.u).
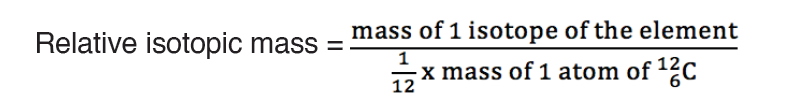
– Atomic mass unit (a.m.u) is a unit of mass used to express “relative
atomic masses”. It is 1/12 of the mass of the isotope of carbon-12 and is equal to 1.66054x10-27kg.
– The relative isotopic masses of all other atoms are obtained by comparison with the mass of a carbon-12 atom.
– On that scale, the relative atomic mass of a proton and that of a neutron are both very close to one unit (1.0074 and 1.0089 units respectively).
Since the relative mass of an electron is negligible (0.0005units), it follows that all isotopic masses will be close to whole numbers.
– However relative atomic masses of elements are not close to whole numbers because natural occurring elements are often mixtures of isotopes.
Application activity 3.2
1. How do you call the members of each of the following pairs?
Explain.
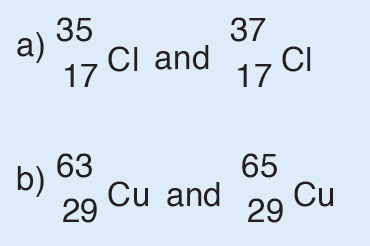
2. Write, using the periodic table, the correct symbols to identify an atom that contains
a) 4 protons, 4 electrons, and 5 neutrons;
b) 23 protons, 23 electrons, and 28 neutrons;
c) 54 protons, 54 electrons, and 70 neutrons; and
d) 31 protons, 31 electrons, and 38 neutrons.
3. Use the list of the words given below to fill in the blank spaces.
Each word will be used once.
Atomic number, Mass number, Protons, Electrons, Isotopes,
Neutron
a) The atomic number tells you how many __________________ and
____________________ are in an atom.
b) ____________________ is the number written as subscript on the
left of the atomic symbol.
c) The total number of protons and neutrons in an atom is called the
_____________________________________.
d) Atoms with the same number of protons but different number of
neutrons are called _____________________________.
e) The subatomic particle that has no charge is called a
________________________.
3.3 Calculations of the relative atomic masses of elements
Activity 3.3
Using textbooks and internet connection, explain the concept of relative
mass and attempt to solve the problems below.
1. Argon has three naturally occurring isotopes: argon-36, argon-38
and argon-40. The reported relative atomic mass of Argon (Ar)
from the periodic table is 39.948, which isotope do you think is the
most abundant in nature? Explain.
2. Calculate the average atomic mass of an element with two naturally
occurring isotopes: 85X (72.15%, 84.9118 amu) and 87X (27.85%,
86.9092 amu). Identify this element.
3. Boron has two naturally occurring isotopes. Find the percent
abundances of 10B and 11B given the isotopic mass of 10B = 10.0129
amu and the isotopic mass of 11B = 11.0093 amu.
Relative atomic mass, symbolized as R.A.M (Ar), is defined as the average
of the relative isotopic masses of the different isotopes weighted in the
proportions in which they naturally occur.
Thus, the different isotopic masses of the same elements and the percentage
abundance of each isotope of an element must be known in order to
accurately calculate the relative atomic mass of an element.
Notice: Remember that mass number is not the same as the relative atomic
mass or isotopic mass! The mass number is the total number of protons and
neutrons while relative atomic mass is the average of the isotopic masses.
Let A1, A2, A3,…, An be an abundance of n isotopes of the same chemical
element with atomic mass M1, M2, M3,…, Mn respectively, the relative atomic
mass (R.A.M) is given by the following equation:
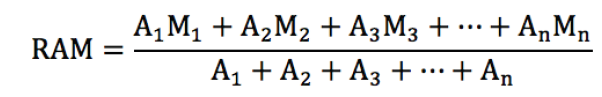
Example 1
Oxygen contains three isotopes 16O, 17O, and 18O. Their respective relative
abundances are 99.76%, 0.04%, and 0.20%. Calculate the relative atomic
mass of oxygen.
Solution:
Relative isotopic mass of 16O is 16 and its relative abundance is 99.76%;
Relative is otopic mass of 17O is 17, abundance 0.04%;
Relative isotopic mass of 18O is 18, abundance 0.20%.
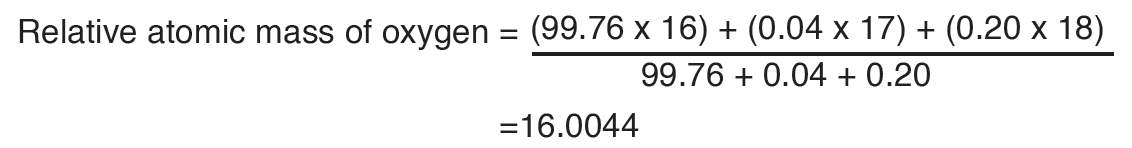
By applying the same formula, the relative abundance of the isotopes may be
calculated knowing the relative atomic mass of the element and the atomic
masses of the respective isotopes.
Example 2
Chlorine contains two isotopes 35Cl and 37Cl, what is the relative abundance
of each isotope in a sample of chlorine if its relative atomic mass is 35.5?
Solution:
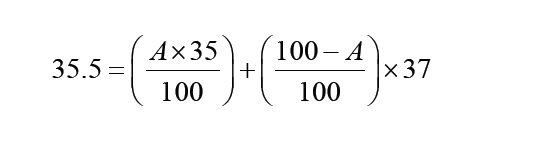
Note that if the abundance of the isotope of atomic mass 35 is A%, the
abundance of the isotope of mass 37 will be (100 – A) %.
0.35A + (100 – A) x 0.37 = 35.5
0.35A– 0.37A =35.5 – 37
– 0.02A = – 1.5
A= 1.5/0.02 = 75
Therefore, the abundance of the isotope of relative atomic mass 35.5 is 75%
while that for the isotope 17Cl is 100 – 75 = 25%.
Application activity 3.3
1. Three isotopes of magnesium occur in nature. Their abundances
and masses are listed in the following table. Use this information
to calculate the relative atomic mass of magnesium.
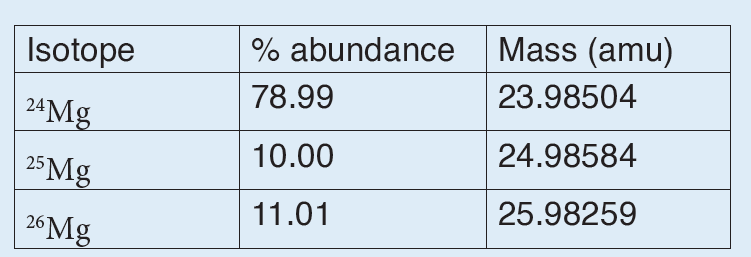
2. The atomic weight of gallium is 69.72 amu. The masses of the naturally occurring isotopes are 68.9257 amu for 69Ga and 70.9249 amu for 71Ga. Calculate the percent abundance of each isotope.
SKILLS LAB 3
Using adequate materials construct any three models of atoms of your
choice. These models show shells and all electrons. These atoms should
be any three of this list: Cl, C, H, O, N, B, F.
End unit assessment 3
I. Multiple choice questions
1. Which of the following is true regarding a typical atom?
a) Neutrons and electrons have the same mass.
b) The mass of neutrons is much less than that of electrons.
c) Neutrons and protons together make the nucleus electrically neutral.
d) Protons are more massive than electrons
2. Which of the following statements is(are) true? For the false statements, correct them.
a) All particles in the nucleus of an atom are charged.
b) The atom is best described as a uniform sphere of matter in which electrons are embedded.
c) The mass of the nucleus is only a very small fraction of the mass of the entire atom.
d) The volume of the nucleus is only a very small fraction of the total volume of the atom.
e) The number of neutrons in a neutral atom must equal the number of electrons.
3. Each of the following statements is true, but Dalton might have
had trouble explaining some of them with his atomic theory. Give explanations for the following statements.
a) Atoms can be broken down into smaller particles.
b) One sample of lithium hydride is 87.4% lithium by mass, while another sample of lithium hydride is 74.9% lithium by mass.
However, the two samples have the same chemical properties.
II. Short and long answer questions
1. What are the three fundamental particles from which atoms are
built? What are their electric charges? Which of these particles
constitute the nucleus of an atom? Which is the least massive
particle of the fundamental particles?
2. Verify that the atomic weight of lithium is 6.94, given the following information:
6Li, mass = 6.015121 u; percent abundance = 7.50%
7Li, mass = 7.016003 u; percent abundance = 92.50%
3. Lithium has 2 naturally occurring isotopes. Lithium-6 has an atomic
mass of 6.015 amu ; lithium-7 has an atomic mass of 7.016 amu.
The atomic mass of lithium 6.941 amu. What is the percentage of
naturally occurring Li-7 ?
4. Observe the table below and complete by the missing data.
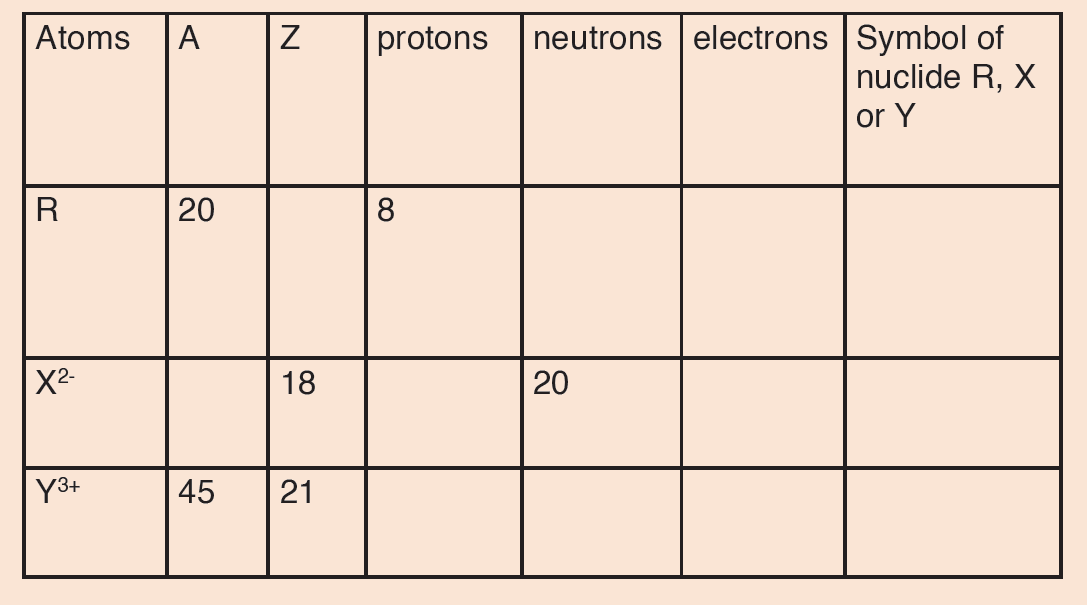
UNIT 4: UNIFORM CIRCULAR MOTION
Key unit competence
Analyse and solve problems related to circular motion.
Introductory Activity 4
Read the following statement and analyse them how it happens thereafter
answer the next questions
a) The rotation of the blades on the ceiling fan.
b) A ball rolling on a floor in a constant velocity.
c) An artificial satellite orbiting the Earth at a constant height.
d) A stone which is tied to a rope and is being swung in circles.
e) A car turning through a curve in a race track.”
1. Refer to the above examples, elaborate other examples on where
circular motion can be applied in real life situation
2. From what you elaborate what are the factors that help the body
to be in circular motion?
3. Observe the following image thereafter answer the question below
What happen for this bicyclist to turn on level ground easily? T
4.1. Definition of key terms in circular motion
Activity 4.1
Study carefully the motion of the ball shown below.
From your observation of below figure, how can a body move in that circular
path? Explain your reason
Circular motion: is movement of an object along the circumference of a
circle, or rotation along a circular path. It is a motion with constant.
Angular displacement: is the angle in radians (degrees, revolutions)
through which a point revolves around a centre or line has been rotated in a
specified sense about a specified axis.
Linear velocity: is themeasure of how fast the dot is moving along the
circumference of the circle
Angular velocity: is the rate at which the central angle swept out by
the object changes as the object moves around the circle, and it is thus
measured in radians per unit time.
Period: The period is the time taken for a full revolution of the motion.
Frequency: is the number of rotation per unit time.
Angular acceleration: also called rotational acceleration, is a quantitative
expression of the change in angular velocity that a spinning object undergoes
per unit time. It is a vector quantity, consisting of a magnitude component
and either of two defined directions or senses.Linear acceleration: is the rate of change of linear velocity
Application activity 4.1
After observing the above pictures, what A, B, C and D represent in circular
motion?
4.2 Relationship between angular and linear parameters
Activity 4.2
Write your observation on the image below
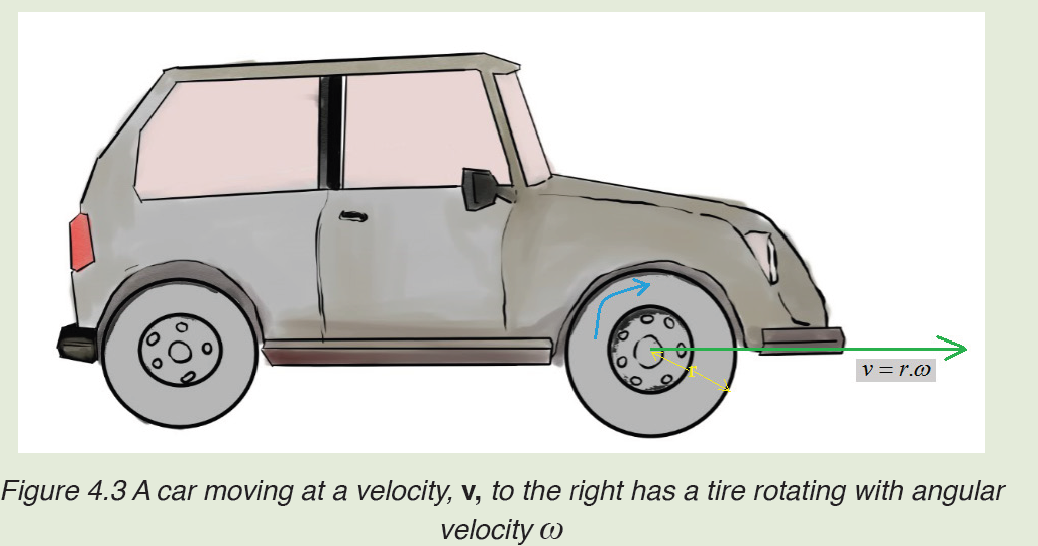
1. What do variables v, ω and r mean in the expression illustrated on the figure 4.3
2. What does ω show in the movement of the wheel in the illustration above.
4.2.1 Angular Velocity
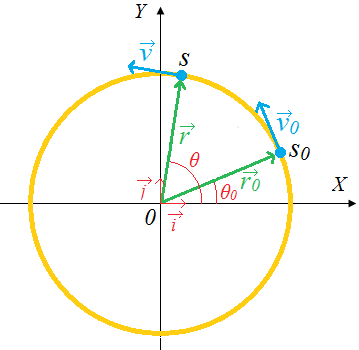
Figure 4.4. Uniform circular motion
To indicate the angular position of a particle, or how far it has rotated we
specify the angle θ of a line joining the centre of the particle and its position
with respect to some reference line, such as the x axis. Consider an object
moving in a circle of radius r with a uniform speed v round a fixed point 0
as centre.
When an object rotates the angular displacement 0 Δθ =θ −θ , the average
angular velocity is defined as
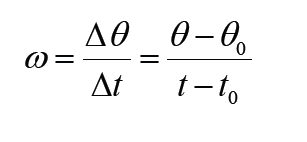
The linear displacement or the Arc of the object along the circle is
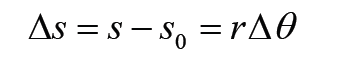
The linear average velocity
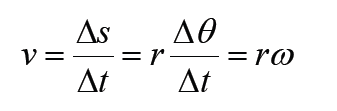
We define the instantaneous angular velocity as the very small angle Δθ ,
through which the object turns in the very short time intervalΔt :
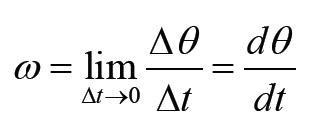
The instantaneous linear velocity
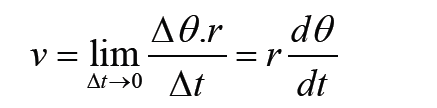
The angular velocity is generally specified in radians per second (rad / s)
whereas the instantaneous linear velocity is expressed in (m / s).
4.2.2 Periodic time and frequency
The period T is the time needed for the object to make one complete revolution.
During this time, the object travels a distance equal to the circumference of
the circle. The frequency f is referred to as the number of revolutions made
by an object in one second. The unit of frequency is Hertz.
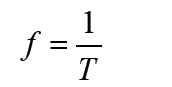
The object’s angular speed is then represented by
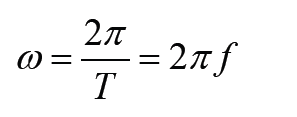
4.2.3 The average angular acceleration
The average angular acceleration is defined as the change in angular velocity
divided by the time required to make this change:
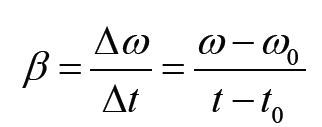
The average linear acceleration
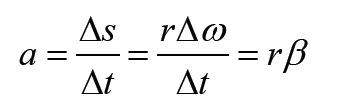
The instantaneous angular acceleration is
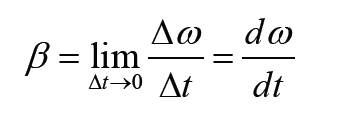
The instantaneous linear acceleration
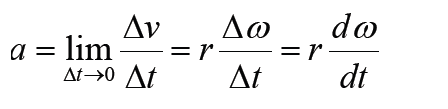
The angular acceleration is generally specified in radians per second (rad / s2)
whereas the instantaneous linear acceleration is expressed in (m / s2).
Application activity 4.2
1. A ball at the end of a string is revolving uniformly in a horizontal
circle of radius 2 meters at constant angular speed 10 rad/s.
Determine the magnitude of the linear velocity of a point located :
a) 0.5 meters from the center
b) 1 meter from the center
c) 2 meters from the center
2. The blades in a blender rotate at a rate of 5000 revolution per minute. Determine the magnitude of the linear velocity :
a) a point located 5 cm from the center
b) a point located 10 cm from the center
3. A point on the edge of a wheel 30 cm in radius, around a circle at constant speed 10 meters/second.
What is the magnitude of the angular velocity?
4. The angular speed of wheel 20 cm in radians is 120 revolutions per minute. What is the distance if the car travels in 10 seconds.
4.3 Acceleration in circular motion
Activity 4.4
A car executing a turn, after a certain speed limit, a car will start drifting.
a) Why at that limitation of the speed the car start travelling? Explain your reasoning
4.3.1. Tangential and Centripetal acceleration
In the circular motion, the tangential acceleration aT always points in the
direction tangent to the circle and changes the rate of velocity in terms of
magnitude because their vectors are always in the same or opposite direction.
The tangential acceleration can be considered as the linear acceleration.
The centripetal acceleration (normal acceleration or radial acceleration)
aN changes the velocity in terms of direction and its vector is perpendicularly
directed inward the circle.
Since the velocity is constant, the tangential component of acceleration doesn’t exist in UCM:
Consequently, the tangential component of the acceleration is also zero. Only, the normal component of the acceleration exists in UCM. Thus, the velocity
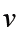 changes in direction but not in magnitude. The figure below shows the representation of the angular velocity
changes in direction but not in magnitude. The figure below shows the representation of the angular velocity using a distant reference frame
using a distant reference frame
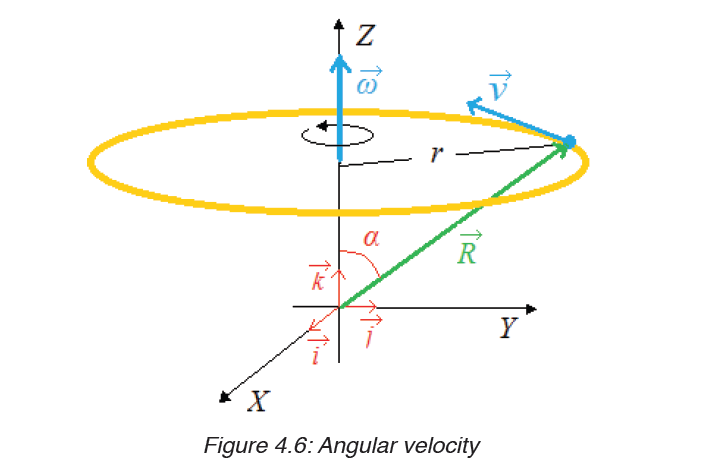
The centripetal component of the acceleration is directed along the radius.
From the above figure we can notice that
This relation indicates that the vector v can be expressed in the vector form by the equation:
It follows that
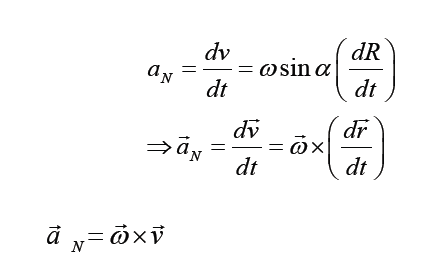
Introducing the equation (1) into (2)
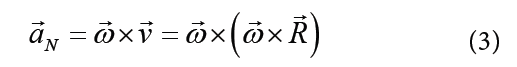
The magnitude of the centripetal acceleration is given by

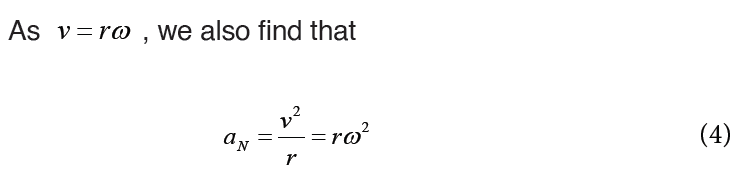
4.3.2. Acceleration in a non-uniform circular motion
Circular motion at a constant speed occurs when the acceleration of the object
is directed toward the centre of the circle (Only the centripetal component is
available). If the acceleration is not directed toward the centre but is at an
angle, as shown in figure below, the acceleration has two components; thecentripetal and the tangential component.
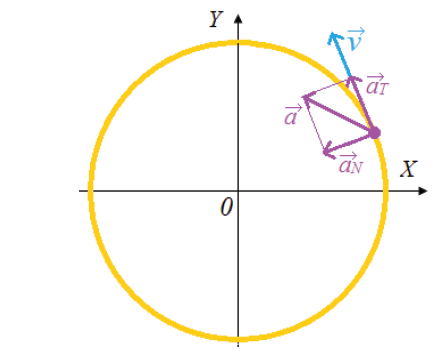
The tangential component of the acceleration is the rate that changes the magnitude of the velocity
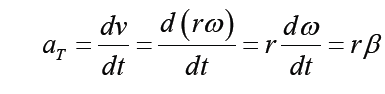
The centripetal acceleration arises from the change in direction of the velocity and, as we have seen, is given by
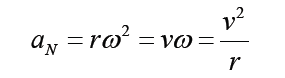


4.3.3. Distance time graph of circular motion
When an object executes a circular motion of constant radius R, its projection on an axis executes a motion of amplitude a that repeats itself back and forth, over the same path.
When M executes a uniform circular motion, its projection on X-axis executes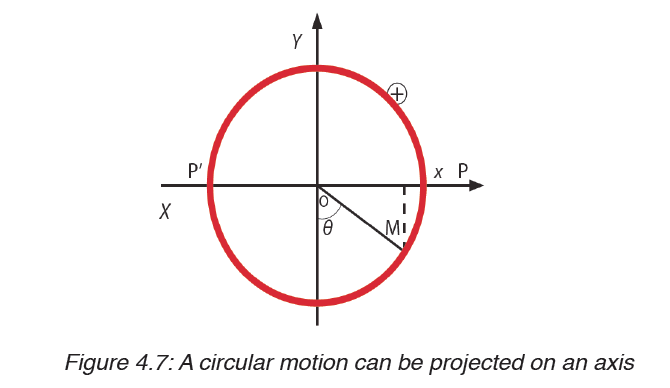
a back and forth motion between positions P and P’ about O.
Considering the displacement and the time, we find the following graph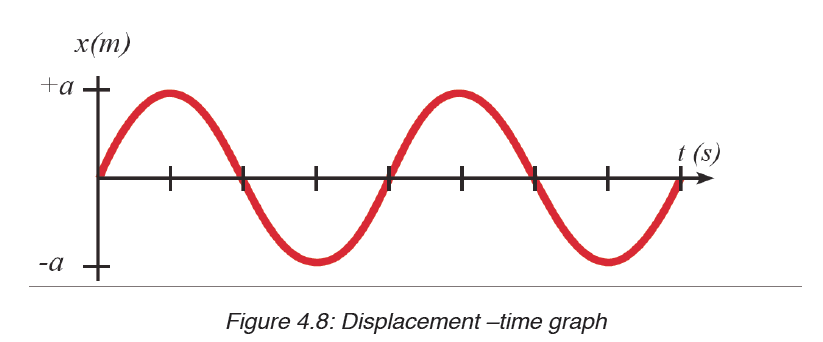
Application activity 4.3
1. A ball is attached to a string that is 1.5m long. It is spun so that it
completes two full rotations every second. What is the centripetalacceleration felt by the ball?
2. Imagine a car driving over a hill at a constant speed. Once the
car has reached the apex of the hill, what is the direction of theacceleration?
4.4 Centripetal force
Activity 4.4
Observe the following figure and answer the question below:
What would happen when a ball is attached to a string and is swung round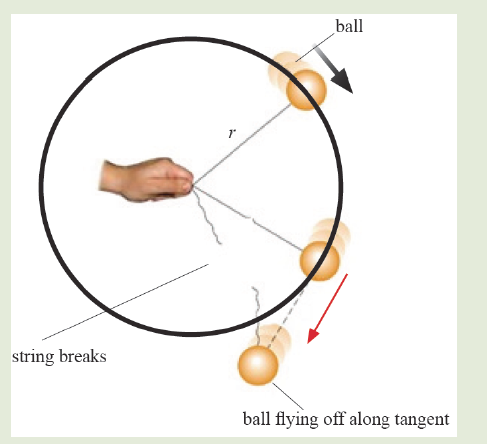
in horizontal circle? Explain your reasoning
If you try to move / run in a circular path, you will finally notice that you keep
moving in a circle even when you try to stop. There is a force that keeps you
more in a circular path called centripetal force. Since a body moving in a
circle (or a circular arc) is accelerating, it follows from Newton’s first law of
motion that there must be force acting on it to cause the acceleration.
This force, like the acceleration, will also be directed toward the centre and
is called the centripetal force. The value F of the centripetal force is given byNewton’s second law, that is:
Application activity 4.4
1. A 3.0 kg mass is tied to a rope and swung in a horizontal circle. If
the velocity of the mass is 4.0 m/s and the radius of the circle is
0.75 m, what is the centripetal force and centripetal acceleration
of the mass?
2. A 200-gram ball, attached to the end of a cord, is revolved in a
horizontal circle with an angular speed of 5 rad s-1. If cord’s length
is 60 cm, what is the centripetal force?
3. A student of mass 50kg decides to go on the ride. The coefficient
of static friction between the student and wall is 0.8. If the diameter
of the ride is 10m, what is the maximum period of the ride’s rotation
that will keep the student pinned to the wall once the floor drops?
g =10m/s2
SKILLS LAB
Go on YouTube, watch video about “Trucks fail to Negotiate Dangerous
Bend in Road”. It is a social issue which relationship with Circular motion.
1. What did you see on the video and what happen?
2. If you are engineer, how you will solve this problem?
3. Brainstorm with your classmates about the property needed to Design asafety road by used scientific knowledge in Circular motion.
End unit assessment 4
1. Why do we need a force to keep a body moving uniformly along a circular path?
2. When an object moves in a circular path the net force is called?
Explain your answer
3. When a particle moves in a circle with constant speed its
acceleration is? Explain your reason
4. Is acceleration constant in circular motion? Defend your reasoning
5. A hot wheels track has a vertical loop with a radius of 20 cm.
a) What is the minimum speed the car can have at the highest point
without falling off of the track?
b) If the actual speed is 1.8 m/s, what is the normal force? (use m=20
grams)
6. A 1200 kg car drives at a constant speed of 14 m/s around a
circular track (r=80.0 m).
a) What is the size of the net force acting on the car?
b) What is the physical agent providing that force?
c) What is the maximum frictional force that can act on the tires if the
static coefficient
of friction is 0.30? Will the car’s tires start slide? If not, how fast can
the car move before it does start sliding?
7. A0.45kg ball, attached to the end of a horizontal cord, is rotated in
a circle of radius 1.3m on a friction less horizontal surface. If the
cord will break when the tension in it exceeds 75N, what is the
maximum speed the ball can have?UNIT 5: CELL STRUCTURE
Key unit competence
Distinguish between the types of microscopy and their principle uses and
relate the structure of cell to their functions.
Introductory ActivityCarefully, analyze the following diagram and the related questions
This is one of materials used in Biology.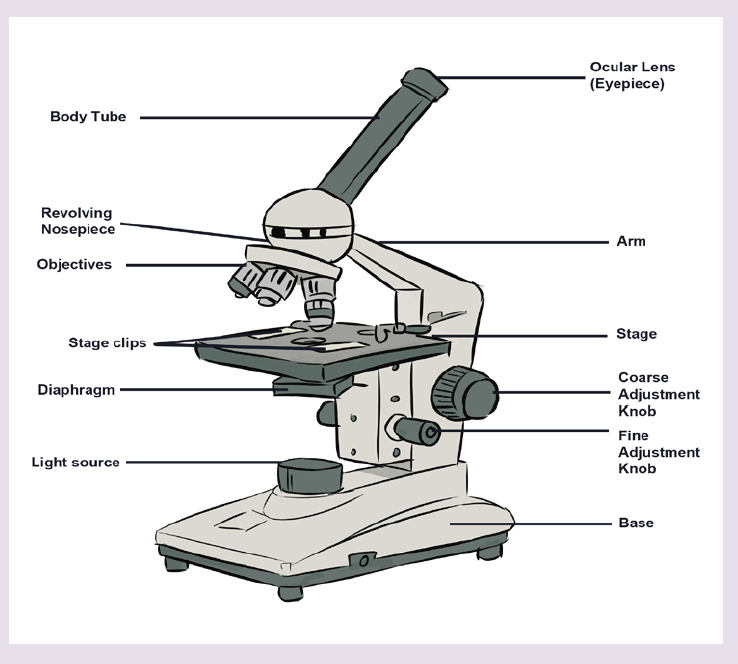
a) Have you ever seen and manipulated it?
b) Observe the parts of the material, discuss and group them into thefollowing:
i. Suporting parts
ii. Magnifying parts
iii. Adjusting parts
iv. Lighting parts
c) Point out scientific activities that require the use of this material in
biology
5.1. Cell theory and microscopes
Activity 5.1
Using your knowledge acquired from ordinary level, textbooks and internet
search
Explain:
a) The meaning of cell theory
b) The functions of the components of the compound light microscope
5.1.1. Cell theory
Cytology is the study of the structure and function of cells. A Cell is the
basic unit of life surrounded by a cell membrane and containing organelles.
All living organisms are made of cells and nothing less than a cell can truly
be said to be alive. The word cell comes from the Latin cellula, meaning
a small room or cubicle, and was first used by Robert Hooke in his book
Micrographia, published in 1665. Hooke was observing slides of cork taken
from the bark of an Oak tree under the compound microscope. He decided
that the slides were made up of a lot of many small chambers that he called
cells that range in size are from 1μm – 1mm.
Living organisms are classified into unicellular organisms made by only one
cell, such as bacteria, whereas animals and plants are composed of many
millions of cells built into tissues and organs. They are called multicellular
organisms. In a multicellular organism, cells divide and thereafter they
undergo differentiation or specialisation for specific functions.
In biology, the historic scientific theory of cells is called cell theory. The cell
theory states that all living organisms are made up of cells, and cells are the
basic unit of structure function in all living organisms. The main principles
of cell theory is that all known living organisms are made up of one or more cells, all cells come from pre-existing cells by division and cells contain the hereditary information that is passed from cell to cell during cell division.
5.1.2. Microscopes: Compound-light microscope and Electron microscopes
Microscopy is the technical field of using microscopes to view objects and
areas of objects that cannot be seen with the naked eye. The microscope
was created by Zacharias Janssen in the late 16th century.
Prior to the invention of the microscope, the details of objects on slides were
limited. Single microscopes were similar to using a magnifying glass such as
hand lens. The invention of the light microscope in the 17th century by Antony
van Leeuwenhoek made cells visible for the first time and, for hundreds of
years afterwards. Electron microscope invented in the 1930s enables the
researchers to see very small organelles which cannot be seen by using
light microscopes. The purpose of the use of a microscope is to magnify
small objects such as cells or to magnify the fine details of a larger object in
order to examine minute specimens that cannot be seen by the naked eye.
a) Compound Light Microscope
The optical microscope, often referred to as light microscope is a type of
microscope which uses visible light and a system of lenses to magnifyimages of small samples.
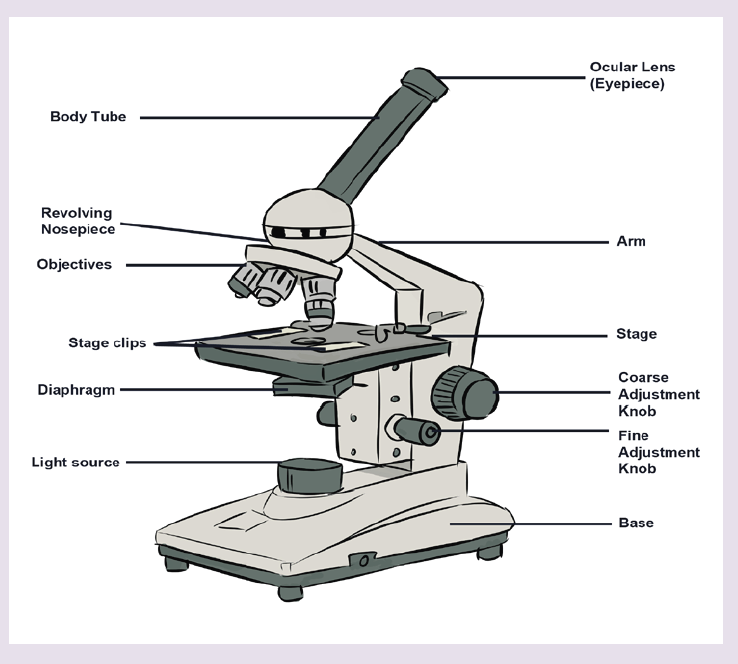
The parts of light microscope and their roles: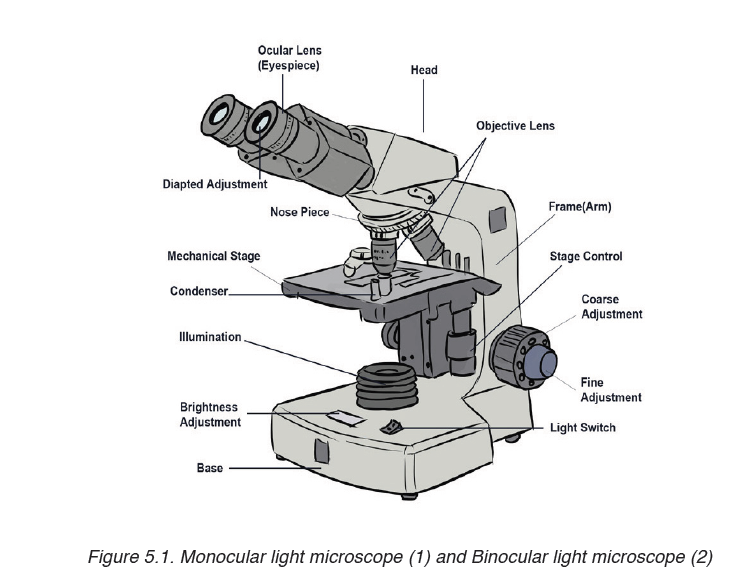
– Base: supports and stabilizes the microscope on the table or any other
working place
– Light source: It is made by lamp or mirror which provides light for
viewing the slide.
– Stage: is a platform used to hold the specimen in position during
observation.
– Stage clips: are pliers used to fix and hold tightly the slide on stage.
– Arm: supports the body tube of microscope
– Body tube: maintains the proper distance between the objective and
ocular lenses
– Arm: used for holding when carrying the microscope and it holds the
body tube which bears the lenses.
– Coarse focus adjustment: moves stage up and down a large amount
for coarse focus
– Fine focus adjustment: moves stage up and down a tiny amount for
fine focus
– Objective lenses: focuses and magnifies light coming through the
slide
– Revolving nosepiece: rotates to allow use of different power objectives- Slide: is a transparent pane on which a specimen is placed.
– Eye piece/ocular lens: magnifies image produced by objective lens
– Condenser: It will gather the light from the illuminator and focus it on
the specimen lying on the stage. The function of the condenser is to
focus the light rays from the light source onto the specimen.
– Iris diaphragm lever: This allows the amount of light passing through
the condenser to be regulated to see the object.
All parts of a microscope work together to magnify a specimen to be observed.
Light from the source is focused on the specimen by the condenser lens.
It then enters the objective lens, where it is magnified to produce a real
image. The real image is magnified again by the ocular lens to produce a
virtual image that is seen by the eye.
Advantages and limitations of the light microscope
Magnification: Most light microscopes can magnify a specimens up to a
maximum of X1500.
Resolution: It is the degree at which it is possible to distinguish clearly
between two objects that are very close together. It is the smallest distance
apart that two separate objects can be seen clearly as two objects. The
resolution for the:
– Human eye is 100μ.
– Light microscope is 200 nm
– Electron microscope is 0.20 nm.
The maximum resolution of the light microscope is 200 nm. This means
that if two objects are closer together than 200 nm, they will be seen as
one object. The light microscopes are used widely in education, laboratory
analysis and research. But because they do not have high resolution, they
cannot give detailed information about internal cell structure.
Specimens: a wide range of specimens are viewed using the light microscope.
These include: unicellular organisms, small sections from large plants and
animals, and smear preparation of blood or cheek cells.
Preparation of specimens for the light microscope
A lot of biological materials are not coloured, so you cannot see details. Also,
some material distorts when you try to cut it into thin sections. Preparation of
slides to overcome these problems involves the following steps:
a) Staining: Coloured stains are chemicals that bind on or in the
specimens. This allows the specimens to be seen. Some stains bind
to specific cell structures. For example, acetic orcein stains DNAdark red, while gentian violet stains bacterial cell walls.
b) Sectioning: Specimens are embedded in wax, where thin sections
are then cut without distorting the structure of the specimen. This is
particularly useful for making sections of soft tissue, such as brain.
Safety measures might be taken. Make sure that hands are washed
with soap and warm water after theexperiment. Use a disinfectant
to wipe down all surfaces where bacteria mayhave been deposited
for example. Be sure that some substances and animalsmight be
harmful to the life.
Measuring cells
Cells and organelles can be measured with a microscope by means of an
eyepiece called graticule. This is a transparent scale, usually having 100
divisions (Figure 5.2, A). The eyepiece graticule is placed in the microscope
eyepiece so that it can be seen at the same time as the object to be measured
(Figure 5.2, B). At this figure (Figure 5.2, B), the cell lies between 40 and 60on the scale, so that it measures 20 eyepiece units in diameter (60 – 40 =20).
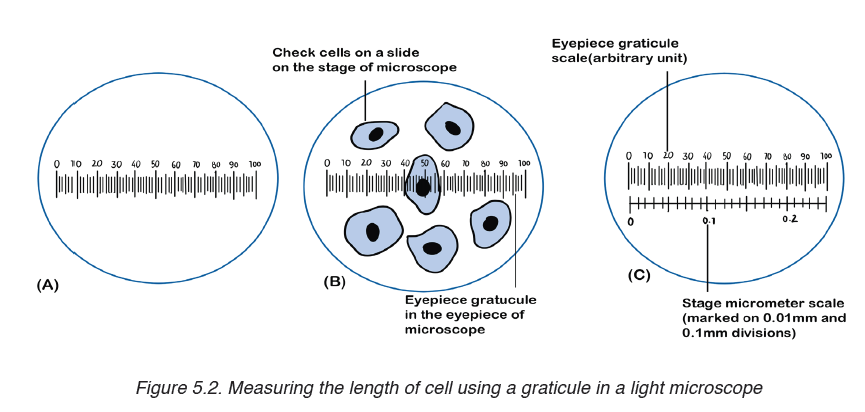
Note that it is not possible to know the actual size of the eyepiece units
until the eyepiece graticule scale is calibrated. To calibrate the eyepiece
graticule scale, a miniature transparent ruler called a stage micrometre scale
is placed on the microscope stage and is brought into focus. This scale may
be fixed onto a glass slide or printed on a transparent film. It commonly has
subdivisions of 0.1 and 0.01 mm. The images of the two scales can then be
superimposed (Figure 5.2, C). If in the eyepiece graticule, 100 units measure
0.25 mm, the value of each eyepiece unit equals
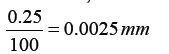
By converting mm to μm, the value of eyepiece equal
The diameter of the cell shown superimposed (Figure 5.2, B) measures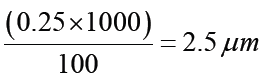
20 eyepiece units. Its actual diameter equals 20 × 2.5 μm = 50 μm. This diameter is greater than that of many human cells because the cell is a flattened epithelial cell.
There is a relationship between the actual size, magnification and image size, where:
Actual size = image size / magnification.
Magnification = image size / actual size.
Image size = Magnification x actual size.
c) Electron microscope
An electron microscopes use a beam of accelerated electrons as a source
of illumination. Electron beams have a much smaller wave length of 0.004
nm, 100 000 times shorter than light wavelength, and therefore have greater
resolving powers and can produce higher effective magnifications than light
microscopes. Electron microscopes are used to study the details of internal
structures (the ultrastructures) of cells. Most modern TEMs can distinguish
objects as small as 0.2nm. This means that they can produce clear images
magnified up to 500,000 times greater than that of the human eye.
There are two types of electron microscopes:
– Transmission electron microscope (TEM).– Scanning electron microscope (SEM)
a) Transmission electron microscope (TEM)
– The electron beam passes through a very thin prepared sample.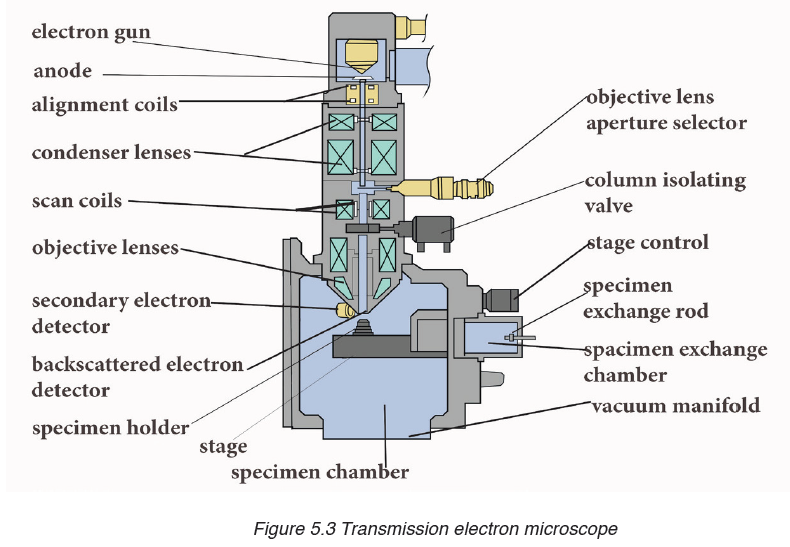
– Electrons pass through the denser parts of the sample less easily, so
giving some contrast.
– The final image produced is two-dimensional.– The magnification possible with a TEM is X500 000.
b) Scanning electron microscope (SEM)
– The electron beam is directed onto a sample. Electrons do not pass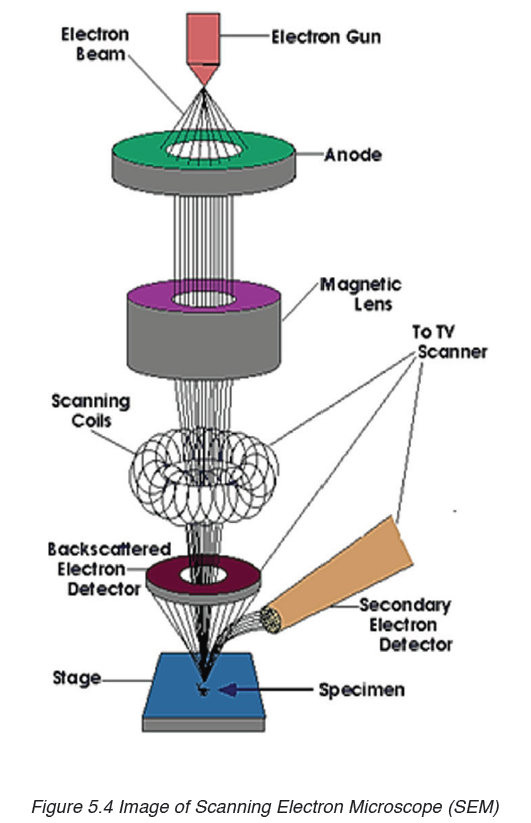
through the specimen.
– They are bounced off the sample.
– The final image produced is a three-dimensional.– The magnification possible with an SEM is about X100 000.
Advantages and disadvantages of the electron microscope
a) Advantages of electron microscope
Light microscope has a higher resolution and are therefore able of a higher
magnification estimated up to 2000 X more than in the light microscope.
Electron microscopes therefore allow for the visualization of too small
structures, such as cell organelles that would normally be not visible by
optical microscopy. The SEM produces a 3D images that can reveal the
detail of cellular and tissue arrangement. This is not possible by using light
microscopes.
b) Disadvantages of electron microscope
Despite the advantages, electron microscope presents a number of
disadvantages and limitations.
– These type of microscope are extremely expensive and the maintenance
costs are high.
– Samples must be completely dry so that it is impossible to observe
living specimens and moving specimens (they are dead).
– It is not possible to observe colors because electrons do not possess a
color. The image is only black-white images/ grey images.
– The energy of the electron beam is very high, the sample is therefore
exposed to high radiation, and therefore not able to live.
– The space requirements are high, so that they may need a whole room.
– Electron beams are deflected by molecules in air, so samples have to
be placed in a vacuum.
Application activity 5.1
1. What is the importance of a light microscope?
2. How can you apply microscope technique rules?
3. Discuss the advantages and disadvantages (limitations) of an
electron microscope.
4. Discuss the advantages and disadvantages of the types of electron
microscopes in medicine and biology research.
5. Make a comparative study between light and electron microscopefocussing on the advantages of each type of microscope.
5.2. Eukaryotic and prokaryotic cell
Activity 5.2
Under microscope, observe mounted slides of bacteria, and plant cells.
Draw and label the parts that are common in both plant and bacterial
specimens
Eukaryotic cells contain membrane-bound organelles, including a true
nucleus enclosed in a nuclear envelope. They include cells of: plants, animals,fungi and protoctista.
Prokaryotes are organisms having cells with no true nuclear envelope.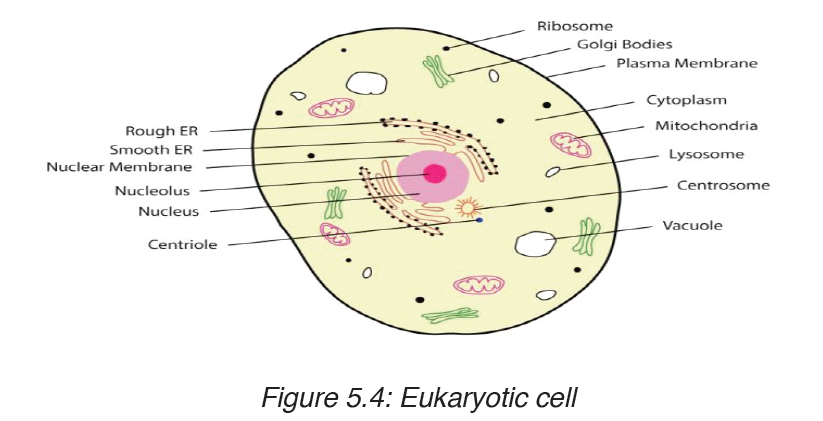
Prokaryotic cells do not contain a nucleus or any other membrane-bound
organelle. Prokaryotes include bacteria and blue-green algae. They makeup the monera kingdom.
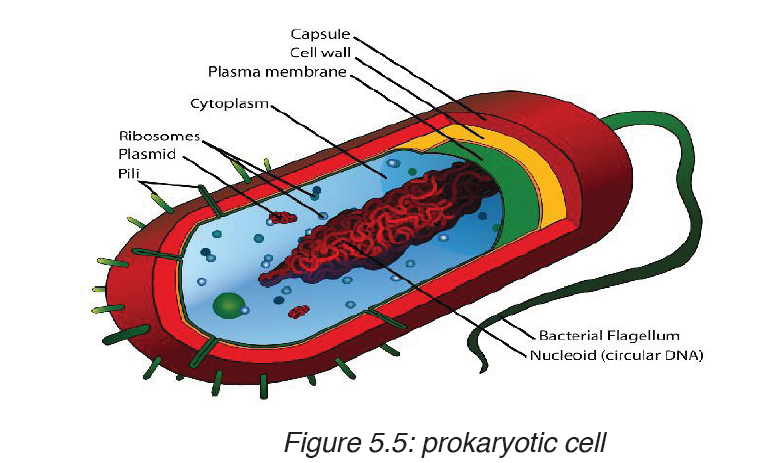
Comparison between prokaryotic and eukaryotic cells
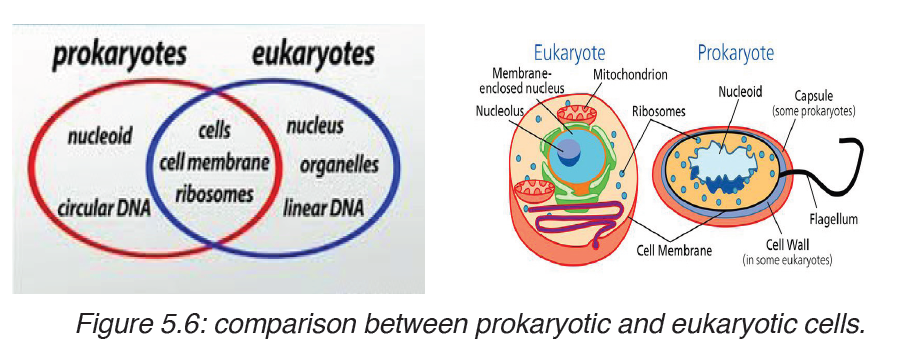
Table 5.3. Comparison between prokaryotic and eukaryotic cells
Application activity 5.2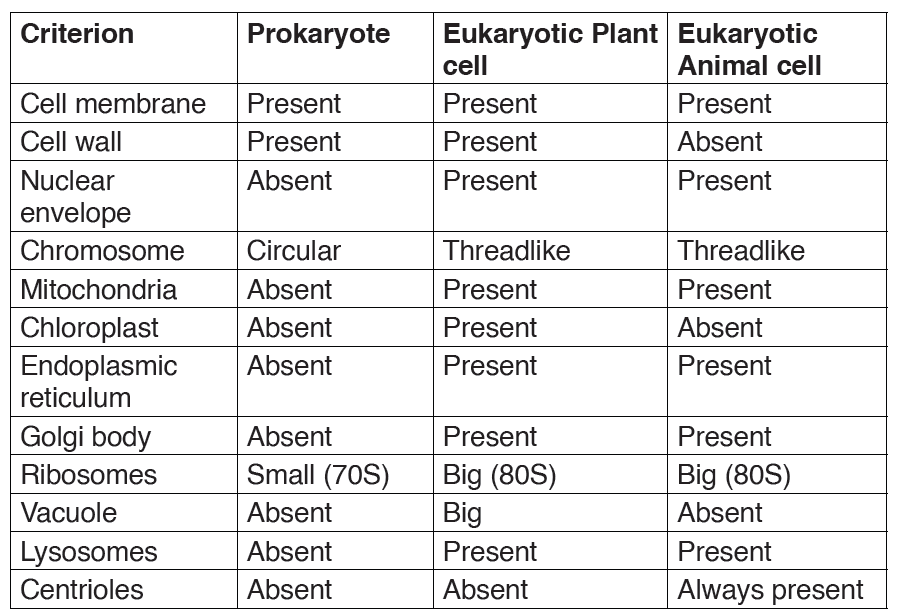
1. Define a prokaryote and eukaryote.
2. In a form of a table differentiate prokaryotic cells from eukaryoticcells.
5.3. Description of plant and animal cell
Activity 5.3
Observe two electron photographs, one containing a plant cell another
an animal cell.Record a description of their features, such as shape andinternal parts.
5.3.1. Structure of animal and plant cells
When viewed under light microscope, the most obvious features observed
are the very large nucleus and a clear cytoplasm surrounded by a cell
membrane. However, under electron microscope, it is possible to identify arange of organelles in plant and animal cells.
Ultrastructure of a plant cell contains different parts like: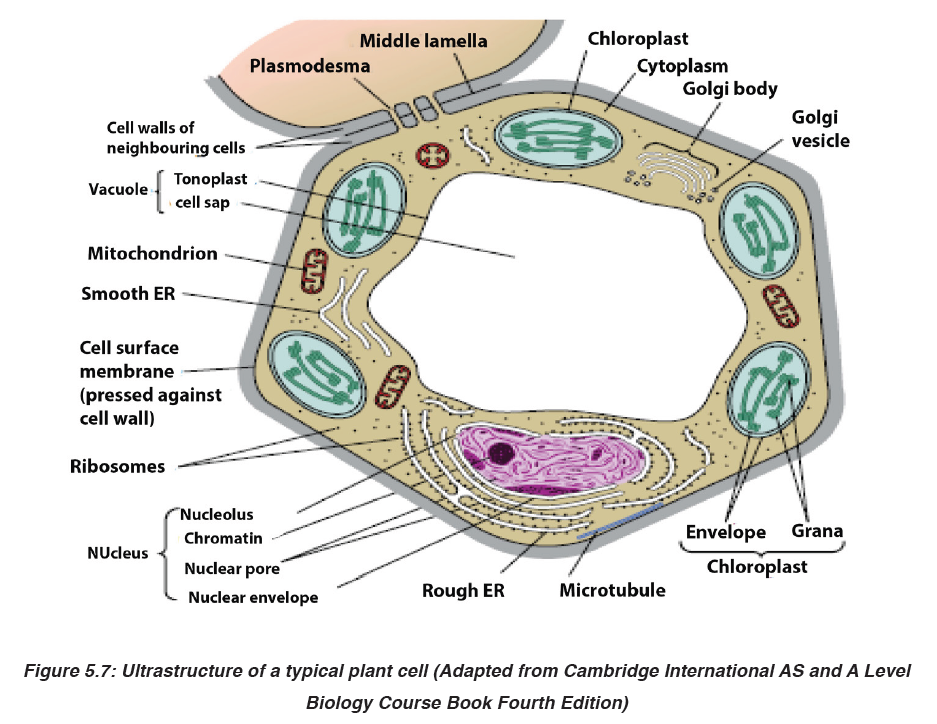
Cell wall, cell membrane, cytoplasm with organelles. Organelles found in the
cytoplasm of a plant cell include: chloroplast, mitochondria, Golgi apparatus,
endoplasmic reticulum, ribosomes, big central vacuole, and the nucleus
which contains chromosomes. The plant cell also has a regular shape, witha relatively bigger size than animal cell.
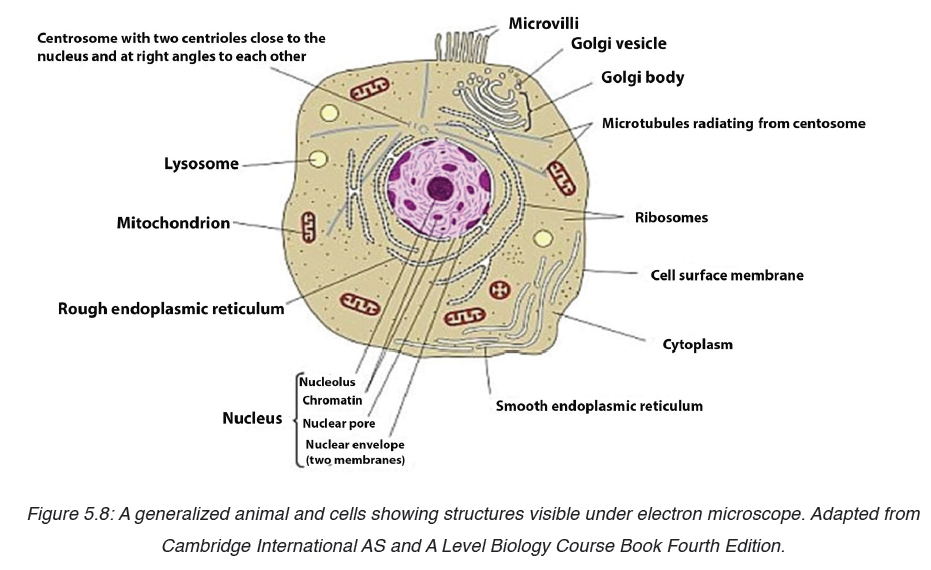 Ultrastructure of an animal cell contains different parts like:The cell nucleus contains nearly all the cell’s DNA with the coded instructions
Ultrastructure of an animal cell contains different parts like:The cell nucleus contains nearly all the cell’s DNA with the coded instructions
Cell membrane, cytoplasm with organelles. Organelles found in the cytoplasm
of an animal cell include: mitochondria, Golgi apparatus, endoplasmic
reticulum, centrioles, ribosomes, small or absent vacuole, and the nucleus
which contains chromosomes. The animal cell also has irregular shape, witha relatively smaller size than a plant cell.
5.3.2. Functions of cell organelles
The previous sections described the structures of plant and animal cells.
This unit will explain the function of each part of both animal cell and plant cell.
1. Organelles surrounded by membranes
a) Nucleus
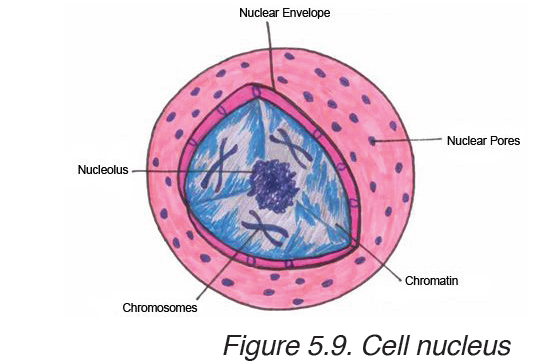
for making proteins and other important molecules. The nucleus is surrounded
by a double nuclear envelope, which allow materials to move into and out
of the nucleus through nuclear pores. The granules found in the nucleus
are called chromatin which consist of DNA bound to protein. When a cell
divides, the chromatin condenses into chromosomes containing the genetic
information that is passed from parents to their offspring. The nucleus
contains a dense spherical structure called nucleolus in which assembly ofribosomes begins. The nucleus controls all activities of the cell.
The ER consists of a series of flattened membrane-bound sacs calledb) Endoplasmic reticulum (ER)
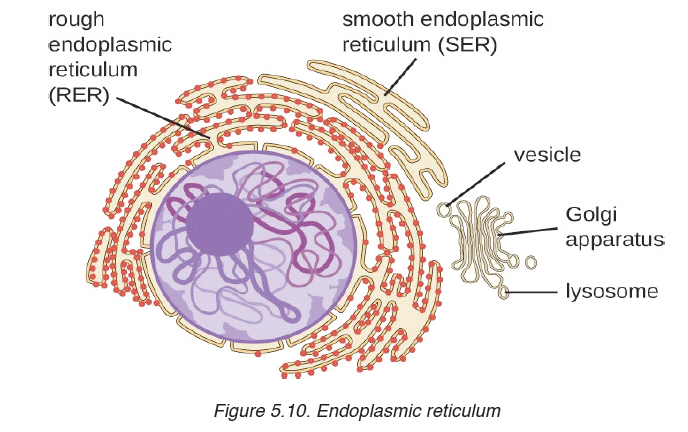
cisternae. The rough ER is surrounded with ribosomes. The rough ER
transports proteins made on attached ribosomes. The smooth ER does not
have ribosomes, and it involves in making lipids that the cell needs.c) Golgi apparatus
The Golgi apparatus is a stack of membrane-bound, flattened sacs, which
receives proteins from the ER and modify them. It may add sugar molecules
to them. The Golgi apparatus then packages the modified substances into
vesicles that can be transported to their final destinations throughout the cellor outside of the cell.
d) Mitochondria
Mitochondrion have two membranes separated by a fluid-filled space. The
inner membrane is highly folded to form cristae. The central part of the
mitochondrion is called matrix. The mitochondria are the site where the
process of cell respiration takes place to produce Adenosine triphosphate
(ATP), a universal energy carrier to be used in cell metabolism.e) Chloroplasts
Chloroplasts are the site of photosynthesis in plant cells. These are found
in plant cells and in cells of some protoctists. They also have two membranes
separated by a fluid-filled space. The inner membrane is continuous, with
thylakoids. A stalk of thylakoids is called a granum (plural: grana).Chlorophyll molecules are present on the thylakoid membranes.
f) Lysosomes
These are spherical sacs surrounded by a single membrane. They contain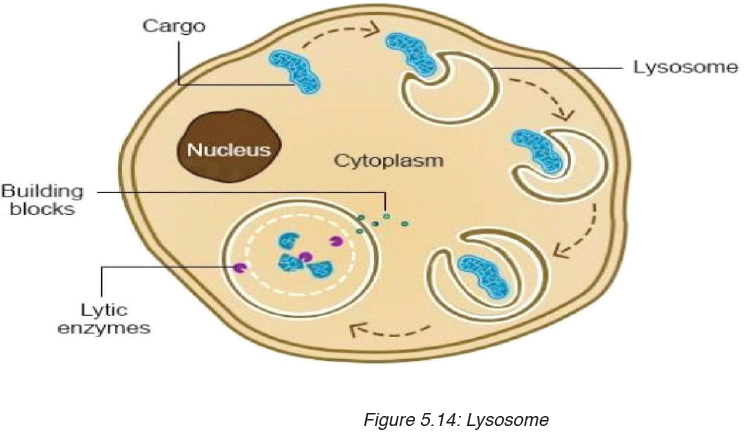
powerful digestive enzymes. Their role is to break down materials such
as white blood cells, and destroy invalid microorganisms. In acrosome,
lysosomes help the sperm to penetrate the egg by breaking down the
material surrounding the egg.Organelles without surrounding membranes
a) Ribosomes
Ribosomes are the site of protein synthesis in the cell. Some are in cytoplasm;
others are bound to ER. Ribosomes consist of two major components (two subunits): the small ribosomal subunit, which reads the RNA, and the large subunit, which joins amino acids to form a polypeptide chain. Each subunit
is composed of one or more ribosomal RNA (rRNA) molecules and a variety of ribosomal proteins (r-protein). Ribosomes act as an assembly line where coded information (mRNA) from the nucleus is used to assemble proteins
from amino acids. Cells that are more active in protein synthesis are often packed with ribosomes.b) Centrioles
Centrioles are small tubes of protein fibres (microtubules), which are involved
in animal cell division. They form fibres, known as spindle, which movechromosomes during nuclear division.
c) Vacuole
A vacuole is a saclike structure that is used to store materials such as water,
salts, proteins, and carbohydrates. In many plant cells there is a single, and
large central vacuole filled with liquid. The pressure of central vacuole in this
cells makes it possible for plants to support heavy structures such as leaves
and flowers. Some animals and some unicellular organisms contain
contractile vacuoles which contract rhythmically to pump excess water outof the cell.
d) Cytoskeleton
Cytoskeleton is a network of protein filaments that helps the cell to maintain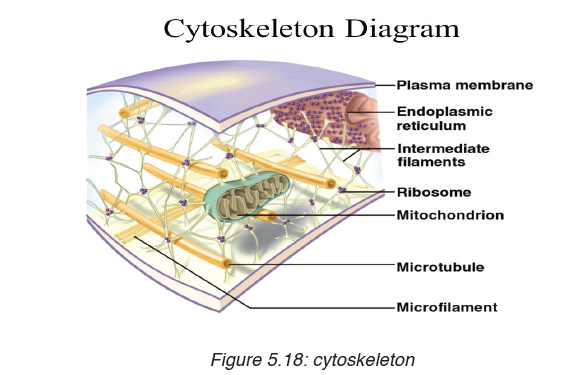
its shape. It is also involved in movement. The main components of
cytoskeleton are microfilaments made of a protein called actin, microtubules
made of a protein called tubulins, and intermediate filaments.
Did you realize that a cell has many organelles with different functions? But
all are important and work together for the survival of the cell. Likewise, in
human society, we can work together in peace and harmony despite of the
difference of our abilities, disabilities or physical appearance.
The structure of the cell membrane is based on fluid mosaic model. The
term fluid mosaic is used to describe the molecular arrangements in
membranes. The main features of the fluid mosaic model are:
– A bilayer of phospholipid molecules forming the basic structure.
– Many protein molecules floating in the phospholipid bilayer. Some are
free, others are bound to other components or to structures within the
cell.
– Some extrinsic proteins are partially embedded in the bilayer on the
inside or the outside face while other intrinsic proteins are completelyspanning the bilayer.
The basic structure of phospholipids has two parts: hydrophilic part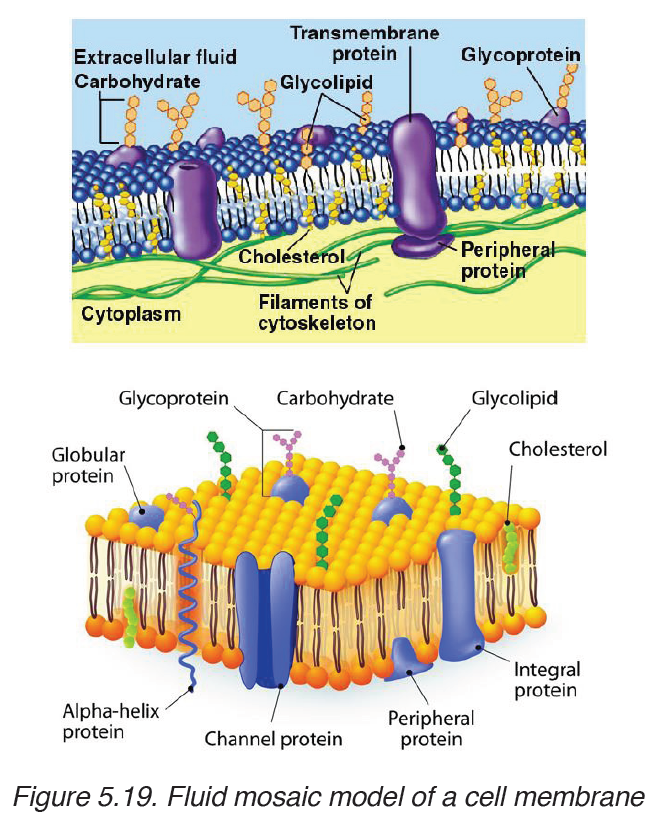
which means water loving and which consists of the phosphate head,and
hydrophobic part which means water hating and which consist of fatty acids.
If phospholipid molecules are completely surrounded by water, a bilayer can
form phosphate heads on each side of the bilayer stick into water, while the
hydrophobic fatty acid tails point towards each other.
Types of protein found in the cell membrane
Various types of proteins are found in the cell membrane. They include:
– Carrier proteins: They fix or attach molecules and facilitate them to
cross through the cell membrane by active transport.
– Channel proteins: they act as pores by pumping substances and
allow facilitated diffusion.
– Receptors: They act as receptors of enzymes and neurotransmitters
– Glycoproteins: They act as receptor proteins which recognize the– Integrated proteins: They define the shape of the cellsubstance to pass through the membrane
– Immune proteins (antigens): found in the membrane on the red blood
cell, they recognize the antibodies.
5.3.3. Roles of different components of cell membrane
a) Cholesterol
– Gives the membranes of some eukaryotic cells the mechanical stability.
– It fits between fatty acid tails and helps make the barrier more complete,
so substances like water molecules and ions cannot pass easily and
directly through the membrane.
b) Channel proteins
– Allow the movement of some substances across the membrane.
– Large molecules like glucose enter and leave the cell using these
protein channels.
c) Carrier proteins
– Actively move some substances across the cell membrane. For
example, magnesium and other mineral ions are actively pumped into
the roots hair cells from the surrounding soil.
– Nitrate ions are actively transported into xylem vessels of plants
d) Receptor sites
– Allow hormones to bind with the cell so that a cell response can be
carried out.
– Glycoproteins and glycolipids may be involved in cells signaling that
they are self to allow recognition by the immune system.
– Some hormone receptors are glycoprotein and some are glycolipid.
e) Enzymes and coenzymes
– Some reactions including metabolic processes in photosynthesis take
place in membranes of chloroplasts.
– Some stages of respiration take place in membranes of mitochondria,
where Enzymes and coenzymes may be bound to these membranes.
– The more membrane there is, the more enzymes and coenzymes it
can hold and this helps to explain why mitochondrial inner membranes
are folded to form cristae, and why chloroplasts contain many stacksof membranes called thylakoids.
Properties of the cell membrane
– It is mainly made of lipids, proteins and carbohydrates.
– It is semi-permeable or partially permeable and allow some substances
to pass through but prevents others to cross depending on their size,
charges and polarity.
– It is positively charged outside and negatively charged inside and has
a hydrophilic pole and a hydrophobic pole
– It is a bilayer, sensitive, flexible, has inorganic ions and its proteins
and lipids may be mobile and contains different types of enzymes and
coenzymes.
– It is perforated of pores and recognizes chemicals messengers
including hormones and neurotransmitters.
Functions of a cell membrane
– The cell membrane acts as a selective barrier at the surface of the cell,
and controls the exchange between the cell and its environment.
– The membrane proteins act as pores where chemicals pass through.
– Glycocalyx including glycoprotein and glycolipid are involved in the
cell protection, the process by which cell adhesions are brought about
and also in the uptake and entry of selected substances.
5.3.4. Comparison between animal and plant cell
By referring to the diagrams which show the structure of animal cell and
plant cell, similarities and differences between animal cell and plant cell can
be identified.
a) Similarities between animal cell and plant cell
– Both animal and plant cells have a cell membrane, a cytoplasm and a
nucleus.
– Both animal and plant cells have a true nucleus bounded by an
envelope.
– Both animal and plant cells have mitochondria, Golgi apparatus,
Reticulum endoplasmic, lysosome, big ribosomes (80S), peroxisome,
microtubules.
– The protoplasm is enveloped by a bounding cell membrane called
plasmalemma.
– The protoplasm is composed of a dense round structure called nucleus
which is surrounded by a less dense jelly-like cytoplasm.
– The cytoplasm contains numerous organelles such as mitochondria,
Golgi bodies, secretory vacuoles, endoplasmic reticulum.
– Mitochondria appear as very small darkly staining, rod-like structures.
– Golgi bodies are semi-transparent irregular, and membrane bound
structures.
– Vacuoles contain secretions, food- particles, or decomposing organic
substances.
– Chemically, both plant and animal cells are made up of water (80-90%),
proteins (7-13%), lipids (1-2%), carbohydrates (1-1.5%) and inorganic
salts.
– The cytoplasmic organelles are suspended in a semi-fluid jelly matrix
called cytosol.
b) Difference between animal and plant cellsTable 5.4: comparison between animal and plant cell
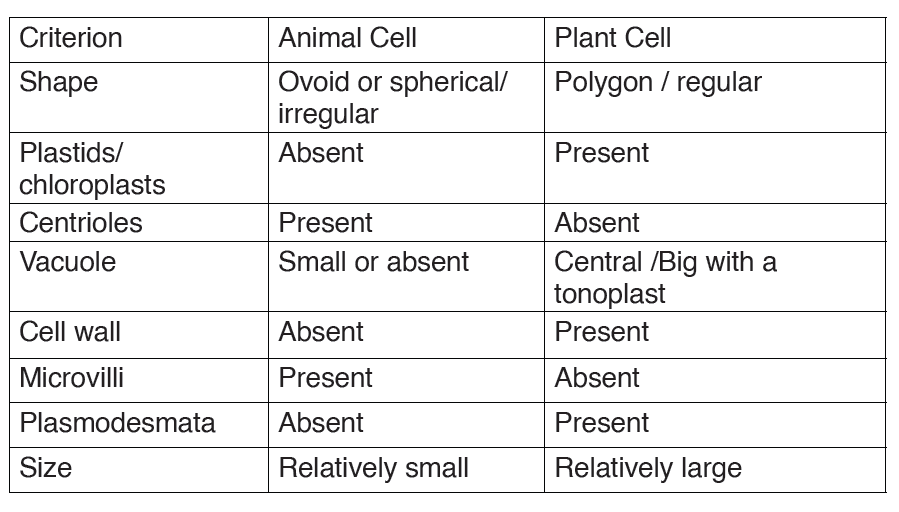
Application activity 5.3
1. Describe the structure of:
a) Animal cell
b) Plant cell
2. The diagram below shows the structures which would be visible ina plant cell examined under an electron microscope.
a) Identify the parts labelled in this plant cell and: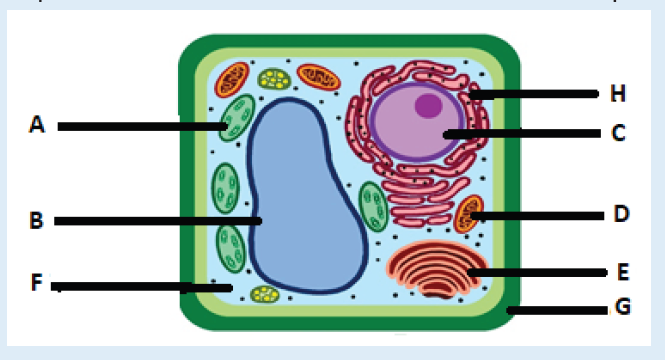
b) State one function for A, B, C, E,D,G, F, and H
c) Explain two functions of the cytoskeleton?
3. What structures do both animal and plant cells have in common?
4. Answer by true or false:
a) All organelles of a cell are well seen through a compound light
microscope.
b) Chloroplasts are found in both animal and plant cells.
c) Mitochondria are found only in animal cells.
5. With your pencil, draw and show the full ultrastructure of bothanimal and plant cells.
5.4 Specialized cells
Activity 5.4
By analyzing the figure, identify the cells on the figure and explain the importanceof those cells
5.4.1: Specialized animal cells and their functions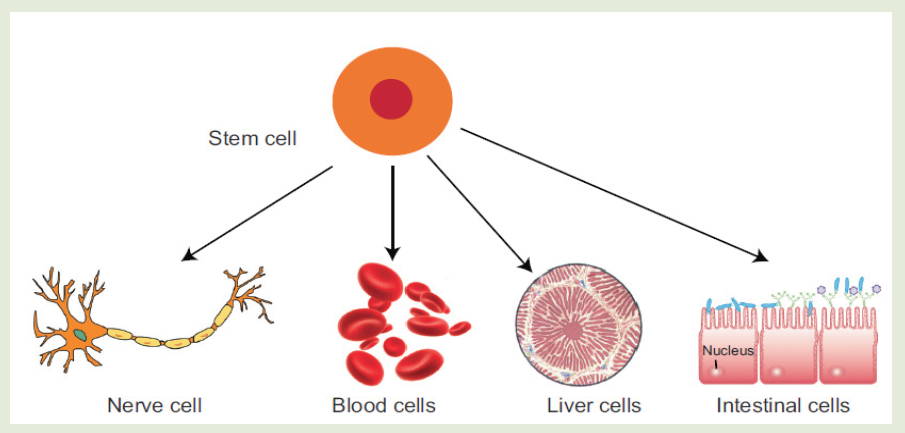
Differentiation refers to the changes occurring in cells of a multicellular
organism so that each different type of cell becomes specialized to
perform a specific function.a) Red blood cells
All blood cells are produced from undifferentiated stem cells in the bone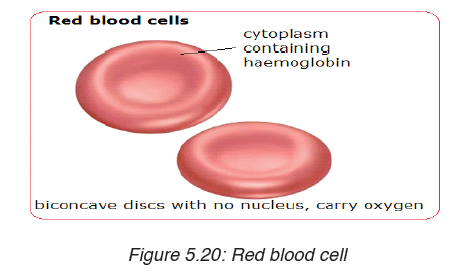
marrow but the cells destined to become erythrocytes (red blood cells)
lose their nucleus, mitochondria, Golgi apparatus and rough endoplasmic
reticulum. They are packed full of the protein called haemoglobin. The shape
of this cells change so that they become biconcave discs, and they are thenable to transport Oxygen in the body.
b) Sperm cell
Sperm cells are specialized to fertilize the egg. Its specialization involves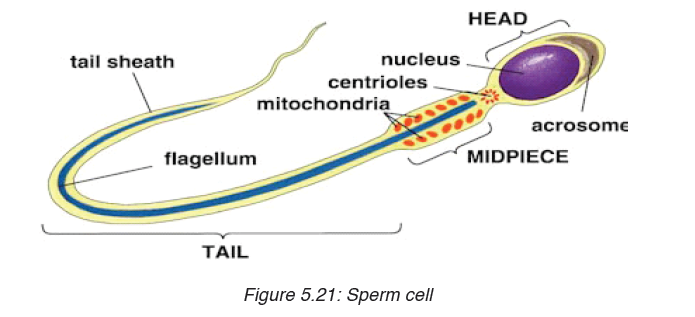
many changes in shape and organelles content.
By shape: the sperm cells are very small, long and thin to help them to move
easily, and they have a flagellum which helps them to move up the uterine
tract towards the egg.
By organelles content: sperm cells contain numerous mitochondria
which generate much energy for their movement. Their acrosome releases
specialized lysosomes contains many enzymes on the outside of the egg.
These enzymes lyse the wall of the egg, and facilitate the sperm nucleus to
penetrate easily. In content, the sperm cell nucleus contains the half number
of chromosomes of the germ cell in order to fulfil its role as a gamete offertilizing the egg.
c) Nerve cells
Nerve cells also known as a neuron are specialized cells to carry nervous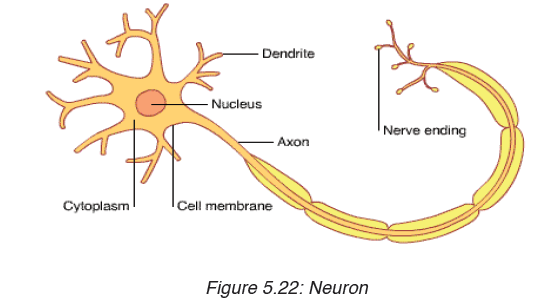
information in the body. It is an electrically excitable cell that receives,
processes, and transmits information through electrical and chemical
signals. These signals between neurons occur via specialized connections
called synapses. Specialized animal cells have different functions. Some ofthem are summarized in the following table.
Table 5.5: Specialized animal cells and their functions.
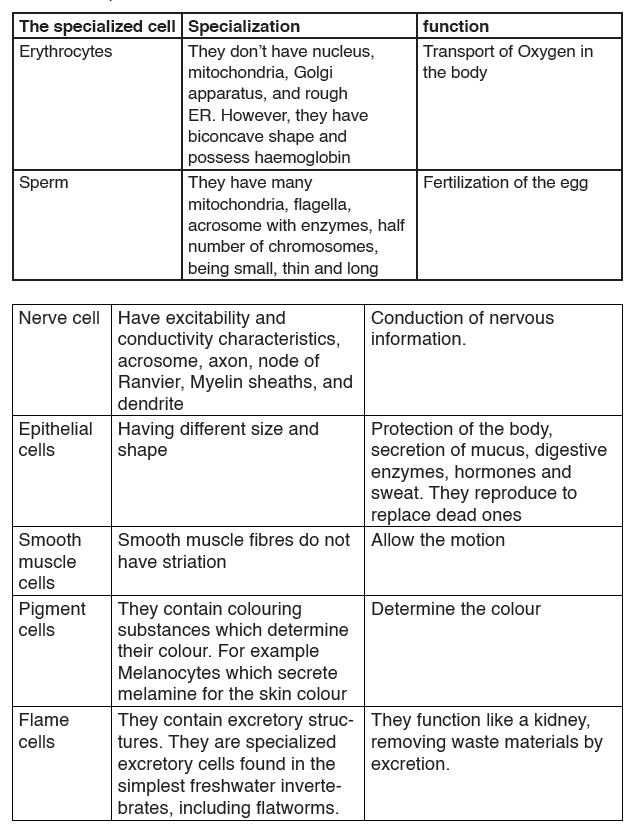
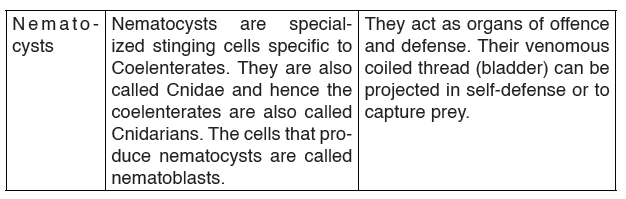
5.5.2: Specialized plant cells and their functions
a) Root hair cells
The root hair cells have hair-like projection from their surface out into the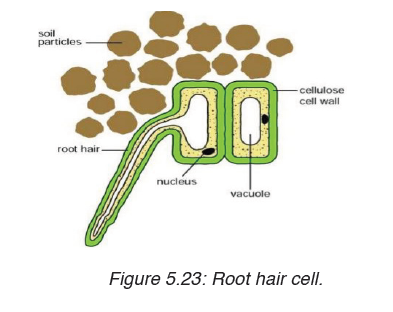
soil. This increase the surface area of root available to absorb water and
minerals from the soil.Root hairs are tip-growing cells that originate from
epidermal cells called trichoblasts. Their role is to extend the surface area ofthe root to facilitate absorption of mineral nutrients and water.
b) Palisade cells
Palisade cells are plant cells located in leaves, right below the epidermis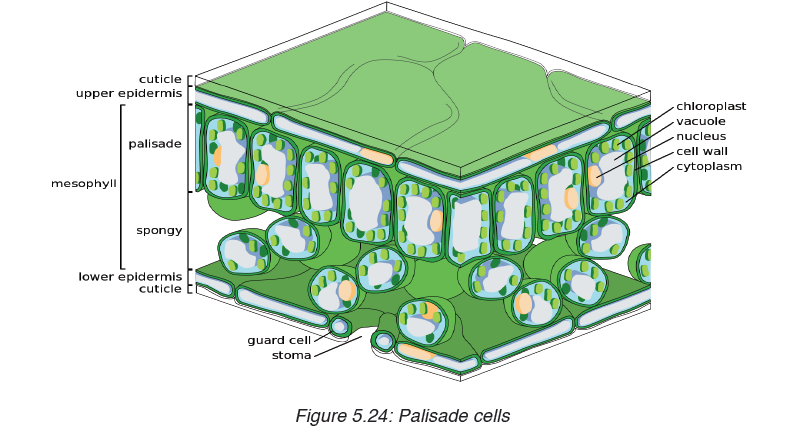
and cuticle. They are vertically elongated, a different shape from the spongy
mesophyll cells beneath them in the leaf. Their big number of chloroplasts
allow them to absorb a major portion of the light energy used by the leaf inthe process of photosynthesis.
c) Parenchyma cells:
Parenchyma cells are alive at maturity. They function in storage,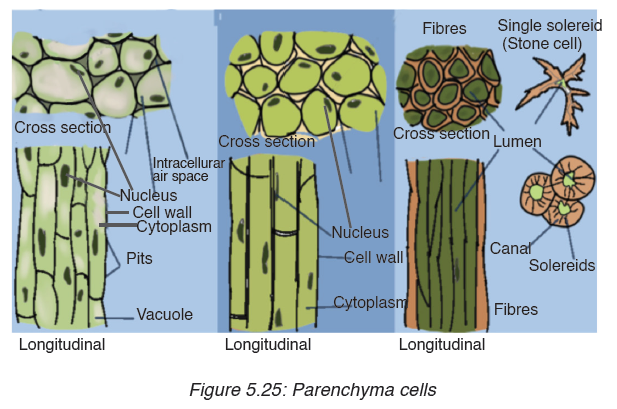
photosynthesis, and as the bulk of ground and vascular tissues. Palisade
parenchyma cells are elongated cells located in many leaves just below
the epidermis. Parenchyma is composed of relatively simple, undifferentiated
parenchyma cells. In most plants, metabolic activity such as respiration,
digestion, and photosynthesis occurs in these cells because they retain
their protoplasts(the cytoplasm, nucleus, and cell organelles) that carry out
these functions. Parenchyma cells are capable of cell division, even afterthey have differentiated into the mature form.
d) Guard cells
Guard cells are cells surrounding each stoma. They help to regulate the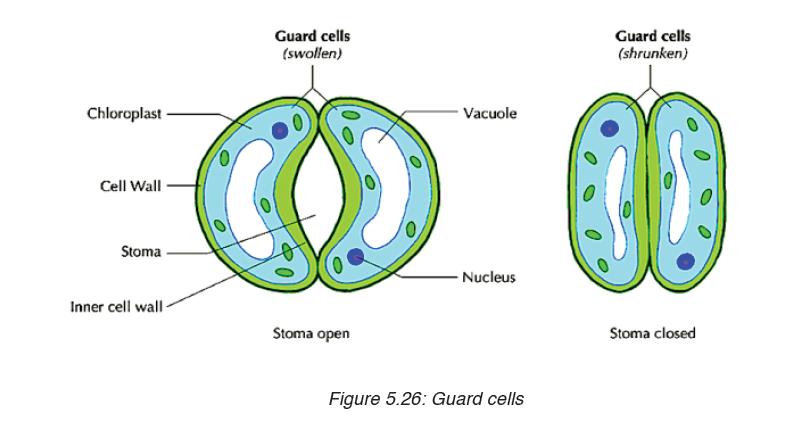
rate of transpiration by opening and closing the stomata. Guard cells are
specialized cells in the epidermis of leaves, stems and other organs that
are used to control gas exchange. They are produced in pairs with a gap
between them that forms a stomatal pore.
Application activity 5.4
1. Explain why differentiation to produce erythrocytes involves a
change in shape.
2. Red blood cells cannot divide as they have no nucleus. State two
other biological processes that red blood cells cannot carry out.
3. Discuss the advantages of cell specialization for living things
SKILLS LAB
After finishing the studies, assuming that the understood the unit 5, student teachers
will be able to use microscope to investigate the presence of
microorganisms that can cause diseases in food and beverages. This will helpin prevention of diseases
End unit assessment 5
Section A. Multiple choice questions
1. Which organelle converts the chemical energy in food into a form that
cells can use?
a) Chromosome
b) Chloroplast
c) Nucleus
d) Mitochondrion
2. The cell membranes are constructed mainly of:
a) Carbohydrate gates
b) Protein pumps
c) Lipid bilayer
d) Free-moving proteins
3. In many cells, the structure that controls the cell’s activities is the:
a) Nucleus
b) Nucleolus
c) Cell membrane
d) Organelle
4. Despite differences in size and shape, all cells have cytoplasm and a
a) Cell wall
b) Cell membrane
c) Mitochondria
d) Nucleus
5. If a cell of an organism contains a nucleus, the organism is a (an)
a) Plant
b) Eukaryote
c) Animald) Prokaryote
Section B: Questions with short answers
1. How does a cell membrane differ from a cell wall?
2. What is meant by the fluid mosaic model of the cell membrane?
3. State the properties of the cell membrane.
4. Discuss at least 4 types of the proteins in the cell membrane and their roles.
5. Explain why muscle cells contain a lot of mitochondria, whereas most fat storage cells do not.
6. What kind of information is contained in chromosomes?
7. You examine an unknown cell under the microscope and discover
that the cell contains chloroplasts. What type of organism could you decide that the cell came from?
Section C: Essay questions
1. Describe the structure and function of the cell membrane and cell wall.
2. Describe the basic structure of the cell membrane.
3. Explain two common characteristics of chloroplasts and mitochondria. Consider both function and membrane structure.4. The diagram below shows the structure of a liver cell as seen using an electron microscope.
a) Name the parts labelled A, B, C and D.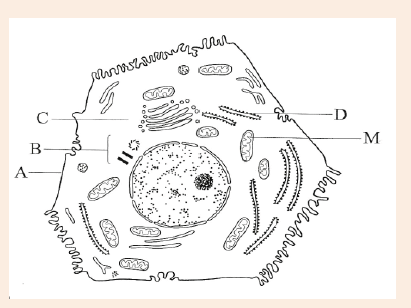
b) The magnification of the diagram above is 12 000. Calculate the
actual length of the mitochondrion labelled M, giving your answer
in μm. Show your working.
c) Explain the advantage to have a division of labour between differentcells in the body.
UNIT 6: CELL AND NUCLEAR DIVISION
Key unit competence
Describe the stages of the cell cycle and explain the significance
of cell and nuclear division in organisms.
Introductory Activity
Analyze the figure of the house built in bricks below. The bricks came from
the valley where they are made, and then have been used to build this
house.
1. Is it possible to build a house like this by using only one brick?
2. Explain what happened for this house to be built at this level?
3. Link the analogous example of building a house by using bricks
with growth of and increasing in size of a human body from the
body size of a one day new baby to the body size of adult person.
4. Is it possible for the body of an adult person to be made by only
one cell?
5. Where are cells which make the adult human body come from?
6. How are they produced?
6.1. Cell cycle
Activity 6.1
Visit your smart classroom/library using search engine or textbooks and
discuss more information about the main phases of the cell cycle and
present them on manila paper.
The cell cycle is a series of events of cellular growth and division that has
five phases such as:
– The first growth phase (G1),
– The synthesis phase (S),
– The second growth phase (G2),
– Mitosis (M),
– Cytokinesis.
The three first phases (G1, S, and G2) of the cell cycle collectively are knownas interphase (the phase between two mitotic divisions).
During the cell cycle, the cell grows, grows its DNA and divides into two daughter cells.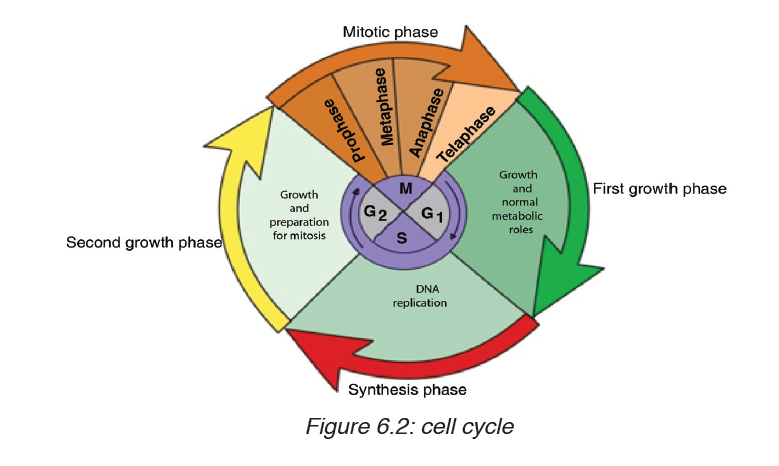
6.1.1. The first growth phase (G1)
During this phase, a cell undergoes rapid growth and the cell performs its
routine functions. The cell spends most of its life in the G1 phase. If the cell
is not dividing, it remains in this phase. The time taken for the completion of
G1 phase varies among species and the type of cells. But on an average, it
takes around 11 hours for the completion of this phase.
6.1.2. The synthesis phase (S)
DNA synthesis or DNA replication takes place in this phase. The S phase
takes around 8 hours to complete.
6.1.3. The second growth phase (G2)
It is the short period, in which the cell continues to grow, making proteins
and manufacturing many organelles necessary for cell division. This phase
serves as an intermediate between the synthesis phase and the mitotic
phase. It takes around four hours to complete
6.1.4. Mitosis
This is the phase of nuclear division in which one nucleus divides through
four phases (prophase, metaphase, anaphase and telophase) and becomes
two nuclei.
6.1.5. Cytokinesis
In this phase, the cytoplasm divides in half, producing two daughter cells,each containing a complete set of genetic material.
Application activity 6.1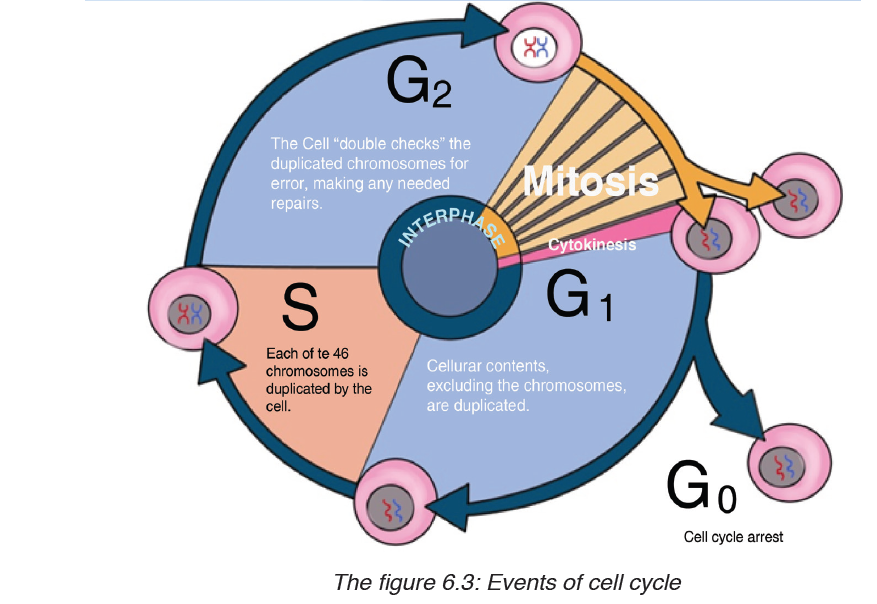
1. What do you understand by the cell cycle?
2. Describe all phases of the cell cycle.
3. Predict what may happen if the cytokinesis does not take place in
the succession of cell cycle.
6. 2. Mitosis and meiosis: stages and results
Activity 6.2
Using your knowledge from ordinary level, textbooks internet, discuss and
present the phases of mitosis and meiosis on manila papers.
6.2.1. Mitosis
Mitosis is a type of cell division that produces two daughter cells having the
same number and kind of chromosomes as the mother cell. It takes place
only in eukaryotes, where the nuclear division (karyokinesis) occurs. This
phase takes around 1 hour to complete. The mitotic cell division is more
rapid at the meristematic region of plant and root tip as it is the growing
region of the plant. The mitotic phase is divided into four steps that includeProphase, Metaphase, Anaphase and Telophase.
6.2.1.1. Prophase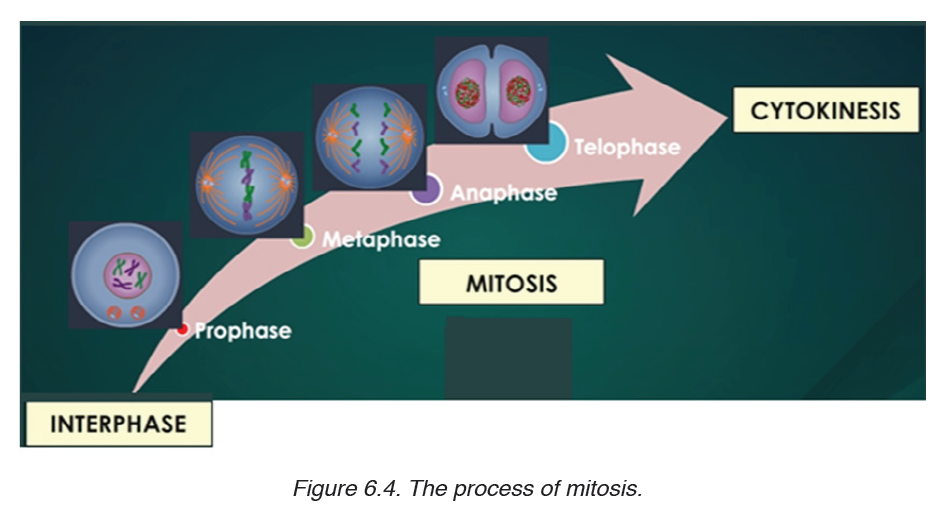
Prophase is the first and longest phase of mitosis. During prophase:
– The DNA and histone proteins coils up into visible chromosomes, each
made up of two sister chromatids held together by the centromere.
– The nucleus disappears as the nuclear envelope and nucleolus break apart.
– The centrioles begin to move to opposite ends, or poles, of the cell.
– As the centrioles migrate, the fiber-like spindle begins to elongate
between the centrioles. In plant cells, the spindle forms without
centrioles. The spindle plays an essential role moving chromosomesand in the separation of sister chromatids.
6.2.1.2. Metaphase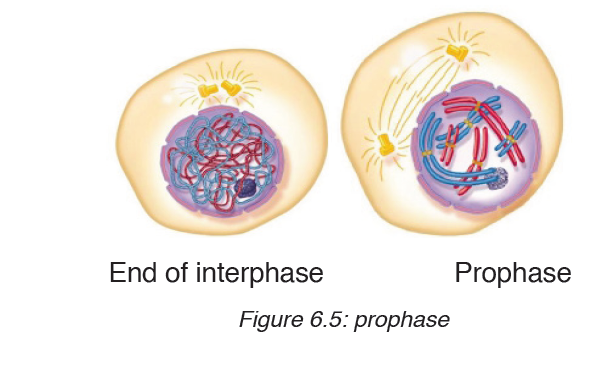
During metaphase, the spindle which attaches to the centromere of each
chromosome helps the chromosomes to line up at the center of the cell by
forming the equatorial plate also known as the metaphase plate. Each sister
chromatid is attached to a separate spindle fiber, with one fiber extending toone pole, and the other fiber extending to the other pole.
6.2.1.3. Anaphase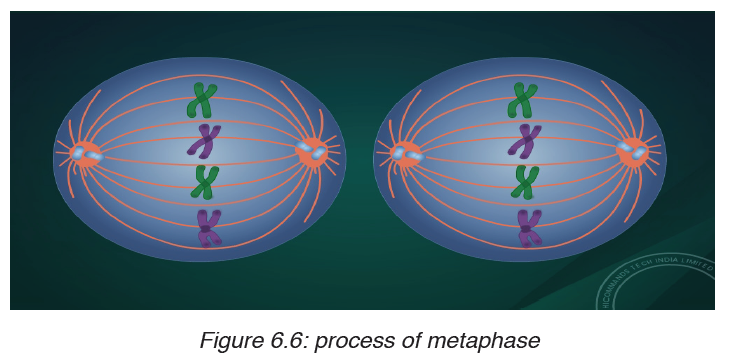
During Anaphase:
– Centromeres divide,
– The sister chromatids separate and pulled apart by the shortening of
the spindles,
– One sister chromatid moves to one pole of the cell, and the other sister
chromatid moves to the opposite pole, (sister chromatids take the name
of chromosomes as soon as they separate).
– At the end of anaphase, each pole of the cell has a complete set of
chromosomes, identical to the amount of DNA at the beginning of G1of the cell cycle.
6.2.1.4. Telophase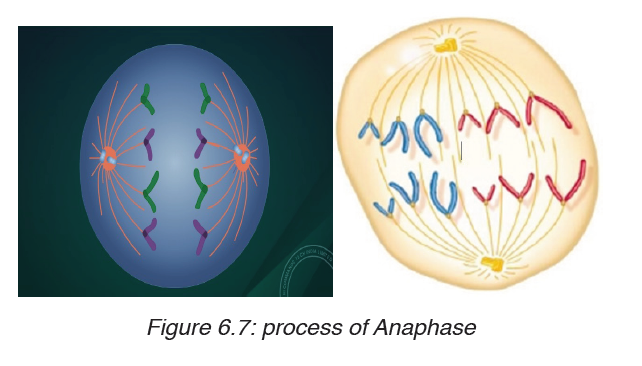
During telophase which is the opposite of prophase:
– The spindle disappears,
– Formation of two nuclei,– The nuclear envelopes surround the two nuclei.
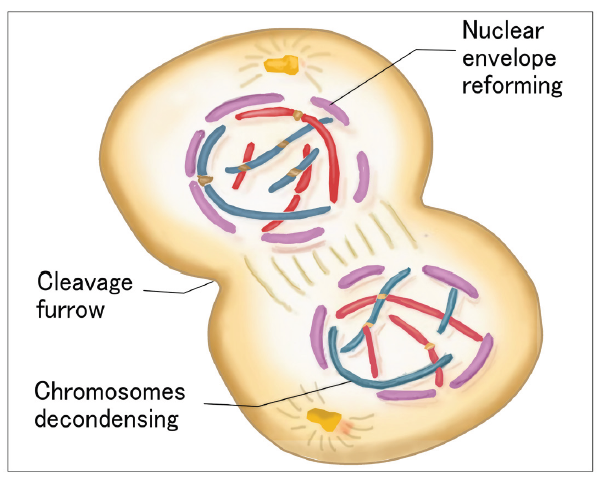
Cytokinesis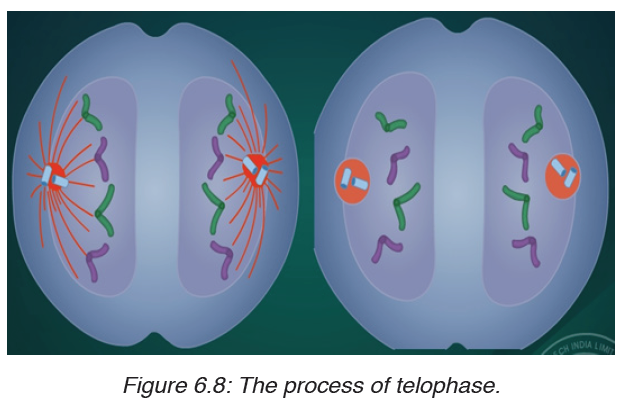
During this phase, the cytoplasm divides in half, producing two daughtercells, each containing a complete set of genetic material as the mother cell.
Table 6.1: A table showing phases of cell cycle with one important event at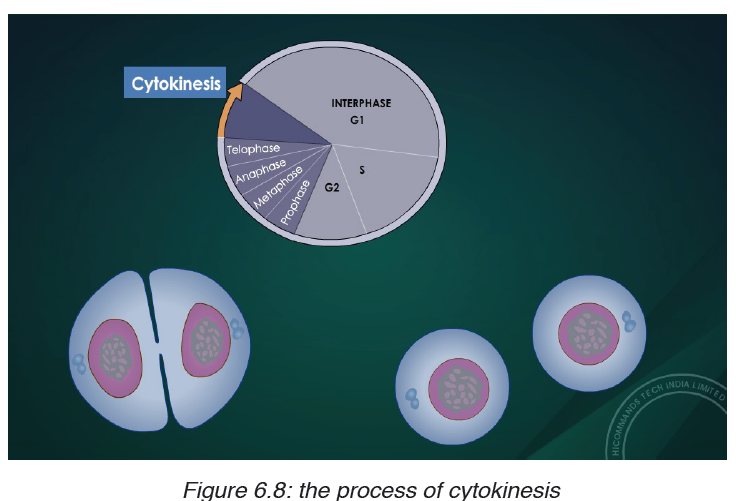
each phase
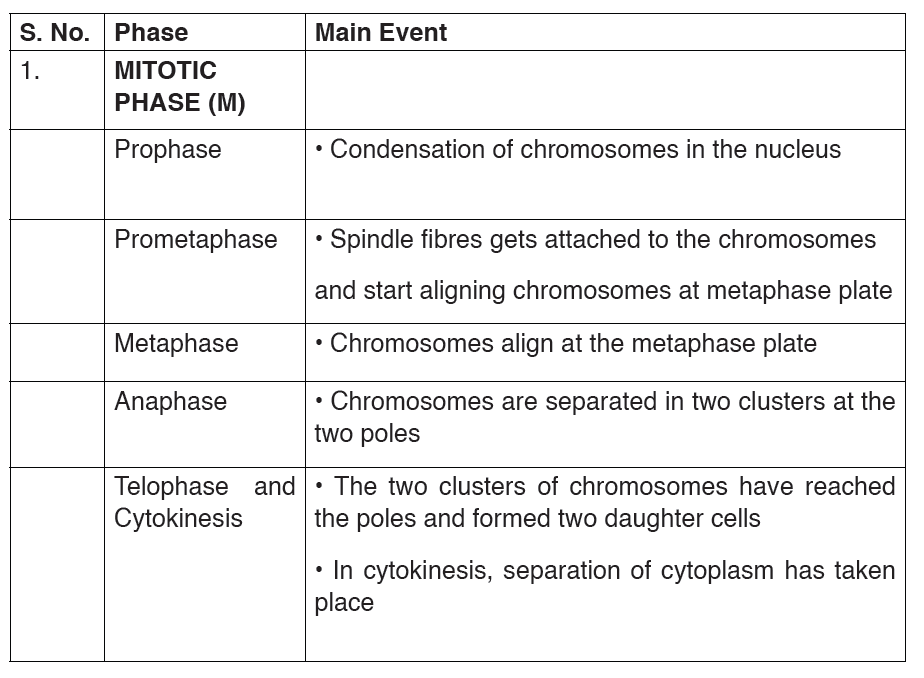
6.2.2 Meiosis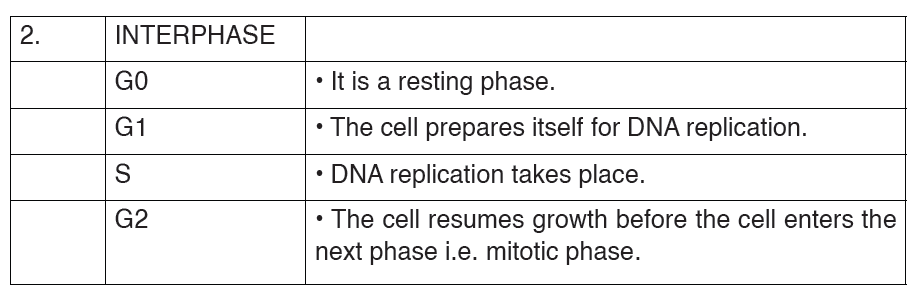
In sexual reproduction, meiosis produces haploid gametes that fuse together
during fertilization to produce a diploid zygote. Meiosis involves two divisions
without an interphase in between, starting with one diploid cell and generating
four haploid cells. Each division, named meiosis I and meiosis II, has fourstages: prophase, metaphase, anaphase, and telophase.
During meiosis the number of chromosomes is reduced from a diploid number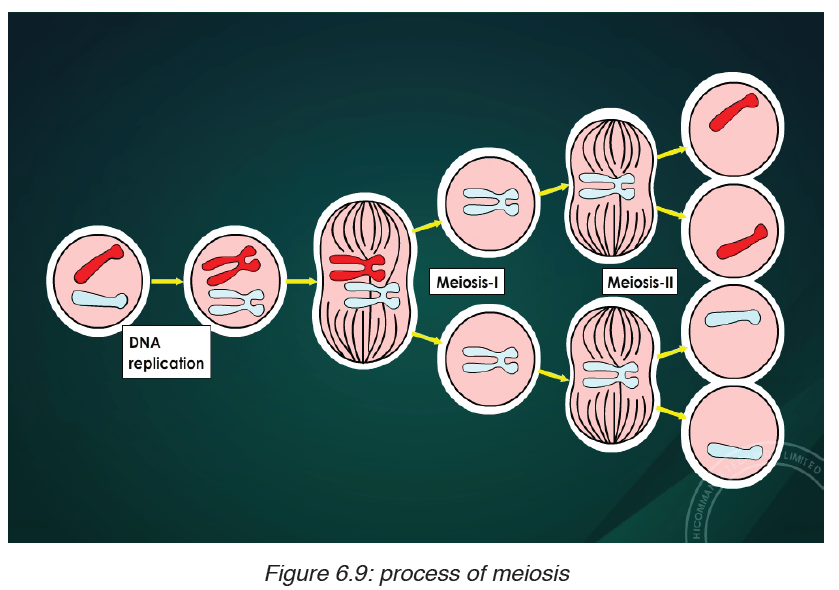
(2n) to a haploid number. During fertilization, haploid gametes come
together to form a diploid zygote, and the original number of chromosomes
(2n) is restored.
8 Stages of meiosis are summarized below:
Prophase I: Prophase I is very similar to prophase of mitosis, but with one
very significant difference. In prophase I, the nuclear envelope breaks down, the chromosomes condense, and the centrioles begin to migrate to opposite
poles of the cell, with the spindle fibers growing between them.
During this time, the homologous chromosomes form pairs. These
homologous chromosomes line up gene-for-gene down their entire length,
allowing the crossing-over to occur.
This process permits the exchange of genetic material between maternal
and paternal chromosomes. Thus, crossing-over results in genetic
recombination by producing a new mixture of genetic material. This is an
important step in creating genetic variation.
Metaphase I: In metaphase I, the 23 pairs of homologous chromosomes
line up along the equator of the cell.
Anaphase I: During anaphase I the spindle fibres shorten, and the
homologous chromosome pairs are separated from each other. One
chromosome from each pair moves toward one pole, with the other moving
toward the other pole, resulting in a nucleus with 23 chromosomes at one
pole and the other 23 at the other pole. The sister chromatids remain attached
at the centromere.
Telophase I: The spindle fibres disappear and the nucleus reforms. This is
quickly followed by cytokinesis and the formation of two haploid cells, each
with a unique combination of chromosomes, some from the father and the
rest from the mother.
After cytokinesis, both cells immediately enter meiosis II, without replication
of the DNA. Meiosis I is described as reductional division as it reduces by
half the number of chromosomes of the mother cell. Meiosis II is equational
division, and it occurs like a normal mitosis, separating the sister chromatids
from each other.
Prophase II: Once again the nuclear membrane breaks down, and the
spindle begins to reform as the centrioles move to opposite sides of the cell.
Metaphase II: The 23 chromosomes, each made out of two sister chromatids,
occupy the equator of the cell.
Anaphase II: The centromere divides and sister chromatids are separated
and move to opposite poles of the cell. As the chromatids separate, each is
known as a chromosome. Anaphase II results in a cell with 23 chromosomes
at each end of the cell; each chromosome contains half as much genetic
material as at the start of anaphase II.
Telophase II: The nucleus reforms and the spindle fibers break down. Each
cell undergoes cytokinesis, producing four haploid cells, each with a uniquecombination of genes and chromosomes.
Comparison of Mitosis and Meiosis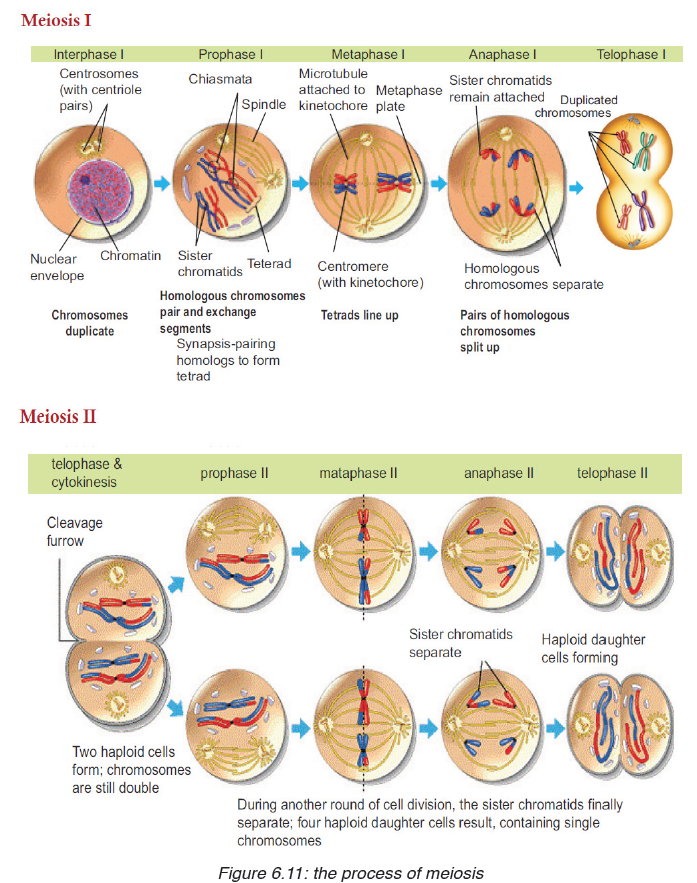
Table 6.2: Comparison between mitosis and meiosis
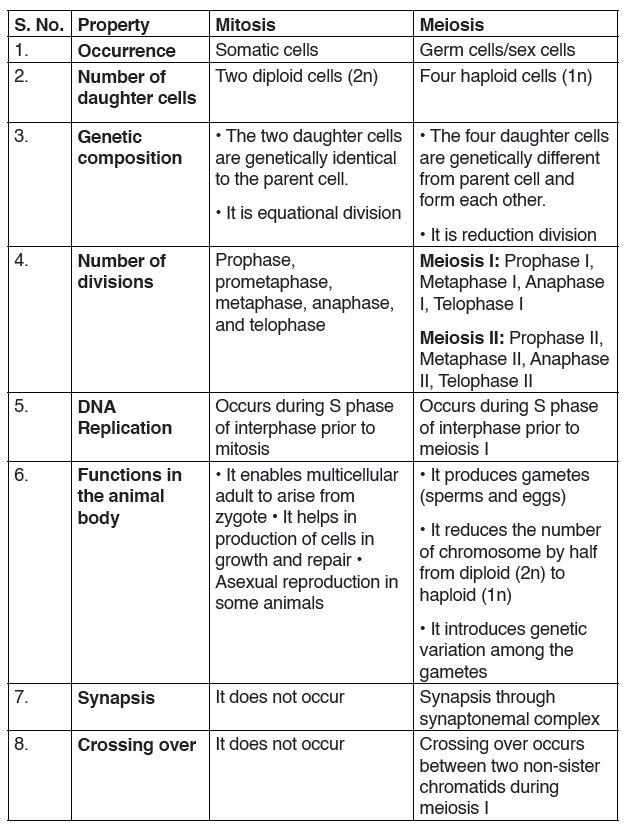
Haploid and diploid conditions of the cell
During the formation of gametes,the number ofchromosomes is reduced
by half, and returned to the full amount when the two gametes fuse during
fertilization. The cells of human beings, most animals and many plants
(except for their gametes) are diploid abbreviated as 2n. They contain two
sets of chromosomes in their nuclei. The haploid cells have only one set of
chromosomes, abbreviated as n. Ploidy is a term referring to the number of
sets of chromosomes.
Organisms with more than two sets of chromosomes are termed polyploid.
Chromosomes that carry the same genes are termed homologous
chromosomes.Meiosis is a special type of nuclear division which segregates
one copy of each homologous chromosome into each new “gamete”. Mitosis maintains the cell’s original ploidy level (for example, one diploid 2n cell
producing two diploid 2n cells; one haploid n cell producing two haploid
n cells; etc.). Meiosis, on the other hand, reduces the number of sets of
chromosomes by half, so that when gametic recombination fertilizationoccurs
the ploidy of the parents will be re-established.
Two successive nuclear divisions occur, Meiosis I (Reduction) and Meiosis II
(Division). Meiosis produces 4 haploid cells. Mitosis produces 2 diploid cells.
The old name for meiosis was reduction/ division. Meiosis I reduces the
ploidy level from 2n to n (reduction) while Meiosis II divides the remaining set
of chromosomes in a mitosis-like process (division). Most of the differences
between the processes occur during Meiosis I.
Application activity 6.2
1. Give two reasons why cells divide.
2. As a cell increases in size, which increases more rapidly, its surface
area or its volume?
3. Describe what happens during each of the four phases of mitosis.
4. Describe what happens during interphase.
5. How is cytokinesis in plant cells similar to cytokinesis in animal cell?
6. The diagram shows meiosis in an animal cell.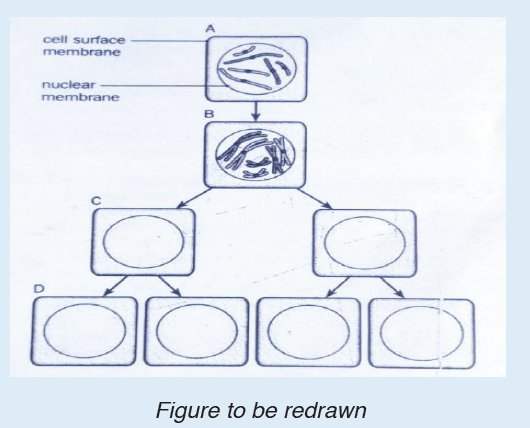
a) What is the diploid number of chromosomes in this cell?
b) Where do you think this cell could be found in an animal?
c) What is the stage of cell division shown at B? Give a reason for your
choice.
6.3. Mitosis and meiosis roles in living organisms
Activity 6.3
Observe figures below and explain the significance of mitosis in livingorganisms
In the early development of an organism, the embryonic cells rapidly proliferate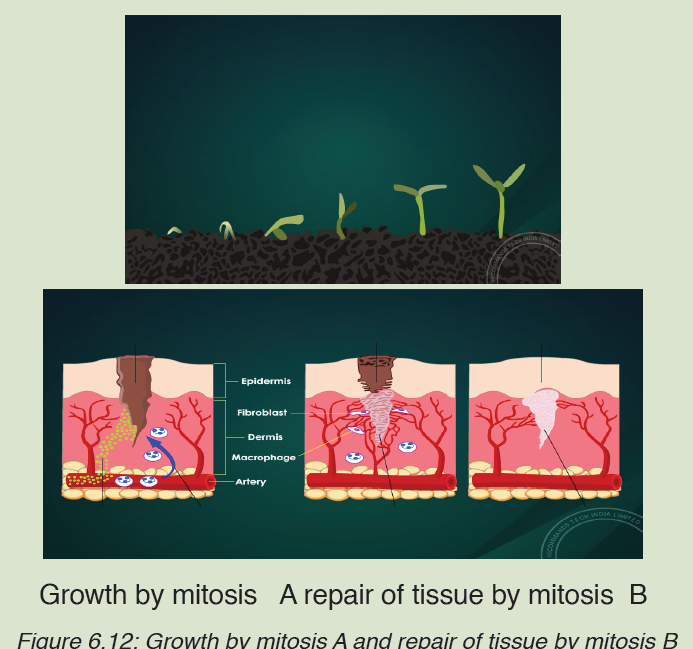
and differentiate into specialized cells of adult tissues and organs. As cells
differentiate from time to time, their rate of proliferation usually decreases.
As a result, most cells in adult animals are arrested at the G0 stage.
Some cells at this phase may resume the cell cycle and proliferate when
they receive certain signals. Some of the differentiated cells enter the Go
resting phase and wait for the signal to resume the cell cycle to repair injured
tissue. There are numerous examples such as skin fibroblast, endothelial
cells, smooth muscle cells, and liver cells. Skin fibroblasts upon receiving
growth factor, skin fibroblast start secreting collagen and help in repairing
cuts or wounds. Most of the fully differentiated cells no longer possess the
capability of cell division. Therefore, they can be replaced by stem cells.
Stem cells are undifferentiated biological cells that can differentiate into
specialized cells and can divide (through mitosis) to produce more stem cells.
The prominent role of stem cells can be seen in—blood cells (hematopoietic system), epithelial cells of the skin, and epithelial cells lining the digestive
tract. All of these cells have short life spans, and they must be replaced
continually by continual cell proliferation in adult animals, which is done by
mitosis.
The life span of blood cells ranges from less than one day to a few months.
All of these cells are derived from the same population of hematopoietic
stem cells. In fact, there are more than 100 billion blood cells that are lost
every day in humans. If there are no stem cells to replace the loss of
these cells, human beings will not be able to survive. Hence, these cells
are continually being replaced by cells produced from hematopoietic stem
cells in the bone marrow by means of mitotic division. Meiosis is necessary
in gametes formation, and this helps organisms to multiply by reproduction.
The Significance of Mitosis in Cell Replacement and Tissue Repair by
Stem Cells
a) Mitosis allows growth: A single cell divides repetitively to produce
all the cells in an adult organism.
b) Mitosis allows to repairing and cell replacement: by producing
new cells to replace ones that have been damaged or worn out.
c) Mitosis is involved in asexual reproduction: a single parent celldivides into two genetically identical offspring.
d) Mitosis allows geneticstability by producing two nuclei which have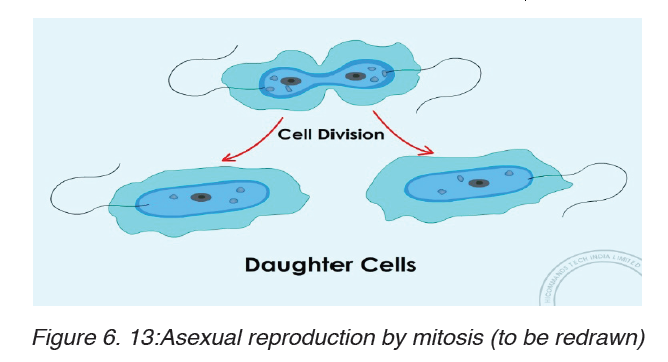
the same number of chromosomes as the parent cell.
e) Regeneration: Some animals are able to regenerate whole parts of
the body, such as legs in crustacean and arms in starfish. Production
of the new cells involves mitosis.
Regulation of the cell cycle
Regulation of the cell cycle helps the living organism to live healthily. The
cell divides by mitosis only when required. When receives signals from
neighboring cells, it responds by dividing or not dividing. The most known
cell cycle regulators include proteins called cyclins, the proteins which
regulate the timing of the cell cycle in eukaryotic cells. There two types of
regulators proteins such as internal regulators, and external regulators
which include growth factors.
Many cancers result from uncontrolled cell division, when the regulation of
the cycle is lost. Cancerous cells divide much more rapidly than healthy
cells. These cells use the blood and nutrients that other cells need and they
can stress the environment of the healthy cells. As cancerous cells do not
provide any useful function to the organism, they are extremely harmful. If
cancerous cells are allowed to grow uncontrolled, they will kill the host
organism.Cancer and uncontrolled cell division
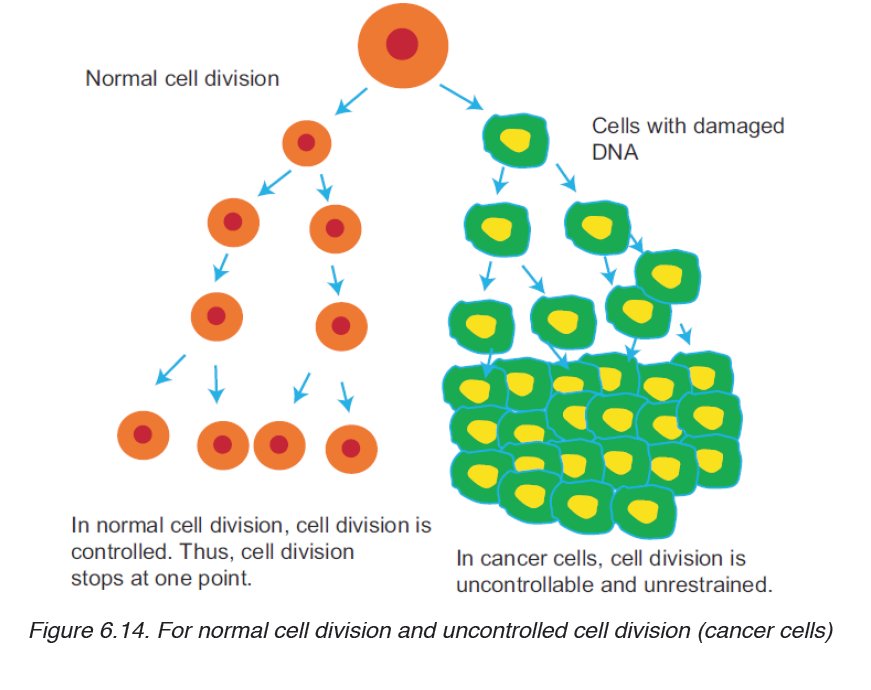
The problem begins when a single cell in a tissue undergoes transformation,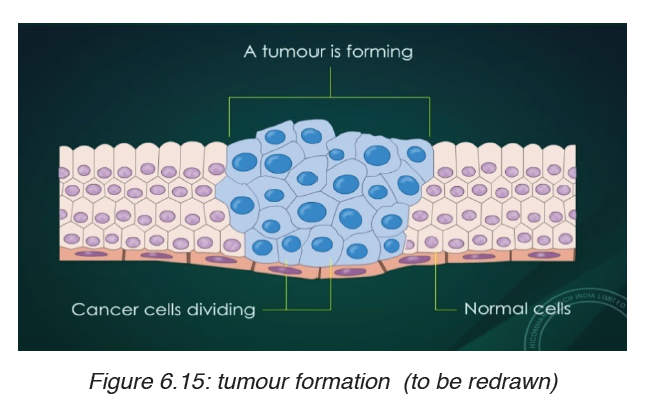
the process that converts a normal cell to a cancer cell. The body’s immune
system normally recognizes a transformed cell asan abnormal and destroys it.
However, if the cell escapes immune system, it may proliferate to form atumor
(a mass of abnormal cells within an otherwise normal tissue). There are
three types of tumors: benign tumors, malignant tumors and metastasis.
i. Benign tumour: it is a lump of the abnormal cells that remains at the
original site. Most benign tumors do not cause serious problems and
can be removed by surgery.
ii. Malignant tumors: these are cells abnormal cells that have become
invasive enough to impair with the functions of one or more organs. An
individual with a malignant tumor is said to have cancer.
iii. Metastasis: Cancer cells may also separate from the original tumor,
enter the blood and lymph vessels, and invade other parts of the body,
where they proliferate to form more tumors. This spread of cancer cells
beyond their original site is called metastasis.
Many cancers can be inherited, such as breast cancer, others are triggered
by viral infections, tobacco smoke (e.g. lung cancer) and radiations (e.g.
skin cancer). All cancers have one thing in common: the control over the cell
cycle has broken down.
Significance of Meiosis
a) The need for reduction prior to fertilization in sexual reproduction
Generally, a cycle of reproduction consists of meiosis and fertilization. Before
sexual reproduction occurs, gametes undergo meiosis and produce haploidcells. Thus during sexual reproduction, one haploid (1n) gamete comes from the paternal side and another haploid (1n) gamete comes from the maternal side; then, they both fuse to form a zygote, which is diploid (2n). The fusion
of gametes to form zygote or new cell is called as fertilization or syngamy. If meiosis does not occur before sexual reproduction, the chromosome number would double up with each fertilization. And after few generations,
the number of chromosomes in each cell would become impossibly large.
For example in humans, in just 10 generations, the 46 chromosomes would
increase to about 47104 (46 × 210).
b) Role and Significance of Meiosis in Producing Gametes
Gametogenesis is a biological process by which diploid cells undergo cell
division and differentiation to form mature haploid gametes. It occurs through
meiosis. In humans, the male gamete (sperm) is produced by a process
called spermatogenesis and the female gamete (egg) is produced by a
process called oogenesis through meiotic division. Here gametes function
takes place soon after meiosis but in plants it happens after gametophyte
formation sexual reproduction of plants starts with spore formation.
Sporophyte is a diploids generation of flowering plant where haploid spores
are produced by meiosis which in turns undergoes mitosis to form multicelled
haploid gametophytes. These haploid gametophytes differentiate to
produce gametes—sperm and egg cells. Similarly, embryo sac is formed
by reduction division. Each of the cells of embryo sac is haploid. Two of the
nuclei fuse to produce diploid nucleus.
c) The Role of Meiosis in Reproduction of Plants
Generally, plants reproducing sexually have life cycle consisting of two
phases
– A multicellular gametophyte or haploid stage:
– It is a haploid stage with n chromosomes. It alternates with a multicellular
sporophyte stage.
– A multicellular sporophyte or diploid stage:
It is a diploid stage with 2n chromosomes, made up of n pairs. A mature
sporophyte produces spores by meiosis, a process which reduces the
number of chromosomes from 2n to 1n.
Alternation of generations (also known as mutagenesis) refers to the
occurrence in the plant life cycle of both a multicellular diploid organism
(sporophyte) and a multicellular haploid organism (gametophyte), each giving
rise to the other. This is in contrast to animals, in which the only multicellular
phase is the diploid organism (such as the human man or woman), whereas
the haploid phase is a single egg or sperm cell. In bryophytes (mosses and liverworts), the dominant generation is haploid, so that the gametophyte
comprises the main plant. On the contrary, in tracheophytes (vascular
plants), the diploid generation is dominant and the sporophyte comprises
the main plant.
d) Independent Assortment of Chromosomes
Specifically at metaphase I, each homologous pair of chromosomes
positioned independently of the other pairs. As a result, each homologous
pair sorts out its maternal and paternal homologue into daughter cells
independently of every other pair. This act of separating homologous pairs
independently is called independent assortment. The random orientation
of homologous pairs of chromosomes due to independent assortment in
meiosis I (metaphase) increases genetic variation in organisms.
e) Crossing Over and Random Fertilization
During crossing over, DNA segments of the two parents-paternal and
maternal are combined into a single chromosome producing recombinant
chromosomes, which are non-identical with their sister chromatids.
In humans, an average of one to three crossing over events occurs per
chromosome pair, depending on the position of their centromeres and on
the size of the chromosome. Thus, crossing over is an important event of
meiosis that brings genetic variation in sexual life cycles.
Besides independent assortment and crossing over, the random fertilization
during sexual reproduction also increases genetic variation in organisms.
During random fertilization, the male gamete and female gamete fuse to
form zygote. The most interesting thing is that this zygote has the possibility
of about 70 trillion diploid combinations. The number 70 trillion comes from
possible combinations of male and female gametes which are 223 × 223 = 70
trillion. The possibility of this enormous number of combinations makes each
and every one of us unique physically and genetically.
f) Non-disjunction of Chromosomes
Proper separation of chromosomes during meiosis is essential for the normal
growth in humans. Any set of chromosomes that do not separate properly
during meiosis results in improper separation of chromosomes or nondisjunction,
which is a serious issue in human genetics. Non-disjunction is
a condition in which the homologues or sister chromatids fail to separate
properly during meiosis. It can lead to the gain or loss of chromosome, a
condition called as aneuploidy. Example: Down syndrome is an autosomal
trisomy. It is also called as trisomy 21, where non-disjunction results in an
embryo with three copies of chromosome 21 instead of the usual two copies of chromosome 21. The origin of trisomy’s condition is through non-disjunction
of chromosome 21 during meiosis. Failure of paired homologues to separate
during either anaphase I or II may lead to gametes with 23 + 1 chromosome
composition instead of the normal 23 gamete chromosome composition.
Therefore, instead of 46 normal chromosomes, Down syndrome patient will
have 47 chromosomes with three copies of chromosome 21 instead of the
normal 2 copies. It was first discovered by John Langdon Down. The chance
of occurrence is one infant in every 800 live births.
The most common symptoms of Down syndrome or trisomy 21are:
– They are short.
– They may also have protruding, furrowed tongues, which causes the
mouth to remain partially open.
– They are mentally retarded.
– They have a prominent epicanthic fold in the corner of each eye; and
typical flat face and round head.
– Usually, there is a wide gap between the first and the second digits on
their feet
Organisms and the significance of cell division
a) Spindle fibres formation,
Spindle fibers are microtubules that move chromosomes during cell division.
They are found in eukaryotic cells. Spindle fibers moves chromosomes during
mitosis and meiosis to ensure that each daughter cell gets the correct number
of chromosomes. The spindle apparatus consists of spindle fibers, motor
proteins, chromosomes, and, in some cells, structures called asters (which
are star-shaped structures form around each pair of centrioles during mitosis.
They help to manipulate chromosomes during cell division to ensure that
each daughter cell has the appropriate complement of chromosomes). In
animal cells, spindle fibers are produced from cylindrical microtubules called
centrioles. Centrioles are separated by asters to generate spindle fibers
during the cell cycle. Centrioles are located in a region of the cell known as
the centrosome.
Synapsis
In prophase I, homologous chromosomes become closely associated in
synapsis. Synapsis includes the formation of an elaborate structure called
the synaptonemal complex, consisting of homologous chromosomes
paired closely along a lattice or zipper-like structure of proteins between
them. The components of synaptonemal complex include a meiosis-specific form of cohesin that helps the two homologous chromosomes to be closely
associated along their length.
Some events that occur along with synapsis are:
– The nuclear envelope breaks down.
– Two pairs of centrosome migrate to opposite poles.
– Spindle fibres formation occurs.
Bivalents
These arethe two homologous chromosomes attached at chiasmata. The
homologous chromosomes consist of two sister chromatids each.
Chiasma formation and movement of chromosomes
Chiasmata is the region of crossing over between two homologous
chromosomes during prophase I of meiosis. At the chiasmata, homologous
chromosomes exchange genes, allowing genetic information from both the
paternal and maternal chromatids to be exchanged, and a recombination
of paternal and maternal genes can be passed down to the progeny. Thisprocess is important in diploid organisms to ensure variation in the progeny.
Process of chiasmata formation.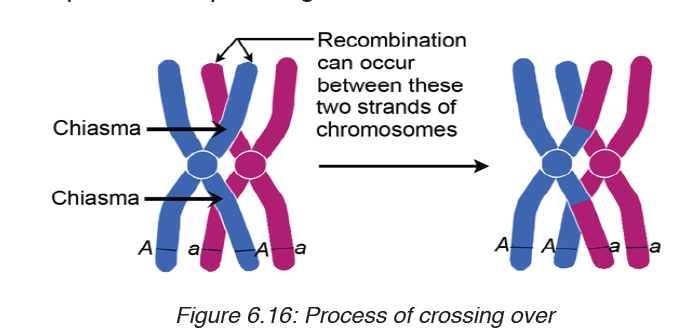
At prophase I of meiosis, after the homologous pair of chromosomes pair up
in the process called synapsis, the non-sister chromatids overlap, forming an
X-shape. They then exchange their alleles at the point of crossing over. The
X-shape the homologous pair together until the cell progresses to anaphase.
Movement of chromosomes.
In early Prophase I, chromosomes change their size and become short and
thick. This is the first movement they make during meiosis
In late Prophase I, homologous chromosomes become fully shortened and
thickened lie side by side (in a process called synapsis).
In metaphase I, Pairs of homologous chromosomes migrate to the equatorial
plane of the cell.
Each chromosome moves independently of all the others and the phenomenon
is called independent assortment.
During anaphase I, the homologous chromosomes separate and begin to
move to the opposite poles of the cell, pulled by the shrinking spindle fibers.
In telophase I, the movement of the homologous chromosomes to the poles
is completed.
During Prophase II chromosomes again become thicker and shorterbegin
to move to the equatorial plane of the cell. Spindle fibers once again begin
to form at the poles.
During metaphase II the chromosomes become aligned on the equatorial
plane and spindle fibers attach to the centromeres.
In anaphase II, the centromeres divide, separating the sister chromatids,
that move to the opposite poles due to the spindle fibers pulling.
In telophase II, the movement of the chromosomes to the poles is completed
and the spindle disappears
Application activity 6.3
1. Discuss possible functions of mitosis in living organisms.
2. Appreciate the role of cyclins in the process of cell cycle.
3. Describe the three types of tumors.
4. Descried the process of tumor formation.
SKILLS LAB
Cell division is essential biological process that contributes to growth and
reproduction that help in well being of all living organisms. Due to this
knowledge of cell division, student-teachers will use different techniques
out of school to make the food producing plants growing rapidly by providingthem food substances increasing the rapid cell division.
End unit assessment 6
I. Choose whether the following statements are True (T) or False (F)
1. A typical cell spends most of its time in interphase.
2. Mitosis is a process where a single cell divides into three identical
daughter cells.
3. Cytokinesis is a division of cytoplasm.
4. The process of mitosis is basically divided into 5 phases.
5. Meiosis is divided into three stages: Meiosis I, Meiosis II and Meiosis III.
6. The unrestrained, uncontrolled growth of cells in human beings results
into a disease called cancer.
7. Cancer occurs due to failure in controlling cell division.
8. Proper separation of chromosomes during meiosis is not essential for
the normal growth in humans.
9. The life span of blood cells ranges from less than one day to a few
months.
II. Multiple Choice Questions
1. In telophase, the nuclear envelope re-forms around the........... set of
haploid daughter chromosomes.
(a) one (b) two (c) three (d) four
2. ............................... is a condition in which the homologues or sister
chromatids fail to separate properly during meiosis.
(a) Disjunction (b) Non-disjunction (c) Down syndrome (d) None of these
3. Which of the event is correct in anaphase
a) Sister chromatids separate and give rise to daughter chromosomes.
b) Chromosomes are aligned at metaphase plate.
c) Cytokinesis starts occurring.
d) Chromosomes begin to uncoil.
4. One round of oogenesis produces
(a) One egg (b) Two eggs (c) Three eggs (d) Four eggs
5. In mitosis, which of the following occurs?
(a) Chiasmata formation (b) DNA replication(c) Synapsis (d) None of these
III. Long Answer Type Questions
1. Describe the main stages of cell cycle.
2. In your own words, explain what is meant by homologous pairs of
chromosomes.
3. In your own words, describe the process of mitosis.
4. In your own words, describe the process of meiosis.
5. Outline the significance of mitosis in cell replacement and tissue repair
by stem cells.
6. In your own words, explain how uncontrolled cell division can result in
the formation of a tumour.
7. What is the need for reduction prior to fertilization in sexual reproduction?
8. In your own words, explain the importance of effective cell division.
9. Outline the role of meiosis in gametogenesis in humans and in the
formation of pollen grain and embryo sacs in flowering plants.
10. Explain how crossing over and random assortment of homologous
chromosomes during meiosis and random fusion of gametes at
fertilization leads to genetic variation, including the expression of rare
recessive alleles.11. Analyse the following diagram and answer the questions below
a) Identify the stage of cell division shown in the figure.
b) Label the structures marked as (1), (2), (3) and (4).
c) Which type of cell is involved in this division?
d) What will happen if the structure marked (3) is not formed?
12. How can you correlate the spread of HIV virus with the process of
Mitosis? Knowing the viral disease and its spread, discuss in brief thestigma and discrimination faced by those affected by HIV and AIDS.
UNIT 7:ELECTRON CONFIGURATIONS OF ATOMS
Key unit competence
Relate Bohr’s model of the atom with hydrogen spectrum and energy levels,
practice writing electronic configurations using s, p, d, f orbitals.
Introductory Activity
At the beginning of this century, it was already known that atoms were
made of protons at the center (the nucleus) and electrons orbiting around
them. Niels Bohr proposed that the energy of an electron in an atom is not
continuous, but quantized.
To understand this better, think about a bookshelf as shown in the Figure
7.1 above.
1. If each of the books were electrons, what will be considered as to
be each shelf?
2. Each shelf is different from the others. What differentiate one shelf
from the other?
3. A book cannot be placed half way between consecutive shelves,
it can only be placed within one shelf. Deduce from this what is
predicted by Bohr, if you remember what shelves and books are
representing.
4. The places corresponding to each of the allowed orbit are referred
to as energy levels. Each shelf is labeled with the number n (n=1,
2, 3, etc). Suppose that this is the same for the atomic model to
be equivalent for the shelf. What is the technical term for these
numbers?
5. Formulate the simple, abbreviated way you can use to represent
the books in each shelf including the number they occupy.
7.1. Bohr’s atomic model and concept of energy levels
Activity 7.1
An atom is known to be further composed by other subatomic particles.
1. State three main subatomic particles.
2. Make a research and reveal the subatomic particles discovered
with the contribution of Rutherford.
3. Draw the structure of how you think boron atom would be looking
like, labeling each of the charged particles. Remember that the
proton number of boron is 5.
4. Suggest the reason why the electrons, in an atom, occupy different
levels.
5. Describe what would happen to the electron to change the level
it occupied.
The Bohr Model has an atom consisting of a small, positively-charged
nucleus orbited by negatively-charged electrons. Here is a closer look at the
Bohr Model, which is sometimes called the Rutherford-Bohr Model.
Overview of the Bohr Model
Niels Bohr proposed the Bohr Model of the Atom in 1915. Because the
Bohr Model is a modification of the earlier Rutherford Model, some people
call Bohr’s Model the Rutherford-Bohr Model. The modern model of the atom
is based on quantum mechanics. The Bohr Model contains some errors, but
it is important because it describes most of the accepted features of atomic
theory in a simple way and tries to answer the following questions failed to
answer by Rutherford.
• Why do atomic spectra consist of discrete (separate) lines?
• Why do atoms absorb or emit light of certain frequencies?
• Why do the spectral lines converge to form a continuum?
Unlike earlier models, the Bohr Model explains the “Rydberg formula for the
spectral emission lines of atomic hydrogen”.
The Bohr Model is a planetary model in which the negatively-charged
electrons orbit a small, positively-charged nucleus similar to the
planets orbiting the Sun(except that the orbits are not planar).
Bohr used the term energy levels (or shells) to describe these orbits of
differing energy. He said that the energy of an electron is quantized, meaning
“electrons can have one energy level or another but nothing in between”
The energy level that an electron normally occupies is called its ground state.
But it can move to a higher-energy, less-stable level, or shell, by absorbing
energy. This higher-energy, less-stable state is called the electron’s excited
state.
After it is done being excited, the electron can return to its original ground
state by releasing the energy it has absorbed, as shown in the diagram
below.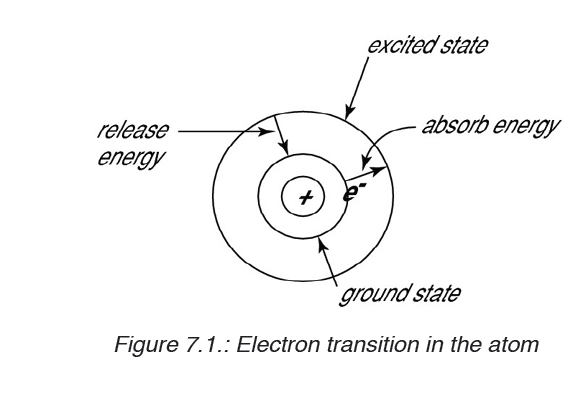
Sometimes the energy released by electrons occupies the portion of
the electromagnetic spectrum (the range of wavelengths of energy) that
humans detect as visible light. Slight variations in the amount of the energy
are seen as light of different colours.
Bohr referred to Max Planck’s recently developed quantum theory, according
to which energy can be absorbed or emitted in certain amounts, like separate
packets of energy, called quanta. The energy change is accompanied by
absorption of radiation energy of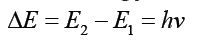 where,
where,
• h is a constant called “Planck’s constant” and
• v is the frequency of radiation absorbed or emitted.
• The value of h is 6.626 x 10-34 Js.
Each of these small “packets” of energy is called photon also called
“quantum of energy”. Energy can be gained or lost only in whole-number
multiples of the quantity hv, that is,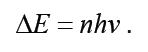
The absorption and emission of light due to electron jumps are measured by
use of spectrometers.
Bohr found that the closer an electron is to the nucleus, the less energy it
needs, but the farther away it is, the more energy it needs. So Bohr numbered
the electron’s energy levels.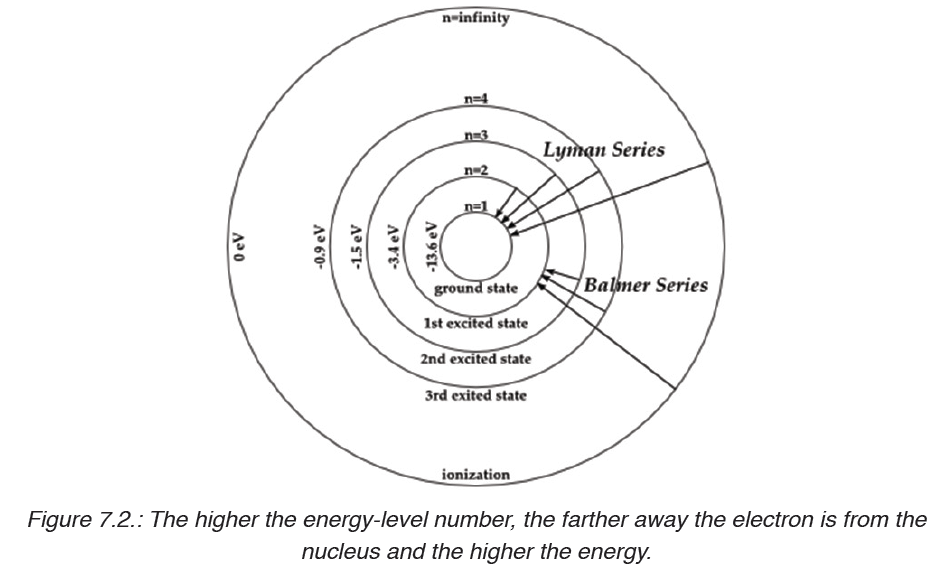
Bohr also found that the various energy levels can hold differing numbers of
electrons: Energy level1 may hold up to 2 electrons,energy level 2 may hold
up to 8 electrons, and so on.
In summary, the postulates of BohrAtomic Model are as follows:
• Electrons revolve around the nucleus in a fixed circular path termed
“orbits” or “shells” or “energy levels”.
• The orbits are termed as “stationary orbits”.
• Every circular orbit will have a certain amount of fixed energy and these
circular orbits were termed orbital shells. The electrons will not radiate
energy as long as they continue to revolve around the nucleus in the
fixed orbital shells.
• The different energy levels are denoted by integers such as n=1 or n=2
or n=3 and so on. These are called as quantum numbers. The range
of quantum number may vary and begin from the lowest energy level
(nucleus side n=1) to highest energy level. Learn the concept of an
Atomic number here.
• The different energy levels or orbits are represented in two ways such
as 1, 2, 3, 4, … or K, L, M, N, … shells. The lowest energy level of the
electron is called the ground state.
• The change in energy occurs when the electrons jump from one
energy level to other. In an atom, the electrons move from lower to
higher energy level by acquiring the required energy. However, when
an electron loses energy it moves from higher to lower energy level.
Bohr Model of Hydrogen
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1)
or for a hydrogen-like ion (Z > 1), in which one negatively-charged electron
orbits a small positively-charged nucleus.
Electromagnetic energy will be absorbed or emitted if an electron moves from
one orbit to another. Only certain electron orbits are permitted. The radius of
the possible orbits increases as n2, where n is the principal quantum number
which represents the number of energy levels in a given atom.
Main Points of the Bohr Model
• Electrons orbit the nucleus in orbits that have a set size and energy.
• The energy of the orbit is related to its size. The lowest energy is found
in the smallest orbit.
• Radiation is absorbed or emitted when an electron moves from one
orbit to another.
Weakness of Bohr’s Model
The Bohr model works well for very simple atoms such as hydrogen (which
has 1 electron) but not for more complex atoms. Although the Bohr model
is still used today, especially in elementary textbooks, a more sophisticated
and complex model (the quantum mechanical model) is used much more
frequently.
Application activity 7.1
1. State any weakness of the Bohr Model.
2. Outline three postulates of Bohr.
3. In Bohr atom, what is represented by the distance between an
orbital and the nucleus of an atom?
4. Explain why some people call Bohr’s Model the Rutherford-Bohr
Model.
5. Give the meaning of each of the following terms:
a) Electromagnetic spectrum
b) Quantized
c) Photon
6.Try to describe the atomic structure in the same way as Bohr can do.
7.2. Absorption and emission spectra and energy
associated
Activity 7.2
Observe the picture below, discuss with your colleagues and answer the
following questions.
1. What do you see on the above photo?
2. State the physical phenomenon which is related to the given
picture.
3. Think of any other means of producing the same pattern. List two
of them.
4. What property can you attribute to light with reference to the above
process?
The colours we see in a rainbow never fail to captivate us! Did you know
that even though we identify the distinct colours of a rainbow, it is actually a
continuous range of colours? A similar range of colours appears when white
light passes through a prism; this range of colours is a spectrum. A rainbow
is a multicoloured arch in the sky, produced by prismatic refraction of light
within droplets of rain in the air or any prismatic refraction of light showing a
spectrum of colours.
7.2.1. Spectrum
Ordinary white light consists of waves of all wavelengths in the visible range.
This is why, when white light passes through a prism, a series of coloured
bands are seen called spectrum. This dispersion of white light demonstrates
that white light contains all the wavelengths of colour and is thus considered
to be continuous. Each colour blends into the next with no discontinuity.
Since the colours merge into each other i.e. violet merges into blue, blue into
green and so on, we call it a “continuous spectrum”.
The interaction of electromagnetic radiation with matter causes the atoms and
molecules to absorb energy and go to a higher energy state. Since this state
is unstable, they need to emit radiations to return to their normal states. This
gives rise to emission and absorption spectra.
7.2.2. Emission and absorption spectra
1. Emission spectrum
Every substance reacts differently when it interacts with light. The material
starts off with being in the ground state, where all molecules are stable and
settled. However when heat, energy or light is applied to a substance, some
of the molecules transition into a higher energy state or an excited state.
During this state the molecules are unstable and try to emit the energy
in order to reach the state of equilibrium. The molecules emit energy in
the form of photons or light. The difference between the substance in ground
state and excited state is then used to determine the emission level of the
substance.
Each element or substance has a unique emission level or the amount of
energy it radiates; this helps the scientists identify elements in unknown
substances. The emission of an element is recorded on an emission spectrum
or atomic spectrum. The emittance of an object measures how much light is
emitted by it. The amount of emission of an object varies depending on the
spectroscopic composition of the object and temperature. The frequencies
on an emission spectrum are recorded in light frequencies, where the
colour of the light determines the frequency.
Emission can happen in the form of light and rays, such as gamma and
radio. The spectrum is a dark wavelength with bands of color on it, which is
used to determine the emission of the object.
The emission spectrum is the spectrum of radiation emitted by a
substance that has absorbed energy. Atoms, molecules, and ions that have
absorbed radiation are called ‘excited‘.
2. Absorption spectrum
The absorption spectrum is the opposite of the emission spectrum. Absorption
can be plotted in a wavelength, frequency or wave number. There are two
types of absorption: atomic absorption spectra and molecular absorption
spectra.
Absorption is used:
• To determine the presence of a particular substance in a sample, or the
quantity of the present substance in the sample.
• In molecular and atomic physics, astronomical spectroscopy and
remote sensing. Absorption is primarily determined by the atomic and
molecular composition of the material.
They can also depend on temperature, electromagnetic field, interaction
between the molecules of the sample, crystal structure in solids and
temperature.
In order to determine the absorption level of a substance, a beam of radiation
is directed at the sample and the absence of light that is reflected through
the object can be used to calculate the absorption. The absorption spectrum
is usually light colored, with dark bands that run through it. These dark bands
are used to determine the absorption of the object.
Absorption spectrum is the plotting of the energy that is absorbed by an
element or substance. It is the spectrum formed by electromagnetic radiation
that has passed through a medium, in which radiation of some frequencies is
absorbed.
Emission and absorption spectra are techniques that are used in chemistry
and physics. Spectroscopy is the study of emission and absorption spectra.
It is the interaction of radiation and matter. Using spectroscopy, a scientist
can figure out the composition of a certain matter. This is really beneficial,
of dealing with unknown substances. Emission spectra and absorption
spectra are different from each other but still related.
7.2.3. Comparison between absorption and emission spectra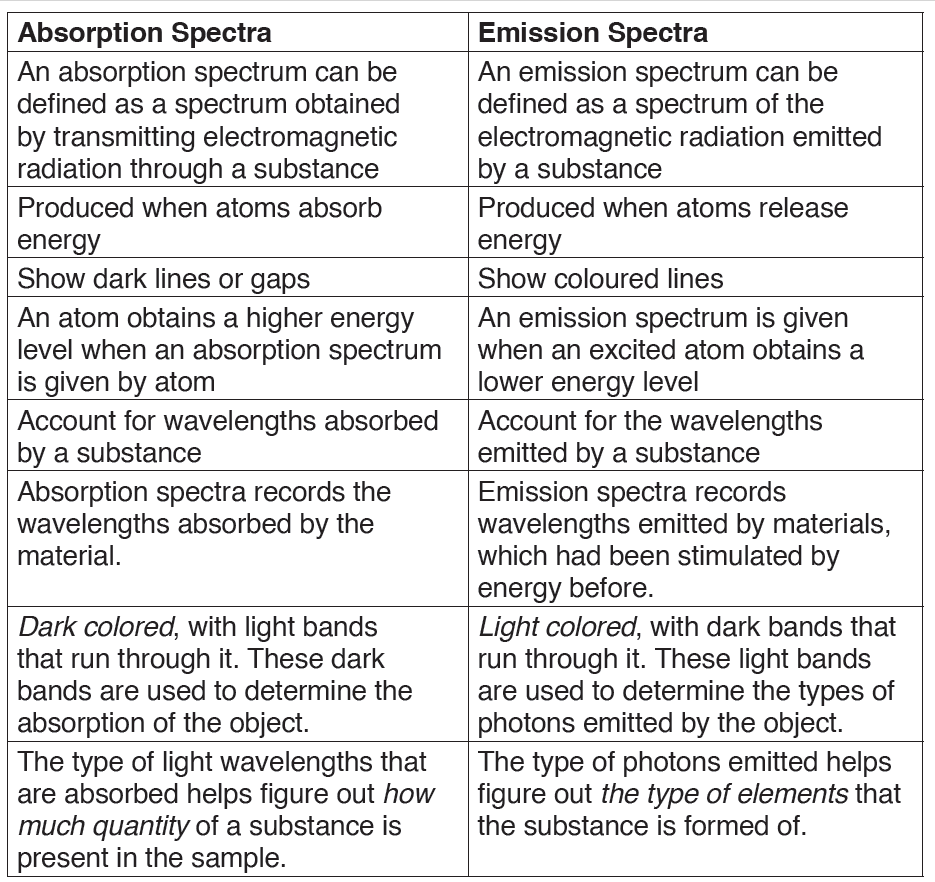
Application activity 7.2
1. State the meaning of the term “Spectrum”.
2. Why do we say that the spectrum of the white light is continuous?
3. Find out some differences between emission and absorption
spectra by filling the table below.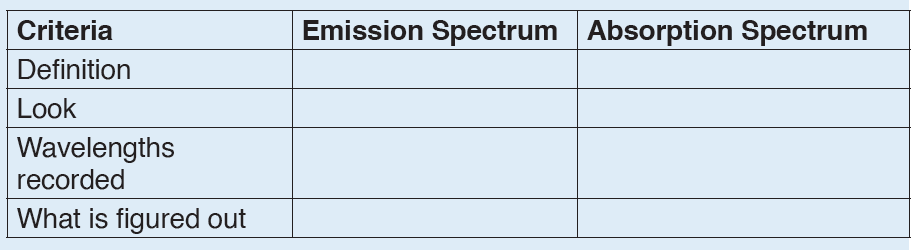
7.3. Hydrogen spectrum and spectral line series
Activity 7.3
Think about any spectrum you have come across with. This might be
composed of vertical lines (that form that spectrum).
1. Formulate the name that can be dedicated to such spectrum.
2. If atoms and molecules are heated to sufficiently high temperatures,
they emit light of certain wavelengths. Do you think the spectrum
drawn to be emission or absorption (spectrum)?
3. Describe the look that spectrum would have.
4. The vertical lines described in the spectrum above are different for
one element to another. How these separate lines can be used to
identify the element?
Unlike visible white light which shows a continuous spectrum of all wavelengths,
the emission spectra of atoms in the gas phase emit light only at specific
wavelengths with dark spaces between them. This is called line spectra or
atomic spectra since the emitted radiation is identified by bright lines in the
spectra. Each element has its own unique line emission spectrum.
Did you know that just the way fingerprints are used to identify people; the
characteristic lines in an atomic spectrum are used to identify unknown atoms!
Line Spectrum of Hydrogen
Hydrogen molecules dissociate when we pass electric discharge through
gaseous hydrogen. Subsequently, the energetically excited H2 atoms emit
electromagnetic radiation of discrete frequencies giving rise to a
spectrum emitted light is analysed with a spectrometer and discrete bright
lines in a dark background are observed.
The well-defined separation of lines is experimental evidence for the
existence of separate, discrete or ‘quantized’ energy levels in the atom.
No two gases give the same exact line spectrum.
The hydrogen spectrum has many series of lines. In 1885, the scientist
Balmer showed that if spectral lines are expressed as wavenumber, thenthe visible lines of the hydrogen spectrum obey the following formula:
The value 109,677 is the Rydberg constant for hydrogen.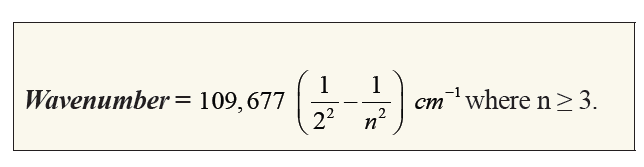
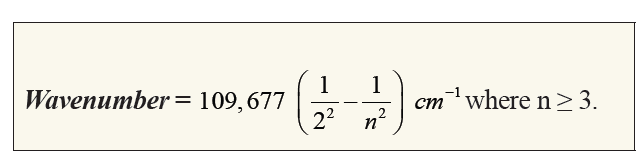
We call this series of lines, Balmer series. These lines are the only lines in
the hydrogen spectrum that appear in the visible region of electromagnetic
radiation. The 3 → 2 transition produces the first line of the Balmer series.
For hydrogen (Z = 1) this produces a photon having wavelength 656 nm (red light).
Johannes Rydberg, a Swedish spectroscopist, showed that all series of linesin the hydrogen spectrum can be described by the formula:

λ is the wavelength;
n1 is the initial energy level
n2 is the final energy level
The lines that correspond to n1 = 1, 2, 3, 4, 5 are called Lyman, Balmer,
Paschen, Brackett and Pfund series, respectively.
The hydrogen atom has the simplest line spectrum of all elements. For heavier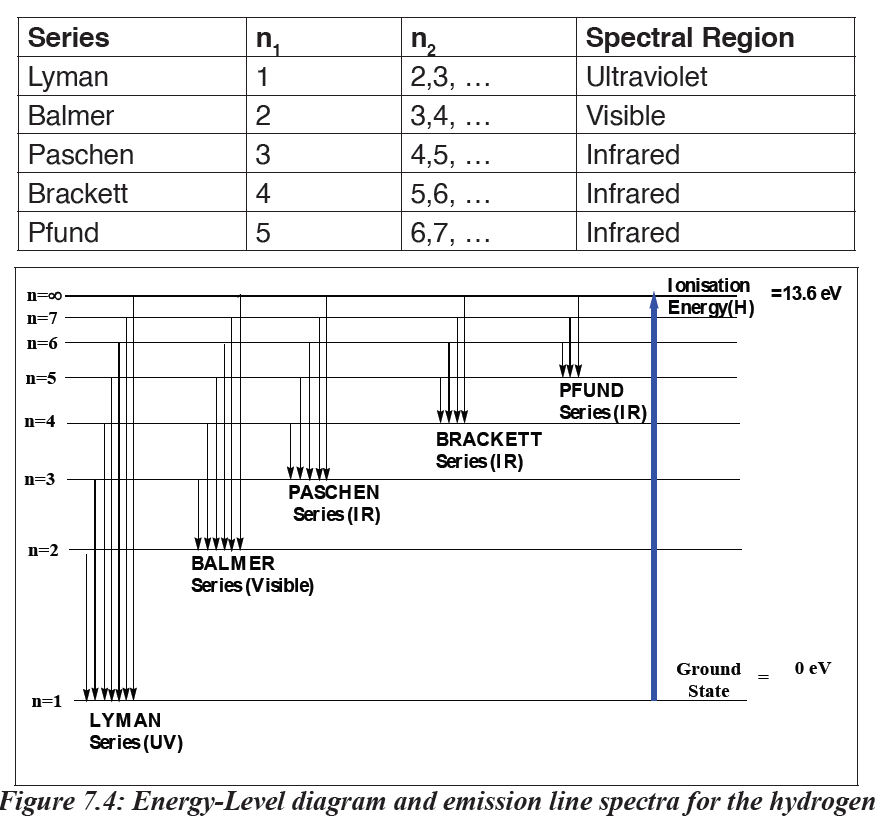
atoms, the line spectrum becomes more and more complex. However, there
are certain features that are common to all line spectra:
• Line spectrum of every element is unique.
• There is regularity in the line spectrum of each element.
Now, that we understand the line spectrum of hydrogen, let us understand the
features of the hydrogen atom, its structure, and its spectrum.
In each series, the intervals between the frequencies of the lines become
smaller and smaller towards the higher frequency end of the spectrum
until the lines run together or converge to form a continuum of light.
Explanation of Line Spectrum of Hydrogen
Bohr’s model can explain the line spectrum of the hydrogen atom. Radiation
is absorbed when an electron goes from orbit of lower energy to higherenergy; whereas radiation is emitted when it moves from higher to lower orbit.
The energy gap between the two orbits is: ΔE = Ef – Ei where:
• f is the final orbit,• i is the initial orbit
The frequency and wavenumber associated with the absorption and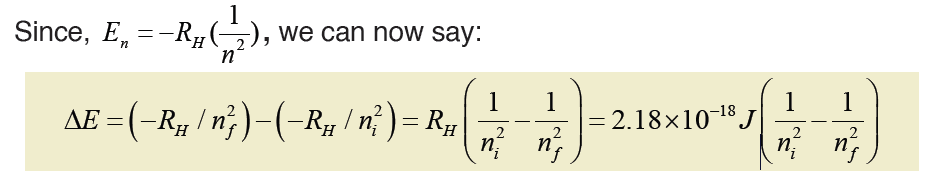
emission of the photon can also be calculated:

Example: Calculate the frequency and after the wavelength of the hydrogen
line that corresponds to the transition of the electron from the n = 4 to the n= 2 states.
Answer:
(The negative frequency or wavelength is physically meaningless, so the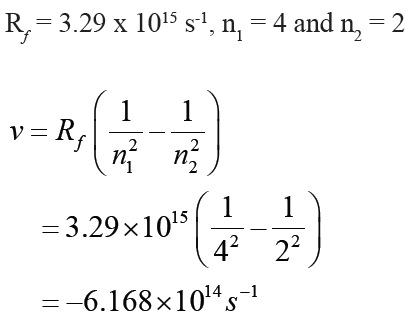
sign is ignored)
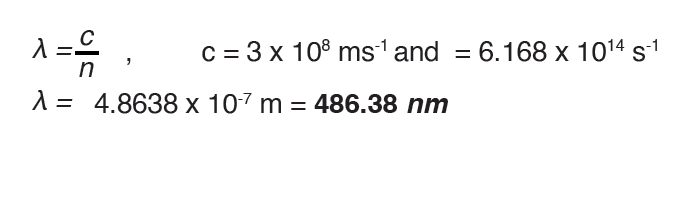
Note: The spectrum of white light ranges from violet (at 7.5 x 1014 Hz) to red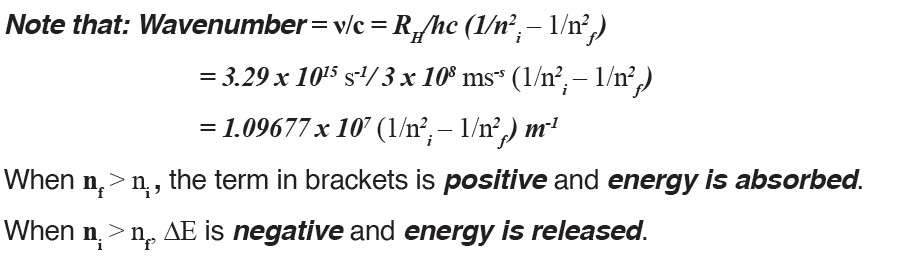
(at 4 x 1014 Hz). When this light passes through an object or medium, the wave
with the shortest wavelength (violet) deviates the more than the one with the
longest wavelength (red).
WORKED EXAMPLES
1. Find the wave length and frequency in Balmer series associated
with a drop of an electron from the fourth orbit.Answer
2. Find the wave length, frequency and energy of the third line in the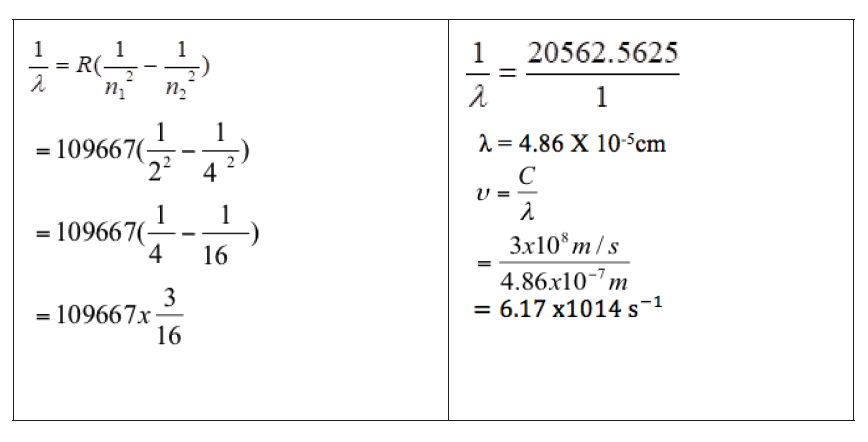
Lyman series.
Answer:
3. A certain source emits radiation of wavelength 500.0 nm. What is
the energy, in kJ, of one mole of photons of this radiation?
Solution:
Convert nm to m: 500.0 nm = 500.0 x 10-9m = 5.000 x 10-7mDetermine the frequency:
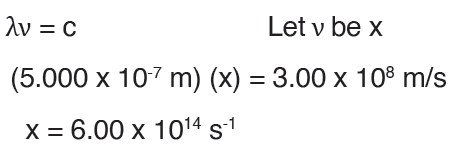
Determine the energy: E = hν
= (6.626 x 10– 34 J s) (6.00 x 1014 s-1)
= 3.9756 x 10–19 J
Important point: this is the energy for one photon.
Determine energy for one mole of photons: (3.9756 x 10–19 J) (6.022 x1023 mol–1) = 239.4 kJ/mol
Note: If you wished to do a direct calculation, you could use this equation:
E = hc / λ. Just make sure that the units for c and λ match.
Application activity 7.3
1. What is the meaning of infinity level in the hydrogen spectral lines?
2. Given a transition of an electron from n=2 to n=5. Calculate
a) Wavelength
b) Frequency
c) Energy
3. Explain how atomic emission spectra arise and how they relate to
each element on the periodic table.
4. How do the lines on the atomic spectrum relate to electron
transitions between energy levels?
5. Explain the difference between atomic absorption and emission
spectra.
6. Describe how the absorption and emission spectra of the gases in
the atmosphere give rise to the Greenhouse Effect.7. Use the figure below to answer the following questions.
a) What colour is the light emitted by hydrogen when an electron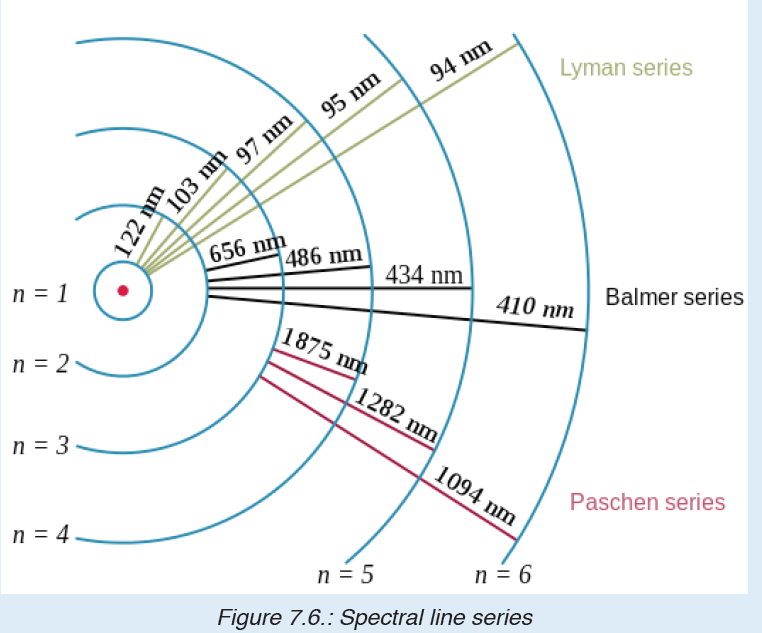
makes the transition from energy level 5 down to energy level 2?
(Use the figure above to find the energy of the released photon.)
b) I have a glass tube filled with hydrogen gas. I shine white light onto
the tube. The spectrum I then measure has an absorption line at
a wavelength of 474 nm. Between which two energy levels did the
transition occur?
8. Calculate the wavelength of a line in the Balmer series that is
associated with energy transition, E4 to E2 (E4 = -1.362×10-19 J, E2= -5.448×10-19J).
7.4. Concept of orbitals and quantum numbers
Activity 7.4
1. Recall the main weakness of the Atomic Bohr’s Model.
2. What do you understand with the term “orbit” in the atomic
structure?
3. Suppose that the orbit you talked about above is subdivided into
other sub-parts, said orbitals. Formulate a definition of an “orbital”.
4. There are numbers used to locate the orbitals. These are of four
types. One usually encountered is qualified to be “principal”.
a) What is the name given to those numbers?
b) Make a research and state them.
c) The principal one gives relevant information about the given atom.
State at least two points that are revealed when this principal
number is given.
We have seen the weakness and critics against the atomic Bohr’s model.
In order to answer the questions not answered by that model, other atomic
models were proposed. One of those models is the Quantum Model that
has been developed by the Australian physicist Erwin Schrödinger (1887-
1961). The model is based on a mathematical equation called Schrödingerequation.
7.4.1. Orbitals
This Quantum Model is based on the following assumptions or hypotheses:
• An electron is in continuous movement around the nucleus but cannot
be localized with precision; only the high probability of finding it in a
certain region around the nucleus can be known.
• The region where the probability of finding electron is high, at more
than 95%, is called “orbital”; in other words, the orbital is the volume
or the space (tridimensional) around the nucleus where there is a high
probability of finding the electron.
The orbitals are of 4 types. They are named s, p, d, f. The s, p, d, and f
stand for sharp, principal, diffuse and fundamental, respectively.1. “s” orbitals are spherically shaped.
2. “p” orbitals are often described as dumb-bell shaped.
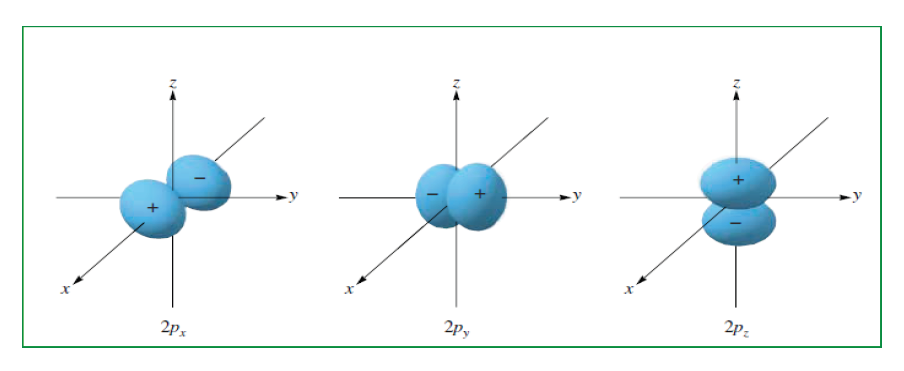
3. “d” orbitals and “f” orbitals are not easily visualized.
7.4.2. Quantum numbers
Without going into the mathematical development of the Schrödinger
equation, we can say that the energy of the electron depends on the orbital
where it is located and an atomic orbital is described by a certain number
of “quantum numbers” according to the solution of Schrodinger equation.
Quantum numbers are a set of numbers that describe the state of an
electron in an atom (and they are derived from quantum mechanical
treatment).
Four numbers, called quantum numbers, were introduced to describe the
characteristics of electrons and their orbitals:
• Principal quantum number: n
• Angular momentum quantum number: ℓ
• Magnetic quantum number: mℓ
• Spin quantum number: ms
1. The Principal Quantum Number
The principal quantum number n describes the average distance of the orbital
from the nucleus (the size of the shell) — and the energy of the electron
in an atom. It can have positive integer (whole number) values: 1, 2, 3, 4,
and so on. Thelarger the value of n, the higher the energy and the larger
the orbital. Chemists sometimes call the orbitals electron shells. The shells
(values of n) can be represented by letters K, L, M, N, O, P.
2. The Angular Momentum Quantum Number
The angular momentum quantum number ℓ is also called Secondary
Quantum number or Azimuthal Quantum Number. It describes the shape
of the orbital, and the shape is limited by the principal quantum number n:
The angular momentum quantum number ℓ can have positive integer values
from 0 to n–1. For example, if the n value is 3, three values are allowed for
ℓ: 0, 1 and 2. l=0(s), l=1(p), l=2(d), l=3(d).
The value of ℓ defines the shape of the orbital, and the value of n defines
the size. Orbitals that have the same value of n but different values of ℓ are
called subshells.
3. The Magnetic Quantum Number
The magnetic quantum number is designated as: mℓ. It describes how thevarious orbitals are oriented in space.
The value of this number depends on the value of ℓ. The values allowed are
integers from – ℓ to 0 to +ℓ. For example, if the value of l = 1 (p orbital), you
can write three values for this number: –1, 0, and +1. This means that thyou
can write three values for this number: –1, 0, and +1. This means that there
are three different p orbitals for the subshells. The orbitals have the same
energy but different orientations in space.
The three p orbitals correspond to magnetic quantum number values of –1,
0, and +1, oriented along the x, y, and z axes.
4. The Spin Quantum Number
The fourth and final quantum number is the spin quantum number, designated
as: ms This number describes the direction the electron is spinning in a
magnetic field — either clockwise or counterclockwise. Only two values are
allowed: +1/2 or –1/2. For each subshell, there can be only two electrons,
one with a spin of +1/2 and another with a spin of –1/2.
Table 7.1.: Relationship between the n, ℓ, mℓ and ms
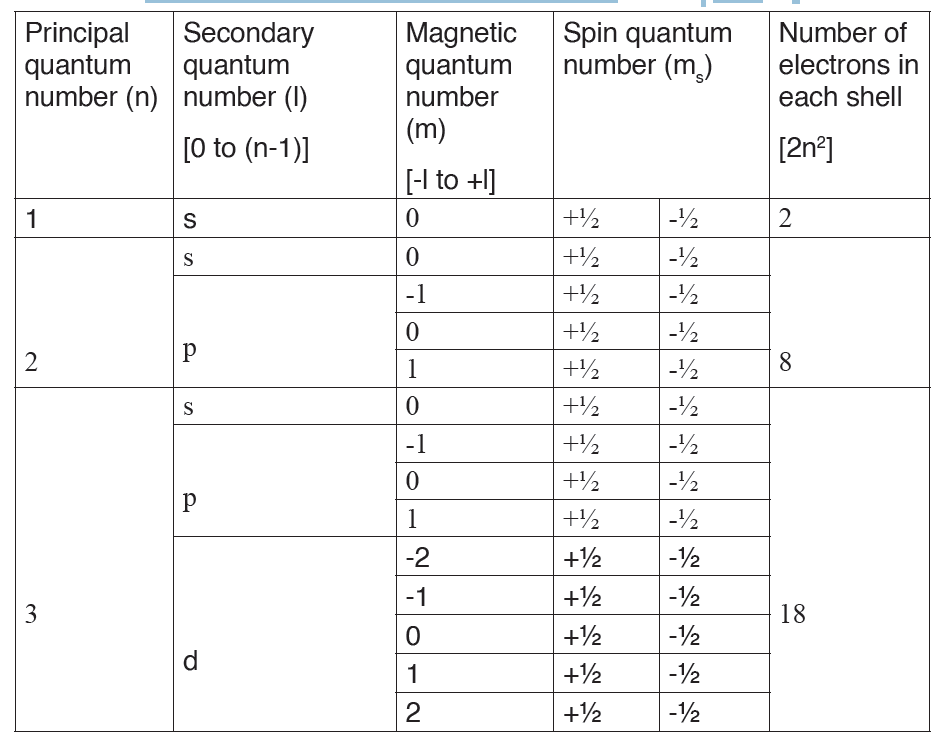
Application activity 7.4
1. Define the following terms:
a) Orbital
b) Quantum number
2. Give the different types of orbitals stating their shapes where it is
possible.
3. We have four quantum numbers. Use the knowledge of quantum
numbers to complete the table below.
4. Which of the following sets of quantum numbers are not allowed?
For each incorrect set, state why it is incorrect.
7.5. Rules governing the electronic configurations
Activity 7.5
1. Write electronic configuration of the following atoms using K, L, M,
N… orbit representations: Ca (z= 20), Cl (Z= 17), Sr (Z=38)
2. Potassium contains 19 electrons while sulphur contains 16. It is
found that the potassium ion (K+) has 18 electrons like the sulphide
ion (S2-).
a) Explain why the two ions contain the same number of electrons.
b) What is the element and its group on the Periodic table which is
isoelectronic with the ions mentioned?
3. State two differences between
a) Calcium atom (Ca) and its ion (Ca2+).
b) Nitrogen (N) and its ion (N3-)
The electron configuration of an atom is the representation of the arrangement
of electrons distributed among the orbital shells and subshells. Commonly,
the electron configuration is used to describe the orbitals of an atom in its
ground state, but it can also be used to represent an atom that has ionized
into a cation or anion by compensating with the loss of or gain of electronsin their subsequent orbitals.
Many of the physical and chemical properties of elements can be correlated
to their unique electron configurations. The valence electrons, electrons
in the outermost shell, are the determining factor for the unique chemistry of
the element.
Before assigning the electrons of an atom into orbitals, one must become
familiar with the basic concepts of electron configurations. Using the
periodic table to determine the electron configurations of atoms is a key,
but also keep in mind that there are certain rules to follow when assigning
electrons to different orbitals. The periodic table is an incredibly helpful tool
in writing electron configurations.
7.5.1. Rules for assigning electron orbitals
Electrons fill orbitals in a way to minimize the energy of the atom.
Therefore, the electrons in an atom fill the principal energy levels in order of
increasing energy (the electrons are getting farther from the nucleus). Theorder of levels filled looks like this:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p
One way to remember this pattern, probably the easiest, is to refer to the
periodic table and remember where each orbital block falls to logically
deduce this pattern. Another way is to make a table like the one below anduse vertical lines to determine which sub-shells correspond with each other.
1. Pauli Exclusion Principle
The Pauli Exclusion Principle states that no two electrons can have the same
four quantum numbers.
As said before, the first three (n, ℓ, and mℓ ) may be the same, but the
fourth quantum number must be different. A single orbital can hold a
maximum of two electrons, which must have opposing spins; otherwise
they would have the same four quantum numbers, which is forbidden. One
electron is spin up (ms = +1/2) and the other would spin down (ms = -1/2).
This tells us that each subshell has double the electrons per orbital. The s
subshell has 1 orbital that can hold up to 2 electrons, the p subshell has 3
orbitals that can hold up to 6 electrons, the d subshell has 5 orbitals that hold
up to 10 electrons, and the f subshell has 7 orbitals with 14 electrons.Example: Hydrogen and Helium
The first three quantum numbers of an electron are n=1, ℓ=0, mℓ=0. Only two
electrons can correspond to these, which would be either ms = -1/2 or ms =+1/2.
As we already know from our studies of quantum numbers and electron
orbitals, we can conclude that these four quantum numbers refer to the 1ssubshell. Visually, this can be represented as:
As shown, the 1s subshell can hold only two electrons and, when filled, the
electrons have opposite spins.
If only one of the ms values are given then we would have 1s1 (denoting
hydrogen); if both are given we would have 1s2 (denoting helium).
2. Hund’s Rule
When assigning electrons in orbitals, each electron will first fill all
the orbitals with similar energy (also referred to as “degenerate”) before
pairing with another electron in a half-filled orbital. Atoms at ground states
tend to have as many unpaired electrons as possible. When visualizing this
process, think about how electrons are exhibiting the same behavior as the
same poles on a magnet would if they came into contact; as the negatively
charged electrons fill orbitals they first try to get as far as possible from each
other before having to pair up.
Example: Oxygen and Nitrogen
If we look at the correct electron configuration of the Nitrogen (Z = 7) atom,a very important element in the biology of plants: 1s2 2s2 2p3
If we look at the element after Nitrogen in the same period, Oxygen (Z = 8)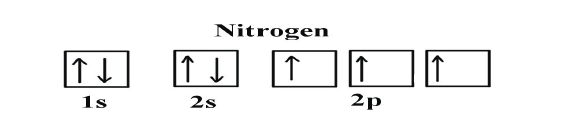
its electron configuration is: 1s2 2s22p4 (for an atom).
Oxygen has one more electron than Nitrogen and as the orbitals are all half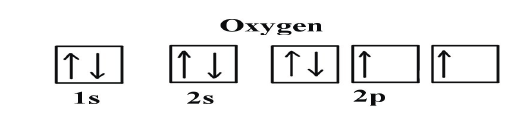
filled, the electron must pair up.
3. The Aufbau Principle
Aufbau comes from the German word “aufbauen” meaning “to build.” When
writing electron configurations, orbitals are built up from atom to atom.
Example: 3rd Row Elements
Following the pattern across a period from B (Z=5) to Ne (Z=10), the number
of electrons increases and the subshells are filled. This example focuses on
the p subshell, which fills from boron to neon.
B (Z=5) configuration: 1s2 2s2 2p1
C (Z=6) configuration: 1s2 2s2 2p2
N (Z=7) configuration: 1s2 2s2 2p3
O (Z=8) configuration: 1s2 2s2 2p4
F (Z=9) configuration: 1s2 2s2 2p5
Ne (Z=10) configuration: 1s2 2s2 2p6
According to the Aufbau Process, when writing the electron configuration
for an atom, orbitals are filled in order of increasing atomicnumber. However, there are some exceptions to this rule.
7.5.2. Writing electron configurations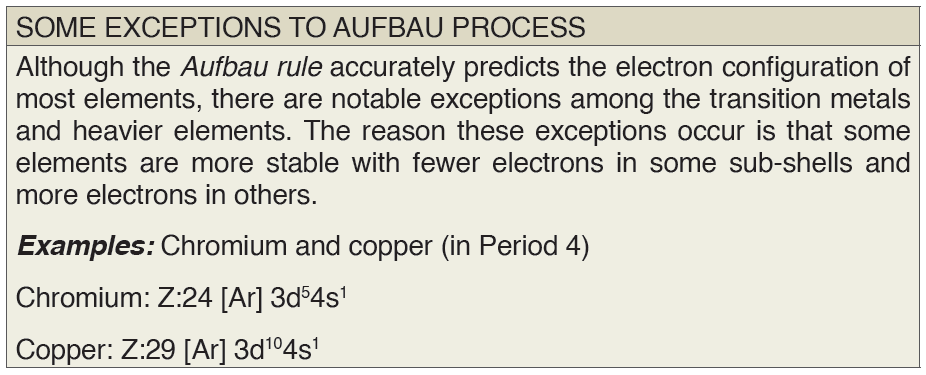
When writing an electron configuration, first write the energy level (the
period), then the subshell to be filled and the superscript, which is the
number of electrons in that sub-shell. The total number of electrons is theatomic number, Z.
The rules above allow one to write the electron configurations for all the
elements in the periodic table. Three methods are used to write electron
configurations:
• Spdf notation
• Orbital diagrams
• Noble gas notation
Each method has its own purpose and each has its own drawbacks.
4. spdf Notation
The most common way to describe electron configurations is to write
distributions in the spdf notation. Although the distributions of electrons
in each orbital are not as apparent as in the diagram, the total number of
electrons in each energy level is described by a superscript that follows the
relating energy level.
To write the electron configuration of an atom, identify the energy level
of interest and write the number of electrons in the energy level as its
superscript as: 1s2. This is the electron configuration of helium; it denotes a
full s orbital. The periodic table is used as a reference to accurately write the
electron configurations of all atoms.
Example:
Potassium has 19 electrons.
– Begin by filling up the 1s sublevel. This gives 1s2. Now the n = 1 level
is filled.
– Since we used 2 electrons, there are 19 − 2 = 17 electrons left
– Next, fill the 2s sublevel. This gives 1s22s2
– Since we used another 2 electrons, there are 17 − 2 = 15 electrons left
– Next, fill the 2p sublevel. This gives 1s22s22p6. Now the n = 2 level is
filled.
– Since we used another 6 electrons, there are 15 − 6 = 9 electrons left
– Next, fill the 3s sublevel. This gives 1s22s22p63s2
– Since we used another 2 electrons, there are 9 − 2 = 7 electrons left
– Next, fill the 3p sublevel. This gives 1s22s22p63s23p6
– Since we used another 6 electrons, there are 7 − 6 = 1 electron left
– Here’s where we have to be careful – right after 3p6!!
– Remember, 4s comes before 3d!The final electron goes into the 4s sublevel. This gives 1s22s22p63s23p64s1
5. Orbital Diagrams
An orbital diagram, like those shown above, is a visual way to reconstruct
the electron configuration by showing each of the separate orbitals and the
spins on the electrons. This is done by first determining the subshell (s,p,d or
f) then drawing in each electron according to the stated rules above.
Example: The atomic number of Iridium (Z) is 77. Write the electron
configuration of Iridium using orbital diagram method.Answer:
Although drawing out each orbital may prove to be helpful in determining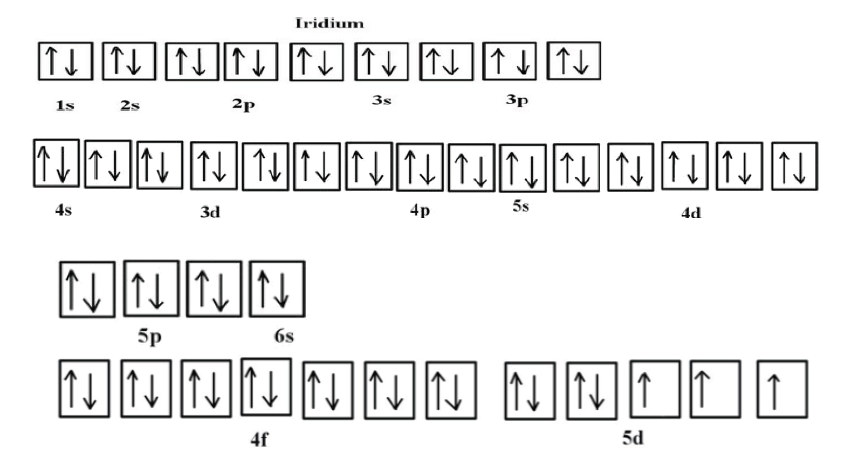
unpaired electrons, it is very time consuming and often not as practical
as the spdf notation, especially for atoms with much longer configurations.
Hund’s rule is also followed, as each electron fills up each 5d orbital before
being forced to pair with another electron.
6. Noble Gas Notation
This brings up an interesting point about elements and electron configurations.
As the p-subshell is filled in the above mentioned example of the period
from B (Z=5) to Ne (Z=10) about the Aufbau principle, it reaches the group
commonly known as the noble gases. The noble gases have the most
stable electron configurations, and are known for being relatively inert. All
noble gases have their subshells filled and can be used them as a shorthand
way of writing electron configurations for subsequent atoms.
This method of writing configurations is called the noble gas notation, in
which the noble gas in the period above the element that is being analyzed
is used to denote the subshells that element has filled and after which the valence electrons (electrons filling orbitals in the outer most shells) are written. This looks slightly different from spdf notation, as the reference noble
gas must be indicated.
Example: Vanadium (V, Z=23)
Vanadium is the transition metal in the fourth period and the fifth group.
The noble gas preceding it is argon (Ar, Z=18), and knowing that vanadium
has filled those orbitals before it, argon is used as the reference noble
gas. The noble gas in the configuration is denoted in brackets.
To find the valence electrons that follow, subtract the atomic numbers: 23 -
18 = 5. Instead of 23 electrons to distribute in orbitals, there are 5. Now there
is enough information to write the electron configuration:
Vanadium, V: [Ar] 4s2 3d3
This method streamlines the process of distributing electrons by showing
the valence electrons, which determine the chemical properties of atoms.
In addition, when determining the number of unpaired electrons in an atom,
this method allows quick visualization of the configurations of the valance
electrons. In the example above, there are one full s orbital and three half
filled d orbitals.
7.5.3. Electron configurations of ions
We already know that ions are formed when atoms gain or lose electrons.
A cation (positively charged ion) forms when one or more electrons are
removed from a parent atom. For main group elements, the electrons that
were added last are the first electrons removed. For transition metals and
inner transition metals, however, electrons in the s orbital are easier to
remove than the d or f electrons, and so the highest ns electrons are lost,
and then the (n – 1)d or (n – 2)f electrons are removed.
An anion (negatively charged ion) forms when one or more electrons are
added to a parent atom. The added electrons fill in the order predicted by
the Aufbau principle.
Example: What is the electron configuration of: Na+, P3–, Al2+, Fe2+ and Sm3+
Solution
• First, write out the electron configuration for each parent atom. We
have chosen to show the full, unabbreviated configurations to provide
more practice for students who want it, but listing the core-abbreviatedelectron configurations is also acceptable.
• Next, determine whether an electron is gained or lost. Remember
electrons are negatively charged, so ions with a positive charge
have lost an electron. For main group elements, the last orbital gains
or loses the electron. For transition metals, the last s orbital loses anelectron before the d orbitals.
Application activity7.5
1. The electron energy levels of a certain element can be represented
as 1s2, 2s2, 2p6, 3s2, 3p4
a) What is the atomic number of the element?
b) What is the name of the element?
2. The element nitrogen forms compounds with metals and nonmetals.
Nitrogen forms a nitride ion with the electron configuration
1s2 2s2 2p6.
a) Write the formula of the nitride ion.
b) An element forms an ion Q with a single negative charge that has
the same electron configuration as the nitride ion. Identify the ionQ.
3. tUsing the noble gas notation, write the electronic configuration of
the following atoms/ions.
a) Ge (Z=32)
b) S (Z=16)
c) Co2+ (Z=27)
d) Br- (Z=35)e) Sr (Z=38)
SKILLS LAB
“ELECTRON CONFIGURATION BINGO ACTIVITY”
Introduction
The wave-mechanical model of the atom states that the exact position of
an electron at any given moment cannot be determined. Instead, electrons
are located in clouds outside the nucleus. These clouds are described by
energy level and type of sublevel. An electron configuration may be written
to identify the placement of electrons in these levels and sublevels.
Objectives
1. Determine electron configurations for given elements.
2. Identify elements given their electron configurations.
Materials: (per student)
• 1 bingo card
• 25 bingo markers
• 1 periodic table
Procedure
1. Choose 25 elements from the provided list.
2. On your bingo card, fill in each box with either the symbol of the
chosen element or its electron configuration. DO NOT WRITEBOTH.
3. Your tutor will call out either the electron configuration or an element.
4. From the question, determine either the element or the electron
configuration. Mark your card appropriately. For example, if the
question is “Oxygen”, you may mark your card only if you have 1s2
2s2 2p4. If the question is “1s2 2s2 2p6”, you may mark your card
only if you have Ne.
5. The winner of the game is the first person to have 5 squares in a row marked.Element List
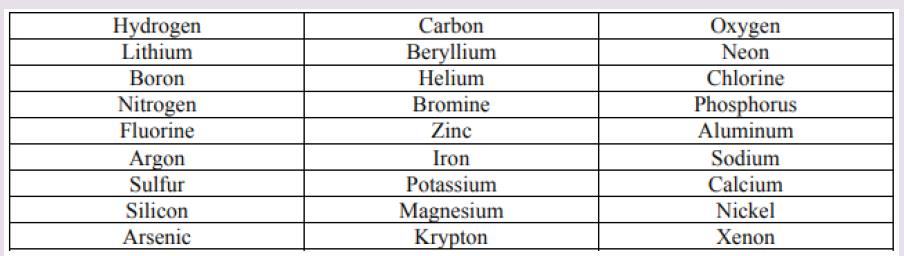
End unit assessment 7
1. For each of the following, choose the letter corresponding to the
best answer.
a) The principal quantum number describes the following, except
i. The size of the shell
ii. The energy of an electron in an atom
iii. The shape of the orbital
iv. The average distance of the orbital from the nucleus
b) On the following list of quantum numbers, the one which is not
correct is:
i. Principal
ii. Spin
iii. Magmatic Quantum
iv. Azimuthal
c) The electron configuration for gallium (Z=31) is:
i. [Ar] 4s24d104p1
ii. [Ar] 4s23d104p1
iii. [Ar] 4s23d103p1
iv. None of the above.
d) The four other spectral line series, in addition to the Balmer series,
are named after their discoverers. They are, except:
i. Lyman
ii. Pfund
iii. Brackettiv. Planck
2. According to the Aufbau principle, which orbital is filled
immediately before each of the following?
a) 3p
b) 4p
c) 4f
d) 5d
3. Hafnium element has 72 electrons. Write its s, p, d, f electron
configuration.
4. Why are the outer-most electrons the only ones included in the
electron dot diagram?
5. The orbital filling diagram has arrows pointing in opposite
directions when two electrons occupy the same orbital. What do
these arrows indicate?
6. The emission spectrum of hydrogen consists of several series
of lines. The series of highest energy is called the Lyman series
(see Figure below). Each line in the series is the result of anelectronic transition between energy levels.
a) State in which direction the energy increases: A to G or G to A.
b) State in which direction the frequency increases A to G or G to A.
c) Explain why the spectrum consists of lines.
d) What do transitions in the same series all have in common?
7. a) Write the electronic configuration of the following elements/
ions:
“Sodium, magnesium ion (Mg2+), aluminium, aluminium ion (Al3+),
oxygen ion (O2-)”
b) Identify the common feature of ions in (a) and why do they have
such feature.
c) Suggest what happened to aluminium atom when it changed to
aluminium ion (Al3+).
d) Identify the group and the period of aluminium, sodium and
oxygen atom.
8. Four possible electron configurations (A, B, C and D) for a nitrogen
atom are
a) Which one is the correct electron configuration?
b) Which configurations violate the Pauli Exclusion Principle?c) Which configurations violate Hund’s rule?
9. Complete the electronic configurations for the sulphur atom, S,
and the sulphide ion, S2-. State the block in the Periodic Table in
which sulphur is placed and explain your answer.10. The diagram below shows the electronic structure of boron.
a) The electrons are represented by arrows. What property of the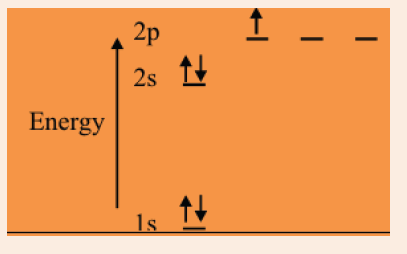
electrons do these ‘up’ and ‘down’ arrows represent?
b) Suggest why electrons which occupy the 2p sub-levels have ahigher energy than electrons in the 2s sub-level.
UNIT 8:KIRCHHOFF’S LAWS IN ELECTRIC CIRCUITS
Key unit competence
Analyze complex electric circuits using Kirchhoff’s laws.
Introductory Activity 8
Look carefully and try to interpret the following illustrations and answer thequestions below:
a) What type of electrical devices available in the illustration above?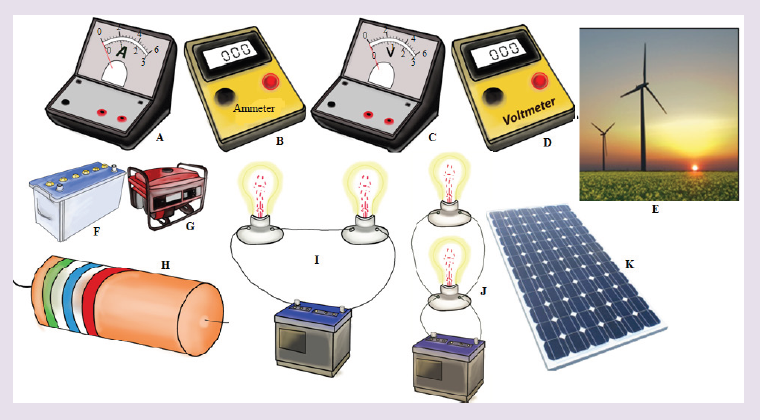
b) Suggest the names of the available devices in the illustration above?
c) Is there any complete circuit in the illustration above? What kind of
electrical circuits identified in the illustration above?
d) Have you ever used or connected these electrical components
somewhere? If yes, what were the difficulties in handling these
electrical components in circuit construction?
e) What can be considered to select the best electrical device(s) to be
used in electrical circuit construction?
f) What can be put in recognition when manipulating these electrical
components to minimize risks in the process of circuit construction?
8.1 Simple electric circuit and its construction
Activity 8.1
Making a simple electric circuit with a battery, bulb(s) , and wires
Task1: Making a series circuitProvided materials: 1 battery,aswitch,3 pieces of copper wire and bulb(s).
Technical procedures: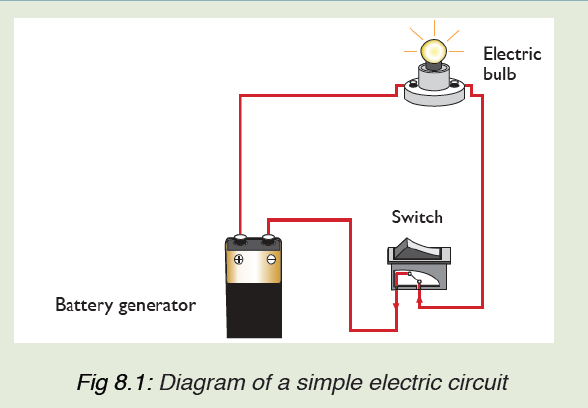
i. Arrange the battery as shown in figure above.
ii. Connect the bulb in series with other components and switch on.
iii. Switch off and explain what happens to the bulb?
iv. Explain what makes the bulb light?
Task 2 :Making a parallel circuit
Provided materials: 1 battery,3 bulbs,Assembled battery holder, 3 bulb
holders and 4 pieces of copper wire.
Technical procedures:
i. Construct a complete circuit with one battery and one bulb.
ii. Using other two wires, add a second bulb as shown in the figurebelow.
iii. What do you notice happened to the first bulb when the second bulb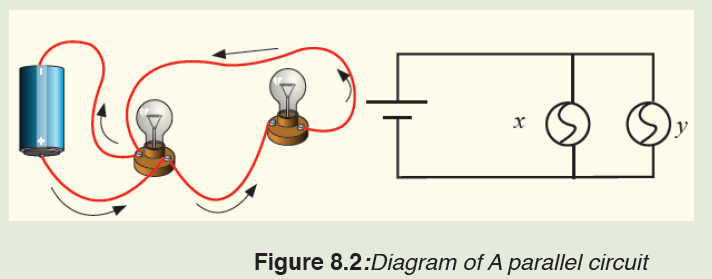
was added?
iv. Look carefully at how a parallel circuit is set up. Write a prediction
of what you think will happen if you unscrew one of the bulbs in the
parallel circuit. By comparing your prediction and the observation,
explain your observation?
v. Unscrew bulb “X”. Describe what happens to bulb “Y”.
vi. Tighten bulb “X” and unscrew bulb “Y”. Describe what happens to
bulb “X”.
vii. Based on the performed experiments for series and parallel circuits,
a) What advantages and disadvantages can you recognize in two
cases above?
b) Identify the characteristics of a series connection and a parallel
connection?
Task 3: Making a simple electric circuit with a bulb, a battery and wires
Provided materials: 2 pieces of copper wire,1 bulb, and 1 battery
Procedure
1. Examine diagrams A-J below. Predict whether the circuit will be
complete, and record your prediction on the chart below.
2. Your tutor, with a helper, demonstrate the arrangements to testyour predictions. Record your results on the chart below
8.1.1 Electric circuit components and their symbols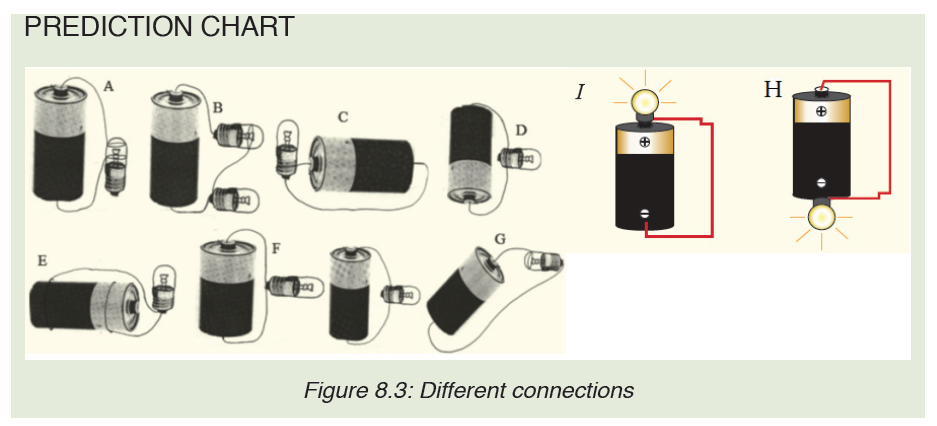
In electric circuit diagrams, we represent the actual components with
symbols. Table below shows some of the components, their symbols anddefinition that are used in electric circuit diagrams.
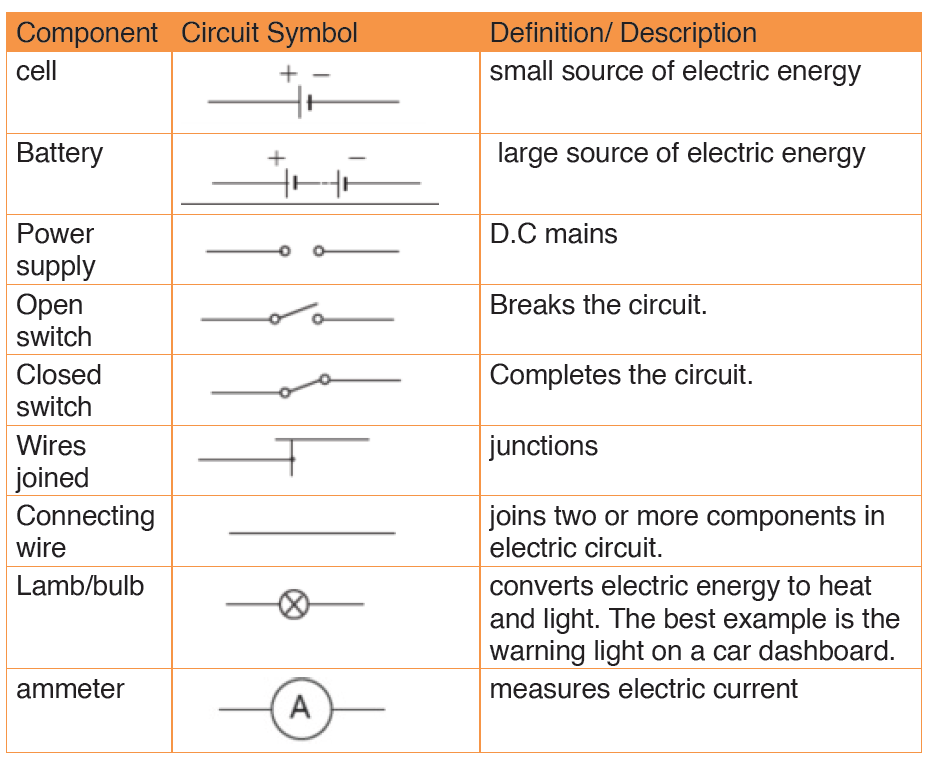
8.1.2 Sample arrangement of electric components in a simple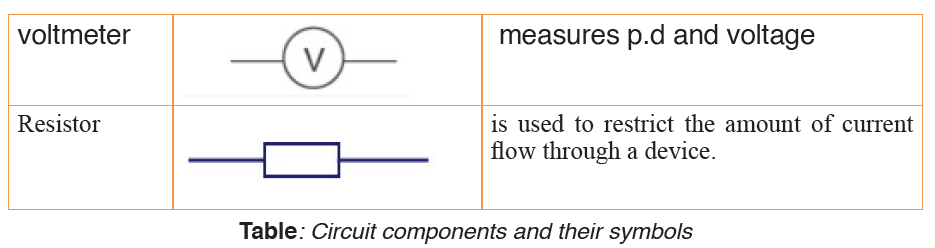
electric circuit.
Remember the cell provides electrical energy needed to light the bulb.
The bulb converts electrical energy into light and heat energy.
A cell is a kind of a ‘pump’ which provides electrical energy needed to drive
charges along a complete path formed by the wire through the bulb switch
and back again to the cell.
When the switch is open, the bulb does not light. This is called an open
circuit. When the bulb lights the circuit is called closed circuit.
In a series circuit, the current is the same at all points; it is not used up. In a
parallel circuit the total current equals the sum of the currents in the separate
branches.• Schematic diagram and its corresponding illustrations:
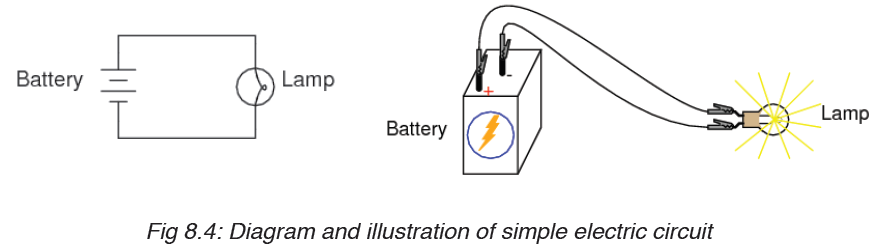
• ILLUSTRATION OF BREAK IN ELECTRIC CIRCUITS
Application activity 8.1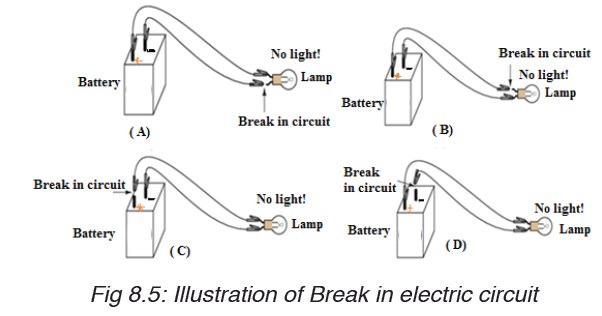
1. Define the term electric circuit.
2. Draw a diagram for a simple circuit using preferable electric
components.
3. What is an open circuit?
4. Draw a labeled diagram of a simple cell.4. The following are some symbols of electric components.
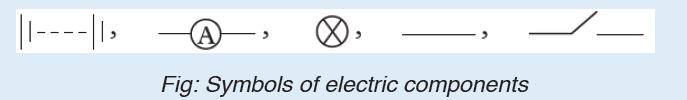
a) Name the electric components represented by these symbol.
b) Using these symbols, draw a simple circuit diagram.
8.2. Voltage or terminal potential and electromotive force in
electric circuit.
Activity 8.2
Provided different dry cells ammeter and voltmeters. Read the value ofvoltage labeled on each dry cell and complete this table.
Any other comment:
………………………………………………………………………….
….………………………………………………………………………………………………
8.2.1 Potential difference (p.d)
Potential difference is defined as the work done in moving one coulomb
of charge from one point to the other in an electrical circuit. The SI unit of
potential difference is the volt (V).
In the electric circuit, the electrons move towards the positive terminal of the
battery. The battery lifts the electrons up through an electrical height. This
electrical height is called a potential.
The positive and the negative terminals have a difference in potential. Thepotential difference is also known as the voltage.
• The volt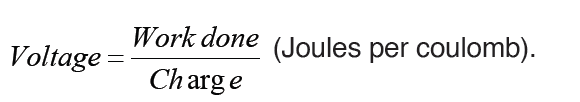
Volt is defined as energy consumption of one joule per electric charge of one
coulomb.
• MEASUREMENT OF VOLTAGE
Figure (A) shows analog voltmeter and (B) shows digital voltmeter the
symbol for a voltmeter. A voltmeter is used to measure voltage across adevice in an electric circuit.
The positive terminal of voltmeter is connected to the wire from the
positive terminal of the cells and the negative terminal to the wire leading
to negative terminal. A voltmeter is always parallel to the device whosevoltage is to be measured.
Voltmeters have uniform scales calibrated in volts or millivolts. The most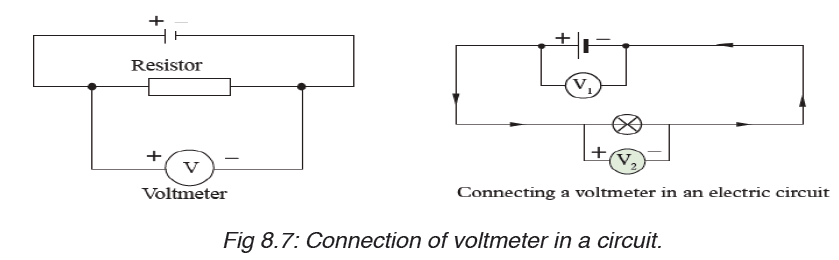
used scales have a range of 0 – 5 V and 0 – 1.5 V. Figure below shows ascale of a voltmeter.
8.2.2 Electromotive force.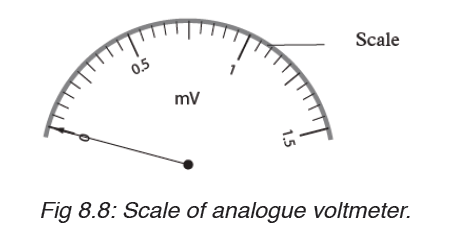
Electromotive force is voltage, or the difference in the electric tension or the
difference in charge between two points that causes an electric current.
The potential difference between two points of a conductor creates an
electromotive force which pushes free electrons in a conducting material
to move towards the positive terminal, creating current.
Electromotive force, or, as it is often written, e.m.f., may be described as that
source of energy which enables electrons to move around an electric circuit.
It is now necessary to define this quantity.
For anything to move from rest, there must be some energy change. To
enable electrons to move round an electrical circuit, they must receive
energy from the source of e.m.f. which is usually a battery or a generator.
Note:
– The terminal potential difference (or voltage) of a battery or generator
when it delivers a current, I is related to its electromotive force, and its
internal resistance r as follows:
1. When delivering current ( on discharge):Terminal voltage = Electromotive force –Voltage drop in internal resistance
V =ε − Ir
2. When receiving current ( on charge):
Terminal voltage = emf + (Voltage drop in internal resistance)
3. When no current exists:
Terminal voltage = Emf of battery or generator.
All voltage sources have two fundamental parts: a source of electrical
energy that has a characteristic electromotive force (emf), and an internal
resistance . The emf is the potential difference of a source when no current
is flowing.
The numerical value of the emf depends on the source of potential difference.
The internal resistance of a voltage source affects the output voltage when
a current flows.
The voltage output of a device is called its terminal voltage and is given by,
where I is the electric current and is positive when flowing away from the
positive terminal of the voltage source.When multiple voltage sources are inseries, their internal resistances add and their emfs add algebraically.
Consider the circuit shown in Figure 8.9.A, consisting of a battery connected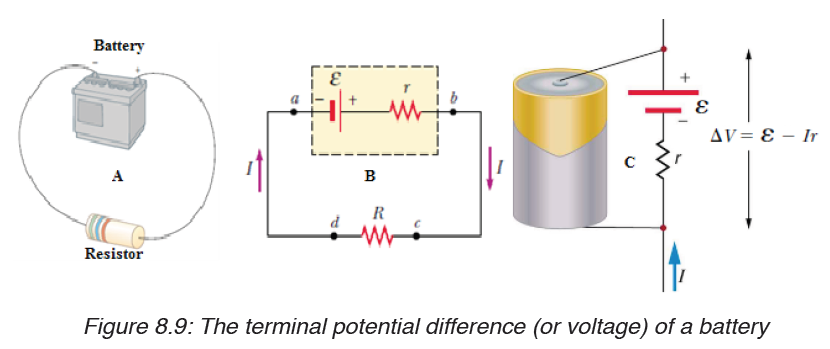
to a resistor. We generally assume that the connecting wires have no
resistance. The positive terminal of the battery is at a higher potential than
the negative terminal. Because a real battery is made of matter, there is
resistance to the flow of charge within the battery. This resistance is called
internal resistance r.
For an idealized battery with zero internal resistance, the potential difference
across the battery (called its terminal voltage) equals its emf. However, for
a real battery, the terminal voltage is not equal to the emf for a battery in a
circuit in which there is a current.
Now imagine moving through the battery from a to b and measuring the
electric potential at various locations. As we pass from the negative terminal
to the positive terminal, the potential increases by an amount . However, as
we move through the resistance r,
the potential decreases by an amount Ir, where I is the current in the circuit.
Thus, the terminal voltage of the battery,
Example 1 :
A battery has an emf of 12.0 V and an internal resistance of 0.05 Ω. Its
terminals are connected to a load resistance of 3.00Ω. Find the current in
the circuit and the terminal voltage of the battery.
Solution:It is possible to calculate current from the equation given by:
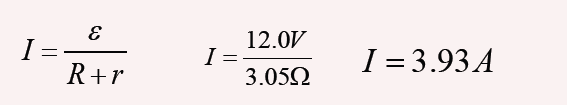
And from equation, we find the terminal voltage; ΔV =ε − Ir ,
ΔV =12.0V − (3.93A)(0.05Ω) =11.8V
To check this result, we can calculate the voltage across the load
resistance R:
ΔV = IR = (3.93 A)(3.0Ω) =11.8V
Example 2
The current in the figure below is 0.125 A in the direction shown. For each
of the following pairs of points, what is their potential difference, and which
point is at the high potential?a) A, B; b) B, C; c) C,D; d) D,E; e) C,E ; f) E,C.
Solution: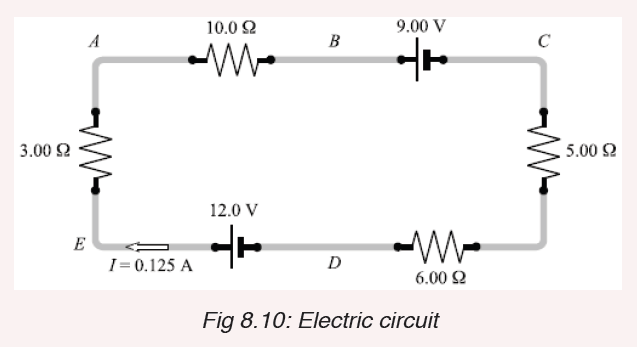
Recall the following facts:
1. The current is the same (0.125 A) at all points in this circuit because
the charge has no other place to flow.
2. Current always flows from high to low potential through a resistor.
3. The positive terminal of a pure emf (the long side of its symbol)
is always the high-potential terminal. Therefore, taking potential
drops as negative, we have the following:
Notice that the answers to e) and f) agree with each other.
Application activity 8.2
1. Define the term potential difference and state its SI units.
2. Name the instrument used to measure voltage.
3. Define a volt.
4. In a circuit, 5 joules are used to drive 2 coulombs of charge across a
bulb in a simple circuit. Find the potential difference across the bulb?
5. Name the instrument used to measure potential difference.
6. Two cells, A and B connected in parallel are in series with a bulb asshown in Figure below.
Copy the diagram and show where the: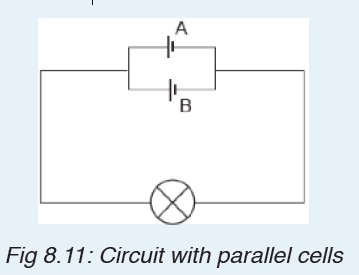
a) ammeter should be connected in order to measure the current
through cell A.
b) voltmeter should be connected to measure the potential difference
across both the bulb and cell B.
7. A dry cell has an emf of 1.52 V. Its terminal potential drops to
zero when a current of 25 A passes through it. What is its internal
resistance?
8.3. Electric receptors and Sources of electric current
Activity 8.3Look and interpret the illustrations and try to answer questions that follow:
a) What is a source of electric current based on the images above?
b) What is another name of electric source of energy?
c) Suggest some of electric sources you have found.
d) On the picture above are some sources of electric energy. Name
them and tell what the common role they have is.
e) Tell what energy is changed in electric energy for each device.
8.3.1 Sources of electric current
There are several different devices that can supply the voltage necessary to
generate an electric current. The two most common sources are generators
and electrolytic cells.
Generators use mechanical energy, such as water pouring through a dam or
the motion of a turbine driven by steam, to produce electricity. The electric
outlets on the walls of homes and other buildings, from which electricity to
operate lights and appliances is drawn, are connected to giant generators
located in electric power stations. Each outlet contains two terminals. The
voltage between the terminals drives an electric current through the appliance
that is plugged into the outlet.
Generator electrolytic cells use chemical energy to produce electricity.
Chemical reactions within an electrolytic cell produce a potential difference
between the cell’s terminals. An electric battery consists of a cell or group of
cells connected together.
There are many sources of electric current other than mechanical generators
and electrolytic cells. Fuel cells or engines, for example, produce electricity
through chemical reactions. Unlike electrolytic cells, however, fuel cells do
not store chemicals and therefore must be constantly refilled.
Certain sources of electric current operate on the principle that some metals
hold onto their electrons more strongly than other metals do. Platinum, for
example, holds its electrons less strongly than aluminum does. If a strip of
platinum and a strip of aluminum are pressed together under the proper
conditions, some electrons will flow from the platinum to the aluminum. As
the aluminum gains electrons and becomes negative, the platinum loses
electrons and becomes positive.
The strength with which a metal holds its electrons varies with temperature.
If two strips of different metals are joined and the joint heated, electrons will
pass from one strip to the other. Electricity produced directly by heating is
called thermoelectricity.
Some substances emit electrons when they are struck by light. Electricity
produced in this way is called photo-electricity. When pressure is applied
to certain crystals, a potential difference develops across them. Electricity
thus produced is called piezoelectricity. Some microphones work on this
principle.
Notice: An electric generator is a device which is used to produce electric
energy, which can be stored in batteries or can be directly supplied to the
homes, shops, offices, etc. Electric generators work on the principle of
electromagnetic induction. A conductor coil (a copper coil tightly wound
onto a metal core) is rotated rapidly between the poles of a horseshoe type
magnet. A conductor coil (a copper coil tightly wound onto a metal core) is
rotated rapidly between the poles of a horseshoe type magnet. The conductor
coil along with its core is known as an armature. The armature is connected
to a shaft of a mechanical energy source such as a motor and rotated. The
mechanical energy required can be provided by engines operating on fuels
such as diesel, petrol, natural gas, etc. or via renewable energy sources
such as a wind turbine, water turbine, solar-powered turbine. When the coil
rotates, it cuts the magnetic field which lies between the two poles of the
magnet. The magnetic field will interfere with the electrons in the conductor
to induce a flow of electric current inside it.
Physical sources such as batteries and generators may be regarded as
approximations to ideal voltage sources. Figure below shows the symbolsfor independent voltage sources.
Cells connected in Series or parallel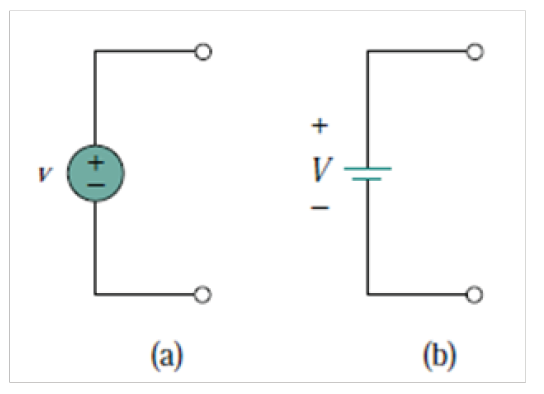
The electromotive force (e.m.f.), ε , of a cell is the p.d. between its terminals
when it is not connected to a load (i.e. the cell is on ‘no load’).
The e.m.f. of a cell is measured by using a high resistance voltmeter
connected in parallel with the cell. The voltmeter must have a high resistance
otherwise it will pass current and the cell will not be on no-load.
The voltage available at the terminals of a cell falls when a load is connected.
This is caused by the internal resistance of the cell which is the opposition
of the material of the cell to the flow of current.
The internal resistance acts in series with other resistances in the circuit. the
figure below shows a cell of e.m.f. ε volts and internal resistance, r , and XYrepresents the terminals of the cell.
When a load (shown as resistance R) is not connected, no current flows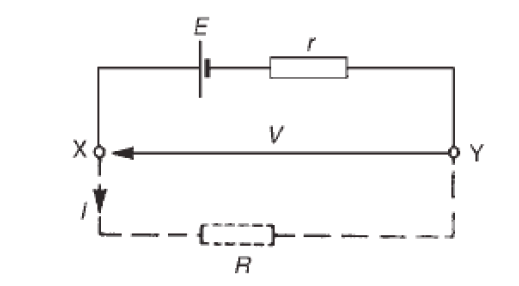
and the terminal p.d., V =ε . When R is connected a current I flows which
causes a voltage drop in the cell, given by r V = Ir . The p.d. available at the
cell terminals is less than the e.m.f. of the cell and is given by:
V =ε − rI
When different values of potential difference V, across a cell or power supply
are measured for different values of current I, a graph may be plotted as
shown in figure below. Since the e.m.f. ε of the cell or power supply is the
p.d. across its terminals on no load (i.e. when I = 0 ), then ε is as shown bythe broken line.
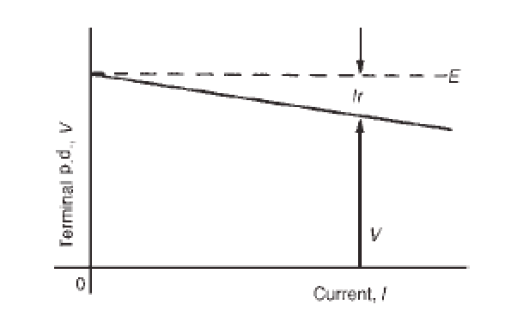
Since V =ε − rI then the internal resistance may be calculated from
When a current is flowing in the direction shown in Figure above the cell is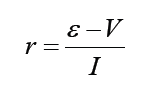
said to be discharging (ε >V )
When a current flows in the opposite direction to that shown in Figure
above the cell is said to be charging ε <V
A battery is a combination of more than one cell. The cells in a battery
may be connected in series or in parallel.
(i) For cells connected in series:Total e.m.f. εeq sum of cell’s e.m.f.
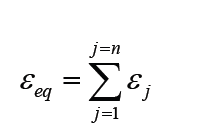
Total internal resistance req = sum of cell’s internal resistances
Example 1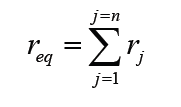
1. Three cells each of emf of 1.5 V and internal resistance 0.6 Ω are connected
in series to form a battery. What current passes or flows when
the battery is connected across a 5 Ωresistance and what is the potential
difference across the terminal of the battery?Answer
The pd across the terminal of battery: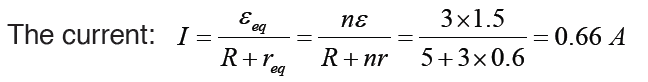
V = nε − nri = 3×1.5 − 3×0.6×0.66 = 3.31V
(ii) For cells connected in parallel:
If each cell has the same e.m.f. and internal resistance:Total e.m.f. ε eq = e.m.f. of one cell i.e.ε eq =ε
Total internal resistance of n cells
Example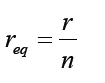
1. For the circuits shown in Fig. the resistors represent the internal resistance
of the batteries. Find, in each case:
i. the total e.m.f. across PQii. the total equivalent internal resistances of the batteries
8.3.2 Electrical receptors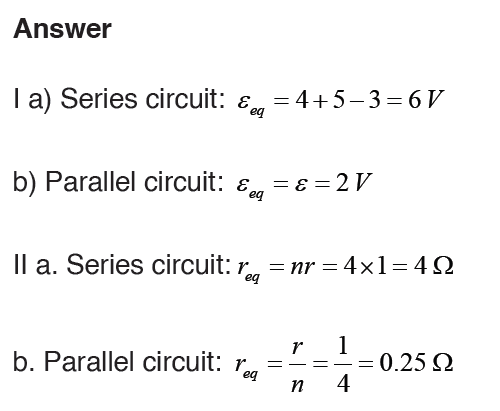
A receptor is any electrical device that can transform electrical energy into
any other form of energy. Example: the lamp, the motor, clippers, iron,
electric cookers.
Passive receptors (thermal receptors) transform electrical energy into
heat energy only by the joule effect. Examples: bulbs, electric irons, electric
heaters.
Active receptors are capable of transforming electric energy not only into
heat energy but also into other forms of energy. Examples: electric motors,
batteries (secondary cells).
An active receptor is characterized by its counter-electromotive force and its
internal resistance.
The counter-electromotive force also known as back electromotive force
(cemf). It is its capacity of transforming a part of energy (electricity) in other
form of energy (except heat). It is also the voltage, or electromotive force, that
pushes against the current which induces it. CEMF is caused by a changing
electromagnetic field. Back electromotive force is a voltage that occurs in
electric motors where there is relative motion between the armature of the
motor and the external magnetic field.
The internal resistance of an active receptor is the measure of its capacity
to absorb heat energy by the joule effect when a current is flowing through it.Back electromotive force of an active receptor is the ratio:
where P is the Power it transforms into energy other than heat and I electric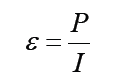
current.
Receptors are often associated in parallel because in series there will be
a big loss of energy. And to associate receptors in series, we will need a
generator which also will be in series.
In Series
If we have n identical receptors characterized by cemf εk and rk for each
receptor, k =1, 2,3,...,n k =1, 2, 3, ......n all in series with a cell (E) and external
resistance R e, the total internal resistances of receptors will be rt= nr k andthe total cemf εk = nεk Then the intensity is
In Parallel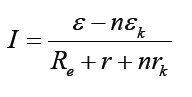
As we know that for parallel the potential difference is the same for each
blanch, here also if we have n receptors in parallel, the c.e.m.f of one is thesame for others. The total internal resistance is
The power consumed by the receptor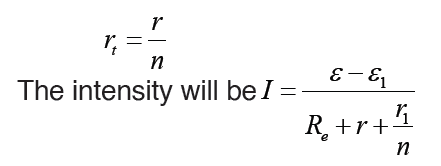
The electric energy produced by the cell to the receptor transforms partially
into heat (Joule’s effect) into chemical energy (voltameter) or into mechanical
energy (motor). The energy produced per second by cell is:P =ε I
Application activity 8.3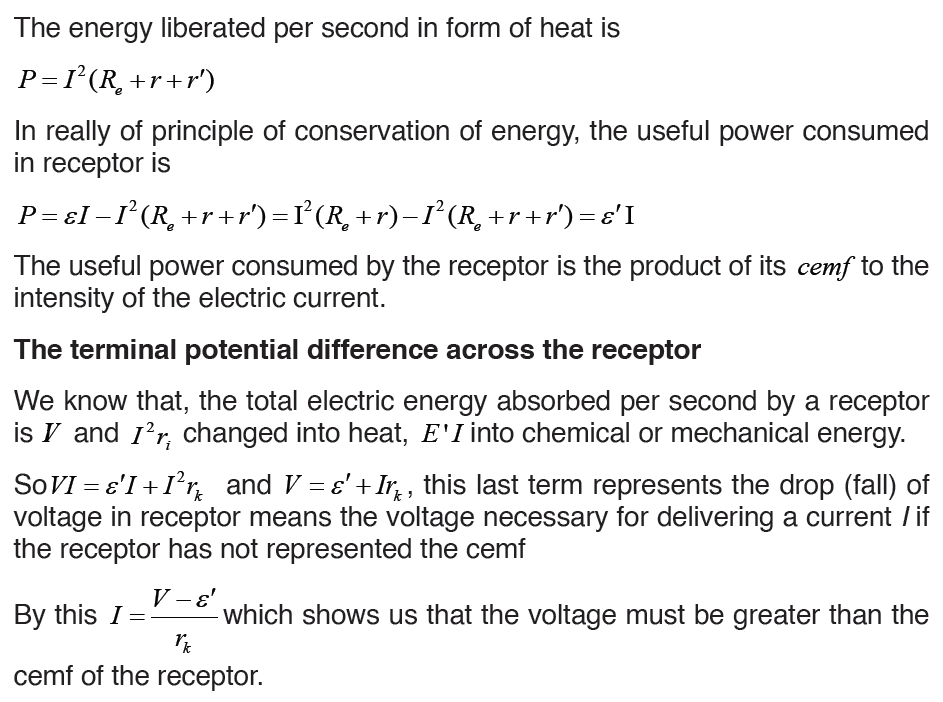
1. Explain the difference between load resistance in a circuit and internal
resistance in a battery.
2. Is the direction of current through a battery always from the negative
terminal to the positive terminal? Explain.
3. A real battery with an emf of provides 50 W to an external resistance
of 4.
a) Find the internal resistance of the battery.
b) For what value of external resistor the supplied power is 100 W?
4. What is the internal resistance of the battery in the following circuit?
5. A battery has an emf of 15.0 V. The terminal voltage of the battery is
11.6 V when it is delivering 20.0 W of power to an external load resistor R.
a) What is the value of R?b) What is the internal resistance of the battery?
8.4. Connection of resistors either in series or parallel or
mix-up
Activity 8.4
Task1: Arrangement of resistors in series circuits.
Provided materials: Battery cells, three torch light bulbs and 4
conducting wires
Technical procedures:
– Arrange the battery cells as shown in figure below and connect all the
two bulbs in series and switch on.
– Remove one bulb and notice what happens based on your
observations.
– Arrange the circuit to have two bulbs, and then to have one bulb andnotice the observation.
Use your observations to answer the following questions: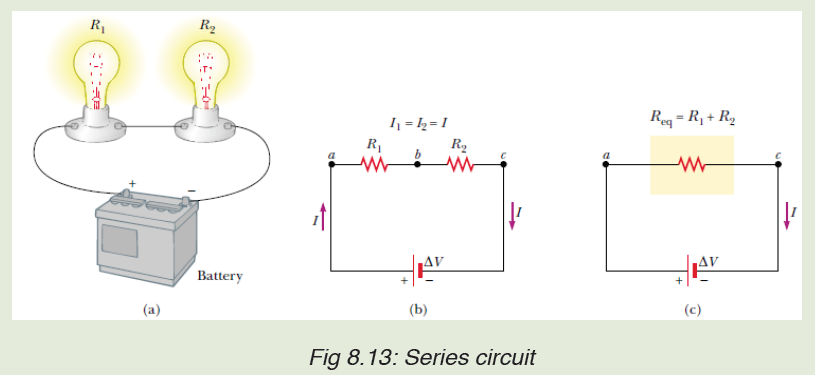
a) What happens in the circuit with three bulbs when one bulb is removed?
b) What happens when the circuit has two bulbs?
c) What happens when the circuit has one bulb only?
Task 2: Arrangement of resistors in series circuits
Technical Procedures:
– Arrange the battery cells as shown in figure below and connect all the three
bulbs in parallel and switch on.
– Remove one bulb and notice your observations. Remove the second bulband notice your observations.
Use your observation to answer to questions below: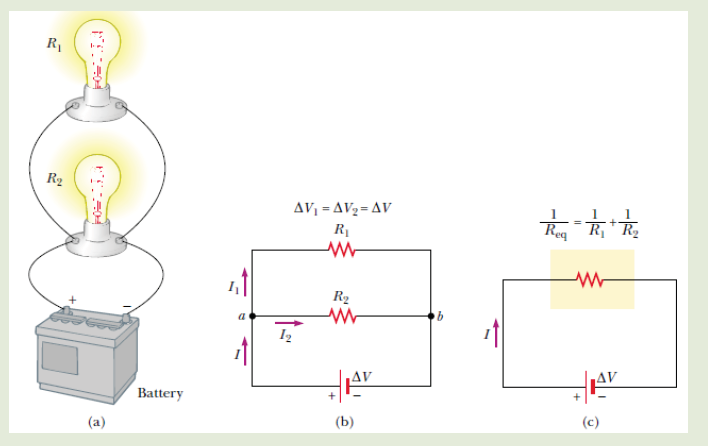
a) Explain what happens in the circuit with two bulbs when one bulb
is removed?
b) What happens when the circuit has two bulbs?
c) What happens when the circuit has one bulb only
Circuits consisting of just one battery and one load resistance are very
simple to analyze, but they are not often found in practical applications.
Usually, we find circuits where more than two components are connected
together. There are two basic ways in which to connect more than two circuit
components: series and parallel.
8.4.1 Resistors in series
The defining characteristic of a series circuit is that there is only one path for
electrons to flow. Consider three resistors R1, R2 and R3 connected in series
across a battery of potential difference V. Across the resistors the potential
difference drops are V1, V2 and V3 but the current flow I is constant due to thesame amount of charges flowing across each resistor.
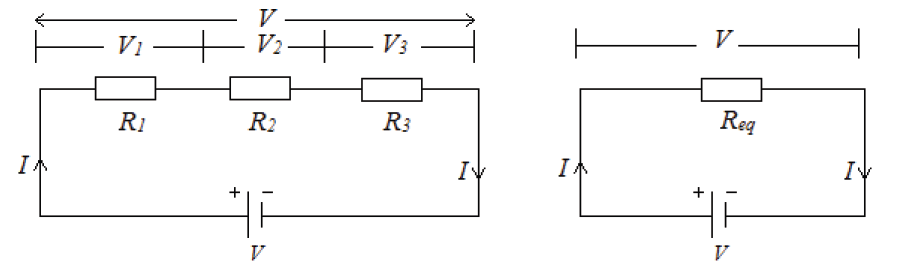
For any n resistors connected in series combination, the effective resistance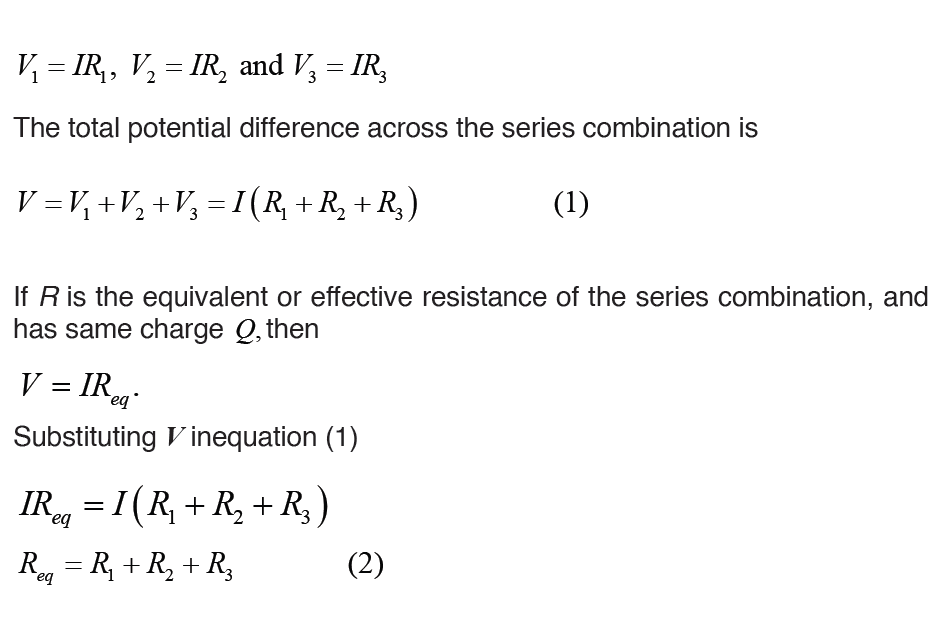
is
Req = R1 + R2 + R3 + R......n
8.4.2 Resistors in parallel
The defining characteristic of a parallel circuit is that all components are
connected between the same set of electrically common points and the
resistors form more than one continuous path for electrons to flow.
Assume three resistors of resistance R1, R2 and R3connected in parallel
across a battery of potential difference V. The potential difference across
each resistor is the same and is equal to the potential difference V across
the battery, but the current flow splits into three parts I1, I2 and I3 due to theseparation of charges
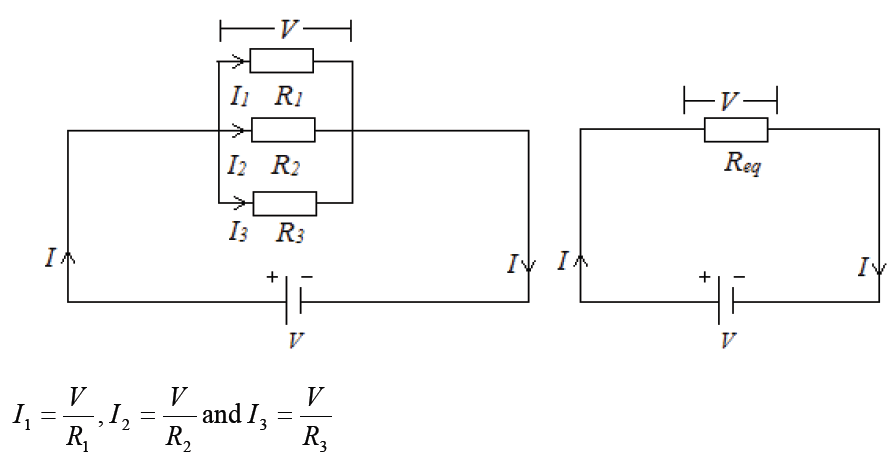
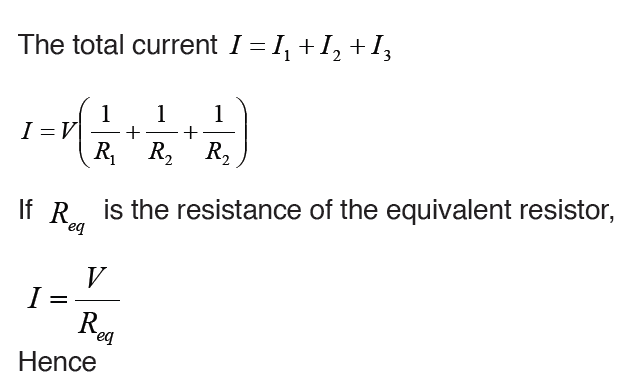
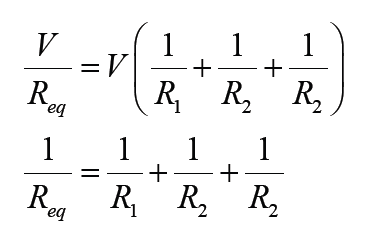
For n resistors connected in parallel combination, the effective resistance is
Household circuits are always wires so that the lights and appliances are
connected in parallel. This way each device operated independently of
the others, so if one is turned off the others remain on. This also had the
advantage that the voltage supplied to each element is the same.Example1: Find the equivalent resistance in the circuit below:
Example 2: A parallel circuit is shown in the figure below. In this case the
current supplied by the battery splits up, and the amount going through
each resistor depends on the resistance.
From the figure below, if the values of the three resistors are
R1 = 8Ω, R2 = 8Ω, and R3 = 4Ω .Determine the total resistance of the circuit.
Solution:
The total resistance R is found by
This gives that R = 2Ω
With a 10 V battery, by V = I R the total current in the circuit is:
Note that the currents add together to 5A, the total current.
Example 3: Interpret the circuit below and determine the total resistanceof the circuit.
Solution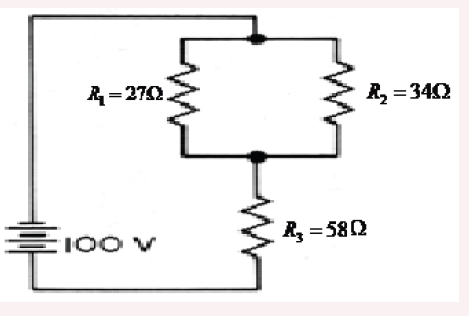
Here we can use the shorter product over sum equation as we only havetwo parallel resistors.
1. Use the concept of equivalent resistance to determine the unknown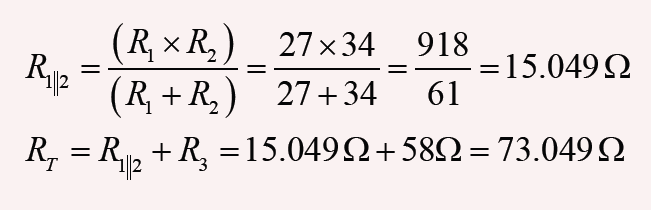
resistance of the identified resistor that would make the circuit’s equivalent.
2. A parallel pair of resistance of value of 3Ω and 6Ωare together connected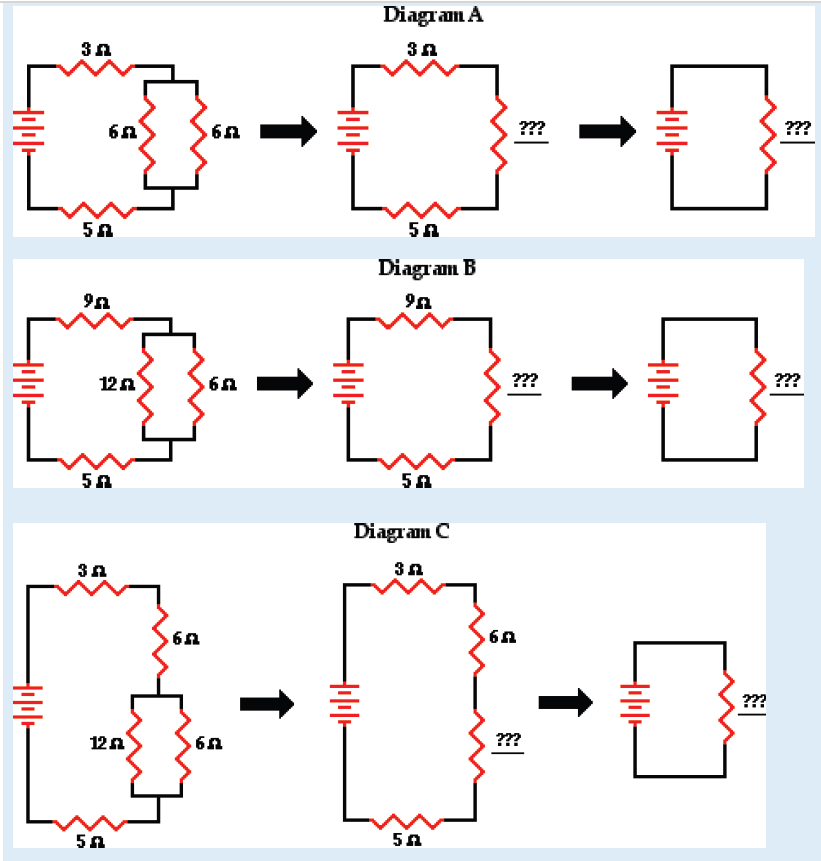
in series with another resistor of value 4 Ω and a battery of e.m.f. 18 V asshown on the fig. (a) below. Calculate the current through each resistor.
3. (a) Find the equivalent resistance between points a and b in Figure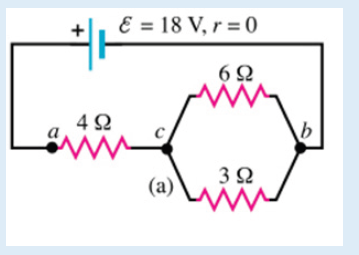
below. (b) A potential difference of 34.0 V is applied between points aand b. Calculate the current in each resistor.
4. Use your understanding of equivalent resistance to complete the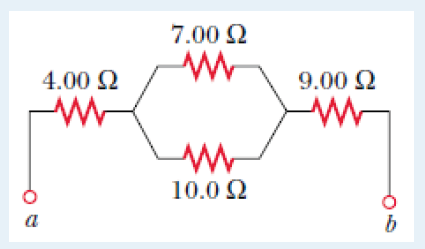
following statements:
i. Two 3 Ω resistors placed in series would provide a resistance which
is equivalent to one ___ Ω resistor.
ii. Three 3 Ω resistors placed in series would provide a resistance
which is equivalent to one ___ Ω resistor.
iii. Three 5 Ω resistors placed in series would provide a resistance
which is equivalent to one ___ Ω resistor.
iv. Three resistors with resistance values of 2 Ω, 4 Ω and 6 Ω are
placed in series. These would provide a resistance which is equivalent
to one ___ Ω resistor.
v. Three resistors with resistance values of 5 Ω, 6 Ω and 7 Ω are
placed in series. These would provide a resistance which is equivalent
to one _____ Ω resistor.
vi. Three resistors with resistance values of ,3 Ω and are
placed in series. These would provide a resistance which is equivalent
to one _____ Ω resistor.
5. As the number of resistors in a series circuit increases, the overall
resistance __________ (increases, decreases, remains the same) and
the current in the circuit __________ (increases, decreases, remains the
same).
6.Imagine that we add a third resistor in series with the first two. Does the
current in the battery (a) increase, (b) decrease, or (c) remain the same?
Does the terminal voltage of the battery (d) increase, (e) decrease, or (f)remain the same?
7. Imagine that we add a third resistor in parallel with the first two. Does the
current in the battery (a) increase, (b) decrease, or (c) remain the same?
Does the terminal voltage of the battery (d) increase, (e) decrease, or (f)
remain the same?
8.5. Kirchhoff’s laws and its applications in solving
problems in complex electric circuits
Activity 8.5
A single-loop circuit contains two resistors and two batteries, as shown in
figure 5.34 (neglect the internal resistances of the batteries). (a) Find the
current in the circuit. (b) What power is delivered to each resistor? Whatpower is delivered by the 12V battery?
8.5.1 Kirchhoff’s laws
Simple circuits can be analyzed using the expression V = IR and the rules for
series and parallel combinations of resistors. Very often, however, it is not
possible to reduce a circuit to a single loop.
The procedure for analyzing more complex circuits is greatly simplified if
we use two principles called Kirchhoff’s rules developed by the German
Physicist Gustav Robert Kirchhoff (1824-1887).
First, here are two terms that we will use often. A junction in a circuit is a
point where three or more conductors meet. Junctions are also called nodes
of branch points. A loop is any closed conducting path.
In figure below, the points a and b are junctions, but points c and d are not.The curved lines show some possible loops in this circuits.
Kirchhoff’s junction rule: “the algebraic sum of the currents into any
junction is zero.” That is,
i.e The sum of the currents entering the junction must equal the sum of the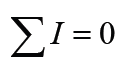
currents leaving the junction.
Kirchhoff’s loop rule: “the algebraic sum of the potential differences in
any loop, including those associated with emfs and those of resistiveelements must equal zero”. That is,
Kirchhoff’s first rule is a statement of conservation of electric charge. All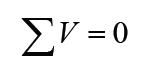
charges that enter a given point in a circuit must leave that point because
charge cannot build up at a point.
Kirchhoff’s second rule follows from the law of conservation of energy.
The sum of the increases in energy as the charge passes through some
circuit elements must equal the sum of the decreases in energy as it passes
through other elements. The potential energy decreases whenever the
charge moves through a potential drop –IR across a resistor or whenever it
When applying Kirchhoff’s second rule in practice, we imagine traveling
around the loop and consider changes in electric potential, rather than the
changes in potential energy.
Problem solving strategy:
1. Junction rule. Assign symbols and directions to the currents in the
various junctions. If you guess the wrong direction for a current it
does not matter. The end result will be a negative answer for that
current and the magnitude will be correct.
2. Loop rule. You must choose a direction for moving around the loop.
As you move around the loop the voltage drops and increases should
be recorded according to the rules (a-d) below.
a) If a resistor is traversed in the direction of the current, the change in
potential across the resistor is –IR.
b) If a resistor is traversed in the direction opposite the current, the
change in potential across the resistor is +IR.
c) If a source of emf is traversed in the direction of the emf (from – to +)
the change in potential is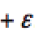
d) If a source of emf is traversed opposite the direction of the emf (from
+ to –) the change in potential is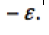
moves in the reverse direction through a source of emf. The potential energy
increases whenever the charge passes through abattery from the negativeterminal to the positive terminal.
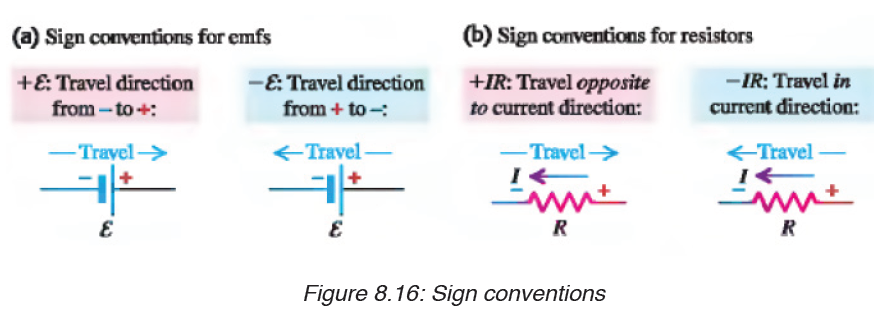
Example
1. In figure below, find I1, I2, and I3 if S is
a) Open
b) Closed
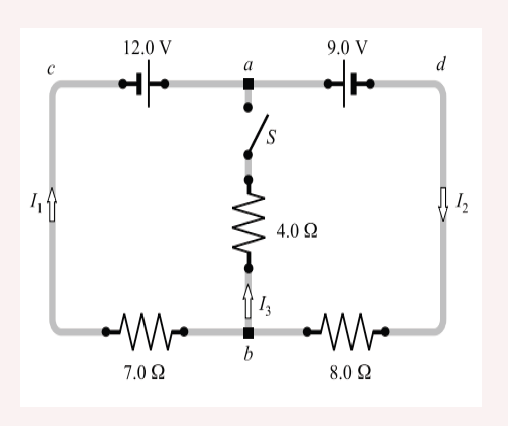
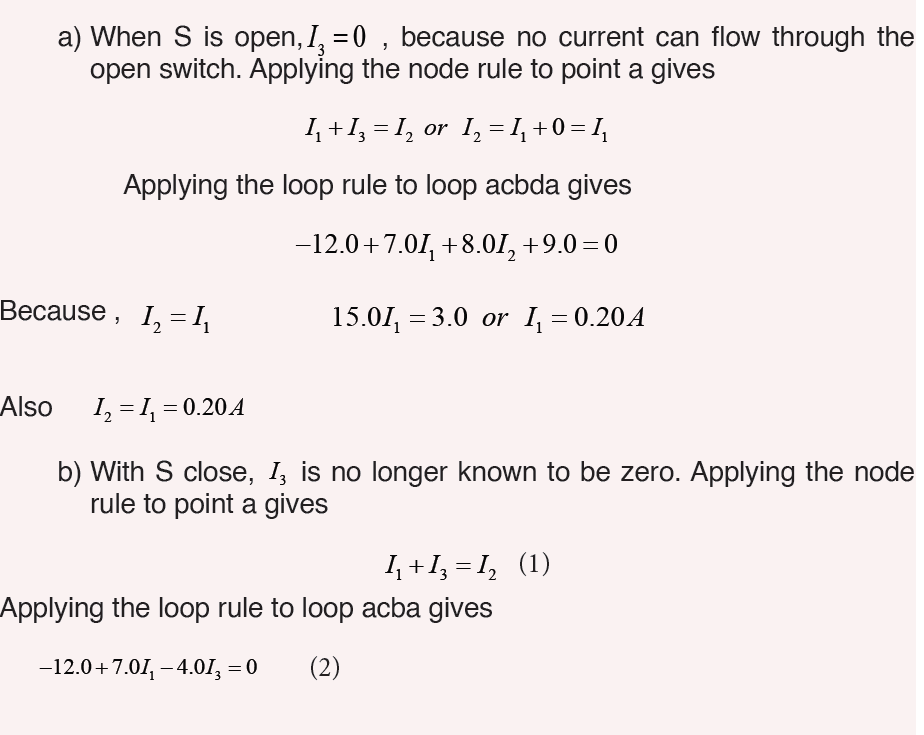
8.5.2 Application of Kirchhoff’s laws in solving problems in
complex electric circuitsExample 1: Find the currents in the circuit given below:
Solution: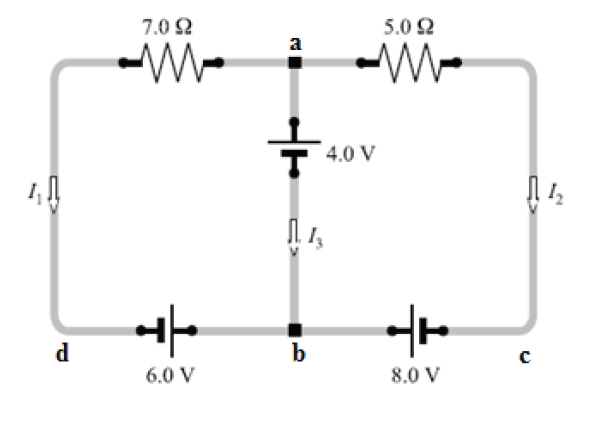
This circuit cannot be reduced further because it contains no resistors in
simple series or parallel combinations. We therefore revert to Kirchhoff’s
rules. If the currents had not been labeled and shown by arrows, we would
do that first. No special care needed to be taken in assigning the current
directions, since those chosen incorrectly will simply give negative numerical
values.
We apply the node rule to node b in the figure above.Current into b = Current out of b.
The minus sign tells us that I3 is opposite in direction to that shown in the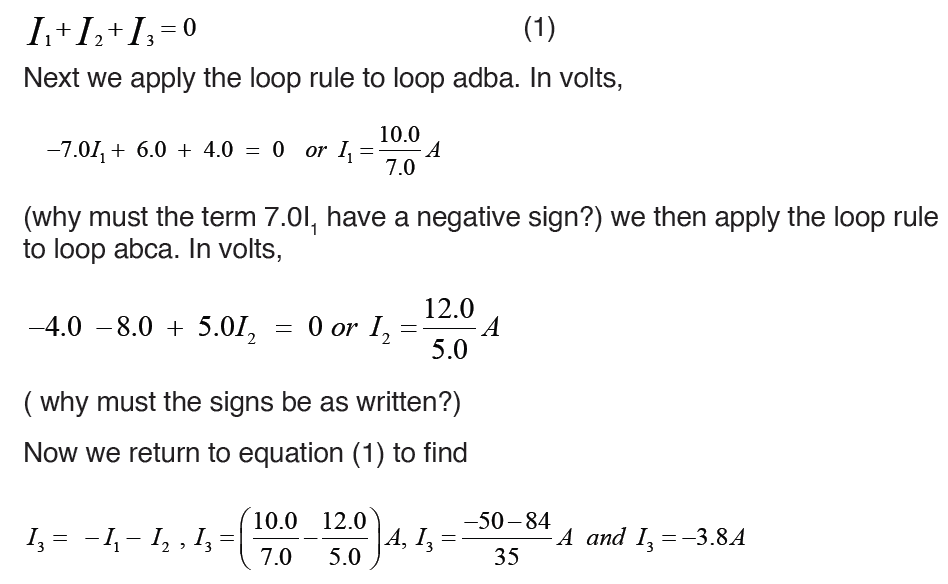
figure.
Example 2: The circuit shown in figure below contains two batteries, each
with an emf and an internal resistance, and two resistors. Find (a) the current
in the circuit (magnitude and direction); (b) the terminal voltage of the 16.0V battery; (c) the potential difference of a with respect to point c.
Solution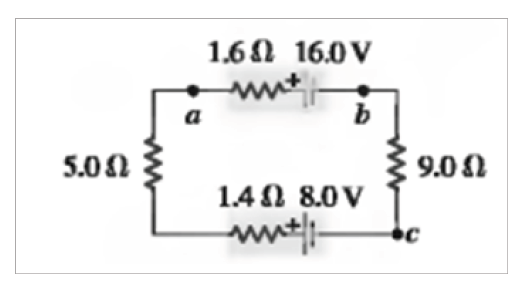
(a) The current is counterclockwise, because the 16 V battery determinesthe direction of current flow.
Example 3: In figure below, the battery has an internal resistance of 0.7Ω .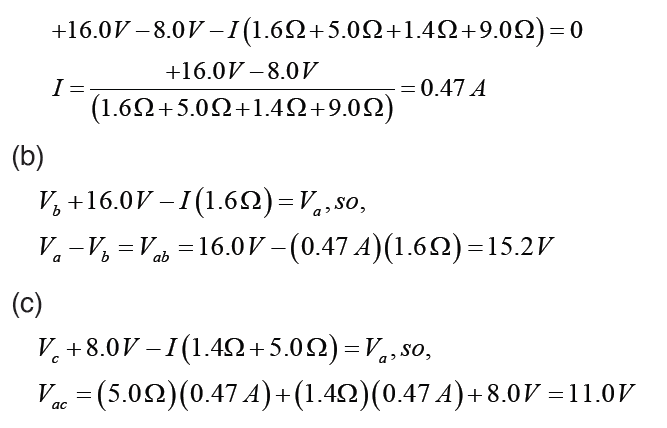
Find
i. the current drawn from battery,
ii. the current in each resistor,iii. The terminal voltage of the battery.
Solution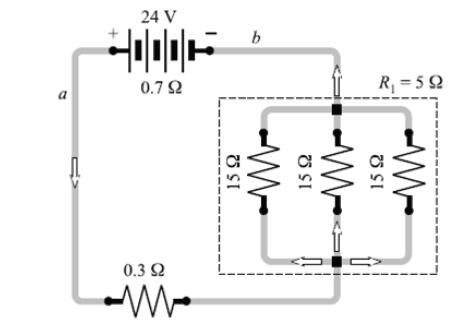
i. For parallel group resistance we have
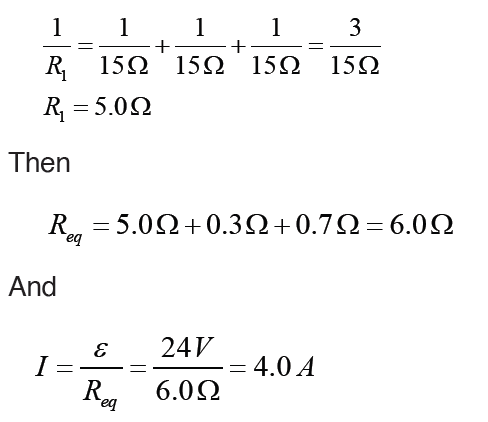
Method 2
In this special case, we know that one – third of the current will go througheach resistor. Hence
Application activity 8.5
In the circuit below, each cell has e.m.f of 1.5 V and zero internal resistance.
Each resistor has a resistance of 10Ω . There are currents I1 and I2 in thebranches as shown.
(a) Use Kirchhoff’s first law to write down an expression for the current in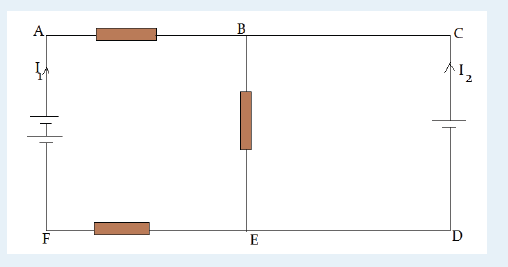
BE, in terms of I1 and I2
(b) Use Kirchhoff’s second law to write down equations for the circuit loops
i) ABEFA ; ii) CBEDCNote that you are not required to solve these equations.
SKILLS LAB
Conduct a survey to find out how people construct electric circuits and apply
Kirchhoff’s laws in analysis of complex electric circuits before installation
process.
Collect and analyze data about when, where, and why people use
Kirchhoff’s laws in dealing with complex electric circuits.
To complete this project you must
• Develop a survey sheet about electric components, demonstration of
Kirchhoff’s laws and complex electric circuit.
• Distribute your survey sheet to other student-teachers, family
members and neighbors.
• Compile and analyze your data.
• Create a report to display your findings in your sheet.
Plan it! To get started, think about the format and content of your survey
sheet. Brainstorm what kinds of questions you will ask. Develop a plan for
involving student-teachers in your class or other classes to gather more
data.
End unit assessment 8
1. State Kirchhoff Current Law and Kirchhoff Voltage Law
2. Distinguish between electric sources of currents from receptors.3. Find the current across the 10V battery
4. The figure below shows four resistors connected in a circuit with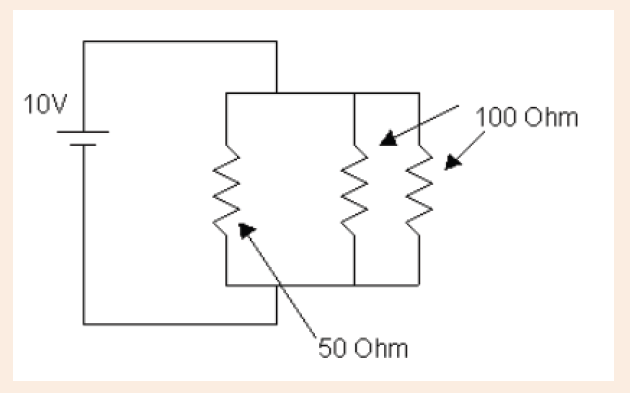
a battery. Which of the following correctly ranks the potentialdifference, ΔV , across the four resistors?
5. Determine the values of the the current flowing through each of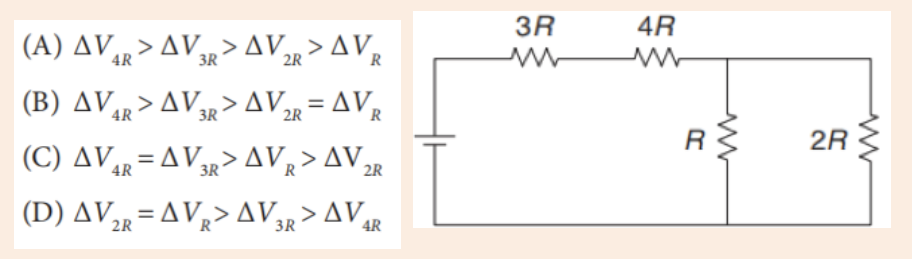
the resistors.
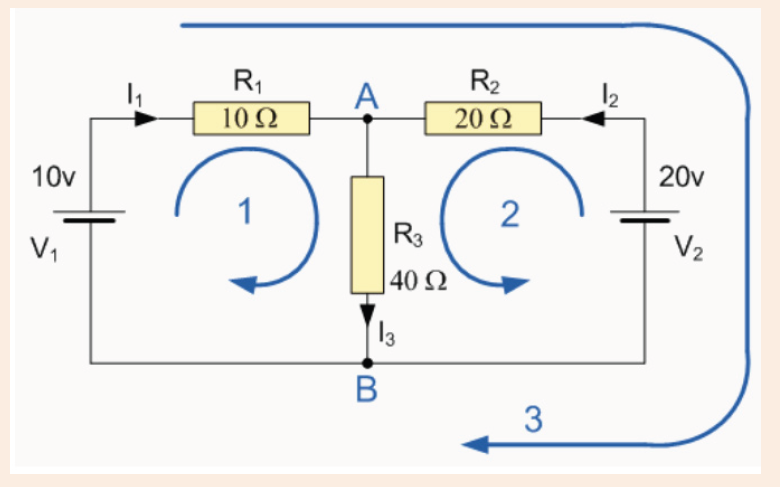
UNIT 9: AUTOTROPHIC NUTRITION
Key unit competence:
Explain photosynthesis as an energy transfer process, its limiting factors
and adaptations.
Introductory Activity
Make a quick lab
Materials: Large clear plastic cup, sodium bicarbonate solution, elodea
plant, large test tube.
Procedure:
– Fill a large clear plastic cup with sodium bicarbonate solution (source
of CO2)
– Place an elodea plant in a large test tube with the cut stem at the
bottom. Fill the tube with sodium bicarbonate solution. Caution: hand
the test tube carefully.
– Hold your thumb over the mouth of the test tube. Turn the tube over,
and lower it to the bottom of the cup. Make sure there is no air trapped
in the tube.
– Place the cup in bright light.
– After at least 20 minutes, look closely at the elodea leaves. Record
your observations.
Analyze and conclude:
a) What do you observe on the elodea leaves?
b) What substance accumulated in the leaves? Should the substance
be considered as a waste product? Explain.
c) What plant organelle carries out photosynthesis and produces thegas?
All organisms require macromolecules like carbohydrates, proteins and
fats for their growth and development. Some organisms produce these
organic compounds from inorganic sources on their own. Such organisms
are called autotrophs or producers and the process of synthesizing complex
compounds from simple inorganic sources is called autotrophic nutrition.
While others including humans are heterotrophs or consumers, which depend
on autotrophs for source of chemical energy. Green plants are autotrophs
and require chlorophyll, sunlight, carbon dioxide, water and minerals for
preparing their own food.
9.1. Types of autotrophic nutrition
Activity 9.1
From what you learnt in previous classes about plant nutrition, differentiate
the types of autotrophic nutrition.
Autotrophic nutrition is a process by which living organisms make their
own food. This process is carried out by photoautotrophs like green plants,
green algae and green bacteria; and chemoautotrophs. Living organisms
which make their own food are called autotrophs, while others, including
humans, which cannot make their own food but depend on autotrophs arecalled heterotrophs.
There are two types of autotrophic nutrition such as chemoautotrophic and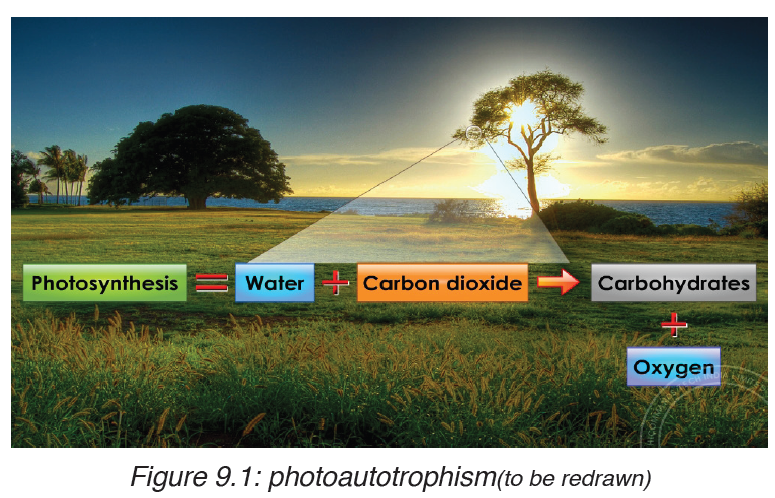
photoautotrophic nutrition.
9.1.1. Chemoautotrophic nutrition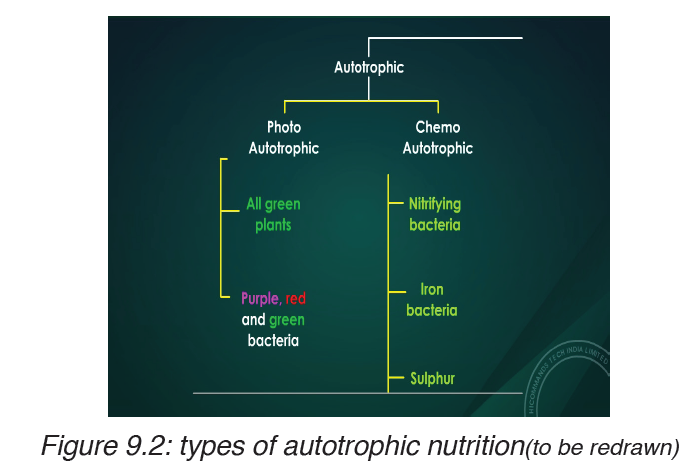
It is an autotrophic nutrition where organisms (mainly bacteria) get energy
from oxidation of chemicals, mainly inorganic substances like hydrogensulphide and ammonia.
9.1.2. Photoautotrophic nutrition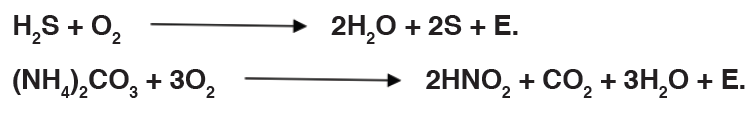
It is an autotrophic nutrition where organisms get energy from sunlight and
convert it into sugars. Green plants and some bacteria like green Sulphur
bacteria can make their own food from simple inorganic substances by a
process called photosynthesis. Photosynthesis is a process by which,
autotrophs make their own food by using inorganic substances in presence
of light energy and chlorophyll.
(Green Sulphur bacteria).(Green plants).
Application activity 9.1
1. Define Photosynthesis
2. Differentiate;
a) Autotrophs and heterotrophs
b) Chemoautotrophs and photoautotrophs.
3. Animals’ life depends on plants. Defend this statement by providing
two convincing reasons.
9.2 Structure adaptation and role of chloroplast in the
process of photosynthesis
Activity 9.2
To show that oxygen is produced during photosynthesis
Requirements:
Two large beakers, two funnels (glass), two test tubes, water with sodium
hydrogen carbonate dissolved in it, splints, match box, water weed e.g.
Elodea or SpirogyraProcedure 1: Prepare two set-ups of apparatus as shown below.
Note: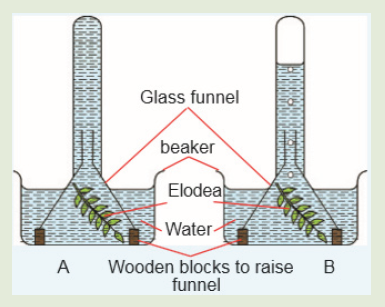
Set up A placed in a dark cupboard Set up B placed in a bright sunshine
2. Observe the set-up in the dark cupboard.
• What did you notice?
3. Observe the set-up in the bright sunshine.• What do you notice?
4. Test any gas produced using a glowing splint.
Study questions
a) Explain the necessity of sodium hydrogen carbonate (sodium
bicarbonate) dissolved in the water?
b) What happens to the glowing splint when it is exposed to the gas
in the test tubes?
• What is your conclusion from the observation?
c) What was the role of the setup that was placed in the dark cupboard?
d) Name the plant cell organelle in which photosynthesis takes place.
Plants are autotrophs because they can make their own food by using
energy from the sun, carbon dioxide and water as raw materials to make
food in a process known as photosynthesis. The chlorophyll contain by
plants traps light energy from the sun. In the process, oxygen is given off as
a by-product.The process of photosynthesis can be summarized as follows:
The chlorophyll arefound in chloroplasts.
Chloroplast is an example of a plastid. It is the organelle in a plant cell where
photosynthesis takes place. Chloroplasts are found in the cytoplasm of the
cells found in either palisade cells mesophyll, spongy mesophyll and guard
cells in a leaf. Cells that have chloroplasts are called photosynthetic
cells. To find out whether the leaf is the site for photosynthesis, we test for
the presence of starch in the leaf.
9.2.1. Structure of the chloroplast
In eukaryotes photosynthesis takes place in chloroplasts which is one of
plant cell organelles. A chloroplast contains many sets of disc like sacs called
thylakoids, which are arranged in stacks known as grana. Each granum
looks like a stack of coins where each coin being a thylakoid. In the thylakoid,
proteins are organized with the chlorophyll and other pigments into clusters
known as photosystems. The photosystems are the light-collecting units ofthe chloroplast.
The function of thylakoids is to hold the chlorophyll molecules in a suitable
position for trapping the maximum amount of light. A typical chloroplast
contains approximatively 60 grana, each consisting of about 50 thylakoids.
The space outside the thylakoid membranes are made by watery matrixcalled stroma. The stroma contains enzymes responsible for photosynthesis.
Note: Photosynthetic prokaryotes have no chloroplasts, but thylakoids often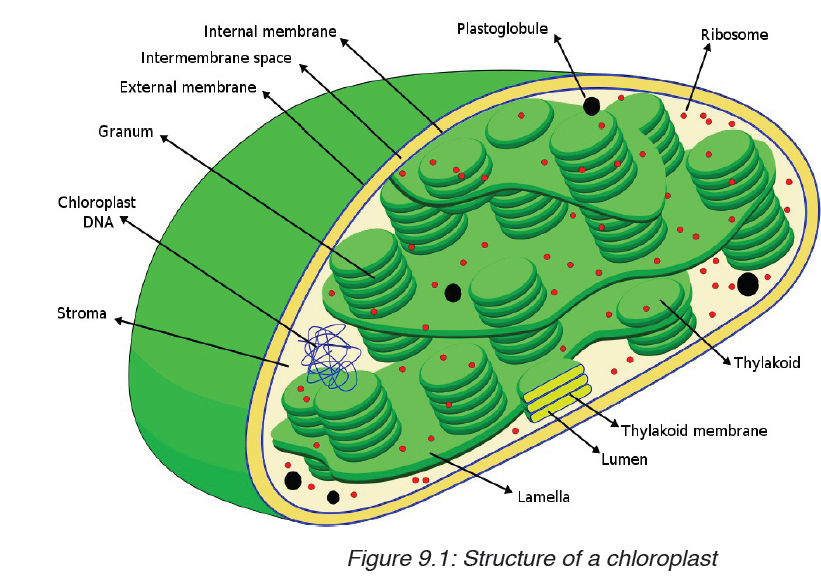
occur as extensions of the plasma membrane and are arranged around the
periphery of the prokaryotic cell.
9.2.2. Adaptations for photosynthesis
Activity 9.2.2
Sample a green leaf and analyze its structure. Observe again the illustration
showing the internal structure of a leaf to describe the adaptations of the
plants and leaf for photosynthesis.
By considering both external and internal structures of the leaf, we canrecognize several adaptations for photosynthesis.
a) Adaptation of leaf for photosynthesis considering to its internalstructure
Note: when stomata are opened, the rate of photosynthesis may be 10
to 20 times as fast as the maximum rate of respiration. If the stomata are
closed, photosynthesis still can continue, using CO2 produced during cell
respiration. The equilibrium can be reached between photosynthesis andcell respiration.
Photosynthesis uses CO2 from respiration, and respiration uses Oxygen
from photosynthesis. However, the rate of photosynthesis under these
circumstances will be much slower than when an external source of CO2 is
available. The stomata cannot remain closed indefinitely, they have to be
open in order to maintain transpiration of the plant.
b) Adaptation of leaf for photosynthesis considering its externalstructure
– Leaves are thin and flat, this facilitate absorption of the maximum
amount of light.
– The cuticle is transparent to allow absorption of light into tissues.
– Presence of a waxy substance on the cuticle to prevent excessive
water loss from photosynthetic tissues.
– Presence of the midrib and veins containing vascular tissues like: the
Xylem which brings water and minerals from soil to photosynthetic
tissues, and Phloem which carry away manufactured organic food from
photosynthetic tissues to other parts (translocation).
– Having the leaf stalk which holds the lamina in a good position to
receive the maximum amount of the light.
9.2.3. Absorption and action spectra
In addition to water and CO2, photosynthesis requires light and chlorophyll.
The chlorophyll pigment is found in the chloroplasts. The light that our eyes
perceive as white light is a mixture of different wavelengths. Most of them
are visible to our eyes and make up the visible spectrum. Our eyes see
different wavelengths of visible spectrum as different colours (violet, blue,
green, yellow, orange and red) except indigo which is not visible to our
eyes. Plants absorb the light energy by using molecules called pigments
such as: chlorophyll a, chlorophyll b, carotene (orange), xanthophyll
(yellow) and phaeophytin (grey) but chlorophyll a is the principle pigment
in photosynthesis.
The chlorophyll absorbs light very well in blue-violet and red regions of
visible spectrum. However, chlorophyll does not absorb well the green
light, instead it allows the green light to be reflected. That is why young
leaves and other parts of the plants containing large amount of chlorophyllappear green.
The chlorophyll a as a principle and abundant pigment, it is directly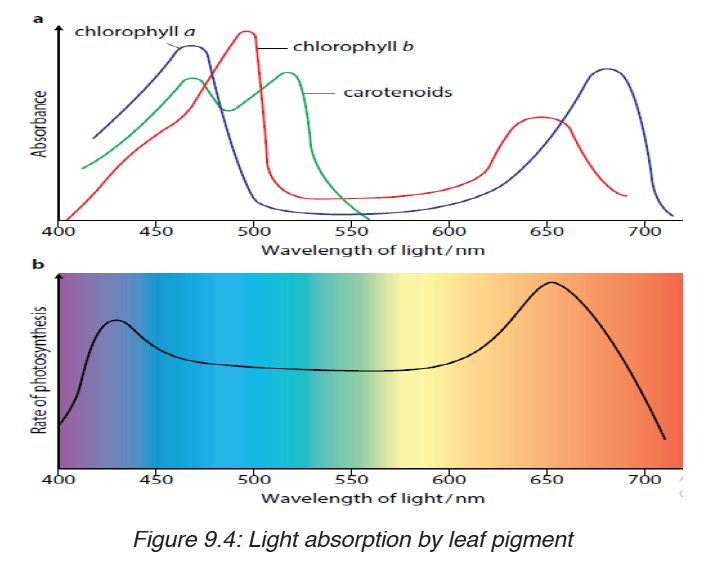
involved in light reactions of photosynthesis. Other pigments (chlorophyll
b, carotene, xanthophyll and phaeophytin) are accessory pigments. They
absorb light colors that chlorophyll a cannot absorb, and this enables plants
to capture more energy from light.
The amount of energy that the pigment can absorb from the light, depends
on its intensity and its wavelengths. So, the greater the intensity of light,
the greater amount of energy will be absorbed by the pigment in a giventime.
9.2.4. Calvin cycle and the process of photosynthesis in C3
plants
a) Stages and sites of photosynthesis in a chloroplast
The process of photosynthesis occurs through two main stages such as:
– The light-dependent reactions: which take place in thylakoids, and
– The light-independent reactions (Calvin cycle): which take place in
stroma.
Table 9.4: Comparison between light-dependent reactions and The light independentreactions (Calvin cycle)
i. The light-dependent reactions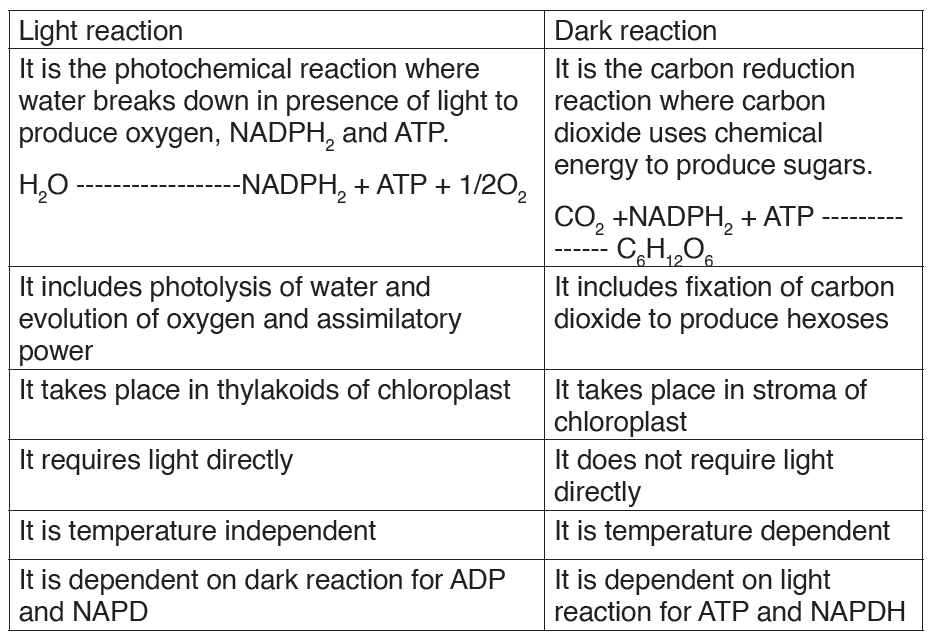
They require light energy and occur in thylakoids. They produce Oxygen
gas and convert ADP and NADP+ into ATP and NADPH.
The light-dependent reactions involve the following steps:
• Photosynthesis begins when the chlorophyll a in photosystem II
absorbs light at different wavelengths of light.
– When the light energy hits the chlorophyll a, the light energy is absorbed
by its electrons, by raising their energy level.
– These electrons with high potential energy (electrons with sufficient
quantum energy) are passed to the electron-transport chain.
– Excited electrons are taken up by an electron acceptor (NADP+:
oxidized Nicotinamide Adenine Dinucleotide Phosphate), and pass
along electron transfer chain from photosystem II to the photosystem
I. (Note: The photosystems are the light-collecting units of the
chloroplast).
• Enzymes in thylakoids and light absorbed by photosystem II are
used to break down a water molecule into energized electrons,hydrogen ions H+, and Oxygen.
– Oxygen produced is released to be used by living things in respiration.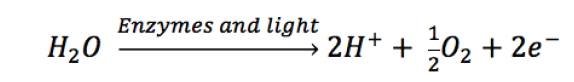
– Electrons and H+ from photolysis of water are used to reduce NADP+
to NADPH (Reduced Nicotinamide Adenine Dinucleotide Phosphate).
– The light-dependent reactions also allow generation of ATP (Adenosine
Triphosphate) by adding inorganic phosphate to ADP+ (AdenosineDiphosphate):
Generally, the light-dependent reactions use light energy, ADP, Pi, NADP+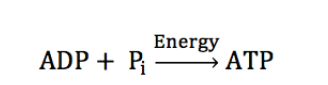
and water to produce ATP, NADPH and Oxygen. Or simply:
Both ATP and NADPH are energy carriers which provide energy to sugars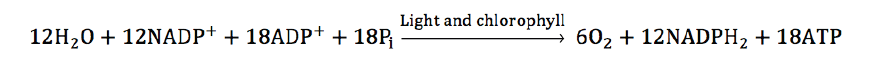
(energy containing sugars) in Light-independent reactions.
ii. The light-independent reactions (Calvin cycle)
The light-independent reactions occur in stroma, and consist of reducing
CO2 into sugars by using ATP and NADPH both coming from light-dependent
reactions in thylakoids. The Calvin cycle involves three main stages such as:
– Carbon fixation in form of CO2.
– Carbon reduction from CO2 to glucose.
– Regeneration of RuBP.
• Carbon fixation (Carboxylation) in form of CO2
Carboxylation: is the process of fixation of carbon in stable organicintermediate, phosphoglyceric acid.
The Calvin cycle begins with a 5-Carbon sugar phosphate called Riburose-1,
5 biphosphate (RuBP) which fixes the CO2 from airThis reaction is
catalyzed by called RuBPcarboxylase-oxygenase (RUBISCO). Rubiscobis-phospahte (RuBP) is the initial acceptor or substrate for dark reaction.
• Carbon reduction from CO2 to glucose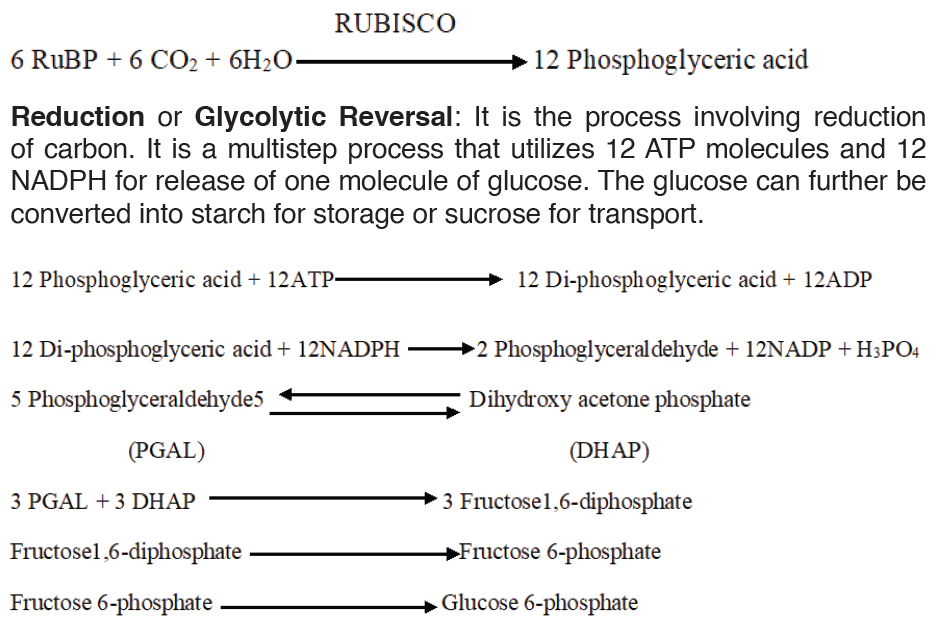
With energy from ATP and reducing power from NADPH, the phosphoglyceric
acid is reduced into 3carbon molecules known as glyceraldehyde-3-
phosphate or phosphoglyceraldehyde (PGAL).
Each molecule of PGA receives an additional phosphate group from ATP,
becoming 1, 3-biphosphoglycerate, and a pair of electrons and H+ from
NADPH reduces the carboxyl group of 3-phosphoglycerate to the aldehyde
group of PGAL which stores more potential energy.
ATP gives one phosphate group becoming ADP+, and NADPH gives H+ and
electrons to become NADP+. Both ADP+, and NADP+ will be used again in
light-dependent reactions.
With 6 turns of Calvin cycle, the plant cell fixes 6CO2 molecules which are
used to synthesize 2 molecules of PGAL which leave the cycle and combine
to make one molecule of glucose or fructose. This glucose can be converted
into:
– Sucrose: when Oxygen combined with fructose. It is a form by which
carbohydrates are transported in plants.
– Polysaccharides like starch for energy storage, and cellulose for
structural support.
– Amino acids when combined with nitrates,
– Nucleic acids when Oxygen combined with phosphates, and– Lipids.
• Regeneration of RuBP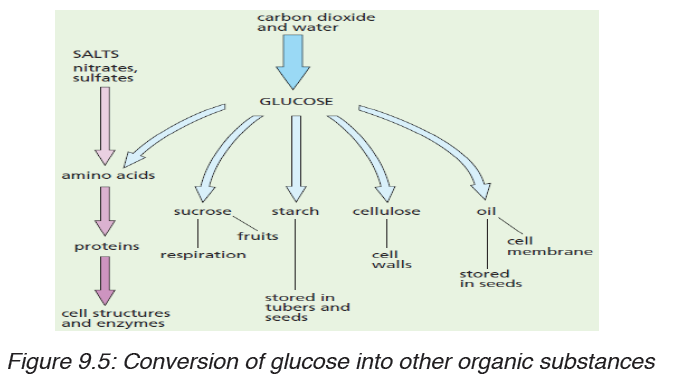
The remaining ten 3-carbon molecules (PGAL) are converted back into six
5-carbon molecules, ready to fix other CO2 molecules for the next cycle. Thelight-independent reactions can be summarized as:
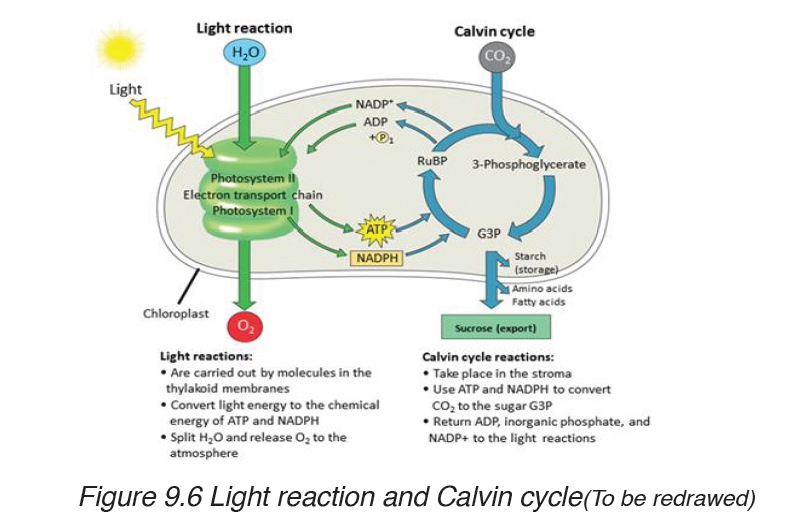
Photorespiration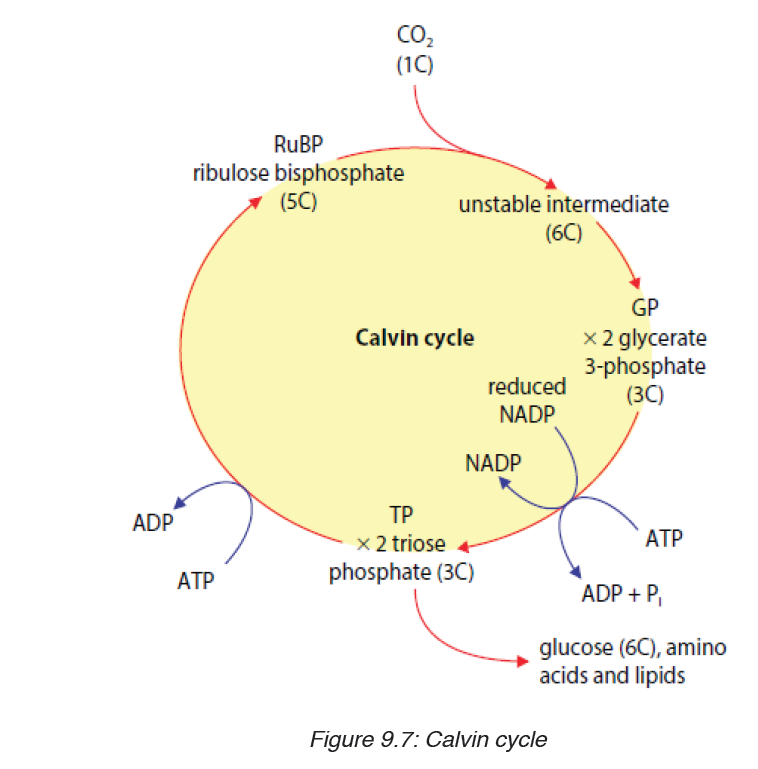
In most plants, initial fixation of carbon occurs via Rubisco, the Calvin cycle
enzyme that adds CO2 to ribulose biphosphate. Such plants are called C3
plants because the first organic product is a three carbon organic compound,
PGA. These plants produce less food when their stomata close on hot and
dry days.
The declining level of CO2 in the leaf starves the Calvin cycle. Making matter
worse, Rubisco can accept O2 in place of CO2. As O2 concentration overtakes
CO2 concentration within the air space, Rubisco adds O2 instead of CO2.
The product splits and one piece, a two-carbon compound is exported from
the chloroplast. Mitochondria then break the two-carbon molecule into CO2.
The process is called photorespiration because it occurs in presence of
light (photo) and consumes O2(respiration). However, unlike normal cellular
respiration, photorespiration generates no ATP, and unlikephotosynthesis,
photorespiration generates no food. In fact, photorespiration decreases
photosynthetic output by using material from the Calvin cycle.
Application activity 9.2
1. Describe the structure of a chloroplast.
2. What may happen to the rate of photosynthesis in a photosynthetic
cell if the thylakoids in chloroplast are damaged completely?
3. Explain adaptations of both thylakoid and stroma for their functions.
4. Relate the internal structure of the leaf with the process of
photosynthesis
5. Explain the involvement of the plant parts bellow in the process of
photosynthesis a) Stomata b) Lamina c) Leaf stalk d)
Leaf cuticle e) Xylem f) Phloem
6. Why are light and chlorophyll needed for photosynthesis?
7. Describe the relationship between the chlorophyll and the color of
plants.
8. How well would a plant grow under pure yellow light? Explain your
answer.
9. Appreciate the presence of accessory pigments in leaves for the
process of photosynthesis.
10. Differentiate the light-dependent stage and light-independent
stage of photosynthesis.
11. Relate the structure of the thylakoid with its function.
12. Explain the stages of the Calvin cycle.
9.3 Rate of photosynthesis: limiting factors of photosynthesis
and importance of autotrophic nutrition.
9.3.1. External factors that affect photosynthesis
Activity 9.3.1.a
Aim: To show effect of carbon dioxide on the rate of photosynthesis.
Materials Required: Elodea, beaker, NaHCO3, lamp.
Procedure: Place a pond weed Elodea upside in a test tube containing
water at 25°C. Place the tube in a beaker of fresh water. Place excess
sodium bicarbonate (NaHCO3) in the water to give a constant saturated
solution of CO2.
Place the lamp at a fixed distance from the plant. Maintain the room
temperature at 20°C. Count the number of oxygen bubbles given off by the
plant in a one minute period.
Observation: The bubbles are formed of oxygen.
Discussion: Discuss why was NaHCO3 added to water.
1. CO2 concentration: Carbon dioxide is the inorganic substrate
for photosynthesis. Increase in concentration up to 0.05% in
atmosphere can cause an increase in CO2 fixation. Carbon dioxide
is the major limiting factor, especially in C-3 plants; C-4 plants are
more productive even at low concentration of CO2. Nevertheless,
both C-3 and C-4 plants show increase in rate of photosynthesis at
high CO2 concentration and high light intensities. The fact that C-3
plants respond to higher CO2 concentration by showing increased
rates of photosynthesis leading to higher productivity has been used
for some green house crops such as tomatoes and bell pepper.
They are allowed to grow in carbon dioxide enriched atmosphere asin glasshouses leading to higher yields.
2. Light: Light is an important factor to carry out photosynthesis. It is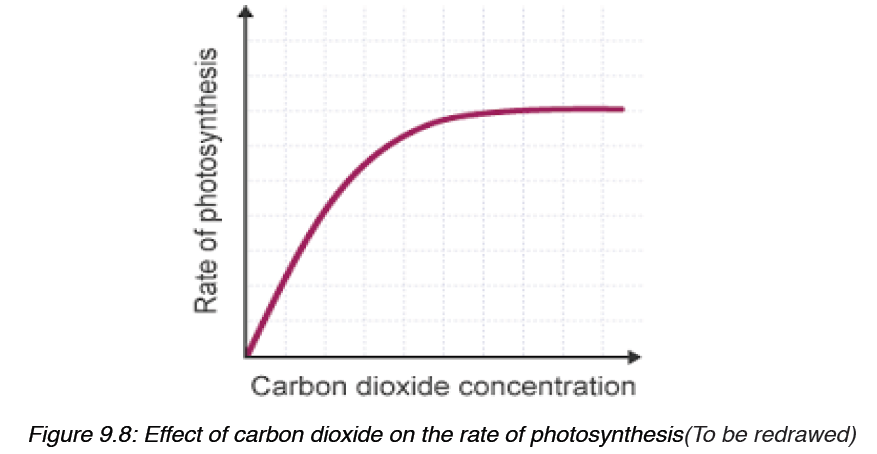
rarely a limiting factor in nature as photosynthesis can occur even at
low light intensities. There is a direct relation between light and CO2
fixation. With increase in light intensity the rate of photosynthesis
increases. However, at higher light intensities, rate does not increase
linearly but light saturation occurs. At very high light intensity, there
is breakdown of chlorophyll molecules called photo-oxidation and
the rate of photosynthesis decreases. The quality of light and time
of exposure also governs photosynthesis. Green plants show high
rate of photosynthesis at red and blue light.Light intensity
3. Temperature: The dark reactions are dependent on temperature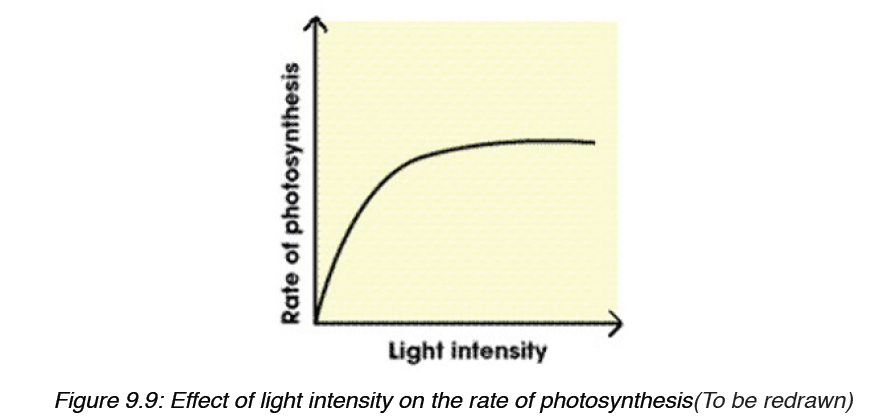
as they are enzymatic. Rate of photosynthesis is best at optimum
temperature. Different plants have different temperature optima thatalso depend on their habitats.
1. Water: Only about 1% of water absorbed by plants is used in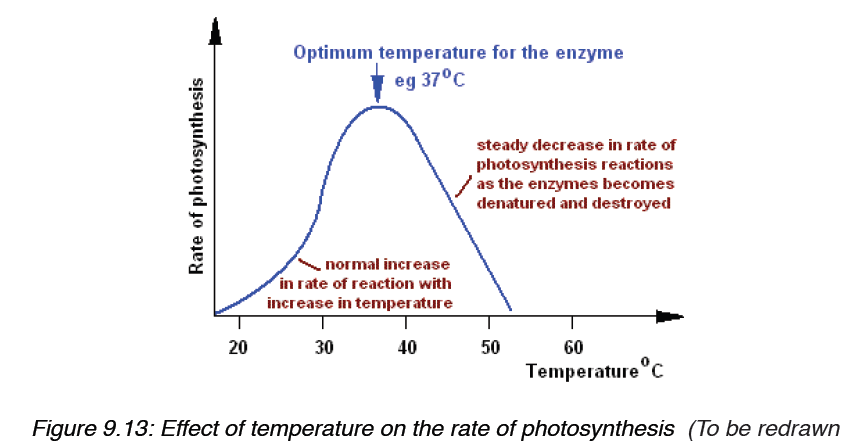
photosynthesis. It is an important factor for various metabolic
processes in plant. Water may not have direct affect on photosynthesis
even though it is one of the reactants in light reaction. In water stress
plants wilt and their stomata close. Thus reducing availability of
carbon dioxide and decreasing the rate of photosynthesis. Water
stress will also alter the hydration of enzymatic proteins, affectingtheir activities.
2. Oxygen concentration: Atmospheric oxygen content affects
photosynthesis directly or indirectly. The decrease in rate of
respiration at high oxygen concentration was first observed by O.
Warburg in 1920 in Chlorella. The phenomenon is called Warburg
effect.
3. Chemical pollutants: Plant growth has been adversely affected by
accumulation of various undesirable chemicals. Heavy metals such
as lead, mercury, cadmium seem to be affecting photosynthesis
through stomata closure. Air pollutants like SO2, NO2 and O3 are
also known to affect photosynthesis at higher concentrations.
9.3.2. Internal Factors
1. Adaptation of leaf: Leaves are arranged on plants to minimize
overlapping. The shape, size, age and orientation of leaf influences
the absorption of light and thus effects photosynthesis. Most leaves are
broad for more absorption of light. The anatomy of leaf is also highly
specialized for absorption of light. The epidermis is transparent and also
acts as convex lens to focus and intensify light reaching mesophyll cells
for maximum absorption.
Application activity 9.3.a
1. Use the graphs to explain how the limiting factors below may
influence the rate of photosynthesis:
a) Temperature
b) Light intensity
c) Concentration of CO2 in air.
2. Student-teacher talked to his Biology group members that:
a) “In Rwanda, the rate of photosynthesis is generally lower at 5:30
AM that it is at 12:30 PM, during a sunny day”. Defend him by
providing two convincing reasons.
b) “The rate of photosynthesis is generally higher in Rwanda during
the sunny day than in Sahara desert”. Defend him with a convincingreason.
9.3.3. Importance of autotrophic nutrition
a) Autotrophic nutrition is a process by which living organisms
(autotrophs: photoautotrophs and chemoautotrophs) make their own
food. The aututrophism is very essential as it allows production of
Oxygen and food for not only themselves but also for heterotrophs.
The roles of autotrophic nutrition include:
b) Independence of green plants from other living organisms to
the nutrition point of view.
This importance relates to their capacity for synthesizing organic
molecules from glucose produced by CO2 and water, this completely
make them independents of the other living organisms to the nutrition
point of view.
c) Synthesis of the organic substances: food for the heterotrophs
(animal and mushrooms): The organic substances produced by
photosynthesis are the food for the heterotrophs which are unable to
synthesize these substances by their own means.
d) Energy storage
The autotrophs like green plants, by the process of photosynthesis
synthesize certain substances like: the cellulose, starch… which are
variables sources of energy.
e) Production of O2 for the living organisms’ respiration
The oxygen produced by the photosynthesis is necessary for the
living organisms’ respiration. Thus without photosynthesis, no
oxygen; without oxygen no respiration; without respiration no life on
Earth.
f) Cleaning the atmosphere
Photoautotrophs absorb carbon dioxide from surrounding air, and
release Oxygen (produced by photosynthesis) in atmosphere.
g) Formation of Ozone layer
Ozone layer is a thick layer in the atmosphere which is formed
Ozone molecule (O3).Oxygen atoms which make ozone molecule
are produced by photosynthesis. Ozone layer protects the Earth
from high solar radiations, and this allows the existence of the life on
the Earth.
Application activity 9.3.b
1. Without autotrophs, the life is impossible on the Earth. By providing
possible reasons, defend or disagree with this statement.
End unit assessment 9
Do all these exercises in your exercise book.
I. Choose whether the given statements are True (T) or False (F)
1. Organisms that are heterotrophic can make their own food.
2. Photosynthesis has two stages—light reaction and dark reaction.
3. Environmental factors improve crop yield.
4. Pigment is a material that changes color of reflected or transmitted
light.
5. Within leaves, chloroplasts are responsible for respiration.
II. Multiple Choice Questions
1. Green plants require which of the following for photosynthesis?
a) Sunlight (b) CO2
b) O2
c) Water
2. What is true about action spectrum?
a) It can be carried out in isolated pigments
b) It gives the function of pigments
c) It is used to identify pigments
d) It does not involve light
3. By looking at which internal structure, you can tell whether a plant
is C-3 or C-4?
a) Mesophyll cell (b) Bundle sheath cells
b) Vascular bundles (d) epidermal cells
4. How many ATP are required to produce 2 molecules of glucose?
a) 12 (b) 24b) 18 (d) 36
5. Autotrophs are commonly called producers because they
a) Produce young plants
b) Produce CO2 from light energy
c) Produce sugars from chemical energy
d) Produce water from light energy
III. Long Answer Type Questions
1. State and explain the types of autotrophic nutrition. Also explain
the role of light in autotrophic nutrition.
2. Analyse and appreciate the importance of photosynthesis as an
energy transfer process.
3. State the role of chloroplast and structure of leaf in photosynthesis.
Giving illustrative diagrams, explain your answer.
4. State the pigments involved in light absorption. Throw light on
absorption and action spectra of chloroplast pigments.
5. Outline the three main stages of Calvin cycle. State the uses of
Calvin cycle intermediaries in plant cell.
6. Summarize the limiting factors affecting photosynthesis. Also state
how this can help yield crop production.
7. Investigate the effect of light intensity or light wavelength on the
rate of photosynthesis.
8. Describe the relationship between the structure and function in the
chloroplast, using diagrams and electron micrographs.
9. Acknowledge the importance of autotrophic nutrition in
sustaining the balance of life on Earth. Also state the ways to
keep the environment sustained. Predict various facts related to
photosynthesis that state the importance of nutrition for all livingbeings.
10. The chart below shows the sequence of events that takes place in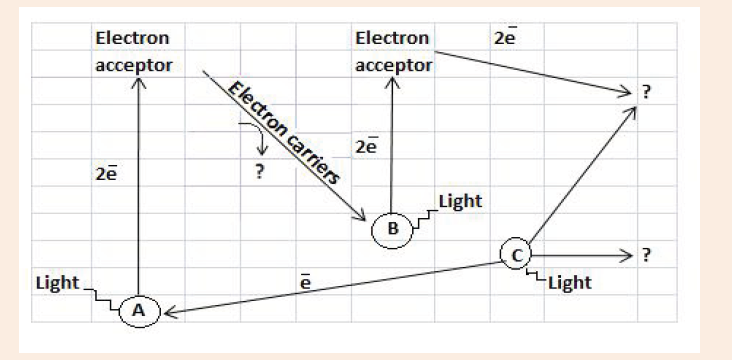
the light dependent reactions.
a) Identify the point A and B
b) What process is taking place at C?
c) What are the products of the light dependent reaction? (They are
indicated by? on the diagram).
11. The diagram below summarizes the movement of materials into
and out of chloroplast. Identify the substances moved, indicatedby labels A-D.
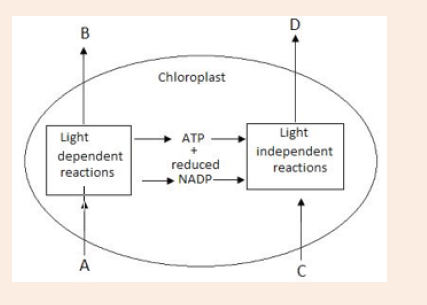
UNIT 10: THE CHEMICAL BASIS OF LIFE
Key unit competence
Explain the use of biological molecules in living organism.Introductory Activity 10
Analyze all foods in the figure and answer to the questions below:
a) Among the foods given in figure, which one do you prefer to eat
every day? Why?
b) Do you think that there are some missing foods to complete all
chemicals of life? Suggest them.
10.1. Biological molecules
Activity 10.1
Discuss chemical elements, sub-units of different types of carbohydrates,
lipids and proteins.
What do you know about the function of water to living organisms?
10.1.1 The chemical elements that make up carbohydrates,
lipids and proteins
a) Carbohydrates
Carbohydrates comprise a large group of organic compounds which contain
carbon, hydrogen and oxygen. The word carbohydrate indicates that these
organic compounds are hydrates of carbon. Their general formula is
Cx(H2O)y.
In carbohydrates the ration hydrogen-oxygen is usually 2:1.
Carbohydrates are divided into three groups including the monosaccharide
(single sugars), disaccharides (double sugars) and polysaccharides (many
sugars). The most common monosaccharide of carbohydrates is glucose
with molecular formula C6H12O6.
b) Lipids
Lipids are a broad group of naturally occurring molecules which include
waxes, sterols, fat soluble vitamins (such as vitamins A, D, E and K),
monoglycerides, diglycerides, triglycerides, Phospholipids and others.
Lipids are made by carbon, hydrogen and oxygen, but the amount of oxygen
in lipids is much smaller than in carbohydrates.
Lipids are grouped into fats which are solid at room temperature and oils
which are liquid at room temperature.
c) Proteins
Proteins are organic compounds of large molecular mass. For example,
the hemoglobin has a molecular mass of 64500. In addition to carbon,
hydrogen and oxygen, proteins always contain nitrogen, usually Sulphur and
sometimes phosphorus.
Water
Living organisms contain between 60% and 90% of water, the remaining
being the dry mass. Water is made up of only two elements, Hydrogen and
Oxygen.
The function of water is defined by its physical and chemical properties thatdiffer from those of most liquids and make it effective in supporting life.
10.1.2. The sub-units that make up biological molecules
a) Sub-units of Carbohydrates
In carbohydrates the following three categories are identified:
Monosaccharides , disaccharides and polysaccharides.
i. Monosaccharides
Monosaccharides are the smallest subunits and are made up of single sugar
molecules. monosaccharides are the sugars like galactose, fructose and
glucose with a general formula C6H12O6, and these typically take on a ringshaped
structure.
All monosaccharides are reducing sugars capable of acting as a reducing
agents because they have a free aldehyde group or a free ketone group.
Sources of Monosaccharides:
Glucose: Fruits and vegetables are natural sources of glucose. It’s also
commonly found in syrups, candy, honey, sports drinks, and desserts.
Fructose: The primary natural dietary source of fructose is fruit, which is
why fructose is commonly referred to as fruit sugar.
Galactose: The main dietary source of galactose is lactose, the sugar in
milk and milk products, such as cheese, butter, and yogurt. https://www.
healthline.com/nutrition/simple-sugars
ii. Oligosaccharides
These are complex carbohydrate chains made up of two to twenty
simple sugars joined together with a covalent bond. The most common
oligosaccharide is the disaccharide, and examples of this include sucrose,
maltose and lactose whose general formula is C12H22O11
A disaccharide is the sugar formed when two monosaccharides are joined
by glycosidic bond. Like monosaccharides, disaccharides are soluble in
water, have sweet taste, and they are reducing sugars because they are
able to reduce Copper II Sulfate of benedict solution directly by heating
into copper II oxide except sucrose which is non-reducing sugar which are
unable to reduce the copper ions in Benedict’s solution. This makes the
color of Benedict’s solution to persist when this sugar is boiled with it.
Sucrose is made up of two monosaccharides Glucose and fructose
Maltose is made up of two monosaccharides Glucose and glucose
Lactose is made up of two monosaccharides Glucose and galactose
In maltose ring, the two rings of glucose are bonded by the -1, 4-glycosidic
bond whilein sucrose the glucose and fructose are bonded by -1, 2-glycosidic bond.
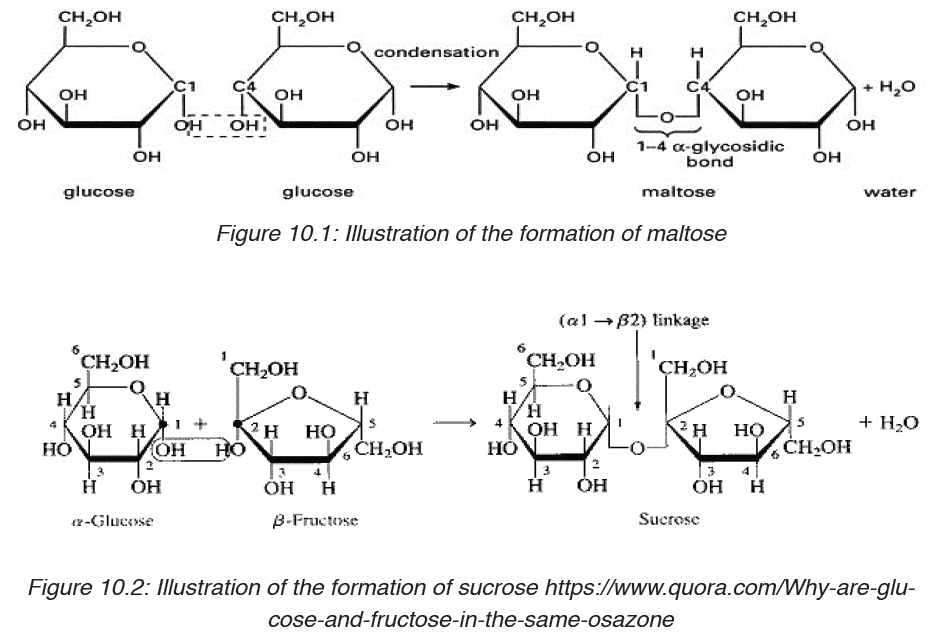
Table 10.1: different groups of Disaccharides, their structure, enzyme and source
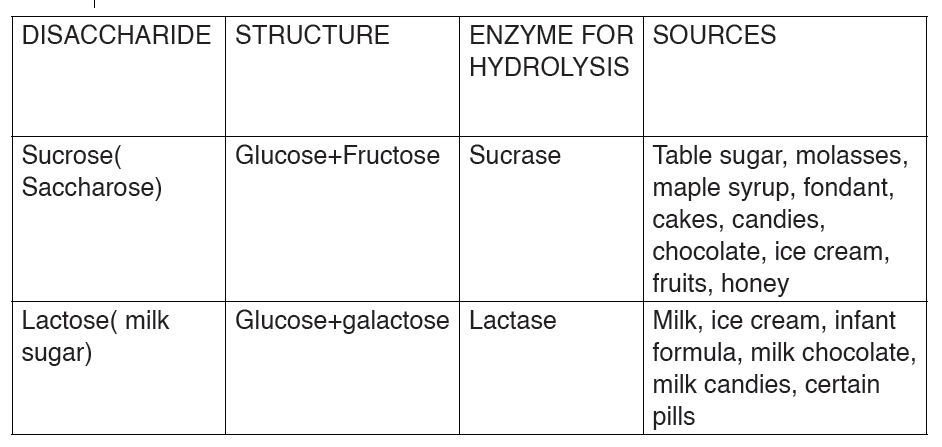
iii. Polysaccharides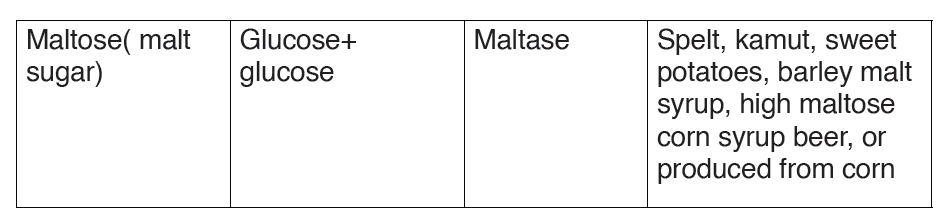
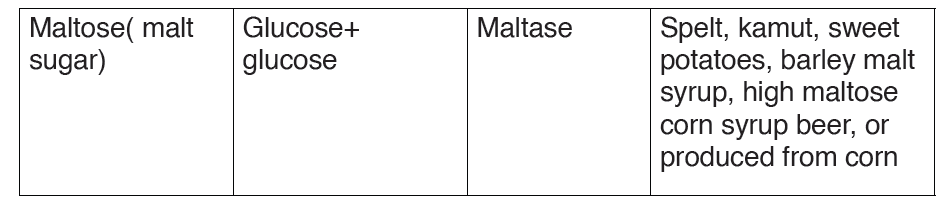
These are known for their ability to store energy and are made up of long
chains of glucose sugars. The most common polysaccharides are starch(
sugar of plant tissues), glycogen ( glucose in the human liver and muscles),
cellulose ( structural polysaccharide in plants; which acts as a dietary fiber
when consumed), chitin( sugar found in exoskeleton of arthropods) and
murein or peptidoglycan( sugar found in the bacteria cell membrane) . http://
www.nutrientsreview.com/carbs/polysaccharides.html
Table 10.2.: different groups of polysaccharides, their composition andsource
b) Sub-units of Proteins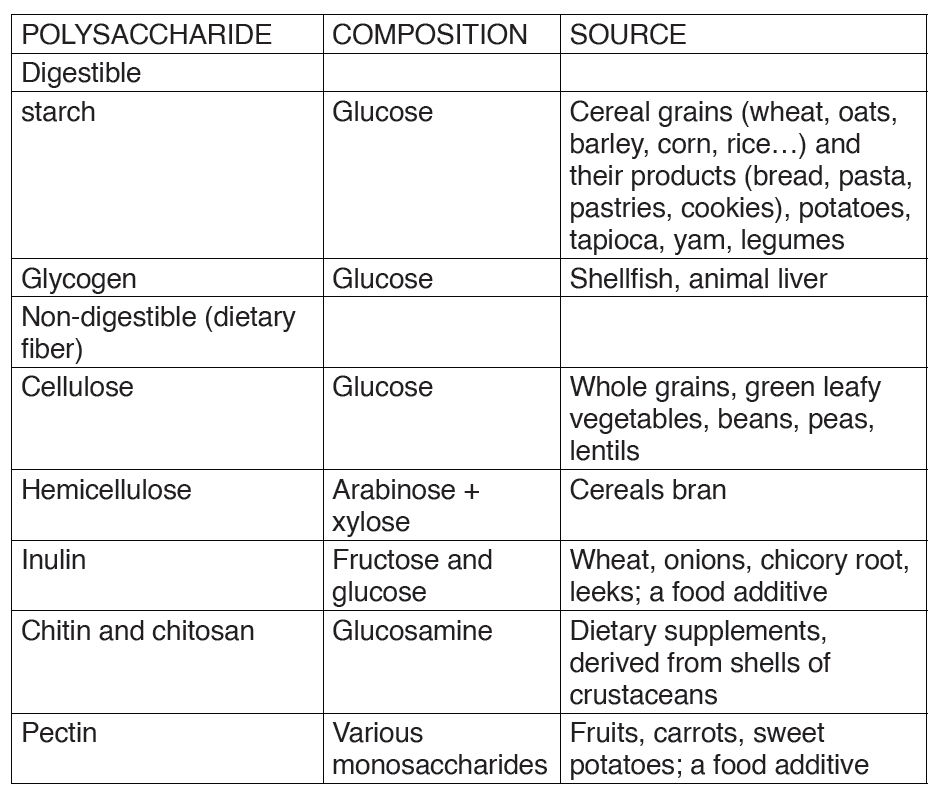
These are also referred to as macro-nutrients. The proteins are also called
body- building food.
The protein molecules are made up of small units called amino acids joinedtogether like links in a chain.
There are 21 different amino acids and each has its own chemical name.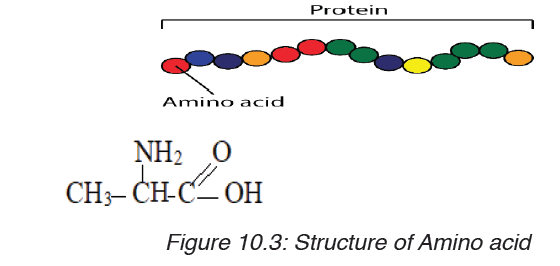
Different proteins are made when different numbers and types of amino
acids combine through a covalent peptide bond. Proteins are therefore
known as polypeptides.
Examples of proteins:
a) Collagen, myosin and elastin found in meat,
b) Caseinogen, lactalbumin, lacto globulin found in milk,
c) valbumin, mucin and liporitellin found in eggs,
d) Zein found in maize
The 21 different amino acids found in protein are
Arginine, Serine,Selenocysteine, Leusine, Histidine,Threonine, Glycine,
Methionine, Lysine, Asparagine, Proline, Phenylalanine, Aspartic acid,
Glutamine, Alanine, Tyrosine, Glutamic acid, Cysteine, Valine, Tryptophan,
Isoleucine.
They are used to repair, to build, to maintain our bodies; to make muscles
and to make breast milk during lactation period. The proteins are classified
into two categories: animal or complete proteins and plant proteins or
incomplete proteins
c) Sub-units of lipids
Lipids are made by two components namely glycerol and fatty acids. The
chemical formula for glycerol is C3H8O3.Structural formula of glycerol is
Sources and classification of lipids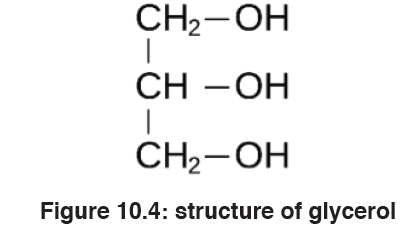
Fats and oils are obtained from both the plants and animals. And fat is
present in food either as visible fat or invisible fat.
Visible fat is the one that is easily seen or detected in food for example; fatin meat, butter, margarine, lard, suet and cooking fat and oil.
Invisible fat is the part of food that is not easily seen for example fat with
in lean meat, egg yolk, flesh of oily fish, groundnuts, soya beans, avocado
and fat found in prepared foods, for example, pastry, cakes, biscuits, Frenchfries, pancakes, croquettes.
Lipids are of different types as it is summarized in the following table
Table 10.3: Lipids, structure, main role and features
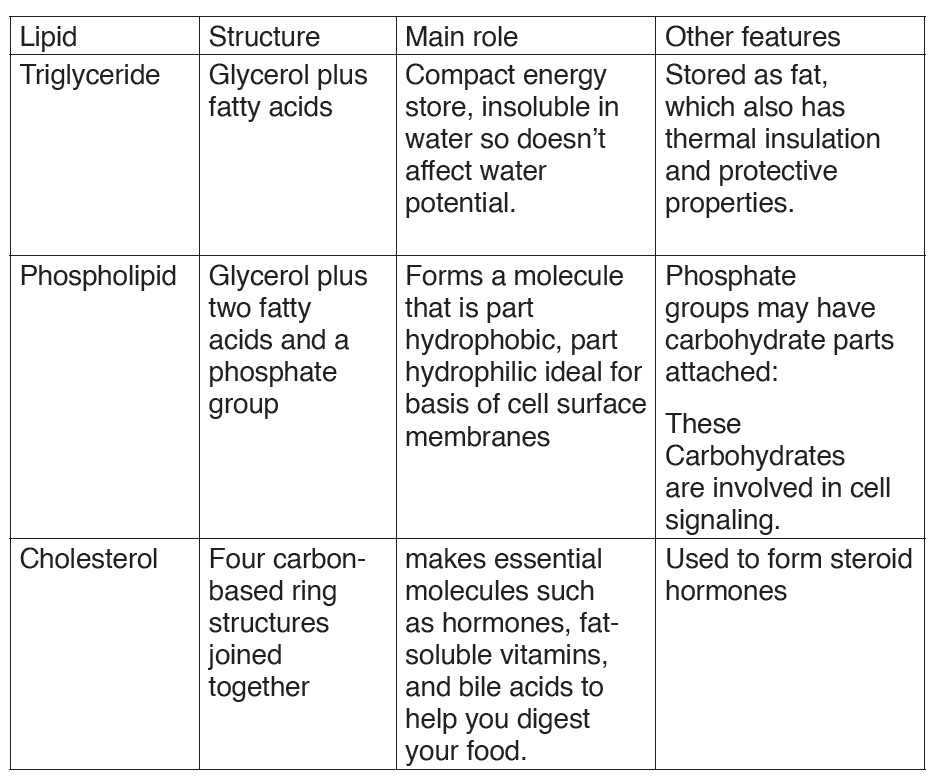
Structure of fatty acid
The following are three main types of lipids: Triglycerides, phospholipids and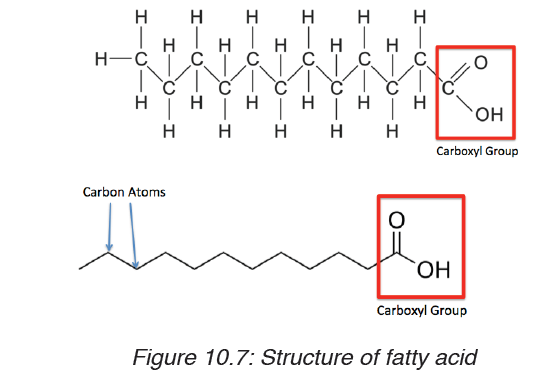
steroids
Triglycerides: these are lipids that are obtained from cooking oils, butter
and animal fat. They are made up with: one molecule of glycerol and three
molecules of fatty acids bonded together by Ester bonds. The triglycerides
play the role like storing energy they have thermal insulation and protectiveproperties
Sterols: these are lipids that include steroid hormones like testosterone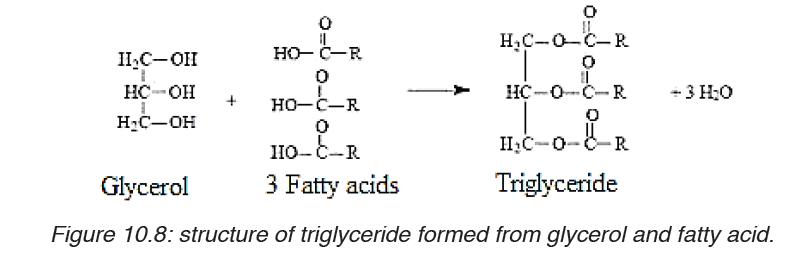
and oestrogen, cholesterol that is formed four carbon-based rings and it
helps in regulation of fluid and strength of the cell membrane.
Phospholipids: They are made up of one molecule of glycerol, two
molecules of fatty acids and one phosphate group. The phospholipids form
a molecule that is part hydrophobic, part hydrophilic, ideal for basis of cellsurface membranes
10.1.3. The proteins and their functions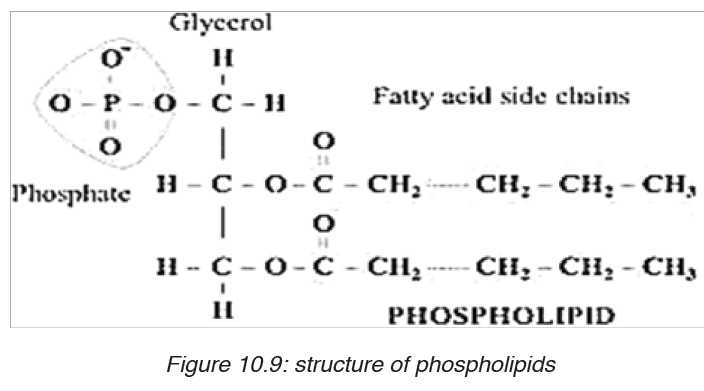
Activity 1.1
1. From the books make a research on proteins and answer to the
following questions:
Differentiate globular proteins and fibrous proteins.
2. Take a plastic cord, create the bulk on it and suppose that those
are monomers of a long chain of polymer (the whole cord), heat
it using a Bunsen burner or another source of fire. Discuss the
change that takes place.
Proteins are organic compounds of large molecular mass ranging up to
40000000 for some viral proteins but more typically several thousand. For
example, the hemoglobin has a molecular mass of 64500. Proteins are
polymers of amino acids and they are not truly soluble in water, but form
colloidal suspensions. In addition to carbon, hydrogen and oxygen, proteins
always contain nitrogen, usually Sulphur and sometimes phosphorus.
Whereas there are relatively few carbohydrates and fats, the number of
proteins is limitless. Coined by a Dutch chemist Mulder the word protein
etymologically means “of the first importance” due to the fundamental role
they play in living cells.
a) Amino acids
Amino acids are group of over a hundred chemicals of which around 20
commonly occur in proteins. They always contain a basic group, the amine
group (-NH2) and an acid group (-COOH) together with -R group side chain
(Figure 10.14). All the amino acid differs one to another by the structure oftheir side chain.
Amino acids are divided into two categories including essential amino acid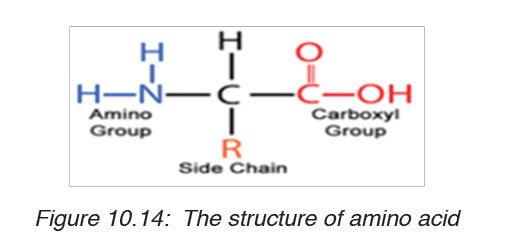
and non-essential amino acid. Essential amino acids are those amino acids
which cannot be synthesized by the body. They include isoleucine, leucine,
lysine, methionine, phenylalanine, threonine, tryptophan, valine, arginine
and histidine. Non –essential amino acids are synthesized by the organism.
They include alanine, asparagine, aspartic acid, cysteine, glutamine,
glutamic acid, glycine, proline, serine and tyrosine. The simplest amino acid
is glycine with H as -R group (Figure 10.15. a). The other one is Alanine with
–CH3 as -R group (Figure 10.15.b). All 21 amino acids can be found in diet
from animals such as meat, eggs, milk, fish…but diet from plant lack one ortwo essential amino acids such plant are beans, soy beans…
When an amino acid is exposed to basic solution, it is deprotonated (release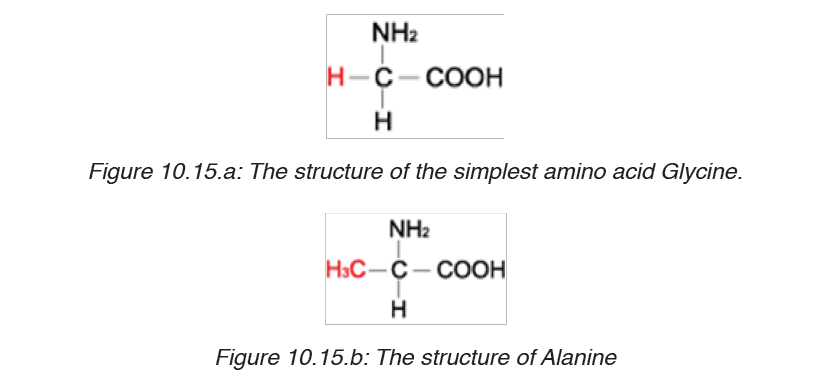
of a proton H+) to became negative carboxylate COO-while in acid solution
it is protonated (gains of a proton H+) to became ammonium positive ion
-NH3+(Figure 10.16.a and Figure 10.16.b).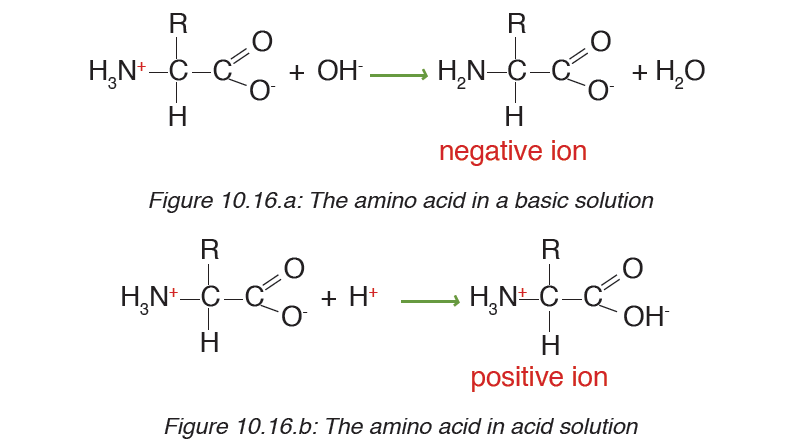
At a physiological pH, usually around 7, the amino acid exists as ZWITTERION
(from German means hermaphrodite) it is a molecule with two differentcharges (positive and negative) at the same time (Figure 10.17).
Formation and breakage of peptide bond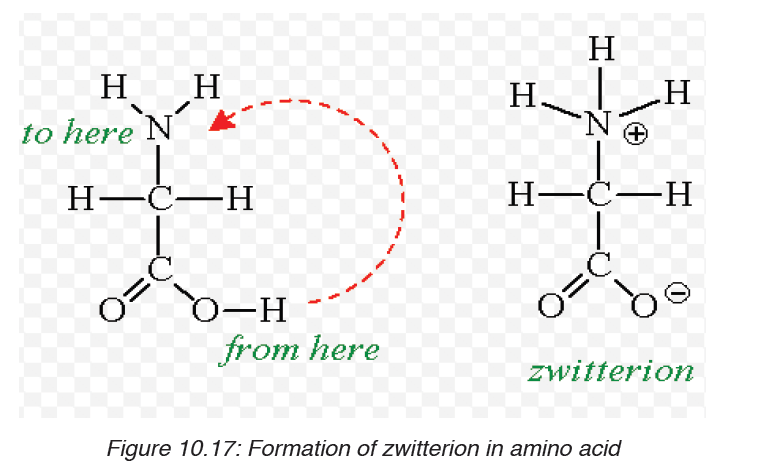
The formation of peptide bond follows the same pattern as the formation
of glycosidic bond in carbohydrates and ester bond in fats. A condensation
reaction occurs between the amino group of one amino acid and the carboxylgroup of another, to form a dipeptide (fig 10.18).
A peptide bond is formed between two amino acids to form a dipeptide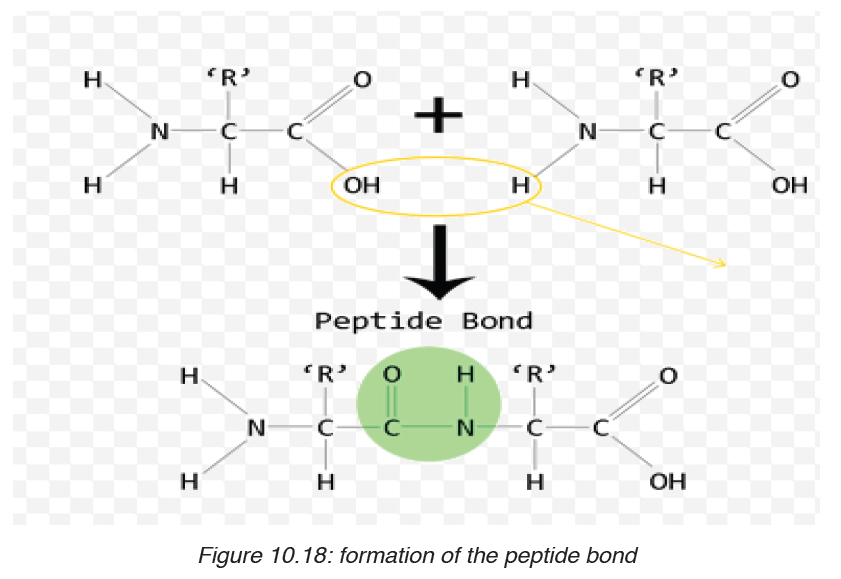
molecule, if three amino acids are assembled together it is a tripeptide, four
amino acids form a tetrapeptide and so on.
A long chain of amino acid it is called a polypeptide. The polypeptide chain
or oligopeptide comprise more than 50 amino acids joined together by a
peptide bond.
During digestion, proteins are hydrolyzed and give their monomer amino
acids small molecules that can be diffused in the wall of intestine to the
organism. In hydrolysis the peptide bond break down by the addition of awater molecule (Figure 10.19).
Functions of proteins.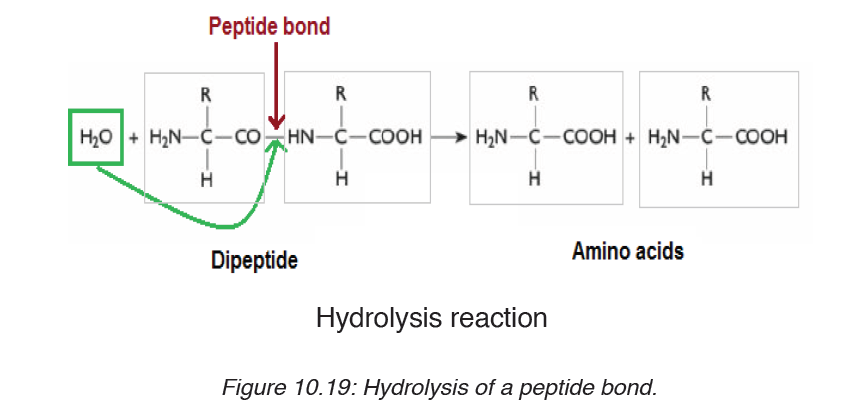
– Proteins such as lipase, pepsin and protease act as enzymes as they
play a crucial role in biochemical reaction where they act as catalysts.
– Proteins play an important role in coordination and sensitivity (hormones
and pigments).
– Proteins have a transport functions. Example: Haemoglobin transport
oxygen
– Proteins in the cell membrane facilitate the transport of substance
across the cell membrane.
– Proteins provide a mechanical support and strength.
– Proteins such as myosin and actin are involved in movement.
– Proteins play the role of defense of the organisms. Example: Antibodies
are proteins
Application activity 10.1
1. Provided with different kinds of biological molecules such as
carbohydrates, proteins, lipids, make a table to show their food
source you always take and suggest their functions.
2. Explain what is meant the essential amino acids
3. Describe the formation of a peptide bond?
4. Alanine is an amino acid with -CH3 as a side chain. Writes its
structural formulae.
5. Most of the plant lacks one or more of the essential amino acids
needed by the body explain how a vegetarian can obtain the
essential amino acids.
6. Use the type of reaction above to form glucose from sucrose
molecule
7. Describe how the glycosidic bond is formed.8. Describe the major types of starch
10.2. Water and enzymes
Activity 10.2
1. You are provided with three groups of enzymes: Group A Group B Group
C Enzymes Maltase and lactase Dehydrogenase and oxidase Pepsinand renin
Make a research to find out: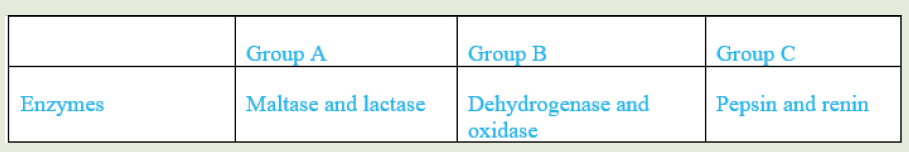
a) specific role of each of the six enzymes mentioned above
b) criterion followed to name enzymes of group A, B and C respectively
2. a. What is the medium of reaction in the organisms?
b. If two people are boiling the same quantity of cooking oil and water,
which one could evaporate first? Explain your choice.
10.2.1. Water
Living organisms contain between 60% and 90% of water, the remaining
being the dry mass. The scientist accepts that life originates from water
and most of animals live in water. The function of water is defined by its
properties mainly: Its physical properties, solvent properties, heat capacity,
surface tension and freezing points. The physical and chemical properties of
water differ from those of most other liquids but make it uniquely effective in
supporting living activities.
a) Physical properties of water
Water has the high boiling point (100°C) compared to other liquid due to the
hydrogen bond that exists among molecules of water. This help the water toexist on the surface in a liquid state otherwise it would evaporate.
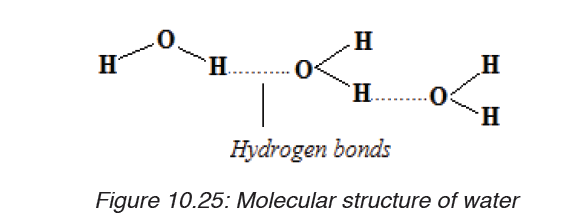
Table 10.4: Biological significance of the physical properties of water
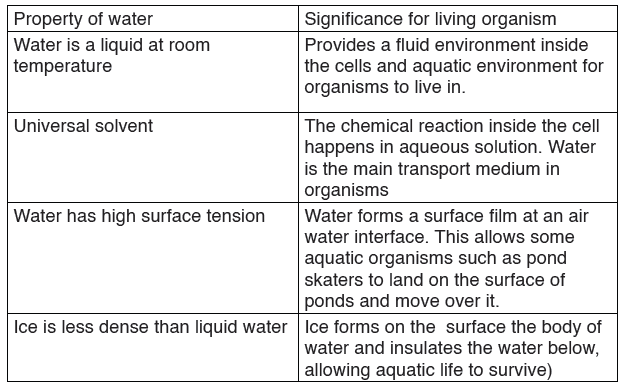
a) Solvent properties of water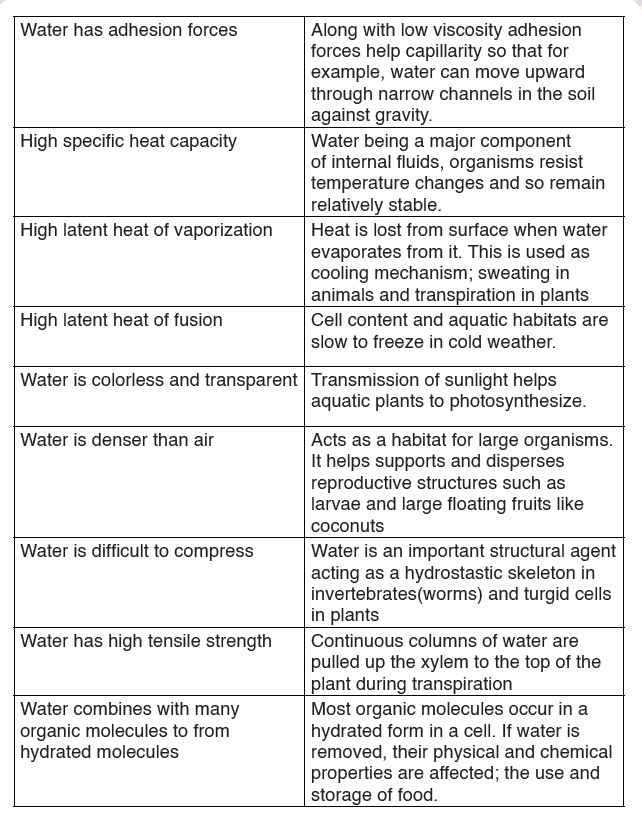
Water is a polar molecule due to its chemical arrangement of hydrogen
and oxygen atom in asymmetric shape instead of being linear. Most of the
substance that are transported in the blood is dissolved in the plasma, the
fluid part of the blood. Water occupies around 92% of the constituents of
plasma. Thus the oxygen atom has a positive charge and hydrogen atom
net positive charges. This is of great importance because all negative and
positive ions are attracted by water. Therefore, water is a good solventbecause the ionic solids and polar molecules are dissolved in it.
b) Heat capacity and latent heat of vaporization
Large changes of heat results in a comparatively small rise in water
temperature this explain why water has a high heat capacity compared to
other liquid. The high heat capacity is defined as the amount of heat required
to raise the temperature of 1gram to 10C.The high thermal capacity of water
make the ideal environment for life in plant and animals because it helps in
maintaining the temperature even if there will be environmental fluctuations
in temperature. The biological importance of this is that the range of
temperatures in which biochemical processes can proceed is narrow.
The latent heat of vaporization is a measure of heat energy needed to cause
the evaporation of a liquid, which means to change from water liquid to
water vapor. During vaporization the energy transferred to water molecules
correspond to the loss of energy in the surroundings which therefore cool
down. During sweating and transpiration living organisms use vaporization
to cool down.
i. Surface tension
The surface tension of water results from its polar nature, and is defined as
the ability of the external surface of the liquid to resist to external force due
to cohesive nature of its molecules. The high surface tension of water and
the cohesion force play a vital role in capillarity thus help the transport of
substance in vessels (tracheid of plant) to the stems and to fulfill the blood in
the cardiovascular vessels. Water being the second liquid with high surface
tension after mercury its surface tension is lowered by the dissolution of ions
and molecules and tend to collect at the interface between its liquid phase
and other.
ii. Freezing points
Oppositely to other liquid water expand as it freezes, under 40 C temperatures
the hydrogen bond becomes more rigid but more open. This explains why
the solid water (ice) is less dense than the liquid water and why the ice floats
over water rather than sinking. When the bodies of water freeze the ice float
over the liquid act as an insulator and prevent water below it from freezing.This protects the aquatic organisms so that they can survive the winter.
10.2.2. Enzymes, their characteristics and actions
a) Criteria for naming enzymes
Activity 10.2.2.aYou are provided with three groups of enzymes:
Make a research from text book or internet to find out: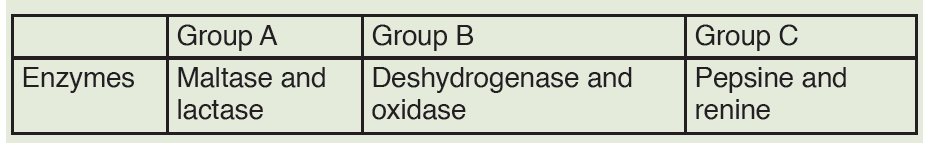
What is the specific role of each of the six enzymes mentioned above?
What criterion was followed to name enzymes of group A, B and C
respectively?
Enzymes are biological catalyst produced by a living organism to control
the speed of specific biochemical reactions (metabolism) by reducing its
activation energy.
First of all, Individual enzymes are named by adding -ase to the name
of the substrate with which they react. The enzyme that controls urea
decomposition is called urease; those that control protein hydrolyses are
known as proteases.
A second way of naming enzymes refers to the enzyme commission number
(EC number) which is a numerical classification scheme for enzymes
based on the chemical reactions they catalyse. As a system of enzyme
nomenclature, every EC number is associated with a recommended name
for the respective enzyme catalysing a specific reaction. They include:
Oxidoreductases catalyse redox reactions by the transfer of hydrogen,
oxygen or electrons from one molecule to another. Example: Oxidasecatalyses the addition of oxygen to hydrogen to form water.
– Transferases catalyze group transfer reactions. The transfer occurs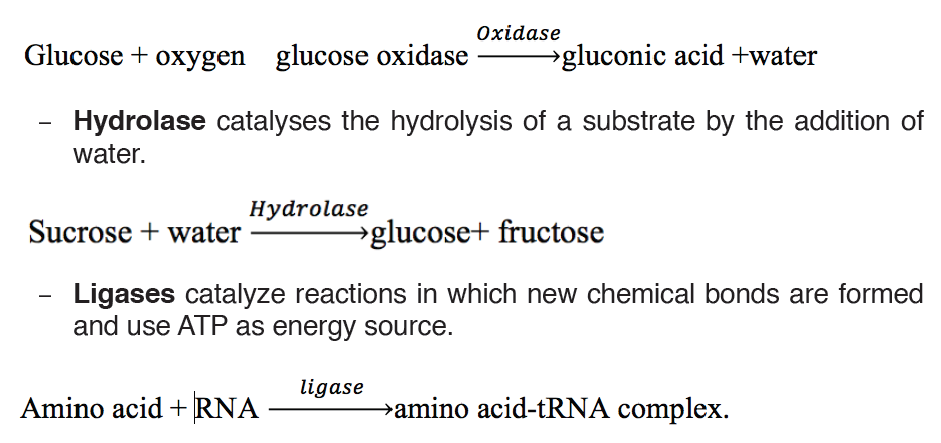
from one molecule that will be the donor to another molecule that will
be the acceptor. Most of the time, the donor is a cofactor that is charged
with the group about to be transferred. Example: Hexokinase used in
glycolysis.
– Lyases catalyze reactions where functional groups are added to break
double bonds in molecules or the reverse where double bonds are
formed by the removal of functional groups. For example: Fructose
bisphosphate aldolase used in converting fructose 1,6-bisphospate to
G3P and DHAP by cutting C-C bond.
– Isomerases catalyze reactions that transfer functional groups within a
molecule so that isomeric forms are produced. These enzymes allow
for structural or geometric changes within a compound. Sometime the
interconverstion is carried out by an intramolecular oxidoreduction.
In this case, one molecule is both the hydrogen acceptor and donor,
so there’s no oxidized product. The lack of a oxidized product is the
reason this enzyme falls under this classification. The subclasses are
created under this category by the type of isomerism. For example:
phosphoglucose isomerase for converting glucose 6-phosphate to
fructose 6-phosphate. Moving chemical group inside same substrate.
A third way of naming enzymes is by their specific names e.g. trypsin
and pepsin are proteases. Pepsin, trypsin, and some other enzymes
possess, in addition, the peculiar property known as autocatalysis,
which permits them to cause their own formation from an inert precursor
called zymogen.
b) Characteristics of enzymes.
Activity 10.2.2.b
Requirement:
Three test tubes, match box, about 1g of liver, 1g of sands, 1% H2O2 and
MnO2 powder.
Procedure:
– Label three test tubes A, B and C respectively.
– Put about 0.1 g of MnO2 powder in test tube A and 1g of liver in tube
B and 0.1g of sand in tube C.
– Pour 5 ml of H2O2 (hydrogen peroxide) in each tube. What do you
observe?
– Place a glowing splint in the mouth parts of each test tube. What do
you observe?
Questions
1. Explain your observations.
2. Write down the chemical equation of the reaction taking place in
tube A and B
3. Carry out your further research to find out the characteristics ofenzymes
The following are main characteristics of enzymes:
– Enzymes are protein in nature: all enzymes are made up of proteins.
– Enzymes are affected by temperature. They work best at specific
temperatures; for example, enzymes found in human bodies work best
at 37oC. This is called the optimum temperature.
– Very low temperatures inactivate enzymes. Therefore enzymes are
not able to catalyse reactions.
– High temperatures beyond the optimum temperature denature
enzymes. The structure of the protein molecule is destroyed by heat.
– Enzymes work best at specific pH. Different enzymes have a given
specific pH at which they act best.
This pH is called optimum pH. Some enzymes work best at low pH
(acidic medium) while others work best at high pH (alkaline medium).
Most enzymes in the human body for instance work best at neutral or
slightly alkaline pH. Examples are: lipases, peptidases and amylase.
A few enzymes like pepsin that digests proteins in the stomach works
best at an acidic pH of 2.
– Enzymes remain unchanged after catalysing a reaction. Enzymes
are catalysts and can therefore be used over and over again in small
amounts without being changed.
– Enzymes catalyse reversible reactions. This means that they can
change a substrate into products and the products back to the originalsubstrate.
– Enzymes are substrate-specific. This means that an enzyme can only
catalyse one reaction involving aparticular substrate. This is because
they have active sites which can only fit to a particular substrate whose
shape complements the active site. For example, pepsin works on
proteins but not on fats or starch.
– Enzymes work rapidly. Enzymes work very fast in converting
substrates into products. The fastest known enzyme is catalase, which
is found in both animal and plant tissues.
– Enzymes are efficient. This is best described by the fact that:
• They are required in very small amounts.
• They are not used up in a reaction and can therefore be used repeatedly.
– Enzymes are globular proteins.
– Enzymes lower the activation energy (Ea) required for reactions
to take place.
In many chemical reactions, the substrate will not be converted to a
product unless it is temporarily given some extra energy. This energy
is called activation energy i.e. the minimum energy required the makea reaction take place.
An enzyme provides a reaction surface for a reaction to take place. This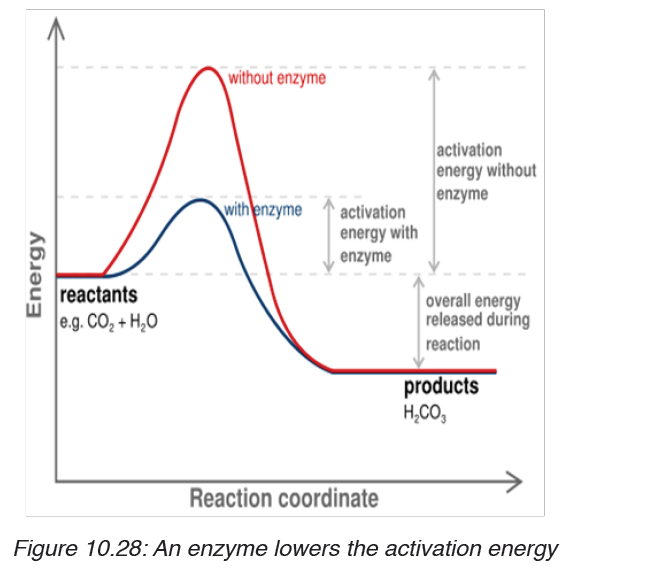
is normally a hollow or cleft in the enzyme which is called the active site,
but it is normally hydrophobic in nature rather than hydrophilic. An enzyme
provides a reaction surface and a hydrophilic environment for the reaction
to take place.
A very small amount of enzymes is needed to react with a large amount of
substrate. The turnover number of an enzyme is the number or reactions
an enzyme molecule can catalyse in one second. Enzymes have a high
turnover number e.g. the turnover number of catalase is 200,000 i.e. one
molecule of enzyme catalase can catalyse the breakdown of about 200,000
molecules of hydrogen peroxide per second into water and oxygen at body
temperature.t
A cofactor is the best general term to describe the non-protein substances
required by an enzyme to function properly. This term covers both organic
molecules and metal ions. A co-enzyme is an organic molecule that acts as
a cofactor. A prosthetic group is a cofactor that is covalently bound to the
enzyme.
Factors affecting enzyme action
Activity 10.2.2.c
You will need
Eight test tubes containing 2 cm3 starch solution, amylase solution, and
cold water (ice) water bath, iodine solution, HCl solution, and droppers
Procedure:1. Label your test tubes A-D as follows:
2. Add 1 cm3 of starch solution to each test tube
3. Keep tube A and B in cold (ice) and tube C and D in the water bath
at 35°C for 5 minutes.
4. Add 1 cm3 of 1M HCl on test tubes B and D, then shake the mixture
to stir.
5. Add 1 cm3 of amylase solution on each test tube. Shake and therefore
keep A and B in cold and C and D in water bath for 10 minutes.
6. Take a sample from each tube and mix it with one drop of iodine.
Use a different tile for each test tube. Record and interpret your
observation and then draw a conclusion.
Enzymes activities can be limited by a number of factors such as the
temperature, the pH, the concentration of the substrate or the enzyme itself
and the presence of inhibitors.
i. Temperature
At zero temperature, the enzyme cannot work because it is inactivated. At
low temperatures, an enzyme-controlled reaction occurs very slowly. The
molecules in solution move slowly and take a longer time to bind to active
sites. Increasing temperature increases the kinetic energy of the reactants.
As the reactant molecules move faster, they increase the number of collisions
of molecules to form enzyme-substrate complex.
At optimum temperature, the rate of reaction is at maximum. The enzyme is
still in active state. The optimum temperature varies with different enzymes.
The optimum temperature for enzymes in the human body is about 37oC.
When the temperature exceeds the optimum level, the enzyme is denatured.
The effect is irreversible. However, some species are thermophilic that is
they better work at high temperatures; others are thermophobic, that is they
better work at low temperatures. For example, some thermophilic algae andbacteria can survive in hot springs of 60oC.
The rate doubles for each 10oC rise in temperature between 0oC and 40oC.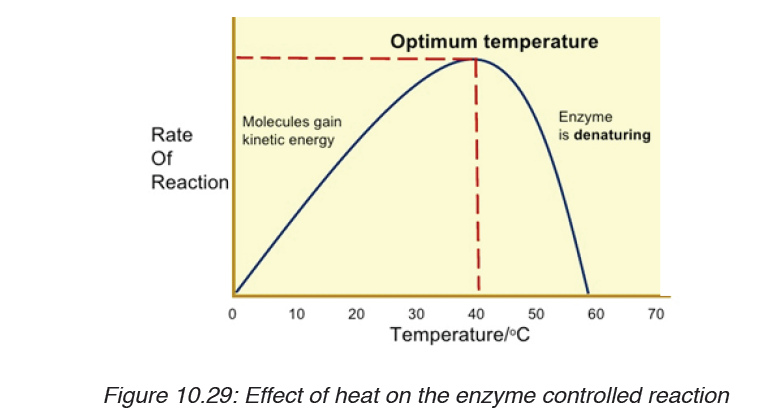
The temperature coefficient Q10 is the number which indicates the effect of
rising the temperature by 10oC on the enzyme-controlled reaction. The Q10
is defined as the increase in the rate of a reaction or a physiological process
for a 10°C rise in temperature. It is calculated as the ratio between rate of
reaction occurring at (X + l0) oC and the rate of reaction at X oC. The Q10 ata given temperature x can be calculated from:
Worked out example
The rate of an enzyme-controlled reaction has been recorded at differenttemperatures as follows:
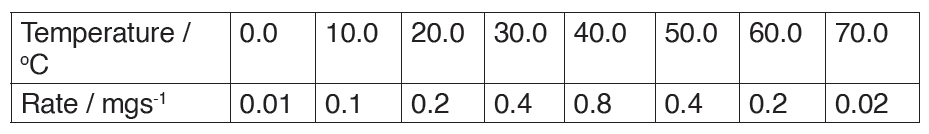
Calculate the Q10 of that reaction at 30 oC
This means that the rate of the reaction doubles if the temperature is raised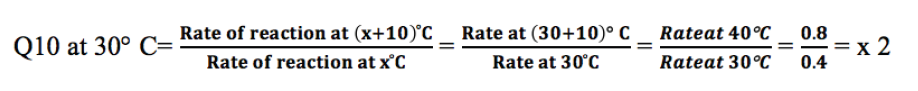
from 30°C to 40°C
Be aware that not all enzymes have an optimum temperature of 40°C. Some
bacteria and algae living in hot springs (e.g. Amashyuza in Rubavu) are
able to tolerate very high temperatures. Enzymes from such organisms are
proving useful in various industrial applications.
ii. The pH
Most enzymes are effective only within a narrow pH range. The optimum pH
is the pH at which the maximum rate of reaction occurs. Below or above the
optimum pH the H+ or OH- ions react with functional groups of amino acids inthe enzyme which loses its tertiary structure and become natured.
Different enzymes have different pH optima (look in the table).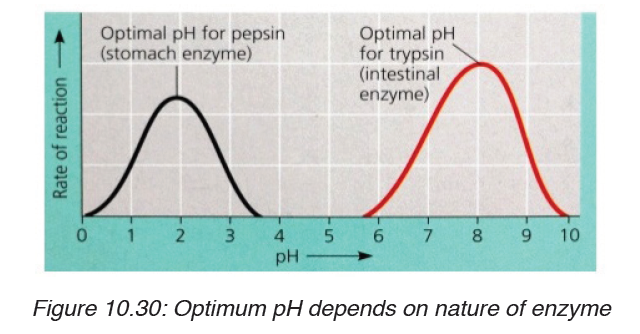
Table 10.5. Optimum pH of some digestive enzymes
Enzyme concentration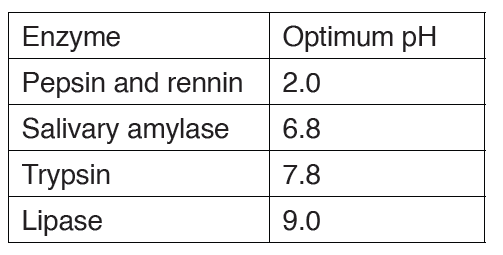
The rate of an enzyme-catalyzed reaction is directly proportional to the
concentration of the enzyme if substrates are present in excess concentrationand no other factors are limiting.
iii. Substrate concentration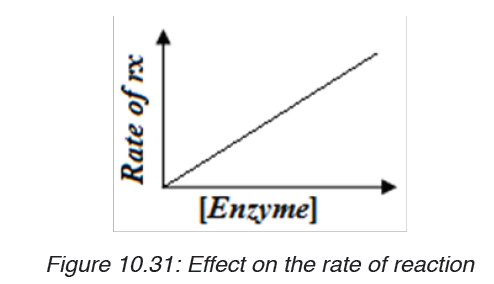
At low substrate concentration, the rate of an enzyme reaction increases with
increasing substrate concentration. The active site of an enzyme molecule
can only bind with a certain number of substrate molecules at a given time.
At high substrate concentration, there is saturation of active sites and thevelocity of the reaction reaches the maximum rate.
iv. Inhibitors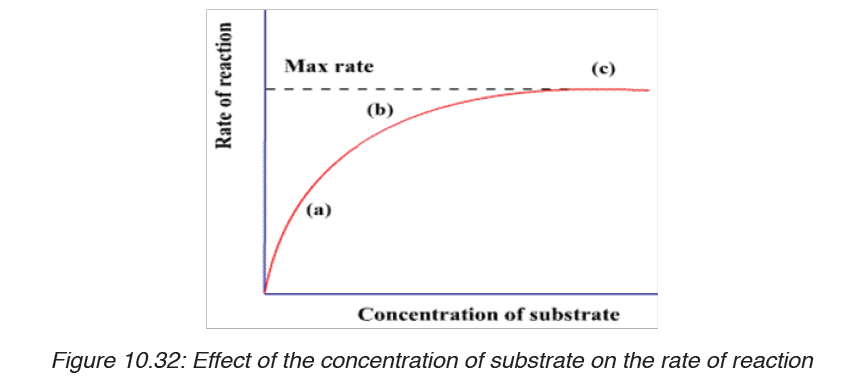
The inhibitors are chemicals or substances that prevent the action of an
enzyme. An inhibitor binds to an enzyme and then decreases or stops its
activity. There are three types of inhibitors:
i. Competitive inhibitors are molecules that have the similar shape
as the substrate. They are competing with the substrate to the activesite of the enzyme e.g. O2 compete with CO2 for the site of RuBPcarboxylase
ii. Non-competitive inhibitors are molecules that can be fixed to the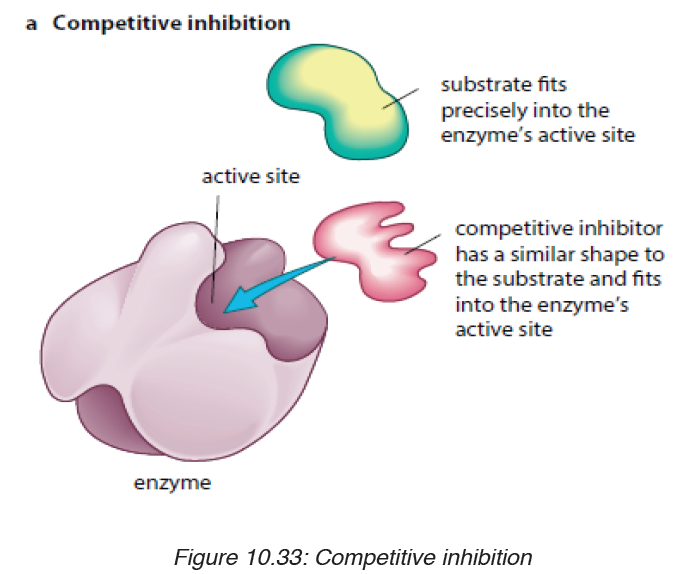
other part of enzyme (not to the active site) so that they change
the shape of active site, due to this the substrate cannot bind tothe active sit of the enzyme.
iii. End product inhibitor Allosteric inhibitor or Allostery.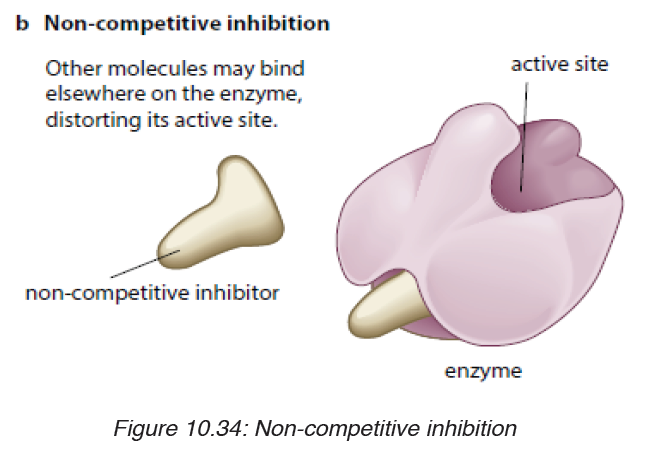
This is a chain enzymatic metabolic pathway where the final end product acts
as an allosteric reversible inhibitor for the first, the second or the third step
in the metabolic pathway. The shape of an allosteric enzyme is altered by
the binding of the end product to an allosteric site. This decreases enzymatic
activity. By acting as allosteric inhibitors of enzymes in an earlier metabolic
pathway, the metabolites can help to regulate metabolism according to the
needs of organisms. This is an example of negative feedback.
This often happen when few enzymes are working on a large number
of substrate e.g. ATP is an end-product inhibitor of the enzyme PFK
(Phosphofructokinase) in glycolysis during cell respiration. The end-productinhibitor leads to a negative feedback.
The products of enzyme-catalysed reactions are often involved in the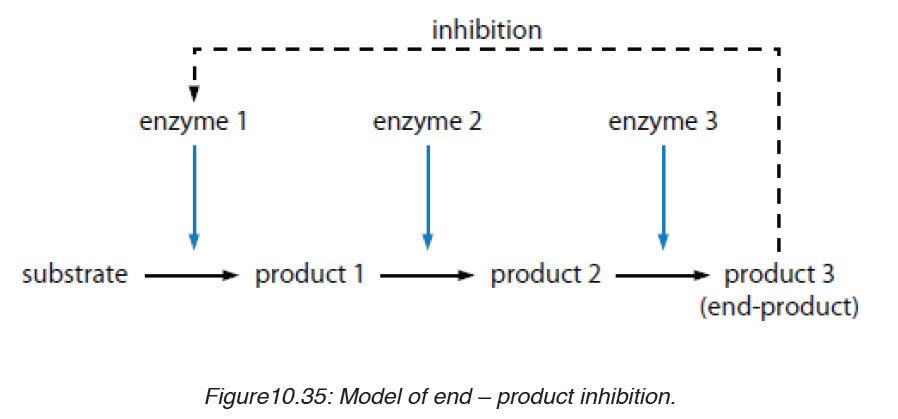
feedback control of those enzymes. Glucose-1-phosphate is the product
formed from this enzyme-catalysed reaction. As its concentration increases,
it increasingly inhibits the enzyme.
Note: Reversible and irreversible inhibition
Competitive inhibitor is reversible inhibitor as it binds temporarily to the
active site. It can be overcome by increasing the relative concentration of
the substrate. Some non-competitive inhibitors are reversible, that is, if the
inhibitor binds temporarily and loosely to the allosteric site. Some inhibitors
have very tightly, often, by forming covalent bonds with enzyme.
The nerve gas DIPF (DiIsopropylPhosphoFluoridate) is an irreversible
inhibitor. It binds permanently with enzyme acetylcholisterase, altering
its shape. The enzyme cannot bind with and break down its substrate
acetylcholine (neurotransmitter). Acetylcholine molecules accumulate in the
synaptic cleft. Nerve impulses cannot be stopped causing continuous muscle
contraction. This leads to convulsions, paralysis and eventually death.
Many pesticides such as organophosphate pesticides act as irreversible
enzyme inhibitors. Exposure to pesticides can produce harmful effects to
the nervous and muscular systems of humans. Heavy metal ions such as
Pb2+, Hg2+, Ag+, As+ and iodine-containing compounds which combine
permanently with sulphydryl groups in the active site or other parts of the
enzyme cause inactivation of enzyme. This usually disrupts disulphide
bridges and cause denaturation of the enzyme.
d) Importance of enzymes in living organisms
Activity 10.2.d
Discuss and present your ideas about the need for different enzymes in
living organisms.
Without enzymes, most of the biochemical reactions in living cells at body
temperature would occur very slowly at not at all. Enzyme can only catalyze
reactions in which the substrate shape fits that of its active site
There are thousands upon thousands of metabolic reactions that happen
in the body that require enzymes to speed up their rate of reaction, or will
never happen. Enzymes are very specific, so nearly each of these chemical
reactions has its own enzyme to increase its rate of reaction. In addition, the
organism has several areas that differ from one another by the pH. Therefore,
the acid medium requires enzymes that work at low pH while other media
are alkaline and therefore require enzymes that work at high pH. In addition
to digestion, enzymes are known to catalyze about 4,000 other chemical
reactions in your body. For example, enzymes are needed to copy genetic
material before your cells divide.
Enzymes are also needed to generate energy molecules called ATP, move
fluid and nutrients around the insides of cells and pump waste material out
of cells. Most enzymes work best at normal body temperature -- about 98
degrees Fahrenheit -- and in an alkaline environment. As such, high fevers
and over-acidity reduce the effectiveness of most enzymes. Some enzymes
need co-factors or co-enzymes to work properly.
10.2.3. Mode of action of enzymes
Activity 10.2.3
There are two main hypotheses that explain the more of action of an
enzyme on its substrate: the lock and key hypothesis and the induced-fit
hypothesis. Carry out a research to find the relevance of each.
Enzymes do not change but substrates are converted to products. A substrate
is a molecule upon which an enzyme acts. In the case of a single substrate,
the substrate binds with the enzyme active site to form an enzyme-substratecomplex. Thereafter the substrate is transformed into one or more products,
which are then released from the active site. This process is summarized asfollows:
Whereby: E = enzyme, S = substrate(s), ES = Complex Enzyme-Substrate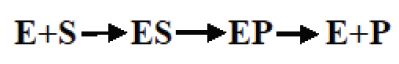
and P= product (s). There are two main hypotheses explaining the mechanism
of enzyme action:
a) The lock and key hypothesis by Emil Fischer
In this hypothesis the substrate is the key and enzyme is the lock. In otherwisethe active site is exactly complementary to the shape of the substrate.
b) The induced-fit hypothesis by Daniel Koshland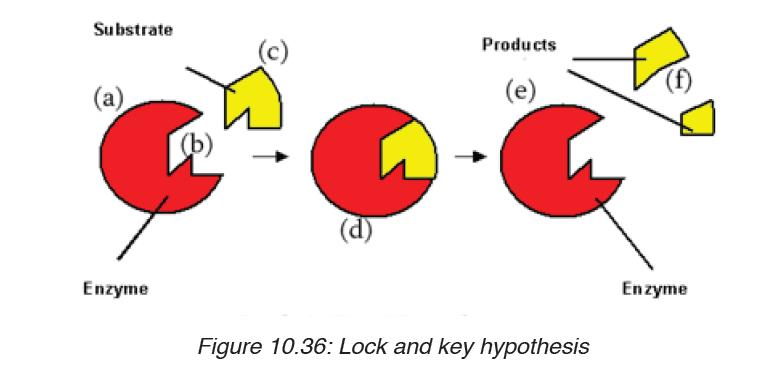
The induced-fit hypothesis is a modified version of the lock and key hypothesis
and is more widely accepted hypothesis. In this hypothesis, the active site
is flexible and is not exactly complementary to the shape of the substrate.
An enzyme collides with the substrate molecule. The substrate binds to the
active site. The bindings induce a slight change in the shape of the enzyme
to enclose the substrate making the fit more precise. The active site now
becomes fully complementary with the substrate as the substrate binds tothe enzyme.
Note that the activity of a given enzyme in vitro (outside a living body) may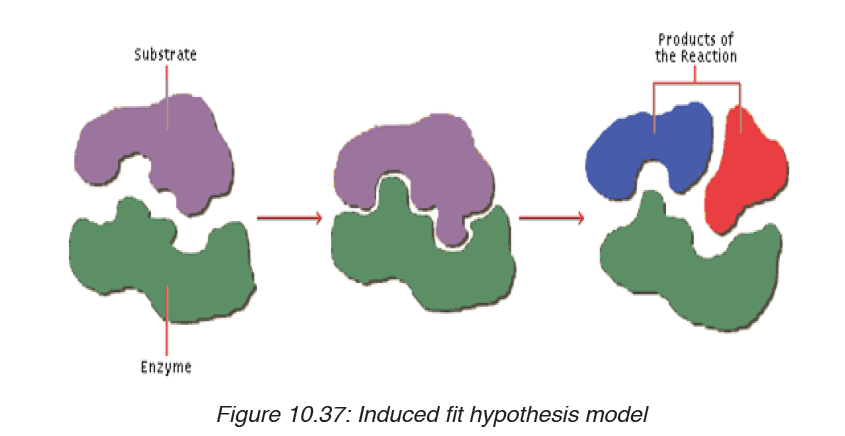
not be necessarily the same in vivo (inside a living body).
Application activity 10.2
Fill the blank with appropriate terms: Enzymes are biological
____________________ produced by ___________________________
cells. Enzymes reduce the amount of ____________________
energy required for reactions to occur. They consist of globular
____________________ with _______________________ structure.
Answer the following questions:
a) What is the main role of enzymes?
b) What would happen if there are no enzymes in the cell? State any
four properties of enzymes.
SKILLS LAB 8
Student-teachers will use the knowledge they acquired in this unit 10 by
testing the components of the food and beverages in their food producing
businesses. Here they will need the knowledge of food test techniques.
End unit assessment 10
1. Biological molecules are divided into:
a) Organic molecules and inorganic molecules
b) Carbohydrates and starch
c) Lipids, carbohydrates and water
d) Carbohydrates, food and potatoes
2. Write the formula of a monosaccharide with 3 atoms of carbon
3. Compare the structure of fat(triglycerides)and the phospholipids
4. Give two examples of how carbohydrates are used in the body.
5. The formula for a hexose is C6H12O6 or (CH2O)6. What would be
the formula of?
a) Triose
b) Pentose
c) Distinguish between:
d) Alpha glucose and beta glucose
e) Glycogen and cellulose
f) Amylopectin and amylose
7. The drug can cleave the covalent bond between two sulfur atoms
of non-adjacent amino acids. Which level of protein can be affected
the most if the drug is mixed with primary, secondary, tertiary and
quaternary structure of proteins.
8. State the property of water that allows each of the following to take
place. In each case, explain its importance:
a) The cooling of skin during sweating
b) The transport of glucose and ions in a mammal
c) Much smaller temperature fluctuations in lakes and oceans than in
terrestrial (land-based) habitats.
9. Construct the table that organize the following terms and label the
columns and rows.
Phosphodiester linkages Monosaccharide polypeptides
Peptide bonds Nucleotides Triacylglycerol
Glycosidic linkages Amino acids Polynucleotides
Ester linkages a ftty acids Polysaccharides
10. Explain what happen during protein denaturation?
11. Enzymes are biocatalysts.
a) What is the meaning of the following terms elated to enzyme
activity?
ii. Catalyst
iii. Activation energy
iv. Lock and key
v. Q10
b) Why are there hundreds of different enzymes in a cell?
c) How do enzymes reduce the activation energy of a reaction?
12. Enzyme activity is related to a number of factors.
a) Explain why enzymes work faster at high temperatures
b) Describe what happens to the enzyme structure if the temperature
is raised well above the optimum temperature.
c) How are enzymes affected by pH?
d) Why do different enzymes have a different optimum pH?
e) What is the difference between a reversible and irreversible enzyme
inhibitor?
13. Some bacteria and algae can survive in the boiling waters of hot
springs. Enzymes from these organisms are used in industrial
processes. Why are these enzymes useful?
14. The following set data show the effect of temperature on the
completion time of an enzyme reaction.
Temperature / 0C 0.0 15 25 35 45 55 65
Rate of reaction / min-1 0.00 0.07 0.12 0.25 0.50 0.28
0.00
a) Plot the data on a graph
b) What is the optimum temperature of this reaction?
c) Describe the shape of the graph between 10 and 400Cd) Calculate the rate of increase between 20 and 300C.
UNIT 11: COVALENT BONDS
Key unit competence
Demonstrate how the nature of the bonding is related to the properties of
covalent compoundsIntroductory Activity 11
As you can see from the picture above, Oxygen is the big buff creature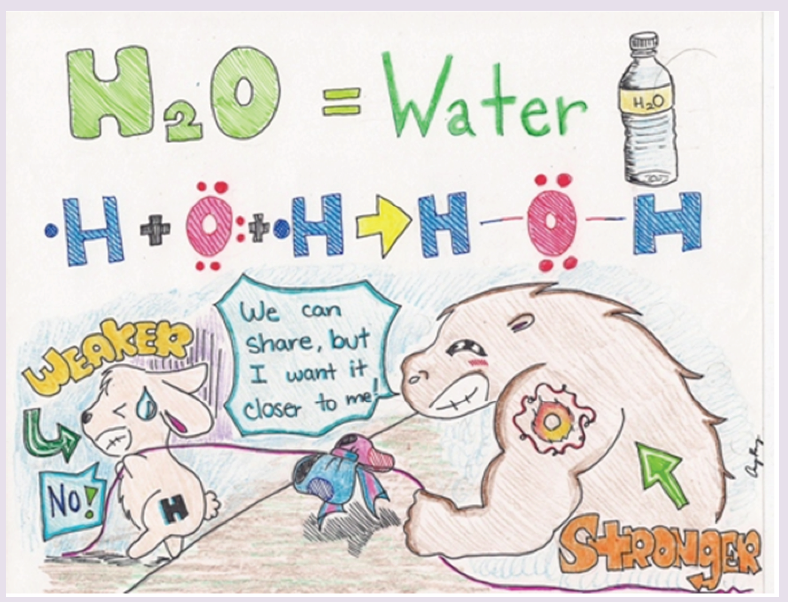
with the tattoo of “O” on its arm. The little bunny represents a Hydrogen
atom. The blue and red bow tied in the middle of the rope, pulled by the
two creatures represent the shared pair of electrons, a single bond.
Because the Hydrogen atom is weaker, the shared pair of electrons will be
pulled closer to the Oxygen atom.
1. Suggest the property used in Chemistry to describe the strength
dedicated to oxygen, the stronger?
2. Suppose that the rope being pulled represents a single covalent
bond, the electron contributed by hydrogen, the weaker will be
transferred to oxygen the stronger?
3. If not, why?
4. Suppose again that we have two oxygens. They have the same
strengths. What will happen to the pulled rope, or the shared pair
of electrons?
5. Suggest a reason why, from the figure, one oxygen needed sharing
with two hydrogens to form water.
6. Conclude about the possible types of covalent bonds.
11.1. Overlap of atomic orbitals to form covalent bonds
Activity 11.1
1. Modern research has shown that an electron moves around
the nucleus in the three dimensional space. What are these
dimensional spaces called?
2. What types of atomic orbital overlapping, what does this overlapping
lead to?
3. Using dots or crosses, give the structure of N(z=7) and Br(z=13)
showing only the electrons on the outermost shell.
Atoms have different ways of combination to achieve the stable octet
electronic structure; two of those ways of combination led to the formation of
ionic bond and metallic bond. But what happens where the two combining
atoms need electrons to complete the octet structure and no one is willing to
donate electrons? For example the combination of 2 hydrogen atoms or the
combination of 2 chlorine atoms?
When this happens, the combining atoms share a pair of electrons where
each atom brings or contributes one electron. In other words there is an
overlapping of two orbitals, one orbital from one atom, each orbital
containing one electron (see Fig.11.1): this bond is called “Covalent bond”.
The attraction between the bonding pair of electrons and the two nuclei holds
the two atoms together. This theory of covalent bond is based on the concept
that electrons are located around the atomic nucleus in orbitals. Then when
two atoms approach each other to share electrons, their two orbitals, each
containing one electron, overlap in the region between the two nuclei to form
a pair of electrons. That pair of electrons is attracted by each nucleus and
this force of attraction maintains the two atoms together; it is this force that
is called chemical bond and in this case, it is qualified as ”covalent”.
Examples:
1. Formation of H2 molecule by overlapping of two 1s orbitals of 2hydrogen atoms:
2. (2) Formation of F2 by overlapping of two p orbitals of 2 fluorine
atoms:
The two examples above have in common that the concentration of the
bonding electrons are on the inter-atomic (inter-nuclei) axis; such bonds are
called “sigma bond”, represented by the symbol “σ”.
As you can observe, p orbitals overlap head-to-head or axially, they form a
σ bond.
1. Formation of O2 molecule (O=O)
When O2 forms, two orbitals in the same orientation, e.g. px, overlap headto-
head to form a σ bond. The other orbitals, e.g. py, will overlap side-by-sideor laterally:
As you notice, the density of bonding electrons is not on the inter-nuclei axis,
it is rather located outside the axis but surrounding it. This kind of covalent
bond is called “ Pi bond”, represented by the symbol “π”. Hence the double
bond O=O is made of two covelent bonds: a σ bond and a π bond.
Due to the position of their electrons density in relation with the two nuclei,
σ bond participates in maintaining the two nuclei together more strongly
than the π bond; that is why σ bond is stronger than π bond. In addition, π
bond cannot exist alone, it exists only where there is a double or triple bond.
Hence, in a double or triple bond, there is one σ bond and one or two π
bonds respectively.
Application activity 11.1
1. Explain the formation of sigma(σ) and pi(π) bonds in:
a) N2
b) Br2
c) NH3
2. Compare the stability of ethane CH3- CH3 and ethene CH2=CH2.
Explain your answer.
3. How many sigma and pi bonds are found in the following molecules:
CH2=CH-CH=CH2 and O2?4. Complete the table that follow by the missing data
11.2. Lewis structures using octet rule (dot and cross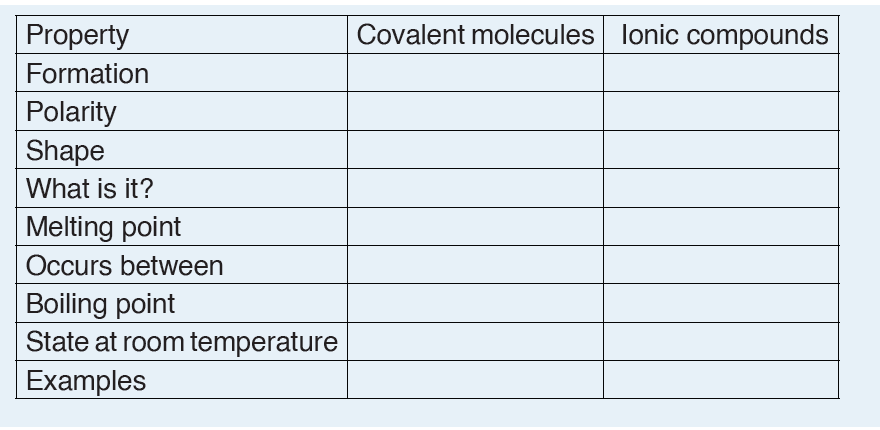
structures)
Activity 11.2
Using available resources, attempt the following:
1. Draw the diagrams indicating only the valence electrons of the
following:
Chlorine molecule (Cl), Carbon atom (C), Phosphorus atom (P),
Nitrogen (N).
2. Draw the diagram to show how all electrons are shared in a
molecule of
i. NH3 indicating all unshared electrons.
ii. HCl (iii) N2
3. Identify the common feature possessed by the diagrams drawn
above in 2.
Lewis structures (also known as Dot and cross structures, Lewis dot
diagrams, Lewis dot formulas, Lewis dot structures, and electrondot
structures) are diagrams that show the bonding between atoms of
a molecule and the lone pairs of electrons that may exist in the molecule. A
Lewis structure can be drawn for any covalently bonded molecule, as well
as coordination compounds.
Lewis structures extend the concept of the electron dot diagram by adding
lines between atoms to represent shared pairs in a chemical bond.
molecule using its chemical symbol. Lines are drawn between atoms that
are bonded to one another (pairs of dots can be used instead of lines).
Excess electrons that form lone pairs are represented as pairs of dots, and
are placed next to the atoms.Examples: Lewis structure of Cl2
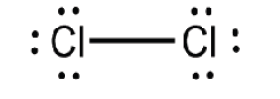
Lewis structure of NH4+
How to draw Lewis Structures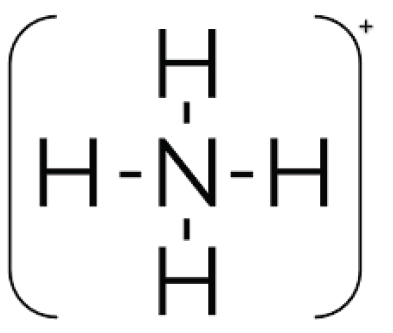
Let us use the nitrate ion (NO3-) as a typical example. An outline of how to
determine the “best” Lewis structure for NO3- is given below:1. Determine the total number of valence electrons in a molecule.
2. Draw a skeleton for the molecule which connects all atoms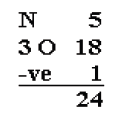
using only single bonds. In simple molecules, the atom with
the most available sites for bonding is usually placed central. The
number of bonding sites is determined by considering the number of
valence electrons and the ability of an atom to expand its octet. As
you become better, you will be able to recognize that certain groupsof atoms prefer to bond together in a certain way!
3. Of the 24 valence electrons in NO3-, 6 were required to make the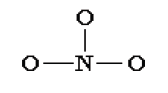
skeleton. Consider the remaining 18 electrons and place them so
as to fill the octets of as many atoms as possible (start with
the most electronegative atoms first then proceed to the moreelectropositive atoms).
4. Are the octets of all the atoms filled? If not then fill the remaining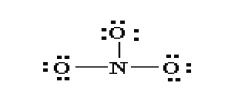
octets by making multiple bonds (make a lone pair of electrons,
located on a more electronegative atom, into a bonding pair ofelectrons that is shared with the atom that is electron deficient).
5. Check that you have the lowest formal charges (F.C.) possible for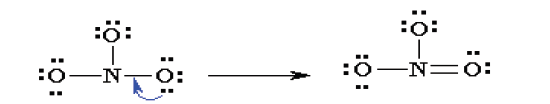
all the atoms, without violating the octet rule; F.C. = (valence e-) -(1/2 bonding e-) - (lone electrons).
6. Thus the Lewis structure of NO3- ion can be written in the following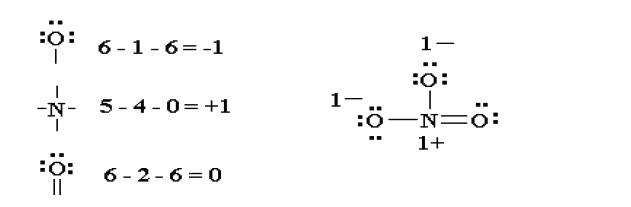
ways:
Lewis structures of unusual compounds that do not obey Octet Rule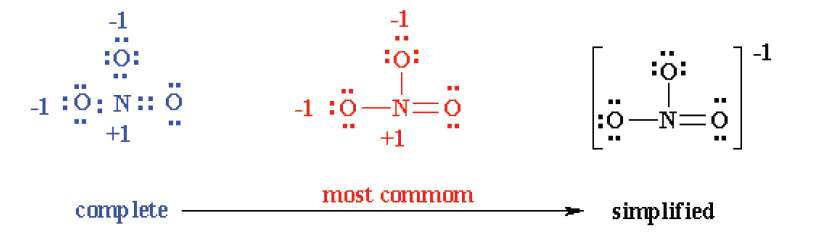
There are three general ways in which the octet rule breaks down:
• Molecules with an odd number of electrons
• Molecules in which an atom has less than an octet
• Molecules in which an atom has more than an octet
1. Odd number of electrons
Consider the example of the Lewis structure for the molecule nitrous oxide
(NO):
• Total electrons: 6 + 5 = 11• Bonding structure:
• Octet on “outer” element:
• Remainder of electrons (11-8 = 3) on “central” atom:
There are currently 5 valence electrons around the nitrogen. A double bond
would place 7 around the nitrogen, and a triple bond would place 9 around
the nitrogen. We appear unable to get an octet around each atom.
2. Less than an octet (most often encountered with elements of Boron
and Beryllium)
Consider the example of the Lewis structure for boron trifluoride (BF3):
• Add electrons (3 x 7) + 3 = 24• Draw connectivities
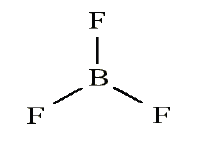
• Add octets to outer atoms:
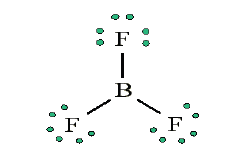
• Add extra electrons (24 – 24 = 0) to central atom:
• Does central electron have octet? No, it has 6 electrons. Add a multiple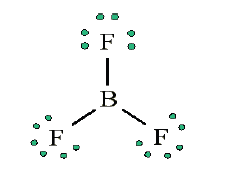
bond (double bond) to see if central atom can achieve an octet:
The central Boron now has an octet (there would be three resonance Lewis structures).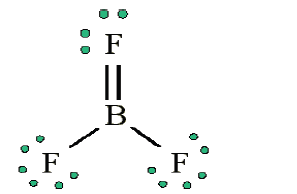
However, in this structure with a double bond the fluorine atom is
sharing extra electrons with the boron.
The fluorine would have a ‘+’ partial charge, and the boron a ‘-’ partial
charge, this is inconsistent with the electronegativities of fluorine and boron.
Thus, the structure of BF3, with single bonds, and 6 valence electrons
around the central boron is the most likely structure.
BF3 reacts strongly with compounds which have an unshared (lone) pair
of electrons which can be used to form a bond with the boron. Example:Reaction of BF3 with ammonia.
3. More than an octet (most common example of exceptions to the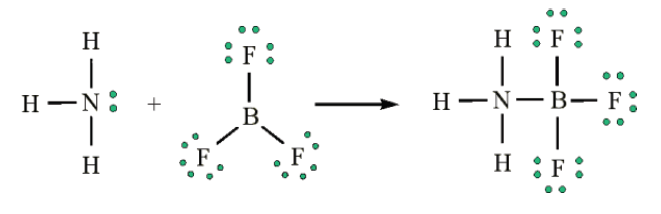
Octet Rule)PCl5 is a legitimate compound, whereas NCl5 is not.
Expanded valence shells are observed only for elements in period 3 (i.e.
n=3) and beyond.
Size is also an important consideration: “The larger the central atom,
the larger the number of electrons which can surround it”. Expanded
valence shells occur most often when the central atom is bonded to small
electronegative atoms, such as F, Cl and O.
Example: Draw the Lewis structure for ICl 4−
• Count up the valence electrons: 7 + (4 x 7) + 1 = 36 electrons• Draw the connectivities:
The ICl4− ion thus has 12 valence electrons around the central Iodine (in the
5d orbitals)
Other examples include: PCl5 and SF6
Application activity 11.2
1. Make a clear definition of the covalent bond.
2. For each of the following, write the electron –dot structures (Lewisstructures) and choose one which violates the Octet Rule?
11.3. Coordinate or dative covalent bond
Activity 11.3
Use your knowledge acquired from the previous lesson and draw the Lewis
structure of ozone (O3), NH4+, H3O+. One of the bonds in these molecules
are special. Explain how they are formed.
A dative covalent bond, or coordinate bond is a type of covalent bonding
(i.e., electron sharing) where the shared electron pair(s) are completely
provided by one of the participants in the union, and not by contributions
from the two of them.
The contributors of these shared electrons are either neutral molecules
which contain lone pair(s) of electrons on one of their atoms, or negatively
charged groups (radicals) with free electrons to donate. Examples of these
are: H2O, NH3 and CN−.
Examples of coordinate bonding: In the reaction
between ammonia and hydrogen chloride a coordinate bond takes place
forming solid ammonium chloride.
NH3 + HCl → NH4Cl
In this reaction the hydrogen ion from the hydrogen chloride leaves its
electrons and gets transferred to the lone pair of electrons on the ammonia
molecule forming ammonium ions (NH4+). This is known as a coordinate
bonding.
Seeing that the hydrogen has left its electron, the chloride will therefore have
a negative charge while the ammonium will have a positive charge. Thediagram below shows the reaction:
Note: The complete compound eventually formed comprises three types of
bonding, i.e., covalent, co-ordinate and electrovalent. In NH4Cl: Formation
of NH3 (covalency); formation of NH4+ (co-ordinate or dative bonding); andformation of NH4Cl (electrovalency).
Dative covalent bonds are represented on drawings as an “arrow”, which
usually points from the atom donating the lone pair to the atom accepting it.
Another example would be the reaction between ammonia and boron
trifluoride. Boron trifluoride is said to be electron deficient meaning it has
3 pairs of electrons at its bonding level but it is capable of having four pairs.
In this reaction the ammonia is used to supply this extra lone pair.
A coordinate bond is formed where the lone pair from the nitrogen moves
toward the boron. The end containing the nitrogen will therefore become
more positive while the boron end will become more negative because it hasreceived electrons.
Application activity 11.3
1. Give the difference and the similarity between a dative covalent
bond and the normal covalent bond.
2. An aluminium chloride molecule reacts with a chloride ion to form
the AlCl4− ion.
a) Name the type of bond formed in this reaction.
b) Explain how this type of bond is formed in the AlCl4− ion.
3. Co-ordinate bonding can be described as dative covalency.
a) In this context, what is the meaning of each of the terms covalency
and dative?
b) Write an equation for a reaction in which a co-ordinate bond is
formed.
11.4. Polarity of the covalent bond
Activity 11.4
The following figures show two types of covalent bond, namely, polar and
non-polar.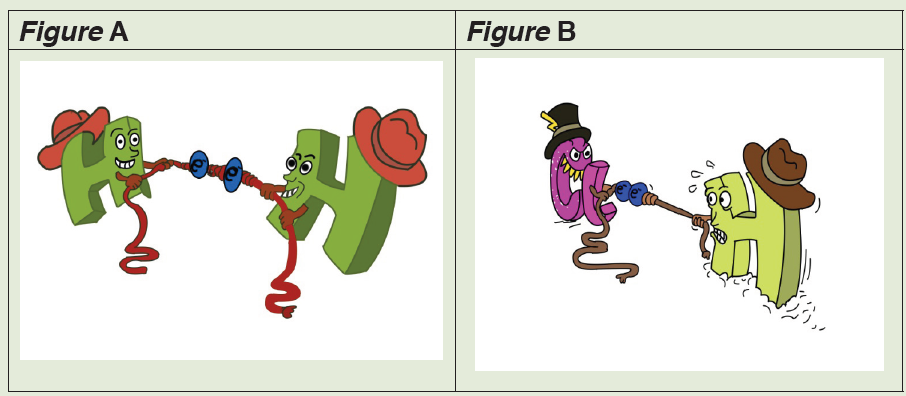
1. Covalent bond is formed between two atoms with similar or close
ability to attract electrons towards themselves, and this is the
reason why they share electrons without being transferred.
a) What is the name of the property used to compare that ability?
b) When the strengths of both atoms are equal, the covalent bond will
be non-polar. Is figure A polar of non-polar?
c) Look at the figure B. The atom, in the zone with more electrons,
will have a partial negative charge. In which zone will be more
electrons?
2. Fill a burette with water. Open the tap and bring a charged ebonite
rod close to the stream of water running from the jet. The water is
deflected from its vertical path towards the charged rod as shownin the figure. Why is this?
A quantity termed ‘electronegativity’ is used to determine the polarity of
the covalent bond; whether a given bond will be non-polar covalent, polar
covalent, or ionic.
Electronegativity is defined as the ability of an atom in a particular
molecule to attract electrons to itself (the greater the value, the greater
the attractiveness for electrons).
Fluorine is the most electronegative element (electronegativity = 4.0),
the least electronegative is Caesium (electronegativity = 0.7).
The bond pair is equally shared in between two atoms when the
electronegativity difference between them is zero or nearer to zero. In
this case, neither of the atoms gets excess of electron density and hence
carry no charge. This is called non-polar covalent bond.
However, when there is a considerable difference in the electronegativity,
the bond pair is no longer shared equally between the atoms. It is shifted
slightly towards the atom with higher electronegativity by creating partial
negative charge (represented by δ-) over it, whereas, the atom with less
electronegativity gets partial positive charge (represented by δ+). This type
of bond is also referred to as polar covalent bond.
We can use the difference in electronegativity between two atoms to
gauge the polarity of the bonding between them.
• In F2 the electrons are shared equally between the atoms, the bond is
non-polar covalent.
• In HF the fluorine atom has greater electronegativity than the hydrogen
atom. The sharing of electrons in HF is unequal: the fluorine atom
attracts electron density away from the hydrogen (the bond is thus apolar covalent bond). The H-F bond can thus be represented as:
The ‘δ+’ and ‘δ-’ symbols indicate partial positive and negative charge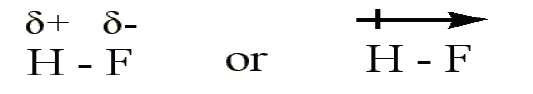
respectively.
The arrow indicates the “pull” of electrons off the hydrogen and towards the
more electronegative atom, fluorine.
• In LiF the much greater relative electronegativity of the fluorine atom
completely strips the electron from the lithium and the result is an ionic
bond (no sharing of the electron).
Note:The following is the general thumb rule for predicting the type of
bond based upon electronegativity differences:
– If the electronegativities are equal (i.e. if the electronegativity difference is 0), the bond is non-polar covalent.
– If the difference in electronegativities between the two atoms is greater
than 0, but less than 2.0, the bond is polar covalent.
– If the difference in electronegativities between the two atoms is 2.0, or
greater, the bond is ionic.Using the examples used above, we can predict the type of bond as follows:
Note: A non-polar molecule is one in which the electrons are distributed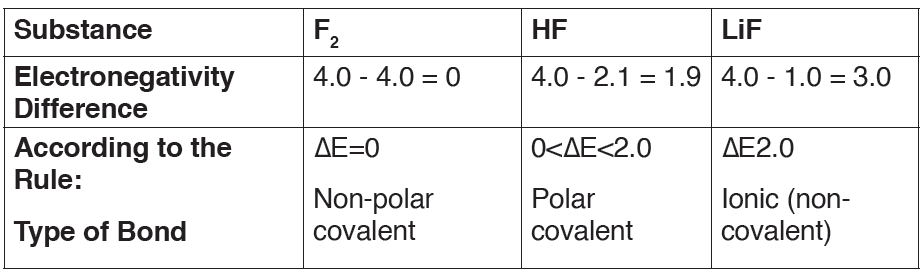
more symmetrically and thus does not have an abundance of charges
at the opposite sides. The charges all cancel out each other. Examples
of non-polar molecules include diatomic molecules, CH4, CO2, C2H4,
cyclohexene, CCl4, etc.
11.5. Physical properties of covalent compounds: simple
molecular structure
Activity 11.5
1. Carbon dioxide (CO2), Bromine (Br2) and SiO2 molecules are all
covalent substances.
a) Give the physical states of these substances at room temperature.
b) Arrange the molecules above in decreasing order of melting and
boiling points knowing their physical states at room temperature.
c) Suggest any reason for the differences in the melting points.
2. Do research and be able to explain physical properties of simple
molecular compounds
Substances composed of relatively small covalently bonded structures
are called Simple Molecular Structures. These contain only a few atoms
held together by strong covalent bonds and can be further categorised into
two types: Individual (which are usually gases like carbon dioxide) and
molecular (which are usually solids like iodine).
The Physical Properties
4. Low melting and boiling points
Simple Molecular Structures tend to have low melting and boiling
points since the forces between molecules are quite weak. Little
energy is required to separate the molecules.
5. Poor electrical conductivity
There are no charged particles (ions or electrons) delocalized
throughout the molecular crystal lattice to conduct electricity. They
cannot conduct electricity in either the solid or molten state.
6. Solubility
Polar compounds are soluble in water (polar) while non-polar
compounds are soluble in nonpolar solvents (oil, hexane…). This
means that substances with the same type of polarity will be soluble
in one another. Moreover, compounds with differing polarities will be
insoluble in one another`
Example:
Hydrogen chloride HCl, Ethanol CH2CH2OH are soluble in water because
there are all polar but they are not soluble in organic solvents which arenonpolar like hexane and heptane.
Application activity 11.5
1. Explain why:
a) Simple molecules have low melting points;
b) Simple molecules have poor conductivity of electricity;
2. Which compounds are soluble or insoluble in water?
SKILLS LAB 11
1. Using adequate materials construct any three models of molecules
of your choice. These models show shells and all electrons.
Electrons which form covalent bonds are highlighted. Molecules to
be made: HCl, CO2, H2, CH4, C2H2, NH3, BF3.
2. Plants contain many chemicals. To extract them from plants for
further studies many solvents such as water, acetone, and hexane
are used. Based on the physical properties especially solubilty do
research and find solvents that should be used to extract some
substances which are found in different plants of your environment.Solvents to be used are: water, hexane and cetone.
End unit assessment 111. Complete the table below by yes or no
2. Choose the best answer. The correct dot formulation for nitrogen
trichloride has:
a) 3 N-Cl bonds and 10 lone pairs of electrons.
b) 3 N=Cl bonds and 6 lone pairs of electrons.
c) 1 N-Cl bond, 2 N=Cl bonds and 7 lone pairs of electrons.
d) 2 N-Cl bonds, 1 N=Cl bond and 8 lone pairs of electrons.
e) 3 N-Cl bonds and 9 lone pairs of electrons.
3. Explain why the boiling point of water is much bigger than that of
methane while their masses are not very different.
4.Choose the correct answer. A (pi) bond is the result of the
a) Overlap of two s orbitals.
b) Overlap of an s and a p orbital.
c) Overlap of two p orbitals along their axes.d) Sidewise overlap of two s orbitals.
5. Show different poles δ- and δ+ in the following molecules between
O, N, Cl and other atoms bonded to them.
a) H3C-Cl b) H3C-O-H c) C2H5-NH2
6. Write the structural formula of propane and propene and compare
their reactivity on the type of bonds in their respective molecules .
7. The equation below shows the reaction between boron trifluoride
and a fluoride ion. BF3 + F− → BF4−
In terms of the electrons involved, explain how the bond between
the BF3 molecule and the F− ion is formed. Name the type of bond
formed in this reaction.
8. The table below shows the electronegativity values of someelements.
a) Define the term electronegativity.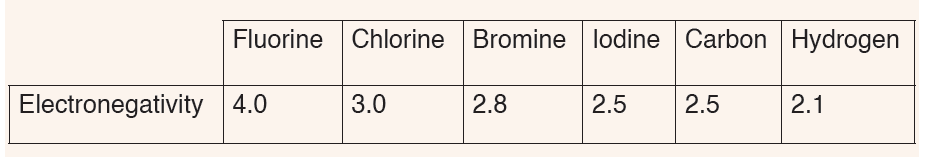
b) Write the formula of hydrogen chloride, hydrogen iodide, hydrogen
fluoride and hydrogen bromide and range them from the most polar
to the least one
9.Draw Lewis structures showing electrons in the outermost shell of
each atom in the following compounds: a) H2O2, b) HCN, c) C2H2,
d)SF6, e) Al2S3
Atomic numbers: H(z=1), O (z= 8), C (z=6), N(z= 7), S (z=16 ), F (z=
9 ), Al (z= 13)
10. How many sigma and pi electrons are contained in the following
molecule?H2C = CH- CH3
UNIT 12: IONIC AND METALLIC BONDS
Key unit competence
Describe how properties of ionic compounds and metals are related to the
nature of their bonding.
Introductory ActivityConsider the following figure 12.1 below.
The figure shows materials commonly used at home. If you reflect back
around your house/home you will see hundreds of objects made from
different kinds of materials.
a) Observe the objects (in picture) and classify them according to the
materials they are made of.
b) Have you ever wondered why the manufacturers choose the
material they did for each item?
c) Why are frying saucepans made of metals and dishes, cups and
plates often made of glass and ceramic?
d) Could dishes be made of metal? And saucepans made of ceramic
and glass?
Of the total number of individual chemically pure substances known, several
million are compounds, formed when two or more elements are chemically
bonded together and less than 100 are elements. Only six of these elements
consist of free, unbounded atoms at room temperature. These are the noble
gases. All other elements exist as individual or giant molecules, or metallic
lattices, in which atoms are chemically bonded to each other.
12.1. Explanations of why atoms of elements form bonds
Activity 12.1
Consider Chlorine (Cl, Z = 17) and Argon (Ar, 18) atoms of the elements of
Period 3 in the Periodic Table.
a) Which of these atoms is more reactive?
b) Suggest the reasons for your answer in (a) in terms of the
electronegativity and electronic structure.
c) Choose, between Chlorine and Argon, which one has lower energy
potential.
The atoms of most elements form chemical bonds because the atoms become
more stable when bonded together. Electric forces attract neighbouring
atoms to each other, making them stick together.
In atoms, electrons are arranged into complex layers called shells. For most
atoms, the outermost shell is incomplete, and the atom shares electrons with
other atoms to fill the shell.
The type of chemical bond maximizes the stability of the atoms that form it.
An ionic bond, where one atom essentially donates an electron to another,
forms when one atom becomes stable by losing its outer electrons and the
other atoms become stable (usually by filling its valence shell) by gaining the
electrons. Covalent bonds form when sharing atoms results in the highest
stability.
Other types of bonds https://www.thoughtco.com/types-of-chemical-bonds-
603984 besides ionic and covalent chemical bonds exist, too.
Atoms with incomplete shells are said to have high potential energy; atoms
whose outer shells are full have low potential energy. In nature, objects with
high potential energy “seek” a lower energy, becoming more stable as a
result. Atoms form chemical bonds to achieve lower potential energy.
Application activity 12.1
Explain why atoms of elements form bonds in terms of stability and potential
energy.
12.2. Gain of stability by losing and gaining electrons
Activity 12.2
The figure below shows how the atoms may gain stability by losing andgaining electrons.
d) State and describe clearly the group on the periodic table that
contains elements that have the same atomic structures as the
species obtained when atom “Big” has received the electron from
atom “Little”.
Like people always relate and connect to others depending on their values,
interests and goals so do unstable atoms. They combine together to achieve
stability. We know that noble gases are the most stable elements in the
periodic table. They have a filled outer electron energy level.
When an atom loses, gains, or shares electrons through bonding to achieve
a filled outer electron energy level, the resulting compound is often more
stable than individual separate atoms.
• Neutral sodium has one valence electron. When it loses this electron
to chlorine, the resulting Na+ cation has an outermost electron energy
level that contains eight electrons. It is isoelectronic (same electronic
configuration) with the noble gas neon.
• On the other hand, chlorine has an outer electron energy level that
contains seven electrons. When chlorine gains sodium’s electron, it
becomes an anion that is isoelectronic with the noble gas argon.
It is easiest to apply the “Octet Rule” to predict whether two atoms will form
bonds and how many bonds they will form. Most atoms need 8 electrons
to complete their outer shell. So, an atom that has 2 outer electrons will
often form a chemical bond with an atom that lacks two electrons to be
“complete”. The octet rule states that elements gain or lose electrons to
attain an electron configuration of the nearest noble gas. Octet comes from
Latin language meaning “eight”.
Note that the “Duet Rule” is also applied. The noble gas HELIUM has two
electrons (a doublet) in its outer shell, which is very stable. Hydrogen only
needs one additional electron to attain this stable configuration, while lithium
needs to lose one.
Low atomic weight elements (the first twenty elements) are most likely to
adhere to the Octet Rule. For example,
• A sodium atom has one lone electron in its outer shell.
• A chlorine atom, in contrast, is short one electron to fill its outer shell.
• Sodium readily donates (loses) its outer electron (forming the
Na+ ion, since it then has one more proton than it has electrons), while
chlorine readily accepts (gains) a donated electrons (making the Cl- ion, since chlorine is stable when it has one more electron than it
has protons).
• Sodium and chlorine form an ionic bond with each other, to form
table salt or sodium chloride.
Application activity 12.2
1) State the following Rule?
a. Octet Rule
b. Duet Rule
2) Answer to the following questions
a. Does sodium need to gain electron than chlorine? (Yes or No)
b. Explain the target of sodium when it is seeking to lose electron and
chlorine to gain electron.
3) Which of the following is stable? Explain why?
i. Na+ iii. Clii. Na iv. Cl-
12.3. Ionic bonding
Activity 12.3
In the Ordinary Level, you learnt that there exist three main types of
chemical bonding namely, covalent, ionic and metallic.
a) Recall the definition of the ionic bond.
b) The bond formed between sodium and chlorine atoms to form the
table salt is an ionic bond. Using experiments performed in daily life
and performing the non-performed ones, state the properties of a
table salt and use it to generalize the properties of ionic compounds
basing on the criteria given in parentheses (Appearance/physical
state, Solubility in water and in organic solvent, Temperature
required to melt, Electrical conductivity of solid and aqueous
solution).
An ionic bond is a chemical bond formed between two ions with opposite
charges. Ionic bonds form when one atom gives up one or more electrons
to another atom. These bonds can form between a pair of atoms or between
molecules and are the type of bond found in salts.
12.3.1. Ionic bond formation
Once the oppositely charged ions formed when electrons are transferred
from one atom to another, they are attracted by their positive and negative
charges (by electrostatic forces) and form an ionic compound. Ionic bonds
are also formed when there is a large electronegativity difference between
two atoms. This difference causes an unequal sharing of electrons such that
one atom completely loses one or more electrons and the other atom
gains one or more electrons. For example, in the creation of an ionic bond
between a metal atom, sodium (electronegativity = 0.93) and a non-metal,
fluorine (electronegativity = 3.98).Let us take a look of how sodium and fluorine bond to form sodium fluoride:
The curved arrow between sodium and fluorine atoms represents the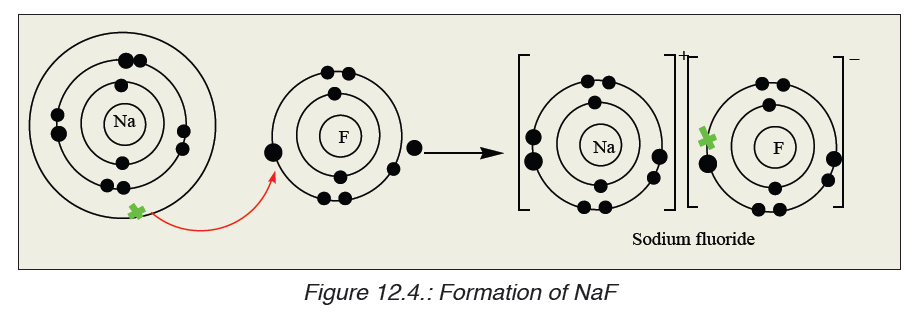
transfer of an electron from a sodium atom to a fluorine atom to form
oppositely charged ions. These two ions are strongly attracted to each
other because of their opposite charges. A bond is now formed and the
resulting compound is called Sodium fluoride.
Another example of ionic bonding formation is the formation of magnesiumoxide.
12.3.2. Properties of ionic compounds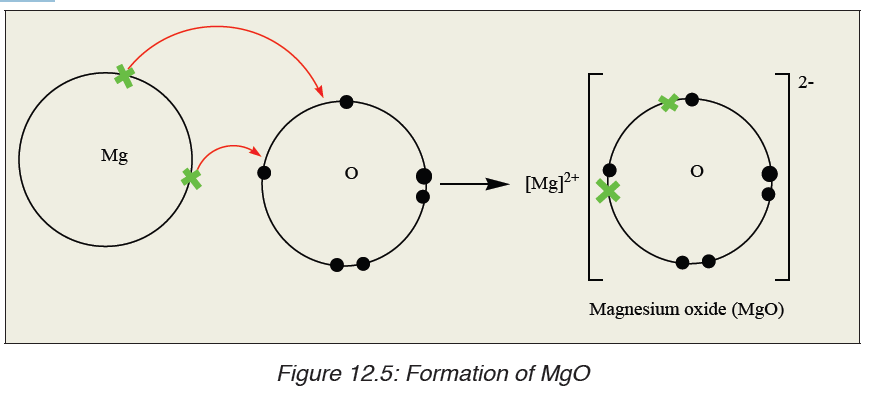
Here are the properties shared by the ionic compounds. Notice that the
properties of ionic compounds relate to how strongly the positive and
negative ions attract each other in an ionic bond.
1. They have high melting points and high boiling points
In an ionic lattice, there are many strong electrostatic attractions between
oppositely charged ions. We therefore expect that ionic solids will have
high melting points. On melting although the regular lattice is broken down,
there will still be significant attractions between the ions in the liquid. This
should result in high boiling points also.
The factors which affect the melting point of an ionic compound are:
• The charge on the ions: «The greater the charge, the greater the
electrostatic attraction, the stronger the ionic bond, the higher the
melting point ». For example, Melting Point of NaCl is 801 oC and that
of MgO is 2,800 oC.
• The size of the ions: «Smaller ions can pack closer together than
larger ions so the electrostatic attraction is greater, the ionic bond is
stronger, the melting point is higher». For example, Melting Point of
NaF is 992 oC and that of CsF is 2,800 oC.
2. Most ionic compounds are soluble in water
This is because the electrostatic forces of the polar water molecules are
stronger than the electrostatic forces keeping the ions together. When
an ionic compound like NaCl is added to water, water molecules attract the
positive and negative salt ions. Water molecules surround each ion and move
the ions apart from each other. The separated ions dissolve in water. There
are several exceptions, however, where the electrostatic forces between the
ions in an ionic compound are strong enough that the water molecules cannot
separate them. Despite these few limitations, water›s ability to dissolve ionic
compounds is one of the major reasons it is so vital to life on Earth. Ionic
compounds are generally insoluble in non-polar solvents like kerosene.
3. They are hard and brittle
Ionic crystals are hard because the positive and negative ions are strongly
attracted to each other and difficult to separate, however, ionic solids are
brittle. When a stress is applied to the ionic lattice, the layers shift slightly.
The layers are arranged so that each cation is surrounded by anions in
the lattice. If the layers shift then ions of the same charge will be brought
closer together. Ions of the same charge will repel each other, so the latticestructure breaks down into smaller pieces.
4. They conduct electricity when molten or dissolved in water
In order for a substance to conduct electricity, it must contain mobile
particles capable of carrying charge.
• Solid ionic compounds do not conduct electricity because the ions
(charged particles) are locked into a rigid lattice or array. The ions
cannot move out of the lattice, so the solid cannot conduct electricity.
• When is molten, the ions are free to move out of the lattice structure.
– Cations (positive ions) move towards the negative electrode (cathode):
M+ + e- → M
– Anions (negative ions) move towards the positive electrode (anode):
X- → X + e-
• When is dissolved in water to form an aqueous solution, the ions are
released from the lattice structure and are free to move so the solution
conducts electricity just like the molten (liquid) ionic compound.
5. They form crystals
Ionic compounds form crystal lattices rather than amorphous solids. Although
molecular compounds form crystals, they frequently take other forms but
molecular crystals typically are softer than ionic crystals. At an atomic
level, an ionic crystal is a regular structure, with the cation and anion
alternating with each other and forming a three-dimensional structure
based largely on the smaller ion evenly filling in the gaps between thelarger ion.
Application activity 12.3
1. The diagram below represents a part of the structure of sodium
chloride. The ionic charge is shown on the centre of only one ofthe ions.
a) On the diagram, mark the charges on the four negative ions.
b) What change occurs to the motion of the ions in sodium chloride
when it is heated from room temperature to a temperature below
its melting point?
c) Sodium chloride can be formed by reacting sodium with chlorine.
A chloride ion has one more electron than a chlorine atom. In the
formation of sodium chloride, from where does this electron come?
2. Draw diagrams to illustrate the formation of ionic compounds in the
following substances:
a) Magnesium chloride
b) Sodium peroxide
c) Iron (III) chloride
d) Sodium sulphide
3. Solid sodium chloride and solid magnesium oxide are both held
together by ionic (electrovalent) bonds.
a) Using s, p, d and f notation, write down the symbol for and the
electronic configuration of (i) a sodium ion; (ii) a chloride ion; (iii) a
magnesium ion; (iv) an oxide ion.
b) Explain what holds sodium and chloride ions together in the solid
crystal
c) Sodium chloride melts at 1074 K; magnesium oxide melts at 3125
K. Both have identical structures. Why is there such a difference intheir melting points?
12.4. Metallic bonding
Activity 12.4
1. Give three examples of substances which are malleable, ductile,
good conductor of heat and electricity, and having a characteristic
luster. Here you can use a dictionary or other searching tools to
find the meaning for any unfamiliar word.
2. Suggest another property, apart from those given, of the substances
you have given in 1.
3. Choose from the examples given in 1, one which is most common
and well known.
a) This substance is seen to be composed by atoms of one element.
Which one?
b) Use a labelled drawing to show the internal structure of that kind of
substance.
A metallic bond is a type of chemical bond formed between positively
charged atoms in which the free electrons are shared among a lattice
of cations. In contrast, covalent and ionic bonds form between two discrete
(separate) atoms.
Metallic bonding is the main type of chemical bonds that forms between
metal atoms (pure metals and alloys and some metalloids). A metal is alattice of positive metal “ions” in a “sea” of delocalised electrons.
12.4.1. Formation of metallic bond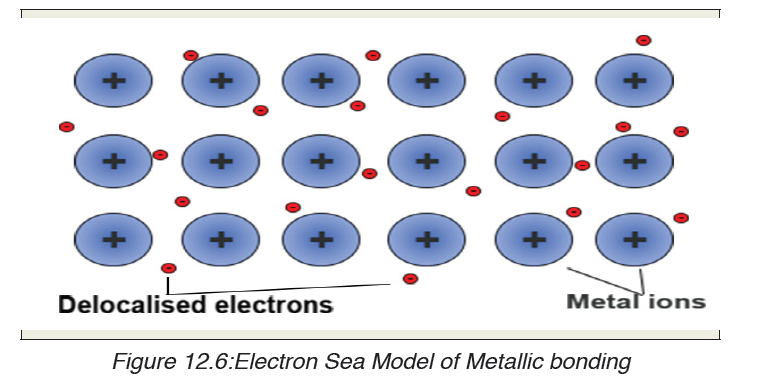
The outer energy levels of metal atoms (the s and p orbitals) overlap. At least
one of the valence electrons participating in a metallic bond is not shared
with a neighbour atom, nor is it lost to form an ion. Instead, the electrons
form what may be termed an «electron sea” in which valence electrons
are free to move from one atom to another. Metallic bonding refers to theinteraction between the delocalised electrons and the metal nuclei.
The electron sea model is an oversimplification of metallic bonding.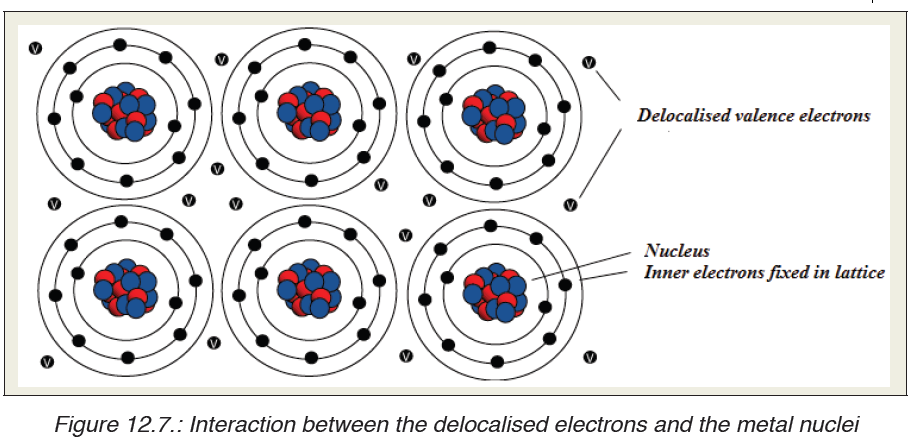
Calculations based on electronic band structure or density functions are
more accurate. Metallic bonding may be seen as a consequence of a
material having many more delocalized energy states than it has delocalized
electrons (electron deficiency), so localized unpaired electrons may become
delocalized and mobile. The electrons can change energy states and move
throughout a lattice in any direction.
Bonding can also take the form of metallic cluster formation, in which
delocalized electrons flow around localized cores. Bond formation depends
heavily on conditions. For example, hydrogen is a metal under high pressure!
As pressure is reduced, bonding changes from metallic to non-polar covalent.
12.4.2. Physical properties of metals
Because electrons are delocalized around positively charged nuclei, metallic
bonding explains many properties of metals. The three main factors that
affect the strength of a metallic bond are:
– Number of protons/ Strength of nuclear attraction: The more
protons the stronger the force of attraction between the positive ions
and the delocalized electrons
– Number of delocalized electrons per atom: The more delocalized
electrons the stronger the force of attraction between the positive ions
and the delocalized electrons
– Size of atom: The smaller the atom, the stronger the force of attraction
between the positive ions and the delocalized electrons and vice-versa,
the larger the atom, the weaker the force of attraction between the
positive ions and the delocalised electrons.
The main physical properties of metallic metals are given below.
1. Electrical Conductivity
Most metals are excellent electrical conductors because the electrons
in the electron sea are free to move and carry charge. For example,
electric wires in our homes are made of aluminium and copper. They are
good conductor of electricity. Electricity flows most easily through gold,
silver, copper and aluminium. Gold and silver are used for fine electrical
contacts in computers.
2. Thermal Conductivity
Metals conduct heat because the free electrons are able to transfer
energy away from the heat source and also because vibrations of atoms
(phonons) move through a solid metal as a wave. Cooking utensils and
water boilers are also made of iron, copper and aluminium, because they aregood conductors of heat.
3. Ductility
Metals tend to be ductile or able to be drawn into thin wires because
local bonds between atoms can be easily broken and also reformed.
Single atoms or entire sheets of them can slide past each other and reform
bonds. Wires are mainly made from copper, aluminium, iron and magnesium.
4. Malleability
Metals are often malleable or capable of being molded or pounded
into a shape, again because bonds between atoms readily break and
reform. This ability to bend or be shaped without breaking occurs because
the electrons simply slide over each other instead of separating. The
binding force between metals is non-directional, so drawing or shaping a
metal is less likely to fracture it. Electrons in a crystal may be replaced
by others. Gold and Silver metals are the most malleable metals. They can
be hammered into very fine sheets. Thin aluminium foils are widely used for
safe wrapping of medicines, chocolates and food material.
5. Metallic Luster
Metals tend to be shiny or display metallic luster. They are opaque
once a certain minimum thickness is achieved. The electron sea reflects
photons off the smooth surface therefore there is an upper frequency limit
to the light that can be reflected. Silver is a very good reflector. It reflects
about 90% of the light falling on it. All modern mirrors contain a thin coating
of metals. Due to their shiny appearance they can be used in jewellery and
decorations.
Application activity 12.4
1. Magnesium has a higher melting and boiling point than sodium. This can
be explained in terms of the electronic structures, the packing, and the
atomic radii of the two elements.
a) Explain why each of these three things causes the magnesium melting
and boiling points to be higher.
b) Explain why metals are good conductors of electricity.
c) Explain why metals are also good conductors of heat.
1. Magnesium has a higher melting and boiling point than sodium. This can
be explained in terms of the electronic structures, the packing, and the
atomic radii of the two elements.
a) Explain why each of these three things causes the magnesium melting
and boiling points to be higher.b) Explain why metals are good conductors of electricity.
c) Explain why metals are also good conductors of heat.
2. Pure metals are usually malleable and ductile.
a) Explain what those two words mean.
b) If a metal is subjected to a small stress, it will return to its original shape
when the stress is removed. However, when it is subjected to a larger
stress, it may change shape permanently. Explain, with the help of simple
diagrams why there is a different result depending on the size of the
stress.
c) When a piece of metal is worked by a blacksmith, it is heated to a high
temperature in a furnace to make it easier to shape. After working it with
a hammer, it needs to be re-heated because it becomes too difficult to
work. Explain what is going on in terms of the structure of the metal.
d) Why is brass harder than either of its component metals, copper andzinc?
SKILLS LAB 12
The figure below shows the electric conductivity of distilled water (A), solid tablesalt (B) and a solution of a table salt (C) respectively.
a) Use the diagrams A, B and C to explain the observations from the set up.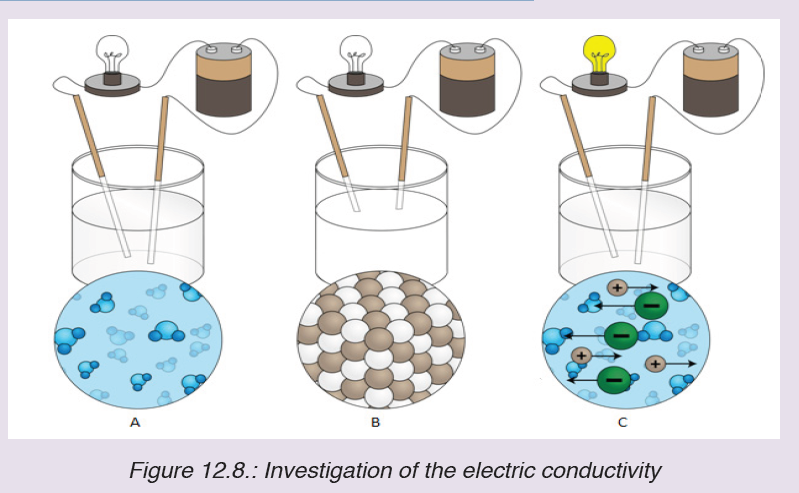
i. No light is given out by bulb in A
ii. No light is given out by bulb in B
iii. Light is given out in C
b) Suppose that you have a 30 cm bar made of table salt. Suggest the
change, if there is any, that can occur and deduce the property related,
when this salt bar is:
i. Dropped from a table of 1 m high to the floor
ii. Immersed in water found in a water bath.c) Dry heated to 100 oC
End unit assessment 12
1. State whether the following statement is True or False. Justify your
answer. “Sodium Chloride has a higher melting point than Magnesium
Oxide”.
2. Why are ionic compounds brittle?
3. Why do ionic compounds have high melting points?
4. What happens when an electric current is passed through a solution of
an ionic compound?
5. This question is about metallic bonding.
a) Describe the bonding that is present in metals.
b) Explain how the bonding and structure lead to the typical metallic
properties of electrical conductivity and malleability.
c) Suggest a reason why aluminium is a better conductor of electricity than
magnesium.
6. Silver and sodium chloride melt at similar temperatures. Give two physical
properties of silver which are different from those of sodium chloride and,
in each case, give one reason why the property of silver is different from
that of sodium chloride.
7. This question is about calcium oxide (CaO).
a) Describe the nature and strength of the bonding in solid calcium oxide.
b) Use the kinetic theory to describe the changes that take place as calcium
oxide is heated from 25°C to a temperature above its melting point.c) State two properties of calcium oxide that depend on its bonding.
UNIT 13: THIN LENSES
Key unit competence
Explain the properties of lenses and image formation by lenses
Introductory Activity 13Observe these images below and answer the following questions
a) Through your observation, Name A,B,C and D and identify where
they can be used in our daily life activities
b) What is the effect of light on A, B, C and D?
c) Analyze the phenomenon in figure C thereafter write your
observation in your notebook
d) How the images are formed on the above figures? Explain your
reason
13.1. Types and characteristics of lenses
Activity 13.1
1. Look closely at the lenses and answer these questions in yournotebook:
a) How are the lenses shaped? Explain your answer
b) How are the lenses different?
c) Classify these lenses according to your observation thereafter
explain the conditions used to classify them.
A lens is an optical device with perfect or approximate axial symmetry which
transmits and refractslight, by converging or diverging the beam. Most lenses
are spherical lenses: their two surfaces are parts of the surfaces of spheres.Lenses are classified by the curvature of the two optical surfaces.
A lens is a piece of glass with one or two curved surfaces. The lens which is
thicker at the centre than at the edges is called a convex lens while the one
which is thinner at its centre is known as a concave lens. The curved surface
of the lens is called a meniscus. The lens in the human eye is thicker in the
centre, and therefore it is a convex lens.
The light rays from the ray box change the direction after passing through the
lens. They are therefore refracted by the lens. Hence, lenses form images of
objects by refracting light.
You can see that the rays from the convex lens are getting closer and closer
to a point. The rays are thus converging, and hence a convex lens is called
a converging lens. You can also see that the refracted rays from the concave
lens are spreading out. This kind of lens is called diverging lens. When light
passes through a lens, refraction occurs at the two lens surfaces.
Using Snell’s law and geometry, you can predict the paths of rays passing
through lenses. To simplify such problems, assume that all refraction occurs
on a plane, called the principal plane that passes through the center of thelens. This approximation, called the thin lens model, applies to all the lenses.
Application activity 13.1
1. The cross sections of four different thin lenses are shown in Figure
below.
a) Which of these lenses, if any, are convex, or converging lenses?
b) Which of these lenses, if any, are concave, or diverging lenses?
c) Why do you make such decision at a and b?d) Where did you see these types of lenses in everyday life?
2. Make a deep research on where this types of lenses are used in
our daily life activities
13.2. Refraction of light through lenses.
Activity 13.2
i. Hold a hand lens about 2 m from the window. Look through the lens.
(CAUTION: Do not look at the sun).
What do you see?
ii. Move the lens farther away from your eye.
What changes do you notice?
iii. Now, hold the lens between the window and a white sheet of paper,
but closer to paper.
iv. Slowly move the lens away from the paper towards the window. Keep
watching the paper.
What do you see? What happens as you move the paper?
Do you see that an inverted image of trees outside is formed on the paper?
How do you think the image is formed?
Lenses can be thought of as a series of tiny refracting prisms, each of
which refracts light to produce an image. These prisms are near each other
(truncated) and when they act together, they produce a bright image focusedat a point.
Each section of a lens acts as a tiny glass prism. The refracting angles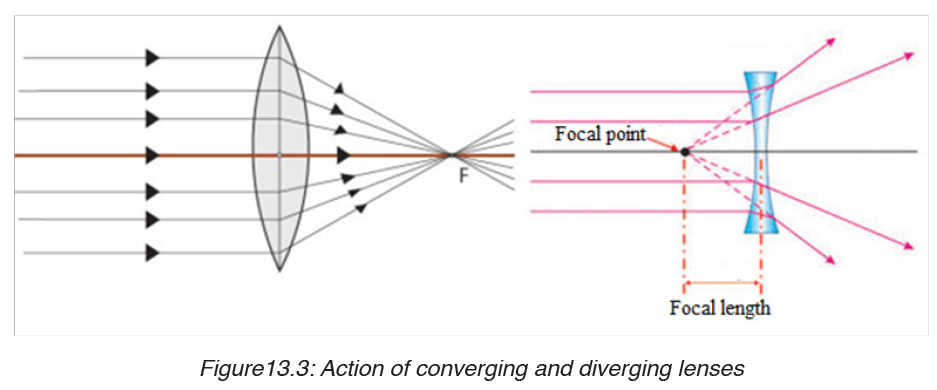
of these prisms decrease from the edges to its centre. As a result, light is
deviated more at the edges than at the centre of the lens.
The refracting angles of the truncated prisms in a converging lens point to
the edges and so bring the parallel rays to a focus.
The truncated prisms of the diverging lens point the opposite way to those of
the converging lens, and so a divergent beam is obtained when parallel rays
are refracted by this lens because the deviation of the light is in the opposite
direction.
The middle part of the lens acts like a rectangular piece of glass and a ray
incident to it strikes it normally, and thus passes without deviation.
13.2.1 Ray diagrams and properties of images formed by lenses
Notice that an image cannot be seen on the screen irrespective of the
position of the object.
The nature of the image formed by a convex lens depends on the position of
the object along the principal axis of the lens.
The principal focus of a lens plays an important part in the formation of
an image by a lens since parallel rays from the object converge to it, and
thus, we consider points F and 2F when describing the nature of the images
formed by the lens.
These images can be larger or smaller than the object or same size as the
object. When an image is larger than the object, we say that it is magnified
and when it is smaller, we say that it is diminished.
Images which can be formed on the screen are Real images. Because light
rays pass through these images, real images can be formed on the screen.
All real images formed by the convex lens are inverted.
To determine an image point, we need to consider only the three rays
indicated if Fig.13.4, which uses an arrow (on the left) as the object, and a
converging lens forming an image to the right. These rays, emanating from
a single point on the object, are drawn as if the lens were infinitely thin, and
we show only a single sharp bend at the centre line of the lens instead ofrefraction at each surface. These three rays are drawn as follows:
The point where these three rays cross is the image point for that object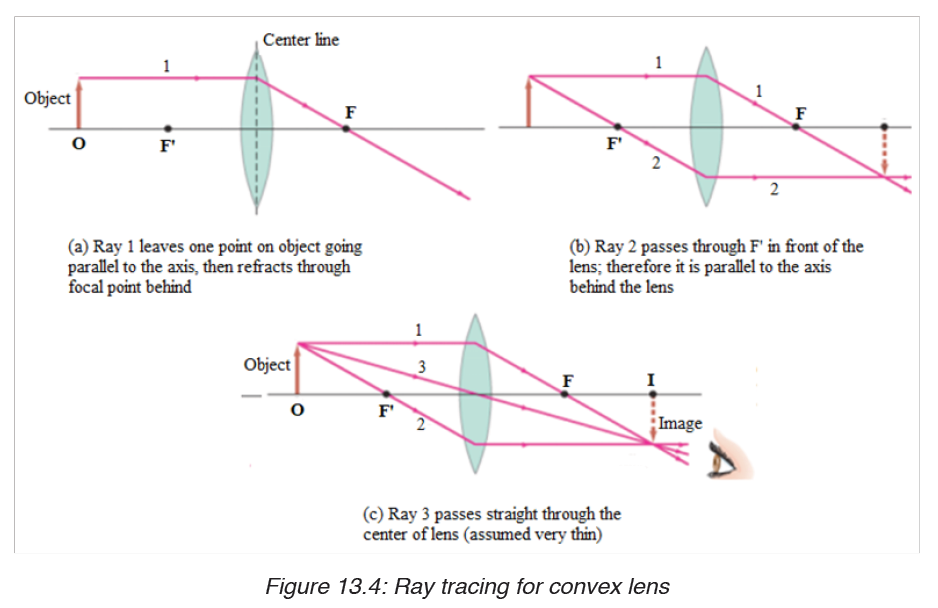
point. Actually, any two of these rays will suffice to locate the image point,
but drawing the third ray can serve as a check.
13.2.2 Graphical construction of images by Converging lens
If the lens is biconvex or plano-convex, a collimated parallel or diverging
beam of light travelling through the lens will be converged to a spot behindthe lens. In this case, the lens is called a converging lens.
When an object is placed at a distance greater than the focal length from the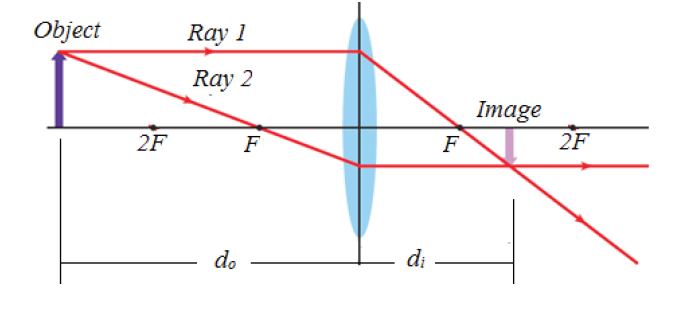
lens, the image is real and inverted on the other side of the lens. When an
object is placed at a distance equal to twice the focal length from the lens,the image is the same size as the object and inverted.
A convex lens forms a virtual image that is upright and larger compared to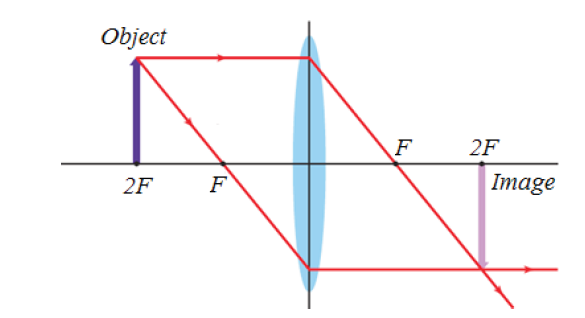
the object when the object is located between the lens and the focal point.
13.2.3 Graphical construction of images by diverging lens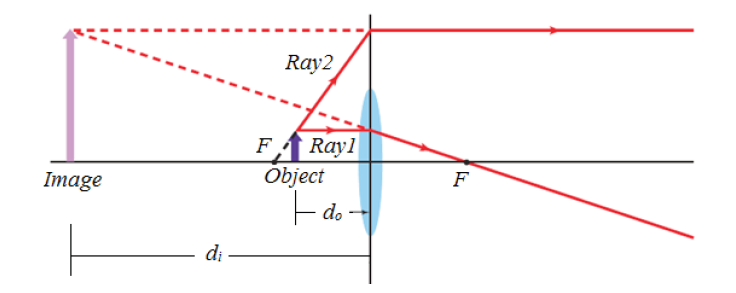
If the lens is biconcave or plano-concave, a beam of light travelling to thelens axis and passing through the lens will be diverged.
Concave lenses produce only virtual images that are upright and smaller
compared to their objects.
When an object is between F and the lens, there is no image formed on
the screen. The image formed is not real and is only seen by removing the
screen and placing an eye in its position. We say that it is a virtual image.
For a virtual image, rays appear to come from its position.
Unlike for a convex lens where the nature of the image depends on the
position of the object, a concave lens gives only an upright, small, virtual
image, and is situated between the principal focus and the lens for allpositions of the object.
Application activity 13.2
1. Design an experiment to study images formed by convex lenses of
various focal lengths. How does the focal length affect the position
and size of the image produced?
2. An object of length 5 cm is placed at a distance 25 cm in front of
a lens of focal length 10 cm.Use a ray diagram to construct the
image of this object and state its properties if the lens is :
a) Converging;
b) Diverging.
13.3. Thin lens equations and determination of focal length
Activity 13.3
Task 1:
i. Place a converging lens on a table while facing a window.
ii. Place a white screen behind the lens. Move the screen to and fro
(forwards and backwards) until a sharp image of a distant object is
seen on the screen.
iii. Discuss and write down the observation in your notebook.
iv. Measure the distance from the lens to the screen. What is thisdistance called?
Task 1:
i. Place a converging lens on a table while facing a window.
ii. Place a white screen behind the lens. Move the screen to and fro
(forwards and backwards) until a sharp image of a distant object is
seen on the screen.
iii. Discuss and write down the observation in your notebook.
iv. Measure the distance from the lens to the screen. What is this
distance called?
Task 2:
i. Draw a ray diagram to determine the nature and position of the image
of an object placed 10cm from a diverging lens of focal length 15cm.
ii. Using the above information, find the nature and position of the
image using a lens formula. (Assign f a negative sign during your
substitution).
iii. What is the location of the image?
13.3.1 Convex lens
We now derive an equation that relates the image distance to the object
distance and focal length of a thin lens. This equation will make the
determination of image position quicker and more accurate than doing ray
tracing. Let be the object distance, the distance of the object from the center
of the lens, and the distance of the image from the center of the lens. And
let and refer to the heights of object and image. Consider two rays shownin Figure below for a converging lens, assumed to be very thin.
The right triangles FI’I and FBA are similar because angle AFB equals angle IFI’; so
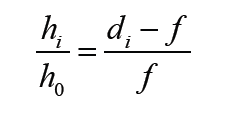
Since length 0 AB = h . Triangles OAO’ and IAI’ are similar as well. Therefore,
We equate the right sides of these equations (the left sides are the same),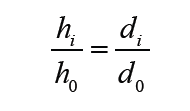
and divide di by to obtain
This is called the thin lens equation. It relates the image distance di to the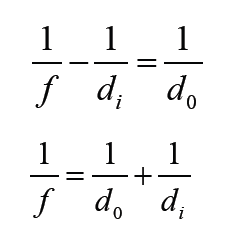
object distance d0 and focal length . It is the most useful equation in geometricoptics. If the object is at infinity, then
length is the image distance for an object at infinity, as mentioned earlier.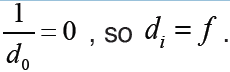 Thus the focal
Thus the focal
13.3.2 Concave lensWe can derive the Lens equation for diverging lens using the following figure.
Triangles IAI’ and OAO’ are similar; and triangles IFI’ and AFB are similar.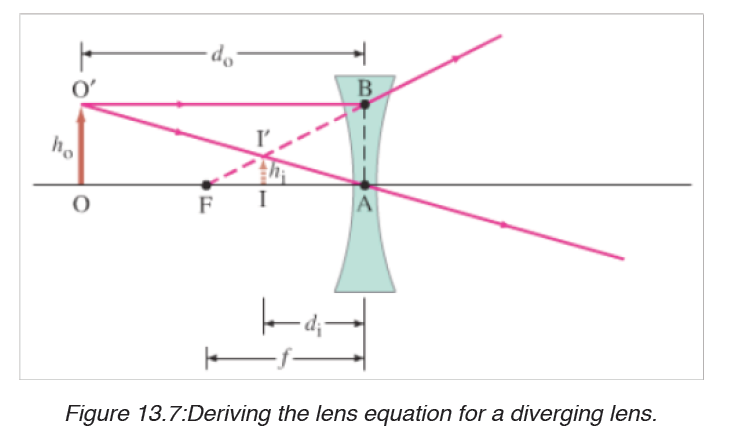
Thus (noting that length AB = h0 )
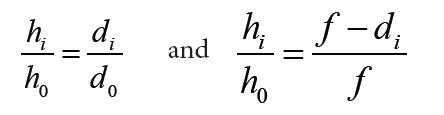
When we equate the right sides of these two equations and simply, we obtain
This equation becomes the same as that of convex lens if we make f and di negative. That is, we take f to be negative for a diverging lens and di negative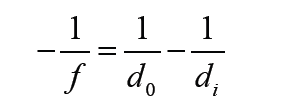
when the image is on the same side of the lens as light comes from.Thus
Will be valid for both converging and diverging lenses, and for all situations,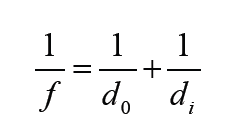
if we use the following sign conventions:
1. The focal length is positive for converging lenses and negative for
diverging lenses.
2. The object distance is positive if the object is on the side of the lens
from which light is coming; otherwise, it is negative.
3. The image distance is positive if the image is on the opposite side
from where light is coming; if it is on the same side, it is negative.
Equivalently, the image distance is positive for real image and
negative for a virtual image.
4. The height of the image, is positive if the image is upright, and
negative if the image is inverted relative to the object. (is always
taken as positive).
13.3.3 Magnification
The magnification, m, of a lens is defined as the ratio of the image heightto object height. From the above figures and the sign conventions, we have
for an upright image the magnification is positive, and for an inverted image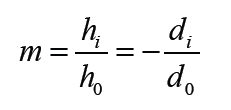
the magnification is negative.
13.3.4 The power of lenses
Whenever a ray of light passes through a lens it bends except when it passes
through the optical centre. The degree of convergence or divergence of a
lens is expressed as power. A lens of short focal length deviates the rays
more while a lens of large focal length deviates the rays less. Thus the power
of a lens is defined as the reciprocal of its focal length.Power of a lens
The unit of power is dioptre (D)1D = 1m−1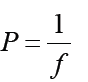
From sign convention 1, it follows that the power of converging lens, in
diopters, is positive, whereas the power of a diverging lens is negative. A
converging lens is sometimes referred to as positive lens, and a diverging
lens as a negative lens.In case the lenses are combined
Example 13.3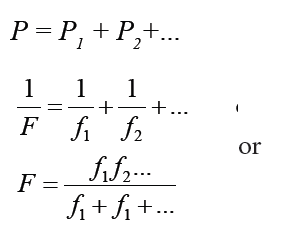
An object is placed 32.0 cm from a convex lens that has a focal length of
8.0 cm.
a) Where is the image?
b) If the object is 3.0 cm high, how tall is the image?
c) What is the orientation of the image?
Solution
1. Analyse and sketch the problem
• Sketch the situation, locating the object and the lens.• Draw the two principal rays
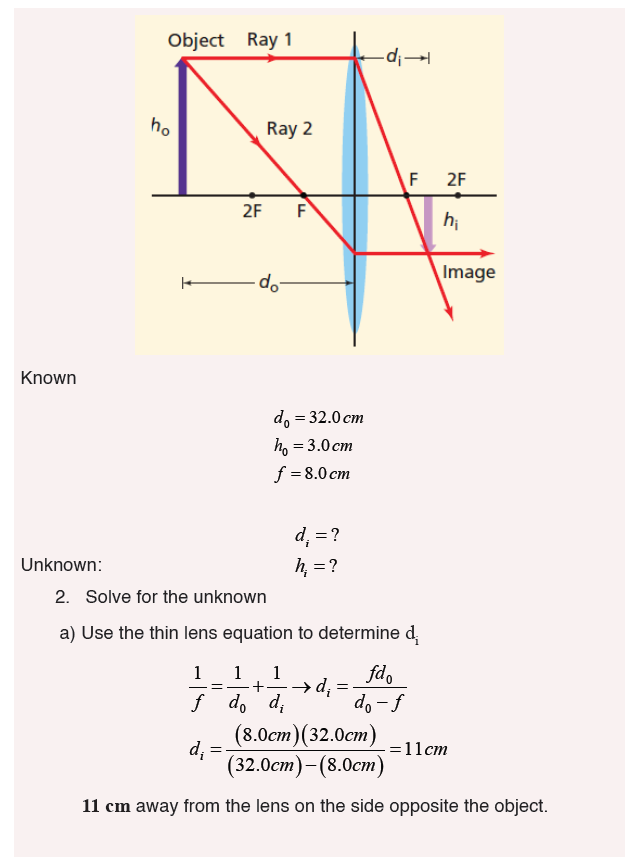
13.3.5 Lens maker formula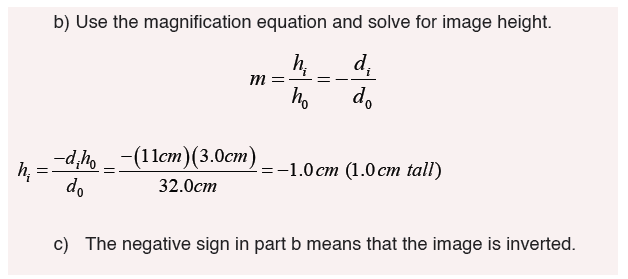
The following assumptions are made for the derivation:
– The lens is thin, so that distances measured from the poles of its
surfaces can be taken as equal to the distances from the optical centre
of the lens.
– The aperture of the lens is small.
– Object point is considered.– Incident and refracted rays make small angles.
Consider a convex lens of absolute refractive index nt to be placed in a rarer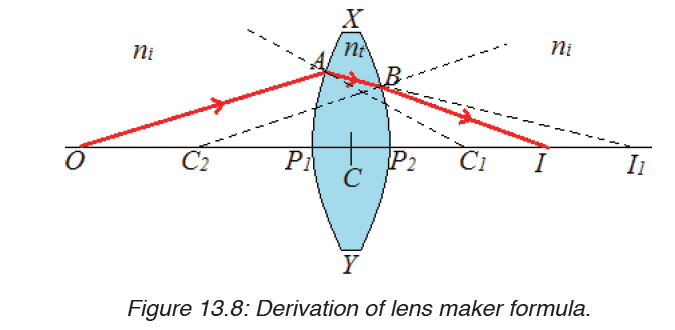
medium of absolute refractive indexni.
Considering the refraction of a point object on the surface XP1Y, the image
is formed at I1 who is at a distance of P1I1.
CI1= P1I1 (as the lens is thin)
CC1 = P1C1=R1
CO = P1O
The refracted ray from A suffers a second refraction on the surface XP2Y
and emerges along BI. Therefore Iis the final real image of O. Here the
object distance is
do = CO ≈ CI1 ≈ R1It follows from the refraction due to convex spherical surface XP1Y
Let CI ≈ P2 I = d be the final image distance. Let R2 be radius of curvature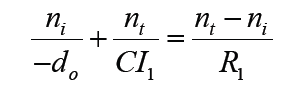
of second surface of the lens.
di = CI ≈ CI1 ≈ R2
It follows from refraction due to concave spherical surface from denser torarer medium that
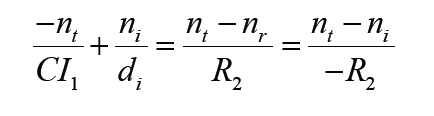
Adding
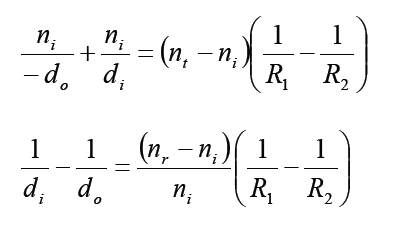
This equation gives the lens maker’s formula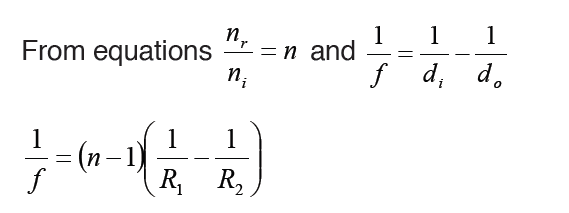
Note:
– The lens maker’s formula indicates that a convex lens can behave like
a diverging one if ni > nt i.e., if the lens is placed in a medium whose
index is greater than the index of lens. Similarly a concave lens can be
made convergent.
– The lens maker’s formula can be derived for a concave lens in the
same way.
Sign convention states that real is positive while virtual is negative. This
should be put under consideration when one is using the lens formula tosolve problems.
Application activity 13.3
1. Compute the composition and focal length of the converging lens
which will project the image lamp, magnified 4 times, upon a screen
10.0 m from the lamp.
2. A lens has a convex surface of radius 20 cm and a concave surface of
radius 40 cm and is made of glass of refractive index 1.54. Compute
the focal length of the lens, and state whether it is a converging or a
diverging lens.
13.4. Defects and correction of lenses
Activity 13.4
i. Place a white sheet of paper on a horizontal ground.
ii. Hold a glass ruler above the paper so as to focus rays from the sun
on to the paper.
iii. Observe carefully the image formed on the sheet of paper.
iv. Repeat the above with the convex lens. What have you observed?
v. Use internet to search for Defects and correction of lenses
Aberration
A lens made of a uniform glass with spherical surface cannot form a perfect
image. The spherical aberration is prominent image defect for a point source
on the optical axis of such a lens. It arises because all rays through the lensare not focused to a common point.
The dependence of index of refraction of wavelength also causes the focal
length, and thereby the image position, to depend on the color of the light.
All simple lenses suffer from such chromatic aberration.
There are several different types of aberration which can cause the image
to be an imperfect replica of the object(Spherical aberration, chromatic
aberration, coma, field curvature, barrel, pincushion distortion, astigmatism,
etc).
Notice that the image has coloured patches. This defect where by an image
formed has coloured patches is called chromatic aberration. There are two
kinds of defects; spherical aberration and chromatic aberration.
13.4.1 Spherical aberration
This arises in lenses of larger aperture when a wide beam of light incident on
the lens, not all rays is brought to one focus.
As a result, the image of the object becomes distorted. The defect is due to
the fact that the focal length of the lens for rays far from the principal axis are
less than for rays closer to a property of a spherical surface and as a result,they converge to a point closer to the lens.
This defect can be minimized (reduced) by surrounding the lens with an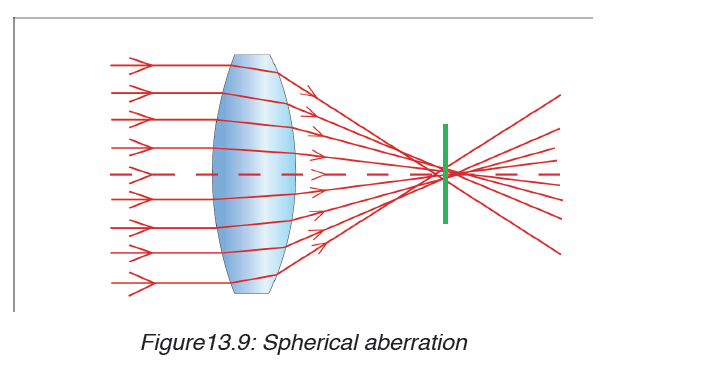
aperture disc having a hole in the middle so that rays fall on the lens at a
point closer to its principal axis. However, this reduces the brightness of the
image since it reduces the amount of light energy passing through the lens.
13.4.2 Chromatic aberration
Chromatic aberration is caused by the dispersion of the lens material. Since,
from the lens formulae, f is dependent upon n, it follows that different coloursof light will be focused to different positions.
Chromatic aberration can be minimised by using an achromatic doublet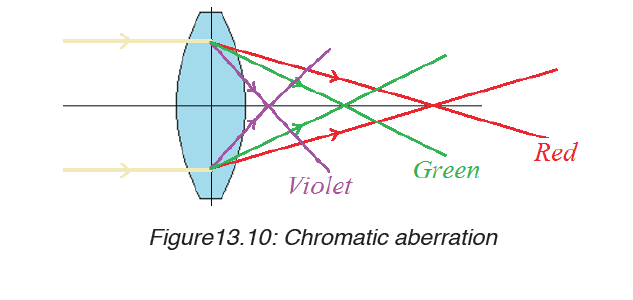
(or achromat) in which two materials with differing dispersion are bondedtogether to form a single lens.
Application activity 13.4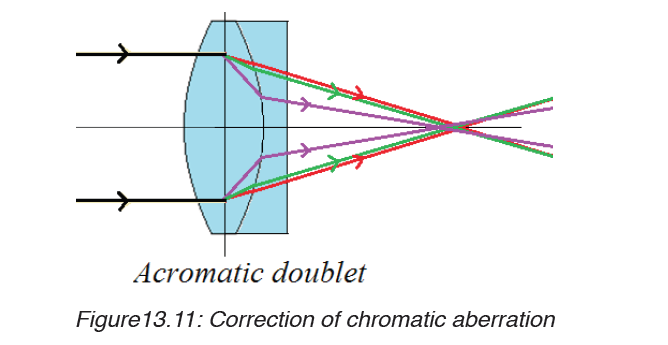
i. How spherical and chromatic aberration can be reduced?
ii. If a plano-convex lens is used as objective lens in a telescope, how
is its convex surface faced to minimize spherical aberration? Explain
13.5. Refraction through prisms
13.5.1. Terms associated with refraction through prism
Activity 13.5
Problem
Have you ever heard of a prism?
How does it look like?
Procedures
a) Consider the shapes of the glasses provided below. Observe them
clearly and identify the shape of a prism.Explain your reasoning.
b) With the help of a teacher, touch, observe and identify the real
shape of the prism.
c) Examine the features of the one selected as a prism.
In optics, a prism is transparent material like glass or plastic that refracts light.
At least two of the flat surfaces must have an angle less than 90o betweenthem. The exact angle between the surfaces depends on the application
From the figure 13.12, the following terms are used in refraction through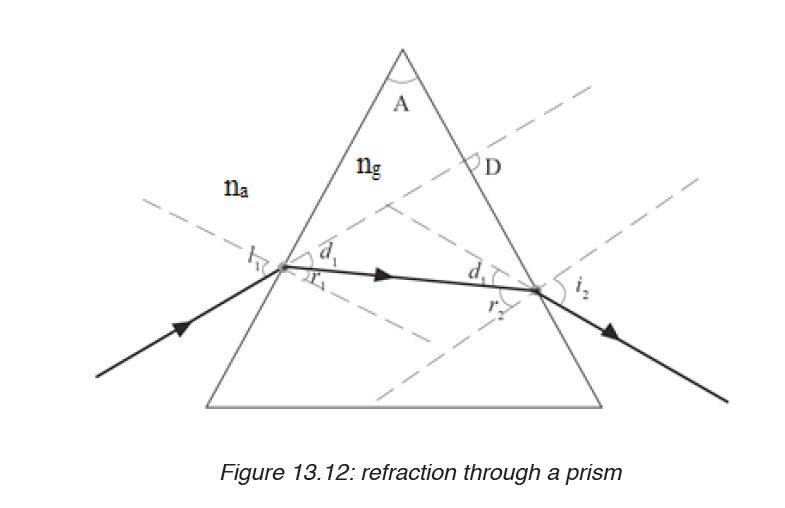
prism.
Angle A: This is called refracting angle or angle of the prism. It is the angle
between the inclined surfaces of the prism.
Angle i: This is the angle of incidence on the first face of the prism.
Angle r1: This is the angle of refraction on the first face of the prism.
Angle r2: This is the angle of refraction on the second face of the prism.
Angle l2: This is the angle of emergence from second face of the prism.
Sometimes this is denoted by letter e.
Angle D: this is the angle of deviation of rays through a prism.
na: is a refractive index in air.
ng:is a refractive index in glass
13.5.2 Dispersion of light by a prism
Dispersion of light is the separation of a beam of light into its constituent
colours. This takes place when a light beam passes through a dispersive
medium. A beam of white light incident on a prism splits into its constituent
colours to form “a visible spectrum”consisted of colours violet, indigo, blue,green, yellow, orange and red.
When a polychromatic light is incident on the first surface of the prism, each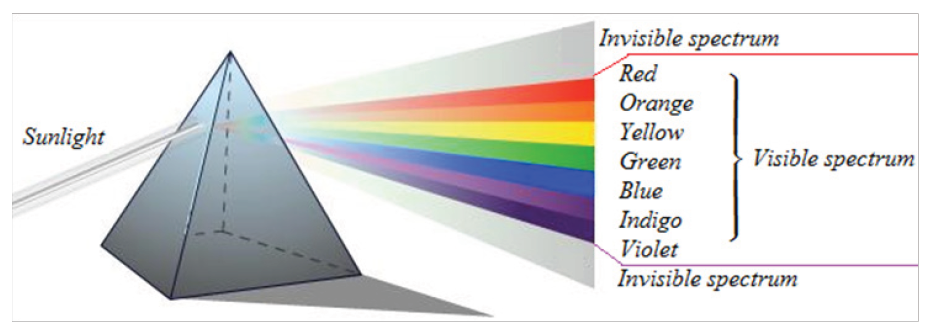
constituent colour gets refracted through a different angle. When these
colours are incident on the second surface of the prism they are again
refracted further.
Application activity 13.5
1. i. Place a prism in the centre of a piece of paper so that its refracting
surface is directly facing the windows in order to receive light from
the sun.
ii. Place a white screen on the far side of the prism so that the
refracted rays hit it.
iii. Observe what is formed on the screen.
iv. In brief, write in your notebook the observation.
2. Do a research to find other terms associated to the refraction light
through prism.
13.6 Deviation by a prism and determination of refractive index
Activity 13.6
1. Have you ever heard of the word deviation? List down in your
notebook at least two ways in which light can be deviated.
2. You are provided with a glass prism of refracting angle 60o, four
optical pins, a white sheet of paper, a soft board and fixing pins.i. Place a prism on a white sheet of paper and mark its outline ABC.
ii. Remove the prism and draw a normal line ON to face AB and draw a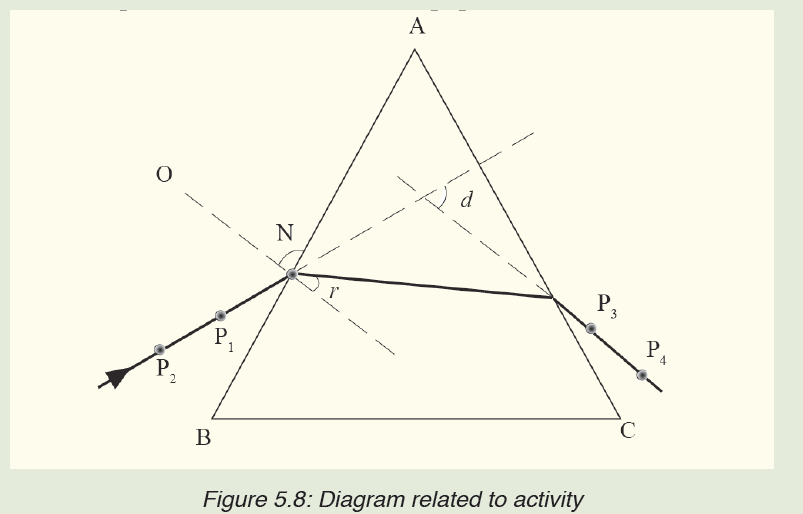
line making an angle of 10o to ON to represent the incident ray.
iii. Place back the prism in its outline and fix pins P1 and P2 along the line.
iv. While looking through the other face AC of the prism, fix pins P3 and
P4 so that they appear in line with images of P1 and P2.
v. Remove the prism and draw a line through P3 and P4 on to face AC
of the prism.
vi. Measure the angle of deviation d.
vii. What is your observation on the direction of incident ray afterrefraction?
In the figure13.13, E F is a ray incident on the refracting surface YX of the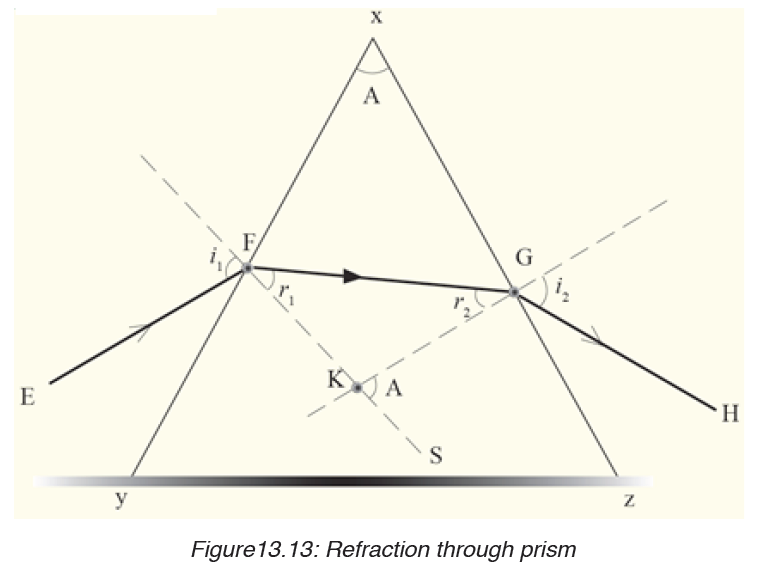
prism XY Z from air and then to air from surface XZ of the prism. KF and KG
are normal at the points of incidence and emergence of the ray respectively.
Now, from the geometry of quadrilateral XFKG,
< X FK + < X GK = 180O and
A + <F K G = 180O ……………………(1)
But since FKS is a straight line,
ΔFKG + ΔGKS = 180O…………….(2)
Comparing equation (1) and (2), it means that, ΔGKS = A.
Using ΔKFG, ΔGKS is an opposite exterior angle of r1 and r2
Thus, r1 + r2 = ΔGKS.
Hence, r1 + r2 = A
Note that given i1, r1 and i2, r2 as angles of incidence and refraction at F and
G as shown and n is the prism refractive index, and then Snells law holds.
That is; Sin i1 = n sin r1, and
Sin i2 = n sin r2
The position and shape of the third side of the prism does not affect the
refraction under consideration and so is shown as an irregular in figure.
Example
A ray of light falls from air to a prism of refracting angle 60o at an angle of
30o. Calculate the angle of emergence on the second face of the prism (Take
refractive index of the material of glass, ng = 1.5).Solution
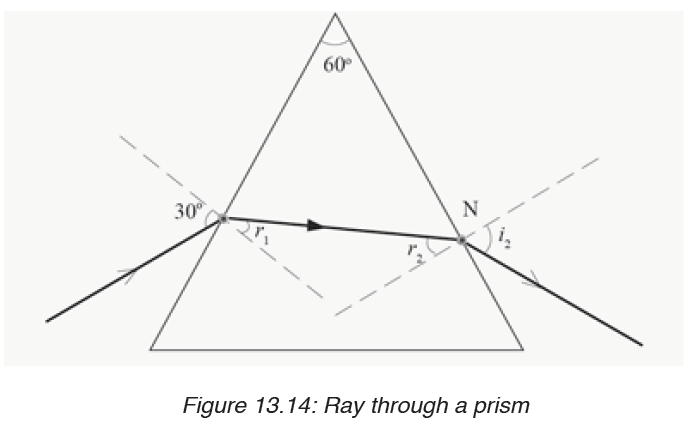
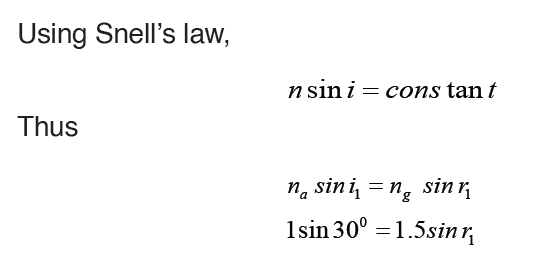
1. Deviation of light by a glass prism
Light can be deviated by reflection and refraction. Since a prism refracts
light, it therefore changes its direction.
A prism deviates light on both faces. These deviations do not cancel out as in
a parallel sided block where the emergent ray, although displaced, is parallel
to the incident ray surface. The total deviation of a ray due to refraction at
both faces of the prism is the sum of the deviation of the ray due to refractionat the first surface and its deviation at the second face.
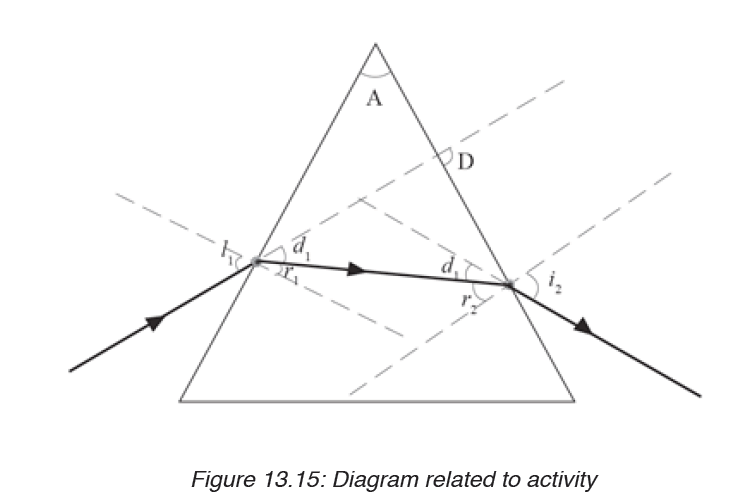
N.B: The deviation D for a small angle prism is D = A (n-1)
The expression D = A (n – 1) shows that for a given angle A, all rays entering
a small angle prism at small angles of incidence suffer the same deviation.
2. Angle of minimum deviation
From the variation figure below, there is one angle of incidence which gives
a minimum deviation. The experiment shows that this minimum deviation
occurs when the angle of emergence is exactly equal to the angle of incidence
and the two internal angles of refraction are equal.
At this value, a ray passes symmetrically through the prism and the rayinside the prism is perpendicular to the directing plane.
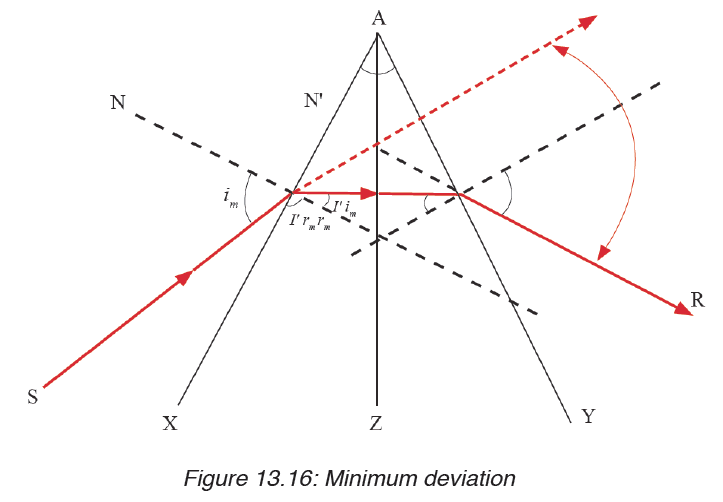
Since the angle of emergence i2 = angle of incidence i1, it follows that
3. Determination of refractive index n of a material of the prism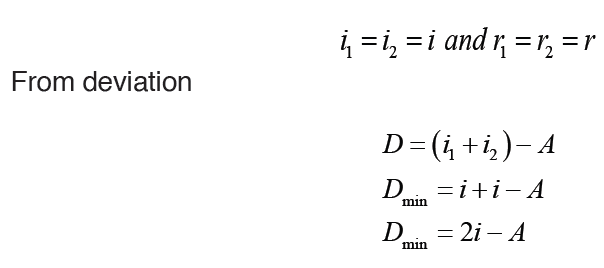
A very convenient formula for refractive index, n, can be obtained in the
minimum deviation case. The ray PQRS then passes symmetrically through
the prism, and the angles made with the normal in the air and in the glass at
Q, R respectively are equal. Suppose the angles are denoted by as shown.Then
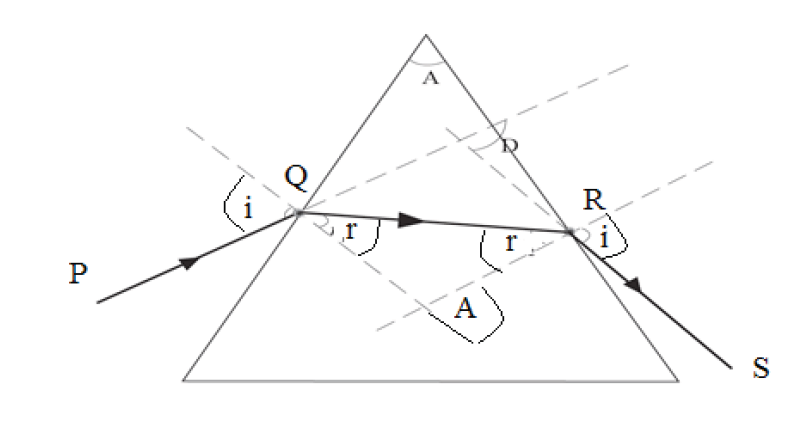
Example
A glass prism of refracting angle 60o has a refractive index of 1.5. Calculate
the angle of minimum deviation for a parallel beam of light passing through it.Solution:
4. Applications of total internal reflection of light by a prism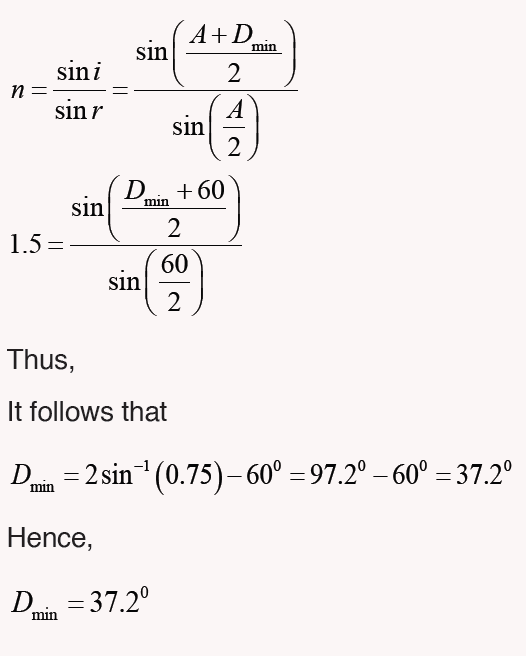
Many optical instruments use right -angled prisms to reflect a beam of light
through 90° or 180° (By total internal reflection) such as cameras, binoculars,periscope and telescope. One of the angles of right angled prism is 90°.
When a ray of light strikes a face of prism perpendicular, it enters the prim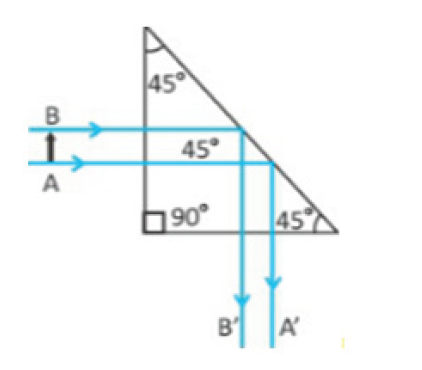
without deviation and strikes the hypotenuse at an angle of 45°. Since the
angle of incidence 45° is greater than critical angle of the glass which is 42°,
the light is totally reflected by the prism through an angle of 90°. Two such
prisms are used in periscope. The light is totally reflected by the prism by an
angle of 180°. Two such prisms are used in binoculars.
Application activity 13.6
1. A ray of light is refracted through a 60 degree prism of ordinary
glass making an incident of 35 degree with the normal i.e incident
ray =35°.What will the angle of emergent ray and the angle of
deviation measure?
2. What are the conditions for minimum deviation when a ray of
light passes through a prism?
3. Find the reflective index of the material of prism, if a thin prism of
angle A is equal to 6 degree, produces a deviation is equal to 3 degree.
4. Show that the deviation produced by a small angled prism is
independent with angle of incidence.
SKILLS LAB 13
DETERMINATION OF THE FOCAL LENGTH OF A LENS
Apparatus required:
1 torch bulb fitted in a bulb holder
1 switch
3 Torch cells
2 Cell holders
Connecting wires about 50cm long.
Wire gauze
Converging lens, of focal length 15cm.
Lens holder,
White screen
Metter rule.
Retort stand with its holding accessories.
Instructions:
In this experiment you will determine the focal length of the converging
lens provided.
a) Mount the on lens the holder and place it facing a window
b) Place the screen behind the lens and adjust the screen until a clear
image of a distant object is obtainedc) Measure and record the distance, x, between the lens and the screen.
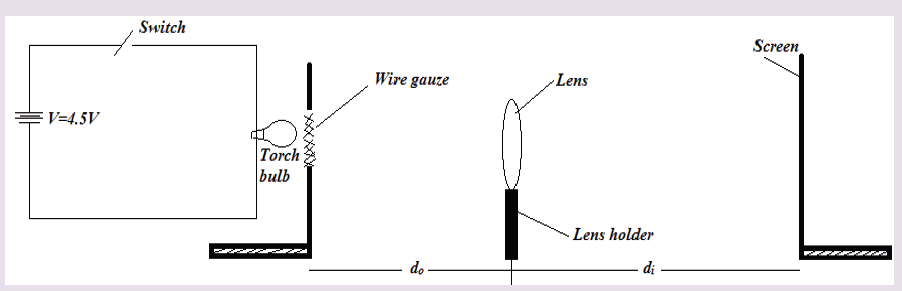
End unit assessment 13
1. Complete the following concept map using the following terms:inverted, larger, smaller, and virtual.
2. Explain the lens defects and their corrections.
3. A sharp image is located 78.0mm behind a 65.0mm-focal-length
converging lens. Find the object distance (a) using a ray diagram,
(b) by calculation.
4. An object is placed 10cm from a lens of 15m of focal length.
Determine the image position.
5. An object of 2cm is placed at 50 cm from a diverging lens of focal
length 10cm. Determine its image height and location.
6. An object located 32.0 cm in front of a lens forms an image on a
screen 8.00 cm behind the lens.
(a) Find the focal length of the lens.
(b) Determine the magnification.
(c) Is the lens converging or diverging?,(d) Determine the power of this lens.
UNIT 14: ASEXUAL AND SEXUALREPRODUCTION IN PLANTS
Key unit competence
Describe modes of reproduction in plants and apply various methods of
asexual and Sexual reproduction as means of increasing crop yield.
Introductory Activity
Task 1
The kingdom Plantae comprises about 260,000 known species including
flowering and non-flowering plants. All plants have a general organization
which includes vegetative and reproductive organs. Plants reproduce through
different ways: Use the books and other source of information to:
1. Write on how lower organisms such unicellular plant and another like
cassava, sugar cane and apple reproduce.
2. Describe the techniques used by people to grow Irish potatoes,
cassava and bananas.
3. Describe each of the following methods of asexual reproduction:
fragmentation, budding and spore formation.
Task 2
1. Observe the following pictures and suggest what is going on.2. How are the pictures below related to reproduction in flowering plants
14.1 Application of artificial propagation in growing
improved varieties of plants
Activity 14.1. a
Using addition resources to your textbook available in your school such
as the books from the school library and search further information from
the internet. Discuss on application of artificial propagation in growing
improved varieties of plants.
Artificial vegetative propagation is the deliberate production of new plants
from parts of old plants by humans. This can be done by following three
methods:Cutting, layering, and grafting.
Artificial vegetative propagation is usually used in agriculture for the
propagation (or reproduction) of those plants which produce either very few
seeds or do not produce viable seeds. Some examples of such plants which
are reproduced by artificial vegetative propagation methods are: Banana,
Pineapple, Orange, Grape, Rose, etc.
Vegetative propagation of particular cultivars that have desirable
characteristics is very common practice. Reasons for preferring vegetative
rather than sexual means of reproduction vary, but commonly include greater
ease and speed of propagation of certain plants, such as many perennial root
crops and vines. Another major attraction is that the resulting plant amounts
to a clone of the parent plant and accordingly is of a more predictable quality
than most seedlings. However, as can be seen in many variegated plants,
this does not always apply, because many plants actually are chimeras and
cuttings might reflect the attributes of only one or some of the parent cell
lines.
Man-made methods of vegetative reproduction are usually enhancements
of natural processes, but they range from rooting cuttings to grafting and
artificial propagation by laboratory tissue culture. In horticulture, a “cutting”
is a piece that has been cut off from a mother plant and then caused to
grow into a whole plant. A popular use of grafting is to produce fruit trees,
sometimes with more than one variety of the same fruit species growing from
the same stem. Rootstocks for fruit trees are either seedlings or propagatedby layering.
The following are methods of vegetative artificial propagation
Activity 14.1. b
Demonstration of asexual reproduction in plants by cuttings
Requirements
Growth medium or moist soil, sweet potatoes vines, elephant grass,
sugarcane or cassava stems, secateurs/sharp knife and rooting hormone.
Procedure
1. Collect clean and healthy stems from cassava, sugarcane or potato plants.
2. Using a secateurs/sharp knife, cut the stem of either cassava,
sugarcane or sweet potato stems into suitable sizes.
3. Place them in either suitable medium of growth or apply rooting
hormone if available or plant them in moist soil in the school garden.
4. Leave the set up for about 13 days, and then observe the
development of roots and leaves at nodes.
Draw and record what you will observe after 13 days on the development
of roots and leaves at nodes.
a) Cutting
This is simple procedure in which part of the plant is removed by cutting and
placed in a suitable medium for grow. The part of the plant which is removed
by cutting it from the parent plant is called a ‘cutting’. In this method oneyear-
old stem of root is cut from a distance of 20 to 30 cm. and is buried in
the moist soil in natural position. After sometime, roots develop from this
cutting and it grows into a new plant. This method is commonly used in rose
and sugar cane. Care is taken that nodes which were lower in parent plant
(morphologically) are put in the soil, while the morphologically higher nodesare kept up. Adventitious roots are given off at the lower nodes.
b) Layering
This method of vegetative propagation is used in those plants whose soft
branches occur near the ground such as jasmine plant. In this method, a
branch of the plant which is near to the ground is pulled towards the ground
and a part of this branch is covered with moist soil leaving the tip of this
branch above the ground. After sometime, roots develop from that part of
the branch which was buried in the soil. This branch is then cut of along with
the roots from the parent plant and develops into a new plant. This method
of asexual reproduction is also used in the production of plants such asBougainvillea, jasmine, guava, strawberries, lemon, China rose etc.
c) Grafting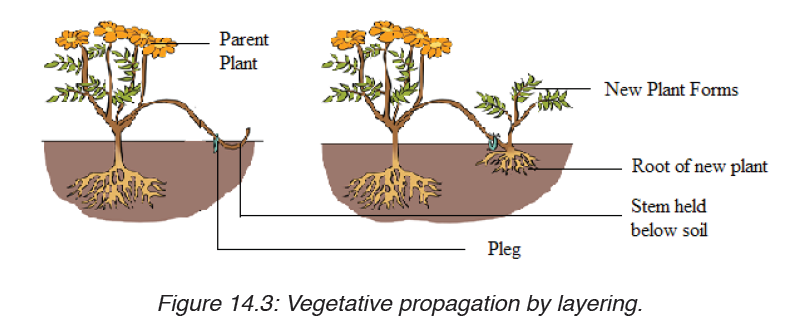
In this method of vegetative propagation the stems of two different plants are
joined together so as to produce a new plant containing the characters of
both plants. Out of the two plants one plant has a strong root system while
the other has a better flower or fruit yield. The plant of which the root system
is taken is called ‘stock’, while the other plant of which the shoot is selected
is known as ‘scion’ or ‘graft’. These two stems i.e. the stock and the scion
are fitted together by making slanting cuts in them and bound tightly with a
piece of cloth and is covered with a polythene sheet.
While joining the scion with the stock care should be taken that the diameter
of the stock and scion chosen for grafting should be equal. Scion gets the
mineral and water from the soil through the stock and develops branches
and produce fruits. This method of propagation is used in mango, apple,banana, pear, grape, pineapple and peach.
Vegetative and artificial reproduction in flowering plants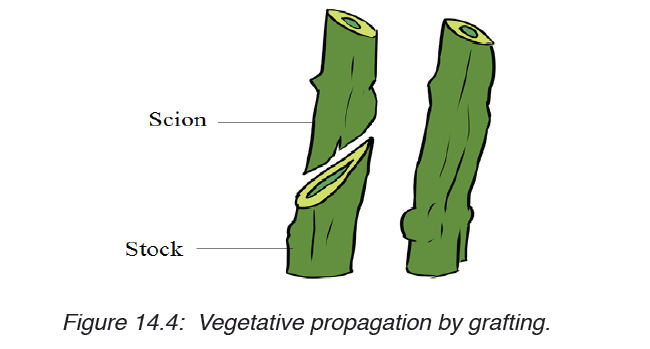
The reproductive part of the plant is a flower. The union of male and female
gametes to form a zygote is called fertilization. The transfer of pollen grains
from the anther to the stigma of the same flower or the different flower is
called pollination. In nature, plants reproduce asexually in a variety of ways.
The vegetative reproductive parts in flowering plant are stem, branches, and
leaves and they have the following characteristics:
Characteristics of Stem:
Stem develops from the plumule of embryo, Stem is generally the ascending;
part of the plant axis, It bears a terminal bud for growth in length, The
stem is differentiated into nodes and internodes, the stem nodes possess
dissimilar appendages called leaves, The young stem is green and capable
of performing photosynthesis, In the mature state it bears flowers and fruits,
Leaves and stem branches develop exogenously, Stem exposes leaves,
flowers and fruits to their most suitable position in the aerial environment
for optimum function, Hair, if present, is commonly multicellular and Stems
are usually positively phototropic, negatively geotropic and negatively
hydrotropic.
Characteristics of Leaf
– It is dissimilar lateral flattened outgrowth of the stem,
– The leaf is exogenous in origin
– It is borne on the stem in the region of a node,
– An axillary bud is often present in the axil of the leaf.
– Leaf has limited growth. An apical bud or a regular growing point is
absent,
– The leaf base may possess two lateral outgrowths called stipules,
– A leaf is differentiated into three parts: leaf base, petiole and lamina.
– The lamina possesses prominent vascular strands called veins,
– It is green and specialized to perform photosynthesis,
– Leaf bears abundant stomata for exchange of gases and it is the major
seat of transpiration.
Characteristics of branches
A branch or tree branch is a woody structural member connected to but not
part of the central trunk of a tree. Large branches are known as boughs
and small branches are known as twigs. Due to a broad range of species of
trees, branches and twigs can be found in many different shapes and sizes.
Application activity 14.1
1. Write on the methods of artificial vegetative propagation.
2. Cassava produces flowers, fruits and seeds. Why people prefer to
grow cassava by cutting rather than germination of seed?
3. Describe the characteristics of vegetative reproductive parts in a
flowering plant
4. Explain the application of artificial propagation in growing improved
varieties of plants.
14.2. Sexual reproduction in plants
Activity 14.2
Collect different forms of flowers from the school compound or around the
school, such as hibiscus, morning glory, sweet potato, or maize flower (use
any type of flower in your community not necessarily the ones mentioned
here)
1. Observe and describe the structures of collected flowers.
2. How do collected flowers differ externally?
3. Cut one of the flowers into two halves, draw and label one half offlower.
14.2.1. Types, structure and functions of flowers
a) Types of flowers
1. According to absence of some reproductive parts of the flower, we
can distinguish:
a) Unisexual flower: is a flower that consists of one type of reproductive
organ. This can be: staminate: unisexual male (with androecium
only), or carpellate: unisexual female (with gynoecium only). E.g.
flower of papaya.
b) Bisexual or hermaphrodite flower: a flower with the two
reproductive organs. It contains both male and female reproductive
organs (androecium and gynoecium). E.g. flowers of beans.
Dioecious plants are plants that have male flowers and female
flowers on separate plants (e.g. papaya/pawpaw) while monoecious
plants are plants that have both male and female flowers on the same
plant (e.g. maize).
2. According to the position of ovary in the point of insertion of calyx,
corolla and stamen, we can distinguish:
a) A flower with inferior ovary: it is when the ovary is located below
the point of insertion of calyx, corolla and stamens.
b) A flower with superior ovary: it is when the ovary is located over
the point of insertion of calyx, corolla and stamens.
c) The semi-infer or semi-super flower: when ovary is neither infer
nor super but in the middle of receptacle which is hollowed.
– When sepals are joined together, the flowers are called gamosepal,
and where are not joined together, the flower is called dialysepal.
– When petals are joined together, the flowers are called gamopetal,
and when are not joined together, the flower is called dialypetal. When
they are absent, the flower is called apetal.
3. According to the shape and symmetry of the flower, we can
distinguish:
i. Zygomorphic or irregular flower: a flower with a bilateral symmetry.
The flower cannot be divided into two similar halves. E.g. flowers of
beans, cassia.
ii. Actinomorphic or regular flower: a flower with a radial symmetry.
The flower can be divided into two or more planes to produce similarhalves. E.g. flowers of coffee, orange.
3. Dichogany: it is when male and female organs of the flower mature
at different times. We can distinguish:
– Protandry: when stamens mature before pistil.
– Protogyny: when pistil matures before stamen.
4. Inflorescence is when two or more flowers borne on a common
stalk.
a) Structure of a typical complete flowerA flower is a reproductive organ of a plant, which produces fruits and seeds.
A typical hermaphrodite or bisexual flower contains the following parts: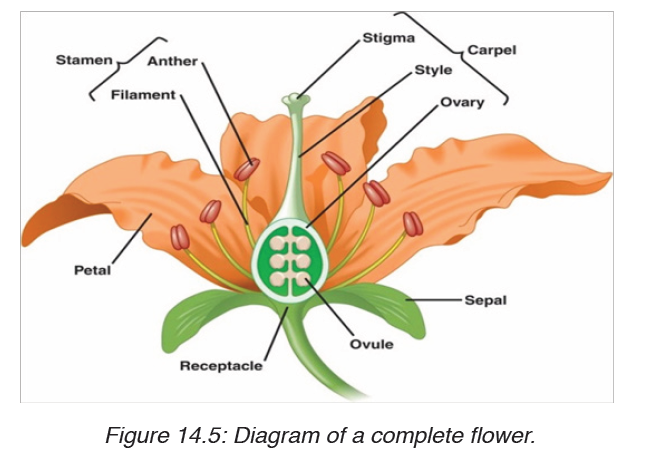
– Pedicel: it is the stalk which attaches the flower on the main floral axis.
– Receptacle: it is the swollen part at the end of the stalk where other
parts of the flower are attached.
– The calyx: it is the set of sepals, generally having green colour. They
protect the internal parts of the flower. In some plants, the sepals are
coloured and are called petaloids.
– The corolla: it is the set of petals, with different colours and nectar
glands that produce sugary substances which participate in attraction
of pollinating agents. In some plants, the petals are green and are called
sepaloids. Both calyx and corolla are collectively called perianth.
They are called floral envelope or accessory organs as they do not
participate directly in reproduction, or in formation of fruits and seeds,
they all insure the protection of internal parts of the flower.
– Androecium: is the male reproductive organs of the flower. It consists
of many stamens. A stamen consists of: the filament which supports
anther, and anther which contains the pollen grains or male gametes.
– Gynoecium/pistil: is the female reproductive organ. It consists of
many carpels, and each carpel is made of: stigma (plural: stigmata),
style and ovary with ovules.
a) The stigma: receive pollen grains from anther during pollination.
b) Style: supports the stigma in a good position to receive pollen
grains.
c) Ovary: a sac where ovules are produced. Ovules become seeds
after fertilisation.
A typical hermaphrodite or bisexual flower contains the following parts:
– Pedicel: it is the stalk which attaches the flower on the main floral axis.
– Receptacle: it is the swollen part at the end of the stalk where other
parts of the flower are attached
– The calyx: it is the set of sepals, generally having green colour. They
protect the internal parts of the flower. In some plants, the sepals are
coloured and are called petaloids.
– The corolla: it is the set of petals, with different colours and nectar
glands that produce sugary substances which participate in attraction
of pollinating agents. In some plants, the petals are green and are called
sepaloids. Both calyx and corolla are collectively called perianth. They
form a floral envelope or accessory organs as they do not participate
directly in reproduction, or in formation of fruits and seeds, they all
insure the protection of internal parts of the flower.
– Androecium: is the male reproductive organs of the flower. It consists
of many stamens. A stamen consists of: the filament which supports
anther, and anther which contains the pollen grains or male gametes.
Gynoecium/pistil: is the female reproductive organ.
It consists of many carpels, and each carpel is made of: stigma (plural:
stigmata), style and ovary with ovules.
• The stigma: receive pollen grains from anther during pollination.
• Style: maintains the stigma in a good position to receive pollen grains.
• Ovary: a sac where ovules are produced. Ovules become seeds after
fertilisation.
Application activity 14.2.1
1. What are the male and female structures of a flower?
2. How might be an advantage for a plant to have many flowers
together in a single structure?
3. Where does the female gametophyte develop?
4. Describe the flower and how it is involved in reproduction.
14.2.2. Pollination and double fertilisation in flowering plants
and events in a flower after fertilisation
Activity 14.2.2
Use library resources to identify different pollinating agents and describe
the process of double fertilization in flowering plants.
1. Pollination
Pollination is transfer of pollen grains from anther to the stigma.
a) Types of pollination: there are two types of pollination such as: self pollination
and cross-pollination.
i. Self-pollination: it is the transfer of pollen grains from anther to the
stigma of the same flower, or of different flowers but of the same plants.
It involves one plant. E.g. flowers of maize and beans.
ii. Cross-pollination: it is the transfer of pollen grains from anther to
the stigma of the flower of another plants. It involves two plants. E.g.flowers of pawpaw.
b) Main Pollinating agents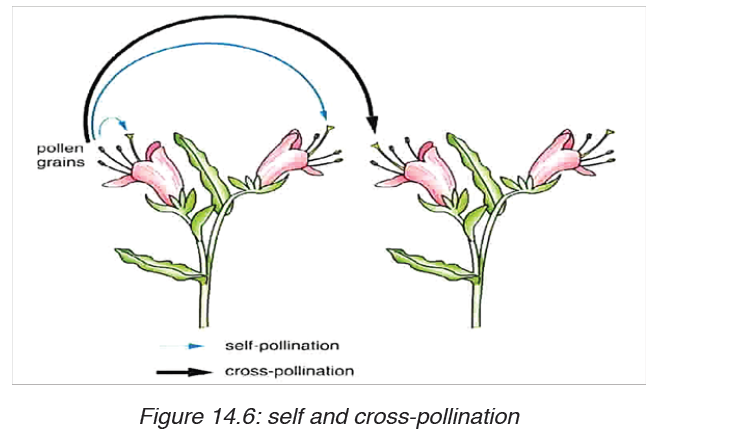
Flower structure is closely related with the way they are pollinated. This
means that flowers are adapted to specific agents or mode of pollination.
The common agents of pollination are: insects (entomophily), wind
(anemophily), water (hydrophily), humans (anthropophily), and birds
(ornithophily).
Characteristics of insect-pollinated flowers: (entomophilous flowers):
– Flowers produce the nectar to attract pollinators.
– Flowers have large brightly coloured corolla to attract pollinators.
– Production of scents to attract pollinators.
– The surface of the stigma should be sticky to hold pollen grains.
– Pollen grains are stickyand rough enough to remain on the surface of
stigma.
Characteristics of wind-pollinated flowers: (enemophilous flowers)
– The flowers have large stigma to hold pollen grains.
– The surface of the stigma should be sticky to hold pollen grains.
– Pollen grains are rough enough to remain on the surface of stigma.
– The flowers are or are not brightly-colored.
– They have or do not have scent.
– They do or do not secrete nectar.
– They produce large quantities of pollen grains, as much of them never
reach the stigmas.
2. Double fertilization and events after fertilization in flowering
plants
Double fertilization is a complex fertilization mechanism of flowering plants
(angiosperms). This process involves the joining of a female gametophyte
(megagametophyte, also called the embryo sac) with two male gametes
(sperm).
a) Development of pollen grains and plant ovules.
i. Development of pollen grains
The pollen grains are produced in the anthers while the ovules are produced
in the ovary.
Pollen grains
Each anther has four pollen sacs which contain many diploid microspore
mother cells that undergo meiosis to form four microspores each. At first,
the four microspores remain together as tetrads. The nucleus of each
microspore then divides by mitosis, forming a generative nucleus and a tube
or vegetative nucleus. At this point, the content of the pollen grain may be
considered as the male gametophyte. A two-layered wall forms around each
pollen grain. The outer wall, the exine is thick and sculptured. The inner wall,
the intine is thin and smooth. There are many pores or apertures in the wallthrough which a pollen tube may emerge.
ii. Development of Plant ovule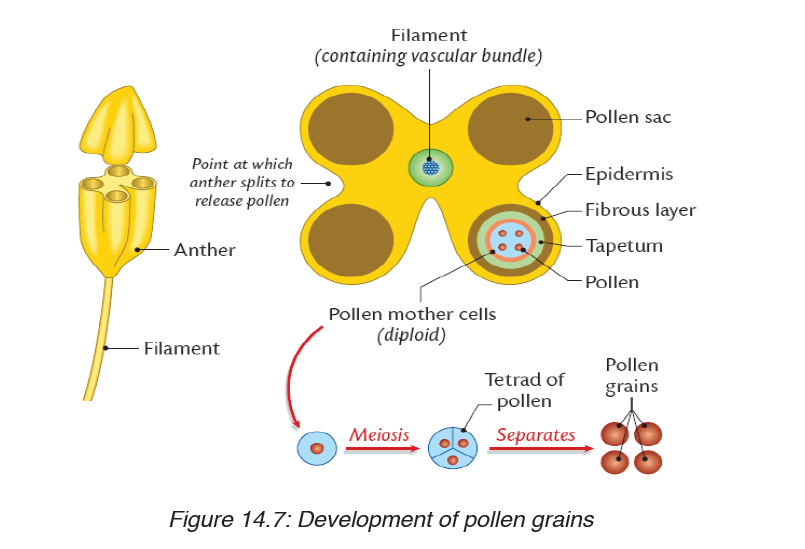
Each ovule is attached to the ovary wall by a short stalk called funicle.
The main tissue in the ovule is the nucellus which is enclosed and protected
by the integuments.
At one end of the ovule, there is a small pore called micropyle. A single
diploid megaspore mother cell in the nucellus undergoes meiosis, producing
four megaspores. Three of the four megaspores degenerate, while the
remaining cell, called the embryo sac, grows to many times its original size.
The nucleus of the embryo sac divides mitotically three times, resulting in
eight haploid nuclei which are arranged in groups of four nuclei at the two
poles. At this point, the contents of the embryo sac may be regarded at the
female gametophyte.
One nucleus from each pole migrates to the centre of the embryo sac. These
two nuclei are called polar nuclei, and they fuse to form a single diploid
nucleus. Meanwhile, cell walls form around the remaining six nuclei and they
form the synergids, antipodals and the egg (ovum). Only the egg functionsas the female gamete.
In summary, the pollen grain: contains two haploid nuclei: one called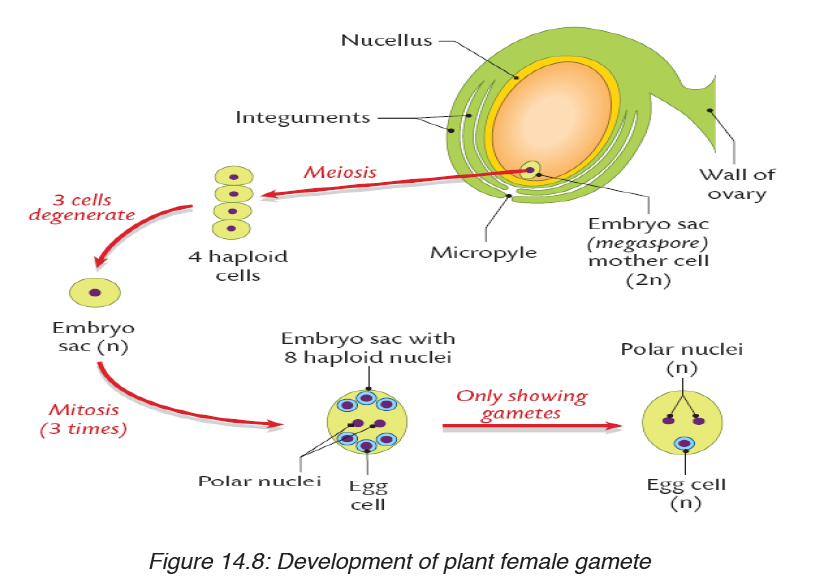
generative nucleus, and the other the tube nucleus.
On the other hand, the ovule or embryonic sac contains eight nuclei: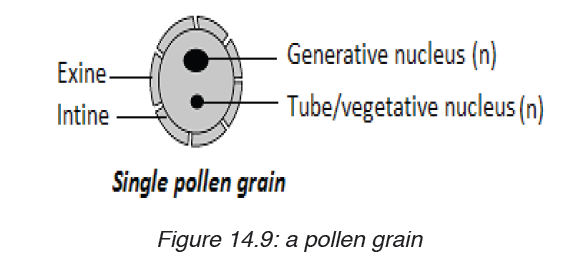
– Three antipodal nuclei/cells at one end
– Two polar nuclei/cells in the middle of ovule
– Two synergids (non-functional nuclei)– One big egg cell.
The process of double fertilization: It begins when a pollen grain adheres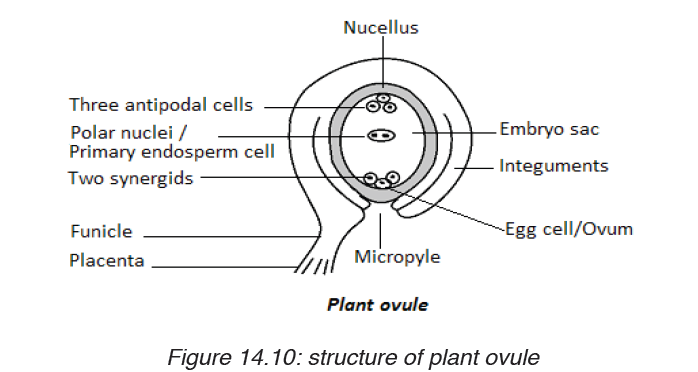
to the stigma of the carpel, the female reproductive structure of a flower. The
pollen grain then takes in moisture and begins to germinate, forming a pollen
tube that extends down toward the ovary through the style.
The growth of the pollen tube is controlled by the pollen tube nucleus.In
the pollen tube, the generative nucleus divides mitotically into two haploid
nuclei which are the male gamete nuclei. These follow behind the tube
nucleus as the pollen tube grows down the style towards the ovule. The tip of
the pollen tube then enters the ovary and penetrates through the micropyle
opening, releasing the two sperms in the megagametophyte or ovule.
The tube nucleus degenerates, leaving a clear passage for the entry of male
nuclei. One nucleus fertilizes the egg cell to form a diploid zygote (2N),
which will grow into a new plant embryo; the other fuses with polar nuclei
to form a triploid nucleus (3N), which will grow into a food-rich tissue knownas endosperm, which nourishes the seedling as it grows.
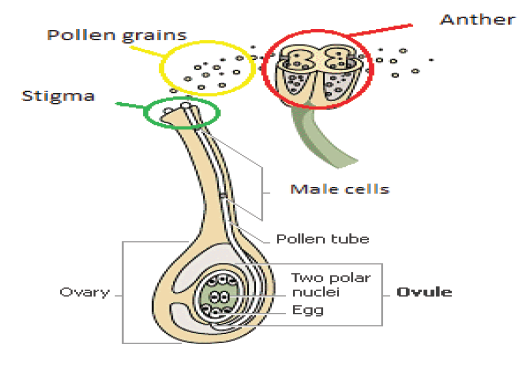
This process is described as double fertilisation and is typical of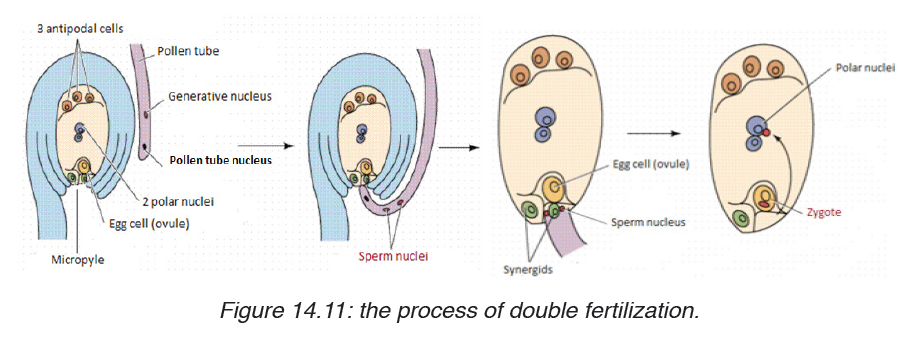
angiosperms. If there is more than one ovule in the ovary, each must be
fertilised by separate pollen grain and hence the fruit will have many seeds
genetically different from each other.
3. Events in a flower after fertilization
After fertilisation, the calyx, corolla, stamens and style may wither gradually
and fall off, but in some flowers the calyx may persist. The ovule forms the
seed, the two integuments of the ovule will form the seed coat, and the ovary
will develop into fruit, with the ovary wall forming the pericarp (fruit wall).
The diploid zygote undergoes cell division to form the embryo, the triploid
primary endosperm nucleus develops into endosperm, a store used by the
developing embryo. This persists in endospermic seeds of monocotyledons.
The micropyle persists as a small hole in the seed coat through which water
is absorbed during germination.Table 14.2: Floral parts and their fate after fertilization
Application activity 14. 2.2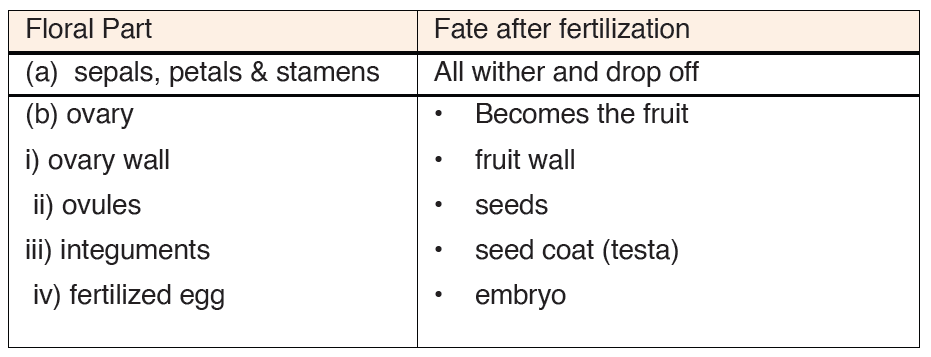
1. Are angiosperms typically wind or animal pollinated? How does
this process occur?
2. What is meant by the term endosperm?
3. How are brightly coloured petals advantageous to the plant?
4. What do you understand by the term double fertilization?
5. What happens to the antipodal cells and synergids cells after
fertilization?
14.2.3 Structures and types of fruits and seeds
Activity 14. 2.3
Observe slides containing micrographs of different fruits and seeds.
According to their characteristics:
a) Differentiate fruits.
b) Draw and show a structure of seed as seen on microscopeBelow are some examples of fruits:
A fruit is a structure formed from the ovary of a flower, usually after the
ovules have been fertilized. In nature, a fruit is normally produced only after
fertilization of ovules has taken place, but in many plants, largely cultivated
varieties such as seedless citrus fruits, grapes, bananas, and cucumbers,
fruit matures without fertilization, a process known as parthenocarpy.
Ovules within fertilized ovaries develop to produce seeds. In unfertilized
varieties, seeds fail to develop, and the ovules remain with their original size.
A fruit consists of two main parts; pericarp (fruit wall) and the seed. The
pericarp has three layers: epicarp or exocarp (outermost), mesocarpe(middle) and endocarp (inner).
The fruit can have a dry pericarp or fleshy pericarp. The fruits with fleshy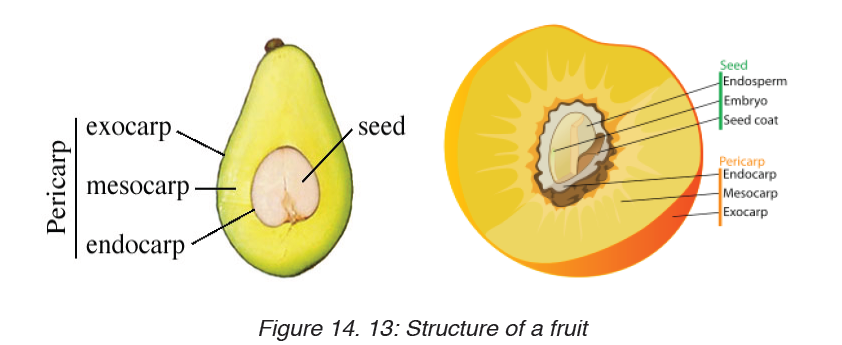
pericarp include: berry and drupe. Drupe is a fleshy fruit with only one seed,E. g. avocado.
Berry is a fleshy fruit having many seeds inside of it. E.g. tomatoes, orange,
and pawpaw.

The fruits with dry pericarp include indehiscent fruit or dehiscent fruit.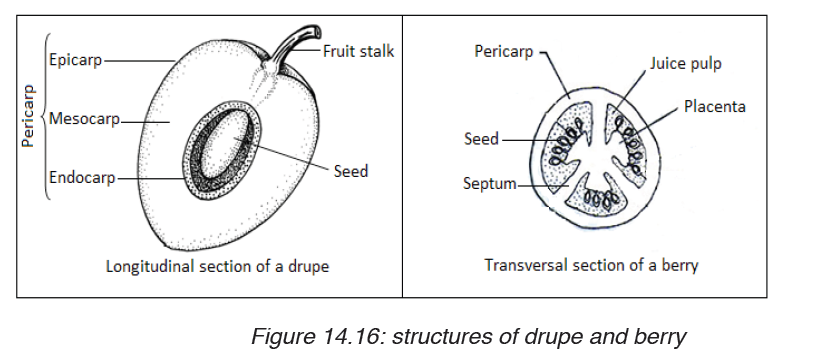
Indehiscent fruits do not open. Seeds remain inside of the fruits. E.g. fruits of
coconuts. Dehiscent fruits open and release seeds. These include: dehiscent
fruits with one carpel, and those with many carpels. Dehiscent fruits with one
carpel include; those which open along one side, e.g. follicle; and those
which open along both sides, e.g. legume (beans). Fruits of eucalyptus areexam of dehiscent fruits with many carpels.
The major function of a fruit is the protection of developing seeds. In many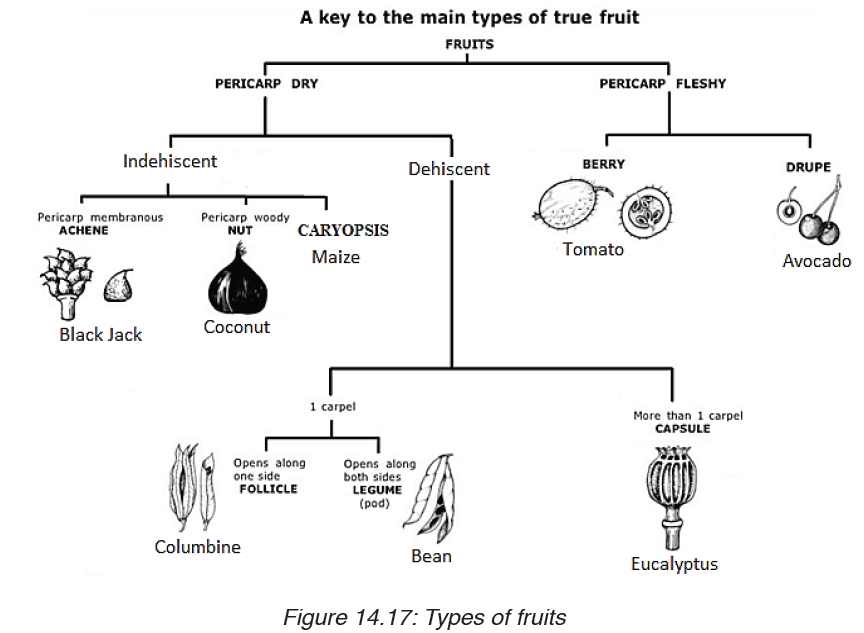
plants, the fruit also aids in seed distribution (dispersal).
Food value
Fruits are eaten raw or cooked, dried, canned, or preserved. Carbohydrates,
including starches and sugars, constitute the principal nutritional material.
Citrus fruits, tomatoes, and strawberries are primary sources of vitamin C,
and most fruits contain considerable quantities of vitamin A and vitamin B.
In general, fruits contain little protein or fat. Exceptions are avocados, nuts,
and olives, which contain large quantities of fat, and grains and legumes,
which contain considerable protein.
A seed is an embryonicplant enclosed in a protective outer covering. The
formation of the seed is part of the process of reproduction in seed plants,
the spermatophytes, including the gymnosperm and angiosperm plants.
Seeds are the product of the ripened ovule, after fertilization by pollen and
some growth within the mother plant. The embryo is developed from thezygote and the seed coat from the integuments of the ovule.
The main components of the seed: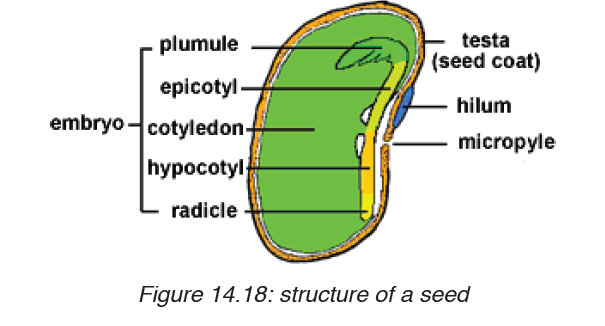
A seed is made up of a seed coat (testa), one or two cotyledons and an
embryonic axis. The embryonic axis is made up of a plumule, an epicotyl,
a hypocotyl and a radical. A seed which has one seed-leaf is described as
monocotyledonous, and one which has two, as dicotyledonous. Maize is
monocotyledonous seed while bean is a dicotyledonous seed.
– The cotyledons, the seed leaves, attached to the embryonic axis. There
may be one (Monocotyledons), or two (Dicotyledons). The cotyledons
are also the source of nutrients in the non-endospermic Dicotyledons,
in this case they replace the endosperm, and are thick and leathery. In
endospermic seeds the cotyledons are thin and papery.
– The epicotyl, the embryonic axis above the point of attachment of the
cotyledon(s).
– The plumule, the tip of the epicotyl, and has a feathery appearance due
to the presence of young leaf primordia at the apex, and will become
the shoot upon germination.
– The hypocotyl, the embryonic axis below the point of attachment of
the cotyledon(s), connecting the epicotyl and the radicle, being the
stem-root transition zone.
– The radicle, the basal tip of the hypocotyl, grows into the primary root.
Monocotyledonous plants have two additional structures in the form of
sheaths. The plumule is covered with a coleoptile that forms the first leaf
while the radicle is covered with a coleorhiza that connects to the primary
root and adventitiousroots form from the sides. Here the hypocotyl is arudimentary axis between radicle and plumule.
Application activity 14.2.3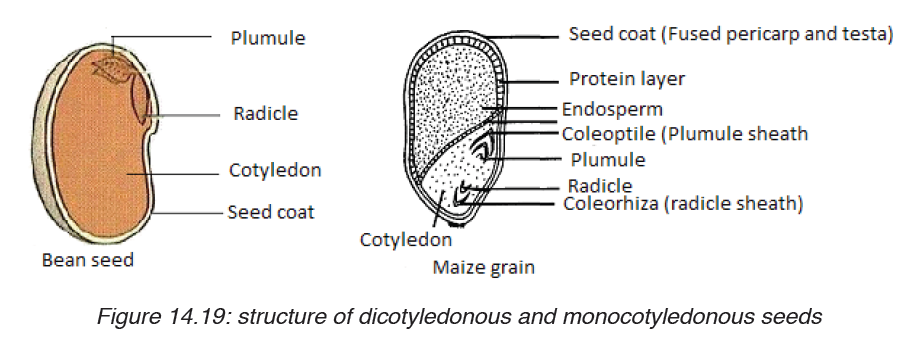
1. Describe the structure of a drupe
2. Differentiate between a drupe and a berry
3. What would happen to the fruit if ovules in the flower did not
develop?
4. Compare the typical structure of seeds that are dispersed by
animals to those dispersed by wind and water.
14.2.4. Fruits and seeds dispersal with their adaptations
Activity 14.2.4
Use library resources like books, internet and search to find answers for
the following questions:
1. Suggest ways of fruits and seeds dispersal.
2. Explain adaptation of fruits dispersed by animals.
Dispersal of fruits and seeds is the scattering of fruits and seeds from their
mother plants. They are four methods of seeds and fruits dispersal such as:
1. Dispersal by Wind
2. Dispersal by Water
3. Dispersal by Animals and
4. Mechanical Dispersal.
Seeds disperses by wind or water are typically lightweight, allowing them
to be carried in air or to float on the surface of water. The wind carries also
small seeds that have wing-like structure. Seeds dispersed by animals
are typically contained in sweet, nutritious flesh fruits. They can be carried
externally on their feet, fur, feathers, or beaks. Those seeds with hooks or
sticky substances rely on the chance that they will attach themselves to a
passing animal. Other seeds are eaten by animals and passed out in the
faeces.
With mechanical Dispersal: all dehiscent fruits scatter the seeds when they
burst. This dehiscence is accompanied by the expression of great force in
many fruits so that seeds are jerked a considerable distance away from the
mother plant. Such fruits are called explosive fruits.
These seeds will germinate where the faeces will be deposited. The dispersal
of seeds is important for the survival of the plant species because:
– It minimises overcrowding of plants growing around the parent plant
that could then result in too much competition for nutrients and light;
– It allows the plant species to colonise new habitats which can offer
suitable conditions.
Application activity 14.2.4
1. Why is it adaptive for some seeds to remain dormant before they
germinate?
2. The seeds of a bishop pine germinate only after they have
undergone a forest fire. Evaluate the significance of this structural
adaptation.3. Evaluate the importance of seed dispersal.
SKILLS LAB
After studies, and completion of this unit 14, student-teachers will use the
acquired knowledge to increase the crop productivity using different modes
of vegetative and artificial propagation. They will also improve the quality
of fruit plants by using for example grafting method.
End unit assessment 14
PART A
A) Multiple choice questions: choose the best answers.
1. In cutting method of vegetative propagation, cuttings are mainly
taken from
a) Leaves of parent plant
b) Roots or stems of parent plant
c) Shoots of parent plant
d) Buds of parent plant
2. Artificial methods of vegetative propagation includes
a) Cloning
b) Grafting
c) Cuttings
d) Both b and c
3. Example of plant in which vegetative propagation is occurred by
leaves is called
a) Cannabis
b) Chrysanthemum
c) Bryophyllumd) Brassica
4. Which of the following is NOT an advantage of asexual reproduction?
a) Rapid reproduction.
b) High genetic diversity.
c) No need for a mate.
d) Low resource investment in offspring.
B) Questions with short and long answers
1. Name the plants which are grown by grafting method.
2. What do you understand by grafting?
3. How will you show that vegetative propagation takes place in
potatoes?
4. Explain the method by which the sugarcane and rose are grown.
5. Give the names the different methods of artificial vegetative
reproduction.
6. Explain the term vegetative reproduction and give one example of
plant which reproduces by using this type of asexual reproduction.
PART B
1. Answer by true or false
a) Seeds that are dispersed by animals are not contained in a flesh sweet
tissue.
b) During pollination, pollen grains move from stigma to anther.
2. Chose the letter that best answers the question or complete
the statement.
a) Which of the following is not part of a flower?
i. Stamens
ii. Petals
iii. Carpels
iv. Stem
v. Sepals.
b) Which flower structure that includes all the others listed below?
i. Stigma
ii. Carpel
iii. Ovary
iv. Style
v. Ovule
c) The thickened ovary wall of a plant may join with other parts of the
flower stem to become the
i. Cotyledon
ii. Fruit
iii. Endosperm
iv. Seed
d) In angiosperms the structures that produce the male gametophyte
are called the
i. Pollen tubes
ii. Stigma
iii. Anthers
iv. Sepals
e) In angiosperms, the mature seed is surrounded by a
i. Flower
ii. Fruit
iii. Cotyledon
iv. Cone
3. Which are more likely to be dispersed by animals- the seeds of
angiosperms or the spores of a fern? Explain your reasoning.
4. Pollination is a process that occurs only in seed plants. What
process in seedless plants is analogous to pollination?
5. Propose a hypothesis to explain why angiosperms have become
the dominant type of plant on the earth.6. Study the structure of the seed bellow
a) Name the parts labelled by: A, B and C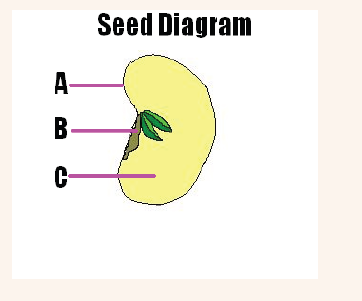
b) What is the importance of the part C for a growing seedling?
7. Many flowers have bright patterns of coloration that directly
surround the reproductive structures. Evaluate the importance of
those bright-coloured patterns to plants.
8. What is the function of endosperm?
9. Some plants form flowers that produce stamens but no carpels.
Could fruit form on one of these flowers? Explain your answer.
10. Distinguish between pollination and fertilization.
11. Give names of letters from A to J, and explain the function of theparts represented by: B, G, and E.
12. Explain why the relationship between bees and flowers is described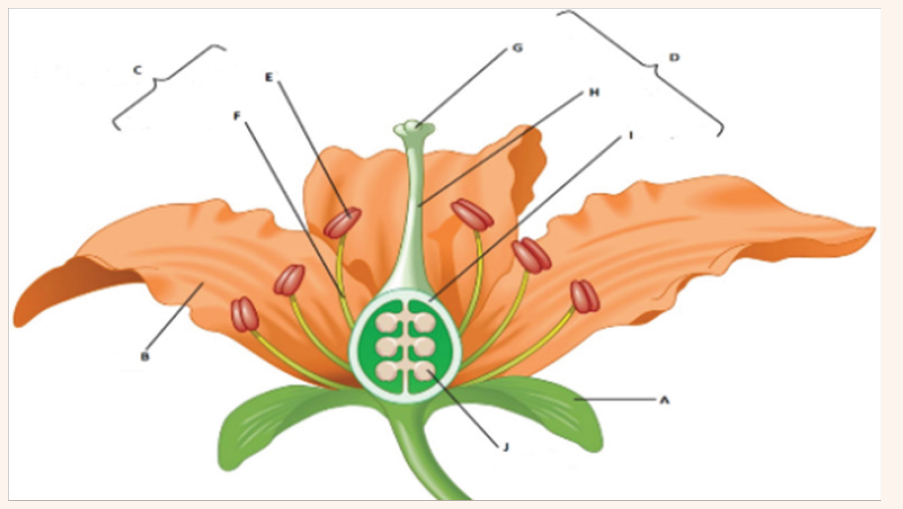
as mutually beneficial.
13. What is the main advantage of cross-pollination?
14. Why are the stamens of wind-pollinated plants and insect-pollinated
plants different?
15. Differentiate wind-pollinated flowers from insect-pollinated flowers.
16. Give one example of a plant that uses each of the following
dispersal mechanism:
a) An explosive device which works by being inflated with water.
b) A winged seed lifted by air currents
c) A buoyant seed carried by sea currents
d) A gluey substance which sticks the seed to an animal.
REFERENCE
1. Kennedy P. and Sochacki F.(2008), OCR Biology, London,British,
Heinemann.
2. Burton S. Guttman (1983), Understanding Biology, New York,
Chicago San Fransisco, Harcout Brace Jovanovich.
3. Virgilio O.K, James K. and all (2013), Biology for Rwanda, Kampala
Uganda.
4. Kenneth R., Miller and Joseph Levine (1993), Biology
5. Antony C., Dennis D. and all (2000), Chemistry fifth edition, Calfornia,
Pearson Education.
6. Theodore L., Bruce E. and Julia R. (2003), Chemistry: the central
science ninth Edition, New Jersy, Pearson Education.
7. Nelkson and Parker (2001), Advanced level Physics 7th edition,
Heineman.
8. Duncan T. (2000), Advanced Physics 5th edition, Hodder Education
an Hachette UK Compay.
9. Biology and Health Sciences For Rwanda Schools Senior Two
Student’s Book by Godfrey Wasswa Benson Mugarura Selah Ndiwa
10. Ramsden, E. (2000). A-Level Chemistry 4th Edition. United Kingdom:
Nelson Thornes.
11. Holman, G. Hill. (2000). Chemistry In Context 5th Edition. United
Kingdom: Nelson Thornes.
12. Huhes, P. Cann. (2002). Chemistry for advanced level. London:
JOHN MURRAY.
13. https://www.hygiene-in-practice.com/pathogen/chlamydiatrachomatis_
en/
14. https://microbenotes.com/habitat-and-morphology-of-neisseriagonorrhoeae/
15. https://en.wikipedia.org/wiki/Kwashiorkorhttps://en.wikipedia.org/
wiki/Kwashiorkor#Signs_and_symptoms
16. https://en.wikipedia.org/wiki/Kwashiorkor#Signs_and_symptoms
17. https://en.wikipedia.org/wiki/Kwashiorkor#Prevention
18. https://www.healthline.com/health/marasmus#causes
19. https://www.healthline.com/health/rickets
20. https://goqii.com/blog/rickets-types-symptoms-and-treatments/
21. https://www.healthline.com/health/beriberi#prevention
22. https://www.hindustantimes.com/fitness/10-vitamin-a-richfoods-
to-include-in-your-diet-for-a-rainbow-of-benefits/storylZ8sTsR7A0UWJqHjtPIEdP.
html
23. https://www.healthline.com/health/pellagra#outlook
24. https://en.wikipedia.org/wiki/Schistosomiasis
25. https://www.mailmyprescriptions.com/praziquantel-600-mg-tabletgeneric-
49884023183.html
26. https://www.google.com/search?client=firefox-b-d&q=Elephantiasis
+lymphatic+filariasis
27. https://www.123rf.com/photo_101015471_stock-illustrationwuchereria-
bancrofti-a-roundworm-nematode-one-of-thecausative-
agents-of-lymphatic-filariasis-3d-ill.html
28. https://www.google.com/search?q=ankilostomiasis&client=firefoxb-
d&sxsrf=ACYBGNR4cYc5MTfYX7hHeDxDkm
29. https://en.wikipedia.org/wiki/Ancylostomiasis#Signs_and_
symptoms
30. https://www.wikihow.com/Get-Rid-of-Hookworms-When-Infected
31. https://www.thoughtco.com/spindle-fibers-373548
32. https://www.quora.com/In-biology-what-is-chiasmata
33. http://cyberbridge.mcb.harvard.edu/mitosis_6.html
34. https://www.mytutor.co.uk/answers/31266/IB/Biology/Describe-theprocess-
of-chiasmata-formation/
35. https://study.com/academy/lesson/what-is-an-energy-level-of-anatom-
definition-equation-quiz.html
36. https://www.clipart.email/clipart/library-bookshelf-clipart-74648.html
37. https://www.thoughtco.com/bohr-model-of-the-atom-603815
38. http://www.okstate.edu/jgelder/bondpage14.html
39. http://www.differencebetween.net/science/difference-betweenionic-
and-covalent-compounds/#ixzz5yqvqXz16
40. https://study.com/academy/lesson/effect-of-intermolecular-forceson-
physical-properties.html
41. https://www.khanacademy.org/test-prep/mcat/chemical-processes/
covalent-bonds/a/intramolecular-and-intermolecular-forces
42. https://www.thoughtco.com/covalent-or-molecular-compoundproperties-
608495
43. https://sciencing.com/difference-between-atoms-ions-5595821.html
44. https://www.adichemistry.com/general/chemicalbond/vsepr/vseprtheory.
html
45. https://www.saps.org.uk/secondary/teaching-resources/157-
measuring-the-rate-of-photosynthesis
46. https://www.biologynotes.site/carbohydrates/
47. (www.learncbse.in/chemical-binding-molecular-structure-cbsenotes-
class-11-chemistry/)48. http://www.nutrientsreview.com/carbs/polysaccharides.html
