UNIT 3: FERTILIZERS
Key Unit Competence:
Analyze the components of quality fertilizers and their benefits, effects ofmisuse and dangers associated with substandard fertilizers.
Introductory activity 3
Observe the pictures below showing some common fertilizers used inagriculture and attempt the following questions:
 i) It is a must for a farmer to use fertilizers. Why?
i) It is a must for a farmer to use fertilizers. Why?
ii) The fertilizers shown above are different. How are they different?
iii) Why a farmer does use different fertilizers (NPK, UREA, …) on only
one same plant?
iv) Can the fertilizers above become hazardous? When? What is
chemical hazard?
v) How the types of fertilizers mentioned above can have effect to the
environment or living things?
vi) Suggest the measures to avoid hazard while using fertilizers.3.1. Classification of fertilizers
Activity 3.1Observe the following fertilizers:
 i) Read the labels of given fertilizers and propose the components ofA fertilizer is any material, organic or inorganic, that is used to supply nutrients
i) Read the labels of given fertilizers and propose the components ofA fertilizer is any material, organic or inorganic, that is used to supply nutrients
each.
ii) Categorize the above fertilizers according to their ways of
manufacturing?
iii) Suggest any other examples of fertilizers you have ever heard or used
at home.
iv) Use engine research or library textbook to describe the nutrients thatplants need in order to grow from fertilizers
to the soil.Fertilizer is a substance added to soil to improve plants’ growth and
yield.Fertilizers replace the chemical components that are taken from the soil
by growing plants. However, they are also designed to improve the growing
potential of soil, and fertilizers can create a better growing environment than
natural soil. They can also be tailored to suit the type of crop that is being grown.
Fertilizers are categorized into natural fertilizers (or organic fertilizers) andartificial fertilizers (or chemical fertilizers)
3.1.1. Natural Fertilizers
The name organic fertilizer refers to materials used as fertilizer that occur
regularly in nature, usually as a by-product or end product of a naturally occurring
process. They are made from remains of dead plants, wastes from animals or
they can be minerals. Examples include manures and minerals. Manure is anorganic material that is used to fertilize land.
Farmyard manure: animal manure that consists of feces.
Green manure: is a term used to describe specific plant or crop varieties that
are grown and turned into the soil to improve its overall quality.
Compost manure: is organic matter that has been decomposed and recycled
as a fertilizer and soil amendment. Minerals: Mineral mined powdered
limestone, rock phosphate and sodium nitrate, are inorganic compounds which
are energetically intensive to harvest and are approved for usage in organicagriculture in minimal amount.
3.1.2. Artificial Fertilizers
Artificial fertilizers are man-made chemical compounds that mimic the soil’s
natural minerals and elements to maximize plant growth. They usually contain
different ratios of nitrogen, phosphorus, potassium, calcium, magnesium andother elements.
Examples: Urea, N.P.K, ammonium dihydrogen phosphate, NH4 (H2PO4), etc.
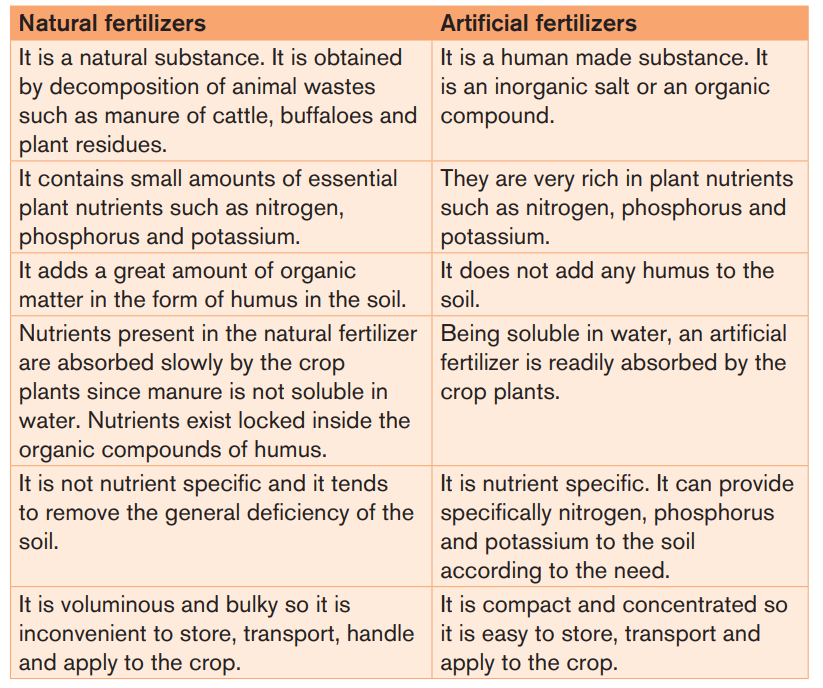
Table 3.1: Differences between natural and artificial fertilizers
3.1.3. Components of a fertilizer
Typically, fertilizers are composed of nitrogen, phosphorus, and potassium
compounds. They also contain trace elements that improve the growth of
plants. The primary components in fertilizers are nutrients which are vital for
plant growth.
First it is important to understand that all industrial Fertilizers, by convention,
regardless of type and specific use, have something called a NPK ratio. The NPK
ratio will be prominently labeled on the package and indicates the percentage
of major (or primary) nutrients the fertilizer contains. Example: Urea is a fertilizer
with an NPK ratio of 46-00-00.
The nutrients of plants are classified into three types namely: major nutrients,secondary nutrients and micronutrients.
a. The major nutrients
The major nutrients for soil are nitrogen (N), phosphorus (P), and potassium
(K). These major nutrients usually are lacking or insufficient in the soil because
plants consume them in large amounts for their growth and survival.
The letter N represents the actual nitrogen content in the fertilizer by percentage
mass while P and K represent the amount of oxide in the form of phosphorus (V)oxide (P2O5) and potassium oxide (K2O) respectively.
Example:
- If a fertilizer is labeled 17-17-17, it means that the fertilizer contains17%
by mass N, 17% by mass P2O5 and 17% by mass K2O.
- If fertilizer is labeled 10-20-20, it means that the fertilizer contains 10% bymass N, 20% by mass P2O5 and 20% by mass K2O.
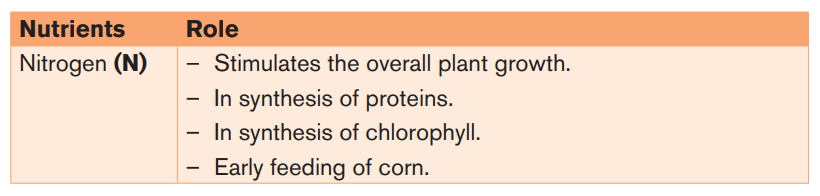
Table 3.2: Role of nutrients

If a fourth number is included on the label of a fertilizer, it indicates the sulphur
content. That fertilizer is NPKS.
b. Secondary nutrients
The category of secondary nutrients, are calcium (Ca), magnesium (Mg), and
Sulphur (S). As, these nutrients are generally enough in the soil, so fertilization
is not always needed. Also, large amounts of Calcium are added when lime
is applied to acidic soils. In fact, Sulphur is usually found in sufficient amounts
from the slow decomposition of soil.
c. Micronutrients
Micronutrients are those elements essential for plant growth which are needed
but in only very small (micro) quantities. These elements are even called minor
elements or trace elements. The common micro nutrients are boron (B), copper
(Cu), iron (Fe), chlorine (Cl), manganese (Mn), molybdenum (Mo) and zinc (Zn).
In fact, recycling organic matter such as grass clippings and tree leaves is anexcellent way of providing micro nutrients to growing plants.
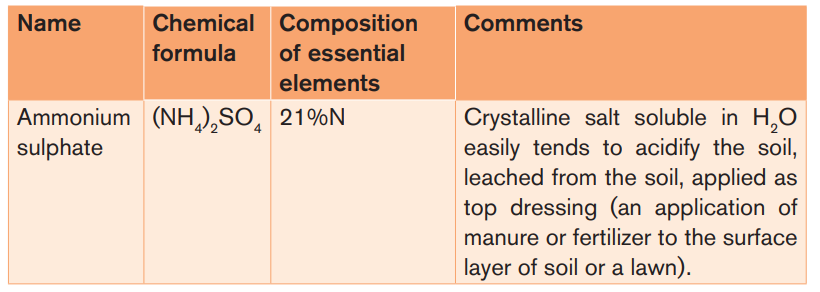
Table 3.3: Characteristics of some common artificial Fertilizers
Depending on the nature of the essential elements that a fertilizer can supply to
the soil, the fertilizers have been classified into the following groups:
– Nitrogenous Fertilizers: N-type Fertilizers
These Fertilizers supply only nitrogen as a major nutrient to the soil. Examples:
ammonium sulphate, urea, sodium nitrate (also called Chile saltpeter or Chile
nitre).
– Phosphorus Fertilizers: P-type Fertilizers
These fertilizers supply phosphorus as major nutrient to the soil. Examples:
Calcium dihydrogen phosphate, Ca (H2PO4)
2H2O, phosphate slag, Ca3
(PO4)
2CaSiO3
– Potassium Fertilizers: (K-type Fertilizers)
These Fertilizers supply only potassium as a major nutrient to the soil. Examples:potassium chloride, potassium sulphate.
– Mixed Fertilizers
Mixed fertilizers are those which can supply more than one essential element to
the soil.
Depending on the nature of the essential element supplied by the fertilizer,
mixed Fertilizers can be classified into the following groups:
- NP Fertilizers: These Fertilizers supply two essential elements,
nitrogen and phosphorus, to the plant. Examples: Ammonium dihydrogen
phosphate, (NH4)(H2PO4),(also called dihydrogen ammoniated
phosphate or ammophos). Calcium dihydrogen phosphate nitrate, Ca
(H2PO4)
22Ca(NO3)
2,(also called calcium superphosphate nitrate or
nitrophosphate).
- PK Fertilizers: It is a mixture of two compounds; containing phosphorus
and the other containing potassium. For example, a mixture of H2PO4)2H2O,
and K2SO4.
- KN fertilizer. Example: KNO3
- NPK Fertilizers: These are Fertilizers that contain %N, %P as P2O5
and %K as K2O.Example: A mixture of (NH4)
2SO4, (N-type fertilizer),(H2PO4)2H2O (P-type fertilizer), and K2SO4(K-type fertilizer).
3.1.4. Characteristics of a good fertilizer
A good fertilizer should have the following characteristics:- It should contain the required nutrients, in such a form that they can be
assimilated by the plants.
- It should be cheap.
- It should be soluble in water.
- It should be stable, so that it may be available for a long time for the
growing plant.
- It should not be injurious to the plants.
- It should be able to correct the acidity of the soil.Application activity 3.1
Andrew is a farmer in rural village. He always used to spray insecticides in
the farm in order to kill insects and use different fertilizers while growing
crops. In his casual work, Andrew uses to combine fertilizers. After getting
advice from the Sector Agronomist, Andrew is suspecting that he has not
used well the fertilizers, contributed to the pollution of atmosphere and has
contributed to water pollution.i) Which fertilizers does Andrew combine while growing his crops and
why?
ii) Referring to the mistakes done above by Andrew, suggest to Andrew
the advice to follow while selecting fertilizer to be used.iii) A NPK fertilizer is labeled 13-13-13. Interpret this labeling.
3.2. Use of organic and inorganic fertilizers\
Activity 3.2
A plot of land has been divided into two parts and in both Irish potatoes has
been cultivated by two cultivators.
One of them harvested 2000 kg of Irish potatoes of big size and the otherharvested 50kg of Irish potatoes of small size.
Given that on both plots of land, the following work has been done at the
same time- Cultivation,Organic fertilizers contain only plant- or animal-based materials that are either
- planting of the same seeds
- weeding (or hoeing)
- spraying with the same chemicals
- Harvesting
i) Suggest reason(s) which caused the difference in the harvest.ii) Provide advice to the cultivator who harvested 50 Kg.
a byproduct or end product of naturally occurring processes, such as manures,
leaves, and compost. Inorganic fertilizer, also referred to as synthetic fertilizer, ismanufactured artificially and contains minerals or synthetic chemicals.
3.2.1. Organic Fertilizers
The use of organic fertilizer may have many advantages but also it may have
some disadvantages
a. Advantages- The manures add organic matter (called humus) to the soil which
restores the soil texture for better retention of water and for aeration of
soil. For example, organic matter present in the manures increases the
water holding capacity in sandy soils and drainage in clay soil.
- The organic matter of manures provides food for the soil organisms
(decomposers such as bacteria, fungi, etc.) which help in making nutrientsavailable to plants.
- Nutrient release: slow and consistent at a natural rate that plants are able
to use. No anger of over concentration of any element, since microbes
must break down the material.
- Trace minerals: typically present in a broad range, providing more balanced
nutrition to the plant.
- They will not burn: safe for all plants with no danger of burning due to salt
concentration.
- Long lasting: does not leach out since the organic matter binds to the soil
particles where the roots have access to it.
- Fewer applications required: once a healthy soil condition is reached, it is
easier to maintain that level with less work- Controlled growth: does not over-stimulate to exceptional growth whichb. Disadvantagescan cause problems and more work.
- Many organic products produce inconsistent results.3.2.2. Inorganic Fertilizers
- The level of nutrients present in organic fertilizer is often low.- The time of their preparation is too long.
The use of inorganic fertilizers may have many advantages but also it may havesome disadvantages.
a. Advantages- Chemical fertilizers are made with synthetic ingredients designed tob. Disadvantages
stimulate plant growth.
- Commercial chemical fertilizers have the advantage of predictability and
reliability
- Formulations are blended with accuracy and you can buy different blends
for different types of plants; commercial formulated fertilizers allow you
to know exactly which nutrients you’re giving your plants, rather thanguessing at the composition of organic formulas.
- They can burn plants
- They require a specific timetable of application and watering because of
fast release of nutrients- On groundwater, artificial fertilizers have the following disadvantages:
– Increased nitrate levels increase the risks of blue baby syndrome, a rare
form of anaemia which affects babies below 6 months of age. The cause
is the oxidation by nitrite ions of Fe2+ in haemoglobin to Fe3+. The oxidized
hemoglobin cannot bind oxygen, and the baby turns blue from lack of
oxygen. Conditions in the digestive tracks of young children are more
favorable to the bacteria which reduce nitrates to nitrites than those in
adults.
– Another hazard of chemical fertilizers is that carcinogenic nitrosoamines
(yellow oil substance) may be formed in the human digestive track by the
conversion of nitrate into nitrite. The nitrite produced in the stomach it
combines with HCl to produce nitrous acid. Nitrous acid can react with any
secondary amine in foods to form nitrosoamines and the reaction of nitritewith amino acids.
- Repeated use or excess use of the same fertilizer producing acidic ionsOther causes of acid soils include:
(NH4
+). Example of such a fertilizer is (NH4)
2SO4.
- Repeated use or excess use of the same fertilizer producing basic ions.
Example of such a fertilizer is CaCO3.
- Warm temperatures and high rain fall: Cations such as Ca++, Mg++, K+
which are essential to living organisms, are leached (dissolved) from the
soil profile, leaving behind more stable materials rich in Fe and Al oxides.This natural weathering process makes soils acid.
- Man-made processes also contribute significantly to soil acidity. For
example, Sulphur dioxide (SO2) and nitrogen oxides (NOx) released
primarily by industrial activities react with water to form acid rain, which
acidifies soils, particularly forest soils with.
- Organic acids from plants during decomposition;- CO2 from root respiration and microbial respiration.
Application activity 3.2
Jane is a farmer in rural village. He always used to spray insecticides in
the farm in order to kill insects and use different fertilizers while growing
crops. In his casual work, Jane uses to combine fertilizers and varies artificial
fertilizers according to plant crop she wants to grow. After getting advice
from the Sector Agronomist, Jane is now using the fertilizers appropriately.i) Provide the advantages of using combined fertilizers while growing
crops.ii) Why do the farmers use specific fertilizer on specific plant crop?
3.3. Dangers of the use of the substandard fertilizers
Activity 3.3
Agriculture practice contributes to pollution of atmosphere and waterpollution. Observe the photos below and answer related questions.
i) What is the situation of living things’ life on the picture above?
ii) In which way agriculture practice can lead to the consequences
observed on the photos?
iii) Suggest the advice to prevent the consequences observe on thephotos.
One of the problems with chemical fertilizers is they seep through the soil into
the groundwater and other water sources, leading to contamination. Now, NPK
in small quantities is non-toxic, but a lot can kill the balance of nature in various
ways. Nitrogen is especially tricky.
Sub-standard fertilizer means any fertilizer which does not conform to therequired NPK ratio.
Example: A fertilizer may be labeled 16-00-00, while the real NPK ratio is for
example 25-00-00, 10-00-05, etc
Using these Fertilizers can lead to:
- Soil pollution (basic soil or acidic soil) due to accumulation of ions which
are acidic or basic- Poor growth of plantsEffects of acid soil
- Poor harvest
- Eutrophication
- Fertilizer burn: leaf scorch resulting from over-fertilization, usually referring
to excess nitrogen salts. Fertilizer burn is the result of desiccation of plant
tissues due to osmotic stress, creating a state of hypertonicity.
Major effects of extremes in pH levels include gaps in nutrient availability andthe presence of high concentrations of minerals that are harmful to plants.
In very alkaline soil, certain micronutrients such as zinc and copper become
chemically unavailable to plants. In very acidic soil, macronutrients such as
calcium, magnesium and phosphorous are not absorbed while others reach
toxic levels,
Acid soil, particularly in the subsurface, will also restrict root access to water
and nutrients.
In addition to affecting how nutrients are dispensed to growing plants, pH levels
also influence microorganism activity that contributes to the decomposition
of organic materials. A neutral pH is ideal for microbial action that produces
chemical changes in soil, making nitrogen, sulfur and phosphorus more available.
A pH that is either too high or too low may also interfere with the effectiveness
of pesticides by changing their basic composition or weakening their ability to
kill unwanted insects.
Plant growth and most soil processes, including nutrient availability and microbialactivity, are favored by a soil pH range of 5.5 – 8.
Example: The optimal pH range for most plants is between 5.5 and 7.0 as it isshown in the table below.
For soils the pH should be maintained at above 5.5 in the topsoil and 4.8 in the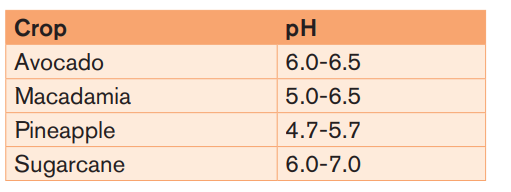
subsurface.
Eutrophication: the undesirable overgrowth of vegetation caused by high
concentration of plants nutrients (Nitrogen and Phosphorous) in bodies of water
(lakes, rivers, etc).
As consequence, water plants (e.g: water hyacinth: amarebe) grow more
vigorously and this prevents the sun light from reaching the water and stops
photosynthesis of aquatic plants which provide oxygen in the water to animals
needed then animals die, deposits of organic matter on the bottom of the lake
build up.
When lake water is enriched with nutrients (e.g.: nitrates and phosphates), algal
flourish, and produce an algae bloom, a green scum with an unpleasant smell.
When algal die they are decomposed by aerobic bacteria. When the oxygencontent falls too low to support aerobic bacteria, anaerobic bacteria take over.
They convert the dead matter into unpleasant-smelling decay products and
debris which falls to the bottom. Gradually, a layer of dead plant material builds
up on the bottom of the lake. The lowering of the oxygen concentration leads tothe death of aquatic animals (fish, crabs, etc).

In order to reduce the effects of substandard fertilizers different measure can
be taken:- Standardization of the fertilizer before use.
- Production of fertilizers in Rwanda, as this will help us to choose good
minerals (where necessary) in producing fertilizers.- Use of chemical fertilizers with coated pellets so that nutrients are releasedApplication activity 3.3slowly.
- Regular watering.
Fertilizers application normally results in increased yield with diminishing
returns until maximum yield is reached. A cultivator James is advised to
use NPK 17-17-17 in growing Irish potatoes. When James reached the
Agrotech, he missed NPK 17-17-17 and bought NPK 16-00-00 and he
used in excess to fit with the fertilizer he wanted.i) Were fertilizers used by James the same? How are they different?
ii) Show how using NPK 16-00-00 will be dangerous than using NPK
17-17-17.iii) What can be done to avoid or minimize those dangers?
Skills Lab 3
Making rich organic fertilizer
The best manure for gardens is properly composted manure. It’s often
called black gold, especially when it contains cow manure. Well rotted
farmyard manure is rich and full of slow releasing natural plant nutrients.
Procedure:
- Select an area in a farm that is protected from strong wind and sun,
for instance, under the shade of a tree.
- Mark the area you intend to locate the compost (the minimum area is
1.25m x 1.25m).
- Dig a shallow trench, same size as the compost heap 20cm deep.
Cover the sides of the trench with water or a mixture of water and
cow dung to prevent moisture and nutrients from leaking from the
compost heap. The shallow trench will become the foundation of
the compost heap. The trench also helps to hold moisture especiallyduring the dry season.
Foundation layer
- Put the dry plants material such as small tree branches, maize stalks
or sorghum stalks. Cut the plant material into small pieces. Spread
the dry material evenly over the bottom of the trench to make a layer
of 15-25cm. Sprinkle with water using a watering can or basin to
ensure all material is moist but not wet.
- Layer 1: put dry plant material such as grass, dry leaves mixed with
top soil, manure and ashes. The layer should be about 20-25cm
thick. Mix the material with soil, manure and ashes and sprinkle water
to make it moist.
- Layer 2: Make another layer of moist (green) material which is fresh or
wilted such as weeds or grass cuttings, stems and vegetable leaves,
tree branch leaves, damaged fruits, or vegetables or even kitchen
waste. Do not sprinkle water in this layer. But you can spread it to
remain even or flat.
- Layer 3: is composed of animal manure collected from fresh or
dried cow dung, chicken waste, donkey manure and sheep or goat
droppings. The animal manure can be mixed with soil, old compost
and some ashes to make a layer that is 5 -10 cm thick. Make a watery
mixture and spread it over as a thin layer about 1-2cm thick.
Covering layer: protect the heap from the sun or animals or anything
that might interrupt with the mixture. The cover should be sealed with
only the ventilation stick.
- Turning the compost: open up the compost heap mixing all the layers
while sprinkling water to make it moist but not wet after three weeks.
- Decomposition progress checking: using the ventilation or temperature
stick, you can keep on checking the decomposition process of your
compost every week by pulling out the stick. If it has a white substance
on it and has a bad smell, it means the decomposition is not going on
well. You can turn the compost further and sprinkle some more water
to make it moist.
- Ready compost: A mature compost heap is about the half the size
of the original heap. Check to ensure the compost has a dark brown
colour or black soil, which has a nice smell. All the original materialshould not be seen if the decomposition process went on well.
End Unit Assessment 3
I. Multiple choice (Choose the best answer)
1. If nitrogen is the main element of fertilizers then fertilizers are classified
asa) Structural fertilizers2. Increased ratio of chemical nutrients in ecosystem is classified as
b) Non-structural fertilizers
c) Nitrogen fertilizers
d) Respiratory fertilizersa) Triplication3. Greenhouse gas which can be emitted from storage of nitrogen-based
b) Eutrophication
c) Crystallization
d) Distillation
Fertilizers isa) Nitrous oxide4. Organic Fertilizers can be derived from
b) Nitric oxide
c) Oxygen
d) Hydroxidea) Animal materialsII. Open-ended questions
b) Carbon materials
c) Plant materials
d) Both (a) and (c)
1. Ammonia itself can be used as a fertilizer but has some disadvantages.
Explain the disadvantages of using ammonia as a fertilizer.
2. Give any two advantages of the use ofa) Natural Fertilizers3. Give any two causes of acid soils
b) Artificial Fertilizers
4. Discuss the advantages and disadvantages of the use of organic and
inorganic Fertilizers.
5. Identify the effects of misusing Fertilizers and the dangers of substandard
fertilizers.
REFERENCES
1. Barbara, A., Jean, B, Niamh, G., Douglas W. (2009). Biology, CK-12
Foundation, California, USA.
2. Benjamin Cummings. 8th Ed. San Francisco, US. Pp. 142-148.
3. Biggs.Hagins.Kapicka.Lundgren.Rillero. Tallman.Zike (2005) The Dynamics
of life, McGraw-Hill, USA
4. Campbell, N.A., Reece, J.B., Urry, A.L., Cain, L.M, Wasserman, A.S.,
Minorksy, V.P and Jackson, B.R. (2008). Biology. 8th edition. Pearson
international, San Francisco, USA.
5. Cram, D. and de Kretser, D. 2002 Genetic Diagnosis: the future, in C. Jonge
and C. Barratt (eds) Assisted
6. Fullick, A. (2006). GCSE Biology. AQA Science Exclusively endorsed and
approved by AQA. Nelson Thornes Ltd. Cheltenham
7. Fullick, A., Mwinshekhe, H., Maddu, M., and Nyabua, S. (2011). A level
Biology. Malaysia Ashton Editorial and Juice Creative, Ashton, Malasia.
8. Kent, M. (2000). Advanced biology. Anew mainstream text for the new
specifications. Oxford university press, New York, USA.
9. Margaret Kabiru and Anne Njenga. (2010) Health, nutrition and care,
Nairobi, Kenya.
10. Reproductive Technology: Accomplishments and New Horizons, Cambridge
University Press, Cambridge.
11. https://healthyeating.sfgate.com/cellular-respiration-making-bread-12156.
html
12. https://www.britannica.com/science/human-reproductive-system
13. https://www.nhs.uk/conditions/pregnancy-and-baby/antenatal-midwifecare-pregnant/
14. https: //www.google.rw/
15. s e a r c h ? e i = R Ya y X a y E M s G 8 9 Q O C 4 I 2 Q B A & q = P u b l i c +
health+services&oq=Public+health+services&gs_l=psy-ab.3.0i67j0i7i30l9.15205.15428.23076.0.2.0.1381.2544.7-2......0...
